IRM de la próstata, con o sin biopsia dirigida por IRM y biopsia sistemática para detectar el cáncer de próstata
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the performance of mpMRI in men with prior‐negative SBx Type of study: retrospective cohort Selection: unclearly reported Enrolled/eligible: 54/58 Inclusion period: not reported, but before April 2013 | ||
| Patient characteristics and setting | Inclusion criteria: men who had ≥ 1 negative SBx and underwent mpMRI (index test) followed by TTMB (reference standard). All men included in the study had either increasing or persistently high PSA level Exclusion criteria: 4 men were excluded from the study as they received limited TTMB (< 20 cores were taken) Setting: London, UK. University hospital Age: median 64 years (range 39‐75) PSA: 10 ng/mL (range 2‐23) Prostate volume: 53 mL (range 19‐136) Previous number of negative Bx: 33 men had 1, 16 had 2, 5 had 3 | ||
| Index tests | Index tests: MRI only, with an MRI‐score 1‐5 with threshold ≥ 3 for positivity. A 1.5 Tesla (Philips Achiva) and 3.0 Tesla (Siemens Avanto) MRI machine, with T2, DWI and DCE sequences were used. Index test performed first, then the reference test. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4, GS ≥ 4+3 and others. Pathology grading before ISUP 2005: GS was based upon most frequent pattern instead of highest grade detected. Reference standard: systematic TTMB with the use of a brachytherapy grid under general anaesthesia, as described by Barzell. Basal and apical cores were obtained routinely, and the minimum number of samples was 20. MRI results were available during TTMB. | ||
| Flow and timing | All men underwent the same reference test. No men were excluded from analysis | ||
| Comparative | |||
| Notes | Study authors provided additional data Although MRI‐TBx were taken in a subset of 15 men, their results are not reported nor are they taken into account in our analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to test diagnostic accuracy of mpMRI and SBx against a reference test, TTMB Type of study: multicentre, paired‐cohort, prospective study Selection: consecutive Enrolled/eligible: 576/740 Inclusion period: May 2012‐November 2015 | ||
| Patient characteristics and setting | Inclusion criteria: PSA ≤ 15 ng/mL within previous 3 months, organ confined disease on DRE Exclusion criteria: previous history of PBx, prostate surgery or treatment for PCa (interventions for benign prostatic hyperplasia/bladder outflow obstruction were accepted. Evidence of a urinary tract infection or history of acute prostatitis within the last 3 months. Contraindication to MRI (e.g. claustrophobia, pacemaker, estimated GFR = 50). Previous history of hip replacement surgery, metallic hip replacement or extensive pelvic orthopedic metal work. Treated using 5‐alpha‐reductase inhibitors at time of registration or during the prior 6 months. Setting: London, UK. University and peripheral hospitals Age: mean 63.4 years (SD 7.6) PSA: mean 7.1 ng/mL (SD 2.9) Prostate volume: not reported | ||
| Index tests | Index test 1: MRI only: at multiple sites, 1.5 Tesla MRI scanners (T1, T2, DWI and DCE sequences) were used. Radiologists were provided with clinical details. A 5‐point Likert radiology reporting scale was used, with score of ≥ 3 designated a suspicious scan. Radiologist had undergone additional centralised training. Index test 2: 10–12 core transrectal SBx. Participants and physicians remained blinded to the mpMRI images and report. Participants first underwent the TTMB, followed by the SBx | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4, GS ≥ 4+3 and others Reference standard: TTMB, blinded for MRI report, with cores taken from every hole in the 5‐mm sampling frame. | ||
| Flow and timing | All men underwent the same reference test. Participants left the study for various reasons: 4 were ineligible, 2 were unblinded, 69 had large prostates (> 100 mL), 5 had T4 or nodal disease, 21 had clinical reasons, 52 did not want to proceed, 11 had other reasons | ||
| Comparative | |||
| Notes | Study authors provided additional data. Number of TTMB cores not reported but estimated | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the potential of a risk‐based strategy including MRI to selectively identify men aged ≥ 70 years with high‐grade PCa Type of study: prospective, 2‐arm, PSA‐screening study: 179 men received 6 core SBx only; 158 received MRI+/‐MRI‐TBx and SBx Selection: consecutive selection based on invitation to participate in a population‐based PSA screening trial Enrolled/eligible: 337/406 (69 participants refused Bx) In the current analysis, only the 158 men in the group receiving MRI and MRI‐TBx are included, of which 85 had a prior‐negative Bx and 74 were Bx‐naïve Inclusion period: Octobr 2013‐April 2016 | ||
| Patient characteristics and setting | Inclusion criteria: PSA ≥ 3.0 ng/mL Exclusion criteria: none Setting: PSA‐screening study. Rotterdam, the Netherlands. University hospital Age: median 73.1 years (IQR 72.4‐73.8)* PSA: median 4.2 ng/mL (IQR 3.4–5.8)* Prostate volume: median 52.9 (IQR 36.8‐70.9)* DRE positive: 14 participants* *of the 158 prior‐negative‐ and Bx‐naïve participants taken together | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI machine (Discovery MR750, General Electric Healthcare) was used, with T2, DWI, and DCE sequences. PI‐RADS version 2 was used, with score 1‐5 and score ≥ 3 for positivity. The Koelis Urostation was used for software fused transrectal MRI‐TBx from all MRI‐positive lesions Index test 2: transrectal extended sextant SBx were taken, blinded for MRI results, before taking the MRI‐TBx | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses (MRI‐pathway vs SBx) study, therefore the reference standard domain is not applicable and disregarded | ||
| Flow and timing | All participants underwent the same reference test. During the study, 69 participants refused Bx. | ||
| Comparative | |||
| Notes | Only the 158 participants in the group receiving MRI and MRI‐TBx are included in the current analysis; the 179 participants with sextant Bx only are excluded from our analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare the PCa detection rate of SBx and mpMRI‐TBx Type of study: prospective cohort Selection: unclear Enrolled/eligible: 206/213 Inclusion period: September 2012‐September 2013 | ||
| Patient characteristics and setting | Inclusion criteria: ≥ 1 prior‐negative SBx session (10–12 cores) and a persistent clinical suspicion of PCa (elevated PSA, an abnormal DRE, or a previous abnormal TRUS image) that warranted a repeat SBx Exclusion criteria: a prior PCa diagnosis, prior prostate mpMRI, or presence of general contraindications for MRI Setting: Herlev, Denmark. University hospital Age: median 65 years (IQR 58‐68) PSA: median 12.8 ng/mL (IQR 8.9‐19.6) Prostate volume: not reported. Instead, PSA‐density: median 0.20 (IQR 0.13‐0.29) DRE positive: 18 men | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Ingenia, Philips) was used, with T2, DWI and DCE sequencing. PI‐RADS version 1 with a Likert 1‐5 score and threshold for positivity of ≥ 2 was used*. Software fusion (Hitachi Ltd, HI‐RVS‐system) MRI‐TBx were taken of all MRI‐positive lesions Index test 2: a 10‐core transrectal SBx. Abnormalities on TRUS were sampled using the standard core for the relevant segment. This was performed first and blinded for MRI results, afterwards the MRI‐TBx were taken | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded | ||
| Flow and timing | All participants underwent the same reference test. During the study, 7 participants were excluded because of technical problems or claustrophobia | ||
| Comparative | |||
| Notes | *Although MRI‐TBx scores 2‐5 were taken, the results for a threshold of ≥ 3 can be distinguished. In our analysis, therefore, we used the threshold of ≥ 3 for a positive MRI and MRI‐TBx. We contacted study authors but they could not provide additional data. Other comparisons were not possible to the lack of detailed data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the diagnostic accuracy and negative predictive value of a novel bpMRI method in Bx‐naïve men in detecting and ruling out significant PCa. Type of study: prospective, single‐institutional, paired diagnostic study Selection: consecutive selection Enrolled/eligible: 1020/1063 (43 participants were excluded for various reasons) Inclusion period: November 2015–June 2017 | ||
| Patient characteristics and setting | Inclusion criteria: Bx‐naïve men with a clinical suspicion of PCa (PSA ≥ 4 ng/mL and/or abnormal DRE results) Exclusion criteria: prior PBx, evidence of acute urinary tract infections, acute prostatitis, general contraindications for MRI, and prior hip replacement surgery or other metallic implants in the pelvic area Setting: Herlev, Denmark, University Hospital Age: median 67 years (IQR 61‐71) PSA: median 8 ng/mL (IQR 5.7‐13) Prostate volume: median 53 mL (IQR 40‐72) DRE positive: 377/1020 (37%) participants | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI machine (Philips) with a pelvic‐phased‐array coil was used with T2, DWI sequences, without DCE (bpMRI). An in‐house modified PI‐RADS version 2 was used with score 1‐5 and score ≥ 3 for positivity. Transrectal software fused MRI‐TBx were performed from all MRI‐positive lesions, using Hitachi (n = 877) and Invivo (n = 143) systems. Index test 2: transrectal 10‐core SBx were taken before the MRI‐TBx, the performers were blinded for the MRI results | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same type of tests. The minimal exclusions were sufficiently explained not leading to relevant bias. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the diagnostic efficacy of cognitive mpMRI‐TBx compared to SBx in Bx‐naïve men Type of study: prospective single‐centre cohort study Selection: consecutive Enrolled/eligible: 168/168 Inclusion period: July 2011‐July 2014 | ||
| Patient characteristics and setting | Inclusion criteria: Bx‐naïve men, with a clinical suspicion of PCa because of elevated PSA levels and/or an abnormal DRE Setting: Madrid, Spain. University Hospital Age: mean 61.4 years (± 7.6) PSA: mean 8.3 ng/mL (± 6.1) Prostate volume: mean 48.9 mL (± 6.7) | ||
| Index tests | Index test 1: MRI‐pathway: mpMRI was performed with a 1.5 Tesla machine (Achieva, Philips Healtcare, Best, the Netherlands) with surface coil, using T1, T2 and DWI. PI‐RADS version 1 was used to assess the MRI by 2 readers independently, with a 1‐5 score and score ≥ 3 for positivity. All PI‐RADS ≥ 3 lesions were targeted cognitively with 2 MRI‐TBx cores. Index test 2: all men underwent transrectal SBx based on the Vienna nomogram (8‐19 biopsy cores depended on age and prostate volume), before MRI‐TBx were taken, by the same urologist. Blinding of MRI results during SBx was not reported | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded | ||
| Flow and timing | All participants underwent the same type of tests No participants were excluded | ||
| Comparative | |||
| Notes | Study authors provided additional data | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Unclear | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to investigate the overall and clinically significant PCa detection rates of MRI‐TBx and SBx in prior‐negative Bx men Type of study: retrospective study Selection: consecutive selection, but performance of MRI according to the physicians’ clinical considerations Enrolled/eligible: 185/185 (65 men underwent MRI and Bx, 120 men underwent only Bx without prior MRI) Inclusion period: March 2012–December 2014 | ||
| Patient characteristics and setting | Inclusion criteria: men with prior‐negative Bx, persistently elevated serum PSA level and normal DRE Exclusion criteria: positive DRE Setting: Taichung, Taiwan. University hospital Age: median 64 years (IQR 60.3‐67.8) PSA: median 10.9 ng/mL (IQR 7.2‐14.7) Prostate volume: median 48 mL (IQR 33.5‐62.5) DRE positive: none | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI machine (Signa HDx, General Electric Healthcare) was used with T2, DWI, and DCE sequences. PI‐RADS version 1 was converted to PI‐RADS version 2, with score 1‐5 and score ≥ 3 for positivity. Transrectal cognitive MRI‐TBx were performed from all MRI‐positive lesions with ≥ 2 cores Index test 2: transrectal SBx were taken with ≥ 16 cores from the peripheral zone and transitional zone, after the MRI‐TBx were taken by the same operator. | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same reference test. No participants were excluded. | ||
| Comparative | |||
| Notes | The 120 participants in the control group who underwent only SBx without prior MRI were excluded from our analysis. Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | No | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| High | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine the detection rate of 3‐Tesla MRI and MRI‐TBx compared to SBx Type of study: prospective cohort Selection: consecutive selection of participants who presented with a suspicion of PCa Enrolled/eligible: 420/429 Inclusion period: June 2008‐December 2013 | ||
| Patient characteristics and setting | Inclusion criteria: abnormal DRE findings and/or persistently elevated PSA levels Exclusion criteria: not reported Setting: Shanghai, China. University hospital Age: median 67 years (range 45‐91) PSA: median 9.7 ng/mL (range 2.4‐35.7) Prostate volume: median 44.8 mL (range 21.2‐83.2) DRE positive: 52 participants | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI machine (Philips Achieva) was used, with T1, T2, T2 spectral presaturation attenuated inversion recovery (SPAIR) and DWI sequences. An in‐house MRI score 1‐5 with threshold ≥ 3 for positivity were used. Cognitive transperineal MRI‐TBx were performed from all MRI‐positive lesions. Index test 2: a 10‐core fan‐shaped transperineal SBx from the peripheral zone with 2‐cores from transition zone was performed, blinded for MRI results, before taking the MRI‐TBx | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same reference test Except for the 9 excluded participants (DWI artifacts due to movement of the participant) all participants were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the clinical benefit of MRI‐TBx over SBx between first‐time and repeat SBx patients with prior (ASAP) Type of study: prospective cohort Selection: unclear Enrolled/eligible: 100/unclear (50 participants with prior‐negative Bx, 50 Bx‐naïve men) Inclusion period: September 2011‐March 2014 | ||
| Patient characteristics and setting | Inclusion criteria: PSA 2‒20 ng/L or DRE abnormalities. Bx‐naïve or ≥ 1 prior Bx with ASAP and ongoing clinical concern for malignancy Exclusion criteria: known PCa diagnosis, previous prostate MRI or contraindication to MRI or SBx Setting: Ontario, Canada. University hospital Age*: mean (SD) 59.4 (7.7); 61.9 (6.5) PSA*: mean (SD) 6.0 ng/mL (3.5); 7.9 (3.9) Prostate volume*: mean (SD) 38 g (18); 56 (27) *for Bx‐naïve men; and previous ASAP men, respectively | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI machine (GE Healthcare) was used, with T2, DWI and DCE sequences. A binary MRI suspicion score was used with a low threshold set to initiate software fusion (Artemis system) transrectal MRI‐TBx from all MRI‐positive lesions. Index test 2: a standard 12‐core transrectal SBx was performed in Bx‐naïve men, 2 additional cores were taken from the transition zone in previous ASAP men. No blinding of MRI results is reported. MRI‐TBx were taken prior to SBx | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants (with the same indication) underwent the same type of tests All participants were included in the analysis | ||
| Comparative | |||
| Notes | Retrospectively, MRI was reassessed using PI‐RADS version 2. The presented Bx data, however, are based on the prospective binary MRI‐score | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Unclear | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the value of MRI and MRI‐TBx added to standard SBx for detecting clinical relevant PCa Type of study: retrospective analysis Selection: retrospective selection of participants meeting inclusion criteria Enrolled/eligible: 38/1053 (of the 1053 participants who had had an MRI, 38 participants met the inclusion criteria) Inclusion period: August 2003‐August 2008 | ||
| Patient characteristics and setting | Inclusion criteria: men with ≥ 2 prior‐negative biopsies who underwent MRI and subsequent SBx complemented with MRI‐TBx of MRI‐suspicious lesions. All men were referred for MRI because of PSA > 4 ng/mL, PSA velocity > 0.75 ng/mL/year or equivocal histopathology from previous Bx Setting: Boston, USA. University hospital Age: mean 64 (range 48‐77) PSA: mean 14.4 (range 1.8‐33.1) Prostate volume: not reported | ||
| Index tests | Index test 1 : MRI‐pathway: a 3 Tesla MRI machine (Genesis Signa LX Excite, GE Healthcare) was used, with T1, T2 and DCE sequences. An in‐house MRI Likert 1‐5 scale was used, grouping score 1‐3 negative and 4‐5 positive. Cognitive MRI‐TBx from MRI‐positive lesions were taken, depending on judgement of urologist Index test 2: transrectal SBx was performed. Sequence of tests and number of cores were dependent on judgement of urologist. A total median of 19 (range 8‐28) cores (MRI‐TBx + SBx) were taken. MRI results were known at time of SBx performance | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same type of tests. All participants were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | No | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| High | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Unclear | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate whether adding 1.5 T magnetic field mpMRI‐TBx improves PCa detection in men undergoing blind 24‐core saturation PBx Type of study: prospective collected data Selection: consecutive selection Enrolled/eligible: 123/123 Inclusion period: January 2013–December 2016 | ||
| Patient characteristics and setting | Inclusion criteria: men who had already undergone a first 10/12‐core PBx with suspected PCa due to an increased PSA level and/or positive DRE Exclusion criteria: > 1 set of 10/12‐core Bx, TURP or other lower urinary tract endoscopic procedures Setting: Padua, Italy. University hospital Age: median 62 years (IQR 57‐68) PSA: median 6.27 ng/mL (IQR 4.75‐8.9) Prostate volume: mean 54.59 mL (range 20‐149) DRE positive: 8.9% (11/123) of the participants | ||
| Index tests | Index tests: MRI only + MRI‐TBx + MRI‐pathway: a 1.5 Tesla MRI machine was used with T2 and DWI sequences. PI‐RADS version 1 was used with score 1‐5 and score ≥ 3 for positivity. Transrectal cognitive MRI‐TBx were performed from all MRI‐positive lesions | ||
| Target condition and reference standard(s) | Target conditions: GS 3+3 = 6, GS ≥ 3+3, GS ≥ 3+4 Reference standard: transrectal 24‐core saturation Bx including 8 anterior biopsies, blinded for MRI results. When a suspicious lesion was present, the operator performed first the MRI‐TBx and then the saturation biopsies (unblinded) | ||
| Flow and timing | All participants underwent the same reference test. No participants were excluded | ||
| Comparative | |||
| Notes | Study authors provided additional data | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare the accuracy of visual MRI‐TBx versus software MRI‐TBx using a rigid or elastic approach Type of study: prospective cohort Selection: consecutive selection, divided into 3 groups: the first 127 participants received visual MRI‐TBx, the next 131 participants had the rigid fusion MRI‐TBx and the last 133 participants had the elastic fusion MRI‐TBx Enrolled/eligible: 391/391 Inclusion period: January 2011‐March 2012 | ||
| Patient characteristics and setting | Inclusion criteria: PSA > 4 ng/mL, and/or suspicious DRE and no previous PBx Exclusion criteria: none Setting: Paris, France. University hospital Age*: mean 62.7 years (SD 7.4); 64.5 (7.9); 64.6 (6.7) PSA*: mean (SD) 8.1 ng/mL (3.7); 9.0 (3.9); 8.3 (4.1) Prostate volume* (SD): 53 mL (25); 58.3 (28.6); 55.7 (35.1) DRE positive*: 20; 16; 16 *For the 3 groups, respectively: visual‐; elastic‐; rigid fusion | ||
| Index tests | Index test 1: MRI‐pathway: a 1.5 Tesla MRI machine was used, with T2, DWI and DCE sequences. An in‐house MRI‐score: 0‐4 score in transitional zone and 0‐10 in peripheral zone were used, with threshold ≥ 2 and ≥ 6 for positivity, respectively. Either cognitive MRI‐TBx or software fusion MRI‐TBx (Koelis, elastic MRI‐TRUS image registration System; Esaote, rigid navigation system) were taken from all positive lesions. Index test 2: 10‐12 core transrectal SBx was performed first. Blinding for MRI results was not reported. Subsequently, MRI‐TBx were taken of suspicious lesions | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same type of tests. All participants were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to analyse the negative predictive value of MRI and PSA density to rule out significant PCa Type of study: prospective cohort Selection: consecutive selection of men with a suspicion of PCa (PSA >4.0 ng/ml and/or suspicious digital rectal examination (DRE)) who were either biopsy‐naïve or after previous negative biopsy. Enrolled/eligible: 1040/1040 (597 Bx‐naïve + 443 prior‐negative Bx men) Inclusion period: October 2012‐December 2015 | ||
| Patient characteristics and setting | Inclusion criteria: suspicion of PCa: PSA > 4.0 ng/mL and/or suspicious DRE, and who were Bx‐naïve or had undergone a prior‐negative Bx Exclusion criteria: none Setting: Heidelberg, Germany. University hospital Age: median 65 years (IQR 60‐71) PSA: median 7.2 ng/mL (IQR 5.3‐10.4) Prostate volume: median 45 mL (IQR 34‐64) DRE positive: 291 | ||
| Index tests | Index tests: MRI only + MRI‐TBx + MRI‐pathway: a 3 Tesla MRI machine (Magnetrom Prisma or Biograph mMR (Siemens Healthcare) was used, with T2, DWI and DCE sequences. The PI‐RADS version 1 Likert 1‐5 score was used, with threshold ≥ 3 for positivity. Transperineal MRI‐TBx were taken from all positive lesions with the Biopsee system (rigid software registration). First MRI‐TBx were taken, subsequently the reference biopsies | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+4 Reference standard: volume‐based systematic transperineal grid‐directed Bx with a median of 24 cores according to the Ginsburg protocol. Bx operators first performed the MRI‐TBx and had access to MRI data during whole procedure. | ||
| Flow and timing | All participants underwent the same reference test. All participants were included in the analysis | ||
| Comparative | |||
| Notes | Results not reported separately for the two participant groups | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the performance of MRI‐TBx in diagnosing clinically significant PCa Type of study: prospective cohort Selection: consecutive selection Enrolled/eligible: 1042/1042 (328 Bx‐naïve‐, 324 prior‐negative Bx‐ and 390 active surveillance men) Inclusion period: September 2009‐February 2015 | ||
| Patient characteristics and setting | Inclusion criteria: elevated PSA level or abnormal DRE or 2) confirmation of low‐risk PCa for men considering active surveillance Exclusion criteria: none reported Setting: Los Angeles, USA. University hospital Age*: median (IQR) 64.4 years (58.5‐69.4); 65.7 (59.3‐70.2) PSA*: median (IQR) 5.8 ng/mL (4.4‐8.1); 7,6 (5‐11.5) Prostate volume*: median (IQR) 45 mL (33‐61.5); 57.7 (39.8‐83.5) *respectively, for the Bx‐naïve‐ and prior‐negative Bx participant groups | ||
| Index tests | Index tests 1: MRI‐pathway: a 3 Tesla MRI machine (Trio Trim/Somatom, Philips) was used, with T2, DWI and DCE sequences. An in‐house Likert 1‐5 score was used, with threshold ≥ 3 for positivity. MRI‐TBx (Artemis fusion device (Eigen, Grass Valley, Calif) were taken first in case of a suspicious lesion, then SBx were taken Index test 2: transrectal 12‐core SBx were taken in all participants, after MRI‐TBx. No blinding for MRI is reported | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same reference test. All participants were included in the analysis. | ||
| Comparative | |||
| Notes | Participants on active surveillance (n = 390) were excluded from our analysis. Although in text 328 participants are reported in the biopsy‐naïve group, in the data tables 329 participants are reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the differences in PCa detection rate and Bx effectiveness between MRI‐TBx and transperineal standard SBx in Bx‐naïve men Type of study: prospective cohort Selection: not explicitly reported Enrolled/eligible: 60/unclear Inclusion period: October 2014‐April 2016 | ||
| Patient characteristics and setting | Inclusion criteria: PSA > 4 ng/mL, a PSA density > 0.18 ng/mL/mL, a PSA velocity > 0.75 ng/mL/year or a pathological DRE Exclusion criteria: previous history of prostate biopsies, prostate surgery or radiotherapy or medical treatment for benign prostate hyperplasia Setting: Reus, Spain. University hospital Age: mean 64.1 years (SD 6.7). PSA: median 7.2 ng/mL (IQR 6‐9.4) Prostate volume: median 47.8 mL (IQR 34.6‐63.2) | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI machine (Signa, GE) was used, with T1, T2 and DWI sequences. The PI‐RADS version 1 Likert 1‐5 score was used, with threshold ≥ 4 for positivity (if no PI‐RADS ≥ 4 lesions were present, also PIRADS 2 and 3 were targeted). (Because study authors provided additional data we were able to use the results for a MRI‐threshold of ≥ 3.) The MRI targets were discussed with radiologist and cognitive fusion transperineal MRI‐TBx on target lesions was performed. Index test 2: 12‐core transperineal SBx in all men: two cores were directed towards the medial segments of the peripheral zone, two towards the lateral segments of the peripheral and two towards the transition zone for each lobe, with blinding for MRI results. Subsequently, MRI‐TBx were taken. | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same reference test. All participants were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. In our analysis we were therefore able to use the results for MRI‐threshold ≥ 3 for positivity. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine the sensitivity and specificity of mpMRI for significant PCa with transperineal sector Bx as the reference standard Type of study: prospective cohort Selection: consecutive patients Enrolled/eligible: 201/205 (83 Bx‐naïve‐, 103 prior‐negative Bx‐, 15 active surveillance participants; 4 participants were excluded due to contraindications to MRI) Inclusion period: July 2012‐November 2013 | ||
| Patient characteristics and setting | Inclusion criteria: a prior‐negative PBx with ongoing suspicion of PCa because of rising PSA levels (n = 103); those undergoing a primary PBx because of raised PSA level or abnormal DRE (n = 83) Exclusion criteria: previous history of PBx, prostate surgery or radiotherapy or medical treatment for benign prostate hyperplasia Setting: London, UK. University hospital Age*: mean (SD) 65 years (7.6); 64.1 (6.8). PSA*: mean (SD) 12.6 ng/mL (13.7); 13.3 (12.1) Prostate volume*: mean (SD) 54 mL (31); 68 (35) *Although test results are reported only for the mix of the 2 participant groups, these basic characteristics are reported for the 2 groups separately (103 prior‐negative Bx‐; Bx‐naïve patients, respectively) | ||
| Index tests | Index test: MRI only. A 1.5 Tesla MRI machine (Signa Excite, GE Healthcare) with T2 and DWI sequences was used. The PI‐RADS version 1 Likert 1‐5 score was used, with threshold ≥ 3 for positivity | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: transperineal sector Bx, with 24‐40 cores (depending on prostate size) with a brachytherapy grid. MRI‐positive lesions were targeted by cognitive registrated MRI‐TBx, but results were not reported separately | ||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | All active surveillance participants (n = 15) were excluded from our analysis after additional information was received from study authors | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the performance of combining a blood‐based biomarker panel and MRI‐TBx for PCa detection Type of study: prospective, multicentre, paired diagnostic study Selection: consecutive selection Enrolled/eligible: 532/727 (195 participants were excluded due to incomplete data) Inclusion period: May 2016–May 2017 | ||
| Patient characteristics and setting | Inclusion criteria: men aged 45‐75 years, no previous PCa, referral for PCa work‐up Exclusion criteria: previous diagnosis of PCa Setting: Stockholm, Sweden; Oslo, Norway; and Tonsberg, Norway. University and peripheral hospitals (cancer centre) Age: Stockholm (n = 160): mean 63 years (6.2); Oslo (n = 236): mean 65 years (7.8); Tonsberg (n = 136): mean 64 years (6.8) PSA: Stockholm (n = 160): median 6.2 ng/mL (IQR 4.8‐8,2) Oslo (n = 236): median 6 ng/mL (IQR 4‐9) Tonsberg (n = 136): median 7.1 ng/mL (IQR 4.7‐11) Prostate volume: Stockholm (n = 160): median 51 mL (IQR 38‐70) Oslo (n = 236): median 42 mL (IQR 32‐54) Tonsberg (n = 136): median 44 mL (IQR 33‐55) DRE positive: not reported | ||
| Index tests | Index test 1: MRI‐pathway, a 1.5 Tesla MRI machine (Avanto and Aera, Siemens) was used with T2, DWI sequences, without DCE. PI‐RADS version 2 was used with score 1‐5 and score ≥ 3 for positivity. Transrectal software fused MRI‐TBx were performed from all MRI‐positive lesions, using several machines. Index test 2: transrectal extended sextant SBx were taken after the MRI‐TBx and therefore not blinded for MRI results. | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests. Men with incomplete data were explained and excluded from the analysis, not leading to relevant bias. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to describe the Ginsburg protocol for transperineal MRI‐TBx supported by mpMRI and TRUS image fusion, and report biopsy results Type of study: prospective cohort study Selection: consecutive patients Enrolled/eligible: 571/571 (107 Bx‐naïve‐, 295 prior‐negative Bx‐ and 169 active surveillance men) Inclusion period: March 2013‐October 2015 | ||
| Patient characteristics and setting | Inclusion criteria: indication for repeat Bx: either rising PSA or ASAP or multifocal high‐grade prostatic intraepithelial neoplasia on a previous Bx Exclusion criteria: previous prostate MRI or a transperineal Bx Setting: Cambridge, UK. University hospital Age: median 65 years (IQR 59‐69) PSA: median 7.8 ng/mL (IQR 60‐12) Prostate volume: median 65 mL (IQR 44‐83) | ||
| Index tests | Index test: MRI only, MRI‐TBx and MRI‐pathway. A 1.5 Tesla (MR450) or a 3 Tesla (Discovery MR750 HDx) machine of GE Healthcare was used with T2, DWI and DCE sequences. The PI‐RADS version 1, Likert 1‐5 score was used, with threshold ≥ 3 for positivity. First transperineal software fusion MRI‐TBx cores were taken (BiopSee platform, Medcom) of every suspicious lesion. Then the reference standard was performed. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: an 18‐24 core systematic transperineal Bx according to the Ginsburg protocol, with 1‐2 cores from each of the 12 sectors, using the BiopSee MRI‐TRUS fusion platform with a brachytherapy grid for guidance. Blinding of MRI results not reported | ||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. In our analysis we excluded the 169 active surveillance participants. Furthermore, we excluded the 106 Bx‐naïve participants because of overlapping data with Hansen 2018. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the detection rates of transperineal MRI‐TBx and SBx for men with previous benign transrectal SBx in 2 high‐volume centres Type of study: prospective cohort study Selection: unclear Enrolled/eligible: 487/487 (200 from centre 1, 287 from centre 2) Inclusion period: October 2013‐November 2015 | ||
| Patient characteristics and setting | Inclusion criteria: indication for repeat Bx: rising PSA or a previous SBx specimen showing suspicion of cancer (ASAP) or multifocal high‐grade prostatic intraepithelial neoplasia Exclusion criteria: none reported Setting: Heidelberg, Germany. University hospital Age: median 66 years (IQR 61‐72) PSA: median 9.7 ng/mL (IQR 7.1‐13.9) Prostate volume: median 52 mL (IQR 36‐75) | ||
| Index tests | Index tests: MRI only,MRI‐TBx and MRI‐pathway. A 3 Tesla MRI machine (Magnetron, Siemens) with T2, DWI and DCE sequences was used. The PI‐RADS version 2, Likert 1‐5 score was used, with threshold ≥ 3 for positivity. First transperineal software fusion MRI‐TBx cores were taken (BiopSee platform, Medcom) of every suspicious lesion. Then template Ginsburg Bx was performed. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: a volume‐based, transperineal template Bx scheme, with a median of 24 cores, according to Ginsburg protocol was performed, using the BiopSee MRI‐TRUS fusion platform with brachytherapy grid for guidance. Blinding of MRI results not reported | ||
| Flow and timing | All participants underwent the same reference test. All participants were included in the analysis. | ||
| Comparative | |||
| Notes | Only participants from centre 1 (Heidelberg, Germany) were included in our analysis, due to overlap with patients of centre 2 (Cambridge, UK) in Hansen 2016b. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to analyse the detection rates of primary MRI‐fusion transperineal PBx using combined targeted and systematic core distribution in 3 tertiary referral centres Type of study: prospective cohort Selection: consecutive patients Enrolled/eligible: 856/807 (163 participants from centre 1, 402 from centre 2* and 242 from centre 3; 49 participants did not comply with the inclusion criteria) Inclusion period: October 2012‐May 2016 | ||
| Patient characteristics and setting | Inclusion criteria: first suspicion of PCa, based on raised PSA levels above age‐related normal range, a suspicious DRE, or other including family history Exclusion criteria: age > 79 years, PSA level > 30 ng/mL, prior‐negative Bx or previous diagnosis or treatment of PCa Setting Centre 1: Cambridge UK, tertiary care hospital Age: median 64 years (IQR 57‐69) PSA: 6.6 ng/mL (IQR 4.6‐9.0) Prostate volume: 44 mL (IQR 33‐55) Positive DRE: 39 participants Setting Centre 2: Heidelberg, Germany, University Hospital (participants from centre 2 in this study were excluded from analyses in this review to prevent overlapping data with the included study Distler 2017*) Age: median 65 years (IQR 60‐70) PSA: 6.9 ng/mL (IQR 5.2‐9.1) Prosate volume: 47 mL (IQR 32‐62) Postive DRE: 94 participants Setting Centre 3: Melbourne, Australia, tertiary care hospital Age: median 65 years (IQR 60‐70) PSA: 5.9 ng/mL (IQR 4.6‐8,0) Prostate volume: 25 mL (IQR 24‐47) Positive DRE: 54 participants | ||
| Index tests | Centre 1: index test: MRI only, a 1.5 Tesla (MR450) or a 3 Tesla (Discovery MR750 HDx) machine of GE Healthcare was used with T2, DWI and DCE sequences. The PI‐RADS version 1 (until 2015) and version 2 (onwards) with a Likert 1‐5 score were used, with threshold ≥ 3 for positivity. Transperineal software fusion MRI‐TBx cores were taken (BiopSee system, Medcom) of every suspicious lesion, followed by template Bx. However, MRI‐TBx results were not reported separately. Centre 3: index test: MRI only, a 3 Tesla Magnetom (Siemens) was used with T2, DWI and DCE sequences. The PI‐RADS version 1 (until 2015) and version 2 (onwards) with a Likert 1‐5 score were used, with threshold ≥ 3 for positivity. Transperineal cognitive MRI‐TBx cores were taken of every suspicious lesion, followed by template Bx. However, MRI‐TBx results were not reported separately. | ||
| Target condition and reference standard(s) | Target condition in both centres: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard in both centres: volume‐based transperineal template Bx with a median of 24 cores according to the Ginsburg protocol. Bx operators had access to MRI data during whole procedure. MRI‐TBx were taken in addition to the template Ginsburg biopsies and included in the reference standard results. Centre 1 used the Biopsee system (Medcom) with a 5‐mm spacing brachytherapy grid Centre 3 used a 5‐mm spacing brachytherapy grid (BK Ultrasound) and a transrectal probe mounted on a stepper | ||
| Flow and timing | All participants underwent same reference standard. No participants were excluded for analysis. | ||
| Comparative | |||
| Notes | *Only the 163 participants from centre 1 and the 242 patients from centre 3 are included in our analysis; we excluded the 402 patients from centre 2 because they are also reported in Distler 2017 | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the diagnostic accuracy of MRI and MRI‐TBx using visual registration Type of study: multicentre study, unclear design Selection: unclear Enrolled/eligible: 55/unclear Inclusion period: April 2011‐March 2013 | ||
| Patient characteristics and setting | Inclusion criteria: PSA > 4 ng/mL on 2 consecutive measurements in the last 6 months Exclusion criteria were:
Setting: Turku, Finland/Bratislava, Slovakia. University hospitals Age: median 66 years (range 47–76) PSA: median 7.4 ng/mL (range 4–14) Prostate volume: median 42 mL (range 17–107) | ||
| Index tests | Index test 1: MRI‐pathway, a 3 Tesla machine (Magnetom Verio 3T, Siemens) was used with T2, DWI, DCE and spectroscopy sequences. An in‐house MRI Likert 1‐5 scale was used, with threshold ≥ 4 for positivity and MRI‐TBx (but small discrete lesions (maximum diameter of 7–9 mm on mpMRI) were also targeted. Cognitive transrectal MRI‐TBx were taken of all suspicious lesions, after SBx Index test 2: transrectal extended sextant SBx were taken, blinded for MRI results | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests. All participants were included in the analysis; except for 2 participants who did not receive MRI‐TBx due to technical problems, which in our current analysis had to be excluded. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the role of a MRI combined with MRI‐TBx for improving risk stratification of men with elevated PSA Type of study: prospective cohort Selection: unclear selection Enrolled/eligible: 161/175 (134 Bx‐naïve, 27 prior‐negative Bx participants and 14 exclusions) Inclusion period: March 2013‐February 2015 | ||
| Patient characteristics and setting | Inclusion criteria: 2 repeated measurements of PSA in the range 2.5–20.0 ng/mL and/or abnormal DRE Exclusion criteria: previous PCa diagnosis, previous Bx within 6 months, prostate surgery, clinical infection or MRI contraindication Setting: Turku, Finland. University hospital Age: mean 64.7 years (SD 6.4) PSA: median 7.5 (IQR 5.7‐9.6). Prostate volume: median 37 (IQR 27.5‐49) | ||
| Index tests | Index tests 1: MRI‐pathway: a 3 Tesla machine (Magnetom Verio 3T, Siemens) was used with T2 and DWI sequences. An in‐house MRI Likert 1‐5 scale was used, with threshold ≥ 3 for positivity and MRI‐TBx. Cognitive transrectal MRI‐TBx were taken of all index lesions, prior to SBx. Index test 2: 12‐core transrectal SBx, without blinding for MRI results (although strictly following the SBx scheme). | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests. Not all participants were included in analysis. 4 withdrew consent before and 7 after MRI, 1 had a non‐diagnostic MRI, 2 had a PSA < 2.5 or > 20 | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate a volume‐based, computer‐assisted method for TOP‐Bx Type of study: prospective cohort Selection: unclear selection Enrolled/eligible: 172/unclear (mix of 95 Bx‐naïve‐, 51 prior‐negative‐ and 26 active surveillance participants) Inclusion period: October 2013‐March 2014 | ||
| Patient characteristics and setting | Inclusion criteria: abnormal PSA or suspicious DRE, persistent suspicion of PCa after prior‐negative Bx Exclusion criteria: none reported Setting: Darmstadt, Germany. University hospital Age*: median 65 years (IQR 58‐71) PSA*: median 7.2 ng/mL (IQR 5.4‐10.2) Prostate volume*: median 46 mL (IQR 36‐60) Positive DRE*: 37 participants | ||
| Index tests | Index tests: MRI only + MRI‐TBx + MRI‐pathway. A 3 Tesla machine (Magnetom, Siemens) was used with T1, T2, DWI and DCE sequences. The PI‐RADS version 1, Likert 1‐5 scale was used, with threshold ≥ 3 for positivity and MRI‐TBx. Software fusion transperineal MRI‐TBx were taken of all index lesions independently of the TOP‐Bx, using the BiopSee MRI‐TRUS fusion platform (Medcom). | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: Novel, volume‐based, automated core‐placement method for TOP‐Bx placement was performed with a needle distribution sampling each conceivable tumour lesion ≥ 0.5 mL in the complete prostate (100%), with a median of 24 (IQR 23‐27) cores, independent of MRI results. | ||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. We excluded the 26 active surveillance participants from our analysis. *However, the basic characteristics are based on all participants (including the active surveillance participants). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine the added value of prostate MRI to the Prostate Cancer Prevention Trial risk calculator Type of study: retrospective study of prospective database Selection: consecutive patients who received prostate MRI prior to Bx Enrolled/eligible: 421/unclear (185 Bx‐naïve‐, 154 prior‐negative Bx and 82 active surveillance participants). Inclusion period: January 2012‐December 2015 | ||
| Patient characteristics and setting | Inclusion criteria: indication for MRI and Bx, no details reported Exclusion criteria not reported Setting: St. Louis, MO, USA. University hospital Age*: mean 63.9 years (SD 7.6) PSA*: mean 10.2 ng/mL (SD 15.1) Prostate volume: not reported Positive DRE*: 48 participants *only reported for the whole group (Bx‐naïve and prior‐negative Bx participants combined) | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla machine (Siemens) was used with T2, DWI and DCE sequences. 2 MRI‐scoring systems were used: in the first 205 participants a binary in‐house score, in the last 194 participants a PI‐RADS version 1 and version 2 Likert 1‐5 score. The MRI‐TBx thresholds for positivity and MRI‐TBx were a comparable triple suspicious (on T2, DWI, DCE) or a PIRADS version 2 4/5 lesion. MRI‐TBx was performed prior to SBx: 70 participants received cognitive MRI‐TBx using the TargetScan system (Best Nomos); 129 with software fusion MRI‐TBx (UroNav system, Invivo). Index test 2: 12‐core transrectal SBx, without blinding for MRI results | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same type of tests and were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. We excluded from our analysis the 82 active surveillance participants. Furthermore we excluded 2 Bx‐naïve participants because only the highest GS was recorded (not differentiating between Bx methods). The remaining 337 (183 Bx‐naïve‐ and 154 prior‐negative Bx‐) participants were included. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to measure the performance characteristics of the MRI suspicion score prior to MRI‐TRUS fusion template transperineal repeat Bx Type of study: retrospective study of prospective data Selection: preselected patients in a MRI‐TRUS fusion template transperineal prostate repeat Bx programme Enrolled/eligible: 39/unclear Inclusion period: February 2012‐June 2012 | ||
| Patient characteristics and setting | Inclusion criteria:
Exclusion criteria: none Setting: Cambridge, UK. University hospital Age: mean 64 (range 47‐77) PSA: median 10 ng/mL (range 1.2‐36) Prostate volume: not reported | ||
| Index tests | Index test: MRI only, MRI‐TBx and MRI‐pathway. A 1.5 or 3 Tesla MRI (MR450, GE healthcare) were used, with T1, T2 and DWI. A PI‐RADS version 1 adapted sum score 1‐10 was used, with a score < 6 = no suspicion, 6 = low suspicion, 7‐8 = intermediate suspicion and 9‐10 = high suspicion, with threshold ≥ 6 for positivity and MRI‐TBx. Transperineal software fused MRI‐TBx were taken of all positive lesions, using the Biopsee system (Medcom), prior to the Ginsburg‐Bx | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 Reference standard: 24‐36 volume‐based transperineal biopsies were taken according to the Ginsburg protocol, without resampling MRI‐TBx trajectories, using the Biopsee system. MRI‐TBx were taken prior to the template biopsies. | ||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | For comparison 1a, we assume that the results of the MRI‐TBx that corresponded to the trajectory of the reference Bx (and thus were not resampled) are also considered results for the template Bx, as it seems it is reported as such. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare PCa detection rates between SBx and mpMRI‐TBx for men with PSA < 10 ng/mL Type of study: retrospective analysis of prospectively collected data Selection: before PBx decision making, mpMRI‐TBx was explained to the participants. Those participants who agreed to the MRI‐TBx pathway (instead of standard SBx) were consecutively selected. Enrolled/eligible: 76/unclear Inclusion period: January 2014‐December 2014 | ||
| Patient characteristics and setting | Inclusion criteria: PSA level < 10 ng/mL, normal DRE and no previous PBx Setting: Yangsan, Korea. University hospital Age: median 65.8 years (range 43‐83) PSA: median 6.4 ng/mL (range 3.3‐9.8) Prostate volume: median 38.8 mL (range 17‐127) | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Intera Achieva, Phillips) was used, with T2 and DWI sequences. A modified 1‐4‐point MRI score was used:
Threshold for positive MRI and MRI‐TBx was score ≥ 2. Cognitive transrectal MRI‐TBx was performed of all positive lesions, prior to SBx. Index test 2: transrectal extended sextant SBx, without blinding for MRI results | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests and were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine the efficacy of cognitive MRI‐TBx using biparametric MRI for men with PSA levels < 10 ng/mL Type of study: retrospective analysis Selection: before PBx, each urologist explained the MRI‐TBx technique to the participants; the final choice regarding the use of the technique (MRI‐TBx or standard SBx) was left to each participant. Hence, all consecutive participants who chose MRI‐TBx were selected. Enrolled/eligible: 123/464 (464 participants underwent PBx. Excluded were: 126 participants with a PSA > 10 ng/mL, 207 participants who chose SBx only, and 8 participants who had a prior‐negative Bx) Inclusion period: 2016 | ||
| Patient characteristics and setting | Inclusion criteria: Bx indication by elevated PSA and choice for MRI‐pathway Exclusion criteria: PSA > 10 ng/mL, previous PBx Setting: Yangsan, Korea. University hospital Age*: mean (SD) 61.8 years (11.7); 62 (7.8) PSA*: mean (SD) 6.7 ng/mL (1.67); 6.19 (1.82) Prostate volume* (SD): 38.6 mL (18.6); 40.2 (18.1) *reported for the mpMRI participants (n = 55) and bpMRI‐participants (n = 68), respectively | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Intera Achieva, Phillips) was used. In 68 participants only T2 and DWI sequences were used, in 55 DCE was also used. A modified 1‐4‐point MRI score was used, based on PI‐RADS version 2:
Threshold for positive MRI and MRI‐TBx was score ≥ 2. Cognitive transrectal MRI‐TBx was performed of all positive lesions, prior to SBx. Index test 2: transrectal extended sextant SBx, without blinding for MRI results | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests and were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the diagnostic accuracy of mpMRI and mpMRI /TRUS fusion‐guided MRI‐TBx against transperineal TSB for the detection of PCa Type of study: retrospective analysis Selection: consecutive selection Enrolled/eligible: 415/415 (163 Bx‐naïve, 86 prior‐negative Bx, 166 previous positive Bx men) Inclusion period: November 2014–September 2016 | ||
| Patient characteristics and setting | Inclusion criteria: men who underwent mpMRI ± MRI‐TBx followed by template Bx Exclusion criteria: previously treated for PCa Setting: Zurich, Switzerland. University hospital Age: Bx‐naïve men: median 63 years (IQR 57‐68) Repeat‐Bx men: median 64 years (IQR 60‐69) PSA: Bx‐naïve men: median 5.8 ng/mL (IQR 4.4‐8.9) Repeat‐Bx men: median 8.6 ng/mL (IQR 5.7‐13) Prostate volume: Bx‐naïve men: median 44.6 mL (IQR 34‐60.1) Repeat‐Bx men: median 53.6 mL (IQR 41‐70) DRE positive: not reported | ||
| Index tests | Index test: MRI only, MRI‐TBx and MRI‐pathway. A 3 Tesla MRI machine (Magnetom Skyra, Siemens) was used with T2, DWI, and DCE sequences. In 16% of participants mpMRI was performed elsewhere. MRI was performed without an endorectal coil in 84% of participants. A Likert score analogous to PI‐RADS version 1 was used, with score 1‐5 and score ≥ 3 for positivity. The Biopsee Pi Medical/MedCom was used for software fused transrectal MRI‐TBx from all MRI‐positive lesions, with 2‐4 cores, after completing the TSB. | ||
| Target condition and reference standard(s) | Target conditions: Bx‐naïve men: GS 3+3 = 6, GS ≥ 3+3, GS ≥ 3+4, GS ≥ 4+3 Repeat‐Bx men: GS 3+3 = 6, GS ≥ 3+3, GS ≥ 3+4 Reference standard: transperineal template saturation prostate biopsies were taken according to the 20 Barzell zones (median 40 cores), not blinded for MRI results. | ||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | The 166 participants with previous positive Bx were excluded from our analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the detection rate of significant PCa by transperineal template‐guided Bx Type of study: partial prospective and retrospective analysis Selection: all men who received MRI prior to template Bx were selected, no criteria reported for performing MRI or template Bx Enrolled/eligible: 200/unclear (9 Bx‐naïve‐, 162 prior‐negative Bx and 29 active surveillance participants). Inclusion period: March 2013‐December 2014 | ||
| Patient characteristics and setting | Inclusion criteria: transperineal template‐guided PBx and MRI prior to Bx Exclusion criteria: previous brachytherapy, previous template biopsies for anorectal abnormalities Setting: Birmingham, UK. Tertiary referral centre Age*: median (range) 68 years (46‐81); 65 (47‐78) PSA*: median (range) 11.5 ng/mL (1.2‐92.5); 10 (2.7‐61). Prostate volume: not reported *reported for template Bx positive (n = 71) and template Bx negative (n = 103) participants, respectively | ||
| Index tests | Index test: MRI only, assessed prior to template Bx. No details for MRI‐acquisition are reported. The PI‐RADS version 1 was used with a 1‐5 score and threshold ≥ 3 for positivity. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: a minimum of 24 sector transperineal prostatic Bx cores were taken in a systematic fashion using a 5 mm brachytherapy template grid, prostate volume depended. Blinding for MRI results was not reported. | ||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. We excluded the 29 active surveillance or other indication participants from this current analysis. The remaining 9 Bx‐naïve‐ and 162 prior‐negative participants were included. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare PCa detection rates between SBx and transperineal template PBx, in Bx‐naïve men Type of study: prospective cohort Selection: unclear Enrolled/eligible: 50/unclear Inclusion period: August 2012‐August 2013 | ||
| Patient characteristics and setting | Inclusion criteria: benign DRE, elevated PSA < 20 ng/mL, > 10 years' life expectancy Exclusion criteria: previous PBx Setting: Leicester, UK. University hospital Age: mean 67 years (range 54‐84) PSA: mean 8 ng/mL (range 4‐18) Prostate volume: mean 58 mL (range 19‐165) | ||
| Index tests | Index test: transrectal 12‐core SBx were taken from the right and left peripheral zones. Index test was taken first, then the reference test, in the same setting. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: 36‐core transperineal template PBx using a brachytherapy grid, after the performance of the SBx | ||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine whether transperineal template PBx is superior to SBx in the detection of PCa Type of study: prospective Selection: not reported Enrolled/eligible: 42/unclear Inclusion period: August 2012‐August 2014 | ||
| Patient characteristics and setting | Inclusion criteria: a history of 1 prior‐negative SBx with benign pathology, benign‐feeling prostate on DRE and a persistently elevated serum PSA more than the age‐specific range but < 20 ng/mL Exclusion criteria: none reported Setting: Leicester, UK. University hospital Age: median 65 years (range 50‐75) PSA: 8.3 ng/mL (range 4.4‐19) Prostate volume: 59 mL (range 21‐152) | ||
| Index tests | Index tests: 12 core transrectal SBx. Index test was taken first, then the reference test, in the same setting. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 Reference standard: 36‐cores transperineal template PBx using a brachytherapy grid | ||
| Flow and timing | All participants underwent the same reference test and were excluded from analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to analyse the contribution of MRI and PCA3 in detecting PCa Type of study: prospective cohort Selection: unclear Enrolled/eligible: 53/unclear Inclusion period: February 2013‐March 2014 | ||
| Patient characteristics and setting | Inclusion criteria: serum PSA level 3‐10 ng/mL participants with normal DRE scheduled for initial PBx Exclusion criteria: none reported Setting: Ankara, Turkey. Single‐centre, university hospital Age: median 62 years (IQR 43‐79) PSA: 5 ng/mL (range 3‐8.9) Prostate volume: median 45 mL (range 17‐93) | ||
| Index tests | Index tests 1: MRI‐pathway: a 1.5 Tesla MRI (Avanto, Siemens) was used, with T2, DWI, DCE and spectroscopy sequencing. A binary MRI score was reported, with additional cognitive transrectal MRI‐TBx taken from all positive lesions. Index test 2: transrectal extended sextant SBx with a mean number of 12.7 cores (including the additional MRI‐TBx only in MRI‐positive men), no further details reported | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests. 1 participant did not undergo MRI for unclear reasons and was not included in analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess whether the proportion of men with clinically significant PCa is higher among men randomised to MRI‐Bx vs those randomised to SBx Type of study: prospective, 2‐armed RCT. Arm 1: MRI +/‐ MRI‐TBx and SBx; arm 2: SBx only. Participants from the SBx‐only arm with a negative Bx result subsequently received MRI +/‐ MRI‐TBx and SBx (with a standard scheme if MRI was positive and a saturation scheme if MRI was negative), therefore we regarded these participants as prior‐negative Bx participants. Selection: consecutive patients meeting the inclusion criteria Enrolled/eligible: 1040/1040 (570 participants in arm 1 and 570 participants in arm 2) Inclusion period: October 2011‐March 2014 | ||
| Patient characteristics and setting | Inclusion criteria: PSA level > 4 ng/mL, PSA density > 0.15, PSA velocity > 0.75 ng/mL/year, free/total PSA ratio < 0.10 when total PSA was 4‐10 ng/mL. The participants needed to meet all 4 inclusion criteria. Exclusion criteria: previous PBx The prior‐negative Bx participants (in arm 2) were not referred in a common clinical way, but selected on the basis of the prior‐negative SBx within the randomised population of Bx‐naïve participants. Setting: Rome, Italy. University hospital Age: median 64 years (range 51‐82) for all 1040 participants PSA: not reported Prostate volume: not reported | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Discovery MR750, GE Healthcare or MAGNETOM Verio, Siemens) was used with T2, DWI and DCE sequencing. PI‐RADS version 1 was used resulting in a Likert 1‐5 scale with threshold ≥ 3 for positivity and MRI‐TBx. All MRI suspicious lesions were cognitively targeted with 2 transrectal MRI‐TBx cores. Index test 2:
Order of index tests unclear, no blinding for MRI results during the Bx procedure reported. | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | In arm 1 (Bx‐naïve participants) all participants received the same tests. In arm 2 (prior‐negative Bx participants) participants received a significantly different type of SBx, depending on MRI‐result. All participants were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. For our analysis, the 115 participants in arm 2 who had an initial positive SBx result were excluded. The remaining 355 participants of arm 2 contributed to our analysis as prior‐negative Bx participants. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare the detection of clinically significant disease by standard SBx vs MRI‐TBx Type of study: prospective Selection: consecutive Enrolled/eligible: 110/129 (14 men with previous Bx and 5 men with contraindications for MRI were excluded) Inclusion period: March 2012‐September 2013 | ||
