Ecografía para el diagnóstico de la discordancia de peso al nacer en embarazos gemelares
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with documented births; GA ≥ 24 weeks at delivery; US within 7 days of delivery | ||
| Patient characteristics and setting | 241 twins, GA: mean 35.8 (SD 2.4) weeks | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | No excluded women indicated. US within 7 days of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | DADC twin pregnancies with EFW within 2 weeks of delivery in the 3rd trimester | ||
| Patient characteristics and setting | 231 twin sets (January 2006 to March 2013); GA: mean 36 weeks 2 days (SD 2 weeks), DADC twins | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | EFW within 2 weeks of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | DADC twins | ||
| Patient characteristics and setting | 336 twin sets (January 2006 March 2013); GA: N/A; DADC twins | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | 1st trimester CRL, 2nd and 3rd trimester EFW | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pairs delivered after 32 weeks' gestation and 1 US within 2 weeks of delivery | ||
| Patient characteristics and setting | 178 twin pregnancies (1 September 1983 to 31 August 1986); GA: discordants: mean 36.4 (SD 2.3) weeks, concordants: mean 37.1 (SD 2.3) weeks; hospital | ||
| Index tests | AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women without AC, FL in both twins, and congenital malformations in either twin. US was within 2 weeks of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with complete sets of US measurements within 2 weeks of delivery delivered at Kaplan Hospital, Rehovot, Israel | ||
| Patient characteristics and setting | 90 twin pregnancies with US within 2 weeks of delivery; GA: mean 36.1 (SD 2.9) weeks; hospital | ||
| Index tests | EFW and AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women who had measurements performed > 2 weeks before delivery and had incomplete measurements | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Live‐born twin pairs born at tertiary centre with GA > 23 weeks, no anomalies and US within 3 weeks of delivery | ||
| Patient characteristics and setting | 242 twin pregnancies; GA: mean 32.8 (SD 4.1) weeks; tertiary centre | ||
| Index tests | EFW or AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | US within 3 weeks of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with last US performed within 7–14 days of delivery | ||
| Patient characteristics and setting | 85 twin pregnancies (enrolled from January 1985 to December 1988); GA at delivery: 36.9 weeks; hospital | ||
| Index tests | EFW using either AC alone or AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women had an interval between US and delivery > 14 days, intrauterine death, BW was not within 6 hours of delivery or EFW was unable to be determined | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin births with GA ≥ 24 weeks, and US within 28 days of delivery | ||
| Patient characteristics and setting | 1257 twin pregnancies (January 1991 to August 2002); GA: mean 35.2 (SD 2.6) weeks; hospital | ||
| Index tests | EFWD using a combination of BPD, AC, HC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women had incomplete data. US was within 28 days of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with FL, BPD and AC within 72 hours of delivery at medical centre | ||
| Patient characteristics and setting | 92 twin gestations; GA: mean 32.3 (SD 4.5) weeks; hospital | ||
| Index tests | EFW using FL, BPD or AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | EFW within 72 hours of delivery. Excluded women were those with fetal death or known structural or chromosomal abnormality | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies | ||
| Patient characteristics and setting | 18 twin pairs; GA: N/A; university | ||
| Index tests | BPD | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women not indicated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | All twin pregnancies registering for antenatal care by 11 weeks | ||
| Patient characteristics and setting | 2161 twin pregnancies (enrolled 2000–2010); large regional cohort of 9 hospitals over ten years | ||
| Index tests | EFW using HC, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women not described | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies registering for routine antenatal care by 11 weeks at antenatal care | ||
| Patient characteristics and setting | 2399 twin pregnancies; GA: N/A; antenatal care in hospital | ||
| Index tests | EFW based on HC, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with termination of pregnancy, structural or chromosomal abnormalities, pregnancies of unknown chorionicity, monochorionic, monoamniotic, and higher‐order multiple gestations | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies > 500 g; GA > 24 weeks: absence of fetal malformations or hydrops | ||
| Patient characteristics and setting | 278 twin pregnancies and 834 pregnancies in control; GA: mean 34.2 (SD 2.6); tertiary centre | ||
| Index tests | EFW using Hadlock equation | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | EFW within 3 days of delivery. Excluded women had pregnancies complicated by gestational or pregestational diabetes | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with delivery ≥ 22 weeks and 1 US within 15 weeks of delivery | ||
| Patient characteristics and setting | 283 participants (3‐year period); GA mean 33.29 (SD 4.12) weeks; tertiary referral centre | ||
| Index tests | EFWD using a combination of formulas including BPD, HC, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women did not have a 1st trimester US, had chromosomal abnormalities or congenital malformations | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies > 24 weeks cared for and delivered in maternal‐fetal medicine practice centre | ||
| Patient characteristics and setting | 343 twin deliveries (August 2005 to June 2010). Delivered by Maternal‐Fetal Medicine practice | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with monoamniotic twins, GA < 24 weeks, pregnancies with didelphic or unicornuate uterus, and those with major fetal anomalies | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with 2 weekly US surveillance from 24 weeks' gestation with surveillance of monochorionic twins 2‐weekly from 16 weeks at an academic perinatal centre | ||
| Patient characteristics and setting | 1001 twin pairs (May 2007 to October 2009); GA: discordant: mean 35.3 (SD 3.0) weeks, concordant: mean 36.3 (SD 2.4) weeks; perinatal centres | ||
| Index tests | EFW based on BPD, HC, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with monochorionic pregnancies with TTTS, a major structural abnormality, fetal aneuploidy or monoamnionicity | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US ≥ 15 weeks | ||
| Patient characteristics and setting | 203 twin gestations; GA: 24.4–39.7 weeks, hospital | ||
| Index tests | EFW from HC and AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women not reported | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US at 18–25 weeks' gestation and 1 US within 14 days prior to delivery | ||
| Patient characteristics and setting | 196 twin pregnancies; GA: median 35.6 (interquartile range 32.4–37.0); university obstetrics/gynaecology department | ||
| Index tests | EFW using BPD, HC, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Fetuses with structural or chromosomal defects were excluded. EFW within 14 days of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies delivered at university hospital with final US within 7 days of delivery | ||
| Patient characteristics and setting | 73 twin pregnancies (1 January 1990 to 31 March 1993); GA: mean 37 (range 19–40) weeks; university hospital | ||
| Index tests | EFW using BPD and AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | US was within 7 days of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with 2 live fetuses and chorionicity determined at the time of NT scan | ||
| Patient characteristics and setting | 2038 diamniotic twin pregnancies (1 January 2004 to 31 December 2006); GA: N/A; Department of Obstetrics/Gynaecology | ||
| Index tests | CRL discordance | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with pregnancies of unknown chorionicity, monochorionic monoamniotic pregnancies and pregnancies with known reduction from a higher number of multiples | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US at 11–14 weeks' gestation | ||
| Patient characteristics and setting | 157 dichorionic twin pregnancies (April 2000 to April 2002); GA: mean 35.5 (SD 2.7) weeks; medical centre | ||
| Index tests | CRL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with monochorionic pregnancies, chromosomal anomalies and the loss of 1 or both fetuses | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with delivery at ≥ 35 weeks' gestations in medical centre | ||
| Patient characteristics and setting | 253 twin pregnancies (January 2007 to December 2013); GA: N/A; medical centre | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women had complicated pregnancies such as preterm labour, premature rupture of membranes, placenta previa, pre‐eclampsia, diabetes, TTTS, monoamniotic twin and congenital fetal anomaly | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Diamniotic twin pregnancies with 2 live births from tertiary care centre | ||
| Patient characteristics and setting | 503 diamniotic twin pregnancies (1 April 1994 to 11 January 2002); GA: growth discordant twins: mean 34.4 (SD 2.9) weeks, growth concordant twins: mean 35.6 (SD 2.9) weeks; tertiary care centre | ||
