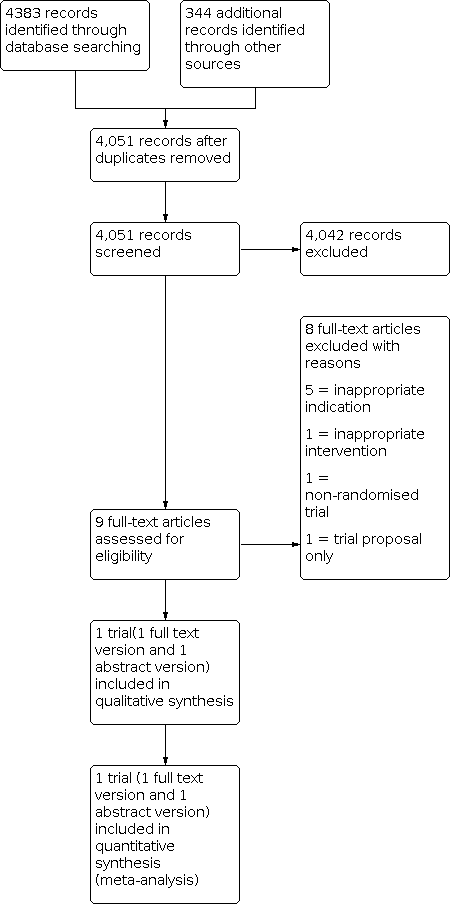
Interventions for preventing distal intestinal obstruction syndrome (DIOS) in cystic fibrosis
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | ||
| Methods | Study design: randomised controlled trial, double‐blind placebo‐controlled Study grouping: cross‐over, each arm lasted for 6 months Carry‐over treatment effect accounted for: yes. Measurements were recorded twice for each 6‐month period. Investigators took an average of the 2 measurements, they then discarded the measurements from the first 3 months, to account for any cumulative effect of the drug. There was no formal washout period | |
| Participants | Inclusion criteria: diagnosis of CF (by sweat test > 60 mmol/L); 1 or more episodes of DIOS in preceding 12 months (based on clinical or radiological evidence and on successful treatment with intestinal lavage, oral N‐acetylcysteine or both) Exclusion criteria: peptic ulcer disease, inflammatory bowel disease, anatomic intestinal obstruction, serious cardiovascular, neurological, renal or hepatic disease, severe pulmonary dysfunction (maximal mid‐expiration flow rate < 25% normal) pregnancy, regular use of metoclopramide, domperidone or anticholinergic drug Pre‐treatment: no significant differences in the clinical characteristics of 2 groups (cross‐over) Baseline characteristics Age, mean (SD), range: 21.0 (5.9) years, 12.9 to 34.9 years old Mean (SD) duration of DIOS symptoms: 4.2 (3.1) years Mean (SD) height percentile: 40.6 (26.3) Mean (SD) weight percentile: 40.3 (23.2) Mean (SD) weight for height: 100.3 (9.5) % of ideal Mean (SD) FVC (% predicted): 80.9 (23.4)% Mean (SD) FEV1 (% predicted): 78.2 (28.1)% | |
| Interventions | Cisapride: oral tablets 7.5 mg 3 times daily (before meals) for participants weighing between 40 kg and 50 kg, 10 mg 3 times daily (before meals) for participants weighing over 50 kg Placebo (identical in appearance to active treatment): oral tablets 7.5 mg 3 times daily (before meals) for participants weighing between 40 kg and 50 kg, 10 mg 3 times daily (before meals) for participants weighing over 50 kg | |
| Outcomes | Gastrointestinal symptoms: a total of 10 symptoms scored 1 to 10 (heartburn, flatulence, regurgitation, fullness, abdominal distension, abdominal pain, diarrhoea, nausea, vomiting, anorexia) and also added to give a total score (20 to 100) where a lower score is better; severity (none, not limiting daily activities, limits daily activities, daily activities not possible) and frequency (never, less than once a week, more than once a week or daily) of symptoms recorded and accounted for in final score for each item Anthrompometric measurements: mid‐arm circumference; skin fold thickness; change in weight; abdominal circumference Alteration in global symptoms: measured by both physician and participant, from 'worse' to 'symptom‐free' using scores from ‐1 to +3 (higher is better) Pulmonary function: FEV1 % predicted, frequency of pulmonary exacerbations, number of hospital admissions Nutrient intake: calories (kcal/day), fat (mmol/day) Stool composition: stool weight (g/day), faecal water content (%), faecal fat (%), calories malabsorbed (%), faecal bile acids (mmol/day), faecal chymotrypsin (10 x 300 units/day) Supine abdominal radiography Intestinal lavage therapy | |
| Identification | Sponsorship source: supported by Janssen Pharmaeutica Inc., Canada. No sponsorship mentioned Country: Canada Setting: single centre (tertiary centre) at the University of Toronto and the research institute at the Hospital for Sick Children, Toronto Comments: approved by the human subjects review committee of the Hospital for Sick Children, Toronto. Janssen Pharmaceutica supplied cisapride and placebo tablets Contact author's name: Sibylle Koletzko Institution: Children's Hospital, Heinrich Heine University, Dusseldorf, Federal Republic of Germany Email: [email protected]‐muenchen.de Address: Lindwurmstraße 4, 80337 München, Germany The authors did not declare any conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated but no specific sequence generation methodology described. |
