Intervensi berdasarkan telefon bimbit bagi meningkatkan kepatuhan ubat yang dipreskripsikan untuk pencegahan primer penyakit kardiovaskular di kalangan orang dewasa.
Abstract
Background
Cardiovascular disease (CVD) is a major cause of disability and mortality globally. Premature fatal and non‐fatal CVD is considered to be largely preventable through the control of risk factors via lifestyle modifications and preventive medication. Lipid‐lowering and antihypertensive drug therapies for primary prevention are cost‐effective in reducing CVD morbidity and mortality among high‐risk people and are recommended by international guidelines. However, adherence to medication prescribed for the prevention of CVD can be poor. Approximately 9% of CVD cases in the EU are attributed to poor adherence to vascular medications. Low‐cost, scalable interventions to improve adherence to medications for the primary prevention of CVD have potential to reduce morbidity, mortality and healthcare costs associated with CVD.
Objectives
To establish the effectiveness of interventions delivered by mobile phone to improve adherence to medication prescribed for the primary prevention of CVD in adults.
Search methods
We searched CENTRAL, MEDLINE, Embase, and two other databases on 21 June 2017 and two clinical trial registries on 14 July 2017. We searched reference lists of relevant papers. We applied no language or date restrictions.
Selection criteria
We included randomised controlled trials investigating interventions delivered wholly or partly by mobile phones to improve adherence to cardiovascular medications prescribed for the primary prevention of CVD. We only included trials with a minimum of one‐year follow‐up in order that the outcome measures related to longer‐term, sustained medication adherence behaviours and outcomes. Eligible comparators were usual care or control groups receiving no mobile phone‐delivered component of the intervention.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. We contacted study authors for disaggregated data when trials included a subset of eligible participants.
Main results
We included four trials with 2429 randomised participants. Participants were recruited from community‐based primary care or outpatient clinics in high‐income (Canada, Spain) and upper‐ to middle‐income countries (South Africa, China). The interventions received varied widely; one trial evaluated an intervention focused on blood pressure medication adherence delivered solely through short messaging service (SMS), and one intervention involved blood pressure monitoring combined with feedback delivered via smartphone. Two trials involved interventions which targeted a combination of lifestyle modifications, alongside CVD medication adherence, one of which was delivered through text messages, written information pamphlets and self‐completion cards for participants, and the other through a multi‐component intervention comprising of text messages, a computerised CVD risk evaluation and face‐to‐face counselling. Due to heterogeneity in the nature and delivery of the interventions, we did not conduct a meta‐analysis, and therefore reported results narratively.
We judged the body of evidence for the effect of mobile phone‐based interventions on objective outcomes (blood pressure and cholesterol) of low quality due to all included trials being at high risk of bias, and inconsistency in outcome effects. Of two trials targeting medication adherence alongside other lifestyle modifications, one reported a small beneficial intervention effect in reducing low‐density lipoprotein cholesterol (mean difference (MD) –9.2 mg/dL, 95% confidence interval (CI) –17.70 to –0.70; 304 participants), and the other found no benefit (MD 0.77 mg/dL, 95% CI –4.64 to 6.18; 589 participants). One trial (1372 participants) of a text messaging‐based intervention targeting adherence showed a small reduction in systolic blood pressure (SBP) for the intervention arm which delivered information‐only text messages (MD –2.2 mmHg, 95% CI –4.4 to –0.04), but uncertain evidence of benefit for the second intervention arm that provided additional interactivity (MD –1.6 mmHg, 95% CI –3.7 to 0.5). One study examined the effect of blood pressure monitoring combined with smartphone messaging, and reported moderate intervention benefits on SBP and diastolic blood pressure (DBP) (SBP: MD –7.10 mmHg, 95% CI –11.61 to –2.59; DBP: –3.90 mmHg, 95% CI –6.45 to –1.35; 105 participants). There was mixed evidence from trials targeting medication adherence alongside lifestyle advice using multi‐component interventions. One trial found large benefits for SBP and DBP (SBP: MD –12.45 mmHg, 95% CI –15.02 to –9.88; DBP: MD –12.23 mmHg, 95% CI –14.03 to –10.43; 589 participants), whereas the other trial demonstrated no beneficial effects on SBP or DBP (SBP: MD 0.83 mmHg, 95% CI –2.67 to 4.33; DBP: MD 1.64 mmHg, 95% CI –0.55 to 3.83; 304 participants).
Two trials reported on adverse events and provided low‐quality evidence that the interventions did not cause harm. One study provided low‐quality evidence that there was no intervention effect on reported satisfaction with treatment.
Two trials were conducted in high‐income countries, and two in upper‐ to middle‐income countries. The interventions evaluated employed between three and 16 behaviour change techniques according to coding using Michie's taxonomic method. Two trials evaluated interventions that involved potential users in their development.
Authors' conclusions
There is low‐quality evidence relating to the effects of mobile phone‐delivered interventions to increase adherence to medication prescribed for the primary prevention of CVD; some trials reported small benefits while others found no effect. There is low‐quality evidence that these interventions do not result in harm. On the basis of this review, there is currently uncertainty around the effectiveness of these interventions. We identified six ongoing trials being conducted in a range of contexts including low‐income settings with potential to generate more precise estimates of the effect of primary prevention medication adherence interventions delivered by mobile phone.
PICO
Ringkasan bahasa mudah
Intervensi melalui telefon bimbit untuk membantu mematuhi pengambilan ubat untuk mencegah penyakit kardiovaskular.
Soalan ulasan
Kami mengkaji semula bukti mengenai kesan intervensi yang disampaikan melalui telefon bimbit untuk membantu orang ramai mengambil ubat mereka untuk mencegah penyakit kardiovaskular (contohnya, sakit jantung dan strok). Kami menemui empat kajian yang melibatkan 2429 peserta.
Latar belakang
Sekitar 17.6 juta orang meninggal dunia disebabkan oleh penyakit kardiovaskular setiap tahun. Ubat‐ubatan boleh mencegah penyakit kardiovaskular; tetapi, ramai yang diberi ubat‐ubat ini tidak sentiasa atau konsisten mengambil ubat seperti yang dicadangkan. Ini bermakna ubat tidak akan berfungsi seperti yang sepatutnya untuk mencegah penyakit kardiovaskular. Intervensi yang disampaikan melalui telefon bimbit, contohnya prom dengan menghantar mesej, mungkin adalah cara kos rendah untuk membantu orang ramai mengambil ubat mereka seperti yang dicadangkan.
Ciri‐ciri kajian
Bukti adalah terkini sehingga Jun 2017. Kami mendapati empat kajian yang menilai intervensi yang disampaikan sekurang‐kurangnya sebahagian melalui telefon bimbt, yang membuat kajian susulan terhadap peserta sekurang‐kurangnya 12 bulan.
Keputusan utama
Kami tidak boleh menggabungkan keputusan daripada empat kajian tersebut kerana intervensi‐intervensi mereka adalah amat berbeza. Kajian‐kajian tersebut mempunyai risiko bias yang tinggi dan keputusan intervensi adalah tidak konsisten apabila dibandingkan, oleh itu penyiasat‐penyiasat tidak yakin terhadap keputusan kajian‐kajian tersebut. Bukti mencadangkan bahawa intervensi yang disampaikan melalui telefon bimbit mungkin dapat membantu orang ramai mengambil ubat, tetapi manfaatnya adalah kecil, dan sesetengah kajian mendapati bahawa intervensi‐intervensi ini tidak bermanfaat. Tidak ada bukti yang mencadangkan sebarang intervensi ini adalah bahaya. Keputusan kajian‐kajian yang sedang dilaksanakan harus memberitahu kami kesan‐kesan intervensi ini dengan lebih tepat, dan harus memberitahu sama ada mereka boleh dilaksanakan dalam konteks yang lebih luas julatnya, termasuk negara‐negara berpendapatan rendah.
Authors' conclusions
Summary of findings
| Mobile phone interventions compared to usual care for improving adherence to medication prescribed for primary prevention of cardiovascular disease | |||
| Patient or population: people prescribed medication for primary prevention of cardiovascular disease | |||
| Outcomes | Impact | № of participants | Quality of the evidence |
| Cholesterol (low‐density lipoprotein) | 1 study found evidence of a small beneficial intervention effect on reducing LDL‐C (–9.20 mg/dL), and 1 study found a very small increase in LDL‐C (0.77 mg/dL) with wide confidence intervals that included no effect. | 893 | ⊕⊕⊝⊝ |
| Systolic blood pressure | 3 of the 4 studies found lower systolic blood pressure with mobile phone interventions, but the size of effect varied. 2 studies showed moderate and large reductions in systolic blood pressure (–7.10 and –12.45 mmHg). 1 multi‐arm trial found small reductions with information‐only text messages (–2.1) and interactive text messaging (–1.6 mmHg) arms. 1 study found a slight increase in blood pressure (0.83 mmHg) but with wide confidence intervals that included no effect. | 2194 | ⊕⊕⊝⊝ |
| Diastolic blood pressure | 2 of 3 studies found lower diastolic blood pressure with mobile phone interventions, but the size of the effect varied. 2 studies showed large and small reductions in diastolic blood pressure (–12.23 and –3.90 mmHg), and 1 study found a slight increase in diastolic blood pressure (1.64 mmHg) but with wide confidence intervals that included no effect. | 998 | ⊕⊕⊝⊝ Lowa,b |
| Combined CVD events | Not reported | (0 studies) | — |
| Adverse events | 1 study reported that there were 0 adverse events attributable to the intervention. 1 study report that there was no difference between groups in experience adverse effects of statins, and that 0 participants reported intervention‐related adverse events. | 1500 | ⊕⊕⊝⊝ |
| Cognitive outcome: satisfaction with treatment | 1 study measured satisfaction with treatment, and found no evidence of a difference between intervention and control arms. | 1190 | ⊕⊕⊝⊝ |
| LDL‐C: low‐density lipoprotein cholesterol; RCT: randomised controlled trial. | |||
| GRADE Working Group grades of evidence | |||
| aDowngraded one level for inconsistency: trial results included large variations in the degree to which the outcome was affected. bDowngraded one level for risk of bias: all trials at unclear risk of bias on multiple domains. cDowngraded one level for imprecision: very low number of events. dDowngraded one level for indirectness: based on a single trial conducted in a single setting (public sector clinic in Cape Town, South Africa). eDowngraded one level for risk of bias: trial at unclear risk of bias on two domains. | |||
Background
Description of the condition
Cardiovascular disease (CVD) is a major cause of disability and mortality throughout the world (Naghavi 2017; WHO 2011; WHO 2016), with an estimated 17.6 million people dying from CVDs in 2016, accounting for 32% of all global deaths (Naghavi 2017). However, premature fatal and non‐fatal CVD is considered to be largely preventable through the control of risk factors (WHO 2011).
Primary prevention of CVD refers to actions taken to reduce the incidence of clinical events due to coronary heart disease (CHD), cerebrovascular disease and peripheral vascular disease, among people with risk factors who have not yet developed clinically manifest CVD (WHO 2007). Primary prevention of CVD consists of lifestyle modifications (e.g. smoking cessation, increasing physical activity) and drug therapy (Piepoli 2016).
Lipid‐lowering and antihypertensive drug therapies for primary prevention are cost‐effective in reducing CVD morbidity and mortality among high‐risk people and are recommended by international guidelines (Piepoli 2016; WHO 2007). Recommendations relating to the use of antiplatelet drugs for primary prevention vary. The European Society of Cardiology (ESC) states that aspirin cannot be recommended in primary prevention due to its increased risk of major bleeding (Piepoli 2016); however, the US Preventive Services Task Force (USPSTF) recommends the use of aspirin when the 10‐year risk of CVD events reaches such a level that the benefits of aspirin, in terms of CVD events prevented, outweigh the potential harm of increased gastrointestinal haemorrhage (USPSTF 2014).
Adherence to long‐term medication is not ideal and results in costs in both health and economic terms (Piepoli 2016). Meta‐analyses have estimated rates of adherence to cardiovascular medications ranging from 50% to 60% (Chowdhury 2013; Naderi 2012), and there is some evidence that adherence is lower for primary prevention (Naderi 2012).
One study of health records of over 430,000 people in UK general practices found that 47% of people prescribed statins for primary prevention discontinued treatment (indicated by a greater than 90‐day gap between prescriptions), among whom, 72% then restarted treatment (Vinogradova 2016). One study of Finnish healthcare registers found that 53% of women prescribed statin therapy for primary prevention were adherent (defined as exceeding 80% of the prescribed regimen) (Lavikainen 2016). It has been estimated that approximately 9% of cases of CVDs in the EU could be attributed to poor adherence to vascular medications (Chowdhury 2013). Improving adherence to medications for the primary prevention of CVD would help to maximise the clinical benefits for the wider population (WHO 2003). Therefore, there is considerable scope for increasing adherence to prescribed medicine, and so, reducing morbidity, mortality and healthcare costs.
Description of the intervention
Mobile phone ownership is almost universal in high‐income countries and estimated to have reached over 90% in low‐ and middle‐income countries (ICT 2016). Given the broad reach of mobile phones and the potential for automation of delivery, interventions delivered by mobile phone are a potentially cost‐effective strategy to improve medication adherence. A range of media can be delivered through mobile phones including text messages, picture messages, interactive‐voice response, telephone calls and, with increasing ownership of smart phones with Internet capabilities (ICT 2016), mobile applications.
How the intervention might work
A wide range of factors have been shown to be associated with medication non‐adherence (DiMatteo 2004; Julius 2009; Kardas 2013; Pound 2005; Vermeire 2001; WHO 2003). Mobile phone‐based interventions have the potential to target a number of these factors. For example, lack of adherence resulting from lack of information regarding the benefits of medication, lack of information about how they work and how to take them, misconceptions about medication adverse effects, complex or unclear advice or poor recall of information provided in consultations may be addressed through text messages providing short and simply worded snippets of information (Julius 2009; Kardas 2013; Pound 2005; Vermeire 2001). Experiences of adverse effects can be targeted through mobile phone‐delivered interventions by providing information about medication and facilitating a link to a healthcare professional for people experiencing problems with their medication. Lack of social support has also been linked to poor medication adherence and previous qualitative research found that the receipt of text message‐based intervention provided social support (Douglas 2013). Mobile phone‐delivered interventions can be designed to target psychological factors such as lack of motivation and low self‐efficacy (Free 2016).
Existing interventions targeting adherence to CVD medication have employed mobile technologies to: deliver medication reminders (Park 2014a); encourage self‐monitoring of medication intake (Park 2014a); encourage habit formation relating to medication‐taking behaviours (Bobrow 2014); provide information (Bobrow 2014; Park 2014a); and facilitate links to healthcare services where required (Bobrow 2014; Piette 2012).
Systematic reviews assessing the effect of mobile health (mhealth) interventions on medication adherence for a range of conditions, including HIV, non‐communicable diseases and prevention of transplant rejection have reported significant improvements (Anglada‐Martinez 2015; Park 2014b), and an RCT found mobile phone messaging to be effective in improving contraceptive use (Smith 2015). Few adverse effects of mobile phone‐based interventions have been reported; potential, but rare, adverse events may include road traffic accidents (Caird 2014).
Why it is important to do this review
Systematic reviews evaluating the effect of mhealth interventions have reported promising but inconclusive results relating to improved medication adherence, including adherence to medication for secondary prevention of heart disease (Adler 2017; Anglada‐Martinez 2015; Park 2014b). However, no systematic review has specifically examined the effect of mobile phone‐based interventions on adherence to medications for the primary prevention of CVD. Mobile phone‐based interventions are of particular interest given their low‐cost and potential for widespread delivery.
Objectives
To establish the effectiveness of interventions delivered by mobile phone to improve adherence to medication prescribed for the primary prevention of CVD in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of parallel group design that randomised by participant or by cluster. We did not include cross‐over trials as this design would be inappropriate for assessing effects on cardiovascular events or mortality, due to the irreversible nature of these events. We only included trials with a minimum of one‐year follow‐up in order that the outcome measures relate to longer‐term, sustained medication adherence behaviours and outcomes. We included studies published as full text and as abstract only, and unpublished data.
