Intervenciones con teléfonos móviles para mejorar la adherencia a la medicación prescrita para la prevención primaria de las enfermedades cardiovasculares en adultos
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: 3‐arm, parallel RCT Setting: outpatient chronic disease services in a public sector clinic, Cape Town, South Africa Duration of study: 12 months | |
| Participants | Number randomised: 1372; group 1 (control): 457; group 2 (informational SMS): 457; group 3 (interactive SMS): 458 Number lost to follow‐up/withdrawn: 176; group 1: 61 (reasons: 3 died; 2 pregnant; 14 lost contact; 12 moved; 25 unable to attend; 5 reason not given); group 2: 51 (reasons: 7 died; 2 pregnant; 7 lost contact; 11 moved; 23 unable to attend; 1 reason not given); group 3: 64 (reasons: 7 died; 5 pregnant; 2 participant decision; 7 lost contact; 14 moved; 29 unable to attend) Number analysed: 1372; group 1: 457; group 2: 457; group 3: 458 (intention‐to‐treat analysis using all data available) Mean age in years (SD): group 1: 54.7 (SD 11.6); group 2: 53.9 (SD 11.2); group 3: 54.2 (SD 11.6) Age range: not stated Gender (% women): group 1: 72; group 2: 72; group 3: 72 Proportion meeting criteria of 'primary prevention': 78.3% (unpublished information received from authors) Proportion prescribed medication for prevention of CVD: 100%; prescribed BP‐lowering medication was an inclusion criterion. Inclusion criteria: aged ≥ 21 years, diagnosed with hypertension by a clinician using local guidelines, prescribed BP‐lowering medication, and with SBP < 220 mmHg and a DBP < 120 mmHg at enrolment. Eligible participants were attending the primary care clinic, resided in 1 of the 2 study communities and had regular access to a mobile phone (and were able to send SMS text messages or could do so with help of a relative). Study enrolled only 1 member per household. Exclusion criteria: requiring specialist care for hypertension at a hospital (in secondary care), women who self‐reported being pregnant or within 3 months postpartum, and people with very high BPs (SBP > 220 mmHg or DBP > 120 mmHg) who had symptoms suggestive of a hypertensive emergency or were otherwise acutely unwell (who were directly referred to the appropriate clinical service). | |
| Interventions | Intervention: all participants received written information about hypertension and continued to receive care from the clinic. Group 2: 'informational SMS texting:' participants received: text messages to motivate collecting and taking medicines and to provide education about hypertension and its treatment. The messages were designed to address a range of common issues with adherence to and persistence with treatment. Additional reminders were sent when medicines were ready for collection or for scheduled clinic appointments. Group 3: 'interactive SMS texting' group: participants received: the same messages as the information‐only group but could also respond to selected messages using free‐to‐user "please call me" requests. These generated an automated series of responses from the text message delivery system offering trial participants a number of options, including cancelling or changing an appointment and changing the timing and language of the text messages. The intervention was specifically designed to primarily focus on medication adherence, with only a few references other lifestyle modifications such as diet and physical exercise. Comparison: control group (group 1) received written information about hypertension and healthy living and continued to receive care from the clinic. The control group only received the texts sent to all trial participants, which were sent no more frequently than 1 text every 4 weeks. The messages were a welcome text, a text confirming enrolment, a text on a birthday and other text messages about participation in the trial. How intervention was developed: the researchers iteratively designed, developed and tested 2 SMS text messaging‐based interventions with clinical staff and participants with high BP working and living in low‐income communities around Cape Town. Behaviour change technique(s) employed: 16 in total: problem solving; goal setting; action planning; review of behavioural goals; behavioural contract; commitment; general social support; practical social support; emotional social support; providing information about health consequences; emphasising salience of consequences; anticipated regret; behavioural rehearsal; behavioural substitution; habit formation; generalisation of target behaviour. Personalised intervention: some texts were personalised to include participants' first or chose name. Information provided not personalised, but reminders of when medications were available for collection and dates of next appointment indicates some personalisation. Additionally, the 'interactive SMS texting' group (group 3) could request further interactions. Frequency and duration of intervention receipt: messages sent weekly at a time selected by participant. Intervention duration: 12 months | |
| Outcomes | Primary outcomes: SBP (mean); proportion of participants achieving a mean SBP < 140 mmHg and a mean DBP < 90 mmHg. Measured at 12 months' postrandomisation Secondary outcomes: medication adherence: 'proportion of days of medication covered' (the proportion of participants with ≥ 80% of days covered with BP‐lowering medication from prescribing and dispensing data routinely recorded in the clinical record, pharmacy record and Chronic Dispensing Unit record); self‐reported adherence to medication using a visual analogue scale (score range, 5–10); health status measured with the EuroQol Group 5‐Dimension Self‐Report Questionnaire; self‐reported satisfaction with treatment. Process outcomes: knowledge about hypertension was measured, but not reported in trial paper. Adverse events: protocol stated recording of those which might reasonably occur as a consequence of the trial and adverse events that might be reasonably related to text messaging including hand or finger pain, or involvement in an accident as a result of sending or receiving a text. | |
| Notes | Funding source: trial supported by the Oxford Centre of Excellence in Medical Engineering funded by the Wellcome Trust and the Engineering and Physical Sciences Research Council. Dr Farmer is a senior NIHR investigator, and Drs Farmer and Tarassenko are supported by funding from the NIHR Oxford Biomedical Research Center. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Conflicts of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants are randomised using a secure, remote, web‐based computer schedule within one week of recruitment [...] minimisation procedure [was] overseen by an independent statistician." |
