Nicht‐medikamentöse Interventionen zur Behandlung von chronischer Prostatitis/ von chronischem Beckenschmerzsyndrom
Abstract
Background
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a common disorder in which the two main clinical features are pelvic pain and lower urinary tract symptoms. There are currently many approaches for its management, using both pharmacological and non‐pharmacological interventions. The National Institute of Health ‐ Chronic Prostatitis Symptom Index (NIH‐CPSI) score is a validated measure commonly used to measure CP/CPPS symptoms.
Objectives
To assess the effects of non‐pharmacological therapies for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS).
Search methods
We performed a comprehensive search using multiple databases, trial registries, grey literature and conference proceedings with no restrictions on the language of publication or publication status. The date of the latest search of all databases was August 2017.
Selection criteria
We included randomised controlled trials. Inclusion criteria were men with a diagnosis of CP/CPPS. We included all available non‐pharmacological interventions.
Data collection and analysis
Two review authors independently classified studies and abstracted data from the included studies, performed statistical analyses and rated quality of evidence (QoE) according to the GRADE methods.
Main results
We included 38 unique studies with 3290 men with CP/CPPS across 23 comparisons.
1. Acupuncture: (three studies, 204 participants) based on short‐term follow‐up, acupuncture probably leads to clinically meaningful reduction in prostatitis symptoms compared with sham procedure (mean difference (MD) in total NIH‐CPSI score ‐5.79, 95% confidence interval (CI) ‐7.32 to ‐4.26, high QoE). Acupuncture may result in little to no difference in adverse events (low QoE). Acupuncture may not reduce sexual dysfunction when compared with sham procedure (MD in the International Index of Erectile Function (IIEF) Scale ‐0.50, 95% CI ‐3.46 to 2.46, low QoE). Acupuncture may also lead to a clinically meaningful reduction in prostatitis symptoms compared with standard medical therapy (MD ‐6.05, 95% CI ‐7.87 to ‐4.24, two studies, 78 participants, low QoE). We found no information regarding quality of life, depression or anxiety.
2. Lifestyle modifications: (one study, 100 participants) based on short‐term follow‐up, lifestyle modifications may be associated with a reduction in prostatitis symptoms compared with control (risk ratio (RR) for improvement in NIH‐CPSI scores 3.90, 95% CI 2.20 to 6.92, very low QoE). We found no information regarding adverse events, sexual dysfunction, quality of life, depression or anxiety.
3. Physical activity: (one study, 85 participants) based on short‐term follow‐up, a physical activity programme may cause a small reduction in prostatitis symptoms compared with control (NIH‐CPSI score MD ‐2.50, 95% CI ‐4.69 to ‐0.31, low QoE). This programme may not reduce anxiety or depression (low QoE). We found no information regarding adverse events, sexual dysfunction or quality of life.
4. Prostatic massage: (two studies, 115 participants) based on short‐term follow‐up, we are uncertain whether the prostatic massage reduces or increases prostatitis symptoms compared with control (very low QoE). We found no information regarding adverse events, sexual dysfunction, quality of life, depression or anxiety.
5. Extracorporeal shockwave therapy: (three studies, 157 participants) based on short‐term follow‐up, extracorporeal shockwave therapy reduces prostatitis symptoms compared with control (NIH‐CPSI score MD ‐6.18, 95% CI ‐7.46 to ‐4.89, high QoE). These results may not be sustained at medium‐term follow‐up (low QoE). This treatment may not be associated with a greater incidence of adverse events (low QoE). This treatment probably improves sexual dysfunction (MD in the IIEF Scale MD 3.34, 95% CI 2.68 to 4.00, one study, 60 participants, moderate QoE). We found no information regarding quality of life, depression or anxiety.
6. Transrectal thermotherapy compared to medical therapy: (two studies, 237 participants) based on short‐term follow‐up, transrectal thermotherapy alone or in combination with medical therapy may decrease prostatitis symptoms slightly when compared with medical therapy alone (NIH‐CPSI score MD ‐2.50, 95% CI ‐3.82 to ‐1.18, low QoE). One included study reported that participants may experience transient adverse events. We found no information regarding sexual dysfunction, quality of life, depression or anxiety.
7. Other interventions: there is uncertainty about the effects of most of the other interventions included in this review. We found no information regarding psychological support or prostatic surgery.
Authors' conclusions
Based on the findings of moderate quality evidence, this review found that some non‐pharmacological interventions such as acupuncture and extracorporeal shockwave therapy are likely to result in a decrease in prostatitis symptoms and may not be associated with a greater incidence of adverse event. The QoE for most other comparisons was predominantly low. Future clinical trials should include a full report of their methods including adequate masking, consistent assessment of all patient‐important outcomes including potential treatment‐related adverse events and appropriate sample sizes.
PICO
Laienverständliche Zusammenfassung
Interventionen zur Behandlung von chronischer Prostatitis und chronischen Beckenschmerzen bei Männern
Fragestellung
Welche Wirkungen erzielen nicht‐medikamentöse Therapien bei Männern mit lang anhaltenden Schmerzen und Beschwerden im Prostata‐ und Beckenbereich, der sogenannten chronischen Prostatitis (CP) bzw. dem sogenannten chronischen Beckenschmerzsyndrom (CPPS)?
Hintergrund
CP/ CPPS ist eine bei Männern häufig auftretende Krankheit, die während des Wasserlassens zu Schmerzen und/oder Beschwerden im Beckenbereich führt. Die Ursache dafür ist unbekannt, und es gibt viele verschiedene Behandlungsmöglichkeiten für diese Erkrankung.
Studienmerkmale
Die Evidenz ist auf dem Stand von August 2017. Wir identifizierten 38 Studien, die zwischen 1993 und 2016 mit insgesamt 3187 Teilnehmern durchgeführt wurden. Dabei wurden 23 Vergleiche zwischen verschiedenen Behandlungen bei Männern mit CP/CPPS angestellt. Die untersuchten Interventionen berücksichtigten für gewöhnlich medizinische Geräte, medizinische Beratung oder Formen von Physiotherapie. In vielen Fällen erhielten die Männer die Therapien ambulant. Die meisten Studien machten keine Angaben zu ihrer Finanzierung; drei Studien berichteten, dass sie von Herstellern medizinischer Geräte finanziert wurden.
Hauptergebnisse
Akupunktur: Wir fanden heraus, dass Akupunktur (eine alternative medizinische Methode, bei der dünne Nadeln an spezifischen Stellen in die Haut gestochen werden) Symptome der Prostatitis wahrscheinlich signifikant vermindert und möglicherweise nicht mit Nebenwirkungen assoziiert ist, wenn mit Schein‐Akupunktur verglichen wird. Möglicherweise reduziert sie jedoch nicht sexuelle Probleme. Im Vergleich zur medizinischen Standardtherapie verringert Akupunktur wahrscheinlich die Symptome. Wir fanden keine Informationen zu den Auswirkungen auf Lebensqualität, Depression oder Angstzustände.
Änderungen im Lebensstil: Wir sind unsicher ob Empfehlungen, den Lebensstil zu verändern die Symptome reduzieren, was aus einem Vergleich mit fortgeführtem Lebensstil hervorgeht. Wir hatten keine Informationen zu Nebenwirkungen, sexuellen Problemen, Lebensqualität, Depression oder Angstzustände.
Körperliche Aktivität: Wir fanden heraus, dass im Vergleich zu einer nicht‐ spezifischen Aktivität als Kontrollgruppe körperliche Aktivitätsprogramme die Symptome möglicherweise reduzieren (kleiner Effekt). Wir haben keine Informationen zu Nebenwirkung, sexuellen Problemen oder Lebensqualität.
Prostatamassage: Wir sind unsicher, ob Prostatamassage im Vergleich zu keiner Massage die Symptome reduziert oder verstärkt. Wir fanden keine Informationen zu Nebenwirkungen, sexuellen Problemen, Lebensqualität, Depression oder Angstzuständen.
Extrakorporale Stoßwellentherapie: Wir fanden heraus, dass die extrakorporale Stoßwellentherapie (bei der Stoßwellen durch die Haut zur Prostata gesendet werden) die Symptome im Vergleich zu einem simulierten Verfahren signifikant verringert. Diese Ergebnisse werden bei fortgesetzter Behandlung möglicherweise nicht andauern. Diese Behandlung ist möglicherweise nicht mit Nebenwirkungen assoziiert. Wir haben keine Informationen zu Lebensqualität, Depression oder Angstzuständen.
Transrektale Thermotherapie im Vergleich zur medizinischen Therapie: Wir fanden heraus, dass transrektale Thermotherapie (die Hitze im Bereich der Prostata und des Beckenmuskels verwendet) allein oder in Kombination mit medizinischer Therapie die Symptome im Vergleich zur alleinigen medizinischen Therapie möglicherweise leicht verringert. Eine der eingeschlossenen Studien berichtete, dass Teilnehmer vorübergehende Nebenwirkungen erleben können. Wir haben keine Informationen zu sexuellen Problemen, Lebensqualität, Depression oder Angstzuständen.
Es gibt Unsicherheit über die Wirkungen anderer Interventionen.
Qualität der Evidenz
Die Qualität der Evidenz war in den meisten Fällen niedrig, d.h. es gibt hinsichtlich der Ergebnisse viel Unsicherheit. Die eingeschlossenen Studien waren nicht gut geplant, hatten wenige Teilnehmer und eine kurze Nachbeobachtungsdauer (gewöhnlich 12 Wochen).
Authors' conclusions
Summary of findings
| Acupuncture compared to sham procedure for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with sham procedure | Risk difference with Acupuncture | ||||
| Prostatitis Symptoms Benefit is indicated by lower scores | 204 | ⊕⊕⊕⊝ | ‐ | The mean prostatitis Symptoms ranged from 17.08 to 22 | MD 5.79 lower |
| Prostatitis Symptoms | 113 | ⊕⊝⊝⊝ | RR 2.49 | Study population | |
| 404 per 1.000 | 596 more per 1000 | ||||
| Adverse events follow up: 6‐8 weeks | 204 | ⊕⊕⊝⊝ | RR 1.33 | Study population | |
| 58 per 1.000 | 19 more per 1000 | ||||
| Sexual dysfunction Benefit is indicated by higher scores | 89 | ⊕⊕⊝⊝ | ‐ | The mean sexual dysfunction was 23 | MD 0.5 lower |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded 1 level due to unclear risk of bias: insufficient information about allocation concealment 2 Downgraded 1 level due to inconsistency: statistical heterogeneity (I2 = 76%). 3 Downgraded 1 level due to imprecision issues: wide confidence interval due to small sample size and few events. 4 Downgraded 1 level for imprecision issues: wide confidence interval includes both appreciable benefit and harm. | |||||
| Acupuncture compared to medical treatment for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: treating chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with medical treatment | Risk difference with acupuncture | ||||
| Prostatitis symptoms (NIH‐CPSI total) Benefit is indicated by lower scores | 78 | ⊕⊕⊕⊝ | ‐ | The mean prostatitis symptoms (NIH‐CPSI total) ranged from 12 to 16 | MD 6.05 lower |
| Prostatitis symptoms: response defined as a 6‐point decrease in NIH‐CPSI score | 24 | ⊕⊕⊝⊝ | RR 3.57 | Study population | |
| 250 per 1000 | 643 more per 1000 | ||||
| Adverse events follow‐up: 12 weeks | 78 | ⊕⊕⊝⊝ | ‐ | There were no adverse events in either group. | |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 1 level due to risk of bias: included studies were not blinded, which affects both detection and performance bias. 2The initial analysis had greater statistical inconsistency (I2 = 70%) and included one study that included people with chronic prostatitis/chronic pelvic pain syndrome using criteria that differed from that recommended by the Research Consensus (Chen 2009). In a sensitivity analysis, we excluded the results from this study and found greater statistical consistency (I2 = 0%), therefore, we chose to report these results in the 'Summary of findings' table. For this reason, we did not downgrade due to inconsistency. 3Downgraded 1 level due to imprecision issues: few events and wide confidence interval. | |||||
| Circumcision plus usual care compared to waiting list plus usual care for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with waiting list for circumcision | Risk difference with early circumcision | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 713 | ⊕⊕⊕⊝ | ‐ | The mean prostatitis symptoms was 15 | MD 3.00 lower |
| Adverse events3 follow‐up: 12 weeks | 713 | ⊕⊕⊝⊝ | RR 1.23 | Study population | |
| 130 per 1000 | 30 more per 1000 | ||||
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 1 level due to high risk of bias: study not blinded (high risk of performance and detection bias). 2Confidence intervals were constructed using transformations described in the Cochrane Handbook for Systematic Reviews of Interventions Section 7.7.3.5. 3All adverse events were minor. 4Downgraded 1 level due to imprecision issues: few events in each group and wide confidence interval. | |||||
| Electromagnetic chair compared to control intervention for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with control intervention | Risk difference with electromagnetic chair | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 57 | ⊕⊝⊝⊝ | ‐ | 1 study found no differences in NIH‐CPSI score measurements at 6 weeks. The other study found a symptom score 16 points (0‐ to 90‐point scale) lower in the intervention group compared to the control group (P value not available) at 12 weeks. | |
| Adverse events follow‐up: 6‐12 weeks | 57 | ⊕⊕⊝⊝ | ‐ | 1 study reported a 0 incidence of adverse events and the other study reported 1 case of transient paraesthesia in the active treatment group. | |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 1 level due to risk of bias: one study not blinded and the other study had high attrition bias and selective outcome reporting bias. 2The two included studies had inconsistent results (see narrative description). 3Downgraded 1 level for imprecision issues: optimal information size not met (OIS for a 4‐point decrease, standard deviation 6, alpha 0.05, beta 0.20 = 74); small sample size in the individual studies (meta‐analysis was not possible). 4Downgraded 1 level due to imprecision issues: rare events and wide confidence interval. | |||||
| Lifestyle modifications compared to control for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with control | Risk difference with lifestyle modifications | ||||
| Prostatitis symptoms: response defined as 6‐point decrease in NIH‐CPSI score Benefit is indicated by lower scores | 100 | ⊕⊝⊝⊝ | RR 3.90 | Study population | |
| 200 per 1000 | 580 more per 1000 | ||||
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 2 levels due to high risk of selection bias (unconcealed allocation), detection and performance bias (study not blinded), missing outcome data and suspected selective outcome reporting (data presented graphically). 2Downgraded 1 level due to imprecision issues: few events and wide confidence interval. | |||||
| Physical activity compared to control intervention procedure for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with sham procedure | Risk difference with physical activity | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 85 | ⊕⊕⊝⊝ | ‐ | The mean prostatitis symptom score was 20 | MD 2.50 lower |
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Anxiety Benefit is indicated by lower scores | 85 | ⊕⊝⊝⊝ | ‐ | The mean anxiety score was 42.1 | MD 2.8 lower |
| Depression Benefit is indicated by lower scores | 85 | ⊕⊝⊝⊝ | ‐ | The mean depression score was 9.3 | MD 0.5 higher |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial; SAI‐Y: State Anxiety Inventory‐Y. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 2 levels: high risk of performance bias and detection bias (study not blinded); high risk of attrition bias at follow‐up. 2Downgraded 1 level due to imprecision issues: wide confidence intervals include both considerable benefits and harms. | |||||
| Prostatic massage compared to control for treating chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with control | Risk difference with prostatic massage | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 44 | ⊕⊝⊝⊝ | ‐ | The mean prostatitis symptom score was 12.4 | MD 1.10 lower |
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 2 levels due to high risk of performance and detection bias (study not blinded), unclear risk of bias in the remaining domains. 2Downgraded 1 level for imprecision issues: optimal information size (OIS) not met (OIS for a 4‐point decrease, standard deviation 6, alpha 0.05, beta 0.20 = 74). | |||||
| Extracorporeal shockwave therapy compared to control procedure for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with control | Risk difference with ESWT | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 157 | ⊕⊕⊕⊕ | ‐ | The mean prostatitis symptom score ranged from 16.8 to 26.81 | MD 6.18 lower |
| Prostatitis symptoms: response defined as a 6‐point decrease in NIH‐CPSI score | 135 | ⊕⊝⊝⊝ | RR 6.20 | Study population | |
| 149 per 1000 | 776 more per 1000 | ||||
| Prostatitis symptoms | 97 | ⊕⊕⊝⊝ | ‐ | The mean prostatitis symptom score ranged from 16.1 to 27 | MD 2.23 lower |
| Adverse events follow‐up: 24 weeks | 195 | ⊕⊕⊝⊝ | RR 1.22 | Study population | |
| 93 per 1000 | 20 more per 1000 | ||||
| Sexual dysfunction Benefit is indicated by higher scores | 60 | ⊕⊕⊕⊝ | ‐ | The mean sexual dysfunction was 16.83 | MD 3.34 higher |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ESWT: extracorporeal shockwave therapy; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Whereas one of the studies was not blinded, which could have posed a high risk of performance and detection bias, we did not downgrade for risk of bias due to the consistency with other studies with low risk of bias. 2Downgraded 1 level due to risk of bias: one of the studies that provided events for this outcome was not blinded. 3Downgraded 1 level due to inconsistency (I2 = 71%). 4Downgraded 1 level due to imprecision issues: few events and wide confidence interval. 5Downgraded 1 level due to inconsistency (I2 = 82%). 6Downgraded 1 level due to imprecision issues: wide confidence interval. | |||||
| Transrectal thermotherapy compared to medical treatment for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with medical treatment | Risk difference with transrectal thermotherapy | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 140 | ⊕⊕⊝⊝ | ‐ | The mean prostatitis symptom score ranged from 14.33 to 17.19 | MD 2.50 lower |
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 2 levels due to high risk of allocation concealment bias, performance and detection bias (study not blinded) and high risk of attrition bias. | |||||
| Transrectal thermotherapy (add‐on) compared to medical treatment alone for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with medical treatment alone | Risk difference with transrectal thermotherapy (add‐on) | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 145 | ⊕⊕⊝⊝ | ‐ | The mean prostatitis symptom score ranged from 14.33 to 17.19 | MD 4.34 lower |
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 2 levels due to high risk of allocation concealment bias, performance and detection bias (study not blinded) and high risk of attrition bias. | |||||
Background
Description of the condition
Prostatitis is a common disorder affecting 10% to 14% of men in Europe and the USA (Bajpayee 2012). This health problem motivates 1% of primary care visits and 8% of urology consultations in the USA (Collins 1998). Only 5% to 10% of prostatitis cases have a bacterial origin (Bartoletti 2007; De La Rosette 1993). This disorder can affect men of all ages and ethnic origins, but it is more common in younger men with a mean age of onset at 42 years old (Schaeffer 2002). The two main clinical features of prostatitis are pelvic pain and lower urinary tract symptoms (LUTS), even though there is a wide range of clinical presentations (Nickel 1999a).
The National Institutes of Health (NIH) classification identifies four types of prostatitis (Nickel 1999a): type I, acute bacterial prostatitis; type II, chronic bacterial prostatitis; type III, chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) and type IV, asymptomatic prostatitis. It remains unclear whether type III can be linked in all cases to prostatic involvement (True 1999), thus the alternate denomination (CPPS). CP/CPPS is subclassified as type IIIa, inflammatory, and type IIIb, non‐inflammatory, depending on the presence of inflammatory cells in prostatic secretions. Before this classification, this entity was denominated chronic abacterial or non‐bacterial prostatitis (similar to type IIIa CP/CPPS) and prostatodynia (similar to type IIIb CP/CPPS) (Krieger 1996). This change in the classification might have changed the epidemiology of this condition (Krieger 2004).
CP/CPPS is defined when pelvic pain is present for at least three of the preceding six months and no other identifiable causes have been detected (Nickel 1999a). Other symptoms include obstructive or irritative voiding difficulties, ejaculatory pain, and haematospermia. Men affected by CP/CPPS have a significantly decreased quality of life (QoL) and the level of pelvic pain is strongly associated with sexual dysfunction (Trinchieri 2007; Walz 2007). CP/CPPS is associated with other functional somatic syndromes, such as irritable bowel syndrome, interstitial cystitis, chronic fatigue syndrome and fibromyalgia (Rodriguez 2009; Suskind 2013). Diagnosis is usually based on patient history, physical examination, urinalysis and the two‐ or four‐glass test (Nickel 2012). Further investigations are performed when considering differential diagnosis.
There are different theories regarding the aetiology and pathophysiology of CP/CPPS, as follows.
-
Infection: bacterial DNA is detected in a significant proportion of men with CP/CPPS (Hou 2012). A previous history of sexually transmitted infection is more frequent in men with CP/CPPS (Pontari 2005). Nevertheless, the isolation of uropathogenic bacteria in prostatic fluids is similar to controls (Nickel 2003a).
-
Inflammation/autoimmunity: elevated concentrations of proinflammatory cytokines (interleukin 1, tumour necrosis factor, interferon‐γ) and of autoimmunity activity (T‐cell proliferation responses to prostate antigens) is found in men with CP/CPPS and in animal models (Pontari 2004).
-
Neuropsychological factors: the central nervous system might be involved through several mechanisms of pain sensitisation (Miller 2002; Yang 2003). Increased stress burden, stress response, pain catastrophising cognitions, poor social functioning and psychiatric comorbidity (anxiety and depression) are contributing factors (Riegel 2014).
-
Dyssynergic voiding associated with bladder neck hypertrophy is detected in men with refractory CP/CPPS (Dellabella 2006; Hruz 2003). Intraprostatic urinary reflux and increased intraprostatic pressure is associated with inflammation in CP/CPPS (Kirby 1982; Mehik 2002).
-
Other theories described for this condition include: adrenal axis abnormalities (Anderson 2008), pelvic floor muscles dysfunction (Hetrick 2006; Shoskes 2008a), pelvic nerve entrapment (Antolak 2002), genetic predisposition to inflammation (Shoskes 2002), and oxidative stress (Arisan 2006).
Description of the intervention
There is a wide variety of interventions for treating CP/CPPS, each one addressing a different pathophysiological or symptomatic framework. The diversity of available interventions reflects the complexity of the condition and how little is known about its determinants.
Management of CP/CPPS involves a multimodal and tailored approach (Rees 2015; Shoskes 2008b). Some of the strategies used alone or in combination are the following.
Pharmacological interventions
-
Alpha‐blockers.
-
5‐alpha reductase inhibitors.
-
Antibiotic therapy (quinolones, tetracyclines and other agents).
-
Analgesics (non‐steroidal anti‐inflammatory drugs (NSAIDs), pregabalin).
-
Phytotherapy (pollen extract and bioflavonoids).
-
Botulinum toxin A.
-
Allopurinol.
-
Traditional medicine (traditional Chinese medicine, etc.).
-
Other pharmacological agents.
Non‐pharmacological interventions
-
Acupuncture and electroacupuncture.
-
Local thermotherapy.
-
Extracorporeal shockwave therapy.
-
Electromagnetic chair.
-
Myofascial trigger point release.
-
Biofeedback.
-
Circumcision.
-
Lifestyle interventions.
-
Physical activity.
-
Psychological support.
-
Prostatic surgery.
-
Other miscellaneous non‐pharmacological therapies.
Multimodal approaches
-
Combination therapy: alpha blockers plus antibiotics, antibiotics plus analgesics, etc.
Adverse events
Common adverse effects of pharmacological regimens include the following (Brunton 2011).
-
Alpha‐blockers: hypotension, ejaculatory dysfunction, headache, dizziness and nasal congestion.
-
5‐alpha‐reductase inhibitors: decreased libido, impotency, and potentiation of hypotension (in combination with alpha‐blockers).
-
Quinolones: gastrointestinal discomfort, headache, dizziness, rash and tendinopathy.
-
Tetracyclines: gastrointestinal discomfort, rash, teeth discolouration and hepatotoxicity.
-
NSAIDs: peripheral oedema, rash, dyspepsia, peptic ulcer and bleeding, renal and hepatic injury, and increased risk of adverse cardiovascular events.
-
Phytotherapy: gastrointestinal discomfort and allergic reactions.
The most common adverse effect in physical therapies is pain worsened during or immediately after the procedure (Fitzgerald 2013).
Clinical phenotyping
Clinical phenotyping is a strategy that was developed to deliver customised treatment in an aetiological framework (Shoskes 2008b). The UPOINT system addresses six domains: Urinary symptoms, Psychosocial dysfunction, Organ‐specific findings, Infection, Neurological dysfunction and Tenderness of muscles, and offers an algorithmic approach for the use of the various available interventions. The number of affected domains holds a significant correlation with the prostatitis symptoms score and the addition of a Sexual dysfunction domain (UPOINT(S)) improves accuracy in stratification of symptom severity (Magri 2010). While in itself it is not an intervention, it serves as a screening tool to select the most appropriate intervention for each patient.
How the intervention might work
Pharmacological interventions
Alpha‐blockers reduce the autonomic sympathetic tone in the bladder neck and prostate, improving urinary flow and LUTS. 5‐alpha‐reductase inhibitors reduce the production of dihydrotestosterone and, consequently, the size of the prostatic gland dependent on the stimulation of this hormone. This might reduce pain and impaired voiding (Brunton 2011).
NSAIDs are antagonists to the cyclo‐oxygenases (COX) enzymes type 1 and 2 and their proinflammatory subproducts (Brunton 2011). Both non‐selective and selective (COX‐2) inhibitors could therefore decrease inflammatory mediated pain in CP/CPPS.
Phytotherapy includes the use of pollen extract and bioflavonoids that appear to have anti‐inflammatory properties, decreasing acinar cell proliferation and the production of interleukin‐6, tumour necrosis factor α, and other proinflammatory cytokines (Capodice 2005; Kamijo 2001).
Even if CP/CPPS is defined when no bacterial cause can be identified, antibiotics have been used to treat it under the assumption of the existence of an occult or undertreated infection (Hou 2012).
Allopurinol would reduce the prostatic secretions of purine and pyrimidine base‐containing metabolites in urine. These metabolites could be responsible for prostatic inflammation through urinary reflux (McNaughton 2002).
Botulinum toxin A has denervating properties and causes reduction in pain mediators when applied to the prostate in animal models. It also causes apoptosis and involution of the prostate gland (Chuang 2006).
Non‐pharmacological interventions
Acupuncture targets specific cutaneous points representing various internal organs using fine needle insertion and sometimes adding electric current to increase stimulation (electroacupuncture). In animal models, electroacupuncture has anti‐inflammatory properties and activates analgesic neurotransmitters (Kim 2006).
Locally induced hyperthermia, using transrectal or transurethral procedures, could decrease oxygen free radicals associated with prostatic inflammation (Gao 2012).
Myofascial trigger point release targets pelvic floor musculature dysfunction as a potential cause or contributor to CP/CPPS (Fitzgerald 2013). Biofeedback also addresses pelvic floor muscle through initial contraction to achieve further relaxation (Capodice 2005).
Extracorporeal shockwave therapy could promote vascularisation of the prostatic tissue and modulate nociceptive nerve impulses and pelvic floor tone (Pajovic 2016).
The length of the foreskin is positively associated with the presence of symptoms of CP/CPPS; therefore, it has been proposed that circumcision could reduce prostatitis symptoms (Zhao 2015).
There are certain risk factors in the lifestyle of men with CP/CPPS, including alcohol consumption and smoking status, among others, that are associated with worse clinical outcomes. Interventions aimed at reducing those risk factors, including those aimed at increasing physical activity, could reduce prostatitis symptoms (Chen 2016).
Prostatic massage has been a classical treatment for CP/CPPS aimed at relieving prostatic congestion, although the mechanisms for its therapeutic effects are controversial (Nickel 1999b).
It has been suggested that psychological treatments could be helpful in all types of chronic pain syndromes and the psychiatric comorbidity associated with the condition (e.g. depression secondary to chronic pain) (Riegel 2014).
Why it is important to do this review
The Cochrane Urology Group undertook an extensive prioritisation exercise to identify a core portfolio of the most clinically important titles. Consequently, this title was identified as a clinically important priority by the urology expert panel for development, maintenance and investment of resources by the editorial base.
CP/CPPS is a prevalent condition among men and it causes significant impairment of QoL. There was a previous Cochrane Review on the same subject but with a different methodological approach (McNaughton 2000). Other non‐Cochrane systematic reviews were also undertaken in previous years: some of them focused on individual interventions (Qin 2016a; Yang 2006; Zhu 2014), while others had a wider scope of interventions (Anothaisintawee 2011; Cohen 2012; Magistro 2016). We consider that a new and updated Cochrane Review is needed to critically summarise the body of evidence for this complex condition using the GRADE approach, thus providing key information about the best estimate of the magnitude of the effect in relative terms and absolute differences for patient‐important outcomes. Previous systematic reviews did not use this approach and had variable adherence to the rigorous methodology recommended by Cochrane.
The protocol for this review was first published in August 2016 with the title 'Interventions for treating chronic prostatitis/chronic pelvic pain syndrome' (Franco 2016). Due to the retrieval of a significant amount of included studies, the review team and the Cochrane Urology Group decided to split the review in two more narrowly defined reviews: 'Non‐pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome' and 'Pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome' (Franco 2017).
Objectives
To assess the effects of non‐pharmacological therapies for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) regardless of their publication status or language of publication.
Types of participants
We included men of all ages, regardless of social condition or ethnic origin, with CP/CPPS according with type III prostatitis of the NIH classification.
If we identified studies in which only a subset of participants was relevant to this review, we included such studies if data were available separately for the relevant subset.
Types of interventions
We investigated the following comparisons of experimental intervention versus comparator intervention. Concomitant interventions had to be the same in the experimental and comparator groups to establish fair comparisons. We performed a condition‐based comprehensive bibliographic search to find all interventions tested so far for CP/CPPS; therefore, some of them might not be listed in this section.
Non‐pharmacological interventions
-
Acupuncture and electroacupuncture.
-
Circumcision.
-
Electromagnetic chair.
-
Lifestyle interventions.
-
Physical activity.
-
Prostatic massage.
-
Extracorporeal shockwave therapy.
-
Local thermotherapy (transurethral, transrectal thermotherapy and external).
-
Biofeedback.
-
Myofascial trigger point release.
-
Laser therapy.
-
Tibial nerve stimulation.
-
Myofascial therapy.
-
Osteopathy.
-
Sono‐electromagnetic therapy.
-
Transelectrical nerve stimulation
-
Transurethral needle ablation.
-
Non‐intrusive ultrasound.
-
Psychological support.
-
Prostatic surgery.
-
Other miscellaneous non‐pharmacological therapies.
Multimodal approaches
-
Combination of pharmacological and non‐pharmacological therapy: acupuncture plus antibiotics, local thermotherapy plus alpha‐blockers, etc.
-
Combination of non‐pharmacological therapies.
Comparator interventions
-
Placebo or sham procedure.
-
No treatment.
-
Other types of interventions: pharmacological and non‐pharmacological.
Comparisons
We performed head‐to‐head comparisons or intervention versus placebo or sham procedure/no treatment comparisons.
We did not include studies evaluating only pharmacological interventions to avoid overlapping with the review 'Pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome' (Franco 2017).
Types of outcome measures
We did not use the measurement of the outcomes assessed in this review as an eligibility criterion.
Primary outcomes
-
Prostatitis symptoms.
-
Adverse events.
Secondary outcomes
-
Sexual dysfunction.
-
Urinary symptoms.
-
Quality of life (QoL).
-
Depression and anxiety.
Method and timing of outcome measurement
We used clinically important difference for the review outcomes to rate overall quality of the evidence in 'Summary of finding' tables (Johnston 2010). When the mean difference (MD) or risk ratio (RR) was equal to or larger than the minimal clinically important difference (MCID), we assumed that many participants may have gained clinically meaningful improvement from treatment; when the MD was at least half of the MCID but less than the MCID, an appreciable number of participants had likely achieved a clinically meaningful improvement; and when the MD was less than one‐half of the MCID, it was unlikely that an appreciable number of participants achieved clinically meaningful improvement (Johnston 2010).
Prostatitis symptoms
-
Measured by the National Institutes of Health ‐ Chronic Prostatitis Symptom Index (NIH‐CPSI) as total score and subscore measurements, when possible, and other validated scales.
-
We considered an MCID in NIH‐CPSI score as a 25% decrease or a 6‐point reduction from baseline (Nickel 2003b). This threshold was used to measure the 'responders rate' (Cates 2015).
Adverse events
-
Defined as treatment intolerance, adverse effects of the interventions at any time after participants were randomised to intervention/comparator groups.
-
There was no established threshold for adverse events. We considered the clinically important differences of adverse events above as relative risk reduction of at least 25% (Guyatt 2011a).
Sexual dysfunction
-
Measured by validated scales (e.g. International Index of Erectile Function, IIEF).
-
We considered the MCID in the erectile function domain score of the IIEF of four (Rosen 2011). We planned to use different thresholds of MCID based on the severity of erectile dysfunction, with a threshold of two for men with mild erectile dysfunction, five with moderate erectile dysfunction and seven with severe erectile dysfunction (Rosen 2011). We also considered IIEF‐5 of over five points as the MCID (Spaliviero 2010).
Urinary symptoms
-
Measured by IPSS (International Prostate Symptom Score) or AUASS (American Urological Association Symptom Score).
-
We considered improvement of the IPSS score of three points as an MCID to assess efficacy and comparative effectiveness (Barry 1995). We planned to use different thresholds of MCID based on the severity of IPSS, with a threshold of three for men with mild LUTS, five for moderate LUTS and eight for severe LUTS (Barry 1995).
Quality of life
-
Assessed by the Medical Outcomes Study Short Form 12 (SF‐12) or other validated scales.
-
We considered an MCID of SF‐12 physical component score to be 8 and SF‐12 mental component score to be 4 (Parker 2013).
Depression and anxiety
-
Assessed by Beck Depression Inventory, State Anxiety Inventory‐Y or other validated scales.
-
We considered an MCID of Beck Depression Inventory to be 11 and State Anxiety Inventory‐Y to be 10 (Button 2015; Corsaletti 2014).
We considered outcomes measured up to and including 12 months after randomisation as short‐term, and later than 12 months as long‐term.
Main outcomes for 'Summary of findings' tables
We presented 'Summary of findings' tables reporting the following outcomes listed according to priority.
-
Prostatitis symptoms.
-
Adverse events.
-
Sexual dysfunction.
-
QoL.
-
Depression and anxiety.
Search methods for identification of studies
We searched for all published and unpublished RCTs meeting our stated inclusion/exclusion criteria, without restrictions on language, publication date or publication status, and in consultation with the Cochrane Urology Information Specialist.
Electronic searches
We identified published, unpublished and ongoing studies by searching the following databases from their inception.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 7) in the Cochrane Library.
-
PubMed (1946 to 11 August 2017).
-
Embase Elsevier (1947 to 11 August 2017).
-
PsycINFO Ovid (1887 to 11 August 2017).
-
CINAHL EBSCO (1937 to 11 August 2017).
-
ClinicalTrials.gov (www.clinicaltrials.gov, 14 August 2017)
-
ISRCTN Registry (BioMed Central; www.isrctn.com/, 14 August 2017).
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch, 14 August 2017).
The search strategies for databases were modelled on the search strategy designed for PubMed (Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6). The PubMed search utilised the Cochrane Highly Sensitive Search Strategy for identifying RCTs in MEDLINE: sensitivity maximising version (2008 revision; Lefebvre 2011). The Embase search utilised the trial filter for therapy, maximising sensitivity developed by the Health Information Research Unit (HIRU) at McMaster University, adapted from Ovid to the Elsevier interface (HIRU 2015). For CENTRAL and clinical trials registries, filters were not applicable. We did not use filters for PsycINFO and CINAHL because the results likely to be obtained were very few.
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials and relevant reviews, meta‐analyses and health technology assessment reports. We contacted authors of included studies to identify any further studies that we may have missed. We contacted drug and device manufacturers for ongoing or unpublished trials. We searched abstract proceedings of the American Urological Association, European Association of Urology and Society of Sexual Medicine from 2015 to 2017 for unpublished studies (Appendix 7).
We searched other grey literature sources such as:
-
Open Grey (www.opengrey.eu/);
-
New York Academy of Medicine Grey Literature Report (www.greylit.org/);
-
Google Scholar.
Data collection and analysis
Selection of studies
We used reference management software (EndNote) and Covidence to identify and remove duplicate records. Three review authors (JVAF, TT, VV) independently scanned in pairs the abstract, title, or both, of remaining records retrieved, to determine which studies should be assessed further. Five review authors (JVAF, TT, SI, YX, VV) investigated all potentially relevant records as full text, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification or ongoing studies in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We used Covidence for title/abstract, and full‐text screening. We resolved any discrepancies through consensus or recourse to a third review author (JHJ). If resolution of a disagreement was not possible, we designated the study as 'awaiting classification' (Characteristics of studies awaiting classification) and we contacted study authors for clarification. We documented reasons for exclusion of studies that may have reasonably been expected to be included in the review in a Characteristics of excluded studies table. We presented an adapted PRISMA flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
We developed a dedicated data abstraction form that we pilot tested ahead of time.
For studies that fulfilled inclusion criteria, six review authors (JVAF, VV, TT, SI, YX, JHJ) independently abstracted in pairs the following information, which is provided in the Characteristics of included studies table.
-
Study design.
-
Study dates (if dates were not available then this was reported as such).
-
Study settings and country.
-
Participant inclusion and exclusion criteria.
-
Participant details, baseline demographics.
-
Number of participants by study and by study arm.
-
Details of relevant experimental and comparator interventions such as dose, route, frequency and duration.
-
Definitions of relevant outcomes, and method and timing of outcome measurement as well as any relevant subgroups.
-
Study funding sources.
-
Declarations of interest by primary investigators.
We further summarised some of the characteristics of the studies, participants and interventions in additional tables (Table 1; Table 2).
| Study ID | Intervention(s) (route, frequency, total dose/day) | Intervention(s) appropriate as applied in a clinical practice setting (description) | Intervention(s) duration | Comparator(s) (route, frequency, total dose/day) | Comparator(s) appropriate as applied in a clinical practice setting (description) | Comparator(s) duration |
| Breathing exercises using 'Karbonik' apparatus (hypercapnic hypoxia) 10‐20 min daily + medical therapy (see comparison). | No information regarding dose‐scaling or contraindications. | 10 days. | Levofloxacin 500 mg/day, tamsulosin 0.4 mg/day, Samprost daily suppository, Serenoa repens fructuum extract 1 capsule/day for 10 days, nimesulide 1‐2 tablets/day for 5‐7 days. | No information regarding dose‐scaling or contraindications. | 10 days. | |
| ESWT + medical therapy: each session had 12‐min duration and 3000 impulses with total energy flow 0.25 mJ/mm2 3 Hz, weekly in supine position without anaesthesia. | No information regarding dose‐scaling or contraindications. | 12 weeks. | Medical therapy with doxazosin 4 mg daily, ibuprofen 400 mg daily and tiocolchicoside 12 mg daily. | Ranitidine was allowed for gastric complaints. No information regarding dose‐scaling or contraindications. | 12 weeks. | |
| Acupuncture group: UB28 bladder meridian Stimulation using disposable acupuncture needles (Hua Long, 25 40 mm Sterile Acupuncture Needles, China) and electrical pulse generator (Agistim Duo, 4 4 mA rms max/ 99 Hz max, France). | No information regarding dose‐scaling. "Localized skin infections concerning the acupoints, bleeding diathesis and use of anticoagulation" were contraindications. | 7 weeks. | Medical therapy levofloxacin 500 mg daily and ibuprofen 200 mg. | Orally administrated twice daily for 7 weeks. No information regarding dose‐scaling or contraindications. | 7 weeks. | |
| Acupuncture, using 2 disposable stainless steel needles (0.3 mm diameter, 60 mm length, Suzhou, Jiangsu, China) that were inserted to a depth of maximum 2.5‐3 cm in 7 acupoints bilaterally: BL33 Zhongliao | Applied weekly for 6 weeks without other treatment modalities. No information regarding dose‐scaling or contraindications. | 6 weeks. | Punctures in sham group performed 1 cm left of each selected acupoint, with same type of needles, of the same duration and frequency. | Applied weekly for 6 weeks. No information regarding dose‐scaling or contraindications. | 6 weeks. | |
| Medical therapy (4 weeks of ciprofloxacin 500 mg bid, 3 months of ibuprofen 400 mg/day, 3 months of tamsulosin 0.4 mg/day) and scheduled for surgery during the same period in each of the sites by study clinicians; given written instructions about postoperative wound care. | No information regarding dose‐scaling or contraindications. | 3 months. | Same medications (4 weeks of ciprofloxacin 500 mg bid, 3 months of ibuprofen 400 mg/day, 3 months of tamsulosin 0.4 mg/day) and remain uncircumcised until end of 3‐month study participation, when they were scheduled to undergo circumcision. | No information regarding dose‐scaling or contraindications. | 3 months. | |
| Individually discussed risk factors detected at history by the completed questionnaire, informed that such risk factors were potential causes of their disease symptoms and it was strongly recommended to avoid them, distributed a vademecum with 13 rules relating to diet, sexual habits and lifestyle. | No information regarding dose‐scaling or contraindications. | 3 months. | Followed same diet, sexual behaviours and lifestyle as those of the previous months. | No information regarding dose‐scaling or contraindications. | 3 months. | |
| Sono‐electromagnetic therapy at home using Sonodyn Device: intensity 100 mW/cm2 with ultrasonic power of 12 mW and frequency 1.9 MHz, electric field force 0.3 V/m and magnetic field force 0.4 A/m; for 10 min, twice daily. | Participants could not see settings or perceive the device. No information regarding dose‐scaling or contraindications. | 12 weeks. | Similar placebo device. Participants applied same procedure, but device was not active during session. | Participants could not see settings or perceive device. No information regarding dose‐scaling or contraindications. | 12 weeks. | |
| Non‐intrusive ultrasound. Output frequency 1.79 MHz; output power: 3.15 W/cm2. Duration: 20 min, administered every 3 days (total of 7 times) + integrated Chinese‐Western medications. | No information regarding dose‐scaling or contraindications. | ‐ | Integrated Chinese‐Western medications alone; QianLieShuTong Capsule, orally 3 times daily, 3 capsules each time, tamsulosin delayed‐release capsule, 0.2 mg, orally 4 times daily. | No information regarding dose‐scaling or contraindications. | 1 month. | |
| ESWT (DUOLITH SD1, Storz Medical, Tägerwilen, Switzerland) once weekly for 4 weeks. Each time 3000 impulses, with 0.25 mJ/mm2 and 3 Hz of frequency were delivered, although 0.5 mJ/mm2 was added in each week (0.3 mJ/mm2 in week 2, 0.35 mJ/mm2 in week 3 and 0.4 mJ/mm2 in week 4). After each 500 pulses, probe position was corrected, using transperineal ultrasound. | No information regarding dose‐scaling or contraindications. | 4 weeks. | In sham group, same protocol applied but with probe being turned off. | No information regarding dose‐scaling or contraindications. | 4 weeks. | |
| 10 × 1‐hour sessions of myofascial physical therapy involved connective tissue manipulation of the abdominal wall, back, buttocks and thighs with connective tissue abnormalities in prone and supine position; double voiding and squatting (as home exercises); transrectal manipulation. | No information regarding dose‐scaling. "The presence of painful scars on lower abdominal wall that according to the health care personnel were unlikely to respond to physical therapy" was used as an exclusion criterion. | ‐ | 10 × 1‐hour sessions of global therapeutic Western massage, including effleurage, petrissage, friction, tapotement, vibration and kneading, in upper and lower limbs, trunk, buttocks, abdomen, head and neck each for prescribed time periods; participants not provided with home exercise programme. | No information regarding dose‐scaling or contraindications. | ‐ | |
| 20,000 ESWT (HB‐ESWT‐01, Haibin Medical Equipment Co. Ltd., China) impulses in 10 sessions, applied directly to perineal area in which pain was localised (from anus to scrotum); starting energy density was 0.06 mJ/mm2 and frequency of 2 Hz was used for all treatments. | Energy density gradually increased until it reached the maximum possible tolerable pain level reported by participant (recorded and used in all subsequent sessions). No information regarding contraindications. | 2 weeks. | Sham ESWT, which was conducted by setting the energy level to 0 (no shockwave energy transmission), under conditions identical to active treatment group. | No information regarding dose‐scaling or contraindications. | 2 weeks. | |
| 60‐min treatment with TRFH (ZRL‐II‐A cavity intervention treatment instrument provided by Shanghai Songhang Industry, Co. Ltd. (Shanghai, China), temperature 40‐43 °C) every day. | No information regarding dose‐scaling or contraindications. | 5 days. | Tamsulosin 0.2 mg once daily + clarithromycin 0.25 g bid. | No information regarding dose‐scaling or contraindications. | 6 weeks. | |
| TRFH + tamsulosin + clarithromycin (6 weeks). | ||||||
| Biofeedback group, participant displayed the electromyography of pelvic floor muscle and was instructed to contract and relax the anus according to instructions on display (20‐min sessions, 5 times weekly for 2 weeks). | No information regarding dose‐scaling or contraindications. All participants received interventions in comparison group. | 2 weeks. | All participants, including those in the "usual care group" instructed to avoid alcohol and spicy food, sitting too long and holding urine, catching a cold. Advised to be physically active and do exercise, have sex regularly, have warm sitz baths. | They were instructed to discontinue antibiotics, alpha‐blockers and other medications during the trial. No information regarding dose‐scaling or contraindications. | ‐ | |
| Electrical stimulation group, anal electrodes using intensity of 6˜23 mA, for 10˜20 s and relaxation for 10˜20 s, (20‐min sessions; 5 times each week for 2 weeks). | ||||||
| Biofeedback + electrical stimulation (factorial design). | ||||||
| Taijiquan (Tai Chi) exercises for 20‐40 min every day. All participants received herbal therapy (no further specifications available) for 1 month after. | No information regarding dose‐scaling or contraindications. | 1 month. | All participants received herbal therapy (no further specifications available) for 1 month after. | No information regarding dose‐scaling or contraindications. | 1 month. | |
| TENS daily for mean of 20 min in painful area, mean frequency 100 Hz, pulse width 100 μs and intensity 25 mA daily, 5 times weekly. | No information regarding dose‐scaling or contraindications. | 1 month. | Same as active treatment group but with machine turned off. | No information regarding dose‐scaling or contraindications. | 1 month. | |
| Advice of hot sitz baths and exercise and 12 × 20‐min sessions of electroacupuncture in 6 weeks: 6 acupoints at bilateral: BL32 zhongliao | No information regarding dose‐scaling or contraindications. | 6 weeks. | Advice and exercise and 12 sessions of sham electroacupuncture, which included the same number and type of needle, duration and frequency of sessions as for the advice and exercise treatment, but treatment was delivered superficially at non‐acupoints 15 mm to the lateral of each corresponding acupoint; points were not stimulated electrically, but sound of pulse generator was heard by the participants. | No information regarding dose‐scaling or contraindications. | 6 weeks. | |
| Acupuncture + warm needle moxibustion. Acupuncture at acupoints: BI18 GanYu For 5 s each; needles were removed afterwards; once daily for 1 month. | For those treated with moxibustion: needles were left afterwards and the tails of which were then covered with 2 cm moxa sticks. Moxa sticks were then ignited. Repeated moxibustion twice more for each acupoint. No information regarding dose‐scaling or contraindications. | 1 month. | Prostat tablet orally twice daily: pollen extract. Course of treatment; 1 month. | No information regarding dose‐scaling or contraindications. | 1 month. | |
| Acupuncture procedures same as acupuncture + warm needle moxibustion. Moxibustion not performed. Treated once daily. Course of treatment: 1 month. | ||||||
| Perineally applied ESWT weekly (3000 pulses each; maximum total energy flow density: 0.25 mJ/mm2; frequency: 3 Hz); position of transducer was changed after every 500 pulses to scan prostatic and pelvic floor region (Duolith SD1, Storz Medical, Tägerwilen, Switzerland); penetration depth 35‐65 mm. | No information regarding dose‐scaling or contraindications. | 4 weeks. | Placebo treatment performed with same therapy head, which was also fitted with a placebo stand‐off. Stand‐off contained shock wave‐absorbing material, layer of air and air‐filled microspheres. | No information regarding dose‐scaling or contraindications. | 4 weeks. | |
| Osteopathic theory of structural dysfunction using direct and indirect techniques of manipulation; including rectal manipulation of the prostate and coccyx; 45‐min sessions for 5 weeks. | No information regarding dose‐scaling or contraindications. | 5 weeks. | Sham exercise program including a warm‐up, stretching, limb, breathing and pelvic floor muscle exercises; 30 min sessions in 5 weeks. | No information regarding dose‐scaling or contraindications. | 5 weeks. | |
| 'Radiofrequency.' | (abstract only) | ‐ | "Placebo therapy." | (abstract only) | ‐ | |
| PTNS applied unilaterally with 26‐gauge stainless steel needles inserted 5 cm cephalad from medial malleolus and posterior to edge of tibia with a ground neutral electrode placed on same leg near arch of foot; both connected to a stimulator at 200 µs with pulse rate 20 Hz (Medtronic Key Point Net, Medtronic), for 30 min. | No information regarding dose‐scaling or contraindications. | 12 weeks. | Same electrode procedure for PTNS; however, stimulator not connected. | No information regarding dose‐scaling or contraindications. | 12 weeks. | |
| TRMT alone for 12 weeks; using a Uro‐DR Device (Somang Medical; Kangreung, Korea), at intrarectal temperature of 43 °C for 30 min, at a medium heating rate. | No information regarding dose‐scaling or contraindications. | 12 weeks. | Medical therapy: ciprofloxacin 500 mg bid and NSAIDs C: TRMT + medical therapy. | No information regarding dose‐scaling or contraindications. | 12 weeks. | |
| 4 acupoints prepared, then sterile, disposable stainless steel needles (Suzhou Huan‐Qiu Acupuncture Medical Supplies, Suzhou, China) placed perpendicularly in 30‐min sessions twice weekly in acupoints: CV1 Guan Yuan | No information regarding dose‐scaling or contraindications. | 10 weeks. | Sham acupuncture included same number, duration and frequency of sessions as acupuncture group at non‐acupoints (15 mm to left). | No information regarding dose‐scaling or contraindications. | 10 weeks. | |
| Antibiotics plus TENS; with high TENS over painful area, daily for mean of 20 min, mean frequency 100 Hz, pulse width 100 μs and intensity 25 mA, 5 times weekly. | Contraindications: loss of skin sensation at and around painful area, cardiac pace maker, previous exposure to TENS and other electroanalgesia. | 4 weeks. | Ofloxacin 300 mg 3 times daily + placebo tablets. | No information regarding dose‐scaling or contraindication. | 4 weeks. | |
| Aerobic exercise group, which included an 18‐week walking programme, 3 times weekly; with a warm up and cool‐down regimen, postural muscle and isometric strengthening exercises, 40 min of fast‐paced walking on 'in‐outdoor' track (achieving 70/80% of predicted maximum heart rate for their age). | Participants with a 'lack of interest,' 'lack of time' and 'lack of confidence' to engage physical activity excluded from study. No information regarding dose‐scaling. | 18 weeks. | Placebo/flexibility and motion exercise programme; with same period and with same frequency of aerobic exercise group, maintaining their heart rate under 100 beats per min. | Participants with a 'lack of interest,' 'lack of time' and 'lack of confidence' to engage physical activity were excluded from the study. No information regarding dose‐scaling. | 18 weeks. | |
| Extracorporeal magnetic innervation using Neoconrol system (Neotonus Inc., Marietta, GA, USA) that generates a magnetic field directed in the seat of chair and concentrated in region of pelvic muscles; 2 sessions weekly lasting 20 min each. | 1st 10 min used 10 Hz field, 2 min rest and then 10 min of 50 Hz field. No information regarding contraindications. | 6 weeks. | Medical therapy with terazosin 4 mg daily. | They started 2 mg daily for 1st 7 days, and continued to receive 4 mg daily for the following 5 weeks. No information regarding contraindications. | 6 weeks. | |
| Participants received antibiotics empirically and prostatic massage (3 times weekly for 4 weeks). Prostate massaged from above and lateral to gland, 6 times on each side, by gentle and firm pressure of finger directed downwards and inwards, followed by a few strokes in middle from above downwards. | No information regarding dose‐scaling or contraindications. | ‐ | Empirical antibiotic therapy alone without prostatic massage. | 'In the 44 participants with negative cultures, antibiotic selection included quinolones (28 participants), clindamycin/metronidazole (11 participants), ampicillin/ | ‐ | |
| Medical therapy with modified BiXieFenQing Drink in morning and evening, 200 mL each + prostate massage once weekly. | No information regarding dose‐scaling or contraindications. | ‐ | Medical therapy alone. | No information regarding dose‐scaling or contraindications. | ‐ | |
| Participant seated in a Neotonus Electromagnetic Chair (Neotonus Inc., Marietta, GA, USA), for 2 consecutive 15‐min periods; 2 sessions weekly. | 1st period 10 Hz, 2nd period 50 Hz. No information regarding contraindications. | 4 weeks. | Participants seated in chair, ventilation mechanism activated, but no active stimulation applied. | No information regarding dose‐scaling or contraindications. | 4 weeks. | |
| He‐Ne laser directed to prostate. Optic fibre was inserted from urethra and located at prostatic urethra; 10 mW output at 18 J each time. | 1 course of treatment: 10 doses of radiation (2 sessions weekly). Participants discontinued all other treatments, except for some of the participants, short‐term sulfa‐drugs were administered temporarily to prevent infection. | ‐ | 'Routine care' for prostatitis including antibiotics, pollen extract, Chinese medicine, physical therapy and lifestyle interventions. | Sulfamethoxazole tablets, 2 tablets, orally bid, 60 days of fluoroquinolones such as levofloxacin 0.2 g, bid, 14 days of pollen drugs such as Prostat; Chinese patent drugs such as salvianolic acid B and saponins of panax notoginseng mixture (SalB/PNS); hot water bath and advise on lifestyle. | ‐ | |
| Transurethral microwave thermotherapy through catheter connected to a Targis System estimated peak intraprostatic temperatures of 55 °C. | No information regarding dose‐scaling or contraindications. | ‐ | Transurethral microwave thermotherapy through catheter connected to a Targis System estimated peak intraprostatic temperatures of 70 °C. | No information regarding dose‐scaling or contraindications. | ‐ | |
| External radiofrequency hyperthermia. | Applied externally: 2 electrodes placed at hip and lower abdomen, 5˜7 cm away from skin, with pubic symphysis as centre (42.5˜43.5 °C), 1˜2 hours each time, course of treatment: 2˜3 times, interval: 1˜2 weeks. No information regarding dose‐scaling or contraindications. | 1˜2 weeks. | Same external radiofrequency hyperthermia + terazosin 2 mg. | Daily for 2 days and then dose was increased to 2 mg bid for 12 weeks. No information regarding contraindications for terazosin. | 12 weeks. | |
| TUNA using 465‐kHz radiofrequency energy and formal needle insertion technique as described by Issa 1996; treatment applied on 2 planes on both lateral lobes of prostate in all participants so target temperature of 50 °C at needle tip was achieved for ≥ 1 min; under spinal analgesia and antibiotic prophylaxis. | No information regarding dose‐scaling or contraindications. | ‐ | 3 sham urethroscopy performed so it was seemingly identical to TUNA in participant's opinion; under spinal analgesia and antibiotic prophylaxis. | No information regarding dose‐scaling or contraindications. | ‐ | |
| TRMT, 6 sessions over 2 weeks each. | No information regarding dose‐scaling or contraindications. | ‐ | Sham procedure. | No information regarding dose‐scaling or contraindications. | ‐ | |
| Transurethral microwave thermotherapy consisted of 1 × 1‐hour treatment with computer‐driven device that elevated prostate interstitial temperatures to 45‐60 °C, a range that does not cause significant necrosis of normal prostatic tissue. | No information regarding dose‐scaling or contraindications. | Single session. | Sham therapy consisted of 1 × 1‐hour session with same device using sham software. | No information regarding dose‐scaling or contraindications. | Single session. | |
| Seaprose S (Flaminase, Formenti) 30 mg 3 times daily in combination with local hyperthermia, total of 7 sessions on alternate days of 60‐min duration, reaching local temperature of 42.5‐43.5 °C. | No information regarding dose‐scaling. Contraindication to those with hypersensitivity to the drug. | ‐ | 7 sessions of local hyperthermia alone. | No information regarding dose‐scaling or contraindications. | ‐ | |
| I1: 1 session of transrectal hyperthermia weekly for 4 weeks. I2: 1 session of transrectal hyperthermia weekly for 6 weeks. I3: 2 sessions of transrectal hyperthermia weekly for 3 weeks. Prostathermer 99D system (Biodan Ltd, Rehovot, Israel), target temperature reached within 1st 10 min of treatment, microwaves at 915 MHz, thermosensors for monitoring rectal temperature, cooling system for anterior rectal wall, thermosensors for urethra temperatures, outpatient basis, required local anaesthesia, with 2% xylocaine jelly before insertion of the catheter. | ||||||
| TRMT, 4 × 1‐hour treatment over 2 or 3 weeks. Temperature raised to 43.8 °C with input of 40 W. | No information regarding dose‐scaling or contraindications. (abstract only) | 2˜3 weeks | Sham group with temperature < 37 °C. | No information regarding dose‐scaling or contraindications. (abstract only) | ‐ | |
‐ denotes not reported.
bid: twice daily; C: comparator; ESWT: extracorporeal shockwave therapy; I: intervention; min: minute; NSAID: non‐steroidal anti‐inflammatory drug; PTNS: posterior tibial nerve stimulation; s: second; TENS: transcutaneous electrical nerve stimulation; TRFH: transrectal radiofrequency hyperthermia; TRMT: transrectal microwave thermotherapy; TUNA: transurethral needle ablation.
| Study ID | Intervention(s) and comparator(s) | Randomised (n) | Analysed (n) | Duration of intervention and follow‐up | Description of participantsa | Baseline NIH‐CPSIb score | Trial period (year to year) | Country | Setting |
| I: hypercapnic hypoxia + medical therapy | 17 | 17 | D: 10 days F: 10 days | Age: mean 37 No specified previous treatment | N/A | N/A | Russia | Outpatient | |
| C: medical therapy | 20 | 20 | |||||||
| I: extracorporeal shockwave therapy + medical care | 30 | 30 | D: 12 weeks F: 24 weeks | Age: mean 39.4 No specified previous treatment | 29.3 | 2013‐2015 | Montenegro | Outpatient | |
| C: medical care | 30 | 30 | 31.06 | ||||||
| I: acupuncture | 28 | 28 | D: 7 weeks F: 10 weeks | Age: mean 33 No previous treatment | 20.36 | 2008‐2009 | Turkey | Outpatient | |
| C: medical care | 26 | 26 | 22.92 | ||||||
| I: acupuncture | 50 | 45 | D: 6 weeks F: 24 weeks | Age: 20‐50 All had received medical therapy | 26.5 | Not available | Turkey | Outpatient | |
| C: sham acupuncture | 50 | 46 | 27.0 | ||||||
| I: early circumcision | 384 | 358 | D: 3 months F: 3 months | Age: mean 33 No specified previous treatment | 21 | 2013‐2014 | China | Outpatient | |
| C: delayed circumcision | 390 | 355 | 21 | ||||||
| I: lifestyle interventions | 50 | 39 | D: 3 months F: 3 months | Age: mean 33‐34 No specified previous treatment | 22.1 | 2012‐2013 | Italy | Outpatient | |
| C: no intervention | 50 | 50 | 21.9 | ||||||
| I: sono‐electromagnetic therapy | 30 | 30 | D: 12 weeks F: 16 weeks | Age: mean 44‐49 All had received medical therapy | 25.1 | 2009‐2012 | Switzerland | Outpatient | |
| C: placebo device | 30 | 30 | 25.2 | ||||||
| I: ultrasound | 35 | 35 | D: 1 month F: 1 month | Age 18‐55 All had received medical therapy | 25.9 | 2013‐2014 | China | Outpatient | |
| C1: medical therapy | 35 | 35 | 26.17 | ||||||
| C2: ultrasound + medical therapy | 35 | 35 | 26.85 | ||||||
| I: extracorporeal shockwave | 20 | N/A | D: 4 weeks F: 12 weeks | Age: 35‐37 No specified previous treatment | 26.5 | 2011‐2012 | Iran | Outpatient | |
| C: sham procedure | 20 | 27.1 | |||||||
| I: extracorporeal shockwave therapy | 40 | 38 | D: 2 weeks F: 12 weeks | Age: mean 46‐49 No specified previous treatment | 30.5 | 2009‐2011 | China | Outpatient | |
| C: sham procedure | 40 | 37 | 29.3 | ||||||
| I: medical therapy | 30 | 30 | D: 6 weeks F: 6 weeks | Age: mean 35‐36 No specified previous treatment | 20.9/20.2 | 2008‐2009 | China | Outpatient | |
| C1: transrectal hyperthermia | 32 | 32 | 20/21.1 | ||||||
| C2: transrectal hyperthermia + medical therapy | 43 | 43 | 22.4/21.7 | ||||||
| I1: biofeedback | 20 | 20 | D: 2 weeks F: 6 weeks | Age: mean 30 All had received medical therapy | 26.92 | 2007 | China | Outpatient | |
| I2: electrical stimulation | 40 | 40 | 26.35 | ||||||
| I3: biofeedback + electrical stimulation | 40 | 40 | 25.30 | ||||||
| C: usual care | 40 | 40 | 25.82 | ||||||
| I: TaiJiQuan | 50 | N/A | D: 1 month F: 1 month | Age: 22‐50 No specified previous treatment | N/A | N/A | China | Outpatient | |
| C: no intervention | 46 | ||||||||
| I: TENS | 20 | 20 | D: 4 weeks F: 4 weeks | Age: 30 to 55 No specified previous treatment | N/A | N/A | Egypt | Outpatient | |
| C: placebo TENS | 20 | 20 | |||||||
| I1: electroacupuncture | 12 | 11 | D: 6 weeks F: 6 weeks | Age: 36‐40 No specified previous treatment | 26.9 | 2007 | Korea | Outpatient | |
| I2: sham procedure | 12 | 10 | 25.5 | ||||||
| C: exercise and advise alone | 12 | 12 | 28 | ||||||
| I1: acupuncture + moxibustion | 42 | 42 | D: 1 month F: 1 month | Age: 21‐52 No specified previous treatment | 22.56 | 2004‐2007 | China | Outpatient | |
| I2: acupuncture | 41 | 41 | 21.97 | ||||||
| C: medical treatment | 42 | 42 | 22.89 | ||||||
| I: extracorporeal shockwave therapy | 30 | 30 | D: 4 weeks F: 12 weeks | Age: 22‐61 No specified previous treatment | 25.07 | N/A | Austria | Outpatient | |
| C: sham procedure | 30 | 30 | 23.3 | ||||||
| I: myofascial trigger point therapy | 10 | 9 | D: 10 weeks F: 12 weeks | Age: mean 41‐45 All had received medical therapy | 33.5 | N/A | US | Outpatient | |
| C: Western massage | 11 | 10 | 25.8 | ||||||
| I: osteopathic therapy | 20 | 20 | D: 8 weeks F: 14 weeks | Age: mean 46‐48 No specified previous treatment | 22.85 | 2003‐2005 | Germany | Outpatient | |
| C: exercise programme | 15 | 13 | 22.95 | ||||||
| I: radiofrequency | N/A (abstract only) | South Korea | Outpatient | ||||||
| C: "placebo therapy" | |||||||||
| I: tibial nerve stimulation | 45 | 45 | D: 12 weeks F: 12 weeks | Age: mean 38‐39 No specified previous treatment | 23.6 | 2006‐2008 | Turkey | Outpatient | |
| C: sham procedure | 44 | 44 | 22.8 | ||||||
| I: medical therapy | 44 | 37 | D: 12 weeks F: 12 weeks | Age: mean 31‐38 No specified previous treatment | 26.27 | 2005‐2010 | South Korea | Outpatient | |
| C1: transrectal thermotherapy | 44 | 41 | 24.59 | ||||||
| C2: transrectal thermotherapy + medical therapy | 44 | 35 | 23.94 | ||||||
| I: acupuncture | 45 | 44 | D: 10 weeks F: 34 weeks | Age: 41‐43 No specified previous treatment | 24.8 | 2004‐2005 | Malaysia and US | Outpatient | |
| C: sham acupuncture | 45 | 45 | 25.2 | ||||||
| I: TENS | 8 | 8 | D: 4 weeks F: 4 weeks | Age: 24‐60 No specified previous treatment | N/A | N/A | Nigeria | Outpatient | |
| C1: analgesic | 8 | 8 | |||||||
| C2: control | 8 | 8 | |||||||
| I: exercise programme | 52 | 41 | D: 18 weeks F: 18 weeks | Age: mean 36‐38 All had received medical therapy | 23.46 | 2002‐2004 | Italy | Outpatient | |
| C: flexibility programme (control intervention) | 51 | 44 | 23.55 | ||||||
| I: extracorporeal magnetic innervation | 21 | 21 | D: 6 weeks F: 6 weeks | Age: mean 42‐49 No specified previous treatment | 21 | 2003‐2004 | South Korea | Outpatient | |
| C: medical therapy | 19 | 19 | 17 | ||||||
| I: medical therapy + prostatic massage | 25 | N/A | D: 4 weeks F: 4 weeks | Age: mean 35 No specified previous treatment | N/A | N/A | Egypt | Outpatient | |
| C: medical therapy | 19 | ||||||||
| I: medical therapy + prostatic massage | 40 | 40 | D: N/A F: after treatment | Age: 20‐46 No specified previous treatment | N/A | 2002‐2005 | China | Outpatient | |
| C: medical therapy | 32 | 32 | |||||||
| I: electromagnetic chair | 11 | 11 | D: 4 weeks F: 1 year | Age: 25‐67 All had received medical therapy | 38.8/100 | N/A | UK | Outpatient | |
| C: sham procedure | 10 | 7 | 39.3/100 | ||||||
| I: He‐Ne laser | 56 | 56 | D: 5 weeks F: "after treatment" | Age: mean 33‐34 No specified previous treatment | N/A | 2002‐2004 | China | Outpatient | |
| C: routine care | 56 | 56 | |||||||
| I: transrectal thermotherapy 55 °C | 42 | 21 | D: once F: 12 months | Age: mean 58‐62 All had received medical therapy | 11.5 | N/A | Chile, Switzerland, UK | Outpatient | |
| C: transrectal thermotherapy 70 °C | 18 | 10.9 | |||||||
| I: radiofrequency hyperthermia | 76 | 76 | D: 12 weeks F: 12 weeks | Age: mean 34.2 Not specified previous treatment | N/A | 1998‐2001 | China | Outpatient | |
| C: radiofrequency hyperthermia + terazosin | 90 | 88 | |||||||
| I: transurethral needle ablation | 25 | 25 | D: once F: 12 months | Age: mean 43‐50 All had received medical therapy | 37.3 | 1998‐2001 | Finland | Outpatient | |
| C: sham procedure | 8 | 8 | 33.6 | ||||||
| I: transrectal thermotherapy | 80 | N/A | D: 2 weeks F: 6 months | N/A (abstract only) | Russia | Outpatient | |||
| C: sham procedure | 20 | ||||||||
| I: transurethral thermotherapy | 10 | 10 | D: 1 session F: 3 months | Age: 45‐46 All had received medical therapy | N/A | N/A | Canada | Outpatient | |
| C: sham procedure | 10 | 10 | |||||||
| I: medical therapy plus local hyperthermia | 10 | N/A | D: 15 days F: 4 weeks | Age: mean 42.5 Not specified previous treatment | N/A | N/A | Italy | Outpatient | |
| C: local hyperthermia alone | 10 | ||||||||
| Transrectal hyperthermia in different regimens | 54 | 54 | D: 3 to 6 weeks F: 26 months | Age: 21‐45 All had received medical therapy | N/A | 1987‐1991 | Italy | Outpatient | |
| I: radiofrequency | 15 | N/A | D: 2‐3 weeks F: 3 months | N/A (abstract only) | UK | Outpatient | |||
| C: sham procedure | 15 | ||||||||
C: comparator; D: duration of intervention; F: duration of follow‐up (might not represent the reported time points); I: intervention; N/A: not available; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; TENS: transcutaneous electric nerve stimulation.
aAge: measurement is in years.
bNIH‐CPSI: scores were mean or median values.
We extracted outcomes data relevant to this Cochrane Review as needed for calculation of summary statistics and measures of variance. For dichotomous outcomes, we attempted to obtain numbers of events and totals of population for a 2 × 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we attempted to obtain means and standard deviations or data necessary to calculate this information. We resolved any disagreements by discussion, or, if required, by consultation with a third review author (SI or JHJ).
We provided information, including trial identifier, about potentially relevant ongoing studies in a Characteristics of ongoing studies table. We attempted to contact authors of included studies to obtain key missing data as needed.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximised yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Six review authors (JVAF, VV, TT, SI, YX, JHJ) assessed the risk of bias of each included study independently in pairs. We resolved disagreements by consensus, or by consultation with a third review author (JVAF or VV).
We assessed risk of bias using the Cochrane 'Risk of bias' assessment tool (Higgins 2011b). We assessed the following domains.
-
Random sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Other sources of bias.
We judged the risk of bias domains as 'low risk,' 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We presented a 'Risk of bias' summary figure to illustrate these findings.
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment), we evaluated the risk of bias separately for each outcome, and we grouped outcomes according to whether they were measured subjectively or objectively when reporting our findings in the 'Risk of bias' table. However, all end points were subjective outcomes.
We assessed attrition bias (incomplete outcome data) on an outcome‐specific basis, and grouped outcomes with like judgements when reporting our findings in the 'Risk of bias' table.
We further summarised the risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome.
Measures of treatment effect
We expressed dichotomous data as risk ratios (RR) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MD) with 95% CIs unless different studies used different measures to assess the same outcome, in which case we expressed data as standardised mean differences (SMD) with 95% CIs.
Unit of analysis issues
The unit of analysis was the individual participant. If we identified cross‐over trials, cluster‐randomised trials or trials with more than two intervention groups for inclusion in the review, we handled these in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Dealing with missing data
We obtained missing data from study authors, if feasible, and performed intention‐to‐treat analyses if data were available; otherwise, we performed available‐case analyses. We investigated attrition rates, such as dropouts, losses to follow‐up and withdrawals, and we critically appraised issues of missing data. We did not impute missing data.
Assessment of heterogeneity
In the event of excessive heterogeneity unexplained by subgroup analyses, we did not report outcome results as the pooled effect estimate in a meta‐analysis, but we provided a narrative description of the results of each study.
We identified heterogeneity (inconsistency) through visual inspection of the forest plots to assess the amount of overlap of CIs, and the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). We interpreted the I2 statistic as follows.
-
0% to 40%: may not be important.
-
30% to 60%: may indicate moderate heterogeneity.
-
50% to 90%: may indicate substantial heterogeneity.
-
75% to 100%: considerable heterogeneity.
When we found heterogeneity, we attempted to determine possible reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We attempted to obtain study protocols to assess for selective outcome reporting.
If we had included 10 studies or more investigating a particular outcome, we would have used funnel plots to assess small‐study effects. Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. Therefore, we would have interpreted the results carefully.
Data synthesis
Unless there was good evidence for homogeneous effects across studies, we summarised data using a random‐effects model. We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects. In addition, we performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). For dichotomous outcomes, we used the Mantel‐Haenszel method; for continuous outcomes, we used the inverse variance method. We used Review Manager 5 (RevMan 2014) software to perform analyses.
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and planned to carry out subgroup analyses with investigation of interactions.
-
Participants' characteristics: symptom severity at recruitment, age, presence of clinical comorbidities (irritable bowel syndrome, fibromyalgia, interstitial cystitis).
-
Duration of the intervention: depending on intervention type, measured in sessions (e.g. one session or repeated sessions) or weeks (e.g. less than 12 weeks or more than 12 weeks).
We planned to use the test for subgroup differences in Review Manager 5 to compare subgroup analyses if there had been sufficient studies (RevMan 2014).
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes.
-
Restricting the analysis by taking into account risk of bias, by excluding studies at 'high risk' or 'unclear risk.'
-
Explore the impact of re‐expressing symptom severity as a dichotomous outcome.
-
Excluding studies that included participants with a diagnosis of chronic non‐bacterial prostatitis or prostatodynia, not filling the criteria of the 1999 Research Consensus (Nickel 1999a).
'Summary of findings' tables
We presented the overall quality of the evidence for each outcome according to the GRADE approach, which takes into account five criteria related to internal validity (risk of bias, inconsistency, imprecision, publication bias), and external validity, such as directness of results (Guyatt 2008). For each comparison, two review authors (JVAF, JHJ) independently rated the quality of evidence for each outcome as 'high,' 'moderate,' 'low' or 'very low' using GRADEpro GDT. We resolved any discrepancies by consensus, or, if needed, by arbitration by a third review author (VV). We presented a summary of the evidence for the main outcomes in the 'Summary of findings' tables, which provide key information about: the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011b; Schünemann 2011). If meta‐analysis was not possible, we presented results in a narrative 'Summary of findings' table. We initially planned to present 'Summary of findings' tables for all comparisons, however, given their multiplicity, we have presented only those most related to clinical practice or containing at least two clinical trials in order to highlight the evidence most relevant to clinicians, patients and other stakeholders (see Differences between protocol and review). Nevertheless, all comparisons were rated using the GRADE approach and are available under the section Effects of interventions.
We used the controlled vocabulary suggested by Glenton 2010 to summarise the findings of the 'Summary of findings' tables in the 'Plain language summary.'
Results
Description of studies
Results of the search
For detailed information of the results of the search see Figure 1. This review shares the search strategy for the protocol 'Pharmacological Interventions for treating chronic prostatitis/chronic pelvic pain syndrome' (Franco 2017). In this section, we described the study flow for the studies relevant to the review question. For this review, we screened 1500 records after removing duplicates. We included two studies from other systematic reviews on this topic (McNaughton 2000). We excluded 1386 records and screened 130 records in the full‐text assessment. We excluded 73 studies (76 records) after full‐text assessment (see Characteristics of excluded studies table). The search identified one protocol of a completed study (Rochester 2011), but we could not retrieve study results (see Characteristics of studies awaiting classification table). We identified four ongoing studies (see Characteristics of ongoing studies table). We included 38 studies (49 records) in this review.

Study flow diagram.
Included studies
We included 38 studies (see Characteristics of included studies table).
Design
All the included studies were RCTs.
Sample sizes
Median sample size was 60 (interquartile range 35 to 100). The smallest sample size was 20 and the largest sample size was 774.
Setting
Since CP/CPPS is usually treated in an outpatient setting, most studies offered ambulatory care. Some studies, depending on the type of intervention (see below), required a temporary stay in hospital, particularly those studies that used therapeutic devices. The studies were conducted in Egypt (Ateya 2006; Samhan 2011), China (Chen 2009; Fang 2005; Gao 2012; Kaikai 2014; Shen 2006; Wang 2002; Yang 2011; Zeng 2012; Zhang 2011a; Zhao 2015), US (Fitzgerald 2013), Italy (Gallo 2014; Giubilei 2007; Montorsi 1993; Muraro 1995), Switzerland (Kessler 2014), Turkey (Kabay 2009; Kucuk 2015; Sahin 2015), Korea (Lee 2009; Oh 2009; Paick 2006; Yoo 2009), Finland (Leskinen 2002), Germany (Marx 2009), Canada (Nickel 1996), Montenegro (Pajovic 2016), UK (Rowe 2005; Shah 1993), Nigeria (Sikiru 2008), Iran (Vahdatpour 2013), Russia (Neimark 2016; Vassily 1999), and Austria (Zimmermann 2009). Two studies were conducted in more than one country (Kastner 2004; Lee 2008).
Participants
The median age of participants was 37 years. Three studies did not provide information regarding age (Oh 2009; Shah 1993; Vassily 1999). These studies were reported in abstract form with few data available. The included studies (except Kastner 2004) did not include participants over 50 years old to avoid symptom overlap with benign prostate hyperplasia. Kastner 2004 included participants with a mean age of 60 years.
All studies referred to diagnostic criteria aimed at the differentiation of CP/CPPS from other forms of prostatitis and other urological diseases. Participants underwent digital rectal examination, urine cultures and 2 or 4 glass Meares‐Stamey test. They excluded participants who had recently undergone prostatic biopsy or surgery, participants with prostate cancer, participants with a recent history of sexually transmitted diseases and participants with concomitant neurological disorders or severe systemic disorders.
Only one study included participants who had not received other previous treatment (Kucuk 2015). Eleven studies specified that participants had previously received medical treatment with antibiotics or alpha blockers (or both) and had not had a positive response (Fitzgerald 2013; Giubilei 2007; Kaikai 2014; Kastner 2004; Kessler 2014; Lee 2008; Montorsi 1993; Nickel 1996; Rowe 2005; Sahin 2015; Yang 2011). The other studies did not specify whether the participants had received previous treatments for this condition. Nevertheless, a common inclusion criterion included a washout period, as stated in a protocol for medical therapy often cited as a consensus for inclusion/exclusion criteria (Propert 2002).
Interventions
We included studies assessing a wide variety of non‐pharmacological interventions.
-
Acupuncture and electroacupuncture (Chen 2009; Kucuk 2015; Lee 2008; Lee 2009; Sahin 2015).
-
Local thermotherapy (Gao 2012; Kastner 2004; Leskinen 2002; Montorsi 1993; Muraro 1995; Nickel 1996; Oh 2009; Shah 1993; Vassily 1999; Wang 2002; Yoo 2009).
-
Extracorporeal shockwave therapy (Pajovic 2016; Vahdatpour 2013; Zeng 2012; Zimmermann 2009).
-
Myofascial trigger point release compared to control intervention (Fitzgerald 2013).
-
Biofeedback with or without electrical stimulation compared to control (Yang 2011).
-
Psychological support: we found no studies for this intervention.
-
Prostatic surgery: we found no studies for this intervention.
-
Other miscellaneous non‐pharmacological therapies:
-
circumcision compared to waiting list (Zhao 2015);
-
electromagnetic chair compared to sham procedure (Paick 2006; Rowe 2005);
-
laser therapy compared to medical treatment (Fang 2005);
-
lifestyle modifications (Gallo 2014);
-
osteopathy (Marx 2009);
-
physical activity (Giubilei 2007);
-
prostatic massage (Ateya 2006; Shen 2006);
-
sono‐electromagnetic therapy (Kessler 2014);
-
TaiJiQuan (Zhang 2011a);
-
transelectrical nerve stimulation (TENS) (Samhan 2011; Sikiru 2008);
-
tibial nerve stimulation (Kabay 2009);
-
ultrasound (Kaikai 2014);
-
hypercapnic hypoxia (Neimark 2016).
-
Outcomes
Almost all studies reported the effects of the interventions on prostatitis symptoms. All but four studies used the NIH‐CPSI score. Leskinen 2002; Nickel 1996; and Rowe 2005 used a 100‐point validated scale (Prostatitis Symptom Severity Index) and Wang 2002 used another validated scale (0 to 12, from Neal 1994).
Other secondary outcomes relevant to this review were reported inconsistently. Five studies did not report any of the prespecified outcomes for this review: three studies were only available as abstracts (Oh 2009; Shah 1993; Vassily 1999), one study reported the evolution of prostatitis symptoms with categorical variables (Muraro 1995), and one study reported global improvement as a composite outcome of symptoms and laboratory findings (Zhang 2011a). One study described the evolution of prostatitis symptoms with categorical variables; however, it reported the incidence of adverse events (Montorsi 1993).
We found only short‐term outcomes for all comparisons.
Funding sources
Most studies (28 studies, 76%) did not specify their funding sources. Three studies received funding from the companies that manufactured the device under evaluation (Kessler 2014; Neimark 2016; Rowe 2005). Three studies stated that they received no funding (Leskinen 2002; Montorsi 1993; Pajovic 2016). Four studies received funding from public institutions (Fitzgerald 2013; Lee 2008; Lee 2009; Zhao 2015).
Excluded studies
We excluded 73 studies for the following reasons (see Characteristics of excluded studies).
Eleven studies evaluated a wrong participant population: eight studies included participants with bacterial prostatitis, with no disaggregated data for CP/CPPS (Barbalias 1998; Feng 2011; Galeone 2012; Glybochko 2014; Golubchikov 2005; Lokshin 2010; Pushkar' 2006; Simmons 1985), and three studies did not use the NIH criteria for CP/CPPS (Nickel 2011; Zhang 2011b; Zhou 2017).
We found 28 studies to have a wrong study design: 25 studies specified that they did not use randomisation or used a non‐random sequence for the allocation of participants (Aliaev 2006; Allen 2017; Colleen 1975; DRKS00009352; Evliyaoglu 2002; Hong 2008; Ikeuchi 1990; ISRCTN43221600; Kalinina 2015; Kamalov 2006; Kogan 2010; Lee 2006; Leng 2007; Lopatkin 2009; Loran 2003; Ma 2015; Osborn 1981; Pavone 2010; Razumov 2005; Stamatiou 2014; Takahashi 2005; Thin 1983; Tkachuk 2006; Tkachuk 2011; Xu 2004); two studies reported the follow‐up of a single arm of RCTs (Kotarinos 2009; Marx 2013); one study was a phase II dose‐finding study with an adaptive design (Wagenlehner 2017).
Additionally, 10 studies were terminated and there were no outcome data available, due to problems in their conduct (Bschleipfer 2007; NCT00194597; NCT00194623; NCT00194636; NCT00301405; NCT00464373; NCT00529386; NCT01678911; NCT01830829; NCT02042651).
Our search strategy identified 24 systematic reviews that we searched for additional studies and some of them were used in the discussion (Aboumarzouk 2012; Anothaisintawee 2011; Capodice 2005; Chambo 2009; Chang 2016; Chen 2006; Chuang 2006; Cohen 2012; Erickson 2008; Jimenez‐Pacheco 2014; Le 2011; Lee 2007; Liu 2016; Magistro 2016; McNaughton 2000; McNaughton 2001; McNaughton 2002; Mishra 2008; Posadzki 2012; Qin 2016a; Qin 2016b; Thakkinstian 2012; Yang 2006; Yang 2008).
Risk of bias in included studies
See Figure 2 for a summary of risk of bias assessments. See Figure 3 for the individual assessments of the included studies. Detailed description of the supporting judgements can be found in the Characteristics of included studies table. Considering a global assessment of risk of bias for the main outcomes of this review, only one study had low risk of bias (Kessler 2014), 10 studies had unclear risk of bias (Lee 2008; Lee 2009; Leskinen 2002; Nickel 1996; Oh 2009; Sahin 2015; Samhan 2011; Vahdatpour 2013; Vassily 1999; Zimmermann 2009), and the remaining 26 studies had at least one domain with high risk of bias.
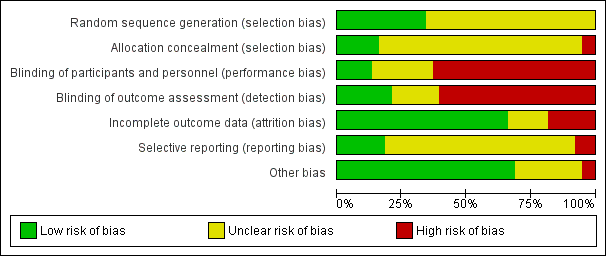
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each study.
Allocation
Random sequence generation
Thirteen studies specified an adequate method of random sequence generation (Chen 2009; Fitzgerald 2013; Kastner 2004; Kessler 2014; Lee 2008; Lee 2009; Marx 2009; Muraro 1995; Pajovic 2016; Rowe 2005; Sahin 2015; Yoo 2009; Zhao 2015). The remaining studies were at unclear risk of bias of random sequence generation.
Allocation concealment
Six studies specified an adequate method of allocation concealment (Fitzgerald 2013; Kastner 2004; Kessler 2014; Marx 2009; Pajovic 2016; Zeng 2012). Two studies specified that they did not conceal the allocation of participants and were deemed at high risk of bias in this domain (Gallo 2014; Yoo 2009). The remaining studies were at unclear risk of bias in allocation concealment.
Blinding
Blinding of participants and personnel
Five studies specified an adequate method for the blinding of participants and personnel (Kessler 2014; Lee 2008; Lee 2009; Sahin 2015; Zimmermann 2009). Twenty‐four studies did not adequately blind participants or personnel and were deemed at high risk of bias, considering that all outcomes were subjective (Ateya 2006; Chen 2009; Fang 2005; Fitzgerald 2013; Gallo 2014; Gao 2012; Giubilei 2007; Kabay 2009; Kaikai 2014; Kucuk 2015; Marx 2009; Montorsi 1993; Muraro 1995; Neimark 2016;Paick 2006; Pajovic 2016; Shen 2006; Sikiru 2008; Wang 2002; Yang 2011; Yoo 2009; Zeng 2012; Zhang 2011a; Zhao 2015). The remaining studies were at unclear risk of bias of blinding of participants and personnel.
Blinding of outcome assessment
All the outcomes of this review were participant‐reported outcomes. Eight studies reported blinding of participants (outcome‐assessors) (Kastner 2004; Kessler 2014; Lee 2008; Lee 2009; Nickel 1996; Rowe 2005; Sahin 2015; Zimmermann 2009). Twenty‐three studies did not adequately blind participants (Ateya 2006; Chen 2009; Fang 2005; Fitzgerald 2013; Gallo 2014; Gao 2012; Giubilei 2007; Kabay 2009; Kaikai 2014; Kucuk 2015; Marx 2009; Montorsi 1993; Muraro 1995; Neimark 2016;Paick 2006; Pajovic 2016; Shen 2006; Sikiru 2008; Wang 2002; Yang 2011; Yoo 2009; Zhang 2011a; Zhao 2015). The remaining studies were at unclear risk of bias of outcome assessment.
Incomplete outcome data
Twenty‐five studies specified that outcome data for all outcomes were available in all or nearly all participants (Chen 2009; Fang 2005; Fitzgerald 2013; Gao 2012; Kabay 2009; Kaikai 2014; Kessler 2014; Kucuk 2015; Lee 2008; Lee 2009; Leskinen 2002; Montorsi 1993; Neimark 2016;Nickel 1996; Paick 2006; Pajovic 2016; Sahin 2015; Samhan 2011; Shen 2006; Sikiru 2008; Wang 2002; Yang 2011; Zeng 2012; Zhao 2015; Zimmermann 2009). Seven studies had unbalanced or high attrition (or both) of outcome data at follow‐up and were deemed at high risk of bias (Gallo 2014; Giubilei 2007; Kastner 2004; Marx 2009; Rowe 2005; Shah 1993; Yoo 2009). The remaining studies were at unclear risk of bias in this domain.
Selective reporting
Seven studies had low risk of reporting bias when comparing their outcomes to their protocols or trial registries (Fitzgerald 2013; Kessler 2014; Lee 2008; Pajovic 2016; Vahdatpour 2013; Zhao 2015; Zimmermann 2009). Three studies reported some of their outcomes graphically or with missing data and were deemed at high risk of bias (Gallo 2014; Rowe 2005; Zeng 2012). The remaining studies were at unclear risk of reporting bias.
Other potential sources of bias
Two studies were at high risk of bias due to large baseline difference in mean symptom scores between groups (Fitzgerald 2013; Sikiru 2008; see Table 2). Seven studies lacked baseline characteristics of participants (Ateya 2006; Montorsi 1993; Neimark 2016;Nickel 1996; Oh 2009; Shah 1993; Vassily 1999), one study had some baseline differences in symptom scores (Leskinen 2002), and two studies did not specify if participants received some additional interventions planned in the protocol (Gallo 2014) or how many participants received the planned intervention (Zhao 2015). The remaining studies were at low risk of other bias.
Effects of interventions
See: Summary of findings for the main comparison Acupuncture compared to sham procedure for treating chronic prostatitis/chronic pelvic pain syndrome; Summary of findings 2 Acupuncture compared to medical treatment for treating chronic prostatitis/chronic pelvic pain syndrome; Summary of findings 3 Circumcision plus usual care compared to waiting list plus usual care for chronic prostatitis/chronic pelvic pain syndrome; Summary of findings 4 Electromagnetic chair compared to control intervention for chronic prostatitis/chronic pelvic pain syndrome; Summary of findings 5 Lifestyle modifications compared to control for chronic prostatitis/chronic pelvic pain syndrome; Summary of findings 6 Physical activity compared to control intervention for chronic prostatitis/chronic pelvic pain syndrome; Summary of findings 7 Prostatic massage compared to no intervention for treating chronic prostatitis/chronic pelvic pain syndrome; Summary of findings 8 Extracorporeal shockwave therapy compared to control procedure for chronic prostatitis/chronic pelvic pain syndrome; Summary of findings 9 Transrectal thermotherapy compared to medical treatment for chronic prostatitis/chronic pelvic pain syndrome; Summary of findings 10 Transrectal thermotherapy (add‐on) compared to medical treatment alone for chronic prostatitis/chronic pelvic pain syndrome
Acupuncture
1. Acupuncture versus sham procedure
Three studies with 204 participants compared acupuncture versus sham procedure for short‐term follow‐up (six to 24 weeks) (Lee 2008; Lee 2009; Sahin 2015). These studies compared the use of acupuncture with a sham procedure in which the acupuncture needles were placed in a point separate from those indicated by the acupuncture technique. One of these studies included the use of electric stimulation (Lee 2009), also called electroacupuncture, in the active treatment group (see Table 1 and Table 2 for further details of the participants and interventions). See Summary of findings table 1.
1.1. Prostatitis symptoms
Three studies with 204 participants reported prostatitis symptoms (Lee 2008; Lee 2009; Sahin 2015). Acupuncture appreciably reduced prostatitis symptoms compared to a sham procedure, measured by NIH‐CPSI score at six to eight weeks' follow‐up (fixed‐effect meta‐analysis; MD ‐5.79, 95% CI ‐7.32 to ‐4.26; Analysis 1.1). These lower scores were observed across all subscores of pain, urinary symptoms and QoL (Analysis 1.2; Analysis 1.3; Analysis 1.4). The quality of evidence was moderate due to unclear risk of bias in one domain (allocation concealment).
Two studies with 113 participants reported the number of participants who achieved an MCID of 6‐point decrease of NIH‐CPSI score at six weeks, defined as "responders" (Lee 2008; Lee 2009). Acupuncture may have resulted in little to no difference in responder rate compared to a sham procedure (random‐effects meta‐analysis; RR 2.49, 95% CI 0.77 to 8.02; Analysis 1.5). The quality of evidence was low due to imprecision issues (small sample size and few events) and inconsistency (statistical heterogeneity I2 = 76%).
One of the studies with 91 participants reported the NIH‐CPSI scores at the 24 weeks' follow‐up (Sahin 2015). Acupuncture reduced prostatitis symptoms in an appreciable number of participants (MD ‐7.36, 95% CI ‐9.93 to ‐4.79; Analysis 1.6). These lower scores were observed across all subscores of pain, urinary symptoms and QoL (see Analysis 1.7; Analysis 1.8; Analysis 1.9). The quality of evidence was high.
1.2. Adverse events
Three studies with 204 participants reported adverse events (Lee 2008; Lee 2009; Sahin 2015). Acupuncture likely resulted in little to no difference in adverse events (RR 1.33, 95% CI 0.51 to 3.46; Analysis 1.10). The quality of evidence was low due to imprecision (small sample size and few events) and unclear risk of bias in one domain (allocation concealment).
1.3. Sexual dysfunction
One study with 89 participants reported sexual dysfunction (Lee 2008). Acupuncture likely resulted in little to no difference in sexual dysfunction (fixed‐effect meta‐analysis; MD ‐0.50, 95% CI ‐3.46 to 2.46; Analysis 1.11). The quality of evidence was low due to imprecision (wide CIs includes both appreciable benefit and harm) and unclear risk of bias in one domain (allocation concealment).
1.4. Urinary symptoms
Two studies with 113 participants reported urinary symptoms (Lee 2008; Lee 2009). Acupuncture may have resulted in a small effect on urinary symptoms (fixed‐effect meta‐analysis; MD ‐2.79, 95% CI ‐4.77 to ‐0.82; Analysis 1.12). The quality of evidence was moderate due to unclear risk of bias in one domain (allocation concealment)..
1.5. Quality of life
None of the studies reported QoL.
1.6. Depression and anxiety
None of the studies reported depression and anxiety.
2. Acupuncture versus medical treatment
Three studies with 245 participants compared acupuncture versus medical treatment (Chen 2009; Kucuk 2015; Lee 2009). These studies compared the use of acupuncture with medical treatment. Medical treatment included the use of pollen extract (Chen 2009), levofloxacin and ibuprofen (Kucuk 2015), or exercise and medical advice (Lee 2009). One of these studies included the use of electric stimulation, also called electroacupuncture, in the active treatment group (Lee 2009; see Table 1 and Table 2 for further details of the participants and interventions). See summary of findings Table 2.
2.1. Prostatitis symptoms
Three studies reported prostatitis symptoms (Chen 2009; Kucuk 2015; Lee 2009). Acupuncture may have reduced prostatitis symptoms compared to medical treatment, measured by NIH‐CPSI score at six to eight weeks' follow‐up (MD ‐4.09, 95% CI ‐6.87 to ‐1.30; I2 = 70%, random‐effects meta‐analysis) (Analysis 2.1). These lower scores were observed across all subscores of pain, urinary symptoms and QoL (Analysis 2.2; Analysis 2.3; Analysis 2.4). Heterogeneity was predominantly attributable to one study (Chen 2009), which included participants with CP/CPPS using criteria that differed from those recommended by the Research Consensus. In a sensitivity analysis (see below), we excluded the results from this study and we found greater statistical consistency (I2 = 0%). Therefore, we chose to report these results in summary of findings Table 2. For this reason, we did not downgrade due to inconsistency. The quality of evidence was moderate due to high risk of bias (the studies were not blinded, which affected both detection and performance bias).
One study with 24 participants reported the number of participants who achieved an MCID of 6‐point decrease of NIH‐CPSI score at six weeks, defined as "responders" (Lee 2009). Acupuncture may have increased the number of responders compared to medical therapy (RR 3.57, 95% CI 1.45 to 8.80; Analysis 2.5). The quality of evidence was low due to high risk of bias (the studies were not blinded, which affected both detection and performance bias) and imprecision (small sample size and few events).
2.2. Adverse events
Two studies with 78 participants reported adverse events (Kucuk 2015; Lee 2009). There were no adverse events in either arm (Analysis 2.6). The quality of evidence was low due to high risk of bias (the studies were not blinded, which affected both detection and performance bias) and imprecision (small sample size and no events).
2.3. Sexual dysfunction
None of the studies reported sexual dysfunction.
2.4. Urinary symptoms
Two studies reported urinary symptoms (Lee 2008; Lee 2009). Acupuncture likely resulted in little to no difference compared to medical therapy, measured by IPSS score at six weeks (MD ‐2.70, 95% CI ‐6.00 to 0.60) (Analysis 2.7). The quality of evidence was moderate due to imprecision (wide CIs that included both appreciable benefit and harm).
2.5. Quality of life
None of the studies reported QoL.
2.6. Depression and anxiety
None of the studies reported depression and anxiety.
3. Acupuncture versus acupuncture with moxibustion
One study with 83 participants compared acupuncture versus acupuncture with moxibustion (Chen 2009) (see Table 1; Table 2 for further details of the participants and interventions).
3.1. Prostatitis symptoms
The study reported prostatitis symptoms. Moxibustion acupuncture probably reduced prostatitis symptoms compared to regular acupuncture, measured by NIH‐CPSI score at eight weeks' follow‐up (MD ‐4.16, 95% CI ‐7.16 to ‐1.16) (Analysis 3.1). The quality of evidence was moderate due to high risk of bias (the study was not blinded, which affected both detection and performance bias).
3.2. Adverse events
The study did not report adverse events.
3.3. Sexual dysfunction
The study did not report sexual dysfunction.
3.4. Urinary symptoms
The study did not report urinary symptoms.
3.5. Quality of life
The study did not report QoL.
3.6. Depression and anxiety
The study did not report depression and anxiety.
4. Circumcision: early versus delayed circumcision
One study with 713 participants compared early versus delayed circumcision (Zhao 2015). This study compared the effects of assigning participants to circumcision at four weeks compared with a waiting list to be circumcised at a delayed interval of three months (see Table 1 and Table 2 for further details of the participants and interventions). See summary of findings Table 3.
4.1. Prostatitis symptoms
Circumcision probably reduced prostatitis symptoms compared to delayed circumcision, measured by NIH‐CPSI score at 12 weeks' follow‐up (MD ‐3.00, 95% CI ‐3.82 to ‐2.18) (Analysis 4.1). These lower scores were observed across all subscores of pain, urinary symptoms and QoL (Analysis 4.2; Analysis 4.3; Analysis 4.4). The quality of evidence was moderate due to high risk of bias (the study was not blinded, which affected both detection and performance bias).
4.2. Adverse events
Circumcision likely resulted in little to no difference in adverse events compared to delayed circumcision group (RR 1.23, 95% CI 0.86 to 1.76) (Analysis 4.5). The quality of evidence was low due to high risk of bias (the study was not blinded, which affected both detection and performance bias) and imprecision (small sample size and few events).
4.3. Sexual dysfunction
The study did not report sexual dysfunction.
4.4. Urinary symptoms
The study did not report urinary symptoms.
4.5. Quality of life
The study did not report QoL.
4.6. Depression and anxiety
The study did not report depression and anxiety.
5. Electromagnetic chair versus control intervention (inactive device)
Two studies with 57 participants compared electromagnetic chair versus control intervention (Paick 2006; Rowe 2005). The control intervention was either the electromagnetic chair that was switched off or medical therapy (see Table 1; Table 2 for further details of the participants and interventions). See summary of findings Table 4.
5.1. Prostatitis symptoms
Both studies reported prostatitis symptoms. The study by Paick 2006 reported that the active treatment group had similar symptoms to the control group, measured by NIH‐CPSI score at six weeks' follow‐up (MD ‐3.00, 95% CI ‐7.75 to 1.75) (Analysis 5.1). This was also observed across all subscores of pain, urinary symptoms and QoL (Analysis 5.2; Analysis 5.3; Analysis 5.4). The study by Rowe 2005 found that the active treatment group had fewer symptoms (mean score 26.4) compared to the control group (mean score 42.4) at 12 weeks, measured by a validated 0 to 90 scale of symptoms for prostatitis. This study did not report information for effect size calculation or P value. This study also reported results at one year' follow‐up: the mean score for the active treatment group was 24 and the mean score in the control group was 33.6. Therefore, we were uncertain about the effects of the electromagnetic chair on prostatitis symptoms. The quality of evidence was very low due to high risk of bias (the studies were not blinded, which affected both detection and performance bias), attrition bias, selective reporting of outcomes for the Rowe 2005 study and small sample size in each study that resulted in imprecision and wide CIs. Additionally, there was inconsistency in the findings. Meta‐analysis of these studies was not possible due to missing information regarding standard deviations in Rowe 2005.
5.2. Adverse events
Electromagnetic chair likely resulted in little to no difference in adverse events compared to the control intervention (RR 2.18, 95% CI 0.10 to 46.92) (Analysis 5.5). The quality of evidence was low due to high risk of bias (the studies were not blinded, which affected both detection and performance bias) and imprecision (small sample size and few events).
5.3. Sexual dysfunction
The studies did not report sexual dysfunction.
5.4. Urinary symptoms
The study by Paick 2006 indicated that the electromagnetic chair may have resulted in no difference in urinary symptoms compared to the control group, measured by IPSS score at six weeks' follow‐up (MD 0.00, 95% CI ‐4.13 to 4.13) (Analysis 5.6). The quality of evidence was low due to high risk of bias (the study was not blinded, which affected both detection and performance bias) and imprecision (small sample size and few events).
5.5. Quality of life
The studies did not report QoL.
5.6. Depression and anxiety
The studies did not report depression and anxiety.
6. Lifestyle modifications versus control (no intervention)
One study with 100 participants compared lifestyle modifications versus control (no intervention) (Gallo 2014). This study compared the effects of instructing participants to change some aspects in their lifestyle related to risk factors for CP/CPPS to no intervention, that is, maintaining the same lifestyle (see Table 1 and Table 2 for further details of the participants and interventions). See summary of findings Table 5.
6.1. Prostatitis symptoms
The study analysed the number of participants who achieved a 6‐point decrease in NIH‐CPSI scores at three months ("responders"). Lifestyle modifications may have increased the number of responders in terms of prostatitis symptoms, but we were very uncertain (RR 3.90, 95% CI 2.20 to 6.92) (Analysis 6.1). The quality of evidence was very low due to high risk of selection bias (unconcealed allocation), detection and performance bias (the study was not blinded), missing outcome data and suspected selective outcome reporting (NIH‐CPSI scores were only presented graphically). Additionally, there were few response events in each group, resulting in imprecision.
6.2. Adverse events
The study did not report adverse events.
6.3. Sexual dysfunction
The study did not report sexual dysfunction.
6.4. Urinary symptoms
The study did not report urinary symptoms.
6.5. Quality of life
The study did not report QoL.
6.6. Depression and anxiety
The study did not report depression and anxiety.
7. Physical activity versus control intervention
One study with 85 participants compared physical activity versus control intervention (Giubilei 2007). This study compared the effects of a regular exercise programme to a control intervention (see Table 1 and Table 2 for further details of the participants and interventions). See summary of findings Table 6.
7.1. Prostatitis symptoms
Physical activity may have reduced prostatitis symptoms, measured by NIH‐CPSI score at six weeks' follow‐up, but we were very uncertain (MD ‐2.50, 95% CI ‐4.69 to ‐0.31) (Analysis 7.1). These lower scores were observed across the subscores of pain and QoL (Analysis 7.2; Analysis 7.4); however, participants in the intervention group had higher urinary symptoms (Analysis 7.3). The quality of evidence was low due to high risk of performance bias and detection bias (the study was not blinded) and high risk of attrition bias.
7.2. Adverse events
The study did not report adverse events.
7.3. Sexual dysfunction
The study did not report sexual dysfunction.
7.4. Urinary symptoms
The study did not report urinary symptoms.
7.5. Quality of life
The study did not report QoL.
7.6. Depression and anxiety
The study measured symptoms of anxiety with the SAI‐Y score and symptoms of depression with the Beck Depression Inventory at six weeks' follow‐up. The scores for anxiety and depression were similar in each group (Analysis 7.5; Analysis 7.6). The quality of evidence was very low due to high risk of performance bias and detection bias (the study was not blinded), high risk of attrition bias and imprecision issues.
8. Prostatic massage versus no intervention
Two studies with 115 participants compared prostatic massage versus no intervention (Ateya 2006; Shen 2006). In Shen 2006, all participants were also treated with traditional Chinese medicine as cointervention (see Table 1 and Table 2 for further details of the participants and interventions). See summary of findings Table 7.
8.1. Prostatitis symptoms
Both studies reported this outcome, however only Ateya 2006 reported the total NIH‐CPSI scores. In this study, prostatic massage may not have reduced prostatitis symptoms compared to no intervention, measured by NIH‐CPSI score at four weeks' follow‐up (MD ‐1.10, 95% CI ‐5.63 to 3.43) (Analysis 8.1). These similar scores were observed across the subscores of pain, urinary symptoms and QoL (Analysis 8.2; Analysis 8.3; Analysis 8.4). The study by Shen 2006 only reported the subscores, and participants who were assigned to prostatic massage had lower subscores for pain, urinary symptoms and QoL compared to those who did not received the intervention. The quality of evidence was very low due to high risk of performance and detection bias (study not blinded), unclear risk of bias in all the remaining domains and imprecision.
8.2. Adverse events
The studies did not report adverse events.
8.3. Sexual dysfunction
The studies did not report sexual dysfunction.
8.4. Urinary symptoms
The studies did not report urinary symptoms.
8.5. Quality of life
The studies did not report QoL.
8.6. Depression and anxiety
The studies did not report depression and anxiety.
9. Extracorporeal shockwave therapy versus control
Four studies with 237 participants compared extracorporeal shockwave therapy (ESWT) versus control (Pajovic 2016; Vahdatpour 2013; Zeng 2012; Zimmermann 2009). In three studies this included a form of blinding using a sham procedure in which the device was turned off (Vahdatpour 2013; Zeng 2012; Zimmermann 2009). In one studies, the control group did not receive any form of ESWT (Pajovic 2016; see Table 1 and Table 2 for further details of the participants and interventions). See summary of findings Table 8.
9.1. Prostatitis symptoms
Three studies with 157 participants reported the NIH‐CPSI scores for prostatitis symptoms (Pajovic 2016; Vahdatpour 2013; Zimmermann 2009). We found that ESWT reduced prostatitis symptoms compared to the control intervention, measured by NIH‐CPSI score at 12 weeks' follow‐up (MD ‐6.18, 95% CI ‐7.46 to ‐4.89; I2 = 34%; random‐effects meta‐analysis) (Analysis 9.1). These lower scores were observed across the subscores of pain, urinary symptoms and QoL (Analysis 9.2; Analysis 9.3; Analysis 9.4). Since one of the studies was not blinded (Pajovic 2016), the evidence could have been downgraded for high risk of detection and performance bias; however, the results of this study were consistent with those with low risk of bias, therefore we did not downgrade the quality of evidence (high‐quality evidence).
Two studies with 135 participants reported prostatitis symptoms analysing the number of participants who achieved a 6‐point decrease in NIH‐CPSI scores at 12 weeks' follow‐up (Zeng 2012; Zimmermann 2009). ESWT may have had little to no effect in the number of responders compared to the control intervention (RR 6.20, 95% CI 0.48 to 79.79; I2 = 71%; random‐effects meta‐analysis) (Analysis 9.5). The quality of evidence was very low due to high risk of detection and performance bias (one study was not blinded), inconsistency and imprecision issues.
Two studies with 97 participants reported prostatitis symptoms at 24 weeks' follow‐up (Pajovic 2016; Vahdatpour 2013). ESWT may have had little to no effect on prostatitis symptoms compared to the control intervention (MD ‐2.23, 95% CI ‐5.98 to 1.53; I2 = 82%; random‐effects meta‐analysis) (Analysis 9.6). These similar scores were observed across the subscores of pain, urinary symptoms and QoL (Analysis 9.7; Analysis 9.8; Analysis 9.9). The quality of evidence was low due to high risk of performance and detection bias (one study was not blinded) and inconsistency.
9.2. Adverse events
Three studies with 195 participants reported adverse events (Pajovic 2016; Zeng 2012; Zimmermann 2009). Two studies reported no adverse events in either group (Zeng 2012; Zimmermann 2009). The numbers of participants who had adverse events were similar in the ESWT and control group in the third study (Pajovic 2016) (RR 1.22, 95% CI 0.59 to 2.51) (Analysis 9.10). The quality of evidence was low due to high risk of performance and detection bias (one study was not blinded) and imprecision issues.
9.3. Sexual dysfunction
One study with 60 participants reported sexual dysfunction (Zimmermann 2009). ESWT probably reduced sexual dysfunction compared to control, measured by the IIEF scale at 12 weeks (MD 3.34, 95% CI 2.68 to 4.00) (Analysis 9.11). For the IIEF scale, higher scores indicated fewer symptoms. The quality of evidence was moderate due to imprecision issues.
9.4. Urinary symptoms
One study with 60 participants reported urinary symptoms (Zimmermann 2009). ESWT probably reduced urinary symptoms compared to control, measured by the IIEF scale at 12 weeks (MD ‐4.50, 95% CI ‐5.14 to ‐3.86) (Analysis 9.12). The quality of evidence was moderate due to imprecision issues.
9.5. Quality of life
The studies did not report QoL.
9.6. Depression and anxiety
The studies did not report depression and anxiety.
10. Transrectal thermotherapy versus medical therapy or as add‐on to medical therapy
Two studies with 237 participants compared transrectal thermotherapy (TRT) versus medical therapy or as add‐on to medical therapy (Gao 2012; Yoo 2009). In both studies, participants were randomised to one of the following groups: medical therapy, TRT or the combination of medical therapy and TRT. Four additional studies with 200 participants evaluated the effects of TRT (Muraro 1995; Oh 2009; Shah 1993; Vassily 1999), however there were no relevant outcome data available for this review. One study with 57 participants evaluated the effects of thermotherapy in three different regimens: one session weekly for four weeks, one session weekly for six weeks and two sessions weekly for three weeks (Montorsi 1993; see Table 1 and Table 2 for further details of the participants and interventions). See summary of findings Table 9 and summary of findings Table 10.
10.1. Prostatitis symptoms
10.1.1. Transrectal thermotherapy versus medical therapy
Two studies compared the effect of TRT versus medical therapy on prostatitis symptoms. TRT may have decreased prostatitis symptoms compared to medical therapy, measured by NIH‐CPSI score at six to 12 weeks' follow‐up (MD ‐2.50, 95% CI ‐3.82 to ‐1.18; I2 = 0%, fixed‐effect meta‐analysis) (Analysis 10.1). Only Yoo 2009 reported the results of the subscores; these lower scores were observed for urinary symptoms and QoL, but not in the pain domain (Analysis 10.2; Analysis 10.3; Analysis 10.4). The quality of evidence was low due to high risk of allocation concealment bias, performance and detection bias (the study was not blinded) and high risk of attrition bias.
10.1.2. Transrectal thermotherapy plus medical therapy versus medical therapy alone
Both studies compared the effect of TRT plus medical therapy versus medical therapy alone on prostatitis symptoms. TRT plus medical therapy may have decreased prostatitis symptoms compared to medical therapy alone, measured by NIH‐CPSI score at six to 12 weeks' follow‐up (MD ‐4.34, 95% CI ‐5.65 to ‐3.04; I2 = 0%, fixed‐effect meta‐analysis) (Analysis 10.1). Only Yoo 2009 reported the results of the subscores, these lower scores were observed across the subscores of pain, urinary symptoms and QoL (Analysis 10.2; Analysis 10.3; Analysis 10.4). The quality of evidence was low due to high risk of allocation concealment bias, performance and detection bias (the study was not blinded), and high risk of attrition bias.
10.2. Adverse events
Montorsi 1993 did not compare the intervention to a control group but compared different regimens. It reported that none of the participants had from "major complications." The other studies did not report adverse effects. Yoo 2009 reported that participants with active treatment had itching and tenesmus, but it was not quantified.
10.3. Sexual dysfunction
The studies did not report sexual dysfunction.
10.4. Urinary symptoms
The studies did not report urinary symptoms.
10.5. Quality of life
The studies did not report QoL.
10.6. Depression and anxiety
The studies did not report depression and anxiety.
11. Biofeedback
One study with 140 participants used biofeedback (Yang 2011). This factorial study had four groups: biofeedback added to usual care (40 participants), biofeedback with electric stimulation added to usual care (40 participants), electrical stimulation added to usual care (40 participants) and usual care alone (20 participants). Usual care included a series of lifestyle modifications (see Table 1; Table 2).
11.1. Prostatitis symptoms
11.1.1. Biofeedback versus usual care
Biofeedback may have decreased prostatitis symptoms compared to usual care, measured by NIH‐CPSI scores one month after treatment (MD ‐10.42, 95% CI ‐11.93 to ‐8.91). These lower scores were observed across all subscores of pain, urinary symptoms and QoL (Analysis 11.2; Analysis 11.3; Analysis 11.4). The quality of evidence was low due to high risk of performance and detection bias (the study was not blinded) and unclear risk of bias in most of the remaining domains.
11.1.2. Electrical stimulation versus usual care
Electrical stimulation may have decreased prostatitis symptoms compared to usual care, measured by NIH‐CPSI scores at one month after treatment (MD ‐10.63, 95% CI ‐12.13 to ‐9.13). These lower scores were observed across all subscores of pain, urinary symptoms and QoL domains (Analysis 11.2; Analysis 11.3; Analysis 11.4). The quality of evidence was low due to high risk of performance and detection bias (the study was not blinded) and unclear risk of bias in most of the remaining domains.
11.1.3. Biofeedback plus electrical stimulation versus usual care
Biofeedback plus electric stimulation may have decreased prostatitis symptoms compared to usual care, measured by NIH‐CPSI scores at one month after treatment (MD ‐15.83, 95% CI ‐17.72 to ‐13.94). These lower scores were observed across all subscores of pain, urinary symptoms and QoL domains (Analysis 11.2; Analysis 11.3; Analysis 11.4). The quality of evidence was low due to high risk of performance and detection bias (the study was not blinded) and unclear risk of bias in most of the remaining domains.
11.2. Adverse events
The study reported that no participants experienced adverse events. The quality of evidence was very low due to high risk of performance and detection bias (the study was not blinded) and unclear risk of bias in most of the remaining domains and imprecision (few events).
11.3. Sexual dysfunction
The study did not report sexual dysfunction.
11.4. Urinary symptoms
The study did not report urinary symptoms.
11.5. Quality of life
The study did not report QoL.
11.6. Depression and anxiety
The study did not report depression and anxiety.
12. External radiofrequency hyperthermia with or without terazosin
One study with 136 participants compared external radiofrequency hyperthermia (ERH) with terazosin versus ERH without terazosin (Wang 2002) (see Table 1; Table 2).
12.1. Prostatitis symptoms
ERH with terazosin may have decreased prostatitis symptoms (validated 0 to 12 score) compared to ERH alone at 12 weeks (MD ‐2.00, 95% CI ‐2.48 to ‐1.52) (Analysis 12.1). The quality of evidence was low due to high risk of performance and detection bias (the study was not blinded) and unclear risk of bias in most of the remaining domains.
12.2. Adverse events
Two participants had dizziness in the group who received the combination of ERH and terazosin in comparison to none in the ERH alone group (RR 5.91, 95% CI 0.29 to 121.23) (Analysis 12.2). The quality of evidence was very low due to high risk of performance and detection bias (the study was not blinded) and unclear risk of bias in most of the remaining domains and imprecision (few events).
12.3. Sexual dysfunction
The study did not report sexual dysfunction.
12.4. Urinary symptoms
The study did not report urinary symptoms.
12.5. Quality of life
The study did not report QoL.
12.6. Depression and anxiety
The study did not report depression and anxiety.
13. Laser therapy applied to the prostate compared with medical care
One study with 112 participants compared laser therapy with medical care (Fang 2005) (see Table 1; Table 2).
13.1. Prostatitis symptoms
Clinical response was defined as a decrease of 60% or more in prostatitis symptoms assessed with NIH‐CPSI at six weeks. Laser therapy may have increased the response rate compared to medical treatment, but we were very uncertain (RR 2.35, 95% CI 1.53 to 3.62) (Analysis 13.1). The quality of evidence was very low due to high risk of performance and detection bias (the study was not blinded) and unclear risk of bias in most of the remaining domains and imprecision (few events).
13.2. Adverse events
None of the participants in the study had adverse events (Analysis 13.2). The quality of evidence was very low due to high risk of performance and detection bias (the study was not blinded) and unclear risk of bias in most of the remaining domains and imprecision (zero events).
13.3. Sexual dysfunction
The study did not report sexual dysfunction.
13.4. Urinary symptoms
The study did not report urinary symptoms.
13.5. Quality of life
The study did not report QoL.
13.6. Depression and anxiety
The study did not report depression and anxiety.
14. Tibial nerve stimulation compared with no intervention
One study with 89 participants compared tibial nerve stimulation with no intervention (Kabay 2009). This study compared participants who received tibial nerve stimulation compared to no active intervention (see Table 1; Table 2).
14.1. Prostatitis symptoms
Tibial nerve stimulation probably reduced prostatitis symptoms measured by NIH‐CPSI compared to no active treatment at 12 weeks (MD ‐11.20, 95% CI ‐12.92 to ‐9.48) (Analysis 14.1). These lower scores were observed across all subscores of pain, urinary symptoms and QoL (Analysis 14.2; Analysis 14.3; Analysis 14.4). The quality of evidence was moderate due to high risk of performance and detection bias.
14.2. Adverse events
The study did not report adverse events.
14.3. Sexual dysfunction
The study did not report sexual dysfunction.
14.4. Urinary symptoms
The study did not report urinary symptoms.
14.5. Quality of life
The study did not report QoL.
14.6. Depression and anxiety
The study did not report depression and anxiety.
15. Sono‐electromagnetic therapy versus placebo intervention
One study with 60 participants compared sono‐electromagnetic therapy versus placebo intervention (Kessler 2014). This study had two groups: participants who were treated at home with a sono‐electromagnetic therapy device and participants treated with a 'placebo' device (see Table 1; Table 2).
15.1. Prostatitis symptoms
Sono‐electromagnetic therapy probably resulted in little to no effect on prostatitis symptoms compared to the placebo device at 16 weeks (MD ‐2.80, 95% CI ‐6.75 to 1.15). These similar scores were observed across all subscores of pain, urinary symptoms and QoL domains (Analysis 17.3; Analysis 17.4; Analysis 17.5). There was a similar number of 'responders' (defined as a drop in 4 points of NIH‐CPSI score) in each group (RR 1.40, 95% CI 0.91 to 2.15). The quality of evidence was moderate due to imprecision issues.
The study authors presented subgroup analysis.
-
Participants aged less than 50 and 50 years or older had similar reductions in NIH‐CPSI scores (P = 0.40).
-
Participants with baseline NIH‐CPSI scores greater than 25 and 25 or less had similar reductions in NIH‐CPSI scores (P = 0.35).
-
Participants with a duration of symptoms greater than 12 months had a lower reduction of NIH‐CPSI scores compared to participants who had a shorter duration of symptoms (P = 0.023). The MDs in NIH‐CPSI score were ‐0.8 (95% CI ‐4.6 to 3.1) with a duration of symptoms greater than 12 months and ‐8.5 (95% CI ‐14.3 to ‐2.6) with a shorter duration of symptoms.
15.2. Adverse events
One participant (1/30) in the active treatment group had worsening of pain symptoms. There were no adverse events observed in the control group. The quality of evidence was low due to imprecision issues (few events).
15.3. Sexual dysfunction
The study did not report sexual dysfunction.
15.4. Urinary symptoms
The study did not report urinary symptoms.
15.5. Quality of life
The study did not report QoL.
15.6. Depression and anxiety
The study did not report depression and anxiety.
16. Myofascial trigger point release therapy compared to control intervention (massage)
One study with 23 men compared myofascial trigger point release therapy with control intervention (massage) (Fitzgerald 2013). This study also included women with interstitial cystitis/painful bladder syndrome, but their results were not included in this review. This study had two groups: participants who were treated with myofascial physical therapy and participants who received therapeutic western massage as a control intervention (see Table 1; Table 2).
16.1. Prostatitis symptoms
Myofascial trigger point release therapy may have resulted in little to no effect compared to the control intervention at 12 weeks (MD 1.00, 95% CI ‐6.45 to 8.45). These similar scores were observed across all subscores of pain, urinary symptoms and QoL (Analysis 15.2; Analysis 15.3; Analysis 15.4). The quality of evidence was very low due to high risk of performance and detection bias (the study was not blinded), the presence of baseline differences and imprecision issues (wide CIs due to small sample size).
16.2. Adverse events
The authors reported the incidence of adverse events globally for men and women. Even though we contacted study authors to obtain the disaggregated data for men with CP/CPPS, we received no additional information.
16.3. Sexual dysfunction
Myofascial trigger point release may have resulted in little to no effect compared to the control intervention, measured by the Sexual Health Inventory for Men (MD ‐2.20, 95% CI ‐9.24 to 4.84) (Analysis 15.5). The quality of evidence was very low due to high risk of performance and detection bias (the study was not blinded), the presence of baseline differences and imprecision issues (wide confidence interval due to small sample size).
16.4. Urinary symptoms
The study did not report urinary symptoms.
16.5. Quality of life
Myofascial trigger point release therapy may have resulted in little to no effect on QoL compared to the control intervention, measured by the SF‐12 Health Status Questionnaire (MD ‐1.30, 95% CI ‐9.54 to 6.94 for the physical domain and 0.80, 95% CI ‐9.25 to 10.85 for the mental domain) (Analysis 15.6; Analysis 15.7). The quality of evidence was very low due to high risk of performance and detection bias (the study was not blinded), the presence of baseline differences and imprecision issues (wide CIs due to small sample size).
16.6. Depression and anxiety
The study did not report depression and anxiety.
17. Osteopathy versus control intervention (exercise programme)
One study with 35 participants compared osteopathy versus control intervention (exercise programme) (Marx 2009) (see Table 1; Table 2).
17.1. Prostatitis symptoms
Osteopathy may have reduced prostatitis symptoms, measured by the NIH‐CPSI score, compared the control intervention at 14 weeks (MD ‐9.67, 95% CI ‐15.15 to ‐4.19) (Analysis 16.1). The authors also reported a decrease in QoL subscore (Analysis 16.2), but did not report the other subscores. The quality of evidence was very low due to high risk of performance and detection bias (the study was not blinded), the unbalanced attrition at follow‐up and imprecision issues (wide CI due to small sample size).
17.2. Adverse events
The study did not report adverse events.
17.3. Sexual dysfunction
The study did not report sexual dysfunction.
17.4. Urinary symptoms
Osteopathy may reduce urinary symptoms, measured by IPSS score, compared to the control intervention (MD ‐8.70, 95% CI ‐12.73 to ‐4.67). The quality of evidence was very low due to high risk of performance and detection bias (the study was not blinded), the unbalanced attrition at follow‐up and imprecision issues (wide CIs due to small sample size).
17.5. Quality of life
The study did not report QoL.
17.6. Depression and anxiety
The study did not report depression and anxiety.
18. Transcutaneous electrical nerve stimulation compared to control intervention
Two studies with 56 participants compared TENS with control (Samhan 2011; Sikiru 2008). In Samhan 2011, the control group received a sham procedure in which the TENS device was switched off, and, in Sikiru 2008, the control group received no intervention (see Table 1; Table 2).
18.1. Prostatitis symptoms
The studies did not report the total NIH‐CPSI scores. They reported the pain subscore at four weeks. TENS may have appreciably reduced prostatitis‐related pain compared to the control intervention, measured by the NIH‐CPSI pain subscore (MD ‐8.60, 95% CI ‐9.71 to ‐7.48; I2 = 97%; random‐effects meta‐analysis) (Analysis 18.1). The quality of evidence was very low due to high risk of performance and detection bias (one study was not blinded), inconsistency and imprecision.
18.2. Adverse events
The studies did not report adverse events.
18.3. Sexual dysfunction
The studies did not report sexual dysfunction.
18.4. Urinary symptoms
The studies did not report urinary symptoms.
18.5. Quality of life
The studies did not report QoL.
18.6. Depression and anxiety
The studies did not report depression and anxiety.
19. Transurethral thermotherapy compared to control intervention
Two studies with 62 participants compared transurethral thermotherapy with control intervention (Kastner 2004; Nickel 1996). These studies assessed transurethral thermotherapy at approximately 50 °C compared to a sham procedure with no temperature elevation (Nickel 1996), or to a transurethral thermotherapy at 70 °C (Kastner 2004) (see Table 1; Table 2).
19.1. Prostatitis symptoms
19.1.1. Transurethral thermotherapy at 50 °C compared to sham procedure
Nickel 1996 recruited 20 participants and reported that participants who received transurethral thermotherapy had fewer symptoms of prostatitis compared to participants who received the sham procedure with no temperature elevation at three months' follow‐up, using the Prostatitis Symptom Severity Index (range 0 to 100) with a mean score of 27.3 with transurethral thermotherapy and 52.9 with sham (P < 0.05). The quality of evidence was low due to severe imprecision.
19.1.2. Transurethral thermotherapy at 55 °C compared to thermotherapy at 70 °C
Kastner 2004 recruited 42 participants and reported that participants who received transurethral thermotherapy at 70 °C had similar symptoms of prostatitis compared to participants who received the procedure at 55 °C at three months' follow‐up, using the NIH‐CPSI score (MD ‐1.10, 95% CI ‐6.50 to 4.30) (Analysis 19.1). These similar scores were observed across all subscores of pain, urinary symptoms and QoL (Analysis 19.2; Analysis 19.3; Analysis 19.4). The quality of evidence was low due to lost to follow‐up (attrition bias) and imprecision.
19.2. Adverse events
Nickel 1996 recruited 20 participants and specified that four participants experienced transient adverse reactions, but there were no specifications whether they were in the active treatment group or the sham intervention group. Kastner 2004 reported that both active treatment (55 °C and 70 °C) groups had genitourinary events that resolved at six weeks, and that the proportion of events was similar across groups. The quality of evidence was very low due to high risk of performance, detection and attrition bias, and imprecision.
19.3. Sexual dysfunction
The studies did not report sexual dysfunction.
19.4. Urinary symptoms
19.4.1. Transurethral thermotherapy at 50 °C compared to sham procedure
Nickel 1996 recruited 20 participants and reported that participants who received transurethral thermotherapy had fewer urinary symptoms compared to participants who received the sham procedure with no temperature elevation at three months' follow‐up, using the American Urology Association Symptom Score (range 0 to 100) with mean scores of 12.8 with transurethral thermotherapy and 21.9 with sham (P value not available).
19.4.2. Transurethral thermotherapy at 55 °C compared to thermotherapy at 70 °C
Kastner 2004 recruited 42 participants and reported that participants who received transurethral thermotherapy at 70 °C had similar urinary symptoms compared to participants who received the procedure at 55 °C at three months' follow‐up (MD ‐2.10, 95% CI ‐6.34 to 2.14) (Analysis 19.5). The quality of evidence was low due to lost to follow‐up (attrition bias) and imprecision.
19.5. Quality of life
The studies did not report QoL.
19.6. Depression and anxiety
The studies did not report depression and anxiety.
20. Transurethral needle ablation compared to sham procedure
One study with 33 participants compared transurethral needle ablation (TUNA) with sham procedure (Leskinen 2002). The sham procedure used urethroscopy but not ablation (see Table 1; Table 2).
20.1. Prostatitis symptoms
TUNA may have had little to no effect on prostatitis symptoms compared to a sham procedure at 12 months' follow‐up, using the Prostatitis Symptom Severity Index (range 0 to 100) (MD 2.30, 95% CI ‐8.02 to 12.62) (Analysis 20.1). The quality of evidence was very low due to severe imprecision and unclear risk of bias in almost all domains.
20.2. Adverse events
The study reported that 10/25 participants in the TUNA group and 3/8 in the sham group experienced dysuria during the first month after the procedure and 3/25 participants in the TUNA group reported transient haematuria. The quality of evidence was very low due to severe imprecision and unclear risk of bias in almost all domains.
20.3. Sexual dysfunction
The study did not report sexual dysfunction.
20.4. Urinary symptoms
TUNA may have had little to no effect on urinary symptoms compared to a sham procedure at 12 months follow‐up, using the IPSS score (MD 0.40, 95% CI ‐5.09 to 5.89) (Analysis 20.2). The quality of evidence was very low due to severe imprecision and unclear risk of bias in almost all domains.
20.5. Quality of life
The study did not report QoL.
20.6. Depression and anxiety
The study did not report depression and anxiety.
21. Ultrasound compared to or as add‐on to medical therapy
One study with 105 participants had three groups that compared non‐intrusive ultrasound alone with Chinese‐Western medicine or non‐intrusive ultrasound plus integrated Chinese‐Western medicine (Kaikai 2014) (see Table 1; Table 2).
21.1. Prostatitis symptoms
21.1.1. Ultrasound versus medical therapy
Ultrasound therapy may have increased prostatitis symptoms compared to medical therapy, measured by NIH‐CPSI scores at one month after treatment (MD 1.09, 95% CI 0.16 to 2.02) (Analysis 21.1). These greater scores were observed across all subscores of pain, urinary symptoms and QoL (Analysis 21.2; Analysis 21.3; Analysis 21.4). The quality of evidence was low due to high risk of performance and detection bias (study not blinded), and imprecision.
21.1.2. Ultrasound plus medical therapy versus medical therapy alone
Ultrasound plus medical therapy may have resulted in fewer prostatitis symptoms compared to medical therapy alone, measured by NIH‐CPSI score at one month after treatment (MD ‐6.67, 95% CI ‐7.62 to ‐5.72) (Analysis 21.1). These lower scores were observed across all subscores of pain, urinary symptoms and QoL (Analysis 21.2; Analysis 21.3; Analysis 21.4). The quality of evidence was low due to high risk of performance and detection bias (study not blinded), and imprecision.
21.2. Adverse events
The study reported five cases of vertigo, six cases of gastrointestinal discomfort and three cases of sleepiness; however, it did not specify which group experienced them. The quality of evidence was low due to high risk of performance and detection bias (study not blinded), and imprecision.
21.3. Sexual dysfunction
The study did not report sexual dysfunction.
21.4. Urinary symptoms
The study did not report urinary symptoms.
21.5. Quality of life
The study did not report QoL.
21.6. Depression and anxiety
The study did not report depression and anxiety.
22. Hypercapnic hypoxia versus no additional intervention
One study with 37 participants compared hypercapnic hypoxia plus medical therapy versus medical therapy alone (Neimark 2016) (see Table 1; Table 2).
22.1. Prostatitis symptoms
The study did not report prostatitis symptoms.
22.2. Adverse events
The study did not report adverse events.
22.3. Sexual dysfunction
The study did not report sexual dysfunction.
22.4. Urinary symptoms
The study reported that at the end of the 10 days of treatment, the mean IPSS score for the hypercapnic hypoxia plus medical therapy group was 9 and the medical therapy alone was 8 (P value not available). The quality of evidence was very low due to high risk of detection and performance bias, and imprecision.
22.5. Quality of life
The study did not report QoL.
22.6. Depression and anxiety
The study did not report depression and anxiety.
23. TaiJiQuan/t'ai chi ch'uan (太極拳) plus usual care versus usual care alone
One study with 96 participants compared TaiJiQuan/t'ai chi ch'uan (太極拳) plus usual care with usual care alone (Zhang 2011a) (see Table 1; Table 2). This study was poorly reported in the methods and results section. None of the predefined outcomes of this review were reported. The study authors reported different levels of clinical "response" (definition not available).
24. Psychological support
We found no studies reporting psychological support.
25. Prostatic surgery
We found no studies reporting prostatic surgery.
Sensitivity analysis
We performed a sensitivity analysis excluding the six studies (Chen 2009; Montorsi 1993; Muraro 1995; Nickel 1996; Shah 1993; Vassily 1999) that did not meet the Research Consensus definition for CP/CPPS (Nickel 1999a).
1. Acupuncture
The study by Chen 2009 was involved in two comparisons.
1.1. Acupuncture versus medical therapy, prostatitis symptoms
The sensitivity analysis excluding Chen 2009 resulted in a reduction of the statistical heterogeneity across the studies (Figure 4). The meta‐analysis of the two remaining studies showed that acupuncture probably reduced appreciably prostatitis symptoms compared with medical therapy (MD ‐6.05, 95% CI ‐7.87 to ‐4.24; 78 participants; 4 studies; I2 = 0%) (Kucuk 2015; Lee 2009). The results of this sensitivity analysis were incorporated in summary of findings Table 2 and the other sections of this review.

Forest plot of comparison: 22 Acupuncture treatments versus medical treatment. Sensitivity analysis, outcome: 22.1 Prostatitis symptoms (NIH‐CPSI total).
1.2. Acupuncture versus acupuncture plus moxibustion
The exclusion of this study resulted in no evidence for this comparison (Chen 2009).
2. Transrectal thermotherapy
The exclusion of three of the studies that did not meet the Research Consensus definition did not affect the results since they did not provide valid outcome measures (Muraro 1995; Shah 1993; Vassily 1999). The exclusion of Montorsi 1993 affected the incidence of adverse events. In Montorsi 1993, there were no adverse events in the participants who received the procedure (all participants received the procedure in different regimens).
3. Transurethral thermotherapy
The exclusion of Nickel 1996 resulted in no evidence for the comparison of transurethral thermotherapy versus sham procedure.
Discussion
Summary of main results
We included 38 unique studies with 3290 men with CP/CPPS across 23 comparisons. We included all comparisons with short‐term follow‐up in the analyses. The median age of the participants was 37 years.
We found moderate quality evidence that acupuncture and extracorporeal shockwave therapy probably leads to a clinically meaningful reduction in prostatitis symptoms based on an NIH‐CPSI reduction of greater than three (but less than six). These interventions may not have been associated with an increased incidence of adverse events. We also found that circumcision probably decreased prostatitis symptoms; however, the effect would be small. We found moderate‐quality evidence that tibial nerve stimulation probably caused an important decrease in prostatitis symptoms, but we have no information regarding adverse events.
Additionally, we found low‐ to very low‐quality evidence that physical activity, biofeedback, ultrasound and transrectal thermotherapy may have reduced prostatitis symptoms. We were uncertain about the effects of lifestyle interventions, use of an electromagnetic chair, sono‐electromagnetic therapy, external radiofrequency hyperthermia, prostatic massage, laser therapy, myofascial trigger point release therapy, osteopathy, TENS, transurethral thermotherapy, transurethral needle ablation, hypercapnic hypoxia and TaiJiQuan. We found no information regarding psychological support or prostatic surgery.
Overall completeness and applicability of evidence
Our review focused on men with CP/CPPS. Almost all the included studies used the consistent inclusion criteria defined by the NIH (Nyberg 1999). These diagnostic criteria are related to clinical practice, since these participants are usually tested for urological diseases that could mimic CP/CPPS. However, some of the impact of the NIH consensus on the diagnosis of CP/CPPS might have changed the classification of participants across time (Krieger 2002), therefore, the results of older trials must be interpreted in caution with the current diagnostic criteria. Nevertheless, we incorporated a sensitivity analysis based on this consideration.
We maintained open inclusion criteria for the included interventions. This decision was based on the poorly understood pathophysiological determinants for CP/CPPS (see Background). This led to the inclusion of a wide variety of interventions, which could relate to clinical practice, considering that people with CP/CPPS usually try different treatment options before achieving some form of relief. However, while this review can be used to discuss the different treatment options with patients, the description of some of these interventions was sometimes insufficient. This was particularly important for more complex interventions such as non‐pharmacological interventions (Hoffmann 2013). This could pose a threat to the open discussion of the implications of implementing a certain intervention in clinical practice.
Our review focused on critical patient‐important outcomes; however, most studies did not report them consistently, especially the incidence of adverse events. This is a common problem in clinical trials (Ioannidis 2001), and poses difficulties when estimating the net benefit of the interventions. Additionally, most of the included studies did not report the effects of their interventions on QoL, sexual function and mental health, considering the important impact of CP/CPPS in these domains (Krsmanovic 2014). We acknowledge that the NIH‐CPSI score includes QoL and urinary symptoms subscores that, alongside the pain subscore, contribute to a total score that has proven to be valid and reliable (Litwin 1999; McNaughton 2001; Propert 2006; Turner 2003); however, these subscores have not been validated for these constructs individually. We acknowledge that the validated scores for QoL, urinary symptoms, sexual dysfunction, and anxiety and depression might have not been validated in the subpopulation of men with CP/CPPS, therefore their values should be carefully interpreted, specially using the assessment of the MCID.
Furthermore, most studies reported results at four to 12 weeks' follow‐up, which might be insufficient considering that men with CP/CPPS generally have a long history of symptoms, usually for years (Clemens 2015).
We had planned to explore the effect of the interventions in subgroups of men with different degrees of disease severity and in subgroups of men with common comorbidities (Gasperi 2017), but the included studies provided insufficient data in this regard.
Some of the included treatments for the main comparisons expressed in the 'Summary of findings' table have not been part of routine care for CP/CPPS, especially circumcision (summary of findings Table 3) and the electromagnetic chair (summary of findings Table 4). For this statement, we used, as an example, the recommendations by the European Association of Urology, available at uroweb.org/guideline/chronic‐pelvic‐pain/ (last accessed December 2017). As the guideline authors stated, careful considerations should be placed when considering the applicability of these interventions in daily practice. The evidence for the electromagnetic chair is inconsistent for the main outcomes (very low‐quality evidence). For circumcision, the effect size was small for the main outcomes (moderate‐quality evidence). Furthermore, circumcision is the only intervention included in this review that entails an irreversible surgical procedure. As the Evidence to Decision Framework stated, a careful evaluation of patient's values and preferences, resource use and equity issues and the acceptability and feasibility of the interventions is warranted when drafting recommendations (Alonso‐Coello 2016).
Quality of the evidence
The main limitations of the body of evidence were the following.
-
Study limitations: most studies had problems when masking the interventions to study personnel and participants. This is particularly important for this review considering that all the predefined outcomes were subjective participant‐reported outcomes. Additionally, most studies poorly reported random sequence generation and allocation concealment and most of the studies had no published protocol or analysis plan to assess the risk of selective reporting. For the comparison of ESWT, we decided not to downgrade due to study limitations since the results from studies with high risk of bias were consistent with those with low/unclear risk of bias.
-
Inconsistency: we performed few meta‐analyses and, in many cases, we found considerable heterogeneity; only in one case we found that the inconsistency was explained to a difference in the criteria for disease definition.
-
Imprecision: we estimated an optimal information size of 74 for the primary outcome 'prostatitis symptoms' and 50% of the studies had a smaller sample size; therefore, most studies had imprecision. Nevertheless, in some cases, meta‐analysis of some of the included studies increased the number of participants for each comparison, overcoming this limitation. This was more difficult to do for the outcome 'adverse events' in which the small number of events (in some cases there was a "zero count") resulted in considerable imprecision.
-
Publication bias: due to the small number of studies in each comparison, we could not assess the risk of publication bias.
Potential biases in the review process
We strictly followed our published protocol to reduce the risk of bias in the conduct of this review. Nevertheless, we had to make further specifications due to the multiplicity of comparisons and available outcomes to review. These specifications did not imply major changes and they were done to provide clarity when summarising multiple comparisons (see Differences between protocol and review), especially when drafting the 'Summary of findings' tables. We acknowledge that we might have made 'Summary of findings' tables of each comparison, but this would not have provided further clarity to the understanding of the findings. We also highlight that even though there were no 'Summary of findings' tables for some comparisons, all the findings were rated using GRADE methods.
We performed a comprehensive search in multiple databases, trial registries and other sources to reduce the risk of meta‐bias in our review. Additionally, we did not restrict the searches or inclusion of studies on the basis of language of publication; for this purpose, we incorporated three authors with expertise in Chinese, Russian and Korean. Nevertheless, we were unable to retrieve some of the studies on acupuncture published in some Chinese journals that were included in other reviews that searched Chinese Databases (see Agreements and disagreements with other studies or reviews). Should we find the report of these studies, they will be evaluated for inclusion in updates of this review, since we have no clear information regarding their eligibility. Nevertheless, the reported results of these studies in the corresponding reviews were consistent with our findings.
We contacted study authors on multiple occasions with a variable rate of response. However, we acknowledge that many of the ratings of 'unclear' risk of bias were due to limitations in the report of the studies, rather than a true risk of bias in the conduct of these trials.
We deleted the 'Clinical Phenotyping' item as an intervention in our review, as suggested by a peer reviewer. We acknowledge that this strategy might not constitute in itself an intervention. However, this change has not affected the results of this review since we found no trials on this subject. We also modified the presentation of some of the methods of this review (assessment of outcomes and GRADE methods), but this did not affect the results of the review either (see Differences between protocol and review).
Agreements and disagreements with other studies or reviews
We found several systematic reviews addressing interventions for CP/CPPS; however, only a few of them incorporated non‐pharmacological interventions.
A systematic review by Erickson 2008 and its update by Le 2011 included some of the studies for non‐pharmacological interventions for CP/CPPS. These reviews indicated that there was no evidence for the use of biofeedback; however, in our review, we included a clinical trial assessing this intervention. These reviews also included some of the evidence for transurethral thermotherapy and prostatic massage with similar results to ours.
Two systematic reviews included both pharmacological and non‐pharmacological interventions (McNaughton 2000; McNaughton 2001). We included two studies from these reviews that were not retrieved by our search strategy (Shah 1993; Vassily 1999); however, we did not include one study on transrectal thermotherapy since the participants in this study did not meet the CP/CPPS criteria (Strohmaier 1988).
The systematic reviews by Cohen 2012; Qin 2016b; and Magistro 2016 included both pharmacological and non‐pharmacological interventions. They included some of the studies in this review addressing the effects of sono‐electromagnetic therapy, ESWT, acupuncture, aerobic exercise and tibial nerve stimulation, with the same results.
The systematic review by Mishra 2008 focused only on prostatic massage and incorporated one of the trials in our review in addition to several case series.
The systematic review by Chang 2016 addressing acupuncture for CP/CPPS incorporated three additional studies from Chinese journals and failed to include one of the trials in our review since the review searched Chinese databases. One of these additional included studies was excluded in our review since it used a quasi‐randomised allocation (Ma 2015). We asked the study authors for the remaining two studies. Nevertheless, the two meta‐analyses reported by this review yielded similar results in terms of reduction of prostatitis symptoms (NIH‐CPSI scores) and an increased response rate with acupuncture in comparison to sham procedures or medical therapy, with a similar incidence of adverse events. Another systematic review by Liu 2016 also focusing on acupuncture for CP/CPPS incorporated four additional studies from Chinese journals (different from those found by Chang 2016) and failed to include one of the trials in our review. One of these additional included studies was also Ma 2015. We asked the study authors for the remaining three studies. Nevertheless, the two meta‐analysis reported by this review yielded similar results in terms of reduction of prostatitis symptoms (NIH‐CPSI scores) and an increased response rate with acupuncture in comparison to sham procedures or medical therapy, with a similar incidence of adverse events. Furthermore, a systematic review by Qin 2016a included three additional trials (one of them not identified in the previous review); however, the reported meta‐analysis yielded similar results in terms of reduction of prostatitis symptoms (NIH‐CPSI scores). We asked the study authors for the remaining studies. Finally, a review by Posadzki 2012 on acupuncture for CP/CPPS included six additional studies (different from those found in Chang 2016; Liu 2016; and Qin 2016a). We asked the study authors for the remaining studies. Nevertheless, the meta‐analysis reported by this review yielded similar results in terms of an increased response rate with acupuncture in comparison to sham procedures or medical therapy, with a similar incidence of adverse events (this review also highlighted the poor reporting of adverse events in the literature).

