نقش داروهای غیر‐استروئیدی ضد‐التهابی (NSAIDs) خوراکی در مدیریت درد ناشی از سرطان در بزرگسالان
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multicentre, randomised, double‐blind, parallel groups Duration: 7 days (multiple‐dose phase) | |
| Participants | Cancer (mostly genitourinary, lung, breast, GI); pain > 1 week, moderate/severe intensity M 43, F 32 | |
| Interventions | Initial single‐dose phase compared placebo, ketorolac tromethamine, paracetamol + codeine | |
| Outcomes | Daily overall evaluation of medication: 1 to 5 (poor to excellent) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) | Low risk | "identical‐appearing capsules"; each dose consisted of two capsules |
| Blinding of outcome assessment (detection bias) | Low risk | "identical‐appearing capsules"; each dose consisted of two capsules |
| Incomplete outcome data (attrition bias) | High risk | 50% (ketorolac) and 32% (paracetamol + codeine) attrition, imputation method not mentioned for multiple dose phase |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but no obvious omissions |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind (double‐dummy), parallel groups Duration: 2 weeks | |
| Participants | Advanced cancer (mainly digestive, lung, breast, male genitourinary tract), requiring step 1 analgesia | |
| Interventions | Nimesulide 2 x 200 mg daily, n = 34 | |
| Outcomes | Daily: | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) | Low risk | "indistinguishable (hydrosoluble granulated)"; double‐dummy method |
| Blinding of outcome assessment (detection bias) | Low risk | "indistinguishable (hydrosoluble granulated)"; double‐dummy method |
| Incomplete outcome data (attrition bias) | High risk | Imputation not mentioned. Pain reduction reported (as average) for participants remaining in treatment (34/68) |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but no obvious omissions |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind (double‐dummy), parallel groups Duration: 10 days | |
| Participants | Cancer pain > 40/100, (mainly lung, breast, GI, with metastases) not receiving opioids | |
| Interventions | All oral Diclofenac Na (Voltaren) 4 x 50 mg daily, n = 33 | |
| Outcomes | Daily: "Responder" at 2 days: ≥ 50% PI reduction or PI < 40/100 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) | Low risk | "diclofenac, nefopam and ASA tablets were put into identical dragees, while codeine phosphate powder or lactose (placebo) powder was put into sachets"; double‐dummy method |
| Blinding of outcome assessment (detection bias) | Low risk | "diclofenac, nefopam and ASA tablets were put into identical dragees, while codeine phosphate powder or lactose (placebo) powder was put into sachets"; double‐dummy method |
| Incomplete outcome data (attrition bias) | High risk | High levels of withdrawal early in the study, and unclearly defined responder analysis after only one or two days of a 10‐day study |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but no obvious omissions |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Single‐centre, randomised, double‐blind, parallel groups Duration: 6 weeks | |
| Participants | Breast cancer (diagnosis > 100 days) and major depression (DSM‐IV‐TR), with score ≤ 18 (17‐item HDRS) to have mild to mod depression, mild to mod pain, needing analgesic | |
| Interventions | Celecoxib 2 x 200 mg daily, n = 28 (26) | |
| Outcomes | PI: 100 mm VAS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computerized random number generator"; carried out by independent group |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered and sealed packages were used to conceal allocation" |
| Blinding of participants and personnel (performance bias) | Low risk | "The participants, the physician who referred the patients, the physician who prescribed the medications, the rater, and the statistician were all blinded to the allocated treatment. Celecoxib and diclofenac capsules were completely identical in their size, shape, color, texture, and odor" |
| Blinding of outcome assessment (detection bias) | Low risk | "The participants, the physician who referred the patients, the physician who prescribed the medications, the rater, and the statistician were all blinded to the allocated treatment. Celecoxib and diclofenac capsules were completely identical in their size, shape, color, texture, and odor" |
| Incomplete outcome data (attrition bias) | Unclear risk | Imputation not mentioned, but few withdrawals; mean data for pain |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but no obvious omissions |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Multicentre, randomised, double‐blind, cross‐over study Duration: 2 x 7 days (single‐ and multiple‐dose phases) | |
| Participants | Cancer pain, moderate or severe | |
| Interventions | Ketorolac 10 mg (single dose phase, 8 h), then 3 x 10 mg daily | |
| Outcomes | Daily PI: 0 to 4 scale (none to extreme) and 10 cm VAS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) | Low risk | "identical‐looking tablets" |
| Blinding of outcome assessment (detection bias) | Low risk | "identical‐looking tablets" |
| Incomplete outcome data (attrition bias) | Unclear risk | Imputation not mentioned; approximately 10% withdrew < 7 days, mainly for lack of efficacy |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but no obvious omissions |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | Multicentre, randomised, double‐blind (double‐dummy), parallel groups Duration: 7 days | |
| Participants | Cancer (mainly lung, breast, bowel, mouth), PI ≥ 70/100 | |
| Interventions | All oral Dipyrone 3 x 1000 mg daily, n = 41 | |
| Outcomes | Daily PI: 100 mm VAS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Interventions not described, but different dosing schedules accounted for: "In order to keep the double‐blind design of the study, patients in the dipyrone groups were given placebo at 4 a.m., noon and midnight" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Interventions not described, but different dosing schedules accounted for: "In order to keep the double‐blind design of the study, patients in the dipyrone groups were given placebo at 4 a.m., noon and midnight" |
| Incomplete outcome data (attrition bias) | Unclear risk | Imputation not mentioned |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but no obvious omissions |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Multicentre, randomised, double‐blind, parallel groups Duration: 7 days | |
| Participants | Bone cancer pain ≥ 40/100, lasting ≥ 10 days, and pain rating index ≥ 10/20 | |
| Interventions | Dexketoprofen trometamol 4 x 25 mg daily, n = 57 | |
| Outcomes | At 3 and 7 days: | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "predetermined computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) | Low risk | "All the medications were identical‐looking tablets" |
| Blinding of outcome assessment (detection bias) | Low risk | "All the medications were identical‐looking tablets" |
| Incomplete outcome data (attrition bias) | Low risk | WOCF for withdrawals due to AE or LoE, otherwise LOCF for missing values |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but no obvious omissions |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | Multicentre, randomised, single‐blind, parallel groups Duration: 14 days | |
| Participants | Somatic or visceral pain due to advanced cancer (mostly lung, head and neck, colorectal), not treated according to WHO guidelines Mean baseline PI moderate | |
| Interventions | Ketorolac 4 x 10 mg daily, n = 50 | |
| Outcomes | Daily Integrated Score (0 to 240) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) | High risk | Personnel not blinded to treatment. Each week participants self‐assessed pain intensity; total hours asleep, without pain and in pain (= 24); and concomitant symptoms |
| Blinding of outcome assessment (detection bias) | High risk | Personnel not blinded to treatment. Decision to move to step II was "according to the opinion of the experimenting physician" |
| Incomplete outcome data (attrition bias) | Unclear risk | Imputation not mentioned |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but no obvious omissions |
| Size | Unclear risk | 50 participants per treatment arm |
| Methods | Randomised, double‐blind (double‐dummy), cross‐over study Duration: 2 x 1 week | |
| Participants | Advanced cancer (mainly rectum/colon, lung) with pain M 15, F 13 Baseline PI not reported; only reported change in PI with treatment | |
| Interventions | Naproxen 2 x 500 mg daily, n = 28 | |
| Outcomes | PI: VAS (0 to 100) and McGill | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Interventions not described; double‐dummy method used but not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Interventions not described; double‐dummy method used but not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Imputation not mentioned |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but no obvious omissions |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, single‐blind, parallel groups Duration: 14 days, or progression to Step II | |
| Participants | Advanced somatic and/or visceral neoplastic pain (mainly digestive tract) Baseline Integrated Pain Score 42/240 | |
| Interventions | Naproxen Na 2 x 550 mg daily, n = 50 | |
| Outcomes | Daily Integrated Score (0 to 240) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) | High risk | Single‐blind; participant‐reported pain and AEs, but personnel aware of allocation and decision to switch to next step made "at experimenter's discretion" |
| Blinding of outcome assessment (detection bias) | High risk | Single‐blind; participant‐reported pain and AEs, but personnel aware of allocation and decision to switch to next step made "at experimenter's discretion" |
| Incomplete outcome data (attrition bias) | Unclear risk | Imputation not mentioned |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but no obvious omissions |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | Randomised, open, cross‐over, 2 x 7 days with 12‐h washout between | |
| Participants | Cancer (mostly breast, lung, colorectal, stomach) pain > 5/10 | |
| Interventions | Diflunisal 2 x 500 mg daily | |
| Outcomes | PI: VAS (0 to 10) worst pain over last 24 h at baseline and end of study | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) | Unclear risk | Imputation not mentioned |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but no obvious omissions |
| Size | High risk | < 50 participants per treatment arm (evaluated) |
AE: adverse event; DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders‐ fourth edition‐ text revisions; F: female; GI: gastrointestinal; HDRS: Hamilton Depression Rating Scale; Hx: history; LOCF: last observation carried forward; LoE: lack of efficacy; M: male; N: number of participants in study; n: number of participants in treatment arm; NSAID: nonsteroidal anti‐inflammatory drug; PGE: Patient Global Evaluation; PI: pain intensity; PR: pain relief; QoL: quality of life; SD: standard deviation; VAS: visual analogue scale; WOCF: worst observation carried forward.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Each treatment period only 2 days | |
| Intravenous route of administration, postoperative pain. Treatment period only 2 days | |
| Treatment period only 4 days | |
| Only 18 cancer patients in cross‐over study | |
| Fewer than 25 participants per treatment group | |
| Fewer than 25 participants per treatment group | |
| Fewer than 25 participants per treatment group | |
| Fewer than 25 participants per treatment group | |
| Each treatment period only 1 day | |
| Treatment duration only 3 hours | |
| Each treatment period only 1 day. Each participant took 2 active and 1 placebo, or 1 active and 2 placebo single doses over 3 days | |
| Fewer than 25 participants per treatment group | |
| Treatment period only 3 days. Compares two doses of naproxen | |
| Small number of participants (26) with high attrition rates and completer analysis, variable opioid dosing | |
| Single‐dose study | |
| Fewer than 25 participants in control arm (morphine) | |
| Single‐dose study | |
| Treatment period only 2 days | |
| Only 13 participants in the treatment arm. Treatment duration not stated | |
| Single‐dose study | |
| Single‐dose study | |
| Treatment period only 2 days | |
| Fewer than 25 participants in control arm (morphine only) | |
| Single‐dose study | |
| Fewer than 25 participants per treatment group | |
| Intramuscular route of administration. Single‐dose study | |
| Single‐dose study | |
| Treatment duration only 6 hours | |
| Single‐dose study | |
| Intramuscular route of administration, each treatment period only 3 days | |
| Single‐dose study | |
| Fewer than 25 participants in control arm (morphine) | |
| Each treatment period only 3 days. Each participant had 2 active and 1 placebo, or 1 active and 2 placebo periods in addition to constant opioid regimen | |
| Single‐dose study | |
| Each treatment period only 2 days |
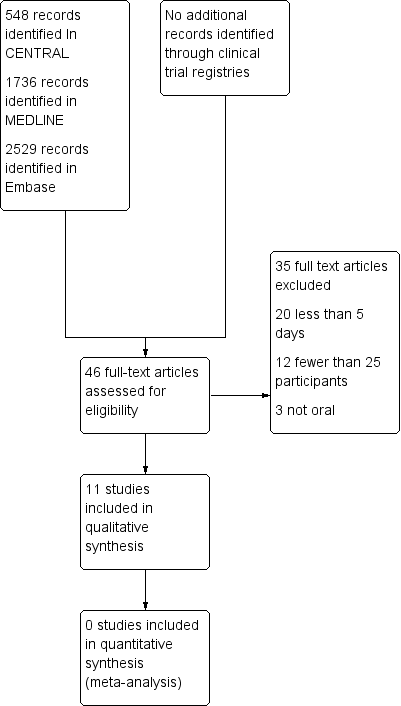
Study flow diagram.
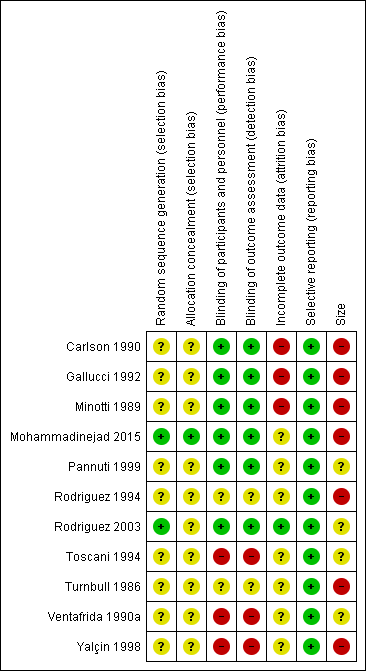
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
| NSAID for cancer pain ‐ non‐controlled data | ||||
| Patient or population: people with cancer pain Settings: inpatient or outpatient Intervention: any NSAID, and dose Comparison: no control ‐ cohort of treated participants | ||||
| Outcomes | Probable outcome with NSAID | No of Participants | Quality of the evidence | Comments |
| Participants with at least 30% or at least 50% reduction in pain | No data | No data | Very low | Limited data, several risks of bias |
| PGIC much or very much improved | No data | No data | Very low | Limited data, several risks of bias |
| Pain no worse than mild at one or two weeks (or equivalent) | Range of estimates from 260 in 1000 to 510 in 1000 | 4 studies 415 participants randomised | Very low | Limited data, several risks of bias |
| Serious adverse events | 2 serious adverse events reported | 11 studies 949 participants | Very low | Limited data, several risks of bias |
| Adverse events | Dry mouth 10% Loss of appetite 4% Somnolence 9% Dyspepsia 9% | Variously reported in studies | Very low | Limited data, several risks of bias |
| Withdrawals | All cause 23% Lack of efficacy 24% Adverse event 5% | Variously reported in studies | Very low | Limited data, several risks of bias |
| Death | 22 deaths, not clearly related to treatment | 11 studies 949 participants | Very low | Limited data, several risks of bias |
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||

