肌肉注射與口服皮質類固醇以減少急性氣喘的急診出院後復發比較
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Search frequency |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify studies for the Cochrane Airways Trials Register
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify studies in other electronic databases
Appendix 2. Search strategy to identify relevant studies from the CAGR
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 prednis*
#6 methylprednis*
#7 dexamethasone
#8 cortisone
#9 hydrocortisone*
#10 medrol
#11 solumedrol
#12 solu‐medrol
#13 betamethasone
#14 triamcinolone
#15 steroid* or corticosteroid* or glucocorticoid*
#16 MeSH DESCRIPTOR Dexamethasone
#17 MeSH DESCRIPTOR Prednisolone Explode All
#18 MeSH DESCRIPTOR Prednisone
#19 MeSH DESCRIPTOR Cortisone
#20 MeSH DESCRIPTOR Injections, Intramuscular
#21 intramuscular* OR intra* NEXT muscular*
#22 IM:ti,ab
#23 injection*
#24 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19
#25 #20 or #21 or #22 or #23
#26 #4 AND #24 AND #25
[Note: in search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, asthma]
Appendix 3. MEDLINE search strategy
Database: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to April 2017>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1. asthma*.mp. or exp Asthma/ [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
2. Respiratory Sounds/
3. wheez*.mp.
4. Bronchial Spasm/
5. bronchospas$.mp.
6. bronchoconstrict*.mp.
7. exp Bronchoconstriction/
8. (bronch$ adj3 constrict$).mp.
9. Bronchial Hyperreactivity/
10. Respiratory Hypersensitivity/
11. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
12. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
13. exp Adrenal Cortex Hormones/
14. exp Glucocorticoids/
15. exp Prednisone/
16. exp Methylprednisolone/
17. corticosteroid*.mp.
18. glucocortico*.mp.
19. cortico‐steroid*.mp.
20. prednis*.mp.
21. deltason.mp.
22. prelone.mp.
23. orapred.mp.
24. pediapred.mp.
25. deltasone.mp.
26. betamethasone.mp.
27. solumedrol.mp.
28. medrol.mp.
29. dexamethasone.mp.
30. methylpred*.mp.
31. solucortef.mp.
32. decadron.mp.
33. triamcinolone.mp.
34. kenalog*.mp.
35. trivaris.mp.
36. or/13‐35
37. exp Injections/ or inject*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
38. (intramuscul* or IM or repository or intra muscul*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
39. 37 or 38
40. oral*.mp.
41. ((drug administration or drug delivery).mp. or exp Drug Administration Routes/) and po.mp.
42. exp Administration, Oral/
43. 40 or 41 or 42
44. (randomi?ed or randomi?ed).ab,ti.
45. placebo*.ti,ab.
46. dt.fs.
47. randomly.ti,ab.
48. trial*.ti,ab.
49. groups.ti,ab.
50. clinical trial/
51. rct.ti,ab.
52. 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51
53. animals/
54. humans/
55. 53 not (53 and 54)
56. 52 not 55
57. 12 and 36 and 39 and 43 and 56
Appendix 4. Embase search strategy
Database: Embase <1974 to 2017 April 17>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1. asthma*.mp. or exp asthma/ [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
2. exp abnormal respiratory sound/
3. wheez*.mp.
4. bronchial spasm/
5. bronchospas$.mp.
6. bronchoconstrict*.mp.
7. exp bronchoconstriction/
8. (bronch$ adj3 constrict$).mp.
9. bronchial hyperreactivity/
10. exp respiratory tract allergy/
11. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
12. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
13. exp acute disease/ or (acute or exacerbat* or emergen*).mp.
14. 12 and 13
15. exp corticosteroids/
16. corticosteroid*.mp.
17. glucocortico*.mp.
18. cortico‐steroid*.mp.
19. prednis*.mp.
20. deltason.mp.
21. prelone.mp.
22. orapred.mp.
23. pediapred.mp.
24. deltasone.mp.
25. betamethasone.mp.
26. solumedrol.mp.
27. medrol.mp.
28. dexamethasone.mp.
29. methylpred*.mp.
30. solucortef.mp.
31. decadron.mp.
32. triamcinolone.mp.
33. kenalog*.mp.
34. trivaris.mp.
35. or/15‐34
36. exp Injection/ or inject*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
37. (intramuscul* or IM or repository or intra muscul*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
38. 36 or 37
39. ((drug administration or drug delivery).mp. or exp drug administration route/) and oral*.ti,ab.
40. ((drug administration or drug delivery).mp. or exp drug administration route/) and po.ti,ab.
41. exp oral drug administration/
42. 39 or 40 or 41
43. clinical trial.pt. or randomi?ed.ab,ti.
44. placebo*.ti,ab.
45. dt.fs.
46. randomly.ti,ab.
47. trial*.ti,ab.
48. groups.ti,ab.
49. controlled clinical trial/
50. rct.ti,ab.
51. 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50
52. animals/
53. humans/
54. 52 not (52 and 53)
55. 51 not 54
56. 14 and 35 and 38 and 42 and 55
Appendix 5. CINAHL search strategy
1. (MH "Asthma+") or (MH "Bronchoconstriction") or (MH "Bronchial Spasm") or asthma* or wheez* or ((bronchia* or respiratory or airway* or lung$) N3 (hypersensitiv* or hyperreactiv* or allerg* or insufficiency or contrict*))
2. (MH "Disease Exacerbation") or (severe or acute* or emergen* or exacerbat*)
3. S1 and S2
4. (MH "Injections, Intramuscular+") or "intra muscular" or "intramuscul* or repository
5. (MH "Administration, Oral+") or (oral N3 (admins*" or delivery or drug or pharmasceutical*))
6. S4 or S5
7. (MH "Adrenal Cortex Hormones+") or glucocorticosteroid* or "cortico steroid*" prednis* or "adrenal cortex hormone*"
8. (corticosteroid* OR "steroids, cortico*" or prelone or orapred or pediapred or deltasone or solumedrol or medrol or betamethasone or dexamethasone or methylpred or solucortef or decahedron or triamcinolone or kenalog or trivaris)
9. S7 OR S8
10. S3 AND S6 AND S9
Appendix 6. Proquest Dissertations and Theses Global search strategy
(all(asthma* AND (emergency OR emergent OR emergencies OR acute* OR exacerbate*)) AND all(corticosteroid* OR "steroids, cortico*" OR corticosteroid* OR prelone OR orapred OR pediapred OR deltasone OR solumedrol OR medrol OR betamethasone OR dexamethasone OR methylpred OR solucortef OR decahedron OR triamcinolone OR kenalog* OR trivaris) AND (oral OR ((im OR po) AND (drug* OR delivery OR administration OR route*)) OR intramuscul*)) AND all(randomiz* OR randomis* OR "clinical trial*" OR randomly OR placebo* OR "controlled trial*" OR RCT*)
Appendix 7. SCOPUS search strategy
1. (asthma*)
2. (emergency OR emergent OR emergencies OR acute* OR exacerbate* )
3. (corticosteroid* OR "steroids,cortico*" OR corticosteroid* OR prelone OR orapred OR pediapred OR deltasone OR solumedrol OR medrol OR betamethasone OR dexamethasone OR methylpred OR solucortef OR decahedron OR triamcinolone OR kenalog* OR trivaris)
4. (randomiz* OR randomis* OR "clinical trial*" OR randomly OR placebo* OR "controlled trial*" OR rct*)
5. ("intra muscul*" OR intramusc* OR inject* OR (im W/5 (drug OR adminst* OR deliver* OR route* ))
6. (oral* OR "by mouth" OR ( op W/5 ( drug OR adminst* OR deliver* OR route* ))
7. 1 AND 2 AND 3 AND 4 AND 5 AND 6
Appendix 8. PROSPERO search strategy
1. (corticosteroid* OR "steroids, cortico*" OR corticosteroid* OR prelone OR orapred OR pediapred OR deltasone OR solumedrol OR medrol OR betamethasone OR dexamethasone OR methylpred OR solucortef OR decahedron OR triamcinolone OR kenalog* OR trivaris)
2. asthma*
3. emergency OR emergent OR emergencies OR acute* OR exacerbate*
4. (oral OR ((im OR po) AND (drug* OR delivery OR administration OR route*)) OR intramuscular* or intra muscular* or repository)
5. #4 and #3 and #1 and #2
6. (randomiz* OR randomis* OR "clinical trial*" OR randomly OR placebo* OR "controlled trial*" OR RCT*)
7. #5 and #6
Appendix 9. Cochrane Library search strategy
1. (asthma* and (emergency or emergent or emergencies or acute* or exacerbate*))
2. (oral or (PO and (drug* or delivery or administration or route*))) and (intramuscul* or "intra muscl*" or (IM and (drug* or delivery or administration or route*)))
3. (corticosteroid* or "steroids, cortico*" or corticosteroid* or prelone or orapred or pediapred or deltasone or solumedrol or medrol or betamethasone or dexamethasone or methylpred or solucortef or decahedron or triamcinolone or kenalog* or trivaris)
4. (randomiz* or randomis* or "clinical trial*" or randomly or placebo* or "controlled trial*" or RCT*)
5. #1 and #2 and #3 and #4
Appendix 10. LILACS search strategy
1. (asthma* and (acute or exacerbat* and emerg*)
2. (oral or (po and (drug* or administration or route* or delivery)))
3. (intramuscular or intra‐muscular or (IM and (drug* or administration or route* or delivery)))
4. (corticosteroid* OR "steroids cortico*" OR "cortico steroid*" OR prelone OR orapred OR pediapred OR deltasone OR solumedrol OR medrol OR betamethasone OR dexamethasone OR methylpred OR solucortef OR decahedron OR triamcinolone OR kenalog* OR trivaris)
5. #1 AND #2 AND #3 AND #4
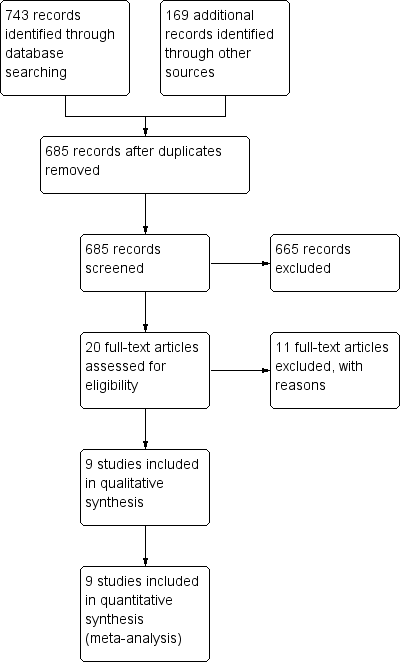
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 1 Relapse.
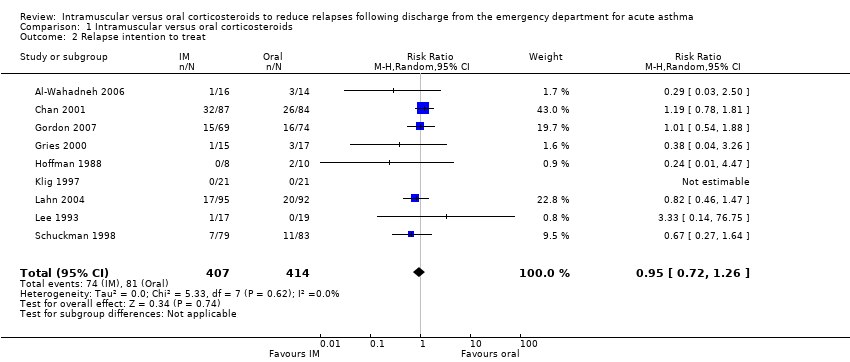
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 2 Relapse intention to treat.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 3 Subgroup analysis: children versus adults.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 4 Subgroup analysis: relapse within 10 days and over 10 days post‐discharge.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 5 Subgroup analysis: mild/moderate versus severe exacerbations.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 6 Sensitivity analysis: risk of bias.
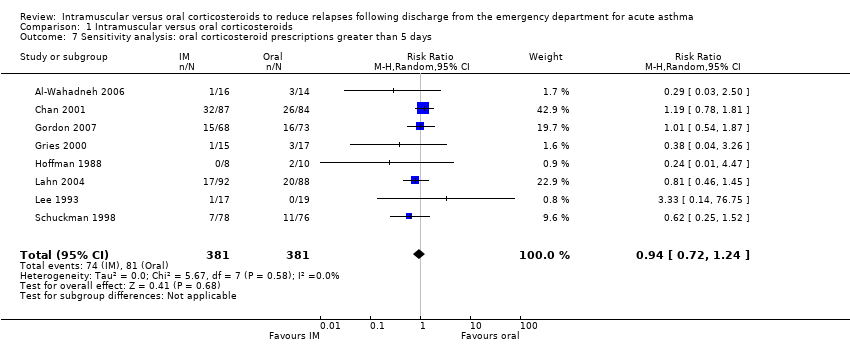
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 7 Sensitivity analysis: oral corticosteroid prescriptions greater than 5 days.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 8 Sensitivity analysis: fixed effects.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 9 Sensitivity analysis: corticosteroids in ED.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 10 Serious adverse events; hospitalization following discharge.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 11 Adverse events.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 12 Adverse events: nausea/vomiting/GI distress.
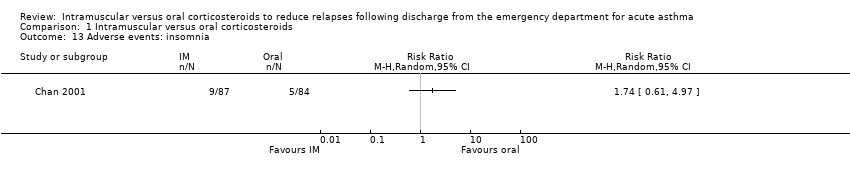
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 13 Adverse events: insomnia.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 14 Adverse events: personality changes.
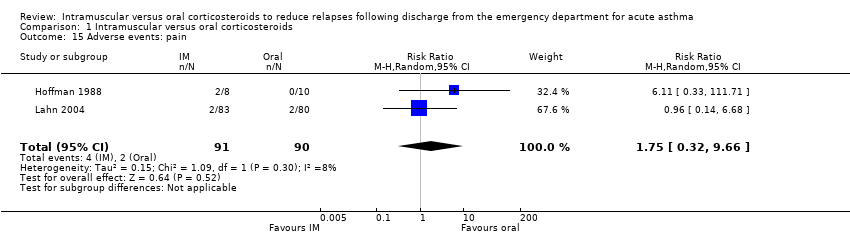
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 15 Adverse events: pain.
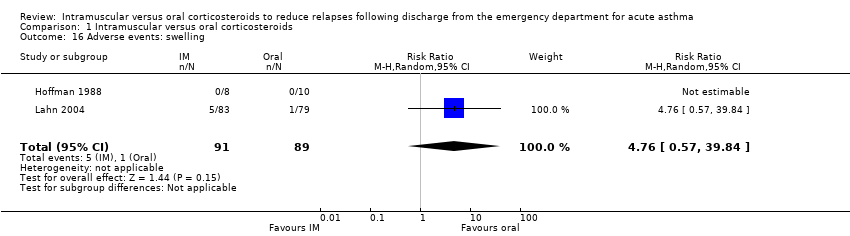
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 16 Adverse events: swelling.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 17 Adverse events: redness.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 18 Pulmonary function: peak expiratory flow (L/min).

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 19 Pulmonary function: FEV₁/FVC (%).

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 20 Symptom persistence.
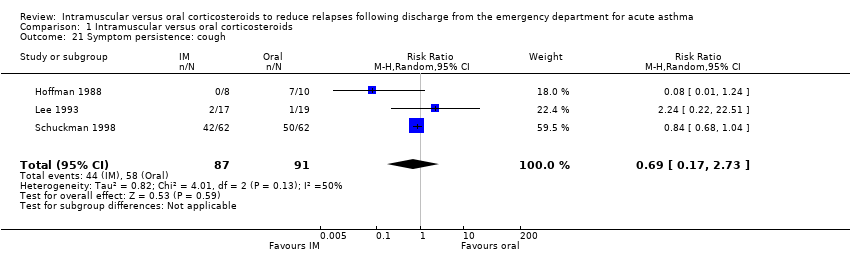
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 21 Symptom persistence: cough.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 22 Symptom persistence: wheezing.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 23 24‐hour beta agonist use.
| Intramuscular corticosteroids compared to Oral corticosteroids for acute asthma | |||||
| Patient or population: patients with acute asthma | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Oral corticosteroids | Intramuscular corticosteroids | ||||
| Relapse | 201 per 1000 | 12 fewer per 1000 | RR 0.94 | 804 | ⊕⊕⊝⊝ |
| Relapse within 10 days post‐discharge | 154 per 1000 | 40 fewer per 1000 (from 75 fewer to 11 more) | RR 0.74 | 742 | ⊕⊕⊕⊝ |
| Relapse occurring after 10 days post‐discharge | 245 per 1000 | 2 fewer per 1000 | RR 0.99 | 556 | ⊕⊕⊝⊝ |
| Adverse events | 294 per 1000 | 50 fewer per 1000 | RR 0.83 | 404 | ⊕⊕⊝⊝ |
| Pulmonary function: Peak expiratory flow | The mean pulmonary function: peak expiratory flow ranged across control groups from | The mean pulmonary function: peak expiratory flow in the intervention groups was | 272 | ⊕⊕⊕⊝ | |
| Symptom persistence | 537 per 1000 | 317 fewer per 1000 | RR 0.41 | 80 | ⊕⊕⊝⊝ |
| 24‐hour beta agonist use | 375 per 1000 | 172 fewer per 1000 | RR 0.54 | 48 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded 1 level for risk of bias. Majority of studies received an unclear risk of bias for random sequence generation and selective outcome reporting | |||||
| Studies | Pulmonary function: Eligibility criteria | Exacerbation severity |
| Severity estimated using modified scoring system based on GINA guidelines. Reported to enrolling patients with mild‐moderate exacerbations, however baseline pulmonary function of the groups was not reported. | Unable to assess | |
| Reported mean baseline PEF greater than 200 L/min: IM group: 270 L/min (SD: 103); oral group: 261 L/min (SD: 104). | Mild/moderate | |
| Reported to enrolling patients identified as moderate exacerbations, however baseline pulmonary function was not reported. | Unable to assess | |
| Applied adapted exacerbation severity score (unspecified). Reported to enrolling patients rated as mild/moderate, however baseline pulmonary function of the groups was not reported. | Unable to assess | |
| Reported baseline mean PEF of enrolled patients of less than 150 L/min: IM group: 129 L/min (SD:14); oral group: 141 L/min (SD: 14). | Severe | |
| Exacerbation severity estimated via pulmonary index score. Study reported to enrolling patients with mild/moderate exacerbations, however baseline pulmonary function was not reported. | Unable to assess | |
| Eligibility criteria required patients to have a PEFR of ≤ 70% predicted with a minimum PEFR of ≥ 40%. Reported PEF of enrolled patients was ≥ 200 L/min: IM group: 205 L/min (SD: 70); oral group: 209 L/min (SD: 72). | Mild/moderate | |
| Reported mean baseline PEF of ≥ 200 L/min: IM group: 210 L/min (SD: 30); oral group: 208 L/min (SD: 26). | Mild/moderate | |
| Reported mean baseline PEF of ≥ 200 L/min: IM group: 243.6 L/min (SD: 64); oral group: 244.7 L/min (SD: 83). | Mild/moderate | |
| Abbreviations: GINA = Global Initiative for Asthma; PEF = Peak expiratory flow; PEFR = Peak expiratory flow rate; IM = intramuscular; SD = standard deviation | ||
| Studies | Location/setting | Co‐interventions | Corticosteroid doses and durations | Methyprednisolone equivalency | Relapse outcome |
| Jordan, ED | Provided in ED: not stated Provided at discharge: SABA | Dexamethasone (IM) 1.7 mg/kg Mean dose: 24 mg Single dose Prednisolone (oral) 2 mg/kg/day for 5 days Mean dose: 19.2 mg per day Total dose: 96 mg | IM Methylprednisone equivalency: 120 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 76.8 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 1/16 Day 21 Oral group 3/14 Day 21 | |
| Canada, ED | Provided in ED: SABA, methylxanthines, supplemental oral/IV corticosteroids Provided at discharge: Methylxanthines, unspecified inhaled beta₂‐agonists, and ICS | Betamethasone (IM) 12 mg Single dose. Received placebo capsules over 7 days. Prednisone (oral) 50 mg a day for 7 days. Received a single placebo injection Total dose: 350 mg | IM Methylprednisone equivalency: 72 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 280 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 12/86 Day 7 Oral group 19/82 Day 7 | |
| United States, Pediatric ED | Provided in ED: SABA, ipratropium bromide. IV corticosteroids for patients who vomited oral corticosteroids. Provided at discharge: Inhaled beta₂‐agonists and ICS | Dexamethasone (IM) 0.6 mg/kg (max 16 mg) Single dose Prednisolone (oral) 2 mg/kg (max 50 mg) daily for 5 days Total: 250 mg | IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12‐36 hours) Oral Methylprednisone equivalency: 200 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 8/69 Day 4 Oral group 11/73 Day 4 IM group 15/68 Day 14 Oral group 16/73 Day 14 | |
| United States, Tertiary medical center | Provided in ED: SABA Provided at discharge: SABA | Dexamethasone (IM) Patients 6 to 12 months old received 16 mg. Patients 13 to 35 months old received 24 mg. Children ≥ 36 months received 36 mg. Single dose Prednisone (oral) 2 mg/kg a day for 5 days Total dose: unclear | IM Methylprednisone equivalency: Unable to assess Oral Methylprednisone equivalency: Unable to assess | IM group 1/15 Day 28 Oral group 3/17 Day 28 | |
| United States, ED | Provided in ED: SABA, epinephrine, methylxanthines, IV corticosteroids Provided at discharge: Methylxanthine and inhaled beta₂‐agonists | Methylprednisonlone (IM) 80 mg Single dose. Received placebo capsules for 7 days Methylprednisolone (oral) Tapering dose over 7 days. Total dose: 216 mg Received placebo injection | IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 216 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 0/8 day Day 7 Oral group 2/10 Day 7 | |
| United States, Pediatric ED | Provided in ED: SABA Provided at discharge: SABA | Dexamethasone (IM) 0.3 mg/kg Total dose: 15 mg Single dose Prednisone (oral) 2 mg/kg a day for 3 days Total dose: 100 mg | IM Methylprednisone equivalency: 75 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 0/21 Day 5 Oral group 0/21 Day 5 | |
| United States, ED | Provided in ED: inhaled beta₂‐agonists, IV corticosteroids Provided at discharge: SABA | Methylprednisolone (IM) 160 mg Single dose. Received placebo capsules for 8 days Methylprednisolone (oral) Tapering dose over 8 days. Total dose: 160 mg (tapering dose 32 mg day 1). Received placebo injection | IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 13/92 Day 10 Oral group 12/88 Day 10 IM group 17/92 Day 21 Oral group 20/88 Day 21 | |
| Taiwan, ED | Provided in ED: SABA, methylxanthines Provided at discharge: Methylxanthine and inhaled beta₂‐agonists | Dexamethasone (IM) 10 mg Single dose. Received placebo capsules for 7 days Dexamethasone (oral) Tapering dose over 7 days. 11.75 mg total. Received placebo injection | IM Methylprednisone equivalency: 50 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 58.8 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 1/17 Day 7 Oral group 0/19 Day 7 | |
| United States, ED | Provided in ED: SABA, oral/IV corticosteroids Provided at discharge: SABA, antibiotics, ICS, cromolyn sodium, ipratropium bromide | Triamcinolone (IM) 40 mg Single dose. Received placebo capsules for 5 days Prednisone (oral) 40 mg a day for 5 days. Total dose: 160 mg Received placebo injection | IM Methylprednisone equivalency: 40 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 160 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 7/78 Day 7 Oral group 11/76 Day 7 | |
| ED = emergency department; SABA = short‐acting beta₂‐agonists; LABA = long‐acting beta₂‐agonists; IV = intravenous; IM = intramuscular; ICS = inhaled corticosteroids | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse Show forest plot | 9 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 2 Relapse intention to treat Show forest plot | 9 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.72, 1.26] |
| 3 Subgroup analysis: children versus adults Show forest plot | 9 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 3.1 Children | 4 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.48, 1.53] |
| 3.2 Adults | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 4 Subgroup analysis: relapse within 10 days and over 10 days post‐discharge Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Within 10 days post‐discharge | 7 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.07] |
| 4.2 Greater than 10 days post‐discharge | 5 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.33] |
| 5 Subgroup analysis: mild/moderate versus severe exacerbations Show forest plot | 5 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.32] |
| 5.1 Mild/moderate exacerbations | 4 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.71, 1.34] |
| 5.2 Severe exacerbations | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.01, 4.47] |
| 6 Sensitivity analysis: risk of bias Show forest plot | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 7 Sensitivity analysis: oral corticosteroid prescriptions greater than 5 days Show forest plot | 8 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 8 Sensitivity analysis: fixed effects Show forest plot | 9 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.69, 1.19] |
| 9 Sensitivity analysis: corticosteroids in ED Show forest plot | 5 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.45, 1.29] |
| 10 Serious adverse events; hospitalization following discharge Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Adverse events Show forest plot | 5 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.64, 1.07] |
| 12 Adverse events: nausea/vomiting/GI distress Show forest plot | 3 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.09, 3.59] |
| 13 Adverse events: insomnia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14 Adverse events: personality changes Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 15 Adverse events: pain Show forest plot | 2 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.32, 9.66] |
| 16 Adverse events: swelling Show forest plot | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 4.76 [0.57, 39.84] |
| 17 Adverse events: redness Show forest plot | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 13.5 [0.77, 235.63] |
| 18 Pulmonary function: peak expiratory flow (L/min) Show forest plot | 4 | 272 | Mean Difference (IV, Random, 95% CI) | ‐7.78 [‐38.83, 23.28] |
| 19 Pulmonary function: FEV₁/FVC (%) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20 Symptom persistence Show forest plot | 3 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.20] |
| 21 Symptom persistence: cough Show forest plot | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.17, 2.73] |
| 22 Symptom persistence: wheezing Show forest plot | 3 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.14, 2.52] |
| 23 24‐hour beta agonist use Show forest plot | 2 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.37] |

