Intervenciones para la prevención del síndrome de obstrucción intestinal distal (SOID) en la fibrosis quística
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012619.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 12 junio 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Fibrosis quística y enfermedades genéticas
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
| Roles and responsibilities | |
| TASK | WHO UNDERTOOK THE TASK |
| Protocol stage: draft the protocol | WC |
| Review stage: select which trials to include (2 + 1 arbiter) | JG + WC + FG as arbiter |
| Review stage: extract data from trials (2 people) | JG + WC |
| Review stage: enter data into RevMan | JG |
| Review stage: carry out the analysis | JG + WC |
| Review stage: interpret the analysis | JG + WC |
| Review stage: draft the final review | JG + WC |
| Update stage: update the review | WC |
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Jessica Green declares no known potential conflict of interest.
Dr Will Carroll declares no known potential conflict of interest.
Dr Francis J Gilchrist declares no known potential conflict of interest.
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We would like to thank Nikki Jahnke (Managing Editor at the Cochrane Cystic Fibrosis and Genetic Disorders Group) for her support and guidance throughout this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2021 Dec 22 | Interventions for preventing distal intestinal obstruction syndrome (DIOS) in cystic fibrosis | Review | Will Carroll, Jessica Green, Francis J Gilchrist | |
| 2018 Jun 12 | Interventions for preventing distal intestinal obstruction syndrome (DIOS) in cystic fibrosis | Review | Jessica Green, Francis J Gilchrist, Will Carroll | |
| 2017 Apr 11 | Interventions for preventing distal intestinal obstruction syndrome (DIOS) in cystic fibrosis | Protocol | Jessica Green, Will Carroll, Francis J Gilchrist | |
Differences between protocol and review
In the protocol, we did not list prokinetic agents as a possible treatment for distal intestinal obstruction syndrome (DIOS). However, we have now included them in the review because there is evidence in the literature for their role in treating constipation and generally increasing colonic transit and motility (Boyle 2009; Colombo 2011). As the treatment of constipation is especially important in the prevention of DIOS, we decided to include them as a possible intervention in this review.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Adult; Humans;
PICO

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Cisapride versus placebo, Outcome 1 Total gastrointestinal symptoms.
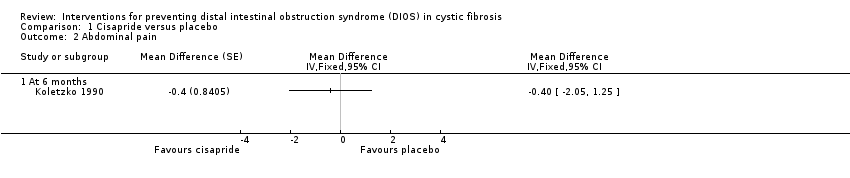
Comparison 1 Cisapride versus placebo, Outcome 2 Abdominal pain.

Comparison 1 Cisapride versus placebo, Outcome 3 Abdominal distension.
| Cisapride compared to placebo for preventing distal intestinal obstruction syndrome (DIOS) in cystic fibrosis | ||||||
| Patient or population: preventing DIOS in cystic fibrosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with cisapride | |||||
| Radiological diagnosis of DIOS (physician‐measured radiological scores) Follow‐up: baseline to 6 months | Trial investigators stated that there was no significant difference between cisapride and placebo. | NA | 17 | ⊕⊝⊝⊝ | Radiologist scored for radiographic signs of DIOS, no numerical data available. | |
| Adverse effects (participant interviews) Follow‐up: 3 to 12 months | No adverse effects were noted in either group. | NA | 17 | ⊕⊝⊝⊝ | No numerical data available. | |
| Total gastrointestinal symptom scores (participant‐reported symptom scores from 20 to 100) Follow‐up: 3 to 12 months | The mean difference was 7.6 lower in the cisapride arm | NA | 17 | ⊕⊝⊝⊝ | Score made up of 10 different gastrointestinal symptoms: heartburn, flatulence, regurgitation, fullness, abdominal distension, abdominal pain, diarrhoea, nausea, vomiting, anorexia. | |
| Hospitalisation for any cause | Outcome not reported. | NA | NA | |||
| Hospitalisation for DIOS | Outcome not reported. | NA | NA | |||
| Quality of life | Outcome not reported. | NA | NA | |||
| Tolerability | Outcome not reported. | NA | NA | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Selective reporting may have occurred with this outcome; allocation concealment and sequence generation was unclear. 2. Cisapride is a prokinetic, not a typical laxative agent (different to protocol). The study was conducted in 1990 when cisapride was still prescribed. It has now been taken off the UK market and other international markets due to its rare but serious cardiac effects. 3. Very small number of participants in the trial does not give sufficient information to give a precise effect estimate. 4. Allocation concealment and sequence generation ranked as unclear risk of bias. | ||||||
| Intervention | Total number of participants | Felt better | Felt the same | Felt worse |
| Cisapride | 17 | 12 | 2 | 3 |
| Placebo | 17 | 3 | 2 | 12 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total gastrointestinal symptoms Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 1.1 At 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Abdominal pain Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2.1 At 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Abdominal distension Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3.1 At 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |