Oralni nesteroidni protuupalni lijekovi za karcinomsku bol u odraslih
Abstract
Background
Pain is a common symptom with cancer, and 30% to 50% of all people with cancer will experience moderate to severe pain that can have a major negative impact on their quality of life. Non‐opioid drugs are commonly used to treat cancer pain, and are recommended for this purpose in the World Health Organization (WHO) cancer pain treatment ladder, either alone or in combination with opioids.
A previous Cochrane review that examined the evidence for nonsteroidal anti‐inflammatory drugs (NSAIDs) or paracetamol, alone or combined with opioids, for cancer pain was withdrawn in 2015 because it was out of date; the date of the last search was 2005. This review, and another on paracetamol, updates the evidence.
Objectives
To assess the efficacy of oral NSAIDs for cancer pain in adults, and the adverse events reported during their use in clinical trials.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase from inception to April 2017, together with reference lists of retrieved papers and reviews, and two online study registries.
Selection criteria
We included randomised, double‐blind, single‐blind, or open‐label studies of five days' duration or longer, comparing any oral NSAID alone with placebo or another NSAID, or a combination of NSAID plus opioid with the same dose of the opioid alone, for cancer pain of any pain intensity. The minimum study size was 25 participants per treatment arm at the initial randomisation.
Data collection and analysis
Two review authors independently searched for studies, extracted efficacy and adverse event data, and examined issues of study quality and potential bias. We did not carry out any pooled analyses. We assessed the quality of the evidence using GRADE and created a 'Summary of findings' table.
Main results
Eleven studies satisfied inclusion criteria, lasting one week or longer; 949 participants with mostly moderate or severe pain were randomised initially, but fewer completed treatment or had results of treatment. Eight studies were double‐blind, two single‐blind, and one open‐label. None had a placebo only control; eight compared different NSAIDs, three an NSAID with opioid or opioid combination, and one both. None compared an NSAID plus opioid with the same dose of opioid alone. Most studies were at high risk of bias for blinding, incomplete outcome data, or small size; none was unequivocally at low risk of bias.
It was not possible to compare NSAIDs as a group with another treatment, or one NSAID with another NSAID. Results for all NSAIDs are reported as a randomised cohort. We judged results for all outcomes as very low‐quality evidence.
None of the studies reported our primary outcomes of participants with pain reduction of at least 50%, and at least 30%, from baseline; participants with Patient Global Impression of Change (PGIC) of much improved or very much improved (or equivalent wording). With NSAID, initially moderate or severe pain was reduced to no worse than mild pain after one or two weeks in four studies (415 participants in total), with a range of estimates between 26% and 51% in individual studies.
Adverse event and withdrawal reporting was inconsistent. Two serious adverse events were reported with NSAIDs, and 22 deaths, but these were not clearly related to any pain treatment. Common adverse events were thirst/dry mouth (15%), loss of appetite (14%), somnolence (11%), and dyspepsia (11%). Withdrawals were common, mostly because of lack of efficacy (24%) or adverse events (5%).
Authors' conclusions
There is no high‐quality evidence to support or refute the use of NSAIDs alone or in combination with opioids for the three steps of the three‐step WHO cancer pain ladder. There is very low‐quality evidence that some people with moderate or severe cancer pain can obtain substantial levels of benefit within one or two weeks.
PICO
Laički sažetak
Oralni nesteroidni protuupalni lijekovi za karcinomsku bol u odraslih
Zaključak
Ne postoje dokazi visoke kvalitete koji potvrđuju da su nesteroidni protuupalni lijekovi koji se uzimaju na usta korisni u liječenju ljudi koji imaju karcinomsku bol (bol povezanu s rakom). Također, nema ni dokaza koji bi pokazali da nisu korisni. Dokazi vrlo niske kvalitete pokazuju da neki ljudi s umjerenom ili ozbiljnom karcinomskom boli imaju znatno smanjenu bol u roku od jednog ili dva tjedna.
Dosadašnje spoznaje
Jedna od dvije ili tri osobe oboljele od karcinoma suočit će se sa boli koja varira u intenzitetu od umjerene do jake. Intenzitet boli se pojačava sa rastom karcinoma. Svjetska zdravstvena organizacija je 1968. preporučila upotrebu lijekova tipa morfina (opijate) za umjerene do jake bolove nastale zbog karcinoma, a lijekove koji nisu opijati, kao što su nesteroidni protuupalni lijekovi, samo za blage do umjerene bolove, ili uz opijate kod onih ljudi koji trpe umjerene do jake bolove. Postoji mnogo različitih vrsta nesteroidnih protuupalnih lijekova (engl. nonsteroidal anti‐inflammatory drugs, NSAID). Uobičajeni NSAID lijekovi su ibuprofen i diklofenak.
Značajke istraživanja
U ovom Cochraneovom sustavnom pregledu pretraženi su svi dokazi o tome koliko dobro su nesteroidni protuupalni lijekovi djelovali (sami ili s lijekovima tipa morfina) kod odraslih osoba s karcinomskom boli. Također smo željeli znati koliko je ljudi imalo nuspojave i koliko su bile ozbiljne.
U travnju 2017. pronađeno je 11 istraživanja sa 949 ispitanika. Uspoređivala su NSAID lijekove sa drugim NSAID lijekovima ili s opioidnim lijekom (morfij ili kodein). Nijedno istraživanje nije proučavalo upotrebu NSAID‐a zajedno s morfinom, iako se u toj kombinaciji često koriste. Istraživanja su bila mala i loše kvalitete. Koristila su različite dizajne i različite načine ispitavanja intenziteta boli. Ishodi važni za ljude s karcinomskom boli često nisu bili praćeni. Testirano je mnogo različitih nesteroidnih protuupalnih lijekova i nije bilo moguće napraviti razumne usporedbe.
Ključni rezultati
Uz NSAID, početna umjerena ili jaka karcinomska bol smanjena je na blagu bol nakon jednog ili dva tjedna kod 1 od 4 (26%) do 1 od 2 (51%) ljudi u četiri istraživanja.
Izvještavanje o nuspojavama bilo je loše. Zabilježene su dvije ozbiljne nuspojave uz uporabu NSAID lijekova, kao i 22 smrti, ali one nisu bile povezane s liječenjem boli. Česte nuspojave bile su žeđ/suha usta (1 od 7; 15%), gubitak apetita (1 od 7; 14%), pospanost (1 od 10; 11%) i žgaravica (1 od 10; 11%). Svaka četvrta osoba prestala je uzimati NSAID jer lijek nije djelovao, a 1 od 20 prestala je zbog nuspojava.
Kvaliteta dokaza
Kvaliteta dokaza bila je vrlo niska. Dokazi vrlo loše kvalitete ukazuju na to da ne možemo biti sigurni o samostalnom učinku NSAID‐a na liječenje boli izazvane karcinomom. Ne znamo ima li korištenje NSAID‐a zajedno s opijatima, kao što su kodein ili morfij, pozitivnog učinka.
Authors' conclusions
Summary of findings
| NSAID for cancer pain ‐ non‐controlled data | ||||
| Patient or population: people with cancer pain Settings: inpatient or outpatient Intervention: any NSAID, and dose Comparison: no control ‐ cohort of treated participants | ||||
| Outcomes | Probable outcome with NSAID | No of Participants | Quality of the evidence | Comments |
| Participants with at least 30% or at least 50% reduction in pain | No data | No data | Very low | Limited data, several risks of bias |
| PGIC much or very much improved | No data | No data | Very low | Limited data, several risks of bias |
| Pain no worse than mild at one or two weeks (or equivalent) | Range of estimates from 260 in 1000 to 510 in 1000 | 4 studies 415 participants randomised | Very low | Limited data, several risks of bias |
| Serious adverse events | 2 serious adverse events reported | 11 studies 949 participants | Very low | Limited data, several risks of bias |
| Adverse events | Dry mouth 10% Loss of appetite 4% Somnolence 9% Dyspepsia 9% | Variously reported in studies | Very low | Limited data, several risks of bias |
| Withdrawals | All cause 23% Lack of efficacy 24% Adverse event 5% | Variously reported in studies | Very low | Limited data, several risks of bias |
| Death | 22 deaths, not clearly related to treatment | 11 studies 949 participants | Very low | Limited data, several risks of bias |
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||
Background
A previous Cochrane review that examined the evidence for nonsteroidal anti‐inflammatory drugs (NSAIDs) or paracetamol, alone or combined with opioids, for cancer pain was withdrawn in 2015 because it was out of date (McNicol 2015); the date of the last search was 2005.
This is one of three reviews on the efficacy and safety of oral non‐opioid medicines to treat cancer pain, in this case focusing on oral NSAIDs in adults. A separate review will examine the efficacy of NSAIDs for cancer pain in children (Cooper 2017). The other review will examine oral paracetamol (acetaminophen) for cancer pain in both adults and children (Wiffen 2017a).
Description of the condition
Cancer is estimated to cause over eight million deaths per annum ‐ approximately 13% of deaths worldwide (IARC 2012). Globally, 32 million people are living with cancer, and detailed information for individual countries is available on the WHO website for the International Agency for Research on Cancer (http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx). In the UK alone in 2014, there were around 350,000 new cases of cancer annually, with around 50% of people surviving for 10 years or more after diagnosis (Cancer Research UK 2016).
Cancer pain is perhaps one of the most feared symptoms associated with the disease. Pain may be the first symptom to cause someone to seek medical advice that leads to a diagnosis of cancer, and 30% to 50% of all people with cancer will experience moderate to severe pain (Portenoy 1999). Pain can occur at any time as the disease progresses, but the frequency and intensity of pain tends to increase as the cancer advances (Portenoy 1999; Van den Beuken‐van Everdingen 2016). For people with advanced cancer, some 75% to 90% will experience pain having a major impact on daily living (Wiffen 2016). Pain had a significant negative correlation with quality of life in people with cancer in China, Japan, and Palestine, for example (Deng 2012; Dreidi 2016; Mikan 2016). A recent systematic review has shown that approximately 40% of patients suffered pain after curative treatment, 55% during cancer treatment, and 66% in advanced disease. Pain related to cancer is frequently described as distressing or intolerable by more than one‐third of patients (Breivik 2009; Van den Beuken‐van Everdingen 2016).
Cancer pain can be the result of the cancer itself, interventions to treat the cancer, and sometimes other underlying pains. Cancer‐related pain is a mosaic of different types of pain generated through different mechanisms. The biology of pain from bone metastasis may well differ from pain due to obstruction of a viscus (an internal organ) or invasion of soft tissue, resulting in differences in responsiveness to analgesics that act via different mechanisms. Prevalence of pain is also linked to cancer type, with head and neck cancer showing the highest prevalence. Age also has an impact, with younger patients experiencing more pain (Prommer 2015). For this review, we will not consider postsurgical pain or specific neuropathic pain conditions.
The current World Health Organization (WHO) cancer pain ladder for adults recommends the use of oral non‐opioid analgesics, including NSAIDs, as the first step on the ladder, with or without an adjuvant (WHO 2017). Non‐opioid analgesics are also to be used on the second and third steps, together with weak or strong opioids. The current National Institute for Health and Care Excellence (NICE) guidelines in the UK advises that non‐opioid analgesics alone be used for treating mild pain (0 to 3 on a 0 to 10 pain scale), together with a weak opioid such as codeine or tramadol for mild to moderate pain (3 to 6), and with a strong opioid such as morphine for severe pain (6 to 10) (NICE 2016). Some authorities have suggested that the second step on the ladder could be removed, and replaced with low doses of strong opioids such as morphine (Twycross 2014).
Description of the intervention
NSAIDs have been prescribed for pain and inflammation for more than 100 years. Salicylic acid and phenazone were produced in a synthetic process in the late 1870s, and salicylic acid, phenazone, and phenacetin were available for the treatment of pain, fever, and inflammation by the turn of the 20th century. The past 60 years has seen the introduction of paracetamol (which is probably a weak NSAID (Hinz 2008)) followed by ibuprofen, diclofenac, and many others (Brune 2004). NSAIDs are used with the aim of providing anti‐inflammatory, antipyretic, and analgesic effects in acute and chronic conditions of pain and inflammation (Dwivedi 2015).
NSAIDs are among the most commonly used analgesics, mostly by prescription for musculoskeletal problems (Laine 2001) or fibromyalgia (Häuser 2012; Wolfe 2014), but also widely used without prescription (Sheen 2002). NSAIDs act by inhibiting the cyclooxygenases (COXs), which are catalysts in the synthesis of prostaglandin. The analgesic and anti‐inflammatory actions of NSAIDs are attributed to the inhibition of cyclooxygenase‐2 (COX‐2), while any anti‐platelet and adverse gastrointestinal effects are attributed to the inhibition of cyclooxygenase‐1 (COX‐1). Many traditional NSAIDs such as ibuprofen are non‐selective.
COX‐2‐selective NSAIDs were therefore developed to reduce adverse gastrointestinal effects, but were later considered to increase the risk of myocardial infarction and stroke (CNT 2014), and some drugs were withdrawn (EMEA 2005; FDA 2004). Whether available drugs increase the risk of cardiovascular effects is a matter of dispute, with the randomised trial evidence pointing to some increased risk for many (CNT 2014), while large‐scale observational studies can point to no increased risk or even a reduced risk of serious harm (Mangoni 2010). There is a fine balance of benefits and risks (Moore 2014b).
How the intervention might work
Anti‐inflammatory, antipyretic, and analgesic effects of NSAIDs are based on the suppression of the COX‐1 and COX‐2 enzymes. By blocking the COX enzymes, vasodilation is reduced and inflammation relieved. Further, the synthesis of prostaglandins is blocked, leading to reduced pain (Dwivedi 2015). NSAIDs block the prostaglandin synthesis as do steroids, but have a different side‐effect profile to steroids. Conventional NSAIDs (aspirin, ibuprofen, diclofenac, indomethacin, naproxen, and piroxicam) block COX‐1 and COX‐2 enzymes to various degrees. Selective COX‐2 inhibiting NSAIDs (celecoxib, etoricoxib) inhibit the COX‐2 enzyme with a 5 to 50 fold selectivity (Brune 2004), and some conventional NSAIDs are selective for COX‐1 (aspirin, for instance).
NSAIDs are responsible for anti‐platelet, gastrointestinal, cardiovascular, renal, and hepatotoxic side effects (Brune 2015). Gastrointestinal adverse events with NSAIDs are the result of blocking of the COX‐1 enzyme, leading to a reduction in mucosal prostaglandin synthesis and its protective effects.
Because there is strong evidence for an important role for increased COX‐2 expression and prostaglandin‐E2 production in colorectal tumorigenesis, drugs that inhibit COX‐2 have been of interest in the potential chemoprevention and therapy of colorectal cancer (Chell 2005; Wender 2015).
Why it is important to do this review
A previous Cochrane review examined the evidence for NSAIDs or paracetamol, alone or combined with opioids, for cancer pain (McNicol 2015), with the last search date in 2005. There have been few subsequent systematic reviews of the evidence. Nabal and colleagues (Nabal 2012) examined the evidence for combinations with opioids, finding little evidence. A review of paracetamol and NSAIDs concluded that the role of these non‐opioid drugs remains controversial (Mercadante 2013).
The evidence of effectiveness of the WHO pain ladder for cancer has been examined several times in the past two decades. These studies report varying degrees of success, typically between 20% and 100% of people with cancer pain achieving good relief (Azevedo São Leão Ferreira 2006; Carlson 2016; Jadad 1995), with some suggesting that as many as 50% of people with cancer pain are undertreated (Deandrea 2008).
In many countries, opioids are severely restricted, if available at all. This means that many people with cancer will have considerable pain and suffering unless non‐opioid analgesics can be used. This review was designed with the intention of informing policy makers such as the WHO on the possible utility of NSAIDs to treat cancer‐related pain. It is hoped that the review will inform patients and carers on the value or otherwise of NSAIDs in this context.
Other relevant Cochrane reviews include an assessment of codeine alone and with paracetamol (Straube 2014), and an evaluation of tramadol alone and with paracetamol (Wiffen 2017b). A number of other reviews have evaluated the evidence for opioids, including buprenorphine (Schmidt‐Hansen 2015a), transdermal fentanyl (Hadley 2013), hydromorphone (Bao 2016), morphine (Wiffen 2016), oxycodone (Schmidt‐Hansen 2015b), and tapentadol (Wiffen 2015).
The standards used to assess evidence in pain trials have changed substantially in recent years, with particular attention being paid to trial duration, withdrawals, and statistical imputation following withdrawal, all of which can substantially alter estimates of efficacy (Moore 2013b). The most important change is the move away from using mean pain scores, or mean change in pain scores, to the number of people who have a large decrease in pain (by at least 50%) (Dworkin 2008; Moore 2013a). Pain intensity reduction of 50% or more correlates with improvements in comorbid symptoms, function, and quality of life generally (Moore 2014a). These standards are set out in the PaPaS author and referee guidance for pain studies of the Cochrane Pain, Palliative and Supportive Care Group (PaPaS 2012).
Three additional issues potentially affect how evidence is evaluated.
The first issue is study size and the overall amount of information available for analysis. There are issues over both random chance effects with small amounts of data, and potential bias in small studies, especially in pain (Dechartes 2013; Dechartres 2014; Fanelli 2017; Moore 1998; Nguyen 2017; Nüesch 2010; Thorlund 2011). Cochrane reviews have been criticised for perhaps over‐emphasising results of underpowered studies or analyses (AlBalawi 2013; Turner 2013). On the other hand, it may be unethical to ignore potentially important information from small studies or to randomise more participants if a meta‐analysis including small, existing studies provided conclusive evidence. In this review, we have therefore chosen to limit analyses to studies with a minimum of 25 participants per treatment group, which we believe has not been done previously.
The second issue is that of study duration. Previous reviews have examined studies of any duration, even in some cases single‐dose studies, or studies lasting one day or less, often with intravenous or intramuscular formulations (McNicol 2015; Mercadante 2013). While short‐term studies and non‐oral formulations may have some relevance in some circumstances, they have little relevance to the vast majority of people with cancer pain who will be treated with oral NSAIDs over weeks, months, or even years. We have therefore chosen to include only studies with five days' duration or longer.
The third issue is that of comparators. Many cancer pain studies involve direct comparisons of one or more formulations of the same drug, as particularly noted for oral morphine (Wiffen 2016). This type of design has limited importance in evaluating the analgesic contribution of a drug, if that is not already well established (McQuay 2005). For that reason, we have limited this review to the two comparators that speak to the efficacy of NSAIDs in cancer pain, namely the comparison of an NSAID versus placebo, and NSAID plus opioid versus the same dose of opioid alone. The latter comparison would be similar to methods used for determining dose‐response of analgesics in acute pain (McQuay 2007), or caffeine as an analgesic adjuvant in acute pain (Derry 2014).
Objectives
To assess the efficacy of oral NSAIDs for cancer pain in adults, and the adverse events reported during their use in clinical trials.
Methods
Criteria for considering studies for this review
Types of studies
To be included, studies had to:
-
be randomised (described as 'randomised' anywhere in the manuscript);
-
ideally be double‐blind, but we included single‐blind or open studies because we expected there to be a limited literature on this important topic, and we wanted to be as inclusive as possible;
-
include a minimum of 25 participants per treatment arm; for cross‐over studies this meant a minimum of 25 participants at the initial randomisation.
-
have a study duration of at least five days of continuous treatment, with outcomes reported at the end of that period.
We excluded non‐randomised studies, studies of experimental pain, case reports and clinical observations. Studies had to be fully published or available as extended abstracts (e.g. from clinical trial websites); we excluded short (usually conference) abstracts as these are often unreliable (PaPaS 2012).
Because dipyrone is known to produce substantial (90% or greater) inhibition of COX‐1 and COX‐2 through its 4‐methyl‐amino‐antipyrine metabolite (Hinz 2007), we have included it in the list of NSAIDs.
Types of participants
We included studies of adults (18 years or older) with cancer pain of any intensity. We did not consider postsurgical pain or specific neuropathic pain conditions.
Types of interventions
Orally administered NSAID for cancer pain where the NSAID alone was compared with placebo or another analgesic (e.g. a different NSAID, paracetamol, or an opioid), or orally administered NSAID combined with an opioid compared with the same dose of opioid alone.
Types of outcome measures
Pain had to be measured using a validated assessment tool. For pain intensity, for example, this could be a 100 mm visual analogue scale (VAS) or 11‐point numerical rating scale (no pain to worst pain imaginable), or a four‐point categorical scale (none, mild, moderate, severe); for pain relief, for example, it could be a 100 mm VAS (no relief to complete relief), or five‐point categorical scale (none, a little, some, a lot, complete or words to that effect). Measures of 30% or greater (moderate) and 50% or greater (substantial) reduction of pain over baseline are recommended outcomes for chronic pain studies from the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) (Dworkin 2008).
A 30% or greater reduction of pain from baseline corresponds to much or very much improved on Patient Global Impression of Change (PGIC), and 50% or greater reduction corresponds to very much or completely improved. We would also use results equivalent to no pain or mild pain, because these are also outcomes acceptable to people with various types of pain (Moore 2013a).
