用于儿童和青少年癌症相关疼痛的非甾体抗炎药(NSAID)
Referencias
References to studies excluded from this review
Ir a:
Additional references
Ir a:
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation: not RCT. | |
| Population: adults. | |
| Intervention: case study, not RCT. | |
| Population: adults. | |
| Intervention: case study, not RCT. | |
| Population: Age range 5 to 69 years. Attempted contact for subunit data. | |
| Allocation/study design: not RCT. | |
| Population: adults. | |
| Allocation/study design: not an RCT. |
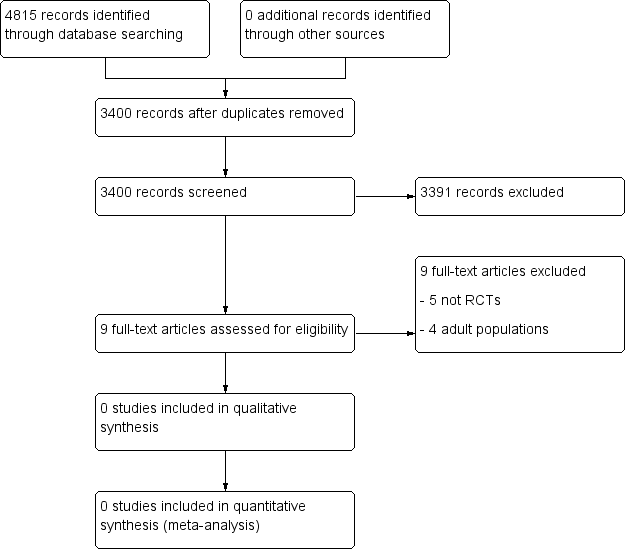
Study flow diagram.

