Antihistamínicos para la cinetosis
Resumen
Antecedentes
La cinetosis (mareo por movimiento) es un síndrome que se produce como resultado del movimiento pasivo del cuerpo en respuesta al movimiento real o la ilusión de movimiento cuando se expone a entornos visuales virtuales y en movimiento. Los síntomas más comunes son las náuseas y los vómitos. Los antihistamínicos se han utilizado durante décadas en el tratamiento de la cinetosis, pero los estudios han mostrado resultados contradictorios en cuanto a su eficacia.
Objetivos
Evaluar la efectividad de los antihistamínicos en la prevención y el tratamiento de la cinetosis en adultos y niños.
Métodos de búsqueda
El documentalista del Grupo Cochrane de Enfermedades de oído, nariz y garganta (Cochrane ENT Group) buscó en el Registro del grupo; en el Registro Cochrane central de ensayos controlados (Central Register of Controlled Trials, CENTRAL); en Ovid MEDLINE; Ovid Embase; Web of Science; ClinicalTrials.gov; ICTRP y en fuentes adicionales para obtener ensayos publicados y no publicados. La fecha de búsqueda fue el 7 de diciembre de 2021.
Criterios de selección
Ensayos controlados aleatorizados (ECA) en adultos y niños susceptibles en los que se indujo la cinetosis en condiciones naturales como el transporte aéreo, marítimo y terrestre. También se incluyeron los estudios en los que se indujo la cinetosis en condiciones experimentales (analizados por separado). Se incluyeron los antihistamínicos independientemente de la clase, la vía de administración o la posología y se compararon con ningún tratamiento, placebo o cualquier otra intervención farmacológica o no farmacológica.
Obtención y análisis de los datos
Se utilizaron los métodos Cochrane estándares. Los desenlaces principales de esta revisión fueron: 1) la proporción de participantes susceptibles que no experimentaron ningún síntoma de cinetosis; 2) la proporción de participantes susceptibles que experimentaron una reducción o resolución de los síntomas existentes. Los desenlaces secundarios fueron 1) medidas fisiológicas (frecuencia cardíaca, temperatura central y taquiarritmia gástrica [electrogastrografía]) y 2) efectos adversos (sedación,disminución cognitiva, visión borrosa). Se utilizó el método GRADE para evaluar la certeza de la evidencia de cada desenlace.
Resultados principales
Se incluyeron nueve ECA (658 participantes). Los estudios se realizaron en siete países, con un intervalo global de edad de 16 a 55 años. La cinetosis se indujo de forma natural en seis estudios y de forma experimental en cuatro (silla giratoria). Todos los estudios inducidos naturalmente evaluaron solo antihistamínicos de primera generación (cinarizina y dimenhidrinato). El riesgo de sesgo entre los estudios varió, con un riesgo mayoritariamente bajo para la generación de la secuencia aleatoria y el ocultamiento de la asignación, y mayoritariamente alto para el informe selectivo. Solo los estudios inducidos experimentalmente midieron parámetros fisiológicos y solo los estudios inducidos naturalmente evaluaron los efectos adversos. No hubo estudios que evaluaran claramente a la población pediátrica.
Antihistamínicos versus placebo o ningún tratamiento
Los antihistamínicos son probablemente más eficaces que el placebo para prevenir los síntomas de cinetosis en condiciones naturales (síntomas prevenidos: 25% placebo; 40% antihistamínicos) (razón de riesgos [RR] 1,81; intervalo de confianza [IC] del 95%: 1,23 a 2,66; tres estudios; 240 participantes) (certeza moderada). La evidencia sobre el efecto de los antihistamínicos en la prevención de la cinetosis en condiciones experimentales es muy incierta (diferencia de medias estandarizada [DME] 0,32; IC del 95%: ‐0,18 a 0,83; dos estudios; 62 participantes) (certeza muy baja). Ningún estudio proporcionó resultados sobre la resolución de los síntomas de cinetosis existentes.
Los antihistamínicos podrían dar lugar a poca o ninguna diferencia en la taquiarritmia gástrica en condiciones experimentales (diferencia de medias [DM] ‐2,2; IC del 95%: ‐11,71 a 7,31; un estudio; 42 participantes) (certeza baja). Ningún estudio proporcionó resultados sobre otras medidas fisiológicas. En comparación con el placebo, los antihistamínicos podrían ser más propensos a causar sedación (sedación: 44% placebo; 66% antihistamínicos) (RR 1,51; IC del 95%: 1,12 a 2,02; dos estudios; 190 participantes) (certeza baja); podrían dar lugar a poca o ninguna diferencia en la visión borrosa (visión borrosa: 12,5% placebo; 14% antihistamínicos) (RR 1,14; IC del 95%: 0,53 a 2,48; dos estudios; 190 participantes) (certeza baja); y podrían dar lugar a poca o ninguna diferencia en términos de disminución cognitiva (disminución de la cognición: 33% placebo; 29% antihistamínicos) (RR 0,89; IC del 95%: 0,58 a 1,38; dos estudios; 190 participantes) (certeza baja).
Antihistamínicos versus escopolamina
La evidencia sobre el efecto de los antihistamínicos en la prevención de la cinetosis en condiciones naturales en comparación con la escopolamina es muy incierta (síntomas prevenidos: 81% escopolamina; 71% antihistamínicos) (RR 0,89; IC del 95%: 0,68 a 1,16; dos estudios; 71 participantes) (certeza muy baja). No se realizaron estudios en condiciones experimentales. Ningún estudio proporcionó resultados sobre la resolución de los síntomas de cinetosis existentes.
La evidencia sobre el efecto de los antihistamínicos en la frecuencia cardíaca en condiciones naturales es muy incierta (informe narrativo, un estudio; 20 participantes; "No hay diferencia en la frecuencia del pulso"; certeza muy baja). Ningún estudio proporcionó resultados sobre otras medidas fisiológicas. En comparación con la escopolamina, la evidencia sobre el efecto de los antihistamínicos en la sedación es muy incierta (sedación: 21% escopolamina; 30% antihistamínicos) (RR 0,82; IC del 95%: 0,07 a 9,25; dos estudios; 90 participantes) (certeza muy baja) y en la visión borrosa (informe narrativo: no hay una diferencias significativas; un estudio; 51 participantes; certeza muy baja). Ningún estudio evaluó la disminución cognitiva.
Antihistamínicos versus antieméticos
Los antihistamínicos podrían dar lugar a poca o ninguna diferencia en la prevención de la cinetosis en condiciones experimentales (DM ‐0,20; IC del 95%: ‐10,91 a 10,51; un estudio; 42 participantes) (certeza baja). La evidencia es de certeza baja debido a la imprecisión ya que el tamaño muestral es pequeño y el intervalo de confianza cruza la línea de ningún efecto. Ningún estudio evaluó los efectos de los antihistamínicos versus los antieméticos en condiciones naturales. Ningún estudio proporcionó resultados sobre la resolución de los síntomas de cinetosis existentes.
Los antihistamínicos podrían dar lugar a poca o ninguna diferencia en la taquiarritmia gástrica (DM 4,56; IC del 95%: ‐3,49 a 12,61; un estudio; 42 participantes) (certeza baja). Ningún estudio proporcionó resultados sobre otras medidas fisiológicas. Ningún estudio evaluó la sedación, la disminución de la cognición ni la visión borrosa.
Un estudio informó acerca de datos fisiológicos para este desenlace, evaluando específicamente la taquiarritmia gástrica. Los antihistamínicos podrían dar lugar a poca o ninguna diferencia en la taquiarritmia gástrica (DM 4,56; IC del 95%: ‐3,49 a 12,61; un estudio; 42 participantes; evidencia de certeza baja). Esta evidencia es de certeza baja debido a la imprecisión ya que el tamaño muestral es pequeño y el intervalo de confianza cruza la línea de ningún efecto.
Antihistamínicos versus acupuntura
La evidencia sobre los efectos de los antihistamínicos en la prevención de la cinetosis en condiciones experimentales cuando se comparan con la acupuntura es muy incierta (RR 1,32; IC del 95%: 1,12 a 1,57; un estudio; 100 participantes) (certeza muy baja). Este estudio no evaluó la prevención de la cinetosis en condiciones naturales, ni la resolución de los síntomas de cinetosis existentes. No se han realizado estudios en condiciones naturales.
No se informaron medidas fisiológicas ni efectos adversos.
Conclusiones de los autores
Es probable que se reduzca el riesgo de desarrollar síntomas de cinetosis en condiciones naturales de movimiento cuando se utilizan antihistamínicos de primera generación, en adultos susceptibles de padecer cinetosis, en comparación con el placebo. Los antihistamínicos podrían ser más propensos a causar sedación en comparación con el placebo. Ningún estudio evaluó el tratamiento de la cinetosis existente, y hay pocos datos sobre el efecto de los antihistamínicos en los niños. La evidencia acerca de todos los demás desenlaces y comparaciones (versus la escopolamina, los antieméticos y la acupuntura) fue de certeza baja o muy baja y, por lo tanto, no están claros los efectos de los antihistamínicos.
PICOs
Resumen en términos sencillos
Antihistamínicos para prevenir y tratar el mareo por movimiento
¿Cuál es el objetivo de esta revisión?
El mareo por movimiento, como el que produce el mar o el coche, es un conjunto de síntomas, a menudo náuseas y vómitos. Su causa es el movimiento pasivo del cuerpo (cuando el cuerpo se mueve sin que lo hagamos conscientemente) en respuesta al movimiento real (por ejemplo, estar en un coche en marcha o en un barco), o a la imagen de movimiento cuando se expone al movimiento virtual (por ejemplo, las simulaciones de realidad virtual). y a los entornos visuales en movimiento (como mirar por la ventana de un tren en marcha). Los antihistamínicos son un tipo de medicamento que se suele administrar a las personas para tratar o prevenir el mareo por movimiento. En este estudio, se quería averiguar si estos medicamentos realmente funcionan para este fin.
Mensaje clave
Se ha descubierto que los antihistamínicos probablemente reducen el riesgo de que una persona sufra síntomas de mareo por movimiento en condiciones naturales de movimiento (como un barco o un avión) en comparación con el placebo (tratamiento ficticio), en adultos propensos a mareo por movimiento. También se determinó que, en comparación con el placebo, los antihistamínicos tienen más probabilidades de provocar somnolencia. No se encontraron estudios que analizaran si los antihistamínicos son eficaces para tratar el mareo por movimiento una vez que ya ha comenzado y hay muy poca información sobre su efecto en niños menores de 18 años. Para todos los demás resultados que se investigaron, no están claros los verdaderos efectos de los antihistamínicos en comparación con otros medicamentos y no medicamentos, ni otros efectos secundarios y efectos sobre las funciones corporales (como la frecuencia cardíaca o los movimientos del estómago).
¿Qué se estudió en la revisión?
Se examinaron los estudios en los que personas que se sabe que se marean con el movimiento reciben un tratamiento con un antihistamínico o con un placebo (tratamiento ficticio). También se analizó a los que se les administró un antihistamínico en comparación con otros medicamentos u otros tipos de tratamiento no farmacológico.
¿Cuáles son los resultados principales de la revisión?
Antihistamínicos comparados con placebo
Los resultados muestran que los antihistamínicos son probablemente más eficaces que el placebo para prevenir los síntomas del mareo en condiciones naturales.
No se sabe con certeza si los antihistamínicos son eficaces para prevenir el mareo o si tienen algún efecto sobre la taquiarritmia gástrica (la forma en que se mueve el interior del estómago), en condiciones experimentales (en un entorno de laboratorio) cuando se comparan con un placebo.
Los antihistamínicos podrían ser más propensos a causar sedación (somnolencia) en comparación con el placebo. No se sabe con certeza si los antihistamínicos provocan visión borrosa (no poder ver con claridad) o disminución de la cognición (no poder pensar con claridad) en comparación con el placebo.
Antihistamínicos frente a escopolamina
No hay certeza sobre la efectividad de los antihistamínicos en la prevención del mareo ni su capacidad para provocar somnolencia en comparación con la escopolamina en condiciones naturales.
Antihistamínicos frente a antieméticos
No hay certeza sobre la efectividad de los antihistamínicos en la prevención del mareo en condiciones naturales o de laboratorio, su efecto sobre los movimientos del estómago ni su capacidad para provocar somnolencia en comparación con los antieméticos.
Antihistamínicos frente a acupuntura
Existe incertidumbre sobre la efectividad de los antihistamínicos para prevenir el mareo en comparación con la acupuntura en condiciones de laboratorio.
¿Qué grado de actualización tiene esta revisión?
Esta revisión está actualizada hasta el 7 de diciembre de 2021.
Authors' conclusions
Summary of findings
| Antihistamines versus placebo for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | Antihistamines | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Assessed by: self‐reported questionnaires6,7 Follow‐up: varied8,9 | Study population | RR 1.81 | 240 | ⊕⊕⊕⊝ | Antihistamines are probably effective at preventing motion sickness symptoms under natural conditions when compared to placebo. | |
| 247 per 1000 | 447 per 1000 | |||||
| Moderate | ||||||
| 313 per 1000 | 567 per 1000 | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Assessed by: rotating chair Follow‐up: varied (7 days; 1 hour 20 minutes) | — | The standardised mean difference in susceptible participants who did not experience any motion sickness symptoms under experimental conditions in the intervention groups was | — | 62 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on preventing motion sickness under experimental conditions when compared to placebo. |
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | No studies in this comparison reported on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate and core temperature | Heart rate and core temperature were not measured in the studies in this comparison. | |||||
| Physiological measures: gastric tachyarrhythmia10 Assessed by: electrogastrography Follow‐up: 1 hour 20 minutes | Mean score: 60.29 | The mean gastric tachyarrhythmia score under experimental conditions in the intervention group was | — | 42 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in gastric tachyarrhythmia when compared to placebo. |
| Adverse effects: sedation Assessed by: adverse effect questionnaire (presence or absence given numerical values) Follow‐up: before departure and after returning from a sea voyage lasting a total duration of 4 to 6 hours in one study, and 5 hours in one study | Study population | RR 1.51 | 190 | ⊕⊕⊝⊝ | Antihistamines may be more likely to cause sedation when compared to placebo. | |
| 438 per 1000 | 661 per 1000 | |||||
| Moderate | ||||||
| 438 per 1000 | 661 per 1000 | |||||
| Adverse effects: impaired cognition Assessed by: adverse effect questionnaire (presence or absence given numerical values) Follow‐up: before departure and after returning from a sea voyage lasting a total duration of 4 to 6 hours in one study, and 5 hours in one study | Study population | RR 0.89 | 190 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in terms of impaired cognition when compared to placebo. | |
| 328 per 1000 | 292 per 1000 | |||||
| Moderate | ||||||
| 328 per 1000 | 292 per 1000 | |||||
| Adverse effects: blurred vision Assessed by: adverse effect questionnaire (presence or absence given numerical values) Follow‐up: before departure and after returning from a sea voyage lasting a total duration of 4 to 6 hours in one study, and 5 hours in one study | Study population | RR 1.14 | 190 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in blurred vision when compared to placebo. | |
| 125 per 1000 | 142 per 1000 | |||||
| Moderate | ||||||
| 125 per 1000 | 142 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to study limitations (risk of bias): incomplete data in one study (95 of 118 participants completed all questionnaires but reasons for this have not been stated); all studies had an unclear risk related to allocation concealment. | ||||||
| Antihistamines versus scopolamine for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: scopolamine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Scopolamine | Antihistamines | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Assessed by: self‐reported questionnaires6,7 Follow‐up: varied8,9 | Study population | RR 0.89 | 71 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on preventing motion sickness under natural conditions when compared to scopolamine. | |
| 806 per 1000 | 717 per 1000 | |||||
| Moderate | ||||||
| 773 per 1000 | 688 per 1000 | |||||
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | No studies in this comparison reported on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate Assessed by: self‐measured (participant measured own pulse rate) Follow‐up: just before flight, every 10 minutes during flight; immediately after flight; and finally at 20 minutes after the flight (flight duration 1 hour) | Results were only presented as a narrative summary in the translation of this study: "No difference in pulse frequency". | 20 (1 study) | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on the heart rate under natural conditions when compared to scopolamine. | ||
| Physiological measures: core temperature and gastric tachyarrhythmia (electrogastrography) | Gastric tachyarrhythmia and core temperature were not measured in the studies in this comparison. | |||||
