Terapias biológicas anti‐factor de necrosis tumoral en el tratamiento del edema macular uveítico (EMU) para la uveítis no infecciosa
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012577.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 18 diciembre 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud ocular y de la visión
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
MT, AD, DM, RB and MC led the development of the protocol. AD, RB and PM provided clinical advice; DM and MC provided methodological advice. NB provided the patient‐public perspective. JM gave input in reviewing the protocol. RB and MT screened and reviewed all papers identified in the database search and drafted the review text. All review authors read and approved the final manuscript.
Sources of support
Internal sources
-
National Institute of Health Research (NIHR), UK.
Melanie Calvert is funded by the NIHR Birmingham Biomedical Research Centre and the NIHR Surgical Reconstruction and Microbiology Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
External sources
-
National Institute for Health Research (NIHR), UK.
-
This review represents an independent research project funded by the NIHR under the Clinical Doctoral Research Fellowship Scheme being undertaken at the University of Birmingham.
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
-
Declarations of interest
RB: none known
MT: none known
NB: none known
JM: none known
PM: none known
MC reports receipt of personal fees from Astellas and Takeda and grants from the NIHR, Macmillan Cancer Support, HDRUK and Innovate UK outside the submitted work.
DM: none known
AD: none known
Acknowledgements
Cochrane Eyes and Vision (CEV) created and executed the electronic search strategies. We thank Anupa Shah, Managing Editor for CEV for help during the review process. We thank Peter Addison and Jennifer Evans for their comments on the protocol and review.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Dec 18 | Anti‐tumour necrosis factor biological therapies for the treatment of uveitic macular oedema (UMO) for non‐infectious uveitis | Review | Robert J Barry, Mohammad O Tallouzi, Nick Bucknall, Jonathan M Mathers, Philip I Murray, Melanie J Calvert, David J Moore, Alastair K Denniston | |
| 2017 Apr 11 | Anti‐tumour necrosis factor biological therapies for the treatment of uveitic macular oedema (UMO) for non‐infectious uveitis | Protocol | Mohammad O Tallouzi, Robert J Barry, Nick Bucknall, Jonathan M Mathers, Philip I Murray, Melanie J Calvert, David J Moore, Alastair K Denniston | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
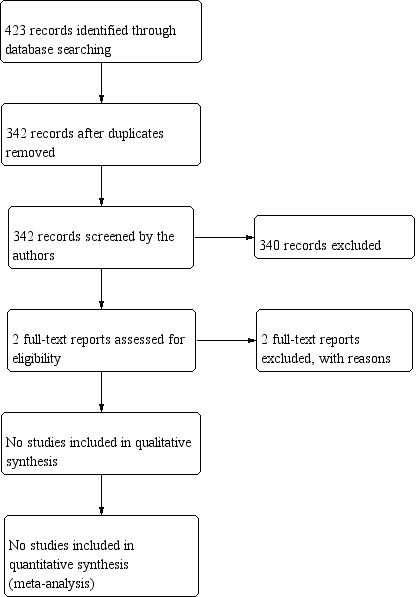
Study flow diagram

