Intervenciones para la infertilidad inexplicada: una revisión sistemática y un metanálisis en red
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics OS
OS‐IUI
Sample size: OS (n = 69); OS‐IUI (n = 44) Included criteria: couples with unexplained infertility: biphasic basal body temperature charts; in‐phase late luteal endometrial biopsy; normal serum levels of thyroid, prolactin, luteinising hormone, and follicle‐stimulating hormone; hysterosalpingogram indicating normal uterine contour and laparoscopy indicating bilateral tubal patency; absence of pelvic adhesions; and endometriosis. All men had normal values on at least 2 standard semen analyses (sperm concentration > 20 million/mL, > 50% motile, and > 50% morphologically normal spermatozoa) and a positive post‐coital test. Tests for immunological causes of infertility for both partners revealed negative results (antisperm antibodies) Excluded criteria: NA Pretreatment: NA | |
| Interventions | Intervention characteristics OS
OS‐IUI
| |
| Outcomes | Clinical pregnancy | |
| Identification | Sponsorship source: Council of Scientific and Industrial Research, New Delhi, India Country: India Setting: Gynecological Outpatients of All India Institute of Medical Sciences (AIIMS), New Delhi Author's name: Sonika Agarwal Institution: Department of Obstetrics Gynaecology, All India Institute of Medical Sciences, New Delhi, India Email: [email protected] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 140 couples were divided into two groups using random number table, 70 in each group and followed over three years" Judgement comment: random numbers table used |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding not possible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blind not likely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Twenty six women in group B and one in group A did not have complete follow up and were thus excluded from the analysis" Judgement comment: 26/70 in OS‐IUI group and 1/70 in OS group lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Judgement comment: outcomes for the 2 groups (live birth, miscarriage, and multiple pregnancy) not reported separately |
| Other bias | Low risk | Judgement comment: no other sources of bias detected |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics OS
OS‐IUI
Sample size: OS (n = 32); OS‐IUI (n = 36) Included criteria: unexplained infertility diagnosed after normal results were obtained from the following tests: basal body temperature measurements and endometrial biopsy in the luteal phase, hysterosalpingography, post‐coital test, and at least 2 semen analyses for the partner. Laparoscopy showed a normal pelvis in all patients Excluded criteria: NA Pretreatment: NA | |
| Interventions | Intervention characteristics OS
OS‐IUI
| |
| Outcomes | Clinical pregnancy | |
| Identification | Sponsorship source: NA Country: Italy Setting: Infertility Unit of the Modern Medical Center, Milan, Italy Authors name: Luisa Arcaini, Luigi Fedele* Institution: Department of Obstetrics and Gynecology, L. Mangiagalli, University of Milan, Milan, Italy Email: NA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: details of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding not possible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding not likely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | High risk | Judgement comment: 14/68 (20.5%) lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Judgement comment: outcomes for the 2 groups (live birth/ongoing pregnancy, miscarriage, and multiple pregnancy) not reported separately |
| Other bias | Low risk | Judgement comment: no sources of bias detected |
| Methods | Study design: randomised controlled trial Study grouping: cross‐over Only data for the first cycle (before cross‐over) were extracted | |
| Participants | Baseline characteristics Overall
Sample size: IUI (n = 16); OS‐IUI (n = 10) Included criteria: for unexplained infertility, all couples exhibited normal semen analysis, negative antisperm antibodies, normal hysterosalpingogram, regular ovulatory cycles (by luteal phase P levels and/or in‐phase endometrial biopsy), and normal laparoscopic findings. Five patients with surgically treated minimal endometriosis without pelvic adhesive disease were included in this diagnostic group Excluded criteria: all patients positive for sperm antibodies by immunobead testing were excluded. Couples unwilling to be randomised were also excluded from the study Pretreatment: NA | |
| Interventions | Intervention characteristics IUI
OS‐IUI
| |
| Outcomes | Clinical pregnancy | |
| Identification | Sponsorship source: NA Country: USA Setting: tertiary academic medical centre Author's name: Aydin Arici Institution: Department of Obstetrics and Gynecology, Yale University School of Medicine Email: NA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "couples were randomized using a computer‐generated random numbers table to one of the two study groups" |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details not reported |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding not possible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding not likely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: original report included 2 subgroups of participants: male infertility and unexplained infertility. Data for participants lost to follow‐up not reported separately |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: live birth and multiple pregnancy not reported |
| Other bias | Unclear risk | Judgement comment: no sufficient information to judge baseline characteristics |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics OS‐IUI
IVF/ICSI
Sample size: OS‐IUI (n = 207); IVF/ICSI (n = 201) Included criteria: couples seeking fertility treatment after at least 12 months of unprotected intercourse were eligible. All couples underwent basic fertility investigations, which included semen analysis, evaluation of ovulation, and tubal patency testing (Chlamydia antibody test, hysterosalpingography, or laparoscopy). Inclusion criteria were age of female partner between 18 and 38 years, unfavourable prognosis for natural conception, and diagnosis of unexplained or mild male subfertility. We classified couples as having unexplained subfertility when fertility investigations showed at least 1 patent fallopian tube, an ovulatory menstrual cycle, and a normal semen analysis (pre‐wash total motile sperm count > 10 million). We considered couples who qualified for intrauterine insemination with donor sperm after at least 6 cycles of artificial intracervical insemination with donor sperm to have unexplained subfertility for the purpose of this study. Mild male subfertility was diagnosed when semen analysis showed a pre‐wash total motile sperm count between 3 and 10 million (according to Dutch guidelines). We defined an unfavourable prognosis for natural conception as a probability of natural conception within the next 12 months of < 30%, as calculated through the validated synthesis model of Hunault. This model encompasses female age, duration of subfertility, whether subfertility is primary or secondary, percentage of motile progressive sperm, and referral status. It is readily available for the use of all clinicians (www.freya.nl/web_bereken/bereken.php) Excluded criteria: anovulation, double‐sided tubal disease, severe endometriosis, premature ovarian failure, known endocrine disorders (such as Cushing’s syndrome or adrenal hyperplasia) Pretreatment: none | |
| Interventions | Intervention characteristics OS‐IUI
IVF/ICSI
| |
| Outcomes | Clinical pregnancy Live birth Multiple pregnancy OHSS | |
| Identification | Sponsorship source: the study was supported by a grant from ZonMW, the Dutch Organization for Health Research and Development (120620027), and a grant from Zorgverzekeraars Nederland, the Dutch Association of Healthcare Insurers (09‐003) Country: Netherlands Setting: 17 fertility clinics in Netherlands Authors' names: A.J. Bensdorp, M. van Wely* Institution: Centre for Reproductive Medicine, Academic Medical Centre, University of Amsterdam, 1100DD Amsterdam, Netherlands Email: [email protected] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed with an online randomisation program, using biased coin minimisation, stratified for study centre" |
| Allocation concealment (selection bias) | Low risk | Quote: "minimisation, stratified for study centre. A web based program generated a unique number with allocation code after entry of the patient’s initials and date of birth. Neither the recruiters nor the trial project group could access the randomisation sequence. " Judgement comment: a web‐based programme generated a unique number with allocation code after entry of the patient’s initials and date of birth. Neither the recruiters nor the trial project group could access the randomisation sequence |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "blinding was not possible owing to the nature of the interventions" |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding not likely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: IVF (SET): 2/201 lost to follow‐up; IVF (NC): 3/194 lost to follow‐up; OS‐IUI: 1/207 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all relevant outcomes reported |
| Other bias | Low risk | Judgement comment: no other sources of bias detected |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics EM
OS
IUI
Sample size: EM (n = 193); OS (n = 194); IUI (n = 193) Included criteria: at least 2 years of infertility, bilateral tubal patency (demonstrated by laparoscopy or hysterosalpingography), ovulation demonstrated by appropriately timed mid‐luteal progesterone, and normal semen variables (according to World Health Organization criteria). Also couples with minimum sperm motility of 20% or minimal endometriosis (rAFS stage 1) Excluded criteria: NA Pretreatment: none | |
| Interventions | Intervention characteristics EM
OS
IUI
| |
| Outcomes | Live birth Clinical pregnancy Multiple pregnancy OHSS | |
| Identification | Sponsorship source: Chief Scientist Office, Scotland Country: UK Setting: 4 teaching hospitals and a district general hospital in Scotland Author's name: S. Bhattacharya Institution: Department of Obstetrics and Gynaecology, University of Aberdeen Email: [email protected] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: central telephone randomisation system used |
| Allocation concealment (selection bias) | Low risk | Quote: "research nurses enrolled participants at each centre and assigned them to their groups using a central telephone randomisation system based in Aberdeen (the coordinating centre)" |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not possible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Non‐blinding not likely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: lost to follow‐up (EM: 0, OS: 2, IUI: 2) |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all outcomes prespecified and adequately reported |
| Other bias | Low risk | Judgement comment: no other bias detected |
| Methods | Study design: randomised controlled trial Study grouping: cross‐over (after the first cycle) | |
| Participants | Baseline characteristics Overall
Sample size: OS (n = 73); OS‐IUI (n = 64); IVF/ICSI (n = 30) Included criteria: (a) women were to be < 38 years of age and must have experienced > 36 months of infertility before study entry; (b) only women with at least 1 macroscopically normal tubo‐ovarian unit, as identified by a recent diagnostic laparoscopy, were included; (c) there must have been evidence of the occurrence of spontaneous ovulation in 2 recent cycles, as judged by plasma progesterone levels in the luteal phase; (d) it was necessary for semen to be classed as 'normal' by WHO criteria; (e) it was mandatory for patients to refrain from sexual activity for 6 days before and 3 days after treatments; (f) there must have been a period of at least 2 months without treatment for infertility before study entry Excluded criteria: NA Pretreatment: no | |
| Interventions | Intervention characteristics OS
OS‐IUI
IVF/ICSI
| |
| Outcomes | Clinical pregnancy | |
| Identification | Sponsorship source: Ares‐Serono (Geneva) Country: France, Greece, Italy, Germany, Belgium, Sweden, Norway, Finland, Austria, and Netherlands Setting: 19 fertility centres in Europe Authors' names: P.G. Crosignani, D.E. Walters* Institution: Department of Obstetrics and Gynaecology, University of Milan, Milan, Italy Email: NA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: details of sequence generation at each centre not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details of allocation concealment at each centre not reported |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding impossible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: details not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: live birth and multiple pregnancy not reported |
| Other bias | Unclear risk | Judgement comment: insufficient information to judge |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics OS‐IUI
IVF/ICSI
Sample size: OS‐IUI (n = 58); IVF/ICSI (n = 58) Included criteria: couples were invited to participate if they were diagnosed with unexplained or mild male subfertility. Couples had to have poor fertility prospects as calculated by the validated model of Hunault. Poor fertility prospects were defined as a chance of natural conception of 30% within 12 months. All couples had undergone a basic fertility workup according to the guidelines of the Dutch Society of Obstetrics and Gynecology. This workup included medical history, cycle monitoring, post‐coital test, semen analysis, and assessment of tubal patency. Mild male subfertility was defined as a total motile count (TMC) of 3 to 10 × 10⁶ spermatozoa/mL. Unexplained subfertility was defined as TMC > 10 × 10⁶ spermatozoa/mL and exclusion of a cervical factor Excluded criteria: other causes of subfertility, including severe male subfertility, cervical factor, and polycystic ovary syndrome; female age > 38 years; prior treatment within this subfertility episode. Age limit was based on concerns that IUI‐COS may compromise pregnancy rates in older women Pretreatment: none detected | |
| Interventions | Intervention characteristics OS‐IUI
IVF/ICSI
| |
| Outcomes | Clinical pregnancy Live birth Multiple pregnancy | |
| Identification | Sponsorship source: Organon, Oss, Netherlands. Country: Netherlands Setting: 3 academic and 6 teaching hospitals in Netherlands Author's name: Inge M. Custers Institution: Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Academic Medical Center, Room H4‐213, Meibergdreef 9, 1105 AZ Amsterdam, Netherlands Email: [email protected] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "couples who gave informed consent were randomized by a central Internet‐based randomization stratified for center" |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details not reported |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding impossible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: zero lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all relevant outcomes reported |
| Other bias | Low risk | Judgement comment: no other sources of bias detected |
| Methods | Study design: randomised controlled trial Study grouping: cross‐over | |
| Participants | Baseline characteristics Overall
Sample size: EM (n = 28); OS‐IUI (n = 23) Included criteria: couples with unexplained infertility or surgically corrected endometriosis Excluded criteria: women with tubal disease Pretreatment: NA | |
| Interventions | Intervention characteristics EM
OS‐IUI
| |
| Outcomes | Clinical pregnancy Multiple pregnancy OHSS Ongoing pregnancy | |
| Identification | Sponsorship source: American College of Obstetricians and Gynecologists, Washington, DC; Mead Johnson Laboratories, Evansville, IN Country: USA Setting: University of Vermont College of Medicine Authors' names: Jeffrey L. Deaton, John R. Brumsted Institution: Department of Obstetrics and Gynecology, University of Vermont, Given C‐252, Burlington, VT 05405 Email: NA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: details not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details not reported |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding not possible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | High risk | Judgement comment: 16/67 participants excluded from analysis due to anovulation, poor semen quality, or inability to follow the treatment protocol. Of the remaining 51 participants, 6 couples did not complete treatment because of illness or relocation. 4/51 dropped out before cross‐over |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: live birth not reported |
| Other bias | Unclear risk | Judgement comment: insufficient data to make a judgement |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics IVF/ICSI
OS‐IUI
Sample size: IVF/ICSI (n = 11); OS‐IUI (n = 33) Included criteria: eligible participants were adults, had primary or secondary infertility of at least 1 year's duration, with evidence of ovulation and tubal patency, and were 18 to 42 years of age if female and 18 to 60 years of age if male Excluded criteria: IUI or IVF treatment in previous 12 months, coital disorder, untreated ovulatory disorders, or endometriosis (American Fertility Society criteria grade 2 to 4), tubal obstruction, abnormal semen analyses (concentration 20*10⁶/mL, progressive motility 25%, abnormal morphology > 95% or positive sperm antibodies), or any contraindication for multiple pregnancy Pretreatment: NA | |
| Interventions | Intervention Characteristics IVF/ICSI
OS‐IUI
| |
| Outcomes | Clinical pregnancy Live birth Multiple pregnancy OHSS | |
| Identification | Sponsorship source: Serono (Geneva, Switzerland) and Melbourne IVF (Melbourne, AUSTRALIA) supported this trial financially Country: Australia Setting: a tertiary level fertility centre at the Royal Women’s Hospital in Melbourne Author's name: Hossam ELZEINY Institution: Reproductive Services, Royal Women’s Hospital, Carlton; Melbourne IVF, 320 Victoria Parade, East Melbourne, Vic 3002, Australia Email: [email protected] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated, adaptive‐biased coin randomisation schedule" |
| Allocation concealment (selection bias) | Low risk | Quote: "allocation was concealed through the use of sequentially numbered opaque sealed envelopes and was held by the research trial manager and opened after the clinician indicated two or three follicles" |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding impossible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: 1/44 not included in the analysis |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all relevant outcomes reported |
| Other bias | Low risk | Judgement comment: no other sources of bias detected |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics EM
OS‐IUI
Sample size: EM (n = 100); OS‐IUI (n = 101) Included criteria: we included women younger than 42 years with body mass index < 35 kg/m² and unexplained infertility, which was defined as normal ovulation (or normal with ovarian stimulation), bilateral patent fallopian tubes as determined by laparoscopy or hysterosalpingography, normal semen analysis (progressive motility ≥ 32% and concentration ≥ 15 million per mL), and a prediction score of natural conception leading to live birth in the next year < 30%. We used the validated Hunault prediction model for natural conception, which includes age, length of infertility, any previous pregnancies, source of referral, and sperm motility. We included women with mild endometriosis (diagnosed by laparoscopy), polycystic ovarian syndrome according to the Rotterdam criteria (providing ovulation was confirmed with or without ovarian stimulation for at least six cycles), and previous IUI or IVF cycles Excluded criteria: couples requiring donor sperm Pretreatment: NA | |
| Interventions | Intervention characteristics EM
OS‐IUI
| |
| Outcomes | Clinical pregnancy Live birth Multiple pregnancy | |
| Identification | Sponsorship source: Auckland Medical Research Foundation, Evelyn Bond Fund of Auckland District Health Board, Mercia Barnes Trust of Royal Australian, and New Zealand College of Obstetricians and Gynaecologists, Maurice and Phyllis Paykel Trust, and The Nurture Foundation for Reproductive Research Country: New Zealand Setting: 2 fertility clinics in New Zealand Author's name: Cynthia M. Farquhar Institution: Department of Obstetrics and Gynaecology, University of Auckland, Auckland 1101, New Zealand Email: [email protected] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we used a computer‐generated randomisation sequence, prepared by an independent statistician, to randomly assign women (1:1) to three cycles of IUI with ovarian stimulation or three cycles of EM in blocks of four, six, and ten, without stratification" |
| Allocation concealment (selection bias) | Low risk | Quote: "allocations were concealed in sequentially numbered, sealed, opaque envelopes, which were opened by the study coordinator at the University of Auckland research department after verification of the inclusion criteria and obtaining written informed consent from each participant" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "the participating couple and the clinicians were informed of treatment allocation" Judgement comment: blinding not possible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "no data were missing for any of the pregnancy, livebirth, or neonatal outcomes" Judgement comment: zero lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all relevant outcomes reported |
| Other bias | Low risk | Judgement comment: no other sources of bias detected |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics Overall
Sample size: OS (n = 76); EM (n = 72) Included criteria: unexplained infertility; primary infertility of 2 or more years' duration; normal history and physical examination; proven ovulation by regular cycles and biphasic basal body temperature charts, serum progesterone (P) > 10 ng/mL in the midluteal phase, or an in‐phase, secretory endometrial biopsy in the late luteal phase; normal hysterosalpingogram; normal laparoscopy done within the last 2 years confirming bilateral tubal patency and no other pelvic pathology; normal serum prolactin; ≥ 2 normal semen analyses fitting the following criteria: volume > 1 cc, count ˜20 × 10⁶ sperm/cc, morphology > 60% normal, motility > 50% Excluded criteria: NA Pretreatment: NA | |
| Interventions | Intervention characteristics OS
EM
| |
| Outcomes | Clinical pregnancy | |
| Identification | Sponsorship source: Medical Research Council of Canada, Ayerst Pharmaceutical Company, Pharmascience, Montreal, Quebec, Canada Country: Canada Setting: 5 Canadian university centres Authors' names: Patricia Fisch, Robert F. Casper* Institution: 6‐240 EN, Toronto General Hospital, 200 Elizabeth Street, Toronto, Ontario, Canada, M5G 2C4 Email: NA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using a computer‐generated random number table" |
| Allocation concealment (selection bias) | Low risk | "assignment of code numbers and distribution of drugs was coordinated by one center" |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear whether outcome assessors were blinded, but this was unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | 22 of 177 couples excluded from analysis for the following reasons: 11 had incomplete data or missed tablets or injections, 7 dropped out, 2 had endometriosis found on review of their records, and 2 were found to have secondary infertility |
| Selective reporting (reporting bias) | Unclear risk | Live births in the 2 groups not reported separately |
| Other bias | Unclear risk | Insufficient information to make a judgement |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics Overall
Sample size: OS (n = 70); EM (n = 70) Included criteria: women with a diagnosis of unexplained infertility Excluded criteria: NA Pretreatment: no statistical difference between the 2 groups in terms of age, presence of medical complications, or side effects of the medication received | |
| Interventions | Intervention characteristics OS
EM
| |
| Outcomes | Clinical pregnancy Live birth Multiple pregnancy | |
| Identification | Sponsorship source: no funding Country: India Setting: single centre Author's name: K. George Institution: Christian Medical Coll, Vellore, India Email: NA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "(computer generated in blocks of 5)" |
| Allocation concealment (selection bias) | Low risk | Quote: "opening consecutively numbered opaque envelopes" Judgement comment: concealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "neither the physician [nor] the patients were aware of the contents of the treatment packets" |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: not sure whether outcomes assessors were blinded, but this was unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: not reported in the abstract |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all relevant outcomes reported |
| Other bias | Unclear risk | Judgement comment: insufficient information to make a judgement |
| Methods | Study design: randomised controlled trial Study grouping: cross‐over | |
| Participants | Baseline characteristics Overall
Sample size: OS (n = 109); EM (n = 105) Included criteria: at least 1 year's infertility, with the following provisions. All women had normal menstrual cycles (21 to 35 days), normal serum prolactin and thyroid hormone levels, normal coital frequency (at least twice weekly), and normal post‐coital sperm‐mucus penetration. Those who failed to conceive within a few months had a laparoscopy to exclude pelvic disease and tubal damage. A blood sample was taken at the midluteal phase in 3 cycles for serum progesterone measurement (timing checked retrospectively as occurring 5 to 10 days before the next menstrual period) Excluded criteria: NA Pretreatment: NA | |
| Interventions | Intervention characteristics OS
EM
| |
| Outcomes | Clinical pregnancy Multiple pregnancy | |
| Identification | Sponsorship source: South Western Regional Health Authority Medical Research Committee for support for Dr. Glazener; Dr. H.C. Masheter of Merrell Pharmaceuticals Ltd., for the supply of clomiphene and matching placebo Country: UK Setting: single centre Author's name: C.M.A. Glazener Institution: Health Services Research Unit, University of Aberdeen Email: NA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: details not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Judgement comment: placebo controlled |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: double‐blind study but unclear whether outcome assessors were blinded; unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: outcome of 1 participant of 109 in CC group not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: live birth not reported |
| Other bias | High risk | Judgement comment: in methods, it was reported that 118 patients were recruited. However, in results, it was reported that 105 patients were treated with placebo and 109 with clomiphene |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics OS‐IUI
IVF/ICSI
Sample size: OS‐IUI (n = 103); IVF/ICSI (n = 51) Included criteria: couples in which the woman was 38 to 42 years of age and sought care for unexplained infertility from August 2004 to November 2009 at Boston IVF and from November 2008 to November 2009 at Brigham and Women's Hospital were screened. Eligibility criteria included 6 months of attempted conception; at least 1 ovary and ipsilateral patent fallopian tube confirmed by hysterosalpingogram or laparoscopy; regular menstrual cycles of 21 to 45 days; and no pelvic pathology, ectopic pregnancy, or previous infertility treatment (except up to 3 cycles of clomiphene without IUI). Acceptable ovarian reserve was demonstrated by a clomiphene challenge test (100 mg clomiphene on cycle days 5 to 9; FSH value 15 mIU/mL on cycle days 3 and 10; and oestradiol value 100 pg/mL on cycle day 3). Normal prolactin and thyroid‐stimulating hormone levels and body mass index (BMI) ≤ 38 in the woman, and sperm concentration ≥ 15 million total motile sperm or ≥ 5 million total motile sperm at reflex IUI preparation in partner required. Only the first 2 cycles were included Excluded criteria: NA Pretreatment: no previous infertility treatment (except up to three cycles of clomiphene without IUI) | |
| Interventions | Intervention characteristics OS‐IUI
IVF/ICSI
| |
| Outcomes | Clinical pregnancy Live birth Multiple pregnancy OHSS | |
| Identification | Sponsorship source: supported by the National Institutes of Health Eunice Kennedy Shriver, National Institute of Child Health and Human Development (grant R01‐HD44547) Country: USA Setting: academic medical centres and private infertility centre in a state with mandated insurance coverage (Boston IVF and Brigham and Women's Hospital) Author's name: Marlene B. Goldman Institution: Department of Obstetrics and Gynecology and Community and Family Medicine, Geisel School of Medicine at Dartmouth and Dartmouth‐Hitchcock Medical Center, Lebanon, New Hampshire Email: [email protected] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomization was performed using permuted blocks of varying sizes, stratified by the woman's age" Judgement comment: but details of sequence generation were not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocation sequence was generated by an independent biostatistician and was implemented by an epidemiologist" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "neither the patients nor their providers were blind to their treatment assignment" Judgement comment: blinding not possible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "all clinical investigators were blinded to the outcome determinations" |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: 6/154 with incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all relevant outcomes reported |
| Other bias | Low risk | Judgement comment: no other sources of bias detected |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics IUI
OS‐IUI
IVF/ICSI
Sample size: IUI (n = 86); OS‐IUI (n = 85); IVF/ICSI (n = 87) Included criteria: couples who had been affected by idiopathic subfertility for at least 3 years, or by male subfertility for at least 1 year, were eligible for the study Excluded criteria: if woman had cycle disorders, untreated endometriosis (American Fertility Society criteria grade 2 to 4), or bilateral occluded tubes, or if a semen sample yielded fewer than 1 million progressively motile spermatozoa after processing by Percoll 40/80 gradient centrifugation; if more than 20% of spermatozoa carried antibodies as tested with an immunobead test after Percoll processing, or if more than 50% of spermatozoa had no acrosome. Patients had undergone extensive investigation of infertility including a basal body temperature chart, a late luteal‐phase endometrial biopsy, a post‐coital test, a hysterosalpingogram, a diagnostic laparoscopy, and ≥ 2 semen analyses. Couples were diagnosed as having idiopathic subfertility if no abnormality was found during the full infertility investigation. Male subfertility was diagnosed if ≥ 3 of 5 semen analyses showed a total motile sperm count of fewer than 20 million progressively motile spermatozoa in the ejaculate, and if the remainder of the infertility investigation revealed no additional abnormalities. In both groups of patients, semen processing by Percoll 40/80 gradient centrifugation yielded a minimum of 1 million progressively motile spermatozoa at least once Pretreatment: none detected | |
| Interventions | Intervention characteristics IUI
OS‐IUI
IVF/ICSI
| |
| Outcomes | Live birth OHSS Multiple pregnancy | |
| Identification | Sponsorship source: this work was financially supported by the Health Insurance Executive Board, Amstelveen, Netherlands Country: Netherlands Setting: single centre Author's name: Angelique J. Goverde Institution: Department of Obstetrics and Gynaecology, University Hospital Vrije Universiteit Email: [email protected] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomisation schedule" |
| Allocation concealment (selection bias) | Low risk | Quote: "administered by numbered masked and sealed envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding impossible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: 13/86, 14/85, and 37/87 participants withdrew during the study in IUI, OS‐IUI, and IVF groups, respectively. Breakdown data unclear in unexplained infertility |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all relevant outcomes reported |
| Other bias | Low risk | Judgement comment: no other sources of bias detected |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics IUI
OS‐IUI
Sample size: IUI (n = 234); OS‐IUI (n = 231) Included criteria: females younger than 40 years of age; negative pregnancy test; normal pelvis and uterine cavity*; “in‐phase” endometrial biopsy; negative serum antisperm antibody test; normal serum follicle‐stimulating hormone and thyrotropin values on days 1 to 5 of cycle; length of 2 of the 3 most recent menstrual cycles between 24 and 40 days; history of infertility for > 1 year. Males younger than 55 years of age; negative serum antisperm antibody test; presence of any motile sperm on screening semen analysis; history of infertility for > 1 year Excluded criteria: females with previous use of in vitro fertilisation or other assisted reproductive technology; previous treatment with gonadotropins; previous intrauterine insemination with current partner; history of chronic disease; history of chemotherapy or radiation to the abdomen or pelvis; history of tubal surgery; extensive tubal adhesions; endometriosis of more than stage II; history of myomectomy, ovarian cystectomy, or unilateral oophorectomy. Males with previous use of in vitro fertilisation or other assisted reproductive technology; previous intrauterine insemination; history of vasovasostomy; varicocelectomy within 6 months before study; history of pelvic node dissection Pretreatment: no pretreatment | |
| Interventions | Intervention characteristics IUI
OS‐IUI
| |
| Outcomes | Clinical pregnancy Live birth | |
| Identification | Sponsorship source: supported in part by Cooperative Agreements with the National Institute of Child Health and Human Development (U10 HD26975, U10HD26981, U10 HD27006, U10 HD27009, U10 HD27001, U10HD27049, U10 HD33172, and U10 HD33173) and by Serono Laboratories Country: USA Setting: 10 clinical sites Authors' names: David S. Guzick, Sandra Ann Carson* Institution: Dr. Carson at Baylor College of Medicine, Department of Obstetrics and Gynecology, 6550 Fannin #801, Houston, TX 77030 Email: [email protected] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: "randomisation was carried out with use of a permuted block procedure, stratified according to center", but details of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details not reported |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding mentioned and seemed impossible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: although there were 22 (9%) in the IUI group and 50 (22%) in the OS‐ IUI group, withdrawal from the study led to numbers of participants with unknown pregnancy outcomes of 1 and 2 in IUI and OS‐IUI groups, respectively |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: data for multiple pregnancy in each group not available |
| Other bias | Low risk | Judgement comment: no other sources of bias detected |
| Methods | Study design: randomised controlled trial Study grouping: cross‐over | |
| Participants | Baseline characteristics Overall
Sample size: EM (n = 15); OS (n = 15) Included criteria: unexplained infertility: semen analysis, post‐coital test, hysterosalpingogram, laparoscopy, immunological tests, and plasma hormone profile (FSH, LH, oestradiol, prolactin, and progesterone) had been found normal. All women had 3 cycles of CC before randomisation Excluded criteria: NA Pretreatment: NA | |
| Interventions | Intervention characteristics EM
OS
| |
| Outcomes | Clinical pregnancy | |
| Identification | Sponsorship source: Merrell U.K., Ltd. Country: Ireland Setting: Rotunda or St. James’s Infertility Clinics Author's name: Robert F. Harrison Institution: Rotunda Hospital, Dublin Email: NA Address: Rotunda Hospital, Dublin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: details not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Judgement comment: placebo‐controlled study |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: placebo‐controlled study; objective outcomes used |
| Incomplete outcome data (attrition bias) | Low risk | All outcome data presented |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: live birth and multiple pregnancy not reported |
| Other bias | Unclear risk | Judgement comment: insufficient information to judge |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics Overall
Sample size: OS (n = 45); OS‐IUI (n = 45) Included criteria: couples presenting with subfertility due to subnormal semen or unexplained infertility Excluded criteria: NA Pretreatment: unclear | |
| Interventions | Intervention characteristics OS
OS‐IUI
| |
| Outcomes | Clinical pregnancy OHSS Multiple pregnancy | |
| Identification | Sponsorship source: NA Country: Hong Kong, China Setting: single centre Author's name: P.C. Ho Institution: Department of Obstetrics and Gynecology, University of Hong Kong, Hong Kong, China Email: NA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: details not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details not reported |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding impossible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding not likely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to make a judgement |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: live birth not reported |
| Other bias | Unclear risk | Judgement comment: insufficient information to make a judgement |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics EM
IVF/ICSI
Sample size: EM (n = 27); IVF/ICSI (n = 24) Included criteria: duration of subfertility > 2 years, defined as no live birth during that time; no previous IVF treatment; female age 18 ± 39 years; willingness to commence either IVF within 6 weeks of allocation or a 3‐month period of observation without intervention; day 3 serum FSH level > 15 IU/L or standard level for inclusion in an individual centre's IVF programme, whichever level was lower; semen analysis available within last 6 months showing an adequate number of sperm to perform ICSI; evidence of Fallopian tube patency, based on a hysterosalpingogram (HSG) or laparoscopy (only data for unexplained infertility were included) Excluded criteria: women with bilateral Fallopian tube occlusion confirmed by HSG or laparoscopy; use of donor sperm; need for sperm recovery procedures; concurrent serious medical illnesses that could be a relative contraindication to IVF Pretreatment: All couples had exhausted appropriate lower intensity treatment options, such as ovulation induction and intrauterine insemination. | |
| Interventions | Intervention characteristics EM
IVF/ICSI
| |
| Outcomes | Live birth Clinical pregnancy | |
| Identification | Sponsorship source: NA Country: Canada Setting: 5 Canadian fertility clinics Author's name: E.G. Hughes Institution: Department of Obstetrics and Gynecology, McMaster University Medical Centre, 1200 Main Street West, Room 4D14, Hamilton, ON L8N 3Z5, Canada Email: [email protected] Address: Department of Obstetrics and Gynecology, McMaster University Medical Centre, 1200 Main Street West, Room 4D14, Hamilton, ON L8N 3Z5, Canada | |
| Notes | Breakdown outcome data of unexplained infertility were extracted from a Cochrane Review (Pandian 2015) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random allocation was based on a blocked schedule using numbered, sealed, opaque envelopes. Randomization was stratified by centre" Judgement comment: but details of random sequence generation not available |
| Allocation concealment (selection bias) | Low risk | Judgement comment: numbered sealed opaque envelopes used |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding impossible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: outcome data of all participants reported |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all relevant outcomes reported |
| Other bias | Low risk | Judgement comment: no other sources of bias detected |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics OS
OS‐IUI
Sample size: OS (n = 36); OS‐IUI (n = 36) Included criteria: couples with a history of more than 3 years of unexplained subfertility Excluded criteria: NA Pretreatment: NA | |
| Interventions | Intervention characteristics OS
OS‐IUI
| |
| Outcomes | Clinical pregnancy | |
| Identification | Sponsorship source: NA Country: Slovakia Setting: not reported Author's name: P. Janko Institution: Department of Gynaecology and Obstetrics, Postgraduate Medidcal School, Limbovn 5, Bratislava 883 07, Slovakia Email: NA Address: Limbova 5, Bratislava 833 07, Slovakia | |
| Notes | Noor Danhof on 4 June 2018, 19:25 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: details not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details not reported |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: not reported; blinding impossible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: unblinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: details not available |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: live birth and multiple pregnancy not reported |
| Other bias | Unclear risk | Judgement comment: insufficient information to judge |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics Overall
Sample size: OS (n = 47); OS‐IUI (n = 32) Included criteria: couples with unexplained infertility including cases with minimal or mild endometriosis according to the American Fertility Society score: (1) duration of the infertility should be at least 2 years; (2) no previous treatment with hMG and/or insemination; (3) woman should be 39 years of age and should have regular ovulatory menstrual cycles with maximum length of 35 days; (4) normal sperm sample according to the World Health Organization criteria and a swim‐up test in hyaluronic acid (Sperm Select; Kabi Pharmacia, Uppsala, Sweden; Select Medical Systems, Williston, VT) using 1‐mL aliquot of semen should result in at least 0.5 × 10⁶/mL progressive motile sperm; (5) laparoscopy and HSG should reveal patent tubes without any adhesions; (6) normal PCT (> 3 progressive motile sperm per high power field) Excluded criteria: NA Pretreatment: NA | |
| Interventions | Intervention characteristics OS
OS‐IUI
| |
| Outcomes | Clinical pregnancy | |
| Identification | Sponsorship source: supported by grant no. B91‐17X‐03495‐20A from The Swedish Medical Research Council, Stockholm, Sweden Country: Sweden Setting: Departments of Obstetrics and Gynecology, Central Hospital, Vasteras and Aka‐demiska Hospital, Uppsala University, Uppsala, Sweden Author's name: Per‐Olof Karlstrom Institution: Department of Obstetrics and Gynecology, Central Hospital, S‐721 89 Viis‐teras, Sweden Email: NA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: details not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details not reported |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding impossible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: this is a factorial design; study authors reported only withdrawals in CC and hMG groups, respectively. Unclear how many in OS and OS‐IUI groups withdrew from the study |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: live birth not reported |
| Other bias | Unclear risk | Judgement comment: insufficient information to judge |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics EM
IUI
Sample size: EM (n = 53); IUI (n = 69) Included criteria: couples included in this trial had at least 2 years of infertility and gave informed consent to participate in the trial. All males except those in the semen defect groups had normal spermiograms on at least 2 occasions. A normal spermiogram consisted of > 40 × 10⁶ sperm/mL, > 45% progressive motility, and > 40% normal morphology. This corresponds to the 15th percentile of a reference population of all men who approached our clinic as potential semen donors. Tubal patency was assessed by laparoscopic tubal dye insufflation, whereas ovulation and cycle endocrinology were assessed in tracking cycles before treatment. Couples selected for the trial had no identifiable cause of infertility (unexplained group) or a single identified cause. The latter categories included cervical mucus hostility, moderate semen defect, and severe semen defect Excluded criteria: NA Pretreatment: NA | |
| Interventions | Intervention characteristics EM
IUI
| |
| Outcomes | Clinical pregnancy | |
