Ligadura con banda versus ninguna intervención para la prevención primaria de la hemorragia digestiva alta en pacientes adultos con cirrosis y várices esofágicas
Resumen
Antecedentes
La presencia de várices esofágicas se asocia con riesgo de hemorragia digestiva alta. La ligadura endoscópica de las várices se utiliza para prevenir que esto ocurra, pero el procedimiento de la ligadura se puede asociar con complicaciones.
Objetivos
Evaluar los efectos beneficiosos y perjudiciales de la ligadura con banda versus ninguna intervención para la prevención primaria de la hemorragia digestiva alta en adultos con cirrosis y várices esofágicas.
Métodos de búsqueda
Se combinaron las búsquedas en el Registro de Ensayos Controlados del Grupo Cochrane Hepato‐Biliar (Cochrane Hepato‐Biliary's Controlled Trials Register), el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), MEDLINE, Embase, LILACS y en Science Citation Index con búsquedas manuales. La última actualización de la búsqueda fue el 9 de febrero 2019.
Criterios de selección
En los análisis de los efectos beneficiosos y perjudiciales, se incluyeron ensayos clínicos aleatorizados que compararon la ligadura con banda versus ninguna intervención, independientemente del estado de publicación, el cegamiento o el idioma, así como estudios observacionales que evaluaron los daños. Los participantes incluidos presentaban cirrosis y várices esofágicas, sin antecedentes de hemorragia de las várices.
Obtención y análisis de los datos
Tres autores de la revisión extrajeron los datos de forma independiente. Las medidas de resultado primarias fueron la mortalidad por todas las causas, la hemorragia digestiva alta y los eventos adversos graves. Se realizaron metanálisis y se presentaron los resultados mediante los riesgos relativos (RR), con intervalos de confianza (IC) del 95% y valores de I2 como un marcador de la heterogeneidad. Además, se calculó el número necesario para tratar para beneficiar (NNTB) para los resultados primarios. Se evaluó el control del sesgo mediante los dominios del Grupo Cochrane Hepatobiliar; se determinó la certeza de la evidencia mediante GRADE; y se realizaron análisis de sensibilidad que incluyeron el análisis secuencial de ensayos.
Resultados principales
Seis ensayos clínicos aleatorizados con 637 participantes cumplieron los criterios de inclusión. Uno de los ensayos incluyó una cantidad pequeña adicional de participantes (< 10% del total) con bloqueo venoso portal/hipertensión portal no cirróticos. Un ensayo se clasificó como bajo riesgo de sesgo para el resultado de mortalidad y como riesgo alto de sesgo para los otros resultados; los cinco ensayos restantes tuvieron un alto riesgo de sesgo para todos los resultados. La calidad de la evidencia se disminuyó a moderada debido al riesgo de sesgo. Se recopilaron los datos sobre todos los resultados primarios de todos los ensayos. Setenta y uno de 320 participantes asignados a ligadura con banda murieron, en comparación con 129 de 317 participantes asignados a ninguna intervención (RR 0,55; IC del 95%: 0,43 a 0,70; I2= 0%; NNTTB = 6 pacientes). Además, la ligadura de banda se asoció con una reducción del riesgo de hemorragia gastrointestinal alta (RR 0,44; IC del 95%: 0,28 a 0,72; seis ensayos, 637 participantes; I2 = 61%; NNTTB = cinco pacientes), eventos adversos graves (RR 0.55, IC del 95%: 0,43 a 0,70; seis ensayos, 637 participantes; I2 = 44%; NNTTB = cuatro pacientes), y hemorragia variceal (RR 0,43, IC del 95%: 0,27 a 0,69; seis ensayos, 637 participantes; I² = 56%; NNTTB = cinco pacientes). Los eventos adversos no graves informados en asociación con la ligadura con banda incluyeron ulceración esofágica, disfagia, odinofagia, dolor retroesternal y de la garganta, pirosis y fiebre, y en el único ensayo que incluyó participantes con várices pequeñas o grandes, la incidencia de efectos secundarios no graves en el grupo de ligadura con banda fue mucho mayor en los que presentaban várices pequeñas; es decir, úlceras: várices pequeñas versus várices grandes 30,5% versus 8,7%; pirosis 39,2% versus 17,4%. Ningún estudio informó sobre la calidad de vida relacionada con la salud.
Dos ensayos no recibieron apoyo de las compañías farmacéuticas; los cuatro ensayos restantes no proporcionaron información sobre este tema.
Conclusiones de los autores
Esta revisión encontró evidencia de certeza moderada de que, en los pacientes con cirrosis, la ligadura con banda de las várices esofágicas reduce la mortalidad, la hemorragia digestiva alta, la hemorragia de las várices y los eventos adversos graves en comparación con ninguna intervención. Es poco probable que se considere ético realizar ensayos adicionales de ligadura con banda versus ninguna intervención.
PICO
Resumen en términos sencillos
Ligadura con banda versus ninguna intervención para la prevención primaria de la hemorragia digestiva alta en pacientes con cirrosis y várices esofágicas
Antecedentes
La cirrosis es un trastorno crónico del hígado. Los pacientes con cirrosis pueden desarrollar venas dilatadas en el esófago que pueden sangrar. La hemorragia de las várices es potencialmente mortal. La ligadura con banda es un procedimiento en el cual un instrumento para visualizar, o endoscopio, se inserta a través de la boca en el esófago y las várices luego se ligan en su base, de modo que se interrumpe el flujo de sangre. Las várices tienen que ser suficientemente grandes para permitir aplicar las bandas. Este procedimiento se puede realizar antes de que las várices del paciente sangren (prevención primaria) o después de que ha ocurrido una hemorragia (prevención secundaria).
Pregunta de la revisión
Se investigaron los efectos beneficiosos y perjudiciales de la ligadura con banda en comparación con ningún tratamiento para la prevención primaria de la hemorragia en pacientes con cirrosis y várices esofágicas al examinar los ensayos clínicos en los que los pacientes se asignaron de manera aleatoria (se seleccionaron al azar) a la ligadura con banda o a ningún tratamiento.
Fecha de la búsqueda
9 febrero 2019.
Fuentes de financiación de los ensayos
Dos de los ensayos incluidos no recibieron financiación o apoyo de compañías con fines de lucro; los cuatro ensayos restantes no proporcionaron información sobre este tema.
Características de los ensayos
Se incluyeron seis ensayos clínicos aleatorizados con 637 participantes. Todos los ensayos clínicos aleatorizados compararon la ligadura con banda con ningún tratamiento. Un ensayo incluyó pacientes con y sin cirrosis. La duración del tiempo que tomó erradicar las várices, cuando se informó, varió como promedio de 28 a 76 días.
Resultados clave
Los análisis demostraron un efecto beneficioso de la ligadura con banda sobre las tasas de muerte, hemorragia y eventos adversos graves en comparación con ningún tratamiento.
Certeza de la evidencia
En pacientes con cirrosis y várices esofágicas, el riesgo de muerte asociado con la hemorragia de las várices es muy alto, al igual que los riesgos de daños graves. La presente revisión encontró que la ligadura con banda reduce los riesgos de estos problemas en comparación con ningún tratamiento. Existe una confianza moderada en los cálculos de los efectos beneficiosos y perjudiciales de la ligadura con banda. Es poco probable que se realicen ensayos adicionales que comparen la ligadura con banda versus ninguna intervención.
Authors' conclusions
Summary of findings
| Band ligation compared to no intervention for primary prevention of upper gastrointestinal bleeding in adults with oesophageal varices | ||||||
| Patient or population: adults with oesophageal varices | ||||||
| Outcomes* | Anticipated absolute effects** (95% CI) | Relative effect (95% CI) | Number of participants (Studies (n)) | Certainty of the evidence | Comments | |
| Risk with no intervention | Risk with band ligation | |||||
| Mortality | Study population | RR 0.55 (0.43 to 0.70) | 637 | ⊕⊕⊕⊝ | Only one trial was at low risk of bias in the overall assessment. | |
| 407 per 1000 | 224 per 1000 | |||||
| Upper gastrointestinal bleeding | Study population | RR 0.44 (0.28 to 0.72) | 637 | ⊕⊕⊕⊝ | All trials were at high risk of bias in the overall assessment. | |
| 410 per 1000 | 180 per 1000 | |||||
| Serious adverse events | Study population | RR 0.55 (0.43 to 0.70) | 637 | ⊕⊕⊕⊝ | All trials were at high risk of bias in the overall assessment. | |
| 634 per 1000 | 349 per 1000 | |||||
| Variceal bleeding | Study population | RR 0.43 (0.27 to 0.69) | 637 | ⊕⊕⊕⊝ | All trials were at high risk of bias in the overall assessment. | |
| 385 per 1000 | 166 per 1000 | |||||
| Non‐serious adverse events | We could not perform meta‐analysis. However, the non‐serious adverse events reported in association with band ligation included oesophageal ulceration, dysphagia, odynophagia, retrosternal and throat pain, heartburn, and fever, and in the one trial involving participants with either small or large varices, the incidence of non‐serious side effects in the banding group was much higher in those with small varices with respect to ulcers: small versus large varices 30.5% versus 8.7%; heartburn 39.2% versus 17.4%. | |||||
| Health‐related quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | None of the six included trials described health‐related quality of life. |
| * All outcomes were assessed at the maximum duration of follow‐up **The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded the certainty of the evidence by one level due to the lack of trials with a low risk of bias. | ||||||
Background
Description of the condition
Portal hypertension is a very common and serious complication of cirrhosis. It develops as a result of increased vascular resistance to portal flow (D'Amico 1999). In people with cirrhosis this resistance develops as a result of an increase in liver stiffness secondary to the development of scar tissue and regenerating nodules within the hepatic parenchyma (Moreau 2006). In addition, changes occurring in the liver sinusoids also play a role. These mechanical factors account for approximately 70% of the increase in hepatic resistance to portal blood flow. The remaining 30% is due to active contraction of sinusoidal stellate cells, myofibroblasts in the portal tract and vascular smooth muscle cells in the hepatic vasculature and sinusoidal endothelial cell dysfunction, which increase vasoconstrictor drive (Bosch 2015; Brunner 2017; Iwakiri 2014). The increased pressure within the portal system causes blood to be redirected through vessels with less vascular resistance, in particular anastomoses or shunts between the portal and systemic vasculature. These 'portal‐systemic collaterals' can develop in several sites within the body; the most important being the lower end of the oesophagus where they appear, on endoscopy, as dilated tortuous submucosal veins or 'varices' protruding into the lumen. Portal hypertension is defined as an hepatic venous pressure gradient of more than 5 mm Hg. However, the risk of developing oesophageal varices does not increase until the pressure reaches 10 mm Hg (Ripoli 2007). Thus, a hepatic venous pressure gradient of 10 mm Hg or higher is termed 'clinically significant portal hypertension'. The most common cause of portal hypertension is cirrhosis but it can also develop in the absence of cirrhosis, a condition referred to as 'non‐cirrhotic portal hypertension.' The best known causes of non‐cirrhotic portal hypertension are vascular changes such as portal vein block or severe hepatic fibrosis short of cirrhosis, as seen in schistosomiasis.
The development of oesophageal varices is one of the most significant consequences of portal hypertension, as these vessels are prone to rupture, resulting in sometimes catastrophic gastrointestinal bleeding with a high associated morbidity and mortality. Approximately 30% of people with cirrhosis have oesophageal varices when diagnosed with liver disease (D'Amico 1995; D'Amico 1999; D'Amico 2007; De Lisi 2010). Varices develop at a rate of 5% to 9% per year in people without varices at presentation (Groszman 2005; Merli 2003 ); the rate of progression from small to large varices is about 10% per year (Merli 2003). Varices are more common in people with severe liver disease; thus, they are found in approximately one‐third of people with well‐compensated cirrhosis but in around 90% of people with severely decompensated disease (Kovalak 2007).
The incidence of variceal haemorrhage in people with cirrhosis and oesophageal varices is approximately 10% to 15% per year (Groszman 2005; NIEC 1988;). A number of risk factors for bleeding have been identified, including: (i) the severity of liver disease; (ii) the size of the varices and their endoscopic appearance ‐ large and pellucid varices with red whale markings (areas of thinning of the variceal wall), are more likely to bleed than small varices (D'Amico 1999; NIEC 1988); and, (iii) the degree of portal hypertension ‐ bleeding is more likely to occur when the hepatic venous pressure gradient is more than 12 mmHg (Groszmann 1990). Without some form of intervention, bleeding usually recurs within one to two years after an incident event (Bosch 2003).
Although the in‐hospital mortality associated with variceal bleeding has decreased in recent years due to improvements in endoscopic therapy and the use of antibiotic prophylaxis, the reported mortality rate still lies between 12% to 44%. The risk of death within six weeks of the initial variceal haemorrhage is below 10% in Child–Pugh Class A and greater than 32% in those in Child–Pugh Class C (Carbonell 2004).
