Penyekat‐beta dan perencat sistem renin‐angiotensin aldosteron untuk kegagalan jantung kronik dengan ejeksi pecahan terpelihara
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: four arm factorial RCT Centres: not reported, assumed one, in Armenia Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: 12 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "III NYHA class chronic ischemic heart failure (CHF) patients (pts) with normal cholesterol who have preserved LV ejection fraction (PEF) and restrictive diastolic filling pattern" Exclusion criteria: not reported Randomised (N): 118 in total, of interest are: carvedilol, no simvastatin (N = 31) versus no carvedilol, no simvastatin (N = 28) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, unspecified): 64.5, 0.3 Sex (% men): not reported Ethnicity (%): not reported Systolic blood pressure: not reported Heart rate: not reported BMI: not reported Serum creatinine: not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF "preserved LV ejection fraction" but not defined NYHA class I (%): 0 NYHA class II (%): 0 NYHA class III (%): 100 NYHA class IV (%): 0 Hypertension: not reported Diabetes: not reported Atrial fibrillation: not reported Hospitalisation for heart failure: not reported Coronary heart disease: not reported Stroke: not reported Diuretic: not reported Digoxin: not reported Beta‐blockers: study drug ACEI: not reported ARB: not reported MRA: not reported | |
| Interventions | Intervention: carvedilol (up to 50 mg), simvastatin, carvedilol and simvastatin Comparator: not receiving carvedilol or simvastatin Concomitant medication: "in addition to ACE inhibitors, aldosterone antagonists and diuretics" | |
| Outcomes | Planned: not reported Reported: "prognosis, left ventricular (LV) diastolic function, plasma BNP level and inflammation status", "Assessment of relation of early (E) and late (A) diastolic filling velocities, deceleration time (DT) of E wave, levels of BNP, interleukin‐6 (IL‐6) and high sensitivity C‐reactive protein (CRP)", mortality, hospitalisation | |
| Notes | Two conference abstracts only. Comparison between carvedilol and no treatment was of interest for this review. No outcome data relevant to this review. Trialists were contacted; no response. Source of funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" but no details given |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | Published as conference abstracts only |
| Methods | Study design: parallel RCT Centres: 10 centres in Germany and Austria Start of enrolment: March 2007 End of enrolment: April 2011 Mean follow‐up: 11.6 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "men and women aged 50 years or older were eligible to participate in the study if they had current heart failure symptoms consistent with New York Heart Association (NYHA) class II or III, left ventricular ejection fraction (LVEF) of 50% or greater, echocardiographic evidence of diastolic dysfunction (grade I) or atrial fibrillation at presentation, and maximum exercise capacity (peak VO2) of 25 mL/kg/min or less." Exclusion criteria: "Major exclusion criteria included prior documented reduced left ventricular ejection fraction (LVEF 40%), significant coronary artery disease (current angina pectoris or ischemia on stress tests; untreated coronary stenosis 50%), myocardial infarction or coronary artery bypass graft surgery 3 months or less prior to enrolment, clinically relevant pulmonary disease (vital capacity 80% or forced expiratory volume in 1 second 80% of reference values on spirometry), significant laboratory abnormalities (potassium 5.1 mmol/L; hemoglobin 11 g/dL; hematocrit 33%; serum creatinine 1.8 mg/dL; or estimated glomerular filtration rate [eGFR] 30 mL/min/1.73 m2 , calculated using the Modification of Diet in Renal Disease formula: 186 [serum creatinine {in micromoles per liter}/ 88.4] 1.154 age [in years] 0.203 1.21 [if patient is black] 0.742 [if patient is female]), known contraindications for spironolactone or known intolerance to or therapy with a mineralocorticoid receptor antagonist within the last 3 months, concomitant therapy with a potassium‐sparing diuretic (eg, triamterene, amiloride), or potassium supplementation." Randomised (N): 422 (213 intervention, 209 control) Withdrawn (N): for reasons other than death 16 (6 intervention, 10 control) Lost to follow‐up (N): 5 (2 intervention, 3 control) Analysed (N): 422 (213 intervention, 209 control) Age (years, mean, SD): intervention: 67, 8; control: 67, 8 Sex (% men): intervention: 48; control: 47 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 135, 18; control: 135, 18 Heart rate (beats/min, mean, SD): intervention: 66, 14; control: 64, 12 BMI (mean, SD): intervention: 28.9, 3.6; control: 28.9, 3.6 Serum creatinine: not reported B‐type natriuretic peptide: not reported NT pro B‐type natriuretic peptide (pg/mL, median, IQR): intervention: 179, 81 to 276; control: 148, 80‐276 LVEF (%, mean, SD): intervention: 67, 8; control: 68, 7 NYHA class I (%): 0 NYHA class II (%): intervention: 85; control: 88 NYHA class III (%): intervention: 15; control: 12 NYHA class IV (%): 0 Hypertension (%): intervention: 92; control: 91 Diabetes (%): intervention: 17; control: 16 Atrial fibrillation (%): intervention: 6; control: 4 Hospitalisation for HF: (%): intervention: 38; control: 36 Coronary heart disease (%): intervention: 43; control: 37 Stroke (%): not reported Diuretic (%); intervention: 55; control: 52 Digoxin (%): not reported Beta‐blocker (%): intervention: 69; control: 75 ACEI (%): intervention: 78; control: 76 ARB (%): nor reported MRA (%): study drug | |
| Interventions | Intervention: spironolactone "The study drug could be decreased temporarily to 25 mg every other day for a potassium level greater than 5.2 mmol/L or in the presence of other reversible, non–life‐threatening adverse effects. For safety reasons, study medication was stopped for relevant hyperkalaemia (serum potassium 5.5 mmol/L) and/or hyperkalaemia‐associated clinical symptoms, significant renal impairment (serum creatinine 2.5 mg/dL; eGFR 20 mL/min/1.73m2), significant breast pain or gynaecomastia, or withdrawal of informed consent; rechallenge was encouraged wherever possible." "mean daily dose of spironolactone was 21.6 mg (95% CI, 20.8‐22.3 mg)" Comparator: matching placebo Concomitant medication: "Standard therapies for risk factor and symptom control were at the discretion of treating physicians and required to be unchanged within the 2 weeks prior to randomization." "concomitant therapy with a potassium‐sparing diuretic (eg, triamterene, amiloride), or potassium supplementation." | |
| Outcomes | Planned: primary outcomes: exercise capacity, left ventricular end‐diastolic pressure. Reported: all‐cause mortality, QoL, diastolic function, exercise capacity, "changes in echocardiographic measures of cardiac function and remodeling, measures of submaximal and maximal exercise capacity, serum biomarkers, and quality of life. Clinical tolerability was assessed as the safety end point. Morbidity and mortality (all‐cause and cardiovascular‐specific) were also predefined exploratory end points." | |
| Notes | Received outcome data for CV mortality, heart failure hospitalisation and hyperkalaemia from investigators. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Pocock minimisation algorithm". |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was implemented remotely via Internet/fax by the Coordination Center for Clinical Trials Leipzig." |
| Blinding of participants and personnel (performance bias) | Low risk | "Patients, the investigator team, individuals performing the assessments, and data analysts remained blinded to the identity of treatment until after database lock". |
| Blinding of outcome assessment (detection bias) | Low risk | "Patients, the investigator team, individuals performing the assessments, and data analysts remained blinded to the identity of treatment until after database lock". |
| Incomplete outcome data (attrition bias) | Low risk | ITT used, except for QoL. |
| Selective reporting (reporting bias) | Low risk | primary outcomes reported as planned, some secondary outcomes not reported as planned, eg all‐cause mortality, cardiovascular mortality. |
| Other bias | Low risk | "Production of identical matching placebo and quality control, packaging, labelling, storage, and dispensing of both spironolactone and placebo were performed by Allphamed PHARBIL." "This work was supported by the German‐Austrian Heart Failure Study Group and the German Competence Network of Heart Failure. AldoDHF was funded by the Federal Ministry of Education and Research Grant 01GI0205 (clinical trial program Aldo‐DHF [FKZ 01KG0506]). The University of Goettingen was the formal sponsor." "The sponsor and supporters of this study had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript." |
| Methods | Study design: RCT. Centres: 46 cardiology centres in Italy. Start of enrolment: September 2002. End of enrolment: July 2005. Follow‐up: 12 months. Run‐in period: not reported. | |
| Participants | Inclusion criteria: aged 18 to 80 years, established evidence of NYHA class II HF, stable, optimised therapy according to European Society of Cardiology criteria, and an LV ejection fraction (EF) ≤ 45%, as measured locally up to 6 months before enrolment. Exclusion criteria: creatinine 2.5 mg/dL; K 5.0 mEq/L; valvular heart disease amenable to surgical treatment; congenital heart disease; unstable angina or acute myocardial infarction or coronary revascularisation procedure within 3 months before enrolment; intravenous therapy with inotropic drugs within 3 months before enrolment; treatment with lithium salts, Kþ‐sparing diuretics, TNF‐a antagonists, or MRA during the last 3 months; history of resuscitated ventricular arrhythmias (unless this occurred within 24 h of a previous acute myocardial infarction or in subjects with an implantable cardioverter defibrillator); other clinical or general conditions contraindicating participation in a clinical trial. Randomised (N): 467 total (225 LVEF > 40%) (231 (116) intervention, 236 (109) control) Withdrawn (N): for reasons other than death 18 (14 intervention,4 control) Lost to follow‐up: not reported Analysed: not reported Age (years, mean, SD): intervention: 62.3, 9.5; control: 62.7, 9.5 Sex (% men): intervention: 81.8; control: 85.2 Ethnicity: not reported Systolic blood pressure (mmHg, mean, SD): intervention: 127.9, 16.2; control: 128.0, 17.2 Heart rate (beats/min, mean, SD): intervention: 68.0, 11.8; control: 65.7, 10.7 BMI (mean, SD): intervention: 26.7, 3.5; control: 26.9, 3.6 Serum creatinine (mg/dL, mean, SD): intervention: 1.1, 0.3; control: 1.1, 0.2 B‐type natriuretic peptide: not reported NT pro B‐type natriuretic peptide: not reported LVEF (%, mean, SD): intervention: 39.9, 8.6; control: 39.7, 8.6 NYHA class: not reported Hypertension (%): intervention: 48.5; control: 42.4 Diabetes (%): intervention: 20.9; control: 19.9 Chronic atrial fibrillation (%): intervention: 7.4; control: 8.5 Hospitalisation for heart failure (%): intervention: 44.6; control: 49.2 Coronary heart disease: not reported Stroke (%): intervention: 1.7; control: 3 Diuretic (%); intervention: 67.8; control: 72 Digoxin: not reported Beta‐blocker (%): intervention: 81.3; control: 77.5 ACEI (%): intervention: 84.9; control: 74.6 ARB (%): intervention: 12.1; control: 24.2 MRA (%): study drug | |
| Interventions | Intervention: canrenone. "The dose of 25 mg/o.d. of canrenone at randomization was increased to 50 mg/o.d. after the first month, if serum Kþ was 5 mEq/L, and in the absence of deterioration in renal function. During follow‐up, if serum Kþ increased up to 5 mEq/L and/or creatinine increased up to 2.5 mg/dL, the dosage of canrenone was reduced to 25 mg/o.d. Subjects requiring down‐titration of study medications were asked to return to the outpatient clinic within 2 weeks for a supplemental visit to evaluate the effectiveness of this change in therapy. If serum Kþ remained .5.5 mEq/L, or if creatinine was 3 mg/dL or had increased by over 1 mg/dL, the study medication was discontinued and the patient managed with conventional treatment only." Comparator: placebo Concomitant medication: "Aspirin, diuretics, digoxin, nitrates, antiarrhythmic agents, oral anticoagulants, and any other therapy were allowed when indicated by the local investigators." | |
| Outcomes | Planned: unclear Reported: "The pre‐specified primary endpoint was the change in echocardiographic LV end‐diastolic volume (LVEDV) over 12 months, measured centrally at the Echocardiographic Reading Centre. Secondary endpoints included changes in EF, estimated diastolic filling pressure, NYHA class, BNP, cardiac mortality, hospitalization for cardiac causes, and the combination of cardiac mortality and hospitalization for cardiac causes" | |
| Notes | Subgroup of participants of interest; response with outcome data for subgroup of participants LVEF > 40% received from trialists; baseline characteristics above are for all trial participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Low risk | double blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | echocardiography data were "read at the end of the study by one experienced independent observer who was blinded to all clinical data and treatment allocation" not reported for other outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT for all outcomes |
| Selective reporting (reporting bias) | Unclear risk | unable to assess as protocol and NCT record published/registered after enrolment completed |
| Other bias | Low risk | "The ANMCO Research Center coordinated the study, managed the data, and undertook analyses, under the supervision of the steering committee, who designed the AREA IN‐CHF study. The funding source (Therabel GiEnne Pharma SpA) had no role in the trial design, conduct, data collection, analyses and data interpretation." |
| Methods | Study design: parallel RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: 3 months Run‐in period: 2 mornings of control period | |
| Participants | Inclusion criteria: "New York Heart Association functional class III CHF associated with prior myocardial infarction and normal LV ejection fraction (>50%) who were able to perform a maximal treadmill exercise test were included in the study". Exclusion criteria: "No patient had valvular heart disease, systolic blood pressure ˜100 mm Hg, lung disease, hepatic disease or renal insufficiency." Randomised (N): 21 (10 intervention, 11 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SD): intervention: 80, 3; control: 79, 4 Sex (% men): 14.3 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 126, 12; control: 127, 10 Heart rate (beats/min, mean, SD): intervention: 85, 6; control: 84, 3 BMI not reported Serum creatinine not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 64, 9; control: 64, 7 NYHA class I (%): 0 NYHA class II (%): 0 NYHA class III (%): 100 NYHA class IV (%): 0 Hypertension not reported Diabetes not reported Atrial fibrillation not reported Hospitalisation for heart failure: not reported Coronary heart disease not reported Stroke not reported Diuretic (%): 100 Digoxin (%): 0 Beta‐blocker (%): 0 ACEI study drug ARB not reported MRA not reported | |
| Interventions | Intervention: enalapril. "The initial dose of enalapril was 2.5 mg/day, which was increased to 5 mg/day during week 2, to 10 mg/day (5 mg twice daily) during week 3, to 15 mg/day (7.5 mg twice daily) during week 4 and up to a maximum of 20 mg (10 mg twice daily) during week 5, tf tolerated. If the patient developed symptomatic hypotension or an increase in serum creatinine level, the dose of enalapril was reduced to the previous dose. At the time of the follow‐up studies, 3 months after beginning enalapril, the dose of enalapril was 2.5 mg/day in 1 patient, 5 mg/day in 1 patient, 10 mg/day in 3 patients, 1.5 mg/day in 2 patients, and 20 mg/day in 3 patients." Comparator: no treatment Concomitant medication: "All patients received diuretic treatment with furosemide for ˜2 weeks before the beginning of the study and a constant dose of furosemide during the study. Digitalis and other cardiac drugs (except enalapril) were not administered to any patient during the study." | |
| Outcomes | Planned: not reported Reported: NYHA class, blood pressure, heart rate, cardiothoracic ratio, treadmill exercise time, LVEF, peak mitral E/A ratio, left ventricular mass | |
| Notes | no outcome data relevant for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Chest roentgenograms were interpreted by a radiologist who was unaware of the study medication. M‐mode, 2‐ dimensional and pulsed‐wave Doppler echocardiograms were interpreted by an experienced echocardiographer (IK) who was unaware of the study medication. Treadmill exercise tests were performed under the guidance of the senior author who was aware of which patients were receiving enalapril." |
| Incomplete outcome data (attrition bias) | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | not reported |
| Other bias | Unclear risk | unable to assess |
| Methods | Study design: parallel RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: 32 months (intervention), 31 months (control) Run‐in period: not reported | |
| Participants | Inclusion criteria: "≥ 62 years of age with New York Heart Association functional class II or III CHF, prior Qwave myocardial infarction, and a LV ejection fraction ≥ 40% after 2 months of treatment with diuretics and ACE inhibitors were included in the study." Exclusion criteria: "No patient had valvular heart disease, systolic blood pressure < 100 mm Hg, lung disease with bronchospasm, hepatic disease, renal insufficiency, sinus bradycardia, greater than first‐degree atrioventricular block, or severe peripheral arterial disease." Randomised (N): 158 (79 intervention, 79 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): 158 (79 intervention, 79 control) Age (years, mean, SD): intervention: 81, 8; control: 81, 7 Sex (% men): intervention: 29; control: 30 Ethnicity (%): not reported Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide (pg/mL): LVEF (%, mean, SD): intervention: 56, 11; control: 57, 11 NYHA class I (%): 0 NYHA class II (%): intervention: 53; control: 51 NYHA class III (%): intervention: 47; control: 49 NYHA class IV (%): 0 Hypertension (%): intervention: 67; control: 65 Diabetes: not reported Atrial fibrillation (%): intervention: 33; control: 34 Hospitalisation for HF: not reported Coronary heart disease (%): 100 Stroke not reported Diuretic (%): 100 Digoxin (%): intervention: 33; control: 34 Beta‐blocker study drug ACEI (%): 100 ARB not reported MRA not reported | |
| Interventions | Intervention: propranolol. "The initial dose of propranolol was 10 mg/day. This dose was increased by 10‐mg increments at 10‐day intervals until a dose of 30 mg 3 times daily was given. All patients treated with propranolol received a final daily dose of propranolol of 30 mg 3 times daily." Comparator: no treatment Concomitant medication: "All patients continued diuretic and ACE inhibitor therapy during the study. Digoxin was administered only if the patient had atrial fibrillation." | |
| Outcomes | Planned: no published protocol or clinical trial registry entry Reported: "total mortality and total mortality plus nonfatal myocardial infarction" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | "LV ejection fraction and LV mass were interpreted by an experienced echocardiographer (IK) who was unaware of the study medications" |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT analyses |
| Selective reporting (reporting bias) | Unclear risk | not reported |
| Other bias | Unclear risk | funding not reported |
| Methods | Study design: RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Median follow‐up: 6 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "with New York Heart Association functional class II or III CHF associated with prior Q‐wave myocardial infarction, a normal LV ejection fraction ( 50%),7 and 30 ventricular premature complexes per hour detected by 24‐hour ambulatory electrocardiograms were included in the study." Exclusion criteria: not reported Randomised (N): 60 (30 intervention, 30 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): 53 completed study (27 intervention, 26 control) Age (years, mean, SD): intervention: 82, 8; control: 82, 7 Sex (% men): intervention: 27; control: 23 Ethnicity (%): not reported Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide not reported LVEF (%, median, IQR): intervention: 61, 7; control: 62, 6 NYHA class not reported Hypertension (%): intervention: 73; control: 70 Diabetes not reported Atrial fibrillation not reported Hospitalisation for HF: Coronary heart disease (%): 100 Stroke not reported Diuretic (%): 100 Digoxin not reported Beta‐blocker not reported ACEI study drug ARB not reported MRA not reported | |
| Interventions | Intervention: benazepril. up to 40 mg/day Comparator: no treatment Concomitant medication: not reported | |
| Outcomes | Planned: unclear Reported: decrease in number of ventricular premature complexes/h, decrease in ventricular couplets/h, decrease in number of runs of ventricular tachycardia/24 h | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The cardiologists interpreting the 24‐hour ambulatory electrocardiograms were blinded to the study medications" |
| Incomplete outcome data (attrition bias) | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess as we are unaware of published protocol or pre‐registered clinical trial registry entry |
| Other bias | Unclear risk | unable to assess |
| Methods | Study design: two‐arm, individual, placebo‐controlled RCT Centres: 8 sites in Germany Start of enrolment: January 2008 End of enrolment: December 2008 Follow‐up: 24 weeks Run‐in period: not reported | |
| Participants | Inclusion criteria: "Male or female patients of at least 45 years of age suffering from a non‐insulin dependent diabetes mellitus type 2 orally treated for at least 3 months and showing normotension or controlled hypertension with sitting systolic blood pressure (sSBP) < 140 mmHg and/or sitting diastolic blood pressure (sDBP) < 90 mmHg. Evidence of an abnormal left ventricular relaxation, diastolic distensibility or diastolic stiffness confirmed by echocardiography under the prerequisite of a preserved Left ventricular ejection fraction (LVEF) ≥ 45%. NT‐proBNP ≥ 250 pg/ml at baseline, NYHA class II or III in stable condition since 3 months, and standard HF‐ therapy with an ACE‐inhibitor alone or with further preparations in a constant regimen since at least 1 month (3 months in terms of β‐blockers). Signed written informed consent available." Exclusion criteria: The following criteria must not be met to enrol a single patient into the study: Impaired renal function (serum creatinine > 2.2 mg/dL or > 194 µmol/l), Known bilateral renal artery stenosis (RAS) or interventional treatment for RAS in the last year, State after kidney transplantation, Serum potassium > 5.5 mmol/l or HbA1C > 9.5 %, Cor pulmonale or primary pulmonary disease with dyspnea at rest, Known disposition to episodes of symptomatic hypotension or sSBP < 95 mmHg at baseline, Acute coronary syndrome or unstable angina pectoris and any coronary artery disease that was not stable during the last 3 months prior to inclusion, CABG or PTCA (incl. stent implantation) within 3 months before inclusion, Myocardial infarction or stroke within 6 months before inclusion, Patients who are dependent on a permanently paced pacemaker (i.e. a patient with a device that is not pacing during the echocardiographic examination can enter the study), Open heart surgery for other reasons than coronary revascularization, Tachycardia at rest > 100 bpm as confirmed by ECG‐recordings, Known clinically relevant rhythm disorders (e.g. tachyarrhythmias, salves of supraventricular or ventricular extrasystoles or atrial fibrillation without ventricular rate control) or symptoms suggesting a significant rhythm disorder (e.g. recurrent syncopes), Primary valvular diseases and/or restrictive or obstructive cardiomyopathy ‐ Existing ventricular assist devices, Relevant liver diseases (cholestasis or ALAT/ASAT > 2xULN or GT > 3xULN), History of primary hyperaldosteronism, of cancer in the last 5 years (exception: nonmetastasizing skin cancer) or of another wasting disease with life expectancy of < 2 years, Known hypersensitivity to Candesartan Cilexetil, Need for maintenance therapy with NSAIDs or Cox‐2‐inhibitors, Use of other ARBs throughout the entire study period, Any history of life‐threatening diseases, History of drug addiction and/or an extensive use of alcohol, Female patients who are pregnant or breast feeding, Sexually active women of childbearing potential not consistently and correctly practicing highly effective birth control with a low failure rate (less than 1% / year) such as implants, injectables, combined oral contraceptives, hormonal intrauterine devices (IUDs), sexual abstinence or vasectomised partner, Psychological and/or emotional problems, which render the informed consent invalid or limit the ability of the patient to comply with the study requirements, Patient is an employee or at least in dependence of the investigator and/or the sponsor or of another institution directly involved in the study or other trials under the investigator's direction, Participation in another clinical investigation within 30 days prior to enrolment or for the course of the present study (incl. studies for compassionate use or experimental medical devices) Randomised (N): 22 (11 intervention, 11 control) Withdrawn (N): for reasons other than death 14 (intervention: 3 premature study termination, 3 adverse events, 1 randomisation /enrolment error, control: 4 premature study termination, 2 adverse events, 1 randomisation / enrolment error) Lost to follow‐up (N): 0 Analysed (N): 22 (11 intervention, 11 control) Age (years, mean, SD): intervention: 67.0, 16.8; control: 69.0, 7.1 Sex (% men): intervention: 64; control: 55 Ethnicity (%): not reported Systolic blood pressure not reported Heart rate not reported BMI (mean, SD): intervention: 31.4, 5.0; control: 30.0, 5.7 Serum creatinine not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF not reported NYHA class: not reported Hypertension (%): not reported Diabetes (%): not reported Atrial fibrillation (%): not reported Hospitalisation for heart failure: not reported Myocardial infarction (%): not reported Stroke (%): not reported Diuretic (%): not reported Digoxin (%): not reported Beta‐blocker (%): not reported ACEI (%): not reported ARB (%): study drug MRA (%): not reported | |
| Interventions | Intervention: candesartan. 8‐32mg as tolerated. "The treatment comprised a titration period of 6 weeks and a period of constant study therapy of at least 18 weeks" Comparator: placebo Concomitant medication: "in an "added" regimen to a constant background‐HF‐therapy with at least ACE‐inhibitors (or further drugs) for the treatment of symptomatic heart failure with diastolic dysfunction in diabetic and hypertensive patients" exclusion criteria: use of other ARB | |
| Outcomes | Planned: primary: mean change from baseline for NT‐proBNP, secondary: QoL, kidney function, NYHA, body weight, BP and echocardiographic measures, adverse events, rate of premature withdrawals Reported: as planned except QoL | |
| Notes | This trial was terminated prematurely and the results are available via a clinical trial registry entry only. No outcome data relevant to this review reported (confirmed by sponsor Takeda via Email). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐blind" but no detail |
| Blinding of outcome assessment (detection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | no information |
| Selective reporting (reporting bias) | Low risk | all outcomes reported as planned except QoL |
| Other bias | High risk | "the study was terminated prematurely as a whole by the sponsor in December 2008 since randomization of patients was very poor until that date (low and slow recruitment (N = 42) with a high number (N = 20) of screening failures)" Takeda funded the study. The study results are unpublished and only available via the clinical trial registry entry. |
| Methods | Study design: parallel RCT Centres: 70, Italy Start of enrolment: December 2005 End of enrolment: May 2008 Mean follow‐up: 48 weeks Run‐in period: not reported | |
| Participants | Inclusion criteria: congestive HF, "Patients aged ≥18 years, of both genders, with stable symptomatic NYHA II‐IV HF and any LVEF measured at screening visit, and who provided a written informed consent were eligible. Patients with LVEF > 40% had to be hospitalized for cardiovascular events during the past 12 months before randomization." Exclusion criteria: "Exclusion criteria were history of prior treatment with ARBs within 2 weeks from screening; severe or malignant hypertension (SBP/DBP > 180/110 mmHg); symptomatic hypotension; prior acute myocardial infarction, stroke or transient ischemic attack (TIA), percutaneous transluminal coronary angioplasty (PTCA) or coronary artery by‐pass graft (CABG) within 1 month from screening; hemodynamically relevant arrhythmias or cardiac valvular defect; prior implant of pacemakers, cardiac resynchronization therapy or cardioverters within 6 months from randomization; constrictive pericarditis or active myocarditis; likelihood of cardiac surgical intervention during the overall treatment period; evidence of angina pectoris in the previous month; poorly controlled diabetes mellitus (blood glucose > 140 mg/mL or HbA1c > 8%); untreated thyroid dysfunction; renal artery stenosis; angio‐edema of any etiology; significant liver (AST, ALT, total bilirubin or alkaline phosphatase > 2x the upper limit of normal range) or renal impairment (serum creatinine > 2.0 mg/dL or serum potassium > 5.0 mmol/L); anemia of any etiology (Hb <10.5 g/dL) or any other clinically relevant hematological disease; pregnant or lactating females or females at risk of pregnancy; any disease with malabsorption; presence of any non‐cardiac disease that is likely to significantly shorten life expectancy; history of chronic alcohol or drug/substance abuse, or presence of other conditions potentially able to affect study subjects’ compliance; known allergy, sensitivity or intolerance to study drugs and/or study drugs’ formulation ingredients; patients unlikely to comply with the protocol or unable to understand the nature, scope and possible consequences of the study; participation in another trial in the month preceding study entry." Randomised (N): 128 Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SD): 66, 12 Sex (% men): 67.2 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): 134, 19 Heart rate (beats/min, mean, SD): 67, 14 BMI (mean, SD): 28.2, 4.5 Serum creatinine (mg/dL, mean, DS): 1.0, 0.3 B‐type natriuretic peptide (pg/mL): 163, 202 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): 48.7, 8.2 NYHA class I (%): 0 NYHA class II (%): 71.9 NYHA class III (%): not reported NYHA class IV (%): not reported Hypertension (%): 59.8 Diabetes (%): 28.1 Atrial fibrillation (%): 21.1 Hospitalisation for HF: not reported Coronary heart disease (%): 30.7 Stroke: not reported Diuretic (%): 86.7 Digoxin (%): 26.6 Beta‐blocker (%): 76.6 ACEI (%): 88.3 ARB (%): study drug MRA (%): 32.8 | |
| Interventions | Intervention: candesartan cilexetil, "1 candesartan cilexetil was administered at an initial dose of 4 mg o.d. (one tablet) and, if tolerated, it was up titrated to 8 mg (one tablet o.d.) after 2 weeks of treatment, to 16 mg (one tablet o.d.) after 4 weeks of treatment, and to 32 mg (two tablets of 16 mg o.d.) after 6 weeks of treatment" Comparator: no treatment Concomitant medication: ongoing standard therapy | |
| Outcomes | Planned: both trial register entries were post‐hoc, unclear what was planned Reported: primary: 3‐month (12‐week) changes of BNP from baseline, "The secondary objectives of the study after a 48‐week treatment period were to assess:1) change of BNP at 48 weeks from baseline values; 2) changes from baseline of aldosterone. Other exploratory analyses included (1) changes from baseline of LVEF, LVIDD, E wave peak velocity/A wave peak velocity (E/A), deceleration time of E wave (E‐DT), atrial dimensions; (2) changes from baseline of BP and heart rate (HR); (3) persistence of active treatment and discontinuation rate; (4) quality of life by Kansas City Cardiomyopathy Questionnaire (KCCQ)." | |
| Notes | Only subgroup of participants with LVEF > 40% of interest to this review. The above data are for this subgroup only. No outcome data reported for this review. Emailed trialists. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "centrally randomized" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | High risk | open label |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Thirty percent of all echocardiographic exams performed during the study were randomly selected and read at the core laboratory by an experienced cardiologist unaware of study group and visit." |
| Incomplete outcome data (attrition bias) | Unclear risk | unable to assess |
| Selective reporting (reporting bias) | Unclear risk | unclear as post‐hoc trial registration and published protocol not identified |
| Other bias | Unclear risk | The study was funded by Takeda Italia Farmaceutici and endorsed by the Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO). "The study was stopped before reaching the target number of patients since an interim analysis by the DSMB showed that an unacceptable number of patients (n=1500 per group) would have been needed to show the observed difference in 3‐month change of BNP with the predefined power of 0.80, when data on 371 patients were available" |
| Methods | Study design: parallel RCT Centres: 618, 26 countries (Australia, Belgium, Canada, Czech Republic, Denmark, Finland, France, Germany, Hungary, Iceland, Italy, Luxembourg, Malaysia, Netherlands, Norway, Poland, Portugal, Russia, Singapore, South Africa, Spain, Sweden, Switzerland, UK/Ireland, USA) Start of enrolment: March 1999 End of enrolment: July 2000 Median follow‐up: 36.6 months Run‐in period: no | |
| Participants | Inclusion criteria: "Eligible patients were aged 18 years or older, had New York Heart Association functional class II–IV of at least 4 weeks’ duration, had a history of hospital admission for a cardiac reason, and had LVEF higher than 40%." Exclusion criteria: Important exclusion criteria for any of the studies include current serum‐creatinine > 265mmol/L (> 3 mg/dL); current serum‐potassium > 5.5 mmol/L (> 5.5 mEq/L) or a history of marked ACE inhibitor‐induced hyperkalemia resulting in either a serum potassium greater than or equal to 6.0 mmol/L (>6.0 mEq/L) or a life‐threatening adverse event; known bilateral renal artery stenosis; current symptomatic hypotension; persistent systolic or diastolic hypertension; stroke, acute myocardial infarction, or open heart surgery within the last 4 weeks; previous heart transplant or heart transplant expected to be performed within the next 6 months; presence of any noncardiac disease (eg, cancer) that is likely to significantly shorten life expectancy to less than 2 years. Randomised (N): 3023 (1514 intervention, 1509 control) Withdrawn (N): for reasons other than death (270 intervention, 204 control) Lost to follow‐up (N): (2 intervention, 1 control) Analysed (N): 3020 (1512 intervention, 1508 control) Age (years, mean, SD): intervention: 67.2, 11.1; control: 67.1, 11.1 Sex (% men): intervention: 60.8; control: 59.0 Ethnicity (%): intervention: European 90.8 , control: European 92.3 Systolic blood pressure (mmHg, mean, SD): intervention: 136.0, 18.6; control: 136.3, 18.3 Heart rate (beats/min, mean, SD): intervention: 71.2, 12.4; control: 71.4, 12.5 BMI (mean, SD): intervention: 29.3, 5.9; control: 29.0, 5.6 Serum creatinine (mg/dL): not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 54.0, 9.4; control: 54.1, 9.4 NYHA class I (%): 0 NYHA class II (%): intervention: 61.5; control: 60.0 NYHA class III (%): intervention: 36.7; control: 38.7 NYHA class IV (%): intervention: 1.8; control: 1.3 Hypertension (%): intervention: 65.0; control: 63.6 Diabetes (%): intervention: 28.7; control: 28.0 Atrial fibrillation (%): intervention: 29.0; control: 29.3 Hospitalisation for heart failure (%): intervention: 69.6; control: 68.8 Coronary heart disease (%): intervention: 45.0; control: 43.7 Stroke (%): intervention: 9.2; control: 8.5 Diuretic (%); intervention: diuretic: 75.2, spironolactone 11.3; control: diuretic 74.3, spironolactone 12.0 Digoxin (%): intervention: 28.5; control: 27.2 Beta‐blocker (%): intervention: 55.9; control: 55.5 ACEI (%): intervention: 19.6; control: 18.6 ARB (%): study drug MRA (%): intervention: 11.3; control: 12.0 | |
| Interventions | Intervention: candesartan. "which could be started at 4 or 8 mg once daily, the assignment code being held by an independent centre and the data safety monitoring board. The treatment dose was doubled every 2 weeks, as tolerated, according to a forced titration protocol, with recommended monitoring of blood pressure, serum creatinine, and potassium. The target dose was 32 mg once daily from 6 weeks onwards." Comparator: "matching placebo" Concomitant medication: "physicians were free to prescribe all treatments other than angiotensin‐receptor blockers." "Initially, angiotensin converting‐enzyme inhibitors were not allowed as concomitant treatment, but after publication of the Heart Outcomes Prevention Evaluation trial results, (The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med 2000; 342: 145–53.) their use was optional in appropriate patients." "By the end of the study, 298 (20%) in the candesartan and 340 (23%) in the placebo group were receiving angiotensin‐converting‐enzyme inhibitors, 712 (47%) and 748 (50%) were receiving blockers, and 136 (9%) and 201 (13%) were receiving spironolactone. Non‐study angiotensin‐receptor blockers were used in 3% of patients in each of the two groups." | |
| Outcomes | Planned: Planned: "The primary outcome was cardiovascular death or unplanned admission to hospital for the management of worsening CHF. Prespecified secondary outcomes were: cardiovascular death, admission to hospital for CHF, or non‐fatal myocardial infarction; cardiovascular death, admission to hospital for CHF, non‐fatal myocardial infarction, or non‐fatal stroke; cardiovascular death, admission to hospital for CHF, non‐fatal myocardial infarction, non‐fatal stroke, or coronary revascularisation; death (any cause) or admission to hospital for CHF; and development of new diabetes." Reported: as planned | |
| Notes | The CHARM program consisted of 3 strands, one of which was CHARM‐Preserved. Contacted investigators for end scores of MLHF QoL by treatment arms. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "We randomly assigned patient" |
| Allocation concealment (selection bias) | Low risk | "the assignment code being held by an independent centre and the data safety monitoring board" |
| Blinding of participants and personnel (performance bias) | Low risk | "We randomly assigned patients, in a double‐blind way", "matching placebo" |
| Blinding of outcome assessment (detection bias) | Low risk | "A committee unaware of treatment assignment and which component of the CHARM programme was being undertaken adjudicated the cause of death, first myocardial infarctions, and first hospital admissions for heart failure." "All final data analyses were done by the sponsor and verified independently by the statistical centre at the London school of Hygiene and Tropical Medicine, London, UK" |
| Incomplete outcome data (attrition bias) | Low risk | "two patients who mistakenly received randomisation numbers but had no other data recorded and never received study medication" "Two candesartan patients and one placebo patient were lost to follow‐up" 207/204 withdrew due to AE "Anaysis was done by intention to treat." |
| Selective reporting (reporting bias) | Low risk | outcomes reported as planned |
| Other bias | Low risk | "MA Pfeffer, K Swedberg, CB Granger, JJV McMurray, and S Yusuf have served as consultants to or received research grants from AstraZeneca and other major cardiovascular pharmaceutical companies. J Östergren has served as a consultant and received research grants from AstraZeneca. P Held, E L Michelson, and B Olofsson are employees of AstraZeneca." "This study was supported by AstraZeneca R&D, Mölndal, Sweden" |
| Methods | Study design: multicenter, double‐blind, placebo controlled, randomised, parallel group trial Centres: 12 in 8 European countries Start of enrolment: not reported End of enrolment: not reported Follow‐up: 6 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "To be included into the study, patients had to fulfil the following criteria: willing and able to sign the informed consent form and comply with the requirements of the study, aged ≥ 40 years, have a documented history of HF and persistent symptoms during effort [New York Heart association (NYHA) class II–III ], an LVEF ≥ 45%, and LV end‐diastolic internal diameter < 3.2 cm/m2 or LV end‐diastolic volume index < 102 mL/m2 by echocardiography, radionuclide ventriculography, or nuclear magnetic imaging, or any abnormality of LV diastolic function documented by echocardiography, according to the guidelines of the European Study Group on Diastolic Heart Failure. This last inclusion criterion was revised in April 2007 following the online publication of the new consensus statement on the diagnosis of HFPEF by the European Society of Cardiology. Accordingly, an E/E ratio > 15 at tissue Doppler echocardiography was required as an inclusion criterion. Patients with an E/E‘ ratio between 8 and 15 could be included when additional abnormalities of diastolic function were found. These included an E/A ratio < 0.5 and/or a deceleration half‐time > 280 ms in patients older than 50 years, and/or a duration of reverse pulmonary vein atrial systole flow–mitral valve atrial wave flow > 30 ms, and/or a left atrial volume index > 40 mL/m2 , and/or an increased LV mass index" Exclusion criteria: "Major exclusion • Patients unable to perform 6‐mi walking test • Planned invasive cardiac procedures or cardiac surgery during the time of the study • Recent (< 3 months) acute coronary syndrome or stroke • Exercise‐induced myocardial ischaemia as main cause of exercise limitation as shown by symptoms (angina) or by previous exams (exercise test, stress echocardiography or myocardial scintigraphy) • Concomitant diseases (COPD, peripheral vasculopathy, orthopaedic disease) as main cause of exercise limitation • Major contraindications to beta‐blocker therapy (sinus bradycardia, \50/min; atrio‐ventricular block, bronchial asthma sensitive to beta‐agonists administration) • Ongoing treatment with beta‐blockers, diltiazem or verapamil • Systolic blood pressure \100 mm Hg • Pregnancy, breast feeding or childbearing potential during the study • History of alcohol or other illicit drug abuse • Expected poor compliance to drug therapy • Participation in any other clinical trial with an investigational product or scheduled to receive any such product during the study or in the 4 weeks following the study • Suffering from any other medical condition that may exclude the patient for safety reasons or interfere with the objective of the study." Randomised (N): 116 (57 intervention, 59 control) Withdrawn (N): for reasons other than death 22 (14 intervention (9 lack of tolerance, 1 protocol violation, 3 consent withdrawal, 1 other), 8 control (consent withdrawal 1, protocol violation 5, 2 other)) Lost to follow‐up (N): 1 (1 intervention, 0 control) Analysed (N): 93 (42 intervention, 51 control) Age (years, mean, SD): intervention: 66.5, 9.8; control: 65.3, 11.3 Sex (% men): intervention: 35; control: 36 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 128, 17 (Table 3 of Conraads), 134, 21 (Table 2); control: 129, 23 (Table 3) 133, 18 (Table 2) Heart rate (beats/min, mean, SD): intervention: 76, 15 (Table 3) 73, 14 (Table 2); control: 78, 13 (Table 3), 73, 11 (Table 2) BMI (mean, SD): intervention: 30.3, 4.5; control: 30.2, 4.9 Serum creatinine (mg/dL, mean, SD): intervention: 88.5, 33.1; control: 85.8, 25.1 B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL, median, range): intervention: 147 (9‐3577); control: 126 (15‐2055) LVEF (%, mean, SD): intervention: 61.9, 7.8; control: 63.2, 9.2 NYHA class I (%): 0 NYHA class II (%): intervention: 77; control: 78 NYHA class III (%): intervention: 21; control: 22 NYHA class IV (%): 0 Hypertension (%): intervention: 86; control: 86.4 Diabetes (%): intervention: 21; control: 20 Atrial fibrillation (%): not reported Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 17; control: 20 Stroke (%): not reported Diuretic (%); intervention: 49; control: 54 Digoxin (%): not reported Beta‐blocker (%): study drug ACEI (%): intervention: 75; control: 80 ARB (%): not reported MRA (%): not reported | |
| Interventions | Intervention: nebivolol. "Nebivolol was started at 2.5 mg/day and gradually up‐titrated to 10 mg/day over a period of 5 weeks. Down‐titration to lower doses was allowed if the higher dose was not tolerated. Treatment at maintenance doses was continued for an additional 21 weeks (6 months of treatment in total)." Comparator: placebo Concomitant medication: "Ongoing treatment with other drugs was maintained throughout the study." | |
| Outcomes | Planned: " The primary endpoint of the study is the change from baseline in the distance walked during the 6‐min walking test (6MWT) after 6 months of treatment with nebivolol versus placebo. Additional secondary endpoints are the changes from baseline after 6 months, with nebivolol versus placebo, in the following measurements: • Symptoms, assessed using a five‐level scale (extremely Reported: as planned | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated 1:1 randomization" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐blinded" |
| Blinding of outcome assessment (detection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Low risk | reasons provided for withdrawal |
| Selective reporting (reporting bias) | Low risk | reported as planned |
| Other bias | Low risk | The trial is funded by a grant from Menarini. "We thank Joachim Klinger Director of Data Management and Statistics, Harrison Clinical Research Deutschland, and Lieven Huysse, who worked at Menarini at the time of the study, for management and statistical support." |
| Methods | Study design: three‐arm, parallel RCT Centres: multicentre, no details Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: 1 year Run‐in period: none | |
| Participants | Inclusion criteria: "The inclusion criteria were age .18 years, clinical history of heart failure within 2 months prior to screening including a chest x ray demonstrating pulmonary congestion, NYHA functional class II – IV, left ventricular ejection fraction .45% by 2D‐echocardiography or a radionuclide technique, and therapy with diuretics with stable dose .14 days prior to recruitment." Exclusion criteria: "NYHA functional class I, myocardial infarction within 3 months, unstable angina within 1 month, significant valvular heart disease, uncontrolled hypertension, serious cardiac arrhythmias, concurrent therapy with calcium channel antagonist, b‐blockers (a‐methyl dopa was used for treating hypertension if required), positive inotropic agents (except digoxin for control of atrial fibrillation) and other angiotensin converting enzyme inhibitors or receptor blockers." Randomised (N): 151 ( intervention R: 45, intervention I: 56, control: 50) Withdrawn (N): for reasons other than death: intervention R: 6 (4 persistent irritating cough, 1 uncontrolled blood pressure, 1 refused to continue), intervention I: 1 due to onset of fast atrial fibrillation, control: 3 (1 uncontrolled high blood pressure, 1 defaulted, 1 refused to continue) Lost to follow‐up (N): 0 Analysed (N): 151 (intervention R: 45, intervention I: 56, control: 50) Age (years, mean, SD): intervention R: 74, 6.1; intervention I: 75, 8.5; control: 73, 8.4 Sex (% men): intervention R: 40; intervention I: 34; control: 42 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention R: 143, 22; intervention I: 145, 19; control: 145, 23 Heart rate (beats/min, mean, SD): intervention R: 79, 13; intervention I: 77, 9; control: 76, 14 BMI (mean, SD): intervention R: 26.8, 3.9; intervention I: 27.2, 4.1; control: 26.8, 4.2 Serum creatinine: not reported B‐type natriuretic peptide (pg/mL, mean, SEM): intervention R: 488, 701; intervention I: 568, 757; control: 566, 944 NT pro B‐type natriuretic peptide: not reported LVEF (%, median, IQR): intervention R: 65, 1; intervention I: 66, 1; control: 69, 2 NYHA class I (%): 0 NYHA class II (%): intervention R: 66.7; intervention I: 67.9; control: 72 NYHA class III (%): intervention R: 33.3; intervention I: 30.4; control: 28 NYHA class IV (%): 0 Hypertension (%): intervention R: 73; intervention I: 71; control: 76 Diabetes (%): intervention R: 22; intervention I: 18; control: 20 Atrial fibrillation (%): intervention R: 16; intervention I: 21; control: 10 Hospitalisation for HF: 100% as it was an inclusion criteria Coronary heart disease (%): intervention R: 18; intervention I: 11; control: 18 Stroke (%): not reported Diuretic (%): intervention R: Hydrocholorthiazide 8.9 furosemide 80 , dyazide 2.2; intervention I: Hydrocholorthiazide 10.7, furosemide 80.4 , dyazide 10.7; control: Hydrocholorthiazide 6, furosemide 68 , dyazide 12 Digoxin (%): not reported Beta‐blocker (%): 0 ACEI (%): study drug (R) ARB (%): study drug (I) MRA (%): not reported | |
| Interventions | Intervention R: "Ramipril was started at 2.5 mg daily and similarly titrated to 10 mg daily" Intervention I: "The initial dose of irbesartan was 18.75 mg daily which was titrated at 4 and 8 weeks to 75 mg daily." Comparator: usual care, "continue with diuretics alone" Concomitant medication: "Exclusion criteria were: ...concurrent therapy with calcium channel antagonist, b‐blockers (a‐methyl dopa was used for treating hypertension if required), positive inotropic agents (except digoxin for control of atrial fibrillation) and other angiotensin converting enzyme inhibitors or receptor blockers." | |
| Outcomes | Planned: planned as per clinical trial registry entry: primary: 1. Number of hospital admissions for heart failure or mortality 2. Quality of life assessed by the Minnesota Quality of life Questionnaire 3. In ambulatory patients the exercise duration assessed by 6 min corridor walk test. Secondary: The incidence of side‐effects, effect on levels of natriuretic peptides, effect on doppler‐echocardiographic derived measurements of left ventricular diastolic function. Reported: cardiovascular mortality, hospitalisation for heart failure, all‐cause mortality, quality of life, 6MWT, blood pressure, NT‐proBNP, peak early diastolic mitral annular velocities, peak systolic velocity, LV mass | |
| Notes | retrospective clinical trial registration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated using computer‐generated random numbers in blocks of 10" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | High risk | "open‐label" |
| Blinding of outcome assessment (detection bias) | Low risk | "All outcomes were reviewed blind to treatment allocation." "with blinded end point design" |
| Incomplete outcome data (attrition bias) | Unclear risk | unable to assess |
| Selective reporting (reporting bias) | Unclear risk | retrospective clinical trial registration, no published protocol identified |
| Other bias | Unclear risk | "None of the authors received any lecture, advisory board, or consultancy fees relating to this study from the sponsors." "This study was initially supported by a small grant from the manufacturers of Irbesartan, who also donated the irbesartan medication (Sanofi‐Synthelabo). Design, conduct, retention of data, analysis and writing were all entirely independent and carried out by the authors only. Data were kept at the Chinese University of Hong Kong and are available for public scrutiny." |
| Methods | Study design: parallel RCT Centres: 293 centres in 25 countries (Argentina, Australia, Belgium, Brazil, Canada, Czech Republic, Denmark, France, Germany, Greece, Hungary, Ireland, Italy, Mexico, The Netherlands, Norway, Poland, Portugal, Russia, South Africa, Spain, Sweden, Switzerland, UK, USA) Start of enrolment: June 2002 End of enrolment: April 2005 Mean follow‐up: mean follow‐up time was 49.5 months, and the trial included 16,798 patient‐years of follow‐up Run‐in period: "Eligible patients were treated with single‐blind placebo for 1 to 2 weeks before randomization" | |
| Participants | Inclusion criteria: "All patients were at least 60 years of age and had heart failure symptoms and a left ventricular ejection fraction of at least 45%. In addition, we required patients to have been hospitalized for heart failure during the previous 6 months and have current New York Heart Association (NYHA) class II, III, or IV symptoms with corroborative evidence; if they had not been hospitalized, they were required to have ongoing class III or IV symptoms with corroborative evidence. Such evidence could include findings of pulmonary congestion on radiography, left ventricular hypertrophy or left atrial enlargement on echocardiography, or left ventricular hypertrophy or left bundle‐branch block on electrocardiography. Treatment with an angiotensin‐converting–enzyme (ACE) inhibitor was permitted only when such therapy was considered essential for an indication other than uncomplicated hypertension." Exclusion criteria: "Exclusion criteria included previous intolerance to an angiotensin‐receptor blocker; an alternative probable cause of the patient’s symptoms (e.g. significant pulmonary disease); any previous left ventricular ejection fraction below 40%; a history of acute coronary syndrome, coronary revascularization, or stroke within the previous 3 months; substantial valvular abnormalities; hypertrophic or restrictive cardiomyopathy; pericardial disease; cor pulmonale or other cause of isolated right heart failure; a systolic blood pressure of less than 100 mm Hg or more than 160 mm Hg or a diastolic blood pressure of more than 95 mm Hg despite antihypertensive therapy; other systemic disease limiting life expectancy to less than 3 years; substantial laboratory abnormalities (such as a hemoglobin level of less than 11 g per deciliter, a creatinine level of more than 2.5 mg per deciliter [221 µmol per liter], or liver‐function abnormalities); or characteristics that might interfere with compliance with the study protocol" Randomised (N): 4128 (2067 intervention, 2061 control) Withdrawn (N): for reasons other than death 1368 (702 intervention, 684 control) Lost to follow‐up (N): 73 (29 intervention, 44 control) Analysed (N): 4128 (2067 intervention, 2061 control) Age (years, mean, SD): intervention: 72, 7; control: 72, 7 Sex (% men): intervention: 41; control: 39 Ethnicity (%): intervention: white 94, control: white 93 Systolic blood pressure (mmHg, mean, SD): intervention: 137, 15; control: 136, 15 Heart rate (beats/min, mean, SD): intervention: 72, 11; control: 71, 10 BMI (mean, SD): intervention: 29.7, 5.2; control: 29.6, 5.3 Serum creatinine (mg/dL, mean, SD): intervention: 1.0, 0.32; control: 1.0, 0.34 B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL, median, IQR): intervention: 360, 139–987; control: 320, 131–946 LVEF (%, mean, SD): intervention: 59, 9; control: 60, 9 NYHA class I (%): 0 NYHA class II (%): intervention: 21; control: 22 NYHA class III (%): intervention: 77; control: 76 NYHA class IV (%): intervention: 3; control: 3 Hypertension (%): intervention: 89; control: 88 Diabetes (%): intervention: 28; control: 27 Atrial fibrillation (%): intervention: 29; control: 29 Hospitalisation for heart failure in the last six months (%): intervention: 44; control: 44 Myocardial infarction (%): intervention: 24; control: 23 Stroke or TIA (%): intervention: 10; control: 10 Diuretic (%): intervention: loop: 52, thiazide: 38, spironolactone 15; control: loop: 52, thiazide: 38, spironolactone 15 Digoxin (%): intervention: 14; control: 13 Beta‐blocker (%): intervention: 59; control: 58 ACEI (%): intervention: 26; control: 25 ARB (%): study drug MRA (%): intervention: 15; control: 15 | |
| Interventions | Intervention: irbesartan. "Patients were started on 75 mg of irbesartan or placebo once daily. The dose was doubled to 150 mg after 1 to 2 weeks and was doubled again to 300 mg after an additional 1 to 2 weeks, according to a forced‐titration protocol as tolerated." "At the end of the titration phase, 84% of the patients in the irbesartan group and 88% of those in the placebo group had reached the 300‐mg dose (mean doses, 275 mg and 284 mg, respectively)." Comparator: "matching placebo" Concomitant medication: "During the study, the proportion of patients receiving an ACE inhibitor rose from 25% in the two groups at baseline to 39% in the irbesartan group and 40% in the placebo group, the use of spironolactone rose from 15% in the two groups at baseline to 28% in the irbesartan group and 29% in the placebo group, and the use of beta‐blockers rose from 59% in the irbesartan group and 58% in the placebo group to 73% in the two groups." | |
| Outcomes | Planned: "The primary end point is defined as time from randomization to the first occurrence of the composite outcome of death (all cause) or cardiovascular hospitalization. [...] The endpoint additionally includes myocardial infarction or stroke occurring during any hospitalization at any point during the study. Secondary endpoints include the effect of irbesartan as compared with placebo in reducing the risk of: cardiovascular death, all‐cause mortality, combined vascular endpoint: cardiovascular death, nonfatal myocardial infarction (MI) or nonfatal stroke; or combined HF endpoint: HF mortality or hospitalizations; [...] quality of life as measured by the Minnesota Living with Heart Failure questionnaire, change in New York Heart Associatioin (NYHA) functional class, change in global assessment of symptoms, N‐terminal B‐type natriuretic peptide levels in blood." (Carson 2005) Reported: all planned outcomes reported | |
| Notes | Emailed trialists to enquire about differing data in different publications and to ask for subgroup data. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using an automated, central randomization system" |
| Allocation concealment (selection bias) | Low risk | "The randomization schedule was implemented with the use of an interactive voice‐response system." |
| Blinding of participants and personnel (performance bias) | Low risk | "All investigators and committee members who were involved in the conduct of the study (except for members of the data and safety monitoring board) were unaware of study‐group assignments." "double‐blind" (Carson 2005) "matching placebo" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "blinded review of event rates in 2004" but blinding of event adjudication not specified overall |
| Incomplete outcome data (attrition bias) | Low risk | "At the end of the study, vital‐status data were not available for 29 patients (1%) in the irbesartan group and 44 patients (2%) in the placebo group. If contact could not be made at end of study, data for these patients were censored from the analysis at the date they were last known to be alive." "Data from all patients who underwent randomization were analyzed according to the intention‐to‐treat principle." |
| Selective reporting (reporting bias) | Low risk | all planned outcomes reported (comparison between published protocol (Carson 2005) and main results paper (Massie 2008) |
| Other bias | Low risk | "Dr. Massie reports receiving grant support from Bristol‐Myers Squibb, Sanofi‐Aventis, and Merck, consulting fees from Bristol‐ Myers Squibb, Sanofi‐Aventis, Merck, Duke Clinical Research Institute, Momentum Research, Novartis, GlaxoSmithKline, Scios–Johnson & Johnson, Corthera, and Niles Therapeutics, and lecture fees from Merck; Dr. Carson, receiving consulting fees from Bristol‐Myers Squibb, Sanofi‐Aventis, and Merck and lecture fees from AstraZeneca and Novartis; Dr. McMurray, receiving support from Bristol‐Myers Squibb (to Glasgow University) for his work on this trial; Dr. Komajda, receiving consulting fees from Bristol‐Myers Squibb and Servier and lecture fees from Servier, Sanofi‐Aventis, and AstraZeneca; Dr. McKelvie, receiving consulting fees from Bristol‐Myers Squibb and Sanofi‐Aventis and lecture fees from Bristol‐Myers Squibb, Sanofi‐Aventis, Pfizer, Merck, and AstraZeneca; Dr. Zile, receiving consulting fees from Bristol‐Myers Squibb and Sanofi‐Aventis; Ms. Anderson, being employed by the Statistical Data Analysis Center at the University of Wisconsin–Madison, which conducted statistical analysis for this trial, supported by Bristol‐Myers Squibb and Sanofi‐Aventis; Drs. Donovan and Ptaszynska, being employees of and having an equity interest in Bristol‐Myers Squibb; and Dr. Staiger, being an employee of and having an equity interest in Sanofi‐Aventis." study sponsors: Bristol‐Myers Squibb and Sanofi‐Aventis. "The sponsors or a contract research organization collected the trial data, which were then analyzed at the Statistical Data Analysis Center at the University of Wisconsin, Madison, independently of the sponsors." |
| Methods | Study design: parallel RCT Centres: "multicenter" but no further details Start of enrolment: May 2004 End of enrolment: March 2009 Mean follow‐up: 3.2 years Run‐in period: no | |
| Participants | Inclusion criteria: "All patients were at least 20 years of age, and had an LVEF of > 40% when diagnosed as having heart failure. Clinical diagnosis of heart failure was based on a slight modification of the Framingham criteria as previously described within the 12 months before study entry. There were no changes in baseline therapy and symptoms of heart failure within a month before study entry in any patients." Exclusion criteria: Current symptomatic hypotension, • Hypertension that has not been controlled to the satisfaction of the investigator by drugs other than β‐blocker • Hemodynamically significant (in the investigators opinion) LV outflow tract obstruction (from either aortic stenosis or ventricular hypertrophy) or mitral valve stenosis • Important aortic or mitral regurgitation in the investigator’s opinion • Heart rate < 50 beats/min • Second‐ or third‐degree heart block without permanent pacemaker in situ • Acute coronary syndrome • Arrhythmogenic right ventricular cardiomyopathy • Primary pulmonary hypertension or pulmonary hypertension not from LV dysfunction • Serious cerebrovascular disease • Acute myocardial infarction within the last 3 months • Patients who require intravenous inotropes • Cerebrovascular accident within the last 6 months • Percutaneous coronary intervention or open heart surgery within the last 3 months • On the waiting list for percutaneous coronary intervention or open heart surgery • Serum creatinine > 3.0 mg/dL or creatinine clearance ≤ 30 mL/min • Known bilateral renal artery stenosis • Serum potassium > 5.5 mEq/L • Serious liver disease • Prescription of β‐blocker within the last month or a history of a life‐threatening adverse event induced by β‐blocker • Any change in cardiovascular drug therapy within a month before randomization • History of chronic obstructive pulmonary disease or restrictive lung disease • Diabetes mellitus that has not been controlled to the satisfaction of the investigator • History of any life‐threatening noncardiac disease (eg, cancer) within 5 years • Other diseases likely to cause death or serious disability within 1 year • Patients unable to walk without personal aid • Arteriosclerosis obliterans with Fontaine Grade II or more. • Severe anemia (hemoglobin ≤ 6.0 g/dL) • Uncontrolled thyroid dysfunction Randomised (N): 245 (120 intervention, 125 control) Withdrawn (N): for reasons other than death (6 intervention, 0 control) Lost to follow‐up (N): (5 intervention, 3 control) Analysed (N): (120 intervention, 125 control) Age (years, mean, SD): intervention: 73, 10; control: 71, 11 Sex (% men): intervention: 57.5; control: 58.4 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 134, 21; control: 133, 21 Heart rate (beats/min, mean, SD): intervention: 72, 11; control: 74, 13 BMI (mean, SD): intervention: 24.2, 4.4; control: 24.1, 4.1 Serum creatinine (mg/dL, mean, SD): intervention: 0.98, 0.37; control: 1.01, 0.45 B‐type natriuretic peptide (pg/mL, mean, SD): intervention: 219.2, 294.9; control: 234.9, 281.6 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 62, 10; control: 63, 11 NYHA class I (%): intervention: 18.3; control: 18.4 NYHA class II (%): intervention: 69.2; control: 75.2 NYHA class III (%): intervention: 10.8; control: 4.8 NYHA class IV (%): intervention: 1.7; control: 1.6 Hypertension (%): intervention: 80.0; control: 80.8 Diabetes (%): intervention: 27.5; control: 33.6 Atrial fibrillation (%): intervention: 50.8; control: 45.6 Hospitalisation for heart failure (%): intervention: 60.0; control: 60.0 Ischaemic heart disease (%): intervention: 28.3; control: 24.0 Stroke (%): intervention: 11.7; control: 12.8 Diuretic (%); intervention: 63.3; control: 56.8 Digoxin (%): intervention: 19.2; control: 21.6 Beta‐blocker (%): study drug ACEI (%): intervention: 24.2; control: 22.4 ARB (%): intervention: 50.8; control: 56.0 MRA (%): intervention: 20.8; control: 25.6 | |
| Interventions | Intervention: carvedilol. "In the carvedilol arm, carvedilol was up‐titrated from 1.25 mg twice daily to the target dose of 10 mg twice daily within 8 weeks based on tolerability. Patients were maintained at the target dose or the maximum tolerated dose for the remainder of the study." Comparator: usual care Concomitant medication: "In both arms, patients were treated with standard cardiovascular therapy excluding beta‐blockers." | |
| Outcomes | Planned: "The primary outcome is a composite of cardiovascular death and unplanned admission to hospital for congestive heart failure. The secondary outcomes are listed as follows: all‐cause mortality; worsening of the symptoms (defined by either a decrease by 1 Mets in the SAS questionnaire score or an increase by 1 class in the New York Heart Association functional class for at least 3 months compared with the baseline); an increase in brain natriuretic peptide by 30% of the value at the randomization in patients with brain natriuretic peptide 200 pg/mL at the randomization; unplanned admission to hospital for congestive heart failure; or a need for modification of the treatment for heart failure (changes in oral medicine for at least 1 month or addition of intravenous inotropes for at least 4 hours)." (Hori 2005) Reported: as planned | |
| Notes | Emailed investigators on 13 November 2017 to ask about data on cardiovascular mortality and all‐cause mortality as different numbers are provided in Table 2 of Yamamoto 2013 (primary reference). No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomized to the arm " but no details |
| Allocation concealment (selection bias) | Unclear risk | no information |
| Blinding of participants and personnel (performance bias) | High risk | "open" |
| Blinding of outcome assessment (detection bias) | Low risk | "Deaths and hospitalizations were adjudicated by a blinded independent Endpoint Committee, using prespecified criteria." "Outcomes were assessed by the Endpoint Committee (see Appendix) where all the committee members were blinded to the allocated group." |
| Incomplete outcome data (attrition bias) | Low risk | "The primary outcome was a composite of cardiovascular death and unplanned hospitalization for heart failure using a time‐to‐first‐event ana‐ lysis and the intention‐to‐treat principle." unspecified for secondary outcomes lost to follow‐up low/similar in both groups (4.2% intervention, 2.4% control) |
| Selective reporting (reporting bias) | Low risk | outcomes reported as planned in published protocol (Hori 2005) |
| Other bias | Low risk | authors CoI: "none declared" funding: "The Ministry of Health, Labor andWelfare, Japan; the Japan Heart Foundation." |
| Methods | Study design: RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Follow‐up: 6 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "patients with chronic heart failure (CHF) with preserved ejection fraction (EF)." "with stable coronary arterial disease (CAD) and mild CHF (no higher IIfunctional class (NYHA)) with preserved systolic function of the LV (EF > 45%)" Exclusion criteria: not reported Randomised (N): 79 Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SD): 54.5, 10.5 Sex (% men): 61 Ethnicity (%): intervention: white , control: white Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF not reported NYHA class: not reported Diabetes not reported Atrial fibrillation not reported Hospitalisation for heart failure: not reported Coronary heart disease not reported Stroke (%): not reported Diuretic (%); not reported Digoxin (%): not reported Beta‐blocker (%): not reported ACEI (%): not reported ARB (%): not reported MRA (%): not reported | |
| Interventions | Intervention: spironolactone. "SPRL group was treated with the standard therapy (ACE inhibitors or angiotensin receptor blockers II, beta‐blockers,statins, antiplatelet agents) plus SPRL (25 mg/day, titrated to 50 mg/day if tolerated)" Comparator: standard therapy Concomitant medication: standard therapy (ACEI, ARB, beta‐blocker, statins, antiplatelet agents) | |
| Outcomes | Planned: unclear Reported: "V posterior wall thickness (LVPWT), intraventricular septal thickness (IVST), relative wall thickness (RWT) and LV mass index (LVMI)" | |
| Notes | Intended to contact trialists to obtain missing details and to enquire whether outcomes of interest to this review were measured. This was not possible as we could not find contact details for trialists. No relevant outcome data for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "were randomly divided" but no further detail |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | could not be assessed |
| Selective reporting (reporting bias) | Unclear risk | could not be assessed |
| Other bias | High risk | reported only as conference abstract |
| Methods | Study design: individual, parallel RCT Centres: 1, Japan Start of enrolment: January 2002 End of enrolment: September 2003 Follow‐up: 6 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "first episode of nonischemic heart failure and preserved LVEF. We confirmed that all patients had symptoms and signs of congestive heart failure in this study."..."they were in New York Heart Association (NYHA) functional class II or III at the time of enrollment, and all had an LVEF > 40%. " Exclusion criteria: "Patients were excluded if they had a history of myocardial infarction, coronary artery disease, congenital heart disease, primary hepatic failure, or active cancer." Randomised (N): 50 (intervention: 25, control: 25) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SD): intervention: 66, 10; control: 67, 8 Sex (% men): intervention: 68; control: 64 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 132, 18; control: 130, 20 Heart rate (beats/min, mean, SD): intervention: 72, 12; control: 74, 14 BMI not reported Serum creatinine not reported B‐type natriuretic peptide (pg/mL): intervention: 202, 125; control: 204, 127 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 54, 7; control: 55, 7 NYHA class I (%): 0 NYHA class II (%): intervention: 64; control: 68 NYHA class III (%): intervention: 36; control: 32 NYHA class IV (%): 0 Hypertension (%): intervention: 64; control: 60 Diabetes (%): not reported Atrial fibrillation (%): not reported Hospitalisation for heart failure (%): 100 Coronary heart disease (%): intervention: 0; control: 0 Stroke (%): not reported Diuretic (%); intervention: 92; control: 88 Digoxin (%): not reported Beta‐blocker (%): intervention: 12; control: 12 ACEI (%): intervention: 92; control: 96 ARB (%): study drug MRA (%): intervention: 16; control: 20 | |
| Interventions | Intervention: candesartan. "the initial daily dose of candesartan was 2 to 4 mg, which was increased to a maintenance dose of 8 to 12 mg/day (mean 10 2 mg/day)." Comparator: placebo Concomitant medication: "in addition to baseline therapy" | |
| Outcomes | Planned: unclear as unaware of published protocol or pre‐registration with a clinical trial registry Reported: hemodynamics, I‐MIBG, echocardiographic findings, NYHA functional class, BNP | |
| Notes | No outcomes reported for relevance to this review. Emailed trialist to ask for outcome data relevant to this review. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly classified" but no further details |
| Allocation concealment (selection bias) | Unclear risk | no information |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐blinded" but no further details |
| Blinding of outcome assessment (detection bias) | Low risk | "assessment was performed in a blinded fashion by two independent observers with no knowledge of the clinical status or medical therapy of the patients." |
| Incomplete outcome data (attrition bias) | High risk | ITT used for all outcomes, but loss to follow‐up and withdrawals not reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess as we are not aware of a published protocol or a pre‐registration in a clinical trial registry |
| Other bias | Unclear risk | funding source not reported |
| Methods | Study design: individual parallel RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Follow‐up: 12 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "As previously described, isolated HFPEF was defined as history, symptoms, and signs of HF; a preserved LVEF (50%); and no evidence of significant coronary, valvular, or pulmonary disease or other medical condition that could mimic HF symptoms, such as anemia or thyroid dysfunction." Exclusion criteria: "Coronary disease was excluded by history, medical records, ECG, and rest and exercise echocardiogram." "Patients were excluded if they had ever been prescribed an ACEI or ARB." Randomised (N): 71 (35 intervention, 36 control) Withdrawn (N): for reasons other than death 12 (10 intervention (3 patient request, 1 pancreatitis, 1 elective rotator cuff surgery, 1 alopecia, 1 worsening cough, 1 hypotension, 1 ankle fracture, exacerbation of knee arthritis, 1 leg and hip pain and fatigue), 2 control (1 elective knee replacement surgery, no details for second participants) Lost to follow‐up (N): not reported Analysed (N): 59 completed study (25 intervention, 34 control) Age (years, mean, SD): intervention: 69, 8; control: 70, 7 Sex (% men): intervention: 20; control: 11 Ethnicity (%): intervention: black 9, control: black 6 Systolic blood pressure (mmHg, mean, SD): intervention: 143, 17; control: 144, 18 Heart rate (beats/min, mean, SD): intervention: 129, 20; control: 133, 16 BMI (mean, SD): intervention: 30, 5; control: 30, 5 Serum creatinine (mg/dL, mean, SD): intervention: 1.1, 0.2; control: 1.1, 0.2 B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 65, 8; control: 65, 7 NYHA class I (%): 0: NYHA class II (%): intervention: 83; control: 75 NYHA class III (%): intervention: 17; control: 25 NYHA class IV (%): 0 Hypertension (%): intervention: 71; control: 75 Diabetes (%): intervention: 9; control: 17 Atrial fibrillation not reported Hospitalisation for heart failure: not reported Coronary heart disease (%): 0 Stroke not reported Diuretic (%); intervention: 49; control: 58 Digoxin (%): 0 Beta‐blocker (%): intervention: 29; control: 39 ACEI (%): study drug ARB not reported MRA not reported | |
| Interventions | Intervention: enalapril. "The study drug was initiated at 2.5 mg BID and titrated up to 10 mg BID as tolerated by the patient within the first 4 weeks of the study." Comparator: placebo Concomitant medication: not reported | |
| Outcomes | Planned: unclear as clinical trial registration was post hoc Reported: exercise capacity, aortic distensibility and LV structure and function, carotid artery stiffness, LV diastolic filling, QoL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "All investigators, staff, and patients were fully blinded to treatment group assignment throughout the entire study period." |
| Blinding of outcome assessment (detection bias) | Low risk | "All investigators, staff, and patients were fully blinded to treatment group assignment throughout the entire study period." |
| Incomplete outcome data (attrition bias) | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | not reported |
| Other bias | Low risk | "Dr Kitzman has served as consultant for and received grant support from Synvista ($10 000), Bristol‐Meyers Squibb ($10 000), Novartis ($10 000), Boston Scientific ($10 000), Relypsa ($10 000), Forest Laboratories, and Medtronic. Dr Little has served as consultant for CorAssist Cardiovascular Ltd, Celladon, Boston Scientific, Medtronic ($10 000), Bio‐Control Medical, CVRx ($10 000), Amylin Pharmaceuticals, Gilead, and BristolMeyers Squibb ($10 000). Drs Hundley, Brubaker, and Morgan; Mr Moore; and Ms Steward report no conflicts." |
| Methods | Study design: parallel RCT Centres: 1 hospital, Houston, Texas Start of enrolment: 2004 End of enrolment: 2008 Follow‐up: 6 months Run‐in period: "patients were treated with 25 mg open‐label spironolactone for 1 week before randomization to ensure drug tolerability, defined as serum potassium < 5 mEq/L and absence of other major side effects." | |
| Participants | Inclusion criteria: "≥ 18 years old with a previous diagnosis of HFpEF. HFpEF was defined as current New York Heart Association (NYHA) functional class II or III HF symptoms or signs, left ventricular ejection fraction (LVEF) ≥ 50% according to echocardiography, diastolic dysfunction with elevated LV filling pressure according to Dopplerechocardiograph" "the subjects had to have a blood pressure of ≤ 150/95 mm Hg for 4 weeks before enrollment and the ability to walk ≥ 50 m at the time of enrollment. Treatment with an ACEI, or ARB if ACEI intolerant, was required for ≥ 4 weeks before enrollment. " Exclusion criteria: "Exclusion criteria included current treatment with spironolactone or epleronone, previous intolerance to spironolactone, creatinine > 2.5 mg/dL, serum potassium > 5.0mEq/L, significant valvular heart disease, pericardial disease, severe chronic lung disease with cor pulmonale, unstable angina or myocardial infarction ≤ 4 weeks before enrollment, severe peripheral vascular disease with claudication that limited walking distance, presence of other severe comorbid conditions with a life expectancy < 6 months, and pregnant or lactating women." Randomised (N): 48 (24 intervention, 24 control) Withdrawn (N): for reasons other than death (3 intervention (hyperkalaemia),0 control) Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SEM): intervention: 66.3, 2.2; control: 76.4, 1.6 Sex (% men): 0 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SEM): intervention: 137.0, 4.1; control: 133.1, 2.8 Heart rate (beats/min, mean, SEM): intervention: 64.2, 2.3; control: 61.1, 1.2 BMI (mean, SEM): intervention: 29.4, 2.2; control: 26.3, 1.2 Serum creatinine not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SEM): intervention: 62.5, 1.2; control: 62.9, 1.2 NYHA class I (%): 0 NYHA class II (%): intervention: 33; control: 42 NYHA class III (%): intervention: 67; control: 58 NYHA class IV (%): 0 Hypertension (%): intervention: 87.5; control: 79.2 Diabetes (%): intervention: 50; control: 25 Atrial fibrillation (%): intervention: 25; control: 25 Hospitalisation for heart failure (%): intervention: 58.3; control: 54.2 Coronary heart disease (%): intervention: 37.5; control: 33.3 Stroke (%): not reported Diuretic (%): intervention: 83.3; control: 75 Digoxin (%): intervention: 12.5; control: 8.3 Beta‐blocker (%): intervention: 62.5; control: 62.5 ACEI (%): intervention: 70.8; control: 66.7 ARB (%): intervention: 29.2; control: 37.5 MRA (%): study drug | |
| Interventions | Intervention: Spironolactone, 25mg once daily Comparator: placebo Concomitant medication: not reported | |
| Outcomes | Planned: no known published protocol or pre‐enrolment clinical trial registry record Reported: 6 min walk distance, clinical composite score, doppler echocardiography, biomarkers, Kansas City Cardiomyopathy Questionnaire clinical summary score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no further details |
| Allocation concealment (selection bias) | Low risk | "subjects were randomly allocated with the use of pharmacy‐controlled concealed randomization methods.", no further details |
| Blinding of participants and personnel (performance bias) | Low risk | double‐blind, placebo controlled, no further details |
| Blinding of outcome assessment (detection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | no able to assess due to lack of pre‐registration in clinical trial registry and published protocol |
| Other bias | High risk | clinical trial registry entry after start of enrolment, was originally marked as an observational study (2005‐2013) and changed to a randomised trial status in 2013. reported as a RCT in 2014 publication. funded by Women’s Fund; Houston, Texas |
| Methods | Study design: RCT Centres: 1 Start of enrolment: not reported End of enrolment: not reported Follow‐up: 12 months Run‐in period: not reported | |
| Participants | Inclusion criteria: heart failure with preserved systolic function, "prior New York Heart Association (NYHA) functional class IV HF admission or symptoms consistent with HF, B‐type natriuretic peptide (BNP) >100 pg/ml, left ventricular ejection fraction >45%, and evidence of diastolic dysfunction on Doppler‐echocardiographic study." Exclusion criteria: "Patients were excluded if they were clinically unstable as defined by any change in diuretic dose a month before enrollment or were already receiving eplerenone or spironolactone therapy. Other exclusion criteria were evidence of significant inflammatory disease, hepatic disease, or metabolic bone disease that may alter parameters of collagen metabolism, serum creatinine >200 mol/l, prior documented left ventricular ejection fraction <45%, hemodynamically significant valvular disease, corpulmonale, hypertrophic, restrictive, or constrictive cardiomyopathy, atrial fibrillation or flutter with resting ventricular rate >120 beats/min, severe anemia, clinically significant pulmonary disease as evidenced by hospitalizations, or use of oral corticosteroids for pulmonary decompensation within 12 months or patients who require home oxygen therapy." Randomised (N): 44 (24 intervention, 20 control) Withdrawn (N): for reasons other than death 0 Lost to follow‐up (N): 2 (0 intervention, 2 control) Analysed (N): 40 (23 intervention, 17 control) Age (years, mean, SD): intervention: 80, 7.7; control: 79, 7.9 Sex (% men): intervention: 38; control: 55 Ethnicity (%): Caucasian: 100 Systolic blood pressure (mmHg, mean, SD): intervention: 140, 20; control: 146, 20 Heart rate (beats/min, mean, SD): intervention: 69, 13; control: 66, 13 BMI (mean, SD): intervention: 31.3, 6.9; control: 31.8, 5.7 Serum creatinine: not reported B‐type natriuretic peptide (pg/mL, median, IQR): intervention: 219 (157‐317); control: 192 (132‐330) NT pro B‐type natriuretic peptide: not reported LVEF (%, mean, SD): intervention: 63, 9.0; control: 64, 9.6 NYHA class I (%): not reported NYHA class II (%): 87% NYHA class III (%): not reported NYHA class IV (%): not reported Hypertension (%): intervention: 92; control: 90 Diabetes (%): intervention: 21; control: 35 Atrial fibrillation (%): intervention: 58; control: 60 Hospitalisation for heart failure: not reported Coronary heart disease (%): not reported Stroke (%): not reported Diuretic (%); intervention: 88; control: 90 Digoxin (%): intervention: 38; control: 30 Beta‐blocker (%): intervention: 62; control: 75 ACEI (%): intervention: 67; control: 60 ARB (%): intervention: 29; control: 40 MRA (%): study drug | |
| Interventions | Intervention: eplerenone. "we evaluated patients with a dose of 25 mg daily for 6 months followed by a dose increment to 50 mg until the 12‐month time point" Comparator: usual heart failure treatment Concomitant medication: not reported | |
| Outcomes | Planned: unable to assess Reported: serum levels of markers of collagen turnover, inflammatory markers, doppler‐echocardiographic indexes, clinical and biochemical measurements, withdrawals, quality of life | |
| Notes | Emailed investigators to ask whether ITT or PP analysis was used. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated" but no further detail |
| Allocation concealment (selection bias) | Unclear risk | nor reported |
| Blinding of participants and personnel (performance bias) | High risk | "open label" |
| Blinding of outcome assessment (detection bias) | Low risk | "all parameters were assessed by persons blinded to treatment" |
| Incomplete outcome data (attrition bias) | Unclear risk | unable to assess |
| Selective reporting (reporting bias) | Unclear risk | unable to assess as unaware of published protocol or pre‐trial registration |
| Other bias | Low risk | Dr. Mak received grant support from the Irish Heart Foundation (The Noel Hickey Bursary) sponsored by Pfizer. Drs. Ledwidge and McDonald have received honoraria from Pfizer. |
| Methods | Study design: RCT Centres: 1, Cardiology Outpatient Department and HTN clinic of Postgraduate Institute of Medical Education and Research, India Start of enrolment: 15 November 2009 (from clinical trial registry) End of enrolment: not reported for pilot study, full study ongoing Follow‐up: 12 weeks Run‐in period: 2 weeks placebo run in (beta‐blocker withdrawn but co‐existing therapies were continued) | |
| Participants | Inclusion criteria: "18 years and above, had New York Heart Association (NYHA) functional Class II–III of at least 4 weeks' duration, LVEF ≥ 50% in a nondilated LV (LV enddiastolic volume < 97 ml/m measured by echocardiography), echocardiographic evidence of LV diastolic dysfunction, and were willing to give written informed consent." Exclusion criteria: "They were excluded if: (1) Clinically unstable as defined by any change in diuretic dose in the month before enrollment, (2) significant valvular heart disease, pericardial disease, hypertrophic or restrictive cardiomyopathy, (3) unstable angina or MI within past 4 weeks, (4) any previous LVEF below 40%, (5) any contraindication to metoprolol use, (6) patients already on beta blockers which cannot be withdrawn, (7) current participation (including prior 30 days) in any other therapeutic trial, and (8) any condition that, in the opinion of investigator, may prevent the participant from adhering to the trial protocol." Randomised (N): 40 (20 intervention, 20 control) Withdrawn (N): for reasons other than death 0 Lost to follow‐up (N): 6 (3 intervention, 3 control) Analysed (N): 40 (20 intervention, 20 control) Age (years, mean, SD): intervention: 55.2, 7.1; control: 57.2, 9.8 Sex (% men): intervention: 45; control: 50 Ethnicity (%): not reported Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide (pg/mL, median (IQR)): intervention: 238.2 (107.7‐2230.2); control: 227.6 (58.3‐3645) LVEF (%, mean, SD): intervention: 62.9, 6.2; control: 62.1, 6.57 NYHA class I (%): 0 NYHA class II (%): intervention: 55; control: 65 NYHA class III (%): intervention: 45; control: 35 NYHA class IV (%): 0 Hypertension (%): not reported Diabetes (%): not reported Atrial fibrillation (%): not reported Hospitalisation for heart failure: not reported Coronary heart disease (%): not reported Stroke (%): not reported Diuretic (%); intervention: 35; control: 40 Digoxin (%): not reported Beta‐blocker (%): intervention: 40; control: 45 ACEI (%): intervention: 15; control: 60 ARB (%): intervention: 15; control: 15 MRA (%): not reported | |
| Interventions | Intervention: metoprolol succinate, 25 mg. "A dose upward titration protocol with monitoring of blood pressure and heart rate (target blood pressure and heart rate as 120/80 mm Hg and 60 beats/min, respectively) was implemented for dose increments up to a maximum dose of 100 mg once daily. For patients not tolerating increased titration of drug, temporary reduction in dosage was done and decision on further escalation made on individual basis by the treating cardiologist." Comparator: placebo Concomitant medication: "During the study, calcium channel blockers were added in three patients (one in placebo and two in metoprolol group) due to high blood pressure records." coexisting therapies were continued | |
| Outcomes | Planned: unable to assess Reported: primary: NYHA class. Secondary: exercise capacity, diastolic dysfunction, change in LV wall thickness, LV mass, NT‐proBNP, PICP, QoL (SF‐36), adverse events, withdrawals | |
| Notes | published results after our search date, identified via search for clinical trial registry number: CTRI/2010/091/000438 which was retrieved by search of the WHO ICTRP register Emailed investigators to ask when completion of full trial is anticipated. Response confirmed that full trial was not conducted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "block randomised" but no further details |
| Allocation concealment (selection bias) | Low risk | "Randomization and allocation sequence generation were done by investigators not directly involved in the evaluation of outcomes" From clinical trial registry: "sequentially numbered, sealed, opaque envelopes" |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐blind" From clinical trial registry entry: "participant and outcome assessor blinded" Unclear whether personnel was blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | "To avoid interobserver variability, all the echocardiographic parameters were evaluated by a single cardiologist who was blinded to study medication and the order of assessment." From clinical trial registry entry: "participant and outcome assessor blinded" |
| Incomplete outcome data (attrition bias) | Unclear risk | pilot study ‐ full study ongoing same numbers lost to follow‐up in both arms ITT and per‐protocol analysis used |
| Selective reporting (reporting bias) | Unclear risk | unable to assess due to lack of published protocol and uncertainty over trial registration date |
| Other bias | Low risk | "The study was supported by Postgraduate Institute of Medical Education and Research, Chandigarh, India." "There are no conflicts of interest" Results from pilot study only so far. |
| Methods | Study design: individual, double‐blind, placebo‐controlled, RCT Centres: 1, Australia Start of enrolment: February 2002 End of enrolment: October 2002 Follow‐up: 6 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "To be eligible, patients had to have hypertension requiring antihypertensive medication and report exertional dyspnea (New York Heart Association class II) but no history of angina or myocardial infarction." Exclusion criteria: "Patients taking angiotensin converting enzyme inhibitors, angiotensin‐receptor blockers, or spironolactone were excluded, as were patients with renal impairment (creatinine 0.20 mmol/dL) or hyperkalemia at baseline." "we excluded patients with evidence of pulmonary disease, ischemic heart disease, abnormal regional or global resting LV systolic function (ejection fraction < 50%), or significant (>mild) valvular dysfunction." Randomised (N): 30 (not reported by treatment arm, assumed 15 in each) Withdrawn (N): not reported Lost to follow‐up (N): 1 (intervention: 1 (migrated overseas); control: 0) Analysed (N): not reported Age (years, mean, SD): intervention: 61, 6; control: 62, 5 Sex (% men): intervention: 40; control: 34 Ethnicity (%): not reported Systolic blood pressure (mmHg): intervention: 199, 18; control: 198, 26 Heart rate (beats/min): intervention: 139, 24; control: 153, 13 BMI: intervention: 29.8, 4.7; control: 31.2, 4.6 Serum creatinine (mg/dL): intervention: 0.07, 0.01; control: 0.07, 0.01 B‐type natriuretic peptide (pg/mL) intervention: 29.3, 26.8; control: 29.7, 27.8 NT pro B‐type natriuretic peptide not reported LVEF: intervention: 68, 5; control: 67, 4 NYHA class not reported Hypertension (%): not reported Diabetes (%): intervention: 7; control: 0 Atrial fibrillation (%): not reported Hospitalisation for heart failure: not reported Coronary heart disease (%): not reported Stroke (%): not reported Diuretic (%); intervention: 40; control: 27 Digoxin (%): not reported Beta‐blocker (%): intervention: 40; control: 20 ACEI (%): not reported ARB (%): not reported MRA (%): study drug | |
| Interventions | Intervention: spironolactone, 25mg/d Comparator: placebo Concomitant medication: not reported | |
| Outcomes | Planned: unable to assess as we are not aware of a published protocol or pre‐registered clinical trial registry entry Reported: mean 24hr ambulatory blood pressure, posterior wall thickness, left atrial area, SR, peak systolic strain, and CVIB | |
| Notes | no outcome data of interest to this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomised, but no details |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Low risk | double blind, matching placebo |
| Blinding of outcome assessment (detection bias) | Low risk | "Investigators remained blinded to the treatment until after analysis of results." |
| Incomplete outcome data (attrition bias) | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | "This work was supported in part by a grant and scholarship from the National Heart Foundation of Australia, Melbourne, Australia, in association with a Centers of Clinical Research Excellence Award, National Health and Medical Research Council, Canberra, Australia. The authors are grateful to the Princess Alexandra Hospital Pharmacy for supervision of randomization and dispensing of active and placebo tablets." |
| Methods | Study design: RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: 13.8 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "Patients with diastolic heart failure attending to Heart Failure Clinic were considered eligible, independently of etiology, if they had history of arterial hypertension (and/or were on antihypertensive treatment), but no history of angina, myocardial infarction or myocardial revascularization (PTCA and / or aortocoronary bypass grafting) during the 3 months previous to recruitment and they referred fatigue, dyspnea on exercise and/or orthopnea." "Diastolic dysfunction was considered when the ejection fraction was over 45%, and shortening fraction = 28%, without severe segmental dyskynesia of the left ventricle, left atrial enlargement, or increased thickness or posterior wall, interventricular septum, and left ventricular mass index." Exclusion criteria: not reported Randomised (N): 28 (14 intervention, 14 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SD): intervention: 63.7, 21.6; control: 64.8, 11.9 Sex (% men): intervention: 28.6; control: 71.4 Ethnicity (%): not reported Blood pressure (mmHg): intervention: 112, 12; control: 114, 8 Heart rate (beats/min): intervention: 86, 4; control: 82, 6 BMI (mean, SD): intervention: 27.5, 9.4; control: 26.9, 4.7 Serum creatinine not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 48.79, 4.65; control: 51.57, 11.71 NYHA class I (%): intervention: 42.9; control: 75.0 NYHA class II (%): intervention: 0; control: 16.7 NYHA class III (%): intervention: 57.1; control: 8.3 NYHA class IV (%): 0 Hypertension (%): intervention: 85.7; control: 92.9 Diabetes (%): intervention: 28.6; control: 64.3 Atrial fibrillation (%): not reported Hospitalisation for heart failure: not reported Ischaemic heart disease (%): intervention: 42.9; control: 57.1 Stroke (%): not reported Diuretic (%): intervention: thiazide: 76.9, loop: 5.1; control: thiazide: 62.3, loop: 13 Digoxin (%): not reported Beta‐blocker (%): intervention: 79.5; control: 79.7 ACEI (%): intervention: 38.5; control: 29 ARB (%): intervention: 69.2; control: 73.9 MRA (%): study drug | |
| Interventions | Intervention: spironolactone, mean dose of 37.5 mg/d (25‐50 mg once a day) Comparator: no treatment Concomitant medication: "In our study, patients with diastolic heart failure were all treated with ACE inhibitors/ARA and Beta blockers." | |
| Outcomes | Planned: We are not aware of a published protocol or pre‐registered clinical trial register entry Reported: echocardiographic parameters, adverse events | |
| Notes | no outcome data of interest to this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) | Low risk | "The echocardiogram was made by a Cardiologist blinded to the clinical evaluation and treatment received." |
| Incomplete outcome data (attrition bias) | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | no funding reported |
| Methods | Study design: RCT Centres: 10 (5 in Germany, 5 in UK) Start of enrolment: December 2002 (from clinical trial registry) End of enrolment: March 2007 (from clinical trial registry) Mean follow‐up: 13.8 weeks Run‐in period: not reported | |
| Participants | Inclusion criteria: "Patients were ≥ 21 years of age and had the following characteristics: symptoms of breathlessness on exertion (based on patient questioning) with normal lung function at rest, an extrapolated maximum oxygen consumption (EMOC) and/or peak oxygen consumption <85% of the age‐corrected normal value on cardiopulmonary exercise testing, preserved systolic function (ejection fraction ≥ 40%) with evidence of diastolic dysfunction on echocardiography (≥ 1 of the following: abnormal flow propagation velocity, prolongation of isovolumic relaxation time, E/A ratio reversal, and abnormal E deceleration time), and ability to exercise for ≥ 3 min on a treadmill." Exclusion criteria: "Uncontrolled hypertension (sitting systolic blood pressure >160 mmHg or sitting diastolic blood pressure > 100 mmHg) Presence of clinically significant asthma or chronic obstructive pulmonary disease Abnormal lung function (forced expiratory volume in 1 s [FEV1]/ forced vital capacity [FVC] ratio < 75%) Treatment with ≥ 2 bronchodilators Exercise limiting symptomatic angina Haemodynamically significant cardiac valvular disease Documented evidence of systolic heart failure (ejection fraction <40%, fractional shortening <25%) Uncontrolled atrial fibrillation (> 100 b.p.m. at rest) History of myocardial infarction, percutaneous transluminal coronary angioplasty, or coronary artery bypass within the previous 3 months Use of ARBs within the previous 1 month" Randomised (N): 152 (70 intervention, 82 control) Withdrawn (N): for reasons other than death 5 (4 intervention (N = 2 adverse events, N = 1 protocol violation, N = 1 withdrew consent), 1 control (N = 1 protocol violation)) Lost to follow‐up (N): 0 Analysed (N): 152 (70 intervention, 82 control), except QoL: 67 intervention, 82 placebo Age (years, mean, SD): intervention: 61.0, 11.5; control: 63.1, 10.3 Sex (% men): intervention: 50; control: 50 Ethnicity (%): intervention: Caucasian: 95.6, Other: 4.4 , control: Caucasian: 93.9, other: 6.1 Systolic blood pressure not reported Heart rate not reported BMI (mean, SD): intervention: 31.0, 4.7; control: 29.3, 5.3 Serum creatinine not reported B‐type natriuretic peptide (pg/mL): intervention: 93.2, 80.2; control: 120.3, 119.5 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 70.48, 11.43; control: 71.52, 12.08 NYHA class not reported Hypertension (%): intervention: 92.2; control: 89.0 Diabetes (%): intervention: 22.1; control: 14.6 Atrial fibrillation (%): intervention: 16.2; control: 9.8 Hospitalisation for heart failure: not reported Coronary heart disease (%): not reported Stroke (%): not reported Diuretic (%): not reported Digoxin (%): not reported Beta‐blocker (%): intervention: 33.8; control: 34.1 ACEI (%): intervention: 41.2; control: 37.8 ARB (%): study drug MRA (%): not reported | |
| Interventions | Intervention: valsartan. 80 mg once daily. "Study medication was force‐titrated between days 5 and 14 (Visit 3) to valsartan 160 mg daily or matching placebo, and between days 10 and 28 (Visit 4) to valsartan 320 mg daily or matching placebo. Up‐titration occurred provided the current dose was adequately tolerated. Down‐titration occurred for any of the following: evidence of persistent symptomatic hypotension, systolic blood pressure <100 mmHg or decrease of >40 mm Hg from baseline, creatinine increase of >50% from baseline, or if the investigators judged the given dose level as potentially harmful to the patient. A safety evaluation was performed between days 15 and 42 (Visit 5). After the dose‐titration period, patients received their maximum tolerated dose through to the end of the study at week 14 (+7 days) (Visit 6)." Comparator: matching placebo Concomitant medication: "Use of other ARBs as concomitant medication was prohibited, but other background medications (e.g. diuretics, calcium channel blockers) were allowed and continued throughout the study. Angiotensin‐converting enzyme inhibitors and beta‐blockers were permitted, although therapy was to be maintained at the same level throughout the study and no new treatment with one of these drugs was permitted during the trial." | |
| Outcomes | Planned: unclear Reported: exercise time, neurohormone levels, echocardiographic parameters, QoL, adverse events | |
| Notes | Response to email enquiring for further details received on 2 December 2017: confirmed that no outcome data are available for heart failure hospitalisation and hyperkalaemia and provided number of centres. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Eligible patients were allocated to either the active treatment group or the placebo group according to a stratified randomization process in order to minimize the differences between study groups. Stratification was based on exercise test time at Visit 2 divided into sections of: 3–6 min, .6 min to 9 min, and .9 min, each stratum being randomized in blocks of 4." No details on how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐blind" but not specified "To maintain blinding, valsartan and placebo capsules were identical in appearance." |
| Blinding of outcome assessment (detection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Low risk | ITT, higher withdrawals in intervention group (5.7%) compared to control (1.2%) |
| Selective reporting (reporting bias) | Unclear risk | unable to assess, unaware of published protocol and clinical trial register entry (Sept 2005) after planned study start (Dec 2002) |
| Other bias | Low risk | "H.K.P. and B.P. have no conflicts of interest; C.D.A., M.W., and P.B. remain in the employ of Novartis Pharma and have no other conflicts of interest; A.D.S. received one honorarium (£1000) from Novartis for intellectual input to the rationale and the design of the study; T.M.MacD: competing interests statement Nov 2008: my department has had research grants from GSK, Aventis, Novartis, AstraZeneca, BMS, Bohringer Ingelheim, Pfizer, and Novartis, I am or have been the principal investigator on trials paid for by Pfizer and Novartis, I have been paid Consulting fees by Pfizer, Novartis, Kaiser Permanante, Takeda, Recordati, Quintiles, and Speedel." "The study was funded by Novartis." |
| Methods | Study design: RCT Centres: 53 centres (Bulgaria (3), Czech Republic (5), Hungary (10), Ireland (1), Poland (26), Russia (1), Slovakia (2), and the UK (5)) Start of enrolment: 2000 End of enrolment: 2003 Mean follow‐up: mean follow‐up 26.2 months (range, excluding deaths 12.0‐54.2) Run‐in period: "A 24‐h open label run in phase, during which patients will receive a single 2‐mg dose of perindopril" | |
| Participants | Inclusion criteria: "Patients had to be aged ≥70 years and treated with diuretics for a clinical diagnosis of CHF due to LV diastolic dysfunction as defined below and to have had a cardiovascular hospitalization within the previous 6 months. Patients had to be able to walk without the aid of another person in order to exclude very frail patients who might not respond to any treatment.""Patients had to be aged 70 years and treated with diuretics for a clinical diagnosis of CHF due to LV diastolic dysfunction as defined below and to have had a cardiovascular hospitalization within the previous 6 months. Patients had to be able to walk without the aid of another person in order to exclude very frail patients who might not respond to any treatment." "As there are no widely agreed criteria for the diagnosis of diastolic heart failure, at least three out of nine clinical and at least two out of four additional echocardiographic criteria were required. Clinical criteria were: exertional breathlessness; orthopnoea or paroxysmal nocturnal dyspnoea; ankle swelling; improved breathlessness with diuretic therapy; increased jugular venous pressure; prior episode of clinical pulmonary oedema; prior MI; cardiothoracic ratio > 0.55; and previous radiological pulmonary oedema. Echocardiographic criteria were: an LV wall motion index of 1.4–1.6 inclusive, roughly equivalent to an LVEF fraction between 40 and 50%, since abnormal diastolic dysfunction is often associated with some impairment of systolic function; a left atrial diameter > 25 mm/m2 body surface area or > 40 mm because chronic elevation of LV filling pressure should lead to atrial dilatation; an interventricular septum or posterior LV wall ≥ 12 mm in thickness suggesting hypertrophy, a common cause of impaired diastolic function or, finally, evidence of impaired LV filling by at least one of the criteria recommended by the European Society of Cardiology Study Group on Diastolic Heart Failure. These included an E/A ratio < 0.5 or deceleration time of > 280 ms from the mitral inflow pattern or an isovolumic relaxation time of > 105 ms. These criteria effectively exclude patients with atrial fibrillation (AF) and therefore, in a protocol modification early in the course of the study, this arrhythmia was counted as equivalent to evidence of impaired LV filling by Doppler." Exclusion criteria: "Patients with a wall motion index of < 1.4, roughly equivalent to an LVEF of 40%, were excluded." "Important exclusion criteria were haemodynamically significant valve disease, stroke within the previous month, sitting systolic arterial pressure < 100 mmHg, serum creatinine > 200 mmol/L or potassium > 5.4mmol/L, history of ACE‐inhibitor intolerance or use of an ACE‐inhibitor or angiotensin receptor blocker within the previous week, potassium‐sparing diuretics (other than low‐dose spironolactone), or potassium supplements." Randomised (N): 850 (424 intervention, 426 control) Withdrawn (N): due to serious adverse events 13 (9 intervention, 4 control) Lost to follow‐up (N): 4 (4 intervention, 0 control) Analysed (N): 846 (420 intervention, 426 control) Age (years, median, IQR): intervention: 75, 72–79; control:75, 72‐79 Sex (% men): intervention: 46; control: 43 Ethnicity (%): not reported Systolic blood pressure (mmHg, median, IQR): intervention: 138, 128–150; control: 140, 129–150 Heart rate (beats/min, median, IQR): intervention: 74, 66 to 81; control: 73, 66 to 82 BMI (median, IQR): intervention: 27.5, 25.1 to 30.0; control: 27.6, 25.3 to 30.7 Serum creatinine (mg/dL, median, IQR, converted from umol/L using http://www.endmemo.com/medical/unitconvert/Creatinine.php): intervention: 1.07, 0.92‐1.24; control: 1.10, 0.95‐1.26 B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): intervention: 335, 160–1014 (for subgroup n =191); control: 453, 206–1045 (for subgroup n = 184) LVEF (%, median, IQR): intervention: 65, 56– 66; control: 64, 56– 66 NYHA class I/II (%): intervention: 77; control: 74 NYHA class III/IV (%): intervention: 23; control: 26 Hypertension (%): intervention: 79; control: 79 Diabetes (%): intervention: 21; control: 20 Atrial fibrillation (%): intervention: 19; control: 22 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 27; control: 26 Stroke not reported Diuretic (%); intervention: loop: 47, thiazide 54, low dose spironolactone 9; control: loop: 44, thiazide 55, low dose spironolactone 11 Digoxin (%): intervention: 11; control: 13 Beta‐blocker (%): intervention: 55; control: 54 ACEI: study drug ARB not reported MRA (%): intervention: 9; control: 11 | |
| Interventions | Intervention: perindopril. "Patients were reviewed weekly for the first 5 weeks to ensure that treatment was tolerated and to check serum potassium and creatinine. The dose of perindopril was increased to 4 mg once daily at the second follow‐up visit if no clinical contraindication, such as hypotension or worsening renal function existed. Study medication was reduced or discontinued if serum creatinine rose to > 250 mmol/L or by > 50 mmol/L from baseline or potassium rose to > 5.5mmol/L. Patients were reviewed at 8, 12, and every 12 weeks thereafter until 1 year follow‐up, then according to the investigator’s judgment until the end of the study." Comparator: placebo Concomitant medication: not reported | |
| Outcomes | Planned: "The primary end‐point of this study will be the time to first occurrence of the combined end‐point of total mortality and unplanned heart failure related hospitalisation." "Secondary 1. Death all causes 2. Death or worsening symptoms and/or signs of CHF requiring hospitalisation or an increase in diuretic treatment for CHF of >40 mg/day of frusemide compared to baseline or equivalent. This will be a time to first event analysis. 3. Cardiovascular mortality. 4. Number of days alive and out of hospital. 5. Number of days alive and not in hospital for cardiovascular reasons including CHF 6. QoL questionnaire change from baseline to 1 year. 7. CHF symptom score change from baseline to 1 year. 8. NYHA heart failure score change from baseline to 1 year." (Cleland 1999) Reported: primary outcome, all‐cause mortality, cardiovascular mortality, HF hospitalisation, days in hospital for cardiovascular reasons, days in hospital for any reason, NYHA class, 6‐min walk distance, plasma concentrations of NTproBNP, cardiovascular death or unplanned HF related hospitalisation, stroke, acute coronary syndrome, blood pressure, serum potassium and creatinine Planned but not reported: QoL | |
| Notes | Protocol (Cleland 1999) mentions subgroup analyses by age and sex but not found in published papers. Emailed investigators. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomly assigned from a computer‐generated list in blocks of four within treatment centres" |
| Allocation concealment (selection bias) | Low risk | "through a centrally administered process, concealed from the study investigators." |
| Blinding of participants and personnel (performance bias) | Low risk | "The study medication was provided in externally indistinguishable tablets." |
| Blinding of outcome assessment (detection bias) | Low risk | "Potential classifying events were independently classified by MT and JGFC, blind to treatment allocation." |
| Incomplete outcome data (attrition bias) | Low risk | 4/850 lost to follow up |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported, but not all secondary outcomes reported as planned (eg QoL) |
| Other bias | Low risk | "Servier funded the trial and provided site monitors for source data verification. The sponsor had access to the database and participated in the analysis under the supervision of an independent statistician (NF). The Steering Committee wrote the manuscript. Servier representatives commented on it prior to submission." |
| Methods | Study design: individual, placebo‐controlled, double‐blind RCT Centres: 1 medical centre, USA Start of study: August 2004 End of Study: October 2007 Follow‐up: 24 weeks Run‐in period: "2‐week open label period of eplerenone 25 mg daily to establish tolerability" | |
| Participants | Inclusion criteria: "All patients were defined as having HFpEF based on the presence of all the following criteria: 1) Clinical HF for ≥ 2 months before the screening visit with New York Heart Association (NYHA) functional Class II or III HF symptoms at enrollment; 2) left ventricular ejection fraction ≥ 50% (by echocardiography, radionuclide ventriculography, or contrast angiography) within 2 months of screening; and 3) B‐type natriuretic peptide (BNP) levels ≥ 100 pg/mL within 2 months of screening. Other inclusion criteria included age ≥ 18 years, systolic blood pressure ≤150, and diastolic blood pressure ≤ 95 mm Hg for 4 weeks before and at enrollment, ability to walk ≥ 50 m, current use of angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), if tolerated, for at least 4 weeks before enrollment. Patients were expected to be euvolemic on clinical examination or all attempts were made to achieve euvolemia with change in diuretic doses prior to enrollment into the study." Exclusion criteria: "Exclusion criteria included the need for eplerenone or spironolactone for treatment of other comorbid illnesses (eg, ascites); hepatic impairment; serum creatinine > 2.5 mg/dL or serum potassium > 5.0 mEq/L; prior intolerance to eplerenone or spironolactone; significant valvular heart disease, pericardial disease or severe chronic lung disease; patients with technically inadequate echocardiographic windows; patients with severe mitral annular calcification; unstable angina or acute myocardial infarction within 4 weeks before enrollment; severe peripheral vascular disease with claudication or other physical conditions limiting the distance walked; pregnant or lactating females; history of active alcohol or substance abuse or history of repeated noncompliance; history of cancer within 3 years (other than resected cutaneous basal or squamous cell carcinoma); and participation in any other drug trial within 30 days before enrollment." Randomised (N): 46 (23 intervention, 23 control) Withdrawn (N): 0 Lost to follow‐up (N): 2 (2 intervention (relocation)) Analysed (N): 44 (21 intervention, 23 control) Age (years, mean, SD): intervention: 72.2, 9.8; control: 68.7, 9.1 Sex (% men): intervention: 95.2; control: 91.3 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 129.7, 12.4; control: 130.6, 10.7 Heart rate (beats/min, mean, SD): intervention: 65.0, 9.3; control: 63.0, 12.1 BMI (mean, SD): intervention: 30.1, 6.1; control: 34.6, 5.8 Serum creatinine (mg/dL, mean, SD): intervention: 1.62, 0.50; control: 1.43, 0.51 B‐type natriuretic peptide (pg/mL): intervention: 254.9, 163.0; control: 283.5, 211.6 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, median, IQR): intervention: 62.1, 5.0; control: 62.5, 7.5 NYHA class I (%): 0 NYHA class II (%): intervention: 66.7; control: 52.2 NYHA class III (%): intervention: 33.3; control: 47.8 NYHA class IV (%): 0 Hypertension (%): 100 Diabetes (%): intervention: 61.9; control: 60.9 Atrial fibrillation (%): intervention: 14.3; control: 13.0 Hospitalisation for heart failure (%): intervention: 42.9; control: 60.9 Coronary heart disease (%): intervention: 66.7; control: 47.8 Stroke (%): nor reported Diuretic (%); intervention: 95.2; control: 100 Digoxin (%): not reported Beta‐blocker (%): intervention: 76.2; control: 82.6 ACEI or ARB (%): intervention: 95.2; control: 100 MRA (%): study drug | |
| Interventions | Intervention: eplerenone. "After randomization, patients received study drug at a dose of 25 mg daily for 2 weeks followed by 50 mg daily for 22 weeks, if tolerated" "Study drug dose was adjusted according to the following algorithm. If the serum K+ was ≥ 5.0 mEq/L but < 5.5 mEq/L, the dose of eplerenone was not increased. If the level was ≥ 5.5 but < 6.0 mEq/L, the dose of eplerenone was reduced to half. If the serum potassium was ≥ 6.0 mEq/L eplerenone was stopped, at least transiently. If an underlying condition that was correctable was identified, the medication could be restarted at the lowest dose once the serum K+ was < 5.0 mEq/L. If no correctable cause was identified for the serum potassium ≥ 6.0 mEq/L, eplerenone was discontinued permanently and serum K+ was followed with adjustments in other medications as indicated. Oral potassium supplements were allowed if the serum potassium was < 4.0 mEq/L after the study drug was started." Comparator: matching placebo Concomitant medication: "Potassium supplements were stopped when eplerenone was initiated." | |
| Outcomes | Planned: in clinical trial register: all of the below, except NYHA class, hospitalisation and mortality Reported: primary: 6MWD. Secondary: echocardiographic measures of diastolic dysfunction, biomarkers including markers of collagen turnover and B‐type natriuretic peptide, HF‐related quality of life measured by the Kansas City Cardiomyopathy Questionnaire, NYHA class, hospitalisation, mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐blind" but no details |
| Blinding of outcome assessment (detection bias) | Low risk | "all end points were evaluated blinded to treatment allocation" |
| Incomplete outcome data (attrition bias) | Low risk | ITT not used, analysis based on participants that completed study, minimal loss to follow‐up (reasons reported) |
| Selective reporting (reporting bias) | Unclear risk | although there is a clinical trial registry record, it's unclear whether this was a pre‐ or post‐registration |
| Other bias | Low risk | "Supported by a VA Clinical Research Service grant # CLIN‐010‐03S (to Dr. Deswal)." "The study was sponsored by the Department of Veterans Affairs (VA). The study drug was provided by Pfizer Pharmaceuticals, but they did not provide any other funding for the study and did not have any role in the conduct and analysis of this study" originally registered as assessing spironolactone, then changed to epleronone |
| Methods | Study design: RCT Centres: 1, India Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: 3‐6 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "Patients with moderate or severe MR on color flow Doppler, LVEF ≥ 55%, and LV end‐systolic dimension < 40 mm were included" Exclusion criteria: "Patients with NYHA class IV symptoms, known coronary artery disease, significant other valvular disease, serum creatinine > 2.5 mg/dL, and hypertension were excluded" Randomised (N): 100 (48 intervention, 52 control) Withdrawn not reported Lost to follow‐up not reported Analysed (N): at 3 months: 100 (48 intervention, 52 control); at 6 months: 75 (39 intervention, 36 control) Age (years, mean, SD): intervention: 30.24, 12.76; control: 29.6, 15.58 Sex (% men): intervention: 35.6; control: 22.7 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 125, 10.1; control: 124.4, 7.4 Heart rate (beats/min, mean, SD): intervention: 90.1, 11.78; control: 88.5, 18.12 BMI: intervention: 21.04, 4.74; control: 19.04, 4.7 Serum creatinine not reported B‐type natriuretic peptide (pg/mL): intervention: 194, 178.5; control: 166, 165.7 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 62.5, 6.5; control: 61.4, 6.9 NYHA class: "most patients were in NYHA class II (77%) while 23% were in NYHA class III" Hypertension (%): intervention: 61.1; control: 62.3 Diabetes (%): intervention: 26.9; control: 25.3 Atrial fibrillation (%): intervention: 33.8; control: 35.5 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 68.9; control: 67.6 Stroke (%): intervention: 0.1; control: 0 Diuretic (%): 92 Digoxin (%): 33 Beta‐blocker (%): study drug ACEI (%): 58 ARB (%): not reported MRA (%): not reported | |
| Interventions | Intervention: metoprolol succinate. "initiated at 12.5–25 mg/day and titrated as tolerated at 2‐week intervals to a maximum of 100 mg/day. Prior to each escalation, care was taken to ensure that resting heart rate was > 60 bpm and systolic BP > 100 mm Hg" Comparator: placebo Concomitant medication: "in addition to ongoing therapy" | |
| Outcomes | Planned: unclear as we did not identify a published protocol or clinical trial registry entry Reported: withdrawals due to adverse events, echocardiographic outcomes, blood pressure, MR grade, NYHA class | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "by a computerized random number generating protocol" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) | Low risk | "Detailed echocardiography […] was performed by two operators who were blinded to the treatment protocol." |
| Incomplete outcome data (attrition bias) | Unclear risk | unclear loss‐to‐follow up of 25 participants at 6 months as no reasons given |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | "The authors declare no conflict of interest" no funding source reported |
| Methods | Study design: RCT Centres: multi‐centre, international (Czech Republic, Hungary, Italy, Ukraine, UK, France, Germany, Romania, Spain, Switzerland, The Netherlands) Start of enrolment: September 2000 End of enrolment: December 2002 Mean follow‐up: 21 months Run‐in period: no | |
| Participants | Inclusion criteria: "To be eligible, patients had to be age ≥ 70 years, provide written informed consent, and have a clinical history of chronic HF with at least 1 of the following features: documented hospital admission within the previous 12 months with a discharge diagnosis of congestive HF or documented LVEF ≤ 35% within the previous 6 months. " Exclusion criteria: "The main exclusion criteria were new drug therapy for heart failure in the 6 weeks prior to randomization, any change in cardiovascular drug therapy in the 2 weeks prior to randomization, heart failure due primarily to uncorrected valvular heart disease, contraindication or previous intolerance to beta‐blockers (e.g. heart rate < 60 beats/min or systolic blood pressure < 90 mmHg), current use of beta‐blockers, significant hepatic or renal dysfunction, cerebrovascular accidents within the previous 3 months, and being on a waiting list for percutaneous coronary intervention or cardiac surgery or other major medical conditions that may have reduced survival during the period of the study." Randomised (N): 2128 (1067 intervention, 1061 control); subgroup of interest: 643 (nebivolol N = 320, placebo N = 323) Withdrawn not reported Lost to follow‐up (N): 37 (16 intervention, 21 control) Analysed (N): 2128 (1067 intervention, 1061 control) Age (years, mean, SD): intervention: 76.1, 4.8; control: 76.1, 4.6 Sex (% men): intervention: 61.6; control: 64.7 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 138.6, 20.1; control: 139.5, 21.1 Heart rate (beats/min, mean, SD): intervention: 79.2, 13.6; control: 78.9, 13.7 BMI: not reported Serum creatinine (mg/dL, mean, SD)*: intervention: 1.2, 0.4; control: 1.2, 0.4 B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 36, 13; control: 36, 12 NYHA class I (%): intervention: 3.0; control: 2.7 NYHA class II (%): intervention: 56.5; control: 56.3 NYHA class III (%): intervention: 38.7; control: 38.7 NYHA class IV (%): intervention: 1.8; control: 2.3 Hypertension (%): intervention: 61.1; control: 62.3 Diabetes (%): intervention: 26.9; control: 25.3 Atrial fibrillation (%): intervention: 33.8; control: 35.5 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 68.9; control: 67.6 Stroke (%): intervention: 0.1; control: 0 Diuretic (%); intervention: 85.8; control: 85.5 Digoxin (%): not reported Beta‐blocker (%): study drug ACEI (%): intervention: 81.7; control: 82.6 ARB (%): intervention: 6.2; control: 7.1 MRA (%): intervention: 28.8; control: 26.4 | |
| Interventions | Intervention: nebivolol "Nebivolol or placebo tablets were provided in identical packaging and tablet appearance. The initial dose was 1.25 mg once daily, and, if tolerated, this was increased to 2.5 and 5 mg, respectively, every 1–2 weeks, reaching a target of 10 mg once daily over a maximum of 16 weeks." Comparator: placebo Concomitant medication: exclusion criteria: current use of beta blockers | |
| Outcomes | Planned: planned in protocol (Shibata 2002): primary: all cause mortality and cardiovascular hospital admissions (time to first event). Secondary: all cause mortality, composite of all cause mortality or all cause hospital admissions, cardiovascular hospital admissions, cardiovascular mortality, functional capacity by NYHA class, functional capacity by 6 min walk test Reported: reported: compliance to treatment, haemodynamics, death or cardiovascular hospital admission, all cause mortality, cardiovascular hospital admissions, total mortality, cardiovascular mortality, all cause hospitalisation | |
| Notes | subgroup of interest, partial outcome data reported baseline data for all participants, outcome data for subgroup only (nebivolol N = 320, placebo N = 323) emailed trialists to ask for details on HF hospitalisation for LVEF > 40%, withdrawal due to AE, hyperkalaemia. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization to nebivolol or placebo on a 1:1 basis was carried out by telephone call to a central office (Clinical Data Care, Lund, Sweden)." |
| Allocation concealment (selection bias) | Low risk | "Patients were allocated a treatment number which corresponded to the appropriate study treatment packs." |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐blind"; no details |
| Blinding of outcome assessment (detection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Low risk | ITT used |
| Selective reporting (reporting bias) | Low risk | primary outcomes reported as planned |
| Other bias | Low risk | "Dr. van Veldhuisen has received lecture fees from Menarini and was a member of the steering committee of the SENIORS trial. Dr. Cohen‐Solal has received lecture and consultancy fees from Menarini, and was a member of the steering committee for the SENIORS trial and received lecture fees. Dr. Böhm has received speaker fees from Menarini. Dr. Anker has received speaking honoraria from Menarini Ricerche SpA, Roche, Merck, and Tanabe. Dr. Babalis’s department has received a grant from Menarini. Dr. Coats has received honoraria from Menarini. Dr. Poole‐Wilson has received honoraria from Menarini for speaking about the SENIORS trial. Dr. Flather has received research grant funding to his institution from Menarini and speaker fees from Menarini for lectures at scientific meetings and symposia. The original SENIORS trial was supported by Menarini Ricerche SpA, Italy. Funding for additional statistical analyses for the present study to the Clinical Trials and Evaluation Unit in London were obtained. All members of the Steering Committee of the SENIORS trial have received honoraria for speaking on aspects of heart failure and beta‐blockers at meetings funded by companies in the pharmaceutical industry." "SENIORS is sponsored by Menarini Ricerche SpA." |
| Methods | Study design: two‐arm, individual, RCT Centres: not reported Start of enrolment: August 2000 End of enrolment: March 2002 Follow‐up: 6‐12 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "Patients were included in the study if they had (1) a history of uncorrected rheumatic heart valvular disease or New York Heart Association (NYHA) functional class III or IV disease, necessitating hospitalization; (2) a cardiothoracic ratio of less than 65%; (3) AF with a resting ventricular rate of 70 beats/ minute or more for at least three months, as depicted on the electrocardiogram (ECG); and (4) an echocardiogram showing a significant mitral stenosis or aortic lesions and mitral valve regurgitation." Exclusion criteria: "Patients were excluded from the study if they had uncorrected congenital heart disease, sustained ventricular tachycardia, severe liver and kidney dysfunction, chronic obstructive pulmonary disease, bronchial asthma, obstructive or restrictive cardiomyopathy or myocarditis, myocardial infarction, or unstable angina within the previous three months. Patients were also ineligible for enrollment if they required intensive care or concurrent intravenous therapy or if they were using calcium‐channel blockers, class I or III antiarrhythmic drugs, monoamine oxidase (MAO)–inhibitors or beta2‐agonists." Randomised (N): 88 (not reported by treatment arm) Withdrawn (N): 20 (did not complete the study) intervention: 11 (5 due to suspected adverse drug effects); control: 9 Lost to follow‐up (N): 14 (excluded from the evaluation at follow‐up ‐ 7 had insufficient quality of echocardiography or difficulties with telephone‐connection) Analysed (N): 67 (intervention: 33; control: 34) Age (years, mean, SD): intervention: 40.6, 6.8; control: 43.5, 7.4 Sex (% male): intervention: 36; control: 35 Ethnicity not reported Systolic blood pressure (mmHg, mean, SD): intervention: 115, 12; control: 121, 14 Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide not reported LVEF not reported NYHA class not reported Hypertension not reported Diabetes not reported Atrial fibrillation not reported Hospitalisation for heart failure: not reported Coronary heart disease not reported Stroke not reported Diuretic not reported Digoxin not reported Beta‐blocker not reported ACEI not reported ARB not reported MRA not reported | |
| Interventions | Intervention: Bisoprolol, "All patients in the treatment group received bisoprolol at the initial dose of 1.25 mg/day. The recommended maximal dose was 10 mg/day. The dose schedule for titration of the selective beta1 blocker was gradually increased over three to five days, by two to three weeks, to as high as 10 mg/day, with adjustments of diuretics and ACE‐inhibitors, as clinically indicated." Comparator: "control" (unspecified) Concomitant medication: "At the discretion of the treating physicians, all patients were given concomitant therapy consisting of one of the following: | |
| Outcomes | Planned: we did not identify a published protocol or pre‐registered clinical trial register record | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "On the basis of admission sequence, patients were randomly assigned to a treatment group or a control group" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | High risk | unclear reporting of withdrawals/loss‐to‐follow up |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | no funding source reported |
| Methods | Study design: two‐arm, individual, RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Follow‐up: 3 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "in ambulatory patients (pts) with arterial hypertension and CHF and preserved systolic left ventricular (LV) function" "According including/exclusion criteria pts have had seated systolic BP(SBP)≤160mmHg and diastolic BP(DBP) ≤ 95mmHg at randomization." "with stable symptomatic CHF (NYHA class II‐III) as a result of arterial hypertension (AH) with preserved LV ejection fraction (EF) ≥ 50%" Exclusion criteria: not reported Randomised (N): 726 (416 intervention, 310 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age not reported Sex not reported Ethnicity not reported Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide not reported LVEF not reported NYHA class not reported Hypertension not reported Diabetes not reported Atrial fibrillation not reported Hospitalisation for heart failure: not reported Coronary heart disease not reported Stroke not reported Diuretic not reported Digoxin not reported Beta‐blocker not reported ACEI not reported ARB not reported MRA not reported | |
| Interventions | Intervention: quinapril Comparator: "conventional treatment, recommended for CHF [congestive heart failure] and AH [arterial hypertension] treatment" Concomitant medication: not reported | |
| Outcomes | Planned: unclear Reported: NYHA, 6MWD, clinical status, QoL (MLHFQ), 2D echocardiography, blood pressure | |
| Notes | Unable to find contact details to ask investigators for end scores for QoL, full publication of results and mortality data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "were randomly assigned" but no detail |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | not reported |
| Other bias | High risk | published conference abstract only |
| Methods | Study design: two‐arm, individual, RCT Centres: Poland, "of each centre" suggests multicentre trial, no details Start of enrolment: Novemer 2011 End of enrolment: February 2015 Mean follow‐up: 6 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "Patients who presented with signs or symptoms of HF (dyspnea, fatigue, and exercise intolerance) consistent with New York Heart Association functional class II or III, with preserved LV ejection fraction (> 50%), and with evidence of diastolic dysfunction, were considered suitable for screening." Exclusion criteria: "Exclusion criteria were: Atrial fibrillation or flutter Resting heart rate > 90 beats/min Ischemic heart disease (defined by a positive coronary angiogram or inducible ischemia during exercise testing) Moderate or worse valvular heart disease Primary myocardial diseases Established or suspected pulmonary diseases (spirometry results < 80% of age‐ and sex‐specific reference values) Hemoglobin ≤ 11 g/dl Adrenocortical, hepatic, rheumatic, neoplastic, skeletal, thyroid, and renal diseases (including renal insufficiency with serum creatinine > 1.5 mg/dl [132 mmol/l]) Hyperkalemia > 5.0 mmol/l Known intolerance or treatment with an MRA within the last 3 months Concomitant therapy with a potassium‐sparing agent Current lithium use Pregnancy" Randomised (N): 150 (75 intervention, 75 control) Withdrawn (N): for reasons other than death 12 (7 intervention, 5 control) Lost to follow‐up (N): 7 (4 intervention, 3 control) Analysed (N): 131 (64 intervention, 67 control) Age (years, mean, SD): intervention: 66.3, 7.7; control: 67.6, 9.1 Sex (% men): intervention: 12; control: 19 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 131, 15; control: 130, 18 Heart rate (beats/min, mean, SD): intervention: 72, 10; control: 73, 10 BMI (mean, SD): intervention: 30.7, 4.5; control: 29.7, 4.6 Serum creatinine (mg/dL, mean, SD): intervention: 0.99, 0.20; control: 1.03, 0.24 B‐type natriuretic peptide (pg/mL, median, IQR): intervention: 40 (26‐63); control: 54 (27‐99) NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, median, IQR): intervention: 72.6 (70.4–74.8); control: 71.4 (69.2–73.5) NYHA class I (%): 0 NYHA class II (%): intervention: 78; control: 79 NYHA class III (%): intervention: 22; control: 21 NYHA class IV (%): 0 Hypertension (%): intervention: 92; control: 91 Diabetes (%): intervention: 39; control: 40 Atrial fibrillation (%): not reported Hospitalisation for heart failure (%): intervention: 17; control: 21 Coronary heart disease (%): significant CAD excluded Stroke (%): not reported Diuretic (%); intervention: thiazides 54, loop 13; control: thiazides 46, loop 18 Digoxin (%): not reported Beta‐blocker (%): intervention: 78; control: 72 ACEI/ARB (%): intervention: 97; control: 95 MRA (%): study drug | |
| Interventions | Intervention: spironolactone, 25mg/day Comparator: matching placebo (120 mg/day of microcellulose) Concomitant medication: "Enrollees continued to receive other prescribed treatments throughout the study period." | |
| Outcomes | Planned: unclear Reported: "Coprimary outcomes were change at 6 months in exercise capacity (assessed by peak VO2) and exertional E/e' (reflecting LVFP). The secondary outcomes included change at follow‐up in exercise blood pressure (BP) response and post‐treatment global longitudinal myocardial deformation (GLS) measured by 2‐dimensional strain." | |
| Notes | Emailed investigator to ask for additional outcome data relevant to this review. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The study coordinator, who was not involved in study procedures, was responsible for drug randomization and dispensing" |
| Allocation concealment (selection bias) | Low risk | "sequentially‐numbered, opaque, sealed envelopes" |
| Blinding of participants and personnel (performance bias) | Low risk | "Patients and investigators performing the assessments and data analysis were blinded to group assignment." |
| Blinding of outcome assessment (detection bias) | Low risk | "core laboratory in Hobart, Australia, for independent adjudication of the primary endpoint" |
| Incomplete outcome data (attrition bias) | Low risk | ITT not used, withdrawals reported with reasons, lost to follow‐up reported, similar numbers for treatment arms |
| Selective reporting (reporting bias) | Unclear risk | unclear, clinical trial registration was post‐hoc |
| Other bias | Low risk | "The authors have reported that they have no relationships relevant to the contents of this paper to disclose" "This study was funded by grants ST‐678 from Wroclaw Medical University and 13‐024 from the Royal Hobart Hospital Foundation." |
| Methods | Study design: parallel, individual RCT Centres: 17, Japan Start of enrolment: October 2006 End of enrolment: March 2010 Median follow‐up: 4.4 years Run‐in period: not reported | |
| Participants | Inclusion criteria: The inclusion criteria of the present study were designed to enroll symptomatic CHF patients with hypertension aged 20 to 79 years who were treated with ACEI or beta‐blocker or both. Inclusion criteria: NYHA Classes II to IV CHF, History of hypertension or treated with anti‐hypertensive medications, Aged 20 or older and, 80 years at the entry, Stable with angiotensin‐converting enzyme inhibitors and/or b‐blockers, Not treated with angiotensin II receptor blockers Exclusion criteria: The exclusion criteria were designed to exclude patients with substantive confounding medical conditions or an inability to meaningfully participate in the SUPPORT trial. Exclusion criteria: Patients who have renal dysfunction (serum creatinine ≥3.0 mg/dL), or those who are under chronic haemodialysis, Drug hypersensitivity to olmesartan, Severe liver dysfunction, History of angioedema, History of malignant tumour or life‐threatening illness of poor prognosis, Pregnant or possibly pregnant patients, Cardiovascular surgery within 6 months prior to the date of the entry, Acute myocardial infarction within 6 months prior to the date of the entry, Percutaneous coronary intervention with or without stent implantation within 6 months prior to the date of the entry. Randomised (N): 1146 (1 patient excluded prior to this for protocol violation) (578 intervention, 568 control) Withdrawn (N): for reasons other than death 9 (1 protocol violation, 8 no LVEF data) Lost to follow‐up (N): not reported Analysed (N): Total 1138 (HFpEF 709, HFrEF 429) (HFpEF 363 intervention, HFpEF 346 control) Age (years, mean, SD): intervention: 66.5, 10.1; control: 65.9, 9.7 Sex (% men): intervention: 70.2; control: 71.1 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 131.5, 17.1; control: 130.1, 17.1 Heart rate (beats/min, mean, SD): intervention: 70.6, 13.2; control: 71.4, 14.9 BMI (mean, SD): intervention: 24.4, 4.2; control: 24.8, 4.2 Serum creatinine (mg/dL, mean, SD): intervention: 0.9, 0.3; control: 0.9, 0.3 B‐type natriuretic peptide (pg/mL, median, IQR): intervention: 71.1 (30.2, 148.0); control: 58.7 (27.5, 139.0) NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 63.8, 8.8; control: 63.1, 8.6 NYHA class I (%): 0 NYHA class II (%): intervention: 94.2; control: 93.4 NYHA class III (%): intervention: 5.5; control: 6.4 NYHA class IV (%): 0 Hypertension (%): 100 Diabetes (%): intervention: 46.6; control: 53.9 Atrial fibrillation (%): not reported Hospitalisation for heart failure (%): intervention: 52.2; control: 44.1 Coronary heart disease (%): intervention: 48.8; control: 45.1 Stroke (%): not reported Diuretic (%); intervention: 45.7; control: 48.0 Digoxin (%): not reported Beta‐blocker (%): intervention: 63.4; control: 65.4 ACEI (%): intervention: 79.9; control: 79.0 ARB (%): study drug MRA (%): intervention: 18.5; control: 22.0 | |
| Interventions | Intervention: olmesartan. "Olmesartan was initiated at a dose of 5–10 mg/day, and then up titrated to 40 mg/ day, if tolerable, in the olmesartan group, while no ARB use was allowed in the control group" Comparator: no treatment Concomitant medication: treated with ACEI and/or beta‐blocker in inclusion criteria, not treated with ARB | |
| Outcomes | Planned: Clinical trial registry entry at point of enrolment: primary outcomes all‐cause death, nonfatal acute myocardial infarction, nonfatal stroke, hospital admission due to congestive heart failure Reported: Primary Endpoint: A composite of the following outcomes: all‐cause death, non‐fatal acute myocardial infarction, non‐fatal stroke, hospital admission due to worsening heart failure | |
| Notes | Emailed investigators to ask for outcome date for participants with LVEF > 40%. No response. Published (and presented above) are baseline characteristics and results for HFpEF as defined by investigators (LVEF ≥ 50%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | High risk | open label |
| Blinding of outcome assessment (detection bias) | Low risk | blinded endpoint study |
| Incomplete outcome data (attrition bias) | Unclear risk | withdrawals reported with reasons, not detailed by treatment arm ITT used, but after exclusion of some randomised patients |
| Selective reporting (reporting bias) | Low risk | primary outcomes reported as planned |
| Other bias | Low risk | "The Department of Evidence‐based Cardiovascular Medicine, Tohoku University Graduate School of Medicine, is supported in part by the unrestricted research grants from Daiichi Sankyo Co, Ltd (Tokyo, Japan), Bayer Yakuhin, Ltd (Osaka, Japan), Kyowa Hakko Kirin Co, Ltd (Tokyo, Japan), Kowa Pharmaceutical Co, Ltd (Tokyo, Japan), Novartis Pharma K.K. (Tokyo, Japan), Dainippon Sumitomo Pharma, Co, Ltd (Osaka, Japan), and Nippon Boehringer Ingelheim Co, Ltd (Tokyo, Japan). H.S. has received lecture fees from Bayer Yakuhin, Ltd (Osaka, Japan), Daiichi Sankyo Co, Ltd (Tokyo, Japan) and Novartis Pharma K.K. (Tokyo, Japan)." "This study was supported in part by the grants‐in‐aid from the Ministry of Health, Labour, and Welfare and those from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. Funding to pay the Open Access publication charges for this article was provided by the author." |
| Methods | Study design: two‐arm, individual, parallel RCT Centres: 12, Sweden Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: not reported Run‐in period: not reported | |
| Participants | Inclusion criteria: "patients with symptoms and/or signs of HF, normal or almost normal systolic function and abnormal DF who did not have a contraindication to receiving therapy with a beta‐ adrenoceptor blocking agent were included into the study." "Major inclusion criteria were a wall motion index (WMI) ≤ 1.2, i.e akinesia of one segment or less or hypokinesia of 2 segments or less, using a 16 segment model with at least 10 segments visible, corresponding to an LVEF > 45%, and evidence of abnormal DF using at least one of the following criteria to assess diastolic dysfunction" Exclusion criteria: "Major exclusion criteria were restrictive or hypertrophic cardiomyopathies, significant uncorrected obstructive or regurgitant valvular diseases, unstable angina, active myocarditis, uncontrolled symptomatic ventricular arrhythmias, history of sick sinus syndrome, second or third degree AV‐block, heart rate less than 60 bpm, systolic blood pressure ‐85 mmHg, uncontrolled hypertension, atrial fibrillation, evidence of obstructive pulmonary disease, unstable diabetes, treatment with beta‐2‐agonists, MAO‐inhibitors, calcium channel blockers or beta‐receptor blockers" Randomised (N): 113 Withdrawn (N): for reasons other than death: "16 patients had echocardiographic data of insufficient quality and were excluded from the evaluation." Lost to follow‐up (N): 2 (reasons not reported) Analysed (N): 97 (47 intervention, 50 control) Age (years, median, IQR): intervention: 67 (48 to 81); control: 66 (48 to 84) Sex (% men): intervention: 59.6; control: 54.0 Ethnicity (%): not reported Systolic blood pressure (mmHg, median, IQR): intervention: 155 (122 to 180); control: 150 (110 to 200) Heart rate (beats/min, median, IQR): intervention: 74 (60 to 95); control: 73 (60 to 101) BMI not reported Serum creatinine not reported B‐type natriuretic peptide (pg/mL): intervention: 67.7, 76.1; control: 67.7, 67.7 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF not reported NYHA class I (%): intervention: 40; control: 26 NYHA class II (%): intervention: 53; control: 53 NYHA class III (%): intervention: 7; control: 21 NYHA class IV (%): 0 Hypertension (%): intervention: 70.2; control: 62 Diabetes (%): intervention: 12.8; control: 16.0 Atrial fibrillation (%): 0 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 17; control: 6 Stroke (%): not reported Diuretic (%); not reported Digoxin (%): not reported Beta‐blocker (%): study drug ACEI (%): not reported ARB (%): not reported MRA (%): not reported | |
| Interventions | Intervention: carvedilol. "carvedilol or placebo twice daily in addition to their conventional treatment" "All patients were uptitrated to the maximum tolerated dose or to the target dose (25 mg b.i.d., or 50 mg b.i.d. in patients weighing 85 kg) of carvedilol or matching placebo. After completion of uptitration they were to continue on double blind medication for a 6 month maintenance period. At study end patients were withdrawn from blinded study medication in a stepwise manner over a 1–3 week period. Optimal therapy for the patient’s condition was then reinstated at the investigator’s discretion" "Overall, carvedilol was well tolerated, with 81% of patients receiving the maximum dose at the end of the uptitration phase (25 mg b.i.d. or 50 mg b.i.d.) and 82% at the end of the study" Comparator: placebo Concomitant medication: "as an addition to conventional treatment" | |
| Outcomes | Planned: not able to assess as we are unaware of a published protocol or pre‐registration in a clinical trial register Reported: primary: diastolic dysfunction. Secondary: Secondary endpoints were the effects of carvedilol as compared to placebo on combined all cause mortality and cardiovascular hospitalisations, combined all‐cause mortality and heart failure hospitalisation, progression of heart failure, individual cardiovascular endpoints and outcome and individual diastolic variables. Additional exploratory analyses on LV dimensions atrial size and WMI were also prespecified." | |
| Notes | Emailed investigators for details for RoB assessment, reasons for 2 participants not completing study, start/end of enrolment, duration of follow‐up. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Low risk | double‐blind but no details, matching placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | "All assessments as to whether the LV diastolic dysfunction had improved, was unchanged, or had worsened were made by two echocardiographers from the core laboratory, who were blinded to the order of the assessment and to the study medication received by the patient." only partial outcome assessment and for outcomes not relevant to this review |
| Incomplete outcome data (attrition bias) | Unclear risk | unable to assess ‐ withdrawals not reported by treatment arm ITT used |
| Selective reporting (reporting bias) | Unclear risk | unable to assess due to lack of published protocol or pre‐registration in a clinical trial register |
| Other bias | Low risk | "The study was investigator‐initiated and was partly funded by F. Hoffmann‐La Roche Ltd." |
| Methods | Study design: parallel RCT Centres: 1, Japan Start of enrolment: April 2000 End of enrolment: March 2001 Mean follow‐up: 12 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "All patients met Framingham criteria for diagnosis of heart failure. LVEF, as assessed by echocardiography using Simpson’s method, was ≥ 45% in each subject at the screening examination." Exclusion criteria: "Patients with primary significant valvular disease, cor pulmonale, thyroid dysfunction, diabetes mellitus with hemoglobin A1C > 8%, alcohol abuse, other systemic diseases, obvious contraindication to carvedilol, or using angiotensin II receptor antagonists or adrenergic blockers were excluded from the initial entry." Randomised (N): 40 (19 intervention, 21 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, median, IQR): intervention: 69.1, 64.4‐73.7; control: 73.1, 69.7–76.4 Sex (% men): intervention: 68; control: 38 Ethnicity (%): not reported Systolic blood pressure (mmHg, median, IQR): intervention: 129.6, 124.5–134.6; control: 138.0, 130.7–145.3 Heart rate (beats/min, median, IQR): intervention: 70.6, 64.3–77.0; control: 68.9, 63.8–74.0 BMI not reported Serum creatinine not reported B‐type natriuretic peptide (pg/mL, median, IQR): intervention: 172, 135–209; control: 150, 114–186 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, median, IQR): intervention: 55.8, 51.4–60.3; control: 57.5, 53.4–61.5 NYHA class I (%): 0 NYHA class II (%): intervention: 63.3; control: 71 NYHA class III (%): intervention: 37; control: 29 NYHA class IV (%): 0 Hypertension not reported Diabetes not reported Atrial fibrillation (%): intervention: 21; control: 38 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 58; control: 48 Stroke not reported Diuretic not reported Digoxin not reported Beta‐blocker study drug ACEI (%): intervention: 79; control: 86 ARB not reported MRA not reported | |
| Interventions | Intervention: carvedilol. "initial daily dosage of 1.25 mg in addition to conventional therapy, and the dose was doubled every week until reaching >= 5 mg/day. The decision to increase carvedilol to > 5 mg/day was made by the attending cardiologists on the basis of subjective symptoms, Comparator: conventional treatment Concomitant medication: not reported | |
| Outcomes | Planned: we are not aware of a published protocol or a pre‐registered clinical trial registry entry Reported: BNP, NYHA, exercise capacity, heart failure hospitalisations, deaths | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | funding not reported |
| Methods | Study design: parallel RCT Centres: "233 sites in 6 countries (1151 participants in the United States, 326 in Canada, 167 in Brazil, 123 in Argentina, 1066 in Russia, and 612 in Georgia)" Start of enrolment: August 2006 End of enrolment: January 2012 Mean follow‐up: 3.3 years Run‐in period: no | |
| Participants | Inclusion criteria: "≥ 50 years old, "had at least one sign and at least one symptom of heart failure on a prespecified list of clinically defined signs and symptoms, a left ventricular ejection fraction of 45% or more as measured at the local site by means of echocardiography or radionuclide ventriculography, controlled systolic blood pressure (defined as a target systolic blood pressure of < 140 mm Hg or ≤ 160 mm Hg if the patient was taking three or more medications to control blood pressure), and a serum potassium level of less than 5.0 mmol per liter. In addition, eligible patients had a history of hospitalization within the previous 12 months, with management of heart failure a major component of the care provided (not adjudicated by the clinical‐events adjudication committee), or an elevated natriuretic peptide level within 60 days before randomization (a brain natriuretic peptide [BNP] level ≥ 100 pg per milliliter or an N‐terminal pro‐BNP [NTproBNP] level ≥360 pg per milliliter)." Exclusion criteria: "severe systemic illness with a life expectancy of less than 3 years, severe renal dysfunction (an estimated glomerular filtration rate [GFR] of <30 ml per minute per 1.73 m2 of body‐surface area or a serum creatinine level that was ≥ 2.5 mg per deciliter [221 μmol per liter]), and specific coexisting conditions, medications, or acute events." Randomised (N): 3445 (1722 intervention, 1723 control) Withdrawn (N): 311 for reasons other than death (160 intervention, 151 control) Lost to follow‐up (N): 132 (67 intervention, 65 control) Analysed (N): 3445 (1722 intervention, 1723 control) Age (years, median, IQR): intervention: 68.7, 61.0 to 76.4; control: 68.7, 60.7 to 75.5 Sex (% men): intervention: 48.4; control: 48.5 Ethnicity (%): intervention: white 88.6, control: white 89.2 Systolic blood pressure (mmHg, median, IQR): intervention: 130 ,120‐139; control: 130, 120‐140 Heart rate (beats/min, median, IQR): intervention: 68, 62‐76; control: 68, 62‐76 BMI (median, IQR): intervention: 31, 27‐36; control: 31, 27‐36 Serum creatinine (mg/dL, median, IQR): intervention: 1.0, 0.9‐1.2; control: 1.1, 0.9‐1.2 B‐type natriuretic peptide (pg/mL): only in subgroup NT pro B‐type natriuretic peptide (pg/mL): only in subgroup LVEF (%, median, IQR): intervention: 56, 51‐61; control: 56, 51‐62 NYHA class I (%): intervention: 3.3; control: 3.1 NYHA class II (%): intervention: 63.3; control: 64.1 NYHA class III (%): intervention: 33.0; control: 32.1 NYHA class IV (%): intervention: 0.4; control: 0.5 Hypertension (%): intervention: 91; control: 92 Diabetes (%): intervention: 33; control: 32 Atrial fibrillation (%): intervention: 35; control: 35 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 26; control: 26 Stroke (%): intervention: 7; control: 8 Diuretic (%); intervention: 81; control: 82 Digoxin not reported Beta‐blocker (%): intervention: 78; control: 77 ACEI or ARB (%): intervention: 84; control: 84 MRA (%): 0 | |
| Interventions | Intervention: spironolactone "Study drugs were initially administered at a dose of 15 mg once daily, which was increased to a maximum of 45 mg daily during the first 4 months after randomization. Subsequent dose adjustments were made as required." Comparator: matching placebo Concomitant medication: "Study patients continued to receive other treatments for heart failure and coexisting illnesses throughout the trial." | |
| Outcomes | Planned: From NCT record 21 April 2006: primary outcomes: cardiovascular mortality, aborted cardiac arrest, composite of hospitalisation for the management of heart failure (ie hospitalisation for non‐fatal myocardial infarction or non‐fatal stroke). Secondary outcomes: all‐cause mortality, composite of cardiovascular mortality or cardiovascular related hospitalization (i.e. hospitalization for non‐fatal myocardial infarction, non‐fatal stroke, or the management of heart failure), hospitalization for the management of heart failure incidence rate, sudden death or aborted cardiac arrest Reported: "composite of death from cardiovascular causes, aborted cardiac arrest, or hospitalization for the management of heart failure; myocardial infarction; stroke; hospitalisation from any cause; hyperkalemia (potassium level, ≥ 5.5 mmol per liter); hypokalemia (potassium level, < 3.5 mmol per liter); an elevated serum creatinine level (≥ 2 times the baseline value and above the upper limit of the normal range); serum creatinine level of 3.0 mg per deciliter (265 μmol per liter) or higher; serious adverse events" | |
| Notes | Kao 2017 mentions subgroup analysis by sex for all‐cause mortality and hospitalisations but no usable data. NCT record reports on QoL but no usable data. Hamo 2015 reports baseline QoL data but not by intervention arm. Solomon 2016 reports data for four LVEF groups for HF hospitalisation, CV death, death (table 2) ‐ 40‐49%, 50‐54.99%, 55‐59.99%, 60% and over. Data for all‐cause mortality, lost to follow up, hyperkalemia differ between Pitt 2014 and NCT results. Emailed investigators to ask for end scores for QoL KCCQ, clarification on withdrawals due to adverse events and subgroup data for primary outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible participants were randomly assigned to receive either spironolactone or placebo in a 1:1 ratio with the use of permuted blocks." "the randomization software will return a Treatment Allocation Code corresponding to either spironolactone or placebo" |
| Allocation concealment (selection bias) | Low risk | "The nurse coordinator will utilize a master list of Treatment Allocation Codes to determine which labelled study drug packet to provide to the subject." |
| Blinding of participants and personnel (performance bias) | Low risk | "Subjects and treating physicians will be blinded to whether subjects are receiving spironolactone or placebo" |
| Blinding of outcome assessment (detection bias) | Low risk | "Data were collected and managed electronically by the New England Research Institutes Clinical Trial Coordinating Center, which also coordinated site monitoring and analyzed the trial results (with independent verification at Brigham and Women’s Hospital)." |
| Incomplete outcome data (attrition bias) | Low risk | "All randomly assigned participants were included in all analyses according to the intention‐to‐treat principle." |
| Selective reporting (reporting bias) | Low risk | reported as planned |
| Other bias | Low risk | "sponsored by National Heart, Lung and Blood Institute, National Institutes of Health" |
| Methods | Study design: parallel, individual, RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Follow‐up: 9 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "HFpEF was defined as history, symptoms, and signs of HF, a preserved LVEF of 50% or greater and no evidence of other medical condition that could mimic HF symptoms" Exclusion criteria: "Coronary disease was excluded according to history, medical record, electrocardiogram, and rest and exercise echocardiogram" "Exclusions included aldosterone antagonist use within the previous 3 months, a known contraindication, concomitant therapy with a potassium‐sparing diuretic or potassium supplementation, baseline serum potassium level greater than 5.0 mEq/L, or serum creatinine level of 2.5 mg/dL or greater." Randomised (N): 80 (42 intervention, 38 control) Withdrawn (N): for reasons other than death 9 (5 intervention (adverse event N = 1, patient choice N = 4), 4 control (patient choice N = 3, death N = 1)) Lost to follow‐up (N): not reported Analysed (N): 71 (37 intervention, 34 control) Age (years, mean, SD): intervention: 70.0, 1.1; control: 72.0, 1.2 Sex (% men): intervention: 19; control: 21 Ethnicity (%): African American: intervention: 21 , control: 37 Systolic blood pressure (mmHg, mean, SD): intervention: 139, 2.7; control: 143, 3.2 Heart rate not reported BMI (mean, SD): intervention: 31.5, 0.8; control: 32.4, 1.2 Serum creatinine not reported B‐type natriuretic peptide (unit not reported): intervention: 55, 46; control: 61, 50 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 62.6, 1.1; control: 62.0, 1.1 NYHA class I (%): 0 NYHA class II (%): intervention: 29; control: 26 NYHA class III (%): intervention: 64; control: 63 NYHA class IV (%): 0 Hypertension (%): intervention: 83; control: 92 Diabetes (%): intervention: 17; control: 29 Atrial fibrillation (%): not reported Hospitalisation for heart failure: not reported Coronary heart disease (%): 0 Stroke (%): not reported Diuretic (%); intervention: 74; control: 71 Digoxin (%): intervention: 2; control: 0 Beta‐blocker (%): intervention: 31; control: 32 ACEI (%): not reported ARB (%): not reported MRA (%): study drug | |
| Interventions | Intervention: spironolactone. "The starting dose of spironolactone was 12.5 mg/d in individuals with baseline creatinine of 2.0 mg/dL or greater or potassium greater than 4.5 mEq/L; in all other participants, the starting dose was 25 mg/d. In participants who initiated therapy with the 12.5‐mg/d dose, the dose was increased to 25 mg/d once creatinine fell below 2.5 mg/dL and potassium fell below 5.0 mEq/L and maintained at that dosage as long as those levels were maintained. Spironolactone was discontinued if 1‐week creatinine was 2.5 mg/ dL or higher or potassium was 5.0 mEq/L or higher. " "The mean daily dose of spironolactone was 24.3 2.9 mg/d." Comparator: matching placebo Concomitant medication: not reported | |
| Outcomes | Planned: July 2005, NCT record: primary outcomes: exercise intolerance, quality of life Reported: exercise performance, aortic distensibility and LV structure and function, carotid artery stiffness, pulse wave velocity, LV diastolic filling, QoL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "The research pharmacy prepared and distributed placebo and active drug using a secure methodology. All investigators, staff, and participants were fully blinded to treatment group assignment throughout the study period" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The research pharmacy prepared and distributed placebo and active drug using a secure methodology. All investigators, staff, and participants were fully blinded to treatment group assignment throughout the study period" |
| Incomplete outcome data (attrition bias) | Unclear risk | unable to assess |
| Selective reporting (reporting bias) | Unclear risk | posthoc clinical trial registration |
| Other bias | High risk | "This study was funded by the National Institutes of Health (NIH; R01AG18915), the Claude D. Pepper Older Americans Independence Center, Wake Forest University (P30AG21332), the Clinical and Translational Science Institute, Wake Forest School of Medicine (NIH UL1TR001420), and the Kermit G. Phillips Chair in Cardiovascular Medicine of Wake Forest School of Medicine" Published as conference abstract only. |
| Methods | Study design: parallel, individual, RCT Centres: 1, Taiwan Start of enrolment: not reported End of enrolment: not reported Follow‐up: at least 3 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "hypertensive pts who had DHF, defined as the presence of HF signs/symptoms, diastolic dysfunction (mitral annular early diastolic velocity (E') < 8 cm/s), and left ventricular (LV) ejection fraction (EF) > 50%" Exclusion criteria: not reported Randomised (N): 36 (19 intervention, 17 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SD): not reported Sex (% men): not reported Ethnicity (%): not reported Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide not reported LVEF (%, mean, SD): intervention: 67, 7; control: 66, 7 NYHA class not reported Hypertension not reported Diabetes not reported Atrial fibrillation not reported Hospitalisation for heart failure: not reported Coronary heart disease not reported Stroke not reported Diuretic not reported Digoxin not reported Beta‐blocker not reported ACEI not reported ARB not reported MRA study drug | |
| Interventions | Intervention: spironolactone. 50 mg/d Comparator: no treatment control Concomitant medication: not reported | |
| Outcomes | Planned: we are not aware of a published protocol or pre‐registered clinical trial registry entry Reported: echo‐parameters, systolic myocardial velocities | |
| Notes | does not contribute outcome data to this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | funding not reported |
| Methods | Study design: parallel, individual, RCT Centres: 1, Turkey Start of enrolment: May 2008 End of enrolment: March 2009 Mean follow‐up: 11 months Run‐in period: not reported | |
| Participants | Inclusion criteria: "HF symptoms. They were ≥ 50 years old, had EF ≥ 50% and echocardiographic diastolic dysfunction." Exclusion criteria: not reported Randomised (N): 108 (54 intervention, 54 control) Withdrawn (N): for reasons other than death 17 (not reported by arm) Lost to follow‐up (N): 3 (not reported by arm) Analysed (N): not reported Age not reported Sex not reported Ethnicity not reported Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide not reported LVEF not reported NYHA class not reported Hypertension not reported Diabetes not reported Atrial fibrillation not reported Hospitalisation for heart failure: not reported Coronary heart disease not reported Stroke not reported Diuretic not reported Digoxin not reported Beta‐blocker not reported ACEI study drug ARB not reported MRA not reported | |
| Interventions | Intervention: perindopril, 10 mg/d Comparator: "standard DHF treatment" Concomitant medication: not reported | |
| Outcomes | Planned: we are not aware of a published protocol or a pre‐registration in a clinical trial register Reported: T‐proBNP values and echocardiography | |
| Notes | no outcome data of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomised, but no details |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | funding not reported |
| Methods | Study design: parallel, individual, RCT Centres: 1, Royal Liverpool and Broadgreen University Hospitals Start of enrolment: 1997 End of enrolment: 1999 Follow‐up: 6 months Run‐in period: mentioned but no details | |
| Participants | Inclusion criteria: "aged 65 years or older, with heart failure" "They all had left ventricular ejection fraction (LVEF) on echocardiography or radionuclide ventriculography equal or greater than 40%. Where a left ventricular ejection fraction could not be measured systolic function had to be preserved or only mildly impaired by direct visualisation of the echocardiograms" Exclusion criteria: "Patients with haemodynamically significant valvular disease, pulmonary hypertension, right ventricular systolic dysfunction, uncontrolled atrial fibrillation or flutter, unstable angina pectoris, hypotension, myocardial infarction within one month, renal failure (serum creatinine >150 mmol/L), renal‐artery stenosis, severe liver or pulmonary disease were excluded. patients treated with tetracyclines, lithium, benzodiazepines, major tranquillisers, anti‐depressants (with the exception of selective serotonin re‐uptake inhibitors) or major psychoactive drugs were also excluded." Randomised (N): 74 (36 intervention, 38 control) Withdrawn (N): for reasons other than death 4 (0 intervention, 4 control (worsening heart failure)) Lost to follow‐up (N): not reported Analysed (N): 74 (36 intervention, 38 control) Age (years, mean, SD): intervention: 77, 7; control: 78, 7 Sex (% men): intervention: 38.9; control: 31.6 Ethnicity (%): not reported Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide not reported LVEF not reported NYHA class I (%): intervention: 5.5; control: 0 NYHA class II (%): intervention: 77.8; control: 73.7 NYHA class III (%): intervention: 16.7; control: 26.3 NYHA class IV (%): 0 Hypertension (%): intervention: 27.8; control: 31.6 Diabetes (%): intervention: 11.1; control: 18.4 Atrial fibrillation (%): intervention: 38.9; control: 31.6 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 55.6; control: 57.9 Stroke (%): not reported Diuretic (%); intervention: 94.4; control: 97.1 Digoxin (%): intervention: 38.9; control: 26.3 Beta‐blocker (%): intervention: 19.4; control: 7.9 ACEI (%): study drug ARB (%): not reported MRA (%): not reported | |
| Interventions | Intervention: quinapril. "Both drugs were titrated at two‐week intervals from 5 mg to 40 mg daily or equivalent within the first six weeks." Comparator: placebo Concomitant medication: "All patients continued concomitant treatment with diuretics, nitrates, digitalis glycosides, calcium channel blockers, and beta‐blockers as appropriate without change of dose except for diuretics. Therapy with ACE inhibitors for heart failure was withdrawn at least two weeks prior to the run‐in period." | |
| Outcomes | Planned: we are not aware of a published protocol or pre‐registered clinical trial registry entry Reported: 6‐minutes walking distance, hypotension, worsening heart failure, changes of electrolytes, adverse events, quality of life, deaths, heart failure hospitalisation, hyperkalaemia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomised, but no further detail |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Low risk | double‐blind, matching placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Low risk | withdrawals due to worsening heart failure reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Low risk | "This study was supported by the grants from Parke Davis & Co. Ltd., UK." |
quotes are from the primary reference unless otherwise stated
* mmol/L converted to mg/dL using online converter
ACEI: angiotensin converting enzyme inhibitor
ARB: angiotensin receptor blocker
BMI: body mass index
CVD: cardiovascular disease
EF: ejection fraction
IQR: interquartile range
ITT: intention‐to‐treat
LV: left ventricular
LVEF: left ventricular ejection fraction
MRA: mineralocorticoid receptor antagonist
N: number of people
NCT: clinicaltrials.gov identifier
QoL: quality of life
RCT: randomised controlled trial
SD: standard deviation
TNF‐a: tumour necrosis factor‐alpha
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Trial registry entry suggested two parts of a trial of which only the second was of interest to this review. Contact with trialists confirmed that the part of interest was registered and reported on separately (ACTRN: 12614000088640, STRUCTURE study). | |
| Wrong patient population | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong comparator | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Subgroup of participants of interest (LVEF = 40‐44%). We did not receive a response from the trialists to our enquiry for details on the subgroup of interest. | |
| Wrong study design | |
| Wrong comparator | |
| Wrong comparator | |
| Wrong comparator | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Turkish paper. Translated methods and data extraction. Unclear if participants had heart failure. We did not receive a response from the investigator when we asked for clarification. | |
| Wrong population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong patient population | |
| Ineligible participants. Emailed trialists to clarify inclusion criteria. Response received: "Our study was confined to individuals with a reduced ejection fraction and the data therefore would not be applicable to your quest." | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong comparator | |
| Wrong patient population | |
| Wrong study design | |
| Wrong patient population | |
| EF not reported. Could not identify current contact details. | |
| Wrong patient population | |
| Wrong study design | |
| EF unclear, unclear whether allocation was random, unable to identify current contact details. | |
| Wrong patient population | |
| Wrong patient population | |
| EF unclear. We did not receive a response to our enquiry for details. | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| subgroup of participants of interest; we did not receive a response from the trialist to our enquiry for details. | |
| unclear EF. contacted trialists. no response | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| EF unclear. We did not receive a response from the trialists to our enquiry for details. | |
| Mean EF suggests a subgroup of eligible participants. We did not receive a response from the trialists to our enquiry. | |
| Wrong study design | |
| EF unclear. Unable to identify current contact details. | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong comparator | |
| Wrong study design | |
| EF unclear. Unable to find current contact details for trialists. | |
| Wrong comparator | |
| Wrong patient population | |
| Wrong study design | |
| EF unclear. We did not receive a response from trialists to our query on the clarification of inclusion criteria. | |
| Wrong comparator | |
| Wrong study design | |
| Wrong study design | |
| Wrong comparator | |
| Wrong study design | |
| subgroup of participants of interest; did not receive a response from trialists to our enquiry. | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Trial registry record states completed but no contact details given an no published results identifiable. Sponsor: South Manchester University Hospital NHS Trust. Emailed sponsor to ask whether results are available. Unclear whether http://www.isrctn.com/ISRCTN77645264 is the same trial. Tried to contact investigator but email was undeliverable. | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong study design | |
| Wrong intervention | |
| Wrong study design | |
| Wrong study design | |
| Potential eligible subgroup. Did not receive a response from trialists to our enquiry for outcome data for subgroup. | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Review | |
| Wrong patient population | |
| EF unclear. Unable to identify current contact details. | |
| Wrong patient population | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| subgroup with HF and LVEF >40%. We did not receive a response from trialists to our enquiry. | |
| Wrong study design | |
| Wrong comparator | |
| Wrong study design | |
| Wrong patient population | |
| Limited information in conference abstract. Response to our enquiry for further details received: "the data you've requested was not collected on this group of patients beyond what has been published in that abstract". As we could not confirm whether the participants meet our inclusion criteria, this study was excluded. | |
| Wrong intervention | |
| Wrong study design | |
| Wrong patient population | |
| Wrong study design | |
| Response from trialists received when asked for outcome data for subgroup of interest: no data specifically for participants in subgroup of interest (LVEF 40‐45%) provided. Confirmed that QoL, mortality and HF hospitalisation were not formal endpoints. Hyperkalaemia was not shown by any participants. | |
| Wrong study design | |
| Wrong study design | |
| HF/EF unclear. No response to our enquiry for details. | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong study design | |
| Wrong comparator | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| No participants with heart failure with preserved ejection fraction (confirmed by trialist via email on 20 November 2017). | |
| Heart failure was not an inclusion criteria (confirmed by trialist via email on 15 November 2017). | |
| EF unclear ("ejection fraction measurement was not part of the protocol") | |
| Wrong patient population | |
| Wrong patient population | |
| wrong study design | |
| Wrong patient population | |
| cross‐over trial | |
| EF unclear. Contacted trialists. Response: data are no longer available. | |
| LVEF unclear, otherwise eligible. Emailed investigator but did not receive a response. | |
| Wrong patient population | |
| EF unclear. Unable to find current contact. | |
| Wrong population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong population | |
| Wrong patient population | |
| EF unclear. unable to find current contact details for trialist. | |
| Wrong patient population | |
| LVEF unclear. Contacted trialists. No response. | |
| LVEF unclear, otherwise eligible. Unable to find current contact details for investigators. | |
| Wrong comparator | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong comparator | |
| Wrong comparator | |
| Wrong study design | |
| wrong participants | |
| Wrong study design | |
| retraction | |
| Wrong patient population | |
| Wrong patient population | |
| terminated due to lack of eligible participants | |
| Trial did not take place as planned (as per information from trialists: "We abandoned this study as we could not adequately recruit. No results to present.") | |
| Completed but no publication with results identified. Emailed trialists to ask for clarification on comparator (placebo or conventional antihypertensive treatment). No response. | |
| Wrong comparator | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong comparator | |
| Wrong patient population | |
| Wrong study design | |
| Wrong patient population | |
| Wrong study design | |
| Wrong patient population | |
| Wrong comparator | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| LVEF unclear, otherwise eligible. Unable to find current contact details for investigators. | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong study design | |
| Wrong patient population | |
| Wrong intervention | |
| Subgroup of interest LVEF 40‐45%. Investigator responded to our enquiry for data: "the mean EF was only 26.9% and I doubt any of the patients were in the group of EF 40% to <45%. [...] I do not have the original data now." | |
| Wrong study design | |
| Wrong study design | |
| LVEF not specified as an inclusion criteria, mean LVEF at baseline 35.4, 4.7%. Could not find current contact details for investigator. | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Portuguese paper. Reported outcomes not of interest but subgroup of participants eligible. Emailed investigators to ask about measured outcomes for subgroup of interest. Response: data not available. | |
| Wrong study design | |
| subgroup of interest LVEF 40‐45%. Contacted investigators. No response. | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong comparator | |
| Wrong study design | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Subgroup of interest (LVEF 40‐45%). Emailed investigators. No response. | |
| Wrong study design | |
| Wrong comparator | |
| Wrong study design | |
| Wrong comparator | |
| Wrong study design | |
| LVEF unclear; response to our enquiry for details: cannot provide data | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| LVEF unclear. Emailed investigators. No response. | |
| Wrong study design | |
| Wrong patient population | |
| Subgroup of interest LVEF 40‐45%. We received a response to our enquiry for more details on the subgroup of interest confirming that the study was conducted in "patients with reduced EF". | |
| Wrong patient population | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| LVEF unclear, otherwise eligible. Unable to find current contact details for investigators. | |
| Wrong population | |
| Wrong study design | |
| LVEF unclear, otherwise eligible. Unable to find current contact details for investigators. | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong patient population |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | No abstract |
| Participants | No abstract |
| Interventions | Eplerenone |
| Outcomes | No abstract |
| Notes | Could not yet obtain full text |
| Methods | Individual, two‐arm, RCT |
| Participants | 42 |
| Interventions | Placebo versus carvedilol |
| Outcomes | QoL |
| Notes | Unclear EF/HF status |
| Methods | Individual, two‐arm, RCT |
| Participants | 80 participants with EHT and COPD of ll‐lll grade of bronchial obstruction (GOLD 2‐3) with chronic heart failure of the II and III NYHA classes and evidence of diastolic dysfunction |
| Interventions | Spironolactone versus standard therapy, 3 months |
| Outcomes | Left ventricular diastolic function, impaired relaxation of left ventricle, adverse events |
| Notes | Have not yet obtained full text |
| Methods | Unclear if RCT |
| Participants | 32 elderly people with chronic heart failure |
| Interventions | Control group and metoprolol group, 8 weeks |
| Outcomes | Left ventricular end‐diastolic diameter and left ventricular ejection fraction, lymphocyte GRK2 mRNA level |
| Notes | Chinese language paper (with translator) |
| Methods | No abstract |
| Participants | Elderly hypertensive people with diastolic heart failure |
| Interventions | Spironolactone |
| Outcomes | No abstract |
| Notes | Chinese language paper. Eligibility criteria for trial unclear regarding LVEF. Emailed investigator. |
| Methods | No abstract |
| Participants | No abstract |
| Interventions | No abstract |
| Outcomes | No abstract |
| Notes | Could not yet retrieve full text |
| Methods | No abstract |
| Participants | No abstract |
| Interventions | No abstract |
| Outcomes | No abstract |
| Notes | Could not yet retrieve full text |
| Methods | Two‐arm, individual, RCT |
| Participants | 76 older people with diastolic heart failure |
| Interventions | Carvediol versus routine treatment |
| Outcomes | Unknown |
| Notes | Chinese language paper. With translator |
EF: ejection fraction
EHT: essential hypertension
HF: heart failure
RCT: randomised controlled trial
LVEF: left ventricular ejection fraction
GRK2: G protein‐coupled receptor kinase 2
mRNA: messenger RNA
COPD: chronic obstructive pulmonary disease
NYHA: New York Heart Association functional Classification of heart failure
QoL: quality of life
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Effects of Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction (HF‐PEF): Cardiac MRI, Echocardiography, Exercise Physiology & Quality of Life Assessment |
| Methods | Study design: RCT, open label, parallel 2‐arm trial Follow‐up: 6 months |
| Participants | Planned inclusion: 60 participants Inclusion criteria: Diagnosis of heart failure with preserved ejection fraction (HF‐PEF), NYHA II‐IV heart failure, Compliance with medical treatment, aged 18 years and over Exclusion criteria: Contra‐indication to undergoing full cardiac magnetic resonance study, Contra‐indication to aldosterone antagonist therapy, Contra‐indication to exercise testing, Unable to give informed consent |
| Interventions | Spironolactone versus placebo |
| Outcomes | Primary: Serial change in ECV, as calculated by T1 map using Cardiac Magnetic Resonance Imaging following treatment with an aldosterone antagonist. Secondary: To determine the effect of spironolactone in HF‐PEF on: Alternative methods of measuring ECV with CMR, Echocardiographically & CMR derived measures of cardiac relaxation, Establish correlation between change in ECV and measures of cardiac relaxation, Exercise tolerance & quality of life |
| Starting date | Unclear. Date of Ethics Committee Opinion: 13 November 2013 |
| Contact information | Sven Plein, [email protected] |
| Notes | Status 'ongoing' in clinical trial registry entry. Emailed trialist to confirm status of trial. Response: "The study is complete and the main manuscript being prepared for submission expected in the next weeks." Emailed trialists to ask for release of results data prior to publication. Response: not possible. |
| Trial name or title | Spironolactone in Atrial Fibrillation (IMPRESS‐AF) |
| Methods | Study design: parallel RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Spironolactone versus placebo |
| Outcomes | Exercise tolerance, QoL, left ventricular diastolic function, exercise tolerance, all‐cause hospitalisations, spontaneous return to sinus rhythm |
| Starting date | January 2015 |
| Contact information | Eduard Shantsila: [email protected] |
| Notes | Contacted trialists to ask about status and anticipated completion date, also queried whether heart failure with preserved ejection fraction was an inclusion criteria. No response. |
| Trial name or title | Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure With Preserved Ejection Fraction, SPIRRIT‐HFPEF |
| Methods | Study design: parallel, open‐label, RCT Anticipated start date: December 2017 Anticipated completion date: June 2022 |
| Participants | Estimated enrolment: 3500 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Spironolactone versus standard care |
| Outcomes | Primary: Time to death from any cause [Time Frame: Collected at data base lock, five (5) years after study start] |
| Starting date | December 2017 |
| Contact information | Inger Ekman ([email protected]) |
| Notes |
| Trial name or title | A Randomized, Double‐blind Controlled Study Comparing LCZ696 to Medical Therapy for Comorbidities in HFpEF Patients (PARALLAX) |
| Methods | Study design: parallel RCT, blinding: participant, care provider, investigator, outcomes assessor Anticipated start date: 29 September 2017 Anticipated completion date: 4 December 2019 |
| Participants | Estimated enrolment: 2200 Inclusion criteria:
Exclusion criteria:
Other protocol‐defined inclusion/exclusion criteria may apply. |
| Interventions | All patients who fulfill the inclusion/exclusion criteria will be stratified before randomization based upon prior therapy for comorbidities to one of 3 strata: ACEi, ARB or no RASi. Patients in the ACEi strata will receive LCZ696 or enalapril. Patients in the ARB strata will receive LCZ696 or valsartan. Patients in the no RASi strata will receive LCZ696 or matching placebo. |
| Outcomes | Primary: Change from baseline in N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) after 12 weeks Secondary:
|
| Starting date | 29 September 29 2017 |
| Contact information | Novartis Pharmaceuticals ([email protected]) |
| Notes | Comparison of interest: LCZ696 or matching placebo Sponsor: Novartis Pharmaceuticals Other identifiers: CLCZ696D2302, 2016‐003410‐28 ( EudraCT Number) |
| Trial name or title | b‐PRESERVE |
| Methods | Study design: "multicentre, prospective, randomized, open‐label, blinded endpoint trial" |
| Participants | "A total of 1200 patients will be randomized to either b‐blocker (metoprolol succinate) or control (n = 600 per group)." "The most essential criteria for HFNEF in this trial are: heart failure symptoms, elevated NT‐proBNP 1500 pg/mL, and LVEF 50%. In addition, age .40 years and a recent hospitalization for heart failure, but not within 3 months prior to enrolment, are required." |
| Interventions | "The follow‐up period is a minimum of 2 years." |
| Outcomes | "The primary endpoint is a composite of hospitalization for heart failure and cardiovascular death. The secondary endpoints include cardiovascular death, heart failure mortality or hospitalization, all‐cause mortality, change in New York Heart Association class, change in left ventricular ejection fraction, increase in NT‐proBNP (by 50% of the value at randomization), b‐blocker tolerance, and premature termination of b‐blocker therapy due to adverse events" |
| Starting date | not reported |
| Contact information | Email: ge.junbo@zs‐hospital.sh.cn or jbge@zs‐hospital.sh.cn |
| Notes | Could not find the entry in the Chinese Clinical Trial Register with ID ChiCTR‐TNC‐00000144. Contacted investigators to clarify status of study. No response. |
ACEI: angiotensin‐converting‐enzyme inhibitor
ARB: angiotensin II receptor blockers
CMR: cardiac magnetic resonance
ECV: extra‐cellular volume
HTN: hypertension
KCCQ: Kansas City Cardiomyopathy Questionnaire
LVEF: left ventricular ejection fraction
NYHA: New York Heart Association Classification of heart failure
QoL: quality of life
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 3 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.99] |
| Analysis 1.1  Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR). | ||||
| 2 Heart failure hospitalisation (RR) Show forest plot | 4 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.13] |
| Analysis 1.2  Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 2 Heart failure hospitalisation (RR). | ||||
| 2.1 Follow‐up < 12 months | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.09, 1.02] |
| 2.2 Follow‐up ≥ 12 months | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] |
| 2.3 Follow‐up unknown | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.31 [0.26, 107.85] |
| 3 All‐cause mortality (RR) Show forest plot | 4 | 1105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.67, 1.00] |
| Analysis 1.3  Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 3 All‐cause mortality (RR). | ||||
| 4 Quality of life (Minnesota) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 4 Quality of life (Minnesota). | ||||
| 5 Withdrawal due to adverse event Show forest plot | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.07 [2.45, 133.04] |
| Analysis 1.5  Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 5 Withdrawal due to adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 3 | 4070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| Analysis 2.1  Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR). | ||||
| 2 Heart failure hospitalisation (RR) Show forest plot | 3 | 3714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| Analysis 2.2  Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 2 Heart failure hospitalisation (RR). | ||||
| 2.1 Follow‐up < 12 months | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.05, 5.61] |
| 2.2 Follow‐up ≥ 12 months | 2 | 3670 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 3 Heart failure hospitalisation (HR) Show forest plot | 2 | 3670 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| Analysis 2.3  Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 3 Heart failure hospitalisation (HR). | ||||
| 4 Hyperkalaemia Show forest plot | 6 | 4291 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.77, 2.51] |
| Analysis 2.4  Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 4 Hyperkalaemia. | ||||
| 5 All‐cause mortality (RR) Show forest plot | 5 | 4207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.06] |
| Analysis 2.5  Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 5 All‐cause mortality (RR). | ||||
| 6 Quality of life Show forest plot | 5 | 603 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.23, 0.34] |
| Analysis 2.6  Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 6 Quality of life. | ||||
| 7 Quality of life (KCCQ) Show forest plot | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐28.02, 26.46] |
| Analysis 2.7  Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 7 Quality of life (KCCQ). | ||||
| 8 Quality of life (Minnesota) Show forest plot | 3 | 511 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐2.30, 3.98] |
| Analysis 2.8  Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 8 Quality of life (Minnesota). | ||||
| 9 Withdrawal due to adverse event Show forest plot | 4 | 3986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.00, 1.21] |
| Analysis 2.9  Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 9 Withdrawal due to adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 2 | 945 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.61, 1.42] |
| Analysis 3.1  Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR). | ||||
| 2 Heart failure hospitalisation (RR) Show forest plot | 3 | 1019 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.15] |
| Analysis 3.2  Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 2 Heart failure hospitalisation (RR). | ||||
| 2.1 Follow‐up < 12 months | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.09, 2.04] |
| 2.2 Follow‐up ≥ 12 months | 2 | 945 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.19] |
| 3 Hyperkalaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 3 Hyperkalaemia. | ||||
| 4 All‐cause mortality (RR) Show forest plot | 4 | 1079 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.38] |
| Analysis 3.4  Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 4 All‐cause mortality (RR). | ||||
| 5 Quality of life (Minnesota) Show forest plot | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐3.66, 3.48] |
| Analysis 3.5  Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 5 Quality of life (Minnesota). | ||||
| 6 Withdrawal due to adverse event Show forest plot | 3 | 1019 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.26, 9.00] |
| Analysis 3.6  Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 6 Withdrawal due to adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 3 | 7254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.14] |
| Analysis 4.1  Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR). | ||||
| 2 Cardiovascular mortality (HR) Show forest plot | 2 | 5087 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.89, 1.13] |
| Analysis 4.2  Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 2 Cardiovascular mortality (HR). | ||||
| 3 Heart failure hospitalisation (RR) Show forest plot | 3 | 7254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.02] |
| Analysis 4.3  Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 3 Heart failure hospitalisation (RR). | ||||
| 4 Heart failure hospitalisation (HR) Show forest plot | 2 | 7148 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.80, 1.01] |
| Analysis 4.4  Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 4 Heart failure hospitalisation (HR). | ||||
| 5 Hyperkalaemia Show forest plot | 2 | 7148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.07, 3.33] |
| Analysis 4.5  Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 5 Hyperkalaemia. | ||||
| 6 All‐cause mortality (RR) Show forest plot | 4 | 7964 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.11] |
| Analysis 4.6  Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 6 All‐cause mortality (RR). | ||||
| 7 All‐cause mortality (HR) Show forest plot | 2 | 4838 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| Analysis 4.7  Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 7 All‐cause mortality (HR). | ||||
| 8 Quality of life (Minnesota) Show forest plot | 3 | 3117 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.86, 1.67] |
| Analysis 4.8  Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 8 Quality of life (Minnesota). | ||||
| 9 Withdrawal due to adverse event Show forest plot | 4 | 7406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.09, 1.36] |
| Analysis 4.9  Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 9 Withdrawal due to adverse event. | ||||

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
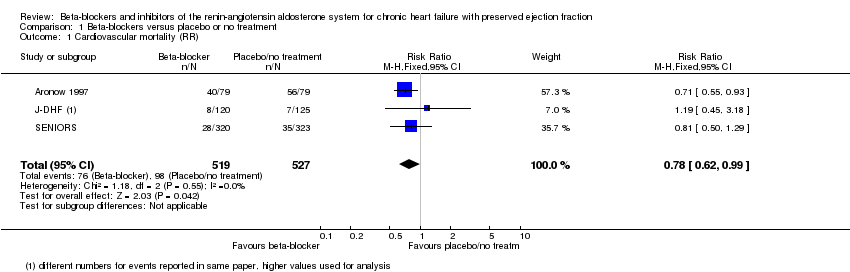
Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR).

Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 2 Heart failure hospitalisation (RR).
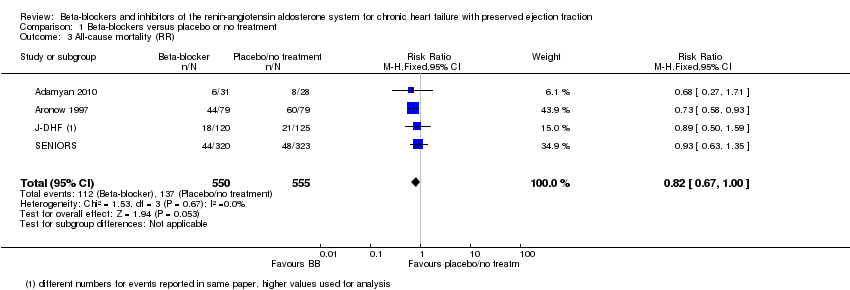
Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 3 All‐cause mortality (RR).
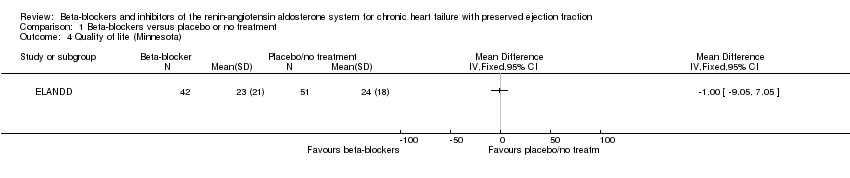
Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 4 Quality of life (Minnesota).
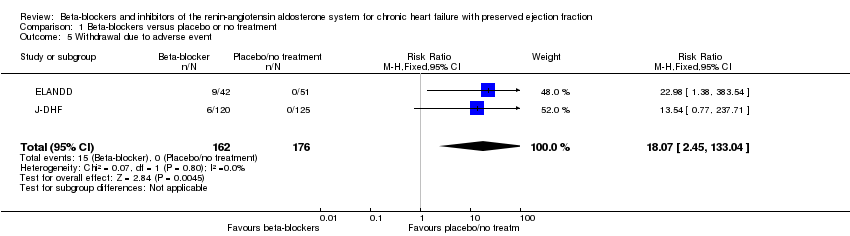
Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 5 Withdrawal due to adverse event.

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR).
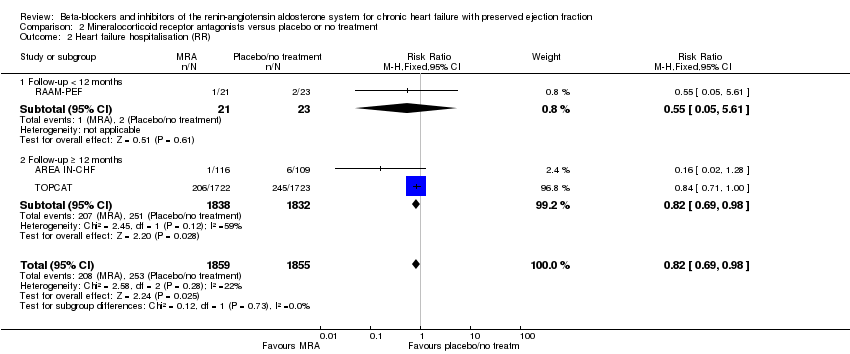
Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 2 Heart failure hospitalisation (RR).
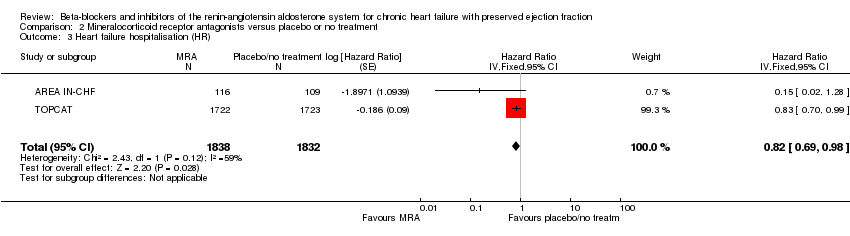
Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 3 Heart failure hospitalisation (HR).

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 4 Hyperkalaemia.
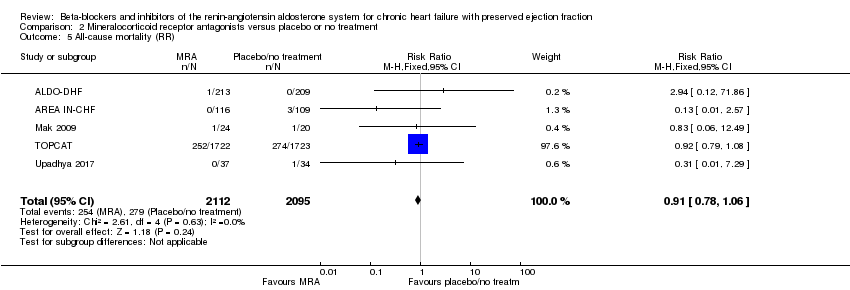
Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 5 All‐cause mortality (RR).

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 6 Quality of life.

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 7 Quality of life (KCCQ).

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 8 Quality of life (Minnesota).

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 9 Withdrawal due to adverse event.

Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR).
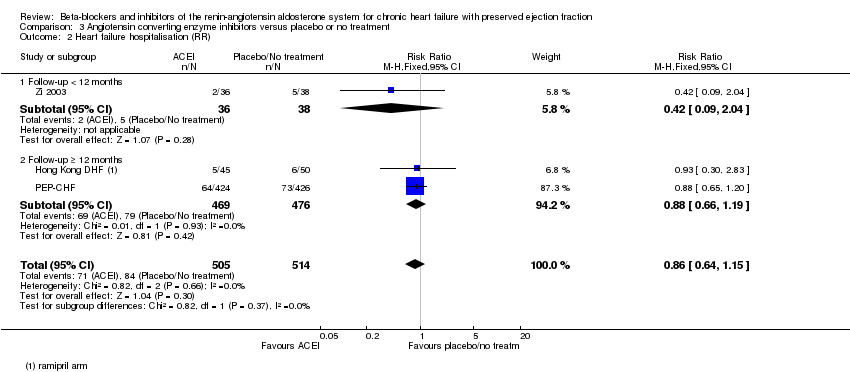
Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 2 Heart failure hospitalisation (RR).

Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 3 Hyperkalaemia.
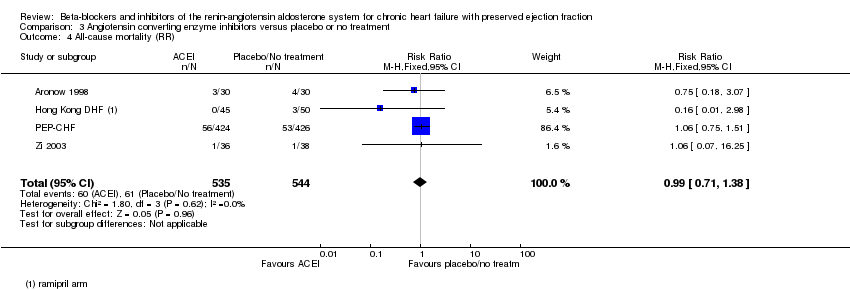
Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 4 All‐cause mortality (RR).

Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 5 Quality of life (Minnesota).

Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 6 Withdrawal due to adverse event.

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR).

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 2 Cardiovascular mortality (HR).

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 3 Heart failure hospitalisation (RR).

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 4 Heart failure hospitalisation (HR).
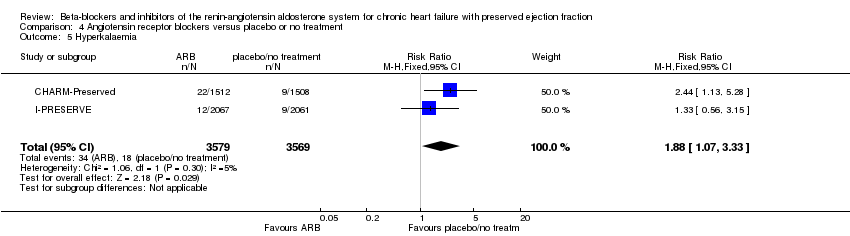
Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 5 Hyperkalaemia.
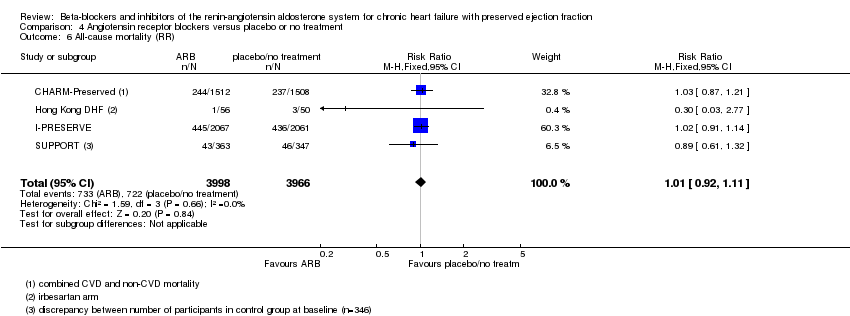
Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 6 All‐cause mortality (RR).
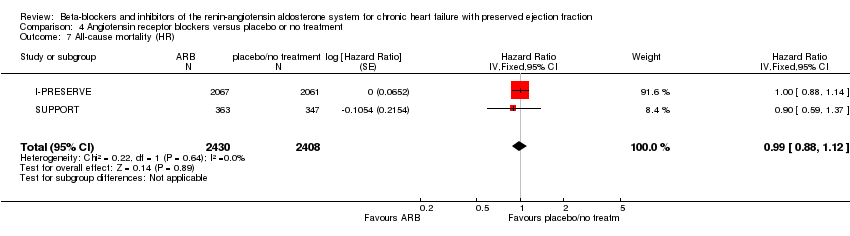
Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 7 All‐cause mortality (HR).

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 8 Quality of life (Minnesota).
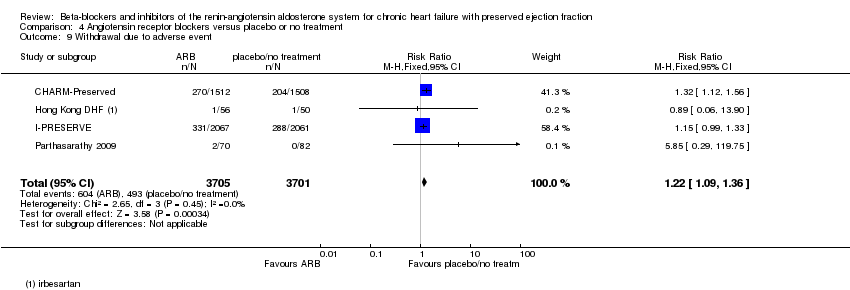
Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 9 Withdrawal due to adverse event.
| Beta‐blockers compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with Beta‐blockers | |||||
| Cardiovascular mortality (RR) | Study population | RR 0.78 | 1046 | ⊕⊕⊝⊝ | Three additional studies (ELANDD; SWEDIC; Takeda 2004) reported that no deaths occurred | |
| 173 per 1000 | 135 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.73 | 449 | ⊕⊝⊝⊝ | Follow‐up unclear for SWEDIC. ELANDD reported that no hospitalisation due to heart failure occurred | |
| 117 per 1000 | 86 per 1000 | |||||
| Hyperkalaemia | 245 (1 RCT) | ⊕⊝⊝⊝ | J‐DHF reported one participant in the intervention group (N = 120) experienced hyperkalaemia but did not report on this outcome for the control group. No further data were available from any of the other studies. | |||
| All‐cause mortality (RR) | Study population | RR 0.82 | 1105 | ⊕⊕⊝⊝ | Follow‐up unclear for Adamyan 2010. ELANDD, SWEDIC and Takeda 2004 reported that no deaths occurred | |
| 243 per 1000 | 199 per 1000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) was 24 | MD 1 lower | ‐ | 93 | ⊕⊝⊝⊝ | Lower = better, 5 point difference considered to be clinically meaningful |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to unclear selection bias in most studies. 2 Downgraded by one level due to concerns about the smaller study being more precise than the larger study. 3 Downgraded by one level due to large variation in size of effect. 4 Downgraded by two levels due to few events and wide CI. 5 Downgraded by two levels due to very small sample size. 6 Suspected publication bias; this is a patient‐relevant outcome that is not reported in most studies. 7 Downgraded by two levels due to incomplete reporting. | ||||||
| MRA compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with MRA | |||||
| Cardiovascular mortality (RR) | Study population | RR 0.90 | 4070 | ⊕⊕⊕⊝ | Two additional trials (RAAM‐PEF, Kurrelmeyer 2014) reported that no deaths occurred | |
| 88 per 1000 | 79 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.82 | 3714 | ⊕⊕⊕⊝ | Three additional trials (ALDO‐DHF ,Kurrelmeyer 2014, Upadhya 2017) reported that no hospitalisation due to heart failure occurred | |
| 136 per 1000 | 112 per 1000 | |||||
| Hyperkalaemia | Study population | RR 2.11 | 4291 | ⊕⊕⊕⊕ | Two trials defined hyperkalaemia ≥ 5.5 mEg/L | |
| 83 per 1000 | 175 per 1000 | |||||
| All‐cause mortality | Study population | RR 0.91 | 4207 | ⊕⊕⊕⊝ | Two additional trials (RAAM‐PEF, Kurrelmeyer 2014) reported that no deaths occurred | |
| 133 per 1000 | 121 per 1000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) ranged from 20 to 25 | MD 0.84 higher | ‐ | 511 | ⊕⊕⊝⊝ | Lower = better, 5 points are considered a clinically significant difference We did not pre‐specify which QoL scale was to be reported in the 'Summary of findings' table. To aid comparisons among 'Summary of findings' tables we chose to include the Minnesota Living with Heart Failure questionnaire and not the SMD across two scales |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to imprecision. 2 Downgraded by one level because one trial was open label. 3 Downgraded by one level due to small sample size. | ||||||
| ACEI compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with ACEI | |||||
| Cardiovascular mortality (RR) | Study population | RR 0.93 | 945 | ⊕⊕⊕⊝ | One additional trial (Kitzman 2010) reported that no deaths occurred | |
| 86 per 1000 | 81 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.86 | 1019 | ⊕⊕⊕⊝ | ||
| 13 per 1000 | 11 per 1000 | |||||
| Hyperkalaemia | 74 (1 RCTs) | ⊕⊝⊝⊝ | One trial (Zi 2003) reported 2 events in the intervention group (N = 36), 0 events in the control group (N = 38) (RR 5.27, 95% CI 0.26 to 106.16) | |||
| All‐cause mortality (RR) | Study population | RR 0.99 | 1079 | ⊕⊕⊕⊝ | One additional trial (Kitzman 2010) reported that no deaths occurred | |
| 119 per 1000 | 119 per 1000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) ranged from 10.9 to 29 | MD 0.09 lower | ‐ | 154 | ⊕⊕⊝⊝ | Scale: 0 to 105, lower = better, 5 point difference considered clinically relevant One trial (SNEGOVIK) reported mean change from baseline of ‐19.8 for intervention and ‐10.7 for control |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to wide CI. 2 Downgraded by one level due to risk of bias (open label). 3 Downgraded by one level due to low sample size. 4 Downgraded by one level due to unclear selection bias. | ||||||
| ARB compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with ARB | |||||
| Cardiovascular mortality (RR) | Study population | RR 1.02 | 7254 | ⊕⊕⊕⊕ | One additional trial (Parthasarathy 2009) reported that no deaths occurred | |
| 131 per 1000 | 133 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.92 | 7254 | ⊕⊕⊕⊕ | ||
| 171 per 1,‐000 | 157 per 1,‐000 | |||||
| Hyperkalaemia | Study population | RR 1.88 | 7148 | ⊕⊕⊕⊕ | ||
| 3 per 1,000 | 5 per 1,000 | |||||
| All‐cause mortality (RR) | Study population | RR 1.01 | 7964 | ⊕⊕⊕⊕ | One additional trial (Parthasarathy 2009) reported that no deaths occurred | |
| 72 per 1000 | 73 per 1,‐000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) ranged from 10.9 to 31.6 | MD 0.41 higher | ‐ | 3117 | ⊕⊕⊕⊕ | Scale: 0 to 105, lower = better, 5 point difference considered clinically relevant |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 3 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.99] |
| 2 Heart failure hospitalisation (RR) Show forest plot | 4 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.13] |
| 2.1 Follow‐up < 12 months | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.09, 1.02] |
| 2.2 Follow‐up ≥ 12 months | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] |
| 2.3 Follow‐up unknown | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.31 [0.26, 107.85] |
| 3 All‐cause mortality (RR) Show forest plot | 4 | 1105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.67, 1.00] |
| 4 Quality of life (Minnesota) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Withdrawal due to adverse event Show forest plot | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.07 [2.45, 133.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 3 | 4070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| 2 Heart failure hospitalisation (RR) Show forest plot | 3 | 3714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 2.1 Follow‐up < 12 months | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.05, 5.61] |
| 2.2 Follow‐up ≥ 12 months | 2 | 3670 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 3 Heart failure hospitalisation (HR) Show forest plot | 2 | 3670 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 4 Hyperkalaemia Show forest plot | 6 | 4291 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.77, 2.51] |
| 5 All‐cause mortality (RR) Show forest plot | 5 | 4207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.06] |
| 6 Quality of life Show forest plot | 5 | 603 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.23, 0.34] |
| 7 Quality of life (KCCQ) Show forest plot | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐28.02, 26.46] |
| 8 Quality of life (Minnesota) Show forest plot | 3 | 511 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐2.30, 3.98] |
| 9 Withdrawal due to adverse event Show forest plot | 4 | 3986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.00, 1.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 2 | 945 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.61, 1.42] |
| 2 Heart failure hospitalisation (RR) Show forest plot | 3 | 1019 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.15] |
| 2.1 Follow‐up < 12 months | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.09, 2.04] |
| 2.2 Follow‐up ≥ 12 months | 2 | 945 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.19] |
| 3 Hyperkalaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 All‐cause mortality (RR) Show forest plot | 4 | 1079 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.38] |
| 5 Quality of life (Minnesota) Show forest plot | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐3.66, 3.48] |
| 6 Withdrawal due to adverse event Show forest plot | 3 | 1019 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.26, 9.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 3 | 7254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.14] |
| 2 Cardiovascular mortality (HR) Show forest plot | 2 | 5087 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.89, 1.13] |
| 3 Heart failure hospitalisation (RR) Show forest plot | 3 | 7254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.02] |
| 4 Heart failure hospitalisation (HR) Show forest plot | 2 | 7148 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.80, 1.01] |
| 5 Hyperkalaemia Show forest plot | 2 | 7148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.07, 3.33] |
| 6 All‐cause mortality (RR) Show forest plot | 4 | 7964 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.11] |
| 7 All‐cause mortality (HR) Show forest plot | 2 | 4838 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 8 Quality of life (Minnesota) Show forest plot | 3 | 3117 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.86, 1.67] |
| 9 Withdrawal due to adverse event Show forest plot | 4 | 7406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.09, 1.36] |

