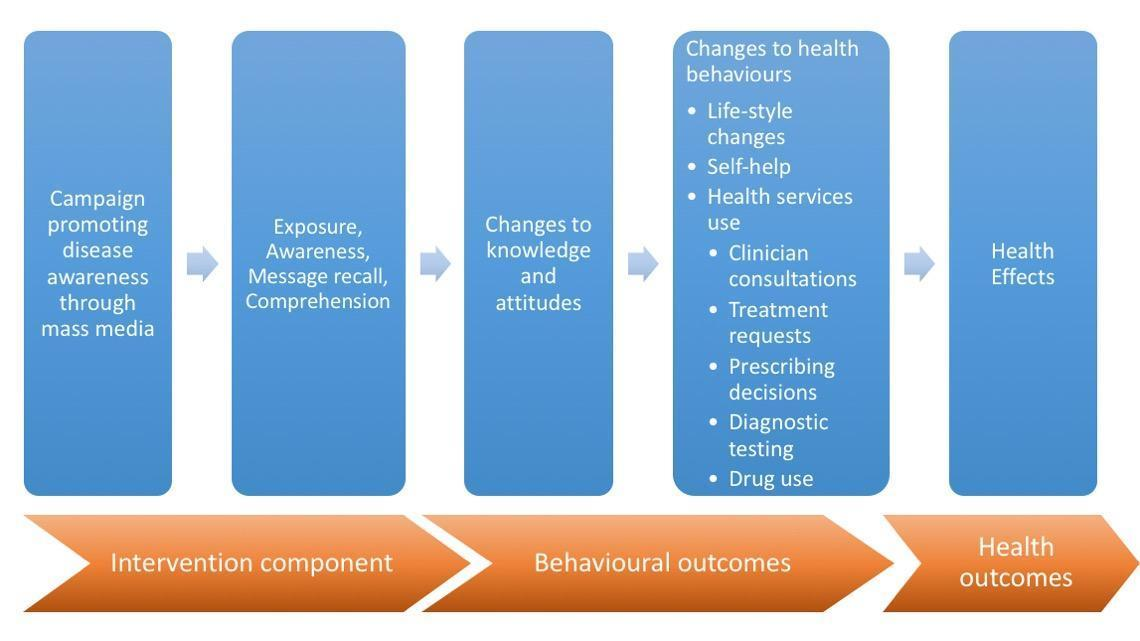
Unbranded advertising of prescription medicines to the public by pharmaceutical companies
Referencias
Additional references
| Key term | Definition |
| Branded direct‐to‐consumer advertising of prescription medicines (DTCA) | Advertising that includes a product's brand name. In the USA, this includes two types of advertising described by the Food and Drug Administration: 1) 'full product advertising', which includes the product name and health claims. Such advertising must also include information on the drug risks; 2) reminder advertising, which states the product's name but makes no health claims. In the USA, this advertising is not allowed for drugs with boxed warnings of serious risks. |
| Unbranded advertising of prescription medicines | Any paid advertising campaign, in any media, by a pharmaceutical manufacturer, with a focus on a condition treated by one or more of its products, but without any mention of brand or generic names. |
| Off‐label promotion | The term 'off‐label promotion' refers to promotion of a medicine for an unapproved use. This type of promotion is generally illegal. Physicians may prescribe a medicine for any use, whether it is approved or not, but manufacturers may not promote medicines for off‐label use. |
| Mixed promotion (both on‐ and off‐label) | 'On‐label promotion' refers to promotion for approved uses. 'Off‐label promotion' is for unapproved uses. Mixed promotion is for both types of uses. |
| Generic drugs or generics | Generic drugs or generics are medicines that have no brand name or registered trademark. Once the patent for a medicine expires, other manufacturers may produce the medicine, and these products are generic drugs. |
| Direct‐to‐consumer advertising | Direct‐to‐consumer advertising (DTCA) refers to advertising of prescription‐only medicines aimed at the public. Such advertising is fully legal only in two countries, the USA and New Zealand. |
| Non‐commercial information sources | We define a non‐commercial information source to be any public or private entity, institution, non‐government organisation, foundation or society involved in distributing information about health and treatment which does not derive a commercial gain from inducing the prescription, supply, purchase and/or use of pharmaceutical products, either directly or indirectly. |
| Third party acting on behalf of pharmaceutical company | Any public relations consultancy, marketing company, professional society, think tank, patient and consumer group, key opinion leader, medical practice or hospital, which has been hired or funded by a pharmaceutical company to promote specific pharmaceutical products. |