| Allocation concealment (selection bias) | Low risk | Quote: "A software algorithm assigned participants independently of the research team." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants cannot be blinded due to nature of intervention. However, "research staff and clinic staff remain blind to the allocated treatment group." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Researchers and clinicians were not aware of randomization assignment, were trained not to ask patients about the content of messages." |
| Incomplete outcome data (attrition bias) | Low risk | 87% follow‐up rate, no evidence of differential follow‐up, ITT analysis accounting for missing data. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported as planned in protocol (the only outcome reported in protocol that was not reported in trial paper was 'hypertension' knowledge). However, this trial began recruiting in June 2012, but details of the protocol were not registered until December 2013. Therefore, we could not be certain what was planned before the trial commenced. |
| Other bias | Low risk | Funded by charity and research council. |
| Methods | Design: 2‐arm, parallel RCT Setting: employees of work units (places of employment) who had been allocated to have a medical examination at the health management centre of a hospital in Guangzhou, China. Duration of study: 1 year | |
| Participants | Number randomised: 589; intervention: 238; control: 351 Number lost to follow‐up/withdrawn: 162 (intervention: 75; reasons: not stated; control: 87; reasons: not stated) Number analysed: 589, intervention: 238; control: 351 (missing data imputed) Mean age in years (SD): intervention: 58.7 (SD 8.9), control: 61.8 (SD 8.8) Age range: not stated Gender (% women): intervention: 41.6; control: 41.9 Proportion meeting criteria of 'primary prevention': 100%; inclusion criteria included having no known CVD. Proportion prescribed medication for prevention of CVD: not reported. Authors contacted for further information and the data for those prescribed medication, but we received no response. Inclusion criteria: aged 45–75 years, without known CVD, willing to participate in the programme Exclusion criteria: history of mental abnormalities; difficulty in communication, such as reading or answering the questionnaire; unable to understand the aim of this study; currently participating in another clinical trial or had done so within the previous 6 months | |
| Interventions | Intervention: participants in the intervention group received a computerised CVD risk evaluation, follow‐up phone calls and text messages targeting reducing the CVD risk in addition to the usual medical examination. The plan included guidance of healthy lifestyle, improvement targets for risk factors and drug treatment goals for those being treated. Participants also received a 15‐minute face‐to‐face counselling with a trained field health worker when they enrolled to the study. Comparison: participants in the control group received the annual medical examination with a usual medical report. This report included the results of physical examination and the normal values of the indicators. How intervention was developed: authors stated, "we developed a mobile phone‐based intervention program to reduce CVD risk, which was assessed by the Chinese cardiovascular disease risk assessment method." Behaviour change technique(s) employed: 7 in total: problem solving; commitment; feedback on behaviour; instruction on how to perform behaviour; providing information about health consequences; emphasising salience of consequences Personalised intervention: yes; individualised electronic health prescription software (IEHPS) calculated participants' overall risk of CVD in the next 10 years which informed participants individualised intervention plan. Frequency and duration of intervention receipt: frequency of phone calls and text messages depended on participants’ individual 10‐year CVD risk. Phone calls (length 5–8 minutes) ranged from twice a month to once a week, text messages ranged from once a month to once a week. Duration: 1 year | |
| Outcomes | Primary outcomes: LDL‐C, TC, HDL‐C, SBP, DBP. All measured at 1‐year postrandomisation. Medical outcomes were presented for entire sample, which included participants not taking medication for primary prevention of CVD. We have contacted authors requested trial data for those participants taking medication for primary prevention of CVD. Secondary outcomes: none reported. Process outcomes: none recorded. Adverse events: none recorded. | |
| Notes | Funding source: Guangdong Provincial Department of Science and Technology (grant No. 2009A030301003) and the Bureau of Health of Guangzhou Municipality (grant No. 2008‐ZDa‐05) Conflicts of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was done via a computerized procedure." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Neither participants nor investigators were masked to group assignment." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessments by medical students; not stated whether they were blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "27.5% of participants failed to attend the follow‐up. Participants who were lost to follow‐up were more likely to be younger, male, current smokers and have a higher level of TC than those who were included in the follow‐up." |
| Selective reporting (reporting bias) | Unclear risk | Protocol not found. Trial appeared to have been registered after recruitment began in October 2012 (www.chictr.org.cn/hvshowproject.aspx?id=7953). |
| Other bias | Low risk | Funded by government body |
| Methods | Design: 2‐arm, parallel RCT Setting: clinics in metropolitan Toronto, Canada Duration of study: 1 year | |
