Niesteroidowe leki przeciwzapalne (NLPZ) w bólu nowotworowym u dzieci i młodzieży
Abstract
Background
Pain is a common feature of childhood and adolescence around the world, and for many young people, that pain is chronic. The World Health Organization (WHO) guidelines for pharmacological treatments for persisting pain in children acknowledge that pain in children is a major public health concern of high significance in most parts of the world. Views on children's pain have changed over time and relief of pain is now seen as important. In the past, pain was largely dismissed and was frequently left untreated, and it was assumed that children quickly forgot about painful experiences.
We designed a suite of seven reviews in chronic non‐cancer pain and cancer pain (looking at antidepressants, antiepileptic drugs, non‐steroidal anti‐inflammatory drugs, opioids, and paracetamol as priority areas) to review the evidence for children's pain using pharmacological interventions.
As one of the leading causes of mortality and morbidity for children and adolescents in the world today, childhood cancer (and its associated pain) is a major health concern. Specific mortality and morbidity data relating to children are not currently identified. All childhood cancer rates are on the rise; for example, in the USA approximately 10,380 children aged under 15 years were expected to be diagnosed with cancer by the end of 2016. However, with survival rates also increasing, over 80% of paediatric cancer patients are expected to survive for five years or more, thus identifying the need to address pain management in this population.
Cancer pain in infants, children, and adolescents is primarily nociceptive pain with negative long term effects. Cancer‐related pain is generally caused directly by the tumour itself such as compressing on the nerve or inflammation of the organs. Cancer‐related pain generally occurs as a result of perioperative procedures, nerve damage caused by radiation or chemotherapy treatments, or mucositis. However, this review focused on pain caused directly by the tumour itself such as nerve infiltration, external nerve compression, and other inflammatory events.
Non‐steroidal anti‐inflammatory drugs (NSAIDs) are used to treat pain, reduce fever, and for their anti‐inflammatory properties. They are commonly used within paediatric pain management. NSAIDs are currently licensed for use in western countries, however not approved for infants aged under three months. Primary adverse effects include gastrointestinal issues and possible renal impairment with long term use. Other adverse effects in children include diarrhoea, headache, nausea, constipation, rash, dizziness, and abdominal pain.
Objectives
To assess the analgesic efficacy, and adverse events, of non‐steroidal anti‐inflammatory drugs (NSAIDs) used to treat cancer‐related pain in children and adolescents aged from birth and 17 years, in any setting.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online, MEDLINE via Ovid, and Embase via Ovid from inception to 21 February 2017. We also searched the reference lists of retrieved studies and reviews, and searched online clinical trial registries.
Selection criteria
Randomised, double‐blind trials of any dose, and any route, treating cancer‐related pain in children and adolescents, comparing NSAIDs with placebo or an active comparator.
Data collection and analysis
Two review authors independently assessed studies for eligibility. We planned to use dichotomous data to calculate risk ratio and number needed to treat for one additional event, using standard methods. We planned to assess GRADE (Grading of Recommendations Assessment, Development and Evaluation) and planned to create a 'Summary of findings' table.
Main results
No studies were eligible for inclusion in this review.
There is no evidence to support or refute the use of NSAIDs for treating cancer‐related pain in children and adolescents.
Authors' conclusions
There is no evidence from randomised controlled trials to support or refute the use of NSAIDs to treat chronic cancer‐related pain in children and adolescents. We are unable to comment about efficacy or harm from the use of NSAIDs to treat chronic cancer‐related pain in children and adolescents.
PICO
Streszczenie prostym językiem
NLPZ w bólu nowotworowym u dzieci i młodzieży
Najważniejsza informacja
Nie jesteśmy pewni, czy NLPZ zapewniają ulgę w bólu związanym z nowotworem u dzieci i młodzieży.
Wprowadzenie
Choroba nowotworowa w dzieciństwie jest obecnie jedną z głównych przyczyn chorobowości i zgonów u dzieci i młodzieży na świecie. Związany z chorobą ból jest głównym problemem zdrowotnym, a szczegółowe dane dla dzieci nie są obecnie dobrze znane.