| Identification | Sponsorship source: supported in part by grant 850294 from the National Health and Medical Research Council of Australia, Canberra, Australia Country: Australia Setting: clinical infertility service Authors' names: Christine A. Kirby; Colin D. Matthews* Institution: Department of Obstetrics and Gynaecology, The University of Adelaide, The Queen Elizabeth Hospital Email: NA Address: Department of Obstetrics and Gynaecology, The University of Adelaide, The Queen Elizabeth Hospital, Woodville, South Australia 5011, Australia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: details not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details not reported |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding impossible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: details not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: protocol not available; live birth and multiple pregnancy not reported |
| Other bias | Unclear risk | Judgement comment: insufficient information to judge |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics Overall
Sample size: IUI (n = 34); OS‐IUI (n = 34) Included criteria: sterility for longer than 3 years, no severe male factors, no tubal damage, moderate oligoasthenospermia, minimal endometriosis, cervical factor, luteal phase defect Excluded criteria: chronic vaginal infection, liver disease, ovarian cyst, 45 years old, uterine malformation, chronic disease Pretreatment: NA | |
| Interventions | Intervention characteristics IUI
OS‐IUI
| |
| Outcomes | Clinical pregnancy | |
| Identification | Sponsorship source: NA Country: Italy Setting: University of Catania Authors' names: V. Leanza, F. Grasso Institution: Department of Surgery, Obstetrics and Gynecology, University of Catania Email: [email protected] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: details not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Judgement comment: placebo‐controlled study |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: objective outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: 9 couples excluded from the analysis |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: protocol not available; live birth not reported |
| Other bias | Unclear risk | Judgement comment: Insufficient information to make a judgement |
| Methods | Study design: randomised controlled trial Study grouping: cross‐over | |
| Participants | Baseline characteristics Overall
Sample size: EM (n = 10); OS (n = 10); IUI (n = 10); OS‐IUI (n = 10) Included criteria: male or idiopathic factor infertility Excluded criteria: NA Pretreatment: NA | |
| Interventions | Intervention characteristics EM
OS
IUI
OS‐IUI
| |
| Outcomes | Clinical pregnancy | |
| Identification | Sponsorship source: Organon International, Oss, Netherlands Country: Netherlands Setting: single centre Author's name: Antonio R. Martinez Institution: Department of Obstetrics and Gynecology, Free University Hospital Email: NA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: details of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding not possible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: no withdrawal in the first cycle |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: live birth and multiple pregnancy not reported |
| Other bias | Unclear risk | Judgement comment: insufficient information to judge |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics OS
OS‐IUI
Sample size: OS (n = 93); OS‐IUI (n = 91) Included criteria: unexplained and mild male factor‐related infertility. All couples underwent evaluation that included at least 2 semen analyses with andrological evaluation, female endocrine profile (FSH, LH, PRL, and T assay during the very early follicular phase), ovulation assessment (P and PRL assays during luteal phase), endometrial biopsy, transvaginal ultrasonography, post‐coital test, hysterosalpingogram, and diagnostic laparoscopy. All couples had undergone 3 cycles of induction of ovulation with clomiphene citrate (CC) associated with timed vaginal intercourse and 3 cycles of induction of ovulation with CC associated with lUI without conceiving before being enrolled in this trial Excluded criteria: couples with severe male factor‐related infertility (sperm concentration 10*10^6/mL, progressive motility 15%, total motility 30%, and normal morphology 30%), tubal damage, anovulatory cycle, polycystic ovary disease, hyperprolactinaemia, uterine fibroids, and endometriosis were treated according to their pathology and were not considered eligible for the study Pretreatment: no significant difference was present between baseline characteristics | |
| Interventions | Intervention characteristics OS
OS‐IUI
| |
| Outcomes | Clinical pregnancy Live birth Multiple pregnancy OHSS | |
| Identification | Sponsorship source: NA Country: Italy Setting: single centre: Infertility Centre of Department of Obstetrics and Gynecology of the University of Cagliari, Cagliari, Italy Author's name: Gian Benedetto Melis Institution: Department of Obstetrics and Gynecology, University of Cagliari, Cagliari, Italy Email: NA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: details not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "numbered sealed envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding not possible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: 16/184 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all relevant outcomes reported |
| Other bias | Unclear risk | Judgement comment: insufficient information to judge |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics OS‐IUI
IVF/ICSI
Sample size: OS‐IUI (n = 101); IVF/ICSI (n = 106) Included criteria: eligible participants were couples with primary or secondary subfertility of minimum 1 year's duration, where the female partner was between 23 and 37 years of age, body mass index (BMI) was 19 to 30, with a regular menstrual cycle of 21 to 35 days, day 2 FSH 10 IU/L, and confirmed bilateral patent tubes. A midluteal serum P level was used to confirm ovulation. The male partner with normal semen parameters (i.e. sperm density > 15 million/mL, progressive motility > 40%, and normal forms > 4% (World Health Organization criteria), or total progressive motile sperm count > 5 million) was included in the trial Excluded criteria: couples not fulfilling the inclusion criteria, with known uterine anomaly or physical disability, or having difficulty in achieving vaginal intercourse, and couples using donor sperm or previous fertility treatment like IUI or IVF. Those with confirmed endometriosis of grade II to IV were also excluded from the trial. However, routine laparoscopy was not performed in all cases to diagnose endometriosis. Self‐funded patients were excluded from the trial due to lack of research funding Pretreatment: No pretreatment | |
| Interventions | Intervention characteristics OS‐IUI
IVF/ICSI
| |
| Outcomes | Clinical pregnancy Live birth Multiple pregnancy OHSS | |
| Identification | Sponsorship source: this trial had no funding Country: UK Setting: single centre Author's name: Anupa Nandi Institution: Fertility Unit, Homerton University Hospital, London, UK Email: [email protected] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "simple randomization procedure was followed" |
| Allocation concealment (selection bias) | Low risk | Quote: "allocation concealment was achieved by using individual, consecutively numbered opaque envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "due to the nature of the trial, blinding was not possible" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "this is unlikely to affect the outcome of the trial, as the outcome was objective" |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: outcome data of all participants reported |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all relevant outcome reported |
| Other bias | Low risk | Judgement comment: no other sources of bias detected |
| Methods | Study design: randomised controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics EM
OS‐IUI
Sample size: EM (n = 126); OS‐IUI (n = 127) Included criteria: couple had not conceived after at least a year of frequent unprotected intercourse; the woman was younger than 39 years; and the woman had a regular menstrual cycle. Couples with unexplained subfertility and an intermediate prognosis of a spontaneous ongoing pregnancy within the next 12 months were eligible for this study. The basic fertility assessment included medical history, cycle monitoring, semen analysis, post‐coital test, and investigation of tubal function Excluded criteria: NA Pretreatment: NA | |
| Interventions | Intervention characteristics EM
OS‐IUI
| |
| Outcomes | Clinical pregnancy Live birth Multiple pregnancy OHSS | |
| Identification | Sponsorship source: this study was supported by grant 945/12/002 from ZonMW (Netherlands Organization for Health Research and Development, The Hague, Netherlands) Country: Netherlands Setting: 26 fertility centres in Netherland Author's name: Pieternel Steures Institution: Centre for Reproductive Medicine, Academic Medical Centre, Amsterdam, Netherlands Email: [email protected] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomisation sequence was computer generated in balanced block multiples of two or four, stratified by centre" |
| Allocation concealment (selection bias) | Low risk | Quote: "the sequence was concealed, and sealed opaque envelopes containing details of the treatment allocation were assembled by an independent person. Clinicians in the participating centres enrolled the couple and subsequently opened the next envelope. The inclusion was then confirmed to the trial coordinator by fax" |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: blinding impossible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: non‐blinding unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: OS‐IUI: 3/127 lost to follow‐up, EM: 2/126 lost to follow‐up, 2 still pregnant |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all relevant outcomes reported |
| Other bias | Low risk | Judgement comment: no other sources of bias detected |
BBT: basal body temperature.
BMI: body mass index.
CC: clomiphene citrate.
EM: expectant management.
ET: embryo transfer.
FSH: follicle‐stimulating hormone.
GnRH: gonadotropin‐releasing hormone.
hCG: human chorionic gonadotropin.
hMG: human menopausal gonadotropin.
HSG: hysterosalpingogram.
ICSI: intracytoplasmic sperm injection.
IQR: interquartile ratio.
IUI: intrauterine insemination.
IUI‐COS: intrauterine insemination with controlled ovarian stimulation.
IVF: in vitro fertilisation.
LH: luteinising hormone.
NA: not applicable.
OHSS: ovarian hyperstimulation syndrome.
OS: ovarian stimulation.
PCT: post‐coital test.
PRL: prolactin.
rAFS: The revised American Fertility Society classification system.
rFSH: recombinant follicle‐stimulating hormone.
SD: standard deviation.
TMC: total motile count.
WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Interventions not of interest | |
| Interventions not of interest | |
| Not a randomised controlled trial | |
| Interventions not of interest | |
| Cross‐over trial but data before cross‐over not available | |
| Interventions not of interest | |
| Cross‐over trial but data before cross‐over not available | |
| Interventions not of interest | |
| Interventions not of interest | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Interventions not of interest | |
| Interventions not of interest | |
| Interventions not of interest | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Cross‐over trial but data before cross‐over not available | |
| Irrelevant population: included women with PCOS and unexplained infertility; breakdown data not available. No response after study authors were contacted |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | IUI vs IVF/ICSI in Women Aged 38‐42 Years: A Prospective Randomized Controlled Trial |
| Methods | Randomised controlled trial; parallel group |
| Participants | Sample size: 138 Inclusion criteria: women between 38 and 42 years of age; use of donor sperm or husband sperm reaching WHO criteria 2010 Exclusion criteria: tubal infertility (even 1 tube); major uterine or ovarian abnormalities; metabolic abnormalities |
| Interventions | 3 consecutive gonadotropin‐stimulated IUI cycles Intervention vs 1 IVF/ICSI with standard antagonist protocol |
| Outcomes | Primary outcome: cumulative ongoing pregnancy |
| Starting date | December 2014 |
| Contact information | Michael De Brucker, MD Universitair Ziekenhuis Brussel, Jette, Belgium Telephone: 024776699 Email: [email protected] |
| Notes | First posted: 25 November 2013; last updated: 27 March 2015 |
| Trial name or title | Comparison of the Efficiency of Intra‐uterine Insemination and In Vitro Fertilization in Women Over 37 Years (AMPAGE) |
| Methods | Randomised controlled trial; parallel group |
| Participants | Sample size: 600 Inclusion criteria: female between 37 and 42 years of age at the time of inclusion; infertility duration ≥ 12 months; normal tubes; no severe endometriosis, at least 1.5*10^6 motile spermatozoa to be inseminated; no previous ART attempt Exclusion criteria: tubal abnormalities; severe endometriosis; less than 1.5*10^6 motile spermatozoa to be inseminated; use of frozen sperm; presence of anti‐spermatozoa antibodies |
| Interventions | IVF (experimental arm) vs IUI (control arm) |
| Outcomes | Primary outcome: delivery rate [Time frame: after 1 year of treatment] |
| Starting date | May 2014 |
| Contact information | Jean PARINAUD, MD Univerisity Hospital, Toulouse, Midi‐Pyrénnées, France, 31059 Telephone: 05 67 77 10 02 ext 33 Email: parinaud.j@chu‐toulouse.fr Caroline PEYROT, CRA University Hospital, Toulouse, Midi‐Pyrénnées, France, 31059 Telephone: 05 61 77 84 86 ext 33 Email: peyrot.c@chu‐toulouse.fr |
| Notes | First posted: 5 December 2013; last update posted: 14 August 2018 |
| Trial name or title | Stimulated Intrauterine Insemination Cycles and Unstimulated Intrauterine Insemination Cycles in Couples With Unexplained Infertility |
| Methods | Randomised controlled trial; parallel group |
| Participants | Sample size: 450 Inclusion criteria: women 20 to 40 years of age; unexplained infertility Exclusion criteria: known allergy to FSH; diabetes; hypertension; known cardiac, renal, or liver disease |
| Interventions | OS‐IUI: ovarian stimulation with hMG followed by IUI IUI: testing of urinary luteinising hormone followed by IUI Timed intercourse: testing of urinary luteinising hormone followed by intercourse |
| Outcomes | Primary outcome: ongoing pregnancy |
| Starting date | June 2015 |
| Contact information | Abdel Gany Hassan, MRCOG, MD Cairo University Hospitals, Cairo, Egypt Telephone: 002 01017801604 Email: [email protected] Nesreen A.A. Shehata, MD BeniSuef University Hospitals Telephone: +2001227866337, BeniSuef, Egypt Email: [email protected] |
| Notes | First posted: 3 June 2015; last updated: 29 July 2016 |
| Trial name or title | Intrauterine Insemination With Letrozole Versus in Natural Cycle |
| Methods | Randomised controlled trial; parallel group |
| Participants | Sample size: 100 Inclusion criteria: being diagnosed with unexplained or mild male subfertility; ≥ 1‐sided tubal patency, established according to local protocol; normal or mild impairment of semen quality defined as TMSC ≥ 3 million based on ≥ 1 semen analysis Exclusion criteria: women with double‐sided tubal pathology; women with irregular cycles, PCOS, or other endocrine disorders; impaired semen quality: pre‐wash TMSC < 3 million |
| Interventions | IUI with ovarian stimulation (letrozole) vs natural cycle IUI |
| Outcomes | Primary outcome: ongoing pregnancy leading to live birth |
| Starting date | March 2018 |
| Contact information | Shuo Huang, PhD Peking University Third Hospital, Beijing, China Telephone: 86‐13601203410 Email: [email protected] |
| Notes | First posted 6 March 2018; last updated 6 March 2018 |
| Trial name or title | Intrauterine Insemination for Unexplained or Mild Male Subfertility ‐ ex IUI |
| Methods | Randomised controlled trial, parallel group |
| Participants | Sample size: 1091 Inclusion criteria: 12 months of unprotected intercourse without conception; females between 18 and 42 years of age; regular ovulatory cycle and ≥ 1 patent fallopian tube. Male partner with no or mild impairment of semen quality with total motile sperm count (TMSC or VCM) > 3 million. Obtained written informed consent. 12‐Month prognosis for natural conception (calculated according to the model of Hunault) ≤ 30%, or 12‐month prognosis > 30% and returning after 6 months of expectant management without conception Exclusion criteria: IUI‐OH with sperm donation; couples with medical contraindication for pregnancy; couples with previous ART in the current treatment episode |
| Interventions | Expectant management (experimental arm) vs OS‐IUI (control arm) |
| Outcomes | Primary outcome: ongoing pregnancy leading to a live birth occurring within 6 months after randomisation Secondary outcomes: number of incomplete/cancelled cycles, clinical pregnancy, ongoing pregnancy, multiple pregnancy, ongoing multiple pregnancy, miscarriage, ectopic pregnancy, time to ongoing pregnancy, pregnancy outcomes, couples preference, quality of life, financial costs |
| Starting date | 10 January 2016 |
| Contact information | F. Mol Centrum voor Voortplantingsgeneeskunde Q3‐119 Academisch Medisch Centrum Amsterdam, Netherlands Telephone: 020 5663557 Email: [email protected] |
| Notes | First posted: 18 December 2015; last updated: 30 April 2017 |
ART: assisted reproductive technology.
FSH: follicle‐stimulating hormone.
hMG: human menopausal gonadotropin.
ICSI: intracytoplasmic sperm injection.
IUI: intrauterine insemination.
IVF: in vitro fertilisation.
OS: ovarian stimulation.
PCOS: polycystic ovarian syndrome.
TMSC: total motile sperm count.
VCM: total motile sperm count calculated as volume (in milliliters) × sperm concentration (106/mL) × percentage forward motility.
WHO: World Health Organization.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 10 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Pairwise meta‐analyses for live birth, multiple pregnancy, and clinical pregnancy, Outcome 1 Live birth. | ||||
| 1.1 OS vs EM | 2 | 527 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.49, 1.31] |
| 1.2 IUI vs EM | 1 | 386 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.87, 2.40] |
| 1.3 OS‐IUI vs EM | 2 | 454 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.36, 9.90] |
| 1.4 IUI vs OS | 1 | 387 | Odds Ratio (M‐H, Random, 95% CI) | 1.85 [1.09, 3.16] |
| 1.5 OS‐IUI vs OS | 1 | 184 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.46, 1.67] |
| 1.6 OS‐IUI vs IUI | 2 | 636 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [1.14, 2.49] |
| 1.7 IVF/ICSI vs OS‐IUI | 3 | 731 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.85, 1.57] |
| 2 Multiple pregnancy Show forest plot | 12 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Pairwise meta‐analyses for live birth, multiple pregnancy, and clinical pregnancy, Outcome 2 Multiple pregnancy. | ||||
| 2.1 OS vs EM/IUI | 3 | 934 | Odds Ratio (M‐H, Random, 95% CI) | 2.04 [0.51, 8.24] |
| 2.2 OS‐IUI vs EM/IUI | 4 | 676 | Odds Ratio (M‐H, Random, 95% CI) | 5.04 [1.24, 20.49] |
| 2.3 OS‐IUI vs OS | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.12, 3.81] |
| 2.5 IVF/ICSI vs OS‐IUI | 3 | 731 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.37, 1.73] |
| 3 Clinical pregnancy Show forest plot | 23 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Pairwise meta‐analyses for live birth, multiple pregnancy, and clinical pregnancy, Outcome 3 Clinical pregnancy. | ||||
| 3.1 OS vs EM | 6 | 939 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.82, 2.10] |
| 3.2 IUI vs EM | 3 | 528 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.47] |
| 3.3 OS‐IUI vs EM | 4 | 525 | Odds Ratio (M‐H, Random, 95% CI) | 2.69 [0.96, 7.55] |
| 3.4 IUI vs OS | 2 | 407 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [1.01, 2.82] |
| 3.5 OS‐IUI vs OS | 8 | 763 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.73, 2.18] |
| 3.6 OS‐IUI vs IUI | 4 | 579 | Odds Ratio (M‐H, Random, 95% CI) | 2.56 [1.72, 3.80] |
| 3.7 IVF/ICSI vs OS‐IUI | 3 | 731 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.95, 1.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OS‐IUI vs EM Show forest plot | 1 | 51 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.1  Comparison 2 Pairwise meta‐analysis for OHSS, Outcome 1 OS‐IUI vs EM. | ||||
| 2 OS‐IUI vs OS Show forest plot | 2 | 274 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.2  Comparison 2 Pairwise meta‐analysis for OHSS, Outcome 2 OS‐IUI vs OS. | ||||
| 3 OS‐IUI vs IUI Show forest plot | 1 | 171 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.3  Comparison 2 Pairwise meta‐analysis for OHSS, Outcome 3 OS‐IUI vs IUI. | ||||
| 4 IVF/ICSI vs IUI Show forest plot | 1 | 173 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.17 [0.36, 140.84] |
| Analysis 2.4  Comparison 2 Pairwise meta‐analysis for OHSS, Outcome 4 IVF/ICSI vs IUI. | ||||
| 5 IVF/ICSI vs OS‐IUI Show forest plot | 5 | 985 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.50 [0.92, 6.76] |
| Analysis 2.5  Comparison 2 Pairwise meta‐analysis for OHSS, Outcome 5 IVF/ICSI vs OS‐IUI. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Data analyses of RCTs that were not included in the network meta‐analysis, Outcome 1 Live birth. | ||||
| 1.1 IVF/ICSI vs EM | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 22.00 [2.56, 189.37] |
| 1.2 IVF/ICSI vs IUI | 1 | 173 | Odds Ratio (M‐H, Random, 95% CI) | 1.49 [0.79, 2.82] |
| 1.3 IVF/ICSI vs OS‐IUI | 3 | 370 | Odds Ratio (M‐H, Random, 95% CI) | 2.23 [0.83, 5.98] |
| 2 Multiple pregnancy Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Data analyses of RCTs that were not included in the network meta‐analysis, Outcome 2 Multiple pregnancy. | ||||
| 2.1 IVF/ICSI vs IUI | 1 | 173 | Odds Ratio (M‐H, Random, 95% CI) | 7.44 [0.90, 61.80] |
| 2.2 IVF/ICSI vs OS‐IUI | 3 | 370 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.37, 1.74] |
| 3 Clinical pregnancy Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Data analyses of RCTs that were not included in the network meta‐analysis, Outcome 3 Clinical pregnancy. | ||||
| 3.1 IVF/ICSI vs EM | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 8.00 [1.89, 33.85] |
| 3.2 IVF/ICSI vs OS | 1 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 2.36 [0.72, 7.72] |
| 3.3 IVF/ICSI vs OS‐IUI | 3 | 292 | Odds Ratio (M‐H, Random, 95% CI) | 2.61 [1.07, 6.37] |
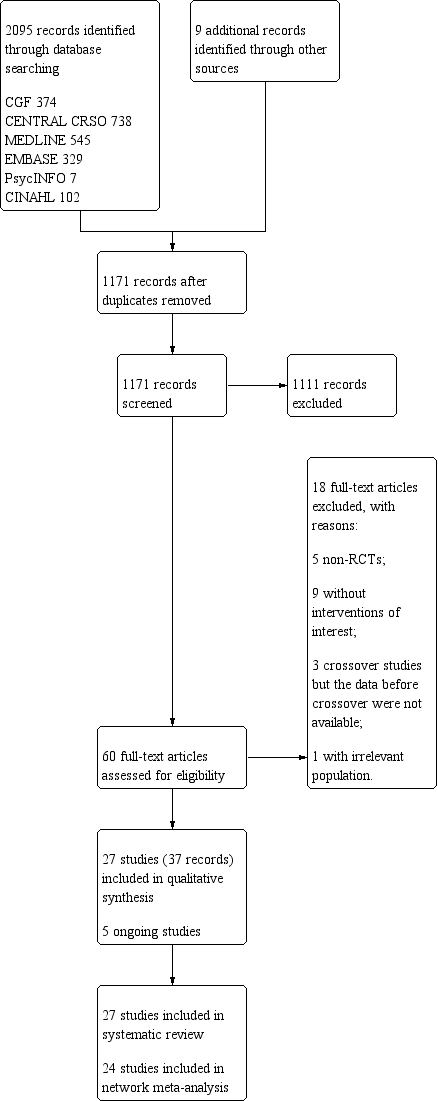
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Box plot for the distribution of means of age in different studies across different comparisons.
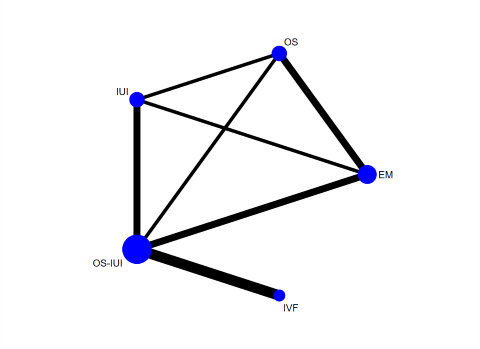
Network plot for live birth.
Each node represents an intervention, and the size of each node is proportional to the number of trials reporting such intervention. The widths of the lines are proportional to the numbers of trials comparing each pair of interventions.

Network meta‐analysis for live birth.
Each diamond represents the estimate summary odds ratio of each comparison; each horizontal line represents the confidence interval of each comparison; blue vertical line represents line of no effect (odds ratio = 1). Odds ratio greater than 1 favours the first intervention; odds ratio less than 1 favours the second intervention.
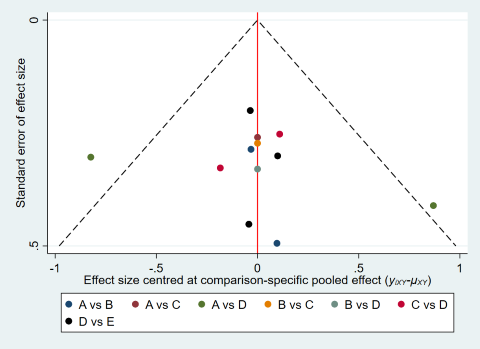
Comparison‐adjusted funnel plot for live birth.
(A: expectant management; B: OS; C: IUI; D: OS‐IUI; E: IVF/ICSI.)

Cumulative rankograms of interventions for live birth.
Each cumulative rankogram illustrates the cumulative probability of each ranking (from the best to the worst rank) for each intervention in terms of live birth.
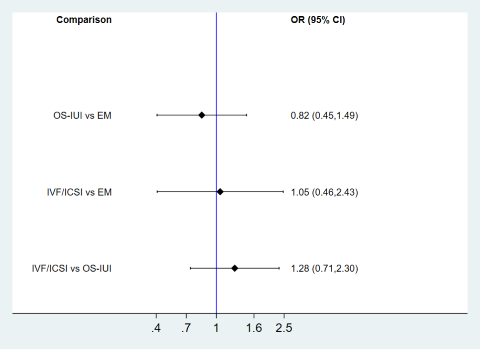
Subgroup analysis for live birth ‐ RCTs with a median duration of infertility ≤ 2 years.
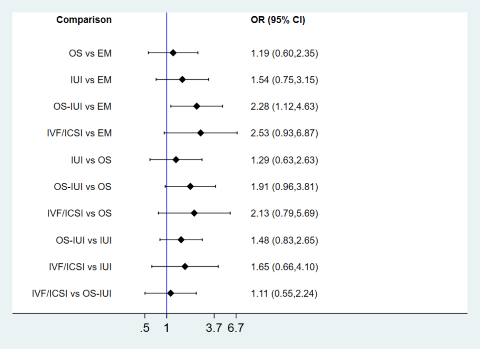
Subgroup analysis for live birth ‐ RCTs with a median duration of infertility > 2 years.

Sensitivity analysis for live birth by exclusion of participants with missing outcome data.
Each diamond represents the estimate summary odds ratio for each comparison; each horizontal line represents the confidence interval for each comparison; blue vertical line represents line of no effect (odds ratio = 1). Odds ratio greater than 1 favours the first intervention; odds ratio less than 1 favours the second intervention.
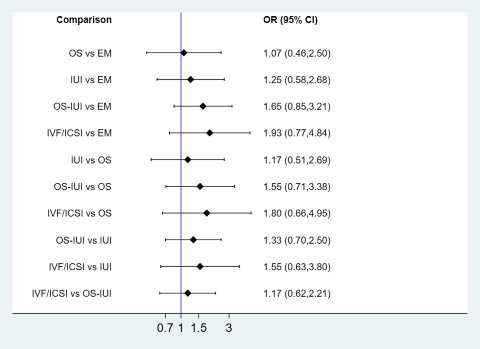
Sensitivity analysis for live birth by exclusion of abstract‐only publications.
Each diamond represents the estimate summary odds ratio for each comparison; each horizontal lines represents the confidence interval for each comparison; blue vertical line represents line of no effect (odds ratio = 1). Odds ratio greater than 1 favours the first intervention; odds ratio less than 1 favours the second intervention.
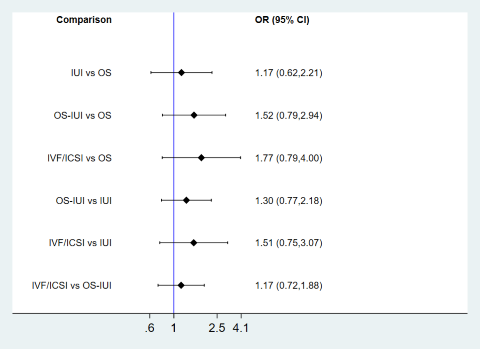
Sensitivity analysis for live birth excluding RCTs involving expectant management from the network.
Each diamond represents the estimate summary odds ratio for each comparison; each horizontal lines represents the confidence interval for each comparison; blue vertical line represents line of no effect (odds ratio = 1). Odds ratio greater than 1 favours the first intervention; odds ratio less than 1 favours the second intervention.

Sensitivity analysis for live birth by limiting to RCTs including couples with poor prognosis of natural conception.
Each diamond represents the estimate summary odds ratio for each comparison; each horizontal lines represents the confidence interval for each comparison; blue vertical line represents line of no effect (odds ratio = 1). Odds ratio greater than 1 favours the first intervention; odds ratio less than 1 favours the second intervention.
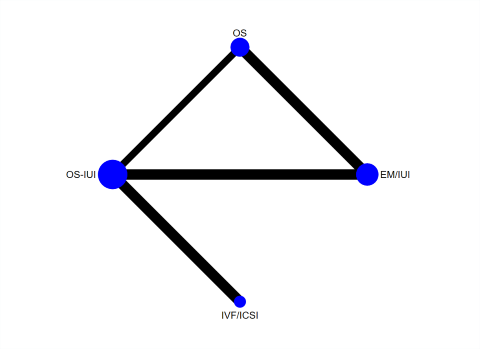
Network plot for multiple pregnancy.
Each node represents an intervention, and the size of each node is proportional to the number of trials reporting such interventions. The widths of lines are proportional to the numbers of trials comparing each pair of interventions.

Network meta‐analysis for multiple pregnancy.
Each diamond represents the estimate summary odds ratio for each comparison; each horizontal line represents the confidence interval for each comparison; blue vertical line represents line of no effect (odds ratio = 1). Odds ratio greater than 1 favours the second intervention; odds ratio less than 1 favours the first intervention.

Cumulative rankograms of interventions for multiple pregnancy.
Each cumulative rankogram illustrates the cumulative probability of each ranking (from the best to the worst rank) for each intervention in terms of multiple pregnancy .
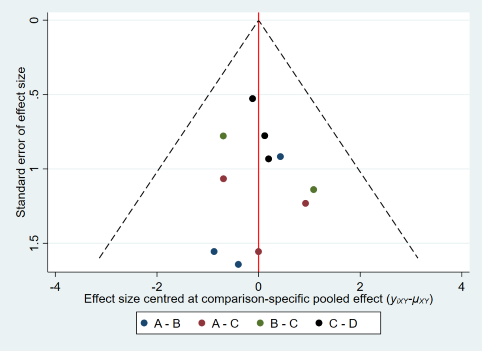
Comparison‐adjusted funnel plot for multiple pregnancy.
(A: expectant management or IUI; B: OS; C: OS‐IUI; D: IVF/ICSI.)
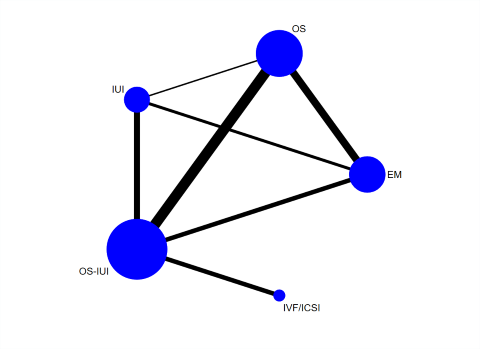
Network plot for clinical pregnancy.
Each node represents an intervention, and the size of each node is proportional to the number of trials reporting such intervention. The widths of the lines are proportional to the numbers of trials comparing each pair of interventions.

Network meta‐analysis for clinical pregnancy.
Each diamond represents the estimate summary odds ratio for each comparison; each horizontal line represents the confidence interval for each comparison; blue vertical line represents line of no effect (odds ratio = 1). Odds ratio greater than 1 favours the first intervention; odds ratio less than 1 favours the second intervention.

Cumulative rankograms of interventions for clinical pregnancy.
Each cumulative rankogram illustrates the cumulative probability of each ranking (from the best to the worst rank) for each intervention in terms of clinical pregnancy.

Comparison‐adjusted funnel plot for clinical pregnancy.(A: expectant management; B: OS; C: IUI; D: OS‐IUI; E: IVF/ICSI.)
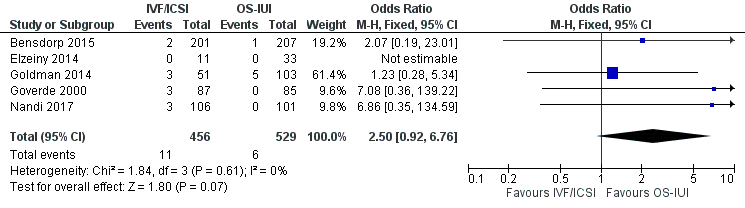
Forest plot of comparison: 2 Pairwise meta‐analysis for OHSS, outcome: 2.5 IVF/ICSI vs OS‐IUI.

Comparison 1 Pairwise meta‐analyses for live birth, multiple pregnancy, and clinical pregnancy, Outcome 1 Live birth.

Comparison 1 Pairwise meta‐analyses for live birth, multiple pregnancy, and clinical pregnancy, Outcome 2 Multiple pregnancy.
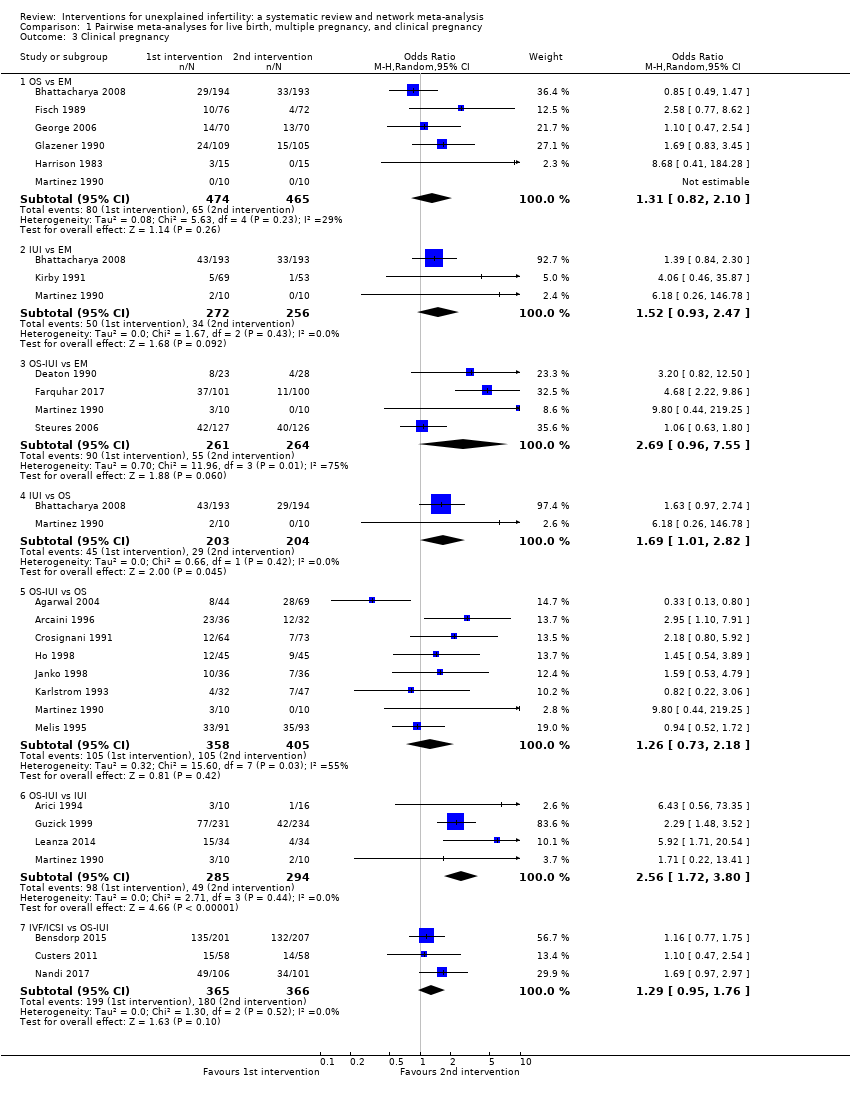
Comparison 1 Pairwise meta‐analyses for live birth, multiple pregnancy, and clinical pregnancy, Outcome 3 Clinical pregnancy.
Comparison 2 Pairwise meta‐analysis for OHSS, Outcome 1 OS‐IUI vs EM.

Comparison 2 Pairwise meta‐analysis for OHSS, Outcome 2 OS‐IUI vs OS.
Comparison 2 Pairwise meta‐analysis for OHSS, Outcome 3 OS‐IUI vs IUI.
Comparison 2 Pairwise meta‐analysis for OHSS, Outcome 4 IVF/ICSI vs IUI.

Comparison 2 Pairwise meta‐analysis for OHSS, Outcome 5 IVF/ICSI vs OS‐IUI.

Comparison 3 Data analyses of RCTs that were not included in the network meta‐analysis, Outcome 1 Live birth.

Comparison 3 Data analyses of RCTs that were not included in the network meta‐analysis, Outcome 2 Multiple pregnancy.

Comparison 3 Data analyses of RCTs that were not included in the network meta‐analysis, Outcome 3 Clinical pregnancy.
| Estimates of effects, confidence intervals, and certainty of the evidence for live birth in couples with unexplained infertility | |||||
| Patient or population: couples with unexplained infertility Intervention: OS, IUI, OS‐IUI, or IVF/ICSI Comparator: expectant management, OS, IUI, or OS‐IUI Outcome: live birth Setting: outpatient | |||||
| All comparisons (10 RCTs, 2725 couples) | Illustrative comparative risks* (95% CI) | Relative effect | Quality of the evidence | ||
| Comparator | Intervention (number of RCTs and number of couples in direct comparison) | Assumed risk with comparator | Corresponding risk with intervention | ||
| Expectant management | OS (2 RCTs, 527 couples) | 166 per 1000 | 167 per 1000 | OR 1.01 | ⊕⊕⊝⊝ LOWa |
| IUI (1 RCT, 386 couples) | 166 per 1000 | 194 per 1000 | OR 1.45 | ⊕⊕⊝⊝ LOWa | |
| OS‐IUI (2 RCTs, 454 couples) | 166 per 1000 | 242 per 1000 | OR 1.61 | ⊕⊕⊝⊝ LOWb | |
| IVF/ICSI (no direct evidence available; only indirect evidence used here) | 166 per 1000 | 272 per 1000 | OR 1.88 | ⊕⊕⊝⊝ LOWa | |
| OS | IUI (1 RCT, 387 couples) | 174 per 1000 | 201 per 1000 | OR 1.20 | ⊕⊕⊝⊝ LOWa |
| OS‐IUI (1 RCT, 184 couples) | 174 per 1000 | 252 per 1000 | OR 1.60 | ⊕⊕⊝⊝ LOWa | |
| IVF/ICSI (no direct evidence available; only indirect evidence used here) | 174 per 1000 | 281 per 1000 | OR 2.63 | ⊕⊕⊝⊝ LOWa | |
| IUI | OS‐IUI (2 RCTs, 636 couples) | 166 per 1000 | 209 per 1000 | OR 1.33 | ⊕⊕⊝⊝ LOWa |
| IVF/ICSI (no direct evidence available; only indirect evidence used here) | 166 per 1000 | 235 per 1000 | OR 1.55 | ⊕⊕⊝⊝ LOWa | |
| OS‐IUI | IVF/ICSI (3 RCTs, 731 couples) | 319 per 1000 | 354 per 1000 | OR 1.17 | ⊕⊕⊝⊝ LOWa |
| CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. *The corresponding risk in the intervention group (and its 95% CI) is based on the mean risk in the comparator group and the relative effect of the intervention (and its 95% CI). **All ORs and 95% CIs are based on network estimates. | |||||
| GRADE Working Group grades of evidence. | |||||
| aDowngraded by two levels for very serious imprecision. bDowngraded by two levels for serious imprecision and serious heterogeneity. | |||||
| Estimates of effects, confidence intervals, and certainty of the evidence for multiple pregnancy in couples with unexplained infertility | |||||
| Patient or population: couples with unexplained infertility Intervention: OS, OS‐IUI, or IVF/ICSI Comparator: expectant management/IUI, OS, or OS‐IUI Outcome: multiple pregnancy Setting: outpatient | |||||
| All comparisons (11 RCTs, 2564 couples) | Illustrative comparative risks* (95% CI) | Relative effect | Quality of the evidence | ||
| Comparator | Intervention (number of RCTs and number of couples in direct comparison) | Assumed risk with comparator | Corresponding risk with intervention | ||
| Expectant management/IUI | OS (3 RCTs, 934 couples) | 6 per 1000 | 17 per 1000 | OR 3.07 | ⊕⊕⊝⊝ LOWa |
| OS‐IUI (3 RCTs, 625 couples) | 6 per 1000 | 18 per 1000 | OR 3.34 | ⊕⊕⊕⊝ MODERATEb | |
| IVF/ICSI (no direct evidence available; only indirect evidence used here) | 6 per 1000 | 15 per 1000 | OR 2.66 | ⊕⊕⊝⊝ LOWc | |
| OS | OS‐IUI (2 RCTs, 274 couples) | 23 per 1000 | 26 per 1000 | OR 1.09 | ⊕⊝⊝⊝ VERY LOWd |
| IVF/ICSI (no direct evidence available; only indirect evidence used here) | 23 per 1000 | 20 per 1000 | OR 0.87 | ⊕⊕⊝⊝ LOWc | |
| OS‐IUI | IVF/ICSI (3 RCTs, 731 couples) | 27 per 1000 | 22 per 1000 | OR 0.80 | ⊕⊕⊝⊝ LOWc |
| CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. *The corresponding risk in the intervention group (and its 95% CI) is based on the mean risk in the comparator group and the relative effect of the intervention (and its 95% CI). **All ORs and 95% CIs are based on network estimates. | |||||
| GRADE Working Group grades of evidence. | |||||
| aDowngraded by two levels for serious imprecision and serious heterogeneity. bDowngraded by one level for serious imprecision. cDowngraded by two levels for very serious imprecision. dDowngraded by three levels for serious study limitations and very serious imprecision. | |||||
| Estimates of effects, confidence intervals, and certainty of the evidence for clinical pregnancy in couples with unexplained infertility | |||||
| Patient or population: couples with unexplained infertility Intervention: OS, IUI, OS‐IUI, or IVF/ICSI Comparator: expectant management, OS, IUI, or OS‐IUI Outcome: clinical pregnancy Setting: outpatient | |||||
| All comparisons (23 RCTs, 3792 couples) | Illustrative comparative risks* (95% CI) | Relative effect | Quality of the evidence | ||
| Comparator | Intervention (number of RCTs and number of couples in direct comparison) | Assumed risk with comparator | Corresponding risk with intervention | ||
| Expectant management | OS (6 RCTs, 939 couples) | 157 per 1000 | 234 per 1000 | OR 1.64 | ⊕⊝⊝⊝ VERY LOWa |
| IUI (3 RCTs, 528 couples) | 157 per 1000 | 182 per 1000 | OR 1.20 | ⊕⊕⊝⊝ LOWb | |
| OS‐IUI (4 RCTs, 525 couples) | 157 per 1000 | 301 per 1000 | OR 2.32 | ⊕⊕⊝⊝ LOWc | |
| IVF/ICSI (no direct evidence available; only indirect evidence used here) | 157 per 1000 | 360 per 1000 | OR 3.03 | ⊕⊕⊝⊝ LOWc | |
| OS | IUI (2 RCTs, 407 couples) | 213 per 1000 | 165 per 1000 | OR 0.73 | ⊕⊝⊝⊝ VERY LOWd |
| OS‐IUI (8 RCTs, 763 couples) | 213 per 1000 | 276 per 1000 | OR 1.41 | ⊕⊝⊝⊝ VERY LOWe | |
| IVF/ICSI (no direct evidence available; only indirect evidence used here) | 213 per 1000 | 332 per 1000 | OR 1.84 | ⊕⊕⊝⊝ LOWf | |
| IUI | OS‐IUI (4 RCTs, 579 couples) | 174 per 1000 | 291 per 1000 | OR 1.94 | ⊕⊝⊝⊝ VERY LOWa |
| IVF/ICSI (no direct evidence available; only indirect evidence used here) | 174 per 1000 | 347 per 1000 | OR 2.52 | ⊕⊕⊝⊝ LOWf | |
| OS‐IUI | IVF/ICSI (3 RCTs, 731 couples) | 344 per 1000 | 437 per 1000 | OR 1.30 | ⊕⊕⊝⊝ LOWb |
| CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. *The corresponding risk in the intervention group (and its 95% CI) is based on the mean risk in the comparator group and the relative effect of the intervention (and its 95% CI). **All ORs and 95% CIs are based on network estimates. | |||||
| GRADE Working Group grades of evidence. | |||||
| aDowngraded by three levels for serious study limitations, imprecision, and heterogeneity. bDowngraded by two levels for very serious imprecision. cDowngraded by two levels for very serious heterogeneity. dDowngraded by three levels for very serious imprecision and serious incoherence. eDowngraded by three levels for very serious study limitations, serious imprecision, and serious heterogeneity. fDowngraded by two levels for serious imprecision and serious heterogeneity. | |||||
| IVF/ICSI compared with OS‐IUI for unexplained infertility | ||||||
| Patient or population: couples with unexplained infertility Settings: outpatient Intervention: IVF/ICSI Comparison: OS‐IUI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| with OS‐IUI | with IVF/ICSI | |||||
| Moderate/severe OHSS | 11 per 1000 | 28 per 1000 | OR 2.50 (0.92 to 6.76) | 958 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by one level for serious imprecision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 10 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 OS vs EM | 2 | 527 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.49, 1.31] |
| 1.2 IUI vs EM | 1 | 386 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.87, 2.40] |
| 1.3 OS‐IUI vs EM | 2 | 454 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.36, 9.90] |
| 1.4 IUI vs OS | 1 | 387 | Odds Ratio (M‐H, Random, 95% CI) | 1.85 [1.09, 3.16] |
| 1.5 OS‐IUI vs OS | 1 | 184 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.46, 1.67] |
| 1.6 OS‐IUI vs IUI | 2 | 636 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [1.14, 2.49] |
| 1.7 IVF/ICSI vs OS‐IUI | 3 | 731 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.85, 1.57] |
| 2 Multiple pregnancy Show forest plot | 12 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 OS vs EM/IUI | 3 | 934 | Odds Ratio (M‐H, Random, 95% CI) | 2.04 [0.51, 8.24] |
| 2.2 OS‐IUI vs EM/IUI | 4 | 676 | Odds Ratio (M‐H, Random, 95% CI) | 5.04 [1.24, 20.49] |
| 2.3 OS‐IUI vs OS | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.12, 3.81] |
| 2.5 IVF/ICSI vs OS‐IUI | 3 | 731 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.37, 1.73] |
| 3 Clinical pregnancy Show forest plot | 23 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 OS vs EM | 6 | 939 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.82, 2.10] |
| 3.2 IUI vs EM | 3 | 528 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.47] |
| 3.3 OS‐IUI vs EM | 4 | 525 | Odds Ratio (M‐H, Random, 95% CI) | 2.69 [0.96, 7.55] |
| 3.4 IUI vs OS | 2 | 407 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [1.01, 2.82] |
| 3.5 OS‐IUI vs OS | 8 | 763 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.73, 2.18] |
| 3.6 OS‐IUI vs IUI | 4 | 579 | Odds Ratio (M‐H, Random, 95% CI) | 2.56 [1.72, 3.80] |
| 3.7 IVF/ICSI vs OS‐IUI | 3 | 731 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.95, 1.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OS‐IUI vs EM Show forest plot | 1 | 51 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 OS‐IUI vs OS Show forest plot | 2 | 274 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 OS‐IUI vs IUI Show forest plot | 1 | 171 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 IVF/ICSI vs IUI Show forest plot | 1 | 173 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.17 [0.36, 140.84] |
| 5 IVF/ICSI vs OS‐IUI Show forest plot | 5 | 985 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.50 [0.92, 6.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 IVF/ICSI vs EM | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 22.00 [2.56, 189.37] |
| 1.2 IVF/ICSI vs IUI | 1 | 173 | Odds Ratio (M‐H, Random, 95% CI) | 1.49 [0.79, 2.82] |
| 1.3 IVF/ICSI vs OS‐IUI | 3 | 370 | Odds Ratio (M‐H, Random, 95% CI) | 2.23 [0.83, 5.98] |
| 2 Multiple pregnancy Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 IVF/ICSI vs IUI | 1 | 173 | Odds Ratio (M‐H, Random, 95% CI) | 7.44 [0.90, 61.80] |
| 2.2 IVF/ICSI vs OS‐IUI | 3 | 370 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.37, 1.74] |
| 3 Clinical pregnancy Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 IVF/ICSI vs EM | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 8.00 [1.89, 33.85] |
| 3.2 IVF/ICSI vs OS | 1 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 2.36 [0.72, 7.72] |
| 3.3 IVF/ICSI vs OS‐IUI | 3 | 292 | Odds Ratio (M‐H, Random, 95% CI) | 2.61 [1.07, 6.37] |