| Allocation concealment (selection bias) | Unclear risk | No method described for concealment of allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind" trial. Placebo tablets were identical in taste and appearance to cisapride. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Only 3 outcomes stated that the investigators were blinded. 1. Gastrointestinal symptom scores ‐ the blinded participants acted as their own assessors. 2. Assessment of supine abdominal radiographs ‐ a paediatric radiologist judged these in a blind fashion. 3. Assessment of nutritional intake and stool collection ‐ the blinded participants recorded their own intake and investigators also worked in a blind fashion. Although blinded participants scored their own global symptoms, physicians assessed them too and we could not make the assumption that the physicians were blinded. There was no mention of blinding for the other outcomes: anthropometric measurements, number of hospital admissions, pulmonary function and frequency of pulmonary infections, laboratory tests, abdominal circumference and intestinal lavage therapy. |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing data and outcomes fully reported: gastrointestinal symptom scores, global symptom scores and intestinal lavage therapy Insufficient information to judge whether outcome data are missing: anthropometric measurements, adverse effects, pulmonary function, frequency of pulmonary exacerbations, number of hospital admissions, radiological signs of DIOS and laboratory values Data missing: for nutritional intake and stool losses, only present data for 10/17 participants, reasons given for missing data but this would have had a big impact on the effect size, since the sample size was small anyway; no mention of ITT analysis, no mention of treating per protocol |
| Selective reporting (reporting bias) | High risk | No access to trial protocol so not possible to compare planned outcomes to outcomes reported in paper. Anthropometric measurements: stated in the methods section that "at the end of each treatment period, difference weight and percentage of ideal weight for height, as well as the difference in weight change during the two periods, were compared by means of t tests", however, these changes and results of t tests were not specifically reported in the results, incomplete report means it can not be entered into a meta‐ analysis. Pulmonary function testing and X‐ray findings: although supposed to be measured "at the end of baseline and 6 month periods" were reported incompletely so cannot be entered into a meta‐analysis. Cisapride levels: in the methods section, it specified that they would determine cisapride levels "at the end of baseline and 6 month periods" but this was not reported in the results. Number of hospital admissions: specified as an outcome measure in the methods section, but not reported in the results. Laboratory test results (blood and urine analysis): incompletely reported so that they could not be entered into a meta‐analysis. |
| Other bias | Unclear risk | None known, but cannot be sure, there is no known sponsorship source but the trial was described as being supported by Janssen Pharmaceuticals. |
CF: cystic fibrosis
DIOS: distal intestinal obstruction syndrome
FEV1: forced expiratory volume at one second
FVC: forced vital capacity
ITT: intention‐to‐treat analysis
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Inappropriate indication: N‐acetylcysteine used for respiratory complications rather than for preventing DIOS | |
| Inappropriate interventions: pancreatic enzyme therapy used for treatment of DIOS instead of prevention of DIOS | |
| Inappropriate indication: N‐acetylcysteine used for respiratory complications rather than for preventing DIOS | |
| Inappropriate indication: N‐acetylcysteine used for respiratory complications rather than for preventing DIOS | |
| Inappropriate indication: N‐acetylcysteine used for respiratory complications rather than for preventing DIOS | |
| Inappropriate indication: N‐acetylcysteine used for malabsorption in CF rather than for preventing DIOS | |
| Non‐randomised and open‐label design | |
| This is a trial proposal regarding a treatment intervention, but the lead author confirmed that the trial was never undertaken due to a lack of approval. |
CF: cystic fibrosis
DIOS: distal intestinal obstruction syndrome
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Total gastrointestinal symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1: Cisapride versus placebo, Outcome 1: Total gastrointestinal symptoms | ||||
| 1.1.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Abdominal pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1: Cisapride versus placebo, Outcome 2: Abdominal pain | ||||
| 1.2.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Abdominal distension Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1: Cisapride versus placebo, Outcome 3: Abdominal distension | ||||
| 1.3.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Cisapride versus placebo, Outcome 1: Total gastrointestinal symptoms

Comparison 1: Cisapride versus placebo, Outcome 2: Abdominal pain

Comparison 1: Cisapride versus placebo, Outcome 3: Abdominal distension
| Cisapride compared to placebo for preventing DIOS in cystic fibrosis | ||||||
| Patient or population: people with cystic fibrosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo | Risk with cisapride | |||||
| Radiological diagnosis of DIOS (physician‐measured radiological scores) Follow‐up: baseline to 6 months | Trial investigators stated that there was no significant difference between cisapride and placebo. | NA | 17 | ⊕⊝⊝⊝ | Radiologist scored for radiographic signs of DIOS, no numerical data available. | |
| Adverse effects (participant interviews) Follow‐up: 3 to 12 months | No adverse effects were noted in either group. | NA | 17 | ⊕⊝⊝⊝ | No numerical data available. | |
| Total gastrointestinal symptom scores (participant‐reported symptom scores from 20 to 100) Follow‐up: 3 to 12 months | The mean difference was 7.6 lower in the cisapride arm | NA | 17 | ⊕⊝⊝⊝ | Score made up of 10 different gastrointestinal symptoms: heartburn, flatulence, regurgitation, fullness, abdominal distension, abdominal pain, diarrhoea, nausea, vomiting, anorexia. | |
| Hospitalisation for any cause | Outcome not reported. | NA | NA |
| ||
| Hospitalisation for DIOS | Outcome not reported. | NA | NA |
| ||
| Quality of life | Outcome not reported. | NA | NA |
| ||
| Tolerability | Outcome not reported. | NA | NA |
| ||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a. Selective reporting may have occurred with this outcome; allocation concealment and sequence generation was unclear. b. Cisapride is a prokinetic, not a typical laxative agent (different to protocol). The study was conducted in 1990 when cisapride was still prescribed. It has now been taken off the UK market and other international markets due to its rare but serious cardiac effects. c. Very small number of participants in the trial does not give sufficient information to give a precise effect estimate. d. Allocation concealment and sequence generation ranked as unclear risk of bias. | ||||||
| Intervention | Total number of participants | Felt better | Felt the same | Felt worse |
|---|---|---|---|---|
| Cisapride | 17 | 12 | 2 | 3 |
| Placebo | 17 | 3 | 2 | 12 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Total gastrointestinal symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Abdominal pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Abdominal distension Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |