Productos biológicos o tofacitinib para pacientes con artritis reumatoide que nunca han recibido metotrexato: una revisión sistemática y un metanálisis en red
Referencias
References to included reviews
References to excluded reviews
Additional references
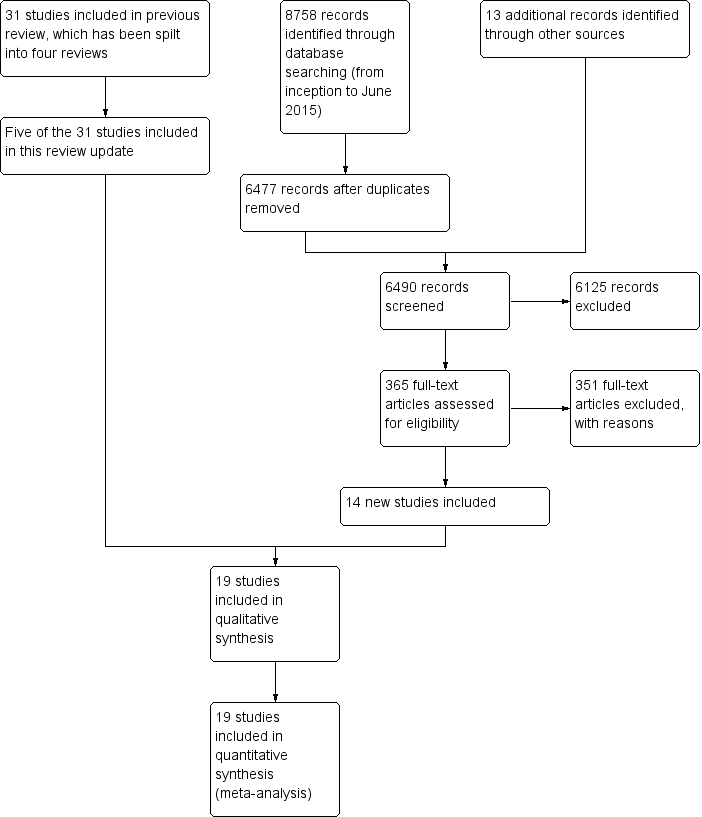
Study flow diagram

Network diagram: ACR50 in people with rheumatoid arthritis who were MTX/other DMARD‐naive
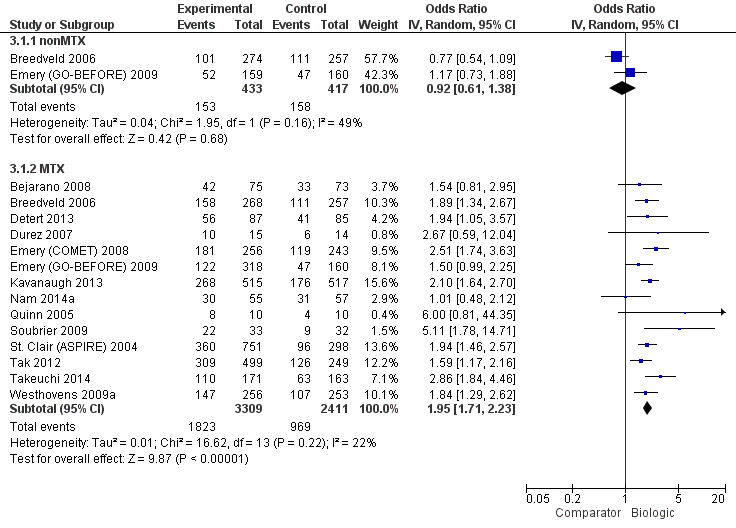
ACR50: biologic (with and without concomitant MTX) versus comparator
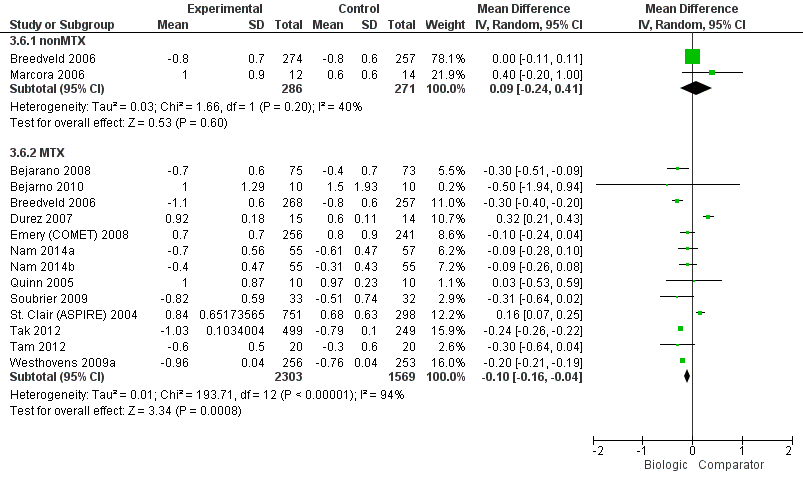
HAQ: biologic (with and without concomitant MTX) versus comparator
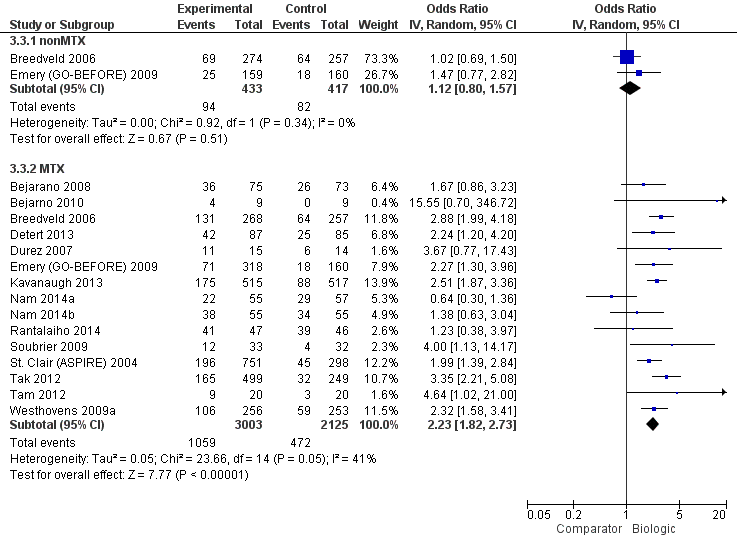
Remission: biologic (with and without concomitant MTX) versus comparator

Radiographic progression: biologic (+MTX) versus comparator

Withdrawals due to adverse events: biologic (with and without concomitant MTX) versus comparator

Serious adverse events: biologic (with and without concomitant MTX) versus comparator
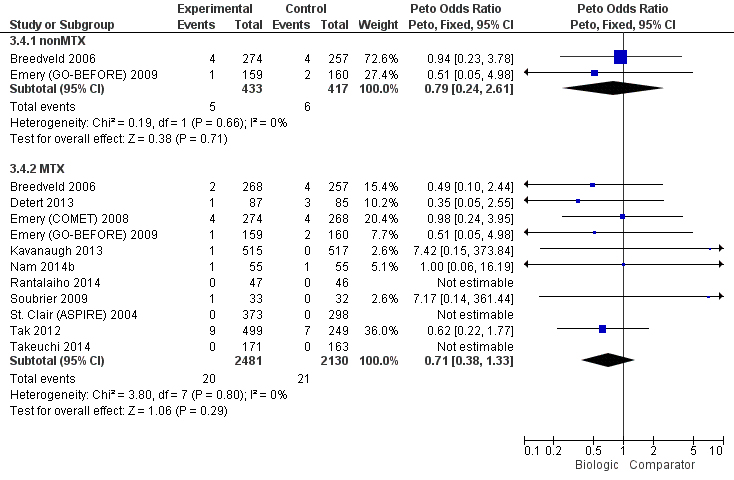
Cancer: biologic (with and without concomitant MTX) versus comparator
| Study name | Biologic(s) | Biologic dose(s) | Number of study arms | Non‐biologic comparator | Concomitant use of MTX | Trial duration | RA duration | Biologic‐naive | Total number of participants |
| Adalimumab | SD | 2 | MTX + PL | Yes | 13 months | Established | Yes | 148 | |
| Infliximab | SD | 2 | MTX + PL | Yes | 8 years | Established | No | 20 | |
| Adalimumab (+/‐ MTX) | SD | 3 | MTX + PL | Yes | 24 months | Established | Yes | 799 | |
| Adalimumab | SD | 2 | MTX + PL | Yes | 12 months | Early | Yes | 172 | |
| Infliximab | SD | 2 | MP + MTX | Yes | 12 months | Early | No | 29 | |
| Emery 2008 (COMET) | Etanercept | SD | 2 | MTX + PL | Yes | 12 months | Established | Yes | 542 |
| Emery 2009 (GO‐BEFORE) | Golilumab (+/‐ MTX) | SD, HD | 4 | MTX + PL | Yes | 12 months | Established | No | 637 |
| Kavanaugh 2013 (OPTIMA) | Adalimumab | SD | 2 | MTX + PL | Yes | 6 months | Established | No | 1032 |
| Etanercept | SD | 2 | MTX | No | 6 months | Unknown | Yes | 26 | |
| Infliximab | SD | 2 | MP + MTX | Yes | 6 months | Early | Yes | 112 | |
| Etanercept | SD | 2 | MTX + PL | Yes | 12 months | Early | Yes | 110 | |
| Infliximab | SD | 2 | MTX + PL | Yes | 12 months | Early | Yes | 20 | |
| Infliximab | SD | 2 | MTX + PL | Yes | 24 months | Early | Yes | 93 | |
| Adalimumab | SD | 2 | MTX + PL | Yes | 12 months | Established | No | 65 | |
| St Clair 2004 (ASPIRE) | Infliximab | SD, HD | 3 | MTX + PL | Yes | 12 months | Early | Yes | 1049 |
| Rituximab | SD, LD | 3 | MTX + PL | Yes | 24 months | Early | Yes | 748 | |
| Adalimumab | SD | 2 | MTX + PL | Yes | 6 months | Early | Yes | 334 | |
| Infliximab | SD | 2 | MTX | Yes | 12 months | Established | Yes | 40 | |
| Abatacept | SD | 2 | MTX + PL | Yes | 24 months | Established | No | 509 | |
| HD: high dose; LD: low dose; MTX: methotrexate; PL: placebo; SD = standard dose | |||||||||
| Comparison | Direct evidence | Network meta‐analysis | ||||||
| Outcome: ACR50 | No. of participants (studies) | RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |
| Biologics + MTX | versus comparator | 5720 (14 studies) | 1.40 (1.30 to 1.49) | 16% (13% to 20%) NNTB = 7 (6 to 8) | ⊕⊕⊕⊖ moderate (downgraded for inconsistency)1 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 850 (2 studies) | 0.94 (0.73 to 1.22) | ‐2% (‐11% to 7%) NNTB = n/a | ⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | 1.00 (0.82 to 1.21) | 0% (‐8% to 9%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 4463 (12 studies) | 1.44 (1.34 to 1.54) | 17% (13% to 21%) NNTB = 6 (5 to 8) | ⊕⊕⊕⊕ high3 | 1.42 (1.30 to 1.54) | 18% (13% to 22%) NNTB = 6 (5 to 8) | ⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) | 1.27 (1.14 to 1.42) | 13% (7% to 19%) NNTB = 8 (6 to 14) | ⊕⊕⊕⊕ high3 | 1.31 (1.11 to 1.52) | 13% (5% to 22%) NNTB = 8 (5 to 22) | ⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Outcome: HAQ score 0‐3 (higher = worse): a measure of function | No. of participants (studies) | Direct evidence | Network meta‐analysis | |||||
| MD (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | MD (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 3872 (13 studies) | ‐0.10 (‐0.16 to ‐0.04) | ‐3.3% (‐5.3% to ‐1.3%) NNTB = 4 (2 to 15) | ⊕⊕⊕⊖ moderate (downgraded for inconsistency)5 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 557 (2 studies) | 0.09 (‐0.24 to 0.41) | 3% (‐8% to 13.7%) NNTB = n/a | ⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | 0.17 (‐0.19 to 0.54) | 5.7% (‐6.3% to 18%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 2615 (11 studies) | ‐0.09 (‐0.26 to 0.07) | ‐3% (‐8.7% to 2.3%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and inconsistency)2,6 | ‐0.08 (‐0.25 to 0.07) | ‐2.7% (‐8.3% to 2.3%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) | ‐0.22 (‐0.26 to ‐0.18) | ‐7.3% (‐8.7% to ‐6%) NNTB = 2 (2 to 3) | ⊕⊕⊕⊖ moderate (downgraded for inconsistency)7 | ‐0.22 (‐0.55 to 0.11) | ‐7.3% (‐18.3% to 3.7%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Outcome: Remission (defined as DAS < 1.6 or DAS28 < 2.6) | No. of participants (studies) | Direct evidence | Network meta‐analysis | |||||
| RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 5128 (15 studies) | 1.62 (1.33 to 1.98) | 15% (11% to 19%) NNTB = 5 (6 to 7) | ⊕⊕⊕⊖ moderate (downgraded for inconsistency)8 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 850 (2 studies) | 1.08 (0.83 to 1.41) | 2% (‐3% to 8%) NNTB = n/a | ⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | 1.02 (0.74 to 1.39) | 1% (‐7% to 11%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 4463 (12 studies) | 1.55 (1.22 to 1.96) | 14% (9% to 19%) NNTB = 7 (5 to 10) | ⊕⊕⊕⊖ moderate (downgraded for inconsistency)9 | 1.62 (1.40 to 1.86) | 18% (12% to 23%) NNTB = 7 (5 to 10) | ⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) | 2.10 (1.45 to 3.04) | 19% (15% to 24%) NNTB = 6 (4 to 9) | ⊕⊕⊕⊖ moderate (downgraded for inconsistency)10 | 1.85 (1.46 to 2.28) | 24% (13% to 35%) NNTB = 6 (4 to 10) | ⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Outcome: Radiographic progression on Sharp/Van der Heijde modification (0‐448 points) | No. of participants (studies) | Direct evidence | Network meta‐analysis | |||||
| MD (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | MD (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 2256 (5 studies) | ‐2.56 (‐6.03 to 0.92) | ‐0.57% (‐1.35% to 0.21%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and inconsistency)2,11 | n/a | ||
| TNF biologic + MTX | versus comparator | 1747 (4 studies) | ‐3.18 (‐6.80 to 0.43) | ‐0.71% (‐1.52% to 959.82%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and inconsistency)2,12 | ‐3.73 (‐5.78 to ‐1.62) | ‐0.83% (‐1.29% to ‐0.36%) NNTB = 3 (3 to 7) | ⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Non‐TNF biologic + MTX | versus comparator | 509 (1 study) | ‐0.43 (‐2.04 to 1.18) | ‐0.22% (‐0.46% to 0.26%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and inconsistency)2,11 | ‐0.42 (‐4.22 to 3.41) | ‐0.09% (‐0.94% to 0.76%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Outcome: Withdrawals due to adverse events | No. of participants (studies) | Direct evidence | Network meta‐analysis | |||||
| RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 5800 (14 studies) | 1.32 (0.89 to 1.97) | 2% (0% to 4%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for inconsistency and imprecision )1,2 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 850 (2 studies) | 1.14 (0.62 to 2.10) | 0% (‐4% to 4%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.93 (0.41 to 1.90) | 0% (‐2% to 3%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 4543 (12 studies) | 1.60 (1.10 to 2.32) | 3% (1% to 4%) NNTH = 35 (17 to 