| Index tests | EFW and AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with chromosomal and structural anomalies, pregnancies complicated by TTTS and intrauterine death of 1 or both fetuses | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US at university medical school | ||
| Patient characteristics and setting | 221 twin pregnancies; GA: mean 35.6 (SD 2.7) weeks; medical school | ||
| Index tests | EFW with HC, AC, BPD and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with fetal malformation, TTTS, fetal death or unknown outcome | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies beyond 28 weeks' gestation, with on 2 US and 1 within 3 weeks of delivery recruited from antenatal clinic | ||
| Patient characteristics and setting | 107 twin pregnancies (1984–1987); GA: mean 36.1 (SD 2.4) weeks; antenatal clinic | ||
| Index tests | EFW using AC formula and AC and BPD formula | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | US within 3 weeks of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies from academic centres with 2‐weekly US from 24 weeks' gestation with surveillance of monochorionic twins 2‐weekly from 16 weeks | ||
| Patient characteristics and setting | 960 twin pairs without TTTS; GA: N/A; academic centres | ||
| Index tests | EFW, BPD, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with twin pairs with TTTS | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Monochorionic twin pregnancies delivered at single referral centre, CRL at 8–10 weeks' gestation | ||
| Patient characteristics and setting | 126 MCDA twin pregnancies; GA: mean 37.4 weeks; single referral centre | ||
| Index tests | CRL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with terminated pregnancies, 1 or both fetal death before 10 weeks, twin‐reversed arterial perfusion sequence, triplets or reduction of high‐order multiples, and anomalies diagnosed before 10 weeks' gestation | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with CRL in 1st trimester, with BPD, HC, AC and FL measured. Included women had 2‐weekly growth scans from 24 weeks' gestation | ||
| Patient characteristics and setting | 1001 twin pregnancies (May 2007 to October 2009); GA: N/A; perinatal centres | ||
| Index tests | EFW, BPD, AC, FL and CRL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women had monoamnionicity, a major structural abnormality or fetal aneuploidy | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US within 2 weeks of delivery, and both twins were alive at antenatal diagnosis centre | ||
| Patient characteristics and setting | 124 twin pairs; GA: N/A; antenatal diagnosis centre | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | US within 2 weeks of delivery. Excluded women not reported | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US at GA > 23 weeks | ||
| Patient characteristics and setting | 78 twin pairs; GA: N/A; general hospital | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with congenital anomalies | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies delivered at university hospital | ||
| Patient characteristics and setting | 80 twin pregnancies; GA: mean 251 (SD 22) days; hospital | ||
| Index tests | EFW using BPD and abdominal diameter | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women not reported | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US at 7–14 week of pregnancy at hospital | ||
| Patient characteristics and setting | 118 twin pregnancies; GA: mean 33.86 (SD 2.36) weeks; hospital | ||
| Index tests | CRL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with monochorionic twins, and with 1st or 2nd trimester pregnancy terminations | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin gestations with US within 2 weeks before birth and both twins were born alive at university hospital | ||
| Patient characteristics and setting | 661 twin pregnancies (1 January 1994 to 30 June 2008); GA: concordant: mean 35.6 (SD 2.2) weeks, appropriate‐for‐gestational‐age: mean 35.7 (SD 1.0) weeks, small‐for‐gestational‐age: mean 33.5 (SD 2.7) weeks; hospital | ||
| Index tests | EFW and AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | US measured within 2 weeks before birth | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies at university health centre, with US every 2 weeks, and US within 2 weeks of delivery | ||
| Patient characteristics and setting | 43 twin pregnancies; GA: 33.5 weeks; university health centre | ||
| Index tests | EFW based on BPD and AC or FL and AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with congenital anomalies. US within 2 weeks of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with antenatal surveillance at university medical centre | ||
| Patient characteristics and setting | 281 twin pregnancies (2008–2011); GA: median 35 + 0 weeks; medical centre | ||
| Index tests | EFW based on HC, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with monoamniotic twin pregnancies, selective feticide, congenital disorders, intrauterine fetal death, GA at birth < 22 weeks, or BW < 500 g | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | MCDA twin pregnancies with EFW at 16, 20 and 26 weeks' gestation, and within 2 weeks of birth at university hospital | ||
| Patient characteristics and setting | 68 MCDA twin pregnancies; GA 35.7 weeks; hospital | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with intrauterine fetal death or twin‐reverse arterial perfusion sequence. EFW within 2 weeks of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with fetal weight within 14 days of delivery | ||
| Patient characteristics and setting | 94 twin pregnancies; GA: mean 33 (SD 2.5) weeks; hospital | ||
| Index tests | BPD, mean abdominal diameters and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with congenital anomalies. EFW within 14 days of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | MCDA twin gestations with NT and CRL measurements on US at 11–13 weeks' gestation | ||
| Patient characteristics and setting | 89 twin pregnancies (August 2003 to August 2012); GA: mean 34.1 (SD) 3.3 weeks; hospital | ||
| Index tests | EFW, CRL and NT | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with lethal anomalies at the time of scan, loss of 1 or both twins prior to scan or altered chorionicity findings | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with MCDA placentation with antenatal care at single academic centre | ||
| Patient characteristics and setting | 73 twin pairs; GA: non‐discordant: mean 34.6 (SD 4.0) weeks, discordant: mean 32.4 (SD 4.6) weeks; academic centre | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with pregnancies complicated by TTTS | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
AC: abdominal circumference; BPD: biparietal diameter; BW: birth weight; CRL: crown rump length; DADC: diamniotic dichorionic; EFW: estimated fetal weight; FL: femur length; GA: gestational age; HC: head circumference; MCDA: monochorionic diamniotic; N/A: not available; NT: nuchal translucency; SD: standard deviation; TTTS: twin‐to‐twin syndrome; US: ultrasound.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Research question did not include the reference standard. | |
| Index test difference in birth weight as reference did not match research question. | |
| Birthweight discordance cut‐off did not match research question. | |
| Research question did not include the reference standard. | |
| Birthweight discordance cut‐off did not match research question. | |
| Research question did not include the reference standard. | |
| Unable to construct 2 × 2 table from data. | |
| Research question did not include the reference standard. | |
| Research question did not include the reference standard. | |
| Index test cut‐off did not match research question. | |
| Study did not address the index test. | |
| Index test cut‐off did not match research question. | |
| Study did not address the index test. | |
| Research question did not include the reference standard. | |
| Study did not address the index test. | |
| Study did not address the index test. | |
| Article was not a published study. | |
| Article was a systematic review and meta‐analysis. | |
| Research question did not include the reference standard. | |
| Study did not address the index test. | |
| Unable to construct 2 × 2 table from data. | |
| Research question did not include the reference standard. | |
| Study did not address the index test. | |
| Research question did not include the reference standard. | |
| Research question did not include the reference standard. | |
| Research question did not include the reference standard. | |
| Research question did not include the reference standard. | |
| Birthweight discordance cut‐off did not match research question. | |
| Birthweight discordance cut‐off did not match research question. | |
| Research question did not include the reference standard. |
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 Cut‐off 20% Show forest plot | 22 | 8005 |
| Test 1  Cut‐off 20% | ||
| 2 20% abdominal circumference Show forest plot | 7 | 2846 |
| Test 2  20% abdominal circumference | ||
| 3 20% femur length Show forest plot | 7 | 2791 |
| Test 3  20% femur length | ||
| 4 Cut‐off 25% Show forest plot | 18 | 6471 |
| Test 4  Cut‐off 25% | ||
| 5 25% abdominal circumference Show forest plot | 7 | 3614 |
| Test 5  25% abdominal circumference | ||
| 6 25% femur length Show forest plot | 4 | 2714 |
| Test 6  25% femur length | ||
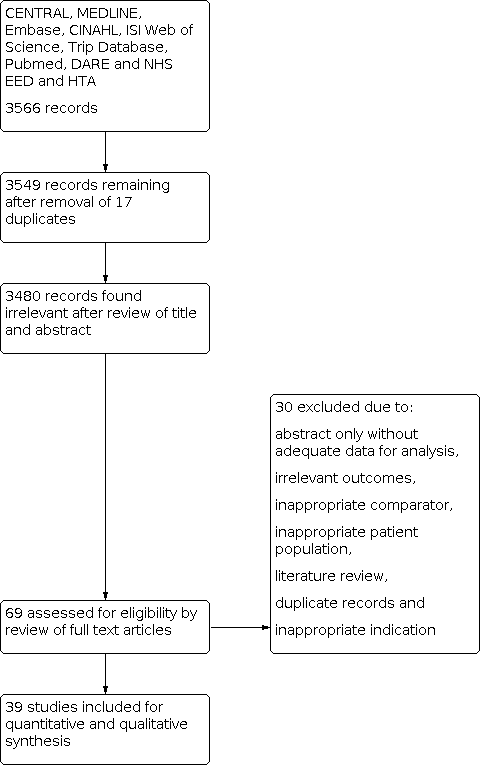
PRISMA flow diagram outlining the study selection process.

QUADAS‐2 risk of bias and applicability concerns graph including review authors' judgements about each domain presented as percentages across included studies.

QUADAS‐2 risk of bias and applicability concerns summary including review authors’ judgements about each domain for each included study.
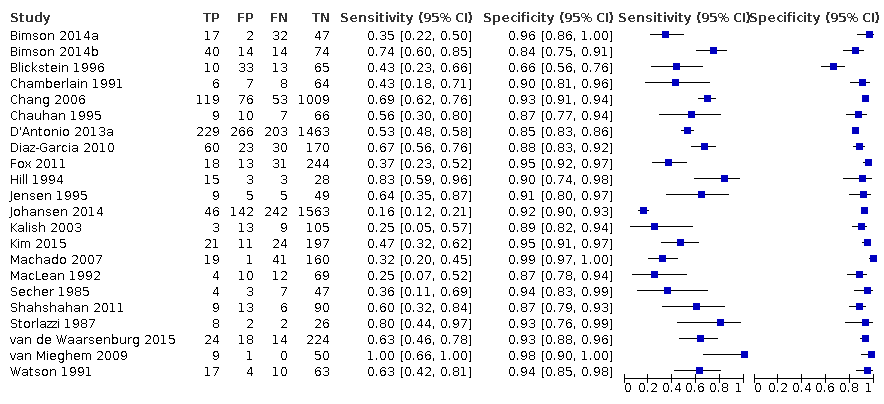
A forest plot representing study level sensitivities and specificities that used an estimated fetal weight discordance (EFWD) of 20%.

Summary receiver operating characteristic plot of studies assessing the accuracy of ultrasound based on an estimated fetal weight discordance (EFWD) of 20%. Each study is represented as an ellipse with size of the ellipse adjusted to the sample size of the study. The filled circle represents the summary point indicating summary sensitivity and specificity of the meta‐analytic estimate. Dotted closed line represents 95% confidence region and the dashed line represents the 95% prediction region around the summary point.

A forest plot representing sensitivities and specificities of the studies that used ultrasound abdominal circumference to detect estimated fetal weight discordance (EFWD) of 20%. FN: false negative; FP: false positive; TN: true negative; TP: true positive.

A forest plot representing sensitivities and specificities of the studies that used femoral length by ultrasound to detect estimated fetal weight discordance (EFWD) of 20%. FN: false negative; FP: false positive; TN: true negative; TP: true positive.

A forest plot representing sensitivities and specificities of all the studies that used an estimated fetal weight discordance (EFWD) of 25%. FN: false negative; FP: false positive; TN: true negative; TP: true positive.