Ból nowotworowy u niemowląt, dzieci i młodzieży to przede wszystkim ból nerwowy (związany z uszkodzeniem nerwów; przyp. tłum.), który prowadzi do długotrwałych negatywnych skutków. Ból nowotorowy jest zazwyczaj spowodowany bezpośrednio przez samego guza prowadząc do nacieczenia nerwu, zewnętrzny ucisk nerwu i inne stany zapalne.
NLPZ są stosowane w leczeniu bólu lub zmniejszaniu gorączki i są powszechnie podawane dzieciom. NLPZ są obecnie zarejestrowane do stosowania w krajach zachodnich, ale nie są dozwolone u niemowląt w wieku poniżej trzech miesięcy. Główne działania niepożądane NLPZ to dolegliwości żołądkowe i ewentualne uszkodzenia nerek w wyniku długotrwałego przyjmowania. Inne działania niepożądane u dzieci to: biegunka, ból głowy, nudności, zaparcia, wysypka, zawroty głowy, wzdęcia, ból brzucha i niestrawność.
Główne wyniki
W lutym 2017 roku poszukiwaliśmy randomizowanych badań klinicznych z grupą kontrolną, w których oceniano stosowanie jakiegokolwiek NLPZ w celu leczenia bólu nowotworowego u osób w wieku od urodzenia do 17 lat. Nie znaleźliśmy żadnych badań, które spełniałyby założone w niniejszym przeglądzie kryteria włączenia. W kilku badaniach oceniano stosowanie NLPZ u dorosłych w przewlekłym bólu, ale żadne nie analizowało uczestników w wieku od urodzenia do 17 lat.
Jakość danych naukowych
Ocenialiśmy jakość danych naukowych używając czterech poziomów: bardzo niska, niska, umiarkowana lub wysoka. Bardzo niska jakość danych naukowych oznacza, że jesteśmy bardzo niepewni co do otrzymanych wyników. Wysoka jakość danych naukowych oznacza, że mamy bardzo dużą pewność co do otrzymanych wyników.
Nie byliśmy w stanie ocenić jakości danych naukowych, ponieważ nie było danych pochodzących z randomizowanych badań z grupą kontrolną, które wspierałyby lub obalały sugestię, że NSAIDs w jakiejkolwiek dawce zmniejszą przewlekły ból związany z rakiem u dzieci lub młodzieży.
Authors' conclusions
Background
Pain is a common feature of childhood and adolescence around the world, and for many young people, that pain is chronic. The World Health Organization (WHO) guidelines for pharmacological treatments for persisting pain in children acknowledge that pain in children is a major public health concern of high significance in most parts of the world (WHO 2012). Views on children's pain have changed over time and relief of pain is now seen as important. In the past, pain was largely dismissed and was frequently left untreated, and it was assumed that children quickly forgot about painful experiences. Since the 1970s, studies comparing child and adult pain management revealed a variety of responses to pain, fuelling the need to focus on paediatric pain in more depth (Caes 2016).
Infants (birth to 12 months), children (1 to 9 years), and adolescents (10 to 18 years) (WHO 2012) account for 27% (1.9 billion) of the world's population (United Nations 2017), and the proportion of those aged up to 14 years varies from 12% (in Hong Kong) to 50% (in Niger) (World Bank 2016). However, we know little about the pain management needs of this population. For example, in the Cochrane Library, approximately 12 reviews produced by the Cochrane Pain, Palliative and Supportive Care (PaPaS) Review Group in the past 18 years have been specifically concerned with children and adolescents, compared to over 100 reviews specific to adults. Additional motivating factors for investigating children's pain include the vast amount of unmanaged pain in the paediatric population and new technologies and treatments being developed. We convened an international group of leaders in paediatric pain to design a suite of seven reviews in chronic pain and cancer pain (looking at antidepressants, antiepileptic drugs, non‐steroidal anti‐inflammatory drugs (NSAIDs), opioids, and paracetamol as priority areas) to review the evidence under a programme grant for children's pain using pharmacological interventions in children and adolescents (Appendix 1).
This review is based on a template for reviews of pharmacotherapies used to relieve pain in infants, children and adolescents. The aim is for all reviews to use the same methods, based on new criteria for what constitutes reliable evidence (Moore 2010a; Moore 2012; Appendix 2). This review focuses on NSAIDs to treat cancer pain.
Description of the condition
This review focused on pain that children and adolescents experience as a result of any type of cancer.
The type of cancer pain in infants, children and adolescents is primarily nociceptive pain (Ljungman 1996), and generally occurs as a result of perioperative procedures and treatments. In addition, nerve damage caused by radiation or chemotherapy (WHO 2012) is also common. However, the tumour itself can also cause nerve infiltration, external nerve compression, and other painful inflammatory events such as distention (WHO 2012).
Whilst diagnostic and perioperative procedures performed for cancer treatment are a known common cause of pain in these patients (Ripamonti 2008), this review did not cover perioperative pain or adverse effects of treatments such as mucositis. We focused on pain caused directly by the tumour itself such as tissue damage, nerve infiltration, external nerve compression and other inflammatory events.
As one of the leading causes of mortality and morbidity in the world today, childhood cancer (and its associated pain) is a major health concern. The WHO predicts 14 to 15 million new cases of cancer across all ages to arise by 2020 (Frankish 2003; Ripamonti 2008), accounting for approximately 8.2 million deaths worldwide (WHO 2011). Specific mortality and morbidity data relating to children were not identified.
Worldwide childhood cancer statistics are difficult to estimate, particularly when examining both developed and developing counties. However, cancer is the leading cause of death in developed countries (WHO 1998). In the European region, leukaemia (34.1%), central nervous system (CNS) tumours (22.6%), and lymphomas (11.5%) are the largest cancer diagnostic groups in the paediatric population (birth to 15 years) (Kaatsch 2010). In the USA, childhood cancer is the second leading cause of death (excluding neonates) (after injury), with leukaemia (30%), CNS tumours or brain and other CNS tumours (26%), and neuroblastoma (6%) as the leading types of diagnosed cancers (ACS 2015). All childhood cancer rates are on the rise; for example, in the USA approximately 10,380 children under the age of 15 years were expected to be diagnosed with cancer by the end of 2016 (ACS 2015). However, with survival rates also increasing, over 80% of paediatric cancer patients are expected to survive for five years or more (ACS 2015). In the developing world, the incidence of cancer is difficult to estimate due to poor reporting, diagnostic facilities and hospital statistics. It is known that Burkitt lymphoma, non‐Hodgkin lymphoma, nephroblastoma, retinoblastoma, and rhabdomyosarcoma are among the most common cancers in children across African regions (Tanko 2009). In Asian regions, leukaemias and CNS tumours are among the most common childhood cancers (IARC 2008).
Description of the intervention
NSAIDs are used for the treatment of pain and fever reduction for their anti‐inflammation properties. They are commonly used within paediatric pain management (Blanca‐López 2015). The two main types of NSAIDs are selective and nonselective, which refer to the ability of the NSAID to inhibit specific types of COX enzymes (Misurac 2013). NSAIDs are currently licensed for use in western countries, but are not approved for infants aged under three months (WHO 2012). NSAIDs are also widely used for patent ductus arteriosus (PDA) closure in neonates.
Currently available NSAIDs include: aceclofenac, acetylsalicylic acid, celecoxib, choline magnesium trisalicylates diclofenac, etodolac, etoricoxib, fenoprofen, ibuprofen, indometacin, ketoprofen, ketorolac, mefenamic acid, meloxicam, nabumetone, naproxen, parecoxib, phenylbutazone, piroxicam, sulindac, tenoxicam, and tiaprofenic acid (BNF 2016).
NSAIDs are used in a variety of doses and are commonly prescribed to children with pain as an oral tablet or liquid formulation. The recommended dose for ibuprofen (for example) is 5 to 10 mg/kg every six to eight hours with a maximum daily dose of 1200 mg. Additionally, for naproxen, a maximum dose of 1000 mg per day is recommended (WHO 2012). The recommendation for paediatric patients is to use the lowest dose, for the shortest duration possible to control symptoms (NICE 2015); hence, NSAIDs are also used in conjunction with paracetamol to reduce the amount administered to children (WHO 2012).
The two primary adverse effects of NSAIDs are renal impairment and gastrointestinal issues (NICE 2015). Common side effects in children include diarrhoea, headache, nausea, constipation, rash, dizziness, flatulence, abdominal pain, and dyspepsia (WHO 2012). Other adverse effects include hepatic function impairment, contraindications with allergic disorders (hypersensitivity to aspirin, asthma, angioedema, urticaria, rhinitis), cardiac impairment, Reye's syndrome, antiplatelet effects, coagulation defects, and dangerous environmental harms (particularly seen in diclofenac). The long‐term safety of the use of NSAIDs in children is unclear (Blanca‐López 2015). However, some safety assessments of ibuprofen in children have been compared with paracetamol and not found a significant increased risk in serious adverse events or main causes of hospitalisation (acute gastrointestinal bleeding, acute renal failure, anaphylaxis, or Reye's syndrome) (Lesko 1995; Lesko 1997; Lesko 1999).
How the intervention might work
One current hypothesis is that damage to the peripheral nerves is followed by an inflammatory reaction that relates to increased production of prostaglandins, amplifying sodium currents and calcium influx in peripheral nociceptive neurons, and enhancing neurotransmitter release in the CNS and depolarisation of second‐order nociceptive neurons (Vo 2009). Preclinical data suggest an immune pathogenesis of neuropathic pain, but clinical evidence of a central role of the immune system is less clear (Calvo 2012). NSAIDs inhibit the production of prostaglandins, and thus could lessen the peripheral and central sensory hypersensitivity that occurs with nerve injury‐associated inflammation. NSAIDs have been shown to reduce sensory hypersensitivity in animal models (Hasnie 2007; Kawakami 2002).
Why it is important to do this review
The paediatric population is at risk of inadequate management of pain (AMA 2013). Some conditions that would be aggressively treated in adults are being managed with insufficient analgesia in the younger populations (AMA 2013). Although there have been repeated calls for best evidence to treat children's pain, such as Eccleston 2003, there are no easily available summaries of the most effective paediatric pain relief.
This Cochrane Review will form part of a Programme Grant to address the unmet needs of people with chronic pain, commissioned by the National Institute for Health Research (NIHR) in the UK. This topic was identified in June 2015 during consultation with experts in paediatric pain. Please see Appendix 1 for full details of the meeting. The standards used to assess evidence in chronic pain trials have changed substantially in recent years, with particular attention being paid to trial duration, withdrawals, and statistical imputation following withdrawal, all of which can substantially alter estimates of efficacy. The most important change is to encourage a move from using average pain scores, or average change in pain scores, to the number of people who have a large decrease in pain (by at least 50%). Pain intensity reduction of 50% or more has been shown to correlate with improvements in comorbid symptoms, function, and quality of life (Moore 2011a). These standards are set out in the reference guide for pain studies (AUREF 2012).
Objectives
To assess the analgesic efficacy, and adverse events, of non‐steroidal anti‐inflammatory drugs (NSAIDs) used to treat cancer‐related pain in children and adolescents aged from birth to 17 years, in any setting.
Methods
Criteria for considering studies for this review
Types of studies
We planned to only include randomised controlled trials (RCTs), with or without blinding, and participant or observer reported outcomes.
Full journal publication was required, with the exception of online clinical trial results, summaries of otherwise unpublished clinical trials and abstracts with sufficient data for analysis. We planned to include studies published in any language. We excluded abstracts (usually meeting reports) or unpublished data, non‐randomised studies, studies of experimental pain, case reports, and clinical observations.
Types of participants
We planned to include studies of infants, children, and adolescents aged from birth to 17 years, who have (one or more) cancer and experience pain directly related to the condition.
We planned to include studies of participants with more than one type of cancer pain, and to analyse results according to the primary condition.
We excluded studies of perioperative pain, short‐term infection pain, short‐term injury or trauma pain, acute pain, functional abdominal pain, burn pain, musculoskeletal pain, headache and migraine, sickle cell disease acute crisis pain, mucositis, or any other chronic non‐cancer pain.
Types of interventions
We planned to include studies reporting interventions prescribing NSAIDs for the relief of cancer pain; by any route, in any dose, with comparison to a placebo or any active comparator.
Types of outcome measures
Studies had to report pain assessment as either a primary or secondary outcome to be eligible for inclusion in this review, as well as meeting the other selection criteria.
We planned to include trials measuring pain intensity and pain relief assessed using validated tools such as numerical rating scale (NRS), visual analogue scale (VAS), Faces Pain Scale ‐ Revised (FPS‐R), Colour Analogue Scale (CAS), or any other validated rating scale.
We were particularly interested in Paediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (PedIMMPACT) definitions for moderate and substantial benefit in chronic pain studies (PedIMMPACT 2008). These are defined as: at least 30% pain relief over baseline (moderate); at least 50% pain relief over baseline (substantial); much or very much improved on Patient Global Impression of Change scale (PGIC; moderate); very much improved on PGIC (substantial).
These outcomes are different from those used in most earlier reviews, concentrating as they do on dichotomous outcomes where pain responses do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50% pain intensity reduction, and ideally having no worse than mild pain (Moore 2013a; O'Brien 2010).
We also planned to record any reported adverse events. We also planned to report the timing of outcome assessments.
Primary outcomes
-
Participant‐reported pain relief of 30% or greater.
-
Participant‐reported pain relief of 50% or greater.
-
PGIC much or very much improved.
In the absence of self‐reported pain, we planned to consider the use of 'other‐reported' pain, typically an observer such as a parent, carer, or healthcare professional (Stinson 2006; Von Baeyer 2007).
Secondary outcomes
We identified the following with reference to the PedIMMPACT recommendations, which suggest core outcome domains and measures for consideration in paediatric acute and chronic/recurrent pain clinical trials (PedIMMPACT 2008):
-
carer global impression;
-
requirement for rescue analgesia;
-
sleep duration and quality;
-
acceptability of treatment;
-
physical functioning as defined by validated scales;
-
quality of life as defined by validated scales;
-
any adverse events;
-
withdrawals due to adverse events; and
-
any serious adverse event. Serious adverse events typically include any untoward medical occurrence or effect that at any dose results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the patient, or may require an intervention to prevent one of the above characteristics or consequences.
Search methods for identification of studies
The authors and Information Specialist developed the search strategy, based on previous strategies used within the PaPaS Review Group, and we carried out the searches. We also sought advice from the Cochrane Childhood Cancer Group.
Electronic searches
We searched the following databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL) (via the Cochrane Library) searched on 21/2/2017;
-
MEDLINE (via Ovid) 1947 to February week 2 2017;
-
Embase (via Ovid) 1974 to 21/2/2017.
We used medical subject headings (MeSH) or equivalent and text word terms. We restricted our search for RCTs and clinical trials. There were no language restrictions. There were no date restrictions. The focus of the key words in our search terms was on cancer pain and NSAIDs. Searches were tailored to individual databases. The search strategies for MEDLINE, Embase and CENTRAL are presented in Appendix 3; Appendix 4 and Appendix 5 respectively.
Searching other resources
We searched clinicaltrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) on 21 February 2017 for ongoing trials. In addition, we checked reference lists of reviews and retrieved articles for additional studies, and performed citation searches on key articles. We planned to contact experts in the field for unpublished and ongoing trials. We planned to contact study authors where necessary for additional information.
Data collection and analysis
We planned to perform separate analyses according to particular types of cancer. We planned to combine different cancer types in analyses for exploratory purposes only.
Selection of studies
Two review authors independently determined eligibility by reading the abstract of each study identified by the search. Independent review authors eliminated studies that clearly did not satisfy inclusion criteria, and obtained full copies of the remaining studies. Two review authors read these studies independently to select relevant studies, and in the event of disagreement, a third author adjudicated. We did not anonymise the studies in any way before assessment. We included a PRISMA flow chart in the full review which shows the status of identified studies (Moher 2009) as recommended in part 2, section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to include studies in the review irrespective of whether measured outcome data were reported in a ‘usable’ way.
Data extraction and management
We planned to obtain full copies of the studies and two authors planned to independently carry out data extraction. Where available, data extraction would include information about the type of cancer, number of participants treated, drug and dosing regimen, study design (placebo or active control), study duration and follow‐up, analgesic outcome measures and results, withdrawals, and adverse events (participants experiencing any adverse event, or serious adverse event). We planned to collate multiple reports of the same study, so that each study rather than each report was to be the unit of interest in the review. We planned to collect characteristics of the included studies in sufficient detail to populate a ‘Characteristics of included studies’ table.
We planned to use a template data extraction form and check for agreement before entry into Cochrane's statistical software Review Manager (version 5.3) (Review Manager 2014).
If a study had more than two intervention arms, we planned to only include in the review intervention groups and control groups that met the eligibility criteria. If multi‐arm studies were included, we planned to analyse multiple intervention groups in an appropriate way to avoid arbitrary omission of relevant groups and double‐counting of participants.
Assessment of risk of bias in included studies
Two authors planned to independently assess risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We planned to complete a 'Risk of bias' table for each included study using the 'Risk of bias' tool in RevMan (Review Manager 2014).
We planned to assess the following for each study. Any disagreements were to be resolved by discussion between review authors and where necessary, a third review author.
-
Random sequence generation (checking for possible selection bias). We planned to assess the method used to generate the allocation sequence as: low risk of bias (ie, any truly random process, for example random number table; computer random number generator); or unclear risk of bias (when the method used to generate the sequence was not clearly stated). We excluded studies at high risk of bias that used a non‐random process (eg, odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We planned to assess the methods as: low risk of bias (eg, telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); or unclear risk of bias (when the method was not clearly stated). We excluded studies that did not conceal allocation and were therefore at a high risk of bias (eg, open list).
-
Blinding of participants and personnel (checking for possible performance bias). We planned to assess any methods used to blind the participants and personnel from knowledge of which intervention a participant received. We planned to assess the methods as: low risk of bias (study stated that the participants and personnel involved were blinded to treatment groups); unclear risk of bias (study did not state either way as to whether participants and personnel were blinded to treatment groups); or high risk of bias (participants or personnel were not blinded) (as stated in Types of studies, we planned to still include trials, with or without blinding, and participant or observer reported outcomes).
-
Blinding of outcome assessment (checking for possible detection bias). We planned to assess any methods used to blind the outcome assessors from knowledge of which intervention a participant received. We planned to assess the methods as: low risk of bias (eg study stated that it was single‐blinded and described the method used to achieve blinding of the outcome assessor); unclear risk of bias (study stated that outcome assessors were blinded but did not provide an adequate description of how it was achieved); or high risk of bias (outcome assessors were not blinded) (as stated in Types of studies, we planned to still include trials, with or without blinding, and participant or observer reported outcomes).
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We planned to assess the methods used to deal with incomplete data as: low risk of bias (ie fewer than 10% of participants did not complete the study or used 'baseline observation carried forward' (BOCF) analysis, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); or high risk of bias (used 'completer' analysis).
-
Selective reporting (checking for possible reporting bias). We planned to assess the methods used to report the outcomes of the study as: low risk of bias (if all planned outcomes in the protocol or methods were also reported in the results); unclear risk of bias (if there was not a clear distinction between planned outcomes and reported outcomes); high risk of bias (if some planned outcomes from the protocol or methods were clearly left out of the results).
-
Size of study (checking for possible biases confounded by small size). We planned to assess studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); or high risk of bias (fewer than 50 participants per treatment arm).
-
Other bias. We planned to assess studies for any additional sources of bias as low, unclear or high, and provide rationale.
Measures of treatment effect
Where dichotomous data were available, we planned to calculate a risk ratio (RR) with 95% confidence intervals (CIs) and meta‐analyse the data as appropriate. We planned to calculate numbers needed to treat for an additional beneficial outcome (NNTBs) where appropriate (McQuay 1998); for unwanted effects the NNTB becomes the number needed to treat for an additional harmful outcome (NNTH) and is calculated in the same manner. Where continuous data were reported, we planned to use appropriate methods to combine these data in the meta‐analysis.
Unit of analysis issues
We planned to accept randomisation to the individual participant only. We planned to split the control treatment arm between active treatment arms in a single study if the active treatment arms were not combined for analysis. We only accepted studies with a minimum 10 participants per treatment arm.
Dealing with missing data
We planned to use intention‐to‐treat (ITT) analysis where the ITT population consisted of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one post‐baseline assessment. We planned to assign missing participants zero improvement wherever possible.
Assessment of heterogeneity
We planned to identify and measure heterogeneity as recommended in chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to deal with clinical heterogeneity by combining studies that examined similar conditions. We planned to undertake and present a meta‐analysis only if participants, interventions, comparisons, and outcomes were judged to be sufficiently similar to ensure an answer that is clinically meaningful. We planned to assess statistical heterogeneity visually (L'Abbé 1987), and with the use of the I² statistic. When I² was greater than 50%, we planned to consider the possible reasons.
Assessment of reporting biases
We planned to assess the risk of reporting bias, as recommended in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The aim of this review was to use dichotomous outcomes of known utility and value to patients (Hoffman 2010; Moore 2010b; Moore 2010c; Moore 2010d; Moore 2013a). The review did not depend on what the authors of the original studies chose to report or not, although clearly difficulties were predicted to arise in studies that failed to report any dichotomous results. We planned to extract and use continuous data, which may reflect efficacy and utility poorly, and may have been useful for illustrative purposes only.
We planned to assess publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean a number needed to treat (NNT) of 10 or higher; Moore 2008).
Data synthesis
We planned to use a fixed‐effect model for meta‐analysis. We planned to use a random‐effects model for meta‐analysis if there was significant clinical heterogeneity and it was considered appropriate to combine studies. We planned to conduct our analysis using the primary outcomes of pain and adverse events, and we planned to calculate the NNTHs for adverse events. We planned to use the Cochrane software program Review Manager 5.3 (Review Manager 2014).
Quality of evidence
To analyse data, two review authors planned to independently rate the quality of each outcome. We planned to use the GRADE approach to assess the quality of the body of the evidence related to each of the key outcomes, and report our judgement on the quality of the evidence in the 'Summary of findings' table (chapter 12, Higgins 2011; Appendix 6).
In addition, there may have been circumstances where the overall rating for a particular outcome needed to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, if there were so few data that the results were highly susceptible to the random play of chance, or if studies used LOCF imputation in circumstances where there were substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by three levels, to very low quality. In circumstances where there were no data reported for an outcome, we planned to report that there was no evidence to support or refute (Guyatt 2013b).
'Summary of findings' table
We planned to include a 'Summary of findings' table as set out in the Cochrane PaPaS Review Group’s author guide (AUREF 2012), and recommended in theCochrane Handbook for Systematic Reviews of Interventions, chapter 4.6.6 (Higgins 2011). We planned to justify and document all assessments of the quality of the body of evidence.
In an attempt to interpret reliability of the findings for this systematic review, we planned to assess the summarised data using the GRADE guidelines (Appendix 6) to rate the quality of evidence (Guyatt 2011) of each of the key outcomes listed in Types of outcome measures (chapter 12, Higgins 2011), as appropriate. Using the explicit criteria against: study design, risk of bias, imprecision, inconsistency, indirectness, and magnitude of effect, we planned to summarise the evidence in an informative, transparent and succinct 'Summary of findings' table or 'Evidence profile' table (Guyatt 2011).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses where a minimum number of data were available (at least 200 participants per treatment arm). We planned to analyse according to age group; type of drug; geographical location or country; type of control group; baseline measures; frequency, dose and duration of drugs; nature of drug.
We planned to investigate whether the results of subgroups were significantly different by inspecting the overlap of confidence intervals and performing the test for subgroup differences available in RevMan.
Sensitivity analysis
We did not plan to carry out any sensitivity analysis because the evidence base was known to be too small to enable reliable analysis; we did not plan to pool results from cancer pain of different origins in the primary analyses. We planned to examine details of dose escalation schedules in the unlikely situation that this could provide some basis for a sensitivity analysis.
Results
Description of studies
Results of the search
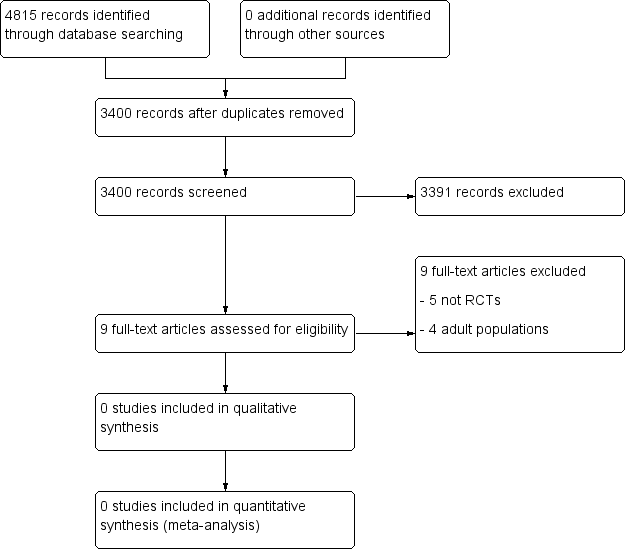
A PRISMA flow diagram of the search results is shown in Figure 1.

Study flow diagram.
Searches of the three main databases revealed 4815 records of titles and abstracts, of which we removed 1417 duplicates. We also searched clinicaltrials.gov and app.who.int/trialsearch/ and found no additional eligible studies.
We screened the remaining 3400 titles and abstracts for eligibility, of which we removed 3391 as ineligible studies.
Of the remaining nine studies we retrieved the full text articles, and excluded all nine. No ongoing studies were identified. No studies fulfilled the eligibility criteria, nor were any eligible to be entered into a quantitative analysis.
Included studies
No studies met our inclusion criteria for this review.
Excluded studies
See Characteristics of excluded studies.
We excluded nine studies in this review.
Upon reading the full texts, we discovered three studies were in adult populations (Cappelaere 1971; Harris 2003; Toscani 1994), and one investigated participants whose ages ranged from 5 to 69 years; however, subunit data were not available (Mercandante 1999). Five studies were not RCTs (Bottner 2001; Gross 2003; Lauretti 1998; Sittl 1991; Zhen 2007).
Risk of bias in included studies
Because no studies were eligible for inclusion we could not assess the efficacy of NSAIDs for treating cancer‐related pain in children and adolescents.
Effects of interventions
No studies were eligible for inclusion in this review, therefore, we were not able to comment on the efficacy or harm from the use of NSAIDs to treat cancer‐related pain in children and adolescents.
Due to the lack of evidence in this field, we were unable to judge the quality of evidence. There are no data and therefore no evidence to support or refute the use of NSAIDs for treating cancer‐related pain in children and adolescents.
Discussion
Summary of main results
We found no randomised controlled trials (RCTs) for inclusion in this review. There are no data from RCTs to inform assessment of the effectiveness of NSAIDs in treating cancer‐related pain in children and adolescents. NSAIDs are used in adults alongside other analgesics as they are thought to be useful for bone pain. There is currently no high quality evidence for their use (Derry 2017).
Overall completeness and applicability of evidence
As no RCTs could be identified we were unable to comment about efficacy or harm from the use of NSAIDs to treat cancer‐related pain in children and adolescents. Similarly, we could not comment on our remaining secondary outcomes: carer global impression of change; requirement for rescue analgesia; sleep duration and quality; acceptability of treatment; physical functioning; and quality of life.
The suite of reviews
This review is part of a suite of reviews on pharmacological interventions for chronic pain and cancer‐related pain in children and adolescents (Appendix 1). Taking a broader view on this suite of reviews, some pharmacotherapies (investigated in our other reviews) are likely to provide more data than others. Thus, the results were as expected considering that RCTs in children are known to be limited. The results have the potential to assist to inform policy making decisions for funding future clinical trials into NSAID treatment of child and adolescent pain, therefore, any results (large or small) are important to capture a snapshot of the current evidence for NSAIDs.
Quality of the evidence
Due to the lack of evidence in this field, we were unable to judge the quality of evidence. There is no evidence to support or refute the use of NSAIDs for treating cancer‐related pain in children and adolescents. We were unable to find any published RCTs to support or refute the use of NSAIDs to treat cancer‐related pain in children and adolescents. We were unable to examine any adverse effects.
This review may represent a case of absence of evidence rather than evidence of absence. While it may be true that the absence of evidence may reflect that NSAIDs per se are inadequately effective and their use as monotherapy analgesics is more likely to cause harm than benefit, the opposite may also pertain as data are lacking. It is difficult to conduct long‐term RCTs in children with cancer‐related pain, and few observational/clinical data have been published.
Potential biases in the review process
We carried out extensive searches of major databases using broad search criteria, and also searched two large clinical trial registries. We think it is unlikely that we have missed relevant studies.
Agreements and disagreements with other studies or reviews
We were not able to identify any published systematic reviews on this topic.
Study flow diagram.

