Cirugía de derivación trabecular ab interno con microstent del canal de Schlemm (Hydrus) para el glaucoma de ángulo abierto
Referencias
References to studies included in this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: parallel, multicentre, single‐masked (participant), randomised controlled trial Unit of randomisation: participant | |
| Participants | Country: conducted at 12 sites in the United States and 8 international sites Total number of participants enrolled: 152 participants (152 eyes) Number of men and women: women 54.7% (Hydrus group), 58.4% (iStent group) Age range: men: 66.9, SD 10 (Hydrus group), 66.5, SD 9.5 (iStent group) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Intervention: Hydrus microstent (N = 75) | |
| Outcomes | Primary outcome Secondary outcomes
Safety outcomes
Length of follow up: 12 months | |
| Notes | Date conducted: Participants were randomised from March 2013 to May 2015. Funding source: Study sponsored by Ivantis, Inc., Irvine, California Declaration of interest: several study authors had received honoraria, grants, consulting fees from Ivantis Inc., as well as other companies. Trial ID: NCT02023242 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation was determined by a computer generated sequence stratified by site and prepared in advance by the study statistician in order to provide balanced study groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed in the operating room by opening a sequentially numbered envelope" Comment: not enough details on how the process was managed. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were masked |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "the investigator at each study site was not masked to treatment randomization during follow‐up examinations." |
| Incomplete outcome data (attrition bias) | Low risk | Two participants were lost in each group |
| Selective reporting (reporting bias) | Low risk | There appears to be no major difference between the published report and details on the protocol in ClinicalTrials.gov; however, visual field was obtained but not reported. |
| Methods | Study design: parallel, multicentre, single‐masked (participant), randomised controlled trial Unit of randomisation: participant | |
| Participants | Country: conducted at 26 sites in the United States and 12 international sites Total number of participants enrolled: 1143 Number (%) of men and women: women 55.8 (intervention group), 56.1 (comparator group) Age range: mean 71.1, SD 7.9 (intervention group), 71.2, SD 7.6 (comparator group) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Intervention: Hydrus microstent + CS with phacoemulsification (N = 369) | |
| Outcomes | Primary outcome
Secondary outcomes
Safety outcomes
Length of follow up: 24 months Adverse events reported: Yes | |
| Notes | Date conducted: Participants "were assessed for study eligibility between February 2012 and April 2015". Study first completion date was June 2017 Sources of funding: Study sponsored by Ivantis, Inc., Irvine, California Declaration of interest: Several study authors had received honoraria, grants, consulting fees from Ivantis Inc., as well as other companies. Trial ID: NCT01539239 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Upon confirmation, eyes were randomized to either Hydrus Microstent implantation (HMS group) or no microstent implantation (NMS group) using an online computer algorithm in a 2:1 allocation ratio." |
| Allocation concealment (selection bias) | Low risk | "Upon confirmation, eyes were randomized to either Hydrus Microstent implantation (HMS group) or no microstent implantation (NMS group) using an online computer algorithm in a 2:1 allocation ratio." |