| Participants | Number randomised: 110; intervention: 55; control: 55 Number lost to follow‐up/withdrawn: 6; intervention group: 2 (reasons: 2 refused BP assessment); control group: 4 (reasons: 3 refused BP assessment; 1 died) Number analysed: 105; intervention group: 54; control group: 51 Mean age in years (SD): intervention group: 62.7 (SD 7.8); control group: 63.1 (SD 9.0) Age range: not stated Gender (% women): intervention group: 51; control group: 38 Proportion meeting criteria of 'primary prevention': intervention group: 79.9%; control group: 78.1%. Paper reported proportion with prior CVD event by CVD event, possible that the same participants had > 1 type of event, therefore percentage stated was minimum estimate of participants meeting criteria of primary prevention. Proportion prescribed medication for prevention of CVD: hypertensive drugs: intervention group: 89.1%; control group: 89.1%; lipid‐lowering drugs: intervention group: 69.1%; control group: 70.9%; aspirin: intervention group: 54.5%; control group: 58.2%. We contacted authors to request data for those prescribed medication, but had no response. Inclusion criteria: aged ≥ 30 years, with diabetes mellitus, with uncontrolled systolic hypertension, defined as a mean daytime SBP of ≥ 130 mmHg on ambulatory BP monitoring. Exclusion criteria: those with severe or end‐stage organ disease (liver, kidney, heart and lung), history of diabetic ketoacidosis, any illness with expected survival < 1 year, severe cognitive impairment, mental illness or disability, clinically significant cardiac arrhythmia, symptomatic orthostatic hypotension, or were pregnant, unsuitable for participation in the opinion of their primary care physician or not fluent in English. | |
| Interventions | Intervention: participants received custom software application running on a BlackBerry smartphone (Research In Motion, Inc, Waterloo, ON, Canada) that was paired with a Bluetooth‐enabled home BP monitoring device. BP readings were automatically transmitted by the smartphone to application servers, which processed the information for trends and applied decisions rules. The reporting and alerting component of the system sent a self‐care message to the screen of the participant's smartphone immediately after each reading. Messages related to the control of hypertension were based on care paths defined by running means of transmitted readings. On the day before the clinic visit to their physician, participants called a dedicated telephone number to initiate the automated process to fax a 1‐page participant summary report to their physician. Self‐care support participants were taught how to use the telemonitoring system, review past readings on their smartphone and the study‐specific website (these activities were optional), and generate a 1‐page participant summary report. They were instructed to take their smartphone to all doctor visits. Comparison: participants in both groups were taught how to measure their BP correctly, instructed to measure their BP 2 days per week twice in the morning and twice in the evening, provided with a validated home BP monitoring device with appropriate‐sized upper arm cuff, and given a booklet with detailed information on the self‐measurement of BP, treatment of hypertension and goals of therapy. Their primary care physician was given an outline of the study's objectives and BP treatment goal, asked to provide relevant medical information and given a copy of the 24‐hour ambulatory BP monitoring report. In both groups, treatment decisions, including medication adjustments and changes in lifestyle, were made by the participant's primary care physician. The control group did not received feedback via smartphone. How intervention was developed: system developed using an iterative process based on feedback from users. A pilot study was undertaken to assess the system's effectiveness in improving BP control in people with diabetes with uncontrolled hypertension, its acceptability to users and the reliability of home BP measurements. Behaviour change technique(s) employed: 3 in total: feedback on behaviour, self‐monitoring, prompts. Personalised intervention: information sent via smartphone was personalised in that it was based on participants' own BP readings. Frequency and duration of intervention receipt: participants were instructed to measure their BP 2 days per week twice in the morning and twice in the evening, and a self‐care message was sent to the participant's smartphone immediately after each reading. Duration: 1 year. | |
| Outcomes | Primary outcomes: mean ambulatory SBP and DBP; proportion achieving guideline recommended target of BP < 130/80 mmHg. Measured at 1 year' postrandomisation. The medical outcomes are presented for entire sample, which included participants not taking medication for primary prevention of CVD. We contacted authors requesting trial data for those participants taking medication for primary prevention of CVD, but had no response. Secondary outcomes: none reported. Process outcomes: adherence rate with home BP measurement schedule (% taking a minimum of 8 readings per week). Adverse events: none recorded. | |
| Notes | Funding source: the Heart and Stroke Foundation of Ontario (ESA 5970) was the sole source of funding for this project and was not involved in any aspect of the study. Conflicts of interest: JAC received funding from Research In Motion, Inc. (makers of the Blackberry mobile telephones) through the National Science and Engineering Research Council Strategic Network Grant Program. PGR received reimbursement of expenses from Research In Motion, Inc., to attend a healthcare advisory meeting. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Group allocation schedule was based on blocks of 4 and 6 patients randomly arranged and administered by a person not directly involved in the study." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants cannot be blinded due to nature of intervention. Unclear whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | > 90% follow‐up, no evidence of differential follow‐up |
| Selective reporting (reporting bias) | Unclear risk | According to trial registry entry (clinicaltrials.gov/ct2/show/NCT00717665), the trial was registered after the first participant was randomised. |
| Other bias | Low risk | Funded by charitable body |
| Methods | Design: 2‐arm, parallel RCT Setting: primary care clinics in 3 health districts of 3 Spanish autonomous communities: Castile‐La Mancha (Albacete), Aragon (Zaragoza) and Galicia (Vigo), Spain Duration of study: 24 months | |
| Participants | Number randomised: 358; intervention group: 179; control group: 179 Number lost to follow‐up/withdrawn: 54 (intervention group: 24 (reasons: 14 withdrew consent; 2 discontinued due to change of residence; 2 discontinued due to disease; 1 discontinued due to other reasons; 5 protocol violation); control group: 30 (reasons: 17 withdrew consent; 1 discontinued due to change of residence; 3 discontinued due to disease; 3 discontinued due to other reasons; 6 protocol violation) Number analysed: 304; intervention group: 155; control group: 149 Mean age in years (SD): intervention group: 58.9 (SD 10.4); control group: 59.3 (SD 8.4) Age range: not stated Gender (% women): intervention group: 56.1; control group: 53.7 Proportion meeting criteria of 'primary prevention': total: 93.1%; intervention group: 91.0%; control group: 95.3% Proportion prescribed medication for prevention of CVD: only statin use stated; total 68.1%; intervention group: 64.5%; control group: 71.8%). We contacted authors requesting trial data for those participants taking medication for primary prevention of CVD, but had no response. Inclusion criteria: aged ≥18 years, previously diagnosed with defined hypercholesterolaemia (TC ≥ 250 mg/dL) who were receiving standard treatment (drug‐based or not) and attending the participating centres. Exclusion criteria: unable to undergo follow‐up during the intervention (due to illiteracy or lack of a mobile telephone), had a physical disability impeding participation, or had a severe organic or psychiatric chronic disease precluding follow‐up. | |
| Interventions | Intervention: participants received the following: written information on the disease and its treatment (provided at each visit); mobile telephone text messages with summaries of recommendations, reminders of dates of next appointments and notifications of new appointments if any previous ones were missed (during between‐visit periods); and self‐completed registration cards on adherence to recommendations (during the entire follow‐up). Intervention group also received the standard recommendations of the European clinical practice guidelines for treatment of hypercholesterolaemia and cardiovascular risk. The intervention targeted lifestyle modifications, including healthy diet and physical activity, alongside medication adherence for those prescribed CVD medication. Comparison: participants received the standard recommendations of the European clinical practice guidelines for treatment of hypercholesterolaemia and CVR. How intervention was developed: not stated Behaviour change technique(s) employed: 6 in total: feedback on behaviour; self‐monitoring; instruction on how to perform behaviour; providing information about health consequences; emphasising salience of consequences; prompts Personalised intervention: information provided not personalised, but reminders of dates of next appointment indicates some personalisation. Frequency and duration of intervention receipt: the disease treatment reminders were sent every 15 days, whereas the attendance reminders for upcoming or missed appointments were sent according to the follow‐up date. Intervention duration: 24 months (although not clear if this relates to all components of the intervention). | |
| Outcomes | Primary outcomes: LDL‐C; TC; HDL‐C; SBP; DBP. All measured 2 years' postrandomisation. The medical outcomes are presented for entire sample, which includes participants not taking medication for primary prevention of CVD. We contacted authors requesting trial data for those participants taking medication for primary prevention of CVD, but had no response. Cardiovascular events in the observation period stated in protocol, but not reported in trial results. Secondary outcomes: self‐report adherence to lipid‐lowering therapy (measured using the Morisky‐Green Test) at 2 years' postrandomisation Process outcomes: satisfaction with intervention (measured using a Likert scale satisfaction questionnaire) at 2 years' postrandomisation Adverse events: adverse effects of statins; intervention‐related adverse effects | |
| Notes | Funding source: funding from the Instituto de Salud Carlos III and the Health Research Project Subprogram of the European Regional Development Fund (PI12/01955), resolution 20 December 2012 Conflicts of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participant randomization was centrally performed according to health care region (Efron randomization) by a researcher who was not involved in the interviews or analysis." |
| Allocation concealment (selection bias) | Unclear risk | Allocation of area was concealed; however, once areas were allocated, participants were allocated according to their area. It is not clear whether recruiting staff may have known to which area the participants belonged and therefore to which group they would be randomised. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants cannot be blinded due to nature of intervention. However, report states "results were evaluated in a blinded manner." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated whether outcome measurements were taken by blinded personnel. |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up rate of 85% and no evidence of differential follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported as planned in protocol, with the exception of cardiovascular events occurring in the trial period which were stated in protocol but not included in trial report. |
| Other bias | Low risk | Funding from government body |
BP: blood pressure; CVD: cardiovascular disease; DBP: diastolic blood pressure; HDL‐C: high‐density lipoprotein cholesterol; ITT: intention to treat; LDL‐C: low‐density lipoprotein cholesterol; NIHR: National Institute for Health Research; RCT: randomised controlled trial; SBP: systolic blood pressure; SD: standard deviation; SMS: short messaging service; TC: total cholesterol.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No mobile phone specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| Follow‐up < 12 months | |
| No mobile phone‐specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| Kidney transplant recipient population | |
| No mobile phone‐specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| Not a randomised controlled trial | |
| No mobile phone‐specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| No mobile phone‐specific intervention delivery | |
| Follow‐up < 12 months | |
| No mobile phone‐specific intervention delivery |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Rationale and design of the Study of a Tele‐pharmacy Intervention for Chronic diseases to Improve Treatment adherence (STIC2IT): a cluster randomized pragmatic trial |
| Methods | Design: 2‐arm, cluster RCT Setting: Harvard Vanguard Medical Associates (medical practice), MA, USA |
| Participants | Expected: 4076 Inclusion criteria: aged 18–85 years; filled and poorly adherent (defined as a PDC < 80%) to medication for hyperlipidaemia, hypertension or diabetes; suboptimal mean adherence to all of the qualifying medications that a participant has filled (defined as combined (mean of means) PDC < 80%); for people with hypertension or diabetes, poor or worsening disease control (according to relevant clinical targets) Exclusion criteria: < 6 months of continuous enrolment in the health plan; no available contact information |