| Patient characteristics and setting | Inclusion criteria: clinical suspicion of PCa due to an abnormal PSA and/or DRE Exclusion criteria: previous PBx, MRI contraindications Setting: Brussels, Belgium. Tertiary care hospital Age: median 65.8 years (IQR 59.5‐70.7) PSA: median 6.9 ng/mL (IQR 4.6‐9.6) Prostate volume: median 44 mL (IQR 35‐59) | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Verio, Siemens) was used, with T2, DWI and DCE sequences. An in‐house MRI score was used resulting in a 1‐4‐point scale (assessment based on PI‐RADS version 1 recommendations): 1 = no suspicious lesions, 2 = low suspicion (0‐1 parameter positive), 3 = moderate suspicion (2 parameters positive, including DWI), 4 = high suspicion (3‐4 parameters positive), with threshold score ≥ 2 for positivity and MRI‐TBx. Transrectal MRI‐TBx were taken with software fusion (Urostation, Koelis), after the performance of SBx. Index test 2: transrectal standard 12 core SBx + 2‐4 additional cores from the transitional zone according to the volume of the prostate. The operator performing SBx was not blinded to MRI results. | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent same tests and were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| High | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate MRI accuracy in PCa diagnosis in men submitted to saturation PBx Type of study: prospective, single‐centre, multi‐departmental study Selection: unclear. Men were selected from a PCa case‐finding protocol (including 14,453 patients) if meeting the inclusion criteria and when having an indication for saturation Bx Enrolled/eligible: 78/unclear Inclusion period: June 2011‐December 2012 | ||
| Patient characteristics and setting | Inclusion criteria: 1 single prior‐negative Bx > 6 months before. Indications for saturation Bx: a persistently high or increasing PSA value, abnormal DRE and PSA > 10 ng/mL or PSA values 4.1‐10 or 2.6‐4 ng/mL with free/total PSA ≤ 25% and ≤ 20%, respectively Setting: Catania, Italy. University Hospital Age: median 63 years (range 49‐72) PSA: median 11 ng/mL (range 3.7‐45) Prostate volume: not reported | ||
| Index tests | Index test: MRI only, MRI‐TBx and MRI‐pathway. A 3 Tesla MRI (Achieva, Philips) was used, with T2, DWI, DCE and spectroscopy sequences. An in‐house binary MRI score was used, with positive lesions cognitively targeted by MRI‐TBx, after the performance of saturation Bx. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 Reference standard: transperineal TSB with a median of 28 cores (range 26‐32) including 4‐6 cores in the transition and anterior zone. MRI results were not blinded during Bx procedure. | ||
| Flow and timing | All participants underwent same reference standard and were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: comparison of the PCa detection rate between SBx versus template Bx Type of study: prospective cohort Selection: consecutive Enrolled/eligible: 2753/2753 Inclusion period: December 2001‐December 2011 | ||
| Patient characteristics and setting | Inclusion criteria: suspicious for PCa, by
Exclusion criteria: none Setting: Créteil, France. Tertiary care hospital Age: mean 64.2 years (SD 7.8) PSA: mean 12.5 ng/mL (SD 7.2) Prostate volume: mean 46.4 mL (SD 25.3) Positive DRE: 318 participants | ||
| Index tests | Index test: transrectal extended sextant 12‐cores SBx, as part of a 21‐core transrectal Bx protocol | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 Reference standard: 21‐core transrectal Bx protocol: first 6 sextant biopsies (standard 45° angle), then 3 Bx in each peripheral zone (80° angle), then 3 Bx in each transition zone, and finally 3 Bx in the midline peripheral zone. The SBx were part of the 21‐core saturation Bx protocol, and therefore the reference standard is not independent of the index test. | ||
| Flow and timing | All participants underwent the same 21‐core Bx protocol. No participants were excluded for analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare the diagnostic efficacy of the MRI‐pathway with SBx Selection: prospective cohort, consecutive series of Bx‐naïve men suspected of having PCa Enrolled/eligible: 223/229 Inclusion period: July 2012‐January 2013 | ||
| Patient characteristics and setting | Inclusion criteria: Bx‐naïve men with concerning PSA levels and/or an abnormal DRE, referred from urologists Exclusion criteria: not reported Setting: prospective single‐centre diagnostic study. Brisbane, Australia, University hospital Age: median 63 years (IQR 57‐68) PSA: median 5.3 ng/mL (IQR 4.1‐6.6) Prostate volume: median 41 mL (IQR 30‐59) | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Skyra, Siemens) was used. PI‐RADS version 1 was used, with score ≥ 3 as threshold for MRI‐TBx MRI was reported before the Bx procedure. In‐bore transrectal MRI‐TBx was performed, independently of reference test as first the MRI‐TBx in case of lesion were taken and subsequently the 12‐core SBx. Index test 2: standard transrectal 12‐core SBx, performed after the MRI‐TBx. The urologist performing 12‐core SBx was blinded to MRI findings and MRI‐TBx procedure. However, the order of the 2 Bx sessions might have made it possible for the urologist to identify the MRI‐TBx tracks and thereby take SBx from the suspicious lesion. | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests. 6 participants were excluded because their PSA normalised, or they refused the MRI or Bx. | ||
| Comparative | |||
| Notes | Study authors provided additional information | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare in the same Bx‐naïve patients the detection rates of ISUP grade group ≥ 2 cancers obtained by 12‐14 core SBx and 3‐6 core MRI‐TBx Type of study: prospective multicentre study Selection: consecutive selection Enrolled/eligible: 251/275 (only participants included with central pathology reading; specimens of 24 participants did not have central reading) Inclusion period: July 2015–August 2016 | ||
| Patient characteristics and setting | Inclusion criteria: primary suspicion of PCa based on elevated PSA, abnormal DRE and/or family history of PCa Exclusion criteria: prior Bx, PSA > 20 ng/mL, T3 disease on DRE, PCa diagnosis, history of hip prosthesis, pelvic radiation Setting: 16 centres in France (11 university hospitals, 2 cancer centres and 3 private hospitals) Age: median 64 years (IQR 59‐68) PSA: median 6.5 ng/mL (IQR 5.6‐9.6) Prostate volume: median 50 mL (IQR 38‐63) DRE positive: 31% (77/251) of the participants | ||
| Index tests | Index test 1: MRI‐pathway: several 1.5 and 3 Tesla MRI machines were used, with or without an endorectal coil, using T2, DWI, and DCE sequences. Both a Likert score based on PI‐RADS version 1, and PI‐RADS version 2 were used with score 1‐5 and score ≥ 3 for positivity. Transrectal cognitive or software fused MRI‐TBx were performed from all MRI‐positive lesions, within 3 months after performing MRI, after taking the SBx. Several machines were used for software fused MRI‐TBx. Index test 2: transrectal extended sextant SBx were taken, blinded for MRI results. | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same reference test. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate mpMRI and MRI‐TRUS fusion MRI‐TBx as a means of detecting clinically significant cancer as well as a potential indicator for avoiding repeat Bx Type of study: retrospective Selection: consecutive Enrolled/eligible: 143/374 (231 participants did not comply with inclusion criteria) Inclusion period: December 2012–June 2015 | ||
| Patient characteristics and setting | Inclusion criteria: indication for repeat PBx Exclusion criteria: Bx‐naïve men, or previous diagnosis of PCa Setting: New Haven, USA. Tertiary care hospital Age: mean 64.1 years (range 47‐82) PSA: mean 11.6 ng/mL (range 0.4‐96.9) Prostate volume: 68.5 mL (range 16.5‐309) | ||
| Index tests | Index test 1: MRI‐pathway: no details reported about the acquisition of the MRI. An in‐house MRI 4‐point suspicion score was used: negative MRI (1), low (2), moderate (3) and high (4) suspicion, with threshold ≥ 2 for positivity and MRI‐TBx. All MRI‐TBx were performed using the Artemis/Pro‐Fuse™ system (Eigen, Grass Valley, California), after the performance of SBx. Index test 2: extended sextant SBx. Blinding of MRI results during the performance of SBx is not reported | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent same reference standard. All participants were included in the analysis. | ||
| Comparative | |||
| Notes | 4 participants were unable to tolerate the complete MRI exam of whom 2 participants had no suspicious lesions during the part of the exam that was completed. The other 2 participants were not specified. We were unable to differentiate and exclude these 4 men from the Bx results. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the accuracy of mpMRI for significant PCa detection before diagnostic Bx in men with an abnormal PSA/DRE Type of study: prospective cohort Selection: consecutive Enrolled/eligible: 344/388 (44 participants were excluded due to refusing informed consent, MRI or Bx) Inclusion period: April 2012‐March 2014 | ||
| Patient characteristics and setting | Inclusion criteria: men > 40 years, scheduled to undergo Bx for abnormal PSA or DRE, with a life expectancy > 10 years and no previous prostate MRI or Bx Exclusion criteria: none Setting: Sydney, Australia, University hospital Age: median 62.9 years (IQR 55.9‐67.1) PSA: median 5.2 ng/mL (IQR 3.7‐7.1) Prostate volume: median 40 mL (IQR 30‐54) | ||
| Index tests | Index test: MRI only. A 1.5 and 3 Tesla MRI (vendor unknown) was used in 2 centres. PI‐RADS version 1 was used, with score ≥ 3 considered positive. MRI was reported before the Bx procedure. Cognitive‐fusion transperineal MRI‐TBx were performed, independent of the reference test. However, the MRI‐TBx results are not reported separately from template Bx results. Therefore the MRI‐TBx could not be assessed as an index test. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: transperineal mapping biopsies (median 30 cores, using a brachytherapy grid, with relative periurethral zone sparing) from 18 template locations. MRI outcomes were known at time of Bx. MRI‐TBx were taken in addition to the TTMB and could not be disaggregated from the TTMB results and were therefore included in the reference standard results. | ||
| Flow and timing | All participants underwent same TTMB technique. 44 participants were excluded: 16 refused consent, 8 refused MRI and 10 refused Bx | ||
| Comparative | |||
| Notes | Study authors provided additional information | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare (TRUS)‐fusion mpMRI‐TBx with routine SBx for overall and clinically significant PCa detection among men with suspected PCa based on PSA values Type of study: prospective RCT, with randomisation 1:1 to the mpMRI or control group. Participants in the mpMRI group underwent pre‐Bx mpMRI followed by SBx and MRI‐TBx; the control group underwent SBx alone. For our current analysis only the mpMRI group is used. Selection: consecutive Enrolled/eligible: 53/65 (of the mpMRI group; 12 participants were excluded because of PSA normalisation, Bx protocol violation or MRI could not be performed) Inclusion period: April 2011‐December 2014 | ||
| Patient characteristics and setting | Inclusion criteria:
Exclusion criteria:
Setting: Oulu, Finland, University hospital Age: median 63 years (IQR 60‐66) PSA: median 6.1 ng/mL (IQR 4.2‐9.9) Prostate volume: median 27.8 mL (IQR 23.5‐36.6) | ||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Skyra, Siemens) was used. An in‐house MRI score on a 1‐4‐point scale was used:
With score ≥ 3 considered a positive MRI and threshold for MRI‐TBx. MRI was reported before the Bx procedure. Cognitive‐fusion transrectal MRI‐TBx were performed independent from the SBx. Index test 2: standard transrectal 12‐core SBx were performed with blinding for the MRI results and before the performance of the MRI‐TBx | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent same tests. Participants with normalised PSA, Bx protocol violation or in which MRI could not be performed were excluded from analysis (n = 12). | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the diagnostic properties of mpMRI in the detection, localisation and characterisation of PCa using 3‐D transperineal TTMB histopathology as the comparator. Selection: retrospective chart review of consecutive men who underwent mpMRI followed by 3‐D TTMB. Enrolled/eligible: 50/unclear Inclusion period: 2011‐2014 | ||
| Patient characteristics and setting | Inclusion criteria: indication for TTMB was either evaluation of elevated PSA with prior‐negative conventional office‐based SBx or restaging of potential candidates for active surveillance of focal therapy. Exclusion criteria: men with prior PCa treatment were excluded Setting: Durham, USA, University hospital Age: median 65 years (range 61‐69) PSA: median 7.1 ng/mL (range 5.1‐13.6) Prostate volume: median 43.9 mL (range 31.8‐64.7) | ||
| Index tests | Index test: MRI only. A 3 Tesla MRI (Signa HDx GE Healthcare of Skyra Siemens) was used. An in‐house MRI 1‐5 Likert score was used, with score ≥ 3 considered positive. MRI was reported before the Bx procedure. No MRI‐TBx were performed. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: TTMB technique, 26 regions were sampled using a 5‐mm grid, independent of MRI results. | ||
| Flow and timing | All participants underwent same tests. No participants were excluded for analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional information. We were able to exclude the 17 active surveillance participants for our current analysis; the remaining 33 participants with a prior‐negative Bx were included. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare the detection rates of clinically significant PCa and insignificant PCa in Bx‐naïve men with PSA levels ≥ 3 ng/mL for an MRI‐pathway and SBx‐pathway; to evaluate the total number of men with a non‐suspicious mpMRI, and the total number of Bx needles needed per pathway Type of study: prospective, multicentre, powered, comparative effectiveness study Selection: consecutive Enrolled/eligible: 626/699 Inclusion period: February 2015‐February 2017 | ||
| Patient characteristics and setting | Inclusion criteria: Bx‐naïve men, aged 50‐75 years with a PSA > 3 ng/mL Exclusion criteria: age < 50 or > 75 years, history of previous PBx or PCa, general contraindications for MRI, use of medications or hormones that are known to affect serum PSA levels, symptoms of urinary tract infection, and a history of invasive treatments for prostate benign hyperplasia Setting: 4 medical centres in the Netherlands (1 university and 3 non‐university centres) Age: median 65 years (IQR 59‐68) PSA: median 6.4 ng/mL (IQR 4.6‐8.2) Prostate volume: mean 55 mL (IQR 41‐77) DRE positive: 176 (28%) clinically significant PCa and insignificant PCa | ||
| Index tests | Index test 1: MRI‐pathway: mpMRI was performed with a 3‐Tesla machine (Magnetom Skyra, Siemens Healthineers, Erlangen, Germany), using T2, DWI and DCE. PI‐RADS version 2 was used to assess the MRI, with a 1‐5 score scale and score ≥ 3 for positivity, co‐read by multiple experienced radiologists. Transrectal in‐bore MRI‐TBx were taken from all positive lesions, with 2‐4 cores per lesion. Index test 2: all men underwent 12‐core transrectalSBx, after MRI‐TBx were taken, by a urologist who was blinded to the imaging results and not informed if a MRI‐TBx procedure was performed. | ||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests. A total of 73 participants were excluded due to several reasons (personal reasons, Bx refusal), possibly leading to verification bias. | ||
| Comparative | |||
| Notes | Study authors provided additional data | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
3‐D: three‐dimensional; ASAP: atypical small acinar proliferation; bpMRI: biparametric magnetic resonance imaging; Bx: biopsy; Bx‐naïve: biopsy‐naïve; DCE: dynamic contrast‐enhanced; DRE: digital rectal exam; DWI: diffusion‐weighted imaging; GFR: glomerular filtration rate; GS: Gleason score; IQR: interquartile range; ISUP: International Society of Urological Pathology; mpMRI: multi‐parametric magnetic resonance imaging; MRI: magnetic resonance imaging; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; PBx: prostate biopsy; PCa: prostate cancer; PCA3: prostate cancer antigen 3; PI‐RADS: Prostate Imaging ‐ Reporting and Data System; PSA: prostate‐specific antigen; RCT: randomised controlled trial; SBx: systematic transrectal ultrasound‐guided biopsy; SD: standard deviation; TBx: target biopsy; TOP‐Bx: transperineal optimised prostate biopsy; TRUS: transrectal ultrasound; TTMB: template‐guided mapping biopsy; TSB: template‐guided saturation biopsy; TURP: transurethral resection of the prostate
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| In group B within this RCT participants received MRI‐TBx and SBx. However, only MRI‐positive participants were investigated thereby not reporting on MRI‐negative participants. | |
| The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. | |
| As for other studies from this author, this study reported on overlapping data with Boesen 2017a, which presented more complete data. | |
| The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. | |
| The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. | |
| The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. | |
| Overlapping data with Hansen 2018. | |
| This RCT did not perform the index tests in the same men but in 2 separate groups, therefore no 2x2 tables could be derived. | |
| The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. | |
| The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. | |
| The reference standard did not comply with our criteria: participants before 2008 underwent 3‐D, 26‐core (transperineal 14 cores plus transrectal 12 cores (n = 203 men); however after 2008, 3‐D, 14‐core Bx (transperineal 8‐core plus transrectal 6 cores) were performed in 102 men. Furthermore, men aged > 75 years or significant comorbidity (n = 46) received a transperineal 14‐core Bx. | |
| Overlapping data with Pepe 2013, which presents more complete data. | |
| The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. | |
| This RCT did not perform the index tests in the same men but in 2 separate groups, therefore no 2x2 tables could be derived. | |
| Overlapping data with Distler 2017, which presents more complete data. | |
| The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4, differentiating between participants with and without a prior PCa diagnosis. | |
| The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. | |
| Overlapping data with Thompson 2016. | |
| The reference standard (12‐region, 48‐core template TRUS‐guided Bx using the TargetScan system) did not sample the whole prostate in all participants. The transition and anterior zones were often only sampled when a MRI lesion was present, often only by MRI‐TBx. Study authors provided additional data. | |
| Pilot study of Boesen 2018. Therefore Boesen 2018 is included, which is more recent and more complete. |
3‐D: three‐dimensional; Bx: biopsy; GS: Gleason score; MRI: magnetic resonance imaging; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; PCa: prostate cancer; RCT: randomised controlled trial; SBx: systematic biopsy; TRUS: transrectal ultrasound
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 Diagnostic accuracy of MRI ‐ G = 1 Show forest plot | 10 | 1764 |
| Test 1  Diagnostic accuracy of MRI ‐ G = 1. | ||
| 2 Diagnostic accuracy of MRI ‐ G ≥ 1 Show forest plot | 10 | 1764 |
| Test 2  Diagnostic accuracy of MRI ‐ G ≥ 1. | ||
| 3 Diagnostic accuracy of MRI ‐ G ≥ 2 Show forest plot | 12 | 3091 |
| Test 3  Diagnostic accuracy of MRI ‐ G ≥ 2. | ||
| 4 Diagnostic accuracy of MRI ‐ G ≥ 3 Show forest plot | 7 | 1438 |
| Test 4  Diagnostic accuracy of MRI ‐ G ≥ 3. | ||
| 5 Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G = 1 Show forest plot | 4 | 834 |
| Test 5  Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G = 1. | ||
| 6 Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 1 Show forest plot | 4 | 834 |
| Test 6  Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 1. | ||
| 7 Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 2 Show forest plot | 5 | 1083 |
| Test 7  Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 2. | ||
| 8 Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 3 Show forest plot | 4 | 834 |
| Test 8  Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 3. | ||
| 9 Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 1 Show forest plot | 3 | 748 |
| Test 9  Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 1. | ||
| 10 Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 2 Show forest plot | 3 | 748 |
| Test 10  Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 2. | ||
| 11 Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 3 Show forest plot | 3 | 748 |
| Test 11  Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 3. | ||
| 12 Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 1 Show forest plot | 8 | 870 |
| Test 12  Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 1. | ||
| 13 Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 2 Show forest plot | 9 | 1157 |
| Test 13  Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 2. | ||
| 14 Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 3 Show forest plot | 4 | 544 |
| Test 14  Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 3. | ||
| 15 Diagnostic accuracy of MRI ‐ Sensitivity analysis with composite reference standard (template‐guided biopsy + MRI‐TBx) ‐ G ≥ 2 Show forest plot | 11 | 3192 |
| Test 15  Diagnostic accuracy of MRI ‐ Sensitivity analysis with composite reference standard (template‐guided biopsy + MRI‐TBx) ‐ G ≥ 2. | ||
| 16 Diagnostic accuracy of TBx ‐ G = 1 Show forest plot | 5 | 497 |
| Test 16  Diagnostic accuracy of TBx ‐ G = 1. | ||
| 17 Diagnostic accuracy of TBx ‐ G ≥ 1 Show forest plot | 6 | 611 |
| Test 17  Diagnostic accuracy of TBx ‐ G ≥ 1. | ||
| 18 Diagnostic accuracy of TBx ‐ G ≥ 2 Show forest plot | 8 | 1553 |
| Test 18  Diagnostic accuracy of TBx ‐ G ≥ 2. | ||
| 19 Diagnostic accuracy of TBx ‐ G ≥ 3 Show forest plot | 3 | 428 |
| Test 19  Diagnostic accuracy of TBx ‐ G ≥ 3. | ||
| 20 Diagnostic accuracy of the MRI‐pathway ‐ G = 1 Show forest plot | 5 | 681 |
| Test 20  Diagnostic accuracy of the MRI‐pathway ‐ G = 1. | ||
| 21 Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 1 Show forest plot | 6 | 844 |
| Test 21  Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 1. | ||
| 22 Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 2 Show forest plot | 8 | 2257 |
| Test 22  Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 2. | ||
| 23 Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 3 Show forest plot | 3 | 604 |
| Test 23  Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 3. | ||
| 24 Diagnostic accuracy of SBx ‐ G = 1 Show forest plot | 4 | 3421 |
| Test 24  Diagnostic accuracy of SBx ‐ G = 1. | ||
| 25 Diagnostic accuracy of SBx ‐ G ≥ 1 Show forest plot | 4 | 3421 |
| Test 25  Diagnostic accuracy of SBx ‐ G ≥ 1. | ||
| 26 Diagnostic accuracy of SBx ‐ G ≥ 2 Show forest plot | 4 | 3421 |
| Test 26  Diagnostic accuracy of SBx ‐ G ≥ 2. | ||
| 27 Diagnostic accuracy of SBx ‐ G ≥ 3 Show forest plot | 2 | 626 |
| Test 27  Diagnostic accuracy of SBx ‐ G ≥ 3. | ||
| 28 MRI‐pathway vs SBx ‐ G = 1 Show forest plot | 21 | 5442 |
| Test 28  MRI‐pathway vs SBx ‐ G = 1. | ||
| 29 MRI‐pathway vs SBx ‐ G ≥ 1 Show forest plot | 24 | 6524 |
| Test 29  MRI‐pathway vs SBx ‐ G ≥ 1. | ||
| 30 MRI‐pathway vs SBx ‐ G ≥ 2 Show forest plot | 25 | 6944 |
| Test 30  MRI‐pathway vs SBx ‐ G ≥ 2. | ||
| 31 MRI‐pathway vs SBx ‐ G ≥ 3 Show forest plot | 21 | 5981 |
| Test 31  MRI‐pathway vs SBx ‐ G ≥ 3. | ||
| 32 MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G = 1 Show forest plot | 17 | 4079 |
| Test 32  MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G = 1. | ||
| 33 MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 1 Show forest plot | 19 | 4799 |
| Test 33  MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 1. | ||
| 34 MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 2 Show forest plot | 20 | 5219 |
| Test 34  MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 2. | ||
| 35 MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 3 Show forest plot | 16 | 4306 |
| Test 35  MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 3. | ||
| 36 MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G = 1 Show forest plot | 8 | 1202 |
| Test 36  MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G = 1. | ||
| 37 MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 1 Show forest plot | 10 | 1564 |
| Test 37  MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 1. | ||
| 38 MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 2 Show forest plot | 10 | 1564 |
| Test 38  MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 2. | ||
| 39 MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 3 Show forest plot | 9 | 1514 |
| Test 39  MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 3. | ||
| 40 MRI‐pathway vs SBx ‐ Positive MRI ‐ G = 1 Show forest plot | 19 | 3460 |
| Test 40  MRI‐pathway vs SBx ‐ Positive MRI ‐ G = 1. | ||
| 41 MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 1 Show forest plot | 20 | 3998 |
| Test 41  MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 1. | ||
| 42 MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 2 Show forest plot | 20 | 3998 |
| Test 42  MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 2. | ||
| 43 MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 3 Show forest plot | 18 | 3902 |
| Test 43  MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 3. | ||
| 44 MRI‐pathway vs SBx ‐ Negative MRI ‐ G = 1 Show forest plot | 19 | 1666 |
| Test 44  MRI‐pathway vs SBx ‐ Negative MRI ‐ G = 1. | ||
| 45 MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 1 Show forest plot | 20 | 1781 |
| Test 45  MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 1. | ||
| 46 MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 2 Show forest plot | 20 | 1781 |
| Test 46  MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 2. | ||
| 47 MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 3 Show forest plot | 18 | 1725 |
| Test 47  MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 3. | ||
| 48 MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G = 1 Show forest plot | 16 | 2682 |
| Test 48  MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G = 1. | ||
| 49 MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 1 Show forest plot | 17 | 2955 |
| Test 49  MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 1. | ||
| 50 MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 2 Show forest plot | 17 | 2955 |
| Test 50  MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 2. | ||
| 51 MRI‐pathway vs. SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 3 Show forest plot | 15 | 2899 |
| Test 51  MRI‐pathway vs. SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 3. | ||
| 52 MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G = 1 Show forest plot | 16 | 1287 |
| Test 52  MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G = 1. | ||
| 53 MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 1 Show forest plot | 17 | 1343 |
| Test 53  MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 1. | ||
| 54 MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 2 Show forest plot | 17 | 1343 |
| Test 54  MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 2. | ||
| 55 MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 3 Show forest plot | 15 | 1297 |
| Test 55  MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 3. | ||
| 56 MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G = 1 Show forest plot | 7 | 655 |
| Test 56  MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G = 1. | ||
| 57 MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 1 Show forest plot | 8 | 920 |
| Test 57  MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 1. | ||
| 58 MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 2 Show forest plot | 8 | 920 |
| Test 58  MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 2. | ||
| 59 MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 3 Show forest plot | 7 | 880 |
| Test 59  MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 3. | ||
| 60 MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G = 1 Show forest plot | 7 | 341 |
| Test 60  MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G = 1. | ||
| 61 MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 1 Show forest plot | 8 | 400 |
| Test 61  MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 1. | ||
| 62 MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 2 Show forest plot | 8 | 400 |
| Test 62  MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 2. | ||
| 63 MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 3 Show forest plot | 7 | 390 |
| Test 63  MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 3. | ||

Clinical pathway flow diagram and study design
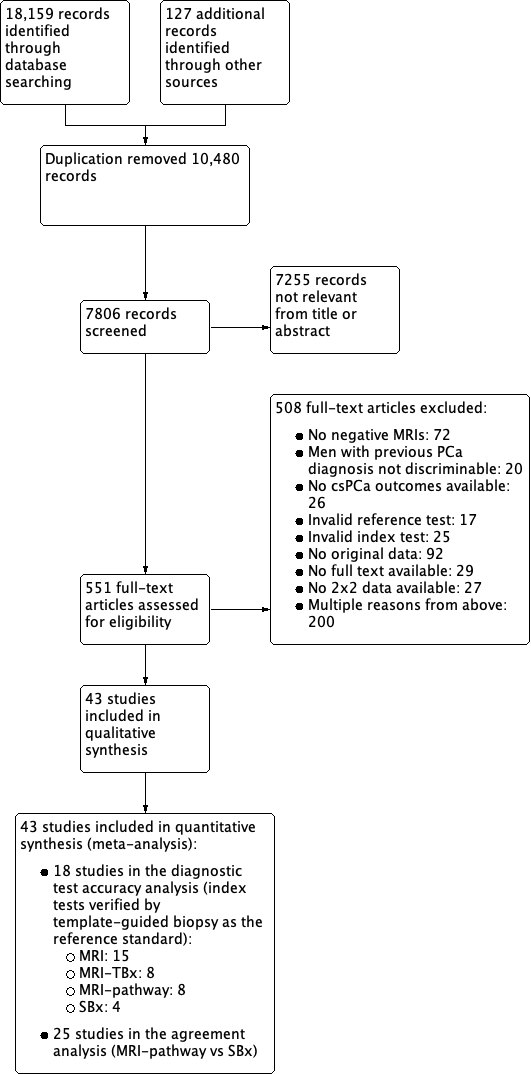
Study flow chart
csPCa: clinically significant prostate cancer; MRI: magnetic resonance imaging; MRI pathway: magnetic resonance imaging with subsequent magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; SBx: systematic biopsy

