Antihistamínicos para la cinetosis
Appendices
Appendix 1. Search strategies
| Cochrane ENT Register | CENTRAL | MEDLINE | Embase |
|---|---|---|---|
| 1 (motion or car or air* or travel* or sea or space or auto* or aviat* or flight or simulator or vehicle or passenger* or train or trains or bus or coach or ship):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 2 (virtual AND reality):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 3 (computer AND simulat*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 4 ((video OR computer) AND game):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 5 (((three or 3) next dimensional) AND (film* or movi* or image*)):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 6 (3D AND (film* or movi* or image*)):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 8 (sick* or nausea or vomit*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 9 #7 AND #8 10 (carsick* or airsick* or seasick* or motionsick* or travelsick* or spacesick*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 11 (kinetosis):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 12 #9 OR #10 OR #11 13 (antihistam* or antiallerg*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 14 (anti next (histam* or alerg*)):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 15 ((histamine* or H1) near (antagonist* or block*)):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 16 (acrivastine* or astemizole* or azelastine* or azatadine* or brompheniramine* or carbinoxamine* or cetirizine* or chlorpheniramine* or chlorphenamine* or clemastine* or cinnarizin* or cyclizine* or cyproheptadine* or dexchlorpheniramine* or dimenhydrinate* or dexbrompheniramine* or desloratadine* or diphenhydramine* or doxylamine* or dimetapp* or drixoral* or dimethindene* or diphenylpyraline* or ebastine* or fexofenadine* or flunarizine* or hydroxyzine* or ketotifen* or levocetirizine* or levocabastine* or loratadine* or methapyrilene* or mequitazine* or methdilazine* or mizolastine* or meclizine* or mepyramine* or oxatomide* or pheniramine* or phenyltoloxamine* or pyrilamine* or promethazine* or terfenadin* or tripelennamine* or triprolidine* or tritoqualine* or dimotane* or zirtek* or clarityn* or neoclarityn* or telfast* or xyzal* or mistamine* or mizollen* or alimemazine* or vallergan* or optimine* or piriton* or tavegil* or periactin* or phenergan* or piriton* or piriteze*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 17 (carebastine or alcaftadine or Antazoline or Astemizole or bamipine or benzonitrile or chloropyramine or dibenzheptropine or embramine or emedastine or epinastine or fonazine or histabudifen or histapendife or Mianserin or mirtazapine or Olopatadine or picumast or protopine or proxicromil or temelastine or tranilast or trimeprazine or Virlix or Zetir or Zyrtec or Reactine or Voltric or Alerlisin or Cetalerg or Ceterifug or Cetiderm or Cetidura or CetiLich or Cetirigamma or Cetirlan or Cinazière or Cinna or Cinnipirine or Cisaken or Dimitronal or Stugeron or Prometazin or Proazamine or Rumergan or Diprazin or Isopromethazine or Phen?rgan or Phensedyl or Pipolfen or Pipolphen or Promet or Prothazin or Pyrethia or Remsed or Atosil or Diphergan or Chlorphenamine or Antihistaminico or chlor or Trimeton or Chlorpro or Chlorspan or chlortab or Efidac or Kloromin or Piriton or Teldrin or doxepi* or Dramamine or Marezine or Aviomarin or Biodramina or "calm X" or Cinfamar or Contramareo or dimen or Dimetabs or dinate or DMH or Dramanate or gravol or Wehamine or "motion aid" or Nausicalm or Reisegold or Reisetabletten or Rodovan or RubieMen or Superpep or "travel well" or triptone or vomex or Vomacur or omisin or Marmine or Benzhydramine or Benhydramin or Benadryl or Benylin or Dormin or Allerdryl or Dimedrol or Parachloramine or Meclozine or Antivert or Bonamine or Bonine or Chiclida or Histametizyn or RuVertM or Agyrax or "d vert" or dvert or Deptran or Desidox or Doneurin or Espadox or Mareen or Prudoxin or Quitaxon or Sin?quan or Zonalon or Xepin or Aponal or ApoDoxepin or Claritin or Clarium or alavert):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 18 #17 OR #16 OR #15 OR #14 OR #13 19 #18 AND #12 | 1 MESH DESCRIPTOR Motion Sickness EXPLODE ALL AND CENTRAL:TARGET 2 ((motion or car or air* or travel* or sea or space or auto* or aviat* or flight or simulator or vehicle or passenger* or train or trains or bus or coach or ship) near (sick* or nausea or vomit*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 3 ((virtual next reality) near (sick* or nausea or vomit*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 4 ((computer next simulat*) near (sick* or nausea or vomit*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 5 (((video or computer) next game* near (sick* or nausea or vomit*))):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 6 (computer next game* near (sick* or nausea or vomit*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 7 (video next game* near (sick* or nausea or vomit*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 8 (3D near (film* or movie* or image*) near (sick* or nausea or vomit*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 9 (((three or 3) next dimensional) near (film* or movie* or image*) near (sick* or nausea or vomit*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 10 (carsick* or airsick* or seasick* or motionsick* or travelsick* or spacesick*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 11 (kinetosis):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 13 MESH DESCRIPTOR Histamine Antagonists EXPLODE ALL AND CENTRAL:TARGET 14 MESH DESCRIPTOR Anti‐allergic Agents EXPLODE ALL AND CENTRAL:TARGET 15 MESH DESCRIPTOR Promethazine EXPLODE ALL AND CENTRAL:TARGET 16 MESH DESCRIPTOR Diphenhydramine EXPLODE ALL AND CENTRAL:TARGET 17 MESH DESCRIPTOR Meclizine EXPLODE ALL AND CENTRAL:TARGET 18 MESH DESCRIPTOR Cinnarizine EXPLODE ALL AND CENTRAL:TARGET 19 MESH DESCRIPTOR Cyclizine EXPLODE ALL AND CENTRAL:TARGET 20 MESH DESCRIPTOR Chlorpheniramine EXPLODE ALL AND CENTRAL:TARGET 21 MESH DESCRIPTOR Cetirizine EXPLODE ALL AND CENTRAL:TARGET 22 MESH DESCRIPTOR Loratadine EXPLODE ALL AND CENTRAL:TARGET 23 MESH DESCRIPTOR Doxepin EXPLODE ALL AND CENTRAL:TARGET 24 (antihistam* or antiallerg*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 25 (anti next (histam* or alerg*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 26 ((histamine* or H1) near (antagonist* or block*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 27 (acrivastine* or astemizole* or azelastine* or azatadine* or brompheniramine* or carbinoxamine* or cetirizine* or chlorpheniramine* or chlorphenamine* or clemastine* or cinnarizin* or cyclizine* or cyproheptadine* or dexchlorpheniramine* or dimenhydrinate* or dexbrompheniramine* or desloratadine* or diphenhydramine* or doxylamine* or dimetapp* or drixoral* or dimethindene* or diphenylpyraline* or ebastine* or fexofenadine* or flunarizine* or hydroxyzine* or ketotifen* or levocetirizine* or levocabastine* or loratadine* or methapyrilene* or mequitazine* or methdilazine* or mizolastine* or meclizine* or mepyramine* or oxatomide* or pheniramine* or phenyltoloxamine* or pyrilamine* or promethazine* or terfenadin* or tripelennamine* or triprolidine* or tritoqualine* or dimotane* or zirtek* or clarityn* or neoclarityn* or telfast* or xyzal* or mistamine* or mizollen* or alimemazine* or vallergan* or optimine* or piriton* or tavegil* or periactin* or phenergan* or piriton* or piriteze*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 28 (carebastine or alcaftadine or Antazoline or Astemizole or bamipine or benzonitrile or chloropyramine or dibenzheptropine or embramine or emedastine or epinastine or fonazine or histabudifen or histapendife or Mianserin or mirtazapine or Olopatadine or picumast or protopine or proxicromil or temelastine or tranilast or trimeprazine or Virlix or Zetir or Zyrtec or Reactine or Voltric or Alerlisin or Cetalerg or Ceterifug or Cetiderm or Cetidura or CetiLich or Cetirigamma or Cetirlan or Cinazière or Cinna or Cinnipirine or Cisaken or Dimitronal or Stugeron or Prometazin or Proazamine or Rumergan or Diprazin or Isopromethazine or Phen?rgan or Phensedyl or Pipolfen or Pipolphen or Promet or Prothazin or Pyrethia or Remsed or Atosil or Diphergan or Chlorphenamine or Antihistaminico or chlor or Trimeton or Chlorpro or Chlorspan or chlortab or Efidac or Kloromin or Piriton or Teldrin or doxepi* or Dramamine or Marezine or Aviomarin or Biodramina or "calm X" or Cinfamar or Contramareo or dimen or Dimetabs or dinate or DMH or Dramanate or gravol or Wehamine or "motion aid" or Nausicalm or Reisegold or Reisetabletten or Rodovan or RubieMen or Superpep or "travel well" or triptone or vomex or Vomacur or omisin or Marmine or Benzhydramine or Benhydramin or Benadryl or Benylin or Dormin or Allerdryl or Dimedrol or Parachloramine or Meclozine or Antivert or Bonamine or Bonine or Chiclida or Histametizyn or RuVertM or Agyrax or "d vert" or dvert or Deptran or Desidox or Doneurin or Espadox or Mareen or Prudoxin or Quitaxon or Sin?quan or Zonalon or Xepin or Aponal or ApoDoxepin or Claritin or Clarium or alavert):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 29 #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 30 #29 AND #12 | 1 exp Motion Sickness/ 2 ((motion or car or air* or travel* or sea or space or auto* or aviat* or flight or simulator or vehicle or passenger* or train or trains or bus or coach or ship or (virtual adj3 reality) or (computer adj3 simulat*)) adj5 (sick* or nausea or vomit*)).ab,ti. 3 ((video or computer) adj3 game* adj5 (sick* or nausea or vomit*)).ab,ti. 4 (carsick* or airsick* or seasick* or motionsick* or travelsick* or spacesick*).ab,ti. 5 kinetosis.ab,ti. 6 (3D adj3 (film* or movie* or image*) adj5 (sick* or nausea or vomit*)).ab,ti. 7 (three adj3 dimensional adj5 (film* or movie* or image*) adj5 (sick* or nausea or vomit*)).ab,ti. 8 ("3" adj3 dimensional adj5 (film* or movie* or image*) adj5 (sick* or nausea or vomit*)).ab,ti. 9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10 exp Histamine Antagonists/ 11 exp Anti‐Allergic Agents/ 12 exp Promethazine/ 13 exp Diphenhydramine/ 14 exp Meclizine/ 15 exp Cinnarizine/ 16 exp Cyclizine/ 17 exp Chlorpheniramine/ 18 exp Cetirizine/ 19 exp Loratadine/ 20 exp Doxepin/ 21 (antihistam* or antiallerg* or (anti adj3 (histam* or alerg*))).ab,ti. 22 ((histamine* or H1) adj5 (antagonist* or block*)).ab,ti. 23 (acrivastine* or astemizole* or azelastine* or azatadine* or brompheniramine* or carbinoxamine* or cetirizine* or chlorpheniramine* or chlorphenamine* or clemastine* or cinnarizin* or cyclizine* or cyproheptadine* or dexchlorpheniramine* or dimenhydrinate* or dexbrompheniramine* or desloratadine* or diphenhydramine* or doxylamine* or dimetapp* or drixoral* or dimethindene* or diphenylpyraline* or ebastine* or fexofenadine* or flunarizine* or hydroxyzine* or ketotifen* or levocetirizine* or levocabastine* or loratadine* or methapyrilene* or mequitazine* or methdilazine* or mizolastine* or meclizine* or mepyramine* or oxatomide* or pheniramine* or phenyltoloxamine* or pyrilamine* or promethazine* or terfenadin* or tripelennamine* or triprolidine* or tritoqualine* or dimotane* or zirtek* or clarityn* or neoclarityn* or telfast* or xyzal* or mistamine* or mizollen* or alimemazine* or vallergan* or optimine* or piriton* or tavegil* or periactin* or phenergan* or piriton* or piriteze*).ab,ti. 24 (carebastine or alcaftadine or Antazoline or Astemizole or bamipine or benzonitrile or chloropyramine or dibenzheptropine or embramine or emedastine or epinastine or fonazine or histabudifen or histapendife or Mianserin or mirtazapine or Olopatadine or picumast or protopine or proxicromil or temelastine or tranilast or trimeprazine or Virlix or Zetir or Zyrtec or Reactine or Voltric or Alerlisin or Cetalerg or Ceterifug or Cetiderm or Cetidura or CetiLich or Cetirigamma or Cetirlan or Cinazi?re or Cinna or Cinnipirine or Cisaken or Dimitronal or Stugeron or Prometazin or Proazamine or Rumergan or Diprazin or Isopromethazine or Phen?rgan or Phensedyl or Pipolfen or Pipolphen or Promet or Prothazin or Pyrethia or Remsed or Atosil or Diphergan or Chlorphenamine or Antihistaminico or chlor or Trimeton or Chlorpro or Chlorspan or chlortab or Efidac or Kloromin or Piriton or Teldrin or doxepi* or Dramamine or Marezine or Aviomarin or Biodramina or "calm X" or Cinfamar or Contramareo or dimen or Dimetabs or dinate or DMH or Dramanate or gravol or Wehamine or "motion aid" or Nausicalm or Reisegold or Reisetabletten or Rodovan or RubieMen or Superpep or "travel well" or triptone or vomex or Vomacur or omisin or Marmine or Benzhydramine or Benhydramin or Benadryl or Benylin or Dormin or Allerdryl or Dimedrol or Parachloramine or Meclozine or Antivert or Bonamine or Bonine or Chiclida or Histametizyn or RuVertM or Agyrax or "d vert" or dvert or Deptran or Desidox or Doneurin or Espadox or Mareen or Prudoxin or Quitaxon or Sin?quan or Zonalon or Xepin or Aponal or ApoDoxepin or Claritin or Clarium or alavert).ab,ti. 25 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 26 9 and 25 | 1 exp motion sickness/ 2 ((motion or car or air* or travel* or sea or space or auto* or aviat* or flight or simulator or vehicle or passenger* or train or trains or bus or coach or ship or (virtual adj3 reality) or (computer adj3 simulat*)) adj5 (sick* or nausea or vomit*)).ab,ti. 3 ((video or computer) adj3 game* adj5 (sick* or nausea or vomit*)).ab,ti. 4 (carsick* or airsick* or seasick* or motionsick* or travelsick* or spacesick*).ab,ti. 5 kinetosis.ab,ti. 6 (3D adj3 (film* or movie* or image*) adj5 (sick* or nausea or vomit*)).ab,ti. 7 (three adj3 dimensional adj5 (film* or movie* or image*) adj5 (sick* or nausea or vomit*)).ab,ti. 8 ("3" adj3 dimensional adj5 (film* or movie* or image*) adj5 (sick* or nausea or vomit*)).ab,ti. 9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10 exp antihistaminic agent/ 11 exp antiallergic agent/ 12 exp promethazine/ 13 exp diphenhydramine/ 14 exp meclozine/ 15 exp cinnarizine/ 16 exp cyclizine/ 17 exp chlorpheniramine/ 18 exp cetirizine/ 19 exp loratadine/ 20 exp doxepin/ 21 (antihistam* or antiallerg* or (anti adj3 (histam* or alerg*))).ab,ti. 22 ((histamine* or H1) adj5 (antagonist* or block*)).ab,ti. 23 (acrivastine* or astemizole* or azelastine* or azatadine* or brompheniramine* or carbinoxamine* or cetirizine* or chlorpheniramine* or chlorphenamine* or clemastine* or cinnarizin* or cyclizine* or cyproheptadine* or dexchlorpheniramine* or dimenhydrinate* or dexbrompheniramine* or desloratadine* or diphenhydramine* or doxylamine* or dimetapp* or drixoral* or dimethindene* or diphenylpyraline* or ebastine* or fexofenadine* or flunarizine* or hydroxyzine* or ketotifen* or levocetirizine* or levocabastine* or loratadine* or methapyrilene* or mequitazine* or methdilazine* or mizolastine* or meclizine* or mepyramine* or oxatomide* or pheniramine* or phenyltoloxamine* or pyrilamine* or promethazine* or terfenadin* or tripelennamine* or triprolidine* or tritoqualine* or dimotane* or zirtek* or clarityn* or neoclarityn* or telfast* or xyzal* or mistamine* or mizollen* or alimemazine* or vallergan* or optimine* or piriton* or tavegil* or periactin* or phenergan* or piriton* or piriteze*).ab,ti. 24 (carebastine or alcaftadine or Antazoline or Astemizole or bamipine or benzonitrile or chloropyramine or dibenzheptropine or embramine or emedastine or epinastine or fonazine or histabudifen or histapendife or Mianserin or mirtazapine or Olopatadine or picumast or protopine or proxicromil or temelastine or tranilast or trimeprazine or Virlix or Zetir or Zyrtec or Reactine or Voltric or Alerlisin or Cetalerg or Ceterifug or Cetiderm or Cetidura or CetiLich or Cetirigamma or Cetirlan or Cinazi?re or Cinna or Cinnipirine or Cisaken or Dimitronal or Stugeron or Prometazin or Proazamine or Rumergan or Diprazin or Isopromethazine or Phen?rgan or Phensedyl or Pipolfen or Pipolphen or Promet or Prothazin or Pyrethia or Remsed or Atosil or Diphergan or Chlorphenamine or Antihistaminico or chlor or Trimeton or Chlorpro or Chlorspan or chlortab or Efidac or Kloromin or Piriton or Teldrin or doxepi* or Dramamine or Marezine or Aviomarin or Biodramina or "calm X" or Cinfamar or Contramareo or dimen or Dimetabs or dinate or DMH or Dramanate or gravol or Wehamine or "motion aid" or Nausicalm or Reisegold or Reisetabletten or Rodovan or RubieMen or Superpep or "travel well" or triptone or vomex or Vomacur or omisin or Marmine or Benzhydramine or Benhydramin or Benadryl or Benylin or Dormin or Allerdryl or Dimedrol or Parachloramine or Meclozine or Antivert or Bonamine or Bonine or Chiclida or Histametizyn or RuVertM or Agyrax or "d vert" or dvert or Deptran or Desidox or Doneurin or Espadox or Mareen or Prudoxin or Quitaxon or Sin?quan or Zonalon or Xepin or Aponal or ApoDoxepin or Claritin or Clarium or alavert).ab,ti. 25 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 26 9 and 25 |
| Web of Science | LILACS | ClinicalTrials.gov | ICTRP |
| #1 TS=(((motion or car or air or airline or travel* or sea or space or auto or automobile or aviat* or flight or simulator or vehicle or passenger* or train or trains or bus or coach or ship or (virtual NEAR/3 reality) or (computer NEAR/3 simulat*)) NEAR/5 (sick or sickness or nausea or vomit*))) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years #2 TS=(((video or computer) NEAR/3 game* NEAR/5 (sick or sickness or nausea or vomit*))) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years #3 TS=(((3D or (three or 3) NEAR/3 dimensional) NEAR/5 (film* or movie* or image*) NEAR/5 (sick or sickness or nausea or vomit*))) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years #4 TOPIC: (carsick* or airsick* or seasick* or motionsick* or travelsick* or spacesick*) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years #5 TOPIC: (kinetosis) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years #6 #5 OR #4 OR #3 OR #2 OR #1 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years #7 TOPIC: (((antihistam* or antiallerg*) or (anti NEAR/3 (histam* or alerg*)))) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years #8 TS=(((histamine* or H1) NEAR/3 (antagonist* or block*))) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years #9 TOPIC: (acrivastine* or astemizole* or azelastine* or azatadine* or brompheniramine* or carbinoxamine* or cetirizine* or chlorpheniramine* or chlorphenamine* or clemastine* or cinnarizin* or cyclizine* or cyproheptadine* or dexchlorpheniramine* or dimenhydrinate* or dexbrompheniramine* or desloratadine* or diphenhydramine* or doxylamine* or dimetapp* or drixoral* or dimethindene* or diphenylpyraline* or ebastine* or fexofenadine* or flunarizine* or hydroxyzine* or ketotifen* or levocetirizine* or levocabastine* or loratadine* or methapyrilene* or mequitazine* or methdilazine* or mizolastine* or meclizine* or mepyramine* or oxatomide* or pheniramine* or phenyltoloxamine* or pyrilamine* or promethazine* or terfenadin* or tripelennamine* or triprolidine* or tritoqualine* or dimotane* or zirtek* or clarityn* or neoclarityn* or telfast* or xyzal* or mistamine* or mizollen* or alimemazine* or vallergan* or optimine* or piriton* or tavegil* or periactin* or phenergan* or piriton* or piriteze*) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years #10 TOPIC: (carebastine or alcaftadine or Antazoline or Astemizole or bamipine or benzonitrile or chloropyramine or dibenzheptropine or embramine or emedastine or epinastine or fonazine or histabudifen or histapendife or Mianserin or mirtazapine or Olopatadine or picumast or protopine or proxicromil or temelastine or tranilast or trimeprazine or Virlix or Zetir or Zyrtec or Reactine or Voltric or Alerlisin or Cetalerg or Ceterifug or Cetiderm or Cetidura or CetiLich or Cetirigamma or Cetirlan or Cinazi?re or Cinna or Cinnipirine or Cisaken or Dimitronal or Stugeron or Prometazin or Proazamine or Rumergan or Diprazin or Isopromethazine or Phen?rgan or Phensedyl or Pipolfen or Pipolphen or Promet or Prothazin or Pyrethia or Remsed or Atosil or Diphergan or Chlorphenamine or Antihistaminico or chlor or Trimeton or Chlorpro or Chlorspan or chlortab or Efidac or Kloromin or Piriton or Teldrin or doxepi* or Dramamine or Marezine or Aviomarin or Biodramina or "calm X" or Cinfamar or Contramareo or dimen or Dimetabs or dinate or DMH or Dramanate or gravol or Wehamine or "motion aid" or Nausicalm or Reisegold or Reisetabletten or Rodovan or RubieMen or Superpep or "travel well" or triptone or vomex or Vomacur or omisin or Marmine or Benzhydramine or Benhydramin or Benadryl or Benylin or Dormin or Allerdryl or Dimedrol or Parachloramine or Meclozine or Antivert or Bonamine or Bonine or Chiclida or Histametizyn or RuVertM or Agyrax or "d vert" or dvert or Deptran or Desidox or Doneurin or Espadox or Mareen or Prudoxin or Quitaxon or Sin?quan or Zonalon or Xepin or Aponal or ApoDoxepin or Claritin or Clarium or alavert) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years #11 #10 OR #9 OR #8 OR #7 OR #6 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years #12 #11 AND #6 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years | Controlled clinical trials (TW:motion OR TW:movim* OR TW:car OR TW:travel OR TW:simulator OR TW:passenger OR TW:space OR TW:sea) AND (TW:sick* OR TW:mareo OR TW:enjoo OR TW:devido) | Search 1: (motion OR car OR travel OR sea OR flight OR simulator OR passenger OR space) AND (sick OR sickness*)) AND Study type: Interventional AND Interventions: antihistamine OR histamine OR cinnarizine OR cetirizine OR loratadine OR cyclizine OR Chlorpheniramine OR fexofenadine OR promethazine Search 2: carsick OR airsick OR seasick OR motionsick OR travelsick OR spacesick OR carsickness OR airsickness OR seasickness OR motionsickness OR travelsickness OR spacesickness AND Study type: Interventional AND Interventions: antihistamine OR (anti AND histamine) OR cinnarizine OR cetirizine OR loratadine OR Hyoscine OR cyclizine OR Chlorpheniramine OR fexofenadine OR promethazine | motion sick* OR car sick* OR travel sick* OR simulator sick* OR passenger sick* OR space sick* OR sea sick* |
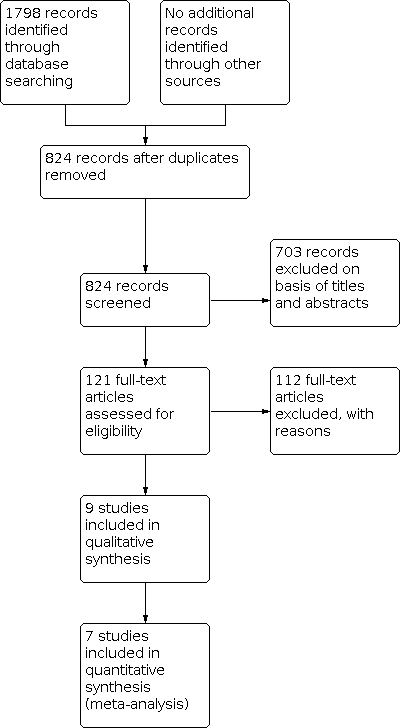
Process for sifting search results and selecting studies for inclusion.

Risk of bias summary: Based on review authors' judgements (performed by NK and RB) about each risk of bias item for each included study.

Risk of bias graph: Based on review authors' judgements (performed by NK and RB) about each risk of bias item presented as percentages across all included studies.

Comparison 1: Antihistamines versus placebo, Outcome 1: Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions

Comparison 1: Antihistamines versus placebo, Outcome 2: Motion sickness symptom severity under experimental conditions

Comparison 1: Antihistamines versus placebo, Outcome 3: Physiological measures under experimental conditions: gastric tachyarrhythmia (electrogastrography)

Comparison 1: Antihistamines versus placebo, Outcome 4: Adverse effects under natural conditions: sedation

Comparison 1: Antihistamines versus placebo, Outcome 5: Adverse effects under natural conditions: impaired cognition

Comparison 1: Antihistamines versus placebo, Outcome 6: Adverse effects under natural conditions: blurred vision

Comparison 2: Antihistamines versus scopolamine, Outcome 1: Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions

Comparison 2: Antihistamines versus scopolamine, Outcome 2: Adverse effects under natural conditions: sedation

Comparison 3: Antihistamines versus antiemetics, Outcome 1: Motion sickness symptom severity under experimental conditions

Comparison 3: Antihistamines versus antiemetics, Outcome 2: Physiological measures under experimental conditions: gastric tachyarrhythmia (electrogastrography)

Comparison 4: Antihistamines versus acupuncture, Outcome 1: Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions
| Antihistamines versus placebo for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | Antihistamines | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Assessed by: self‐reported questionnaires6,7 Follow‐up: varied8,9 | Study population | RR 1.81 | 240 | ⊕⊕⊕⊝ | Antihistamines are probably effective at preventing motion sickness symptoms under natural conditions when compared to placebo. | |
| 247 per 1000 | 447 per 1000 | |||||
| Moderate | ||||||
| 313 per 1000 | 567 per 1000 | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Assessed by: rotating chair Follow‐up: varied (7 days; 1 hour 20 minutes) | — | The standardised mean difference in susceptible participants who did not experience any motion sickness symptoms under experimental conditions in the intervention groups was | — | 62 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on preventing motion sickness under experimental conditions when compared to placebo. |
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | No studies in this comparison reported on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate and core temperature | Heart rate and core temperature were not measured in the studies in this comparison. | |||||
| Physiological measures: gastric tachyarrhythmia10 Assessed by: electrogastrography Follow‐up: 1 hour 20 minutes | Mean score: 60.29 | The mean gastric tachyarrhythmia score under experimental conditions in the intervention group was | — | 42 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in gastric tachyarrhythmia when compared to placebo. |
| Adverse effects: sedation Assessed by: adverse effect questionnaire (presence or absence given numerical values) Follow‐up: before departure and after returning from a sea voyage lasting a total duration of 4 to 6 hours in one study, and 5 hours in one study | Study population | RR 1.51 | 190 | ⊕⊕⊝⊝ | Antihistamines may be more likely to cause sedation when compared to placebo. | |
| 438 per 1000 | 661 per 1000 | |||||
| Moderate | ||||||
| 438 per 1000 | 661 per 1000 | |||||
| Adverse effects: impaired cognition Assessed by: adverse effect questionnaire (presence or absence given numerical values) Follow‐up: before departure and after returning from a sea voyage lasting a total duration of 4 to 6 hours in one study, and 5 hours in one study | Study population | RR 0.89 | 190 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in terms of impaired cognition when compared to placebo. | |
| 328 per 1000 | 292 per 1000 | |||||
| Moderate | ||||||
| 328 per 1000 | 292 per 1000 | |||||
| Adverse effects: blurred vision Assessed by: adverse effect questionnaire (presence or absence given numerical values) Follow‐up: before departure and after returning from a sea voyage lasting a total duration of 4 to 6 hours in one study, and 5 hours in one study | Study population | RR 1.14 | 190 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in blurred vision when compared to placebo. | |
| 125 per 1000 | 142 per 1000 | |||||
| Moderate | ||||||
| 125 per 1000 | 142 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to study limitations (risk of bias): incomplete data in one study (95 of 118 participants completed all questionnaires but reasons for this have not been stated); all studies had an unclear risk related to allocation concealment. | ||||||
| Antihistamines versus scopolamine for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: scopolamine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Scopolamine | Antihistamines | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Assessed by: self‐reported questionnaires6,7 Follow‐up: varied8,9 | Study population | RR 0.89 | 71 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on preventing motion sickness under natural conditions when compared to scopolamine. | |
| 806 per 1000 | 717 per 1000 | |||||
| Moderate | ||||||
| 773 per 1000 | 688 per 1000 | |||||
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | No studies in this comparison reported on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate Assessed by: self‐measured (participant measured own pulse rate) Follow‐up: just before flight, every 10 minutes during flight; immediately after flight; and finally at 20 minutes after the flight (flight duration 1 hour) | Results were only presented as a narrative summary in the translation of this study: "No difference in pulse frequency". | 20 (1 study) | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on the heart rate under natural conditions when compared to scopolamine. | ||
| Physiological measures: core temperature and gastric tachyarrhythmia (electrogastrography) | Gastric tachyarrhythmia and core temperature were not measured in the studies in this comparison. | |||||
| Adverse effects: sedation Assessed by: self‐reported Follow‐up: every 1 to 2 hours for a total sea voyage lasting 7 to 8 hours in one study; unspecified in one study | Study population | RR 0.82 | 90 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on sedation when compared to scopolamine. | |
| 213 per 1000 | 174 per 1000 | |||||
| Moderate | ||||||
| 206 per 1000 | 169 per 1000 | |||||
| Adverse effects: impaired cognitive function | No studies evaluated impaired cognition in this comparison. | |||||
| Adverse effects: blurred vision Assessed by: self‐reported Follow‐up: every 1 to 2 hours for a total sea voyage lasting 7 to 8 hours | Results were only presented as a narrative summary: "Use of transdermal scopolamine resulted in some side effects before motion, including dry mouth, drowsiness, and blurred vision, but only the incidence of dry mouth was statistically significant (P = 0.001)". | 51 (1 study) | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on blurred vision when compared to scopolamine. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to study limitations (risk of bias): studies had an unclear risk related to allocation concealment and random sequence generation. | ||||||
| Antihistamines versus antiemetics for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: antiemetic | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Antiemetic | Antihistamine | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Assessed by: calculated based on head movements tolerated (rotating chair); MSAQ Follow‐up: 1 hour and 20 minutes | 22.3 | The mean proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions in the intervention groups was | — | 42 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in the prevention of motion sickness under experimental conditions when compared to an antiemetic |
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | No studies in this comparison reported on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate and core temperature | Heart rate and core temperature were not measured in the studies in this comparison. | |||||
| Physiological measures: gastric tachyarrhythmia2 Assessed by: electrogastrography Follow‐up: 1 hour and 20 minutes | Mean score: 53.53 | The mean gastric tachyarrhythmia score under experimental conditions in the intervention groups was | — | 42 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in gastric tachyarrhythmia when compared to an antiemetic. |
| Adverse effects: impaired cognition and blurred vision | No studies in this comparison evaluated impaired cognition or blurred vision. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded two levels due to imprecision: overall confidence interval crosses the line of no effect; small sample size. | ||||||
| Antihistamines versus acupuncture for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: acupuncture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Acupuncture | Antihistamines | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Assessed by: Graybiel motion sickness scale Follow‐up: before and after treatment (exact time not specified) | Study population | RR 1.32 | 100 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of antihistamines on the prevention of motion sickness under experimental conditions when compared to acupuncture. | |
| 740 per 1000 | 977 per 1000 | |||||
| Moderate | ||||||
| 740 per 1000 | 977 per 1000 | |||||
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | The study in this comparison did not report on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate, core temperature and gastric tachyarrhythmia (electrogastrography) | Heart rate, core temperature and gastric tachyarrhythmia (electrogastrography) were not measured in the study in this comparison. | |||||
| Adverse effects: sedation, impaired cognitive function, blurred vision | The study in this comparison did not evaluate sedation, impaired cognitive function or blurred vision. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by two levels due to study limitations (risk of bias): incomplete data. (While the study appears to have complete outcome data, the authors have specified that participants were eliminated from the study based on the following criteria: poor compliance and inability to complete the treatment according to the test plan, serious adverse effects, serious deterioration of the participants' condition during the study, and participants who dropped out of the study due to "subjective and objective reasons". The number of participants who were eliminated has not been stated and it is unclear if these participants were replaced in order to complete the study with the same number of participants that were originally enrolled); unclear risk related to allocation concealment and random sequence generation; unblinded. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Show forest plot | 3 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.23, 2.66] |
| 1.2 Motion sickness symptom severity under experimental conditions Show forest plot | 2 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.18, 0.83] |
| 1.3 Physiological measures under experimental conditions: gastric tachyarrhythmia (electrogastrography) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐11.71, 7.31] |
| 1.4 Adverse effects under natural conditions: sedation Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.12, 2.02] |
| 1.5 Adverse effects under natural conditions: impaired cognition Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.38] |
| 1.6 Adverse effects under natural conditions: blurred vision Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.53, 2.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Show forest plot | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.16] |
| 2.2 Adverse effects under natural conditions: sedation Show forest plot | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.07, 9.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Motion sickness symptom severity under experimental conditions Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐10.91, 10.51] |
| 3.2 Physiological measures under experimental conditions: gastric tachyarrhythmia (electrogastrography) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 4.56 [‐3.49, 12.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.12, 1.57] |

