Fármacos antiinflamatorios no esteroideos (AINE) para el dolor relacionado con el cáncer en niños y adolescentes
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012563.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 24 julio 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
TC and CE registered the title.
TC, PW and CE wrote the template protocol for the suite of children's reviews of which this review is a part.
All authors contributed to writing the protocol and all authors agreed on the final version.
All authors were responsible for data extraction, analysis, and writing of the discussion for the full review.
All authors will be responsible for the completion of updates.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
NIHR Programme Grant, Award Reference Number: 13/89/29 (Addressing the unmet need of chronic pain: providing the evidence for treatments of pain)
Declarations of interest
CE: none known. Since CE is an author as well as the PaPaS Co‐ordinating Editor at the time of writing, we acknowledge the input of Leontien Kremer, of the Cochrane Childhood Cancer Group, who acted as Sign Off Editor for this review. CE had no input into the editorial decisions or processes for this review.
TC: none known.
BA: none known; BA is a specialist anaesthetist and intensive care physician and manages the perioperative care of children requiring surgery and those critically ill requiring intensive care.
MCG: none known; MCG is a specialist paediatric pain and palliative care physician and treats patients with complex pain.
LH: none known.
GL: none known; GL is a specialist paediatric oncologist and paediatric pain physician and manages patients with cancer and cancer pain.
Acknowledgements
We acknowledge the contribution of Phil Wiffen to the template protocol.
We thank Gillian Dickson, EAH Loeffen and Andrew Moore for peer reviewing.
Cochrane Review Group funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). Disclaimer: the views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jul 24 | Non‐steroidal anti‐inflammatory drugs (NSAIDs) for cancer‐related pain in children and adolescents | Review | Tess E Cooper, Lauren C Heathcote, Brian Anderson, Marie‐Claude Grégoire, Gustaf Ljungman, Christopher Eccleston | |
| 2017 Feb 21 | Non‐steroidal anti‐inflammatory drugs (NSAIDs) for cancer‐related pain in children and adolescents | Protocol | Tess E Cooper, Lauren C Heathcote, Brian Anderson, Marie‐Claude Grégoire, Gustaf Ljungman, Christopher Eccleston | |
Differences between protocol and review
We altered the MEDLINE search strategy after some discussion with the Cochrane Childhood Cancer Group. The search strategies for Embase and CENTRAL were then modelled on the minor changes.
Minor changes to background wording and details of examples.
Studies with < 10 participants per treatment arm were not considered for inclusion in this review, as is standard practice for this group.
Notes
A restricted search in January 2019 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Child; Child, Preschool; Humans; Infant; Infant, Newborn;
PICO
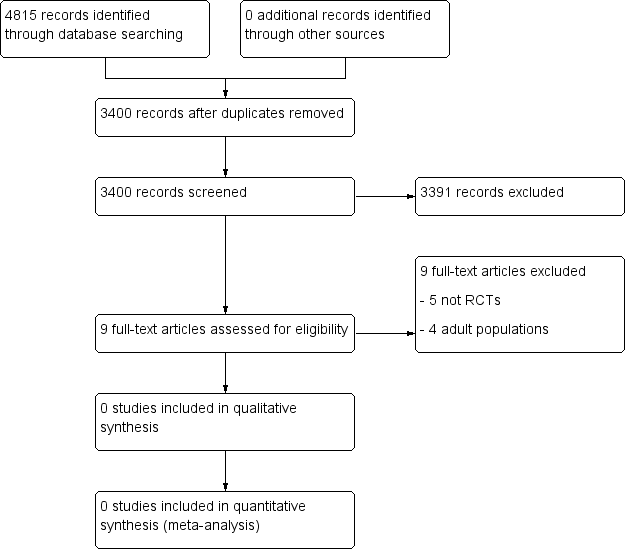
Study flow diagram.