| Blinding of participants and personnel (performance bias) | Low risk | "Study subjects remained masked to treatment assignment throughout the course of the study." |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The tonometry protocol utilized a 2‐person method: an observer and a reader who was masked to study treatment." "Despite multiple measures to minimize bias, it was not possible to mask the surgeon to treatment group during postoperative examinations." |
| Incomplete outcome data (attrition bias) | Unclear risk | "After accounting for the randomization ratio and 10% annual attrition, the study size was calculated to be 558 subjects." 556 participants were randomized (so trial investigators did try to avoid attrition bias). 3% were lost to follow‐up and 2% died or could not return owing to non‐study‐related critical illness. No details given on losses in each study arm or methods used to account for missing data. |
| Selective reporting (reporting bias) | Unclear risk | There appears to be no major differences between the published report and details on the protocol in ClinicalTrials.gov; however, worsening of visual field was not listed as an outcome measure, but was obtained since visual field loss by 2.5 dB or more was reported as an adverse event (4.3% for Hydrus and 5.3% for iStent). |
| Methods | Study design: Parallel, multicentre, randomised, single‐masked (participant), controlled clinical trial Unit of randomisation: participant Only 1 eye per participant was eligible for treatment, although both eyes could be screened for inclusion. "Before surgery, participants were washed out of all hypotensive medications in the study eye for a variable period, depending on the class of medication in use at the time of screening. The washout protocol is described in the Ocular Hypertension Treatment Study. At the completion of the washout, a preoperative baseline diurnal IOP (DIOP) value was obtained by averaging 3 Goldmann tonometry measurements obtained 4 hours apart between 8AM and 4PM. The tonometry protocol used a 2‐person system (an observer and a reader), and 2 readings were obtained at each time point during the day. If the difference in the 2 measurements was more than 2 mmHg, a third measurement was obtained. The average of 2 measurements or the median value of 3 was used for the time point, and the average of the IOP measurements at all 3 time points was the mean DIOP. The DIOP value was required to be between 21 and 36 mmHg for study inclusion." | |
| Participants | Country: study conducted at 7 European sites: Germany, Spain, the Netherlands, Italy Total number of participants randomised: N = 100 Number of men and women: 40% men (intervention Group), 58% (comparator group). Inclusion criteria: People with concurrent cataract and open‐angle glaucoma ("IOP of 24 mmHg or less with no more than 4 hypotensive medications, Shaffer grade III or IV chamber angle in all quadrants and Humphrey visual field changes characteristic of glaucoma or glaucomatous optic nerve damage confirmed by ophthalmoscopy and nerve fiber layer imaging"). Exclusion criteria: "Clinical exclusion criteria included angle‐closure glaucoma, secondary glaucomas except pseudoexfoliation or pigment dispersion syndromes, exudative age‐related macular degeneration (AMD), proliferative diabetic retinopathy, or significant risk of glaucomatous vision loss because of washout of IOP‐lowering medications. Anatomic exclusion criteria were narrow angle or other angle abnormality visible on gonioscopy, central corneal thickness of less than 480 mm or more than 620 mm, or clinically significant corneal dystrophy. participants with prior corneal surgery, argon laser trabeculoplasty, cycloablation, or any incisional glaucoma procedure, such as trabeculectomy, tube shunts, deep sclerectomy, or canaloplasty, also were excluded." | |
| Interventions | Intervention: Hydrus microstent + CS with phacoemulsification (Hydrus + CS) N = 50 | |
| Outcomes | Primary outcome
Secondary outcomes
Safety outcomes
Length of follow up: 24 months Intervals at which outcomes assessed: Follow‐up examinations were conducted per protocol at 1 day, 1 week, and 1, 3, 6, 12, 18, and 24 months. Loss to follow‐up Adverse events reported: Yes | |
| Notes | Date conducted: Participants randomised to the study from July 2011 to April 2012 Sources of funding: Study sponsored by Ivantis, Inc., Irvine, California Declaration of interest: The trial investigators have declared their financial disclosures in the trial report including financial support from Ivantis Inc.; Transcend; Glaukos, Innfocus and Alcon. Trial registration: NCT01818115 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ". . . were assigned randomly in a 1:1 ratio according to a computer‐generated listing just before surgery" |
| Allocation concealment (selection bias) | Low risk | Quote: ". . . were assigned randomly in a 1:1 ratio according to a computer‐generated listing just before surgery" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Subjects remained masked to treatment assignment for the course of the study." Comment: Single‐masked study where the personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | "Masking the surgeon to the assigned treatment was not possible, and because the microstent is visible on the slit lamp with gonioscopic examination, masking the treatment group from the IOP assessor during follow‐up visits also was not possible." |
| Incomplete outcome data (attrition bias) | Low risk | All participants lost to follow‐up were accounted for. |
| Selective reporting (reporting bias) | Low risk | Outcomes match those reported on ClinicalTrials.gov; however, Humphrey visual field was collected but not listed as an outcome measure and not reported in the manuscript. |
CS: cataract surgery
IOP: intraocular pressure
MDIOP: modified diurnal intraocular pressure
POAG: primary angle glaucoma
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial |
| Participants | People with cataract and open angle glaucoma |
| Interventions | Micro incision cataract surgery (MICS) phaco with Express P50 implant under scleral flap Phacoemulsification with the new trabecular stent (Hydrus) implant |
| Outcomes | Endothelial cell loss |
| Notes | Authors have been contacted but no response as yet |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A prospective, multicenter, randomized comparison of the Hydrus microstent to the iStent for lowering intraocular pressure in glaucoma patients undergoing cataract surgery |
| Methods | Randomised, parallel assignment, single‐masked (participant) |
| Participants | Listed locations: United States Total number of participants enrolled: 300 Age: 21 years and older Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Intervention: Hydrus microstent Comparator: iStent trabecular micro‐bypass stent |
| Outcomes | Primary outcome (current): IOP at 24 months following surgery Secondary outcome (current): proportion of eyes with IOP greater than 5 and less than or equal to 19 mmHg at 24 months Secondary outcome (original): proportion of participants requiring supplemental medication for pressure control at 12 months Other outcomes (current): loss of BCVA at 24 months Other outcomes (original): 1. Proportion of eyes with IOP greater than 5 and less than or equal to 19 mmHg at 12 months 2. Loss of BCVA at 12 months |
| Starting date | August 2011 |
| Contact information | Principal investigator: Iqbal K Ahmed, Canada |
| Notes | Sponsor: Ivantis Inc. |
BCVA: best‐corrected visual acuity
IOP: intraocular pressure
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion drop‐free: short‐term (6 to 18 months) Show forest plot | 2 | 639 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.39, 1.83] |
| Analysis 1.1  Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 1 Proportion drop‐free: short‐term (6 to 18 months). | ||||
| 2 Proportion drop‐free: medium‐term (18 to 36 months) Show forest plot | 2 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.40, 1.88] |
| Analysis 1.2  Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 2 Proportion drop‐free: medium‐term (18 to 36 months). | ||||
| 3 Mean change in IOP measured using Goldmann applanation tonometry: medium‐term (18 to 36 months) Show forest plot | 2 | 619 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐2.69, ‐1.31] |
| Analysis 1.3  Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 3 Mean change in IOP measured using Goldmann applanation tonometry: medium‐term (18 to 36 months). | ||||
| 4 Mean change in IOP‐lowering drops instilled per day: medium‐term (18 to 36 months) Show forest plot | 2 | 619 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.56, ‐0.27] |
| Analysis 1.4  Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 4 Mean change in IOP‐lowering drops instilled per day: medium‐term (18 to 36 months). | ||||
| 5 Proportion of participants requiring additional glaucoma surgery or laser Show forest plot | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.86] |
| Analysis 1.5  Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 5 Proportion of participants requiring additional glaucoma surgery or laser. | ||||
| 6 Adverse events: loss of 2+ VA lines Show forest plot | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.14, 1.50] |
| Analysis 1.6  Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 6 Adverse events: loss of 2+ VA lines. | ||||
| 7 Adverse events: IOP spike > 10 mmHg Show forest plot | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.12, 1.24] |
| Analysis 1.7  Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 7 Adverse events: IOP spike > 10 mmHg. | ||||
| 8 Adverse events: bleeding Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 8 Adverse events: bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion drop‐free: short‐term (6 to 18 months) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 1 Proportion drop‐free: short‐term (6 to 18 months). | ||||
| 2 Mean change in IOP measured using Goldmann applanation tonometry: short‐term (6 to 18 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 2 Mean change in IOP measured using Goldmann applanation tonometry: short‐term (6 to 18 months). | ||||
| 3 Mean change in IOP‐lowering drops instilled per day: short‐term (6 to 18 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 3 Mean change in IOP‐lowering drops instilled per day: short‐term (6 to 18 months). | ||||
| 4 Proportion of participants with IOP < 21 mmHg Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 4 Proportion of participants with IOP < 21 mmHg. | ||||

Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
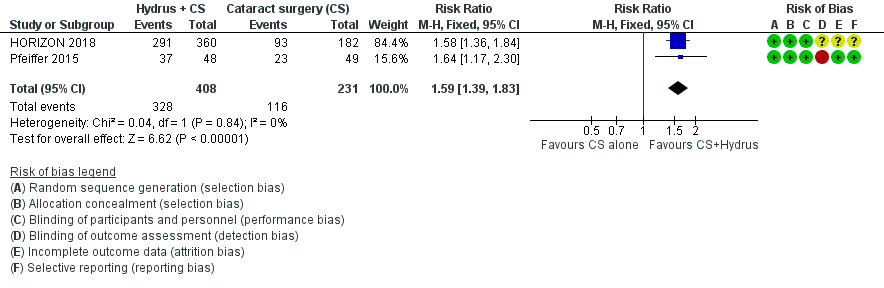
Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs. cataract surgery (CS) alone, outcome: 1.1 Proportion drop‐free: short term

Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs cataract surgery (CS) alone, outcome: 1.2 Proportion drop‐free: medium term
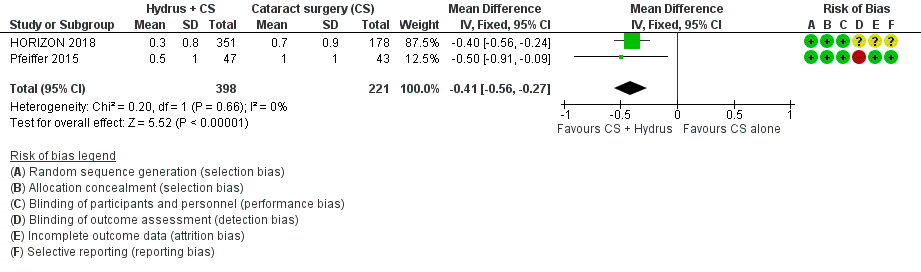
Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs cataract surgery (CS) alone, outcome: 1.4 Mean change in IOP‐lowering drops taken per day: medium term

Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs cataract surgery (CS) alone, outcome: 1.3 Mean change in IOP measured using Goldmann applanation tonometry: medium term

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 1 Proportion drop‐free: short‐term (6 to 18 months).
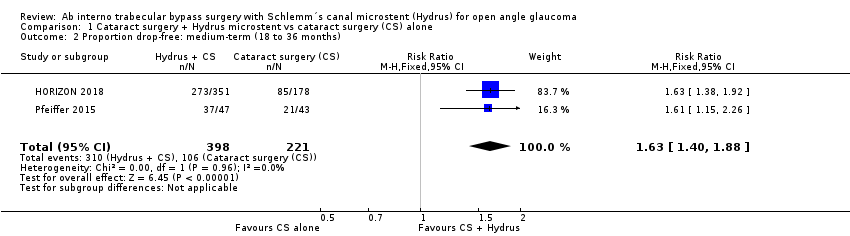
Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 2 Proportion drop‐free: medium‐term (18 to 36 months).
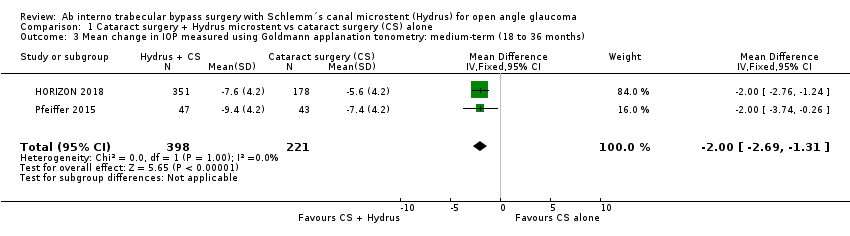
Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 3 Mean change in IOP measured using Goldmann applanation tonometry: medium‐term (18 to 36 months).
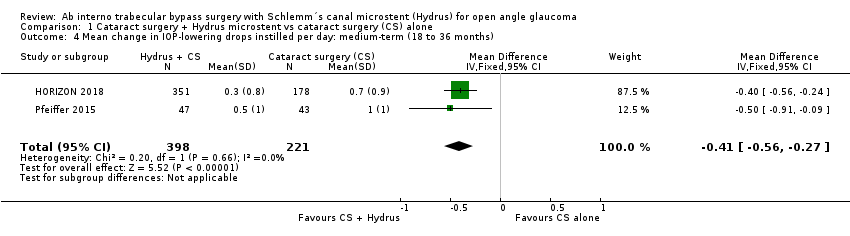
Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 4 Mean change in IOP‐lowering drops instilled per day: medium‐term (18 to 36 months).

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 5 Proportion of participants requiring additional glaucoma surgery or laser.
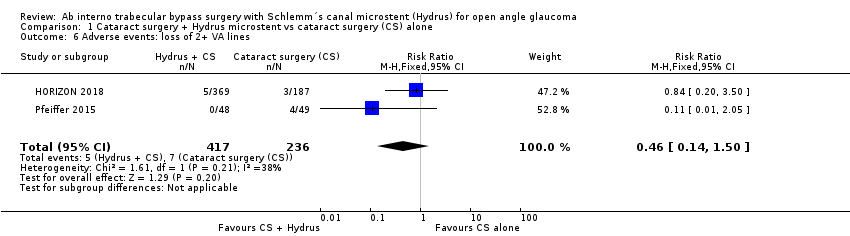
Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 6 Adverse events: loss of 2+ VA lines.

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 7 Adverse events: IOP spike > 10 mmHg.

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 8 Adverse events: bleeding.

Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 1 Proportion drop‐free: short‐term (6 to 18 months).

Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 2 Mean change in IOP measured using Goldmann applanation tonometry: short‐term (6 to 18 months).
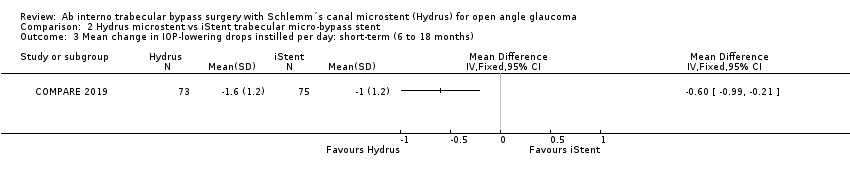
Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 3 Mean change in IOP‐lowering drops instilled per day: short‐term (6 to 18 months).

Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 4 Proportion of participants with IOP < 21 mmHg.
| Cataract surgery with Hydrus microstent compared to cataract surgery alone | ||||||
| Patient or population: people with cataracts and open angle glaucoma, many of whom had mild or moderate glaucoma, which was well‐controlled with medication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with cataract surgery alone | Risk with cataract surgery with Hydrus | |||||
| Proportion of participants who were medication‐free (not using eye drops) medium‐term follow‐up at 24 months | Study population | RR 1.63 | 619 | ⊕⊕⊕⊝ | ||
| 480 per 1000 | 782 per 1000 | |||||
| Mean change in unmedicated IOP (after washout) measured using Goldmann applanation tonometry medium‐term follow‐up at 24 months | The mean change in unmedicated IOP in the cataract surgery group was ‐5.95 mmHg | The MD in the cataract surgery plus Hydrus group was 2 mmHg lower | ‐ | 619 | ⊕⊕⊕⊝ | |
| Mean change in the number of IOP‐lowering drops instilled per day medium‐term follow‐up at 24 months | The mean change in the number of IOP‐lowering drops instilled per day in the cataract surgery group was ‐0.76 drops | The MD in the cataract surgery plus Hydrus group was 0.41 drops lower | ‐ | 619 | ⊕⊕⊝⊝ | |
| Proportion of participants who required further glaucoma surgery, including laser | Study population | RR 0.17 (0.03 to 0.86) | 619 | ⊕⊕⊝⊝ | ||
| 25 per 1000 | 4 per 1000 | |||||
| Visual field progression | No data available | |||||
| Mean change in health‐related quality of life | No data available | |||||
| Proportion of participants experiencing intraoperative or postoperative complications medium‐term follow‐up at 24 months | Intraoperative: device malposition (1.6%) or hyphaema obscuring the surgeons view (1.1%) only occurred with Hydrus implantation Postoperative: Intraocular bleeding, loss of 2 or more VA lines, and IOP spikes of 10 mmHg or more were rare in both groups. There were no cases of endophthalmitis in either group | ⊕⊕⊝⊝ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aUnclear or high risk of bias for most domains (‐1 for risk of bias) | ||||||
| Hydrus microstent compared to iStent trabecular micro‐bypass stent | ||||||
| Patient or population: people with open angle glaucoma, many of whom had mild or moderate glaucoma, which was well‐controlled with medication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with iStent | Risk with Hydrus | |||||
| Proportion of participants who were medication‐free (not using eye drops) short‐term follow‐up at 12 months | Study population | RR 1.94 | 148 | ⊕⊕⊝⊝ | ||
| 240 per 1000 | 466 per 1000 | |||||
| Mean change in unmedicated IOP (after washout) measured using Goldmann applanation tonometry short‐term follow‐up at 12 months | The mean change in unmedicated IOP in the iStent group was ‐5.1 mmHg | The MD in the Hydrus group was 3.1 lower | ‐ | 148 | ⊕⊕⊕⊝ | |
| Mean change in number of IOP‐lowering drops instilled per day short‐term follow‐up at 12 months | The mean change in the number of IOP‐lowering drops instilled per day in the iStent group was 0 | The MD in the Hydrus group was 0.6 lower | ‐ | 148 | ⊕⊕⊝⊝ | |
| Proportion of participants who required further glaucoma surgery, including laser | Study population | not analysed | 148 | ⊕⊝⊝⊝ | ||
| 0/74 | 2/76 | |||||
| Visual field progression | No data available | |||||
| Mean change in health‐related quality of life | No data available | |||||
| Proportion of participants experiencing intraoperative or postoperative complications short‐term follow‐up at 12 months | No intraoperative complications reported. Postoperative: no cases of intraocular bleeding or endophthalmitis in either group. Hydrus: 2/74 cases of VA loss of 2 or more lines, 3/74 IOP spikes > 10 mmHg iStent: 1/76 cases of VA loss of 2 or more lines, 4/76 IOP spikes > 10 mmHg | not analysed | 148 | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aUnmasked investigator | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion drop‐free: short‐term (6 to 18 months) Show forest plot | 2 | 639 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.39, 1.83] |
| 2 Proportion drop‐free: medium‐term (18 to 36 months) Show forest plot | 2 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.40, 1.88] |
| 3 Mean change in IOP measured using Goldmann applanation tonometry: medium‐term (18 to 36 months) Show forest plot | 2 | 619 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐2.69, ‐1.31] |
| 4 Mean change in IOP‐lowering drops instilled per day: medium‐term (18 to 36 months) Show forest plot | 2 | 619 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.56, ‐0.27] |
| 5 Proportion of participants requiring additional glaucoma surgery or laser Show forest plot | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.86] |
| 6 Adverse events: loss of 2+ VA lines Show forest plot | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.14, 1.50] |
| 7 Adverse events: IOP spike > 10 mmHg Show forest plot | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.12, 1.24] |
| 8 Adverse events: bleeding Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion drop‐free: short‐term (6 to 18 months) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Mean change in IOP measured using Goldmann applanation tonometry: short‐term (6 to 18 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Mean change in IOP‐lowering drops instilled per day: short‐term (6 to 18 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Proportion of participants with IOP < 21 mmHg Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