| Interventions | Intervention: brief telephonic consultation with a clinical pharmacist using behavioural interviewing techniques tailored to participant's level of health activation and progress reports of medication taking and disease control. Based on the barriers identified during the initial telephone consultation, participants will be offered more intensive support including reminder and motivational text messages, video visits and pill boxes. Control group: usual care |
| Outcomes | Primary outcome: medication adherence at 12 months (mean PDC for medications to treat eligible conditions) Secondary outcomes: disease control at 12 months (proportion of participants achieving good disease control for all eligible conditions); disease control at 12 months (proportion of participants achieving good disease control for ≥ 1 eligible condition); healthcare utilisation at 12 months (rates of resource utilisation) |
| Starting date | August 2015 |
| Contact information | Niteesh K Choudhry, MD, PhD; Niteesh K Choudhry, MD, PhD, Associate Professor, Harvard Medical School, Brigham and Women's Hospital |
| Notes | ClinicalTrials.gov, NCT02512276 |
| Trial name or title | Telemonitoring and/or self‐monitoring of blood pressure in hypertension (TASMINH4): protocol for a randomised controlled trial |
| Methods | Design: 3‐arm, parallel RCT Setting: UK. 144 practices recruited from the following NIHR Clinical Research Networks: Thames Valley, West Midlands, East of England, West of England, Kent Surrey and Sussex, North West Coast, North West London. |
| Participants | Expected: 1010 Inclusion criteria: willing and able to give informed consent for participation in the trial; men or women, aged ≥ 35 years; on practice hypertension register, not already taking > 3 antihypertensive agents and above clinic target BP (i.e. = 140/90 mmHg) at baseline (mean of 2nd/3rd readings); stable dose of current antihypertensive medication for ≥ 4 weeks prior to trial entry; in the Investigator's opinion, is able and willing to comply with all trial requirements or has a carer able to help sufficiently (e.g. in the case of physical issues with self‐monitoring); willing to allow his or her GP to be notified of participation in the trial Exclusion criteria: BP below target at baseline (i.e. < 140/90 mmHg on clinic measurement at baseline visit); already taking > 3 antihypertensive agents; orthostatic hypotension: > 20 mmHg SBP drop after standing for 1 minute; diagnosed atrial fibrillation; unwilling to self‐monitor; BP managed outside of primary care (including secondary hypertension); unable to provide consent; dementia or score > 10 on the short orientation memory concentration test (and with no carer support); women pregnant, lactating or planning pregnancy during the course of the trial; partner or spouse of an individual already randomised in the trial; CKD Grade 4 or worse, any grade of CKD with proteinuria; any other significant disease or disorder which, in the opinion of the Investigator, may either put the participants at risk because of participation in the trial, or may influence the result of the trial, or the participants ability to participate in the trial (e.g. terminal illness, house bound and unable to attend baseline and follow‐up clinics); participants who have participated in another research trial involving an antihypertensive medication in the past 4 weeks. |
| Interventions | Intervention:Group 1: self‐monitoring alone: participants will monitor their BP twice each morning and evening (i.e. 4 times in all) for the 1st week of each month. A paper record sheet will be used for communication between paticipant and healthcare professionals in the self‐monitoring alone group. GPs and nurses will be advised to calculate the mean self‐monitored BP and to use this to titrate antihypertensive medication. Group 2: telemonitoring: the frequency of self‐monitoring will be identical to the self‐monitoring alone group but BP readings will be transmitted to a secure centralised database from which the GP/nurse can review the records. Readings will be transmitted by free SMS text message. A mean BP will be automatically calculated. High or low readings will trigger alerts to paticipant to contact their surgery for a BP check. GPs and nurses will be advised to use the mean self‐monitored BP to titrate antihypertensive medication. Control: usual care: usual care guided by clinic BP measured by the GP/practice nurse without further instruction. |
| Outcomes | Primary outcome: SBP (mean of 2nd and 3rd BP readings) at 12 months Secondary outcomes: SBP and DBP at 6 and 12 months; costs, health sector resource use, and acceptability at 12 months; MARS adherence questionnaires and prescribing data at 12 months; questionnaire data on lifestyle factors at 12 months; comparison between trial outcome data and that from clinical databases at 12 months |
| Starting date | 1 September 2014 |
| Contact information | Richard McManus: [email protected] Nuffield Department of Primary Care, Oxford University, Oxford, UK |
| Notes | Trial identifier: ISRCTN 83571366 |
| Trial name or title | Educational intervention to improve effectiveness in treatment and control of patients with high cardiovascular risk in low‐resource settings in Argentina: study protocol of a cluster randomised controlled trial |
| Methods | Design: 2‐arm, cluster RCT Setting: 10 public PCCs (low‐resource settings) in Argentina |
| Participants | Expected: 357 Inclusion criteria (for PCCs): clinic is affiliated with the Remediar programme; clinic located in a poor urban area according to 2010 census data; clinic has ≥ 800 outpatient adult visits each month (to ensure recruitment of enough participants); physician visits and statins are available free‐of‐charge to participants at the point of care; minimum distance between PCCs is 10 km (different catchment area) and they do not share health professionals (to minimise intervention bias); good performance of the PCCs (and their pharmacy) according to the reports of Remediar programme. Inclusion criteria (for participants): aged ≥ 40 years and < 75 years who have received primary care at the participating PCCs with ≥ 1 of the following criteria: arteriosclerotic CVD (defined as acute coronary syndrome; history of myocardial infarction, stable or unstable angina, coronary revascularisation, stroke or transient ischaemic attack presumed to be of atherosclerotic origin or revascularisation); or high CVD risk according to the WHO charts adapted by the National MoH (estimated 10‐year CVD risk ≥ 20%); or LDL‐C level ≥190 mg/dL; or type 2 diabetes Exclusion criteria: statin treatment; pregnant women; bed‐bound people; unable to give informed consent; history of end‐stage chronic kidney disease treated with dialysis, HIV/AIDS, alcohol or drug abuse, or active tuberculosis |