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
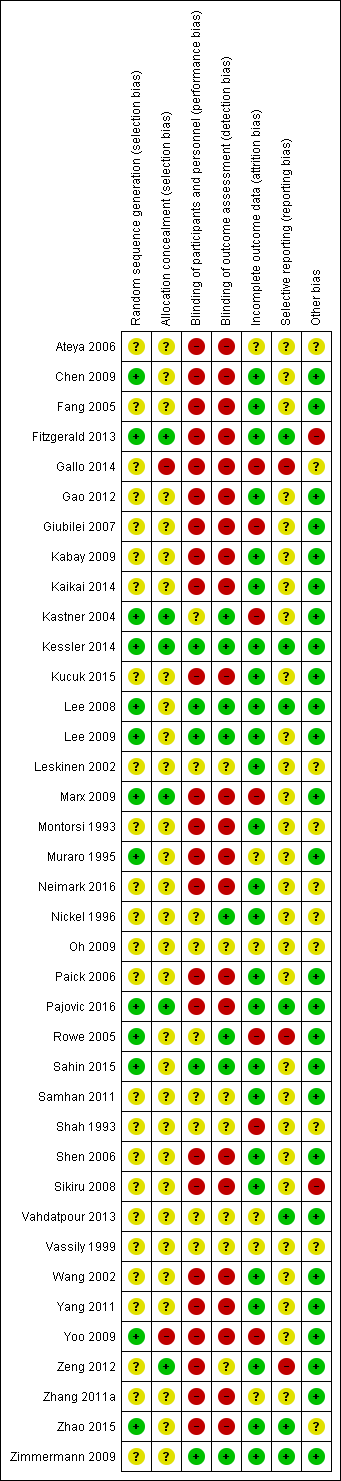
Risk of bias summary: review authors' judgements about each risk of bias item for each study.
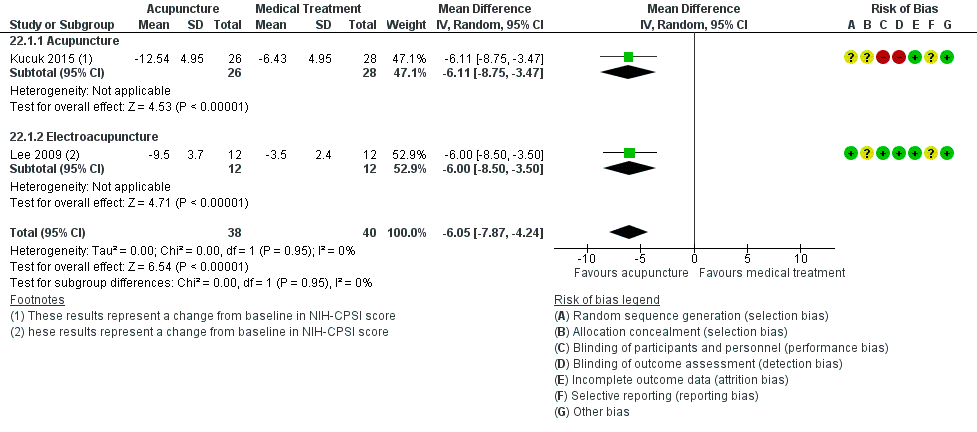
Forest plot of comparison: 22 Acupuncture treatments versus medical treatment. Sensitivity analysis, outcome: 22.1 Prostatitis symptoms (NIH‐CPSI total).

Comparison 1 Acupuncture versus sham procedure, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).
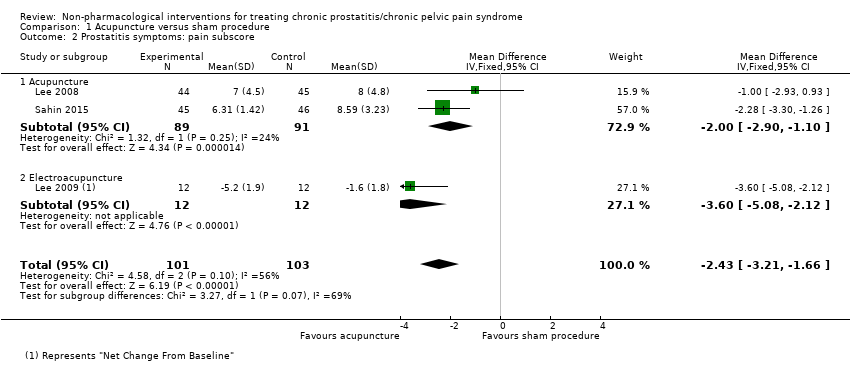
Comparison 1 Acupuncture versus sham procedure, Outcome 2 Prostatitis symptoms: pain subscore.
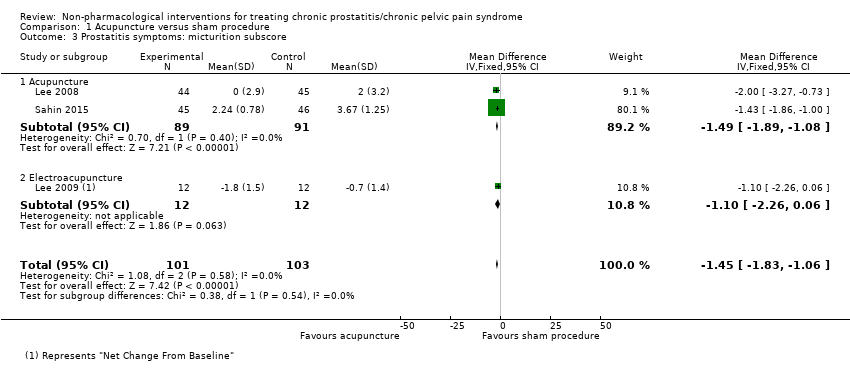
Comparison 1 Acupuncture versus sham procedure, Outcome 3 Prostatitis symptoms: micturition subscore.

Comparison 1 Acupuncture versus sham procedure, Outcome 4 Prostatitis symptoms: quality of life subscore.
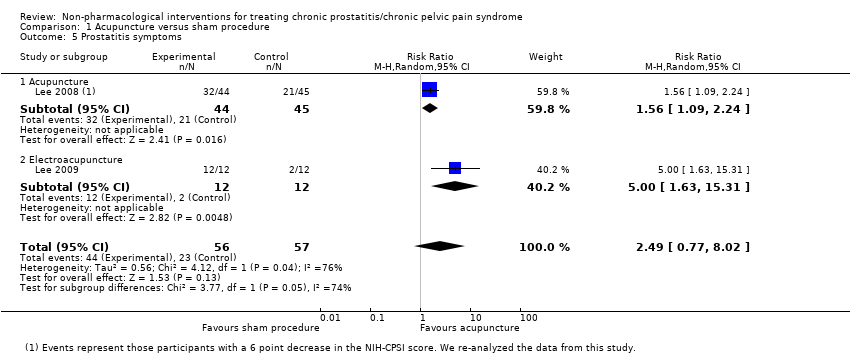
Comparison 1 Acupuncture versus sham procedure, Outcome 5 Prostatitis symptoms.

Comparison 1 Acupuncture versus sham procedure, Outcome 6 Prostatitis symptoms (NIH‐CPSI total) ‐ medium term.
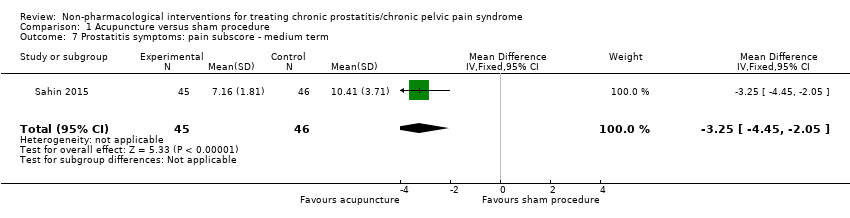
Comparison 1 Acupuncture versus sham procedure, Outcome 7 Prostatitis symptoms: pain subscore ‐ medium term.

Comparison 1 Acupuncture versus sham procedure, Outcome 8 Prostatitis symptoms: micturition subscore ‐ medium term.

Comparison 1 Acupuncture versus sham procedure, Outcome 9 Prostatitis symptoms: quality of life subscore ‐ medium term.
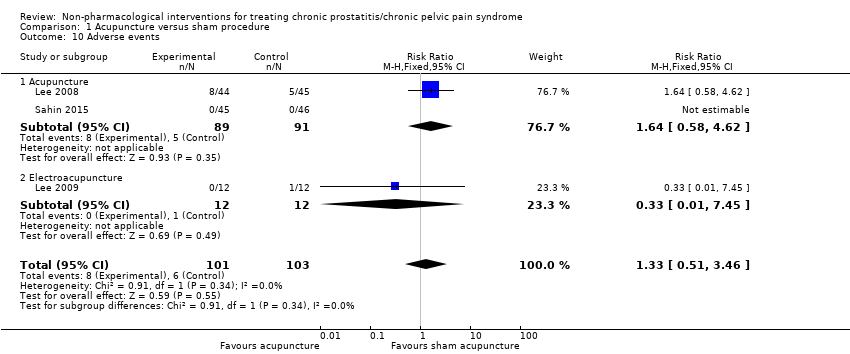
Comparison 1 Acupuncture versus sham procedure, Outcome 10 Adverse events.
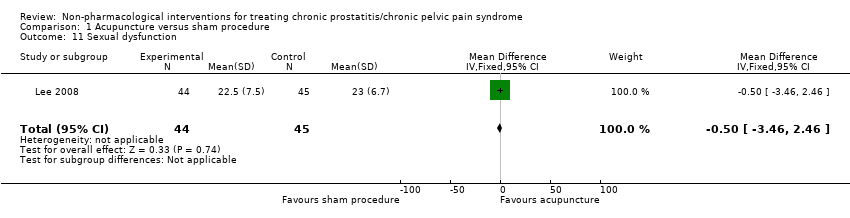
Comparison 1 Acupuncture versus sham procedure, Outcome 11 Sexual dysfunction.

Comparison 1 Acupuncture versus sham procedure, Outcome 12 Urinary symptoms.

Comparison 2 Acupuncture treatments versus medical treatment, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).

Comparison 2 Acupuncture treatments versus medical treatment, Outcome 2 Prostatitis symptoms: pain subscore.

Comparison 2 Acupuncture treatments versus medical treatment, Outcome 3 Prostatitis symptoms: micturition subscore.

Comparison 2 Acupuncture treatments versus medical treatment, Outcome 4 Prostatitis symptoms: quality of life subscore.

Comparison 2 Acupuncture treatments versus medical treatment, Outcome 5 Prostatitis symptoms.

Comparison 2 Acupuncture treatments versus medical treatment, Outcome 6 Adverse events.

Comparison 2 Acupuncture treatments versus medical treatment, Outcome 7 Urinary symptoms.

Comparison 3 Acupuncture with or without moxibustion, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).

Comparison 4 Circumcision versus waiting list, Outcome 1 Prostatitis symptoms.
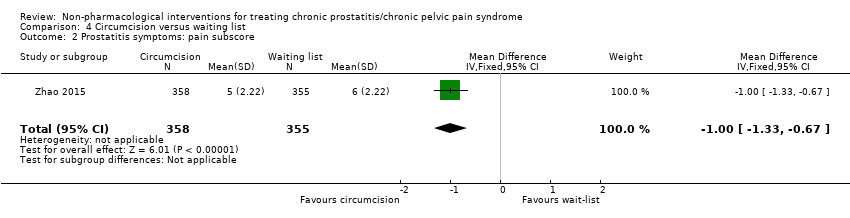
Comparison 4 Circumcision versus waiting list, Outcome 2 Prostatitis symptoms: pain subscore.
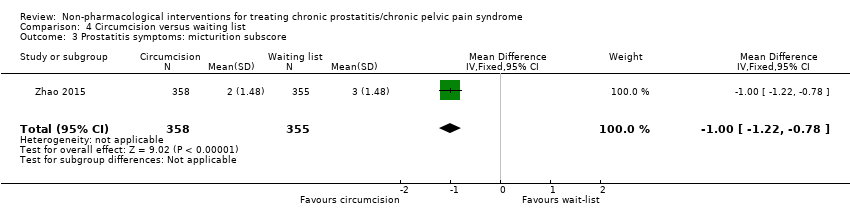
Comparison 4 Circumcision versus waiting list, Outcome 3 Prostatitis symptoms: micturition subscore.

Comparison 4 Circumcision versus waiting list, Outcome 4 Prostatitis symptoms: quality of life subscore.

Comparison 4 Circumcision versus waiting list, Outcome 5 Adverse events.

Comparison 5 Electromagnetic chair versus sham procedure, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).

Comparison 5 Electromagnetic chair versus sham procedure, Outcome 2 Prostatitis symptoms: pain subscore.

Comparison 5 Electromagnetic chair versus sham procedure, Outcome 3 Prostatitis symptoms: micturition subscore.

Comparison 5 Electromagnetic chair versus sham procedure, Outcome 4 Prostatitis symptoms: quality of life subscore.

Comparison 5 Electromagnetic chair versus sham procedure, Outcome 5 Adverse events.

Comparison 5 Electromagnetic chair versus sham procedure, Outcome 6 Urinary symptoms.
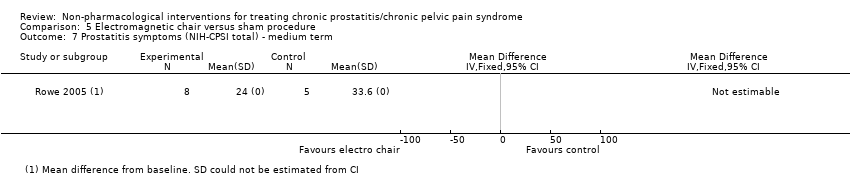
Comparison 5 Electromagnetic chair versus sham procedure, Outcome 7 Prostatitis symptoms (NIH‐CPSI total) ‐ medium term.

Comparison 5 Electromagnetic chair versus sham procedure, Outcome 8 Prostatitis symptoms: pain subscore ‐ medium term.

Comparison 6 Lifestyle modifications versus control, Outcome 1 Prostatitis symptoms.

Comparison 7 Physical activity versus control, Outcome 1 Prostatitis symptoms.

Comparison 7 Physical activity versus control, Outcome 2 Prostatitis symptoms: pain subscore.

Comparison 7 Physical activity versus control, Outcome 3 Prostatitis symptoms: micturition subscore.

Comparison 7 Physical activity versus control, Outcome 4 Prostatitis symptoms: quality of life subscore.
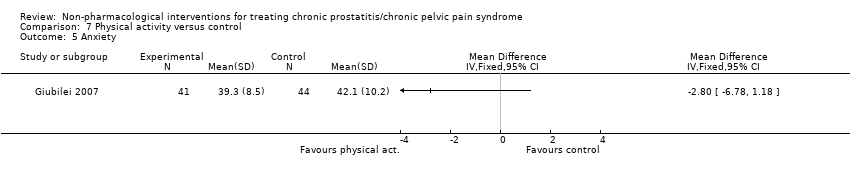
Comparison 7 Physical activity versus control, Outcome 5 Anxiety.

Comparison 7 Physical activity versus control, Outcome 6 Depression.

Comparison 8 Prostatic massage versus control, Outcome 1 Prostatitis symptoms.

Comparison 8 Prostatic massage versus control, Outcome 2 Prostatitis symptoms: pain subscore.
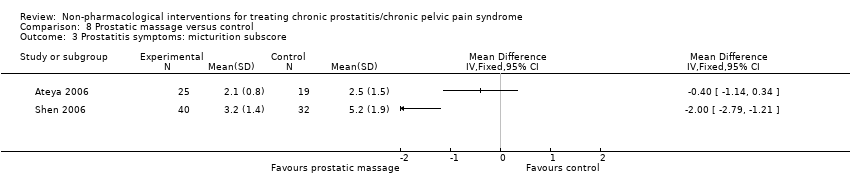
Comparison 8 Prostatic massage versus control, Outcome 3 Prostatitis symptoms: micturition subscore.

Comparison 8 Prostatic massage versus control, Outcome 4 Prostatitis symptoms: quality of life subscore.

Comparison 9 Extracorporeal shockwave therapy (ESWT) versus control, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).
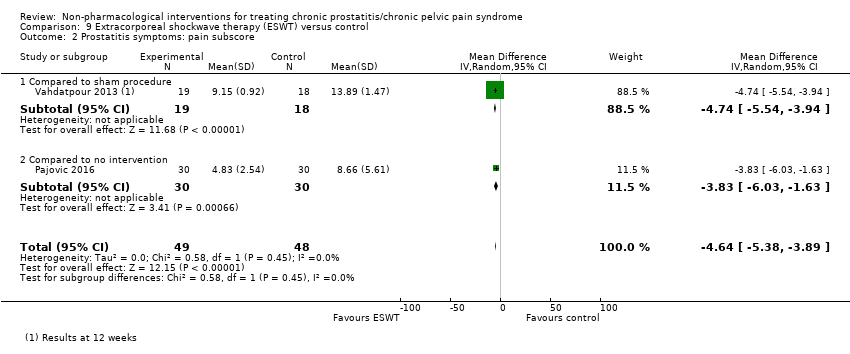
Comparison 9 Extracorporeal shockwave therapy (ESWT) versus control, Outcome 2 Prostatitis symptoms: pain subscore.

Comparison 9 Extracorporeal shockwave therapy (ESWT) versus control, Outcome 3 Prostatitis symptoms: micturition subscore.
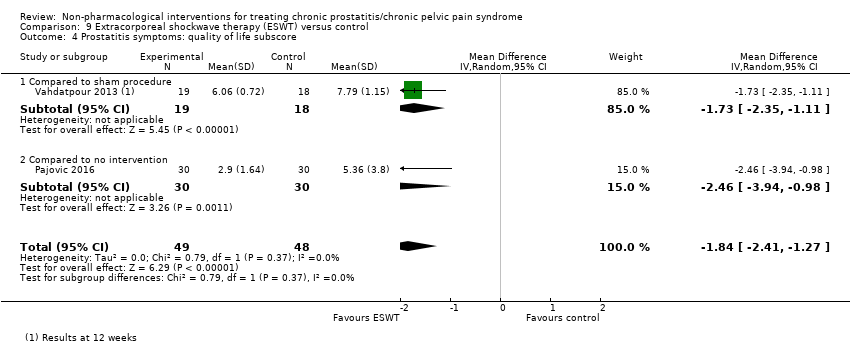
Comparison 9 Extracorporeal shockwave therapy (ESWT) versus control, Outcome 4 Prostatitis symptoms: quality of life subscore.
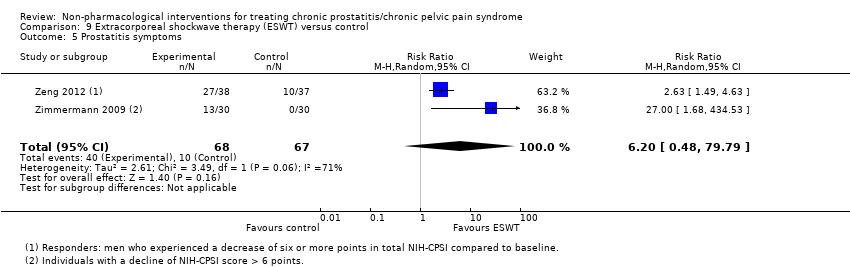
Comparison 9 Extracorporeal shockwave therapy (ESWT) versus control, Outcome 5 Prostatitis symptoms.

Comparison 9 Extracorporeal shockwave therapy (ESWT) versus control, Outcome 6 Prostatitis symptoms (total score) ‐ long term.

Comparison 9 Extracorporeal shockwave therapy (ESWT) versus control, Outcome 7 Prostatitis symptoms: pain subscore ‐ long term.

Comparison 9 Extracorporeal shockwave therapy (ESWT) versus control, Outcome 8 Prostatitis symptoms: micturition subscore ‐ long term.
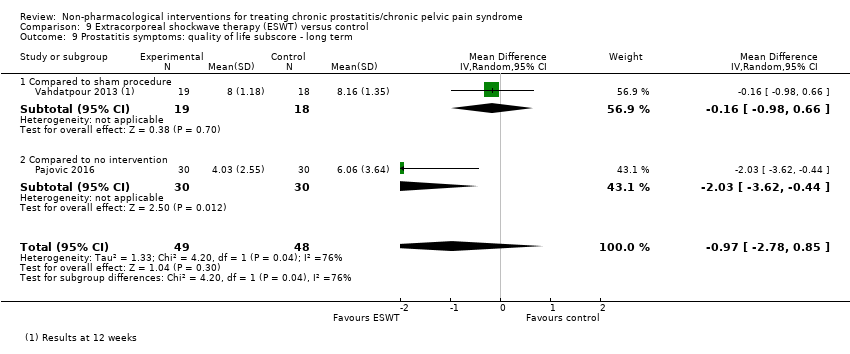
Comparison 9 Extracorporeal shockwave therapy (ESWT) versus control, Outcome 9 Prostatitis symptoms: quality of life subscore ‐ long term.
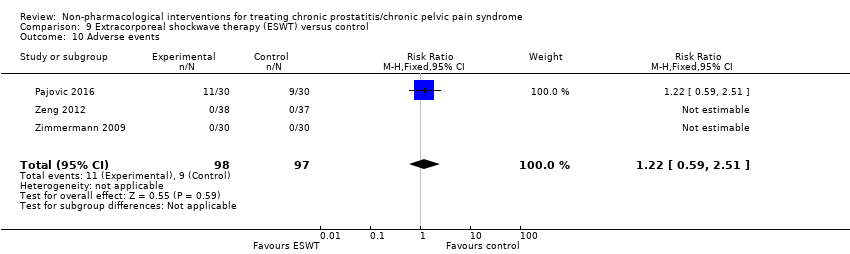
Comparison 9 Extracorporeal shockwave therapy (ESWT) versus control, Outcome 10 Adverse events.

Comparison 9 Extracorporeal shockwave therapy (ESWT) versus control, Outcome 11 Sexual dysfunction.

Comparison 9 Extracorporeal shockwave therapy (ESWT) versus control, Outcome 12 Urinary symptoms.

Comparison 10 Transrectal thermotherapy (TRT) versus medical treatment, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).

Comparison 10 Transrectal thermotherapy (TRT) versus medical treatment, Outcome 2 Prostatitis symptoms: pain subscore.
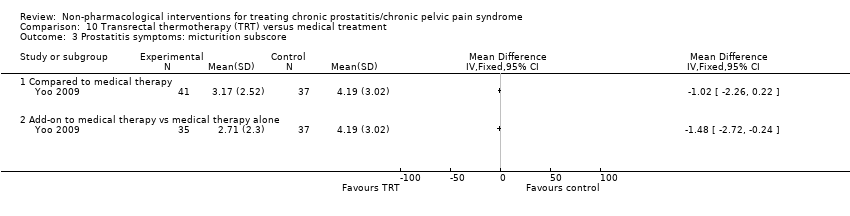
Comparison 10 Transrectal thermotherapy (TRT) versus medical treatment, Outcome 3 Prostatitis symptoms: micturition subscore.

Comparison 10 Transrectal thermotherapy (TRT) versus medical treatment, Outcome 4 Prostatitis symptoms: quality of life subscore.

Comparison 11 Biofeedback with or without electrical stimulation versus usual care, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).
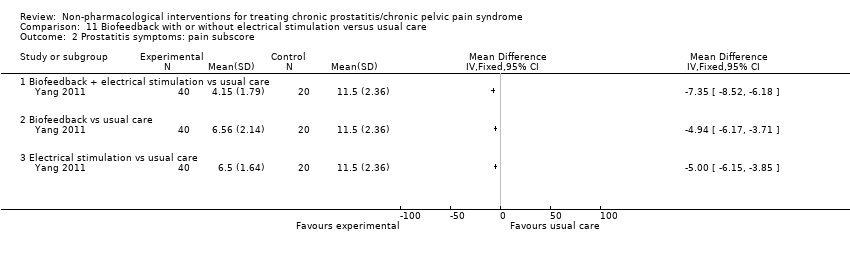
Comparison 11 Biofeedback with or without electrical stimulation versus usual care, Outcome 2 Prostatitis symptoms: pain subscore.

Comparison 11 Biofeedback with or without electrical stimulation versus usual care, Outcome 3 Prostatitis symptoms: micturition subscore.

Comparison 11 Biofeedback with or without electrical stimulation versus usual care, Outcome 4 Prostatitis symptoms: quality of life subscore.

Comparison 12 External radiofrequency hyperthermia with or without terazosin, Outcome 1 Prostatitis symptoms.

Comparison 12 External radiofrequency hyperthermia with or without terazosin, Outcome 2 Adverse events.

Comparison 13 Laser therapy versus medical treatment, Outcome 1 Prostatitis symptoms.

Comparison 13 Laser therapy versus medical treatment, Outcome 2 Adverse events.

Comparison 14 Tibial nerve stimulation versus no intervention, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).
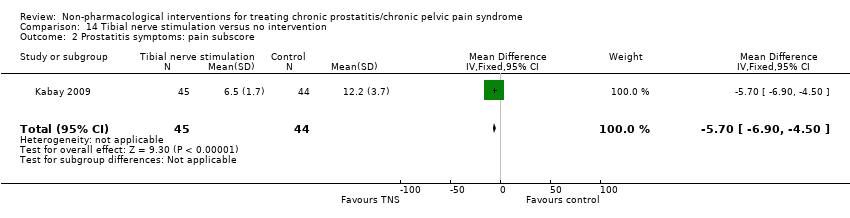
Comparison 14 Tibial nerve stimulation versus no intervention, Outcome 2 Prostatitis symptoms: pain subscore.

Comparison 14 Tibial nerve stimulation versus no intervention, Outcome 3 Prostatitis symptoms: micturition subscore.

Comparison 14 Tibial nerve stimulation versus no intervention, Outcome 4 Prostatitis symptoms: quality of life subscore.
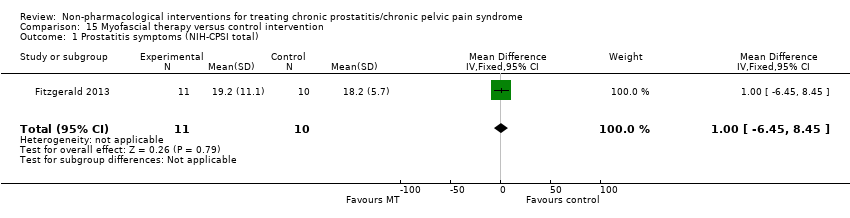
Comparison 15 Myofascial therapy versus control intervention, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).

Comparison 15 Myofascial therapy versus control intervention, Outcome 2 Prostatitis symptoms: pain subscore.
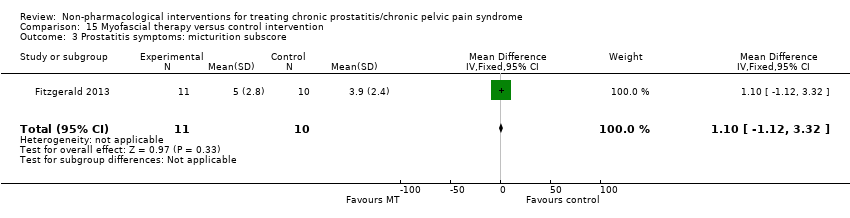
Comparison 15 Myofascial therapy versus control intervention, Outcome 3 Prostatitis symptoms: micturition subscore.

Comparison 15 Myofascial therapy versus control intervention, Outcome 4 Prostatitis symptoms: quality of life subscore.
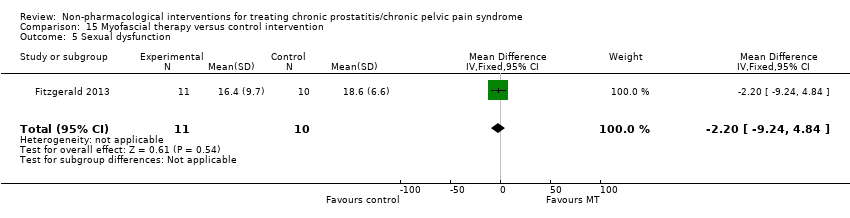
Comparison 15 Myofascial therapy versus control intervention, Outcome 5 Sexual dysfunction.
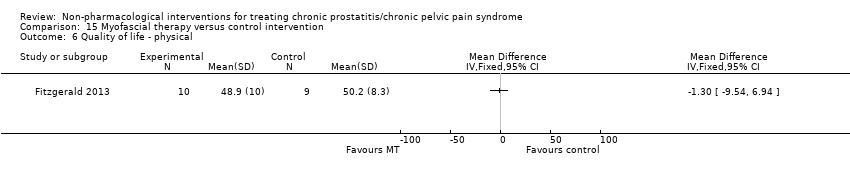
Comparison 15 Myofascial therapy versus control intervention, Outcome 6 Quality of life ‐ physical.

Comparison 15 Myofascial therapy versus control intervention, Outcome 7 Quality of life ‐ mental.

Comparison 16 Osteopathy versus sham procedure, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).
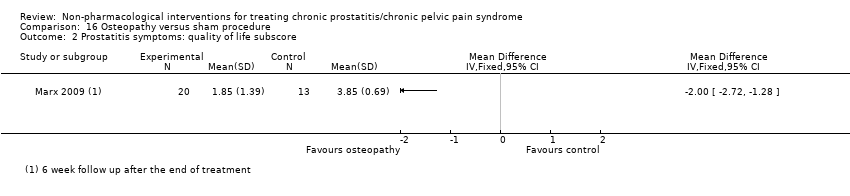
Comparison 16 Osteopathy versus sham procedure, Outcome 2 Prostatitis symptoms: quality of life subscore.
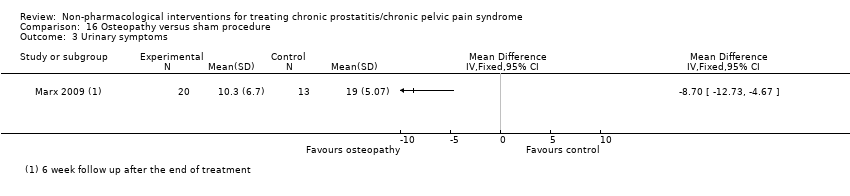
Comparison 16 Osteopathy versus sham procedure, Outcome 3 Urinary symptoms.
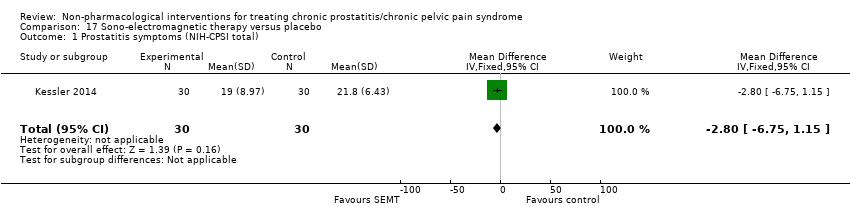
Comparison 17 Sono‐electromagnetic therapy versus placebo, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).

Comparison 17 Sono‐electromagnetic therapy versus placebo, Outcome 2 Prostatitis symptoms.

Comparison 17 Sono‐electromagnetic therapy versus placebo, Outcome 3 Prostatitis symptoms: pain subscore.

Comparison 17 Sono‐electromagnetic therapy versus placebo, Outcome 4 Prostatitis symptoms: micturition subscore.

Comparison 17 Sono‐electromagnetic therapy versus placebo, Outcome 5 Prostatitis symptoms: quality of life subscore.

Comparison 18 Transelectrical nerve stimulation (TENS) versus control, Outcome 1 Prostatitis symptoms: pain subscore.

Comparison 19 Transurethral microwave thermotherapy, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).

Comparison 19 Transurethral microwave thermotherapy, Outcome 2 Prostatitis symptoms: pain subscore.
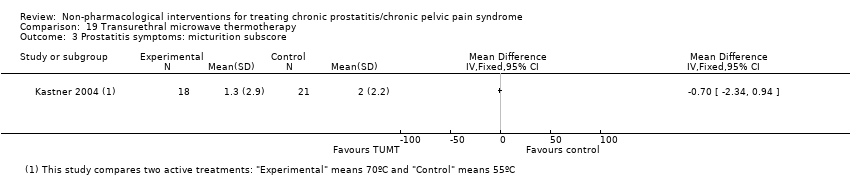
Comparison 19 Transurethral microwave thermotherapy, Outcome 3 Prostatitis symptoms: micturition subscore.
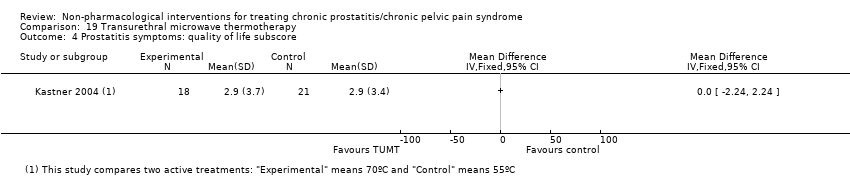
Comparison 19 Transurethral microwave thermotherapy, Outcome 4 Prostatitis symptoms: quality of life subscore.

Comparison 19 Transurethral microwave thermotherapy, Outcome 5 Urinary symptoms.
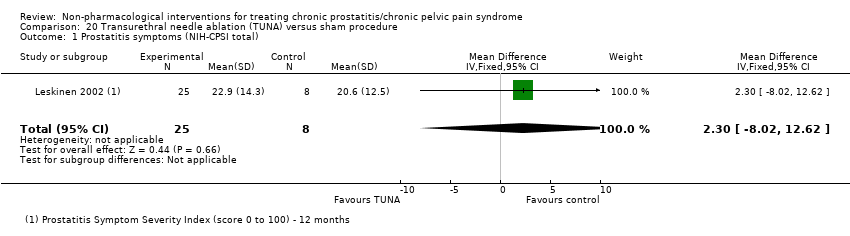
Comparison 20 Transurethral needle ablation (TUNA) versus sham procedure, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).

Comparison 20 Transurethral needle ablation (TUNA) versus sham procedure, Outcome 2 Urinary symptoms.

Comparison 21 Ultrasound (non‐intrusive), Outcome 1 Prostatitis symptoms (NIH‐CPSI total).
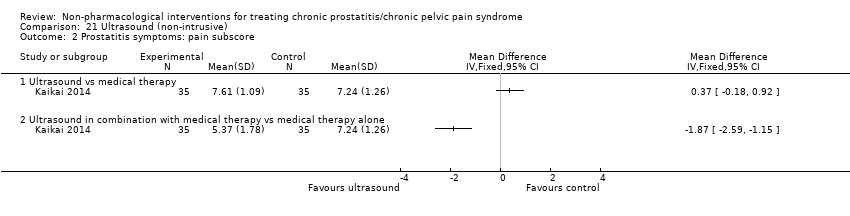
Comparison 21 Ultrasound (non‐intrusive), Outcome 2 Prostatitis symptoms: pain subscore.
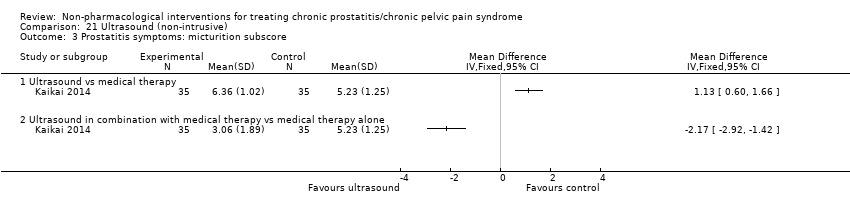
Comparison 21 Ultrasound (non‐intrusive), Outcome 3 Prostatitis symptoms: micturition subscore.
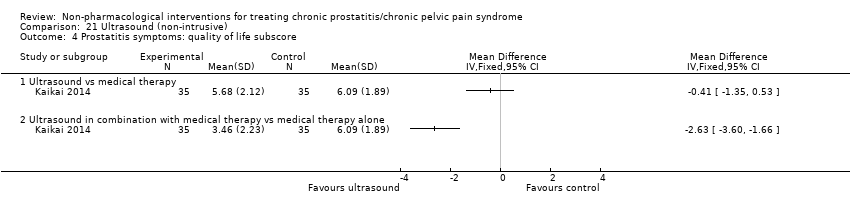
Comparison 21 Ultrasound (non‐intrusive), Outcome 4 Prostatitis symptoms: quality of life subscore.
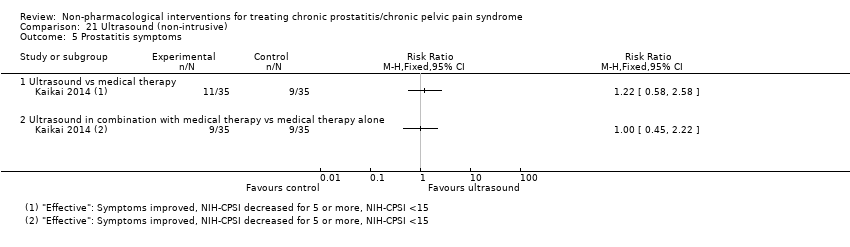
Comparison 21 Ultrasound (non‐intrusive), Outcome 5 Prostatitis symptoms.

Comparison 22 Acupuncture treatments versus medical treatment ‐ sensitivity analysis, Outcome 1 Prostatitis symptoms (NIH‐CPSI total).
| Acupuncture compared to sham procedure for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with sham procedure | Risk difference with Acupuncture | ||||
| Prostatitis Symptoms Benefit is indicated by lower scores | 204 | ⊕⊕⊕⊝ | ‐ | The mean prostatitis Symptoms ranged from 17.08 to 22 | MD 5.79 lower |
| Prostatitis Symptoms | 113 | ⊕⊝⊝⊝ | RR 2.49 | Study population | |
| 404 per 1.000 | 596 more per 1000 | ||||
| Adverse events follow up: 6‐8 weeks | 204 | ⊕⊕⊝⊝ | RR 1.33 | Study population | |
| 58 per 1.000 | 19 more per 1000 | ||||
| Sexual dysfunction Benefit is indicated by higher scores | 89 | ⊕⊕⊝⊝ | ‐ | The mean sexual dysfunction was 23 | MD 0.5 lower |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded 1 level due to unclear risk of bias: insufficient information about allocation concealment 2 Downgraded 1 level due to inconsistency: statistical heterogeneity (I2 = 76%). 3 Downgraded 1 level due to imprecision issues: wide confidence interval due to small sample size and few events. 4 Downgraded 1 level for imprecision issues: wide confidence interval includes both appreciable benefit and harm. | |||||
| Acupuncture compared to medical treatment for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: treating chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with medical treatment | Risk difference with acupuncture | ||||
| Prostatitis symptoms (NIH‐CPSI total) Benefit is indicated by lower scores | 78 | ⊕⊕⊕⊝ | ‐ | The mean prostatitis symptoms (NIH‐CPSI total) ranged from 12 to 16 | MD 6.05 lower |
| Prostatitis symptoms: response defined as a 6‐point decrease in NIH‐CPSI score | 24 | ⊕⊕⊝⊝ | RR 3.57 | Study population | |
| 250 per 1000 | 643 more per 1000 | ||||
| Adverse events follow‐up: 12 weeks | 78 | ⊕⊕⊝⊝ | ‐ | There were no adverse events in either group. | |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 1 level due to risk of bias: included studies were not blinded, which affects both detection and performance bias. 2The initial analysis had greater statistical inconsistency (I2 = 70%) and included one study that included people with chronic prostatitis/chronic pelvic pain syndrome using criteria that differed from that recommended by the Research Consensus (Chen 2009). In a sensitivity analysis, we excluded the results from this study and found greater statistical consistency (I2 = 0%), therefore, we chose to report these results in the 'Summary of findings' table. For this reason, we did not downgrade due to inconsistency. 3Downgraded 1 level due to imprecision issues: few events and wide confidence interval. | |||||
| Circumcision plus usual care compared to waiting list plus usual care for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with waiting list for circumcision | Risk difference with early circumcision | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 713 | ⊕⊕⊕⊝ | ‐ | The mean prostatitis symptoms was 15 | MD 3.00 lower |
| Adverse events3 follow‐up: 12 weeks | 713 | ⊕⊕⊝⊝ | RR 1.23 | Study population | |
| 130 per 1000 | 30 more per 1000 | ||||
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 1 level due to high risk of bias: study not blinded (high risk of performance and detection bias). 2Confidence intervals were constructed using transformations described in the Cochrane Handbook for Systematic Reviews of Interventions Section 7.7.3.5. 3All adverse events were minor. 4Downgraded 1 level due to imprecision issues: few events in each group and wide confidence interval. | |||||
| Electromagnetic chair compared to control intervention for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with control intervention | Risk difference with electromagnetic chair | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 57 | ⊕⊝⊝⊝ | ‐ | 1 study found no differences in NIH‐CPSI score measurements at 6 weeks. The other study found a symptom score 16 points (0‐ to 90‐point scale) lower in the intervention group compared to the control group (P value not available) at 12 weeks. | |
| Adverse events follow‐up: 6‐12 weeks | 57 | ⊕⊕⊝⊝ | ‐ | 1 study reported a 0 incidence of adverse events and the other study reported 1 case of transient paraesthesia in the active treatment group. | |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 1 level due to risk of bias: one study not blinded and the other study had high attrition bias and selective outcome reporting bias. 2The two included studies had inconsistent results (see narrative description). 3Downgraded 1 level for imprecision issues: optimal information size not met (OIS for a 4‐point decrease, standard deviation 6, alpha 0.05, beta 0.20 = 74); small sample size in the individual studies (meta‐analysis was not possible). 4Downgraded 1 level due to imprecision issues: rare events and wide confidence interval. | |||||
| Lifestyle modifications compared to control for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with control | Risk difference with lifestyle modifications | ||||
| Prostatitis symptoms: response defined as 6‐point decrease in NIH‐CPSI score Benefit is indicated by lower scores | 100 | ⊕⊝⊝⊝ | RR 3.90 | Study population | |
| 200 per 1000 | 580 more per 1000 | ||||
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 2 levels due to high risk of selection bias (unconcealed allocation), detection and performance bias (study not blinded), missing outcome data and suspected selective outcome reporting (data presented graphically). 2Downgraded 1 level due to imprecision issues: few events and wide confidence interval. | |||||
| Physical activity compared to control intervention procedure for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with sham procedure | Risk difference with physical activity | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 85 | ⊕⊕⊝⊝ | ‐ | The mean prostatitis symptom score was 20 | MD 2.50 lower |
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Anxiety Benefit is indicated by lower scores | 85 | ⊕⊝⊝⊝ | ‐ | The mean anxiety score was 42.1 | MD 2.8 lower |
| Depression Benefit is indicated by lower scores | 85 | ⊕⊝⊝⊝ | ‐ | The mean depression score was 9.3 | MD 0.5 higher |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial; SAI‐Y: State Anxiety Inventory‐Y. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 2 levels: high risk of performance bias and detection bias (study not blinded); high risk of attrition bias at follow‐up. 2Downgraded 1 level due to imprecision issues: wide confidence intervals include both considerable benefits and harms. | |||||
| Prostatic massage compared to control for treating chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with control | Risk difference with prostatic massage | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 44 | ⊕⊝⊝⊝ | ‐ | The mean prostatitis symptom score was 12.4 | MD 1.10 lower |
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 2 levels due to high risk of performance and detection bias (study not blinded), unclear risk of bias in the remaining domains. 2Downgraded 1 level for imprecision issues: optimal information size (OIS) not met (OIS for a 4‐point decrease, standard deviation 6, alpha 0.05, beta 0.20 = 74). | |||||
| Extracorporeal shockwave therapy compared to control procedure for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with control | Risk difference with ESWT | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 157 | ⊕⊕⊕⊕ | ‐ | The mean prostatitis symptom score ranged from 16.8 to 26.81 | MD 6.18 lower |
| Prostatitis symptoms: response defined as a 6‐point decrease in NIH‐CPSI score | 135 | ⊕⊝⊝⊝ | RR 6.20 | Study population | |
| 149 per 1000 | 776 more per 1000 | ||||
| Prostatitis symptoms | 97 | ⊕⊕⊝⊝ | ‐ | The mean prostatitis symptom score ranged from 16.1 to 27 | MD 2.23 lower |
| Adverse events follow‐up: 24 weeks | 195 | ⊕⊕⊝⊝ | RR 1.22 | Study population | |
| 93 per 1000 | 20 more per 1000 | ||||
| Sexual dysfunction Benefit is indicated by higher scores | 60 | ⊕⊕⊕⊝ | ‐ | The mean sexual dysfunction was 16.83 | MD 3.34 higher |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ESWT: extracorporeal shockwave therapy; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Whereas one of the studies was not blinded, which could have posed a high risk of performance and detection bias, we did not downgrade for risk of bias due to the consistency with other studies with low risk of bias. 2Downgraded 1 level due to risk of bias: one of the studies that provided events for this outcome was not blinded. 3Downgraded 1 level due to inconsistency (I2 = 71%). 4Downgraded 1 level due to imprecision issues: few events and wide confidence interval. 5Downgraded 1 level due to inconsistency (I2 = 82%). 6Downgraded 1 level due to imprecision issues: wide confidence interval. | |||||
| Transrectal thermotherapy compared to medical treatment for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with medical treatment | Risk difference with transrectal thermotherapy | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 140 | ⊕⊕⊝⊝ | ‐ | The mean prostatitis symptom score ranged from 14.33 to 17.19 | MD 2.50 lower |
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 2 levels due to high risk of allocation concealment bias, performance and detection bias (study not blinded) and high risk of attrition bias. | |||||
| Transrectal thermotherapy (add‐on) compared to medical treatment alone for chronic prostatitis/chronic pelvic pain syndrome | |||||
| Patient or population: participants with chronic prostatitis/chronic pelvic pain syndrome | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with medical treatment alone | Risk difference with transrectal thermotherapy (add‐on) | ||||
| Prostatitis symptoms Benefit is indicated by lower scores | 145 | ⊕⊕⊝⊝ | ‐ | The mean prostatitis symptom score ranged from 14.33 to 17.19 | MD 4.34 lower |
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sexual dysfunction ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Depression and anxiety ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 2 levels due to high risk of allocation concealment bias, performance and detection bias (study not blinded) and high risk of attrition bias. | |||||
| Study ID | Intervention(s) (route, frequency, total dose/day) | Intervention(s) appropriate as applied in a clinical practice setting (description) | Intervention(s) duration | Comparator(s) (route, frequency, total dose/day) | Comparator(s) appropriate as applied in a clinical practice setting (description) | Comparator(s) duration |
| Breathing exercises using 'Karbonik' apparatus (hypercapnic hypoxia) 10‐20 min daily + medical therapy (see comparison). | No information regarding dose‐scaling or contraindications. | 10 days. | Levofloxacin 500 mg/day, tamsulosin 0.4 mg/day, Samprost daily suppository, Serenoa repens fructuum extract 1 capsule/day for 10 days, nimesulide 1‐2 tablets/day for 5‐7 days. | No information regarding dose‐scaling or contraindications. | 10 days. | |
| ESWT + medical therapy: each session had 12‐min duration and 3000 impulses with total energy flow 0.25 mJ/mm2 3 Hz, weekly in supine position without anaesthesia. | No information regarding dose‐scaling or contraindications. | 12 weeks. | Medical therapy with doxazosin 4 mg daily, ibuprofen 400 mg daily and tiocolchicoside 12 mg daily. | Ranitidine was allowed for gastric complaints. No information regarding dose‐scaling or contraindications. | 12 weeks. | |
| Acupuncture group: UB28 bladder meridian Stimulation using disposable acupuncture needles (Hua Long, 25 40 mm Sterile Acupuncture Needles, China) and electrical pulse generator (Agistim Duo, 4 4 mA rms max/ 99 Hz max, France). | No information regarding dose‐scaling. "Localized skin infections concerning the acupoints, bleeding diathesis and use of anticoagulation" were contraindications. | 7 weeks. | Medical therapy levofloxacin 500 mg daily and ibuprofen 200 mg. | Orally administrated twice daily for 7 weeks. No information regarding dose‐scaling or contraindications. | 7 weeks. | |
| Acupuncture, using 2 disposable stainless steel needles (0.3 mm diameter, 60 mm length, Suzhou, Jiangsu, China) that were inserted to a depth of maximum 2.5‐3 cm in 7 acupoints bilaterally: BL33 Zhongliao | Applied weekly for 6 weeks without other treatment modalities. No information regarding dose‐scaling or contraindications. | 6 weeks. | Punctures in sham group performed 1 cm left of each selected acupoint, with same type of needles, of the same duration and frequency. | Applied weekly for 6 weeks. No information regarding dose‐scaling or contraindications. | 6 weeks. | |
| Medical therapy (4 weeks of ciprofloxacin 500 mg bid, 3 months of ibuprofen 400 mg/day, 3 months of tamsulosin 0.4 mg/day) and scheduled for surgery during the same period in each of the sites by study clinicians; given written instructions about postoperative wound care. | No information regarding dose‐scaling or contraindications. | 3 months. | Same medications (4 weeks of ciprofloxacin 500 mg bid, 3 months of ibuprofen 400 mg/day, 3 months of tamsulosin 0.4 mg/day) and remain uncircumcised until end of 3‐month study participation, when they were scheduled to undergo circumcision. | No information regarding dose‐scaling or contraindications. | 3 months. | |
| Individually discussed risk factors detected at history by the completed questionnaire, informed that such risk factors were potential causes of their disease symptoms and it was strongly recommended to avoid them, distributed a vademecum with 13 rules relating to diet, sexual habits and lifestyle. | No information regarding dose‐scaling or contraindications. | 3 months. | Followed same diet, sexual behaviours and lifestyle as those of the previous months. | No information regarding dose‐scaling or contraindications. | 3 months. | |
| Sono‐electromagnetic therapy at home using Sonodyn Device: intensity 100 mW/cm2 with ultrasonic power of 12 mW and frequency 1.9 MHz, electric field force 0.3 V/m and magnetic field force 0.4 A/m; for 10 min, twice daily. | Participants could not see settings or perceive the device. No information regarding dose‐scaling or contraindications. | 12 weeks. | Similar placebo device. Participants applied same procedure, but device was not active during session. | Participants could not see settings or perceive device. No information regarding dose‐scaling or contraindications. | 12 weeks. | |
| Non‐intrusive ultrasound. Output frequency 1.79 MHz; output power: 3.15 W/cm2. Duration: 20 min, administered every 3 days (total of 7 times) + integrated Chinese‐Western medications. | No information regarding dose‐scaling or contraindications. | ‐ | Integrated Chinese‐Western medications alone; QianLieShuTong Capsule, orally 3 times daily, 3 capsules each time, tamsulosin delayed‐release capsule, 0.2 mg, orally 4 times daily. | No information regarding dose‐scaling or contraindications. | 1 month. | |
| ESWT (DUOLITH SD1, Storz Medical, Tägerwilen, Switzerland) once weekly for 4 weeks. Each time 3000 impulses, with 0.25 mJ/mm2 and 3 Hz of frequency were delivered, although 0.5 mJ/mm2 was added in each week (0.3 mJ/mm2 in week 2, 0.35 mJ/mm2 in week 3 and 0.4 mJ/mm2 in week 4). After each 500 pulses, probe position was corrected, using transperineal ultrasound. | No information regarding dose‐scaling or contraindications. | 4 weeks. | In sham group, same protocol applied but with probe being turned off. | No information regarding dose‐scaling or contraindications. | 4 weeks. | |
| 10 × 1‐hour sessions of myofascial physical therapy involved connective tissue manipulation of the abdominal wall, back, buttocks and thighs with connective tissue abnormalities in prone and supine position; double voiding and squatting (as home exercises); transrectal manipulation. | No information regarding dose‐scaling. "The presence of painful scars on lower abdominal wall that according to the health care personnel were unlikely to respond to physical therapy" was used as an exclusion criterion. | ‐ | 10 × 1‐hour sessions of global therapeutic Western massage, including effleurage, petrissage, friction, tapotement, vibration and kneading, in upper and lower limbs, trunk, buttocks, abdomen, head and neck each for prescribed time periods; participants not provided with home exercise programme. | No information regarding dose‐scaling or contraindications. | ‐ | |
| 20,000 ESWT (HB‐ESWT‐01, Haibin Medical Equipment Co. Ltd., China) impulses in 10 sessions, applied directly to perineal area in which pain was localised (from anus to scrotum); starting energy density was 0.06 mJ/mm2 and frequency of 2 Hz was used for all treatments. | Energy density gradually increased until it reached the maximum possible tolerable pain level reported by participant (recorded and used in all subsequent sessions). No information regarding contraindications. | 2 weeks. | Sham ESWT, which was conducted by setting the energy level to 0 (no shockwave energy transmission), under conditions identical to active treatment group. | No information regarding dose‐scaling or contraindications. | 2 weeks. | |
| 60‐min treatment with TRFH (ZRL‐II‐A cavity intervention treatment instrument provided by Shanghai Songhang Industry, Co. Ltd. (Shanghai, China), temperature 40‐43 °C) every day. | No information regarding dose‐scaling or contraindications. | 5 days. | Tamsulosin 0.2 mg once daily + clarithromycin 0.25 g bid. | No information regarding dose‐scaling or contraindications. | 6 weeks. | |
| TRFH + tamsulosin + clarithromycin (6 weeks). | ||||||
| Biofeedback group, participant displayed the electromyography of pelvic floor muscle and was instructed to contract and relax the anus according to instructions on display (20‐min sessions, 5 times weekly for 2 weeks). | No information regarding dose‐scaling or contraindications. All participants received interventions in comparison group. | 2 weeks. | All participants, including those in the "usual care group" instructed to avoid alcohol and spicy food, sitting too long and holding urine, catching a cold. Advised to be physically active and do exercise, have sex regularly, have warm sitz baths. | They were instructed to discontinue antibiotics, alpha‐blockers and other medications during the trial. No information regarding dose‐scaling or contraindications. | ‐ | |
| Electrical stimulation group, anal electrodes using intensity of 6˜23 mA, for 10˜20 s and relaxation for 10˜20 s, (20‐min sessions; 5 times each week for 2 weeks). | ||||||
| Biofeedback + electrical stimulation (factorial design). | ||||||
| Taijiquan (Tai Chi) exercises for 20‐40 min every day. All participants received herbal therapy (no further specifications available) for 1 month after. | No information regarding dose‐scaling or contraindications. | 1 month. | All participants received herbal therapy (no further specifications available) for 1 month after. | No information regarding dose‐scaling or contraindications. | 1 month. | |
| TENS daily for mean of 20 min in painful area, mean frequency 100 Hz, pulse width 100 μs and intensity 25 mA daily, 5 times weekly. | No information regarding dose‐scaling or contraindications. | 1 month. | Same as active treatment group but with machine turned off. | No information regarding dose‐scaling or contraindications. | 1 month. | |
| Advice of hot sitz baths and exercise and 12 × 20‐min sessions of electroacupuncture in 6 weeks: 6 acupoints at bilateral: BL32 zhongliao | No information regarding dose‐scaling or contraindications. | 6 weeks. | Advice and exercise and 12 sessions of sham electroacupuncture, which included the same number and type of needle, duration and frequency of sessions as for the advice and exercise treatment, but treatment was delivered superficially at non‐acupoints 15 mm to the lateral of each corresponding acupoint; points were not stimulated electrically, but sound of pulse generator was heard by the participants. | No information regarding dose‐scaling or contraindications. | 6 weeks. | |
| Acupuncture + warm needle moxibustion. Acupuncture at acupoints: BI18 GanYu For 5 s each; needles were removed afterwards; once daily for 1 month. | For those treated with moxibustion: needles were left afterwards and the tails of which were then covered with 2 cm moxa sticks. Moxa sticks were then ignited. Repeated moxibustion twice more for each acupoint. No information regarding dose‐scaling or contraindications. | 1 month. | Prostat tablet orally twice daily: pollen extract. Course of treatment; 1 month. | No information regarding dose‐scaling or contraindications. | 1 month. | |
| Acupuncture procedures same as acupuncture + warm needle moxibustion. Moxibustion not performed. Treated once daily. Course of treatment: 1 month. | ||||||
| Perineally applied ESWT weekly (3000 pulses each; maximum total energy flow density: 0.25 mJ/mm2; frequency: 3 Hz); position of transducer was changed after every 500 pulses to scan prostatic and pelvic floor region (Duolith SD1, Storz Medical, Tägerwilen, Switzerland); penetration depth 35‐65 mm. | No information regarding dose‐scaling or contraindications. | 4 weeks. | Placebo treatment performed with same therapy head, which was also fitted with a placebo stand‐off. Stand‐off contained shock wave‐absorbing material, layer of air and air‐filled microspheres. | No information regarding dose‐scaling or contraindications. | 4 weeks. | |
| Osteopathic theory of structural dysfunction using direct and indirect techniques of manipulation; including rectal manipulation of the prostate and coccyx; 45‐min sessions for 5 weeks. | No information regarding dose‐scaling or contraindications. | 5 weeks. | Sham exercise program including a warm‐up, stretching, limb, breathing and pelvic floor muscle exercises; 30 min sessions in 5 weeks. | No information regarding dose‐scaling or contraindications. | 5 weeks. | |
| 'Radiofrequency.' | (abstract only) | ‐ | "Placebo therapy." | (abstract only) | ‐ | |
| PTNS applied unilaterally with 26‐gauge stainless steel needles inserted 5 cm cephalad from medial malleolus and posterior to edge of tibia with a ground neutral electrode placed on same leg near arch of foot; both connected to a stimulator at 200 µs with pulse rate 20 Hz (Medtronic Key Point Net, Medtronic), for 30 min. | No information regarding dose‐scaling or contraindications. | 12 weeks. | Same electrode procedure for PTNS; however, stimulator not connected. | No information regarding dose‐scaling or contraindications. | 12 weeks. | |
| TRMT alone for 12 weeks; using a Uro‐DR Device (Somang Medical; Kangreung, Korea), at intrarectal temperature of 43 °C for 30 min, at a medium heating rate. | No information regarding dose‐scaling or contraindications. | 12 weeks. | Medical therapy: ciprofloxacin 500 mg bid and NSAIDs C: TRMT + medical therapy. | No information regarding dose‐scaling or contraindications. | 12 weeks. | |
| 4 acupoints prepared, then sterile, disposable stainless steel needles (Suzhou Huan‐Qiu Acupuncture Medical Supplies, Suzhou, China) placed perpendicularly in 30‐min sessions twice weekly in acupoints: CV1 Guan Yuan | No information regarding dose‐scaling or contraindications. | 10 weeks. | Sham acupuncture included same number, duration and frequency of sessions as acupuncture group at non‐acupoints (15 mm to left). | No information regarding dose‐scaling or contraindications. | 10 weeks. | |
| Antibiotics plus TENS; with high TENS over painful area, daily for mean of 20 min, mean frequency 100 Hz, pulse width 100 μs and intensity 25 mA, 5 times weekly. | Contraindications: loss of skin sensation at and around painful area, cardiac pace maker, previous exposure to TENS and other electroanalgesia. | 4 weeks. | Ofloxacin 300 mg 3 times daily + placebo tablets. | No information regarding dose‐scaling or contraindication. | 4 weeks. | |
| Aerobic exercise group, which included an 18‐week walking programme, 3 times weekly; with a warm up and cool‐down regimen, postural muscle and isometric strengthening exercises, 40 min of fast‐paced walking on 'in‐outdoor' track (achieving 70/80% of predicted maximum heart rate for their age). | Participants with a 'lack of interest,' 'lack of time' and 'lack of confidence' to engage physical activity excluded from study. No information regarding dose‐scaling. | 18 weeks. | Placebo/flexibility and motion exercise programme; with same period and with same frequency of aerobic exercise group, maintaining their heart rate under 100 beats per min. | Participants with a 'lack of interest,' 'lack of time' and 'lack of confidence' to engage physical activity were excluded from the study. No information regarding dose‐scaling. | 18 weeks. | |
| Extracorporeal magnetic innervation using Neoconrol system (Neotonus Inc., Marietta, GA, USA) that generates a magnetic field directed in the seat of chair and concentrated in region of pelvic muscles; 2 sessions weekly lasting 20 min each. | 1st 10 min used 10 Hz field, 2 min rest and then 10 min of 50 Hz field. No information regarding contraindications. | 6 weeks. | Medical therapy with terazosin 4 mg daily. | They started 2 mg daily for 1st 7 days, and continued to receive 4 mg daily for the following 5 weeks. No information regarding contraindications. | 6 weeks. | |
| Participants received antibiotics empirically and prostatic massage (3 times weekly for 4 weeks). Prostate massaged from above and lateral to gland, 6 times on each side, by gentle and firm pressure of finger directed downwards and inwards, followed by a few strokes in middle from above downwards. | No information regarding dose‐scaling or contraindications. | ‐ | Empirical antibiotic therapy alone without prostatic massage. | 'In the 44 participants with negative cultures, antibiotic selection included quinolones (28 participants), clindamycin/metronidazole (11 participants), ampicillin/ | ‐ | |
| Medical therapy with modified BiXieFenQing Drink in morning and evening, 200 mL each + prostate massage once weekly. | No information regarding dose‐scaling or contraindications. | ‐ | Medical therapy alone. | No information regarding dose‐scaling or contraindications. | ‐ | |
| Participant seated in a Neotonus Electromagnetic Chair (Neotonus Inc., Marietta, GA, USA), for 2 consecutive 15‐min periods; 2 sessions weekly. | 1st period 10 Hz, 2nd period 50 Hz. No information regarding contraindications. | 4 weeks. | Participants seated in chair, ventilation mechanism activated, but no active stimulation applied. | No information regarding dose‐scaling or contraindications. | 4 weeks. | |
| He‐Ne laser directed to prostate. Optic fibre was inserted from urethra and located at prostatic urethra; 10 mW output at 18 J each time. | 1 course of treatment: 10 doses of radiation (2 sessions weekly). Participants discontinued all other treatments, except for some of the participants, short‐term sulfa‐drugs were administered temporarily to prevent infection. | ‐ | 'Routine care' for prostatitis including antibiotics, pollen extract, Chinese medicine, physical therapy and lifestyle interventions. | Sulfamethoxazole tablets, 2 tablets, orally bid, 60 days of fluoroquinolones such as levofloxacin 0.2 g, bid, 14 days of pollen drugs such as Prostat; Chinese patent drugs such as salvianolic acid B and saponins of panax notoginseng mixture (SalB/PNS); hot water bath and advise on lifestyle. | ‐ | |
| Transurethral microwave thermotherapy through catheter connected to a Targis System estimated peak intraprostatic temperatures of 55 °C. | No information regarding dose‐scaling or contraindications. | ‐ | Transurethral microwave thermotherapy through catheter connected to a Targis System estimated peak intraprostatic temperatures of 70 °C. | No information regarding dose‐scaling or contraindications. | ‐ | |
| External radiofrequency hyperthermia. | Applied externally: 2 electrodes placed at hip and lower abdomen, 5˜7 cm away from skin, with pubic symphysis as centre (42.5˜43.5 °C), 1˜2 hours each time, course of treatment: 2˜3 times, interval: 1˜2 weeks. No information regarding dose‐scaling or contraindications. | 1˜2 weeks. | Same external radiofrequency hyperthermia + terazosin 2 mg. | Daily for 2 days and then dose was increased to 2 mg bid for 12 weeks. No information regarding contraindications for terazosin. | 12 weeks. | |
| TUNA using 465‐kHz radiofrequency energy and formal needle insertion technique as described by Issa 1996; treatment applied on 2 planes on both lateral lobes of prostate in all participants so target temperature of 50 °C at needle tip was achieved for ≥ 1 min; under spinal analgesia and antibiotic prophylaxis. | No information regarding dose‐scaling or contraindications. | ‐ | 3 sham urethroscopy performed so it was seemingly identical to TUNA in participant's opinion; under spinal analgesia and antibiotic prophylaxis. | No information regarding dose‐scaling or contraindications. | ‐ | |
| TRMT, 6 sessions over 2 weeks each. | No information regarding dose‐scaling or contraindications. | ‐ | Sham procedure. | No information regarding dose‐scaling or contraindications. | ‐ | |
| Transurethral microwave thermotherapy consisted of 1 × 1‐hour treatment with computer‐driven device that elevated prostate interstitial temperatures to 45‐60 °C, a range that does not cause significant necrosis of normal prostatic tissue. | No information regarding dose‐scaling or contraindications. | Single session. | Sham therapy consisted of 1 × 1‐hour session with same device using sham software. | No information regarding dose‐scaling or contraindications. | Single session. | |
| Seaprose S (Flaminase, Formenti) 30 mg 3 times daily in combination with local hyperthermia, total of 7 sessions on alternate days of 60‐min duration, reaching local temperature of 42.5‐43.5 °C. | No information regarding dose‐scaling. Contraindication to those with hypersensitivity to the drug. | ‐ | 7 sessions of local hyperthermia alone. | No information regarding dose‐scaling or contraindications. | ‐ | |
| I1: 1 session of transrectal hyperthermia weekly for 4 weeks. I2: 1 session of transrectal hyperthermia weekly for 6 weeks. I3: 2 sessions of transrectal hyperthermia weekly for 3 weeks. Prostathermer 99D system (Biodan Ltd, Rehovot, Israel), target temperature reached within 1st 10 min of treatment, microwaves at 915 MHz, thermosensors for monitoring rectal temperature, cooling system for anterior rectal wall, thermosensors for urethra temperatures, outpatient basis, required local anaesthesia, with 2% xylocaine jelly before insertion of the catheter. | ||||||
| TRMT, 4 × 1‐hour treatment over 2 or 3 weeks. Temperature raised to 43.8 °C with input of 40 W. | No information regarding dose‐scaling or contraindications. (abstract only) | 2˜3 weeks | Sham group with temperature < 37 °C. | No information regarding dose‐scaling or contraindications. (abstract only) | ‐ | |
| ‐ denotes not reported. bid: twice daily; C: comparator; ESWT: extracorporeal shockwave therapy; I: intervention; min: minute; NSAID: non‐steroidal anti‐inflammatory drug; PTNS: posterior tibial nerve stimulation; s: second; TENS: transcutaneous electrical nerve stimulation; TRFH: transrectal radiofrequency hyperthermia; TRMT: transrectal microwave thermotherapy; TUNA: transurethral needle ablation. | ||||||
| Study ID | Intervention(s) and comparator(s) | Randomised (n) | Analysed (n) | Duration of intervention and follow‐up | Description of participantsa | Baseline NIH‐CPSIb score | Trial period (year to year) | Country | Setting |
| I: hypercapnic hypoxia + medical therapy | 17 | 17 | D: 10 days F: 10 days | Age: mean 37 No specified previous treatment | N/A | N/A | Russia | Outpatient | |
| C: medical therapy | 20 | 20 | |||||||
| I: extracorporeal shockwave therapy + medical care | 30 | 30 | D: 12 weeks F: 24 weeks | Age: mean 39.4 No specified previous treatment | 29.3 | 2013‐2015 | Montenegro | Outpatient | |
| C: medical care | 30 | 30 | 31.06 | ||||||
| I: acupuncture | 28 | 28 | D: 7 weeks F: 10 weeks | Age: mean 33 No previous treatment | 20.36 | 2008‐2009 | Turkey | Outpatient | |
| C: medical care | 26 | 26 | 22.92 | ||||||
| I: acupuncture | 50 | 45 | D: 6 weeks F: 24 weeks | Age: 20‐50 All had received medical therapy | 26.5 | Not available | Turkey | Outpatient | |
| C: sham acupuncture | 50 | 46 | 27.0 | ||||||
| I: early circumcision | 384 | 358 | D: 3 months F: 3 months | Age: mean 33 No specified previous treatment | 21 | 2013‐2014 | China | Outpatient | |
| C: delayed circumcision | 390 | 355 | 21 | ||||||
| I: lifestyle interventions | 50 | 39 | D: 3 months F: 3 months | Age: mean 33‐34 No specified previous treatment | 22.1 | 2012‐2013 | Italy | Outpatient | |
| C: no intervention | 50 | 50 | 21.9 | ||||||
| I: sono‐electromagnetic therapy | 30 | 30 | D: 12 weeks F: 16 weeks | Age: mean 44‐49 All had received medical therapy | 25.1 | 2009‐2012 | Switzerland | Outpatient | |
| C: placebo device | 30 | 30 | 25.2 | ||||||
| I: ultrasound | 35 | 35 | D: 1 month F: 1 month | Age 18‐55 All had received medical therapy | 25.9 | 2013‐2014 | China | Outpatient | |
| C1: medical therapy | 35 | 35 | 26.17 | ||||||
| C2: ultrasound + medical therapy | 35 | 35 | 26.85 | ||||||
| I: extracorporeal shockwave | 20 | N/A | D: 4 weeks F: 12 weeks | Age: 35‐37 No specified previous treatment | 26.5 | 2011‐2012 | Iran | Outpatient | |
| C: sham procedure | 20 | 27.1 | |||||||
| I: extracorporeal shockwave therapy | 40 | 38 | D: 2 weeks F: 12 weeks | Age: mean 46‐49 No specified previous treatment | 30.5 | 2009‐2011 | China | Outpatient | |
| C: sham procedure | 40 | 37 | 29.3 | ||||||
| I: medical therapy | 30 | 30 | D: 6 weeks F: 6 weeks | Age: mean 35‐36 No specified previous treatment | 20.9/20.2 | 2008‐2009 | China | Outpatient | |
| C1: transrectal hyperthermia | 32 | 32 | 20/21.1 | ||||||
| C2: transrectal hyperthermia + medical therapy | 43 | 43 | 22.4/21.7 | ||||||
| I1: biofeedback | 20 | 20 | D: 2 weeks F: 6 weeks | Age: mean 30 All had received medical therapy | 26.92 | 2007 | China | Outpatient | |
| I2: electrical stimulation | 40 | 40 | 26.35 | ||||||
| I3: biofeedback + electrical stimulation | 40 | 40 | 25.30 | ||||||
| C: usual care | 40 | 40 | 25.82 | ||||||
| I: TaiJiQuan | 50 | N/A | D: 1 month F: 1 month | Age: 22‐50 No specified previous treatment | N/A | N/A | China | Outpatient | |
| C: no intervention | 46 | ||||||||
| I: TENS | 20 | 20 | D: 4 weeks F: 4 weeks | Age: 30 to 55 No specified previous treatment | N/A | N/A | Egypt | Outpatient | |
| C: placebo TENS | 20 | 20 | |||||||
| I1: electroacupuncture | 12 | 11 | D: 6 weeks F: 6 weeks | Age: 36‐40 No specified previous treatment | 26.9 | 2007 | Korea | Outpatient | |
| I2: sham procedure | 12 | 10 | 25.5 | ||||||
| C: exercise and advise alone | 12 | 12 | 28 | ||||||
| I1: acupuncture + moxibustion | 42 | 42 | D: 1 month F: 1 month | Age: 21‐52 No specified previous treatment | 22.56 | 2004‐2007 | China | Outpatient | |
| I2: acupuncture | 41 | 41 | 21.97 | ||||||
| C: medical treatment | 42 | 42 | 22.89 | ||||||
| I: extracorporeal shockwave therapy | 30 | 30 | D: 4 weeks F: 12 weeks | Age: 22‐61 No specified previous treatment | 25.07 | N/A | Austria | Outpatient | |
| C: sham procedure | 30 | 30 | 23.3 | ||||||
| I: myofascial trigger point therapy | 10 | 9 | D: 10 weeks F: 12 weeks | Age: mean 41‐45 All had received medical therapy | 33.5 | N/A | US | Outpatient | |
| C: Western massage | 11 | 10 | 25.8 | ||||||
| I: osteopathic therapy | 20 | 20 | D: 8 weeks F: 14 weeks | Age: mean 46‐48 No specified previous treatment | 22.85 | 2003‐2005 | Germany | Outpatient | |
| C: exercise programme | 15 | 13 | 22.95 | ||||||
| I: radiofrequency | N/A (abstract only) | South Korea | Outpatient | ||||||
| C: "placebo therapy" | |||||||||
| I: tibial nerve stimulation | 45 | 45 | D: 12 weeks F: 12 weeks | Age: mean 38‐39 No specified previous treatment | 23.6 | 2006‐2008 | Turkey | Outpatient | |
| C: sham procedure | 44 | 44 | 22.8 | ||||||
| I: medical therapy | 44 | 37 | D: 12 weeks F: 12 weeks | Age: mean 31‐38 No specified previous treatment | 26.27 | 2005‐2010 | South Korea | Outpatient | |
| C1: transrectal thermotherapy | 44 | 41 | 24.59 | ||||||
| C2: transrectal thermotherapy + medical therapy | 44 | 35 | 23.94 | ||||||
| I: acupuncture | 45 | 44 | D: 10 weeks F: 34 weeks | Age: 41‐43 No specified previous treatment | 24.8 | 2004‐2005 | Malaysia and US | Outpatient | |
| C: sham acupuncture | 45 | 45 | 25.2 | ||||||
| I: TENS | 8 | 8 | D: 4 weeks F: 4 weeks | Age: 24‐60 No specified previous treatment | N/A | N/A | Nigeria | Outpatient | |
| C1: analgesic | 8 | 8 | |||||||
| C2: control | 8 | 8 | |||||||
| I: exercise programme | 52 | 41 | D: 18 weeks F: 18 weeks | Age: mean 36‐38 All had received medical therapy | 23.46 | 2002‐2004 | Italy | Outpatient | |
| C: flexibility programme (control intervention) | 51 | 44 | 23.55 | ||||||
| I: extracorporeal magnetic innervation | 21 | 21 | D: 6 weeks F: 6 weeks | Age: mean 42‐49 No specified previous treatment | 21 | 2003‐2004 | South Korea | Outpatient | |
| C: medical therapy | 19 | 19 | 17 | ||||||
| I: medical therapy + prostatic massage | 25 | N/A | D: 4 weeks F: 4 weeks | Age: mean 35 No specified previous treatment | N/A | N/A | Egypt | Outpatient | |
| C: medical therapy | 19 | ||||||||
| I: medical therapy + prostatic massage | 40 | 40 | D: N/A F: after treatment | Age: 20‐46 No specified previous treatment | N/A | 2002‐2005 | China | Outpatient | |
| C: medical therapy | 32 | 32 | |||||||
| I: electromagnetic chair | 11 | 11 | D: 4 weeks F: 1 year | Age: 25‐67 All had received medical therapy | 38.8/100 | N/A | UK | Outpatient | |
| C: sham procedure | 10 | 7 | 39.3/100 | ||||||
| I: He‐Ne laser | 56 | 56 | D: 5 weeks F: "after treatment" | Age: mean 33‐34 No specified previous treatment | N/A | 2002‐2004 | China | Outpatient | |
| C: routine care | 56 | 56 | |||||||
| I: transrectal thermotherapy 55 °C | 42 | 21 | D: once F: 12 months | Age: mean 58‐62 All had received medical therapy | 11.5 | N/A | Chile, Switzerland, UK | Outpatient | |
| C: transrectal thermotherapy 70 °C | 18 | 10.9 | |||||||
| I: radiofrequency hyperthermia | 76 | 76 | D: 12 weeks F: 12 weeks | Age: mean 34.2 Not specified previous treatment | N/A | 1998‐2001 | China | Outpatient | |
| C: radiofrequency hyperthermia + terazosin | 90 | 88 | |||||||
| I: transurethral needle ablation | 25 | 25 | D: once F: 12 months | Age: mean 43‐50 All had received medical therapy | 37.3 | 1998‐2001 | Finland | Outpatient | |
| C: sham procedure | 8 | 8 | 33.6 | ||||||
| I: transrectal thermotherapy | 80 | N/A | D: 2 weeks F: 6 months | N/A (abstract only) | Russia | Outpatient | |||
| C: sham procedure | 20 | ||||||||
| I: transurethral thermotherapy | 10 | 10 | D: 1 session F: 3 months | Age: 45‐46 All had received medical therapy | N/A | N/A | Canada | Outpatient | |
| C: sham procedure | 10 | 10 | |||||||
| I: medical therapy plus local hyperthermia | 10 | N/A | D: 15 days F: 4 weeks | Age: mean 42.5 Not specified previous treatment | N/A | N/A | Italy | Outpatient | |
| C: local hyperthermia alone | 10 | ||||||||
| Transrectal hyperthermia in different regimens | 54 | 54 | D: 3 to 6 weeks F: 26 months | Age: 21‐45 All had received medical therapy | N/A | 1987‐1991 | Italy | Outpatient | |
| I: radiofrequency | 15 | N/A | D: 2‐3 weeks F: 3 months | N/A (abstract only) | UK | Outpatient | |||
| C: sham procedure | 15 | ||||||||
| C: comparator; D: duration of intervention; F: duration of follow‐up (might not represent the reported time points); I: intervention; N/A: not available; NIH‐CPSI: National Institutes of Health ‐ Chronic Prostatitis Symptom Index; TENS: transcutaneous electric nerve stimulation. aAge: measurement is in years. bNIH‐CPSI: scores were mean or median values. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐5.79 [‐7.32, ‐4.26] |
| 1.1 Acupuncture | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐5.71 [‐7.50, ‐3.91] |
| 1.2 Electroacupuncture | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐8.92, ‐3.08] |
| 2 Prostatitis symptoms: pain subscore Show forest plot | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐2.43 [‐3.21, ‐1.66] |
| 2.1 Acupuncture | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐2.90, ‐1.10] |
| 2.2 Electroacupuncture | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐3.6 [‐5.08, ‐2.12] |
| 3 Prostatitis symptoms: micturition subscore Show forest plot | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐1.45 [‐1.83, ‐1.06] |
| 3.1 Acupuncture | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐1.89, ‐1.08] |
| 3.2 Electroacupuncture | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐1.1 [‐2.26, 0.06] |
| 4 Prostatitis symptoms: quality of life subscore Show forest plot | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐3.09, ‐1.71] |
| 4.1 Acupuncture | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐2.62 [‐3.37, ‐1.86] |
| 4.2 Electroacupuncture | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐1.3 [‐1.00, 0.40] |
| 5 Prostatitis symptoms Show forest plot | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 2.49 [0.77, 8.02] |
| 5.1 Acupuncture | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.09, 2.24] |
| 5.2 Electroacupuncture | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [1.63, 15.31] |
| 6 Prostatitis symptoms (NIH‐CPSI total) ‐ medium term Show forest plot | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐7.36 [‐9.93, ‐4.79] |
| 7 Prostatitis symptoms: pain subscore ‐ medium term Show forest plot | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐3.25 [‐4.45, ‐2.05] |
| 8 Prostatitis symptoms: micturition subscore ‐ medium term Show forest plot | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐1.54, ‐0.50] |
| 9 Prostatitis symptoms: quality of life subscore ‐ medium term Show forest plot | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐3.07 [‐4.14, ‐2.00] |
| 10 Adverse events Show forest plot | 3 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.51, 3.46] |
| 10.1 Acupuncture | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.58, 4.62] |
| 10.2 Electroacupuncture | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.45] |
| 11 Sexual dysfunction Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐3.46, 2.46] |
| 12 Urinary symptoms Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐2.79 [‐4.77, ‐0.82] |
| 12.1 Acupuncture | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐4.98, ‐0.02] |
| 12.2 Electroacupuncture | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐6.56, ‐0.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 3 | 203 | Mean Difference (IV, Random, 95% CI) | ‐4.09 [‐6.87, ‐1.30] |
| 1.1 Acupuncture | 2 | 116 | Mean Difference (IV, Random, 95% CI) | ‐2.97 [‐9.36, 3.43] |
| 1.2 Electroacupuncture | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐8.50, ‐3.50] |
| 1.3 Acupuncture (Moxibustion) | 1 | 63 | Mean Difference (IV, Random, 95% CI) | ‐3.74 [‐7.41, ‐0.07] |
| 2 Prostatitis symptoms: pain subscore Show forest plot | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐4.05, ‐1.76] |
| 2.1 Acupuncture | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.76 [‐4.57, ‐0.95] |
| 2.2 Electroacupuncture | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐4.48, ‐1.52] |
| 3 Prostatitis symptoms: micturition subscore Show forest plot | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐1.96, ‐0.35] |
| 3.1 Acupuncture | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐2.03, 0.13] |
| 3.2 Electroacupuncture | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐1.4 [‐2.60, ‐0.20] |
| 4 Prostatitis symptoms: quality of life subscore Show forest plot | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐2.41, ‐0.41] |
| 4.1 Acupuncture | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐2.52, 0.16] |
| 4.2 Electroacupuncture | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐3.19, ‐0.21] |
| 5 Prostatitis symptoms Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [1.45, 8.80] |
| 5.1 Electroacupuncture | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [1.45, 8.80] |
| 6 Adverse events Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Acupuncture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Electroacupuncture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Urinary symptoms Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐2.7 [‐4.00, 0.60] |
| 7.1 Electroacupuncture | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐2.7 [‐4.00, 0.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐4.16 [‐7.16, ‐1.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms Show forest plot | 1 | 713 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐3.82, ‐2.18] |
| 2 Prostatitis symptoms: pain subscore Show forest plot | 1 | 713 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.33, ‐0.67] |
| 3 Prostatitis symptoms: micturition subscore Show forest plot | 1 | 713 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.22, ‐0.78] |
| 4 Prostatitis symptoms: quality of life subscore Show forest plot | 1 | 713 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐3.38, ‐2.62] |
| 5 Adverse events Show forest plot | 1 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.86, 1.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐7.75, 1.75] |
| 2 Prostatitis symptoms: pain subscore Show forest plot | 2 | 57 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐1.07, 0.19] |
| 3 Prostatitis symptoms: micturition subscore Show forest plot | 2 | 57 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.40, 0.84] |
| 4 Prostatitis symptoms: quality of life subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Adverse events Show forest plot | 2 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.10, 46.92] |
| 6 Urinary symptoms Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐4.13, 4.13] |
| 7 Prostatitis symptoms (NIH‐CPSI total) ‐ medium term Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Prostatitis symptoms: pain subscore ‐ medium term Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.9 [2.20, 6.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms Show forest plot | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐4.69, ‐0.31] |
| 2 Prostatitis symptoms: pain subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Prostatitis symptoms: micturition subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Prostatitis symptoms: quality of life subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Anxiety Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Depression Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms Show forest plot | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐5.63, 3.43] |
| 2 Prostatitis symptoms: pain subscore Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Prostatitis symptoms: micturition subscore Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Prostatitis symptoms: quality of life subscore Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 3 | 157 | Mean Difference (IV, Random, 95% CI) | ‐6.18 [‐7.46, ‐4.89] |
| 1.1 Compared to sham procedure | 2 | 97 | Mean Difference (IV, Random, 95% CI) | ‐6.14 [‐7.87, ‐4.41] |
| 1.2 Compared to no intervention | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐6.64 [‐10.17, ‐3.11] |
| 2 Prostatitis symptoms: pain subscore Show forest plot | 2 | 97 | Mean Difference (IV, Random, 95% CI) | ‐4.64 [‐5.38, ‐3.89] |
| 2.1 Compared to sham procedure | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐4.74 [‐5.54, ‐3.94] |
| 2.2 Compared to no intervention | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐3.83 [‐6.03, ‐1.63] |
| 3 Prostatitis symptoms: micturition subscore Show forest plot | 2 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [1.00, ‐0.07] |
| 3.1 Compared to sham procedure | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐1.79 [‐2.59, ‐0.99] |
| 3.2 Compared to no intervention | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.47, 0.67] |
| 4 Prostatitis symptoms: quality of life subscore Show forest plot | 2 | 97 | Mean Difference (IV, Random, 95% CI) | ‐1.84 [‐2.41, ‐1.27] |
| 4.1 Compared to sham procedure | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐2.35, ‐1.11] |
| 4.2 Compared to no intervention | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐2.46 [‐3.94, ‐0.98] |
| 5 Prostatitis symptoms Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 6.20 [0.48, 79.79] |
| 6 Prostatitis symptoms (total score) ‐ long term Show forest plot | 2 | 97 | Mean Difference (IV, Random, 95% CI) | ‐2.23 [‐5.98, 1.53] |
| 6.1 Compared to sham procedure | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.42, 0.24] |
| 6.2 Compared to no intervention | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐4.47 [‐7.60, ‐1.34] |
| 7 Prostatitis symptoms: pain subscore ‐ long term Show forest plot | 2 | 97 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐6.25, 2.09] |
| 7.1 Compared to sham procedure | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐1.26, 1.24] |
| 7.2 Compared to no intervention | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐4.27 [‐6.15, ‐2.39] |
| 8 Prostatitis symptoms: micturition subscore ‐ long term Show forest plot | 2 | 97 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.19, ‐0.10] |
| 8.1 Compared to sham procedure | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.50, 0.80] |
| 8.2 Compared to no intervention | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.35, ‐0.11] |
| 9 Prostatitis symptoms: quality of life subscore ‐ long term Show forest plot | 2 | 97 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐2.78, 0.85] |
| 9.1 Compared to sham procedure | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.98, 0.66] |
| 9.2 Compared to no intervention | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐2.03 [‐3.62, ‐0.44] |
| 10 Adverse events Show forest plot | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.59, 2.51] |
| 11 Sexual dysfunction Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.34 [2.68, 4.00] |
| 12 Urinary symptoms Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.50 [‐5.14, ‐3.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Compared to medical therapy | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐3.82, ‐1.18] |
| 1.2 Add‐on to medical therapy vs medical therapy alone | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐4.34 [‐5.65, ‐3.04] |
| 2 Prostatitis symptoms: pain subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Compared to medical therapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Add‐on to medical therapy vs medical therapy alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Prostatitis symptoms: micturition subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Compared to medical therapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Add‐on to medical therapy vs medical therapy alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Prostatitis symptoms: quality of life subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Compared to medical therapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Add‐on to medical therapy vs medical therapy alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Biofeedback + electrical stimulation vs usual care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Biofeedback vs usual care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Electrical stimulation vs usual care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Prostatitis symptoms: pain subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Biofeedback + electrical stimulation vs usual care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Biofeedback vs usual care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Electrical stimulation vs usual care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Prostatitis symptoms: micturition subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Biofeedback + electrical Stimulation vs usual care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Biofeedback vs usual care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Electrical stimulation vs usual care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Prostatitis symptoms: quality of life subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Biofeedback + electrical stimulation vs usual care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Biofeedback vs usual care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Electrical stimulation vs usual care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events Show forest plot | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐11.2 [‐12.92, ‐9.48] |
| 2 Prostatitis symptoms: pain subscore Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐5.70 [‐6.90, ‐4.50] |
| 3 Prostatitis symptoms: micturition subscore Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐3.77, ‐2.63] |
| 4 Prostatitis symptoms: quality of life subscore Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐4.6 [‐5.27, ‐3.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐6.45, 8.45] |
| 2 Prostatitis symptoms: pain subscore Show forest plot | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐5.04, 4.64] |
| 3 Prostatitis symptoms: micturition subscore Show forest plot | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [‐1.12, 3.32] |
| 4 Prostatitis symptoms: quality of life subscore Show forest plot | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.90, 3.10] |
| 5 Sexual dysfunction Show forest plot | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐9.24, 4.84] |
| 6 Quality of life ‐ physical Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Quality of life ‐ mental Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐9.25, 10.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Prostatitis symptoms: quality of life subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Urinary symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐6.75, 1.15] |
| 2 Prostatitis symptoms Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.91, 2.15] |
| 3 Prostatitis symptoms: pain subscore Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐3.44, 0.84] |
| 4 Prostatitis symptoms: micturition subscore Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.26, 1.26] |
| 5 Prostatitis symptoms: quality of life subscore Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐2.76, ‐0.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms: pain subscore Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Compared to sham procedure | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐15.25 [‐17.71, ‐12.79] |
| 1.2 Compared to no intervention | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐6.88 [‐8.13, ‐5.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 70 °C vs 55 °C | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐6.50, 4.30] |
| 1.2 Compared to control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Prostatitis symptoms: pain subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Prostatitis symptoms: micturition subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Prostatitis symptoms: quality of life subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Urinary symptoms Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 70 °C vs 55 °C | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐6.34, 2.14] |
| 5.2 Compared to control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [‐8.02, 12.62] |
| 2 Urinary symptoms Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐5.09, 5.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Ultrasound vs medical therapy | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 1.09 [0.16, 2.02] |
| 1.2 Ultrasound in combination with medical therapy vs medical therapy alone | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐6.67 [‐7.62, ‐5.72] |
| 2 Prostatitis symptoms: pain subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Ultrasound vs medical therapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Ultrasound in combination with medical therapy vs medical therapy alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Prostatitis symptoms: micturition subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Ultrasound vs medical therapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Ultrasound in combination with medical therapy vs medical therapy alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Prostatitis symptoms: quality of life subscore Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Ultrasound vs medical therapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Ultrasound in combination with medical therapy vs medical therapy alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Prostatitis symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Ultrasound vs medical therapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Ultrasound in combination with medical therapy vs medical therapy alone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prostatitis symptoms (NIH‐CPSI total) Show forest plot | 2 | 78 | Mean Difference (IV, Random, 95% CI) | ‐6.05 [‐7.87, ‐4.24] |
| 1.1 Acupuncture | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐6.11 [‐8.75, ‐3.47] |
| 1.2 Electroacupuncture | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐8.50, ‐3.50] |

