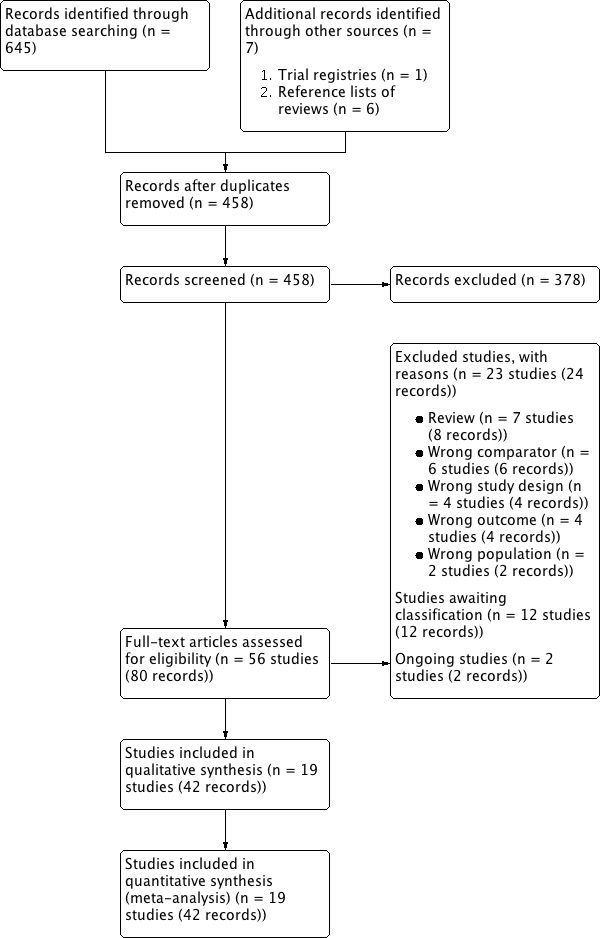
نقش سیلودوزین در درمان نشانههای دستگاه ادراری تحتانی در مردان مبتلا به هیپرپلازی خوشخیم پروستات
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع مطالعات در حال انجام
منابع اضافی
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: double‐blind, placebo‐ and active‐controlled, parallel‐group clinical study Setting/Country: 72 hospital clinics and inpatient units in 11 countries/Europe Dates when study was conducted: May 2006‐May 2007 | |
| Participants | Inclusion criteria: men ≥ 50 years with LUTS (defined by a stable IPSS total score ≥ 13 points), bladder outlet obstruction (defined by a Qmax 4 mL/s‐15 mL/s, with a minimum voided volume of ≥ 125 mL), and the evidence of satisfactory compliance with study medication (80%–120% during the placebo run‐in period) Exclusion criteria: men with improvement in the IPSS total score ≥ 25% in the run‐in period, PVR ≥ 250 mL, intravesical obstruction from any cause other than BPH, history of any procedure considered an intervention for BPH, active urinary tract infection or history of recurrent urinary tract infections, current prostatitis or diagnosis of chronic prostatitis, history of prostate or invasive bladder cancer, significant postural hypotension, use of 5‐ARIs within 6 months, or use of an a‐blocker or phytotherapy within 2 weeks before entry Total number of participants randomly assigned: 955 Group A (Silodosin)
Group B (Tamsulosin)
Group C (Placebo)
| |
| Interventions | Run‐in period: 4 weeks, single‐blind, placebo run‐in period Group A: silodosin 8 mg once daily Group B: tamsulosin 0.4 mg once daily Group C: placebo once daily Duration: 12 weeks | |
| Outcomes | Primary outcome
How measured: IPSS questionnaire Time points measured: at screening, at baseline, and after 7, 14, 28, 56, and 84 d of treatment Time points reported: baseline and end of study Secondary outcome
How measured: IPSS questionnaire, uroflowmetry Time points measured: IPSS: see primary outcome/Qmax: at the same time periods as IPSS Time points reported: baseline and end of study Safety outcomes How measured: adverse events, physical examination (BP, heart rate), laboratory tests, and a 12‐lead electrocardiogram Time points measured: at each visit Time points reported: at each visit Subgroup: a post hoc analysis was conducted in the subgroup of participants with nocturia at baseline (defined as at least two voids per night), as assessed by question 7 of the IPSS | |
| Funding sources | Recordati Ireland Ltd | |
| Declarations of interest | Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher R. Chapple and Francesco Montorsi are consultants and researchers for Recordati. Teuvo L.J. Tammela, Manfred Wirth, Evert Koldewijn, and Eldiberto Fernandez Fernandez are researchers for Recordati | |
| Notes | Language of publication: English Data (unadjusted IPSS and IPSS‐QoL, AUR, and surgical intervention) were given by contact with study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned (in a ratio of 2:2:1, with stratification by center, with blocks of five assigned to each center, produced and managed centrally by an international contract research organization)" |
| Allocation concealment (selection bias) | Low risk | Quote: "managed centrally by an international contract research organization" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "A multicenter double‐blind, placebo‐ and active‐controlled parallel group clinical study, all study personnel and participants were blinded to treatment assignment for the entire duration of the study" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement: no information given |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: 10/381 (2.6%) in silodosin, 8/384 (2.1%) in tamsulosin, and 5/190 (2.6%) in placebo group were not included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Selective reporting (reporting bias) | High risk | Judgement: Protocol (NCT00359905) was revised after completion of study (2009) and percentage of treatment responders was added as secondary outcome. All primary and secondary endpoints were measured at 7, 14, 28, 56, 84 day, but only reported at 12 weeks Unadjusted data for analysis for IPSS and QoL were not reported |
| Other bias | Unclear risk | Judgement: 4 weeks' placebo run‐in period |
| Methods | Study design: open‐label, randomized comparative study Setting/Country: not reported/South Korea Dates when study was conducted: not reported | |
| Participants | Inclusion criteria: sexually active men with BPH Exclusion criteria: not reported Total number of participants randomly assigned: 138 Group A (Silodosin)
Group B (Tamsulosin)
Group C (Alfusocin)
| |
| Interventions | Run‐in period: none Group A: silodosin 8 mg once daily Group B: tamsulosin 0.2 mg once daily Group C: alfuzosin 10 mg once daily Duration: 4 weeks | |
| Outcomes |
How measured: IPSS, MSHQ Time points measured: baseline and on the 4th week of medication Time points reported: baseline and on the 4th week of medication Subgroup: none | |
| Funding sources | None | |
| Declarations of interest | Not reported | |
| Notes | Language of publication: English Publication status: abstract (full text has not been published; study author reply) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement: no information given |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no information given |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open‐label, randomized comparative study" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "open‐label, randomized comparative study" |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: the number of participants excluded in analysis was not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: the number of participants excluded in analysis was not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement: protocol was not published and no information related to the review outcomes given |
| Other bias | Unclear risk | Judgement: abstract only |
| Methods | Study design: randomized, double‐blind, placebo‐controlled study Setting/Country: 88 centers/outpatient/Japan Dates when study was conducted: not reported | |
| Participants | Inclusion criteria: men ≥ 50 years with total IPSS of ≥ 8, an associated QoL score of ≥ 3, prostate volume (measured by transabdominal or transrectal US) of ≥ 20 mL, a Q max of < 15 mL/s with a voided volume of ≥ 100 mL and a PVR of < 100 mL Exclusion criteria: men who had antiandrogen preparations for 1 year before the study or had a prostatectomy, intrapelvic radiation therapy or prostatic hyperthermia (transurethral microwave hyperthermia or transurethral needle ablation). Men who had prostate cancer or suspected prostate cancer, neurogenic bladder, bladder neck constriction, urethral stricture, bladder calculus, severe bladder diverticulum, active urinary tract infection requiring medical treatment, renal impairment (serum creatinine ≥ 2.0 mg/dL) and other complications considered likely to affect micturition, severe hepatic disorders, severe cardiovascular disease and a history of orthostatic hypotension Total number of participants randomly assigned: 457 Group A (Silodosin)
Group B (Tamsulosin)
Group C (Placebo)
| |
| Interventions | Run‐in period: none Group A: oral silodosin 4 mg twice daily Group B: oral tamsulosin 0.2 mg once daily Group C: oral placebo twice daily Duration: 12 weeks | |
| Outcomes | Primary outcome
How measured: IPSS questionnaire Time points measured: at the end of wash‐out period and at 1, 2, 4, 8 and 12 weeks during the treatment period Time points reported: at the end of wash‐out period and at 1, 2, and 12 weeks during the treatment period Secondary outcome
How measured: IPSS questionnaire, uroflowmetry Time points measured: at the end of wash‐out period and at 1, 2, 4, 8 and 12 weeks during the treatment period Time points reported: at the end of wash‐out period and 12 weeks Safety outcomes How measured: adverse events, physical examinations (BP and heart rate), clinical laboratory tests (hematology, blood chemistry and urine analysis) Time points measured: at the start of the observation period and at 4 and 12 weeks of treatment (follow‐up visit) Time points reported: at the start of the observation period and at 12 weeks of treatment Subgroup: analysis based on LUTS severity, post hoc investigation in Qmax among the 3 treatment groups in the overall subgroup of participants with a change in voided volume of < 50% before and after treatment | |
| Funding sources | None | |
| Declarations of interest | None | |
| Notes | Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized, double‐blind, placebo controlled study" Judgement: the randomization method was not defined |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no information given |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "randomized, double‐blind, placebo controlled study" Judgement: placebo‐controlled study, but double‐blind was not clear who was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "randomized, double‐blind, placebo controlled study" Judgement: double‐blind was not clear who was blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: 1/176 (0.5%) in silodosin group, 0/192 (0%) in tamsulosin, and 0/89 (0%) were not included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: 1/176 (0.5%) in silodosin group, 0/192 (0%) in tamsulosin, and 0/89 (0%) were not included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: 1/176 (0.5%) in silodosin group, 0/192 (0%) in tamsulosin, and 0/89 (0%) were not included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: 1/176 (0.5%) in silodosin group, 0/192 (0%) in tamsulosin, and 0/89 (0%) were not included in analysis |
| Selective reporting (reporting bias) | High risk | Judgement: protocol was not published and additional post hoc subgroup investigation was not defined in method section |
| Other bias | High risk | Judgement: baseline imbalance in QoL; primary endpoint was adjusted analysis by baseline QoL. Drug administration times were different between groups |
| Methods | Study design: prospective, randomized, comparative, open‐label study Setting/Country: tertiary care hospital/India Dates when study was conducted: June 2013‐June 2014 | |
| Participants | Inclusion criteria: men ≥ 45 years with symptomatic BPH with IPSS of ≥ 8, QoL of ≥ 3, and Qmax of < 15 mL/s, but > 4 mL/s with a voided volume of > 100 mL Exclusion criteria: severe hepatic or renal insufficiency, urinary tract infections, urethral stricture, neurogenic bladder, PSA ≥ 5 ng/mL, history of urethral or prostatic surgery, hypotension or severe untreated hypertension, history of esophageal or intestinal obstruction, history of multiple drug abuse, significant psychiatric problems, serious disease or malignancy, increased risk of QTc (corrected QT Interval in the heart's electrical cycle) prolongation (e.g. hypokalemia, concomitant use of Class Ia and III antiarrhythmics, antipsychotics, tricyclic antidepressants, etc.), concomitant use of 5‐ARIs and strong cytochrome P450 3A4 inhibitors, likelihood of requiring catheterization within next 3 months Total number of participants randomly assigned: 90 Group A (Silodosin)
Group B (Tamsulosin)
Group C (Alfuzosin)
| |
| Interventions | Run‐in period: none Group A: silodosin capsule 8 mg once daily in the morning (before breakfast) Group B: tamsulosin tablet/capsule 0.4 mg (before meals) once daily at bedtime Group C: alfuzosin sustained release tablet 10 mg (immediately after meals) once daily Duration: 12 weeks | |
| Outcomes | Primary outcome
How measured: IPSS questionnaire Time points measured: baseline, after 2 weeks (visit 1), 4 weeks (visit 2), 8 weeks (visit 3), and 12 weeks (visit 4) Time points reported: baseline, after 2 weeks (visit 1), 4 weeks (visit 2), 8 weeks (visit 3), and 12 weeks (visit 4) Secondary outcome
How measured: uroflowmetry, IPSS questionnaire Time points measured: baseline, after 2 weeks (visit 1), 4 weeks (visit 2), 8 weeks (visit 3), and 12 weeks (visit 4) Time points reported: baseline, after 2 weeks (visit 1), 4 weeks (visit 2), 8 weeks (visit 3), and 12 weeks (visit 4) Safety outcomes How measured: side effects, cardiovascular parameters, pulse rate, and BP (supine and standing) Time points measured: baseline, after 2 weeks (visit 1), 4 weeks (visit 2), 8 weeks (visit 3), and 12 weeks (visit 4) Time points reported: cumulative results from follow‐up visits Subgroup: none | |
| Funding sources | None | |
| Declarations of interest | None | |
| Notes | Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "prospective, randomized". Judgement: random number table in protocol |
| Allocation concealment (selection bias) | Low risk | Judgement: no information in article, but "Sequentially numbered, sealed, opaque envelopes" in protocol |
| Blinding of participants and personnel (performance bias) | High risk | Judgement: open‐label randomized study |
| Blinding of outcome assessment (detection bias) | High risk | Judgement: open‐label randomized study |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "there were no drop‐outs or protocol violations during the study and all the ninety subjects were included in the analysis" |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "there were no drop‐outs or protocol violations during the study and all the ninety subjects were included in the analysis" |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "there were no drop‐outs or protocol violations during the study and all the ninety subjects were included in the analysis" |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "there were no drop‐outs or protocol violations during the study and all the ninety subjects were included in the analysis" |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: the number of participants with sexual adverse events in tamsulosin and alfuzosin group were not reported |
| Selective reporting (reporting bias) | Low risk | Judgement: protocol (Clinical Trials Registry‐India (CTRI/2013/07/003805)) was published and the prespecified outcomes were well described. |
| Other bias | High risk | Judgement: probably, baseline imbalance in IPSS score, PSA, PVR (silodosin group has markedly lower) and prostate volume (alfuzosin group has markedly higher prostate volume). |
| Methods | Study design: parallel‐group, double‐blind, randomized study Setting/Country: multicenter/USA Dates when study was conducted: May 2005‐August 2006 | |