| Interventions | Intervention: multi‐faceted educational intervention targeting physicians and pharmacist assistants to improve detection, treatment and control of hypercholesterolaemia among uninsured participants with moderate‐high cardiovascular risk in Argentina. Physicians belonging to the PCC randomised to the intervention group receive a 3‐component intervention: education workshop, educational outreach visits and a mHealth application uploaded to their smartphones. In addition, 2 intervention support tools are used at the intervention clinics: 1. a web‐based platform that is tailored to send SMS messages for lifestyle modification, and prompts and reminders for clinic appointments are used to improve medication adherence for participants; 2. on‐site training to pharmacist assistants at the first educational outreach visit is given by physician trainers focused on counselling to improve medication adherence among participants initiating statin therapy and at each participant visit to the clinic to refill drug prescriptions. Control: usual care |
| Outcomes | Primary outcome: cholesterol level (net change in LDL‐C levels from baseline to month 12 between intervention and usual care groups among all study participants) Secondary outcomes: global cardiovascular risk at 1 year (net change in 10‐year‐CVD Framingham risk score before and after the implementation of the programme); clinical practice guidelines compliance at 1 year (proportion of participants with high CVD risk who are on statins, and are receiving an appropriate dose according to the clinical practice guideline); cholesterol reduction at 1 year (proportion of participants with moderate‐high CVD risk who have reduced their LDL‐C by 30%, and by 50%); treatment compliance at 1 year (level of treatment adherence evaluated through questionnaire; costs of the intervention (cost‐effectiveness of the intervention programme) |
| Starting date | April 2015 |
| Contact information | Adolfo Rubinstein, MD, MSc, PhD Institute for Clinical Effectiveness and Health Policy; [email protected] |
| Notes | ClinicalTrials.gov, NCT02380911 |
| Trial name or title | mWellcare trial: a multi‐centre, cluster randomised, 12‐month, controlled trial to compare the effectiveness of mWellcare, an mHealth system for an integrated management of patients with hypertension and diabetes, versus enhanced usual care in India |
| Methods | Design: 2‐arm, cluster RCT Setting: India (1 southern state and 1 northern state), 40 community health centres |
| Participants | Recruited: 3702 Inclusion criteria: participants aged ≥ 30 years intending to reside in the catchment area of community health centres for at least next 12 months. Participants were included if they were diagnosed case of hypertension with BP measuring ≥140/90 mmHg or type 2 diabetes mellitus with fasting blood sugar ≥ 140 mg/dL or postprandial blood sugar ≥ 200 mg/dL and if they provided informed consent Exclusion criteria: pregnant women, type 1 diabetes, requiring immediate referral to tertiary care due to accelerated hypertension or diabetic complications, learning difficulties or vision or hearing impairments (or a combination of these), malignancy or life‐threatening disease with death probable in 4 years and not residing in the catchment area of the community health centre |
| Interventions | Intervention: nurses and physicians will provide treatment and follow‐up using mWellcare. mWellcare system is an Android‐based mobile application designed to generate algorithm‐based clinical management prompts for treating hypertension and diabetes and also capable of storing health records. The system also sends SMS reminders for adherence to medication and follow‐up visits to participants. Control: enhanced care arm. Nurse and physicians are provided 'refresher' training on the clinical management guidelines for hypertension and diabetes. Charts on management of these conditions are provided to the facilities for prominent display at the outpatient department. Physicians in the enhanced care arm provide the management plan based on their assessment of clinical parameters of the participants. Nurse provides lifestyle advice brochure (in local language) and explains the same to each participant. |
| Outcomes | Primary outcomes: difference in mean change (from baseline to 1 year) in SBP; difference in mean change (from baseline to 1 year) HbA1c Secondary outcomes: difference in mean change (from baseline to 1 year) of fasting plasma glucose, TC and predicted 10‐year risk of CVD using recalibrated Framingham risk score; differences in risk factors such as depression/anxiety, smoking behaviour, BMI and alcohol uses; comparison of costs associated with delivering the mWellcare intervention arm with respect to enhanced care |
| Starting date | April 2016 |
| Contact information | Dr Dorairaj Prabhakaran; [email protected] |
| Notes | Clinicaltrial.gov, NCT02480062 |
| Trial name or title | A randomised controlled trial of a consumer‐focused e‐health strategy for cardiovascular risk management in primary care: the Consumer Navigation of Electronic Cardiovascular Tools (CONNECT) |
| Methods | Design: 2‐arm, parallel RCT Setting: 65 Australian General Practices and Aboriginal Community Controlled Health Services |
| Participants | Expected: 2000 Inclusion criteria: consenting adults (> 18 years) with access to the Internet at least once a month via mobile phone, tablet or computer who are at moderate‐to‐high risk of a CVD event will be included. Moderate‐to‐high CVD risk is defined as any of the following: 1. 5‐year CVD risk ≥ 10% using the Framingham risk equation; 2. a clinically high‐risk condition (Aboriginal/Torres Strait Islander and aged > 75 years, diabetes and age > 60 years, diabetes and albuminuria, epidermal growth factor receptor 7.5 mmol); 3. an established CVD diagnosis (ischaemic heart disease, stroke/transient ischaemic attack and peripheral vascular disease) Exclusion criteria: severe intellectual disability or if they have insufficient English knowledge to provide written informed consent. |
| Interventions | Intervention: CONNECT programme, a consumer‐focused e‐health strategy aimed at assisting with the management and prevention of CVD in addition to usual care. Programme components focus on cardiovascular risk assessment, medication adherence, lifestyle change and seamless patient‐provider communication. Control group: usual healthcare. No access to the portal; however, at the end of study, all participants (control and intervention) will be offered portal access for a maximum of 12 months |
| Outcomes | Primary outcome: proportion of participants meeting the Australian guideline BP and lipid targets; BP 140/90 mmHg for all except those with CVD, diabetes or albuminuria for whom the target BP is 130/80 mmHg. Secondary outcomes: proportion meeting guideline‐recommended BP and LDL‐C targets separately; difference in mean SBP and DBP at the end of study; difference in mean cholesterol levels at end of study (TC, LDL‐C and HDL‐C); difference in mean BMI and waist circumference at the end of study; difference in health literacy scores (HLQ51 and the eHEALS52) at end of study; cardiovascular and renal events, new onset diabetes ‐ self report and confirmed with medical records; physical activity ‐ WHO Global Physical Activity Questionnaire; point abstinence in smoking (≤ 5 cigarettes in the previous 7 days or recent smoking according to assessment using carbon monoxide meter); fruit and vegetable intake, fish, salt and saturated fat intake ‐ self‐report portions consumed in 7 days prior and compared with published guidelines recommendations; cardioprotective medication adherence ‐ self‐report and verified by medical record and pharmaceutical benefits scheme data; all‐cause mortality ‐ medical record; hospital readmissions ‐ self‐report and verified by medical record; health‐related quality of life ‐ EQ5D (version 5L with Australian standardised weights) |
| Starting date | 17 October 2014 |
| Contact information | Dr Julie Redfern; [email protected] |
| Notes | Clinical Trials registration number ACTRN12613000715774. |
| Trial name or title | A coordinated PCP‐Cardiologist Telemedicine Model (PCTM) in China’s community hypertension care: study protocol for a randomized controlled trial |
| Methods | Design: 3‐arm, parallel RCT Setting: 4 CHCsin XuHui District in Shanghai, China |
| Participants | Expected: 330 Inclusion criteria: aged ≥ 21 years; clinical diagnosis of hypertension with uncontrolled BP in the previous 3 months, currently taking or about to take antihypertensive medications; received high school or above level of education; active user of smartphone (Android or Apple) and mobile Apps; mean of 3 BP measurements during the screening visit at the CHC ≥ 140/90 mmHg, or ≥ 130/80 mmHg if the person has diabetes or renal diseases; being able to give informed consent Exclusion criteria: acute coronary syndrome; heart failure; cardiac arrhythmia; stroke within the past 3 months; renal failure; cancer; dementia, severe or acute psychiatric illness; pregnancy or intention to be pregnant in the next 18 months; hospitalisation within 3 months; participation in another clinical trial; arm circumference > 32 cm that may affect the accuracy of BP measurement due to cuff size limit of the telemonitors and unwillingness to comply with the 12‐month intervention duration |
| Interventions | Intervention:Group 1: 'Self‐management' (BP telemonitor and App‐based self‐management supports; patient proficiency training) Group 2: 'PCTM intervention' (BP telemonitor and App‐based self‐management supports; patient proficiency training; PCP and cardiologist training of using Web‐based analytics; proactive and interactive care by PCPs and cardiologists) Control group: management by PCPs at the registered CHCs as usual |
| Outcomes | Primary outcome: changes in mean SBP from baseline to 12 months measured using the BP telemonitor (Bliss BL928). The 12‐month BP readings will be determined by taking the mean of 3 BP measurements at the follow‐up visit to the CHC. Secondary outcomes: changes in mean DBP from baseline to 12 months; hypertension control rate from baseline to 6 and 12 months; hypertension control rate defined as BP < 140/90 mmHg or < 130/80 mmHg (people with diabetes or renal diseases) following the national guidelines; changes in measures related to hypertension complications (HbA1c, BMI and lipid levels) from baseline to 6 and 12 months; antihypertensive medication adherence at baseline and 12 months assessed by self‐report, 8‐item Morisky Medication Adherence Scale modified to focus on BP drugs |
| Starting date | September 2016 |
| Contact information | Contact: Lei Xu, Master; +86‐21‐32260806; [email protected] Contact: Kai Liu, Doctor; +86‐18918656956; [email protected] |
| Notes | ClinicalTrials.gov, NCT02919033 |
BMI: body mass index; BP: blood pressure; CHC: community healthcare centre; CKD: Chronic Kidney Disease; CVD: cardiovascular disease; DBP: diastolic blood pressure; GP: general practitioner; HbA1c: glycated haemoglobin; HDL‐C: high‐density lipoprotein cholesterol; LDL‐C: low‐density lipoprotein cholesterol; MARS: Medication Adherence Report Scale; mHealth: mobile health; MoH: Minister of Health; NIHR: National Institute for Health Research; PCC: primary care centre; PCP: primary care physician; PDC: proportion of days covered; RCT: randomised controlled trial; SBP: systolic blood pressure; SMS: short messaging service; TC: total cholesterol; WHO: World Health Organization.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in low‐density lipoprotein cholesterol (mg/dL) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Mobile phone intervention versus control, Outcome 1 Change in low‐density lipoprotein cholesterol (mg/dL). | ||||
| 2 Change in total cholesterol (mg/dL) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Mobile phone intervention versus control, Outcome 2 Change in total cholesterol (mg/dL). | ||||
| 3 Change in high‐density lipoprotein cholesterol (mg/dL) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Mobile phone intervention versus control, Outcome 3 Change in high‐density lipoprotein cholesterol (mg/dL). | ||||
| 4 Change in systolic blood pressure (mmHg) Show forest plot | 4 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Mobile phone intervention versus control, Outcome 4 Change in systolic blood pressure (mmHg). | ||||
| 5 Change in diastolic blood pressure (mmHg) Show forest plot | 3 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Mobile phone intervention versus control, Outcome 5 Change in diastolic blood pressure (mmHg). | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
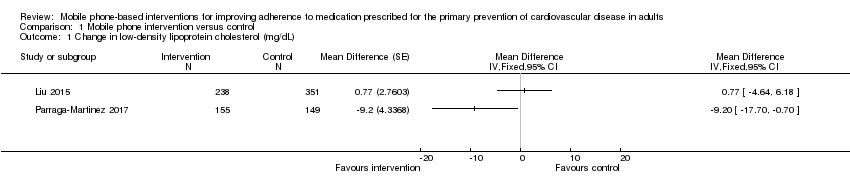
Comparison 1 Mobile phone intervention versus control, Outcome 1 Change in low‐density lipoprotein cholesterol (mg/dL).