183) | ⊕⊕⊕⊖ moderate (downgraded for imprecision)14 | 1.68 (1.16 to 2.56) | 3% (1% to 5%) NNTH = 31 (14 to 138) | ⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) | 0.56 (0.31 to 1.01) | ‐2% (‐5% to 1%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.56 (0.25 to 1.29) | ‐2% (‐3% to 1%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Outcome: Serious adverse events | No. of participants (studies) | Direct evidence | Network meta‐analysis | |||||
| RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 4850 (12 studies) | 1.05 (0.87 to 1.26) | 1% (‐1% to 3%) NNTH = n/a | ⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 319 (1 study) | 0.46 (0.16 to 1.29) | ‐4% (‐8% to 1%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.52 (0.16 to 1.30) | ‐5% (‐9% to 3%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 3593 (10 studies) | 1.14 (0.92 to 1.42) | 1% (‐1% to 3%) NNTH = n/a | ⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | 1.16 (0.90 to 1.51) | 2% (‐1% to 4%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) | 0.87 (0.64 to 1.18) | ‐1% (‐5% to 2%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.87 (0.57 to 1.34) | ‐1% (‐4% to 3%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Outcome: Cancer (note: Peto OR used but can interpret as RR due to low event rate) | No. of participants (studies) | Direct evidence | Network meta‐analysis | |||||
| RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 4611 (11 studies) | 0.71 (0.38 to 1.33) | 0% (0% to 0%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 850 (2 studies) | 0.79 (0.24 to 2.61) | 0% (‐2% to 1%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.94 (0.25 to 3.18) | 0% (‐1% to 2%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 3863 (10 studies) | 0.77 (0.35 to 1.69) | 0% (0% to 0%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.81 (0.36 to 1.73) | 0% (‐1% to 0%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Non‐TNF biologic + MTX | versus comparator | 748 (1 study) | 0.62 (0.22 to 1.77) | 0% (‐3% to 1%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.64 (0.20 to 2.12) | 0% (‐1% to 1%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Comparator = MTX and/or DMARD GRADE Working Group grades of evidence CI: confidence interval; CrI; credible interval; DAS: Disease Activity Score; DMARD: disease‐modifying anti‐rheumatic drug; MTX: methotrexate; n/a: not available; NNTB/NNTH: number needed to treat for an additional beneficial/harmful outcome; OR: odds ratio; RR: risk ratio; TNF: tumor necrosis factor 1Downgraded for inconsistency: I2= 51%. | ||||||||