| Participants | Inclusion criteria: men aged ≥ 50 years with an IPSS of ≥ 13, a Qmax of 4 mL/s‐15 mL /s and a PVR < 250 mL Exclusion criteria: men with intravesical obstruction unrelated to BPH; bladder calculi; history of or current condition affecting bladder function; prior surgical intervention to relieve BPH or bladder neck obstruction; active urinary tract infection or history of recurrent urinary tract infection within the past 2 years; prostatitis within the past 3 months; BPH‐unrelated urinary retention within the past 3 months; and a history of recurring prostatitis (> 3 times within the past year), prior or current prostate cancer or PSA > 10 ng/mL; prior invasive bladder cancer; bladder catheterization or bladder or prostate instrumentation within the past 30 days and history of or current significant postural hypotension, including changes in systolic (> 30 mm Hg) or diastolic (> 20 mm Hg) BP or heart rate (more than 20 beats per minute), and lightheadedness, fainting, blurred vision, profound weakness or syncope upon change in position Total number of participants randomly assigned: 923 Group A (Silodosin)
Group B (Placebo)
| |
| Interventions | Run‐in period: single‐blind, placebo run in for 4 weeks Group A: silodosin 8 mg once daily with breakfast Group B: placebo once daily with breakfast Duration: 12 weeks | |
| Outcomes | Primary outcome
How measured: IPSS questionnaire Time points measured: at weeks 0 (baseline), 0.5, 1, 2, 4 and 12 Time points reported: at weeks 0 (baseline), 0.5, 1, 2, 4 and 12 Secondary outcome
How measured: uroflow assessed by study investigator and a blinded central reader Time points measured: at baseline, 2‐6 h after the first dose, and at weeks 1, 2, 4 and 12 Time points reported: at baseline, 2‐6 h after the first dose, and at weeks 1, 2, 4 and 12 Safety outcomes How measured: adverse event/additional safety assessments included 12‐lead electrocardiograms, clinical laboratory tests and vital sign measurements including postural hypotension tests and physical examinations Time points measured: at every visit except at post‐randomization week 0.5 Time points reported: cumulative results from follow‐up visits Subgroup: none | |
| Funding sources | Allergan, American Medical Systems, Astellas, Bayer, Beckman Coulter, Diagnostic Ultrasound, GTX, GlaxoSmithKline, Gen‐Probe, Indevus, Light Sciences Oncology, Lilly/ICOS, Merck, Novartis, Onconome, Pfizer, Sanofi, Solvay, Watson, National Institutes of Health, CapCURE, Pardee Foundation and Seder Foundation | |
| Declarations of interest | Allergan, American Medical Systems, Astellas, Bayer, Beckman Coulter, Diagnostic Ultrasound, GTX, GlaxoSmithKline, Gen‐Probe, Indevus, Light Sciences Oncology, Lilly/ICOS, Merck, Novartis, Onconome, Pfizer, Sanofi, Solvay, Watson, National Institutes of Health, CapCURE, Pardee Foundation and Seder Foundation | |
| Notes | Language of publication: English Study author reply: "Pharmaceutics only have the data". We cannot contact study supporter in pharmaceutics due to wrong email address | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned (1:1) to double‐blind treatment, randomization schedule using PROC PLAN in SAS." |
| Allocation concealment (selection bias) | Low risk | Judgement: no information in article, but "Sequentially numbered, sealed, opaque envelopes" in protocol |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Blinding was maintained throughout the study by the use of identical medication packaging with placebo matching silodosin in size and external appearance. Emergency information labels that indicated the patient’s assigned treatment were available to the investigator should knowledge of treatment assignment be needed to ensure the patient’s well‐being. If unblinding of the investigator, site personnel or the patient was required in a particular case that patient was to be discontinued from the study." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Blinding was maintained throughout the study by the use of identical medication packaging with placebo matching silodosin in size and external appearance. Emergency information labels that indicated the patient’s assigned treatment were available to the investigator should knowledge of treatment assignment be needed to ensure the patient’s well‐being. If unblinding of the investigator, site personnel or the patient was required in a particular case that patient was to be discontinued from the study." |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: discontinued/lost to follow‐up were 53/466 (11.4%) and 38/457 (8.3%), Loss of follow‐up 6/466 (1.3%) and 3/457 (0.3%), but discrepancies in the reason of discontinuation (47/466, 35/457) between intervention and placebo group. Last observations were carried forward to impute values missing for week 12 |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Selective reporting (reporting bias) | Low risk | Judgement: protocols (NCT00224107, NCT00224120) were published and the prespecified outcomes were well described |
| Other bias | High risk | Judgement: single‐blind placebo run‐in for 4 weeks; participants with ≥ 30% decrease in IPSS or an increase in Qmax of ≥ 3 mL/s during the run‐in period were excluded from randomization |
| Methods | Study design: randomized, cross‐over study Setting/Country: multicenter/Japan Dates when study was conducted: November 2009‐March 2011 | |
| Participants | Inclusion criteria: men ≥ 50 years with prostate estimated volume of > 20 cm3, IPSS of ≥ 8, a QoL score of ≥ 3 points Exclusion criteria: men with organic diseases other than BPH (prostate cancer, bladder tumor, prostatitis, urethral stricture, etc.), transurethral resection of prostate or minimally invasive treatment, under catheterization and under self‐donating urination, active urinary tract infections, combined neuropathic bladder and nervous system disorder, received hormone‐based prostatic hyperplasia treatment medicine 6 months before the start of the study, received androgen‐receptor blocking agent 6 weeks before the study, and those judged unsuitable by the attending physician Total number of participants randomly assigned: 92 Group A (Silodosin)
Group B (Naftopidil)
| |
| Interventions | Run‐in period: none Group A: silodosin 2 mg‐4 mg twice a day for 2 weeks in the morning and evening, and 4 mg twice daily in the morning and evening for 4 more weeks, if there were no problems such as side effects Group B: naftopidil 50 mg‐75 mg once in the morning for 2 weeks, and if there were no problems such as side effects 75 mg was administered once in the morning for 4 more weeks Duration: 6 weeks (before cross‐over)/total 12 weeks | |
| Outcomes | Primary outcome
How measured: IPSS questionnaire Time points measured: before and after treatment (6 weeks and 12 weeks) Time points reported: before and after treatment (6 weeks and 12 weeks) Secondary outcome
How measured: IPSS: IPSS questionnaire/OABSS: OABSS questionnaire/PVR: abdominal US/patient preference: questionnaire survey Time points measured: before administration of the drug and 4 weeks, 8 weeks Time points reported: before administration of the drug and 4 weeks, 8 weeks Safety outcomes How measured: adverse events Time points measured: not reported Time points reported: at the end of study (12 weeks) Subgroup: none | |
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes | Language of publication: Japanese No wash‐out period between cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "all patients who got consent were assigned in turn to 'Naf‐Silo group' and 'Silo‐Naf group' alternately" |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no information given |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement: no information given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement: no information given |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | Judgement: 14/44 (31.9%) and 14/48 (29.2%) participants in the silodosin and naftopidil group were not included in the analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: not available (cross‐over trial) |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 5/88 (5.7%) and 17/96 (17.8%) participants in the silodosin and tamsulosin group were not included in the analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 5/88 (5.7%) and 17/96 (17.8%) participants in the silodosin and tamsulosin group were not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Judgement: prespecified study outcomes were well described but protocol was not published |
| Other bias | Unclear risk | Judgement: no wash‐out period |
| Methods | Study design: prospective, open‐label, randomized study Setting/Country: 52 urologists participated at a total of 44 investigational sites/outpatients/Japan Dates when study was conducted: May 2012‐September 2013 | |
| Participants | Inclusion criteria: men with total IPSS 8 or greater, IPSS QoL score 3 or greater, total OABSS 3 or greater, 1 or more urinary urgency episodes per week, prostate volume 20 ml or greater on transabdominal US, Qmax less than 15 ml per second at a voided volume of 100 ml or greater and PVR less than 150 ml, and age 50 years or greater Exclusion criteria: men received oral treatment with a1‐blockers, anticholinergic agents, 5a‐reductase inhibitors, antidepressants, anti‐anxiety agents or sex hormonal agents, they had neurogenic bladder dysfunction, bladder calculi or active urinary tract infection, severe cardiac disease, renal dysfunction (serum creatinine levels 2 mg/dl or more) or hepatic dysfunction (aspartate and alanine aminotransferase concentrations more than twice normal values) Total number of participants randomly assigned: 350 Group A (Silodosin)
Group B (Naftopidil)
| |
| Interventions | Run‐in period: none Group A: silodosin 4 mg per day for 4 weeks, followed by 8 mg per day for 8 weeks Group B: naftopidil 50 mg per day for 4 weeks, followed by 75 mg per day for 8 weeks Duration: 12 weeks | |
| Outcomes | Primary outcome
How measured: IPSS and OABSS questionnaire Time points measured: baseline 4 and 12 weeks after the start of treatment Time points reported: baseline 4 and 12 weeks after the start of treatment Secondary outcome
How measured: uroflowmetry Time points measured: baseline 4 and 12 weeks after the start of treatment Time points reported: baseline 4 and 12 weeks after the start of treatment Safety outcomes How measured: not reported Time points measured: not reported Time points reported: not reported Subgroup: none | |
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes | Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple randomization using a random number table" |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no information given |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "prospective, open label, randomized" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "prospective, open label, randomized" |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 18/175 (10.2%) and 18/175 (10.2%) participants in silodosin and naftopidil group were not included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all subjects who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all subjects who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: information was partly reported (the number of withdrawal due to cardiovascular adverse events were only reported) |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Selective reporting (reporting bias) | Unclear risk | Judgement: prespecified outcomes were well described, but protocol was not published |
| Other bias | Low risk | Judgement: not detected |
| Methods | Study design: randomized, cross‐over study Setting/Country: multicenter/Japan Dates when study was conducted: May 2006‐July 2007 | |
| Participants | Inclusion criteria: men with IPSS ≥ 8 points; QoL score ≥ 3 points; prostate volume measured by US ≥ 20 mL; void volume ≥ 100 mL; and Qmax < 15 mL/s Exclusion criteria: already used any a1‐blocker for the treatment of hypertension; taking vardenafil hydrochloride hydrate; and otherwise judged by an attending physician to be inappropriate Total number of participants randomly assigned: 97 Group A (Silodosin)
Group B (Tamsulosin)
| |
| Interventions | Run‐in period: none Group A: silodosin 8 mg/d (4 mg/dose, twice daily) Group B: tamsulosin 0.2 mg/d (0.2 mg/dose, once daily) Duration: 4 weeks (before cross‐over)/total 8 weeks | |
| Outcomes | Primary outcome
How measured: IPSS questionnaire Time points measured: before administration of the drug and 1, 2, 4, 6 and 8 weeks after initiation of administration Time points reported: before administration of the drug, and 4 weeks and 8 weeks Secondary outcome
How measured: not reported Time points measured: before administration of the drug, and 4 weeks and 8 weeks Time points reported: before administration of the drug, and 4 weeks and 8 weeks Safety outcomes How measured: adverse events Time points measured: throughout the study period Time points reported: throughout the study period Subgroup: none | |
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes | Language of publication: English No wash‐out period between cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement: no information given |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no information given |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement: no information given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement: no information given |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | Judgement: 12/46 (26.1%) and 20/51 (39.3%) participants in the silodosin and tamsulosin group were not included in the analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: not available (cross‐over trial) |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Judgement: prespecified outcomes were well described, but protocol was not published |
| Other bias | Unclear risk | Judgement: no wash‐out period |
| Methods | Study design: parallel, randomized Setting/Country: tertiary hospital/Tamil Nadu, India Dates when study was conducted: August 2013‐April 2015 | |
| Participants | Inclusion criteria: men > 50 years with bothersome LUTS from BPH and IPSS > 7 Exclusion criteria: history of LUTS but not BPH, AUR in past 6 months, raised PSA level at baseline, serious co‐morbidity of vital organs, use of concomitant medication having anticholinergic, androgenic or estrogenic influence, on other α‐adrenergic antagonists or diuretics or with a history of prostatic or per urethral surgery or substance abuse Total number of participants randomly assigned: 57 Group A (Silodosin)
Group B (Tamsulosin)
| |
| Interventions | Run‐in period: none Group A: silodosin 8 mg capsule once daily after dinner Group B: tamsulosin 0.4 mg controlled‐release capsule once daily after dinner Duration: 12 weeks | |
| Outcomes | Primary outcome
How measured: IPSS questionnaire Time points measured: baseline and 12 weeks Time points reported: baseline and 12 weeks Secondary outcome
How measured: IPSS, US by blinded radiologist, uroflowmetry by blinded operator Time points measured: baseline and 12 weeks Time points reported: symptom free and change in Qmax not reported; change in prostate size, baseline and 12 weeks Safety outcomes How measured: adverse affects Time points measured: at follow‐up visits (4, 8, 12 weeks) Time points reported: cumulative results from follow‐up visits Subgroup: none | |
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes | Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement: no information given |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no information given |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement: no information given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement: no information given |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Selective reporting (reporting bias) | High risk | Quote: "Silodosin showed a significant decrease in IPSS versus tamsulosin at 2 weeks" Judgement: protocol was not published and there was no information of 2 weeks' follow‐up. Secondary endpoints (proportion of participants who became completely or relatively symptom free (IPSS < 8), changes in Qmax) were not reported |
| Other bias | Unclear risk | Judgement: no data on baseline characteristics |
| Methods | Study design: parallel, randomized, double‐blinded study Setting/Country: NR Dates when study was conducted: January 2009‐October 2009 | |
| Participants | Inclusion criteria: men in good general health and ≥ 50 years, with symptoms of moderate‐severe BPH and nocturia (≥ 2 episodes per night) Exclusion criteria: men with 1) medical conditions that would confound the efficacy evaluation 2) medical conditions in which it would be unsafe to use an alpha‐blocker 3) the use of concomitant drugs that would confound the efficacy evaluation 4) the use of concomitant drugs that would be unsafe with this alpha‐blocker Total number of participants randomly assigned: 209 Group A (Silodosin)
Group B (Placebo)
| |
| Interventions | Run‐in period: not reported Group A: silodosin 8 mg daily Group B: 1 placebo capsule daily Duration: 12 weeks | |
| Outcomes | Primary outcome
How measured: not reported Time points measured: before and 12 weeks after treatment Time points reported: before and 12 weeks after treatment Secondary outcomes
How measured: not reported Time points measured: before and 12 weeks after treatment Time points reported: before and 12 weeks after treatment Subgroup: not reported | |
| Funding sources | Watson Pharmaceuticals | |
| Declarations of interest | Watson Pharmaceuticals | |
| Notes | Language of publication: English Publication status: NCT00793819 (results in clinicaltrials.gov) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement: no information given |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no information given |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "parallel randomized double blinded (Participant, Investigator)" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "parallel randomized double blinded (Participant, Investigator)" |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Selective reporting (reporting bias) | Low risk | Judgement: protocol (NCT00793819) was published and the prespecified outcomes were well described |
| Other bias | Unclear risk | Judgement: protocol only |
| Methods | Study design: single‐blind, parallel‐group, randomized, controlled trial Setting/Country: outpatient/tertiary care hospital/Kolkata, India Dates when study was conducted: July 2012‐June 2013 | |
| Participants | Inclusion criteria: ambulatory, treatment naïve, men > 50 years with bothersome LUTS from BPH and IPSS > 7 Exclusion criteria: history of LUTS but not BPH, AUR in past 6 months, raised PSA level at baseline, serious co‐morbidity of vital organs, use of concomitant medication having anticholinergic, androgenic or estrogenic influence, on other α‐adrenergic antagonists or diuretics or with a history of prostatic or per urethral surgery or substance abuse Total number of participants randomly assigned: 61 Group A (Silodosin)
Group B (Tamsulosin)
| |
| Interventions | Run‐in period: none Group A: silodosin 8 mg capsule once daily Group B: tamsulosin 0.4 mg controlled‐release capsule once daily Duration: 12 weeks | |
| Outcomes | Primary outcome
How measured: IPSS questionnaire Time points measured: at 4, 8 and 12 weeks Time points reported: at 4, 8 and 12 weeks Secondary outcome
How measured: IPSS questionnaire, US by blinded radiologist, uroflowmetry by blinded investigator Time points measured: baseline and 12 weeks Time points reported: baseline and 12 weeks Safety outcomes How measured: vital signs, treatment‐emergent adverse events, postural hypotension and retrograde ejaculation, laboratory investigations (complete blood count, fasting plasma glucose, routine liver function tests and creatinine level) Time points measured: lab ‐ at baseline and study end/others ‐ each visit Time points reported: lab ‐ at baseline and study end/others ‐ cumulative results from follow up visits Subgroup: none | |
| Funding sources | M/s Cipla Limited, Mumbai, donated both study drugs and reimbursed part of the cost of laboratory investigations on request | |
| Declarations of interest | None | |
| Notes | Language of publication: English Data (IPSS, QoL, AUR, surgical intervention, cardiovascular, sexual adverse events) were given by contact with study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized in 1:1 ratio to one of the two study groups in fixed blocks of 8, using computer generated random number list" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was achieved using the serially numbered, opaque, sealed envelope technique" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "single blind, parallel group, randomized, controlled trial", "The capsules were removed from their commercial blister strip packaging and repackaged in air‐tight, screw cap containers and suitably labelled and coded as trial medication. Repackaging was done with the help of residents not otherwise involved in the study. Capsule identity was not revealed to the patients who received the total medication in three installments" Judgement: participants were blinded, but investigator and observer might be not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "single blind, parallel group, randomized, controlled trial", "The capsules were removed from their commercial blister strip packaging and repackaged in air‐tight, screw cap containers and suitably labelled and coded as trial medication. Repackaging was done with the help of residents not otherwise involved in the study. Capsule identity was not revealed to the patients who received the total medication in three installments" Judgement: participants were blinded, but investigator and observer might be not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 6/32 (18.7%) in silodosin, 2/29 (6.9%) in tamsulosin lost to follow‐up: missing data were imputed by last observation carried forward strategy |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis. We received the data after contacting the study author. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Selective reporting (reporting bias) | Low risk | Judgement: protocol (Clinical Trials Registry‐India (CTRI/2014/01/004366)) was published and the prespecified outcomes were well described. Data (AUR, surgical intervention, cardiovascular, sexual adverse events) were given by contact with study author |
| Other bias | Low risk | Judgement: not detected |
| Methods | Study design: randomized, open‐label, controlled, multicenter study Setting/Country: Kobe University School or other collaborating institutions/Japan Dates when study was conducted: July 2007‐March 2011 | |
| Participants | Inclusion criteria: men with: BPH/ LUTS with a total IPSS ≥ 8 points; QoL index ≥ 3 points; Qmax < 15 mL/s; prostate volume ≥ 20 mL; and either without a history of using any a1‐receptor blocker (hereafter, drug‐naive group) or who had continued to use tamsulosin (0.2 mg once daily) for ≥ 3 months and wanted to switch the medication to another oral drug (hereafter, drug‐switching group) Exclusion criteria: other diseases such as prostate cancer, bladder tumor, cystolithiasis, prostatitis, urethral stricture, or active urinary tract infection; complication of neurogenic bladder or disease suspected of neurogenic bladder; and whose participation in the study was deemed inappropriate by their primary physician(s) Total number of participants randomly assigned: 121 Group A (Silodosin)
Group B (Naftopidil)
| |
| Interventions | Run‐in period: none Group A: silodosin 4 mg twice daily Group B: naftopidil 50 mg once daily Duration: 8 consecutive weeks | |
| Outcomes |
How measured: IPSS questionnaire Time points measured: at baseline, 4 and 8 weeks Time points reported: at baseline, 4 and 8 weeks Safety outcomes How measured: adverse events Time points measured: not reported Time points reported: cumulative results from follow‐up visits Subgroup: drug‐naïve/drug‐switching | |
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes | Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned (using a random number table)" |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no information given |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "randomized, open‐label, controlled multicenter study" Judgement: no one was blinded (protocol) |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "randomized, open‐label, controlled multicenter study" Judgement: no one was blinded (protocol) |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: 5/61 (8.2%) and 4/60 (6.7%) participants in silodosin and naftopidil were not included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | 2/61 (3.3%) and 3/60 (5.0%) participants in silodosin and naftopidil were not included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: 2/61 (3.3%) and 3/60 (5.0%) participants in silodosin and naftopidil were not included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: 2/61 (3.3%) and 3/60 (5.0%) participants in silodosin and naftopidil were not included in analysis |
| Selective reporting (reporting bias) | Low risk | Judgement: protocol (UMIN000008331) was published. While the results were shown separately in participants with drug naïve and switching group, review outcomes were well described |
| Other bias | Low risk | Not detected |
| Methods | Study design: randomized, cross‐over study Setting/Country: 4 community‐based hospitals/Japan Dates when study was conducted: February 2011‐July 2014 | |
| Participants | Inclusion criteria: men aged ≥ 50 years with LUTS/BPH, an IPSS of ≥ 8, QoL score of ≥ 3, and US‐estimated prostatic volume of ≥ 20 mL Exclusion criteria: those that had taken any alpha‐blocker within the previous 28 days; those currently taking phosphodiesterase type 5 inhibitors, 5‐ARIs, or antiandrogens; those with severe renal dysfunction (estimated glomerular filtration rate < 30 mL/min per 1.73 m2); and those judged to be inappropriate by the attending physicians Total number of participants randomly assigned: 34 Group A (Silodosin)
Group B (Tamsulosin)
| |
| Interventions | Run‐in period: none Group A: silodosin 4 mg once daily in the morning Group B: tamsulosin 0.2 mg once daily in the morning Duration: 4 weeks (before cross‐over)/total 8 weeks | |
| Outcomes | Primary outcome
How measured: IPSS questionnaire Time points measured: baseline to weeks 4 and 8 Time points reported: baseline to weeks 4 and 8 Secondary outcome
How measured: participant preference for the drug by self‐administered questionnaire Time points measured: before administration of the drug, and 4 weeks and 8 weeks Time points reported: before administration of the drug, and 4 weeks and 8 weeks Safety outcomes How measured: adverse events Time points measured: baseline to weeks 4 and 8 Time points reported: baseline to weeks 4 and 8 Subgroup: none | |
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes | Language of publication: English No wash‐out period between cross‐over Data (IPSS, QoL, treatment withdrawal, cardiovascular, sexual adverse events) were given by contact with study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement: author reply "random number table" |
| Allocation concealment (selection bias) | Low risk | Judgement: author reply "sealed opaque envelope" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open‐label, randomized crossover study" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "open‐label, randomized crossover study" |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 2/18 (11.2%) and 2/16 (12.5%) participants in the silodosin and tamsulosin group were not included in the analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: not available (cross‐over trial) |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Selective reporting (reporting bias) | Low risk | Judgement: protocol (JPRN‐UMIN000004918) was published and prespecified, study outcomes were analyzed as planned. Data (IPSS, QoL, treatment withdrawal, cardiovascular, sexual adverse events) were provided after contact with study author |
| Other bias | Unclear risk | Judgement: no wash‐out period |
| Methods | Study design: randomized, cross‐over study Setting/Country: 3 institutions/Japan Dates when study was conducted: February 2008‐September 2009 | |
| Participants | Inclusion criteria: men with LUTS associated with BPH and had an IPSS ≥ 8 and an IPSS‐QoL score ≥ 2 Exclusion criteria: not reported Total number of participants randomly assigned: 102 Group A (Silodosin)
Group B (Tamsulosin)
| |
| Interventions | Run‐in period: none Group A: silodosin 4 mg, orally, twice daily Group B: tamsulosin 0.2 mg, orally, once daily Duration: 4 weeks (before cross‐over)/total 8 weeks | |
| Outcomes | Primary outcome
How measured: questionnaire and open question Time points measured: 8 weeks Time points reported: 8 weeks Secondary outcome
How measured: not reported Time points measured: baseline and at 4 and 8 weeks Time points reported: baseline and at 4 and 8 weeks Safety outcomes How measured: adverse events Time points measured: not reported Time points reported: during the study Subgroup: age ≥ 70/IPSS ≥ 20 | |
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes | Language of publication: English No wash‐out period between cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement: no information given |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no information given |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open‐label study" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "open‐label study" |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 9/51 (17.7%) and 9/51 (17.7%) participants in the silodosin and tamsulosin group were not included in the analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: not available (cross‐over trial) |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 14/102 (13.8%) and 11/102 (10.8%) participants in the silodosin and tamsulosin group were not included in the analysis (study author reported the results after cross‐over) |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 14/102 (13.8%) and 11/102 (10.8%) participants in the silodosin and tamsulosin group were not included in the analysis (study author reported the results after cross‐over) |
| Selective reporting (reporting bias) | Unclear risk | Judgement: no protocol available |
| Other bias | Unclear risk | Judgement: no wash‐out period |
| Methods | Study design: parallel, randomized trial Setting/Country: Nihon University School of Medicine/Japan Dates when study was conducted: December 2007‐November 2010 | |
| Participants | Inclusion criteria: men with BPH≥ 50 years with significant LUTS and deteriorated QoL, with IPSS of ≥ 8 and its QoL score of ≥ 3 Exclusion criteria: established prostate cancer, neurogenic bladder and any other complications that affect micturitional status; underwent prostate surgery, intervention or radiation therapy Total number of participants randomly assigned: 109 Group A (Silodosin)
Group B (Naftopidil)
| |
| Interventions | Run‐in period: none Group A: silodosin 8 mg/d Group B: naftopidil 75 mg/d Duration: 12 weeks | |
| Outcomes |
How measured: questionnaire, uroflowmetry Time points measured: before and 4, 8, and 12 weeks after treatment Time points reported: IPSS, QoL, IIEF‐5: before and 4, 8, and 12 weeks after treatment/Qmax, PVR: before and 12 weeks after treatment Subgroup: none | |
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes | Language of publication: English Data (IPSS, QoL, treatment withdrawal, AUR, surgical intervention, cardiovascular adverse events) were given by contact with study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement: author reply "random number table envelope method" |
| Allocation concealment (selection bias) | Unclear risk | Judgement: author reply "random number table envelope method" |
| Blinding of participants and personnel (performance bias) | High risk | Judgement: author reply "not blinded" |
| Blinding of outcome assessment (detection bias) | High risk | Judgement: author reply "not blinded" |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | Judgement: 17/58 (29.3%) and 13/51 (25.5) participants in silodosin and naftopidil group were not included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis. We received the data after contacting the study author |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis. We received the data after contacting the study author |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis. We received the data after contacting the study author |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis. We received the data after contacting the study author |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all sexually active subjects (silodosin: 23/53 (44%), naftopidil: 21/44 (47%)) who were randomized in each group were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Judgement: data (treatment withdrawal, AUR, and surgical intervention) were given by contact with study author, but protocol was not published |
| Other bias | Low risk | Judgement: not detected |
| Methods | Study design: parallel, randomized trial Setting/Country: not reported Dates when study was conducted: not reported | |
| Participants | Inclusion criteria: men with IPSS total score ≥ 8, Qmax < 15 mL/s, total prostate volume measured by US > 20 mL Exclusion criteria: prostate cancer, urethral stricture, apparent neurogenic bladder and those on medication that might affect voiding function such as alpha‐blockers, anticholinergics and/or antiandrogen drugs Total number of participants randomly assigned: 149 Group A (Silodosin)
Group B (Tamsulosin)
| |
| Interventions | Run‐in period: none Group A: silodosin 4 mg twice daily Group B: tamsulosin 0.2 mg‐0.4 mg daily Duration: 12 months | |
| Outcomes |
How measured: questionnaire, uroflowmetry Time points measured: before, and at 1, 3, 6 and 12 months after the therapy Time points reported: before, and at 1, 3, 6 and 12 months after the therapy Subgroup: none | |
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes | Language of publication: English Publication status: abstract (full text has not been published; study author reply) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement: no information given |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no information given |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement: no information given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement: no information given |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | Judgement: 19/75 (25.3%) and 30/74 (40.5%) participants in silodosin and tamsulosin were not included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Selective reporting (reporting bias) | Unclear risk | Judgement: protocol was not published and no information related to the review outcomes given |
| Other bias | Unclear risk | Judgement: abstract only |
| Methods | Study design: parallel, randomized trial Setting/Country: Kawasaki Medical School/Japan Dates when study was conducted: June 2007‐December 2008 | |
| Participants | Inclusion criteria: men with LUTS aged 50–80 years and with IPSS ≥ 8 Exclusion criteria: received oral treatment with 5‐ARIs, anticholinergic agents, antidepressants, or sex hormonal agents, had neurogenic bladder dysfunction, bladder calculi, or active urinary tract infection, or had severe cardiac disease, renal dysfunction (serum creatinine > 2 mg/dL), or hepatic dysfunction Total number of participants randomly assigned: 136 Group A (Silodosin)
Group B (Tamsulosin)
Group C (Naftopidil)
| |
| Interventions | Run‐in period: none Group A: silodosin 4 mg twice a day Group B: tamsulosin 0.2 mg once a day Group C: naftopidil 50 mg once a day Duration: 12 weeks | |
| Outcomes |
How measured: questionnaire (IPSS, QoL, IIEF), uroflowmetry, US (PVR) Time points measured: before, and 1 and 3 months Time points reported: before, and 1 and 3 months Subgroup: none | |
| Funding sources | Not reported | |
| Declarations of interest | None (study author reply) | |
| Notes | Language of publication: English Data (IPSS, QoL, AUR, and surgical intervention) from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement: study author reply "Computer generated central randomization" |
| Allocation concealment (selection bias) | Low risk | Judgement: study author reply "Computer generated central randomization" |
| Blinding of participants and personnel (performance bias) | High risk | Judgement: study author reply "participants were not blinded" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement: no information given |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 4/45 (8.9%), 6/45 (13.3%), and 4/46 (8.7) participants in silodosin, tamsulosin, and naftopidil group were not included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 4/45 (8.9%), 6/45 (13.3%), and 4/46 (8.7) participants in silodosin, tamsulosin, and naftopidil group were not included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 4/45 (8.9%), 6/45 (13.3%), and 4/46 (8.7) participants in silodosin, tamsulosin, and naftopidil group were not included in analysis. We received the data after contacting the study author |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 4/45 (8.9%), 6/45 (13.3%), and 4/46 (8.7) participants in silodosin, tamsulosin, and naftopidil group were not included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all sexually active subjects (silodosin: 11/45 (24.4%), tamsulosin 12/45 (26.6%), naftopidil: 15/46 (31.9%)) were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Judgement: prespecified outcomes were well described and data were given by study author, but protocol was not published |
| Other bias | High risk | Judgement: drug administration times were different among groups. Baseline imbalance in PVR, but the fact that silodosin group had much higher PVR may underestimate the effect size |
| Methods | Study design: randomized, cross‐over study Setting/Country: single center/Japan Dates when study was conducted: June 2008‐March 2010 | |
| Participants | Inclusion criteria: men aged 50 years who had a total IPSS ≥ 8 and a QoL index ≥ 3 Exclusion criteria: prostate cancer, neurogenic bladder, urethral stricture, active urinary tract infection and other complications considered likely to affect micturition Total number of participants randomly assigned: 46 Group A (Silodosin)
Group B (Tamsulosin)
| |
| Interventions | Run‐in period: none Group A: silodosin 4 mg twice daily Group B: tamsulosin 0.2 mg once daily Duration: 3 months (before cross‐over)/1 month wash‐out/3 months (after cross‐over)/total 7 months | |
| Outcomes |
How measured: IPSS questionnaire and transabdominal US (PVR) Time points measured: before and after treatment (not reported in method) Time points reported: before and after treatment (1 months, 3 months, 4 months, and 7 months) Safety outcomes How measured: adverse events Time points measured: not reported Time points reported: not reported Subgroup: none | |
| Funding sources | None | |
| Declarations of interest | None | |
| Notes | Language of publication: English One month wash‐out period between cross‐over Data (IPSS, QoL, treatment withdrawal for any reason, method of random sequence generation, allocation concealment, and blinding) were given by contact with study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement: study author reply "random number table" |
| Allocation concealment (selection bias) | Unclear risk | Judgement: study author reply "sealed envelope" |
| Blinding of participants and personnel (performance bias) | High risk | Judgement: study author reply "participants were blinded" |
| Blinding of outcome assessment (detection bias) | High risk | Judgement: study author reply "participants were blinded" |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 5/46 (10.9%) participants were not included in the analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: not available (cross‐over trial) |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Judgement: prespecified outcomes are well described and data were given by study author, but protocol was not published (study author confirmed via email) |
| Other bias | Low risk | Judgement: not detected |
| Methods | Study design: parallel, randomized, double‐blinded Setting/Country: 9 medical centers/Taiwan Dates when study was conducted: July 2007‐September 2008 | |
| Participants | Inclusion criteria: men aged ≥ 40 years with an IPSS of ≥ 13, a health‐related QoL score of ≥ 3, a prostate volume of ≥ 20 mL, and a Qmax of < 15 mL/s with a voided volume of ≥ 100 mL Exclusion criteria: men with a history of previous prostate surgery, prostate cancer, neurogenic bladder, bladder neck constriction, urethral stricture, bladder calculus, active urinary tract infection, a PVR of > 250 mL, exposure to sex hormone within 3 months prior to the wash‐out period, renal dysfunction (serum creatinine of > 2.0 mg/dL), a history of severe liver impairment, severe cardiovascular diseases, severe hypotension, and known hypersensitivity or history of active substance abuse (including alcohol) within the past 2 years Total number of participants randomly assigned: 209 Group A (Silodosin)
Group B (Tamsulosin)
| |
| Interventions | Run‐in period: 7‐day wash‐out and 7‐day observation periods Group A: silodosin 4 mg twice daily Group B: tamsulosin 0.2 mg in the morning and one placebo capsule in the evening Duration: 12 weeks | |
| Outcomes | Primary outcome
How measured: IPSS questionnaire Time points measured: 0 (initiation of treatment), 2, 4, 8, and 12 weeks Time points reported: 0 (initiation of treatment), 2, 4, 8, and 12 weeks in figure Secondary outcome
How measured: uroflowmetry, IPSS questionnaire Time points measured: 0, 4 and 12 weeks/0 (initiation of treatment), 2, 4, 8, and 12 weeks Time points reported: 0 and 12 weeks Safety outcomes How measured: all adverse events/BP/pulse rate/laboratory tests Time points measured: 0 (initiation of treatment), 2, 4, 8, and 12 weeks/0, 4 and 12 weeks/follow‐up visits Time points reported: 0 and 12 weeks/cumulative results from follow‐up visits (adverse events) Subgroup: none | |
| Funding sources | Synmosa pharmaceutical company | |
| Declarations of interest | All authors were study investigators for Synmosa | |
| Notes | Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement: no information given |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no information given |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "both investigators and patients were ‘blinded’ to treatment" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "both investigators and patients were ‘blinded’ to treatment" |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement: objective outcomes are not likely affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 18/105 (17.1%), 21/104 (20.2%) participants who were randomized in silodosin, tamsulosin group were not included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: all participants who were randomized in each group were included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: no information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 18/105 (17.1%), 21/104 (20.2%) participants who were randomized in silodosin, tamsulosin group were not included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement: 18/105 (17.1%), 21/104 (20.2%) participants who were randomized in silodosin, tamsulosin group were not included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Judgement: protocol was published, but protocol was not found due to the absence of protocol number |
| Other bias | High risk | Judgement: serious baseline imbalance in age, IPSS, prostate volume, health‐related QoL, Qmax in as treated analysis. Two weeks' observation run‐in period |
5‐ARIs: 5‐alpha reductase inhibitors; AUR: acute urinary retention; BP: blood pressure; BPH: benign prostatic hyperplasia; IIEF: International Index of Erectile Function; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; OABSS: Overactive Bladder Symptom Score; PSA: prostate‐specific antigen; PVR: postvoid residual; Qmax; maximum flow rate; QoL: quality of life; US: ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Review | |
| Review | |
| Wrong study design (non randomized trial) | |
| Wrong study design (study design not described, silodosin versus silodosin plus Serenoa repens) | |
| Wrong comparator (silodosin versus transurethral incision of prostate) | |
| Wrong study design (integrated safety analysis) | |
| Review | |
| Wrong comparator (silodosin 4 mg once daily versus silodosin 4 mg twice daily) | |
| Wrong study design (non randomized study) | |
| Wrong outcome (semen parameters) | |
| Wrong outcome (semen parameters) | |
| Wrong outcome (cost effectiveness) | |
| Wrong comparator (silodosin versus silodosin and propiverine) | |
| Wrong comparator (silodosin and dutasteride versus dutasteride) | |
| Wrong study population (participants with ≥ 2 voids per night ) | |
| Review | |
| Review | |
| Review (medical article) | |
| Review (medical article) | |
| Wrong study population (participants with retrograde ejaculation as adverse event) | |
| Wrong comparator (silodosin versus phosphodiesterase 5 inhibitor) | |
| Wrong comparator (silodosin versus silodosin and propiverine) | |
| Wrong outcome (pharmacokinetics and adverse events (not available)) |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomized, parallel‐group, active, controlled trial |
| Participants | Inclusion criteria
|
| Interventions | Group A: silodosin capsule 8 mg Group B: tamsulosin ER capsules 0.4 mg Duration: 12 weeks |
| Outcomes |
|
| Notes | Funding source: MSN Laboratories Ltd Publication status: CTRI/2010/091/000526 (final publication status has not been clarified) |
| Methods | Open‐label, parallel study |
| Participants | Inclusion criteria: men with symptomatic BPH aged 50‐80 years, with IPSS > 8 or QoL score > 2 Exclusion criteria: not reported |
| Interventions | Group A: silodosin 8 mg/d Group B: tamsulosin 0.4 mg/d Group C: alfuzosin 10 mg/d Duration: 1 month |
| Outcomes |
|
| Notes | Publication status: abstract (final publication status has not been clarified) |
| Methods | Parallel, randomized study |
| Participants | Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | Group A: silodosin 8 mg daily at night Group B: tamsulosin 0.4 mg daily at night Duration: 6 months |
| Outcomes |
|
| Notes | Publication status: abstract (final publication status has not been clarified) |
| Methods | Open‐label, parallel, randomized study |
| Participants | Inclusion criteria: male patients with newly diagnosed LUTS according to the treatment algorithm of the Clinical Practice Guidelines for male LUTS Exclusion criteria: men who meet any of the following criteria are excluded:
|
| Interventions | Group A: silodosin 8 mg daily Group B: tamsulosin 0.2 mg daily Duration: not reported |
| Outcomes |
|
| Notes | Funding source: none Publication status: JPRN‐UMIN000003125 (final publication status has not been clarified) |
| Methods | Parallel, randomized study |
| Participants | Inclusion criteria Men aged ≥ 50 years with previously‐untreated BPH with total IPSS ≥ 8 and QoL score ≥ 2 |
| Interventions | Group A: silodosin 4 mg twice daily Group B: tamsulosin hydrochloride 0.2 mg daily Duration: not reported |
| Outcomes |
|
| Notes | Funding source: none Publication status: JPRN‐UMIN000005151 (final publication status has not been clarified) |
| Methods | Parallel, randomized study |
| Participants | Inclusion criteria Men aged 50‐90 years 1) a total IPSS of ≥ 8 2) Qmax of ≤ 15 mL/s as evaluated by uroflowmetry 3) prostate volume of ≥ 15 mL as measured by prostatic US |
| Interventions | Group A: silodosin Group B: tamsulosin Duration: not reported |
| Outcomes |
|
| Notes | Funding source: Dokkyo Medical University Publication status: JPRN‐UMIN000008538 (final publication status has not been clarified) |
| Methods | Parallel, randomized study |
| Participants | Inclusion criteria Men aged ≥ 50 years
|
| Interventions | Group A: silodosin 4 mg, once daily after breakfast Group B: tamsulosin 0.2 mg, once daily after breakfast Duration: not reported |
| Outcomes |
|
| Notes | Funding source: Kissei Pharmaceutical Co., Ltd Publication status: JPRN‐UMIN000011556 (final publication status has not been clarified) |
| Methods | Open‐label, parallel study |
| Participants | Inclusion criteria: men with symptomatic BPH aged 50‐80 years with IPSS > 8 or QoL score > 2 Exclusion criteria: other causes of bladder outlet obstruction, prostate and bladder malignancy, renal failure, hepatic failure and prior history of prostatectomy |
| Interventions | Group A: silodosin 8 mg/d Group B: tamsulosin 0.4 mg/d Group C: alfuzosin 10 mg/d Duration: 1 month |
| Outcomes |
|
| Notes | Publication status: abstract (final publication status has not been clarified) |
| Methods | Parallel, randomized study |
| Participants | Inclusion criteria: patients of LUTS in BPH Exclusion criteria: not reported |
| Interventions | Group A: silodosin 8 mg once/d Group B: tamsulosin 0.4 mg once/d Group C: alfuzosin 10 mg once/d Duration: 12 weeks |
| Outcomes |
|
| Notes | Publication status: abstract (final publication status has not been clarified) |
| Methods | Parallel, randomized study |
| Participants | Inclusion criteria: men with untreated BPH
Exclusion criteria: not reported |
| Interventions | Group A: silodosin 4 mg twice daily Group B: tamsulosin 0.2 mg once daily Duration: 1 week |
| Outcomes |
|
| Notes | Publication status: abstract (final publication status has not been clarified) |
| Methods | Parallel, randomized, double‐blind, placebo‐controlled study |
| Participants | Inclusion criteria: men with BPH and LUTS Exclusion criteria: history of prostatectomy, intrapelvic radiation therapy, thermotherapy of prostate or prostatic hyperthermia, prostate cancer or suspected prostate cancer, any clinically relevant cardiovascular, hepatic or renal disorder |
| Interventions | Group A: not reported Group B: not reported Duration: 12 weeks |
| Outcomes |
|
| Notes | Funding source: Kissei Pharmaceutical Co., Ltd Publication status: NCT01222650 (final publication status has not been clarified) |
| Methods | Parallel, randomized study |
| Participants | Inclusion criteria: men > 45 years with symptomatic BPH (increased daytime frequency, urgency and nocturia and or voiding symptoms (hesitancy, incomplete voiding, impaired stream or interruption of stream), increased daytime micturition, nocturia > 2, maximum flow rate < 15 mL/sec with a voided volume of ≥ 150 mL, PVR < 100 mL by abdominal US, IPSS > 13 points, international prostatic symptom bother score > 3 points Exclusion criteria: not reported |
| Interventions | Group A: silodosin 8 mg once daily Group B: tamsulosin 0.2 mg once daily Duration: 12 weeks |
| Outcomes |
|
| Notes | Publication status: abstract (final publication status has not been clarified) |
BPH: benign prostatic hyperplasia; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; PSA: prostate specific antigen; PVR: postvoid residual; Qmax; maximum flow rate; QoL: quality of life; US: ultrasound
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A study to find out the medication which offers maximum health benefits with minimum spending by the patient among the three commonly used medications in the treatment of benign prostatic hyperplasia, a disease in aging men which results in bothersome urinary problems |
| Methods | Randomized, parallel group trial |
| Participants | Inclusion criteria
|
| Interventions | Group A: silodosin oral, 8 mg once daily Group B: tamsulosin oral, 0.4 mg once daily Group C: alfuzosin oral, 10 mg once daily Duration: 12 weeks |
| Outcomes | Treatment success is defined as ≥ 30% improvement in IPSS from baseline and its maintenance over the study period |
| Starting date | September 2013 |
| Contact information | |
| Notes | Funding source: none |
| Trial name or title | The effects of selective alpha‐1‐adrenergic receptor antagonists in patients with lower urinary tract symptoms caused by benign prostatic hyperplasia who failed to obtain sufficient efficacy by previous alpha‐1‐blockades. A comparison of naftopidil or silodosin |
| Methods | Parallel, randomized |
| Participants | Inclusion criteria Men with LUTS caused by BPH who failed to obtain sufficient efficacy by naftopidil 50 mg and with IPSS of ≥ 9, a QoL score of ≥ 2, a prostate volume of ≥ 20 mL are eligible for enrolment |
| Interventions | Group A: silodosin 8 mg Group B: naftopidil 75 mg Duration: not reported |
| Outcomes |
|
| Starting date | May 2010 |
| Contact information | Email address was not given |
| Notes | Funding source: none |
BPH: benign prostatic hyperplasia; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; Qmax; maximum flow rate; QoL: quality of life
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urologic symptom scores (short term) Show forest plot | 3 | 1743 | Mean Difference (IV, Random, 95% CI) | ‐2.65 [‐3.23, ‐2.08] |
| Analysis 1.1  Comparison 1 Silodosin versus placebo, Outcome 1 Urologic symptom scores (short term). | ||||
| 2 Quality of life (short term) Show forest plot | 2 | 820 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.71, ‐0.13] |
| Analysis 1.2  Comparison 1 Silodosin versus placebo, Outcome 2 Quality of life (short term). | ||||
| 3 Treatment withdrawal due to any reason (short term) Show forest plot | 3 | 1703 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.70, 1.66] |
| Analysis 1.3  Comparison 1 Silodosin versus placebo, Outcome 3 Treatment withdrawal due to any reason (short term). | ||||
| 4 Treatment withdrawal due to adverse events (short term) Show forest plot | 4 | 1967 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [1.41, 3.72] |
| Analysis 1.4 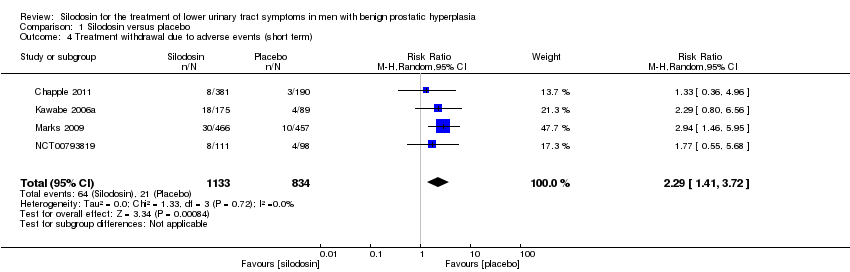 Comparison 1 Silodosin versus placebo, Outcome 4 Treatment withdrawal due to adverse events (short term). | ||||
| 5 Cardiovascular adverse events (short term) Show forest plot | 4 | 1967 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.67, 2.45] |
| Analysis 1.5  Comparison 1 Silodosin versus placebo, Outcome 5 Cardiovascular adverse events (short term). | ||||
| 6 Sexual adverse events (short term) Show forest plot | 4 | 1967 | Risk Ratio (M‐H, Random, 95% CI) | 26.07 [12.36, 54.97] |
| Analysis 1.6  Comparison 1 Silodosin versus placebo, Outcome 6 Sexual adverse events (short term). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urologic symptom scores (short term) Show forest plot | 10 | 1708 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐1.31, 1.24] |
| Analysis 2.1  Comparison 2 Silodosin versus tamsulosin, Outcome 1 Urologic symptom scores (short term). | ||||
| 2 Quality of life (short term) Show forest plot | 10 | 1707 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.53, 0.22] |
| Analysis 2.2  Comparison 2 Silodosin versus tamsulosin, Outcome 2 Quality of life (short term). | ||||
| 3 Treatment withdrawal due to any reason (short term) Show forest plot | 10 | 1573 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.62, 1.69] |
| Analysis 2.3  Comparison 2 Silodosin versus tamsulosin, Outcome 3 Treatment withdrawal due to any reason (short term). | ||||
| 4 Treatment withdrawal due to adverse events (short term) Show forest plot | 9 | 1572 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.12, 3.44] |
| Analysis 2.4 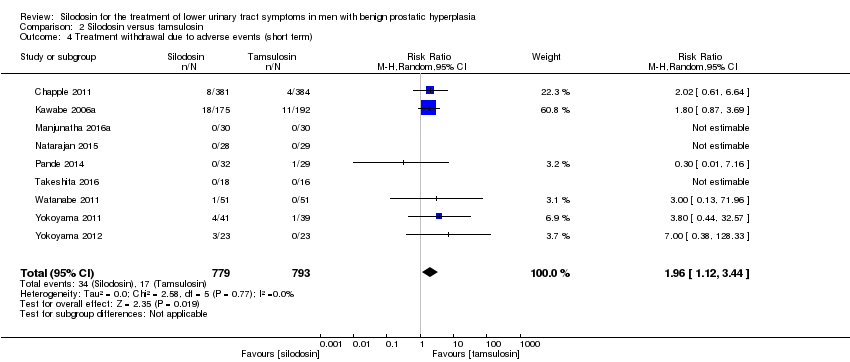 Comparison 2 Silodosin versus tamsulosin, Outcome 4 Treatment withdrawal due to adverse events (short term). | ||||
| 5 Cardiovascular adverse events (short term) Show forest plot | 11 | 1955 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.53, 1.12] |
| Analysis 2.5 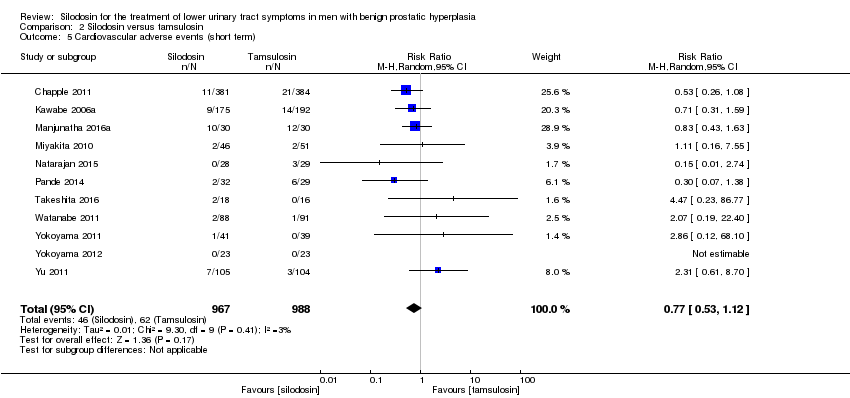 Comparison 2 Silodosin versus tamsulosin, Outcome 5 Cardiovascular adverse events (short term). | ||||
| 6 Sexual adverse events (short term) Show forest plot | 10 | 1849 | Risk Ratio (M‐H, Random, 95% CI) | 6.05 [3.55, 10.31] |
| Analysis 2.6 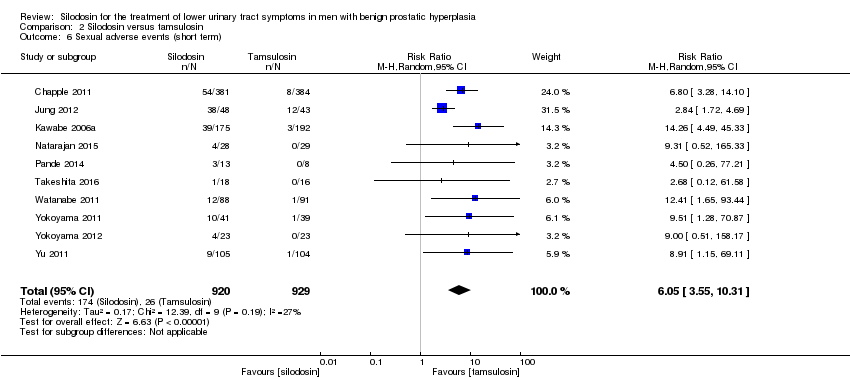 Comparison 2 Silodosin versus tamsulosin, Outcome 6 Sexual adverse events (short term). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urologic symptom scores (short term) Show forest plot | 5 | 652 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐2.57, 0.87] |
| Analysis 3.1  Comparison 3 Silodosin versus naftopidil, Outcome 1 Urologic symptom scores (short term). | ||||
| 2 Quality of life (short term) Show forest plot | 5 | 652 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.60, 0.27] |
| Analysis 3.2  Comparison 3 Silodosin versus naftopidil, Outcome 2 Quality of life (short term). | ||||
| 3 Treatment withdrawal due to any reason (short term) Show forest plot | 4 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.81, 1.93] |
| Analysis 3.3 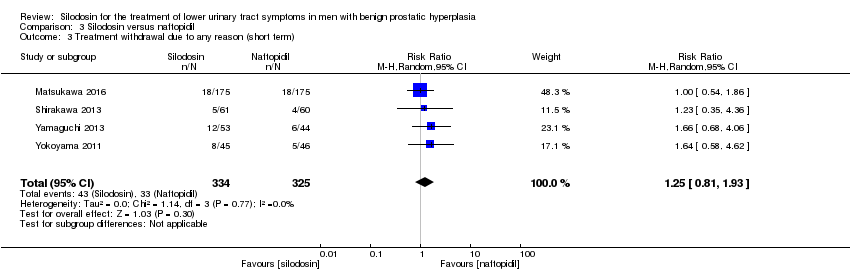 Comparison 3 Silodosin versus naftopidil, Outcome 3 Treatment withdrawal due to any reason (short term). | ||||
| 4 Treatment withdrawal due to adverse events (short term) Show forest plot | 5 | 738 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.66, 2.89] |
| Analysis 3.4 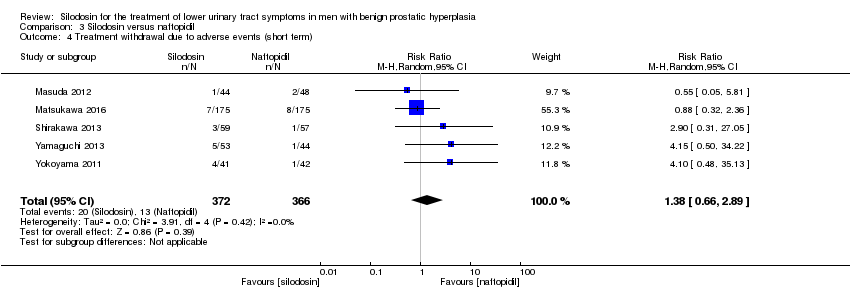 Comparison 3 Silodosin versus naftopidil, Outcome 4 Treatment withdrawal due to adverse events (short term). | ||||
| 5 Cardiovascular adverse events (short term) Show forest plot | 5 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.41, 2.56] |
| Analysis 3.5  Comparison 3 Silodosin versus naftopidil, Outcome 5 Cardiovascular adverse events (short term). | ||||
| 6 Sexual adverse events (short term) Show forest plot | 4 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 5.93 [2.16, 16.29] |
| Analysis 3.6  Comparison 3 Silodosin versus naftopidil, Outcome 6 Sexual adverse events (short term). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urologic symptom scores (short term) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 3.83 [0.12, 7.54] |
| Analysis 4.1  Comparison 4 Silodosin versus alfuzosin, Outcome 1 Urologic symptom scores (short term). | ||||
| 2 Quality of life (short term) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.46, 0.74] |
| Analysis 4.2  Comparison 4 Silodosin versus alfuzosin, Outcome 2 Quality of life (short term). | ||||
| 3 Cardiovascular adverse events (short term) Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.36, 1.24] |
| Analysis 4.3  Comparison 4 Silodosin versus alfuzosin, Outcome 3 Cardiovascular adverse events (short term). | ||||
| 4 Sexual adverse events (short term) Show forest plot | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 37.21 [5.32, 260.07] |
| Analysis 4.4  Comparison 4 Silodosin versus alfuzosin, Outcome 4 Sexual adverse events (short term). | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Silodosin versus placebo, Outcome 1 Urologic symptom scores (short term).

Comparison 1 Silodosin versus placebo, Outcome 2 Quality of life (short term).

Comparison 1 Silodosin versus placebo, Outcome 3 Treatment withdrawal due to any reason (short term).

Comparison 1 Silodosin versus placebo, Outcome 4 Treatment withdrawal due to adverse events (short term).

Comparison 1 Silodosin versus placebo, Outcome 5 Cardiovascular adverse events (short term).

Comparison 1 Silodosin versus placebo, Outcome 6 Sexual adverse events (short term).

Comparison 2 Silodosin versus tamsulosin, Outcome 1 Urologic symptom scores (short term).

Comparison 2 Silodosin versus tamsulosin, Outcome 2 Quality of life (short term).
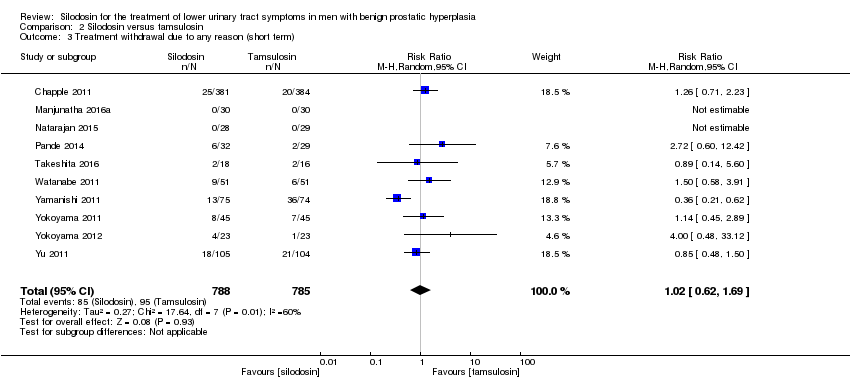
Comparison 2 Silodosin versus tamsulosin, Outcome 3 Treatment withdrawal due to any reason (short term).

Comparison 2 Silodosin versus tamsulosin, Outcome 4 Treatment withdrawal due to adverse events (short term).

Comparison 2 Silodosin versus tamsulosin, Outcome 5 Cardiovascular adverse events (short term).
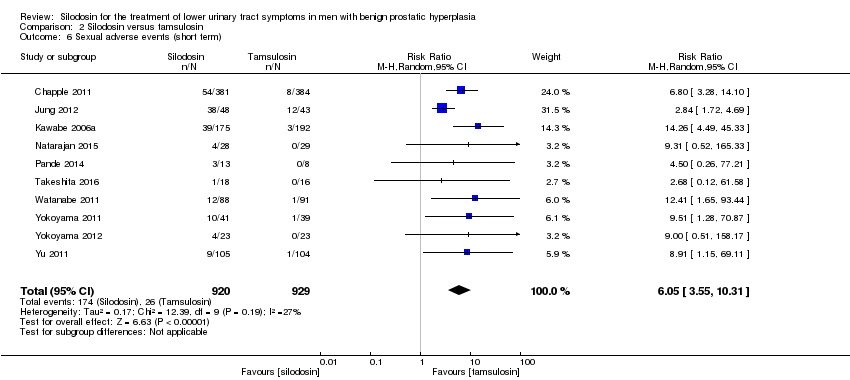
Comparison 2 Silodosin versus tamsulosin, Outcome 6 Sexual adverse events (short term).

Comparison 3 Silodosin versus naftopidil, Outcome 1 Urologic symptom scores (short term).
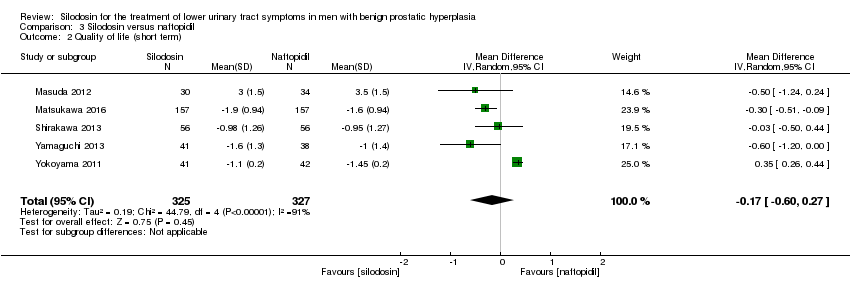
Comparison 3 Silodosin versus naftopidil, Outcome 2 Quality of life (short term).

Comparison 3 Silodosin versus naftopidil, Outcome 3 Treatment withdrawal due to any reason (short term).

Comparison 3 Silodosin versus naftopidil, Outcome 4 Treatment withdrawal due to adverse events (short term).
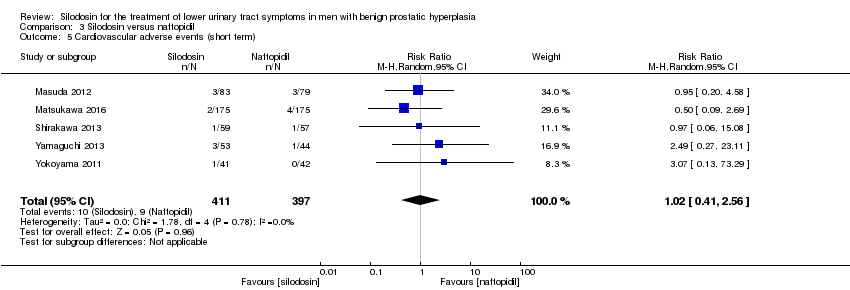
Comparison 3 Silodosin versus naftopidil, Outcome 5 Cardiovascular adverse events (short term).

Comparison 3 Silodosin versus naftopidil, Outcome 6 Sexual adverse events (short term).

Comparison 4 Silodosin versus alfuzosin, Outcome 1 Urologic symptom scores (short term).

Comparison 4 Silodosin versus alfuzosin, Outcome 2 Quality of life (short term).

Comparison 4 Silodosin versus alfuzosin, Outcome 3 Cardiovascular adverse events (short term).

Comparison 4 Silodosin versus alfuzosin, Outcome 4 Sexual adverse events (short term).
| Silodosin compared to placebo for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia (short term) | |||||
| Participants: men with lower urinary tract symptoms suggesting benign prostatic hyperplasia Setting: likely outpatients Intervention: silodosin Comparator: placebo | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk difference with silodosin | ||||
| Urologic symptom scores | 1743 | ⊕⊕⊝⊝ | ‐ | The mean change of urologic symptom scores ranged from ‐5.30 to ‐3.50 | MD 2.65 lower |
| QoL | 820 | ⊕⊕⊕⊝ | ‐ | The mean change of QoL ranged from ‐1.10 to ‐0.80 | MD 0.42 lower |
| Treatment withdrawal due to any reason | 1703 | ⊕⊕⊝⊝ | RR 1.08 | Study population | |
| 83 per 1000 | 7 more per 1000 | ||||
| Cardiovascular adverse events | 1967 | ⊕⊝⊝⊝ | RR 1.28 | Study population | |
| 42 per 1000 | 12 more per 1000 | ||||
| Assumed baseline riske | |||||
| 61 per 1000 | 17 more per 1000 | ||||
| Sexual adverse events | 1967 | ⊕⊕⊝⊝ | RR 26.07 | Study population | |
| 7 per 1000 | 180 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level for study limitations: unclear or high risk of bias for one or more domains among the included studies. | |||||
| Silodosin compared to tamsulosin for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia (short term) | |||||
| Participants: men with lower urinary tract symptoms suggesting benign prostatic hyperplasia Setting: likely outpatients Intervention: silodosin Comparator: tamsulosin | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with tamsulosin | Risk difference with silodosin | ||||
| Urologic symptom scores | 1708 | ⊕⊕⊝⊝ | ‐ | The mean change of urologic symptom scores ranged from ‐15.60 to ‐4.60 | MD 0.04 lower |
| QoL | 1707 | ⊕⊕⊝⊝ | ‐ | The mean change of QoL ranged from ‐3.60 to ‐0.90 | MD 0.15 lower |
| Treatment withdrawal due to any reason | 1573 | ⊕⊝⊝⊝ | RR 1.02 | Study population | |
| 121 per 1000 | 2 fewer per 1000 | ||||
| Cardiovascular adverse events | 1955 | ⊕⊕⊝⊝ | RR 0.77 | Study population | |
| 63 per 1000 | 14 fewer per 1000 | ||||
| Sexual adverse events | 1849 | ⊕⊕⊕⊝ | RR 6.05 | Study population | |
| 28 per 1000 | 141 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level for study limitations: unclear or high risk of bias for one or more domains among the included studies. | |||||
| Silodosin compared to naftopidil for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia (short term) | |||||
| Participants: men with lower urinary tract symptoms suggesting benign prostatic hyperplasia Setting: likely outpatients Intervention: silodosin Comparator: naftopidil | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with naftopidil | Risk difference with silodosin | ||||
| Urologic symptom scores | 652 | ⊕⊕⊝⊝ | ‐ | The mean change of urologic symptom scores ranged from ‐7.52 to ‐3.56 | MD 0.85 lower |
| QoL | 652 | ⊕⊕⊝⊝ | ‐ | The mean change of QoL ranged from ‐1.60 to ‐0.95 | MD 0.17 lower |
| Treatment withdrawal due to any reason | 659 | ⊕⊕⊝⊝ | RR 1.25 | Study population | |
| 102 per 1000 | 25 more per 1000 | ||||
| Cardiovascular adverse events | 808 | ⊕⊕⊝⊝ | RR 1.02 | Study population | |
| 23 per 1000 | 0 more per 1000 | ||||
| Sexual adverse events | 405 | ⊕⊕⊕⊝ | RR 5.93 | Study population | |
| 15 per 1000 | 74 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level for study limitations: unclear or high risk of bias for one or more domains among the included studies. | |||||
| Silodosin compared to alfuzosin for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia (short term) | |||||
| Participants: men with lower urinary tract symptoms suggesting benign prostatic hyperplasia Setting: likely outpatients Intervention: silodosin Comparator: alfuzosin | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with alfuzosin | Risk difference with silodosin | ||||
| Urologic symptom scores | 60 | ⊕⊕⊝⊝ | ‐ | The mean change of urologic symptom scores was ‐16.93 | MD 3.83 higher |
| QoL | 60 | ⊕⊕⊕⊝ | ‐ | The mean change of QoL was ‐4.27 | MD 0.14 higher |
| Treatment withdrawal due to any reason | 60 | ⊕⊕⊝⊝ | Not estimable | Study population | |
| ‐ | ‐ | ||||
| Cardiovascular adverse events | 60 | ⊕⊕⊝⊝ | RR 0.67 | Study population | |
| 500 per 1000 | 165 fewer per 1000 | ||||
| Assumed baseline riskd | |||||
| 44 per 1000 | 15 fewer per 1000 | ||||
| Sexual adverse events | 95 | ⊕⊕⊝⊝ | RR 37.21 | Study population | |
| 21 per 1000 | 770 more per 1000 | ||||
| Assumed baseline riskd | |||||
| 6 per 1000 | 217 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level for study limitations: unclear or high risk of bias for one or more domains in the included study. | |||||
| Study name | Trial | Country | Setting | Description of participants | Intervention(s) | Duration of | Age | Prostate volume | IPSS |
| 2006‐2007 | Europe | 72 hospital clinics and inpatient units in 11 countries | Men ≥ 50 years with LUTS (defined by a stable IPSS total score ≥ 13 points), bladder outlet obstruction (defined by a Qmax between 4 and 15 mL/s, with a minimum voided volume of ≥ 125 mL) | Silodosin 8 mg once daily | 12 weeks | 65.8 ± 7.70 | NR | 19.1 ± 4.2 | |
| Tamsulosin 0.4 mg once daily | 65.9 ± 7.41 | 18.9 ± 4.3 | |||||||
| Placebo once daily | 66.0 ± 7.37 | 19.3 ± 4.3 | |||||||
| NR | South Korea | NR | Sexually active men with BPH | Silodosin 8 mg once daily | 4 weeks | NR | NR | NR | |
| Tamsulosin 0.2 mg once daily | |||||||||
| Alfuzosin 10 mg once daily | |||||||||
| NR | Japan | 88 centers/outpatient | Men ≥ 50 years with LUTS (IPSS of ≥ 8, an associated QoL score of ≥ 3) and prostate volume of ≥ 20 mL | Silodosin 4 mg twice daily | 12 weeks | 65.4 ± 7.0 | 36.0 ± 16.9 | 17.1 ± 5.7 | |
| Tamsulosin 0.2 mg once daily | 65.6 ± 7.0 | 35.7 ± 14.4 | 17.0 ± 5.7 | ||||||
| Placebo twice daily | 65.0 ± 6.9 | 35.2 ± 16.0 | 17.1 ± 6.1 | ||||||
| 2013‐2014 | India | Tertiary care hospital | Men ≥ 45 years with symptomatic BPH with LUTS (IPSS of ≥ 8, QoL of ≥ 3, and Qmax of < 15 mL/s, but > 4 mL/s with a voided volume of > 100 mL) | Silodosin 8 mg once daily | 12 weeks | 64.00 ± 11.14 | 40.57 ± 16.45 | 15.93 ± 6.03 | |
| Tamsulosin 0.4 mg once daily | 63.60 ± 9.05 | 40.33 ± 21.55 | 21.63 ± 7.63 | ||||||
| Alfuzosin 10 mg once daily | 63.43 ± 8.91 | 44.43 ± 27.72 | 19.2 ± 9.6 | ||||||
| 2005‐2006 | USA | Multicenter | Men ≥ 50 years with IPSS ≥ 13, Qmax 4 mL/s‐15 mL/s and a PVR < 250 mL | Silodosin 8 mg once daily | 12 weeks | 64.6 ± 8.1 | NR | 21.3 ± 5.1 | |
| Placebo once daily | 64.7 ± 8.1 | 21.3 ± 4.9 | |||||||
| 2009‐2011 | Japan | NR | Men ≥ 50 years with prostate estimated volume of > 20 mL, IPSS ≥ 8, QoL score ≥ 3 points | Silodosin 2 mg‐4 mg twice/d for 2 weeks, followed by 4 mg twice/d for 4 weeks | 6 weeks (before cross‐over)/total 12 weeks | 66.5 ± 5.6 | 38.8 ± 13.1 | 18.6 ± 5.5 | |
| Naftopidil 50 mg‐75 mg once/d for 2 weeks, followed by 75 mg once/d for 4 weeks | 68.5 ± 5.7 | 45.7 ± 17.8 | 17.6 ± 5.0 | ||||||
| 2012‐2013 | Japan | 52 urologists participated at a total of 44 investigational sites/outpatients | Men with LUTS (IPSS ≥ 8, IPSS QoL score 3) and prostate volume ≥ 20 mL | Silodosin 4 mg/d for 4 weeks, followed by 8 mg/d for 8 weeks | 12 weeks | 70.6 ± 7.8 | 39.6 ± 16.7 | 18.8 ± 6.2 | |
| Naftopidil 50 mg/d for 4 weeks, followed by 75 mg/d for 8 weeks | 70.3 ± 7.8 | 38.6 ± 14.8 | 18.9 ± 6.1 | ||||||
| 2006‐2007 | Japan | Multicenter | Men with IPSS ≥ 8 points; QoL score ≥ 3 points; prostate volume measured by ultrasonographic method ≥ 20 mL; void volume ≥ 100 mL; and maximal urinary flow rate (Qmax) < 15 mL/s | Silodosin 4 mg twice daily | 4 weeks (before cross‐over)/total 8 weeks | 68.2 ± 8.6 | 41.3 ± 25.3 | 16.6 ± 5.2 | |
| Tamsulosin 0.2 mg once daily | 70.1 ± 8.9 | 37.8 ± 16.3 | 18.2 ± 5.8 | ||||||
| 2013‐2015 | India | Tertiary hospital | Men > 50 years with bothersome LUTS from BPH and IPSS > 7 | Silodosin 8 mg once daily | 12 weeks | 61 ‐ 62 | NR | NR | |
| Tamsulosin 0.4 mg once daily | |||||||||
| 2009 | NR | NR | Men ≥ 50 years, with symptoms of moderate‐severe BPH and nocturia (≥ 2 episodes/night) | Silodosin 8 mg daily | 12 weeks | 64.6 ± 8.03 | NR | NR | |
| Placebo once daily | 64.2 ± 8.92 | ||||||||
| 2012‐2013 | India | Tertiary care hospital/ outpatient | Men > 50 years with bothersome LUTS from BPH and IPSS > 7 | Silodosin 8 mg once daily | 12 weeks | 61.4 ± 7.88 | 42.0 ± 19.96 | 18.4 ± 3.32 | |
| Tamsulosin 0.4 mg once daily | 62.6 ± 7.55 | 35.6 ± 9.56 | 18.4 ± 3.94 | ||||||
| 2007‐2011 | Japan | Kobe University School or other collaborating institutions | Men with LUTS (total IPSS ≥ 8, QoL index ≥ 3) and prostate volume ≥ 20 mL | Silodosin 4 mg twice daily | 8 weeks | 70.98 ± 6.69 | 38.24 ± 12.94 | 17.53 ± 5.4 | |
| Naftopidil 50 mg once daily | 70.50 ± 6.58 | 39.39 ± 25.96 | 17.56 ± 6.7 | ||||||
| 2011‐2014 | Japan | Four community‐based hospitals | Men aged ≥ 50 years with LUTS/BPH, an IPSS of ≥ 8, QoL score of ≥ 3, and ultrasound‐estimated prostatic volume of ≥ 20 mL | Silodosin 4 mg once daily | 4 weeks (before cross‐over)/total 8 weeks | 69.6 ± 5.4 | 38.7 ± 11.6 | 17.1 ± 7.3 | |
| Tamsulosin 0.2 mg once daily | 69.4 ± 7.0 | 47.3 ± 30.4 | 15.2 ± 7.0 | ||||||
| 2008‐2009 | Japan | Three institutions | Men with LUTS associated with BPH and had an IPSS ≥ 8 and an IPSS‐QoL score ≥ 2 | Silodosin 4 mg twice daily | 4 weeks (before cross‐over)/total 8 weeks | 69.3 ± 8.3 | 36.6 ± 18.3 | 16.4 ± 5.0 | |
| Tamsulosin 0.2 mg once daily | 69.9 ± 8.4 | 35.1 ± 13.0 | 18.1 ± 6.2 | ||||||
| 2007‐2010 | Japan | Nihon University School of Medicine | Men with BPH, ≥ 50 years with significant LUTS (IPSS ≥ 8, QoL score ≥ 3) | Silodosin 8 mg/ day | 12 weeks | 69.3 ± 7.8 | 33.2 ± 21.2 | 16.9 ± 5.5 | |
| Naftopidil 75 mg/ day | 70.0 ± 7.0 | 39.5 ± 18.0 | 18.9 ± 7.0 | ||||||
| NR | NR | NR | Men with LUTS (IPSS total score ≥ 8, Qmax < 15 mL/s) and prostate volume > 20 mL | Silodosin 4 mg twice daily | 12 months | 71.3 ± 8.2 | 42.0 ± 23.7 | 18.8 ± 7.3 | |
| Tamsulosin 0.2 ‐ 0.4 mg daily | 72.2 ± 7.6 | 41.2 ± 23.0 | 17.8 ± 6.4 | ||||||
| NR | Japan | Kawasaki Medical School | Men aged 50–‐80 years and with IPSS ≥ 8 | Silodosin 4 mg twice daily | 12 weeks | 70.2 ± 0.9 | 33.3 ± 2.3 | 18.7 ± 0.7 | |
| Tamsulosin 0.2 mg once daily | 71.5 ± 1.1 | 32.5 ± 2.0 | 18.0 ± 1.1 | ||||||
| Naftopidil 50 mg once daily | 69.1 ± 1.2 | 35.0 ± 3.1 | 17.4 ± 0.8 | ||||||
| 2008‐2010 | Japan | Single center | Men aged 50 years who had a total IPSS ≥ 8 and a QoL index ≥ 3 | Silodosin 4 mg twice daily | 3 months (before cross‐over)/1 month wash‐out/3 months (after cross‐over)/total 7 months | 68.9 ± 5.6 | 35.0 ± 18.4 | 19.3 ± 4.9 | |
| Tamsulosin 0.2 mg once daily | 70.0 ± 6.8 | 36.1 ± 15.5 | 21.1 ± 6.8 | ||||||
| 2007‐2008 | Taiwan | Nine medical centers | Men aged ≥ 40 years with an IPSS of ≥ 13 and prostate volume of ≥ 20 mL | Silodosin 4 mg twice daily | 12 weeks | 67.5 ± 9.3 | 44.8 ± 24.2 | 19.3 ± 4.5 | |
| Tamsulosin 0.2 mg and one placebo | 65.0 ± 8.8 | 38.2 ± 16.7 | 19.8 ± 4.5 | ||||||
| BPH: benign prostatic hyperplasia; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; NR: not reported; PVR: postvoid residual; Qmax: maximum flow rate; QoL: quality of life | |||||||||
| Study name | Intervention(s) and comparator(s) | Screened/eligible (N) | randomized (N) | Analysed (N) | Finishing trial (N (%)) |
| Silodosin 8 mg | 1228/955 | 381 | 346 | 356 (93.4) | |
| Tamsulosin 0.4 mg | 384 | 347 | 364 (94.7) | ||
| Placebo | 190 | 168 | 172 (90.5) | ||
| Total | 955 | 861 | 892 (93.4) | ||
| Silodosin 8 mg | NR/138 | 48 | 48 | 48 (100.0) | |
| Tamsulosin 0.2 mg | 43 | 43 | 43 (100.0) | ||
| Alfuzosin 10 mg | 47 | 47 | 47 (100.0) | ||
| Total | 138 | 138 | 138 (100.0) | ||
| Silodosin 8 mg | NR/457 | 176 | 175 | 175 (99.4) | |
| Tamsulosin 0.2 mg | 192 | 192 | 192 (100.0) | ||
| Placebo | 89 | 89 | 89 (100.0) | ||
| Total | 457 | 456 | 456 (99.7) | ||
| Silodosin 8 mg | NR/90 | 30 | 30 | 30 (100.0) | |
| Tamsulosin 0.4 mg | 30 | 30 | 30 (100.0) | ||
| Alfuzosin 10 mg | 30 | 30 | 30 (100.0) | ||
| Total | 90 | 90 | 90 (100.0) | ||
| Silodosin 8 mg | 2849/923 | 466 | 466 | 413 (88.6) | |
| Placebo | 457 | 457 | 419 (91.6) | ||
| Total | 923 | 923 | 832 (90.1) | ||
| Silodosin 4 mg or 8 mg | NR/92 | 44 | 30/83a | 30 (68.1) | |
| Naftopidil 50 mg or 75 mg | 48 | 34/79a | 34 (70.8) | ||
| Total | 92 | 64/162a | 64 (69.5) | ||
| Silodosin 4 mg followed by 8 mg | NR/350 | 175 | 157 | 157 (89.7) | |
| Naftopidil 50 mg followed by 75 mg | 175 | 157 | 157 (89.7) | ||
| Total | 350 | 314 | 314 (89.7) | ||
| Silodosin 8 mg | NR/97 | 46 | 34 | 34 (73.9) | |
| Tamsulosin 0.2 mg | 51 | 31 | 31 (60.7) | ||
| Total | 97 | 65 | 65 (67.0) | ||
| Silodosin 8 mg | NR/57 | 28 | NR | NR | |
| Tamsulosin 0.4 mg | 29 | NR | NR | ||
| Total | 57 | NR | NR | ||
| Silodosin 8 mg | 215/209 | 111 | 111 | 97 (87.3) | |
| Placebo | 98 | 98 | 89 (90.8) | ||
| Total | 209 | 209 | 186 (88.9) | ||
| Silodosin 8 mg | 102/61 | 32 | 26 | 26 (81.2) | |
| Tamsulosin 0.4 mg | 29 | 27 | 27 (93.1) | ||
| Total | 61 | 53 | 53 (86.8) | ||
| Silodosin 8 mg | NR/121 | 61 | 56/59a | 56 (91.8) | |
| Naftopidil 50 mg | 60 | 56/57a | 56 (93.3) | ||
| Total | 121 | 112/116a | 112 (92.5) | ||
| Silodosin 4 mg | NR/34 | 18 | 16 | 16 (88.8) | |
| Tamsulosin 0.2 mg | 16 | 14 | 14 (87.5) | ||
| Total | 34 | 30 | 30 (88.2) | ||
| Silodosin 4 mg | NR/102 | 51 | 42/88a | 42 (82.3) | |
| Tamsulosin 0.2 mg | 51 | 42/91a | 42 (82.3) | ||
| Total | 102 | 84/179a | 42 (82.3) | ||
| Silodosin 8 mg | 109/109 | 58 | 53 | 53 (91.3) | |
| Naftopidil 75 mg | 51 | 44 | 44 (86.2) | ||
| Total | 109 | 97 | 97 (88.9) | ||
| Silodosin 8 mg | NR/149 | 75 | NR | NR | |
| Tamsulosin 0.2 ‐ 0.4 mg | 74 | NR | NR | ||
| Total | 149 | NR | NR | ||
| Silodosin 8 mg | 136/136 | 45 | 41 | 41 (91.1) | |
| Tamsulosin 0.2 mg | 45 | 39 | 39 (86.6) | ||
| Naftopidil 50 mg | 46 | 42 | 42 (91.3) | ||
| Total | 136 | 122 | 122 (89.7) | ||
| Silodosin 8 mg | NR/46 | 23 | 23 | 23 (100.0) | |
| Tamsulosin 0.2 mg | 23 | 23 | 23 (100.0) | ||
| Total | 46 | 46 | 46 (100.0) | ||
| Silodosin 8 mg | NR/209 | 105 | 87 | 87 (82.8) | |
| Tamsulosin 0.2 mg | 104 | 83 | 83 (79.8) | ||
| Total | 209 | 170 | 170 (81.3) | ||
| Grand total | Interventions: silodosin | 1955 | 1684b | ||
| Compartors: placebo | 834 | 769 (92.2) | |||
| Compartors: tamsulosin | 1049 | 888b | |||
| Compartors: naftopidil | 380 | 333 (87.6) | |||
| Comparator: alfuzosin | 77 | 77 (100.0) | |||
| Overall | 4295 | 3751b | |||
| N: number; NR: not reported a Efficacy analysis/safety analysis. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urologic symptom scores (short term) Show forest plot | 3 | 1743 | Mean Difference (IV, Random, 95% CI) | ‐2.65 [‐3.23, ‐2.08] |
| 2 Quality of life (short term) Show forest plot | 2 | 820 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.71, ‐0.13] |
| 3 Treatment withdrawal due to any reason (short term) Show forest plot | 3 | 1703 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.70, 1.66] |
| 4 Treatment withdrawal due to adverse events (short term) Show forest plot | 4 | 1967 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [1.41, 3.72] |
| 5 Cardiovascular adverse events (short term) Show forest plot | 4 | 1967 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.67, 2.45] |
| 6 Sexual adverse events (short term) Show forest plot | 4 | 1967 | Risk Ratio (M‐H, Random, 95% CI) | 26.07 [12.36, 54.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urologic symptom scores (short term) Show forest plot | 10 | 1708 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐1.31, 1.24] |
| 2 Quality of life (short term) Show forest plot | 10 | 1707 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.53, 0.22] |
| 3 Treatment withdrawal due to any reason (short term) Show forest plot | 10 | 1573 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.62, 1.69] |
| 4 Treatment withdrawal due to adverse events (short term) Show forest plot | 9 | 1572 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.12, 3.44] |
| 5 Cardiovascular adverse events (short term) Show forest plot | 11 | 1955 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.53, 1.12] |
| 6 Sexual adverse events (short term) Show forest plot | 10 | 1849 | Risk Ratio (M‐H, Random, 95% CI) | 6.05 [3.55, 10.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urologic symptom scores (short term) Show forest plot | 5 | 652 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐2.57, 0.87] |
| 2 Quality of life (short term) Show forest plot | 5 | 652 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.60, 0.27] |
| 3 Treatment withdrawal due to any reason (short term) Show forest plot | 4 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.81, 1.93] |
| 4 Treatment withdrawal due to adverse events (short term) Show forest plot | 5 | 738 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.66, 2.89] |
| 5 Cardiovascular adverse events (short term) Show forest plot | 5 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.41, 2.56] |
| 6 Sexual adverse events (short term) Show forest plot | 4 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 5.93 [2.16, 16.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urologic symptom scores (short term) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 3.83 [0.12, 7.54] |
| 2 Quality of life (short term) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.46, 0.74] |
| 3 Cardiovascular adverse events (short term) Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.36, 1.24] |
| 4 Sexual adverse events (short term) Show forest plot | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 37.21 [5.32, 260.07] |