Primary outcomes
-
Number of participants with pain reduction of 50% or greater from baseline.
-
Number of participants with pain reduction of 30% or greater from baseline.
-
Number of participants with pain no worse than mild (Moore 2013a).
-
Number of participants with PGIC of much improved or very much improved (or equivalent wording).
Secondary outcomes
-
Quality of life.
-
Use of rescue medication.
-
Participant satisfaction or preference.
-
Serious adverse events, including death.
-
Other adverse events, particularly reports of effects of treatment on somnolence, appetite, or thirst, because these are of particular interest (Wiffen 2014).
-
Withdrawals due to lack of efficacy, adverse events, or any cause.
Search methods for identification of studies
Electronic searches
We searched the following databases, without language or date restrictions.
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (via CRSO) on 4 April 2017.
-
MEDLINE (via Ovid) from 1946 to 4 April 2017.
-
Embase (via Ovid) from 1974 to 4 April 2017.
We used a combination of MeSH (or equivalent) and text word terms and tailored search strategies to individual databases. The search strategy for CENTRAL, MEDLINE, and Embase are in Appendix 1, Appendix 2, and Appendix 3, respectively.
Searching other resources
We searched the metaRegister of controlled trials in ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) for ongoing and unpublished trials. In addition, we checked reference lists of reviews and retrieved articles for additional studies and performed citation searches on key articles. We did not contact authors for additional information.
Data collection and analysis
Selection of studies
Two review authors (RAM, SD) independently read the abstract of each study identified by the search, eliminated studies that clearly did not satisfy inclusion criteria, and obtained full copies of the remaining studies. Two review authors (RAM, SD) read these studies independently to select relevant studies for inclusion. In the event of disagreement, a third review author (PW) was available to adjudicate. We did not anonymise the studies before assessment. We have included a PRISMA flow chart in the review to show the status of identified studies as recommended in Section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included studies in the review, irrespective of whether measured outcome data were reported in a 'usable' way.
Data extraction and management
Two review authors (RAM, SD) independently extracted data using a standard form and checked for agreement before entry into Review Manager (RevMan) (RevMan 2014). We included information about the number of participants treated and demographic details, type of cancer, drug and dosing regimen, study design (placebo or active control) and methods, study duration and follow‐up, analgesic outcome measures and results, withdrawals and adverse events. We collated multiple reports of the same study, so that each study rather than each report is the unit of interest in the review. We collected characteristics of the included studies in sufficient detail to complete a Characteristics of included studies table.
Assessment of risk of bias in included studies
Two review authors (RAM, SD) independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8, Higgins 2011) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We completed a 'Risk of bias' table for each included study, using the 'Risk of bias' tool in RevMan (RevMan 2014).
We assessed the following for each study.
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated); high risk of bias where study did not conceal allocation (e.g. open list).
-
Blinding of participants and personnel (checking for possible performance bias). We assessed the methods used to blind study participants and personnel from knowledge of which intervention a participant received as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, such as identical tablets matched in appearance or smell, or a double‐dummy technique); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved); high risk of bias (study participants or personnel, or both, not blinded).
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received as: low risk of bias (study had a clear statement that outcome assessors were unaware of treatment allocation, and ideally described how this was achieved); unclear risk of bias (study stated that outcome assessors were blind to treatment allocation but lacked a clear statement on how it was achieved); high risk of bias (outcome assessment not blinded).
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (fewer than 10% of participants did not complete the study or used ‘baseline observation carried forward’ analysis, or both); unclear risk of bias (used 'last observation carried forward' analysis); high risk of bias (used 'completer' analysis).
-
Reporting bias due to selective outcome reporting (reporting bias). We checked if an a priori study protocol was available and if all outcomes of the study protocol were reported in the publications of the study. We assessed the methods used to deal with incomplete data as: low risk of reporting bias if the study protocol was available and all of the study’s prespecified (primary and secondary) outcomes that were of interest in the review were reported in the pre‐specified way, or if the study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon); high risk of reporting bias if not all of the study’s pre‐specified primary outcomes were reported; one or more primary outcomes was reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting was provided, such as an unexpected adverse effect); one or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis; the study report did not include results for a key outcome that would be expected to have been reported for such a study; and unclear risk of bias risk’ of bias if insufficient information is available to permit judgement of ‘Low risk’ or ‘High risk’.
-
Size of study (checking for possible biases confounded by small size (Dechartes 2013; Dechartres 2014; Moore 1998; Nüesch 2010; Thorlund 2011)). We assessed studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (fewer than 50 participants per treatment arm).
Measures of treatment effect
We planned to use dichotomous data to calculate risk difference (RD) or risk ratio (RR) with 95% confidence intervals (CIs) using a fixed‐effect model, and calculate numbers needed to treat for one additional beneficial outcome (NNT) as the reciprocal of the absolute risk reduction (McQuay 1998). For unwanted effects, the number needed to treat (NNT) becomes the number needed to treat for one additional harmful outcome (NNH), and is calculated similarly.
We planned to use the following terms to describe adverse outcomes in terms of harm or prevention of harm.
-
When significantly fewer adverse outcomes occurred with NSAIDs than with control (placebo or active control), we used the term number needed to treat to prevent one event (NNTp).
-
When significantly more adverse outcomes occurred with NSAIDs compared with control (placebo or active control) we used the term number needed to treat for an additional harmful outcome or cause one event (NNH).
We did not plan to use continuous data for the primary outcome because it is inappropriate where there is an underlying skewed distribution, as is usually the case with analgesic response.
Unit of analysis issues
The unit of randomisation was the individual participant.
Dealing with missing data
We planned to use intention‐to‐treat (ITT) analyses: participants who were randomised, took the study medication, and gave a minimum of one post‐baseline assessment. We have reported per‐protocol data in the absence of ITT data.
Assessment of heterogeneity
We planned to assess statistical heterogeneity using L'Abbé plots, a visual method for assessing differences in results of individual studies (L'Abbé 1987), and by use of the I2 statistic. We anticipated that there could be an effect of differences between participants, environment (inpatient versus outpatient), and outcome measures. We planned to explore these with subgroup and sensitivity analyses where there were sufficient data, recognising the difficulties of assessing heterogeneity with small numbers of small studies (Gavaghan 2000; IntHout 2015). In the event, there were insufficient data to assess heterogeneity.
Assessment of reporting biases
We planned to use dichotomous data of known utility (Moore 2010a; Moore 2013a). The review would not depend on what authors of the original studies chose to report or not.
We planned to undertake an assessment of publication bias if there were sufficient data for meta‐analysis, using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean an NNT of 10 or higher) (Moore 2008). In the event, there were insufficient data to assess publication bias.
Data synthesis
We planned to undertake a quantitative synthesis and present data in forest plots if there were sufficient data. In the event of substantial clinical heterogeneity, we would switch off the totals in the forest plots.
-
We would undertake a meta‐analysis only if participants, interventions, comparisons, and outcomes were judged to be sufficiently similar to ensure an answer that is clinically meaningful.
-
We would undertake a meta‐analysis only where there were data from at least two studies and 200 participants for analysis.
-
We planned to use RevMan for meta‐analysis (RevMan 2014) and Excel for NNT and NNH.
In the event, there were insufficient data for pooling of data for any outcome.
Quality of the evidence
We used the GRADE system to assess the quality of the evidence related to the key outcomes listed in Types of outcome measures, as appropriate (Appendix 4). Two review authors (RAM, SD) independently rated the quality of each outcome.
We paid particular attention to inconsistency, where point estimates vary widely across studies or confidence intervals (CIs) of studies show minimal or no overlap (Guyatt 2011), and to the potential for publication bias, based on the amount of unpublished data required to make the result clinically irrelevant (Moore 2008).
In addition, there may be circumstances where the overall rating for a particular outcome needs to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, if there are so few data that the results are highly susceptible to the random play of chance, or if a study uses last observation carried forward (LOCF) imputation in circumstances where there are substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by three levels, to very low quality. In circumstances where there were no data reported for an outcome, we have reported the level of evidence as very low quality (Guyatt 2013b).
In addition, we are aware that many Cochrane reviews are based largely or wholly on small underpowered studies, and that there is a danger of making conclusive assessments of evidence based on inadequate information (AlBalawi 2013; Brok 2009; Roberts 2015; Turner 2013).
'Summary of findings' table
We have included a 'Summary of findings' table as set out in the Pain, Palliative and Supportive Care Review Group author guide (PaPaS 2012) and recommended in Chapter 4.6.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We present the main findings in a simple tabular format, to include key information concerning the quality of evidence, the magnitude of effect of the interventions examined, the sum of available data on the outcomes of at least 30% and at least 50% pain relief, PGIC much or very much improved, adverse events, and serious adverse events. In addition, we have also included other measures of efficacy or harm (quality of life or well‐being at end of treatment, use of rescue medication, patient satisfaction or preference, withdrawals, and death).
For the 'Summary of findings' table we used the following descriptors for levels of evidence (EPOC 2015).
High: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low.
Moderate: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate.
Low: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high.
Very low: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high.
† Substantially different: a large enough difference that it might affect a decision.
Subgroup analysis and investigation of heterogeneity
We planned several possible subgroup analyses, depending on the availability of data.
-
Because we expected that many studies would have a cross‐over design that could impede meta‐analysis (Elbourne 2002), we planned to examine cross‐over and parallel‐group designs separately.
-
We planned to investigate whether subgroup analysis by individual drug or dose was possible.
-
We planned to analyse separately studies with NSAID alone, and NSAID combined with opioid. We anticipated that these studies might also reflect different levels of initial pain intensity.
We planned no other subgroup analyses because the data were expected to be sparse, with small numbers of small trials. In the event, there were insufficient data for any subgroup analysis.
Sensitivity analysis
We did not plan any sensitivity analyses because the data were expected to be sparse, with small numbers of small trials.
Results
Description of studies
Results of the search
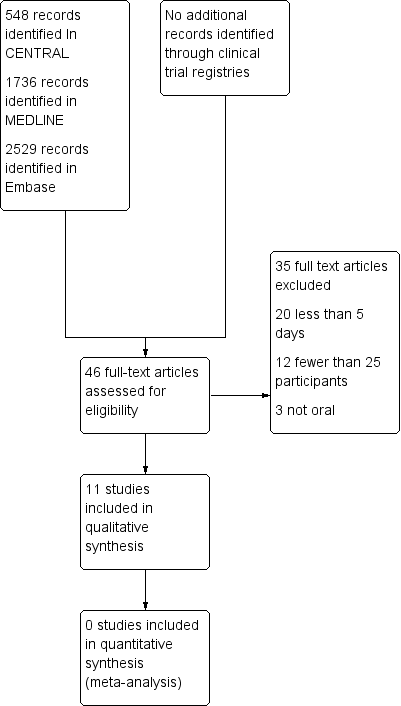
Searches identified 548 articles in CENTRAL, 1736 articles in MEDLINE, 2529 articles in Embase, and no additional relevant reports in clinical trial registries. We reassessed all studies included in the earlier review (McNicol 2015). After screening and assessment of relevant full texts, we included 11 studies and excluded 35 studies (Figure 1).
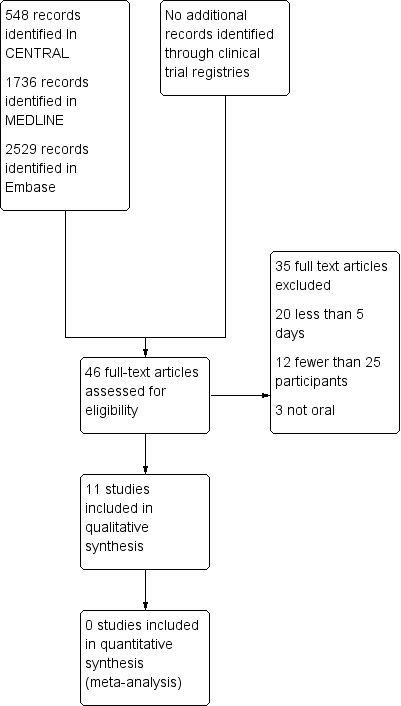
Study flow diagram.
Included studies
We included 11 studies, all of which were randomised Carlson 1990; Gallucci 1992; Minotti 1989; Mohammadinejad 2015; Pannuti 1999; Rodriguez 2003; Rodriguez 1994; Toscani 1994; Turnbull 1986; Ventafrida 1990a; Yalçin 1998). Two were not in the earlier review (Mohammadinejad 2015; Rodriguez 2003). Eight studies were double‐blind, two were single‐blind, and one was open‐label. None had a placebo only control. They initially randomised 949 participants, although not all contributed to outcomes.
Three studies used a cross‐over design with treatment periods of seven days (Pannuti 1999; Turnbull 1986; Yalçin 1998). Yalçin 1998 specified a 12‐hour washout between treatment phases, but there was no washout reported in the other two studies. Eight studies used a parallel‐group design, with treatment periods mostly between seven and 14 days, and one study (primarily investigating depression) lasting six weeks.
No included study compared an NSAID with placebo, or compared an NSAID plus opioid with the same dose of opioid alone.
Nine studies included treatment arms comparing one NSAID with a different NSAID (Gallucci 1992; Minotti 1989; Mohammadinejad 2015; Pannuti 1999; Rodriguez 2003; Toscani 1994; Turnbull 1986; Ventafrida 1990a; Yalçin 1998), one of which was reported in three articles (Gallucci 1992). Three studies compared an NSAID with an opioid (morphine; Rodriguez 1994), or an oral opioid combination (paracetamol plus codeine; Carlson 1990, and aspirin plus codeine; Minotti 1989).
Ten studies included both men and women, with a slight preponderance of men (60% overall). These studies each included participants with various types of cancer, except one that exclusively enrolled participants with bone cancer (Rodriguez 2003). The other study included women with breast cancer (Mohammadinejad 2015). Where reported, the mean age was 52 to 70 years (age range 30 to 89 years).
Most studies required participants to have moderate or severe pain levels at study entry, or reported baseline pain levels of at least moderate intensity. Mohammadinejad 2015 required mild to moderate pain as an entry criterion, and reported mean baseline levels of 60 (± 7)/100. Gallucci 1992 recruited participants who "needed analgesic treatment according to the first step of WHO scale" and reported a mean baseline Integrated Pain Score of 40 to 50, which equates to at least moderate pain (De Conno 1994). Turnbull 1986 reported only changes in pain levels, not absolute values.
Doses of NSAIDs used were within recommended dosing schedules, and doses of oral morphine were 60 mg to 180 mg daily (Rodriguez 1994), and of oral codeine in combination with a non‐opioid were 160 mg or 240 mg daily (Carlson 1990; Minotti 1989).
Further details are in the Characteristics of included studies table.
Excluded studies
We excluded 35 studies, 31 of which were included in the earlier review (Bjorkman 1993; Bosek 1994; Chary 1994; Corli 1993; Dellemijn 1994; Estapé 1990; Ferrer‐Brechner 1984; Frankendal 1973; Johnson 1994; Lauretti 1999; Levick 1988; Lomen 1986; Martino 1976; Minotti 1998a; Minotti 1998b; Moertel 1971; Moertel 1974; Sacchetti 1984; Saxena 1994; Stambaugh 1988a; Stambaugh 1988b; Staquet 1989; Staquet 1993; Strobel 1992; Sunshine 1988; Tonachella 1985; Ventafridda 1975; Ventafridda 1990b; Weingart 1985; Wool 1991; Yalcin 1997), and four were newly identified (Chen 2003; Dutre Souza 2007; Mercadante 2002; Shen 2003).
We excluded studies because the treatment duration was less than five days (20), they had fewer than 25 participants per treatment arm (12), or used a non‐oral route of administration (3).
Other studies had been excluded from the earlier review because the drug had been discontinued due to serious adverse events, and we did not consider these for inclusion.
Risk of bias in included studies
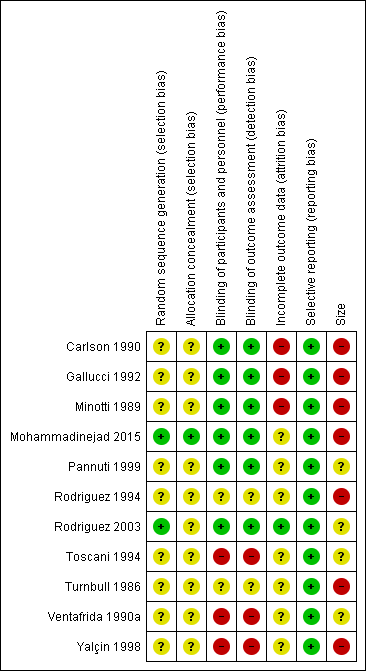
Only two studies were without at least one high risk of bias (Pannuti 1999; Rodriguez 2003; Figure 2).
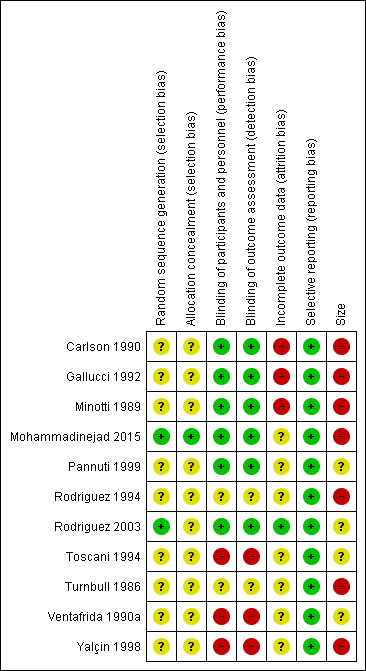
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
All studies were described as randomised, but only two adequately described the method used to generate the random sequence (Mohammadinejad 2015; Rodriguez 2003; low risk of bias). One study adequately described how the allocation of the sequence was concealed (Mohammadinejad 2015; low risk of bias).
Blinding
We judged three studies at high risk of bias for blinding because one was open‐label (Yalçin 1998), and two were single‐blind (Toscani 1994; Ventafrida 1990a). Two studies did not adequately describe the methods of blinding (Rodriguez 1994; Turnbull 1986; unclear risk of bias), and the remainder we judged at low risk.
Incomplete outcome data
We judged three studies at high risk of bias for incomplete outcome data because they did not report how withdrawals were handled in a situation where there were very high levels of attrition (Carlson 1990; Gallucci 1992; Minotti 1989). We judged Rodriguez 2003 at low risk because it used and described an appropriate method of imputation. We judged the remaining studies at unclear risk because they did not adequately report on how withdrawals were handled in analyses.
Selective reporting
Although protocols were not available for any of the included studies, we judged all of the studies to be at low risk of bias because they reported the outcomes stated in their Methods sections. Outcomes reported frequently did not include the efficacy outcomes of interest to this review.
Other potential sources of bias
We judged seven studies at high risk of bias because they had fewer than 50 participants in each treatment arm. The remaining four studies had between 50 and 200 participants per treatment arm, but most groups included fewer than 60 participants and the largest included 72; not all of the participants contributed to outcomes.
Effects of interventions
See: Summary of findings for the main comparison
A summary of results for efficacy outcomes in individual studies is available in Appendix 5, and for rescue medication, adverse events and withdrawals in Appendix 6. summary of findings Table for the main comparison includes several of the main outcomes.
Efficacy
No included study compared an NSAID with placebo, or compared an NSAID plus opioid with the same dose of opioid alone.
Primary outcomes
Few studies reported any of our primary outcomes: participants with pain reduction of at least 50%, and at least 30%, from baseline; participants with pain no worse than mild at the end of the treatment period; participants with PGIC of much improved or very much improved (or equivalent wording).
NSAID versus NSAID
Minotti 1989 defined a "responder" as a participant who experienced ≥ 50% reduction in pain intensity or had pain of < 40/100 at the end of the study, but did not report this or any pain outcome for the intended 10 days of treatment. Non‐responders at two days were withdrawn from the study (pain intensity > 40/100 at two days: 16/33 diclofenac and 14/33 nefopam), and withdrawal rates were typically above 60%.
Pannuti 1999 reported a Patient Global Evaluation of "moderate, good or complete" in 83/128 participants with ketorolac 30 mg daily and 74/129 with diclofenac 150 mg daily after seven days. We judged this equivalent to PGIC of much improved or very much improved (≥ moderate benefit). A Patient Global Evaluation of "good or complete" (≥ substantial benefit) was reported in 34/128 participants with ketorolac 30 mg daily and 33/129 with diclofenac 150 mg daily (26% response rate at seven days with NSAID).
Rodriguez 2003 reported the number of participants with pain intensity < 30/100 at the end of the study; 31/56 with dexketoprofen trometamol 100 mg daily, and 27/57 with ketorolac 40 mg daily. With NSAID, therefore, 58/113 (51%) participants had a pain intensity below 30/100 after seven days.
There were insufficient data to compare one NSAID with another. We assessed this as very low‐quality evidence.
NSAID versus opioid
Minotti 1989 defined a "responder" as a participant who experienced ≥ 50% reduction in pain intensity or had pain of < 40/100 at the end of the study, but did not report this or any pain outcome for the intended 10 days of treatment. Non‐responders at two days were withdrawn from the study (pain intensity > 40/100 at two days: 16/33 diclofenac, 14/33 nefopam, 10/33 aspirin plus codeine), and withdrawal rates were typically above 60%.
Rodriguez 1994 reported the number of participants experiencing at least 50% improvement in pain at five days as 5/41 with dipyrone 3000 mg daily, 15/38 with dipyrone 6000 mg daily, and 10/42 with morphine 60 mg daily. In an overall rating of efficacy, the numbers reporting "good or excellent" (judged equivalent to PGIC of much improved or very much improved) were 16/41 with dipyrone 3000 mg daily, 17/38 with dipyrone 6000 mg daily, and 19/42 with morphine 60 mg daily (42% response rate at seven days with NSAID). The evaluation of pain improvement appeared to show a difference between the two doses of dipyrone (higher was better), but not between the higher dose of dipyrone and morphine. The evaluation of overall efficacy did not replicate this difference.
There were insufficient data to compare one NSAID with an opioid. We assessed this as very low‐quality evidence.
Other pain outcomes
NSAID versus NSAID
Gallucci 1992 reported that in participants who completed two weeks of treatment (43/68), the mean Integrated Pain Score was reduced by 65% with nimesulide 400 mg daily and by 70% with naproxen 1000 mg daily. We estimate that the mean baseline Integrated Pain Score of 40 to 50 was equivalent to moderate pain, and the mean 14‐day score of approximately 15 was equivalent to mild pain (De Conno 1994). It seems likely that most participants who could tolerate treatment had no worse than mild pain at 14 days.
Minotti 1989 reported a physician assessment of therapeutic efficacy of very good in 1/33 (3%) participants with diclofenac and 3/33 (10%) with nefopam.
Mohammadinejad 2015 reported that mean pain was reduced from about 60/100 to about 45/100 after six weeks with both celecoxib 400 mg daily and diclofenac 100 mg daily. It is likely that most participants still had moderate pain levels.
Turnbull 1986 did not report baseline pain levels, but results suggest that pain was reduced by an average of about 15/100 with both naproxen 1000 mg daily and aspirin 3600 mg daily, with very large standard deviations.
Ventafrida 1990a reported that the mean Integrated Pain Score decreased by about 25 points (> halved) in both groups, to 16 with naproxen sodium 1100 mg daily and 17 with diclofenac sodium 200 mg daily. This probably equates to moderate pain reduced to mild pain for most participants as the mean integrated pain score for weeks one and two was equivalent to all‐day slight pain or below (score of 1 for 24 hours = 24).
Yalçin 1998 reported a mean reduction in pain intensity of 4.7/10 with diflunisal 1000 mg daily and 3.3/10 with dipyrone 1500 mg daily, but with large standard deviations.
These results using mean data for the most part indicate that NSAIDs reduce pain to an acceptable level (≤ mild) in a proportion of participants, but the large standard deviations show that the response is variable, and some participants will not achieve good pain relief.
We assessed this as very low‐quality evidence because of high risk of bias and small size.
NSAID versus opioid
Carlson 1990 reported that mean daily pain relief was greater for the combination of paracetamol and codeine than ketorolac over seven days, with statistical significance on the second and fourth days of treatment.
Minotti 1989 reported a physician assessment of therapeutic efficacy of very good occurred in 1/33 participants with diclofenac, 3/33 with nefopam, and 1/33 with codeine plus aspirin.
We assessed this as very low‐quality evidence because of high risk of bias and small size.
Secondary outcomes
None of the studies reported on quality of life or participant satisfaction or preference in cross‐over studies.
Use of rescue medication
Two studies reported on the number of participants who used rescue medication during treatment periods.
In Rodriguez 2003, 40/57 participants taking dexketoprofen trometamol 100 mg daily and 42/58 taking ketorolac 40 mg daily used rescue medication; the median number of tablets (paracetamol + codeine 500 mg/30 mg) taken over the whole study period was three with dexketoprofen and six with ketorolac.
In Rodriguez 1994, 17/41 participants taking dipyrone 3000 mg daily, 11/38 taking dipyrone 6000 mg daily, and 12/42 taking morphine 60 mg daily used rescue medication.
We assessed this as very low‐quality evidence because of high risk of bias and small size.
Harm
Serious adverse events and deaths
Nine studies did not report specifically on serious adverse events.
Rodriguez 2003 reported two serious adverse events, each with dexketoprofen trometamol 100 mg daily and ketorolac 40 mg daily. One event, gastrointestinal haemorrhage with ketorolac was considered treatment‐related.
Turnbull 1986 reported that there were no adverse events during the study.
Deaths were reported separately from adverse events in these studies, probably because most were due to progression of the cancer and not considered relevant to the study drugs.
Twenty‐two deaths were reported in the 11 studies. Gallucci 1992 reported 5/34 deaths with nimesulide and 9/58 with naproxen, Ventafrida 1990a reported 2/50 deaths with naproxen and 2/50 with diclofenac, and Turnbull 1986 reported two deaths, judged unrelated to treatment (group not given). These studies enrolled participants with advanced cancers. Rodriguez 2003 reported one death with dexketoprofen, and Yalçin 1998 reported one death (group not given) due to progression of the disease.
We assessed this as very low‐quality evidence because of high risk of bias and small size.
Specific adverse events
For cancer patients we were particularly interested in somnolence, loss of appetite (or anorexia), and thirst (or dry mouth), which have been highlighted as of concern (Wiffen 2014). Where results were reported at one week and two weeks, we have used the one week data for consistency with other studies.
Turnbull 1986 reported that there were no adverse events with either treatment in 28 participants. While all the remaining studies reported some information on specific adverse events, the categories that were reported (e.g. the most common, those that were of "high intensity") differed, as did the descriptions of the events. It was often difficult to determine which events could be counted together (e.g. somnolence, tiredness, sleepiness, fatigue; or dyspepsia, stomach pain, gastralgia). There were no obvious differences between treatment groups within a study.
There were 47 somnolence events reported in 543 participants (9%) in seven comparisons of NSAID versus NSAID, and 22 amongst 115 participants (19%) taking an opioid (or opioid combination) in three comparisons with NSAIDs.
There were 11 anorexia events reported in 313 participants (4%) in four comparisons of NSAID versus NSAID, and two amongst 82 participants (2%) taking an opioid (or opioid combination) in two comparisons with NSAIDs.
There were 23 dry mouth events reported in 234 participants (10%) in three comparisons of NSAID versus NSAID, and two amongst 40 participants (5%) taking an opioid (or opioid combination) in one comparison with an NSAID.
Since gastrointestinal events are of concern with NSAIDs, we looked for evidence of a difference in frequency between NSAIDs and between NSAIDs and opioids (or opioid combinations). There were no obvious differences between treatment groups within a study.
Dyspepsia or epigastric pain was one of the more common specific gastrointestinal events reported. There were 83 events in 971 participants (9%) in 10 comparisons of NSAID versus NSAID, and nine amongst 115 participants (8%) taking an opioid (or opioid combination) in three comparisons with NSAIDs.