Types of participants
We included adults (aged 18 years and over) who have been prescribed medication for the primary prevention of CVD. As this review focused on the primary prevention of CVD, we only included studies involving participants who had not had a prior CVD event, defined as: a previous myocardial infarction, stroke, revascularisation procedure (coronary artery bypass grafting or percutaneous coronary intervention), people with angina, and people with angiographically defined CHD. Where we identified trials that included a subset of eligible participants, we contacted the authors to request data for only those participants of interest. When we were unable to access these data, we applied a cut‐off whereby only trials in which at least 75% of participants met the criteria for primary prevention were included.
Types of interventions
We included trials of interventions delivered wholly or partly by mobile phone to improve adherence to cardiovascular medications prescribed for the primary prevention of CVD. We included interventions targeting adherence to antihypertensive drugs (thiazide‐like diuretic, angiotensin‐converting enzyme inhibitor, calcium channel blocker, beta‐blocker); lipid‐lowering drugs (statins); and antiplatelet drugs (low‐dose aspirin, non‐aspirin antiplatelet drugs). We only included trials targeting adherence to at least one of these medications. We also included trials of interventions that targeted medication adherence alongside other lifestyle modifications.
Intervention
Any mobile phone‐specific delivery mechanism, including short messaging service (SMS), multimedia messaging (MMS), applications (apps) and Interactive Voice Response. We included interventions employing a mix of delivery mechanisms of which at least one was mobile phone‐based, for example, interventions delivered by mobile phones in combination with traditional methods such as face‐to‐face communication and links to other types of support (e.g. healthcare support worker, telephone calls, Internet pages).
Comparator
Usual care and active controls where the control group intervention had no component delivered by a mobile phone‐specific delivery mechanism.
Types of outcome measures
Primary outcomes
-
Objective measures of adherence to treatment (low‐density lipoprotein cholesterol (LDL‐C), total cholesterol (TC) and high‐density lipoprotein cholesterol (HDL‐C), for the effect of statins; blood pressure for antihypertensive drugs; heart rate for the effect of atenolol; urinary 11‐dehydrothromboxane B2 for the antiplatelet effects of aspirin).
-
Combined CVD events (fatal or non‐fatal events).
-
Adverse effects including self‐reported road traffic accidents.
Secondary outcomes
-
Indirect measures of adherence to treatment (self‐report, tablet counts, medication event monitoring systems, pharmacy prescription data).
-
Fatal cardiovascular events.
-
Non‐fatal cardiovascular events (CHD, stroke).
-
Health‐related quality of life assessed using validated instruments (e.g. 36‐Item Short Form Health Survey (SF‐36), EQ‐5D).
-
Cognitive outcomes (any measures of: satisfaction with treatment, medication‐taking self‐efficacy, autonomy related to medication, attitudes (e.g. concerns about medicine adverse effects)).
-
Costs.
We also reported on the following process measures: extent of intervention received (e.g. number of text messages received, measures of use of allocated mobile application) and acceptability of intervention.
Reporting one or more of the outcomes listed here in the trial was not an inclusion criterion for the review.
Where outcomes (primary or secondary) were measured at multiple time points, we extracted data for the final point of measurement.
Search methods for identification of studies
Electronic searches
We identified trials through systematic searches of the following bibliographic databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL) (2017, Issue 6);
-
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 21 June 2017);
-
Embase (Ovid, 1980 to 2017 week 25);
-
CINAHL Plus (EBSCOhost, 1937 to 21 June 2017);
-
Conference Proceedings Citation Index‐Science (CPCI‐S) on Web of Science (Thomson Reuters, 1990 to 21 June 2017).
The search strategies are presented in Appendix 1. The Cochrane sensitivity‐precision maximising RCT filter was applied to MEDLINE (Ovid) and adaptations of it to the other databases, except CENTRAL (Lefebvre 2011).
We carried out a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch/) for ongoing or unpublished trials on 14 July 2017.
We imposed no restriction on date or language of publication.
We did not perform a separate search for adverse effects of mobile phone‐based interventions targeting medication adherence. We considered adverse effects described in included studies only.
Searching other resources
We checked the reference lists of all included studies and reviewed relevant articles for additional references. We also examined relevant retraction statements and errata for included studies.
Data collection and analysis
Selection of studies
Two review authors (MP and SB) independently screened the titles and abstracts of all identified potential studies to decide whether to retrieve the full text (eligible or potentially eligible/unclear studies) or to discard the study. Two review authors (MP and SB) independently screened the retrieved full texts to identify studies for inclusion and identify and record reasons for exclusion of the ineligible studies in the Characteristics of excluded studies table. We resolved any disagreements though discussion, and where necessary, a third review author (CF) arbitrated. We excluded any duplicates. We collated multiple reports of the same RCT into a single entry. We completed a PRISMA flow diagram (Liberati 2009).
Data extraction and management
We used a standardised, prepiloted form to extract data from the included studies for assessment of study quality and evidence synthesis. We contacted chief investigators for additional information where necessary. We extracted the following information.
-
Methods: study design; total duration of study; study setting and date of study.
-
Participants: number randomised; number lost to follow‐up/withdrawn; number analysed; mean age; age range; gender; proportion meeting criteria of 'primary prevention'; and inclusion criteria and exclusion criteria.
-
Interventions: intervention; comparison; concomitant medications; excluded medications; intervention delivery mechanism (text messages/MMS/mobile application/combined); how intervention was developed; behaviour change technique(s) employed; if intervention was personalised; and frequency and duration of intervention receipt.
-
Outcomes: primary and secondary outcomes specified and collected; adverse effects; and time points reported.
-
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (MP and SB) independently extracted data and resolved any differences by returning to the original study reports and discussion with a third review author (CF) where necessary. One review author (MP) transferred data into the Review Manager 5 (Review Manager 2014). To ensure that there were no errors in data entry, one review author (SB) checked that the data entered into Review Manager 5 were consistent with those in the data extraction form.
Assessment of risk of bias in included studies
Two review authors (MP and SB) independently assessed the risk of bias for each study using the criteria detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For each of the following domains, we graded the potential bias as high, low or unclear.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other biases.
We resolved disagreements by discussion. Where necessary, we consulted a third review author (CF) to arbitrate. We constructed a 'Risk of bias' table including justifications for our judgements. Where information relating to the risk of bias came from unpublished data or correspondence with an author, we noted this. We summarised the risk of bias judgements across different studies for each of the domains listed. When considering treatment effects, we accounted for the risk of bias for the studies that contributed to that outcome.
Given the nature of the interventions included in this review, it is likely that blinding of participants and personnel would be impossible, therefore, we expected trials to be categorised at high risk of bias on this domain. For the overall study assessment, we categorised a trial as being at low risk of bias if it was rated as low risk in all the domains listed above (with the exception of blinding of participants and personnel). Trials that were at high or unclear risk of bias on any of the domains (except blinding of participants and personnel) were categorised as being at high risk of bias.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and report any deviations from it in the Differences between protocol and review section (Palmer 2017).
Measures of treatment effect
We planned to analyse dichotomous outcome data as risk ratios (RR) with 95% confidence intervals (CI). We planned to analyse continuous outcome data as mean differences (MD) with 95% CIs, or if a continuous outcome had been measured in multiple ways, as a standardised mean difference (SMD) with 95% CIs. If it had been applicable, we would have entered data presented as a scale with a consistent direction of effect. If it had been applicable, we would have reported any skewed data identified as medians and interquartile ranges.
Unit of analysis issues
We did not carry out a meta‐analysis because of the heterogeneity of the included studies' intervention content and delivery mechanisms; as a result, we had no unit of analysis issues. Had we conducted meta‐analyses, we would have included RCTs with a parallel design, and if we had identified any cluster randomised trials, we would have analysed the data accounting for clustering using the intracluster coefficient. If we had identified multi‐arm trials for inclusion in meta‐analyses, where there was more than one relevant intervention arm but only one control arm, we would have pooled the intervention arms for a single pair‐wise comparison as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to exclude intervention arms not appropriate for this review.
Dealing with missing data
We contacted investigators to obtain further information where necessary (e.g. when the study included a mixed population of participants who met the criteria for primary prevention and participants who met the criteria for secondary prevention, and when only a subset of participants had been prescribed CVD preventive medication). We also planned to contact investigators or study sponsors to obtain missing data (e.g. when a study was identified as abstract only). We planned that where this was not possible, and the missing data were considered a potential source of serious bias, we would conduct a sensitivity analysis to explore the impact of including such studies in the overall assessment of results.
Assessment of heterogeneity
We considered the included trials to be too methodologically heterogeneous to pool the data in a meta‐analysis. Therefore, we described the studies narratively. We planned to use the I2 statistic to measure heterogeneity across the trials for the analysis of each outcome. In constructing the narrative forest plots for those outcomes reported by multiple studies, we calculated the I2 statistic and reported this. Had we considered the trials methodologically similar enough to pool, and had we identified there to be moderate to substantial heterogeneity (an I2 statistic between 30% and 100%), we would have reported it and examined possible causes according to our prespecified subgroup analyses, subject to having a sufficient number of studies.
Assessment of reporting biases
We did not use a funnel plot to explore possible small‐study biases for the primary outcomes as we only included four studies which were too heterogeneous to pool in a meta‐analysis. We planned that if the results from more the 10 trials could be pooled, we would use a funnel plot to explore possible small‐study biases for the primary outcomes.
Data synthesis
We planned to carry out meta‐analyses only if it was meaningful to do so (i.e. if the interventions, participants and outcome measures were similar enough for pooling to make sense). We did not undertake meta‐analyses as the included studies were too heterogeneous in the content and delivery of their interventions. We presented the effect estimates for outcomes reported by multiple studies on forest plots (without pooling); it should be noted that in transferring effect estimates from papers into Review Manager 5 using the generic inverse variance method, some CIs differed from those reported in the original paper by a decimal place.
Should more studies become available in future updates of this review which enable meaningful meta‐analyses, we plan to use fixed‐effect models. In the presence of heterogeneity (an I2 statistic in excess of 30%), we plan to examine whether this heterogeneity can be explained through our prespecified subgroup analyses. If these analyses account for the heterogeneity, we would only present the subgroup pooled effect estimates. If these subgroup analyses did not explain the heterogeneity, we would present results narratively. We intended to use fixed‐effect meta‐analysis and apply a conservative I2 threshold to identify heterogeneity in this review to avoid overweighting smaller studies. This is because we consider that the heterogeneity observed in these behaviour change trials will primarily be a result of differences in the content of the interventions and differences in risk of bias.
'Summary of findings' table
We created a 'Summary of Findings' table of narrative results for the following outcomes: objective measures of adherence to treatment, combined CVD events (fatal and non‐fatal events), adverse events and cognitive outcomes. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it related to the studies that contributed data for each outcome. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro software (GRADEpro GTD 2015). We justified decisions to downgrade the quality of studies using footnotes and made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We had planned to conduct the following subgroup analyses for the primary outcome of adherence to treatment if there had been sufficient studies:
-
income region (by World Bank income group) (World Bank 2017);
-
how text messages were developed (i.e. theory‐based, incorporating user views and based on evidence relating to factors influencing behaviour‐targeted versus other);
-
intervention content (number behaviour change technique employed coded according to the taxonomy developed by Michie and colleagues (Michie 2015));
-
delivery mechanisms (i.e. mobile phone messaging only, mobile applications only, combined mobile phone messaging and application, combined application and other).
Due to the limited number of studies, we were unable to conduct subgroup analyses. Should more trials become available for future updates of this review, we will re‐examine the planned subgroup analyses.
Sensitivity analysis
We planned to carry out a sensitivity analysis by only including studies with low risk of bias. As we were unable to carry out a meta‐analysis, no sensitivity analysis was conducted.
Results
Description of studies
Results of the search
The search of the databases retrieved 7287 records, and the search of the clinical trial registers retrieved an additional 32 records. After deduplication, we screened 4166 title and abstract records and excluded 4115 records. We assessed 51 full texts and excluded 32 references (23 studies). Six studies (eight references) were identified as ongoing and four studies (11 references) were eligible for inclusion. The flow diagram of search results is shown in Figure 1.

Study flow diagram.
Included studies
The Characteristics of included studies table presents details of the design, methods, participants, intervention, comparison and outcome measures for the studies included in this review. Four studies were identified for inclusion, which were relatively heterogeneous with particular variation in terms of the nature (content and delivery) of the intervention, and the population.
Participants
The sample sizes of included studies range from 110 (Logan 2012) to 1372 (Bobrow 2016), with a total of 2429 participants across all four included studies, of which 2031 participants completed follow‐up assessments.
Liu 2015 specified that participants must have had "no known cardiovascular disease" as an inclusion criterion, and therefore included 100% participants meeting the criteria for primary prevention. The other included studies had a mix of participants: Parraga‐Martinez 2017 included 93% primary prevention participants and Logan 2012 included at least 79% primary prevention participants. Bobrow 2016 did not specifically report the proportion of participants who met the criteria of primary prevention in the published report; however, after contact with trial authors they confirmed 78.3% of participants met the criteria for primary prevention.
There was heterogeneity between trials in the proportion of participants who were taking medication for the primary prevention of CVD. Bobrow 2016 prescribed medication to all participants. Logan 2012 included at least 89.1% of participants prescribed medication (hypertensive drugs or lipid‐lowering drugs or aspirin, or a combination of these); and Parraga‐Martinez 2017 stated that 68.1% of their sample had been prescribed lipid‐lowering medication (but did not mention other types of CVD prevention drugs). Liu 2015 did not report the proportion of participants prescribed medication, but explicitly stated that the intervention targeted adherence to medication among those on treatment.
The mean age of participants varied from 54.4 years (Bobrow 2016) to 62.9 years (Logan 2012). The proportion of women in the trial samples ranged from 42% (Liu 2015) to 72% (Bobrow 2016).
Settings
All studies recruited from healthcare settings. Logan 2012 recruited from the offices or clinics of physicians practicing in metropolitan Toronto, Canada. Bobrow 2016 recruited from an outpatient chronic disease service in a single, large, public sector clinic in Cape Town, South Africa. Parraga‐Martinez 2017 recruited participants from primary care clinics in three health districts of three Spanish autonomous communities. Liu 2015 recruited from a health management centre in a hospital in Guangzhou, China.
Intervention
The content and delivery of the interventions varied across studies. The intervention evaluated by Bobrow 2016 was specifically designed to primarily focus on medication adherence, with only a few references to other lifestyle modifications such as diet and physical exercise. In two trials, the interventions targeted a combination of behaviours such as lifestyle modifications including healthy diet and physical activity, alongside medication adherence for those prescribed CVD medication (Liu 2015; Parraga‐Martinez 2017). The intervention tested by Logan 2012 was primarily a blood pressure monitoring and feedback (via smartphone) intervention, which could be considered to implicitly target adherence to treatment as well as other health behaviours important for the control blood pressure.
Bobrow 2016 delivered the intervention solely through mobile phone text messages, and the intervention evaluated by Logan 2012 combined blood pressure monitoring with feedback messages delivered via smartphone. In the other studies, the intervention included additional components alongside the mobile delivery component, such as written information and self‐completion cards for participants to record adherence to recommendations (Parraga‐Martinez 2017), and a computerised CVD risk evaluation and a face‐to‐face counselling session (Liu 2015). Three of the studies tested interventions which were delivered only to the participant (Bobrow 2016; Liu 2015; Parraga‐Martinez 2017), while Logan 2012 evaluated an intervention which involved home blood pressure monitoring and feedback to participants' smartphones, alongside an automated fax providing detailed information on the participants' status to their physicians on the day before their next scheduled appointment.
Two studies involved potential users in developing the interventions (Bobrow 2016; Liu 2015), and none of the interventions were developed based on a specific theory. The interventions employed a minimum of three (Logan 2012) to a maximum of 16 (Bobrow 2016) behaviour change techniques. The behaviour change techniques applied in the greatest number of studies were: 'providing feedback on behaviour' (Liu 2015; Logan 2012; Parraga‐Martinez 2017), 'providing information about health consequences' and 'emphasising the salience of consequences' (Bobrow 2016; Liu 2015; Parraga‐Martinez 2017).
Three studies had a duration of the intervention of one year (Bobrow 2016; Liu 2015; Logan 2012). One study had a follow‐up at two years, but it was unclear whether the intervention was delivered throughout the entire study period (Parraga‐Martinez 2017).
Two studies had a control group that received standard care (Liu 2015; Parraga‐Martinez 2017). The control group in Logan 2012 received the same home blood pressure monitoring equipment as the intervention group and a booklet containing information on the measurement of blood pressure, treatment of hypertension and goals of therapy. The control in group in Bobrow 2016 received written information about hypertension and healthy living, and only received text messages that were sent to all trial participants, which were primarily related to trial participation.
Outcomes
All studies reported at least one objective measure related to medication adherence. All four studies measured blood pressure, and two studies measured cholesterol levels (LDL‐C, HDL‐C, TC) (Liu 2015; Parraga‐Martinez 2017). No studies reported outcome data relating to combined CVD events (fatal or non‐fatal). One study reported adverse events, specifically adverse effects of statins and intervention‐related adverse events (Parraga‐Martinez 2017).
Two studies reported indirect measures of adherence to treatment (our secondary outcomes). One study included outcome data on self‐report adherence to lipid‐lowering therapy, measured using the Morisky‐Green Test (Parraga‐Martinez 2017). One trial included self‐reported adherence to medication measured using a visual analogue scale, in addition to a measure of 'proportion of days of medication covered' (defined as the proportion of participants with 80% or more days covered with blood pressure‐lowering medication from prescribing and dispensing data routinely recorded in the clinical record, pharmacy record and Chronic Dispensing Unit record) (Bobrow 2016). This trial also included a measure of quality of life (health status measured with the EuroQol Group 5‐Dimension Self‐Report Questionnaire) and reported deaths (including those caused by CVD events) occurring during the trial (Bobrow 2016). Two trials reported data relating to our process measures including satisfaction with the intervention (Parraga‐Martinez 2017), and adherence to the intervention home blood pressure monitoring schedule (Logan 2012).
Funding
All four studies reported the source of funding; these were charitable body and research council (Bobrow 2016), government body and EU (Parraga‐Martinez 2017), charitable body (Logan 2012), and government body (Liu 2015).
Further information requested
Three of the trials identified for inclusion in this review included participants who had, and participants who had not, been prescribed CVD prevention medication (Liu 2015; Logan 2012; Parraga‐Martinez 2017). We contacted trial authors to request the trial data for only these participants, but received no responses. Therefore, we extracted primary outcome data of objective measures of medication adherence (e.g. blood pressure, LDL‐C, etc.) for these mixed populations. We also contacted authors of one trial for information relating to the proportion of participants who had previously experienced a CVD event and received this information (Bobrow 2016).
Excluded studies
See Characteristics of excluded studies table for details of excluded studies.
Ongoing studies
We identified six ongoing studies (see Characteristics of ongoing studies table). Three of these studies are being conducted in high‐income settings (Australia, 2000 participants (Redfern 2014); USA, 4076 participants (Choudhry 2016); UK, 1010 participants (Franssen 2017)). One study is being carried out in 'low resource settings' in Argentina (an upper‐ to middle‐income country; expected 357 participants) (Gulayin 2017), one study in China, an upper‐ to middle‐income country (330 participants) (Xu 2017), and one study in India (low‐ to middle‐income country; 3702 participants) (Jha 2017).
Risk of bias in included studies
Details of the risk of bias assessments for each of the included studies are presented in the 'Risk of Bias' tables in the Characteristics of included studies table, and in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
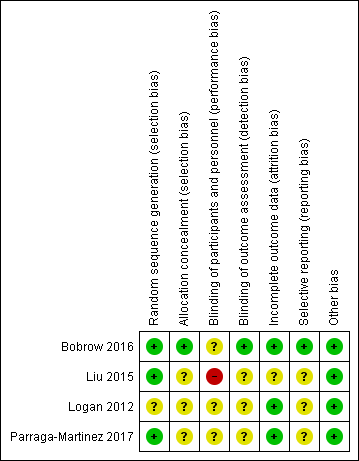
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three studies reported adequate random sequence generation and were at low risk of bias for this domain (Bobrow 2016; Liu 2015; Parraga‐Martinez 2017). One study did not provide sufficient information and therefore was at unclear risk of bias for random sequence generation (Logan 2012).
One study described their allocation concealment adequately and was at low risk of bias in this domain (Bobrow 2016). The other three studies did not provide sufficient information on their allocation procedures and therefore were at unclear risk of bias for allocation concealment (Liu 2015; Logan 2012; Parraga‐Martinez 2017).
Blinding
In all four included studies the nature of the interventions precluded blinding of participants. However, blinding of personnel may have been possible. One study specifically stated that personnel were not blinded to group assignment (Liu 2015). Two studies stated that personnel were blinded (Bobrow 2016; Parraga‐Martinez 2017), and in one study it was not clear whether personnel were blinded (Logan 2012). No trials were at low risk of bias for blinding of both personnel and participants.
For the blinding of outcome assessment domain, one study provided sufficient detail relating to the blinding of outcome assessors and the use of automated outcome measurements with data transmitted directly to the trial database and as a result, was at low risk of bias on this domain (Bobrow 2016). The remaining three studies did not provide sufficient details for this domain and were judged as being at unclear risk of bias (Liu 2015; Logan 2012; Parraga‐Martinez 2017).
Incomplete outcome data
Three studies had high rates of follow‐up (85% or greater) with no evidence of differential loss to follow‐up and were at low risk of bias on the incomplete outcome data domain (Bobrow 2016; Logan 2012; Parraga‐Martinez 2017). One study reported that 27.5% of participants did not attend for follow‐up, and that they differed from those who did attend for follow‐up based on several characteristics. The study also reported that these missing values were likely to have little impact on the primary outcome based on sensitivity analyses. However, it is unclear whether this may have affected other outcomes, and so this study was judged as being at unclear risk of bias on this domain (Liu 2015).
Selective reporting
One study reported outcomes as planned in their protocol, with the exception of one outcome that was reported in protocol, but not in the trial report (‘hypertension knowledge'). This trial began recruiting in June 2012, but details of the protocol were not registered until December 2013, and so we cannot be certain as to what was planned before the trial commenced. Therefore, we judged this study at unclear risk of bias on the selective reporting domain (Bobrow 2016). Two of the other trials also appeared to have been registered after recruitment had begun, and therefore were also judged at unclear risk of bias (Liu 2015; Logan 2012). One study reported all outcomes as planned in the protocol with the exception of cardiovascular events occurring during the study period. This was considered an important outcome; however, it was not clear whether this outcome was not reported because no events occurred. Therefore, this trial was at unclear risk of bias on this domain (Parraga‐Martinez 2017).
Other potential sources of bias
All four trials were at low risk of 'other' bias; all studies were funded by government bodies, charitable bodies or research councils (Bobrow 2016; Liu 2015; Logan 2012; Parraga‐Martinez 2017).
Effects of interventions
We did not pool results in a meta‐analysis as the content and delivery mechanisms of the interventions were heterogeneous. The intervention assessed by Bobrow 2016 was designed to focus on medication adherence and delivered solely through SMS. The intervention tested by Logan 2012 was a blood pressure monitoring and feedback (via smartphone) intervention. The Parraga‐Martinez 2017 intervention targeted a combination of lifestyle modifications, alongside medication adherence for those prescribed CVD medication and was delivered through text messages, written information pamphlets and self‐completion cards for participants. Finally, the intervention evaluated by Liu 2015 targeted healthy lifestyle alongside treatment regimens with a multi‐component intervention comprising of text messages, a computerised CVD risk evaluation and face‐to‐face counselling. Based on these differences, we considered that pooling data from these trials would not have been appropriate.
In generating the narrative forest plots, we also checked heterogeneity statistically (I2 greater than 90% for systolic blood pressure (SBP) and diastolic blood pressure (DBP); I2 = 0% for TC; I2 = 73% for LDL‐C; I2 = 0% for HDL‐C). Based on these findings, we considered pooling results from the two studies which reported on TC and HDL outcomes; however, we still considered the interventions too distinct to warrant meaningful pooling (specifically, one intervention included face‐to‐face counselling (Liu 2015), whereas the other consisted of written information and text messages (Parraga‐Martinez 2017)).
We present results narratively, below, and in Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5.
Primary outcomes
Objective measures of adherence to treatment
Cholesterol
Two trials reported LDL‐C levels (Analysis 1.1), one of which showed a reduction in LDL‐C (MD in reduction: 9.20 mg/dL, 95% CI 0.70 to 17.70, P = 0.034; 304 participants) (Parraga‐Martinez 2017), while the other demonstrated no evidence of intervention effect on LDL‐C (MD 0.77 mg/dL, 95% CI –4.64 to 6.18; 589 participants) (Liu 2015) (note: we converted mmol/L cholesterol to mg/dL using a multiplier of 38.67 as recommended by Rugge 2011). We judged the evidence relating to the intervention effect on LDL‐C to be of low quality due to both trials contributing to this comparison being at unclear risk of bias across multiple domains, and the inconsistency in effect estimates across studies.
These two trials also reported TC finding evidence of intervention benefit (Analysis 1.2). Parraga‐Martinez 2017 showed an MD in the reduction of TC of 9.7 mg/dL (95% CI 0.30 to 19.10; P = 0.041) for the intervention compared with control group, and Liu 2015 recorded an MD in reduction of TC of 10.05 mg/dL (95% CI –17.01 to –3.09).
Neither trial found evidence for an adverse effect on HDL‐C (Analysis 1.3) (MD 1.16 mg/dL, 95% CI –1.55 to 3.87 (Liu 2015); MD 0.10 mg/dL, 95% CI –2.60 to 2.80 (Parraga‐Martinez 2017)).
Blood pressure
All four studies reported data for blood pressure, of which three trials showed a beneficial intervention effect (Analysis 1.4; Analysis 1.5). We judged the evidence relating to SBP of low quality due inconsistent outcome effects, and because all four of the trials were at unclear risk of bias across multiple domains. Three trials measured DBP as an outcome and we considered this to constitute low‐quality evidence due to all three trials being at unclear risk of bias across multiple domains, and inconsistency between studies in the degree to which the outcome was affected.
Bobrow 2016 (1372 participants) reported a greater reduction in mean SBP from baseline to 12‐month follow‐up in the intervention group receiving information‐only text messages compared with the control group (MD –2.2 mmHg, 95% CI –4.4 to 0.00; P = 0.046), but no difference between the intervention group receiving interactive text messaging and the control group (MD –1.6 mmHg, 95% CI –3.70 to 0.50, P = 0.16). Bobrow 2016 also presented the proportion of participants achieving SBP and DBP less than 140/90 mmHg. They found evidence of benefit for both the information‐only text messaging intervention group (65% with information‐only text messaging versus 58% with control; odds ratio (OR) 1.42, 95% CI 1.03 to 1.95; P = 0.033), and the interactive text messaging group (65% with interactive text messaging versus 58% with control; OR 1.41, 95% 1.02 to 1.95; P = 0.038), compared with the control group receiving usual care (Bobrow 2016).
Logan 2012 showed a greater reduction in SBP and DBP in the intervention group compared with control group at 12 months for: 24‐hour blood pressure and daytime ambulatory blood pressure (mean between‐group difference in change (standard error (SE)): 24‐hour SBP: –6.8 mmHg (SE 2.4); P = 0.005; 24‐hour DBP: –3.6 mmHg (SE 1.3); P = 0.006; daytime SBP: –7.10 mmHg (SE 2.3); P = 0.003; daytime DBP: –3.9 mmHg (SE 1.3) P = 0.003)). However, there was at best only weak evidence of a benefit for change in night‐time blood pressure (SBP: –4.7 mmHg (SE 2.8); P = 0.098; DBP: –2.3 mmHg (SE 1.6); P = 0.16) (105 participants) (Logan 2012).
Liu 2015 also found evidence of a beneficial intervention effect on blood pressure at 12 months, with an MD between the intervention and control group for SBP of –12.45 mmHg (95% CI –15.02 to –9.88) and for DBP of –12.23 (95% CI –14.03 to –10.43) (589 participants).
However, Parraga‐Martinez 2017 found no evidence of a benefit of their intervention for reducing blood pressure at two years, with an MD in change of 0.83 mmHg (95% CI –2.67 to 4.33) for SBP, and 1.64 mmHg (95% CI –0.55 to 3.83) for DBP (304 participants).
Heart rate
No studies reported heart rate.
Urinary 11‐dehydrothromboxane B2
No studies reported urinary 11‐dehydrothromboxane B.
Combined cardiovascular disease event (fatal or non‐fatal events)
No studies reported on combined CVD events.
Adverse effects
Based on two trials, we found low‐quality evidence that the mobile phone‐based interventions under study did not lead to adverse events. The evidence was of low quality due to the studies being at unclear risk of bias across multiple domains, and the potential for imprecision in effect estimates resulting from the very low number of events. One study (1372 participants) reported no adverse events attributable to the intervention (Bobrow 2016). The other study (304 participants) reported that there were no differences between groups in experiencing adverse effects of statins (intervention group: seven events; control group: 10 events), and no participants reported intervention‐related adverse events (Parraga‐Martinez 2017). The other two trials did not report on adverse events (Liu 2015; Logan 2012).
Secondary outcomes
Indirect measures of adherence to treatment
An overview of the trial results relating to indirect measures of medication adherence is presented in Table 1. Bobrow 2016 (1372 participants) presented 12‐month outcome data for the median difference in the proportion of days covered by dispensed medication, finding evidence of a modest benefit for both the information‐only text messaging intervention group (83.3% with intervention versus 79.2% with control; median difference 5.2, quartiles 1‐3: 1.5 to 8.9; P = 0.006), and the interactive text messaging group (83.3% with intervention versus 79.2% with control; median difference: 3.8, quartiles 1‐3: 0.03 to 7.6; P = 0.048), compared with the control group receiving usual care (Bobrow 2016). There were similar results for the outcome of achieving 80% or more days covered (information‐only text messaging group versus control: OR 1.86, 95% CI 1.39 to 2.49; P < 0.001; interactive text messaging group versus control: OR 1.60, 95% CI 1.20 to 2.16; P = 0.002) (it is not clear how the underlying proportions compared as the authors did not report the proportion achieving 80% or more days covered for the control group). However, there was no evidence of benefit for the outcome of self‐reported medication adherence (information‐only text messaging group versus control: median difference 0.04, quartiles 1‐3: –0.1 to 0.2; P = 0.70; interactive text messaging group versus control: median difference 0.02, quartiles 1‐3: –0.2 to 0.2, P = 0.80).
| Trial | Outcome measure | Comparison | Intervention | Number (intervention) | Control | Number (Control) | Narrative results |
| (1‐year follow‐up) | Proportion of days covered by dispensed medicine | Information‐only SMS vs control | 83.3% (95% CI 69.3 to 91.7) | 457 | 79.2% (95% CI 64.6 to 91.4) | 458 | Median difference 5.2, quartiles 1‐3: 1.5 to 8.9; P = 0.006 |
| Interactive SMS vs control | 83.3% (95% CI 66.7 to 91.7) | 457 | 79.2% (95% CI 64.6 to 91.4) | 458 | Median difference 3.8; quartiles 1‐3: 0.03 to 7.6; P = 0.048 | ||
| Proportion of participants with proportion of days covered ≥ 80% | Information‐only SMS vs control | 63% | 457 | 49.4% | 458 | Adjusted odds ratio 1.86, 95% CI 1.39 to 2.49; P < 0.001 | |
| Interactive SMS vs control | 60% | 457 | 49.4% | 458 | Adjusted odds ratio 1.60, 95% CI 1.20 to 2.16; P = 0.002 | ||
| Self‐reported medication adherence (score range 5–10) | Information‐only SMS vs control | 10 (quartiles 1‐3: 9 to 10) | 457 | 10 (quartiles 1‐3: 9 to 10) | 458 | Median difference 0.04, 95% CI –0.1 to 0.2; P = 0.70 | |
| Interactive SMS vs control | 10 (quartiles 1‐3: 9 to 10) | 457 | 10 (quartiles 1‐3: 9 to 10) | 458 | Median difference 0.02, 95% CI –0.2 to 0.2; P = 0.80 | ||
| (2‐year follow‐up) | Proportion adherent according to self‐reported medication adherence (measured using 'adapted Morisky‐Green test') | — | 77.2% | Disaggregated not reported | 64.1% | Disaggregated not reported | P = 0.029 220 in total, not reported by group |
CI: confidence interval; SMS: short messaging service.
Parraga‐Martinez 2017 also reported outcome data for self‐reported adherence to treatment (specifically to lipid‐lowering therapy) measured using the Morisky‐Green Test, among those participants prescribed lipid‐lowering therapy. This study found evidence of benefit for the outcome of proportion of participants reporting adherence at two years' postrandomisation (77.2% with intervention versus 64.1% with control; P = 0.029, 220 participants).
Fatal cardiovascular events
Bobrow 2016 (1372 participants) reported that two participants in the information‐only text messaging group died due to ischaemic heart disease, two participants in the interactive text messaging group died due to congestive cardiac failure and there were no deaths in the control group known to be due to CVD. There were slightly more participants in the usual care arm who were lost to follow‐up due to the reason of 'lost contact' (14 participants), compared to the information SMS arm (seven participants), and the interactive SMS arm (seven participants). Therefore, it is possible that this differential lost to follow‐up due to lost contact could have underestimated deaths, including those due to CVD, in the usual care arm.
Non‐fatal cardiovascular events
No studies reported non‐fatal cardiovascular events.
Health‐related quality of life assessed using validated instruments
Bobrow 2016 reported the median difference in quality of life as measured by the Euro‐Qol 5‐Dimension Index, finding no effect of the information‐only text messages (median difference 0.01, quartiles 1‐3: –0.01 to 0.02; P = 0.50) or the interactive text messages (median difference: 0.003, quartiles 1‐3: –0.02 to 0.02; P = 0.73) compared with the control group.
Cognitive outcomes
Bobrow 2016 measured satisfaction with treatment and found no evidence of difference between intervention arms and control arm (information‐only text messaging group versus control: median difference 0, quartiles 1‐3: –0.3 to 0.3; P > 0.99; interactive text messaging group versus control: median difference 0, quartiles 1‐3: –0.3 to 0.3; P > 0.99).
Costs
No studies reported costs.
Process measures
Parraga‐Martinez 2017 recorded satisfaction with the intervention, finding that 90.8% (95% CI 85.9 to 95.7) of the 155 intervention group participants reported being satisfied or very satisfied with the intervention at two years' postrandomisation. Logan 2012 recorded a 65.4% (standard deviation 30) adherence rate to the home blood pressure measurement schedule (taking a minimum of eight readings per weeks) in the intervention group. Bobrow 2016 reported that 50% of participants allocated to the interactive SMS intervention arm responded to messaging. No studies reported on other process indicators such as measures relating to the proportion of intervention received/used.
Discussion
Summary of main results
This review provided low‐quality evidence regarding the effects of adherence interventions delivered by mobile phone, with some trials reporting small benefits and other reporting no benefits. There was low‐quality evidence that the interventions did not cause harm. In our review, we identified four trials, none of which were at low risk of bias. One trial evaluated an intervention targeting medication adherence via text messaging and one trial assessed a blood pressure monitoring system which delivered feedback to participants via smartphone messaging. The remaining two trials were of interventions targeting healthy lifestyle modifications more generally, including adherence to medication, one of which was delivered through text messages, written information pamphlets and self‐completion cards for participants, and the other through a combination of text messages, a computerised CVD risk evaluation and face‐to‐face counselling. Due to these differences in content and delivery of the interventions, we did not pool results in a meta‐analysis.
We considered the body of evidence relating to the effect of mobile phone‐based interventions to be of low quality for outcomes relating to blood pressure and cholesterol due to the trials being at high risk of bias across multiple domains, and inconsistent outcome effects. The trial of the text messaging‐based intervention targeting adherence showed a small reduction in SBP for the intervention arm which delivered information‐only text messages, but no evidence of a benefit for the second intervention arm that provided interactivity in addition to the information‐based text messages. Bobrow 2016 reported that only 50% of participants allocated to the interactive SMS intervention arm responded to messages, which may be indicative of relatively low engagement with this feature. Both arms demonstrated an increase in the proportion of participants achieving the recommended threshold for SBP and DBP, with a modest risk difference between the intervention and control groups of 7% (Bobrow 2016). One of two indirect measures of adherence also showed improvements and there was no difference in CVD‐related deaths, health‐related quality of life or cognitive outcomes (satisfaction with treatment) (Bobrow 2016). The study examining the effect of blood pressure monitoring, and messaging via smartphone, reported a modest intervention benefit on four of their six outcome measures of blood pressure (Logan 2012). There was mixed evidence of benefit in two trials targeting medication adherence alongside other lifestyle advice. Liu 2015 reported benefits in SBP and DBP, but Parraga‐Martinez 2017 reported no such effects. Both trials reported a beneficial effect of their intervention on lowering TC (Liu 2015; Parraga‐Martinez 2017); however, only Parraga‐Martinez 2017 found an effect on LDL‐C. Only one trial included an indirect measure of adherence reporting a benefit in self‐reported medication adherence (Parraga‐Martinez 2017). In both of these trials the contribution of increased adherence to the reductions in cholesterol and blood pressure reported was unclear due to their inclusion of a mix of participants who had and had not been prescribed CVD medication (Liu 2015; Parraga‐Martinez 2017).
Two trials reported on adverse events and provided low‐quality evidence that the interventions did not cause harm (Bobrow 2016; Parraga‐Martinez 2017).
Overall completeness and applicability of evidence
The generalisability of this review was limited by the small number of trials identified for inclusion. Given that one of our inclusion criteria was trials having a minimum of one‐year follow‐up, we can be confident that our results are applicable to longer‐term, sustained medication adherence behaviours and outcomes. No studies reported on non‐fatal cardiovascular events, meaning we were unable to establish whether the modest benefits observed in individual trials for cholesterol and blood pressure translated into such patient‐relevant outcomes. Two studies were conducted in high‐income settings and two in upper‐ to middle‐income settings, meaning that the applicability of these results to other settings including low‐income settings is unclear. Four of the six ongoing studies identified are being carried out in high‐income countries, however, one trial is being conducted in 'low resource settings' in Argentina, and one in a low‐ to middle‐income county (India), which may provide greater information on the applicability of results across settings (Gulayin 2017).
Quality of the evidence
Using GRADE methodology we assessed the quality of the evidence for our narrative synthesis of objective outcomes of medication adherence (LDL‐C, SBP and DBP), cognitive outcomes and adverse events. The evidence was of low quality across all outcomes. The quality of the evidence relating to objective outcomes of medication adherence were downgraded one level as a result of inconsistency in effect estimates which spanned both clinically meaningful improvements and null effects. The quality of the evidence relating to all five outcomes considered were downgraded one level because none of the included studies were at low risk of bias. Three of the four studies were at unclear risk of bias on at least four of the domains, indicating poor quality of reporting of the trial methods in these studies which limited our ability to make clear judgements about the level of risk of bias. Finally, the evidence relating to the cognitive outcome of satisfaction with treatment was also downgraded for indirectness, because this was based on one trial conducted in a single setting.
Two trials of interventions targeting broader lifestyle modifications, including medication adherence, included a mixture of participants who had and had not been prescribed CVD prevention medication, and therefore, in both of these trials the contribution of increased adherence to the reductions in cholesterol and blood pressure reported was unclear (Liu 2015; Parraga‐Martinez 2017).
Potential biases in the review process
We were limited in the outcome data we could extract due to our inability to procure further information and data for subsets of specific participants in the included trials from the study authors. It is unclear whether the additional data would have altered the overall findings of this review. Our inability to conduct a meta‐analysis means that this review cannot benefit from examining pooled effect estimates based on larger sample sizes than the individual trials. Furthermore, publication bias, whereby trials with positive findings are more likely to be published, may have biased the selection of included studies in this review. However, efforts were made to overcome this through searching clinical trial registries for prospectively registered trials. The decision was taken to only include trials with a minimum of one‐year follow‐up in order that results were applicable to longer‐term sustained behaviour change in adherence, which would therefore be more important in improving health status. This means that we are unable to comment on the effectiveness of mobile phone‐based interventions for short‐term adherence to medication prescribed for the primary prevention of CVD.
Agreements and disagreements with other studies or reviews
Our findings of mixed evidence of the effects of mobile phone‐delivered interventions to increase adherence to medication prescribed for the primary prevention of CVD and no reported harms are consistent with those of a Cochrane Review examining the effectiveness of text‐messaging interventions to improve adherence to medication prescribed for the secondary prevention of CVD (Adler 2017). These findings are broadly consistent with systematic reviews concerned with mhealth interventions to improve medication adherence across conditions, although these reviews included short‐term studies and non‐RCT designs, which are subject to bias (Anglada‐Martinez 2015; Park 2014b). One systematic review examining RCTs of monitoring and messaging interventions targeting medication adherence for the management of type 2 diabetes found no evidence for an improvement in medication adherence in their pooled meta‐analyses of five trials (Farmer 2016). Our finding that one intervention delivered by text messaging alone reported small benefits, some of which achieved statistical significance, is consistent with the findings from trials employing SMS alone targeting adherence to HIV medication which also report small benefits of borderline clinical and statistical significance (da Costa 2012; Orrell 2015; Pop‐Eleches 2011; Sabin 2015). The reported benefits of the monitoring and SMS intervention is consistent with the modest benefits of monitoring interventions in general (Carrasco 2008; Lim 2011; McKinstry 2013; Yoo 2009). The small or modest benefits reported may reflect the challenges involved in improving adherence, and overall inconclusive findings relating to adherence interventions in general, which have previously been noted in a Cochrane Review of all adherence interventions (Nieuwlaat 2014).

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
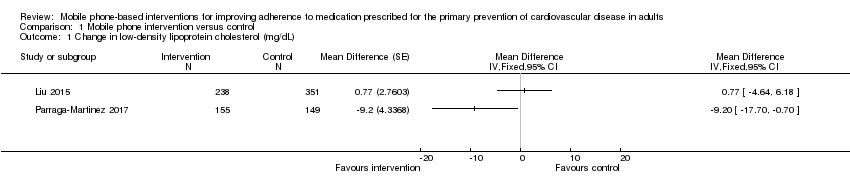
Comparison 1 Mobile phone intervention versus control, Outcome 1 Change in low‐density lipoprotein cholesterol (mg/dL).

Comparison 1 Mobile phone intervention versus control, Outcome 2 Change in total cholesterol (mg/dL).

Comparison 1 Mobile phone intervention versus control, Outcome 3 Change in high‐density lipoprotein cholesterol (mg/dL).

Comparison 1 Mobile phone intervention versus control, Outcome 4 Change in systolic blood pressure (mmHg).

Comparison 1 Mobile phone intervention versus control, Outcome 5 Change in diastolic blood pressure (mmHg).
| Mobile phone interventions compared to usual care for improving adherence to medication prescribed for primary prevention of cardiovascular disease | |||
| Patient or population: people prescribed medication for primary prevention of cardiovascular disease | |||
| Outcomes | Impact | № of participants | Quality of the evidence |
| Cholesterol (low‐density lipoprotein) | 1 study found evidence of a small beneficial intervention effect on reducing LDL‐C (–9.20 mg/dL), and 1 study found a very small increase in LDL‐C (0.77 mg/dL) with wide confidence intervals that included no effect. | 893 | ⊕⊕⊝⊝ |
| Systolic blood pressure | 3 of the 4 studies found lower systolic blood pressure with mobile phone interventions, but the size of effect varied. 2 studies showed moderate and large reductions in systolic blood pressure (–7.10 and –12.45 mmHg). 1 multi‐arm trial found small reductions with information‐only text messages (–2.1) and interactive text messaging (–1.6 mmHg) arms. 1 study found a slight increase in blood pressure (0.83 mmHg) but with wide confidence intervals that included no effect. | 2194 | ⊕⊕⊝⊝ |
| Diastolic blood pressure | 2 of 3 studies found lower diastolic blood pressure with mobile phone interventions, but the size of the effect varied. 2 studies showed large and small reductions in diastolic blood pressure (–12.23 and –3.90 mmHg), and 1 study found a slight increase in diastolic blood pressure (1.64 mmHg) but with wide confidence intervals that included no effect. | 998 | ⊕⊕⊝⊝ Lowa,b |
| Combined CVD events | Not reported | (0 studies) | — |
| Adverse events | 1 study reported that there were 0 adverse events attributable to the intervention. 1 study report that there was no difference between groups in experience adverse effects of statins, and that 0 participants reported intervention‐related adverse events. | 1500 | ⊕⊕⊝⊝ |
| Cognitive outcome: satisfaction with treatment | 1 study measured satisfaction with treatment, and found no evidence of a difference between intervention and control arms. | 1190 | ⊕⊕⊝⊝ |
| LDL‐C: low‐density lipoprotein cholesterol; RCT: randomised controlled trial. | |||
| GRADE Working Group grades of evidence | |||
| aDowngraded one level for inconsistency: trial results included large variations in the degree to which the outcome was affected. bDowngraded one level for risk of bias: all trials at unclear risk of bias on multiple domains. cDowngraded one level for imprecision: very low number of events. dDowngraded one level for indirectness: based on a single trial conducted in a single setting (public sector clinic in Cape Town, South Africa). eDowngraded one level for risk of bias: trial at unclear risk of bias on two domains. | |||
| Trial | Outcome measure | Comparison | Intervention | Number (intervention) | Control | Number (Control) | Narrative results |
| (1‐year follow‐up) | Proportion of days covered by dispensed medicine | Information‐only SMS vs control | 83.3% (95% CI 69.3 to 91.7) | 457 | 79.2% (95% CI 64.6 to 91.4) | 458 | Median difference 5.2, quartiles 1‐3: 1.5 to 8.9; P = 0.006 |
| Interactive SMS vs control | 83.3% (95% CI 66.7 to 91.7) | 457 | 79.2% (95% CI 64.6 to 91.4) | 458 | Median difference 3.8; quartiles 1‐3: 0.03 to 7.6; P = 0.048 | ||
| Proportion of participants with proportion of days covered ≥ 80% | Information‐only SMS vs control | 63% | 457 | 49.4% | 458 | Adjusted odds ratio 1.86, 95% CI 1.39 to 2.49; P < 0.001 | |
| Interactive SMS vs control | 60% | 457 | 49.4% | 458 | Adjusted odds ratio 1.60, 95% CI 1.20 to 2.16; P = 0.002 | ||
| Self‐reported medication adherence (score range 5–10) | Information‐only SMS vs control | 10 (quartiles 1‐3: 9 to 10) | 457 | 10 (quartiles 1‐3: 9 to 10) | 458 | Median difference 0.04, 95% CI –0.1 to 0.2; P = 0.70 | |
| Interactive SMS vs control | 10 (quartiles 1‐3: 9 to 10) | 457 | 10 (quartiles 1‐3: 9 to 10) | 458 | Median difference 0.02, 95% CI –0.2 to 0.2; P = 0.80 | ||
| (2‐year follow‐up) | Proportion adherent according to self‐reported medication adherence (measured using 'adapted Morisky‐Green test') | — | 77.2% | Disaggregated not reported | 64.1% | Disaggregated not reported | P = 0.029 220 in total, not reported by group |
| CI: confidence interval; SMS: short messaging service. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in low‐density lipoprotein cholesterol (mg/dL) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Change in total cholesterol (mg/dL) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 Change in high‐density lipoprotein cholesterol (mg/dL) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 Change in systolic blood pressure (mmHg) Show forest plot | 4 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Change in diastolic blood pressure (mmHg) Show forest plot | 3 | Mean Difference (Fixed, 95% CI) | Totals not selected | |