Diagnostic test accuracy of magnetic resonance imaging (MRI) verified by template‐guided biopsy: risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study

Diagnostic test accuracy of magnetic resonance imaging‐targeted biopsy (MRI‐TBx) in MRI‐positive men: risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study

Diagnostic test accuracy of the MRI pathway: risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
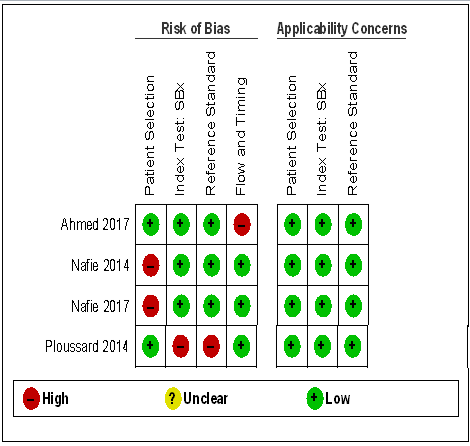
Diagnostic test accuracy of systematic biopsy (SBx): risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study

Agreement analyses between the MRI pathway and systematic biopsy (SBx): risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
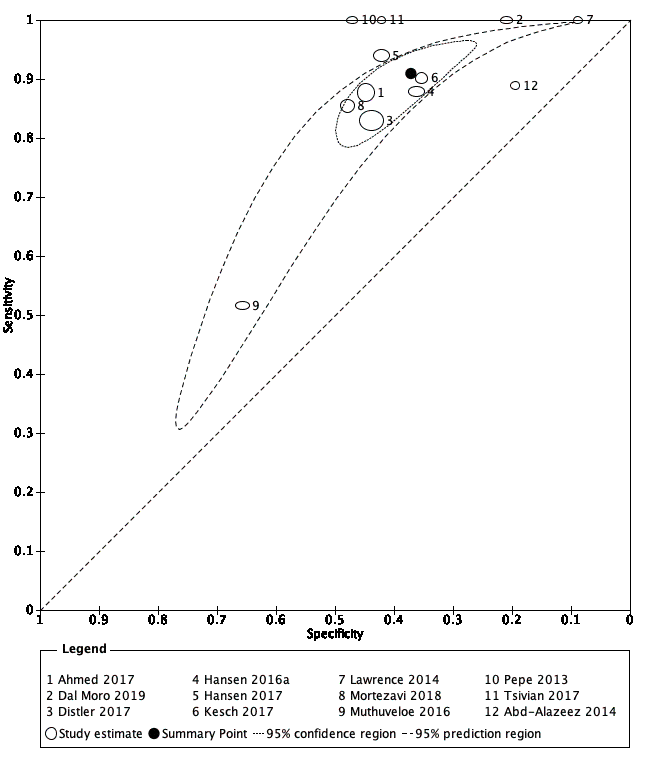
Diagnostic test accuracy of MRI for indicating grade 2 and higher prostate cancer.
Summary ROC plot of MRI verified by template‐guided biopsy. The 95% confidence region illustrates the uncertainty around the pooled summary point; the 95% prediction region illustrates the heterogeneity
MRI: magnetic resonance imaging
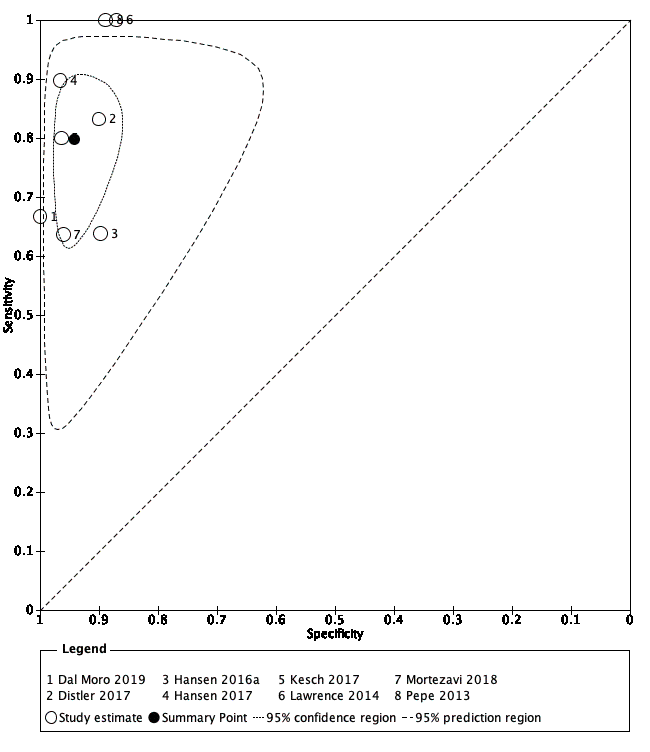
Diagnostic test accuracy of MRI‐targeted biopsy for detecting grade 2 and higher prostate cancer
Summary ROC plot of MRI‐targeted biopsy (in an MRI‐positive population) verified by template‐guided biopsy. The 95% confidence region illustrates the uncertainty around the pooled summary point; the 95% prediction region illustrates the heterogeneity
MRI: magnetic resonance imaging

Diagnostic test accuracy of the MRI pathway for detecting grade 2 and higher prostate cancer
Summary ROC plot of the MRI pathway verified by template‐guided biopsy. The 95% confidence region illustrates the uncertainty around the pooled summary point; the 95% prediction region illustrates the heterogeneity
MRI: magnetic resonance imaging; MRI pathway: MRI with or without MRI‐targeted biopsy

Test consequence graphic showing results that would be obtained if a hypothetical cohort of 1000 men were tested for prostate cancer using the MRI pathway.

Diagnostic test accuracy of systematic biopsy for detecting grade 2 and higher prostate cancer
Summary ROC plot of systematic biopsy verified by template‐guided biopsy

Test consequence graphic showing results that would be obtained if a hypothetical cohort of 1000 men were tested for prostate cancer using systematic biopsy.

Comparison of diagnostic test accuracy between MRI and the MRI pathway for detecting grade 2 and higher prostate cancer.
Summary ROC plot of MRI and the MRI pathway verified by template‐guided biopsy
G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI pathway: MRI with or without MRI‐targeted biopsy
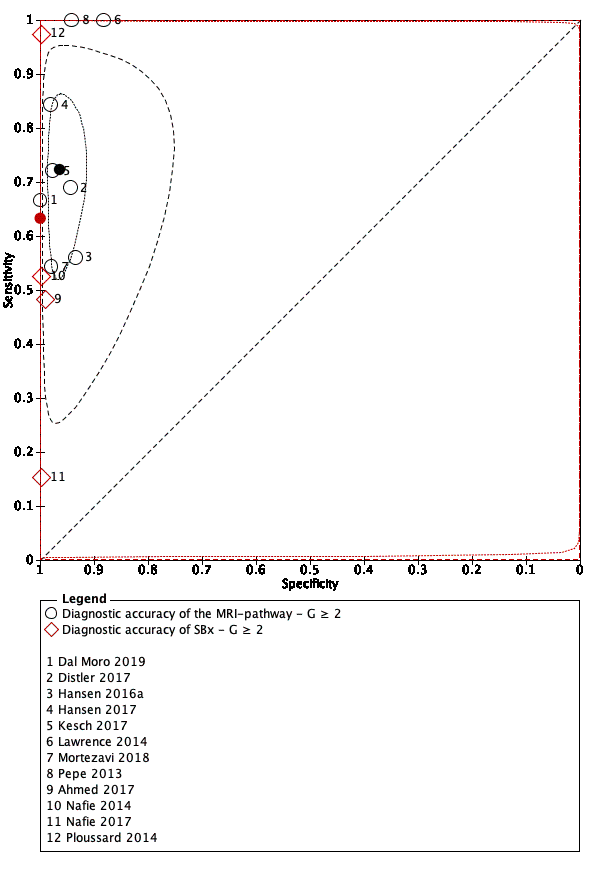
Comparison of diagnostic test accuracy between the MRI pathway and systematic biopsy for detecting grade 2 and higher prostate cancer.
Summary ROC plot of the MRI pathway versus systematic biopsy, verified by template‐guided biopsy
G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI pathway: MRI with or without MRI‐targeted biopsy; SBx: systematic biopsy
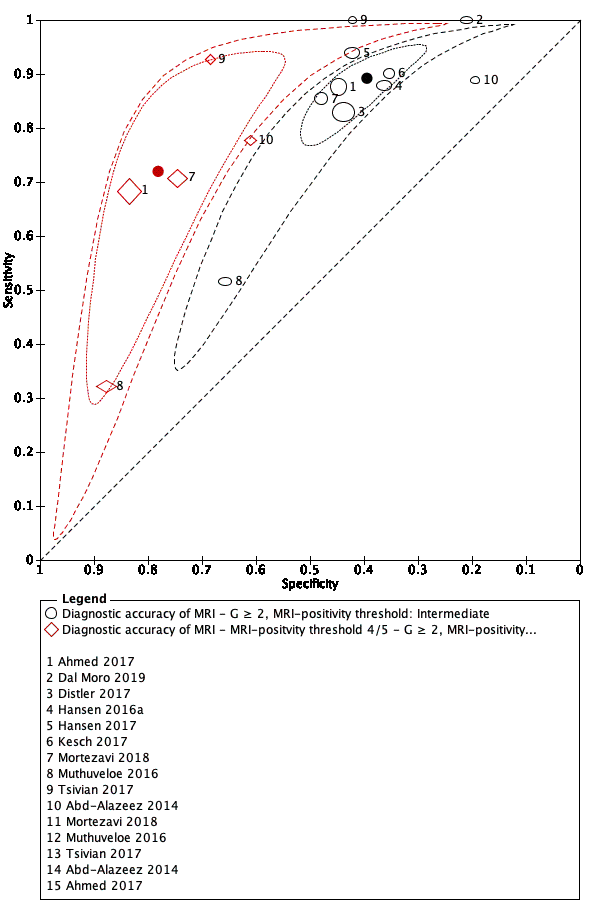
MRI‐positivity threshold effect for indicating grade 2 and higher prostate cancer.
Summary ROC plot of MRI verified by template‐guided biopsy, with different thresholds for positivity: intermediate (3/5) vs high (4/5)
G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging

MRI‐positivity threshold effect for indicating grade 3 and higher prostate cancer.
Summary ROC plot of MRI verified by template‐guided biopsy, with different thresholds for positivity: intermediate (3/5) vs high (4/5)
G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging

Forest plots of the agreement analysis (MRI pathway vs systematic biopsy) for detecting grade 2 and higher prostate cancer
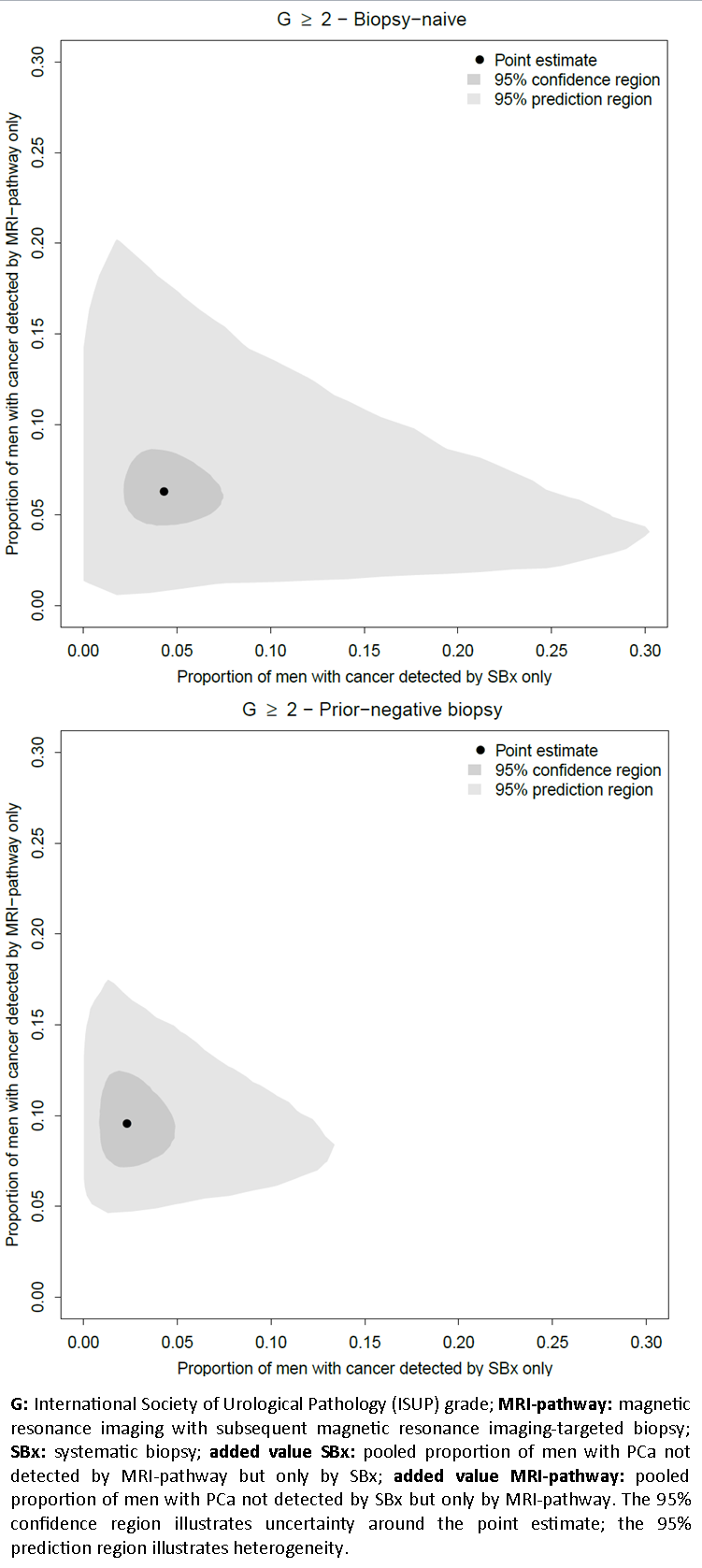
Added value of systematic biopsy plotted against the added value of the MRI pathway per population type in the agreement analysis, for detecting grade 2 and higher prostate cancer

Diagnostic accuracy of MRI ‐ G = 1.

Diagnostic accuracy of MRI ‐ G ≥ 1.

Diagnostic accuracy of MRI ‐ G ≥ 2.
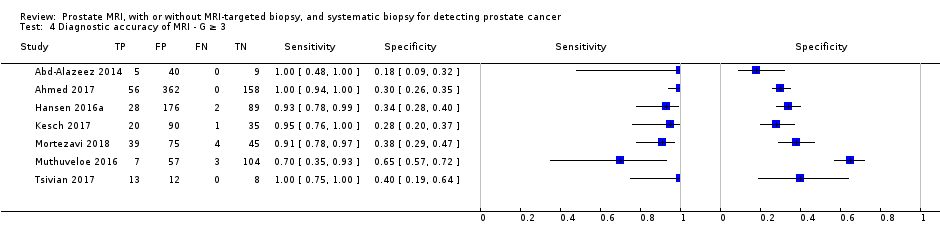
Diagnostic accuracy of MRI ‐ G ≥ 3.

Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G = 1.

Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 1.
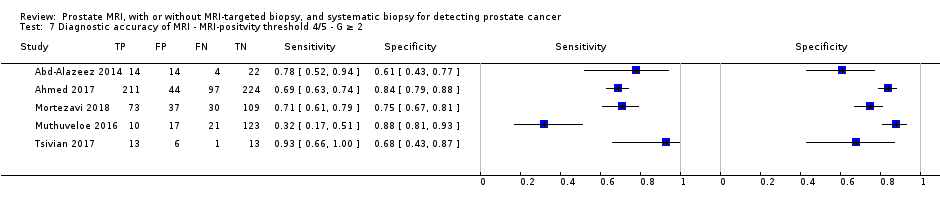
Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 2.

Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 3.

Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 1.

Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 2.

Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 3.

Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 1.

Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 2.
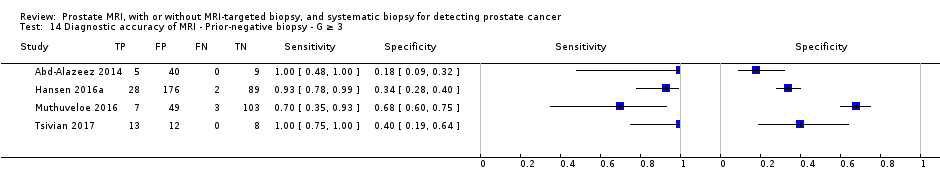
Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 3.
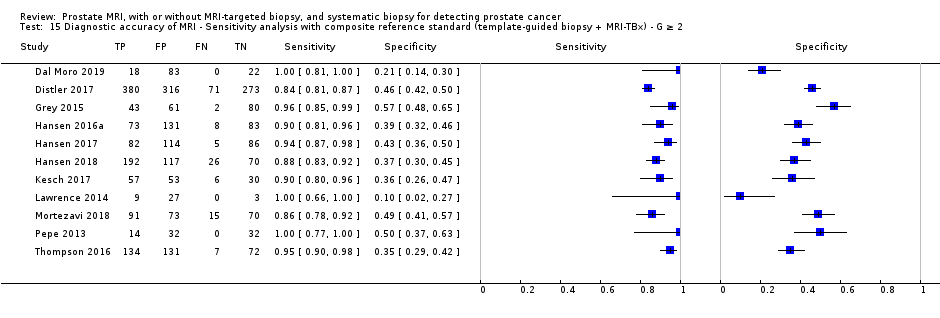
Diagnostic accuracy of MRI ‐ Sensitivity analysis with composite reference standard (template‐guided biopsy + MRI‐TBx) ‐ G ≥ 2.

Diagnostic accuracy of TBx ‐ G = 1.

Diagnostic accuracy of TBx ‐ G ≥ 1.

Diagnostic accuracy of TBx ‐ G ≥ 2.

Diagnostic accuracy of TBx ‐ G ≥ 3.

Diagnostic accuracy of the MRI‐pathway ‐ G = 1.

Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 1.

Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 2.

Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 3.

Diagnostic accuracy of SBx ‐ G = 1.

Diagnostic accuracy of SBx ‐ G ≥ 1.

Diagnostic accuracy of SBx ‐ G ≥ 2.

Diagnostic accuracy of SBx ‐ G ≥ 3.

MRI‐pathway vs SBx ‐ G = 1.
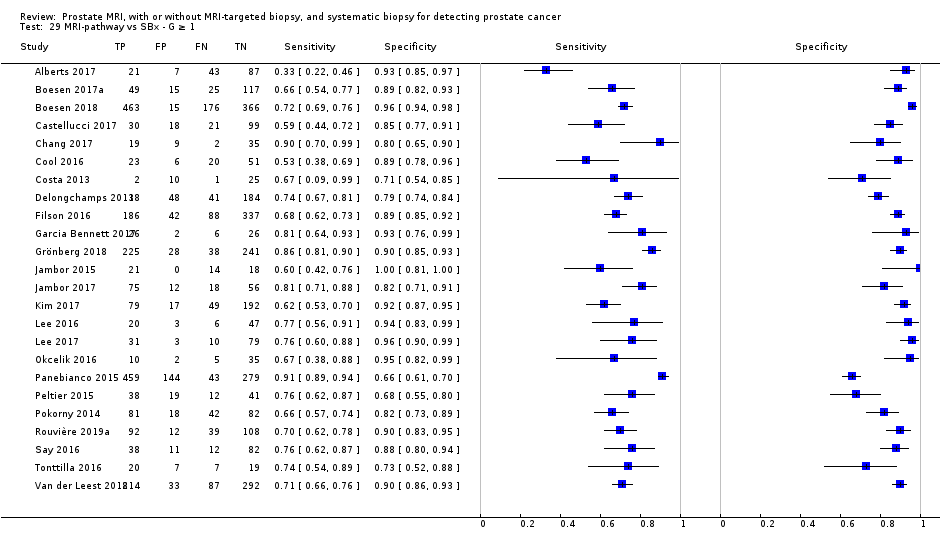
MRI‐pathway vs SBx ‐ G ≥ 1.

MRI‐pathway vs SBx ‐ G ≥ 2.

MRI‐pathway vs SBx ‐ G ≥ 3.

MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G = 1.

MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 1.

MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 2.

MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 3.

MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G = 1.

MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 1.

MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 2.

MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 3.

MRI‐pathway vs SBx ‐ Positive MRI ‐ G = 1.

MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 1.
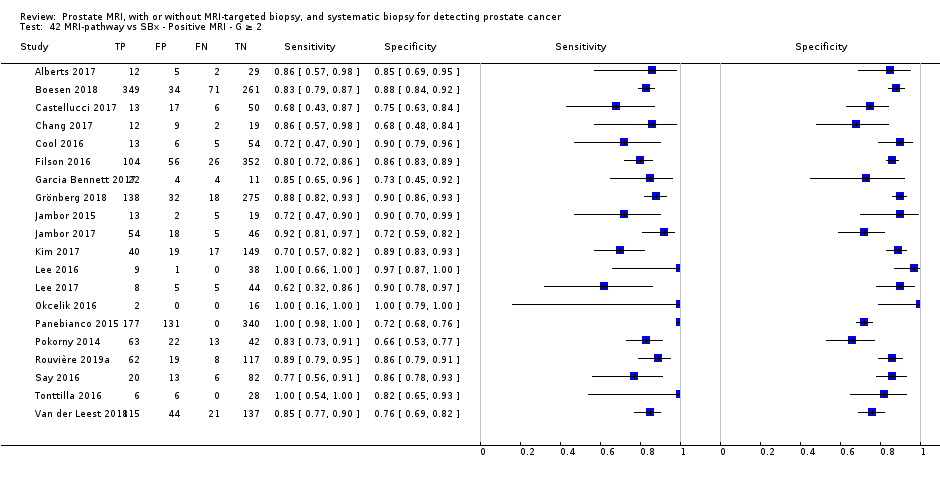
MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 2.
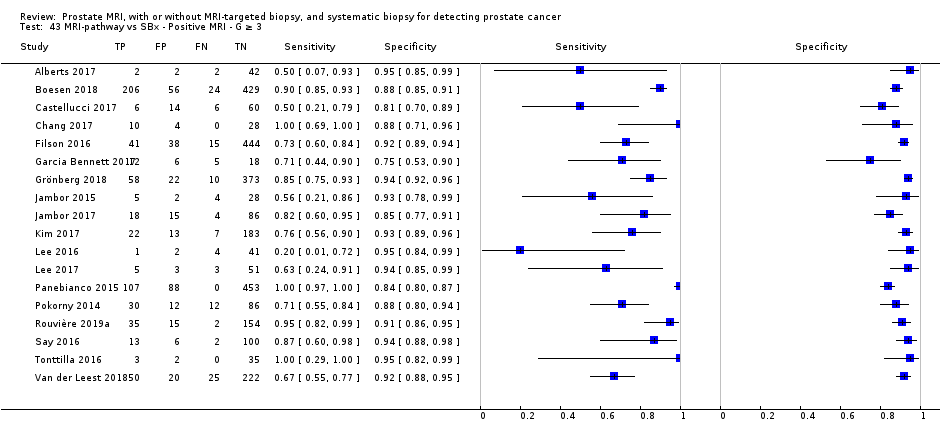
MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 3.

MRI‐pathway vs SBx ‐ Negative MRI ‐ G = 1.
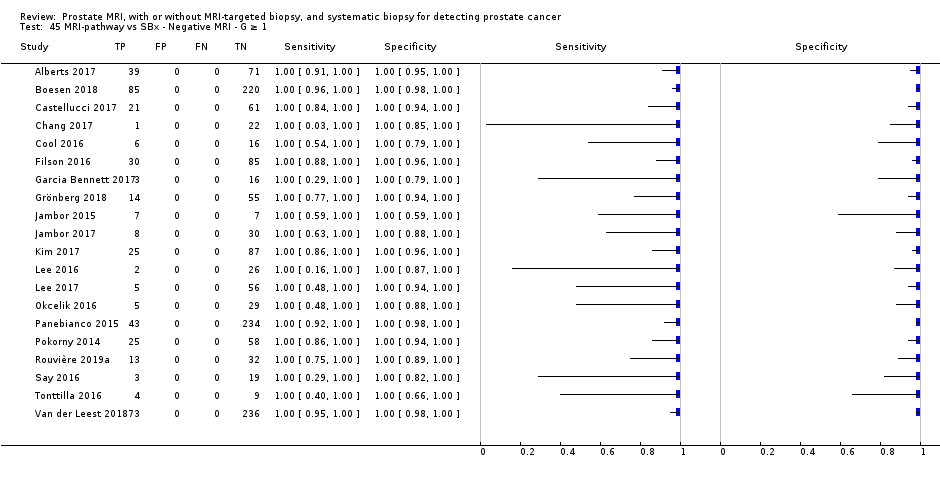
MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 1.
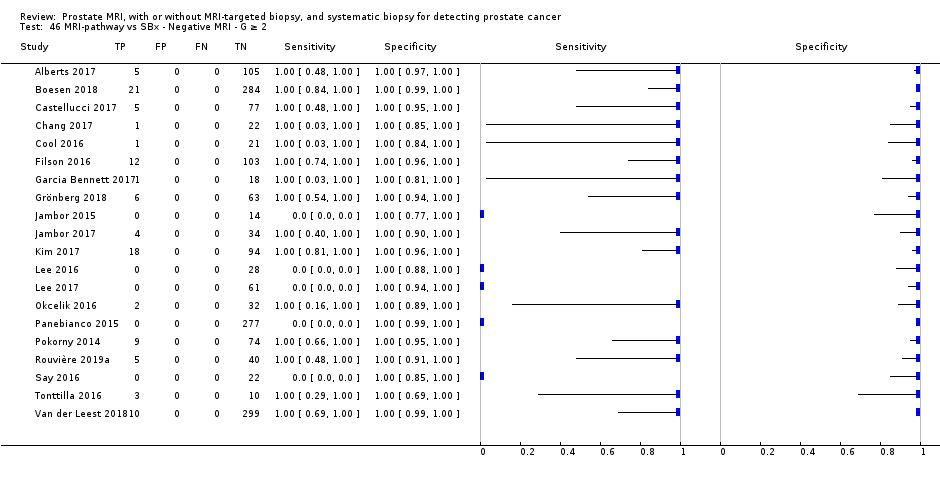
MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 2.

MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 3.

MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G = 1.
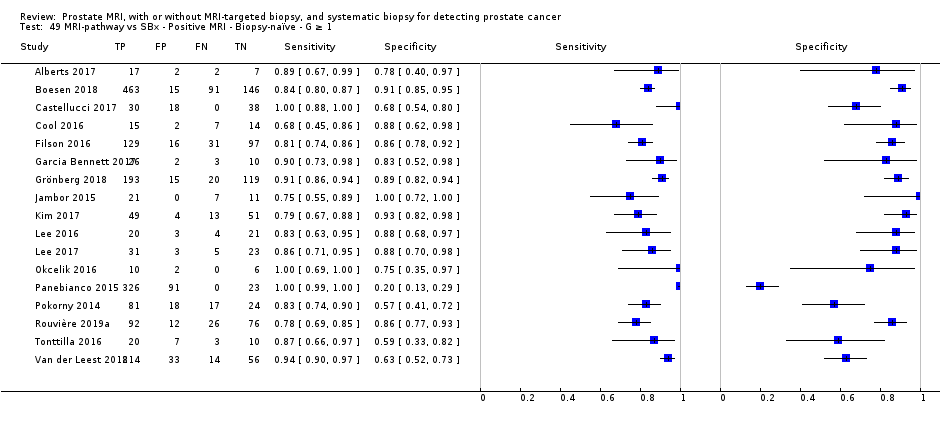
MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 1.
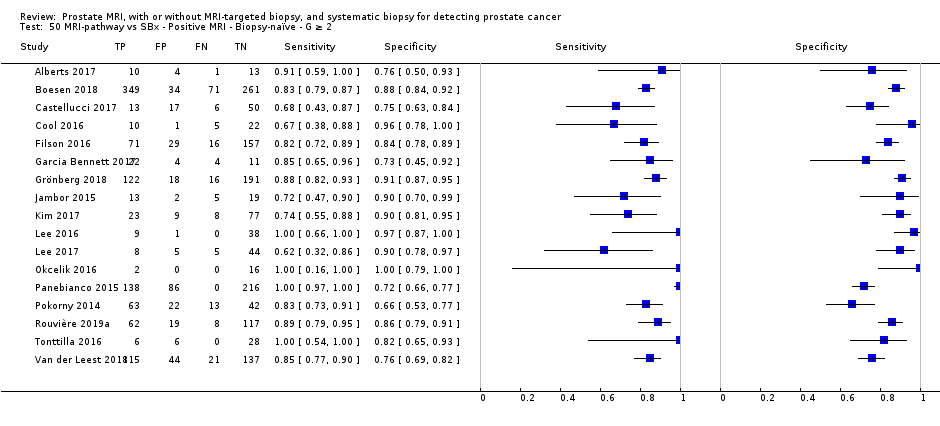
MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 2.
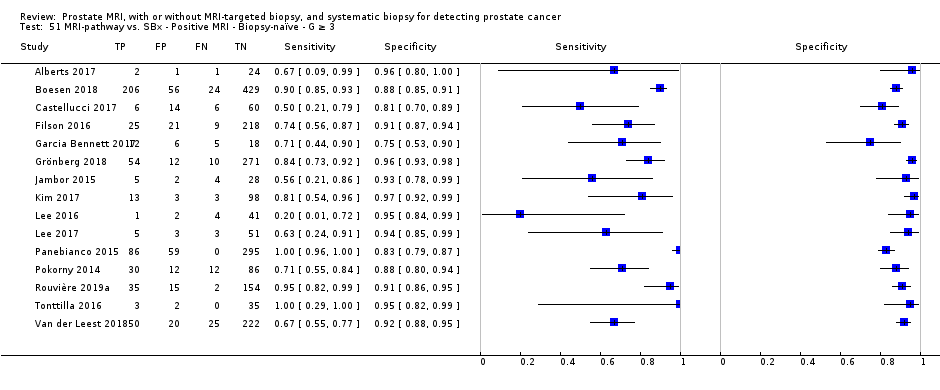
MRI‐pathway vs. SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 3.

MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G = 1.

MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 1.
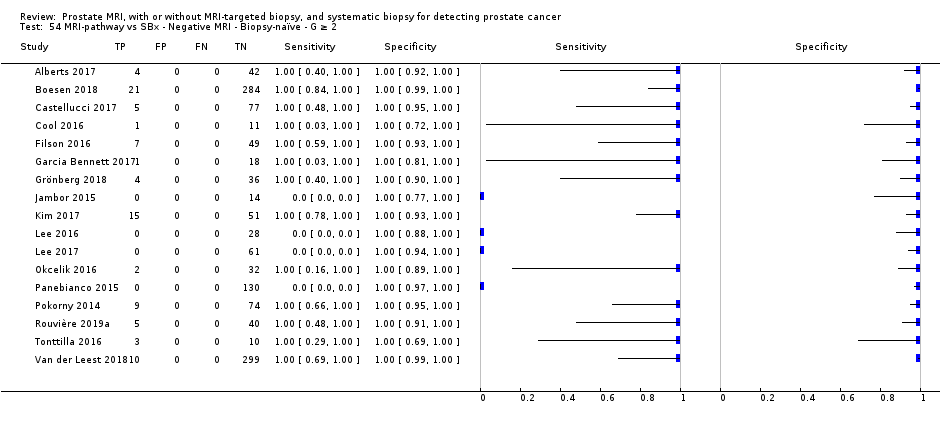
MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 2.

MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 3.

MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G = 1.
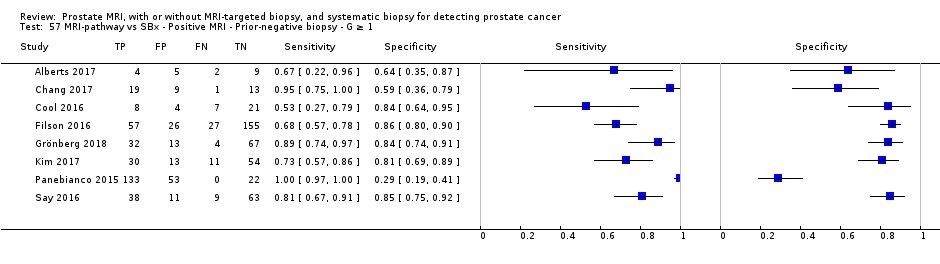
MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 1.

MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 2.

MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 3.

MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G = 1.

MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 1.

MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 2.

MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 3.
| Detecting ISUP grade 2 or higher prostate cancer by MRI, MRI‐targeted biopsy, MRI pathway and systematic biopsy | ||||||||||
| Population | 13,770 men with a suspicion of prostate cancer (PSA‐ or DRE‐based) undergoing their first biopsy (biopsy‐naïve men) or a repeat biopsy (prior‐negative biopsy men) | |||||||||
| Setting | University hospitals and specialized care centers | |||||||||
| Index tests | MRI; MRI‐targeted biopsy (MRI‐TBx) in men with a positive MRI; the MRI pathway (MRI with or without MRI‐TBx); and systematic biopsy (SBx) | |||||||||
| Reference standard | Template‐guided biopsy, which comprehensively samples all zones of the prostate | |||||||||
| Tests | Population type (biopsy‐naïve, prior‐negative biopsy, or mixed) | Summary | Summary | Detection | Missed grade 2 or higher prostate cancer per 1000 men (95% CI)a | Number of | Number of studies with a | |||
| Participant | Index | Reference | Flow and timing | |||||||
| MRI | Mixed | 0.91 | 0.37 | NA | 27 | 3091 (12) | 7 | 0 | 11 | 2 |
| MRI‐TBx | Mixed | 0.80 | 0.94 | NA | 60 | 1553 (8) | 4 | 0 | 6 | 0 |
| MRI pathway | Mixed | 0.72 | 0.96 | NA | 84 | 2257 (8) | 4 | 0 | 6 | 0 |
| SBx | Mixed | 0.63 | 1.00 | NA | 111 | 3421 (4) | 2 | 1 | 1 | 1 |
| MRI pathwayvs SBx | Mixed | NA | NA | 1.12 | MRI pathway missed 12% (2 to 23) less than SBx | 6944 (25) | 13 | 15 | NA | 8 |
| Biopsy‐naïve | NA | NA | 1.05 | MRI pathway missed 5% (−5 to 16) less than SBx | 5219 (20) | 9 | 12 | NA | 7 | |
| Prior‐negative biopsy | NA | NA | 1.44 | MRI pathway missed 44% (19 to 75) less than SBx | 1564 (10) | 5 | 6 | NA | 1 | |
| DRE: digital rectal exam; ISUP: International Society of Urological Pathology; MRI: magnetic resonance imaging; MRI‐TBx: MRI‐targeted biopsy; MRI pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; PSA: prostate‐specific antigen; SBx: systematic biopsy. | ||||||||||
| Detecting ISUP grade 1 prostate cancer by MRI, MRI‐targeted biopsy, MRI pathway and systematic biopsy | ||||||||||
| Population | 10,051 men with a suspicion of prostate cancer (PSA‐ or DRE‐based) undergoing their first biopsy (biopsy‐naïve men) or a repeat biopsy (prior‐negative biopsy men) | |||||||||
| Setting | University hospitals and specialized care centers | |||||||||
| Index tests | MRI; MRI‐targeted biopsy (MRI‐TBx) in men with a positive MRI; the MRI pathway (MRI with or without MRI‐TBx); and systematic biopsy (SBx) | |||||||||
| Reference standard | Template‐guided biopsy, which comprehensively samples all zones of the prostate | |||||||||
| Tests | Population type (biopsy‐naïve, prior‐negative biopsy, or mixed) | Summary | Summary | Detection | Avoided | Number of | Number of studies with a | |||
| Participant | Index | Reference | Flow and timing | |||||||
| MRI | Mixed | 0.70 | 0.27 | NA | 63 | 1764 (10) | 5 | 0 | 5 | 1 |
| MRI‐TBx | Mixed | 0.51 | 1.00 | NA | 103 | 497 (5) | 3 | 0 | 3 | 0 |
| MRI pathway | Mixed | 0.34 | 1.00 | NA | 139 | 681 (5) | 3 | 0 | 3 | 0 |
| SBx | Mixed | 0.55 | 0.99 | NA | 95 | 3421 (4) | 2 | 1 | 1 | 1 |
| MRI pathwayvs SBx | Mixed | NA | NA | 0.61 | MRI pathway | 5442 (21) | 11 | 11 | NA | 8 |
| Biopsy‐naïve | NA | NA | 0.63 | 4079 (17) | 9 | 9 | NA | 7 | ||
| Prior‐negative biopsy | NA | NA | 0.62 | 1202 (8) | 5 | 5 | NA | 2 | ||
| DRE: digital rectal exam; ISUP: International Society of Urological Pathology; MRI: magnetic resonance imaging; MRI‐TBx: MRI‐targeted biopsy; MRI pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; PSA: prostate‐specific antigen; SBx: systematic biopsy. aAt the representative pre‐test probability of 21% of having grade 1 prostate cancer, based on prevalence findings in the test accuracy analysis (proportion avoided = [prevalence*1000]*[1‐sensitivity]). | ||||||||||
| Question: Should MRI be used to diagnose ISUP grade 2 or higher prostate cancer in men suspected of having clinically significant prostate cancer? | |||||
| Population: men suspected of having clinically significant prostate cancer undergoing their first biopsy (biopsy‐naïve men) or a repeat biopsy (prior‐negative biopsy men) | |||||
| Setting: university hospitals and specialized care centers | |||||
| New test: MRI only | Cut‐off value: MRI score ≥ 3 out of 5 | |||||
| Reference test: template‐guided biopsy, which comprehensively samples all zones of the prostate | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Pooled sensitivity: 0.91 (95% CI: 0.83 to 0.95) | Pooled specificity: 0.37 (95% CI: 0.29 to 0.46) | |||||
| Test result | Number of results per 1,000 men tested (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence 10% | Prevalence 30% | Prevalence 40% | |||
| True positives | 9 (83 to 95) | 273 (249 to 285) | 364 (332 to 380) | 3091 (12) | ⊕⊕○○ LOWa, b |
| False negatives | 9 (5 to 17) | 27 (15 to 51) | 36 (20 to 68) | ||
| True negatives | 333 (261 to 414) | 259 (203 to 322) | 222 (174 to 276) | 3091 (12) | ⊕⊕○○ LOWa, b |
| False positives | 567 (486 to 639) | 441 (378 to 497) | 378 (324 to 426) | ||
| MRI: magnetic resonance imaging; ISUP: International Society of Urological Pathology; CI: confidence interval aA considerable number of studies had a high or unclear risk of bias, mainly in the participant selection and reference standard domains. | |||||
| Question: Should MRI‐targeted biopsy be used to diagnose ISUP grade 2 or higher prostate cancer in men suspected of having clinically significant prostate cancer? | |||||
| Population: men with a positive MRI suspected of having clinically significant prostate cancer undergoing their first biopsy (biopsy‐naïve men) or a repeat biopsy (prior‐negative biopsy men) | |||||
| Setting: university hospitals and specialized care centers | |||||
| New test: MRI‐targeted biopsy | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Reference test: template‐guided biopsy, which comprehensively samples all zones of the prostate | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Pooled sensitivity: 0.80 (95% CI: 0.69 to 0.87) | Pooled specificity: 0.94 (95% CI: 0.90 to 0.97) | |||||
| Test result | Number of results per 1,000 men tested (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence 10% | Prevalence 30% | Prevalence 40% | |||
| True positives | 80 (69 to 87) | 240 (207 to 261) | 320 (276 to 348) | 1553 (8) | ⊕⊕○○ LOWa, b |
| False negatives | 20 (13 to 31) | 60 (39 to 93) | 80 (52 to 124) | ||
| True negatives | 846 (810 to 873) | 658 (630 to 679) | 564 (540 to 582) | 1553 (8) | ⊕⊕○○ LOWa, b |
| False positives | 54 (27 to 90) | 42 (21 to 70) | 36 (18 to 60) | ||
| MRI: magnetic resonance imaging; ISUP: International Society of Urological Pathology; CI: confidence interval aA considerable number of studies had a high or unclear risk of bias, mainly in the participant selection and reference standard domains. | |||||
| Question: Should an MRI pathway be used to diagnose ISUP grade 2 or higher prostate cancer in men suspected of having clinically significant prostate cancer? | |||||
| Population: men suspected of having clinically significant prostate cancer undergoing their first biopsy (biopsy‐naïve men) or a repeat biopsy (prior‐negative biopsy men) | |||||
| Setting: university hospitals and specialized care centers | |||||
| New test: MRI pathway | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Reference test: template‐guided biopsy, which comprehensively samples all zones of the prostate | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Pooled sensitivity: 0.72 (95% CI: 0.60 to 0.82) | Pooled specificity: 0.96 (95% CI: 0.94 to 0.98) | |||||
| Test result | Number of results per 1,000 men tested (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence 10% | Prevalence 30% | Prevalence 40% | |||
| True positives | 72 (60 to 82) | 216 (180 to 246) | 288 (240 to 328) | 2257 (8) | ⊕⊕○○ LOWa, b |
| False negatives | 28 (18 to 40) | 84 (54 to 120) | 112 (72 to 160) | ||
| True negatives | 864 (846 to 882) | 672 (658 to 686) | 576 (564 to 588) | 2257 (8) | ⊕⊕○○ LOWa, b |
| False positives | 36 (18 to 54) | 28 (14 to 42) | 24 (12 to 36) | ||
| MRI pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; ISUP: International Society of Urological Pathology; CI: confidence interval aA considerable number of studies had a high or unclear risk of bias, mainly in the participant selection and reference standard domains. | |||||
| Question: Should systematic biopsy be used to diagnose ISUP grade 2 or higher prostate cancer in men suspected of having clinically significant prostate cancer? | |||||
| Population: men suspected of having clinically significant prostate cancer undergoing their first biopsy (biopsy‐naïve men) or a repeat biopsy (prior‐negative biopsy men) | |||||
| Setting: university hospitals and specialized care centers | |||||
| New test: systematic biopsy | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Reference test: template‐guided biopsy, which comprehensively samples all zones of the prostate | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Pooled sensitivity: 0.63 (95% CI: 0.19 to 0.93) | Pooled specificity: 1.00 (95% CI: 0.91 to 1.00) | |||||
| Test result | Number of results per 1,000 men tested (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence 10% | Prevalence 30% | Prevalence 40% | |||
| True positives | 63 (19 to 93) | 189 (57 to 279) | 252 (76 to 372) | 3421 (4) | ⊕⊕⊕○ MODERATEa, b, c |
| False negatives | 37 (7 to 81) | 111 (21 to 243) | 148 (28 to 324) | ||
| True negatives | 900 (819 to 900) | 700 (637 to 700) | 600 (546 to 600) | 3421 (4) | ⊕⊕○○ LOWa, b, c |
| False positives | 0 (0 to 81) | 0 (0 to 63) | 0 (0 to 54) | ||
| ISUP: International Society of Urological Pathology; CI: confidence interval aA considerable number of studies had a high or unclear risk of bias, mainly in the participant selection and reference standard domains. | |||||
| Domain 1: Participant selection | |
| SQ 1: Was a consecutive or random sample of participants enrolled? | Yes: if stated that participants were consecutively or randomly selected No: if one of these criteria was not met Unclear: if insufficient information to make a judgement |
| SQ 2: Did the study avoid inappropriate exclusions? | Yes: if stated that the study did not exclude men 1) aged between 50 and 70 years, 2) with PSA values between 4 and 10 ng/mL, or 3) with an abnormal DRE No: if one of these criteria was not met Unclear: insufficient information to make a judgement |
| Risk of bias Could the selection of participants have introduced bias? | Low risk: if ‘Yes’ for all SQ's High risk: if ‘No’ for at least 1 SQ Unclear risk: if 'Unclear' for at least 1 SQ |
| Concerns for applicability Are there concerns that the included participants and setting do not match the review question? | Low concern: the participants were referred because of a suspicion of prostate cancer. High concern: the participants were not referred because of a suspicion of prostate cancer, e.g. PSA‐screening trials are less applicable to the current clinical practice. Unclear concern: insufficient information to make a judgement |
| Domain 2: Index texts | |
| SQ 1: If applicable, was the MRI assessed without knowledge of the results of the reference (or other index) biopsies? | Yes: if stated that the radiologist was unaware of all biopsy results; or, if the order of testing was MRI before all biopsies for every participant No: if stated that the radiologist was aware of any biopsy results during MRI assessment Unclear: insufficient information to make a judgement |
| SQ 2: If applicable, were the MRI‐targeted biopsies performed independently of the performance and the results of the reference (or other index) biopsies? | Yes: if stated that the performance of MRI‐targeted biopsies was not influenced by the performance or trajectory of reference (or other index) biopsies No: if stated that MRI‐targeted biopsies were not, or differently, taken from locations already hit by the reference (or other index) biopsies; or, if the performance of MRI‐targeted biopsies was dependent on the judgement of the same operator that also performed the reference (or other index) biopsies without blinding Unclear: insufficient information to make a judgement |
| SQ 3: If applicable, were the systematic biopsies taken independently of the performance and the results of the reference (of other index) biopsies? | Yes: if stated that the systematic biopsies were taken blinded for
No: if stated that the systematic biopsy operator was not blinded for MRI results, or was the same operator that also performed the reference (or other index) biopsies without blinding Unclear: insufficient information to make a judgement |
| Risk of bias Could the conduct or interpretation of the index test have introduced bias? | Low risk: ‘Yes’ for all applicable SQs High risk: ‘No’ for at least one applicable SQ Unclear risk: ‘Unclear’ for at least one applicable SQ |
| Concerns for applicability Are there concerns that the index tests, their conduct or their interpretation differ from the review question? | Low concern: if stated that, when applicable,
High concern: the index test did not meet the criteria above Unclear concern: insufficient information to make a judgement |
| Domain 3: Reference standard | |
| SQ1: Is the reference standard likely to correctly classify the target condition? (i.e. Is histological diagnosis made from appropriately sampled tissue?) | Yes: if stated that the whole prostate was comprehensively sampled by a full 5‐mm transperineal TTMB, or by a equivalently well described transperineal template‐guided biopsy method with a prostate volume based median of ≤ 20 biopsy cores. No: one of these criteria was not met (i.e. in‐house transperineal saturation biopsy or transrectal saturation biopsy are less likely to appropriately sample the whole prostate). Unclear: insufficient information to make a judgement |
| SQ2: Was the reference standard performed independent of the index test? | Yes: if stated that the reference biopsies were taken without knowledge of the MRI‐score and location of target lesions; and, if incorporation was avoided (i.e. the index test was not part of the reference standard). No: one of these criteria was not met Unclear: insufficient information to make a judgement |
| Risk of bias Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk: 'Yes’ for all SQs High risk: ’No’ for at least 1 of the 3 SQs Unclear risk: ’Unclear’ for at least 1 SQ |
| Concerns for applicability Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern: data were presented for GS ≥ 3+4 without any volume criteria (ISUP grade ≥ 2), if necessary after requesting additional data from study authors High concern: data were presented for an alternative target condition definition and study authors did not provide additional data. Unclear: insufficient information to make a judgement |
| Domain 4: Flow and timing | |
| SQ1: Did all participants receive the same biopsy methods (i.e. was differential verification avoided)? | Yes: if stated that all participants received the same type of index test(s) and reference standard, prostate volume dependency was allowed. No: if one of these criteria was not met Unclear: if insufficient information to make a judgement |
| SQ2: Were all enrolled participants included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes: if stated that all eligible participants were enrolled and included in the final analyses; or, if reasons to excluded participants did not cause a relevant bias (e.g. participants with claustrophobia who refused MRI). No: one of these criteria was not met. Unclear: if insufficient information to make a judgement |
| Risk of bias Could the participant flow have introduced bias? | Low risk: ’Yes’ for all SQs High risk: ’No’ for at least 1 SQ Unclear risk: ’Unclear’, for at least 1 SQ |
| DCE: dynamic contrast‐enhanced; DRE: digital rectal examination; DWI: diffusion‐weighted imaging; MRI: magnetic resonance imaging; PSA: prostate‐specific antigen; QUADAS: Quality Assessment of Diagnostic Accuracy Studies; SQ: signalling question; TTMB: template‐guided mapping biopsy; ISUP: International Society of Urological Pathology | |
| Study | MRI | Index biopsy | Reference standard | Target | |||||
| Study | Consecutive | N of | Index | MRI‐scale; | MRI‐TBx | Technique | Median N | Independence | ISUP |
| No | 54 | MRI | 1‐5; ≥ 3 | Cognitive/transperineal | TTMB | 45 (21‐137) | No | G = 1 | |
| Yes | 576 | MRI, SBx | 1‐5; ≥ 3 | NA/transrectal | TTMB | > 40b | Yes | G = 1 | |
| Yes | 123 | MRI, | 1‐5; ≥ 3 | Cognitive/transrectal | TSBc | 24d | Yes | G = 1 | |
| Yes | Bx‐naïve: 597 | MRI, | 1‐5; ≥ 3 | Software/transperineal | TSBe | 24 (22‐25) | No | G ≥ 2 | |
| Yes | Bx‐naïve: 83 | MRI | 1‐5; ≥ 3 | Cognitive/transperineal | TSBe | (24‐40) | No | G = 1 | |
| Yes | 295 | MRI, | 1‐5; ≥ 3 | Software/transperineal | TSBe | (18‐24) | Unclear | G = 1 | |
| Yes | Centre 1: 163 | MRI | 1‐5; ≥ 3 | Software, | TSBe | 24 (22‐26f), | No | G = 1 | |
| Unclear | 287 | MRI, | 1‐5; ≥ 3 | Software/transperineal | TSBe | 24 (24‐25) | Unclear | G ≥ 2 | |
| Unclear | Bx‐naïve: 95 | MRI, | 1‐5; ≥ 3 | Software/transperineal | TSBg | 24 (23‐27f) | Yes | G = 1 | |
| No | 39 | MRI, | 1‐4; ≥2 | Software/transperineal | TSBe | 24 (14‐34) | No | G = 1 | |
| Yes | 163 | MRI, | 1‐5; ≥ 3 | Software/Transrectal | TSB | 40 (30‐55) | No | G = 1 | |
| Unclear | 9 | MRI | 1‐5; ≥ 3 | NA | TSBh | 24 (24–28) | Unclear | G = 1 | |
| Unclear | 78 | MRI, | 0‐1: ≥1 | Cognitive/transrectal | TSBh | 28 (26‐32) | No | G = 1 | |
| Yes | 344 | MRI | 1‐5; ≥ 3 | Software, | TTMB | 30 | No | G = 1 | |
| Unclear | 33 | MRI | 1‐5; ≥ 3 | NA | TTMB | 55 (42‐63f) | Yes | G = 1 | |
| Unclear | 50 | SBx | NA | NA/transrectal | TSBh | 36 | Yes | G = 1 | |
| Unclear | 42 | SBx | NA | NA/transrectal | TSBh | 36 | Yes | G = 1 | |
| Yes | 2753 | SBx | NA | NA/transrectal | TSBc | 21 | No | G = 1 | |
| Bx: biopsy; ISUP G : International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; PI‐RADS v1, v2: Prostate Imaging Reporting Data System version 1 or 2; SBx: systematic biopsy; TSB: transperineal saturation biopsy; TTMB: transperineal template mapping biopsy | |||||||||
| aIncluded participants were part of the same study cohort (no randomised populations were included). | |||||||||
| Patient characteristics of the included diagnostic test accuracy studies | ||||
| Study | Population | Median age | Median PSA | Median prostate |
| Prior‐negative Bx | 64 (39‐75) | 10 (2‐23) | 53 (19‐136) | |
| Bx‐naïve | 63 (7.6)a | 7.1 (2.9)a | NR | |
| Prior‐negative Bx | 62 (57‐68b) | 6.3 (4,8‐8,9b) | 55 (20‐149)a | |
| Mixedc | 65 (60‐71b) | 7.2 (5.3‐10.4b) | 45 (34‐64b) | |
| Mixedc | 64 (6.8)a | 13.3 (12,1)a | 68 (35)a | |
| Prior‐negative Bx | 65 (59‐69b) | 7.8 (6.0‐12b) | 65 (44‐83b) | |
| Bx‐naïve | 64 (57‐69b) | 6.6 (4.6‐9.0b) | 44 (33‐55b) | |
| Prior‐negative Bx | 66 (61‐72b) | 9.7 (7.1‐13.9b) | 52 (36‐75b) | |
| Mixedc | 65 (58‐71b) | 7.2 (5.4‐10.2b) | 46 (36‐60b) | |
| Prior‐negative Bx | 64 (47‐77)a | 10 (1.2‐36) | NR | |
| Bx‐naïve | 63 (57‐68b) | 5.8 (4.4‐8.9b) | 44 (34‐60b) | |
| Bx‐naïve | 68 (46‐81) | 11.5 (1.2‐92.5) 10 (2.7‐61)d | NR | |
| Prior‐negative Bx | 63 (49‐72) | 11 (3.7‐45) | NR | |
| Bx‐naïve | 63 (56‐67b) | 5.2 (3.7‐7.1b) | 40 (30‐54b) | |
| Prior‐negative Bx | 65 (61‐69b) | 7.1 (5.1‐13.6b) | 44 (32‐65b) | |
| Bx‐naïve | 67 (54‐84)a | 8 (4‐18)a | 58 (19‐165)a | |
| Prior‐negative Bx | 65 (50‐75)a | 8.3 (4.4‐19)a | 59 (21‐152)a | |
| Bx‐naïve | 64 (8)a | 12.5 (7.2)a | 46 (25)a | |
| Bx: biopsy; NR: not reported; PSA: prostate specific antigen | ||||
| aMean (standard deviation or range) (as opposed to median (range)). | ||||
| Study | MRI | Index biopsy | Target | ||||||
| Study | Consecutive | N of | Index tests | MRI‐scale; | MRI‐TBx | SBx | MRI‐TBx & | ISUP | |
| Technique | Median N | Independence | Route | ||||||
| Yes | Bx‐naïve: 74 | MRI‐pathway | 1‐5; ≥ 3 | Software | 12 (12‐12b) | Yes | Transrectal | G = 1 | |
| Unclear | 206 | MRI‐pathway | 1‐5; ≥ 3 | Software | 10 (10‐10) | Yes | Transrectal | G = 1 | |
| Yes | 1020 | MRI‐pathway | 1‐5; ≥ 3 | Software | 10c | Yes | Transrectal | G = 1 | |
| Yes | 168 | MRI‐pathway | 1‐5; ≥ 3 | Cognitive | (8‐19) | Unclear | Transrectal | G = 1 | |
| Yes | 65 | MRI‐pathway | 1‐5; ≥ 3 | Cognitive | 18 (16.2‐19.8b) | No | Transrectal | G = 1 | |
| Yes | 420 | MRI‐pathway | 1‐5; ≥ 3 | Cognitive | 12d | Yes | Transperineal | G ≥ 2 | |
| Unclear | Bx‐naïve: 50 | MRI‐pathway | Other | Software | 12‐14e | Unclear | Transrectal | G = 1 | |
| No | 38 | MRI‐pathway | 1‐5; ≥4 | Cognitive | NR | No | Transrectal | G ≥ 2 | |
| Yes | 391 | MRI‐pathway | TZ: 0‐4; ≥2 | Software Cognitive | 12 (10‐12) | Unclear | Transrectal | G ≥ 2 | |
| Yes | Bx‐naïve: 329 | MRI‐pathway | 1‐5; ≥ 3 | Software | 12 | Unclear | Transrectal | G ≥ 2 | |
| Unclear | 60 | MRI‐pathway | 1‐5; ≥ 3 | Cognitive | 12 | Yes | Transperineal | G = 1 | |
| Yes | Bx‐naïve: 387 | MRI‐pathway | 1‐5; ≥ 3 | Software | 11 (10‐12) | No | Transrectal | G = 1 | |
| Unclear | 53 | MRI‐pathway | 1‐5; ≥4 | Cognitive | 12 | Yes | Transrectal | G = 1 | |
| Unclear | Bx‐naïve: 134 | MRI‐pathway | 1‐5; ≥ 3 | Cognitive | 12c | No | Transrectal | G = 1 | |
| Unclear | Bx‐naïve: 183 | MRI‐pathway | 1‐5; ≥4 | Software Cognitive | 14c | No | Transrectal | G = 1 | |
| Unclear | 76 | MRI‐pathway | 1‐4; ≥2 | Cognitive | 12 (12‐12) | No | Transrectal | G = 1 | |
| Unclear | 123 | MRI‐pathway | 1‐4; ≥2 | Cognitive | 12 | No | Transrectal | G = 1 | |
| Unclear | 52 | MRI‐pathway | 0‐1: ≥1 | Cognitive | NR | Unclear | Transrectal | G = 1 | |
| Yes | Bx‐naïve: 570 | MRI‐pathway | 1‐5; ≥ 3 | Cognitive | 10, 14 or 45f | Unclear | Transrectal | G = 1 | |
| Yes | 110 | MRI‐pathway | 1‐4; ≥2 | Software | 15 | No | Transrectal | G = 1 | |
| Yes | 223 | MRI‐pathway | 1‐5; ≥ 3 | In‐bore | 12 | Unclear | Transrectal | G = 1 | |
| Yes | 251 | MRI‐pathway | 1‐5; ≥ 3 | Software Cognitive | 12.2c | Yes | Transrectal | G = 1 | |
| Yes | 143 | MRI‐pathway | 1‐4; ≥2 | Software | 12c | Unclear | Transrectal | G = 1 | |
| Yes | 53 | MRI‐pathway | 1‐4; ≥2 | Cognitive | 12 (12‐14) | Yes | Transrectal | G = 1 | |
| Yes | 626 | MRI‐pathway | 1‐5; ≥ 3 | In‐bore | 12c | Yes | Transrectal | G = 1 | |
| Bx: biopsy; ISUP G : International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; PI‐RADS v1, v2: Prostate Imaging Reporting Data System version 1 or 2; PZ: peripheral zone; SBx: systematic biopsy; TSB: transperineal saturation biopsy; TTMB: transperineal template mapping biopsy; TZ: transition zone | |||||||||
| aIncluded participants were part of the same study cohort (no randomised populations were included). | |||||||||
| Study | Population | Median age | Median PSA | Median prostate |
| Bx‐naïve | 73 (72‐74b) | 4.2 (3.4–5.8b) | 53 (37‐71b) | |
| Prior‐negative Bx | 65 (58‐68b) | 12.8 (8.9‐19.6b) | NR | |
| Bx‐naïve | 67 (61‐71b) | 8 (5.7‐13b) | 53 (40‐72b) | |
| Bx‐naïve | 61 (8)c | 8.3 (6.1)c | 49 (7)c | |
| Prior‐negative Bx | 64 (60‐68b) | 10.9 (7.2‐14.7b) | 48 (34‐63b) | |
| Bx‐naïve | 67 (45‐91) | 9.7 (2.4‐35.7) | 45 (21‐83) | |
| Bx‐naïve | 59 (8)c | 6.0 (3.5)c | 38 (18)c | |
| Prior‐negative Bx | 64 (48‐77)c | 14.4 (1.8‐33.1)c | NR | |
| Bx‐naïve | 64 (7)c | 8.5 (3.9)c | 56 (30)c | |
| Bx‐naïve | 64 (59‐69b) | 5.8 (4.4‐8.1b) | 45(33‐62b) | |
| Bx‐naïve | 64 (6.7)c | 7.2 (6‐9.4b) | 48 (35‐63b) | |
| Bx‐naïve | 64 (45–74)c | 6.3 (4.4b) | (32‐70)d | |
| Bx‐naïve | 66 (47‐76) | 7.4 (4‐14) | 42 (17‐107) | |
| Mixed | 65 (6)c | 7.5 (5.7‐9.6b) | 37 (28‐49b) | |
| Bx‐naïve | 64 (7)c | 10.2 (15.1)c | NR | |
| Bx‐naïve | 66 (43‐83) | 6.4 (3.3‐9.8) | 39 (17‐127) | |
| Bx‐naïve | 62 (10)c | 6.4 (1.8)c | 40 (18)c | |
| Bx‐naïve | 62 (43‐79) | 5 (3‐8.9) | 45 (17‐93) | |
| Bx‐naïve | 64 (51‐82) | NR | NR | |
| Bx‐naïve | 65 (7)c | 8.4 (6.3)c | 49 (22)c | |
| Bx‐naïve | 63 (57‐68b) | 5.3 (4.1‐6.6b) | 41 (30‐59b) | |
| Bx‐naïve | 64 (59‐68b) | 6.5 (5.6‐9.6b) | 50 (38‐63b) | |
| Prior‐negative Bx | 64 (47‐82)c | 11.59 (0.4‐96.9)c | 69 (17‐309)c | |
| Bx‐naïve | 63 (60‐66b) | 6.1 (4.2‐9.9b) | 28 (24‐37b) | |
| Bx‐naïve | 65 (59‐68b) | 6.4 (4.6‐8.2b) | 55 (41‐77b) | |
| Bx: biopsy; NR: not reported; PSA: prostate specific antigen | ||||
| aResults not reported per population type. | ||||
| Diagnostic accuracy of the index tests verified by template‐guided biopsy as the reference standard | |||||||
| Index test | MRI | Target | N participants | Proportion | Sensitivity | Specificity | P value |
| MRI | Positive + negative | G = 1 | 1764 (10) | 0.28 (0.20 to 0.38) | 0.70 (0.59 to 0.80) | 0.27 (0.19 to 0.37) | P < 0.01b |
| G ≥ 1 | 1764 (10) | 0.39 (0.30 to 0.50) | 0.84 (0.74 to 0.90) | 0.39 (0.30 to 0.50) | NA | ||
| G ≥ 2 | 3091 (12) | 0.29 (0.22 to 0.37) | 0.91 (0.83 to 0.95) | 0.37 (0.29 to 0.46) | P < 0.01b | ||
| G ≥ 3 | 1438 (7) | 0.31 (0.21 to 0.42) | 0.95 (0.87 to 0.99) | 0.35 (0.26 to 0.46) | ID | ||
| MRI‐TBx | Positive | G = 1 | 497 (5) | NA | 0.51 (0.21 to 0.81) | 1.00 (0.77 to 1.00) | NA |
| G ≥ 1 | 611 (6) | NA | 0.71 (0.61 to 0.80) | 0.93 (0.87 to 0.96) | NA | ||
| G ≥ 2 | 1553 (8) | NA | 0.80 (0.69 to 0.87) | 0.94 (0.90 to 0.97) | NA | ||
| G ≥ 3 | 428 (3) | NA | ID | ID | ID | ||
| MRI‐pathway | Positive + negative | G = 1 | 681 (5) | 0.24 (0.16 to 0.36) | 0.34 (0.19 to 0.53) | 1.00 (0.90 to 1.00) | P = 0.52c |
| G ≥ 1 | 844 (6) | 0.28 (0.21 to 0.35) | 0.58 (0.52 to 0.65) | 0.96 (0.92 to 0.98) | NA | ||
| G ≥ 2 | 2257 (8) | 0.29 (0.24 to 0.35) | 0.72 (0.60 to 0.82) | 0.96 (0.94 to 0.98) | P = 0.06c | ||
| G ≥ 3 | 604 (3) | 0.29 (0.26 to 0.33) | ID | ID | ID | ||
| SBx | NA | G = 1 | 3421 (4) | NA | 0.55 (0.25 to 0.83) | 0.99 (0.81 to 1.00) | NA |
| G ≥ 1 | 3421 (4) | NA | 0.65 (0.31 to 0.88) | 1.00 (0.88 to 1.00) | NA | ||
| G ≥ 2 | 3421 (4) | NA | 0.63 (0.19 to 0.93) | 1.00 (0.91 to 1.00) | NA | ||
| G ≥ 3 | 626 (2) | NA | ID | ID | ID | ||
| CI: confidence interval; G: International Society of Urological Pathology grade; ID: inadequate data; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; SBx: systematic biopsy | |||||||
| aData did not allow differentiation between the mix of included participants (biopsy‐naïve and prior‐negative biopsy men). | |||||||
| Predictive values of the index tests and prostate cancer prevalences | ||||||
| Test | MRI | Target | N participants | Prevalenceb | NPVc | PPVc |
| MRI | Positive + negative | G = 1 | 1764 (10) | 0.20 (0.17 to 0.23) | 0.79 (0.74 to 0.82) | 0.20 (0.18 to 0.21) |
| G ≥ 2 | 3091 (12) | 0.29 (0.22 to 0.38) | 0.91 (0.86 to 0.94) | 0.37 (0.35 to 0.39) | ||
| G ≥ 3 | 1438 (7) | 0.14 (0.08 to 0.23) | 0.98 (0.95 to 0.99) | 0.19 (0.17 to 0.21) | ||
| MRI‐TBx | Positive | G = 1 | 497 (5) | 0.22 (0.19 to 0.26) | 0.88 (0.78 to 0.94) | 0.98 (0.23 to 1.00) |
| G ≥ 2 | 1553 (8) | 0.34 (0.24 to 0.46) | 0.90 (0.85 to 0.93) | 0.88 (0.80 to 0.92) | ||
| G ≥ 3 | 428 (3) | 0.21 (0.12 to 0.35) | ID | ID | ||
| MRI‐pathway | Positive + negative | G = 1 | 681 (5) | 0.21 (0.18 to 0.24) | 0.85 (0.81 to 0.88) | 0.95 (0.38 to 1.00) |
| G ≥ 2 | 2257 (8) | 0.26 (0.18 to 0.36) | 0.91 (0.87 to 0.94) | 0.88 (0.80 to 0.92) | ||
| G ≥ 3 | 604 (3) | 0.16 (0.09 to 0.27) | ID | ID | ||
| SBx | NA | G = 1 | 3421 (4) | 0.20 (0.16 to 0.25) | 0.90 (0.81 to 0.95) | 0.94 (0.37 to 1.00) |
| G ≥ 2 | 3421 (4) | 0.34 (0.21 to 0.51) | 0.84 (0.60 to 0.95) | 1.00 (0.76 to 1.00) | ||
| G ≥ 3 | 626 (2) | 0.10 (0.08 to 0.12) | ID | ID | ||
| CI: confidence interval; G: International Society of Urological Pathology grade; ID: inadequate data; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; NA: not applicable; NPV: negative predictive value; PPV: positive predictive value; SBx: systematic biopsy | ||||||
| aData did not allow differentiation between the mix of included participants (biopsy‐naïve and prior‐negative biopsy men). | ||||||
| MRI‐positivity threshold effect, verified by template‐guided biopsy as the reference standard, with threshold ≥ 3and ≥ 4 out of 5 for identifying prostate cancer | |||||
| MRI threshold | Target | N participants | Proportion | Sensitivity | Specificity |
| ≥ 3/5 | G = 1 | 1647 (8) | 0.29 (0.21 to 0.40) | 0.68 (0.57 to 0.77) | 0.28 (0.19 to 0.39) |
| G ≥ 2 | 2974 (10) | 0.30 (0.23 to 0.38) | 0.89 (0.82 to 0.94) | 0.39 (0.32 to 0.47) | |
| G ≥ 3 | 1438 (7) | 0.31 (0.21 to 0.42) | 0.96 (0.87 to 0.99) | 0.35 (0.26 to 0.46) | |
| ≥ 4/5 | G = 1 | 834 (4) | 0.60 (0.38 to 0.78) | 0.26 (0.16 to 0.40) | 0.57 (0.36 to 0.76) |
| G ≥ 2 | 1083 (5) | 0.59 (0.43 to 0.74) | 0.72 (0.52 to 0.86) | 0.78 (0.68 to 0.86) | |
| G ≥ 3 | 834 (4) | 0.60 (0.38 to 0.78) | 0.86 (0.51 to 0.97) | 0.68 (0.51 to 0.81) | |
| CI: confidence interval; G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; N: number | |||||
| aData did not allow differentiation between the mix of included participants (biopsy‐naïve and prior‐negative biopsy men). | |||||
| Population | Target condition | N participants (studies) | Proportion prostate cancer detected in % (95% CI) | Detection ratiob | Difference | ||||
| Biopsy status | MRI, proportion in % (95% CI)a | MRI‐pathway and SBx combined (total cancer detected) | MRI‐pathway | SBx | MRI‐pathway versus SBx | P value | |||
| Mixedd | Positive + negative | G = 1 | 5442 (21) | 25.6 (22.8 to 28.8) | 12.3 (10.1 to 15.1) | 20.8 (18.0 to 24.1) | 0.61 (0.52 to 0.71) | P < 0.01 | NA |
| G ≥ 1 | 6524 (24) | 50.2 (46.4 to 54.3) | 37.9 (33.4 to 42.6) | 43.3 (39.1 to 47.8) | 0.88 (0.81 to 0.95) | P < 0.01 | NA | ||
| G ≥ 2 | 6944 (25) | 26.7 (23.3 to 30.7) | 22.9 (19.5 to 26.8) | 19.4 (15.9 to 23.5) | 1.12 (1.02 to 1.23) | P = 0.01 | NA | ||
| G ≥ 3 | 5981 (21) | 15.0 (12.7 to 18.0) | 12.7 (10.5 to 15.6) | 9.7 (7.5 to 12.7) | 1.20 (1.06 to 1.36) | P < 0.01 | NA | ||
| Positive | G = 1 | 3460 (19) | 29.5 (26.0 to 33.8) | 18.8 (15.2 to 23.4) | 22.4 (18.9 to 26.9) | 0.85 (0.75 to 0.97) | P = 0.01 | NA | |
| G ≥ 1 | 3998 (20) | 68.0 (62.3 to 73.5) | 61.1 (54.1 to 67.7) | 58.9 (51.5 to 65.9) | 1.03 (0.95 to 1.10) | P = 0.52 | NA | ||
| G ≥ 2 | 3998 (20) | 42.6 (37.6 to 48.1) | 37.9 (32.7 to 43.7) | 31.6 (26.2 to 37.9) | 1.17 (1.07 to 1.28) | P < 0.01 | NA | ||
| G ≥ 3 | 3902 (18) | 24.2 (20.9 to 28.1) | 21.0 (17.8 to 24.8) | 16.3 (13.1 to 20.3) | 1.24 (1.11 to 1.38) | P < 0.01 | NA | ||
| Biopsy‐naïve | Positive + negative | G = 1 | 4079 (17) | 27.2 (23.9 to 31.1) | 13.5 (10.7 to 17.2) | 22.4 (19.1 to 26.3) | 0.63 (0.54 to 0.74) | P < 0.01 | P = 0.91 |
| G ≥ 1 | 4799 (19) | 53.2 (48.7 to 57.9) | 41.0 (35.8 to 46.4) | 47.8 (42.8 to 52.9) | 0.85 (0.77 to 0.93) | P < 0.01 | P = 0.12 | ||
| G ≥ 2 | 5219 (20) | 27.7 (23.7 to 32.6) | 23.4 (19.3 to 28.1) | 21.4 (17.2 to 26.5) | 1.05 (0.95 to 1.16) | P = 0.35 | P < 0.01 | ||
| G ≥ 3 | 4306 (16) | 15.5 (12.6 to 19.5) | 12.7 (9.9 to 16.5) | 10.8 (8.0 to 14.8) | 1.09 (0.94 to 1.26) | P = 0.27 | P < 0.01 | ||
| Positive | G = 1 | 2682 (16) | 31.8 (27.7 to 36.9) | 21.3 (17.0 to 26.9) | 23.7 (19.6 to 29.1) | 0.85 (0.74 to 0.98) | P = 0.03 | P = 0.35 | |
| G ≥ 1 | 2955 (17) | 70.9 (65.0 to 76.6) | 63.7 (56.3 to 70.6) | 63.8 (56.2 to 70.7) | 0.99 (0.92 to 1.08) | P = 0.88 | P = 0.05 | ||
| G ≥ 2 | 2955 (17) | 44.2 (38.6 to 50.4) | 39.2 (33.3 to 45.7) | 34.4 (28.3 to 41.3) | 1.12 (1.01 to 1.23) | P = 0.03 | P < 0.01 | ||
| G ≥ 3 | 2899 (15) | 24.8 (21.0 to 29.6) | 21.2 (17.4 to 25.7) | 17.5 (13.8 to 22.3) | 1.16 (1.02 to 1.31) | P = 0.02 | P < 0.01 | ||
| Prior‐negative biopsy | Positive + negative | G = 1 | 1202 (8) | 23.0 (18.0 to 30.2) | 10.9 (7.9 to 15.3) | 17.8 (12.7 to 25.2) | 0.62 (0.44 to 0.88) | P < 0.01 | P = 0.91 |
| G ≥ 1 | 1564 (10) | 40.7 (35.1 to 47.2) | 30.0 (24.1 to 37.0) | 30.3 (24.3 to 37.5) | 0.97 (0.85 to 1.11) | P = 0.70 | P = 0.12 | ||
| G ≥ 2 | 1564 (10) | 22.8 (20.0 to 26.2) | 20.5 (17.7 to 23.5) | 13.2 (10.8 to 16.4) | 1.44 (1.19 to 1.75) | P < 0.01 | P < 0.01 | ||
| G ≥ 3 | 1514 (9) | 12.6 (10.5 to 15.6) | 11.5 (9.4 to 14.2) | 6.3 (4.4 to 9.1) | 1.64 (1.27 to 2.11) | P < 0.01 | P < 0.01 | ||
| Positive | G = 1 | 655 (7) | 27.9 (22.1 to 36.2) | 18.2 (12.8 to 26.7) | 18.9 (13.3 to 27.5) | 1.03 (0.89 to 1.18) | P = 0.71 | P = 0.35 | |
| G ≥ 1 | 920 (8) | 54.8 (44.6 to 66.4) | 48.5 (37.0 to 61.5) | 39.4 (27.1 to 53.9) | 1.16 (1.02 to 1.32) | P = 0.02 | P = 0.05 | ||
| G ≥ 2 | 920 (8) | 31.3 (27.4 to 36.1) | 28.6 (24.7 to 33.1) | 18.3 (15.1 to 22.5) | 1.49 (1.22 to 1.82) | P < 0.01 | P < 0.01 | ||
| G ≥ 3 | 880 (7) | 17.9 (14.3 to 22.9) | 16.7 (13.1 to 21.5) | 9.4 (6.4 to 14.2) | 1.65 (1.30 to 2.09) | P < 0.01 | P < 0.01 | ||
| CI: confidence interval; G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; N: number; SBx: systematic biopsy | |||||||||
| aProportion of participants with a positive or negative magnetic resonance imaging result, based on the studies reporting grade 2 or higher. | |||||||||
| Population | Target | N participants | Proportion prostate cancer detected in % (95% CI) | ||||||
| Biopsy status | MRI, | MRI‐pathway and SBx combined (total cancer detected) | MRI‐pathway | SBx | Both MRI‐pathway and SBx | Only MRI‐pathway (added valueb) | Only SBx (added valueb) | ||
| Mixedc | Positive + negative | G = 1d | 5442 (21) | 19.5 (16.9 to 22.7) | 10.3 (8.1 to 13.1) | 16.8 (14.2 to 19.9) | 7.6 (5.5 to 10.2) | 2.7 (1.8 to 4.0) | 9.2 (7.4 to 11.4) |
| G ≥ 1 | 6524 (24) | 50.2 (46.4 to 54.3) | 37.9 (33.4 to 42.6) | 43.3 (39.1 to 47.8) | 30.9 (26.3 to 36.0) | 6.9 (5.2 to 9.2) | 12.4 (10.2 to 14.9) | ||
| G ≥ 2 | 6944 (25) | 26.7 (23.3 to 30.7) | 22.9 (19.5 to 26.9) | 19.4 (15.9 to 23.6) | 15.6 (12.2 to 19.6) | 7.3 (5.9 to 9.0) | 3.8 (2.5 to 5.7) | ||
| G ≥ 3 | 5981 (21) | 15.0 (12.7 to 18.0) | 12.7 (10.5 to 15.6) | 9.7 (7.5 to 12.7) | 7.4 (5.3 to 10.2) | 5.3 (4.3 to 6.5) | 2.3 (1.4 to 3.7) | ||
| Positive | G = 1d | 3460 (19) | 19.7 (15.9 to 24.7) | 15.8 (12.2 to 20.7) | 15.8 (12 to 20.8) | 12.0 (8.4 to 16.8) | 3.9 (2.6 to 5.7) | 3.8 (2.3 to 6.2) | |
| G ≥ 1 | 3998 (20) | 68.0 (62.3 to 73.5) | 61.1 (54.1 to 67.7) | 58.9 (51.5 to 65.9) | 52.0 (43.6 to 59.9) | 9.1 (5.9 to 13.5) | 6.9 (4.6 to 10.1) | ||
| G ≥ 2 | 3998 (20) | 42.6 (37.6 to 48.1) | 37.9 (32.7 to 43.7) | 31.6 (26.2 to 37.9) | 27.0 (21.4 to 33.4) | 10.9 (8.5 to 13.9) | 4.6 (2.9 to 7.2) | ||
| G ≥ 3 | 3902 (18) | 24.2 (20.9 to 28.1) | 21 (17.8 to 24.8) | 16.3 (13.1 to 20.3) | 13.2 (10.1 to 16.9) | 7.9 (6.3 to 9.7) | 3.1 (1.9 to 5.2) | ||
| Negative | G = 1d | 1666 (19) | 16.8 (12.9 to 21.6) | NA | 16.8 (12.9 to 21.6) | NA | NA | 16.8 (12.9 to 21.6) | |
| G ≥ 1 | 1781 (20) | 23.1 (19.7 to 26.9) | NA | 23.1 (19.7 to 26.9) | NA | NA | 23.1 (19.7 to 26.9) | ||
| G ≥ 2 | 1781 (20) | 7.2 (5.3 to 9.8) | NA | 7.2 (5.3 to 9.8) | NA | NA | 7.2 (5.3 to 9.8) | ||
| G ≥ 3 | 1725 (18) | 2.7 (1.6 to 4.6) | NA | 2.7 (1.6 to 4.6) | NA | NA | 2.7 (1.6 to 4.6) | ||
| Biopsy‐naïve | Positive + negative | G = 1d | 4079 (17) | 20.9 (18.0 to 24.7) | 11.2 (8.4 to 14.9) | 18.5 (15.6 to 22.2) | 8.8 (6.2 to 12.3) | 2.4 (1.4 to 4.0) | 9.8 (8.0 to 11.8) |
| G ≥ 1 | 4799 (19) | 53.2 (48.7 to 57.9) | 41.0 (35.8 to 46.4) | 47.8 (42.8 to 52.9) | 35.6 (30.2 to 41.2) | 5.4 (3.6 to 8.0) | 12.2 (8.7 to 16.7) | ||
| G ≥ 2 | 5219 (20) | 27.7 (23.7 to 32.6) | 23.4 (19.4 to 28.2) | 21.4 (17.2 to 26.5) | 17.1 (13.0 to 22) | 6.3 (4.8 to 8.2) | 4.3 (2.6 to 6.9) | ||
| G ≥ 3 | 4306 (16) | 15.5 (12.6 to 19.5) | 12.7 (9.9 to 16.5) | 10.8 (8.0 to 14.8) | 8.0 (5.4 to 11.6) | 4.7 (3.5 to 6.3) | 2.8 (1.7 to 4.8) | ||
| Positive | G = 1d | 2682 (16) | 21.1 (16.7 to 27.1) | 17.0 (12.6 to 22.9) | 17.7 (13.3 to 23.8) | 13.6 (9.3 to 19.5) | 3.4 (2.1 to 5.3) | 4.1 (2.5 to 6.7) | |
| G ≥ 1 | 2955 (17) | 70.9 (65.0 to 76.6) | 63.7 (56.3 to 70.6) | 63.8 (56.2 to 70.7) | 56.6 (47.7 to 64.6) | 7.1 (4.2 to 11.9) | 7.2 (4.7 to 10.8) | ||
| G ≥ 2 | 2955 (17) | 44.2 (38.6 to 50.4) | 39.2 (33.3 to 45.7) | 34.4 (28.3 to 41.3) | 29.5 (23.2 to 36.5) | 9.8 (7.1 to 13.2) | 4.9 (2.8 to 8.3) | ||
| G ≥ 3 | 2899 (15) | 24.8 (21.0 to 29.6) | 21.2 (17.4 to 25.7) | 17.5 (13.8 to 22.3) | 13.9 (10.3 to 18.3) | 7.3 (5.4 to 9.7) | 3.7 (2.2 to 6.1) | ||
| Negative | G = 1 | 1287 (16) | 18.4 (14.2 to 23.7) | NA | 18.4 (14.2 to 23.7) | NA | NA | 18.4 (14.2 to 23.7) | |
| G ≥ 1 | 1343 (17) | 25.5 (20.7 to 30.9) | NA | 25.5 (20.7 to 30.9) | NA | NA | 25.5 (20.7 to 30.9) | ||
| G ≥ 2 | 1343 (17) | 8.1 (5.6 to 11.6) | NA | 8.1 (5.6 to 11.6) | NA | NA | 8.1 (5.6 to 11.6) | ||
| G ≥ 3 | 1297 (15) | 3.0 (1.6 to 5.5) | NA | 3.0 (1.6 to 5.5) | NA | NA | 3.0 (1.6 to 5.5) | ||
| Prior‐negative biopsy | Positive + negative | G = 1d | 1202 (8) | 17.6 (13.0 to 25.0) | 9.8 (6.9 to 14.3) | 13.5 (8.9 to 21.0) | 5.8 (3.2 to 10.0) | 4.1 (2.6 to 6.2) | 7.7 (3.9 to 14.8) |
| G ≥ 1 | 1564 (10) | 40.7 (35.1 to 47.2) | 30.0 (24.1 to 37.0) | 30.3 (24.3 to 37.5) | 19.6 (13.7 to 27.1) | 10.3 (7.5 to 13.9) | 10.7 (7.4 to 15) | ||
| G ≥ 2 | 1564 (10) | 22.8 (20.0 to 26.2) | 20.5 (17.7 to 23.5) | 13.2 (10.8 to 16.4) | 10.9 (8.7 to 13.5) | 9.6 (7.7 to 11.8) | 2.3 (1.2 to 4.5) | ||
| G ≥ 3 | 1514 (9) | 12.6 (10.5 to 15.6) | 11.5 (9.4 to 14.2) | 6.3 (4.4 to 9.1) | 5.1 (3.4 to 7.7) | 6.3 (5.2 to 7.7) | 1.1 (0.5 to 2.6) | ||
| Positive | G = 1d | 655 (7) | 19.5 (13.9 to 28.8) | 16.5 (11.0 to 25.2) | 12.4 (7.2 to 21.6) | 9.4 (4.6 to 17.9) | 7.1 (4.1 to 11.8) | 3.0 (1.0 to 8.0) | |
| G ≥ 1 | 920 (8) | 54.8 (44.6 to 66.4) | 48.5 (37.0 to 61.5) | 39.4 (27.1 to 53.9) | 33.1 (20.1 to 48.7) | 15.4 (8.2 to 26.4) | 6.3 (3.8 to 9.8) | ||
| G ≥ 2 | 920 (8) | 31.3 (27.4 to 36.1) | 28.6 (24.7 to 33.1) | 18.3 (15.1 to 22.5) | 15.7 (12.7 to 19.1) | 13.0 (9.7 to 17.0) | 2.7 (1.2 to 5.7) | ||
| G ≥ 3 | 880 (7) | 17.9 (14.3 to 22.9) | 16.7 (13.1 to 21.5) | 9.4 (6.4 to 14.2) | 8.2 (5.2 to 12.6) | 8.5 (6.1 to 11.5) | 1.2 (0.4 to 3.2) | ||
| Negative | G = 1 | 341 (7) | 14.2 (5.9 to 30.2) | NA | 14.2 (5.9 to 30.2) | NA | NA | 14.2 (5.9 to 30.2) | |
| G ≥ 1 | 400 (8) | 19.5 (12.9 to 28.3) | NA | 19.5 (12.9 to 28.3) | NA | NA | 19.5 (12.9 to 28.3) | ||
| G ≥ 2 | 400 (8) | 5.3 (3.1 to 8.9) | NA | 5.3 (3.1 to 8.9) | NA | NA | 5.3 (3.1 to 8.9) | ||
| G ≥ 3 | 390 (7) | 3.3 (1.7 to 6.3) | NA | 3.3 (1.7 to 6.3) | NA | NA | 3.3 (1.7 to 6.3) | ||
| CI: confidence interval; G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy: N: number; NA: not applicable; SBx: systematic biopsy | |||||||||
| aProportion of participants with a positive or negative MRI result, based on the studies reporting grade 2 or higher. | |||||||||
| Agreement analysis: number needed to biopsy by systematic biopsy to detect one extra prostate cancer not detected by the MRI‐pathway | |||
| Population | Target | NNBa | |
| Biopsy status | MRI | ||
| Biopsy‐naïve | Positive | G = 1 | 24 (15 to 40) |
| G ≥ 2 | 20 (12 to 36) | ||
| G ≥ 3 | 27 (16 to 45) | ||
| Negative | G = 1 | 5 (4 to 7) | |
| G ≥ 2 | 13 (9 to 18) | ||
| G ≥ 3 | 33 (18 to 63) | ||
| Prior‐negative biopsy | Positive | G = 1 | 33 (13 to 100) |
| G ≥ 2 | 37 (18 to 83) | ||
| G ≥ 3 | 83 (31 to 250) | ||
| Negative | G = 1 | 7 (3 to 17) | |
| G ≥ 2 | 19 (11 to 32) | ||
| G ≥ 3 | 31 (16 to 63) | ||
| CI: confidence interval; G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; NNB: number needed to biopsy; SBx: systematic biopsy | |||
| aNumber needed to biopsy by systematic biopsy is 100 divided by the added value of systematic biopsy. | |||
| Heterogeneity exploration in the agreement analysis: detection ratio MRI‐pathway vs systematic biopsy for G ≥ 2 prostate cancer | ||||
| Covariate | Category | N participants | Detection ratio | P value |
| Population | Biopsy‐naïve | 5219 (20) | 1.05 (0.95 to 1.16) | 0.002 |
| Prior to negative biopsy | 1564 (10) | 1.44 (1.19 to 1.75) | ||
| Field strength | 3T | 5407 (19) | ID | ID |
| 1.5T | 1143 (4) | ID | ID | |
| Endorectal coil | Yes | 1815 (6) | 1.42 (1.07 to 1.88) | 0.008 |
| No | 4082 (14) | 1.03 (0.94 to 1.12) | ||
| MRI pulse sequence | mpMRI | 4941 (16) | 1.18 (1.05 to 1.33) | 0.233 |
| bpMRI | 1775 (6) | 1.03 (0.91 to 1.17) | ||
| mpMRI + spectroscopy | 105 (2) | ID | ID | |
| MRI risk threshold | Low | 605 (6) | 1.18 (1.03 to 1.35) | 0.556 |
| Intermediate | 5859 (15) | 1.14 (1.03 to 1.26) | ||
| High | 428 (3) | ID | ID | |
| MRI‐TBx technique | Software | 3313 (9) | 1.15 (0.99 to 1.33) | 0.483 |
| Cognitive | 2194 (12) | 1.17 (1.00 to 1.36) | ||
| In‐bore | 849 (2) | ID | ID | |
| Route index test | Transrectal | 6464 (23) | ID | ID |
| Transperineal | 480 (2) | ID | ID | |
| bpMRI: biparametric magnetic resonance imaging; CI: confidence interval; G: International Society of Urological Pathology grade; ID: inadequate data; mpMRI: multiparametric magnetic resonance imaging; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; N: number; SBx: systematic biopsy | ||||
| aDetection ratio is the detection rate of MRI‐pathway divided by detection rate of systematic biopsy; the detection rate = the pooled number of positive results of the test divided by the pooled total number of positive results from both tests. | ||||
| Sensitivity analyses of the diagnostic test accuracy of MRI and the MRI‐pathway for detecting G ≥ 2 prostate cancer, verified by template‐guided biopsy as the reference standard | ||||||||
| Covariate | Category | MRI | MRI‐pathwaya | |||||
| N | Sensitivity | Specificity | N | Sensitivity | Specificity | |||
| Main analyses (as reference) | No selection | 12 | 0.91 (0.83 to 0.95) | 0.37 (0.29 to 0.46) | 8 | 0.72 (0.60 to 0.82) | 0.96 (0.94 to 0.98) | |
| QUADAS domains | Participant selection | Only low risk of bias | 5 | 0.86 (0.83 to 0.88) | 0.39 (0.31 to 0.47) | 4 | 0.61 (0.54 to 0.69) | 0.97 (0.92 to 0.99) |
| Only low applicability concern | 11 | 0.91 (0.83 to 0.96) | 0.36 (0.28 to 0.46) | 7 | 0.69 (0.60 to 0.77) | 0.97 (0.94 to 0.98) | ||
| Index test | Only low risk of bias | 12 | 0.91 (0.83 to 0.95) | 0.37 (0.29 to 0.46) | 8 | 0.72 (0.60 to 0.82) | 0.96 (0.94 to 0.98) | |
| Only low applicability concern | 9 | 0.90 (0.85 to 0.94) | 0.37 (0.31 to 0.43) | 6 | 0.68 (0.59 to 0.77) | 0.97 (0.94 to 0.99) | ||
| Reference standard | Only low risk of bias | 4 | 0.93 (0.82 to 0.98) | 0.34 (0.24 to 0.45) | 2 | ID | ID | |
| Only low applicability concern | 12 | 0.91 (0.83 to 0.95) | 0.37 (0.29 to 0.46) | 8 | 0.72 (0.60 to 0.82) | 0.96 (0.94 to 0.98) | ||
| Flow and timing | Only low risk of bias | 11 | 0.91 (0.83 to 0.96) | 0.36 (0.28 to 0.46) | 8 | 0.72 (0.60 to 0.82) | 0.96 (0.94 to 0.98) | |
| Additional analyses | MRI positivity threshold | Only threshold 3/5 | 10 | 0.89 (0.82 to 0.94) | 0.39 (0.32 to 0.47) | 6 | 0.68 (0.59 to 0.77) | 0.97 (0.94 to 0.98) |
| MRI positivity threshold effect | MRI positivity threshold 3/5 (only studies with also 4/5) | 5 | 0.87 (0.73 to 0.94) | 0.45 (0.33 to 0.57) | 0 | ID | ID | |
| MRI positivity threshold 4/5 (only studies with also 3/5) | 5 | 0.72 (0.52 to 0.86) | 0.78 (0.68 to 0.86) | 0 | ID | ID | ||
| MRI vs MRI‐pathway | Only MRI and MRI‐pathway in the same men (paired data) | 8 | 0.92 (0.83 to 0.96) | 0.35 (0.27 to 0.44) | 8 | 0.72 (0.60 to 0.82) | 0.96 (0.94 to 0.98) | |
| Reference standard | Only TTMB, TSB or TOP | 9 | 0.90 (0.84 to 0.93) | 0.36 (0.29 to 0.44) | 6 | 0.69 (0.58 to 0.78) | 0.96 (0.93 to 0.97) | |
| Template‐guided biopsy + MRI‐TBx (composite reference standard) | 11 | 0.94 (0.91 to 0.96) | 1.00 (1.00 to 1.00) | 8 | 0.72 (0.63 to 0.80) | 1.00 (1.00 to 1.00) | ||
| Experience of radiologist | Only high experience | 10 | 0.91 (0.85 to 0.95) | 0.34 (0.27 to 0.42) | 7 | 0.69 (0.60 to 0.77) | 0.97 (0.94 to 0.98) | |
| CI: confidence interval; G: International Society of Urological Pathology grade; ID: inadequate data; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; QUADAS: Quality Assessment of Diagnostic Accuracy Studies; SBx: systematic biopsy; TOP: transperineal optimised prostate biopsy;TSB: Ginsburg transperineal saturation biopsy; TTMB: transperineal template mapping biopsy | ||||||||
| aThe diagnostic test accuracy analyses of magnetic resonance imaging‐targeted biopsy are based on the same studies as the MRI‐pathway. | ||||||||
| Sensitivity analyses of the agreement between the MRI‐pathway vs systematic biopsy for detecting G ≥ 2 prostate cancer | ||||
| Covariate | Category | N | Detection ratio | |
| Main analyses (as reference) | Mixed population | 25 | 1.12 (1.02 to 1.23) | |
| QUADAS domains | Patient selection | Only low risk of bias | 12 | 1.08 (1.00 to 1.17) |
| Only low applicability concern | 23 | 1.09 (1.01 to 1.17) | ||
| Index test (MRI‐pathway) | Only low risk of bias | 24 | 1.11 (1.02 to 1.22) | |
| Only low applicability concern | 14 | 1.13 (1.01 to 1.26) | ||
| Index test (SBx) | Only low risk of bias | 10 | 1.04 (0.94 to 1.15) | |
| Only low applicability concern | 20 | 1.07 (0.99 to 1.15) | ||
| Flow and timing | Only low risk of bias | 17 | 1.10 (1.00 to 1.22) | |
| Additional analyses | MRI positivity threshold | Only threshold 3/5 | 15 | 1.14 (1.03 to 1.26) |
| Population | Biopsy‐naïve (only studies with also prior‐negative biopsy men) | 6 | 0.98 (0.76 to 1.28)b | |
| Prior‐negative biopsy (only studies with also biopsy‐naïve men) | 6 | 1.42 (1.03 to 1.95)b | ||
| Experience of radiologist | Only high experience | 21 | 1.13 (1.03 to 1.24) | |
| CI: confidence interval; G: International Society of Urological Pathology grade; MRI‐pathway: magnetic resonance imaging (MRI) with or without MRI‐targeted biopsy; N: number; QUADAS: Quality Assessment of Diagnostic Accuracy Studies; SBx: systematic biopsy | ||||
| aDetection ratio is the detection rate of the MRI‐pathway divided by detection rate of systematic biopsy; the detection rate is the pooled number of positive results of the test divided by the pooled total number of positive results from both tests. | ||||
| Test | No. of studies | No. of participants |
| 1 Diagnostic accuracy of MRI ‐ G = 1 Show forest plot | 10 | 1764 |
| 2 Diagnostic accuracy of MRI ‐ G ≥ 1 Show forest plot | 10 | 1764 |
| 3 Diagnostic accuracy of MRI ‐ G ≥ 2 Show forest plot | 12 | 3091 |
| 4 Diagnostic accuracy of MRI ‐ G ≥ 3 Show forest plot | 7 | 1438 |
| 5 Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G = 1 Show forest plot | 4 | 834 |
| 6 Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 1 Show forest plot | 4 | 834 |
| 7 Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 2 Show forest plot | 5 | 1083 |
| 8 Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 3 Show forest plot | 4 | 834 |
| 9 Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 1 Show forest plot | 3 | 748 |
| 10 Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 2 Show forest plot | 3 | 748 |
| 11 Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 3 Show forest plot | 3 | 748 |
| 12 Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 1 Show forest plot | 8 | 870 |
| 13 Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 2 Show forest plot | 9 | 1157 |
| 14 Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 3 Show forest plot | 4 | 544 |
| 15 Diagnostic accuracy of MRI ‐ Sensitivity analysis with composite reference standard (template‐guided biopsy + MRI‐TBx) ‐ G ≥ 2 Show forest plot | 11 | 3192 |
| 16 Diagnostic accuracy of TBx ‐ G = 1 Show forest plot | 5 | 497 |
| 17 Diagnostic accuracy of TBx ‐ G ≥ 1 Show forest plot | 6 | 611 |
| 18 Diagnostic accuracy of TBx ‐ G ≥ 2 Show forest plot | 8 | 1553 |
| 19 Diagnostic accuracy of TBx ‐ G ≥ 3 Show forest plot | 3 | 428 |
| 20 Diagnostic accuracy of the MRI‐pathway ‐ G = 1 Show forest plot | 5 | 681 |
| 21 Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 1 Show forest plot | 6 | 844 |
| 22 Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 2 Show forest plot | 8 | 2257 |
| 23 Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 3 Show forest plot | 3 | 604 |
| 24 Diagnostic accuracy of SBx ‐ G = 1 Show forest plot | 4 | 3421 |
| 25 Diagnostic accuracy of SBx ‐ G ≥ 1 Show forest plot | 4 | 3421 |
| 26 Diagnostic accuracy of SBx ‐ G ≥ 2 Show forest plot | 4 | 3421 |
| 27 Diagnostic accuracy of SBx ‐ G ≥ 3 Show forest plot | 2 | 626 |
| 28 MRI‐pathway vs SBx ‐ G = 1 Show forest plot | 21 | 5442 |
| 29 MRI‐pathway vs SBx ‐ G ≥ 1 Show forest plot | 24 | 6524 |
| 30 MRI‐pathway vs SBx ‐ G ≥ 2 Show forest plot | 25 | 6944 |
| 31 MRI‐pathway vs SBx ‐ G ≥ 3 Show forest plot | 21 | 5981 |
| 32 MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G = 1 Show forest plot | 17 | 4079 |
| 33 MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 1 Show forest plot | 19 | 4799 |
| 34 MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 2 Show forest plot | 20 | 5219 |
| 35 MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 3 Show forest plot | 16 | 4306 |
| 36 MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G = 1 Show forest plot | 8 | 1202 |
| 37 MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 1 Show forest plot | 10 | 1564 |
| 38 MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 2 Show forest plot | 10 | 1564 |
| 39 MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 3 Show forest plot | 9 | 1514 |
| 40 MRI‐pathway vs SBx ‐ Positive MRI ‐ G = 1 Show forest plot | 19 | 3460 |
| 41 MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 1 Show forest plot | 20 | 3998 |
| 42 MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 2 Show forest plot | 20 | 3998 |
| 43 MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 3 Show forest plot | 18 | 3902 |
| 44 MRI‐pathway vs SBx ‐ Negative MRI ‐ G = 1 Show forest plot | 19 | 1666 |
| 45 MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 1 Show forest plot | 20 | 1781 |
| 46 MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 2 Show forest plot | 20 | 1781 |
| 47 MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 3 Show forest plot | 18 | 1725 |
| 48 MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G = 1 Show forest plot | 16 | 2682 |
| 49 MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 1 Show forest plot | 17 | 2955 |
| 50 MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 2 Show forest plot | 17 | 2955 |
| 51 MRI‐pathway vs. SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 3 Show forest plot | 15 | 2899 |
| 52 MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G = 1 Show forest plot | 16 | 1287 |
| 53 MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 1 Show forest plot | 17 | 1343 |
| 54 MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 2 Show forest plot | 17 | 1343 |
| 55 MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 3 Show forest plot | 15 | 1297 |
| 56 MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G = 1 Show forest plot | 7 | 655 |
| 57 MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 1 Show forest plot | 8 | 920 |
| 58 MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 2 Show forest plot | 8 | 920 |
| 59 MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 3 Show forest plot | 7 | 880 |
| 60 MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G = 1 Show forest plot | 7 | 341 |
| 61 MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 1 Show forest plot | 8 | 400 |
| 62 MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 2 Show forest plot | 8 | 400 |
| 63 MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 3 Show forest plot | 7 | 390 |

