Antihistamines for motion sickness
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012715.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 17 octubre 2022see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Enfermedades de oído, nariz y garganta
- Copyright:
-
- Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Nadine Karrim: drafted, reviewed and edited the protocol; prepared the manuscript and contributed as the primary review author; obtained copies of studies; selected studies for inclusion; extracted data; entered data into RevMan 5 and GRADEpro; performed the data analysis and interpretation of data; provided a clinical and statistical perspective; and drafted, reviewed, edited and approved the final document.
Ryan Byrne: selected studies for inclusion; extracted data; performed the meta‐analysis and the interpretation of data; provided a clinical and statistical perspective; and reviewed, edited and approved the final document.
Nombulelo Magula: reviewed and edited the protocol; provided a clinical and statistical perspective; reviewed and approved the final document.
Yougan Saman: reviewed and edited the protocol; provided a clinical perspective; reviewed and approved the final document; will be responsible for future updates.
Sources of support
Internal sources
-
No sources of support provided
External sources
-
National Institute for Health Research, UK
Infrastructure funding for Cochrane ENT
Declarations of interest
Nadine Karrim: none known.
Ryan Byrne: none known.
Nombulelo Magula: none known.
Yougan Saman: none known.
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We would like to acknowledge Samantha Cox for designing with the search strategy, assisting with obtaining copies of studies and facilitating translations for studies that were not conducted in English. We would also like to thank Jenny Bellorini for copy editing and reviewing the protocol and completed review and for always being available and willing to help. We thank them both for their unwavering support throughout the process of this review.
We are indebted to Karren Komitas, Huisi Wang and Ksenia Aaron for translating and excluding primary studies for this review; as well as Arne Liebau and Haralampos Gouveris for completing data extractions and risk of bias assessments for Reimann 1981 and Offenloch 1986, Ksenia Aaron for completing data extractions and risk of bias assessments for Salenko 2006, Huisi Wang for completing data extractions and risk of bias assessments for Gao 2015, and Dr HM Abdulazeem for translation of the papers in German (Brandt 1976; von Lieven 1970).
We are also grateful to biostatistician, Partson Tinarwo, for his feedback on our meta‐analysis.
Editorial and peer reviewer contributions
Cochrane ENT supported the authors in the development of this review.
The following people conducted the editorial process for this article:
-
Sign‐off Editor (final editorial decision): Professor Martin Burton, Cochrane ENT.
-
Managing Editor (selected peer reviewers, collated peer reviewer comments, provided editorial guidance to authors, edited the article): Jenny Bellorini, Cochrane ENT.
-
Copy Editor (copy editing and production): Jenny Bellorini, Cochrane ENT.
-
Peer reviewers (provided comments and recommended an editorial decision): Professor Adrian James, Cochrane ENT Editor (clinical/content review), Dr. Vincent A van Vugt, Department of General Practice, Amsterdam UMC (clinical/content review), Sabeeh Kamil (consumer review), Nuala Livingstone, Cochrane Central Editorial Service (methods review).
Version history
| Published | Title | Stage | Authors | Version |
| 2022 Oct 17 | Antihistamines for motion sickness | Review | Nadine Karrim, Ryan Byrne, Nombulelo Magula, Yougan Saman | |
| 2017 Jul 07 | Antihistamines for motion sickness | Protocol | Nadine Karrim, Nombulelo Magula, Yougan Saman | |
Differences between protocol and review
-
An additional author was added as the second review author (RB) and authorship contributions changed.
-
We did not complete an intention‐to‐treat analysis and did not convey the pooled results as absolute numbers (as number needed to treat), as the relevant data were not available in the included studies
-
Although our protocol stated that cross‐over studies would be excluded, Shupak 1994, a cross‐over study, was included ‐ however, only the sea limb of the study was included in this review and the author confirmed that the participants in each limb of the study were not the same.
-
A planned subgroup analysis between experimental studies and natural conditions studies was not completed; this analysis was included in error as we decided at protocol stage that these different types of studies would not be pooled.
-
In the experimental studies, the motion stimulus was deliberately applied until participants experienced motion sickness symptoms. Symptom severity was reported using a validated scale and was presented as continuous data across all the included studies. Therefore these results are presented here as the standardised mean difference in motion sickness severity (before and after treatment), rather than as a proportion of participants with symptoms.
Notes
The Cochrane ENT Information Specialist changed the sources to search after the protocol was published, but before the first search was run. The following were removed:
-
EBSCO CINAHL (1982 to date);
-
Ovid CAB abstracts (1910 to date);
-
IndMed (search to date);
-
PakMediNet (search to date);
-
ISRCTN, www.isrctn.com (search to date);
-
Google (search to date).
The following was added:
-
CNKI, www.cnki.com.cn (search via Google Scholar).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Adult; Child; Humans; Middle Aged; Young Adult;
PICO
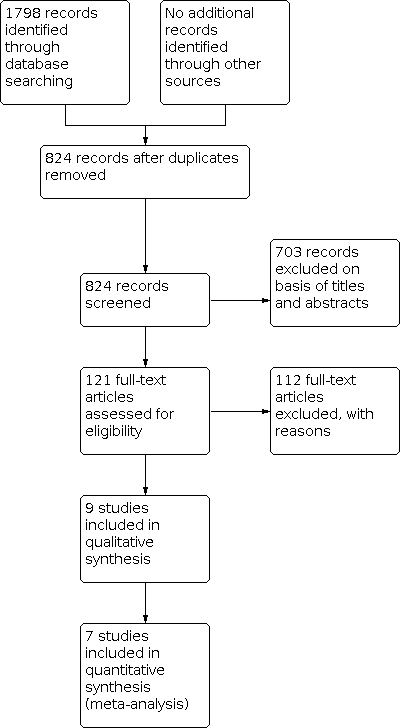
Process for sifting search results and selecting studies for inclusion.