Description of the intervention
As the incidence of variceal bleeding in people with cirrhosis and oesophageal varices is approximately 10% to 15% per year and the mortality rate associated with a first bleed is 12% to 44% then it is clear that prophylactic regimens to prevent bleeding are important (Garcia‐Tsao 2007; Garcia‐Tsao 2008). Drugs that reduce the portal flow or the hepatic vascular resistance, or both, such as non‐selective beta‐blockers will reduce the azygos blood flow and variceal pressure and have been shown to effectively prevent variceal bleeding and to reduce bleeding‐associated mortality (Lebrec 1981; Poynard 1991). However, approximately 15% of people with cirrhosis may have absolute or relative contraindications to the use of non‐selective beta‐blockers, for example, peripheral vascular diseases, diabetes mellitus, chronic obstructive pulmonary disease and asthma. Adverse effects, such as fatigue, weakness, and shortness of breath are common, and may result in the need to reduce the dose or even to discontinue the drug in a further 15% of people with cirrhosis (Longacre 2008). In addition, a long‐term satisfactory haemodynamic response is only obtained in 33% to 50% of treated patients ( Albillos 2007; Bosch 2003; García‐Pagán 1990; Reiberger 2013). Endoscopic obliteration of the varices provides an alternative management option (Gluud 2007; Tripathi 2007; (van Buuren 2003). Variceal sclerotherapy, which involves injecting a strong and irritating sclerosant or glue, is associated with serious adverse events including severe bleeding and oesophageal strictures (Schmitz 2001). Band ligation may provide a safer option (Gluud 2007).
How the intervention might work
Banding devices use a means of capturing the target tissue, in this case an oesophageal varix, while a small diameter circular band is deployed around its base (ASGE 2008). The band may be rubber, latex, or a similar material. The ligation procedure results in tight compression with vascular compromise leading to thrombosis, necrosis, and sloughing. As sufficient tissue needs to be captured in order to allow placement of the bands, this technique can not be used successfully in people with small varices and so is only used in people with medium to large varices. Previous banding devices used an overtube for the repeated intubation, allowing the placement of multiple bands (Collins 2001). The insertion of an overtube is associated with adverse events including perforation of the oesophagus (Gluud 2007; Gluud 2012; Wong 2000). At present, multiband devices (without an overtube) are used, resulting in considerably fewer adverse events (ASGE 2008). Several sessions of banding are normally required to completely eradicate the varices and are undertaken over a period of weeks; in addition, ongoing long‐term endoscopic surveillance is required to check for variceal recurrence.
Why it is important to do this review
The annual risk of people with cirrhosis developing varices, in European countries, is 7% to 8%, and the annual risk of bleeding from these varices is 5% to 15% (Asrani 2013). A number of pharmacological and endoscopic interventions have improved prognosis in patients with variceal haemorrhage, but six‐week mortality rates remain high at 15% to 20% ( Carbonell 2004; Chalasani 2003; D'Amico 2003; Hobolth 2010). The management of people with variceal bleeding is expensive, hence it is important to identify interventions that are both clinically effective and cost‐effective (Thabut 2007).
Several randomised clinical trials have found that band ligation has beneficial effects on a number of important outcomes, including mortality and gastrointestinal bleeding, when used for primary prevention in people with cirrhosis when compared to no intervention (Chen 1997; Lay 1997; Lo 1999; Sarin 1996; Svoboda 1999; Triantos 2005). Although these results have been confirmed in a number of meta‐analyses (Bedi 2000; Imperiale 2001; Triantos 2005; Vlachogiannakos 2000), these have invariably included trials involving a high proportion of participants with non‐cirrhotic portal hypertension. Thus, the benefit of band ligation for the primary prevention of gastrointestinal bleeding in patients with cirrhosis and oesophageal varices remains unclear. This systematic review with meta‐analyses aims to clarify this situation.
Objectives
To assess the beneficial and harmful effects of band ligation versus no intervention for primary prevention of upper gastrointestinal bleeding in adults with cirrhosis and oesophageal varices.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials regardless of their publication status, blinding or language in our primary analyses. If, during the selection of trials, we identified observational studies (i.e. quasi‐randomised studies; cohort studies; or case series) which reported adverse events caused by, or associated with, the interventions under review, and which included comparative control data, we included them for that purpose. We did not specifically search for observational studies for inclusion in this review, which is a known limitation.
Types of participants
We included adult participants (> 18 years) with cirrhosis and endoscopically verified oesophageal varices that had not bled, irrespective of the size of the varices or the hepatic venous pressure gradient. We did not include participants with non‐cirrhotic portal hypertension unless they comprised < 10% of the total population or separate analyses were provided for this participants subgroup.
Types of interventions
We compared band ligation versus no intervention. It would be very difficult to adequately double‐blind the banding procedure, and as the use of sham procedures would not be of any benefit to participants and might have an associated morbidity, their use might be considered unethical. We did not compare band ligation versus non‐selective beta‐blockers due to overlap with another review (Gluud 2012). We allowed effective cointerventions if administered equally to the intervention and control groups.
Types of outcome measures
We assessed all outcomes at the maximum duration of follow‐up.
Primary outcomes
-
All‐cause mortality
-
Upper gastrointestinal bleeding, using the definitions applied by primary investigators
-
Serious adverse events. We defined adverse events as any untoward medical occurrence and considered adverse events as serious if they resulted in death, were life‐threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, or resulted in persistent or significant disability or incapacity (ICH‐GCP 1997). In this review, serious adverse events included mortality and upper gastrointestinal bleeding, and we analysed them as a composite outcome (hbg.cochrane.org/information‐authors).
Secondary outcomes
-
Variceal bleeding
-
Non‐serious adverse events, defined as all adverse events that did not fulfil the criteria for serious adverse events (ICH GCP 1997)
-
Health‐related quality of life
Search methods for identification of studies
We combined the electronic and manual searches.
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (hbg.cochrane.org/specialised‐register; February 2019), the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 2) in the Cochrane Library, MEDLINE Ovid (1946 to February 2019), Embase Ovid (1974 to February 2019), LILACS (Bireme; 1982 to February 2019), Science Citation Index Expanded (Web of Science; 1900 to February 2019), and Conference Proceedings Citation Index – Science (Web of Science; 1990 to February 2019) (Royle 2003), using the strategies described in Appendix 1.
We did not have access to Chinese, Russian, or Japanese databases. We plan to search these additional databases in future updates, should they become available via the Cochrane Hepato‐Biliary Group.
Searching other resources
We searched the reference lists of papers identified in the electronic searches and the 2000 to 2018 conference proceedings of the British Society for Gastroenterology (BSG), the British Association for the Study of the Liver (BASL), the European Association for the Study of the Liver (EASL), the United European Gastroenterology Week (UEGW), the American Gastroenterological Association (AGA), and the American Association for the Study of Liver Diseases (AASLD). We wrote to the principal authors of randomised clinical trials and the device companies for additional information about completed randomised clinical trials and for information about any ongoing randomised clinical trials. We also searched online trial registries such as ClinicalTrials.gov (clinicaltrials.gov/), European Medicines Agency (EMA) (www.ema.europa.eu/ema/), World Health Organization (WHO) International Clinical Trial Registry Platform (www.who.int/ictrp), and the Food and Drug Administration (FDA) (www.fda.gov), for ongoing or unpublished trials. In addition, we searched Google Scholar using the terms (band* OR ligat*) AND bleed* AND varic* AND cirrhosis.
Data collection and analysis
We performed the review following the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), the Cochrane Hepato‐Biliary Group (hbg.cochrane.org/), and the MECIR guidelines (MECIR 2018).
Selection of studies
All review authors participated in the literature searches, identified trials eligible for inclusion, and participated in the decisions regarding the eligibility of trials for consideration. We reached the final selection through discussion; LGG acted as ombudsman where agreement could not be reached through discussion. We listed the excluded trials with the reason for their omission. For randomised clinical trials reported in more than one publication, we selected the paper reporting the longest duration of follow‐up as the primary reference.
Data extraction and management
Three review authors (SV, CWKY and MYM) independently extracted data from the included trials data and evaluated bias. The collected data included information on the following.
-
Trials: design (cross‐over or parallel), settings (number of clinical sites; outpatient or inpatient; inclusion period), country of origin; publication status; funding sources.
-
Participants: mean age, proportion of men, aetiology of cirrhosis, proportion with Child‐Pugh A/B/C; endoscopic findings; classification of varices (based on the primary authors' definition).
-
Interventions: equipment used; operator experience; technique; endpoint and whether achieved and within what time scale; number of banding sessions, number of bands used per session; cointerventions.
-
Outcomes: including definitions used in the assessment and duration of follow‐up; number of participants included in the assessment of outcomes (number of losses to follow‐up/withdrawals); outcomes included in the meta‐analyses.
We gathered the primary and secondary outcome data, including the criteria used in the definition of high and low risk varices, methods and definitions used to assess bleeding.
If we could not find the relevant data in the published trial reports, we wrote to the primary investigators to ask for the necessary information.
Assessment of risk of bias in included studies
We followed Cochrane Hepato‐Biliary Group recommendations for assessing the risk of bias in the included trials, based on the definitions described below (hbg.cochrane.org/information‐authors). We assessed each domain separately as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), and combined the domains into an overall score. We classified trials as low risk of bias only if none of the domains was designated as being at unclear or high risk of bias.
Allocation sequence generation
-
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
-
Uncertain risk of bias: the method of sequence generation was not specified.
-
High risk of bias: the sequence generation method was not random.
Allocation concealment
-
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
-
Uncertain risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
-
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants and personnel
-
Low risk of bias: blinding of participants and personnel performed adequately using a placebo. We defined lack of blinding as not likely to affect the evaluation of mortality (Savović 2012a; Savović 2012b).
-
Unclear risk of bias: insufficient information to assess blinding.
-
High risk of bias: no blinding or incomplete blinding.
Blinding of outcome assessors
-
Low risk of bias: blinding of outcome assessors performed adequately using a placebo. We defined lack of blinding as not likely to affect the evaluation of mortality (Savović 2012a; Savović 2012b).
-
Unclear risk of bias: there was insufficient information to blinding.
-
High risk of bias: no blinding or incomplete blinding.
Incomplete outcome data
-
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, were employed to handle missing data.
-
Uncertain risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
-
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
-
Low risk: the trial reported the following predefined primary outcomes ‐ mortality, upper gastrointestinal bleeding, and adverse events. If the original trial protocol was available, the outcomes should be those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.clinicaltrials.gov), the outcomes sought were those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes were not considered to be reliable.
-
Unclear risk: not all predefined outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
-
High risk: one or more predefined outcomes were not reported.
Other bias
-
Low risk of bias: the trial appeared to be free of other bias domains, including vested interests that could put it at risk of bias.
-
Uncertain risk of bias: the trial may or may not have been free of other domains that could put it at risk of bias.
-
High risk of bias: there were other factors in the trial that could put it at risk of bias.
Overall bias risk assessment
-
Low risk of bias: all domains were at low risk of bias, using the definitions described above.
-
High risk of bias: one or more of the bias domains were of unclear or high risk of bias.
Measures of treatment effect
We analysed dichotomous data using risk ratios (RRs) and continuous outcomes using mean differences (MDs), both with 95% confidence intervals (CIs). For meta‐analyses with statistically significant outcomes (based on the 95% CI), we calculated the number needed to treat for an additional beneficial outcome (NNTB) as 1/control risk*(1‐RR). We considered P values < 0.05 as significant.
Unit of analysis issues
We included randomised clinical trials using a parallel group design. In multiarmed trials, we analysed separate pair‐wise comparisons of the intervention of interest. We did not identify any cross‐over trials. However, if such trials are identified in future updates, we will only use data from the first treatment period (Higgins 2011).
Dealing with missing data
We extracted data on all randomised participants in order to allow intention‐to‐treat analyses. We undertook analyses to evaluate the influence of missing data (Higgins 2008); including, worst‐case scenario analysis, and extreme worst‐case and best‐case scenario analyses in which we include missing outcome data as treatment failures in the intervention group and successes in the control group and vice versa (hbg.cochrane.org/information‐authors).
Assessment of heterogeneity
We evaluated heterogeneity as I2 values using the following thresholds: 0% to 40% (unimportant), 40% to 60% (moderate), 60% to 80% (substantial), and more than 80% (considerable). We included this information in summary of findings Table for the main comparison.
Assessment of reporting biases
We planned to use visual inspection of funnel plots and regression analyses to evaluate reporting biases if our analysis included at least 10 trials with reported events (Egger 1997; Harbord 2006), However, our review did not reach this number threshold.
Data synthesis
Meta‐analysis
We performed our meta‐analyses and regression analyses using Review Manager 5 (Review Manager 2014), and STATA version 15 (STATA). We performed random‐effects and fixed‐effect meta‐analyses. The estimates of the random‐effects and fixed‐effect meta‐analyses were similar for all analyses so we assumed that any small trial effects had little influence on the intervention effect estimates. In random‐effects models, precision decreases with increasing heterogeneity and confidence intervals widen correspondingly. Accordingly, the random‐effects model provides a more conservative estimate of the intervention effect. Thus, we only report the results of the random‐effects meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses to evaluate the effect of banding in trials:
-
assessed as having a low risk compared to a high risk of bias;
-
involving participants with high risk compared to low risk varices;
-
involving participants in whom the oesophageal varices were completely obliterated compared to those in whom they were not.
We assessed all but one of the studies at high risk of bias for the outcome, mortality (Triantos 2005). However, this trial was stopped prematurely because the rate of bleeding in the intervention group was higher than expected ‐ at this point only 52 participants of the 214 needed for the trial to be adequately powered had been included. The trial was conducted single‐blind and was only classified at low risk for mortality because mortality is robust to blinding. We did not think a subgroup analysis was warranted. We assessed all of the studies at high risk of bias for non‐mortality outcomes and so we did not conduct subgroup analyses for these outcomes either. We were, however, able to analyse the differential effects of banding in people with small or large varices in one trial which provided the data separately. We also undertook post hoc subgroup analyses of trials involving participants with portal hypertension secondary to cirrhosis compared to one trial which also involved participants with non‐cirrhotic portal hypertension/portal vein block. We were not able to undertake post hoc subgroup analyses of trials by the degree and severity of the underlying liver injury because of significant inter‐trial heterogeneity and a lack of the necessary data.
Sensitivity analysis
We undertook a worst‐case scenario analysis and extreme worst‐case and best‐case scenario analyses as described in the section Dealing with missing data.
We compared our GRADE assessment of imprecision with the assessments obtained from the Trial Sequential Analysis.
Trial Sequential Analysis
We performed Trial Sequential Analysis of our primary outcomes to evaluate the risk of random error associated with sparse data and cumulative testing, and to evaluate futility (Higgins 2008; Wetterslev 2008). We defined the required information size (also known as the 'diversity‐adjusted required information size') as the number of participants needed to detect or reject an intervention effect based on the relative risk reduction (RRR) and the control group risk (CGR). The analyses show firm evidence if the Z‐curve crosses the monitoring boundary (also known as the 'trial sequential monitoring boundary ') before reaching the required information size. We used the upper CI to determine the RRR and the observed event rate in the control group. Based on the Cochrane Hepato‐Biliary Group recommendations, we set alpha to 2.5%, because of three primary outcomes, set power to 90%, and used model‐based heterogeneity.
'Summary of findings' tables
We used GRADEpro GDT 2015 to generate a 'Summary of findings' table with information about mortality, upper gastrointestinal bleeding, serious adverse events, variceal bleeding, non‐serious adverse events, and quality of life. The GRADE approach appraises the certainty of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The certainty of a body of evidence considers within‐study risk of bias, indirectness of the evidence (population, intervention, control, outcomes), unexplained inconsistency (heterogeneity) of results (including problems with subgroup analyses); imprecision of results, and risk of publication bias.
We defined the levels of evidence as 'high', 'moderate', 'low', or 'very low'. These grades are defined as follows.
-
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
-
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
We included six randomised clinical trials evaluating band ligation versus no intervention for primary prevention of variceal bleeding in 637 adults with cirrhosis (Chen 1997; Lay 1997; Lo 1999; Sarin 1996; Svoboda 1999; Triantos 2005). One randomised clinical trial included six additional participants (9% of the total number included) with non‐cirrhotic portal hypertension (Sarin 1996; Characteristics of included studies).
We excluded one observational study (Lim 2009), and two randomised clinical trials (Gameel 2005; Omar 2000; Characteristics of excluded studies).
Results of the search
We identified 2398 potentially relevant references in the electronic searches and 15 additional references in the manual searches. After excluding duplicates and records that were clearly irrelevant, we retrieved nine articles for detailed assessment. We excluded two randomised clinical trials because the majority of the participants had non‐cirrhotic portal hypertension (Gameel 2005; Omar 2000), and one further study because it was observational (Lim 2009). The remaining six randomised clinical trials fulfilled our inclusion criteria and we included them in the review (Chen 1997; Lay 1997; Lo 1999; Sarin 1996; Svoboda 1999; Triantos 2005). We displayed the results of the search in a flow diagram (Figure 1), as recommended (PRISMA 2009).

Study flow diagram.
Included studies
We included six randomised clinical trials, five published as full papers (Lay 1997; Lo 1999; Sarin 1996; Svoboda 1999; Triantos 2005), and one as an abstract (Chen 1997). One trial involving 52 participants with small or large varices was terminated prematurely because there were more upper gastrointestinal bleeding episodes in the intervention group than expected (Triantos 2005). The countries of origin were Taiwan (Chen 1997; Lay 1997; Lo 1999), India (Sarin 1996), the Czech Republic (Svoboda 1999), and Greece (Triantos 2005).
Two trials did not receive for‐profit funding (Svoboda 1999; Triantos 2005). The remaining four trials did not report on funding sources (Chen 1997; Lay 1997; Lo 1999; Sarin 1996).
Participants
Five randomised clinical trials involved 569 participants with cirrhosis and oesophageal varices that had not previously bled. The remaining randomised clinical trial, involved 68 participants with varices that had not bled, of whom six (9%) had non‐cirrhotic portal hypertension or portal vein block (Sarin 1996); the trial report did not describe outcomes for participants without (or with) cirrhosis separately. The mean age of the included participants ranged from 40.6 in Sarin 1996 to 61.5 years in Triantos 2005. The proportion of men ranged from 73.5% in Triantos 2005 to 84.3% in Lo 1999. Participants with alcohol‐related cirrhosis comprised 18.3% in Lay 1997 to 67.7% of cases in Svoboda 1999. Participants with Child‐Pugh class C made up 11.8% in Svoboda 1999 to 42.2% of the study cohort in Triantos 2005. Five trials included participants with medium to large oesophageal varices (Chen 1997; Lay 1997; Lo 1999; Sarin 1996; Svoboda 1999), while one study (Triantos 2005), included participants with small or large oesophageal varices. Three trials used the Japanese Research Society for Portal Hypertension system of variceal classification (Lay 1997; Lo 1999; Sarin 1996); one trial used the Paquet classification system (Svoboda 1999); one trial used an open forceps and oesophageal insufflation to measure variceal diameter and classified them as small (< 5mm) or large (> 5mm); no information was provided on variceal classification in the final study (Chen 1997; Table 1). Three trials assessed the risk of variceal bleeding (Lay 1997; Lo 1999; Sarin 1996), using a standardised system (Beppu 1981).
| Trial | Inclusion criteria | Assessment of varices | Randomisation by variceal characteristics | Gastric varices or portal hypertensive gastropathy |
| Not reported | Not stipulated | Not reported | Not reported | |
| Participants were assessed for risk of bleeding (Beppu 1981), using criteria defined by the Japanese Research Society for Portal Hypertension (Inokuchi 1980). The included participants had blue varices of at least F2 or F3 size with at least one of the following: cherry‐red spots (++, +++), red wale markings (++, +++), haematocystic spots (+) | Not stipulated | Not described | Participants with gastric or ectopic varices at recruitment were excluded. During follow‐up, 4 (6%) participants in the banding group and 3 (5%) in the control group developed gastric varices. | |
| F2 or F3, associated with a moderate degree of red colour signs (red wale markings, cherry‐red spots or haematocystic spots (Beppu 1981)) | Not stipulated | F2 Banding: 27/64 (42%) No intervention: 30/63 (48%) F3 Banding: 37/64 (58%) No intervention: 33/63 (52%) Red colour signs moderate Banding: 33/64 (52%) No intervention: 36/63 (57%) Red colour signs severe Banding: 31/64 (48%) No intervention: 27/63 (43%) | Participants with gastric varices at recruitment were excluded. During follow‐up, 8 (12%) participants in the banding group and 3 (5%) in the no intervention group developed gastric varices. During follow‐up, 1 (1.6%) patient in the banding group and 2 (3.2%) in the no intervention group developed gastropathy. | |
| Participants with large varices > 5 mm were assessed for risk of bleeding (Beppu 1981), using criteria defined by the Japanese Research Society for Portal Hypertension (Inokuchi 1980). The included participants had blue varices of at least F2 or F3 size with one or more red colour signs; (cherry‐red spots, red wale markings or haematocystic spots) | Variceal size and grade assessed by two independent observers | Not reported | The presence/absence of gastric varices was recorded at initial assessment; no further mention and so absence is assumed. Portal hypertensive gastropathy present in 3 (8.6%) participants at inclusion and developed in a further two postbanding. | |
| Grade III or IV, or grade II with signs of high risk, classified using the Paquet’s system (Paquet 1978) | Not stipulated | Grade II Banding: 2/52 (4%) Control: 1/50 (2%) Grade III Banding: 36/52 (69%) No intervention: 38/50 (76%) Grade IV Banding: 14/52 (27%) No intervention: 11/50 (22%) | Not described | |
| Varices of any size: Small varices: < 5 mm diameter Large varices: diameter of largest varix > 5 mm Measured with open forceps and not disappearing on oesophageal insufflation | Assessed endoscopically by two independent observers | Small varices Banding: 14/25 (56%) No intervention: 17/27 (63%) Large varices Banding: 11/25 (44%) No intervention: 10/27 (37%) Red spots Banding: 9/25 (36%) No intervention: 8/27 (30%) | Gastric varices present at inclusion Banding: 2/25 (8%) No intervention: 1/27 (4%) No further participants in the banding group developed gastric varices during follow‐up; two participants in whom the varices were obliterated appear to have developed portal hypertensive gastropathy from which they bled. |
Japanese Research Society for Portal Hypertension classification (Inokuchi 1980) (Form: F1‐ straight varices; F2‐ enlarged tortuous varices; F3‐ largest sized varices; fundamental colour: Cw ‐ white varices; Cb ‐ blue varices: red colour signs: RC(‐) ‐ red colour signs negative; RC(+) ‐ red colour signs positive; red wale marks: RWM ‐ (+), (++), (+++); cherry‐red spots: CRS ‐ (+), (++), (+++); haematocystic spot: HCS: diffuse redness: DR; Group A: both red wale markings and cherry‐red spots were negative or mild (+); Group B: both red wale markings and cherry‐red spots were moderate (++) or severe (+++). Location: li ‐ locus inferior; Lm ‐ locus medialis; Ls ‐ locus superior
Paquet classification: 0 ‐ no varices; I ‐ varices that disappear with insufflation; II ‐ larger, usually straight, visible varices that disappear with insufflation; III ‐ more prominent coil‐shaped varices, occupying part of the lumen; IV ‐ tortuous varices occupying the lumen (Paquet 1978).
Interventions and comparators
All six trials evaluated band ligation of oesophageal varices (Table 2). Four randomised clinical trials used an overtube (Lay 1997; Lo 1999; Svoboda 1999; Sarin 1996), while one used a multiband device (Triantos 2005). One study did not specify the type of ligator used (Chen 1997). In all six trials, the banding sessions were repeated until the varices were eradicated or were too small to ligate. The comparator was no intervention.
| Banding | ||||||
| Equipment | Not described | Endoscopic ligating device (Bard Interventional Products, Billerica, MA, USA) with a 25 cm overtube (Olympus XQ 20, Tokyo, Japan) | Endoscopic ligating device (Bard Interventional Products, Billerica, MA, USA) with a 25 cm overtube (Olympus XQ 20, Tokyo, Japan) | Endoscopic ligating device and a 25 cm overtube (Bard Inteventional Products, Tewksbury MA, USA) | Endoscopic ligation device (Suction oesophageal varices | Multiband Ligator 6 shooter (Wilson‐Cook, Limerick, Ireland) |
| Operator experience | Not described | Ligation was performed by two | Ligation was performed by two | Not described | Ligation was performed by two experienced endoscopists; each of whom had performed | Ligation was performed by four experienced endoscopists each of whom had performed 100 ligation |
| Technique | ‐Variceal ligation performed at 2‐ to 3‐ week intervals | ‐ Ligation was performed at 1 cm to 5 cm above the gastroesophageal junction; each varix was ligated with 1 to 3 rubber bands to a maximum of 10 bands/session ‐ Procedure repeated weekly for the first 3 weeks, if possible and then every 2 weeks ‐ Follow‐up endoscopy repeated every 3 months after eradication | ‐ Ligation was performed at 1 cm to 5 cm above the gastroesophageal junction; each varix was ligated with 1 to 2 rubber bands ‐ Procedure repeated at intervals of 3 weeks ‐ Follow‐up endoscopy repeated every 3 months after eradication | ‐ Varices ligated 1 cm to 2 cm above the gastroesophageal junction; 1 to 2 bands applied to each variceal column between the lower 4 cm to 5 cm of the oesophagus; every variceal column was ligated at each session ‐ Procedure repeated at 7‐ to 10‐day intervals ‐ Follow‐up endoscopy repeated every 3 months after eradication | ‐ The largest number possible (up to ‐ The first three therapeutic sessions were performed at 2‐week intervals then monthly ‐ Follow‐up endoscopy repeated every 3 months after eradication ‐ Participants in the no treatment group were endoscoped every 3 months | ‐ Bands were placed starting at the gastroesophageal junction and then proximally in a helical fashion for approximately 5 cm, putting at least one band on each varix ‐ Subsequent sessions scheduled at 14‐day intervals ‐ Participants in the no intervention group were endoscoped yearly |
| Endpoint | Variceal eradication | Variceal eradication | Varices obliterated or too small to be ligated | Variceal obliteration or decreasing the size to grade 1 (not possible to suck in varix for band ligation) | Varices too small to treat | Eradication or varices too small to ligate (no effect of suction) |
| Achievement of endpoint | 71/80 (88.7%) | 62/62 (100%) | 55/64 (86%) | Banding successful in all participants, except for those who died before complete eradication (numbers not specified) | 42/52 (81%) (includes 8 eradicated, 34 too small to band) | 20/25 (80%) |
| Reasons for failure | Not reported | Not applicable | Reluctance (3) Asthenia (2) Aspiration pneumonia (1) Encephalopathy (1) Hepatic failure (2) | Death due to hepatic coma or bleeding (numbers not specified) | Not reported | Bleeding (3) |
| Mean (± 1 SD) number of sessions to achieve obliteration | 2.9 ± 0.7 | 3.6 ± 1.7 Mean examinations 5.1 ± 2.8 | 2.9 ± 0.5 (range 2 to 5) | 3.2 ± 1.2 | 4.8 ± 1.8 | Median 2 (1 to 4) Small varices, median 1 (1 to 4) Large varices, median 2 (1 to 3) |
| Mean (± 1 SD) time to achieve obliteration | Not reported | 75.6 ± 28.4 days | 40 ± 4 days | 4.9 ± 2.2 weeks | Not reported | Median 28 (14 to 101) days |
| Number of bands each session | Not reported | Maximum did not exceed 10 bands per treatment session | 1 to 2 per varix (mean not specified) | Each variceal column ligated with one to two bands (mean not specified) | Up to 6 | Median: 4 (2 to 7) per session |
| Recurrent varices | Not reported | 26/62 (42%) (of which 4 had a second recurrence) | 12 (21.8%) | 10 (28.6%) | 16 (31.0%) | 7 (35%) 3/11 with small varices and 4/8 with large |
Japanese Research Society for Portal Hypertension classification (Inokuchi 1980) (Form: F1‐ straight varices; F2‐ enlarged tortuous varices; F3‐ largest sized varices; fundamental colour: Cw ‐ white varices; Cb ‐ blue varices: red colour signs: RC(‐) ‐ red colour signs negative; RC(+) ‐ red colour signs positive; red wale marks: RWM ‐ (+), (++), (+++); cherry‐red spots: CRS ‐ (+), (++), (+++); haematocystic spot: HCS: diffuse redness: DR; Group A: both red wale markings and cherry‐red spots were negative or mild (+); Group B: both red wale markings and cherry‐red spots were moderate (++) or severe (+++). Location: li ‐ locus inferior; Lm ‐ locus medialis; Ls ‐ locus superior
Paquet classification: 0 ‐ no varices; I ‐ varices that disappear with insufflation; II ‐ larger, usually straight, visible varices that disappear with insufflation; III ‐ more prominent coil‐shaped varices, occupying part of the lumen; IV ‐ tortuous varices occupying the lumen (Paquet 1978).
In one trial, participants in both groups were given the oral angiotensin‐converting enzyme (ACE) inhibitor enalapril, and later quinapril, to reduce portal pressure (Svoboda 1999). In two studies, participants in the banding group were given sucralfate (Lay 1997; Lo 1999).
Outcomes
All six trials reported on mortality, upper gastrointestinal bleeding, variceal bleeding and severe adverse events. Four of the six trials reported non‐serious events in the band ligation group (Lo 1999; Sarin 1996; Svoboda 1999; Triantos 2005), but only one reported non‐serious events in the no intervention group for comparison (Svoboda 1999). No trials reported on health‐related quality of life.
Four studies reported the mean duration of follow‐up which ranged from 13 months in Lay 1997 to 25 months in Svoboda 1999. Two studies reported the median duration of follow‐up as 29 months in Lo 1999 and 32 months in Chen 1997.
Excluded studies
We excluded one observational study evaluating the use of band ligation for primary prevention of variceal bleeding in people with cirrhosis who were on the waiting list for liver transplantation (Lim 2009). The study included 300 participants, of whom 258 did not have a history of variceal bleeding; 101 participants were deemed to have high risk varices and underwent primary prophylaxis. Data was retrospectively collected from patient notes and endoscopy databases. There were two reported variceal bleeds and three episodes of ulcer‐related bleeds. One participant developed a mild oesophageal stricture. There were no reported deaths.
We excluded two randomised clinical trials because the majority of the included participants had portal hypertension secondary to schistosomal liver disease. The first of these trials involved 74 participants with non‐alcoholic cirrhosis and/or schistosomal hepatic fibrosis and large oesophageal varices (Omar 2000). The proportion of participants without cirrhosis was not reported. Thirty‐six participants were randomised to band ligation while 38 participants received no intervention. There were no bleeding‐related deaths. There was no significant difference in the occurrence of variceal bleeding in the two groups during the 14‐month follow‐up period; one participant in the ligation group and four in the no intervention group had a bleeding episode. Complications of band ligation were minor and included variceal ulcers (44.4%), retrosternal pain (33.3%), and low‐grade fever (19.4%). No major complications were reported.
The second trial involved a three‐way comparison of band ligation, sclerotherapy and no intervention in 50 participants with schistosomal portal hypertension and high risk varices, 42 (84%) of whom also had cirrhosis secondary to chronic viral hepatitis (Gameel 2005). One liver‐related death occurred in the ligation group and one bleeding‐related death in the control group. Two further bleeding episodes were reported in the control group. Two participants in the banding group developed oesophageal ulceration.
Risk of bias in included studies
Based on our overall assessment, we classified one trial at low risk of bias for the assessment of mortality but at high risk of bias for the remaining outcomes (Triantos 2005), while we assessed the remaining five trials at high risk of bias for all outcomes (Chen 1997; Lay 1997; Lo 1999; Sarin 1996; Svoboda 1999; Figure 2; Figure 3).
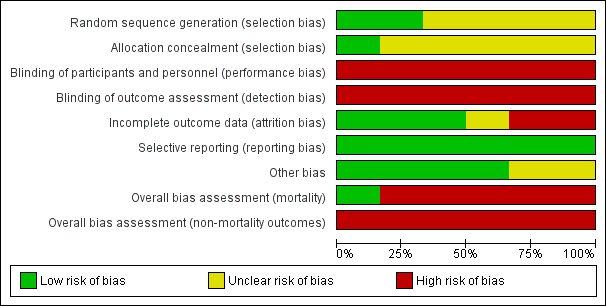
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In two randomised clinical trials, investigators used a computer (Lo 1999), or table of random numbers (Triantos 2005), to generate the allocation sequence. One trial used sealed opaque envelopes, opened in a numbered sequence, to conceal allocation (Triantos 2005); one used sealed envelopes to conceal the allocation but did not stipulate if they were opaque or serially numbered (Lay 1997). The remaining trials did not describe the allocation sequence generation or allocation concealment (Chen 1997; Svoboda 1999; Sarin 1996).
Blinding
All six randomised clinical trials were open without blinding; in none was the outcome assessment blinded.
Incomplete outcome data
In two trials (Lay 1997; Sarin 1996), there were no missing outcome data and all the participants were included in the analyses. In one trial (Triantos 2005), two participants randomised to band ligation refused to undergo the procedure but were still included in the primary analyses but were excluded from the reporting of the complications of banding. Two trials (Lo 1999; Svoboda 1999), described losses to follow‐up but excluded them from the analyses. One study did not describe participant losses (Chen 1997).
Selective reporting
Clinically relevant outcomes were defined and reported in all six trials. We did not have access to the protocols for any of the included trials.
Other potential sources of bias
In two of the six randomised clinical trials (Lay 1997; Lo 1999), sucralfate was given to the patients in the banding group; this is not an effective medication for preventing upper gastrointestinal bleeding in patients with oesophageal varices but its use may be associated with improved healing of iatrogenic ulceration (Yang 1998)
Effects of interventions
All randomised clinical trials (637 participants) reported mortality (Analysis 1.1); upper gastrointestinal bleeding (Analysis 1.3); variceal bleeding (Analysis 1.7), and serious adverse events (Analysis 1.5). Non‐serious adverse events were inconsistently reported and hence not amenable to meta‐analysis (Table 3). None of the trials reported on health‐related quality of life.
| Trial | Participants allocated to band ligation (n) | Participants allocated to no intervention (n) | Non‐serious adverse event in participants allocated to band ligation |
| 80 | 76 | Not reported | |
| 62 | 64 | Not reported | |
| 66* | 67* | Banding: oesophageal ulceration without bleeding (n = 16 (24%)), transient dysphagia (n = 7 (11%)), retrosternal pain (n = 5 (8%)), pleural effusion (n = 2 (3%)), fever > 38oC (n = 2 (3%)) No intervention: not reported | |
| 35 | 33 | Banding: oesophageal ulceration without bleeding (n = 24 (69%)), throat pain (n = 12 (34%)); retrosternal pain (n = 8 (23%)), dysphagia (n = 6 (17%)), fever (n = 4 (11%)) No intervention: not reported | |
| 52 | 50 | Banding: ulcer (n = 2 (4%)), dysphagia (n = 3 (6%)), odynophagia (n = 1 (2%)), others (n = 4 (8%)) No intervention: ulcer (n = 0), dysphagia (n = 4 (8%)), odynophagia (n = 2 (4%)), others (n = 1 (2%)) | |
| 25** | 27 | Banding: small varices: ulcers (n = 7 (30.5%)), dysphagia (n = 5 (21.7%)), heartburn (n = 9 (39.2%)), chest pain (n = 3 (13.0%)) large varices: ulcers (n = 2 (8.7%)), dysphagia (n = 5 (21.7%)), heartburn (n = 4 (17.4%)), chest pain (n = 2 (8.7%)), fever (n = 1 (8.7%)) No intervention: not reported |
* Three participants in the banding and three participants in the no intervention groups were lost to follow‐up. The authors omitted these 6 participants providing data on only 63 in the banding and 64 in the no intervention groups in their main analyses; we have included the number randomised in our analyses and recalculated the percentages of people with non‐serious adverse effects using the full data set.
** Two participants allocated to band ligation refused the procedure; the authors provide data on the complications which arose in the 23 participants who did undergo the procedure.
Mortality
Band ligation had a beneficial effect on mortality when all six trials were included; overall 71 of 320 participants undergoing band ligation died compared to 129 of 317 participants in the no intervention group (risk ratio (RR) 0.55, 95% confidence interval (CI) 0.43 to 0.70; I2 = 0%; Analysis 1.1). The number needed to treat to benefit (NNTB) to avoid one death is six persons. Subgroup analyses found no differences between the five trials including participants with cirrhosis and the one trial including participants with cirrhosis or non‐cirrhotic portal hypertension/portal vein block (test for subgroup differences P = 0.78; Analysis 1.1), or between the five trials involving participants with medium to large varices and the one trial involving participants with small or large varices (test for subgroup differences P = 0.56; Analysis 1.2)). Worst‐case and extreme worst‐case scenario analyses of all six randomised clinical trials found a beneficial effect of band ligation on mortality (Analysis 2.1), as did the Trial Sequential Analysis when setting the relative risk reduction (RRR) at 30%, control group risk (CGR) at 40%, and the heterogeneity correction at 0% (Figure 4).
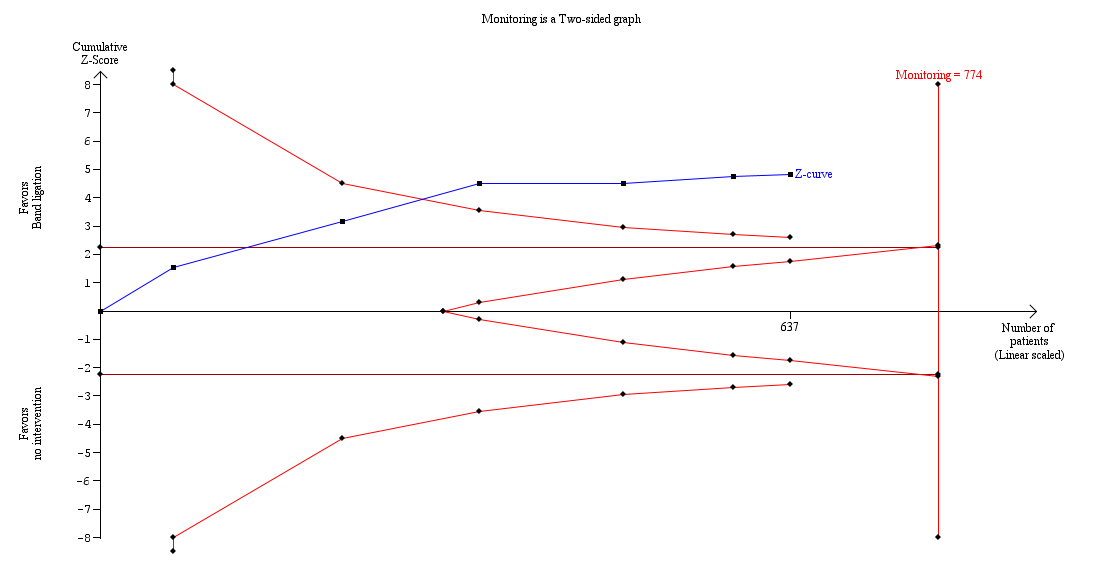
Trial Sequential Analysis of meta‐analysis including six randomised clinical trials evaluating the effect of band ligation versus no intervention on mortality (RR 0.55, 95% CI 0.43 to 0.70; 637 participants; I2 = 0%).
The analysis found that the blue Z‐curve crossed the trial monitoring boundary.
Upper gastrointestinal bleeding
Band ligation had a beneficial effect on upper gastrointestinal bleeding when all six trials were included (RR 0.44, 95% CI 0.28 to 0.72; I2 = 61%; NNTB = 5 persons; Analysis 1.3). Subgroup analyses found no difference between the five trials including participants with cirrhosis and the one trial including participants with cirrhosis or non‐cirrhotic portal hypertension/portal vein block (test for subgroup differences P = 0.22; Analysis 1.3), but identified a difference between the five trials involving participants with medium to large varices where the risks were reduced, and the one trial involving participants with small or large varices where no risk reduction was seen (test for subgroup differences P = 0.006; Analysis 1.4 ).
The worst‐case and extreme worst‐case analyses, including all six trials showed that, on balance, band ligation reduced upper gastrointestinal bleeding (Analysis 2.2). In the Trial Sequential Analysis, including all six trials, and setting the RRR at 28%, CGR at 41%, and the heterogeneity correction at 67%, the Z‐curve crossed the monitoring boundary, suggesting that band ligation has a beneficial effect on upper gastrointestinal bleeding (Figure 5).

Trial Sequential Analysis including six randomised clinical trials evaluating the effect of band ligation versus no intervention on upper gastrointestinal bleeding (RR 0.44, 95% CI 0.28 to 0.72; participants = 637; I2 = 61%).
The analysis showed that the Z‐curve crossed the trial monitoring boundary.
Serious adverse events
Overall, 108 of 320 participants who underwent band ligation experienced a serious adverse event compared to 201 of the 317 participants who received no intervention (RR 0.55, 95% CI 0.43 to 0.70; I2 = 44%; NNTB = 4 persons; Analysis 1.5). Subgroup analyses found no difference in the five trials involving participants with cirrhosis and the one trial involving participants with cirrhosis or non‐cirrhotic portal hypertension/portal vein block (test for subgroup differences (P = 0.28; Analysis 1.5), or in the five trials involving participants with medium to large varices and the one trial involving participants with small or large varices (test for subgroup differences P = 0.30; Analysis 1.6). Worst‐case and extreme worst‐case scenario analyses including all six trials showed that band ligation reduced serious adverse events (Analysis 2.3). In the Trial Sequential Analysis, including all six trials and setting the RRR at 30%, CGR at 60%, and the heterogeneity correction at 79%, the Z‐curve crossed the monitoring boundary, suggesting that band ligation has a beneficial effect on serious adverse events (Figure 6).

Trial Sequential Analysis including six randomised clinical trials evaluating the effect of band ligation versus no intervention on serious adverse events (RR 0.55, 95% CI 0.43 to 0.70; 637 participants; I2 = 44%).
The analysis showed that the Z‐curve crossed the trial monitoring boundary.
Variceal bleeding
Band ligation was associated with a lower risk of variceal bleeding (RR 0.43, 95% CI 0.27 to 0.69; I2 = 56%; NNTB = 5 persons; Analysis 1.7). Subgroup analysis found no difference between the five trials involving participants with cirrhosis and the one trial involving participants with cirrhosis or non‐cirrhotic portal hypertension/portal vein block (test for subgroup differences P = 0.24; Analysis 1.7), but found a difference between the five trials involving participants with medium to large varices where the risks were reduced and the one trial involving participants with small or large varices where no risk reduction was seen (test for subgroup differences P = 0.01; Analysis 1.8). The worst‐case and extreme worst‐case scenario analyses found a beneficial effect of band ligation on variceal bleeding (Analysis 2.4).
Non‐serious adverse events
Non‐serious adverse events were reported in participants in the band ligation group in four trials (Lo 1999; Sarin 1996; Svoboda 1999; Triantos 2005), but events in the no treatment group were only reported in one (Svoboda 1999). We were not able to undertake a meta‐analysis of these data. The non‐serious adverse events reported in association with band ligation included: oesophageal ulceration, dysphagia, odynophagia, retrosternal and throat pain, heartburn and fever (Table 3). In the one study involving participants with either small or large varices (Triantos 2005), the incidence of non‐serious side effects in the banding group was much higher in those with small varices in relation to ulcers: small versus large varices 30.5% versus 8.7%; heartburn 39.2% versus 17.4%.
Health‐related quality of life
None of the six included trials described health‐related quality of life outcomes.
Sensitivity analyses
We found no differences between our GRADE assessment of imprecision and that of the Trial Sequential Analysis.
'Summary of findings' table
We presented the results of mortality, upper gastrointestinal bleeding, serious adverse events, variceal bleeding, non‐serious adverse events, and health‐related quality of life in summary of findings Table for the main comparison. We downgraded the certainty of the evidence, for all outcomes, by one level to 'moderate' due to a high risk of bias.
Discussion
Summary of main results
Our review found a beneficial effect of band ligation on mortality, upper gastrointestinal bleeding, including variceal bleeding, and serious adverse events. Reporting of non‐serious adverse events was inconsistent and often incomplete across trials, generally unclear and potentially subject to reporting bias. No information was available on health‐related quality of life. None of the trials was conducted double‐blind and the certainty of the evidence was moderate due to the lack of trials with adequate bias control.
In spite of the fact that many trials were undertaken several years ago using banding devices with an overtube, the analyses found an effect on mortality. The analyses of upper gastrointestinal bleeding and variceal bleeding showed between‐trial heterogeneity, which could reflect the inclusion of participants with small varices in one trial (Triantos 2005).
Overall completeness and applicability of evidence
Our review included six randomised clinical trials, involving 637 people, published between 1996 and 2005. We were able to extract primary outcome data from all six trials.
There were inter‐trial differences in the aetiology and severity of the liver disease and this may have affected outcomes. The proportion of participants with alcohol‐related liver disease, in the five trials which provided details, ranged from 18.3% in Lay 1997 to 67.7% in Svoboda 1999. None of the trials reported on drinking behaviour during the follow‐up period which is a significant determinant of outcome (Lucey 2008; Saunders 1981; Xie 2014). In the five trials with available data, the proportion of participants with Child's Grade C cirrhosis ranged from 11.8% in Svoboda 1999 to 42.3% in Triantos 2005. The risk of death within six weeks of the initial variceal haemorrhage increases with the severity of liver disease reaching 32% in people with the most severely decompensated disease (Carbonell 2004). Unfortunately, outcomes were not stratified by either the aetiology or severity of the underlying liver disease in the individual trial reports, thus precluding any form of meaningful subgroup analyses.
The classification system used to stratify variceal size also differed between trials. Of the five trials which provided this information three used the Japanese Research Society for Portal Hypertension (JRSPH) classification system (Lay 1997; Lo 1999; Sarin 1996), whilst one study used the Paquet classification (Svoboda 1999). While these classifications are valuable in helping to predict the risk of variceal haemorrhage (with risk increasing with variceal size and preponderance of red signs), there have been no head‐to‐head comparisons between the systems (Rigo 1992). Of note, the Paquet system has not been validated since its formulation. The fifth trial classified the varices in relation to their size measured with an open forceps and their response to oesophageal insufflation (Triantos 2005); varices with a diameter of < 5 mm were classified as small while those with a diameter of > 5mm were classified as large. The authors comment on the presence or absence of red signs but do not provide information on how these were defined.
The majority of participants in the included trials had varices considered to be at high risk of bleeding (Chen 1997; Lay 1997; Lo 1999; Sarin 1996; Svoboda 1999). However, in one study (Triantos 2005), 59.6% of participants had small varices likely to be at low risk of bleeding. Indeed, the incidence of upper gastrointestinal bleeding was significantly higher in participants with large rather than small varices (29% versus 3%; Fischer's exact test P = 0.013), but the risk of bleeding did not differ between the banding and no intervention groups (large varices 57% versus 25%, P = 0.64; small varices 7% versus 0%, P = 0.45). This study was stopped prematurely because of the higher than expected bleeding rate in the banding group. At the time of trial cessation, however, there was no significant difference in bleeding rates in the banding (20%) and no intervention (7.4%) groups. Two of the five participants in the banding group who bled had developed portal hypertensive gastropathy following successful obliteration of their varices and this was the source of the bleeding in both; one further patient bled following insertion of the endoscope during a banding session while the final participants developed banding ulceration, although the authors still classified the bleed as variceal. Other factors, such as severity of liver disease, may have been important as this study included the highest proportion of participants with Child's Grade C cirrhosis (42%). In addition, all the participants had contraindications to or were intolerant of beta‐blocker therapy. Thus, increased bleeding rates may reflect pathophysiological differences in this particular trial population. Nevertheless, the authors suggest that most of the bleeding was probably iatrogenic.
Band ligation techniques and protocols varied across the trials. One study did not specify the ligation device used (Chen 1997). In four of the remaining five trials (Lay 1997; Lo 1999; Sarin 1996; Svoboda 1999), an overtube was used in all participants or in an unspecified proportion of the study cohort. In one study (Svoboda 1999), a multiband ligation device without an overtube was used in a proportion of the patients, while a similar device was used exclusively in the latest trial (Triantos 2005). Ligation devices with an overtube are recognised to have a worse adverse effect profile and to be associated with higher rates of oesophageal injury, including varix rupture (Wong 2000). However, the authors of the trial in which banding was undertaken, either with a ligation device with an overtube or with a multiband device without an overtube, did not comment on differences in outcomes between procedures (Svoboda 1999), and the trial that used the multiband device exclusively (Triantos 2005), did not provide data on the adverse events profile in the no intervention group.
Endoscopic variceal ligation is an operator‐dependent procedure (Bohnacker 2000), although the extent to which it is so is unclear (Stiegmann 1989; Triantos 2006). Operator experience before the start of the trials varied significantly from 10 ligation sessions in Lay 1997 and Lo 1999 to ≥ 300 sessions of endoscopic interventions in Svoboda 1999. Operator experience was not described in the remaining two trials (Chen 1997; Sarin 1996;). However, there were no significant differences in the frequency of serious adverse events in the trials with the less experienced operators at 40% in Lay 1997 and 39% in Lo 1999, and the trial with the most experienced operators at 42% (Svoboda 1999). Likewise there did not appear to be any significant difference in the frequency of non‐serious adverse events between trials employing less experienced operators in Lo 1999 or more experienced operators in Svoboda 1999. (See Table 3).
The number of bands applied per varix, the maximum number of bands applied per session and the number of sessions undertaken were inconsistently reported, and where reported, differed between trials (Table 2). In the majority of trials, one to three bands were applied to each varix or variceal column at each session; the maximum number of bands applied was only specified in two studies as six in Svoboda 1999 and 10 in Lay 1997. Sessions were undertaken at varying time intervals, ranging from every seven to 10 days in Sarin 1996 to every three weeks in Lay 1997 and Lo 1999. Banding was performed until varices were eradicated or were too small to ligate in all six trials. This endpoint was reached in two trials (Lay 1997; Sarin 1996). Eradication rates ranged from 80% in Triantos 2005 to 89% in Chen 1997 in the remaining four trials (Chen 1997; Lo 1999; Svoboda 1999; Triantos 2005). The mean number of sessions required to reach this endpoint ranged from two in Triantos 2005 to 4.8 in Svoboda 1999. The differences in success rates for variceal obliteration may reflect differences in operator proficiency, ligation protocol or participant willingness to comply (Table 2). Follow‐up endoscopy was undertaken every three months after the finish of the band ligation in the four trials which provided this information (Lay 1997; Lo 1999; Sarin 1996; Svoboda 1999).
The duration of follow‐up varied between trials from a mean of 13 months in Lay 1997 to a median of 32 months in Chen 1997. As most episodes of variceal haemorrhage occur within the first two years (Burroughs 1986), follow‐up schedules shorter than this may underestimate the effect size of any benefit afforded by ligation prophylaxis.
Sucralfate was given to participants undergoing band ligation in two trials (Lay 1997; Lo 1999). The use of sucralfate is associated with improved oesophageal ulcer healing in patients undergoing endoscopic variceal sclerotherapy, but there is no evidence that it affects bleeding rates (Yang 1998). There are no trials on the use of sucralfate in people undergoing variceal band ligation so we included these two trials as the use of sucralfate was unlikely to affect any of our primary outcomes.
We planned to undertake a series of subgroup analyses. However, none was possible. Thus, apart from one trial which we classified at low risk of bias for mortality (Triantos 2005), we otherwise assessed the included trials at high risk of bias, so we could not explore outcomes in relation to the stratification of risk bias. We were also unable to perform subgroup analyses based on the characteristics of the varices in relation to their risk of bleeding as all but one of the trials included patients with medium to large varices judged to be at high risk. We were, however, able to analyse the differential effects of banding in people with small or large varices in one trial (Triantos 2005). This showed that although the incidence of upper gastrointestinal bleeding was significantly higher in participants with large rather than small varices, the risk of bleeding did not differ between the banding and no treatment groups. However, this study was terminated prematurely because the number of bleeding episodes in the banding group was higher than expected. Finally, we were unable to undertake subgroup analyses based on the completeness of the variceal obliteration as insufficient data were provided within trials and because the variceal recurrence rates were high, ranging from 21.8% in Lo 1999 to 42.0% in Lay 1997, nor were we able to undertake subgroup analyses based on the aetiology and severity of the liver disease.
This review included participants with portal hypertension secondary to chronic liver disease. We excluded studies in people with portal hypertension associated with schistosomiasis, portal/splenic vein thrombosis, Budd‐Chiari syndrome and other rarer conditions of pre‐ or postsinusoidal block. However, one included trial contained a small number of participants, amounting to < 10% of the total, with non‐cirrhotic portal hypertension/portal vein block (Sarin 1996). We undertook a post hoc subgroup analyses of trials involving participants with portal hypertension secondary to cirrhosis compared to this one trial and found no essential differences in our primary outcomes. The Baveno guidelines state that there are insufficient data on whether beta‐blockers or endoscopic therapy should be preferred for primary prophylaxis in people with extrahepatic portal vein block/idiopathic portal hypertension and suggest that the guidelines for cirrhosis should be applied (Baveno VI 2015).
This review showed that when used for primary prophylaxis for upper gastrointestinal bleeding in patients with cirrhosis, who have not bled previously, band ligation has beneficial effects on mortality, upper gastrointestinal bleeding, including variceal bleeding, and serious adverse events compared to no intervention. Current recommendation stipulates that the benefit of ligation extends only to the treatment of medium to large varices.
Certainty of the evidence
The main reason for downgrading the evidence in this review is bias. As recommended, we combined the individual bias domains in an overall assessment (hbg.cochrane.org/information‐authors). We identified potential biases in all of the included trials. We defined mortality, but not serious adverse events, as an outcome that is robust to performance and detection bias (Savović 2012a; Savović 2012b). This decision can be questioned, as lack of blinding is not likely to influence the assessment of events such as upper gastrointestinal bleeding.
Several trials lacked information about allocation methods, which is one of the most important bias domains. Although, none reported clear differences between intervention and control groups, we cannot exclude the possibility of selection bias. In addition, all trials were open without blinding and hence were at high risk of bias for this domain. Only three trials provided full outcome data and included all participants in their analyses and we consequently classified them at low risk for attrition bias ( Lay 1997; Sarin 1996; Triantos 2005); two trials did not account for all participants and thus had a high risk for attrition bias (Lo 1999; Svoboda 1999); the risk in the remaining trial was unclear (Chen 1997). All trials reported outcome data for all of the primary outcome measures and so were at low risk for reporting bias. One trial was at low risk of bias for mortality (Triantos 2005), while the remaining trials were at high risk of bias for this overall domain. As none of the trials were double‐blind they were all at high risk of bias in the assessment of bleeding and serious adverse events.
We registered for‐profit funding (hbg.cochrane.org/information‐authors). Two trials were not in receipt of this type of funding (Svoboda 1999;Triantos 2005), but the remaining four trials did not report on funding sources. Thus, we were not able to undertake a post hoc subgroup analysis based on for‐profit funding.
We found little heterogeneity when assessing the trials clinically. The included participants had cirrhosis, mainly due to alcohol or viral hepatitis and the clinical settings were similar in all six trials. The publication year varied, which indicates that the collateral interventions may have varied, e.g. in terms of antibiotic use and the type of banding. On the other hand, our analyses showed little to moderate between‐trial heterogeneity.
Only one trial described a sample size calculation (Triantos 2005); the authors of this trial determined that they would need to enrol 214 participants and observe 37 events for adequate power. However, the trial was stopped after the inclusion of 52 participants because the number of bleeding events in the band ligation group was double that expected, although the difference in bleeding rates between the band ligation and no treatment groups was not significant at that time (20% versus 7.4%). Considering the sample size calculation in the Triantos trial, it is possible that the trials included in our review were underpowered. Nevertheless, both the pair‐wise and the Trial Sequential Analysis still found a beneficial effect of band ligation on outcomes compared to no intervention.
Potential biases in the review process
We undertook the review based on current recommendations for bias control (hbg.cochrane.org/information‐authors;Higgins 2017). We attempted to minimise possible selection bias (Page 2014), by using a comprehensive search strategy; we combined searches in electronic databases with handsearches of the biographies of identified trials and the conference proceedings and abstract books from relevant national and international society meetings. We consider it unlikely that we failed to identify any published trials.
Agreements and disagreements with other studies or reviews
The present review includes five randomised clinical trials published as full papers (Lay 1997; Lo 1999; Sarin 1996; Svoboda 1999; Triantos 2005), and one published abstract (Chen 1997), reporting the use of band ligation for primary prophylaxis of upper gastrointestinal bleeding compared to no treatment in participants with cirrhosis. We excluded two primary prophylaxis trials, both published in abstract form because the majority of the included participants had non‐cirrhotic portal hypertension (Gameel 2005; Omar 2000) .
We identified five previous meta‐analyses of trials of band ligation compared to no treatment in patients with oesophageal varices that had not bled. Three were published as full papers (Imperiale 2001; NICE 2016; Vlachogiannakos 2000); one was included as part of the discussion in an original randomised clinical trial published in full (Triantos 2005), and one was published as an abstract (Bedi 2000). They all include varying combinations of the trials we included or excluded; none of the previous meta‐analyses included trials we had not identified or considered.
The first of the meta‐analyses (Bedi 2000), included three randomised clinical trials and one abstract. No further details of the included trials are provided, not even the names of the first authors. Pooled results from the three trials published as full papers showed that use of band ligation had a favourable effect on all‐cause mortality (odds ratio (OR) 0.41, 95% confidence interval (CI) 0.25 to 0.65), bleeding‐related mortality (OR 0.25, 95% CI 0.12 to 0.56), and variceal bleeding (OR 0.25, 95% CI 0.15 to 0.42). Including data from the trial reported in abstract form did not significantly affect the results of the meta‐analyses.
The second meta‐analysis (Vlachogiannakos 2000), included four trials published as full papers (Lay 1997; Lo 1999; Sarin 1996; Svoboda 1999), and two published as abstracts (Chen 1997; Gameel 2005). It found that band ligation reduced both the risk of mortality (pooled OR 2.44, 95% CI 1.7 to 3.51) and of the first variceal bleed (pooled OR 4.26, 95% CI 2.85 to 6.37).
The third meta‐analysis (Imperiale 2001), included three trials published as full papers (Lay 1997; Lo 1999; Sarin 1996), and two published as abstracts (Chen 1997; Gameel 2005), and found that, compared to no treatment, band ligation reduces all‐cause mortality (risk ratio (RR) 0.55, 95% CI 0.43 to 0.71), bleeding‐related mortality (RR 0.20, 95% CI 0.11 to 0.39), and first variceal bleed (RR 0.35, 95% CI 0.26 to 0.50).
The fourth meta‐analysis was included in the discussion of an original publication of this topic (Triantos 2005); it was intended to place the findings of this study, which was stopped prematurely because the rate of bleeding in the band ligation group was greater than expected, in context with the findings in the other trials conducted to date. The authors included five trial papers published in full, including their own (Lay 1997; Lo 1999; Sarin 1996; Svoboda 1999; Triantos 2005), and three trials published in abstract form (Chen 1997; Gameel 2005; Omar 2000; ). They concluded that, in comparison to no treatment, band ligation reduces the risk of death (OR 0.43, 95% CI 0.30 to 0.60) and of the first variceal bleed (OR 0.31, 95% CI 0.17 to 0.53). Of note the findings in one of the trials included in this meta‐analysis (Gameel 2005), are reported incorrectly as showing no deaths in the no treatment group.
The final meta‐analysis (NICE 2016), included five trials published as full papers (Lay 1997; Lo 1999; Sarin 1996; Svoboda 1999; Triantos 2005). The authors undertook time‐to‐event analyses using the data available from two studies with 253 participants (Lay 1997; Lo 1999), and found moderate‐certainty evidence of a clinically important benefit of band ligation, over no intervention, on survival and variceal bleeding in people with medium to large varices. They found very low‐certainty evidence that band ligation was associated with a reduction in overall mortality compared to no treatment (RR 0.57, 95% CI 0.33 to 0.97; 2 trials, 170 participants) and very low‐certainty evidence of a benefit of band ligation on upper gastrointestinal bleeding (RR 0.49, 95% CI 0.31 to 0.76; 5 trials, 444 participants). Although they include all five trials in the analysis of upper gastrointestinal bleeding, they only included two studies in their analysis of overall mortality (Sarin 1996; Svoboda 1999); this is unexplained, as mortality data were extractable from all five of the included trials.
The majority of the previous meta‐analyses included one or more of the trials we excluded because the majority of the participants had non‐cirrhotic portal hypertension (Gameel 2005; Omar 2000). In addition, whereas we used outcome and complication data from the actual trial period, two meta‐analyses (Triantos 2005; Vlachogiannakos 2000), used the 2‐year cumulative mortality and bleeding rates provided in one included study (Lay 1997), while another (NICE 2016), used the time‐to‐event data for mortality and variceal bleeding provided in two included studies (Lay 1997; Lo 1999). Nevertheless, despite these participant and procedural differences, the results of our meta‐analysis are in accord with the findings in earlier meta‐analyses that, compared to no treatment, band ligation reduces the risk of death and of serious adverse events, including variceal bleeding, in people with oesophageal varices who have not previously bled.

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
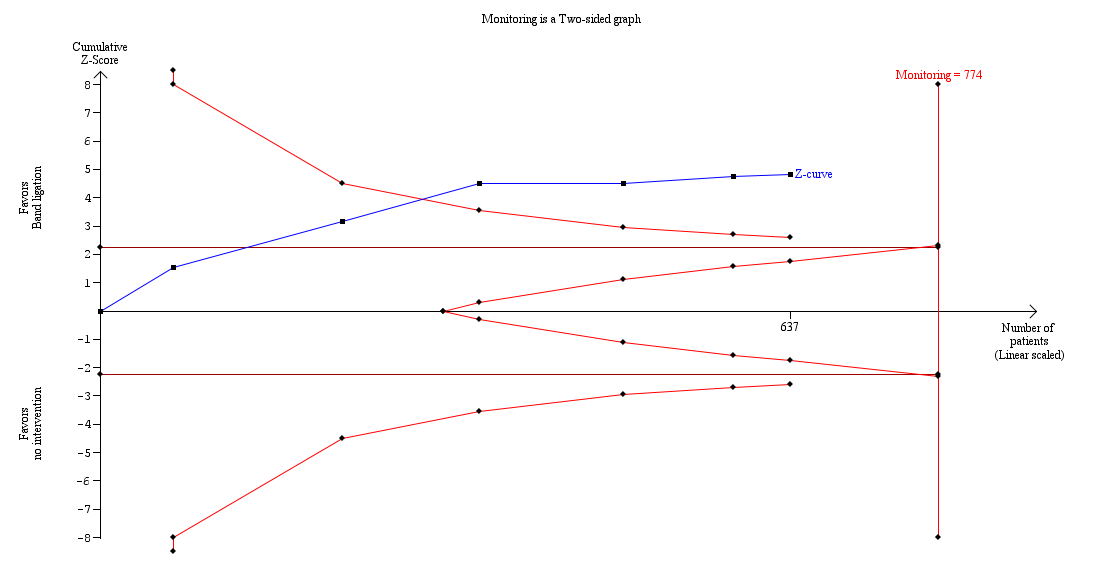
Trial Sequential Analysis of meta‐analysis including six randomised clinical trials evaluating the effect of band ligation versus no intervention on mortality (RR 0.55, 95% CI 0.43 to 0.70; 637 participants; I2 = 0%).
The analysis found that the blue Z‐curve crossed the trial monitoring boundary.

Trial Sequential Analysis including six randomised clinical trials evaluating the effect of band ligation versus no intervention on upper gastrointestinal bleeding (RR 0.44, 95% CI 0.28 to 0.72; participants = 637; I2 = 61%).
The analysis showed that the Z‐curve crossed the trial monitoring boundary.

Trial Sequential Analysis including six randomised clinical trials evaluating the effect of band ligation versus no intervention on serious adverse events (RR 0.55, 95% CI 0.43 to 0.70; 637 participants; I2 = 44%).
The analysis showed that the Z‐curve crossed the trial monitoring boundary.
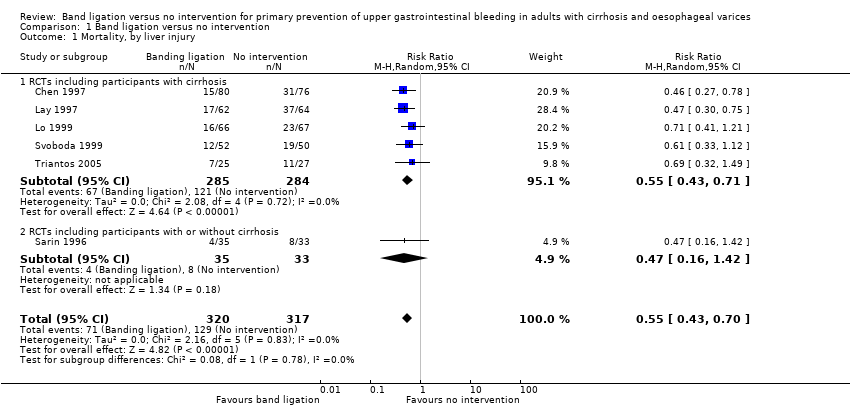
Comparison 1 Band ligation versus no intervention, Outcome 1 Mortality, by liver injury.
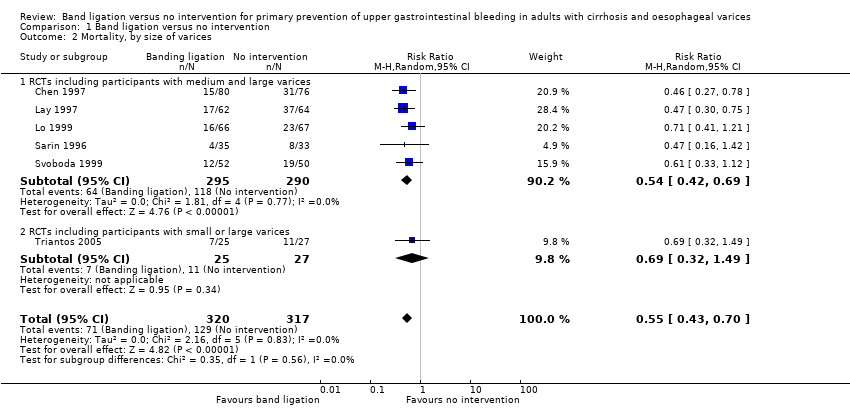
Comparison 1 Band ligation versus no intervention, Outcome 2 Mortality, by size of varices.
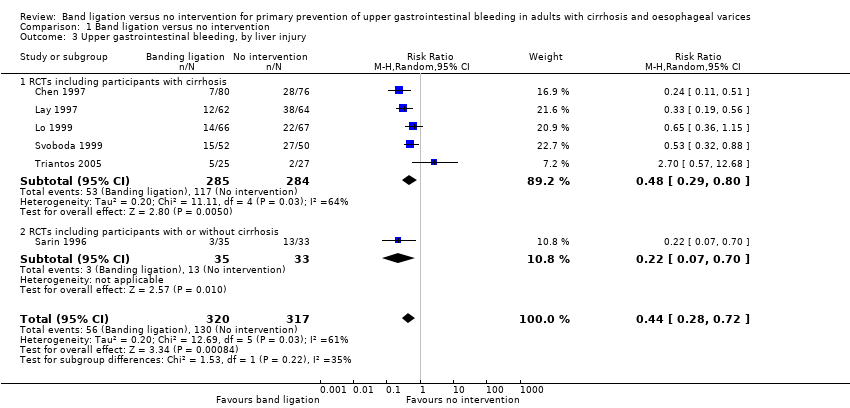
Comparison 1 Band ligation versus no intervention, Outcome 3 Upper gastrointestinal bleeding, by liver injury.

Comparison 1 Band ligation versus no intervention, Outcome 4 Upper gastrointestinal bleeding, by size of varices.

Comparison 1 Band ligation versus no intervention, Outcome 5 Serious adverse events, by liver injury.
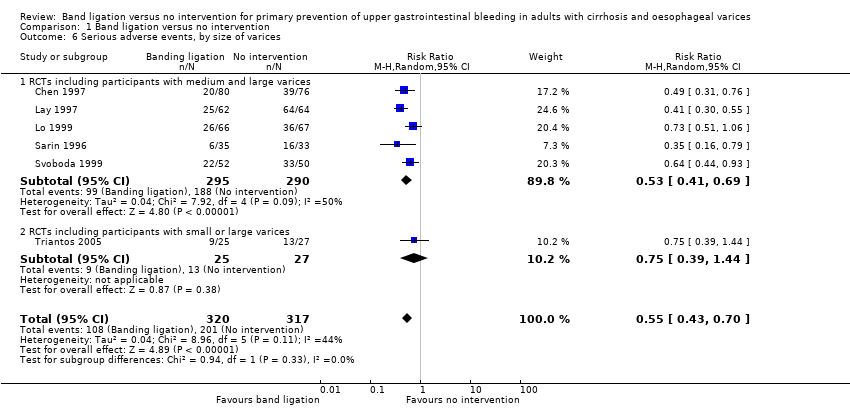
Comparison 1 Band ligation versus no intervention, Outcome 6 Serious adverse events, by size of varices.
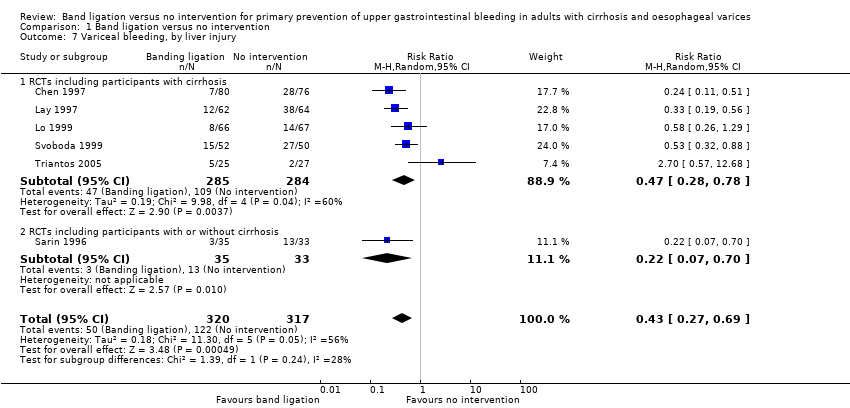
Comparison 1 Band ligation versus no intervention, Outcome 7 Variceal bleeding, by liver injury.

Comparison 1 Band ligation versus no intervention, Outcome 8 Variceal bleeding, by size of varices.

Comparison 2 Band ligation versus no intervention: worst‐case, extreme worst‐case, and extreme best‐case scenario analyses, Outcome 1 Mortality.
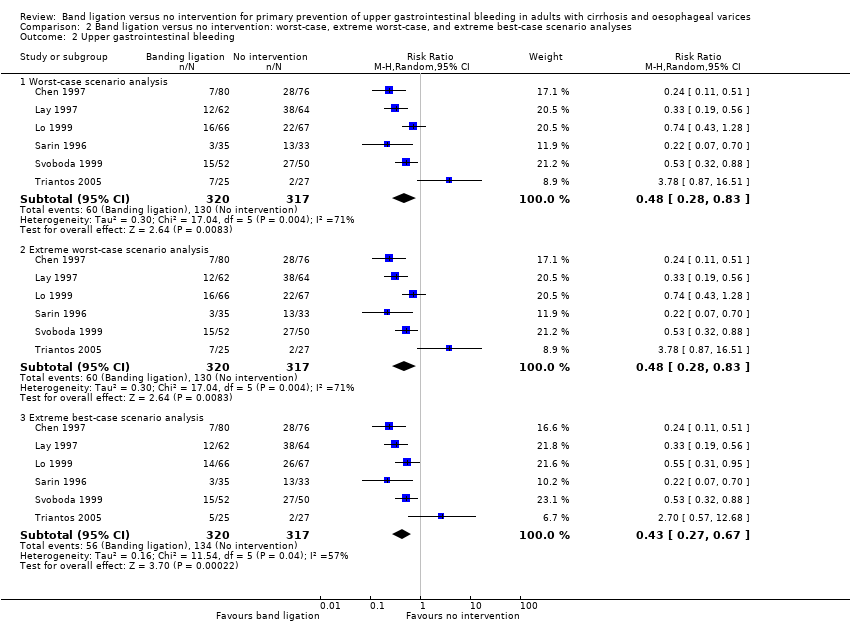
Comparison 2 Band ligation versus no intervention: worst‐case, extreme worst‐case, and extreme best‐case scenario analyses, Outcome 2 Upper gastrointestinal bleeding.
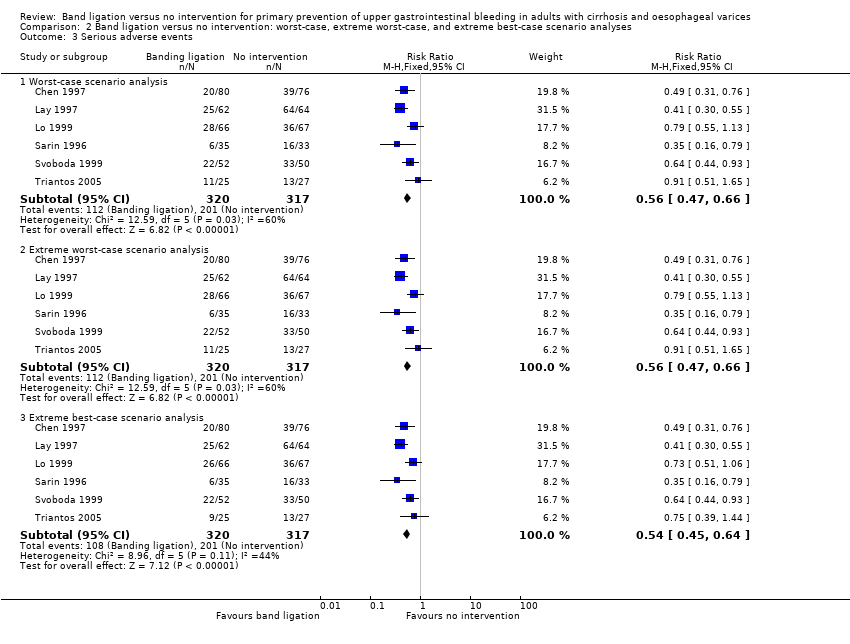
Comparison 2 Band ligation versus no intervention: worst‐case, extreme worst‐case, and extreme best‐case scenario analyses, Outcome 3 Serious adverse events.
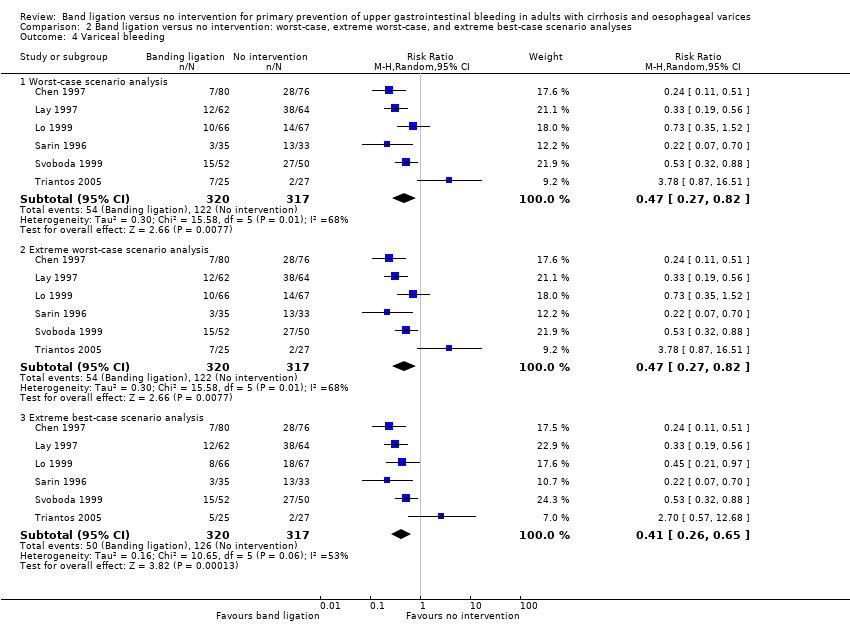
Comparison 2 Band ligation versus no intervention: worst‐case, extreme worst‐case, and extreme best‐case scenario analyses, Outcome 4 Variceal bleeding.
| Band ligation compared to no intervention for primary prevention of upper gastrointestinal bleeding in adults with oesophageal varices | ||||||
| Patient or population: adults with oesophageal varices | ||||||
| Outcomes* | Anticipated absolute effects** (95% CI) | Relative effect (95% CI) | Number of participants (Studies (n)) | Certainty of the evidence | Comments | |
| Risk with no intervention | Risk with band ligation | |||||
| Mortality | Study population | RR 0.55 (0.43 to 0.70) | 637 | ⊕⊕⊕⊝ | Only one trial was at low risk of bias in the overall assessment. | |
| 407 per 1000 | 224 per 1000 | |||||
| Upper gastrointestinal bleeding | Study population | RR 0.44 (0.28 to 0.72) | 637 | ⊕⊕⊕⊝ | All trials were at high risk of bias in the overall assessment. | |
| 410 per 1000 | 180 per 1000 | |||||
| Serious adverse events | Study population | RR 0.55 (0.43 to 0.70) | 637 | ⊕⊕⊕⊝ | All trials were at high risk of bias in the overall assessment. | |
| 634 per 1000 | 349 per 1000 | |||||
| Variceal bleeding | Study population | RR 0.43 (0.27 to 0.69) | 637 | ⊕⊕⊕⊝ | All trials were at high risk of bias in the overall assessment. | |
| 385 per 1000 | 166 per 1000 | |||||
| Non‐serious adverse events | We could not perform meta‐analysis. However, the non‐serious adverse events reported in association with band ligation included oesophageal ulceration, dysphagia, odynophagia, retrosternal and throat pain, heartburn, and fever, and in the one trial involving participants with either small or large varices, the incidence of non‐serious side effects in the banding group was much higher in those with small varices with respect to ulcers: small versus large varices 30.5% versus 8.7%; heartburn 39.2% versus 17.4%. | |||||
| Health‐related quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | None of the six included trials described health‐related quality of life. |
| * All outcomes were assessed at the maximum duration of follow‐up **The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded the certainty of the evidence by one level due to the lack of trials with a low risk of bias. | ||||||
| Trial | Inclusion criteria | Assessment of varices | Randomisation by variceal characteristics | Gastric varices or portal hypertensive gastropathy |
| Not reported | Not stipulated | Not reported | Not reported | |
| Participants were assessed for risk of bleeding (Beppu 1981), using criteria defined by the Japanese Research Society for Portal Hypertension (Inokuchi 1980). The included participants had blue varices of at least F2 or F3 size with at least one of the following: cherry‐red spots (++, +++), red wale markings (++, +++), haematocystic spots (+) | Not stipulated | Not described | Participants with gastric or ectopic varices at recruitment were excluded. During follow‐up, 4 (6%) participants in the banding group and 3 (5%) in the control group developed gastric varices. | |
| F2 or F3, associated with a moderate degree of red colour signs (red wale markings, cherry‐red spots or haematocystic spots (Beppu 1981)) | Not stipulated | F2 Banding: 27/64 (42%) No intervention: 30/63 (48%) F3 Banding: 37/64 (58%) No intervention: 33/63 (52%) Red colour signs moderate Banding: 33/64 (52%) No intervention: 36/63 (57%) Red colour signs severe Banding: 31/64 (48%) No intervention: 27/63 (43%) | Participants with gastric varices at recruitment were excluded. During follow‐up, 8 (12%) participants in the banding group and 3 (5%) in the no intervention group developed gastric varices. During follow‐up, 1 (1.6%) patient in the banding group and 2 (3.2%) in the no intervention group developed gastropathy. | |
| Participants with large varices > 5 mm were assessed for risk of bleeding (Beppu 1981), using criteria defined by the Japanese Research Society for Portal Hypertension (Inokuchi 1980). The included participants had blue varices of at least F2 or F3 size with one or more red colour signs; (cherry‐red spots, red wale markings or haematocystic spots) | Variceal size and grade assessed by two independent observers | Not reported | The presence/absence of gastric varices was recorded at initial assessment; no further mention and so absence is assumed. Portal hypertensive gastropathy present in 3 (8.6%) participants at inclusion and developed in a further two postbanding. | |
| Grade III or IV, or grade II with signs of high risk, classified using the Paquet’s system (Paquet 1978) | Not stipulated | Grade II Banding: 2/52 (4%) Control: 1/50 (2%) Grade III Banding: 36/52 (69%) No intervention: 38/50 (76%) Grade IV Banding: 14/52 (27%) No intervention: 11/50 (22%) | Not described | |
| Varices of any size: Small varices: < 5 mm diameter Large varices: diameter of largest varix > 5 mm Measured with open forceps and not disappearing on oesophageal insufflation | Assessed endoscopically by two independent observers | Small varices Banding: 14/25 (56%) No intervention: 17/27 (63%) Large varices Banding: 11/25 (44%) No intervention: 10/27 (37%) Red spots Banding: 9/25 (36%) No intervention: 8/27 (30%) | Gastric varices present at inclusion Banding: 2/25 (8%) No intervention: 1/27 (4%) No further participants in the banding group developed gastric varices during follow‐up; two participants in whom the varices were obliterated appear to have developed portal hypertensive gastropathy from which they bled. | |
| Japanese Research Society for Portal Hypertension classification (Inokuchi 1980) (Form: F1‐ straight varices; F2‐ enlarged tortuous varices; F3‐ largest sized varices; fundamental colour: Cw ‐ white varices; Cb ‐ blue varices: red colour signs: RC(‐) ‐ red colour signs negative; RC(+) ‐ red colour signs positive; red wale marks: RWM ‐ (+), (++), (+++); cherry‐red spots: CRS ‐ (+), (++), (+++); haematocystic spot: HCS: diffuse redness: DR; Group A: both red wale markings and cherry‐red spots were negative or mild (+); Group B: both red wale markings and cherry‐red spots were moderate (++) or severe (+++). Location: li ‐ locus inferior; Lm ‐ locus medialis; Ls ‐ locus superior Paquet classification: 0 ‐ no varices; I ‐ varices that disappear with insufflation; II ‐ larger, usually straight, visible varices that disappear with insufflation; III ‐ more prominent coil‐shaped varices, occupying part of the lumen; IV ‐ tortuous varices occupying the lumen (Paquet 1978). | ||||
| Banding | ||||||
| Equipment | Not described | Endoscopic ligating device (Bard Interventional Products, Billerica, MA, USA) with a 25 cm overtube (Olympus XQ 20, Tokyo, Japan) | Endoscopic ligating device (Bard Interventional Products, Billerica, MA, USA) with a 25 cm overtube (Olympus XQ 20, Tokyo, Japan) | Endoscopic ligating device and a 25 cm overtube (Bard Inteventional Products, Tewksbury MA, USA) | Endoscopic ligation device (Suction oesophageal varices | Multiband Ligator 6 shooter (Wilson‐Cook, Limerick, Ireland) |
| Operator experience | Not described | Ligation was performed by two | Ligation was performed by two | Not described | Ligation was performed by two experienced endoscopists; each of whom had performed | Ligation was performed by four experienced endoscopists each of whom had performed 100 ligation |
| Technique | ‐Variceal ligation performed at 2‐ to 3‐ week intervals | ‐ Ligation was performed at 1 cm to 5 cm above the gastroesophageal junction; each varix was ligated with 1 to 3 rubber bands to a maximum of 10 bands/session ‐ Procedure repeated weekly for the first 3 weeks, if possible and then every 2 weeks ‐ Follow‐up endoscopy repeated every 3 months after eradication | ‐ Ligation was performed at 1 cm to 5 cm above the gastroesophageal junction; each varix was ligated with 1 to 2 rubber bands ‐ Procedure repeated at intervals of 3 weeks ‐ Follow‐up endoscopy repeated every 3 months after eradication | ‐ Varices ligated 1 cm to 2 cm above the gastroesophageal junction; 1 to 2 bands applied to each variceal column between the lower 4 cm to 5 cm of the oesophagus; every variceal column was ligated at each session ‐ Procedure repeated at 7‐ to 10‐day intervals ‐ Follow‐up endoscopy repeated every 3 months after eradication | ‐ The largest number possible (up to ‐ The first three therapeutic sessions were performed at 2‐week intervals then monthly ‐ Follow‐up endoscopy repeated every 3 months after eradication ‐ Participants in the no treatment group were endoscoped every 3 months | ‐ Bands were placed starting at the gastroesophageal junction and then proximally in a helical fashion for approximately 5 cm, putting at least one band on each varix ‐ Subsequent sessions scheduled at 14‐day intervals ‐ Participants in the no intervention group were endoscoped yearly |
| Endpoint | Variceal eradication | Variceal eradication | Varices obliterated or too small to be ligated | Variceal obliteration or decreasing the size to grade 1 (not possible to suck in varix for band ligation) | Varices too small to treat | Eradication or varices too small to ligate (no effect of suction) |
| Achievement of endpoint | 71/80 (88.7%) | 62/62 (100%) | 55/64 (86%) | Banding successful in all participants, except for those who died before complete eradication (numbers not specified) | 42/52 (81%) (includes 8 eradicated, 34 too small to band) | 20/25 (80%) |
| Reasons for failure | Not reported | Not applicable | Reluctance (3) Asthenia (2) Aspiration pneumonia (1) Encephalopathy (1) Hepatic failure (2) | Death due to hepatic coma or bleeding (numbers not specified) | Not reported | Bleeding (3) |
| Mean (± 1 SD) number of sessions to achieve obliteration | 2.9 ± 0.7 | 3.6 ± 1.7 Mean examinations 5.1 ± 2.8 | 2.9 ± 0.5 (range 2 to 5) | 3.2 ± 1.2 | 4.8 ± 1.8 | Median 2 (1 to 4) Small varices, median 1 (1 to 4) Large varices, median 2 (1 to 3) |
| Mean (± 1 SD) time to achieve obliteration | Not reported | 75.6 ± 28.4 days | 40 ± 4 days | 4.9 ± 2.2 weeks | Not reported | Median 28 (14 to 101) days |
| Number of bands each session | Not reported | Maximum did not exceed 10 bands per treatment session | 1 to 2 per varix (mean not specified) | Each variceal column ligated with one to two bands (mean not specified) | Up to 6 | Median: 4 (2 to 7) per session |
| Recurrent varices | Not reported | 26/62 (42%) (of which 4 had a second recurrence) | 12 (21.8%) | 10 (28.6%) | 16 (31.0%) | 7 (35%) 3/11 with small varices and 4/8 with large |
| Japanese Research Society for Portal Hypertension classification (Inokuchi 1980) (Form: F1‐ straight varices; F2‐ enlarged tortuous varices; F3‐ largest sized varices; fundamental colour: Cw ‐ white varices; Cb ‐ blue varices: red colour signs: RC(‐) ‐ red colour signs negative; RC(+) ‐ red colour signs positive; red wale marks: RWM ‐ (+), (++), (+++); cherry‐red spots: CRS ‐ (+), (++), (+++); haematocystic spot: HCS: diffuse redness: DR; Group A: both red wale markings and cherry‐red spots were negative or mild (+); Group B: both red wale markings and cherry‐red spots were moderate (++) or severe (+++). Location: li ‐ locus inferior; Lm ‐ locus medialis; Ls ‐ locus superior Paquet classification: 0 ‐ no varices; I ‐ varices that disappear with insufflation; II ‐ larger, usually straight, visible varices that disappear with insufflation; III ‐ more prominent coil‐shaped varices, occupying part of the lumen; IV ‐ tortuous varices occupying the lumen (Paquet 1978). | ||||||
| Trial | Participants allocated to band ligation (n) | Participants allocated to no intervention (n) | Non‐serious adverse event in participants allocated to band ligation |
| 80 | 76 | Not reported | |
| 62 | 64 | Not reported | |
| 66* | 67* | Banding: oesophageal ulceration without bleeding (n = 16 (24%)), transient dysphagia (n = 7 (11%)), retrosternal pain (n = 5 (8%)), pleural effusion (n = 2 (3%)), fever > 38oC (n = 2 (3%)) No intervention: not reported | |
| 35 | 33 | Banding: oesophageal ulceration without bleeding (n = 24 (69%)), throat pain (n = 12 (34%)); retrosternal pain (n = 8 (23%)), dysphagia (n = 6 (17%)), fever (n = 4 (11%)) No intervention: not reported | |
| 52 | 50 | Banding: ulcer (n = 2 (4%)), dysphagia (n = 3 (6%)), odynophagia (n = 1 (2%)), others (n = 4 (8%)) No intervention: ulcer (n = 0), dysphagia (n = 4 (8%)), odynophagia (n = 2 (4%)), others (n = 1 (2%)) | |
| 25** | 27 | Banding: small varices: ulcers (n = 7 (30.5%)), dysphagia (n = 5 (21.7%)), heartburn (n = 9 (39.2%)), chest pain (n = 3 (13.0%)) large varices: ulcers (n = 2 (8.7%)), dysphagia (n = 5 (21.7%)), heartburn (n = 4 (17.4%)), chest pain (n = 2 (8.7%)), fever (n = 1 (8.7%)) No intervention: not reported | |
| * Three participants in the banding and three participants in the no intervention groups were lost to follow‐up. The authors omitted these 6 participants providing data on only 63 in the banding and 64 in the no intervention groups in their main analyses; we have included the number randomised in our analyses and recalculated the percentages of people with non‐serious adverse effects using the full data set. ** Two participants allocated to band ligation refused the procedure; the authors provide data on the complications which arose in the 23 participants who did undergo the procedure. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality, by liver injury Show forest plot | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.43, 0.70] |
| 1.1 RCTs including participants with cirrhosis | 5 | 569 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.43, 0.71] |
| 1.2 RCTs including participants with or without cirrhosis | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.16, 1.42] |
| 2 Mortality, by size of varices Show forest plot | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.43, 0.70] |
| 2.1 RCTs including participants with medium and large varices | 5 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.42, 0.69] |
| 2.2 RCTs including participants with small or large varices | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.32, 1.49] |
| 3 Upper gastrointestinal bleeding, by liver injury Show forest plot | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.28, 0.72] |
| 3.1 RCTs including participants with cirrhosis | 5 | 569 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.29, 0.80] |
| 3.2 RCTs including participants with or without cirrhosis | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.07, 0.70] |
| 4 Upper gastrointestinal bleeding, by size of varices Show forest plot | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.28, 0.72] |
| 4.1 RCTs including participants with medium and large varices | 5 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.27, 0.59] |
| 4.2 RCTs including participants with small or large varices | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 2.7 [0.57, 12.68] |
| 5 Serious adverse events, by liver injury Show forest plot | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.43, 0.70] |
| 5.1 RCTs including participants with cirrhosis | 5 | 569 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.44, 0.73] |
| 5.2 RCTs including participants with or without cirrhosis | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.16, 0.79] |
| 6 Serious adverse events, by size of varices Show forest plot | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.43, 0.70] |
| 6.1 RCTs including participants with medium and large varices | 5 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.41, 0.69] |
| 6.2 RCTs including participants with small or large varices | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.44] |
| 7 Variceal bleeding, by liver injury Show forest plot | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.27, 0.69] |
| 7.1 RCTs including participants with cirrhosis | 5 | 569 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.28, 0.78] |
| 7.2 RCTs including participants with or without cirrhosis | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.07, 0.70] |
| 8 Variceal bleeding, by size of varices Show forest plot | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.27, 0.69] |
| 8.1 RCTs including participants with medium and large varices | 5 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.26, 0.55] |
| 8.2 RCTs including participants with small or large varices | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 2.7 [0.57, 12.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Worst‐case scenario analysis | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.46, 0.74] |
| 1.2 Extreme worst‐case scenario analysis | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.46, 0.74] |
| 1.3 Extreme best‐case scenario analysis | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.42, 0.68] |
| 2 Upper gastrointestinal bleeding Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Worst‐case scenario analysis | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.28, 0.83] |
| 2.2 Extreme worst‐case scenario analysis | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.28, 0.83] |
| 2.3 Extreme best‐case scenario analysis | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.27, 0.67] |
| 3 Serious adverse events Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Worst‐case scenario analysis | 6 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.47, 0.66] |
| 3.2 Extreme worst‐case scenario analysis | 6 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.47, 0.66] |
| 3.3 Extreme best‐case scenario analysis | 6 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.45, 0.64] |
| 4 Variceal bleeding Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Worst‐case scenario analysis | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.27, 0.82] |
| 4.2 Extreme worst‐case scenario analysis | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.27, 0.82] |
| 4.3 Extreme best‐case scenario analysis | 6 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.26, 0.65] |