Comparison 1 Mobile phone intervention versus control, Outcome 2 Change in total cholesterol (mg/dL).

Comparison 1 Mobile phone intervention versus control, Outcome 3 Change in high‐density lipoprotein cholesterol (mg/dL).

Comparison 1 Mobile phone intervention versus control, Outcome 4 Change in systolic blood pressure (mmHg).

Comparison 1 Mobile phone intervention versus control, Outcome 5 Change in diastolic blood pressure (mmHg).
| Mobile phone interventions compared to usual care for improving adherence to medication prescribed for primary prevention of cardiovascular disease | |||
| Patient or population: people prescribed medication for primary prevention of cardiovascular disease | |||
| Outcomes | Impact | № of participants | Quality of the evidence |
| Cholesterol (low‐density lipoprotein) | 1 study found evidence of a small beneficial intervention effect on reducing LDL‐C (–9.20 mg/dL), and 1 study found a very small increase in LDL‐C (0.77 mg/dL) with wide confidence intervals that included no effect. | 893 | ⊕⊕⊝⊝ |
| Systolic blood pressure | 3 of the 4 studies found lower systolic blood pressure with mobile phone interventions, but the size of effect varied. 2 studies showed moderate and large reductions in systolic blood pressure (–7.10 and –12.45 mmHg). 1 multi‐arm trial found small reductions with information‐only text messages (–2.1) and interactive text messaging (–1.6 mmHg) arms. 1 study found a slight increase in blood pressure (0.83 mmHg) but with wide confidence intervals that included no effect. | 2194 | ⊕⊕⊝⊝ |
| Diastolic blood pressure | 2 of 3 studies found lower diastolic blood pressure with mobile phone interventions, but the size of the effect varied. 2 studies showed large and small reductions in diastolic blood pressure (–12.23 and –3.90 mmHg), and 1 study found a slight increase in diastolic blood pressure (1.64 mmHg) but with wide confidence intervals that included no effect. | 998 | ⊕⊕⊝⊝ Lowa,b |
| Combined CVD events | Not reported | (0 studies) | — |
| Adverse events | 1 study reported that there were 0 adverse events attributable to the intervention. 1 study report that there was no difference between groups in experience adverse effects of statins, and that 0 participants reported intervention‐related adverse events. | 1500 | ⊕⊕⊝⊝ |
| Cognitive outcome: satisfaction with treatment | 1 study measured satisfaction with treatment, and found no evidence of a difference between intervention and control arms. | 1190 | ⊕⊕⊝⊝ |
| LDL‐C: low‐density lipoprotein cholesterol; RCT: randomised controlled trial. | |||
| GRADE Working Group grades of evidence | |||
| aDowngraded one level for inconsistency: trial results included large variations in the degree to which the outcome was affected. bDowngraded one level for risk of bias: all trials at unclear risk of bias on multiple domains. cDowngraded one level for imprecision: very low number of events. dDowngraded one level for indirectness: based on a single trial conducted in a single setting (public sector clinic in Cape Town, South Africa). eDowngraded one level for risk of bias: trial at unclear risk of bias on two domains. | |||
| Trial | Outcome measure | Comparison | Intervention | Number (intervention) | Control | Number (Control) | Narrative results |
| (1‐year follow‐up) | Proportion of days covered by dispensed medicine | Information‐only SMS vs control | 83.3% (95% CI 69.3 to 91.7) | 457 | 79.2% (95% CI 64.6 to 91.4) | 458 | Median difference 5.2, quartiles 1‐3: 1.5 to 8.9; P = 0.006 |
| Interactive SMS vs control | 83.3% (95% CI 66.7 to 91.7) | 457 | 79.2% (95% CI 64.6 to 91.4) | 458 | Median difference 3.8; quartiles 1‐3: 0.03 to 7.6; P = 0.048 | ||
| Proportion of participants with proportion of days covered ≥ 80% | Information‐only SMS vs control | 63% | 457 | 49.4% | 458 | Adjusted odds ratio 1.86, 95% CI 1.39 to 2.49; P < 0.001 | |
| Interactive SMS vs control | 60% | 457 | 49.4% | 458 | Adjusted odds ratio 1.60, 95% CI 1.20 to 2.16; P = 0.002 | ||
| Self‐reported medication adherence (score range 5–10) | Information‐only SMS vs control | 10 (quartiles 1‐3: 9 to 10) | 457 | 10 (quartiles 1‐3: 9 to 10) | 458 | Median difference 0.04, 95% CI –0.1 to 0.2; P = 0.70 | |
| Interactive SMS vs control | 10 (quartiles 1‐3: 9 to 10) | 457 | 10 (quartiles 1‐3: 9 to 10) | 458 | Median difference 0.02, 95% CI –0.2 to 0.2; P = 0.80 | ||
| (2‐year follow‐up) | Proportion adherent according to self‐reported medication adherence (measured using 'adapted Morisky‐Green test') | — | 77.2% | Disaggregated not reported | 64.1% | Disaggregated not reported | P = 0.029 220 in total, not reported by group |
| CI: confidence interval; SMS: short messaging service. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in low‐density lipoprotein cholesterol (mg/dL) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Change in total cholesterol (mg/dL) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 Change in high‐density lipoprotein cholesterol (mg/dL) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 Change in systolic blood pressure (mmHg) Show forest plot | 4 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Change in diastolic blood pressure (mmHg) Show forest plot | 3 | Mean Difference (Fixed, 95% CI) | Totals not selected | |

