Trachealabsaugung bei der Geburt bei deprimierten Neugeborenen, die mit mekoniumhaltiges Fruchtwasser geboren wurden
Abstract
Background
Neonates born through meconium‐stained amniotic fluid (MSAF) are at risk of developing meconium aspiration syndrome (MAS). Neonates who are non‐vigorous due to intrapartum asphyxia are at higher risk of developing MAS. Clearance of meconium from the airways below the vocal cords by tracheal suction before initiating other steps of resuscitation may reduce the risk of development of MAS. However, conducting tracheal suction may not only be ineffective, it may also delay effective resuscitation, thus prolonging and worsening the hypoxic‐ischaemic insult.
Objectives
To evaluate the efficacy of tracheal suctioning at birth in preventing meconium aspiration syndrome and other complications among non‐vigorous neonates born through meconium‐stained amniotic fluid.
Search methods
We used the standard search strategy of Cochrane Neonatal to search Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 11) in the Cochrane Library; Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to 25 November 2020) for randomised controlled trials (RCTs) and quasi‐randomised trials. We also searched clinical trials databases and the reference lists of retrieved articles for RCTs and quasi‐randomised trials (up to November 2020).
Selection criteria
We included studies enrolling non‐vigorous neonates born through MSAF, if the intervention being tested included tracheal suction at the time of birth with an intent to clear the trachea of meconium before regular breathing efforts began. Tracheal suction could be performed with an endotracheal tube or a wide‐gauge suction catheter. Neonates in the control group should have been resuscitated at birth with no effort made to clear the trachea of meconium.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data, consulting with a third review author about any disagreements. We used standard Cochrane methodological procedures, including assessment of risk of bias for all studies. Our primary outcomes were: MAS; all‐cause neonatal mortality; and incidence of hypoxic‐ischaemic encephalopathy (HIE). Secondary outcomes included: need for mechanical ventilation; incidence of pulmonary air leaks; culture‐positive sepsis; and persistent pulmonary hypertension. We used the GRADE approach to assess the certainty of evidence.
Main results
We included four studies (enrolling 581 neonates) in the review. All four studies were conducted in tertiary care hospitals in India. Three of the four studies included neonates born at and beyond term gestation, whereas one included neonates born at and beyond 34 weeks of gestation. Due to the nature of the intervention, it was not possible to blind the healthcare personnel conducting the intervention.
Tracheal suction compared to no suction in non‐vigorous neonates born through MSAF
In non‐vigorous infants, no differences were noted in the risks of MAS (RR 1.00, 95% CI 0.80 to 1.25; RD 0.00, 95% CI ‐0.07 to 0.08; 4 studies, 581 neonates) or all‐cause neonatal mortality (RR 1.24, 95% CI 0.76 to 2.02; RD 0.02, 95% CI ‐0.03 to 0.07; 4 studies, 575 neonates) with or without tracheal suctioning. No differences were reported in the risk of any severity HIE (RR 1.05, 95% CI 0.68 to 1.63; 1 study, 175 neonates) or moderate to severe HIE (RR 0.68, 95% CI 0.43 to 1.09; 1 study, 152 neonates) among non‐vigorous neonates born through MSAF. We are also uncertain as to the effect of tracheal suction on other outcomes such as incidence of mechanical ventilation (RR 0.99, 95% CI 0.68 to 1.44; RD 0.00, 95% CI ‐0.06 to 0.06; 4 studies, 581 neonates), pulmonary air leaks (RR 1.22, 95% CI 0.38 to 3.93; RD 0.00, 95% CI ‐0.02 to 0.03; 3 studies, 449 neonates), persistent pulmonary hypertension (RR 1.29, 95% CI 0.60 to 2.77; RD 0.02, 95% CI ‐0.03 to 0.06; 3 studies, 406 neonates) and culture‐positive sepsis (RR 1.32, 95% CI 0.48 to 3.57; RD 0.01, 95% CI ‐0.03 to 0.05; 3 studies, 406 neonates). All reported outcomes were judged as providing very low certainty evidence.
Authors' conclusions
We are uncertain about the effect of tracheal suction on the incidence of MAS and its complications among non‐vigorous neonates born through MSAF. One study awaits classification and could not be included in the review. More research from well‐conducted large trials is needed to conclusively answer the review question.
PICOs
Zusammenfassung in einfacher Sprache
Trachealabsaugung bei der Geburt bei deprimierten Neugeborenen, die mit mekoniumhaltiges Fruchtwasser geboren wurden
Fragestellung des Reviews
Bestimmung der Wirksamkeit der Reinigung der Luftröhre von Mekonium durch Einführen eines Schlauchs in die Luftröhre (Intubation) und Absaugen bei der Geburt bei Neugeborenen, die durch mekoniumbelastetes Fruchtwasser geboren werden und bei der Geburt deprimiert sind (erkennbar an fehlender Atmung, Schlaffheit oder niedriger Herzfrequenz).
Hintergrund
Mekonium ist eine dicke, grüne, teerartige Substanz, die den Darm des Babys während der Schwangerschaft auskleidet. Mekonium enthält verschiedene Darmenzyme und Substanzen (Blut, Hautzellen usw.), die der Fötus aufgenommen hat. Mekonium wird in der Regel innerhalb von 24 Stunden nach der Geburt erstmals ausgeschieden. Unter bestimmten Umständen, wenn die Blut‐ oder Sauerstoffversorgung des Fötus beeinträchtigt ist oder die Schwangerschaft über den normalen Zeitraum von 40 Wochen hinausgeht, kann es jedoch zu einem Mekoniumausscheidung vor der Geburt kommen. Wenn das Mekonium einmal ausgeschieden ist, kann es über das Fruchtwasser in die Atemwege eingeatmet (aspiriert) werden. Dies kann entweder vor der Geburt oder mit den ersten Atemzügen nach der Geburt passieren. Dies kann zu einer Blockierung der Atemwege und einer Entzündung des Lungengewebes durch Giftstoffe führen (chemische Lungenentzündung). Nahezu 10 % bis 25 % der Geburten werden durch den Ausfluss von Mekonium vor der Geburt erschwert. Von diesen Neugeborenen entwickeln 5 % bis 12 % ein Mekoniumaspirationssyndrom (MAS). Der Schweregrad eines MAS kann von leichter Atemnot bis hin zum lebensbedrohlichen Atemstillstand reichen. Ein Ansatz zur Vorbeugung von MAS besteht darin, Säuglinge zu identifizieren, die bei der Geburt deprimiert sind; und bei diesen das Mekonium aus den Atemwegen zu entfernen, bevor das Baby seinen ersten Atemzug macht. Dabei wird ein Endotrachealtubus (auch Trachealtubus) in die obere Luftröhre eingeführt und herausgezogen, während die Luftröhre abgesaugt wird. Dies kann die MAS jedoch nicht verhindern, wenn das Baby bereits vor der Geburt Mekonium aspiriert hat. Außerdem müssen die meisten Säuglinge, die für diesen Eingriff in Frage kommen, sofort wiederbelebt werden; und die Durchführung einer Trachealabsaugung kann Schaden anrichten, weil sie die Einleitung der künstlichen Beatmung verzögert.
Studienmerkmale
Wir schlossen vier Studien (581 Neugeborene) ein, die in Krankenhäusern in Indien durchgeführt wurden. Drei Studien schlossen Neugeborene ein, die am und nach dem Geburtstermin geboren wurden, während eine Studie Neugeborene einschloss, die während und nach 34 Schwangerschaftswochen geboren wurden. In allen vier Studien wurden die in Frage kommenden Neugeborenen anhand von mindestens einem der folgenden Merkmale bei der Geburt identifiziert: keine Atmung oder kein Weinen, geringer Muskeltonus und eine Herzfrequenz von weniger als 100 Schlägen pro Minute. Der Eingriff bestand in einer trachealen Absaugung zum Zeitpunkt der Geburt mit dem Ziel, die Luftröhre von Mekonium zu befreien, bevor die regulären Atemversuche beginnen. Die Neugeborenen in der Kontrollgruppe wurden bei der Geburt reanimiert, ohne dass Anstrengungen unternommen wurden, die Luftröhre von Mekonium zu befreien.
Die Suche wurde bis zum 25. November 2020 aktualisiert.
Hauptergebnisse
Wir sind uns nicht sicher, ob die tracheale Absaugung das Risiko einer MAS verringert. Von 1000 Neugeborenen, bei denen eine Trachealabsaugung durchgeführt wird, wird MAS zwischen 70 Neugeborenen weniger und bis zu bei 80 Neugeborenen mehr beobachtet. Ebenso ist die Auswirkung der Trachealabsaugung auf das Sterberisiko vor der Entlassung aus dem Krankenhaus ungewiss (22 weniger bis 92 Fälle mehr pro 1000 Neugeborene). Wir wissen auch nicht genau, wie sich das Absaugen der Luftröhre auf das Risiko anderer Faktoren auswirkt, wie z. B. die Notwendigkeit fortgeschrittener Wiederbelebungsmaßnahmen, Enzephalopathie (Hirnschädigung oder ‐erkrankung) aufgrund von Asphyxie (Sauerstoffmangel, der zu Bewusstlosigkeit und oft zum Tod führt), die Notwendigkeit oder Dauer der mechanischen Beatmung, die Notwendigkeit nicht‐invasiver Atemunterstützung (Maske), die Dauer der Sauerstofftherapie und die Dauer des Krankenhausaufenthalts. Bei diesen und andere Komplikationen des MAS waren kein Unterschied zwischen dem Vorgehen mit oder ohne Trachealabsaugung festzustellen.
Vertrauenswürdigkeit der Evidenz
Die vier Studien, die in diesen Review einbezogen wurden, erbrachten nur sehr niedrige Vertrauenswürdigkeit der Evidenz. Erstens waren in den meisten Studien die Gesundheitsfachpersonen, das die klinische Versorgung durchführte oder über das Vorhandensein von Symptomen entschied, über die Zuweisung der Neugeborenen zur Studiengruppe informiert. Dies erhöht das Risiko für Bias. Zweitens hatten wir aufgrund der geringen Studiengrösse und der geringen Häufigkeit der Ergebnisse wenig Vertrauen in den Ausschluss von klinisch bedeutsamen Vorteilen oder Schäden, wenn eine Trachealabsaugung durchgeführt wurde. Eine Studie muss noch klassifiziert werden und konnte nicht in den Review einbezogen werden. Zur endgültigen Beantwortung der Forschungsfrage sind weitere Untersuchungen in gut durchgeführten großen Studien erforderlich.
Authors' conclusions
Summary of findings
| Tracheal suction compared to no suction in non‐vigorous neonates born through meconium‐stained amniotic fluid (MSAF) | ||||||
| Patient or population: non‐vigorous neonates born through meconium‐stained amniotic fluid | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with no tracheal suction | Risk with Tracheal suction | |||||
| Meconium aspiration syndrome (MAS) | Study population | RR 1.00 | 581 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of meconium aspiration syndrome. | |
| 346 per 1000 | 346 per 1000 | |||||
| All‐cause neonatal mortality assessed with: all‐cause neonatal deaths before discharge from hospital | Study population | RR 1.24 | 575 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of death before hospital discharge. | |
| 90 per 1000 | 112 per 1000 | |||||
| Moderate to severe hypoxic‐ischaemic encephalopathy (HIE) | Study population | RR 0.68 | 152 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of moderate to severe hypoxic‐ischaemic encephalopathy. | |
| 390 per 1000 | 265 per 1000 | |||||
| Any hypoxic‐ischaemic encephalopathy (HIE) | Study population | RR 1.05 | 175 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of any hypoxic‐ischaemic encephalopathy. | |
| 307 per 1000 | 322 per 1000 | |||||
| Need for mechanical ventilation assessed with: clinical and laboratory assessment of gas exchange before discharge from hospital | Study population | RR 0.99 | 581 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the need for mechanical ventilation. | |
| 154 per 1000 | 153 per 1000 | |||||
| Pulmonary air leaks (PAL) assessed with: clinical and radiological assessment before discharge from hospital | Study population | RR 1.22 | 449 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of pulmonary air leaks. | |
| 22 per 1,000 | 27 per 1,000 | |||||
| Persistent pulmonary hypertension (PPHN) assessed with: clinical or echocardiographic diagnosis before discharge from hospital | Study population | RR 1.29 | 406 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of persistent pulmonary hypertension. | |
| 54 per 1000 | 70 per 1000 | |||||
| Culture‐positive sepsis assessed with: blood culture before discharge from hospital | Study population | RR 1.32 | 406 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of culture‐positive sepsis. | |
| 29 per 1000 | 39 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by three levels because of very serious risk of bias, serious inconsistency and serious imprecision (wide confidence interval crossing the line of no significance). Outcome assessors were masked to the study intervention in only two studies. Care providers were masked to the study intervention in only one of the two studies which reported this outcome. There was no allocation concealment in one study. | ||||||
Background
Description of the condition
Meconium is found in the gastrointestinal tract of the foetus as early as 14 to 16 weeks of gestation. It is a complex substance, composed of water (nearly 75%), gastric secretions, lanugo, blood, pancreatic enzymes, free fatty acids, and squamous cells (Wiswell 1993; Wiswell 1999). It accumulates in the gastrointestinal tract of the foetus throughout pregnancy and usually is first passed within 24 hours after birth. Factors that prevent meconium passage in utero include a viscous terminal cap, a contracted anal sphincter, and the absence of propulsive forces. However, meconium passage may occur in utero in post‐term pregnancies and in response to foetal hypoxia, acidaemia, or infection (Miller 1981; Usher 1988).
Once passed, meconium may be aspirated from amniotic fluid into the airways either in utero or with the first few breaths after birth. Breathing movements are observed in utero but are shallow. Therefore, amniotic fluid is not drawn into the distal airway; rather, the net flow of alveolar lung fluid moves outward. However, fetal distress due to hypoxia or ischaemia may induce deep gasping efforts resulting in aspiration of amniotic fluid containing meconium. Aspiration of meconium can cause airway obstruction, alveolar collapse, ventilation‐perfusion mismatch, secondary surfactant deficiency, and chemical pneumonitis (Tyler 1978).
Nearly 10% to 25% of births are complicated by meconium passage before birth (Wiswell 1993; Wiswell 1999). Among the total births complicated by meconium passage, 5% to 12% of neonates develop meconium aspiration syndrome (MAS) (Berkus 1994; Carson 1976). MAS is diagnosed in a neonate who is born through meconium‐stained amniotic fluid (MSAF), develops respiratory distress shortly after birth, and whose symptoms cannot be otherwise explained (Fanaroff 2008). MAS can present with varying degrees of severity, from mild distress to life‐threatening respiratory failure. The incidence of MAS is lower among preterm neonates (Tybulewicz 2004). The International Liaison Committee on Resuscitation (ILCOR) guidelines for the treatment of neonates born through meconium‐stained amniotic fluid do not differentiate between term and preterm neonates (Wyckoff 2015).
Description of the intervention
A combined obstetric and neonatal approach that aimed to clear meconium from the upper airway before it could be aspirated with the infant's first breaths was widely adopted and became part of neonatal resuscitation guidelines after it was shown to be effective in reducing the incidence of death and MAS in a cohort study (Carson 1976). The first component of this approach ‐ the intrapartum suctioning of the oropharynx and nasopharynx after delivery of the head but before delivery of the shoulders and chest (Carson 1976) ‐ was removed from the guidelines after a large randomised controlled trial (RCT) reported no reduction in the incidence of MAS (Vain 2004). The second neonatal component of this combined approach consists of direct laryngoscopic visualisation of vocal cords, intubation, and endotracheal suctioning. A large RCT (Wiswell 2000), and a subsequent Cochrane Review (Halliday 2001), revealed that the attempt to clear the airway of meconium by intubation and endotracheal suctioning is ineffective in reducing the incidence of MAS if the neonate is not depressed (or is vigorous) at birth. However, the evidence is insufficient to support or refute intubation and endotracheal suctioning immediately after birth for non‐vigorous neonates (defined as the presence of any of the following at the time of birth: no or gasping breathing efforts, poor muscle tone, and heart rate < 100 beats per minute) (Wyckoff 2015). For a non‐vigorous infant, the upper airways are suctioned and cleared of meconium; an endotracheal tube then is passed through the vocal cords and pulled out while the trachea is being suctioned. If meconium is aspirated from the trachea, the procedure is repeated until meconium is cleared or severe bradycardia ensues.
How the intervention might work
Neonates born through MSAF are often depressed and need prompt resuscitation. With the start of breathing efforts, meconium present in the upper airways and trachea can move into the distal airways, resulting in MAS. The clearance of meconium from the airways before the start of breathing may decrease the risk of MAS.
Why it is important to do this review
Observational studies evaluating the effectiveness of tracheal suction at birth in neonates born through MSAF have described variable results, with some studies suggesting the decreased likelihood of respiratory complications after tracheal suction (Gregory 1974; Suresh 1994), and others suggesting no difference (Daga 1994), or increased risk (Linder 1988; Yoder 1994). Although a large RCT has demonstrated the futility of tracheal suctioning for preventing MAS in vigorous neonates (Wiswell 2000), no similar conclusive evidence has been found for non‐vigorous neonates. After examining the evidence, the ILCOR concluded in 2010 that available evidence neither supports nor refutes tracheal suctioning for prevention of MAS in depressed infants born through MSAF (Perlman 2010).
Clearing of the larger airways before the meconium can be aspirated distally into the lungs with the first few breaths seems a logical step in neonatal resuscitation. However, this intervention would be futile if deep gasping efforts, which usually precede the apnoea, have already resulted in the aspiration of meconium in utero. In addition, the time taken to intubate and perform tracheal suctioning can delay resuscitation and further aggravate hypoxic‐ischaemic injury in a depressed neonate. This issue is of special relevance for low‐ and middle‐income countries, where most of the births complicated by MSAF take place. The incidence of MSAF and MAS remains high, but a nurse or physician with the skills needed for endotracheal intubation and tracheal suctioning may not be available for every delivery (Chettri 2015).
Objectives
To evaluate the efficacy of tracheal suctioning at birth in preventing meconium aspiration syndrome and other complications among non‐vigorous neonates born through meconium‐stained amniotic fluid.
Methods
Criteria for considering studies for this review
Types of studies
We included in the review randomised controlled trials (RCTs) comparing tracheal suction with no tracheal suction at birth in non‐vigorous neonates born through MSAF. We did not apply any language or sex restrictions. Trials reported in abstract form were eligible for inclusion if methods were reported that allowed assessment of eligibility for inclusion and risk of bias. We planned to exclude cross‐over trials.
Types of participants
We included studies enrolling neonates born at term or at late preterm gestation in the review.
Eligible studies should have enrolled non‐vigorous neonates born through MSAF. Studies may have shown some variation in the way they defined 'non‐vigorous'. Most studies were likely to define non‐vigorous according to ILCOR/Neonatal Resuscitation Program (NRP) guidelines, as “the presence of any of the following at the time of birth: no or gasping breathing efforts, poor muscle tone, or bradycardia” (Wyckoff 2015). We planned to note variations in the definition used by the included studies.
Types of interventions
The intervention consisted of tracheal suction of enrolled neonates at the time of birth with an intent to clear the trachea of meconium before regular breathing efforts began. Tracheal suction could be performed with an endotracheal tube or a wide‐gauge suction catheter. We did not consider suction of the upper airway (above the vocal cords) alone as an intervention. Neonates in the control group should have been resuscitated at birth with no effort made to clear the trachea of meconium. Neonates undergoing suction of the oropharynx or the nasopharynx or both without tracheal suction belonged to the control group.
Types of outcome measures
We included the following outcomes in this review.
Primary outcomes
-
Incidence of meconium aspiration syndrome (proportion). Meconium aspiration syndrome is diagnosed when respiratory distress develops soon after birth in an infant born through meconium‐stained amniotic fluid with compatible radiological findings that cannot be otherwise explained.
-
Incidence of all‐cause neonatal mortality (proportion) defined as all‐cause neonatal death (death before 28 days)
-
Incidence of hypoxic‐ischaemic encephalopathy (proportion) (Sarnat 1976; Thompson 1997)
Secondary outcomes
-
Need for mechanical ventilation (proportion), defined as the need for mechanical ventilation (proportion) during the first 48 hours after birth
-
Duration of oxygen therapy (hours/days), defined as the number of days of oxygen supplementation during the hospital stay
-
Duration of mechanical ventilation (hours/days), defined as the number of days of mechanical ventilation (invasive or non‐invasive) during the hospital stay
-
Need for non‐invasive ventilation (proportion)
-
Incidence of pulmonary air leaks (proportion), defined as the proportion of conditions such as pulmonary interstitial emphysema, pneumothorax, pneumomediastinum, or pneumopericardium during the hospital stay
-
Duration of hospitalisation (hours)
-
Incidence of neurodevelopmental delay (proportion), defined as the incidence of major neurodevelopmental disability at 18 months or older (major neurodevelopmental disabilities among all participants or survivors (cerebral palsy, developmental delay (Bayley or Griffith assessment > 2 standard deviations (SD) below the mean) or intellectual impairment (IQ > 2 SD below the mean), blindness (vision < 6/ 60 in both eyes), or sensorineural deafness requiring amplification)
Secondary resuscitation outcomes
-
Need for chest compressions during resuscitation
-
Need for epinephrine during resuscitation
-
Apgar score less than seven at five minutes after birth
Search methods for identification of studies
Electronic searches
We conducted a comprehensive search in November 2020 including: Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 11) in the Cochrane Library; Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to 25 November 2020). We have included the search strategies for each database in Appendix 1. We did not apply language restrictions.
We searched clinical trial registries for ongoing or recently completed trials. We searched the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/), and the United States' National Library of Medicine’s ClinicalTrials.gov (clinicaltrials.gov), via Cochrane CENTRAL. Additionally, we searched the ISRCTN Registry (www.isrctn.com/), for any unique trials not found through the Cochrane CENTRAL search.
Searching other resources
We cross‐referenced relevant literature including identified trials and existing review articles. We searched the following additional resources.
-
Reference lists from the above sources and from review articles.
-
Personal communication with primary authors from the above sources to retrieve unpublished data related to published articles.
-
Proceedings of annual meetings of the European Society for Pediatric Research and from the Society for Pediatric Research and Pediatric Academic Societies Annual Meeting. These are available at Abstracts2view from the year 2000 onward, up to and including 2020 (www.aap.org/en-us/professional-resources/Research/research-findings/Pages/PAS-Abstracts.aspx).
-
Proceedings of the Perinatal Society of Australia and New Zealand (PSANZ).
Data collection and analysis
We used the standard methods of Cochrane and Cochrane Neonatal.
Selection of studies
Two review authors (AT and DC) independently identified the studies for inclusion using the Covidence interface. We resolved disagreements through discussion.
Data extraction and management
Two review authors (AT and DC) independently extracted the data from the included studies using a pretested data extraction form. We resolved any disagreements through discussion. We deferred to the third review author (SN) if we were unable to reach an agreement.
