Positron emission tomography (PET) and magnetic resonance imaging (MRI) for assessing tumour resectability in advanced epithelial ovarian, fallopian tube and/or primary peritoneal cancer
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012567Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 03 marzo 2017see what's new
- Tipo:
-
- Diagnostic
- Etapa:
-
- Protocol
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer ginecológico, neurooncología y otros cánceres
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
The study protocol was written by review authors JFR, JPH, RS, RJPMS and RPZ and subsequently revised by all authors.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
KCE Belgian healthcare knowledge centre, Belgium.
Practical support during all stages of the review process.
Declarations of interest
All review authors declare they have no conflicts of interest relevant to the presented research.
Acknowledgements
We thank Jo Morrison for clinical and editorial advice, Jo Platt for designing the search strategy and Clare Jess, Gail Quinn and Tracey Bishop for their contribution to the editorial process.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Oct 08 | Positron emission tomography (PET) and magnetic resonance imaging (MRI) for assessing tumour resectability in advanced epithelial ovarian/fallopian tube/primary peritoneal cancer | Review | Joline F Roze, Jacob P Hoogendam, Fleur T van de Wetering, René Spijker, Leen Verleye, Joan Vlayen, Wouter B Veldhuis, Rob JPM Scholten, Ronald P Zweemer | |
| 2017 Mar 03 | Positron emission tomography (PET) and magnetic resonance imaging (MRI) for assessing tumour resectability in advanced epithelial ovarian, fallopian tube and/or primary peritoneal cancer | Protocol | Jacob P Hoogendam, Joline F Roze, Fleur T van de Wetering, René Spijker, Leen Verleye, Joan Vlayen, Wouter B Veldhuis, Rob JPM Scholten, Ronald P Zweemer | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Diffusion Magnetic Resonance Imaging;
- Fallopian Tube Neoplasms [*diagnostic imaging, pathology, surgery];
- Feasibility Studies;
- *Fluorodeoxyglucose F18;
- Neoplasm, Residual [diagnostic imaging];
- Ovarian Neoplasms [*diagnostic imaging, pathology, surgery];
- Peritoneal Neoplasms [*diagnostic imaging, pathology, surgery];
- *Positron Emission Tomography Computed Tomography;
- *Radiopharmaceuticals;
- Randomized Controlled Trials as Topic;
- Sensitivity and Specificity;
- Tomography, X-Ray Computed;
Medical Subject Headings Check Words
Female; Humans;
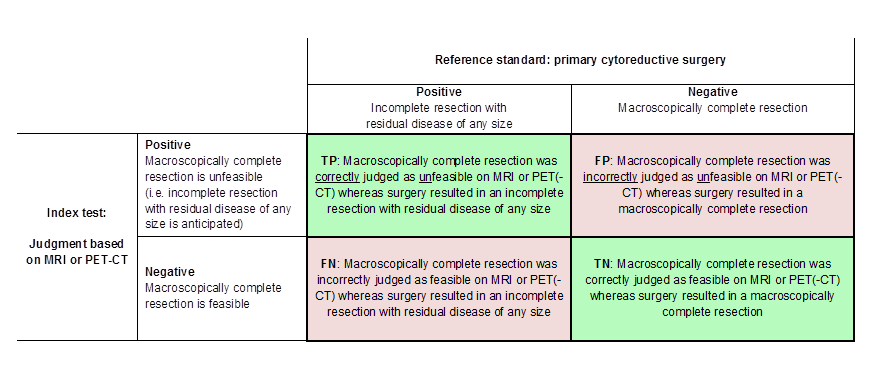
Definitions of the two by two table, wherein the index tests are tabulated against the reference standard outcome, on the analysis: macroscopic debulking versus incomplete debulking with residual disease of any size (i.e. consisting of deposits ≤ 1 cm and > 1 cm in diameter ). TP= true positive, FP= false positive, FN= false negative, TN=true negative.
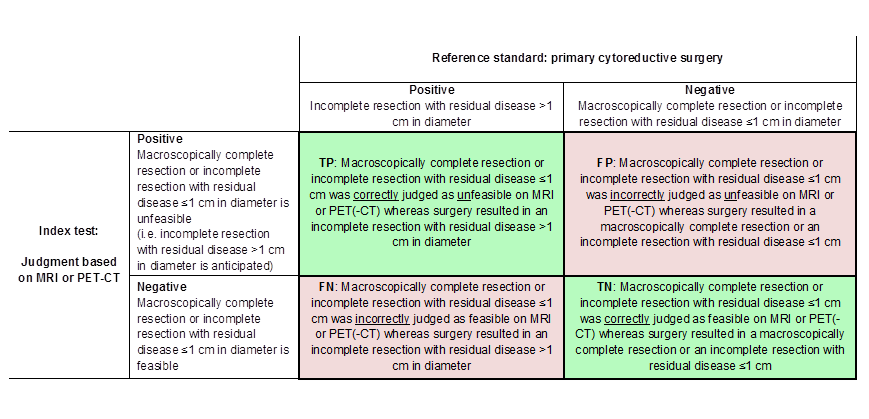
Definitions of the two by two table, wherein the index tests are tabulated against the reference standard outcome, on the analysis: macroscopic debulking or incomplete debulking with residual disease ≤ 1 cm in diameter versus incomplete resection with residual disease > 1 cm in diameter. TP= true positive, FP= false positive, FN= false negative, TN=true negative.