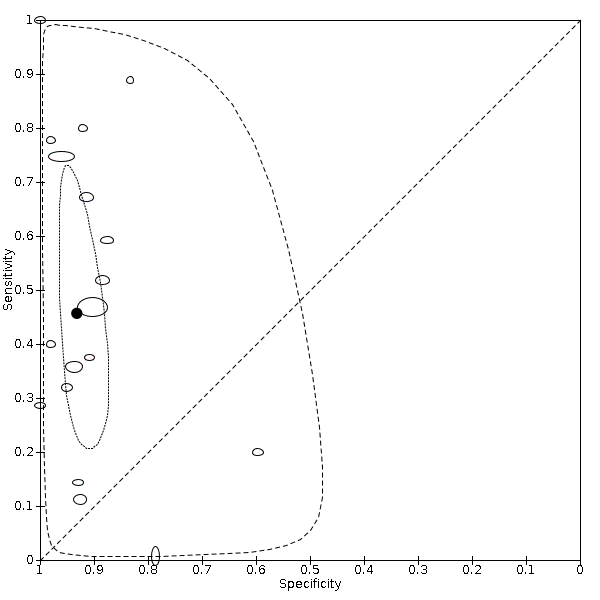
Summary receiver operating characteristic plot of studies assessing the accuracy of ultrasound based on an estimated fetal weight discordance (EFWD) of 25%. Each study is represented as an ellipse with size of the ellipse adjusted to the sample size of the study. The filled circle represents the summary point indicating summary sensitivity and specificity of the meta‐analytic estimate. Dotted closed line represents 95% confidence region and the dashed line represents the 95% prediction region around the summary point.
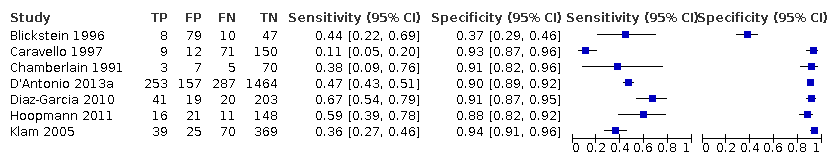
A forest plot representing sensitivities and specificities of the studies that used ultrasound abdominal circumference to detect estimated fetal weight discordance (EFWD) of 25%. FN: false negative; FP: false positive; TN: true negative; TP: true positive.

A forest plot representing sensitivities and specificities of the studies that used ultrasound femoral length to detect estimated fetal weight discordance (EFWD) of 25%. FN: false negative; FP: false positive; TN: true negative; TP: true positive.

20% abdominal circumference
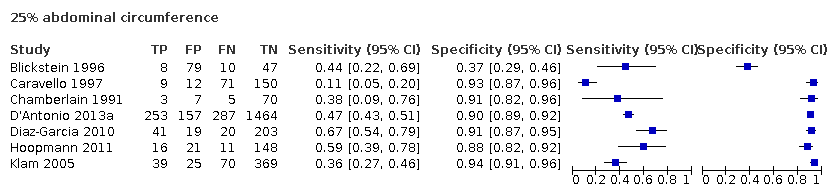
25% abdominal circumference
| Sensitivity | 0.51 (95% CI 0.42 to 0.60) | Prevalencesa | 18% | 15% | 28% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Specificity | 0.91 (95% CI 0.89 to 0.93) | ||||||||||
| Outcome | No. of studies and participants | Study design | Factors that may decrease certainty | Effect per 1000 women tested | |||||||
| Risk of bias | Indirectness | Inconsistency | Imprecision | Publication bias | Pretest probability of 18% | Pretest probability of 15% | Pretest probability of 28% | Test accuracy (certainty of the evidence) | |||
| True positives (women with diagnosis of birth weight discordance) | 22 studies, | Cohort‐type studies with delayed verification (cohort type accuracy study) | Very seriousb | Not serious | Very seriousc | Not serious | None | 92 (76 to 108) | 77 (63 to 90) | 143 (118 to 168) | ⊕⊝⊝⊝ |
| False negatives (women incorrectly classified as not having diagnosis of birth weight discordance) | 88 (72 to 104) | 73 (60 to 87) | 137 (112 to 162) | ||||||||
| True negatives | 22 studies, 6453 participants | Cohort‐type studies with delayed verification (cohort type accuracy study) | Very seriousb | Not serious | Very seriousc | Not serious | None | 746 (730 to 763) | 774 (757 to 791) | 655 (641 to 670) | ⊕⊝⊝⊝ |
| False positives | 74 (57 to 90) | 76 (59 to 93) | 65 (50 to 79) | ||||||||
| CI: confidence interval. | |||||||||||
| Sensitivity | 0.46 (95% CI 0.26 to 0.66) | Prevalencesa | 19% | 9% | 27% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Specificity | 0.93 (95% CI 0.89 to 0.96) | ||||||||||
| Outcome | No. of studies and participants | Study design | Factors that may decrease certainty | Effect per 1000 women tested | |||||||
| Risk of bias | Indirectness | Inconsistency | Imprecision | Publication bias | Pretest probability of 19% | Pretest probability of 9% | Pretest probability of 27% | Test accuracy (certainty of the evidence) | |||
| True positives (women with diagnosis of birth weight discordance) | 18 studies, | Cohort‐type studies with delayed verification (cohort type accuracy study) | Very seriousb | Not serious | Very seriousc | Not serious | None | 87 (49 to 125) | 41 (23 to 59) | 124 (70 to 178) | ⊕⊝⊝⊝ |
| False negatives (women incorrectly classified as not having diagnosis of birth weight discordance) | 103 (65 to 141) | 49 (31 to 67) | 146 (92 to 200) | ||||||||
| True negatives | 18 studies | Cohort‐type studies with delayed verification (cohort type accuracy study) | Very seriousb | Not serious | Very seriousc | Not serious | None | 753 (721 to 778) | 846 (810 to 874) | 679 (650 to 701) | ⊕⊝⊝⊝ |
| False positives | 57 (32 to 89) | 64 (36 to 100) | 51 (29 to 80) | ||||||||
| CI: confidence interval. | |||||||||||
| Groups | No. of studies | Sensitivity (95% CI) | Specificity (95% CI) | LR+ | LR– |
|---|---|---|---|---|---|
| BWD of 20% | 22 | 0.51 (0.42 to 0.60) | 0.91 (0.89 to 0.93) | 5.9 (4.3 to 8.1) | 0.53 (0.44 to 0.64) |
| EFWD by AC | 7 | 0.57 (0.48 to 0.66) | 0.84 (0.72 to 0.92) | 3.6 (1.8 to 7.4) | 0.51 (0.38 to 0.68) |
| EFWD by FL | 7 | 0.60 (0.53 to 0.67) | 0.87 (0.84 to 0.90) | 4.6 (3.4 to 6.1) | 0.46 (0.38 to 0.56) |
| BWD of 25% | 18 | 0.46 (0.26 to 0.66) | 0.93 (0.89 to 0.96) | 6.7 (3.0 to 14.9) | 0.58 (0.39 to 0.88) |
| EFWD by AC | 7 | 0.42 (0.27 to 0.58) | 0.88 (0.76 to 0.94) | 3.3 (1.6 to 6.9) | 0.67 (0.51 to 0.87) |
| EFWD by FL | 4 | 0.55 (0.44 to 0.65) | 0.91 (0.89 to 0.92) | 5.8 (4.3 to 7.9) | 0.50 (0.40 to 0.64) |
| AC: abdominal circumference; BWD: birth weight discordance; CI: confidence interval; EFWD: estimated fetal weight discordance; FL: femur length; LR+: likelihood ratio of a positive test; LR–: likelihood ratio of a negative test. | |||||
| Test | No. of studies | No. of participants |
| 1 Cut‐off 20% Show forest plot | 22 | 8005 |
| 2 20% abdominal circumference Show forest plot | 7 | 2846 |
| 3 20% femur length Show forest plot | 7 | 2791 |
| 4 Cut‐off 25% Show forest plot | 18 | 6471 |
| 5 25% abdominal circumference Show forest plot | 7 | 3614 |
| 6 25% femur length Show forest plot | 4 | 2714 |