Productos biológicos o tofacitinib para pacientes con artritis reumatoide que nunca han recibido metotrexato: una revisión sistemática y un metanálisis en red
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012657Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 08 mayo 2017see what's new
- Tipo:
-
- Overview
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud musculoesquelética
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
JS ‐ study concept
JS, GW ‐ protocol development
JS, PT, GW, ETG ‐ protocol editing
JS, GW, ETG ‐ data extraction
JS, AM ‐ study quality rating
AH, GW, JS, AM‐ data analysis
JS ‐ first draft of the review and NMA
All authors ‐ revision of the manuscript, and approval of the final version
Sources of support
Internal sources
-
The Oak Foundation, Switzerland.
provide support for The Parker Institute: Musculoskeletal Statistics Unit.
-
Birmingham VA Medical Center, Other.
JAS is also supported by the resources and the use of facilities at the VA Medical Center at Birmingham, Alabama, USA.
External sources
-
Jasvinder Singh, USA.
This work was supported in part by resources provided to the UAB Cochrane NMA Satellite by the Rheumatology division at University of Alabama at Birmingham (UAB). JAS is supported by grants from the Agency for Health Quality and Research Center for Education and Research on Therapeutics (AHRQ CERTs) U19 HS021110, National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS) P50 AR060772 and U34 AR062891, National Institute of Aging (NIA) U01 AG018947, National Cancer Institute (NCI) U10 CA149950, and research contract CE‐1304‐6631 from the Patient Centered Outcomes Research Institute (PCORI). JAS is also supported by the resources and the use of facilities at the VA Medical Center at Birmingham, Alabama, USA.
Declarations of interest
JS ‐ JS has received research grants from Takeda and Savient and consultant fees from Savient, Takeda, Regeneron, Merz, Iroko, Bioiberica, Crealta and Allergan pharmaceuticals, WebMD, UBM LLC and the American College of Rheumatology. JS serves as the principal investigator for an investigator‐initiated study funded by Horizon pharmaceuticals through a grant to DINORA, Inc., a 501 (c)(3) entity. JS is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology and receives arms‐length funding from 36 companies; a member of the American College of Rheumatology's (ACR) Annual Meeting Planning Committee (AMPC); Chair of the ACR Meet‐the‐Professor, Workshop and Study Group Subcommittee; and a member of the Veterans Affairs Rheumatology Field Advisory Committee.
AH ‐ none
AM ‐ none
EG ‐ none
RB ‐ RB is a Principal Investigator and Chair of the Management Committee of the Australian Rheumatology Association Database (ARAD). The Australian Rheumatology Association receives ongoing unrestricted educational grants from Abbvie, AstraZeneca, Bristol‐Myers Squibb, Celgene, Pfizer and Sanofi to support ARAD
LM ‐ none
MSA ‐ research grant from Pfizer. Consultant fees from Pfizer
PT ‐ grants/honoraria from Bristol Myers, Chiltern International, and UCB
GW ‐ research grant and consultant fee from Bristol‐Myers Squibb
Acknowledgements
We thank Shahrzad Noorbaloochi and Tyler Cullis at Boston University for contributing to abstract/title review and data abstraction, and TC for double‐checking the data; Tamara Rader of University of Ottawa for performing the searches.
This work was supported in part by resources provided to the University of Alabama at Birmingham (UAB) Cochrane NMA Satellite by the Rheuamtology division at UAB.
JS is supported by the resources and the use of facilities at the VA Medical Center at Birmingham, Alabama, USA.
RB is funded by an Australian National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 May 08 | Biologics or tofacitinib for people with rheumatoid arthritis naive to methotrexate: a systematic review and network meta‐analysis | Review | Jasvinder A Singh, Alomgir Hossain, Amy S Mudano, Elizabeth Tanjong Ghogomu, Maria E Suarez‐Almazor, Rachelle Buchbinder, Lara J Maxwell, Peter Tugwell, George A Wells | |
Notes
We used risk ratios in the Abstract, 'Summary of findings' table and Plain language summary for ease of interpretation. Throughout the rest of the review and NMA, we used odds ratios derived from the NMA.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Abatacept [therapeutic use];
- Adalimumab [therapeutic use];
- Antibodies, Monoclonal [therapeutic use];
- Antirheumatic Agents [*therapeutic use];
- Arthritis, Rheumatoid [*drug therapy];
- Bayes Theorem;
- Biological Products [*therapeutic use];
- Etanercept [therapeutic use];
- Infliximab [therapeutic use];
- Methotrexate [*therapeutic use];
- Methylprednisolone [therapeutic use];
- Network Meta‐Analysis;
- Piperidines [*therapeutic use];
- Pyrimidines [*therapeutic use];
- Pyrroles [*therapeutic use];
- Randomized Controlled Trials as Topic;
- Rituximab [therapeutic use];
Medical Subject Headings Check Words
Adult; Humans;
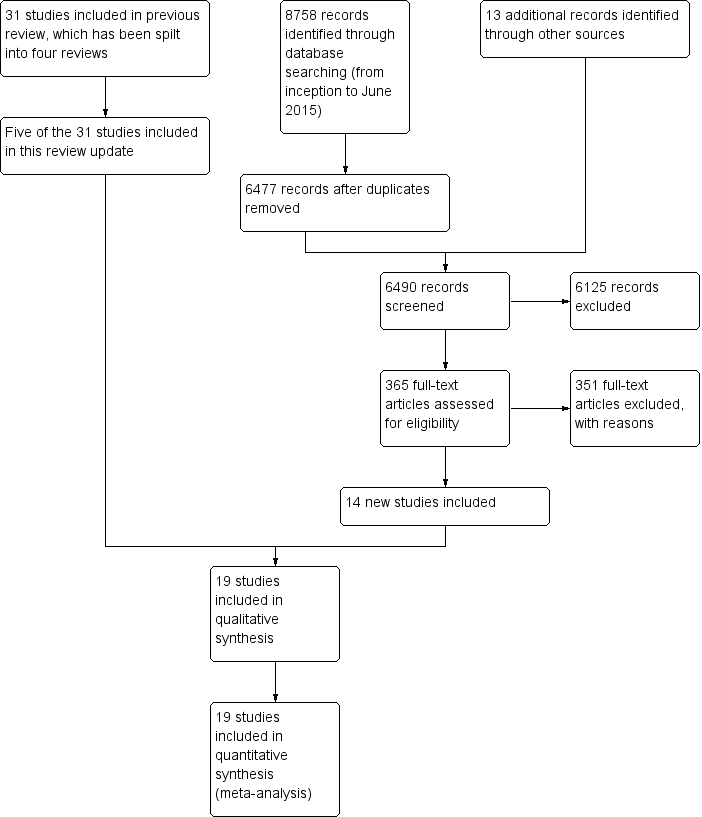
Study flow diagram

Network diagram: ACR50 in people with rheumatoid arthritis who were MTX/other DMARD‐naive
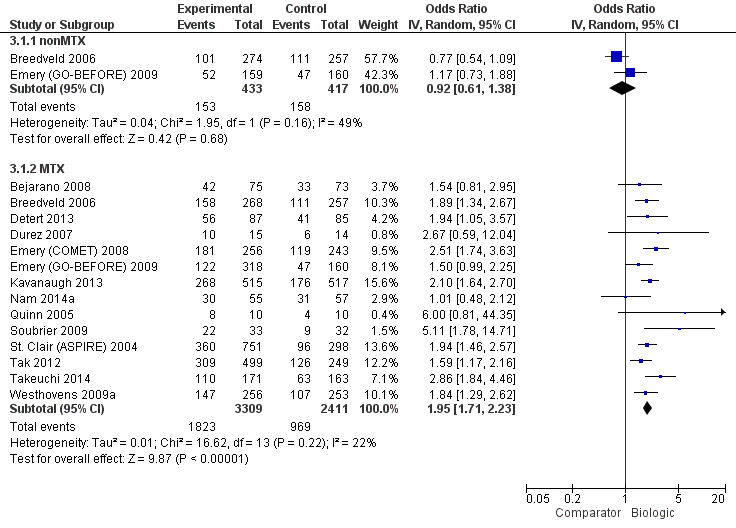
ACR50: biologic (with and without concomitant MTX) versus comparator
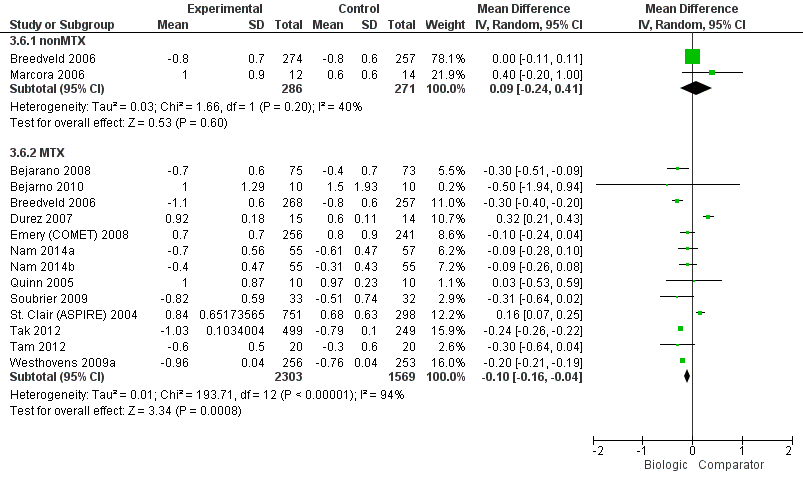
HAQ: biologic (with and without concomitant MTX) versus comparator
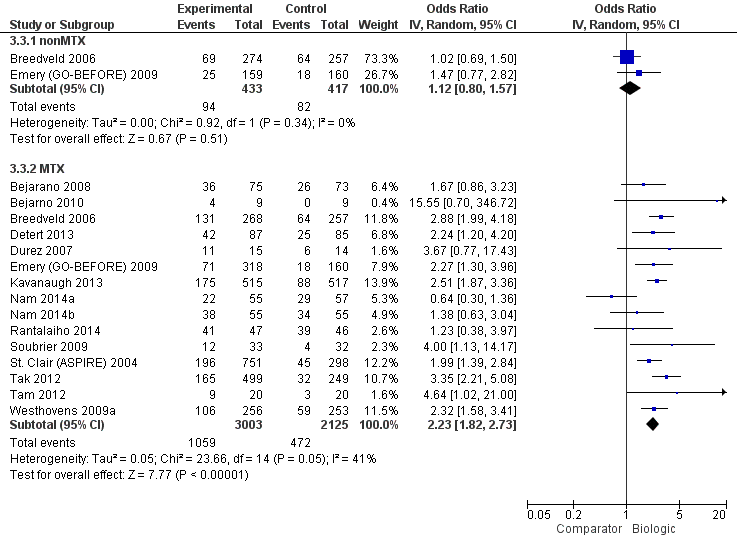
Remission: biologic (with and without concomitant MTX) versus comparator

Radiographic progression: biologic (+MTX) versus comparator

Withdrawals due to adverse events: biologic (with and without concomitant MTX) versus comparator

Serious adverse events: biologic (with and without concomitant MTX) versus comparator
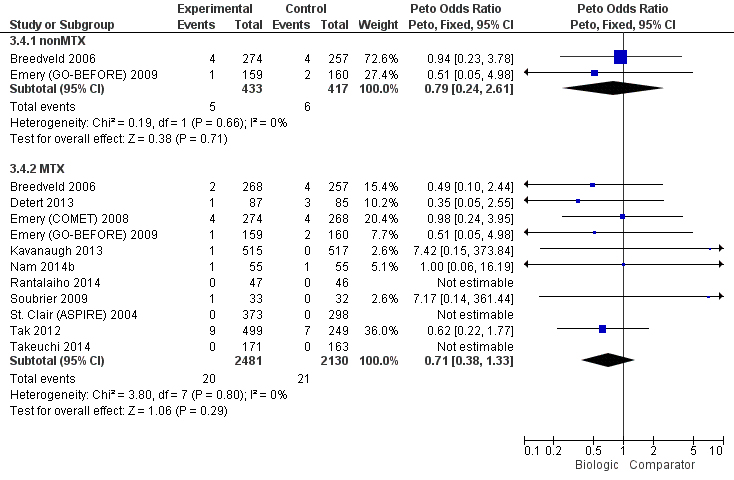
Cancer: biologic (with and without concomitant MTX) versus comparator
| Study name | Biologic(s) | Biologic dose(s) | Number of study arms | Non‐biologic comparator | Concomitant use of MTX | Trial duration | RA duration | Biologic‐naive | Total number of participants |
| Adalimumab | SD | 2 | MTX + PL | Yes | 13 months | Established | Yes | 148 | |
| Infliximab | SD | 2 | MTX + PL | Yes | 8 years | Established | No | 20 | |
| Adalimumab (+/‐ MTX) | SD | 3 | MTX + PL | Yes | 24 months | Established | Yes | 799 | |
| Adalimumab | SD | 2 | MTX + PL | Yes | 12 months | Early | Yes | 172 | |
| Infliximab | SD | 2 | MP + MTX | Yes | 12 months | Early | No | 29 | |
| Emery 2008 (COMET) | Etanercept | SD | 2 | MTX + PL | Yes | 12 months | Established | Yes | 542 |
| Emery 2009 (GO‐BEFORE) | Golilumab (+/‐ MTX) | SD, HD | 4 | MTX + PL | Yes | 12 months | Established | No | 637 |
| Kavanaugh 2013 (OPTIMA) | Adalimumab | SD | 2 | MTX + PL | Yes | 6 months | Established | No | 1032 |
| Etanercept | SD | 2 | MTX | No | 6 months | Unknown | Yes | 26 | |
| Infliximab | SD | 2 | MP + MTX | Yes | 6 months | Early | Yes | 112 | |
| Etanercept | SD | 2 | MTX + PL | Yes | 12 months | Early | Yes | 110 | |
| Infliximab | SD | 2 | MTX + PL | Yes | 12 months | Early | Yes | 20 | |
| Infliximab | SD | 2 | MTX + PL | Yes | 24 months | Early | Yes | 93 | |
| Adalimumab | SD | 2 | MTX + PL | Yes | 12 months | Established | No | 65 | |
| St Clair 2004 (ASPIRE) | Infliximab | SD, HD | 3 | MTX + PL | Yes | 12 months | Early | Yes | 1049 |
| Rituximab | SD, LD | 3 | MTX + PL | Yes | 24 months | Early | Yes | 748 | |
| Adalimumab | SD | 2 | MTX + PL | Yes | 6 months | Early | Yes | 334 | |
| Infliximab | SD | 2 | MTX | Yes | 12 months | Established | Yes | 40 | |
| Abatacept | SD | 2 | MTX + PL | Yes | 24 months | Established | No | 509 | |
| HD: high dose; LD: low dose; MTX: methotrexate; PL: placebo; SD = standard dose | |||||||||
| Comparison | Direct evidence | Network meta‐analysis | ||||||
| Outcome: ACR50 | No. of participants (studies) | RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |
| Biologics + MTX | versus comparator | 5720 (14 studies) | 1.40 (1.30 to 1.49) | 16% (13% to 20%) NNTB = 7 (6 to 8) | ⊕⊕⊕⊖ moderate (downgraded for inconsistency)1 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 850 (2 studies) | 0.94 (0.73 to 1.22) | ‐2% (‐11% to 7%) NNTB = n/a | ⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | 1.00 (0.82 to 1.21) | 0% (‐8% to 9%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 4463 (12 studies) | 1.44 (1.34 to 1.54) | 17% (13% to 21%) NNTB = 6 (5 to 8) | ⊕⊕⊕⊕ high3 | 1.42 (1.30 to 1.54) | 18% (13% to 22%) NNTB = 6 (5 to 8) | ⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) | 1.27 (1.14 to 1.42) | 13% (7% to 19%) NNTB = 8 (6 to 14) | ⊕⊕⊕⊕ high3 | 1.31 (1.11 to 1.52) | 13% (5% to 22%) NNTB = 8 (5 to 22) | ⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Outcome: HAQ score 0‐3 (higher = worse): a measure of function | No. of participants (studies) | Direct evidence | Network meta‐analysis | |||||
| MD (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | MD (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 3872 (13 studies) | ‐0.10 (‐0.16 to ‐0.04) | ‐3.3% (‐5.3% to ‐1.3%) NNTB = 4 (2 to 15) | ⊕⊕⊕⊖ moderate (downgraded for inconsistency)5 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 557 (2 studies) | 0.09 (‐0.24 to 0.41) | 3% (‐8% to 13.7%) NNTB = n/a | ⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | 0.17 (‐0.19 to 0.54) | 5.7% (‐6.3% to 18%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 2615 (11 studies) | ‐0.09 (‐0.26 to 0.07) | ‐3% (‐8.7% to 2.3%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and inconsistency)2,6 | ‐0.08 (‐0.25 to 0.07) | ‐2.7% (‐8.3% to 2.3%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) | ‐0.22 (‐0.26 to ‐0.18) | ‐7.3% (‐8.7% to ‐6%) NNTB = 2 (2 to 3) | ⊕⊕⊕⊖ moderate (downgraded for inconsistency)7 | ‐0.22 (‐0.55 to 0.11) | ‐7.3% (‐18.3% to 3.7%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Outcome: Remission (defined as DAS < 1.6 or DAS28 < 2.6) | No. of participants (studies) | Direct evidence | Network meta‐analysis | |||||
| RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 5128 (15 studies) | 1.62 (1.33 to 1.98) | 15% (11% to 19%) NNTB = 5 (6 to 7) | ⊕⊕⊕⊖ moderate (downgraded for inconsistency)8 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 850 (2 studies) | 1.08 (0.83 to 1.41) | 2% (‐3% to 8%) NNTB = n/a | ⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | 1.02 (0.74 to 1.39) | 1% (‐7% to 11%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 4463 (12 studies) | 1.55 (1.22 to 1.96) | 14% (9% to 19%) NNTB = 7 (5 to 10) | ⊕⊕⊕⊖ moderate (downgraded for inconsistency)9 | 1.62 (1.40 to 1.86) | 18% (12% to 23%) NNTB = 7 (5 to 10) | ⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) | 2.10 (1.45 to 3.04) | 19% (15% to 24%) NNTB = 6 (4 to 9) | ⊕⊕⊕⊖ moderate (downgraded for inconsistency)10 | 1.85 (1.46 to 2.28) | 24% (13% to 35%) NNTB = 6 (4 to 10) | ⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Outcome: Radiographic progression on Sharp/Van der Heijde modification (0‐448 points) | No. of participants (studies) | Direct evidence | Network meta‐analysis | |||||
| MD (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | MD (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 2256 (5 studies) | ‐2.56 (‐6.03 to 0.92) | ‐0.57% (‐1.35% to 0.21%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and inconsistency)2,11 | n/a | ||
| TNF biologic + MTX | versus comparator | 1747 (4 studies) | ‐3.18 (‐6.80 to 0.43) | ‐0.71% (‐1.52% to 959.82%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and inconsistency)2,12 | ‐3.73 (‐5.78 to ‐1.62) | ‐0.83% (‐1.29% to ‐0.36%) NNTB = 3 (3 to 7) | ⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Non‐TNF biologic + MTX | versus comparator | 509 (1 study) | ‐0.43 (‐2.04 to 1.18) | ‐0.22% (‐0.46% to 0.26%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and inconsistency)2,11 | ‐0.42 (‐4.22 to 3.41) | ‐0.09% (‐0.94% to 0.76%) NNTB = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Outcome: Withdrawals due to adverse events | No. of participants (studies) | Direct evidence | Network meta‐analysis | |||||
| RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 5800 (14 studies) | 1.32 (0.89 to 1.97) | 2% (0% to 4%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for inconsistency and imprecision )1,2 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 850 (2 studies) | 1.14 (0.62 to 2.10) | 0% (‐4% to 4%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.93 (0.41 to 1.90) | 0% (‐2% to 3%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 4543 (12 studies) | 1.60 (1.10 to 2.32) | 3% (1% to 4%) NNTH = 35 (17 to 183) | ⊕⊕⊕⊖ moderate (downgraded for imprecision)14 | 1.68 (1.16 to 2.56) | 3% (1% to 5%) NNTH = 31 (14 to 138) | ⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) | 0.56 (0.31 to 1.01) | ‐2% (‐5% to 1%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.56 (0.25 to 1.29) | ‐2% (‐3% to 1%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Outcome: Serious adverse events | No. of participants (studies) | Direct evidence | Network meta‐analysis | |||||
| RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 4850 (12 studies) | 1.05 (0.87 to 1.26) | 1% (‐1% to 3%) NNTH = n/a | ⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 319 (1 study) | 0.46 (0.16 to 1.29) | ‐4% (‐8% to 1%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.52 (0.16 to 1.30) | ‐5% (‐9% to 3%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 3593 (10 studies) | 1.14 (0.92 to 1.42) | 1% (‐1% to 3%) NNTH = n/a | ⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | 1.16 (0.90 to 1.51) | 2% (‐1% to 4%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) | 0.87 (0.64 to 1.18) | ‐1% (‐5% to 2%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.87 (0.57 to 1.34) | ‐1% (‐4% to 3%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Outcome: Cancer (note: Peto OR used but can interpret as RR due to low event rate) | No. of participants (studies) | Direct evidence | Network meta‐analysis | |||||
| RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 4611 (11 studies) | 0.71 (0.38 to 1.33) | 0% (0% to 0%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 850 (2 studies) | 0.79 (0.24 to 2.61) | 0% (‐2% to 1%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.94 (0.25 to 3.18) | 0% (‐1% to 2%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 3863 (10 studies) | 0.77 (0.35 to 1.69) | 0% (0% to 0%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.81 (0.36 to 1.73) | 0% (‐1% to 0%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Non‐TNF biologic + MTX | versus comparator | 748 (1 study) | 0.62 (0.22 to 1.77) | 0% (‐3% to 1%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.64 (0.20 to 2.12) | 0% (‐1% to 1%) NNTH = n/a | ⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Comparator = MTX and/or DMARD GRADE Working Group grades of evidence CI: confidence interval; CrI; credible interval; DAS: Disease Activity Score; DMARD: disease‐modifying anti‐rheumatic drug; MTX: methotrexate; n/a: not available; NNTB/NNTH: number needed to treat for an additional beneficial/harmful outcome; OR: odds ratio; RR: risk ratio; TNF: tumor necrosis factor 1Downgraded for inconsistency: I2= 51%. | ||||||||