Risk of bias summary: Based on review authors' judgements (performed by NK and RB) about each risk of bias item for each included study.

Risk of bias graph: Based on review authors' judgements (performed by NK and RB) about each risk of bias item presented as percentages across all included studies.

Comparison 1: Antihistamines versus placebo, Outcome 1: Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions

Comparison 1: Antihistamines versus placebo, Outcome 2: Motion sickness symptom severity under experimental conditions

Comparison 1: Antihistamines versus placebo, Outcome 3: Physiological measures under experimental conditions: gastric tachyarrhythmia (electrogastrography)

Comparison 1: Antihistamines versus placebo, Outcome 4: Adverse effects under natural conditions: sedation

Comparison 1: Antihistamines versus placebo, Outcome 5: Adverse effects under natural conditions: impaired cognition

Comparison 1: Antihistamines versus placebo, Outcome 6: Adverse effects under natural conditions: blurred vision

Comparison 2: Antihistamines versus scopolamine, Outcome 1: Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions

Comparison 2: Antihistamines versus scopolamine, Outcome 2: Adverse effects under natural conditions: sedation

Comparison 3: Antihistamines versus antiemetics, Outcome 1: Motion sickness symptom severity under experimental conditions

Comparison 3: Antihistamines versus antiemetics, Outcome 2: Physiological measures under experimental conditions: gastric tachyarrhythmia (electrogastrography)

Comparison 4: Antihistamines versus acupuncture, Outcome 1: Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions
| Antihistamines versus placebo for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | Antihistamines | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Assessed by: self‐reported questionnaires6,7 Follow‐up: varied8,9 | Study population | RR 1.81 | 240 | ⊕⊕⊕⊝ | Antihistamines are probably effective at preventing motion sickness symptoms under natural conditions when compared to placebo. | |
| 247 per 1000 | 447 per 1000 | |||||
| Moderate | ||||||
| 313 per 1000 | 567 per 1000 | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Assessed by: rotating chair Follow‐up: varied (7 days; 1 hour 20 minutes) | — | The standardised mean difference in susceptible participants who did not experience any motion sickness symptoms under experimental conditions in the intervention groups was | — | 62 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on preventing motion sickness under experimental conditions when compared to placebo. |
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | No studies in this comparison reported on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate and core temperature | Heart rate and core temperature were not measured in the studies in this comparison. | |||||
| Physiological measures: gastric tachyarrhythmia10 Assessed by: electrogastrography Follow‐up: 1 hour 20 minutes | Mean score: 60.29 | The mean gastric tachyarrhythmia score under experimental conditions in the intervention group was | — | 42 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in gastric tachyarrhythmia when compared to placebo. |
| Adverse effects: sedation Assessed by: adverse effect questionnaire (presence or absence given numerical values) Follow‐up: before departure and after returning from a sea voyage lasting a total duration of 4 to 6 hours in one study, and 5 hours in one study | Study population | RR 1.51 | 190 | ⊕⊕⊝⊝ | Antihistamines may be more likely to cause sedation when compared to placebo. | |
| 438 per 1000 | 661 per 1000 | |||||
| Moderate | ||||||
| 438 per 1000 | 661 per 1000 | |||||
| Adverse effects: impaired cognition Assessed by: adverse effect questionnaire (presence or absence given numerical values) Follow‐up: before departure and after returning from a sea voyage lasting a total duration of 4 to 6 hours in one study, and 5 hours in one study | Study population | RR 0.89 | 190 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in terms of impaired cognition when compared to placebo. | |
| 328 per 1000 | 292 per 1000 | |||||
| Moderate | ||||||
| 328 per 1000 | 292 per 1000 | |||||
| Adverse effects: blurred vision Assessed by: adverse effect questionnaire (presence or absence given numerical values) Follow‐up: before departure and after returning from a sea voyage lasting a total duration of 4 to 6 hours in one study, and 5 hours in one study | Study population | RR 1.14 | 190 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in blurred vision when compared to placebo. | |
| 125 per 1000 | 142 per 1000 | |||||
| Moderate | ||||||
| 125 per 1000 | 142 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to study limitations (risk of bias): incomplete data in one study (95 of 118 participants completed all questionnaires but reasons for this have not been stated); all studies had an unclear risk related to allocation concealment. | ||||||
| Antihistamines versus scopolamine for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: scopolamine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Scopolamine | Antihistamines | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Assessed by: self‐reported questionnaires6,7 Follow‐up: varied8,9 | Study population | RR 0.89 | 71 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on preventing motion sickness under natural conditions when compared to scopolamine. | |
| 806 per 1000 | 717 per 1000 | |||||
| Moderate | ||||||
| 773 per 1000 | 688 per 1000 | |||||
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | No studies in this comparison reported on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate Assessed by: self‐measured (participant measured own pulse rate) Follow‐up: just before flight, every 10 minutes during flight; immediately after flight; and finally at 20 minutes after the flight (flight duration 1 hour) | Results were only presented as a narrative summary in the translation of this study: "No difference in pulse frequency". | 20 (1 study) | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on the heart rate under natural conditions when compared to scopolamine. | ||
| Physiological measures: core temperature and gastric tachyarrhythmia (electrogastrography) | Gastric tachyarrhythmia and core temperature were not measured in the studies in this comparison. | |||||
| Adverse effects: sedation Assessed by: self‐reported Follow‐up: every 1 to 2 hours for a total sea voyage lasting 7 to 8 hours in one study; unspecified in one study | Study population | RR 0.82 | 90 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on sedation when compared to scopolamine. | |
| 213 per 1000 | 174 per 1000 | |||||
| Moderate | ||||||
| 206 per 1000 | 169 per 1000 | |||||
| Adverse effects: impaired cognitive function | No studies evaluated impaired cognition in this comparison. | |||||
| Adverse effects: blurred vision Assessed by: self‐reported Follow‐up: every 1 to 2 hours for a total sea voyage lasting 7 to 8 hours | Results were only presented as a narrative summary: "Use of transdermal scopolamine resulted in some side effects before motion, including dry mouth, drowsiness, and blurred vision, but only the incidence of dry mouth was statistically significant (P = 0.001)". | 51 (1 study) | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on blurred vision when compared to scopolamine. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to study limitations (risk of bias): studies had an unclear risk related to allocation concealment and random sequence generation. | ||||||
| Antihistamines versus antiemetics for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: antiemetic | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Antiemetic | Antihistamine | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Assessed by: calculated based on head movements tolerated (rotating chair); MSAQ Follow‐up: 1 hour and 20 minutes | 22.3 | The mean proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions in the intervention groups was | — | 42 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in the prevention of motion sickness under experimental conditions when compared to an antiemetic |
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | No studies in this comparison reported on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate and core temperature | Heart rate and core temperature were not measured in the studies in this comparison. | |||||
| Physiological measures: gastric tachyarrhythmia2 Assessed by: electrogastrography Follow‐up: 1 hour and 20 minutes | Mean score: 53.53 | The mean gastric tachyarrhythmia score under experimental conditions in the intervention groups was | — | 42 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in gastric tachyarrhythmia when compared to an antiemetic. |
| Adverse effects: impaired cognition and blurred vision | No studies in this comparison evaluated impaired cognition or blurred vision. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded two levels due to imprecision: overall confidence interval crosses the line of no effect; small sample size. | ||||||
| Antihistamines versus acupuncture for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: acupuncture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Acupuncture | Antihistamines | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Assessed by: Graybiel motion sickness scale Follow‐up: before and after treatment (exact time not specified) | Study population | RR 1.32 | 100 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of antihistamines on the prevention of motion sickness under experimental conditions when compared to acupuncture. | |
| 740 per 1000 | 977 per 1000 | |||||
| Moderate | ||||||
| 740 per 1000 | 977 per 1000 | |||||
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | The study in this comparison did not report on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate, core temperature and gastric tachyarrhythmia (electrogastrography) | Heart rate, core temperature and gastric tachyarrhythmia (electrogastrography) were not measured in the study in this comparison. | |||||
| Adverse effects: sedation, impaired cognitive function, blurred vision | The study in this comparison did not evaluate sedation, impaired cognitive function or blurred vision. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by two levels due to study limitations (risk of bias): incomplete data. (While the study appears to have complete outcome data, the authors have specified that participants were eliminated from the study based on the following criteria: poor compliance and inability to complete the treatment according to the test plan, serious adverse effects, serious deterioration of the participants' condition during the study, and participants who dropped out of the study due to "subjective and objective reasons". The number of participants who were eliminated has not been stated and it is unclear if these participants were replaced in order to complete the study with the same number of participants that were originally enrolled); unclear risk related to allocation concealment and random sequence generation; unblinded. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Show forest plot | 3 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.23, 2.66] |
| 1.2 Motion sickness symptom severity under experimental conditions Show forest plot | 2 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.18, 0.83] |
| 1.3 Physiological measures under experimental conditions: gastric tachyarrhythmia (electrogastrography) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐11.71, 7.31] |
| 1.4 Adverse effects under natural conditions: sedation Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.12, 2.02] |
| 1.5 Adverse effects under natural conditions: impaired cognition Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.38] |
| 1.6 Adverse effects under natural conditions: blurred vision Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.53, 2.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Show forest plot | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.16] |
| 2.2 Adverse effects under natural conditions: sedation Show forest plot | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.07, 9.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Motion sickness symptom severity under experimental conditions Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐10.91, 10.51] |
| 3.2 Physiological measures under experimental conditions: gastric tachyarrhythmia (electrogastrography) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 4.56 [‐3.49, 12.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.12, 1.57] |