Assessment of risk of bias in included studies
Two review authors (AT and DC) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane risk of bias tool (Higgins 2011), for these domains:
-
sequence generation (selection bias);
-
allocation concealment (selection bias);
-
blinding of participants and personnel (performance bias);
-
blinding of outcome assessment (detection bias);
-
incomplete outcome data (attrition bias);
-
selective reporting (reporting bias);
-
any other bias.
We resolved any disagreements through discussion or by consulting the third review author (SN). See Appendix 2 for a more detailed description of the risk of bias for each domain.
Measures of treatment effect
For dichotomous data, we calculated relative risk (RR) and risk difference (RD), along with 95% confidence intervals (CIs). We analysed continuous data using the mean difference (MD) along with its 95% CI.
Unit of analysis issues
We compared non‐vigorous neonates born through MSAF who underwent tracheal suction with those who did not receive tracheal suction. We did not include the repeated measurements in a group or the measurements done in a cross‐over design. We planned to include RCTs and cluster‐randomised trials that compared tracheal suction with no tracheal suction at birth in non‐vigorous neonates born through MSAF. We planned to use the inverse variance method (IVM) by analysing the effect estimate from each individual cluster trial and calculate the standard errors. We planned to provide an additional table of actual data used in the analysis (numerators and denominators, or means and SDs) for each treatment group. We did not plan to perform a combined meta‐analysis of the cluster and non‐cluster trials.
Dealing with missing data
In cases of missing data, we planned to contact original investigators with a request to provide the data if feasible. We contacted authors of one study awaiting classification for any published or presented version of the study results (CTRI/2012/08/002920). However, results of the study have not been published. Authors of the four studies included in the review were not contacted for any missing data.
Assessment of heterogeneity
We examined heterogeneity between trials first by assessing differences in trial methods and clinical heterogeneity. We used the following cut‐offs and labels for results of the I2 test:
-
0% to 25%: no heterogeneity;
-
25% to 49%: low heterogeneity;
-
50% to 74%: moderate heterogeneity;
-
75% to 100%: high heterogeneity.
We planned to inspect forest plots and quantify the impact of heterogeneity by using the I2 statistic. If heterogeneity was noted, we planned to explore possible causes of statistical heterogeneity by performing pre‐specified subgroup analysis (e.g. differences in study quality, participants, intervention regimens, outcome assessments). We used a fixed‐effect model for meta‐analyses.
Assessment of reporting biases
We planned to use funnel plots to assess publication bias where there were a sufficient number of studies (> 10) reporting the same outcome. This was not possible as we included only four studies in our review.
Data synthesis
For dichotomous outcomes, we provide RR and RD with 95% CIs. If the RD was significant, we reported the typical number needed to treat for an additional beneficial outcome (NNTB), or number needed to treat for an additional harmful outcome (NNTH). We report mean difference (MD) with 95% CIs for continuous outcomes.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses to consider:
-
gestational age (GA) at birth: term neonates (≥ 37 weeks' GA) and late preterm neonates (34 to 36 weeks' GA);
-
meconium consistency: 'thick' (if the fluid was viscous and tenacious and contained large amounts of particulate material), 'moderate' (if the fluid was thicker and darker in colour), or 'thin' (if the fluid was normal except for greenish colouring);
-
setting: low‐ and middle‐income countries;
-
type of suctioning: endotracheal tube and suction catheter;
-
type of delivery: vaginal and caesarean section.
However, we could not conduct any of the planned subgroup analyses as separate data for the subgroups were not reported in any of the included studies.
Sensitivity analysis
We planned to perform sensitivity analyses to test the robustness of decisions if we identified a sufficient number of trials. We planned to perform a sensitivity analysis to determine if findings were affected by inclusion limited to studies using adequate methods, defined as adequate randomisation and allocation concealment, blinding of intervention and measurement, and less than 10% loss to follow‐up. However, we did not perform a sensitivity analysis as the four studies included in the review had similar methodological rigour.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for these (clinically relevant) outcomes:
-
meconium aspiration syndrome;
-
all‐cause neonatal mortality;
-
the incidence of hypoxic‐ischaemic encephalopathy;
-
need for mechanical ventilation;
-
incidence of pulmonary air leaks;
-
culture‐positive sepsis;
-
persistent pulmonary hypertension.
Two authors (AT and DC) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, the directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create summary of findings Table 1 to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades:
-
high certainty: further research is very unlikely to change our confidence in the estimate of effect;
-
moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate;
-
low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate;
-
very low certainty: we are very uncertain about the estimate.
Results
Description of studies
Studies included in the review are described below and in the Included studies section.
Results of the search
We ran the search in November 2020. Of 88 studies imported for screening in Covidence, we found 79 to be not relevant to this review (Figure 1). We assessed nine studies for eligibility for inclusion in the review. Of these nine studies, we excluded four for various reasons (see Characteristics of excluded studies), and one study is awaiting classification as the study is completed but not published (see Characteristics of studies awaiting classification). We included four studies in this systematic review (see Characteristics of included studies).
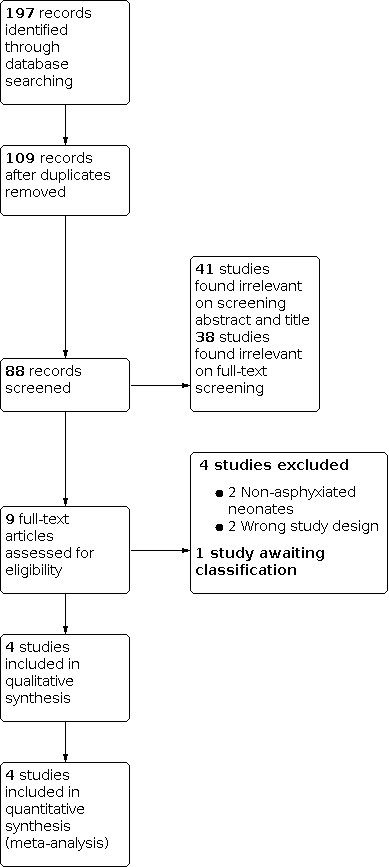
PRISMA flow diagram
Included studies
We included four studies (581 neonates) in the review (Chettri 2015; Kumar 2019; Nangia 2016; Singh 2018). All four studies were conducted in a single country: India. All four included studies enrolled non‐vigorous neonates born through MSAF and identified 'non‐vigorousness' using identical criteria – the presence of any of the following: heart rate below 100 beats/min, poor muscle tone, and no breathing or crying. All four studies randomised the enrolled neonates to tracheal suction versus no‐suction groups. The timing of enrolment (before versus immediately after birth) and sequence of events in the tracheal suction group (upper airway clearance before or after tracheal suction) varied between the trials, as discussed below.
Chettri 2015 enrolled 122 non‐vigorous neonates born through MSAF at 37 or more weeks of gestation. Randomisation and study group allocation occurred before birth on the detection of meconium in the amniotic fluid. Neonates found to be vigorous at birth were excluded from the study. Neonates found to be non‐vigorous at birth were randomised to suction or no‐suction groups. In neonates who were randomised to the suction group, intubation was performed before initial steps and the endotracheal tube was used for suctioning. A maximum of two suction procedures was allowed if the first suctioning yielded meconium and there was bradycardia (heart rate < 60). In neonates who were randomised to the no‐suction group, oropharyngeal suction was done to clear meconium from the upper airway. The primary outcome was MAS, defined as the presence of respiratory distress in a neonate born through MSAF, presence of characteristic radiological findings, and no other explanation for respiratory distress. The authors also reported survival and developmental outcome at nine months of age.
Kumar 2019 enrolled 132 non‐vigorous neonates born through MSAF at 34 or more weeks of gestation. Women with MSAF were screened and approached for consent before birth. However, randomisation and study group allocation occurred immediately after birth when the neonate was found to be non‐vigorous. While oropharyngeal suction was being performed to clear meconium from the upper airway, eligible neonates were randomised to suction or no‐suction groups. In neonates who were randomised to the suction group, oropharyngeal suction was followed by endotracheal suction. The endotracheal suction was repeated until no meconium was returned from the trachea. Once the trachea was clear of meconium, the initial steps of resuscitation were completed. In neonates who were randomised to the no‐suction group, oropharyngeal suction was followed by initial steps. The primary outcome was MAS, defined as persistence of respiratory distress beyond two hours of age and the presence of characteristic radiological findings.
Nangia 2016 enrolled 175 non‐vigorous neonates born through MSAF at 37 to 41 weeks of gestation with cephalic presentation. Women with MSAF were screened and approached for consent before birth. However, randomisation and study group allocation occurred immediately after birth when the neonate was found to be non‐vigorous. Neonates found to be non‐vigorous at birth were randomised to suction or no‐suction groups. In neonates who were randomised to the suction group, first oropharyngeal and nasal suctioning was done using a 12 to 14 French suction catheter, and then tracheal suctioning was done “as recommended by neonatal resuscitation program 2010 guidelines”. In neonates who were randomised to the no‐suction group, only oropharyngeal and nasal suction was done to clear meconium from the upper airway. The primary outcome was MAS or death, or both. MAS was defined as the presence of respiratory distress in a neonate born through MSAF with compatible radiological findings, and no other explanation for the symptoms.
Singh 2018 enrolled 155 non‐vigorous neonates born through MSAF at 37 or more weeks of gestation. While informed consent for enrolment was obtained before the birth of the baby, enrolment occurred after the baby was found to be non‐vigorous after birth. To do this, the random number sequence list was kept open and available to all. Using this list, neonates found to be non‐vigorous at birth were randomised to suction or no‐suction groups. In neonates who were randomised to the suction group, intubation was performed before initial steps and the endotracheal tube was pulled out while performing suction with the help of a meconium aspirator. A maximum of two suction procedures was allowed if the first suctioning yielded meconium and there was bradycardia (heart rate < 60). Oropharyngeal and nasal suctioning was performed after the tracheal suction was complete. In neonates who were randomised to the no‐suction group, oropharyngeal and nasal suctioning was done to clear meconium from the upper airway. The primary outcome was MAS, defined as the presence of early‐onset respiratory distress in a neonate born through MSAF, presence of characteristic radiological findings, and no other explanation for the symptoms.
Further details of the four included studies are provided in the Characteristics of included studies table.
Excluded studies
We excluded four studies (Daga 1994; Linder 1988; Ting 1975; Yoder 1994).
Daga 1994 and Linder 1988 enrolled non‐asphyxiated neonates and randomised them to receive only oropharyngeal suction or oropharyngeal suction with tracheal suction. We excluded both these studies as only non‐asphyxiated neonates were enrolled.
In a retrospective study, Ting 1975 evaluated the effect of tracheal suctioning in neonates born through MSAF and admitted to the newborn intensive‐care unit for observation. We excluded this study because it was a retrospective analysis.
In a prospective non‐randomised study, Yoder 1994 compared respiratory outcomes in neonates with moderate to thick meconium who were selectively not suctioned versus a control group of neonates. We excluded this study as details of intervention and method of allocation of intervention were not clear.
Risk of bias in included studies
The risk of bias is discussed below and summarised in the Characteristics of included studies, Figure 2, and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All four included studies reported the appropriate generation of random number sequences. Adequate allocation concealment was reported by three studies (Chettri 2015; Kumar 2019: Nangia 2016). No allocation concealment was done in one study (Singh 2018), which kept the randomisation sequence open. The baseline characteristics of the two study groups were similar in each of the four included studies.
Blinding
Due to the nature of the intervention, blinding of the team performing resuscitation was not possible in any of the included studies. However, the review authors could not identify any reported deviation from the intended intervention that arose because of the trial context. None of the four studies reported any protocol deviation in delivering the assigned intervention. The primary outcome of MAS was defined in an identical manner in the four studies. However, blinding of the outcome assessors was explicitly stated by only one study (Nangia 2016), and was probably done in another study (Chettri 2015).
Incomplete outcome data
Data about the main outcomes of this review are available for all the enrolled participants in all four studies. The longest follow‐up data for survival and developmental outcomes are reported by Chettri 2015 until nine months of age. About 14% of those discharged alive were reported lost to follow‐up by nine months of age.
Selective reporting
All the important outcomes, as identified in the registered trial protocols, have been reported by all four included studies.
Other potential sources of bias
We did not identify any other potential source of bias in the included studies. All four studies were academic trials, not funded by any public or private organisation.
Effects of interventions
Comparison 1. Tracheal suction compared to no suction in non‐vigorous neonates born through meconium‐stained amniotic fluid (MSAF)
All four included studies (581 neonates) compared tracheal suction with no tracheal suction at birth in non‐vigorous neonates born through MSAF (Chettri 2015; Kumar 2019; Nangia 2016; Singh 2018). See summary of findings Table 1.
Primary outcomes
Meconium aspiration syndrome (MAS)
All four included studies reported this outcome. All four studies defined MAS similarly, as respiratory distress developing soon after birth in an infant born through MSAF with compatible radiological findings that cannot be otherwise explained. Incidence of MAS varied from 27.3% (Kumar 2019), to 49.3% (Singh 2018). We are uncertain as to the effect of tracheal suction on the risk of MAS (RR 1.00, 95% CI 0.80 to 1.25; RD 0.00, 95% CI ‐0.07 to 0.08; I² = 49%; 4 studies, 581 neonates; Analysis 1.1). We graded the certainty of evidence as very low due to lack of masking of the care providers in three of the four studies (Chettri 2015; Kumar 2019; Singh 2018), lack of masking of outcome assessors in two of the four studies (Chettri 2015; Kumar 2019), lack of allocation concealment in one of the four studies (Singh 2018), moderate heterogeneity, and wide confidence interval of the relative effect estimate.
All‐cause neonatal mortality
All four included studies reported this outcome. The endpoint in all four studies was discharge from hospital. However, in one study (Chettri 2015), three neonates in the two study groups left the hospital against medical advice (LAMA) and all three died within a day of leaving the hospital. These deaths were included while pooling the event rate in this systematic review. In another study (Singh 2018), only combined event rate of death or LAMA is given which has been used for the pooled risk calculation.
Neonatal mortality varied from 7.2% (Singh 2018), to 16.4% (Chettri 2015). We are uncertain as to the effect of tracheal suction on overall incidence of in‐hospital neonatal mortality (RR 1.24, 95% CI 0.76 to 2.02; RD 0.02, 95% CI ‐0.03 to 0.07; I² = 12%; 4 studies, 575 neonates; Analysis 1.2). We graded the certainty of evidence as very low due to lack of masking of the care providers in three of the four studies (Chettri 2015; Kumar 2019; Singh 2018), lack of allocation concealment in one of the four studies (Singh 2018), and upper and lower bounds of the 95% CI of the pooled risk ratio reaching points of clinically significant reduction or increase in the outcome.
Hypoxic‐ischaemic encephalopathy (HIE)
The incidence of HIE was reported in two trials (Nangia 2016; Singh 2018).
Nangia 2016 reported the incidence of any‐grade HIE while Singh 2018 reported the incidence of moderate or severe HIE. We are uncertain as to the effect of tracheal suction on any‐grade HIE (RR 1.05, 95% CI 0.68 to 1.63; 1 study, 175 neonates; g; Analysis 1.3). We are uncertain as to the effect of tracheal suction on moderate or severe HIE (RR 0.68, 95% CI 0.43 to 1.09; 1 study, 152 neonates; heterogeneity not applicable; Analysis 1.3). We graded the certainty of evidence as very low due to lack of allocation concealment and lack of masking of the care providers in one of the two studies which reported this outcome (Singh 2018), inconsistency of results between the two studies, and upper and lower bounds of the 95% CI of the pooled risk ratio reaching points of clinically significant reduction or increase in the outcome.
Two other studies did not report the incidence of HIE but did report the incidences of perinatal asphyxia and neonatal seizures (Chettri 2015; Kumar 2019).
Secondary outcomes
Need for mechanical ventilation
The need for mechanical ventilation was reported by all four included studies. The incidence of this outcome varied, from the lowest of 10.9% (Nangia 2016), to the highest of 23.7% (Chettri 2015). The pooled incidence of the need for mechanical ventilation was not different with or without tracheal suction (RR 0.99, 95% CI 0.68 to 1.44; RD 0.00, 95% CI ‐0.06 to 0.06; I² = 0%; 4 studies, 581 neonates; Analysis 1.7). We graded the certainty of evidence as very low due to lack of masking of the care providers in three of the four studies (Chettri 2015; Kumar 2019; Singh 2018), lack of masking of outcome assessors in two of the four studies (Chettri 2015; Kumar 2019), lack of allocation concealment in one of the four studies (Singh 2018), and upper and lower bounds of the 95% CI of the pooled risk ratio reaching points of clinically significant reduction or increase in the outcome.
Duration of oxygen therapy
Only Nangia 2016 reported the duration of oxygen therapy. The mean duration of oxygen therapy was not different with or without tracheal suctioning (MD 1 hour, 95% CI ‐0.83 to 2.83 hours; 1 study, 175 neonates; Analysis 1.8). We graded the certainty of evidence as very low due to lack of masking of care providers to the study intervention and a single study reporting this outcome (lack of optimum information size).
Duration of mechanical ventilation
Two studies reported this outcome (Nangia 2016; Singh 2018). The number of neonates who needed mechanical ventilation was small in the two studies: 10.9% in the study by Nangia 2016 and 15.8% in the study by Singh 2018. Overall, the duration of mechanical ventilation was not different with or without tracheal suctioning (MD ‐4.04 hours, 95% CI ‐20.41 to 12.34 hours; I² = 0%; 2 studies, 43 neonates; Analysis 1.9). We graded the certainty of evidence as very low due to very serious risk of bias in the studies reporting this outcome and the wide confidence interval of the effect estimate.
Need for non‐invasive ventilation
The need for non‐invasive ventilation was reported by Kumar 2019. It was not different with or without tracheal suction (RR 1.36, 95% CI 0.68 to 2.74; 1 study, 132 neonates; Analysis 1.10). We graded the certainty of evidence as very low due to lack of masking of care providers to the study intervention and a single study reporting this outcome (lack of optimum information size).
Incidence of pulmonary air leaks
The incidence of pulmonary air leaks was reported by three studies (Chettri 2015, Nangia 2016; Singh 2018).
The pooled incidence of pulmonary air leaks was not different with or without tracheal suction (RR 1.22, 95% CI 0.38 to 3.93; RD 0.00, 95% CI ‐0.02 to 0.03; I² = 0%; 3 studies, 449 neonates; Analysis 1.11). We graded the certainty of evidence as very low due to lack of allocation concealment in one study (Singh 2018), lack of masking of the care providers in two studies (Chettri 2015; Singh 2018), lack of masking of outcome assessors in one study (Chettri 2015), and upper and lower bounds of the 95% CI of the pooled risk ratio reaching points of clinically significant reduction or increase in the outcome.
Duration of hospitalisation (hours)
A wide variation was noted in the duration of hospital stay within and across the three studies which reported this outcome (Kumar 2019; Nangia 2016; Singh 2018). The pooled estimate with heterogeneity (I² = 64%) shows a similar duration of hospital stay with or without tracheal suction (MD ‐2.24 hours, CI ‐9.27 to 4.79 hours; 3 studies, 459 neonates; Analysis 1.12). We graded the certainty of evidence as very low due to very serious risk of bias and substantial heterogeneity.
Incidence of neurodevelopmental delay
None of the included studies reported neurodevelopmental delay at 18 months old or older. Chettri 2015 reported the developmental index at nine months of age. Mental development index (RR 0.65, 95% CI 0.23 to 1.84; 1 study, 86 neonates; Analysis 1.13), and motor development index (RR 0.90, 95% CI 0.33 to 2.45; 1 study, 86 neonates; Analysis 1.14), assessed at nine months of age by the Development Assessment Scale for Indian Infants (DASII, an adaptation of the Bayley Scale of Infant Development (Phatak 1996)) were similar in the two groups. We graded the certainty of evidence as very low due to lack of masking of care providers to the study intervention and a single study reporting this outcome (lack of optimum information size).
Persistent pulmonary hypertension
The incidence of persistent pulmonary hypertension was reported by three studies (Chettri 2015; Kumar 2019; Singh 2018). The pooled incidence of persistent pulmonary hypertension was not different with or without tracheal suction (RR 1.29, 95% CI 0.60 to 2.77; RD 0.02, 95% CI ‐0.03 to 0.06; I² = 0%; 3 studies, 406 neonates; Analysis 1.15). We graded the certainty of evidence as very low due to lack of allocation concealment in one study (Singh 2018), lack of masking of the care providers in all three studies (Chettri 2015; Kumar 2019; Singh 2018), lack of masking of outcome assessors in two studies (Chettri 2015; Kumar 2019), and upper and lower bounds of the 95% CI of the pooled risk ratio reaching points of clinically significant reduction or increase in the outcome.
Proven blood‐stream infection (culture‐positive sepsis)
The incidence of culture‐positive sepsis was reported by three studies (Chettri 2015; Kumar 2019; Singh 2018). The pooled incidence of sepsis was not different with or without tracheal suction (RR 1.32, 95% CI 0.48 to 3.57; RD 0.01, 95% CI ‐0.03 to 0.05; I² = 0%; 3 studies, 406 neonates; Analysis 1.16). We graded the certainty of evidence as very low due to lack of allocation concealment in one study (Singh 2018), lack of masking of the care providers in all three studies (Chettri 2015; Kumar 2019; Singh 2018), lack of masking of outcome assessors in two studies (Chettri 2015; Kumar 2019), and upper and lower bounds of the 95% CI of the pooled risk ratio reaching points of clinically significant reduction or increase in the outcome.
Secondary resuscitation outcomes
Need for chest compressions during resuscitation
The need for chest compressions during resuscitation was reported by all four included studies. The pooled incidence of the outcome was not different with or without tracheal suction (RR 0.93, 95% CI 0.42 to 2.06; RD 0.00, 95% CI ‐0.03 to 0.03; I² = 0%; 4 studies; 581 neonates; Analysis 1.4). We graded the certainty of evidence as very low due to lack of masking of outcome assessors in all four included studies, lack of allocation concealment in one of the four studies (Singh 2018), and upper and lower bounds of the 95% CI of the pooled risk ratio reaching points of clinically significant reduction or increase in the outcome.