We assessed all evidence on specific adverse events as very low quality because of high risk of bias and small size.
Withdrawals
All‐cause
There were 204 withdrawals for any reason reported amongst 874 participants (23%) in 10 comparisons of NSAID versus NSAID, and 48 amongst 73 participants (66%) taking an opioid (or opioid combination) in two comparisons with NSAIDs. It was not always clear whether reported withdrawals included participants who progressed to step II analgesics. There were no obvious differences between treatment groups.
Lack of efficacy
There were 136 withdrawals due to lack of efficacy reported amongst 567 participants (24%) in 14 comparisons of NSAID versus NSAID, and 17 amongst 73 participants (23%) taking an opioid (or opioid combination) in two comparisons with NSAIDs. There were no obvious differences between treatment groups. Some studies reported lack of efficacy withdrawals, some reported participants progressing to step II (we judged probably due to lack of efficacy), some reported neither, and others reported both. It is difficult to interpret these numbers in a situation where the natural course of the disease is likely to require progression to step II.
Adverse events
Withdrawals due to adverse events were not common; 39 events were reported for 718 participants (5%) in 17 comparisons of NSAID versus NSAID, and 10 events reported for 115 participants taking an opioid (9%) or opioid combination) in three comparisons with NSAIDs. There were no obvious differences between treatment groups, and too few events for analysis.
We assessed all evidence on withdrawals as very low quality because of high risk of bias and small size..
Discussion
Summary of main results
Few studies met the criteria for inclusion, and together with the nature of the efficacy results presented, the results of the available studies for NSAIDs for cancer pain are insufficient to allow any conclusions to be drawn about efficacy or harm. The evidence is insufficient to support or refute their use.
No studies were found for the use of NSAIDs alone in mild or moderate cancer pain, as suggested for the first step of the WHO analgesic ladder (WHO 2017). Included studies typically had initial pain that was moderate or severe in intensity. No studies were found examining the additional analgesic efficacy of NSAIDs combined with strong opioids.
The 11 included studies randomised 949 participants with cancer pain, predominantly of moderate or severe intensity. Studies typically compared one NSAID with another, though a few compared an NSAID with an opioid or opioid combination. In this circumstance no comparative analyses were possible. What was possible was an assessment of the proportion of participants who had a pain score of no or mild pain (or some equivalent measure) around one or two weeks following the start of NSAID treatment. This was highly variable, with assessments of 3% for a physician assessment of very good efficacy (Minotti 1989) or 7% for participant evaluation of complete relief (Pannuti 1999), to 26% for complete or good relief (Pannuti 1999), 52% for overall analgesic efficacy good or excellent (Rodriguez 1994), and 51% for pain intensity of below 30/100 mm on the final day of treatment (Rodriguez 2003). One other study, while providing no comparable results, also reported a mean integrated pain score equivalent to all‐day slight pain or below at one and two weeks (Ventafrida 1990a). This impression of efficacy was also seen in a study excluded for its small size for individual NSAIDs, but which was essentially a randomised cohort study of NSAIDs given to 65 participants with moderate or severe cancer pain (Ventafridda 1990b); pain scores were below 30/100 at one and two weeks for seven of eight NSAIDs tested.
Reporting of harms provided no useful information, and could not be expected to do so given the small size of the studies, individually and together. There are limits on how comparable adverse event information can be, but for particular adverse events, such as somnolence (11%), loss of appetite (14%), or thirst or dry mouth (15%), rates with NSAIDs were similar to those found with opioids (somnolence 23%, anorexia 17%, dry mouth 17%) (Wiffen 2014).
A large number of studies were excluded, mainly because of short treatment duration or their very small size limiting applicability.
Where there are small numbers of small studies, there is a situation where a positive bias in favour of a therapy might be found (Dechartes 2013; Dechartres 2014; Fanelli 2017; Nguyen 2017; Nüesch 2010) even by the random play of chance (Brok 2009; Moore 1998; Thorlund 2011), and overemphasising results of underpowered studies or analyses has been criticised (AlBalawi 2013; Roberts 2015; Turner 2013). Because of the small number of studies and participants in this review, any reported positive effect of NSAIDs in cancer pain should be treated with caution.
NSAIDs are known to have good efficacy in acute postoperative pain (Moore 2015a), migraine (Rabbie 2013), and dysmenorrhoea (Edwards 2004). In chronic pain, they have efficacy in musculoskeletal conditions like arthritis (van Walsem 2015), but not in neuropathic pain (Moore 2015b) or fibromyalgia (Derry 2017).
Overall completeness and applicability of evidence
This review highlights our lack of knowledge about the effectiveness of NSAIDs for cancer pain. The WHO ladder recommends non‐opioid analgesics for all three steps of the WHO ladder (WHO 2017), and NSAIDs in many countries are the mainstay of the first two steps.
Not all of the studies reported on primary outcomes of efficacy known to be important to people with pain (Moore 2013a).
Quality of the evidence
Our GRADE judgement was very low quality for all outcomes. Very low quality means that this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different is very high.
A number of individual studies had high risk of bias for issues such as incomplete outcome data and small size, and none of the trials was unequivocally at low risk of bias for all criteria.
Potential biases in the review process
We are unaware of any biases in the review process. A number of the authors prescribe or have prescribed NSAIDs for cancer pain, or have been involved with its use in people with cancer pain.
Agreements and disagreements with other studies or reviews
Our findings are in broad agreement with a previous Cochrane review (McNicol 2015) and other reviews in adults (Mercadante 2013; Nabal 2012). In this review we included only studies with a minimum treatment period of five days, excluding some studies of short duration in these earlier reviews, but allowing any pain‐relieving effects of NSAIDs that might be seen in clinical practice to emerge. We also excluded very small studies. That meant that we excluded some studies in these previous reviews.
Study flow diagram.
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
| NSAID for cancer pain ‐ non‐controlled data | ||||
| Patient or population: people with cancer pain Settings: inpatient or outpatient Intervention: any NSAID, and dose Comparison: no control ‐ cohort of treated participants | ||||
| Outcomes | Probable outcome with NSAID | No of Participants | Quality of the evidence | Comments |
| Participants with at least 30% or at least 50% reduction in pain | No data | No data | Very low | Limited data, several risks of bias |
| PGIC much or very much improved | No data | No data | Very low | Limited data, several risks of bias |
| Pain no worse than mild at one or two weeks (or equivalent) | Range of estimates from 260 in 1000 to 510 in 1000 | 4 studies 415 participants randomised | Very low | Limited data, several risks of bias |
| Serious adverse events | 2 serious adverse events reported | 11 studies 949 participants | Very low | Limited data, several risks of bias |
| Adverse events | Dry mouth 10% Loss of appetite 4% Somnolence 9% Dyspepsia 9% | Variously reported in studies | Very low | Limited data, several risks of bias |
| Withdrawals | All cause 23% Lack of efficacy 24% Adverse event 5% | Variously reported in studies | Very low | Limited data, several risks of bias |
| Death | 22 deaths, not clearly related to treatment | 11 studies 949 participants | Very low | Limited data, several risks of bias |
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||