| Adverse effects: sedation Assessed by: self‐reported Follow‐up: every 1 to 2 hours for a total sea voyage lasting 7 to 8 hours in one study; unspecified in one study | Study population | RR 0.82 | 90 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on sedation when compared to scopolamine. | |
| 213 per 1000 | 174 per 1000 | |||||
| Moderate | ||||||
| 206 per 1000 | 169 per 1000 | |||||
| Adverse effects: impaired cognitive function | No studies evaluated impaired cognition in this comparison. | |||||
| Adverse effects: blurred vision Assessed by: self‐reported Follow‐up: every 1 to 2 hours for a total sea voyage lasting 7 to 8 hours | Results were only presented as a narrative summary: "Use of transdermal scopolamine resulted in some side effects before motion, including dry mouth, drowsiness, and blurred vision, but only the incidence of dry mouth was statistically significant (P = 0.001)". | 51 (1 study) | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on blurred vision when compared to scopolamine. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to study limitations (risk of bias): studies had an unclear risk related to allocation concealment and random sequence generation. | ||||||
| Antihistamines versus antiemetics for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: antiemetic | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Antiemetic | Antihistamine | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Assessed by: calculated based on head movements tolerated (rotating chair); MSAQ Follow‐up: 1 hour and 20 minutes | 22.3 | The mean proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions in the intervention groups was | — | 42 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in the prevention of motion sickness under experimental conditions when compared to an antiemetic |
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | No studies in this comparison reported on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate and core temperature | Heart rate and core temperature were not measured in the studies in this comparison. | |||||
| Physiological measures: gastric tachyarrhythmia2 Assessed by: electrogastrography Follow‐up: 1 hour and 20 minutes | Mean score: 53.53 | The mean gastric tachyarrhythmia score under experimental conditions in the intervention groups was | — | 42 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in gastric tachyarrhythmia when compared to an antiemetic. |
| Adverse effects: impaired cognition and blurred vision | No studies in this comparison evaluated impaired cognition or blurred vision. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded two levels due to imprecision: overall confidence interval crosses the line of no effect; small sample size. | ||||||
| Antihistamines versus acupuncture for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: acupuncture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Acupuncture | Antihistamines | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Assessed by: Graybiel motion sickness scale Follow‐up: before and after treatment (exact time not specified) | Study population | RR 1.32 | 100 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of antihistamines on the prevention of motion sickness under experimental conditions when compared to acupuncture. | |
| 740 per 1000 | 977 per 1000 | |||||
| Moderate | ||||||
| 740 per 1000 | 977 per 1000 | |||||
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | The study in this comparison did not report on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate, core temperature and gastric tachyarrhythmia (electrogastrography) | Heart rate, core temperature and gastric tachyarrhythmia (electrogastrography) were not measured in the study in this comparison. | |||||
| Adverse effects: sedation, impaired cognitive function, blurred vision | The study in this comparison did not evaluate sedation, impaired cognitive function or blurred vision. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by two levels due to study limitations (risk of bias): incomplete data. (While the study appears to have complete outcome data, the authors have specified that participants were eliminated from the study based on the following criteria: poor compliance and inability to complete the treatment according to the test plan, serious adverse effects, serious deterioration of the participants' condition during the study, and participants who dropped out of the study due to "subjective and objective reasons". The number of participants who were eliminated has not been stated and it is unclear if these participants were replaced in order to complete the study with the same number of participants that were originally enrolled); unclear risk related to allocation concealment and random sequence generation; unblinded. | ||||||
Background
Description of the condition
Definition
Motion sickness is a syndrome that occurs as a result of passive body movement in response to actual motion, or the illusion of motion when exposed to virtual and moving visual environments. It generally occurs as a physiological response in a healthy person with an intact vestibular system; however, the presentation may be modulated by various pathologies (Bertolini 2016; Murdin 2015).
Presentation
Symptoms can include nausea, vomiting, loss of appetite, gastric awareness, increased sensitivity to odours, headaches (including migraines), dizziness, sweating, pallor, sensations of bodily warmth, increased salivation, bradycardia, arterial hypotension, general malaise, repetitive yawning and sopite syndrome (which includes fatigue, drowsiness and lethargy) (Bertolini 2016; Golding 2015). Space motion sickness differs from general motion sickness and is characterised by sudden projectile vomiting within minutes of weightlessness (Thornton 2013). Symptoms produced by motion sickness may be severe enough to have a negative impact on cognition and performance (Matsangas 2014).
Epidemiology
Historically, motion sickness was first described in seafarers (Hippocrates). A recent study undertaken on expedition ships to Antarctica has shown that motion sickness was the most common reason for consultation, with 150 out of a total of 680 physician consultations for prophylaxis followed by an additional 142 visits (27%, 4.2 per 1000 person‐days) for treatment (Schutz 2014).
Car sickness can affect most people with varying degrees of severity, under the right circumstances (Wada 2015), and is worse in passengers than drivers (Dong 2011). In one study it occurred in 25.9% of experienced rally co‐drivers, while reading and while seated as rear‐seat passengers (Perrin 2013). It may also prove a significant factor in the use of autonomous cars (Diels 2016), and on tilting trains, but can be influenced by compensation strategies (Förstberg 1998). Space motion sickness affects 50% of astronauts within the first 24 to 72 hours of weightlessness (Thornton 2013). Virtual reality has been shown to induce motion sickness (Nishiike 2013), and an incidence of up to 56% has been demonstrated with the use of video games (Stoffregen 2008). Amongst cinema patrons, 54.8% experienced motion sickness after viewing a 3D movie compared to 14.1% after viewing a 2D movie (Solimini 2013).
Motion sickness is rare in children under the age of two, but increases through childhood with a peak incidence at age nine, followed by a progressive decline through adolescence and adulthood (Henriques 2014). There is a slight preponderance in females (Henriques 2014; Paillard 2013; Perrin 2013).
Ménière’s disease and vestibular migraines are associated with increased motion sensitivity (Sharon 2014). A similar association between patients with vestibular migraines and those with migraines without vestibular symptoms has been shown (Murdin 2015). Benign paroxysmal positional vertigo and vestibular neuritis show no association with motion sickness (Golding 2015). Bilateral vestibular failure has a protective effect against the susceptibility to motion sickness, although this is not seen with unilateral vestibular failure (Murdin 2015).
Aetiology/pathophysiology
The sensory conflict or mismatch theory suggests that conflict arises between one's visual, proprioceptive and vestibular systems when the actual motion experienced differs from the anticipated motion (Reason 1978). Oman 1990 suggested that the difference between all the true sensory input and all the expected sensory information results in the conflict vector. The larger this vector, the greater the likelihood and severity of motion sickness. Bles 1998 further postulated that only vertical input is responsible for motion sickness, suggesting an alternate theory known as the subjective vertical conflict theory, while Holly 1996 expanded this to include all translations. Another hypothesis suggests a link between motion sickness and the time constant of velocity storage (Cohen 2003).
A genetic predisposition showed concordance of 70% in childhood and 50% in adulthood in monozygotic and dizygotic twins (Reavley 2006).
Diagnosis
The Reason and Brand Motion Sickness Susceptibility Questionnaire remains the most widely used tool to assess susceptibility to motion sickness (Golding 1998). Once symptoms have been established, Graybiel's diagnostic criteria may be used to grade the severity of motion sickness (Graybiel 1968). There is no laboratory test that is pathognomonic of motion sickness. Electrogastrography (Cevette 2014), vestibular evoked myogenic potentials (Tal 2013), vestibulo‐ocular reflexes (Tanguy 2008), caloric testing (Sharon 2014), computerised dynamic posturography (Tal 2010), neurochemical markers (ACTH, epinephrine, norepinephrine) (Kohl 1985), and measurements of autonomic activity (Cowings 1986) have all been used to evaluate and study motion sickness.
Management
Habituation is an effective countermeasure to motion sickness (Cowings 2000). It is influenced by the intensity and frequency of exposure to the stimulus, and it is potentiated by controlled breathing (Yen Pik Sang 2005). While playing video games, passive restraint (Chang 2013) and being in control reduce the onset of motion sickness. Reducing passive head movements and postural instability by viewing the horizon and widening one's stance have been shown to be protective (Stoffregen 2013), although the same is not true for artificial horizons (Tal 2012). Optokinetic training reduced sea sickness in 71.4% of participants compared to 12% in the control group (Ressiot 2013). Stroboscopic illumination may also be protective against motion sickness, possibly by reducing retinal slip (Webb 2013). Other methods such as galvanic vestibular stimulation in synchrony with the visual field (Cevette 2014), acupuncture, acupressure, transcutaneous electrical nerve stimulation (Chu 2012), ginger (Lien 2003), and music (Keshavarz 2014) have all been used to control motion sickness.
Pharmacological therapy for the management of motion sickness primarily involves the use of anticholinergics and antihistamines (Murdin 2011). Scopolamine is the most commonly used anticholinergic, and is effective compared to placebo in the prevention of motion sickness; however, there are insufficient data regarding its treatment of established symptoms. The side effects include dry mouth, blurred vision, dilated pupils and bradycardia (Spinks 2011). Other pharmacological agents include antiemetics (Muth 2007), neuroleptics such as phenytoin (Woodard 1993), µ‐opiate receptor agonists (Otto 2006), sympathomimetics (Weerts 2014a), and various combinations of all of these drugs.
Current approaches to countering space motion sickness include the combination of pre‐training in an altered gravity environment in combination with the use of promethazine (Karmali 2016).
Future measures to control the incidence of motion sickness may involve engineering the expected stimulus to be less provocative.
Description of the intervention
Antihistamines have been used in the management of motion sickness for decades (Brand 1967), alone or in combination with other interventions (Weerts 2014a). H1‐antihistamines are available as over‐the‐counter preparations, as well as by prescription (Simons 2004). For the control of motion sickness, routes of administration and dosages vary depending on the specific drug used (Zajonc 2006).
H1‐antihistamines may be classified according to their functional class (generation), or by their sedative effect. First‐generation H1‐antihistamines are generally sedating, while second‐ and third‐generation antihistamines are non‐sedating. This may be due to the fat‐soluble nature of first‐generation antihistamines, which allows them to cross the blood–brain barrier, while second‐ and third‐generation antihistamines do not. In addition, first‐generation antihistamines exhibit anticholinergic properties (Mahdy 2014). Wood 1970b suggested this as the reason for their protective effect against motion sickness. Typically, after a single oral dose of an H1‐antihistamine, the onset of action is between two to three hours for first‐generation antihistamines, and one to two hours for second‐generation antihistamines. The duration of action may be up to 24 hours (Simons 2004).
Side effects that limit the use of H1‐antihistamines in certain professions (such as astronauts) include drowsiness, fatigue, dizziness and impairment of cognitive function, memory and psychomotor performance (Weerts 2014b). Other reported adverse effects include dystonia, dyskinesia, agitation, confusion, hallucinations and cardiac toxicity. Additionally, first‐generation antihistamines may produce side effects related to their anticholinergic activity, such as blurred vision, dry mouth, dilated pupils and urinary retention. Second‐generation H1‐antihistamines have been relatively free of adverse effects. However, two early second‐generation antihistamines, astemizole and terfenadine, have been withdrawn due to cardiac toxicity (Simons 2004).
Antihistamines have been compared to scopolamine (Gil 2012; Pingree 1994); however, the comparative effectiveness in the management of motion sickness was found to be inconclusive in a Cochrane Review (Spinks 2011).
How the intervention might work
Acetylcholine (ACh) is a vestibular neurotransmitter and has been identified in all vestibular nuclei. Histamine may be a vestibular neurotransmitter or neuromodulator, acting on histamine receptors (H1‐H3 are expressed in the vestibular system), but this remains unclear (Soto 2010). First‐generation antihistamines are ACh and H1 receptor antagonists, thus inhibiting their effects on the vestibular system. Second‐generation antihistamines do not possess any anticholinergic properties but inhibit histaminergic activity only (Mahdy 2014). Cheung 2003 concluded that second‐generation agents are not effective in the management of motion sickness and suggested that the anticholinergic and sedative effects of first‐generation agents may be the reason for their apparent success.
Why it is important to do this review
When motion sickness was first described by Hippocrates in 400 BC, land and sea travel were the main sources of passive motion. Now, for the general population, this includes motor vehicles, trains, buses, cruise liners and other smaller vessels, and passenger aircraft. Additionally, in this age of rapid technological advancement, new sources of motion sickness inducing stimuli have emerged, including virtual reality, 3D visual effects, 4D experiences, video games, driverless cars and commercial space flight. Apart from the daily life and recreational aspects, occupational exposure to motion sickness inducing stimuli has increased over time. This includes but is not limited to paramedics in helicopters and ambulances, military personnel on naval vessels and in the air force, pilots, seafarers, and astronauts during space flight and training.
While habituation is effective and has no side effects, it lacks immediacy. Antihistamines have been the most commonly used pharmacological therapy (Weerts 2014b), however studies reveal conflicting results regarding their efficacy in the management of motion sickness (Buckey 2004; Cheung 2003). This review aimed to potentially resolve this conflict and to facilitate advancement of future research in the field of motion sickness.
Objectives
To assess the effectiveness of antihistamines in the prevention and treatment of motion sickness in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
We included all published and unpublished randomised controlled trials (RCTs), including cluster‐randomised trials. We excluded cross‐over studies. There were no time or language limitations on included studies.
Types of participants
Participants included susceptible adults and children (the age limit to define children was 18 years and under), of any gender and ethnicity, with no vestibular, visual or neurological co‐morbidities.
We included:
-
susceptible participants in whom motion sickness was induced under natural conditions such as air, sea and land transportation.
Susceptibility was defined as:
-
previous experience of motion sickness; and/or
-
motion sickness susceptibility based on the result of any validated scale.
We included studies in which motion sickness was induced under experimental conditions but we analysed the data from these studies separately.
Types of interventions
The main intervention was all antihistamines regardless of:
-
class (first‐ or second‐generation);
-
route of administration; or
-
dosage.
Comparison interventions included:
-
no treatment;
-
placebo;
-
any other pharmacological interventions (for example, scopolamine, phenytoin, ondansetron, metoclopramide); and
-
any non‐pharmacological interventions (for example, acupuncture, transcutaneous electrical nerve stimulation, habituation techniques).
The main comparison was:
-
antihistamine versus no treatment or placebo.
Other possible comparison pairs included:
-
antihistamine versus scopolamine;
-
antihistamine versus antiemetics;
-
antihistamine versus neuroleptics;
-
antihistamine versus µ‐opiate receptor agonists;
-
antihistamine versus sympathomimetics;
-
antihistamine versus acupuncture;
-
antihistamine versus acupressure;
-
antihistamine versus autogenic feedback training exercises;
-
antihistamine versus transcutaneous electrical nerve stimulation.
Concurrent use of other medication was only acceptable if used equally in each group.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Primary outcomes
-
Proportion of susceptible participants who did not experience any motion sickness symptoms (based on subjective reporting of nausea and/or vomiting or the use of a validated scale).
-
Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms (based on subjective reporting of nausea and/or vomiting or the use of a validated scale).
Secondary outcomes
-
Physiological measures: heart rate, core temperature and gastric tachyarrhythmia (electrogastrography).
-
Adverse effects (type, duration and severity): sedation, impaired cognitive function, blurred vision.
We intended to divide the secondary outcomes into short‐term (less than or equal to 24 hours) and long‐term (over 24 hours) outcomes, however no studies evaluated long‐term physiological measures or adverse effects.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 7 December 2021.
Electronic searches
The Information Specialist searched:
-
the Cochrane ENT Trials Register (searched via the Cochrane Register of Studies 7 December 2021);
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (searched via the Cochrane Register of Studies 7 December 2021);
-
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 7 December 2021);
-
Ovid EMBASE (1974 to 7 December 2021);
-
LILACS, lilacs.bvsalud.org (searched 7 December 2021);
-
Web of Knowledge, Web of Science (1945 to 7 December 2021);
-
CNKI, www.cnki.com.cn (searched via Google Scholar 7 December 2021);
-
ClinicalTrials.gov, (searched via the Cochrane Register of Studies 7 December 2021);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), www.who.int/ictrp (searched 7 December 2021).
In searches prior to 2019 we also searched KoreaMed to July 2017.
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Higgins 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
Data collection and analysis
Selection of studies
Two review authors (NK and RB) independently sifted through the initial search results and identified studies that appeared to meet our inclusion criteria. We then obtained full‐text articles for the studies on this preliminary list. We then independently examined these studies and selected those that met all our inclusion criteria. We resolved discrepancies by reviewing and discussing the reasons for including or excluding the original studies and where an agreement could not be arrived at, we consulted a third review author (YS).
Data extraction and management
Two review authors (NK and RB) independently extracted data using standardised forms. For any studies with missing or incomplete data, we contacted the study authors, where possible.
We extracted the following:
-
study design features (double‐/single‐/non‐blinded; cluster/parallel‐group);
-
setting;
-
sample size;
-
participant (baseline) characteristics (age, gender, susceptibility to motion sickness and how this was assessed, co‐morbidities);
-
inclusion criteria;
-
exclusion criteria;
-
method of induction of motion sickness;
-
duration of motion;
-
type of antihistamine used (name, class, route, dosage);
-
comparison intervention;
-
outcomes;
-
funding sources;
-
study author declarations of interest.
Assessment of risk of bias in included studies
NK and RB independently assessed the risk of bias of the included studies. This was determined using Cochrane's tool for assessing the risk of bias as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We considered the following domains and assigned a judgement based on the following criteria:
Random sequence generation
-
Low risk: Study authors describe a random component in the sequence generation process such as referring to a random number table, using a computerised random number generation, coin tossing, shuffling cards or envelopes, throwing dice or drawing of lots.
-
High risk: Study authors describe a non‐random component in the sequence generation process (such as allocation based on geographic location, hospital number, date of birth).
-
Unclear risk: Study authors have not specified the sequence generation process.
Concealment of allocation prior to assignment
-
Low risk: Participants and/or investigators could not foresee drug allocation due to concealed allocation (such as the use of central allocation, or sequentially numbered, opaque envelopes or drug containers).
-
High risk: Participants and/or investigators could foresee drug allocation due to an inadequate concealment process.
-
Unclear risk: Insufficient information is given on the allocation concealment process.
Blinding of provider, participant and outcome assessor
-
Low risk: Blinding of treatment provider, participant or outcome assessor undertaken.
-
High risk: Blinding not undertaken.
-
Unclear risk: Study does not state whether blinding was undertaken or not.
Incomplete outcome data
-
Low risk: No incomplete outcome information, or the reason for incomplete outcome data is unrelated to the study's outcomes (for example: a participant dropped out of the study due to relocating to a new geographic location).
-
High risk: Incompleteness of outcome data is related to the study's outcomes (for example: a participant dropped out of the study due to severe nausea).
-
Unclear risk: Reason for missing data unspecified.
Selective outcome reporting
-
Low risk: The study protocol is available and all of the study's pre‐specified outcomes have been reported in the pre‐specified manner.
-
High risk: Not all the primary outcomes have been reported, or one or more of the primary outcomes were reported using methods of analysis that were not pre‐specified, or one or more of the primary outcomes were not pre‐specified, or one or more of the primary outcomes were reported incompletely.
-
Unclear risk: Insufficient information available to assign a judgement.
Other bias
-
Low risk: Study appears free of other sources of bias.
-
High risk: Other source of bias noted by review authors.
We classified studies that have been categorised as high risk on the basis of random sequence generation and/or concealment of allocation of treatment and/or incomplete outcome data as having a high overall risk of bias. We did not consider studies that have been categorised as high risk in one or more of the other domains to have a high overall risk of bias.
Measures of treatment effect
For dichotomous data, we calculated individual and pooled statistics as risk ratios (RR) with 95% confidence intervals (95% CI). We assessed continuous data (for example, heart rate) using the mean difference (MD) for outcomes measured on the same scale. We used the standardised mean difference (SMD) for outcomes measured on different scales. We did not use a change from baseline analysis and did not complete an intention‐to‐treat analysis, as the relevant data were not available in the included studies.
Unit of analysis issues
For multi‐arm studies, we established which comparisons were relevant to this review and included data from the respective arms. In cases where more than one treatment arm is relevant, we included data from both arms, provided the participants were different in each arm and there was no treatment overlap. We did not include cross‐over studies.
Dealing with missing data
We attempted to contact study authors by email for studies with missing data. Whenever we were unable to contact the author and/or if the author was unable to provide the relevant information, we assumed the missing data to be 'missing at random' and conducted the data analysis using only the available data.
Assessment of heterogeneity
We assessed clinical, methodological and statistical heterogeneity. We measured statistical heterogeneity using the Chi² test and the I² statistic. For the latter, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), a value of > 50% suggests substantial heterogeneity.
Assessment of reporting biases
We addressed publication bias (between‐study reporting bias) by searching for published, unpublished and ongoing studies in the specified trial databases. We ensured data from all the available outcomes across all papers are recorded, taking care not to duplicate results. In the event of potentially eligible but unpublished studies being identified, our intention was to contact the authors to acquire the full study results and/or to enquire why the results had not been published. However, no potentially eligible unpublished studies were identified. For ongoing studies, we intended to include results available until the date of publication of this review, however no potentially eligible ongoing studies were identified. We addressed language bias by including studies in all languages and we obtained English translations where possible. We addressed outcome (within‐study) reporting bias by ensuring results have been presented as indicated in our protocol, which was published beforehand (Karrim 2017). We assessed between‐study reporting bias as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
For comparable data, we combined data to give a summary measure of effect using the methods set out in Measures of treatment effect. We used the available data to perform a meta‐analysis using Review Manger 5.4 (RevMan 2020). We tested for heterogeneity using the I2 statistic and we assumed significant heterogeneity if the I2 was greater than 50% (i.e. more than 50% of the variability in outcome between studies could not be explained by sampling variation) (Higgins 2011). We used a fixed‐effect model if there was no statistical heterogeneity (i.e. I2 < 50%), and a random‐effects model if heterogeneity was present (i.e. I2 > 50%).
Subgroup analysis and investigation of heterogeneity
If there had been sufficient studies available, we intended to conduct the following subgroup analysis in RevMan 5, using the formal test for subgroup differences (RevMan 2020):
-
age (adults versus children).
This subgroup analysis was chosen as adults and children may report symptoms differently and antihistamines may have differing effects on each group (for example, children may be more susceptible to the side effects of antihistamines). However, only one study included children and a subgroup analysis was not possible.
Sensitivity analysis
Two review authors (NK and RB) independently conducted a sensitivity analysis by identifying studies with a high risk of bias using the Cochrane risk of bias tool as detailed in the Assessment of risk of bias in included studies.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to rate the overall certainty of the evidence. The certainty of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high certainty of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low certainty implies that any estimate of effect obtained is very uncertain. The GRADE approach rates evidence from RCTs that do not have serious limitations as being of high certainty. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
-
study limitations (risk of bias);
-
inconsistency;
-
indirectness of evidence;
-
imprecision; and
-
publication bias.
We have included the relevant summary of findings tables, constructed according to the recommendations described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). These show the following comparisons:
-
antihistamines versus placebo;
-
antihistamines versus scopolamine;
-
antihistamines versus antiemetics;
-
antihistamines versus acupuncture.
See summary of findings Table 1; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
After a complete search as detailed above, we identified 1798 articles that met our initial search criteria at the time of our most recent search in December 2021. After duplicates were removed, we were left with 824 articles. Of these, we discarded 703 on the basis of their titles and abstracts (see Figure 1).
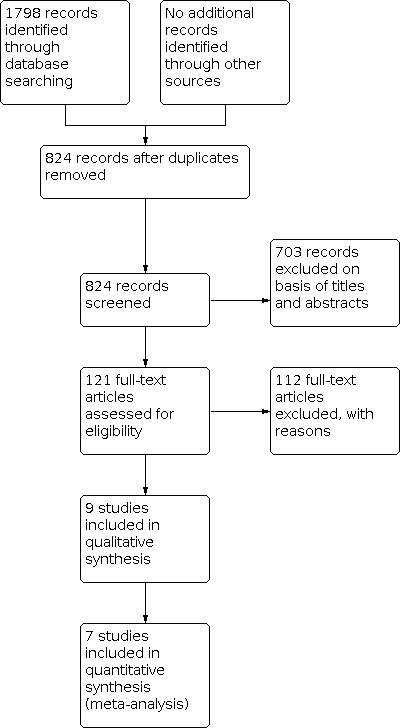
Process for sifting search results and selecting studies for inclusion.
We considered the remaining 121 studies potentially relevant and performed a full‐text analysis. Of these, we excluded 112 based on the full‐text review. We were unable to obtain a full‐text copy of one article, so in this case, we contacted the author who confirmed that the paper was not a randomised controlled trial (Kohl 1985).
The remaining nine studies met all our inclusion criteria were included in our review (Doweck 1994; Gao 2015; Hargreaves 1982; Kohl 1987; Muth 2007; Offenloch 1986; Price 1981; Salenko 2006; Shupak 1994).
Included studies
We included nine randomised controlled trials in this review, with a total of 658 participants (Doweck 1994; Gao 2015; Hargreaves 1982; Kohl 1987; Muth 2007; Offenloch 1986; Price 1981; Salenko 2006; Shupak 1994). Full details of the studies are shown in the Characteristics of included studies table.
Design
Eight studies were parallel‐group randomised controlled trials and one was a cross‐over study (Shupak 1994). Although our protocol stated that cross‐over studies would be excluded, we included Shupak 1994 as the author confirmed that the participants in each arm of the study were not the same ‐ therefore the sea arm of the study was included in this review as it met out inclusion criteria. Four studies were two‐arm trials (Gao 2015; Hargreaves 1982; Kohl 1987; Offenloch 1986), and five studies were three‐arm trials (Doweck 1994; Muth 2007; Price 1981; Salenko 2006; Shupak 1994). Six studies were double‐blinded (Doweck 1994; Kohl 1987; Muth 2007; Offenloch 1986; Price 1981; Shupak 1994), one study mentioned that it was blinded but was not specific on the degree of blinding (Salenko 2006), one study made no mention of blinding (Hargreaves 1982), and one study was unblinded (Gao 2015). Duration was unspecified in two studies (Hargreaves 1982; Salenko 2006). The duration of the remaining seven studies ranged from 60 minutes to five weeks, with a maximum follow‐up time of one year.
Sample sizes
The total sample size for all the included studies was 658 participants. There were 325 participants in the studies where motion sickness was induced under natural conditions (Doweck 1994; Hargreaves 1982; Offenloch 1986; Price 1981; Shupak 1994) and 333 in the experimentally induced motion sickness studies (Gao 2015; Kohl 1987; Muth 2007; Salenko 2006). Within studies, the sample size ranged from 20 (Kohl 1987; Offenloch 1986) to 150 (Salenko 2006).
Setting
Of the nine included studies, motion sickness was induced under natural conditions in five (Doweck 1994; Hargreaves 1982; Offenloch 1986; Price 1981; Shupak 1994), and under experimental conditions in four (Gao 2015; Kohl 1987; Muth 2007; Salenko 2006). Four of the five natural studies were conducted at sea (Doweck 1994; Hargreaves 1982; Price 1981; Shupak 1994), and one was conducted on a helicopter flight (Offenloch 1986). The experimentally induced studies were all conducted using a rotational chair in a laboratory setting.
The naturally induced studies ranged in date with the earliest study being in 1980 to the most recent in 1994. The experimentally induced studies ranged from 1987 to 2013. These years refer to the year of publication as the dates over which the actual studies were conducted were rarely mentioned. Overall, five studies were conducted in the 1980s, one study in the 1990s (Shupak 1994), and three studies in the 2000s (Gao 2015; Muth 2007; Salenko 2006).
The studies were conducted in six countries ‐ Germany, Russia, UK, China, two studies in Israel and three in the USA. All but two studies were single‐centre, one was conducted out of two centres (Salenko 2006), and in one this was not specified (Gao 2015).
Participants
The majority of studies recruited "healthy young males" with an overall ratio of approximately 2:1 in favour of male participants. The age distribution ranged from 16 to 55 years. Five studies specified that the participants were healthy and had no co‐morbidities (Doweck 1994; Gao 2015; Kohl 1987; Price 1981; Shupak 1994), one study clarified that participants had no gastrointestinal, neurological or cardiovascular co‐morbidities, and three studies made no mention of the presence or absence of co‐morbidities amongst participants (Hargreaves 1982; Offenloch 1986; Salenko 2006).
Interventions
Antihistamines
Antihistamines were the main intervention that we evaluated, regardless of class (first‐ or second‐generation), route of administration or dosage. The antihistamines used in the included studies were cinnarizine, dimenhydrinate and astemizole. No other antihistamines (e.g. promethazine) were evaluated in the studies that met our criteria. Nine of the 10 studies evaluated first‐generation antihistamines. All studies performed under natural conditions used only first‐generation antihistamines. Only one study tested a second‐generation antihistamine (Kohl 1987), and this was performed under experimental conditions.
Of the five studies performed under natural conditions, three made use of cinnarizine, with a total of 171 participants receiving the drug (Doweck 1994; Hargreaves 1982; Shupak 1994), and two made use of dimenhydrinate, with a total of 35 participants receiving the drug (Offenloch 1986; Price 1981). The antihistamine used in three of the experimental studies was dimenhydrinate in a total of 100 participants (Gao 2015; Muth 2007; Salenko 2006), and one study made use of astemizole in 10 participants (Kohl 1987).
Cinnarizine
Cinnarizine is a first‐generation H1‐antihistamine. Three studies used cinnarizine as an orally ingested formulation (Doweck 1994; Hargreaves 1982; Shupak 1994). No alternate routes of administration are available for this drug. The dose and timing of administration varied across all four studies. The highest reported dose across studies was 50 mg. Timing of administration ranged from 30 minutes to two hours prior to the onset of motion. Once‐off dosing was used in all but one study (Hargreaves 1982), which repeated the dose after six to eight hours.
A breakdown of the doses and timing of administration of oral cinnarizine across the three studies is as follows:
Hargreaves 1982: cinnarizine was given as two 15 mg tablets (total 30 mg dose) administered one to two hours before motion was induced (departure out to sea), and repeated six‐ to eight‐hourly for the duration of the voyage (total duration was not specified in the published manuscript).
Shupak 1994: cinnarizine was given in two arms of the study, one as two 25 mg tablets (total 50 mg dose) and in the other arm as a single 25 mg tablet (total 25 mg dose), administered two hours before motion was induced (departure out to sea).
Doweck 1994: cinnarizine was given in two arms of the study, one as two 25 mg tablets (total 50 mg dose) and in the other arm as a single 25 mg tablet (total 25 mg dose), administered one hour before motion was induced (departure out to sea).
In all of the studies that used cinnarizine, motion sickness was induced under natural conditions in the form of sea voyages.
Dimenhydrinate
Dimenhydrinate is a first‐generation H1‐antihistamine. Five studies used dimenhydrinate as an oral formulation, four of which were in tablet form (Gao 2015; Muth 2007; Price 1981; Salenko 2006) and one as an oral lozenge ("dragee") (Offenloch 1986). No other routes of administration were used for this drug. The dose of dimenhydrinate was 100 mg in one study (Muth 2007) and 50 mg in two studies (Gao 2015; Salenko 2006). Two studies did not report the dose of dimenhydrinate that was used (Offenloch 1986; Price 1981). The timing of administration varied across four studies, ranging from 30 minutes to 90 minutes prior to onset of motion. One study did not report the time at which the drug was given in relation to the onset of motion (Salenko 2006). Once‐off dosing was used in all but one study (Price 1981), which repeated the dose after 2.5 hours.
A breakdown of the doses and timing of administration of oral dimenhydrinate across the five studies is as follows:
Gao 2015: dimenhydrinate was given as a 50 mg tablet, administered 30 minutes before motion was induced (rotational chair).
Muth 2007: dimenhydrinate was given as a 100 mg tablet, administered 60 minutes before motion was induced (rotational chair).
Offenloch 1986: dimenhydrinate was given as a lozenge of unspecified dosage, administered 90 minutes before motion was induced (flight).
Price 1981: dimenhydrinate was given as a tablet of unspecified dosage, administered 90 minutes before motion was induced (sea voyage), and repeated 2.5 hours into the sea voyage.
Salenko 2006: dimenhydrinate was given as a 50 mg tablet, administered at an unspecified time in relation to the initiation of motion (rotational chair).
In three of the studies that used dimenhydrinate, motion sickness was induced under experimental conditions by way of rotational chair. In two studies, motion sickness was induced under natural conditions – one study was performed at sea and one study used flight as the method of induction as detailed above.
Astemizole
Astemizole is a second‐generation antihistamine, which was used in a single study (Kohl 1987), given orally at a dose of 30 mg, administered daily for seven consecutive days. Motion was then induced under experimental conditions (rotational chair) on day seven, two hours after the final 30 mg dose was administered. Astemizole has since been withdrawn from the market due to potentially fatal side effects.
Comparison interventions
Our planned comparison interventions included no treatment, placebo, any other pharmacological interventions (for example, scopolamine, phenytoin, ondansetron, metoclopramide) and any non‐pharmacological interventions (for example, acupuncture, transcutaneous electrical nerve stimulation, habituation techniques). Of these, placebo, scopolamine, ondansetron and acupuncture were the only comparison interventions used.
None of the included studies compared antihistamines to neuroleptics, µ‐opiate receptor agonists, sympathomimetics, acupressure, autogenic feedback training exercises or transcutaneous electrical nerve stimulation.
Antihistamine versus no treatment or placebo
Antihistamines were compared to placebo in six studies (Doweck 1994; Kohl 1987; Muth 2007; Price 1981; Salenko 2006; Shupak 1994). An additional study made use of a placebo, however this was used in order to perform blinding, and was not evaluated as an independent comparator (Offenloch 1986).
Three studies compared placebo to dimenhydrinate (Muth 2007; Price 1981; Salenko 2006), two studies compared placebo to cinnarizine (Doweck 1994; Shupak 1994), and one compared placebo to astemizole (Kohl 1987).
Antihistamines versus scopolamine
Three studies compared antihistamines to scopolamine (Hargreaves 1982; Offenloch 1986; Price 1981). Two studies used transdermal mode of delivery (Offenloch 1986; Price 1981), and one study administered the drug orally (Hargreaves 1982). The dose of scopolamine ranged from 0.3 mg to 0.5 mg. Timing of administration was described across the three studies as "17:30 the evening before" (no time of flight recorded for the following day), 13.5 hours prior to the onset of motion (sea voyage), and one to two hours prior to the onset of motion (sailing). Once‐off dosing was used in all but one study (Hargreaves 1982), which repeated the dose after six to eight hours.
A breakdown of the dose, timing and route of administration of scopolamine across the three studies is as follows:
Price 1981 applied a 0.5 mg transdermal scopolamine patch 13.5 hours prior to the onset of motion (sea voyage).
Offenloch 1986 applied a transdermal scopolamine patch that delivered 1.5 mg per day over three days. This was applied at 17:30 the day before the flight, with an estimated delivered dose of 0.5 mg at the time of the onset of motion (sea voyage).
Hargreaves 1982 administered a 0.3 mg tablet of scopolamine one to two hours prior to the onset of motion (sailing), and the dose was repeated six‐ to eight‐hourly for the duration of the voyage.
In all the studies that used scopolamine, motion sickness was induced under natural conditions in the form of sea voyages.
All three studies compared scopolamine to first‐generation antihistamines. Two studies compared scopolamine to dimenhydrinate (Offenloch 1986; Price 1981), and one study compared scopolamine to cinnarizine (Hargreaves 1982).
Antihistamines versus antiemetics
One study compared a first‐generation antihistamine (dimenhydrinate) to an antiemetic (ondansetron) (Muth 2007). The antiemetic was delivered orally (once‐off dosing). Ondansetron was given as 3 x 8 mg tablets (total dose of 24 mg), administered 60 minutes before motion was induced under experimental conditions (rotational chair).
Antihistamines versus acupuncture
One study compared a first‐generation antihistamine (dimenhydrinate) to acupuncture (Gao 2015). The regimen involved five‐point acupuncture, retained for 20 minutes and repeated once. This was performed twice a week for a total of five weeks. Motion sickness was induced under experimental conditions (rotating chair).
Antihistamines versus homeopathic remedies
One study compared a first‐generation antihistamine (dimenhydrinate) to Avia‐Sea, a homeopathic remedy (Salenko 2006). Avia‐sea was given as a single tablet (dose unspecified), administered every 30 minutes until five tablets had been administered in total, at an unspecified time in relation to the initiation of motion. Motion sickness was induced under experimental conditions (rotating chair).
Rescue medication
Price 1981 offered rescue medication to participants who became unwell during the sea voyage. This was given in the form of intramuscular scopolamine (0.2 mg).
Outcomes
Primary outcomes
Proportion of susceptible participants who did not experience any motion sickness symptoms (based on subjective reporting of nausea and/or vomiting or the use of a validated scale)
All nine studies evaluated the prevention of motion sickness symptoms in their outcomes. Five studies reported the prevention of nausea and vomiting amongst participants (Doweck 1994; Gao 2015; Kohl 1987; Price 1981; Shupak 1994), two studies reported only the prevention of nausea with no mention of vomiting (Muth 2007; Offenloch 1986). Two studies reported outcomes that did not evaluate the prevention of either nausea or vomiting (Hargreaves 1982; Salenko 2006).
Five studies made use of validated scales to assess the severity of motion sickness symptoms. Of these, two studies used the scale described by Graybiel 1968 (Gao 2015; Kohl 1987) and one study (Muth 2007) used the Motion Sickness Assessment Questionnaire (MSAQ) score (Gianaros 2001) and nausea profile score (Muth 1995b). Two studies, Shupak 1994 and Doweck 1994, made use of the motion sickness scale described by Wiker 1979, however both of these studies transformed the original seven‐point seasickness scale into a three‐point scale based on participants' responses. A summary of the modified scale categories I, II and III is as follows: I ‐ Wiker score of 0 to 3; II ‐ Wiker score of 4 to 6; III ‐ Wiker score of 7. Three studies used self‐defined scales as outlined below (Hargreaves 1982; Offenloch 1986; Price 1981).
-
-
Do you think the tablets helped?: not at all, a little, very much, drowsiness.
-
-
-
Self‐reported scale: 1) nausea: graded 1 to 5; 2) stomach ache: yes/no; 3) dizziness: grade 1 to 3; 4) headache: yes/no; 5) paleness: yes/no (reported by investigator).
-
This scale was then interpreted by the authors and transformed into an effectiveness rating of: very good, good, weak or none.
-
-
-
Stage of motion sickness severity: no symptoms – 0; stomach awareness or discomfort – 1; mild nausea – 2; moderate nausea – 3; severe nausea – 4; retching – 5; vomiting – 6.
-
Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms (based on subjective reporting of nausea and/or vomiting or the use of a validated scale)
No studies reported the outcome resolution of existing motion sickness symptoms.
Secondary outcomes
In this review we assessed secondary outcomes as short‐term (less than or equal to 24 hours) or long‐term (over 24 hours). With regard to the treatment duration and follow‐up of the nine included studies, five were of a short‐term duration (Doweck 1994; Muth 2007; Offenloch 1986; Price 1981; Shupak 1994), two were of a long‐term duration (Gao 2015; Kohl 1987), and two did not specify the duration (Hargreaves 1982; Salenko 2006). Of the two long‐term studies (over 24 hours), neither Gao 2015 nor Kohl 1987 reported outcomes in line with our secondary outcomes (physiological measures and side effects). Therefore all the outcomes described below are short‐term outcomes.
Physiological measures: heart rate, core temperature and gastric tachyarrhythmia (electrogastrography)
Physiological outcomes were measured in two out of the nine included studies (Muth 2007; Offenloch 1986).
Heart rate: Offenloch 1986 assessed participants' pulse rate before the flight to obtain a baseline recording and then again throughout the flight phase. Results were reported as a median range. No other studies measure the heart rate of participants.
Core temperature: this was not measured in any of the included studies.
Electrogastrography: the measure of significance from this test is the presence of gastric tachyarrhythmia. Muth 2007 performed an electrogastrography on participants with four‐minute, 75% overlapping windows and the results were averaged across the windows for the entire period. Results were reported as a baseline recording and a rotational recording. No other studies measured electrogastrography.
Adverse effects (type, duration and severity): sedation, impaired cognitive function, blurred vision
Five of the nine included studies provided information about the incidence of adverse effects (Doweck 1994; Hargreaves 1982; Offenloch 1986; Price 1981; Shupak 1994).
Adverse effects were self‐reported by the participants in all five studies. In three of the five studies, the participants were required to complete a checklist that included a pre‐determined list of adverse effects from them to choose from (Hargreaves 1982; Price 1981; Shupak 1994). Two studies were unclear in their method of collection of adverse effect data (Doweck 1994; Offenloch 1986). None of the studies clearly reported that participants were allowed to list/describe their adverse effects using their own terminology.
The duration of each adverse effect was not reported in any of the included studies. Severity of the adverse effects included in our review was examined by one study (Doweck 1994); however, Doweck 1994 converted the severity scale into a "No" if the participant reported no adverse effects or mild adverse effects, or "Yes" if the participant reported moderate or severe adverse effects. The remaining studies categorised adverse effects as either present or absent.
The adverse effects of interest in our review are sedation, impaired cognition and blurred vision.
All five studies reported on sedation, three studies reported on blurred vision (Doweck 1994; Price 1981; Shupak 1994), and two studies reported on impaired cognition (Doweck 1994; Shupak 1994).
A breakdown of the manner in which the adverse effects relevant to this review were measured in each study is as follows:
Sedation:
-
Shupak 1994: an adverse effect questionnaire was completed after the voyage with a score of 0 for non‐existence or 1 for existence.
-
Price 1981: participants reported the occurrence of drowsiness before and after motion.
-
Doweck 1994: an adverse effect questionnaire was completed after the voyage, which made use of a four‐point classification: 0 ‐ no side effects; 1 – mild; 2 – moderate; 3 – severe. These data were then converted into two categories: "No" (score 0 or 1) or "Yes" (score 2 or 3).
-
Hargreaves 1982: diary cards were completed by the participants after the voyage to note the presence or absence of drowsiness.
-
Offenloch 1986: it was unclear how the adverse effects were reported and when the data were collected.
Blurred vision:
-
Shupak 1994: an adverse effect questionnaire was completed after the voyage with a score of 0 for non‐existence or 1 for existence.
-
Price 1981: participants reported the occurrence of blurred vision before and after motion.
-
Doweck 1994: an adverse effect questionnaire was completed after the voyage, which made use of a four‐point classification: 0 ‐ no side effects; 1 – mild; 2 – moderate; 3 – severe. These data were then converted into two categories: "No" (score 0 or 1) or "Yes" (score 2 or 3).
Impaired cognition:
-
Shupak 1994: an adverse effect questionnaire was completed after the voyage with a score of 0 for non‐existence or 1 for existence.
-
Doweck 1994: an adverse effect questionnaire was completed after the voyage, which made use of a four‐point classification: 0 ‐ no side effects; 1 – mild; 2 – moderate; 3 – severe. These data were then converted into two categories: "No" (score 0 or 1) or "Yes" (score 2 or 3).
Outcomes not relevant to this review
One study did not use any outcomes that were relevant to this review: Salenko 2006 measured the amount of time tolerated by the participant on the rotating chair until symptoms developed and the time to recovery from symptoms after the chair, but did not clarify which symptoms were assessed as the endpoints.
Excluded studies
We excluded 112 studies. The decision to exclude was made on the basis of the treatment used, participant characteristics, study design, outcomes measured or the absence of a motion sickness susceptibility assessment. See the Characteristics of excluded studies table for details.
Risk of bias in included studies
The complete results of the risk of bias assessment are contained in the Characteristics of included studies section. These results are based on independent assessments by two review authors (NK and RB).

Risk of bias summary: Based on review authors' judgements (performed by NK and RB) about each risk of bias item for each included study.

Risk of bias graph: Based on review authors' judgements (performed by NK and RB) about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
All studies stated that they were "randomised", with only four studies supplying more detail about the process (Doweck 1994; Muth 2007; Offenloch 1986; Shupak 1994). Doweck 1994 and Shupak 1994 specify "breaking the code" to determine which agent the participant received, Muth 2007 describes participants being randomised in groups of six and Offenloch 1986 mentions a randomisation list but the details thereof were not specific enough to pass a definite judgement. In view of this, we considered only Doweck 1994, Muth 2007 and Shupak 1994 to be low risk. The remaining six studies were unclear in terms of their risk of selection bias.
Allocation concealment
Muth 2007 was the only study that provided adequate detail about the allocation concealment process and is therefore the only study that we assessed as low risk for bias in this category. In this study, an external pharmacy associated with the project handled the treatment randomisation process and allocation concealment. All the other included studies provided insufficient information in their published manuscripts to allow for a clear assessment and have therefore all been assessed as having unclear risks of bias in terms of allocation concealment.
Blinding
Performance bias
All but one study (Hargreaves 1982) in the naturally induced group were double‐blinded (Doweck 1994; Offenloch 1986; Price 1981; Shupak 1994), and we considered them to have a low risk of performance bias. Hargreaves 1982 made no mention of blinding, but states that the test drugs were identically presented, suggesting possible blinding of the participants and personnel, however the author then states that those given cinnarizine received two 15 mg tablets and those given hyoscine received only one tablet. As a result of these contradictory statements, there is insufficient information to make a definite assessment and therefore we assessed the risk of performance bias as being unclear in this study. Of the experimentally induced studies, two specified double‐blinding (Muth 2007; Kohl 1987) and we assessed them as low risk. One study states that it was "blinded" but does not go into further detail about this so it remains unclear if this was a single‐blinded or double‐blinded study and thus it has an unclear risk of performance bias (Salenko 2006). One study could not perform any degree of blinding due to the invasive nature of the comparison intervention (acupuncture) ‐ this study therefore has a high risk of performance bias (Gao 2015).
Detection bias
We assessed five studies as having a low risk of detection bias (Doweck 1994; Muth 2007; Offenloch 1986; Price 1981; Shupak 1994). We assessed two studies as having an unclear risk of bias as they did not provide adequate information about the blinding process (Hargreaves 1982; Salenko 2006). Kohl 1987 stated that the outcome assessors were blinded to the study test state, however the plasma drug concentrations of the test drug were determined each day for all test participants and it is unclear if the results of these test were available immediately or only after the full test protocol to maintain blinding. We have therefore made an assessment of unclear risk of detection bias. One study could not perform any degree of blinding due to the invasive nature of the comparison intervention (acupuncture) and was therefore at high risk of detection bias (Gao 2015).
Incomplete outcome data
In six studies, all participants completed the study and the outcome data were complete. We therefore assessed these six studies as having a low risk of attrition bias (Doweck 1994; Hargreaves 1982; Kohl 1987; Muth 2007; Offenloch 1986; Price 1981). Of the remaining three studies, two had an unclear risk of bias (Salenko 2006; Shupak 1994), and one had a high risk of bias (Gao 2015). Shupak 1994 reports that 95 of the 118 participants completed the study, however no reason for this was given. In Salenko 2006, no detail was given on the attrition of participants and the risk of bias remains unclear.
While Gao 2015 appears to have complete outcome data, the authors have specified that participants were eliminated from the study based on the following criteria: poor compliance and inability to complete the treatment according to the test plan, serious adverse effects, serious deterioration of the participants' condition during the study, and participants who dropped out of the study due to "subjective and objective reasons". The number of participants who were eliminated was not stated and it is unclear if these participants were replaced in order to complete the study with the same number of participants that were originally enrolled.
Selective reporting
Selective outcome reporting bias is high risk in seven studies (Doweck 1994; Gao 2015; Hargreaves 1982; Offenloch 1986; Price 1981; Shupak 1994; Salenko 2006), and unclear in two studies (Kohl 1987; Muth 2007). In both Doweck 1994 and Shupak 1994, there was a discrepancy between the planned outcomes noted in the methodology, compared with the outcomes reported in the results. Outcome data were originally collected using a Seasickness Severity Scale (Wiker 1979), which graded motion sickness severity on a scale of 0 to 7; however, at the time of outcome reporting, this scale was "transformed" to a three‐point scale based on participants' responses. Gao 2015 states that participants were eliminated from the study based on the following criteria: poor compliance and inability to complete the treatment according to the test plan, serious adverse effects, serious deterioration of the participants' condition during the study, and participants who dropped out of the study due to "subjective and objective reasons". The number of participants who were eliminated was not stated. Hargreaves 1982 does not specify a time point at which the outcomes were measured, the duration of the sea voyage, the presence of co‐morbidities or the age or gender of the participants. Offenloch 1986 does not specify the dose of dimenhydrinate and complete side effect data were not reported (i.e. number of participants experiencing sedation in each group). Price 1981 does not specify the dose of dimenhydrinate used. In addition, the side effects experienced with the use of placebo or scopolamine were not clearly reported. In Salenko 2006, the timing of administration of dimenhydrinate in relation to the induction of motion was not specified. In the studies by Kohl 1987 and Muth 2007 a protocol was not available to make an assessment.
Other potential sources of bias
Shupak 1994 had no other potential sources of bias and we therefore deemed it low risk. Two studies, Muth 2007 and Hargreaves 1982, had an unclear risk of bias. Muth 2007 acknowledges GlaxoSmithKline for funding the study, however the authors have noted that the company was not involved in the design of the study, the collection of the data, the analysis of results, the preparation of the manuscript or the decision to publish the article. Hargreaves 1982 did not comment on the presence or absence of co‐morbidities amongst participants.
The remaining six studies had a high risk of bias from other potential sources (Doweck 1994; Gao 2015; Kohl 1987; Offenloch 1986; Price 1981; Salenko 2006).
The drugs used in the study by Kohl 1987 were provided by Janssen Pharmaceuticals. In addition, this pharmaceutical company also made suggestions regarding the methodology and experimental design of the study.
Doweck 1994 notes that there were more highly susceptible participants in the group that received cinnarizine 25 mg compared to the other two groups, as well as worse sea conditions.
Hargreaves 1982, Offenloch 1986 and Salenko 2006 did not specify the presence or absence of co‐morbidities in the participants.
In Gao 2015, the study inclusion criteria were adopted from a textbook on motion sickness in paediatrics, however the participant population was aged 18 to 26. In addition, the study had inclusion criteria that included a "positive attitude" and being "co‐operative". The outcome measure of motion sickness was vertigo, which is not a typical symptom of motion sickness. The treatment was still considered "effective" if vertigo was relieved even if one of the other "typical" symptoms of motion sickness ("dizziness, nausea, excessive salivation, cold sweat, pale complexion, etc") appeared; and remission was defined as the relief of vertigo but the presence of two "typical" symptoms of motion sickness. The results reported in the tables contradict the text ‐ in the tabulated results box, the control group (dimenhydrinate) was more effective overall, however in the discussion this was reported the other way around (this was double‐checked with the translator).
For the non‐English papers, Gao 2015, Offenloch 1986 and Salenko 2006, all assessments of the studies were based on information extracted by translators who assisted with translation of these papers from Mandarin, German and Russian to English, respectively.
Effects of interventions
See: Summary of findings 1 Antihistamines versus placebo for motion sickness; Summary of findings 2 Antihistamines versus scopolamine for motion sickness; Summary of findings 3 Antihistamines versus antiemetics for motion sickness; Summary of findings 4 Antihistamines versus acupuncture for motion sickness
Comparison 1: Antihistamines versus placebo or no treatment
See summary of findings Table 1.
Primary outcomes
Proportion of susceptible participants who did not experience any motion sickness symptoms (based on subjective reporting of nausea and/or vomiting or the use of a validated scale)
The primary outcome for this review was the effectiveness of antihistamines in the prevention of motion sickness when compared to placebo. We divided the studies into two comparisons – those performed under natural conditions and those performed under experimental conditions. The data from these two comparisons are not pooled, as planned in the protocol, due to differences in statistical methodology and are therefore discussed separately. There were insufficient data available to perform a planned subgroup analysis of adults versus children.
Natural conditions
Combining the results of three studies (Doweck 1994; Price 1981; Shupak 1994), we found that antihistamines probably reduce the risk of developing motion sickness symptoms under natural conditions when compared to placebo (risk ratio (RR) 1.81, 95% confidence interval (CI) 1.23 to 2.66; 3 studies; 240 participants; moderate‐certainty evidence). This data set has an I2 of 0%, demonstrating no heterogeneity (Analysis 1.1).
Experimental conditions
We combined the results of two studies (Kohl 1987; Muth 2007). These studies did not report the presence or absence of motion sickness symptoms. Instead they reported the severity of motion sickness symptoms using different validated scales. These results have therefore been pooled using the standardised mean difference (SMD), as both studies reported this outcome measure. The evidence is very uncertain about the effect of antihistamines on preventing motion sickness under experimental conditions when compared to placebo. The SMD in motion sickness symptoms in the intervention groups was 0.32 standard deviations higher (better) in the intervention group but the confidence intervals were very wide, leading to uncertainty in the result (95% CI ‐0.18 to 0.83; 2 studies; 62 participants; very low‐certainty evidence). The pooled data had an I2 of 0%, demonstrating no heterogeneity (Analysis 1.2).
Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms (based on subjective reporting of nausea and/or vomiting or the use of a validated scale)
No studies reported outcomes on the resolution of existing motion sickness symptoms.
Secondary outcomes
Physiological measures: heart rate, core temperature and gastric tachyarrhythmia (electrogastrography)
Heart rate
No studies reported heart rate for this comparison.
Core temperature
This was not measured in any of the included studies.
Gastric tachyarrhythmia (electrogastrography)
One study evaluated gastric tachyarrhythmia under experimental conditions (Muth 2007). Antihistamines may result in little or no difference in gastric tachyarrhythmia when compared to placebo (MD ‐2.20, 95% CI ‐11.71 to 7.31; 1 study; 42 participants; low‐certainty evidence) (Analysis 1.3).
Adverse effects: sedation, impaired cognitive function, blurred vision
Sedation
We pooled the data from two studies (Doweck 1994; Shupak 1994). Antihistamines may be more likely to cause sedation than placebo (RR 1.51, 95% CI 1.12 to 2.02; 2 studies; 190 participants; low‐certainty evidence). The pooled data had an I2 of 12%, demonstrating little heterogeneity (Analysis 1.4).
Impaired cognition
We pooled the data from two studies (Doweck 1994; Shupak 1994). Antihistamines may result in little or no difference in terms of impaired cognition when compared to placebo (RR 0.89, 95% CI 0.58 to 1.38; 2 studies; 190 participants; low‐certainty evidence). The pooled data had an I2 of 0%, demonstrating no heterogeneity (Analysis 1.5).
Blurred vision
We pooled the data from two studies (Doweck 1994; Shupak 1994). Antihistamines may result in little or no difference in blurred vision when compared to placebo (RR 1.14, 95% CI 0.53 to 2.48; 2 studies; 190 participants; low‐certainty evidence). The pooled data had an I2 of 0%, demonstrating no heterogeneity (Analysis 1.6).
Comparison 2: Antihistamines versus scopolamine
See summary of findings Table 2.
Primary outcomes
Proportion of susceptible participants who did not experience any motion sickness symptoms (based on subjective reporting of nausea and/or vomiting or the use of a validated scale)
Natural conditions
We pooled the results of two studies (Offenloch 1986; Price 1981). The evidence is very uncertain about the effect of antihistamines on preventing motion sickness under natural conditions when compared to scopolamine (RR 0.89, 95% CI 0.68 to 1.16; 2 studies; 71 participants; very low‐certainty evidence). This data set has an I2 of 0%, demonstrating no heterogeneity (Analysis 2.1).
There were no studies that were performed under experimental conditions for this comparison.
Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms (based on subjective reporting of nausea and/or vomiting or the use of a validated scale)
No studies reported the outcome resolution of existing motion sickness symptoms.
Secondary outcomes
Physiological measures: heart rate, core temperature and gastric tachyarrhythmia (electrogastrography)
Heart rate
Offenloch 1986 evaluated this outcome, but inadequate statistical data were provided for a data analysis (no mean, standard deviation or standard error available in the published manuscript). Results were only presented as a narrative summary in the translation of this study: "No difference in pulse frequency". The evidence is very uncertain about the effect of antihistamines on heart rate under natural conditions when compared to scopolamine (1 study; 20 participants; very low‐certainty evidence).
Core temperature
This was not measured in any of the included studies.
Gastric tachyarrhythmia (electrogastrography)
No studies reported this outcome in this comparison.
Adverse effects: sedation, impaired cognitive function, blurred vision
Sedation
We pooled the data from two studies (Hargreaves 1982; Price 1981). The evidence is very uncertain about the effect of antihistamines on sedation when compared to scopolamine (RR 0.82, 95% CI 0.07 to 9.25; 2 studies; 90 participants; very low‐certainty evidence). The pooled data had an I2 of 65%, demonstrating significant heterogeneity (Analysis 2.2).
Impaired cognition
No studies evaluated this outcome in this comparison.
Blurred vision
Price 1981 evaluated this adverse effect but provided no statistical data. Results were only presented as a narrative summary: "Use of transdermal scopolamine resulted in some side effects before motion, including dry mouth, drowsiness, and blurred vision, but only the incidence of dry mouth was statistically significant (P = 0.001)". The evidence is very uncertain about the effect of antihistamines on blurred vision when compared to scopolamine (1 study; 51 participants; very low‐certainty evidence).
There were insufficient data available to perform a planned subgroup analysis of adults versus children.
Comparison 3: Antihistamines versus antiemetics
See summary of findings Table 3.
Primary outcomes
Proportion of susceptible participants who did not experience any motion sickness symptoms (based on subjective reporting of nausea and/or vomiting or the use of a validated scale)
Natural conditions
No studies compared antihistamines versus antiemetics under natural conditions.
Experimental conditions
One study assessed the severity of motion sickness symptoms using a scale (Muth 2007). Antihistamines may result in little or no difference in the prevention of motion sickness under experimental conditions when compared to an antiemetic (MD ‐0.20, 95% CI ‐10.91 to 10.51; 1 study; 42 participants; low‐certainty evidence) (Analysis 3.1).
Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms (based on subjective reporting of nausea and/or vomiting or the use of a validated scale)
No studies in this comparison reported the outcome resolution of existing motion sickness symptoms.
Secondary outcomes
Physiological measures: heart rate, core temperature and gastric tachyarrhythmia (electrogastrography)
Heart rate
No studies reported this outcome in this comparison.
Core temperature
This was not measured in any of the included studies.
Gastric tachyarrhythmia (electrogastrography)
One study evaluated gastric tachyarrhythmia under experimental conditions (Muth 2007). Antihistamines may result in little or no difference in gastric tachyarrhythmia when compared to an antiemetic (MD 4.56, 95% CI ‐3.49 to 12.61; 1 study; 42 participants; low‐certainty evidence) (Analysis 3.2).
Adverse effects: sedation, impaired cognitive function, blurred vision
Sedation
No studies evaluated this outcome in this comparison.
Impaired cognition
No studies evaluated this outcome in this comparison.
Blurred vision
No studies reported this outcome in this comparison.
There were insufficient data available to perform a planned subgroup analysis of adults versus children.
Comparison 4: Antihistamines versus acupuncture
See summary of findings Table 4.
Primary outcomes
Proportion of susceptible participants who did not experience any motion sickness symptoms (based on subjective reporting of nausea and/or vomiting or the use of a validated scale)
Experimental conditions
One study compared antihistamines versus acupuncture and assessed this outcome (Gao 2015). The evidence is very uncertain about the effects of antihistamines on the prevention of motion sickness under experimental conditions when compared to acupuncture (RR 1.32, 95% CI 1.12 to 1.57; 1 study; 100 participants; very low‐certainty evidence) (Analysis 4.1).
The included study in this comparison did not assess outcomes under natural conditions.
Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms (based on subjective reporting of nausea and/or vomiting or the use of a validated scale)
The included study did not report the resolution of existing motion sickness symptoms.
Secondary outcomes
Physiological measures: heart rate, core temperature and gastric tachyarrhythmia (electrogastrography)
Heart rate
The included study in this comparison did not report this outcome.
Core temperature
This was not measured in any of the included studies.
Gastric tachyarrhythmia (electrogastrography)
The included study in this comparison did not report this outcome.
Adverse effects: sedation, impaired cognitive function, blurred vision
Sedation
The included study in this comparison did not report this outcome.
Impaired cognition
The included study did not report this outcome.
Blurred vision
The included study did not report this outcome.
There were insufficient data available to perform a planned subgroup analysis of adults versus children.
Antihistamines versus homeopathic remedies
Salenko 2006 compared antihistamines to Avia‐Sea, a homeopathic remedy, but did not measure any of the outcomes of interest in this review.
Discussion
Summary of main results
We included nine studies reporting on five different comparisons (Doweck 1994; Gao 2015; Hargreaves 1982; Kohl 1987; Muth 2007; Offenloch 1986; Price 1981; Salenko 2006; Shupak 1994).
-
Six studies compared antihistamines to placebo (Comparison 1) (Doweck 1994; Kohl 1987; Muth 2007; Price 1981; Salenko 2006; Shupak 1994).
-
Three studies compared antihistamines to scopolamine (Comparison 2) (Hargreaves 1982; Offenloch 1986; Price 1981).
-
One study compared antihistamines to antiemetics (Comparison 3) (Muth 2007).
-
One study compared antihistamines to acupuncture (Comparison 4) (Gao 2015)
-
One study compared antihistamines to homeopathic remedies (Comparison 5) (Salenko 2006).
Due to the choice of outcome measures used in Salenko 2006, we were not able to evaluate Comparison 5 and we also excluded this study from Comparison 1. In addition, due to the chosen method of outcome reporting in Hargreaves 1982, we were unable to include data from this study related to our primary outcome, but we did include the data related to adverse effects for Comparison 2. The following is a summary of the key findings for the comparisons:
Comparison 1: Antihistamines versus placebo or no treatment
See summary of findings Table 1.
For this comparison we included the results of five studies that evaluated the effectiveness of antihistamines in the prevention of motion sickness when compared to placebo (Doweck 1994; Kohl 1987; Muth 2007; Price 1981; Shupak 1994). Three studies with a total of 240 participants were performed under natural conditions (Doweck 1994; Price 1981; Shupak 1994). Shupak 1994 and Doweck 1994 both compared placebo to oral cinnarizine at doses of 25 mg and 50 mg in two arms, which we combined for the data analysis in this review. Outcomes (which included nausea and vomiting) were reported immediately after motion using the Wiker 1979 scale. Price 1981 compared oral dimenhydrinate to placebo at an unspecified dose, with outcomes (which included nausea and vomiting) reported one‐ to two‐hourly throughout the seven‐ to eight‐hour duration of motion, measured using a self‐defined scale.
When we pooled the results of these three studies we found that antihistamines probably reduce the risk of developing motion sickness symptoms under natural conditions when compared to placebo (risk ratio (RR) 1.81, 95% confidence interval (CI) 1.23 to 2.66; 3 studies; 240 participants; moderate‐certainty evidence). The evidence is of moderate certainty due to the risk of bias as described earlier (see Assessment of risk of bias in included studies).
Two studies were performed under experimental conditions with a total of 62 participants (Kohl 1987; Muth 2007). Muth 2007 compared oral dimenhydrinate at a dose of 100 mg to placebo. Outcomes (which included nausea) were reported using the MSAQ and nausea profile score (Gianaros 2001; Muth 1995b). Kohl 1987 compared oral astemizole at a dose of 30 mg daily for seven days to placebo. Outcomes (which included nausea and vomiting) were measured on day seven, two hours after the last dose was given, and were reported using the Graybiel 1968 scale. When we pooled the results of these two studies we found that the evidence is very uncertain about the effect of antihistamines on preventing motion sickness under experimental conditions when compared to placebo. The standardised mean difference in motion sickness symptoms under experimental conditions in the intervention groups was 0.32 standard deviations higher (better) (95% CI ‐0.18 to 0.83; 2 studies; 62 participants; very low‐certainty evidence). The evidence is of very low certainty due to the risk of bias as described earlier (see Risk of bias in included studies) and imprecision as a result of the small sample size and the overall confidence interval crossing the line of no effect.
In terms of physiological measurements, Muth 2007 evaluated gastric tachyarrhythmia under experimental conditions. Antihistamines may result in little or no difference in gastric tachyarrhythmia when compared to placebo (MD ‐2.20, 95% CI ‐11.71 to 7.31; 1 study; 42 participants; low‐certainty evidence). This evidence is of low certainty due to imprecision as the sample size is small and the confidence interval crosses the line of no effect.
Two studies evaluated adverse effects in this comparison, with a total of 190 participants (Doweck 1994; Shupak 1994). When we pooled the data, we found that antihistamines may be more likely to cause sedation than placebo (RR 1.51, 95% CI 1.12 to 2.02; 2 studies; 190 participants; low‐certainty evidence). Antihistamines may result in little or no difference in blurred vision when compared to placebo (RR 1.14, 95% CI 0.53 to 2.48; 2 studies; 190 participants; low‐certainty evidence). Antihistamines may result in little or no difference in terms of impaired cognition when compared to placebo (RR 0.89, 95% CI 0.58 to 1.38; 2 studies; 190 participants; low‐certainty evidence). With regard to sedation, this evidence is of low certainty due to risk of bias as a result of incomplete data and a small sample size. For the latter two adverse effects (blurred vision and impaired cognition) the evidence is of low certainty due to risk of bias (incomplete data) and imprecision as the confidence interval crosses the line of no effect.
No studies reported the resolution of existing motion sickness symptoms or any other physiological measures in this comparison.
Comparison 2: Antihistamines versus scopolamine
See summary of findings Table 2.
We included the results of two studies that evaluated the effectiveness of antihistamines in the prevention of motion sickness when compared to scopolamine, under natural conditions, with a total of 71 participants (Offenloch 1986; Price 1981). Both studies compared transdermal scopolamine 0.5 mg to oral dimenhydrinate at an unspecified dose given 1.5 hours before motion. Price 1981 measured outcomes (which included nausea and vomiting) using a self‐defined scale, every one to two hours over the seven‐ to eight‐hour duration of motion. Offenloch 1986 measured outcomes (which included nausea) using a self‐defined questionnaire every 10 minutes for the duration of motion, immediately after motion had ceased, and made a final measurement 20 minutes after motion had ceased. The evidence is very uncertain about the effect of antihistamines on preventing motion sickness under natural conditions when compared to scopolamine (RR 0.89, 95% CI 0.68 to 1.16; 2 studies; 71 participants; very low‐certainty evidence). The evidence is of very low certainty due to risk of bias and imprecision as the sample size is small and the confidence interval crosses the line of no effect.
Two studies reported adverse effect data for sedation specifically, with a total of 90 participants (Hargreaves 1982; Price 1981). The evidence is very uncertain about the effect of antihistamines on sedation when compared to scopolamine (RR 0.82, 95% CI 0.07 to 9.25; 2 studies; 90 participants; very low‐certainty evidence). This evidence is of very low certainty due to a high overall risk of bias, inconsistency (high heterogeneity, I2 = 65%) and imprecision as the sample size is small and the confidence interval crosses the line of no effect.
The were no studies that were performed under experimental conditions in this comparison and the other outcomes were either not measured or poorly reported.
Comparison 3: Antihistamines versus antiemetics
See summary of findings Table 3.
We included one study that evaluated the effectiveness of an antihistamine compared to an antiemetic in the prevention of motion sickness (Muth 2007).
One study, with 42 participants, was performed under experimental conditions and compared oral dimenhydrinate at a dose of 100 mg to oral ondansetron at a dose of 24 mg (Muth 2007). Outcomes (which included nausea) were reported using the MSAQ and nausea profile score (Gianaros 2001; Muth 1995b). Antihistamines may result in little or no difference in the prevention of motion sickness under experimental conditions when compared to an antiemetic (MD ‐0.20, 95% CI ‐10.91 to 10.51; 1 study; 42 participants; low‐certainty evidence). The evidence is of low certainty due to imprecision as the sample size is small and the confidence interval crosses the line of no effect.
The study reported physiological data for this outcome, evaluating gastric tachyarrhythmia specifically. Antihistamines may result in little or no difference in gastric tachyarrhythmia when compared to an antiemetic (MD 4.56, 95% CI ‐3.49 to 12.61; 1 study; 42 participants; low‐certainty evidence). This evidence is of low certainty due to imprecision as the sample size is small and the confidence interval crosses the line of no effect.
In this comparison, no studies reported the resolution of existing motion sickness symptoms, or the remaining adverse effects or physiological effects.
Comparison 4: Antihistamines versus acupuncture
See summary of findings Table 4.
We included one study that evaluated the effectiveness of antihistamines compared to acupuncture in the prevention of motion sickness (Gao 2015). This study was performed under experimental conditions, with a total number of 100 participants, comparing oral dimenhydrinate 50 mg to acupuncture. Outcomes (which included nausea and vomiting) were reported after motion ceased using the Graybiel 1968 scale. The evidence is very uncertain about the effects of antihistamines on the prevention of motion sickness under experimental conditions when compared to acupuncture (RR 1.32, 95% CI 1.12 to 1.57; 1 study; 100 participants; very low‐certainty evidence). The evidence is of very low certainty as a result of a high overall risk of bias (see Risk of bias in included studies) and the small sample size.
The included study in this comparison did not report the prevention of motion sickness under natural conditions, the resolution of existing motion sickness symptoms, adverse effects or physiological effects.
Overall completeness and applicability of evidence
Completeness
The number of high‐quality studies that met our criteria was limited and the overall completeness was variable for each outcome. All the studies reported outcomes on the prevention of motion sickness symptoms, however only half of the studies made use of validated scales. Among the studies that did not use validated scales, some measured nausea and vomiting, some measured only nausea or only vomiting and some measured neither. No studies reported on the treatment of established motion sickness symptoms, highlighting a significant gap in the overall body of evidence. For the secondary outcomes, only two studies reported physiological outcomes, with one reporting electrogastrography findings and the other reporting heart rate, however the reported data for the latter were incomplete. None of the included studies reported core temperature. Five studies evaluated adverse effects: two studies reported data on impaired cognition; three studies evaluated blurred vision (but only two reported their data); and five studies evaluated sedation (but only four reported their data). None of the studies assessed any long‐term adverse effects of antihistamines.
There was variability in the type and dose of the antihistamines used. None of the studies evaluated second‐generation antihistamines under natural conditions. Astemizole is a second‐generation antihistamine that was evaluated under experimental conditions, however this drug is no longer available commercially. All the included studies tested antihistamines that were given orally. No studies looked at antihistamines delivered via alternate routes of administration (e.g. intramuscular, rectal, intravenous).
Only one study potentially evaluated motion sickness in children, however the age group was defined as "16 to 55", with no clear indication of how many participants fell between the age of 16 and 18 (with under 18 being the definition of children in this review). All the other studies included adult participants only.
Participants' susceptibility to motion sickness was predominantly self‐reported, however between‐study and within‐study motion sickness susceptibility of study participants was inconsistent.
Overall, we graded only one of the reported outcomes as having moderate‐certainty evidence, which means that more evidence is needed to strengthen the certainty of this outcome. The remaining outcomes had either low‐ or very low‐certainty evidence, which means that more evidence may change the overall result for these outcomes.
Applicability
Overall, the participants in these studies are representative of the expected at‐risk adult population: an age range of 16 to 55, males and females (although 2:1 in favour of males), with variable susceptibility to motion sickness. These studies do not adequately represent the paediatric population.
The drugs were administered in variable doses but all in keeping with manufacturer recommendations. The applicability is limited in terms of the mode of delivery of the drug (all were orally administered) and the class of antihistamines used. Only first‐generation antihistamines (dimenhydrinate and cinnarizine) were used in the studies performed under natural motion conditions. Astemizole is the only second‐generation antihistamine that was used and this drug has since been withdrawn form the market in most countries due to rare but potentially fatal side effects.
The majority of studies were performed under naturally occurring conditions of motion, making these applicable to the likely exposures experience in real‐life situations. The experimental studies are less applicable from this point of view, which is why we had chosen to evaluate them separately at the outset.
The results are applicable to the use of antihistamines to prevent motion sickness, which makes the outcomes applicable to those who have a known susceptibility to motion sickness. However, for those who are being exposed to various motion sickness stimuli for the first time, there were no studies evaluating the treatment of established motion sickness symptoms. In addition to this, there were no studies evaluating the second‐generation antihistamines that are currently available and we are therefore unable to draw conclusions on this class of antihistamines.
Quality of the evidence
Using the GRADE approach, we assessed the certainty of the available evidence (see summary of findings Table 1; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4). Overall, the evidence for the majority of outcomes was of low certainty. One outcome was of moderate certainty and four were of very low certainty. The primary reasons for downgrading the certainty of the available evidence were the risk of bias and imprecision due to small sample sizes. Details related to the specific outcomes are described below.
Antihistamines versus placebo
Primary outcomes
For the primary outcomes, the evidence was of moderate certainty for one outcomes in studies performed under natural conditions, and of very low certainty for outcomes in studies performed under experimental conditions. We downgraded the evidence due to study limitations (risk of bias) as there were incomplete data, which was not explained, and an unclear risk of bias related to allocation concealment and random sequence generation. We also downgraded the evidence for imprecision due to the small sample size and because the overall confidence interval crossed the line of no effect.
Secondary outcomes
For the secondary outcomes, the evidence was of low certainty for all the outcomes. We downgraded the evidence due to study limitations (risk of bias) as data were incomplete in one of the studies, which was not explained, and most studies had an unclear risk of bias related to allocation concealment. We also downgraded the evidence due to imprecision, predominantly as a result of small sample sizes and because the overall confidence intervals crossed the line of no effect.
Antihistamines versus scopolamine
Primary outcomes
For the primary outcomes, the evidence was of very low certainty. We downgraded the evidence due to study limitations as there was an unclear risk of bias related to allocation concealment and random sequence generation. We also downgraded the evidence due to imprecision as a result of a small sample size and because the overall confidence interval crossed the line of no effect.
Secondary outcomes
For the secondary outcomes, the evidence was of very low certainty. We downgraded the evidence due to study limitations as all studies had an unclear risk of bias related to allocation concealment and random sequence generation. One study also had an unclear risk of bias related to blinding (no mention of blinding at all). We further downgraded the evidence due to imprecision, as a result of a small sample size and because the overall confidence interval crossed the line of no effect. Statistical heterogeneity was also high and we downgraded the evidence further due to inconsistency.
Antihistamines versus antiemetics
Primary outcomes
For the primary outcomes, the evidence was of low certainty. We downgraded the evidence due to study limitations as there was an unclear risk of bias related to allocation concealment and random sequence generation. We also downgraded the evidence for imprecision due to a small sample size and because the overall confidence interval crossed the line of no effect.
Secondary outcomes
For the secondary outcomes, the evidence was of low certainty. We downgraded the evidence due to study limitations as there were incomplete data, and an unclear risk of bias related to allocation concealment and random sequence generation. We further downgraded the evidence due to imprecision, as a result of the small sample size and because the overall confidence interval crossed the line of no effect.
Antihistamines versus acupuncture
Primary outcomes
For the primary outcomes, the evidence was of very low certainty. We downgraded the evidence due to study limitations as a result of incomplete data, an unclear risk related to allocation concealment and random sequence generation, and there was no blinding.
There were no secondary outcomes for this comparison.
Potential biases in the review process
In order to reduce the risk of introducing bias into the review process, the protocol for this review was published prior to conducting the search and data collection (Karrim 2017). All methodology was outlined in the protocol to avoid introducing any bias once studies were identified.
Search strategies were comprehensive and included all the main databases, as outlined in Search methods for identification of studies. The search terms were comprehensive and cast a wide net. While our review included studies in any language, our search was conducted using English, which may have excluded any studies that did not have an English translation of either the title, abstract or full‐text article. We addressed publication bias (between‐study reporting bias) by searching for published, unpublished and ongoing trials in the specified trial databases.
Two studies were not reported in English (Gao 2015; Salenko 2006), and the data extraction process was only performed by one translator (as opposed to two independent translators or review authors), therefore the interpretation and translation of these papers may have introduced bias.
The drug administration protocols varied widely across studies, in terms of drug dosing, timing of administration and once‐off dosing versus repeated dosing. The duration of motion stimulus also varied across studies.
Across all the sea studies, sea conditions were variable, providing an inconsistent motion stimulus (within‐study and between‐study inconsistencies). All the experimental studies used different motion stimulus protocols, again providing an inconsistent motion stimulus (between‐study). In addition, the duration of motion stimulus varied from one to eight hours (or was not specified).
For the reporting of motion sickness symptoms, validated scales were used by some studies, while other used self‐defined scales. The validated scales used differed across the studies as well. In terms of specific symptoms, some studies asked about nausea and vomiting, some only nausea and some only vomiting. To provide the best possible data for analysis, we examined each reporting tool used, and did not include any non‐validated tools that made no mention of either nausea or vomiting.
Agreements and disagreements with other studies or reviews
There have been a number of clinical summaries and literature reviews on motion sickness. Some of the most recent of these include Murdin 2011, Zajonc 2006, Zhang 2016b, Brainard 2014 and Leung 2019, which have been performed within the last 15 years. These summaries have all been descriptive narratives of evidence with no statistical analysis or a clear and thorough evaluation of the entire body of evidence. In addition, the use of antihistamines has not clearly been divided into prevention versus treatment. These summaries have also identified specific antihistamines and characterise their efficacy at an individual level and not as a class as a whole. As a result it was difficult to draw comparisons between our findings and those reported in these papers. Other than a single Cochrane systematic review on the use of scopolamine for motion sickness (Spinks 2011), there have been no other previous exhaustive systematic reviews and meta‐analyses performed for the prevention and treatment of motion sickness in the last 50 years. The results of the review by Spinks et al stated that no conclusions could be made on the comparative effectiveness of scopolamine and antihistamines. This is in keeping with the findings of this review, that the evidence is very uncertain about the effect of antihistamines on preventing motion sickness when compared to scopolamine. In addition, Spinks et al found no studies that evaluated the treatment of existing motion sickness symptoms, which correlated with the findings of this review. The majority of the previous literature does also agree that there is limited evidence for the use of antihistamines to treat or prevent motion sickness in children. Clinical guidelines, such as BMJ Best Practice and UpToDate have made recommendations on the treatment and prevention of motion sickness. BMJ Best Practice has recommended antihistamines as a first‐line adjunct to non‐pharmacological therapy. UpToDate notes that antihistamines are effective for the prevention of motion sickness but cause more sedation than placebo, which is in agreement with our findings.

Process for sifting search results and selecting studies for inclusion.

Risk of bias summary: Based on review authors' judgements (performed by NK and RB) about each risk of bias item for each included study.

Risk of bias graph: Based on review authors' judgements (performed by NK and RB) about each risk of bias item presented as percentages across all included studies.

Comparison 1: Antihistamines versus placebo, Outcome 1: Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions

Comparison 1: Antihistamines versus placebo, Outcome 2: Motion sickness symptom severity under experimental conditions

Comparison 1: Antihistamines versus placebo, Outcome 3: Physiological measures under experimental conditions: gastric tachyarrhythmia (electrogastrography)

Comparison 1: Antihistamines versus placebo, Outcome 4: Adverse effects under natural conditions: sedation

Comparison 1: Antihistamines versus placebo, Outcome 5: Adverse effects under natural conditions: impaired cognition

Comparison 1: Antihistamines versus placebo, Outcome 6: Adverse effects under natural conditions: blurred vision

Comparison 2: Antihistamines versus scopolamine, Outcome 1: Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions

Comparison 2: Antihistamines versus scopolamine, Outcome 2: Adverse effects under natural conditions: sedation

Comparison 3: Antihistamines versus antiemetics, Outcome 1: Motion sickness symptom severity under experimental conditions

Comparison 3: Antihistamines versus antiemetics, Outcome 2: Physiological measures under experimental conditions: gastric tachyarrhythmia (electrogastrography)

Comparison 4: Antihistamines versus acupuncture, Outcome 1: Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions
| Antihistamines versus placebo for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | Antihistamines | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Assessed by: self‐reported questionnaires6,7 Follow‐up: varied8,9 | Study population | RR 1.81 | 240 | ⊕⊕⊕⊝ | Antihistamines are probably effective at preventing motion sickness symptoms under natural conditions when compared to placebo. | |
| 247 per 1000 | 447 per 1000 | |||||
| Moderate | ||||||
| 313 per 1000 | 567 per 1000 | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Assessed by: rotating chair Follow‐up: varied (7 days; 1 hour 20 minutes) | — | The standardised mean difference in susceptible participants who did not experience any motion sickness symptoms under experimental conditions in the intervention groups was | — | 62 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on preventing motion sickness under experimental conditions when compared to placebo. |
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | No studies in this comparison reported on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate and core temperature | Heart rate and core temperature were not measured in the studies in this comparison. | |||||
| Physiological measures: gastric tachyarrhythmia10 Assessed by: electrogastrography Follow‐up: 1 hour 20 minutes | Mean score: 60.29 | The mean gastric tachyarrhythmia score under experimental conditions in the intervention group was | — | 42 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in gastric tachyarrhythmia when compared to placebo. |
| Adverse effects: sedation Assessed by: adverse effect questionnaire (presence or absence given numerical values) Follow‐up: before departure and after returning from a sea voyage lasting a total duration of 4 to 6 hours in one study, and 5 hours in one study | Study population | RR 1.51 | 190 | ⊕⊕⊝⊝ | Antihistamines may be more likely to cause sedation when compared to placebo. | |
| 438 per 1000 | 661 per 1000 | |||||
| Moderate | ||||||
| 438 per 1000 | 661 per 1000 | |||||
| Adverse effects: impaired cognition Assessed by: adverse effect questionnaire (presence or absence given numerical values) Follow‐up: before departure and after returning from a sea voyage lasting a total duration of 4 to 6 hours in one study, and 5 hours in one study | Study population | RR 0.89 | 190 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in terms of impaired cognition when compared to placebo. | |
| 328 per 1000 | 292 per 1000 | |||||
| Moderate | ||||||
| 328 per 1000 | 292 per 1000 | |||||
| Adverse effects: blurred vision Assessed by: adverse effect questionnaire (presence or absence given numerical values) Follow‐up: before departure and after returning from a sea voyage lasting a total duration of 4 to 6 hours in one study, and 5 hours in one study | Study population | RR 1.14 | 190 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in blurred vision when compared to placebo. | |
| 125 per 1000 | 142 per 1000 | |||||
| Moderate | ||||||
| 125 per 1000 | 142 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to study limitations (risk of bias): incomplete data in one study (95 of 118 participants completed all questionnaires but reasons for this have not been stated); all studies had an unclear risk related to allocation concealment. | ||||||
| Antihistamines versus scopolamine for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: scopolamine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Scopolamine | Antihistamines | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Assessed by: self‐reported questionnaires6,7 Follow‐up: varied8,9 | Study population | RR 0.89 | 71 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on preventing motion sickness under natural conditions when compared to scopolamine. | |
| 806 per 1000 | 717 per 1000 | |||||
| Moderate | ||||||
| 773 per 1000 | 688 per 1000 | |||||
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | No studies in this comparison reported on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate Assessed by: self‐measured (participant measured own pulse rate) Follow‐up: just before flight, every 10 minutes during flight; immediately after flight; and finally at 20 minutes after the flight (flight duration 1 hour) | Results were only presented as a narrative summary in the translation of this study: "No difference in pulse frequency". | 20 (1 study) | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on the heart rate under natural conditions when compared to scopolamine. | ||
| Physiological measures: core temperature and gastric tachyarrhythmia (electrogastrography) | Gastric tachyarrhythmia and core temperature were not measured in the studies in this comparison. | |||||
| Adverse effects: sedation Assessed by: self‐reported Follow‐up: every 1 to 2 hours for a total sea voyage lasting 7 to 8 hours in one study; unspecified in one study | Study population | RR 0.82 | 90 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on sedation when compared to scopolamine. | |
| 213 per 1000 | 174 per 1000 | |||||
| Moderate | ||||||
| 206 per 1000 | 169 per 1000 | |||||
| Adverse effects: impaired cognitive function | No studies evaluated impaired cognition in this comparison. | |||||
| Adverse effects: blurred vision Assessed by: self‐reported Follow‐up: every 1 to 2 hours for a total sea voyage lasting 7 to 8 hours | Results were only presented as a narrative summary: "Use of transdermal scopolamine resulted in some side effects before motion, including dry mouth, drowsiness, and blurred vision, but only the incidence of dry mouth was statistically significant (P = 0.001)". | 51 (1 study) | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of antihistamines on blurred vision when compared to scopolamine. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to study limitations (risk of bias): studies had an unclear risk related to allocation concealment and random sequence generation. | ||||||
| Antihistamines versus antiemetics for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: antiemetic | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Antiemetic | Antihistamine | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Assessed by: calculated based on head movements tolerated (rotating chair); MSAQ Follow‐up: 1 hour and 20 minutes | 22.3 | The mean proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions in the intervention groups was | — | 42 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in the prevention of motion sickness under experimental conditions when compared to an antiemetic |
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | No studies in this comparison reported on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate and core temperature | Heart rate and core temperature were not measured in the studies in this comparison. | |||||
| Physiological measures: gastric tachyarrhythmia2 Assessed by: electrogastrography Follow‐up: 1 hour and 20 minutes | Mean score: 53.53 | The mean gastric tachyarrhythmia score under experimental conditions in the intervention groups was | — | 42 | ⊕⊕⊝⊝ | Antihistamines may result in little or no difference in gastric tachyarrhythmia when compared to an antiemetic. |
| Adverse effects: impaired cognition and blurred vision | No studies in this comparison evaluated impaired cognition or blurred vision. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded two levels due to imprecision: overall confidence interval crosses the line of no effect; small sample size. | ||||||
| Antihistamines versus acupuncture for motion sickness | ||||||
| Patient or population: patients with motion sickness Comparison: acupuncture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Acupuncture | Antihistamines | |||||
| Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Assessed by: Graybiel motion sickness scale Follow‐up: before and after treatment (exact time not specified) | Study population | RR 1.32 | 100 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of antihistamines on the prevention of motion sickness under experimental conditions when compared to acupuncture. | |
| 740 per 1000 | 977 per 1000 | |||||
| Moderate | ||||||
| 740 per 1000 | 977 per 1000 | |||||
| Proportion of susceptible participants who experienced a reduction or resolution of existing motion sickness symptoms | The study in this comparison did not report on the resolution of existing motion sickness symptoms. | |||||
| Physiological measures: heart rate, core temperature and gastric tachyarrhythmia (electrogastrography) | Heart rate, core temperature and gastric tachyarrhythmia (electrogastrography) were not measured in the study in this comparison. | |||||
| Adverse effects: sedation, impaired cognitive function, blurred vision | The study in this comparison did not evaluate sedation, impaired cognitive function or blurred vision. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by two levels due to study limitations (risk of bias): incomplete data. (While the study appears to have complete outcome data, the authors have specified that participants were eliminated from the study based on the following criteria: poor compliance and inability to complete the treatment according to the test plan, serious adverse effects, serious deterioration of the participants' condition during the study, and participants who dropped out of the study due to "subjective and objective reasons". The number of participants who were eliminated has not been stated and it is unclear if these participants were replaced in order to complete the study with the same number of participants that were originally enrolled); unclear risk related to allocation concealment and random sequence generation; unblinded. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Show forest plot | 3 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.23, 2.66] |
| 1.2 Motion sickness symptom severity under experimental conditions Show forest plot | 2 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.18, 0.83] |
| 1.3 Physiological measures under experimental conditions: gastric tachyarrhythmia (electrogastrography) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐11.71, 7.31] |
| 1.4 Adverse effects under natural conditions: sedation Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.12, 2.02] |
| 1.5 Adverse effects under natural conditions: impaired cognition Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.38] |
| 1.6 Adverse effects under natural conditions: blurred vision Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.53, 2.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Proportion of susceptible participants who did not experience any motion sickness symptoms under natural conditions Show forest plot | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.16] |
| 2.2 Adverse effects under natural conditions: sedation Show forest plot | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.07, 9.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Motion sickness symptom severity under experimental conditions Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐10.91, 10.51] |
| 3.2 Physiological measures under experimental conditions: gastric tachyarrhythmia (electrogastrography) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 4.56 [‐3.49, 12.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Proportion of susceptible participants who did not experience any motion sickness symptoms under experimental conditions Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.12, 1.57] |