Need for epinephrine during resuscitation
The need for epinephrine during resuscitation was reported by all four included studies. The pooled incidence of the outcome was not different with or without tracheal suction (RR 1.21, 95% CI 0.37 to 3.92; RD 0.00, 95% CI ‐0.02 to 0.03; I² = 0%; 4 studies, 581 neonates; Analysis 1.5). We graded the certainty of evidence as very low due to lack of masking of outcome assessors in all four included studies, lack of allocation concealment in one of the four studies (Singh 2018), and upper and lower bounds of the 95% CI of the pooled risk ratio reaching points of clinically significant reduction or increase in the outcome.
Apgar score less than seven at five minutes after birth
The Apgar score less than seven at five minutes after birth was reported by all four included studies. The pooled incidence of the outcome was not different with or without tracheal suction (RR 1.11, 95% CI 0.87 to 1.42; RD 0.03, 95% CI ‐0.04 to 0.10; I² = 0%; 4 studies, 581 neonates; Analysis 1.6). We graded the certainty of evidence as very low due to lack of masking of outcome assessors in all four included studies, lack of allocation concealment in one of the four studies (Singh 2018), and upper and lower bounds of the 95% CI of the pooled risk ratio reaching points of clinically significant reduction or increase in the outcome.
Discussion
Summary of main results
In this Cochrane Review, we have evaluated the efficacy of tracheal suction at birth in reducing morbidity and mortality among non‐vigorous neonates born through meconium‐stained amniotic fluid. The review includes four RCTs, all conducted in a single country: India (Chettri 2015; Kumar 2019; Nangia 2016; Singh 2018). All four included trials enrolled non‐vigorous infants born through MSAF, used identical criteria to define eligibility (non‐vigorousness) of neonates for inclusion, and randomised the enrolled neonates to tracheal suction versus no‐suction groups. Three of the four studies included neonates born at, and beyond, term gestation (Chettri 2015; Nangia 2016: Singh 2018), whereas one study included neonates born at and beyond 34 weeks of gestation (Kumar 2019).
We are uncertain from the evidence from four studies included in this review whether tracheal suction at birth in non‐vigorous neonates born through MSAF reduces the incidence of MAS. We are uncertain whether tracheal suction improves all‐cause mortality, need for mechanical ventilation, any hypoxic‐ischaemic encephalopathy (HIE) or moderate to severe HIE, or culture‐positive sepsis. None of the included RCTs reported long‐term neurodevelopmental outcomes at 18 to 24 months of age. Only one study reported developmental index at nine months which was not different between the two groups (Chettri 2015). However, the certainty of the evidence for the review outcomes was very low due to imprecision in the effect estimates and lack of blinding of the outcome assessors.
Overall completeness and applicability of evidence
The available evidence is not sufficient to prove or disprove the efficacy or safety of tracheal suction at birth in non‐vigorous neonates born through MSAF. For important review outcomes such as MAS, death, the need for mechanical ventilation, and HIE, the 95% CIs of both relative and absolute measures of effectiveness cross the boundaries of clinically important effect size. A 20% relative or 7% absolute reduction (lower bounds of the 95% CI of risk ratio and risk difference respectively) or a 25% relative or 8% absolute increase (upper bounds of the 95% CI of risk ratio and risk difference respectively) in the incidence of MAS would mean significant benefit or harm of the intervention.
About one‐third (Chettri 2015; Kumar 2019; Nangia 2016), to half (Singh 2018), of the neonates enrolled in the included studies developed MAS. Although observational studies from middle and high‐income countries show a lower incidence of MAS, this can be explained by the accompanying lower incidence of birth asphyxia, the most important risk factor of MAS (Dargaville 2006). When restricted to non‐vigorous neonates, studies from middle and high‐income countries report an incidence of MAS similar to the studies included in this review (Vain 2004).
Apart from possible lack of benefit, two reasons given against routine tracheal suctioning include delay in resuscitation and injury caused due to the procedure. Two trials reported the incidence of HIE (Nangia 2016; Singh 2018). We are uncertain as to the effect of tracheal suction on any‐grade HIE (RR 1.05, 95% CI 0.68 to 1.63; 1 study, 175 neonates), or moderate or severe HIE (RR 0.68, 95% CI 0.43 to 1.09; 1 study, 152 neonates; heterogeneity not applicable). We are uncertain as to the effect of tracheal suction on Apgar score at five minutes after birth, and the need for advanced resuscitative measures such as chest compressions or medications. Chettri 2015 reported vocal cord injury with bleeding in one (1.6%) neonate who was intubated only for meconium suction. Other studies did not report any outcomes related to local injury.
Quality of the evidence
We judged the included studies to be at low risk of bias. However, the overall certainty of evidence was very low due to imprecision and inconsistency in the result estimates (summary of findings Table 1). The main reasons for downgrading the evidence included lack of blinding of the personnel providing the intervention in all four included studies, of health care personnel providing care in the neonatal intensive care unit (NICU) in three studies, and of outcome assessors in two studies. One study did not implement allocation concealment. Lastly, the upper and lower bounds of the CIs reach clinically significant reduction or increase for most of the review outcomes, indicating significant imprecision.
Potential biases in the review process
It is unlikely that the literature search strategy used in this review would have missed any relevant RCT. One trial (CTRI/2012/08/002920), identified from a clinical trial registry, is awaiting classification; although completed, the results have not been published in the literature or presented at any conference. We did not exclude any other potentially relevant study and there were no marginal decisions affecting the inclusion of any study in the review. However, due to the limited number of eligible studies, we are not able to evaluate publication bias using a funnel plot. We have used the standard methods recommended by Cochrane Neonatal to minimise the risk of bias in conducting this review. Two review authors independently assessed the eligibility of the studies for inclusion in the review, extracted data, and performed the risk of bias rating.
Agreements and disagreements with other studies or reviews
Two other systematic reviews were published while we were working on this review (Phattraprayoon 2020; Trevisanuto 2020). Phattraprayoon 2020 included the same four studies included in this review. No statistically significant difference was found for MAS (RR 0.98, 95% CI 0.71 to 1.35) in the first systematic review (Phattraprayoon 2020). Trevisanuto 2020 also included a fifth observational study (Chiruvolu 2018), from a high‐resource setting. The pooled estimate for survival was not significantly different for the group treated with tracheal suctioning (RR 1.01, 95% CI 0.96 to 1.06; P = 0.69; absolute risk reduction (ARR) 0.9%, 95% CI 3.7 to 5.6). Survival at discharge was reported in one observational study involving a total of 231 non‐vigorous newborns delivered through MSAF with no deaths in either group (Chiruvolu 2018). A recent observational study evaluated NICU admissions for MAS before and after a Neonatal Resuscitation Program (NRP) suctioning guideline change (Edwards 2019). This study suggested that NICU admissions for MAS and tracheal suctioning decreased after the introduction of the new guideline and there was no subsequent increase in severe respiratory distress among infants with and without a MAS diagnosis (Edwards 2019).

Risk of bias graph: review authors' judgements about each risk of bias item

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 1: Meconium aspiration syndrome

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 2: All‐cause neonatal mortality

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 3: Hypoxic‐ischaemic encephalopathy

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 4: Need for chest compressions (during resuscitation)

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 5: Need for epinephrine (during resuscitation)

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 6: Apgar score less than 7 at 5 minutes after birth

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 7: Mechanical ventilation

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 8: Duration of oxygen therapy

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 9: Duration of mechanical ventilation (hours)

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 10: Non‐invasive ventilation

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 11: Pulmonary air leaks

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 12: Duration of hospital stay (hours)

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 13: Severe delay in mental development index at 9 months

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 14: Severe delay in motor development index at 9 months

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 15: Persistent pulmonary hypertension

Comparison 1: Tracheal suction versus no tracheal suction, Outcome 16: Culture positive sepsis
| Tracheal suction compared to no suction in non‐vigorous neonates born through meconium‐stained amniotic fluid (MSAF) | ||||||
| Patient or population: non‐vigorous neonates born through meconium‐stained amniotic fluid | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with no tracheal suction | Risk with Tracheal suction | |||||
| Meconium aspiration syndrome (MAS) | Study population | RR 1.00 | 581 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of meconium aspiration syndrome. | |
| 346 per 1000 | 346 per 1000 | |||||
| All‐cause neonatal mortality assessed with: all‐cause neonatal deaths before discharge from hospital | Study population | RR 1.24 | 575 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of death before hospital discharge. | |
| 90 per 1000 | 112 per 1000 | |||||
| Moderate to severe hypoxic‐ischaemic encephalopathy (HIE) | Study population | RR 0.68 | 152 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of moderate to severe hypoxic‐ischaemic encephalopathy. | |
| 390 per 1000 | 265 per 1000 | |||||
| Any hypoxic‐ischaemic encephalopathy (HIE) | Study population | RR 1.05 | 175 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of any hypoxic‐ischaemic encephalopathy. | |
| 307 per 1000 | 322 per 1000 | |||||
| Need for mechanical ventilation assessed with: clinical and laboratory assessment of gas exchange before discharge from hospital | Study population | RR 0.99 | 581 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the need for mechanical ventilation. | |
| 154 per 1000 | 153 per 1000 | |||||
| Pulmonary air leaks (PAL) assessed with: clinical and radiological assessment before discharge from hospital | Study population | RR 1.22 | 449 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of pulmonary air leaks. | |
| 22 per 1,000 | 27 per 1,000 | |||||
| Persistent pulmonary hypertension (PPHN) assessed with: clinical or echocardiographic diagnosis before discharge from hospital | Study population | RR 1.29 | 406 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of persistent pulmonary hypertension. | |
| 54 per 1000 | 70 per 1000 | |||||
| Culture‐positive sepsis assessed with: blood culture before discharge from hospital | Study population | RR 1.32 | 406 | ⊕⊝⊝⊝ | We are uncertain whether tracheal suction has an effect on the incidence of culture‐positive sepsis. | |
| 29 per 1000 | 39 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by three levels because of very serious risk of bias, serious inconsistency and serious imprecision (wide confidence interval crossing the line of no significance). Outcome assessors were masked to the study intervention in only two studies. Care providers were masked to the study intervention in only one of the two studies which reported this outcome. There was no allocation concealment in one study. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Meconium aspiration syndrome Show forest plot | 4 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.80, 1.25] |
| 1.2 All‐cause neonatal mortality Show forest plot | 4 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.76, 2.02] |
| 1.3 Hypoxic‐ischaemic encephalopathy Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Any hypoxic‐ischaemic encephalopathy | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.68, 1.63] |
| 1.3.2 Moderate to severe hypoxic‐ischaemic encephalopathy | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.43, 1.09] |
| 1.4 Need for chest compressions (during resuscitation) Show forest plot | 4 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.42, 2.06] |
| 1.5 Need for epinephrine (during resuscitation) Show forest plot | 4 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.37, 3.92] |
| 1.6 Apgar score less than 7 at 5 minutes after birth Show forest plot | 4 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.87, 1.42] |
| 1.7 Mechanical ventilation Show forest plot | 4 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.68, 1.44] |
| 1.8 Duration of oxygen therapy Show forest plot | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐0.83, 2.83] |
| 1.9 Duration of mechanical ventilation (hours) Show forest plot | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐4.04 [‐20.41, 12.34] |
| 1.10 Non‐invasive ventilation Show forest plot | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.68, 2.74] |
| 1.11 Pulmonary air leaks Show forest plot | 3 | 449 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.02, 0.03] |
| 1.12 Duration of hospital stay (hours) Show forest plot | 3 | 459 | Mean Difference (IV, Fixed, 95% CI) | ‐2.24 [‐9.27, 4.79] |
| 1.13 Severe delay in mental development index at 9 months Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.23, 1.84] |
| 1.14 Severe delay in motor development index at 9 months Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.33, 2.45] |
| 1.15 Persistent pulmonary hypertension Show forest plot | 3 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.60, 2.77] |
| 1.16 Culture positive sepsis Show forest plot | 3 | 406 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |

