Degarelix for treating advanced hormone‐sensitive prostate cancer
Abstract
Background
Degarelix is a gonadotropin‐releasing hormone antagonist that leads to medical castration used to treat men with advanced or metastatic prostate cancer, or both. It is unclear how its effects compare to standard androgen suppression therapy.
Objectives
To assess the effects of degarelix compared with standard androgen suppression therapy for men with advanced hormone‐sensitive prostate cancer.
Search methods
We searched multiple databases (CENTRAL, MEDLINE, Embase, Scopus, Web of Science, LILACS until September 2020), trial registries (until October 2020), and conference proceedings (until December 2020). We identified other potentially eligible trials by reference checking, citation searching, and contacting study authors.
Selection criteria
We included randomized controlled trials comparing degarelix with standard androgen suppression therapy for men with advanced prostate cancer.
Data collection and analysis
Three review authors independently classified studies and abstracted data from the included studies. The primary outcomes were overall survival and serious adverse events. Secondary outcomes were quality of life, cancer‐specific survival, clinical progression, other adverse events, and biochemical progression. We used a random‐effects model for meta‐analyses and assessed the certainty of evidence for the main outcomes according to GRADE.
Main results
We included 11 studies with a follow‐up of between three and 14 months. We also identified five ongoing trials.
Primary outcomes
Data to evaluate overall survival were not available.
Degarelix may result in little to no difference in serious adverse events compared to standard androgen suppression therapy (risk ratio (RR) 0.80, 95% confidence interval (CI) 0.62 to 1.05; low‐certainty evidence; 2750 participants). Based on 114 serious adverse events in the standard androgen suppression group, this corresponds to 23 fewer serious adverse events per 1000 participants (43 fewer to 6 more). We downgraded the certainty of evidence for study limitations and imprecision.
Secondary outcomes
Degarelix likely results in little to no difference in quality of life assessed with a variety of validated questionnaires (standardized mean difference 0.06 higher, 95% CI 0.05 lower to 0.18 higher; moderate‐certainty evidence; 2887 participants), with higher scores reflecting better quality of life. We downgraded the certainty of evidence for study limitations.
Data to evaluate cancer‐specific survival were not available.
The effects of degarelix on cardiovascular events are very uncertain (RR 0.15, 95% CI 0.04 to 0.61; very low‐certainty evidence; 80 participants). We downgraded the certainty of evidence for study limitations, imprecision, and indirectness as this trial was conducted in a unique group of high‐risk participants with pre‐existing cardiovascular morbidities.
Degarelix likely results in an increase in injection site pain (RR 15.68, 95% CI 7.41 to 33.17; moderate‐certainty evidence; 2670 participants). Based on 30 participants per 1000 with injection site pain with standard androgen suppression therapy, this corresponds to 440 more injection site pains per 1000 participants (192 more to 965 more). We downgraded the certainty of evidence for study limitations.
We did not identify any relevant subgroup differences for different degarelix maintenance doses.
Authors' conclusions
We did not find trial evidence for overall survival or cancer‐specific survival comparing degarelix to standard androgen suppression, but serious adverse events and quality of life may be similar between groups. The effects of degarelix on cardiovascular events are very uncertain as the only eligible study had limitations, was small with few events, and was conducted in a high‐risk population. Degarelix likely results in an increase in injection site pain compared to standard androgen suppression therapy. Maximum follow‐up of included studies was 14 months, which is short. There is a need for methodologically better designed and executed studies with long‐term follow‐up evaluating men with metastatic prostate cancer.
PICO
Plain language summary
Degarelix for newly diagnosed advanced prostate cancer
Review question
How does degarelix, a newer drug that treats prostate cancer by lowering male sex hormone levels, compare to existing medications for newly diagnosed advanced prostate cancer?
Background
There is no cure if prostate cancer has spread outside of the prostate gland to lymph nodes or to the bones. In such a situation, hormonal therapy that lowers levels of the male sex hormone testosterone can slow down cancer growth. Testosterone levels are regulated by complicated mechanisms that involve a hormone known as gonadotropin‐releasing hormone (GnRH), which is present in men at different levels at different times of the day. It is understood that giving men with prostate cancer high levels of medications that increase GnRH levels first raises testosterone levels, and then drops them to very low levels. These medications are commonly used to treat men with prostate cancer that has spread outside the prostate. Degarelix is a newer drug known as a GnRH antagonist, which blocks receptors in the brain and thereby lowers testosterone levels immediately.
Study characteristics
We included randomized controlled trials (studies in which participants are assigned to one of two or more treatment groups using a random method) comparing degarelix and standard hormonal therapy in men with newly diagnosed advanced prostate cancer. The evidence is current to September 2020 for electronic databases, to October 2020 for trial registries, and to December 2020 for conference proceedings.
Key results
We found 11 studies that were eligible for inclusion in the review, but none of these studies evaluated the risk of dying from any cause or dying from prostate cancer. There may be no difference between degarelix and standard hormonal therapy in serious unwanted effects and quality of life. The effects of degarelix on cardiovascular issues such as the risk of a heart attack or stroke are uncertain; while one study suggested that the risk may be reduced with degarelix, it had major issues, in particular that it was conducted in men at high risk for such problems. We found that degarelix therapy likely results in an increase in the occurrence of pain at the injection site.
Certainty of the evidence
The certainty of evidence for the various outcomes ranged from moderate to very low. There is a need for additional, better designed studies to further understand the effects of degarelix for newly diagnosed advanced prostate cancer.
Authors' conclusions
Summary of findings
| Degarelix compared to standard androgen suppression therapy for treating advanced hormone‐sensitive prostate cancer | ||||||
| Patient or population: hormone‐sensitive prostate cancer | ||||||
| Outcomes | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) |
What happens | |
|---|---|---|---|---|---|---|
| Risk with standard androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy) | Risk difference with degarelix | |||||
| Overall survival | ‐ | ‐ | ‐ | ‐ | N/A | We do not know the effect of degarelix on overall survival. |
| Serious adverse events | 2750 | ⊕⊕⊝⊝ | RR 0.80 | Study population | Degarelix may have little to no effect on serious adverse events. | |
| 114 per 1000 | 23 fewer per 1000 | |||||
| Quality of life | 2887 | ⊕⊕⊕⊝ | ‐ | The mean quality of life was 0. | SMD 0.06 higher | Degarelix probably has little to no effect on quality of life. |
| Cancer‐specific survival | ‐ | ‐ | ‐ | ‐ | N/A | We do not know the effect of degarelix on cancer‐specific survival. |
| Cardiovascular events | 80 | ⊕⊕⊝⊝ | RR 0.15 | General population4 | The effect of degarelix on cardiovascular events is very uncertain. | |
| 300 per 1000 | 255 fewer per 1000 | |||||
| Injection site pain | 2670 | ⊕⊕⊕⊝ | RR 15.68 | Study population | Degarelix probably increases the occurrence of injection site pain. | |
| 30 per 1000 | 440 more per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level for study limitations (performance or detection bias, or both). | ||||||
Background
Description of the condition
Worldwide, prostate cancer is the second most common cancer in men, with 1.3 million newly diagnosed people in 2018 (GLOBOCAN 2018). This tumor type is associated with significant mortality, leading to an estimated 359,000 prostate cancer deaths in 2018, making it the fifth‐leading cause of death from cancer in men (GLOBOCAN 2018). Prostate cancer that is limited to the prostate gland, or that has spread locally outside it but not to more distant organs, is considered to be a potentially curable disease. However, prostate cancer that is disseminated to regional lymph nodes or that has metastasized to bones or to other areas is currently only amenable for palliative therapy such as androgen suppression therapy (EAU 2020).
The androgen testosterone is important for the growth and survival of the prostate as well as prostate cancer cells. This dependency forms the basis for systemic androgen deprivation therapy, which is the mainstay of treatment for metastatic prostate cancer (EAU 2020). Androgen suppression therapy inhibits or eliminates testicular testosterone production and decreases circulating testosterone in the blood to very low, so‐called castrate levels. The suppression of testosterone slows prostate cancer disease progression and leads to a decrease in prostate‐specific antigen (PSA).
There are different therapy options available to achieve androgen suppression.
Standard systemic androgen suppression therapy includes surgical or medical castration, an antiandrogen monotherapy, or a combination of both treatment options. While surgical castration (bilateral orchiectomy or subcapsular orchiectomy) removes the source of testicular androgen production, medical castration using gonadotropin‐releasing hormone (GnRH) agonists (e.g. leuprorelin, goserelin, buserelin, and triptorelin) induces castration by drug, administered as depot preparations subcutaneously or intramuscularly at defined intervals (e.g. four weeks, three months, or six months) (EAU 2020). GnRH agonists bind to the GnRH receptors on gonadotropin‐producing cells in the pituitary, causing an initial release of both luteinizing hormone (LH) and follicle stimulating hormone (FSH), which causes a subsequent temporary increase in testosterone production from testicular Leydig cells. In the long term, GnRH receptors are downregulated on the gonadotropin‐producing cells, resulting in a decline in pituitary production of LH and FSH and a reduction of serum testosterone to castration levels.
Surgical and medical castrations are recommended as standard initial treatment options for advanced stages of prostate cancer (EAU 2020).
Antiandrogens are administered orally or as depot preparations and work by blockade of the androgen receptor. A Cochrane Review has demonstrated the reduced effectiveness of this drug class when compared to systemic androgen deprivation therapy in the form of surgical or medical castration (Kunath 2014). While its use in combination with surgical or medical castration is not recommended due to increased side effects and costs at only marginal benefits, it is used as a first‐line form of secondary hormonal treatment for men who progress to systemic androgen therapy (EAU 2020).
Description of the intervention
Degarelix is a GnRH antagonist that competitively binds to receptors in the pituitary gland, leading to immediate castration (Damber 2012b). Degarelix is administered subcutaneously as a depot preparation with a starting dose of 240 mg, and 80 mg or 160 mg maintenance doses every four weeks thereafter or tri‐monthly 480 mg subcutaneous maintenance doses. The standard dosage is 240 mg in the first month, followed by monthly injections of 80 mg (EAU 2020). Abarelix is another GnRH antagonist which is not part of this review.
Adverse effects of the intervention
Surgical castration achieves fast androgen suppression. However, it might cause psychological distress, and some men consider it to be unacceptable because of its irreversibility (EAU 2020). For this reason, more attention has been paid to the medical use of androgen suppression therapies, especially with the evolvement of GnRH antagonists, GnRH agonists, and antiandrogens. However, these therapies have potential adverse events such as injection side effects, gynecomastia, breast pain, hot flushes, and cardiovascular events. A pooled analysis of individual participant data of five randomized controlled trials found differences regarding survival and PSA progression, as well as musculoskeletal and urinary tract events, favoring degarelix when compared to GnRH agonists (Klotz 2014). Furthermore, degarelix may also decrease the risk of death and the incidence of cardiovascular events in men with pre‐existing cardiovascular disease (Klotz 2014).
How the intervention might work
Androgens are necessary for the growth of prostate cancer cells. The secretion of the androgen testosterone is regulated by the hypothalamic‐pituitary‐gonadal axis. The hypothalamus secretes gonadotropin‐releasing hormone (GnRH; also known as luteinizing hormone‒releasing hormone (LHRH)), which stimulates the release of luteinizing hormone (LH) and follicle‐stimulating hormone (FSH) from the anterior pituitary gland. The distribution of LH stimulates the Leydig cells of the testes to secrete testosterone, which is then converted within the prostate cells by 5‐α‐reductase enzyme to dihydrotestosterone (Gibbs 1996). Dihydrotestosterone is important for the development, growth and differentiation of cells of the prostate gland, as well as prostate cancer. Androgen suppression therapy aims to reduce or prevent testosterone secretion, thereby slowing down disease progression (Huggins 2002). The suppression of testosterone also leads to a decrease of prostate‐specific antigen (PSA).
Surgical castration (bilateral orchiectomy or subcapsular orchiectomy) removes the source of testicular androgen production, leading to immediate castration.
GnRH agonists suppress androgen production through a negative feedback mechanism. The continuous exposure of GnRH from the hypothalamus leads to a desensitization of GnRH receptors in the anterior pituitary gland causing a downregulation of LH and testosterone production. The initial exposure of GnRH results in a surge of LH and testosterone levels (also known as flare phenomenon). This surge can induce an exacerbation of clinical symptoms, such as bone pain, ureteral obstruction, and spinal cord compression in men with advanced prostate cancer. The simultaneous short‐term administration of antiandrogens can prevent this testosterone surge. A combination of GnRH agonists with antiandrogens is known as maximal androgen suppression therapy.
Non‐steroidal antiandrogens (e.g. bicalutamide, flutamide, and nilutamide) or steroidal antiandrogens (e.g. cyproterone acetate) compete with testosterone and dihydrotestosterone at the receptor level in the prostate cell nucleus, leading to an androgen suppression.
GnRH antagonists bind competitively to GnRH receptors in the pituitary gland leading to an immediate reduction of LH and testosterone levels without provoking an LH or testosterone surge (Broqua 2002; Damber 2012b).
Why it is important to do this review
A former meta‐analysis on individual patient data including five randomized controlled trials suggested that degarelix is an alternative to standard androgen suppression therapies (Klotz 2014). The GnRH antagonist may have beneficial effects on lower urinary tract symptoms, testosterone suppression, and PSA progression compared to standard androgen suppression (Klotz 2014; Kunath 2015). However, the current European guideline on prostate cancer indicates surgical castration as the 'gold standard' for androgen suppression, and long‐acting GnRH agonists are currently the main forms of androgen suppression therapy (EAU 2020). The current American Urological Association guideline strongly recommends that clinicians should offer androgen suppression therapy with either GnRH agonists or antagonists or surgical castration in men with metastatic hormone‐sensitive prostate cancer (AUA 2020). However, the effect of degarelix compared to standard androgen suppression therapy remains unclear (EAU 2020). Since publication of the systematic review of Kunath 2015, further randomized controlled trials have been published. We therefore expect this review to yield meaningful new insights into the effects of this agent to inform clinical and health policy decision‐making.
Objectives
To assess the effects of degarelix compared with standard androgen suppression therapy for men with advanced hormone‐sensitive prostate cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐group randomized controlled trials comparing degarelix with standard androgen suppression therapy for men with advanced prostate cancer. There was no restriction on publication status or language of publication.
Types of participants
We initially planned to include men with advanced stages of prostate cancer who were not previously treated with androgen suppression therapy. We defined advanced prostate cancer as any of the following diagnoses.
-
Men with documented disease spread outside the prostate either to the lymph nodes or other organs (N+/M0 or M1a‐c) (TNM 2005).
-
Men with locally advanced disease who have not undergone surgery or radiation with no spread outside the prostate either to the lymph nodes or other organs (T3‐4/N0 or Nx/M0) (TNM 2005).
-
Men who have undergone local treatment with curative intent (such as local radiation therapy, radical surgery, or cryotherapy) with biochemical evidence of failure as documented by an elevated or rising PSA in the absence of spread outside the prostate either to the lymph nodes or other organs (T3‐4/N0 or Nx/M0) (TNM 2005).
We post hoc included men with localized disease (defined as prostate cancer within the prostate gland; T1‐2 N0 M0; TNM 2005; see Differences between protocol and review).
There were no restrictions on age or ethnicity of men.
Types of interventions
We included trials with the following comparisons of experimental versus comparator intervention.
Experimental intervention
Degarelix 240 mg subcutaneous (s.c.) given as a starting dose and 80 mg s.c. maintenance doses every four weeks thereafter (or the following maintenance doses: 160 mg s.c. monthly, 480 mg s.c. tri‐monthly).
Comparator interventions
Standard androgen suppression therapy included surgical or medical castration monotherapy, non‐steroidal or steroidal antiandrogen monotherapy, or maximal androgen blockade (combination therapy of surgical or medical castration with antiandrogens).
Bilateral surgical castration included total and subcapsular techniques.
Medical castration monotherapy was defined as an androgen suppression therapy using leuprorelin, goserelin, buserelin, or triptorelin. Antiandrogen therapy included non‐steroidal antiandrogens (e.g. bicalutamide, flutamide, and nilutamide) or steroidal antiandrogens (e.g. cyproterone acetate).
Androgen suppression therapies using estrogens or 5‐α‐reductase inhibitors or combination therapies of medical/surgical castration and newer androgen suppression therapies such as abiraterone, enzalutamide, darolutamide, or apalutamide were not part of this review.
Comparisons
Degarelix versus standard androgen suppression therapy.
Minimum duration of intervention
We included studies evaluating degarelix therapy with at least one administration.
Minimum duration of follow‐up
We included studies evaluating degarelix therapy with a minimum follow‐up of at least 30 days, because androgen suppression arises after this time in almost all men.
Types of outcome measures
Measurement of outcomes assessed in this review was not an eligibility criterion.
Primary outcomes
-
Overall survival
-
Serious adverse events
Secondary outcomes
-
Quality of life
-
Cancer‐specific survival
-
Clinical progression
-
Other adverse events
-
Biochemical progression
Method and timing of outcome measurement
-
Overall survival: defined as the time from randomization to the date of death.
-
Serious adverse events: defined as adverse events during the study requiring hospitalization or that were life‐threatening or fatal, or that were reported as serious adverse events by the authors of the original publication, measured at six months, one year, two years, or at the longest reported follow‐up.
-
Cancer‐specific survival: defined as the time from randomization to the date of cancer‐related death.
-
Clinical progression: defined as the date from randomization to disease progression, determined by the appearance of new—or an increase in existing—bone or extraskeletal metastases confirmed by imaging or physical examination.
-
Quality of life: assessed using validated generic and disease‐specific questionnaires, measured at baseline, six months, one year, two years, or at the longest reported follow‐up.
-
Other adverse events: injection site pain, cardiovascular events, total non‐serious adverse events, back pain, gynecomastia, constipation, diarrhea, vomiting, cardiac arrest, hypertension, myocardial infarction, libido decrease, erectile dysfunction, fatigue, hot flushes, anemia, hepatic enzyme increase, hepatic failure, dyspnea, gastritis, urinary tract infection, hematuria and urinary retention, defined as any new adverse events during the study (after the first dose of study medication until 30 days after the last dose), measured at six months, one year, two years, or at the longest reported follow‐up.
-
Biochemical progression: defined as the date from randomization to PSA progression; determined by an increase of more than 25% in the serum PSA concentration from the nadir value on two evaluations.
Post hoc analyses
We included the following outcomes post hoc; for details see Differences between protocol and review.
-
Mortality during study conduction, as a further adverse event outcome
-
Discontinuation due to adverse events
-
Total non‐serious adverse events
Main outcomes for summary of findings table
We have presented a summary of findings table reporting the following outcomes.
-
Overall survival
-
Serious adverse events
-
Quality of life
-
Cancer‐specific survival
-
Cardiovascular events
-
Injection site pain (see Differences between protocol and review)
Search methods for identification of studies
We performed a comprehensive systematic search with no restrictions on language of publication or publication status.
Electronic searches
We searched the following sources from inception of each database.
-
The Cochrane Central Register of Controlled Trials (CENTRAL; last searched 15 September 2020)
-
MEDLINE (via OvidSP; 1946 onwards to 15 September 2020)
-
Embase (initial search in March 2017 via Elsevier's Embase.com, update searches via OvidSP, 1947 onwards to 15 September 2020)
-
Web of Science (Clarivate Analytics; 1970 onwards to 15 September 2020)
-
Scopus (last update search on 15 September 2020)
-
LILACS (Latin American and Caribbean Health Science Information database; 1982 onwards to 15 September 2020)
Two review authors (FK, SS) developed the search strategy after input and feedback from the research team. The search strategy is adapted from the version of the previous published systematic review (Kunath 2015). We used controlled vocabulary, such as Medical Subject Headings (MeSH) and Emtree terms, in combination with keywords for the concepts of prostatic neoplasms, degarelix and androgen suppression therapies, including specific drug names. We made an effort to account for plurals, acronyms, and synonyms. For details on the search strategy, see Appendix 1.
We also searched the following trial registries (last searched 20 October 2020).
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/)
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/)
We used the following keywords for this search: ‘degarelix,’ ‘firmagon,’ 'FE200486,' 'FE 200486.' We checked every included study for a trial registry entry (see Characteristics of included studies tables).
Searching other resources
We identified other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials and reviews. We contacted the study authors of trials and representatives of the manufacturing company Ferring Pharmaceuticals for further studies and missing information. We included correspondence information in the Characteristics of included studies tables.
We searched the electronically available abstract books of the following conferences for unpublished studies.
-
American Society of Clinical Oncology (ASCO; jco.ascopubs.org/; 2004 until 2020; last searched 4 December 2020)
-
European Association of Urology (EAU; www.sciencedirect.com/journal/european-urology-supplements/issues; Annual EAU Congress; 2004 until 2020; last searched 4 December 2020)
-
American Urological Association (AUA; www.auajournals.org/; 2008 until 2020; last searched 4 December 2020)
We used the following keywords for this search: ‘degarelix,’ ‘firmagon,’ 'FE200486,' 'FE 200486.'
Data collection and analysis
Selection of studies
We used EndNote reference management software to collate references and remove potential duplicate records (EndNote 2019). Three review authors (JJJ, FK, FZ) independently screened the abstracts or titles (or both) of the remaining records for studies that were considered to be potentially eligible and assessed as full texts. The same three review authors assessed the full texts, mapped records to studies, and classified studies as included studies, excluded studies, or ongoing studies in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Any discrepancies were resolved through consensus or recourse to a fourth review author (CS or SS). We documented the reasons for exclusion of studies in a Characteristics of excluded studies table. A PRISMA flow diagram illustrating the process of study selection is shown in Figure 1 (Liberati 2009).
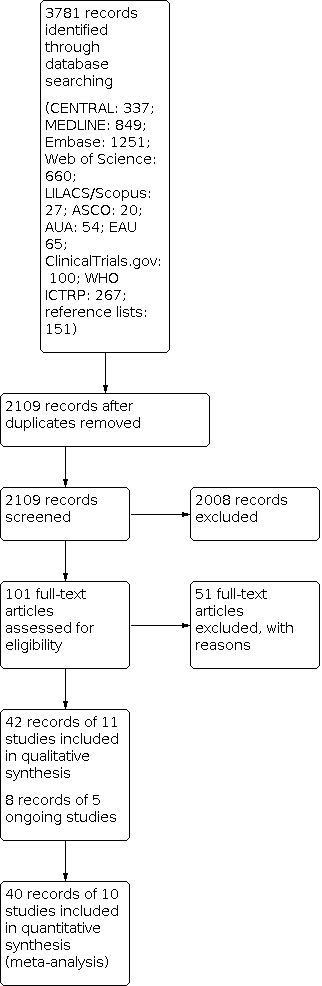
Study flow diagram.
Data extraction and management
We used a data abstraction form that had been pilot tested (Kunath 2015).
Three review authors (JJJ, FK, FZ) independently abstracted the following information from the included studies, which is presented in the Characteristics of included studies table.
-
Study design
-
Study dates
-
Study settings and country
-
Participant inclusion and exclusion criteria
-
Participant details, such as baseline demographics and disease characteristics
-
Number of participants by study and by study arm
-
Details of relevant experimental and comparator interventions such as dose, route, frequency, and duration
-
Definitions of relevant outcomes, method and timing of outcome measurement, as well as any relevant subgroups
-
Study funding sources
-
Declarations of interest by primary investigators
We extracted outcome data relevant to this review as needed for calculation of summary statistics and measures of variance. We did not assess time‐to‐event outcomes because no studies reported the respective endpoints. For dichotomous outcomes, we used numbers of events and totals for population of a 2 × 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we used means and standard deviations or data necessary to calculate this information. Any disagreements were resolved by discussion or by consultation with a fourth review author (AB) if required.
We contacted authors of the included studies to obtain key missing data as needed; we included information on any correspondence in the Characteristics of included studies tables.
Information regarding any potentially relevant ongoing studies, including trial identifier, is provided in the Characteristics of ongoing studies table.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary study, we maximized yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Three review authors (JJJ, FK, FZ) independently assessed the risk of bias of each included study. Any disagreements were resolved by consensus or by consultation with a fourth review author (SS, JJM, or CS) if required.
We assessed risk of bias using Cochrane's risk of bias tool for randomized controlled trials (Higgins 2017). We assessed the following risk of bias domains.
-
Random sequence generation (selection bias)
-
Allocation concealment (selection bias)
-
Blinding of participants and personnel (performance bias)
-
Blinding of outcome assessment (detection bias)
-
Incomplete outcome data (attrition bias)
-
Selective reporting (reporting bias)
-
Other sources of bias
We judged risk of bias domains as 'low risk,' 'high risk,' or 'unclear risk' as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), and present a risk of bias summary figure to illustrate these findings.
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment), we evaluated risk of bias separately for each outcome and grouped outcomes according to whether they were measured subjectively or objectively, as described in Blinding (performance bias and detection bias).
We assessed attrition bias (incomplete outcome data) on an outcome‐specific basis, and grouped outcomes with judgments when reporting our findings in the Characteristics of included studies tables.
We further summarized the risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome.
We defined the following endpoints as subjective outcomes as determined by their susceptibility to detection bias and the importance of blinding of outcome assessors.
-
Serious adverse events
-
Cancer‐specific survival
-
Clinical progression
-
Quality of life
-
Other adverse events
-
Biochemical progression
We defined the following endpoint as an objective outcome.
-
Overall survival
Concomitant interventions had to be the same in the experimental and comparator groups to establish valid comparisons.
Measures of treatment effect
We did not assess data for time‐to‐event outcomes.
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs).
We expressed quality of life data (continuous data) as standardized mean difference (SMD) with 95% CIs. Before standardization, we multiplied the mean values from Crawford 2013 (CS37) by −1 to correct for differences in the direction of the scale.
Unit of analysis issues
The unit of analysis is the individual participant. In the case of trials with more than two intervention groups, we handled these in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Dealing with missing data
We obtained missing data from study authors and included information regarding any correspondence with study authors in the Characteristics of included studies tables. We investigated attrition rates (e.g. dropouts, losses to follow‐up, and withdrawals) and critically appraised issues regarding missing data. We did not impute missing data.
Assessment of heterogeneity
We identified heterogeneity (inconsistency) through visual inspection of the forest plots to assess the amount of overlap of CIs, and the I² statistic, which quantifies heterogeneity across studies (Higgins 2002; Higgins 2003). We interpreted I² as follows:
-
0% to 40%: may not be important;
-
30% to 60%: may indicate moderate heterogeneity;
-
50% to 90%: may indicate substantial heterogeneity;
-
75% to 100%: considerable heterogeneity.
When we found heterogeneity, we attempted to determine possible reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We obtained study protocols to assess for selective outcome reporting. We included fewer than 10 studies investigating any given outcome, and therefore did not use funnel plots to assess small‐study effects.
Data synthesis
We summarized data using a random‐effects model. We interpreted random‐effects meta‐analyses with consideration of the whole distribution of effects. In addition, we performed statistical analyses according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We used the Mantel‐Haenszel method for dichotomous outcomes, and the inverse variance method for continuous outcomes. We did not assess time‐to‐event outcomes. We used Review Manager 5 software to perform analyses (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analysis.
-
Degarelix 240 mg s.c. given as a starting dose and 80 mg s.c. maintenance doses every four weeks thereafter versus degarelix 240 mg s.c. given as a starting dose and 160 mg s.c. maintenance doses every four weeks thereafter versus degarelix 240 mg s.c. given as a starting dose and tri‐monthly 480 mg maintenance doses s.c.
We were not able to perform the following subgroup analyses due to lack of data for the predefined subgroups.
-
Different standard androgen suppression therapies (surgical castration versus medical castration versus antiandrogen monotherapy versus combination of medical castration and antiandrogen therapy).
-
Different stages of advanced hormone‐sensitive prostate cancer (non‐metastatic versus metastatic disease).
Sensitivity analysis
We performed sensitivity analyses to explore the influence of the following factors on effect sizes.
-
Restricting the analysis by taking into account risk of bias, by excluding studies at 'high risk' or 'unclear risk' (one of the criteria 'high risk' or two of the criteria 'unclear risk').
Summary of findings and assessment of the certainty of the evidence
We assessed the overall certainty of the evidence for each outcome according to the GRADE approach, which takes into account five criteria not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results (Guyatt 2008). For each comparison, two review authors (JJJ, FZ/FK) independently rated the certainty of evidence for each outcome as 'high,' 'moderate,' 'low,' or 'very low' using GRADEpro GDT (GRADEpro GDT), with any discrepancies resolved by consensus or through arbitration by a third review author (AB or CS) if required. We have presented a summary of the evidence for the main outcomes in a summary of findings table, which provides key information about the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011; Schünemann 2019a).
Interpreting results and drawing conclusions
We used the recommendations of Schünemann 2019b for drawing and phrasing conclusions according to the individual GRADE domains.
Results
Description of studies
We included 11 randomized controlled trials (for details see Characteristics of included studies; Table 1). We additionally identified five ongoing studies (for details see Characteristics of ongoing studies).
| Study name | Intervention(s) and comparators (s) | Follow‐up | Number of participants | Study dates | Stage of disease |
|---|---|---|---|---|---|
| Degarelix 240/80 mg1 | 12 weeks | 27 | 2009 to 2010 | Localized/locally advanced: 9 (22.5%) Metastatic: 14 (35%) Unclear: 17 (42.5%) | |
| GnRH agonist with flare protection (goserelin 3.6 mg s.c. every 28 days with bicalutamide 50 mg orally per day for 14 days) | 13 | ||||
| Degarelix 240/80 mg1 | 12 weeks | 82 | 2009 to 2011 | Localized: 56 (31%) Advanced: 106 (59%) Unclear: 17 (9%) | |
| GnRH agonist with flare protection (goserelin 3.6 mg s.c. every 28 days with bicalutamide 50 mg orally per day for 28 days) | 97 | ||||
| Degarelix 240/80 mg2 (intermittent; data not included) | 14 months | 175 | 2009 to 2012 | Unclear (not reported) | |
| Degarelix 240/80 mg1 | 50 | ||||
| GnRH agonist with flare protection (leuprolide 7.5 mg i.m. monthly, maintenance dose 22.5 mg i.m. 3‐monthly with bicalutamide 50 mg orally per day for 28 days on Investigator's discretion) | 178 | ||||
| Degarelix 240/160 mg (degarelix starting dose of 240 mg s.c. with maintenance doses of 80 mg s.c. every 28 days) | 364 days | 202 | 2006 to 2007 | Localized: 191 (31%) Locally advanced: 178 (29%) Metastatic: 125 (20%) Not classifiable: 116 (19%) | |
| Degarelix 240/80 mg1 | 207 | ||||
| GnRH agonist (leuprolide 7.5 mg i.m. monthly) | 201 | ||||
| Degarelix 240/80 mg s.c.1 | 12 months | 41 | 2015 to 2019 | Localized: 59 (74%) Metastatic: 21 (26%) | |
| GnRH agonist 3‐monthly (at the discretion of the treating urologist/oncologist) | 28 | ||||
| Degarelix 240/80 mg1 | 12 weeks | 180 | 2009 to 2011 | Localized: 152 (62%) Advanced: 83 (34%) Unclear: 9 (4%) | |
| GnRH agonist with flare protection (goserelin 3.6 mg s.c. every 28 days with bicalutamide 50 mg orally per day for 14 days) | 64 | ||||
| Degarelix 240/480 mg (starting dose of 240 mg s.c. with maintenance doses of 480 mg s.c. every 84 days) | 12 months | 117 | 2013 to 2016 | Localized: 124 (53%) Locally advanced: 63 (27%) Metastatic: 44 (19%) Unclear: 3 (1%) | |
| GnRH agonist (goserelin 3.6 mg s.c. with maintenance dose 10.8 mg s.c. every 84 days) | 117 | ||||
| Degarelix 240/80 mg1 | 6 months | 50 | 2016 to 2018 | Localized: 76 (76%) Locally advanced and/or metastatic: 24 (24%) | |
| GnRH agonist (leuprolide 3.75 mg every 28 days) | 50 | ||||
| Degarelix 240/80 mg1 | 12 weeks | 13 | 2012 to 2015 | Localized: 10 (26%) Locally advanced: 15 (60%) Node positive: 6 (24%) PSA failure (> 0.2 ng/mL) or use of adjuvant androgen suppression/radiotherapy: 8 (21%)3 | |
| Degarelix 240/80 mg s.c. 2‐monthly + bicalutamide 50 mg orally per day (data not included) | 14 | ||||
| GnRH agonist + bicalutamide (leuprorelin 22.5 mg, leuprolide 22.5 mg, or goserelin acetate 10.8 mg 3‐monthly and bicalutamide 50 mg orally per day) | 12 | ||||
| Degarelix 240/480 mg (starting dose of 240 mg s.c. with maintenance doses of 480 mg s.c. every 3 months) | 13 months | 565 | 2009 to 2011 | Unclear (not reported) | |
| GnRH agonist (goserelin 3.6 mg s.c. with maintenance doses of 10.8 mg s.c. 3‐monthly) | 283 | ||||
| Degarelix 240/80 mg1 | 364 days | 143 | 2013 to 2015 | Unclear (not reported) | |
| GnRH agonist (goserelin 3.6 mg s.c. monthly) | 142 |
Abbreviations: GnRH: gonadotropin‐releasing hormone; i.m.: intramuscular; PSA: prostate‐specific antigen; s.c.: subcutaneous
1Degarelix starting dose of 240 mg s.c. with maintenance doses of 80 mg s.c. every 28 days.
2Degarelix starting dose of 240 mg s.c. with maintenance doses of 80 mg s.c. every 28 days. Six maintenance doses of degarelix 80 mg per month at Days 28 to 168 were administered. If a participant had PSA ≥ 2 ng/mL at any visit, additional doses of degarelix 240 mg followed by 80 mg maintenance dose(s) were administered. Degarelix treatment provided for first seven months (one starting dose and six maintenance doses) followed by no treatment for next seven‐month period.
3Multiple entries possible.
Results of the search
We identified 3781 records through electronic database searching. After removal of duplicates, we screened the titles and abstracts of 2109 records, and excluded 2008 records. We reviewed 101 full‐text articles and excluded 51 with reasons (see Characteristics of excluded studies). We included 50 records of 16 studies: 42 records of 11 included studies and 8 records of 5 ongoing studies (see Characteristics of included studies; Characteristics of ongoing studies). The flow of literature through the assessment process is shown in the PRISMA flow chart (Figure 1).
Included studies
Source of data
All trials were identified through the literature search. We identified multiple abstracts and conference proceedings for most of the included trials.
Study design and settings
We included 11 parallel‐group randomized controlled trials (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sawazaki 2019; Sayyid 2017 (DEG_PRE‐OP); Shore 2012 (CS35); Xie 2016 (PANDA)). None of the included trials had a cross‐over design. The included studies were reported as 'open‐label' with no blinding of participants or personnel, and were multicenter studies that included outpatients. Countries contributing to the enrollment of study participants are summarized in the Characteristics of included studies tables.
Study duration with outcome assessment was less than 14 months in all trials, as follows: 3 months: Anderson 2013 (CS28); Axcrona 2012 (CS31); Mason 2013 (CS30); Sayyid 2017 (DEG_PRE‐OP); 6 months: Sawazaki 2019; 12 months: Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35); Xie 2016 (PANDA); and 14 months: Crawford 2013 (CS37).
We found five ongoing studies (000108 (PRONOUNCE); JPRN‐UMIN000014243; NCT01542021; NCT02799706; NCT04182594). For details, see Characteristics of ongoing studies.
Participants
We included a total of 2777 randomized participants: 1629 participants received degarelix, and 1148 received standard androgen suppression therapy. All studies included men aged over 18 years. In the Anderson 2013 (CS28) trial, the percentage of participants with locally advanced or metastatic prostate cancer was less than 80%. All of the other included trials involved mainly men with localized prostate cancer (percentage of participants with advanced prostate cancer: Axcrona 2012 (CS31) 59%, Klotz 2008 (CS21) 50%, Margel 2019 (0102‐15‐RMC) 26%, Mason 2013 (CS30) 35%, Ozono 2018 (3550‐CL‐0010) 46%, Sawazaki 2019 24%, Sayyid 2017 (DEG_PRE‐OP) 24%). Three trials did not report the stage of disease of the included participants (Crawford 2013 (CS37); Shore 2012 (CS35); Xie 2016 (PANDA)). Margel 2019 (0102‐15‐RMC) included participants with pre‐existing cardiovascular morbidity.
Interventions and comparators
Degarelix was administered as a subcutaneous (s.c.) starting dose of 240 mg (two 120 mg s.c. injections), followed by monthly maintenance doses of 80 mg, in the following trials: Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Mason 2013 (CS30); Sawazaki 2019; Sayyid 2017 (DEG_PRE‐OP); Xie 2016 (PANDA). In the Ozono 2018 (3550‐CL‐0010) trial, participants received an initial degarelix dose of 240 mg s.c. followed by maintenance doses of 480 mg s.c. every 84 days. Klotz 2008 (CS21) had an additional treatment arm with starting dose of 240 mg s.c., followed by a monthly intensified maintenance dose of 160 mg s.c. Participants in Shore 2012 (CS35) received starting degarelix dose of 240 mg s.c. followed by a maintenance dose of 480 mg s.c. after one month with further administrations after 4, 7, and 10 months. In Crawford 2013 (CS37), degarelix was administered continuously (group 1) or intermittently (group 2); only participants treated continuously were included in the review. Sayyid 2017 (DEG_PRE‐OP) had an additional treatment arm with starting dose of 240 mg s.c. followed by two monthly maintenance doses of 80 mg each combined with the non‐steroidal antiandrogen bicalutamide once daily 50 mg. We did not include this treatment arm in our analyses.
Standard androgen suppression therapy was performed using: goserelin 3.6 mg s.c. with maintenance therapy using goserelin 10.8 mg s.c. every 84 days (Ozono 2018 (3550‐CL‐0010), Shore 2012 (CS35)); goserelin 3.6 mg s.c. every 28 days (Anderson 2013 (CS28); Axcrona 2012 (CS31); Mason 2013 (CS30); Xie 2016 (PANDA)); leuprolide 7.5 mg intramuscular (i.m.) every 28 days (Klotz 2008 (CS21)); leuprolide 7.5 mg i.m. with maintenance therapy using leuprolide 22.5 mg i.m. every 3 months (Crawford 2013 (CS37)); leuprorelin 22.5 mg every 3 months or goserelin 10.8 mg every 3 months (Sayyid 2017 (DEG_PRE‐OP)); and leuprolide 3.75 mg every 28 days (Sawazaki 2019).
The following studies combined gonadotropin‐releasing hormone (GnRH) agonist therapy with bicalutamide 50 mg orally for flare protection: Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Mason 2013 (CS30); Sawazaki 2019. One study used maximum androgen suppression therapy (Sayyid 2017 (DEG_PRE‐OP)). One trial did not further specify androgen suppression therapy and stated that men were treated using a GnRH agonist at the discretion of the treating urologist/oncologist (Margel 2019 (0102‐15‐RMC)). We identified no trials comparing degarelix with surgical castration or antiandrogen monotherapy.
Outcomes
We did not find data for overall survival, cancer‐specific survival, or clinical progression. One study reported survival data, but this outcome was not prespecified in the protocol, was post hoc analyzed, and follow‐up of study was 12 months (Klotz 2008 (CS21)). We considered these data as a further adverse event outcome and referred to it as 'mortality during study conduction' (see analysis of adverse events: Analysis 1.20; Types of outcome measures; Differences between protocol and review).
The co‐primary outcome 'serious adverse events' was reported in the following trials: Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35); Xie 2016 (PANDA). Sayyid 2017 (DEG_PRE‐OP) reported treatment‐emergent adverse events; however, it was unclear which of the reported adverse events met the definition of serious adverse events according to our predefined definition, therefore we did not include the results of this trial in the review.
Two trials reported data for biochemical progression (Klotz 2008 (CS21); Xie 2016 (PANDA)).
The following trials evaluated adverse event outcomes: Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sayyid 2017 (DEG_PRE‐OP); Shore 2012 (CS35); Xie 2016 (PANDA).
We included the quality of life assessment of three studies (Crawford 2013 (CS37); Klotz 2008 (CS21); Shore 2012 (CS35)), using data from the following scales: EORTC QLQ‐C30 mapped to EORTC‐8D (Klotz 2008 (CS21)), 36‐item Short Form Health Survey (SF‐36; Shore 2012 (CS35)), and Functional Assessment of Cancer Therapy‐Prostate (FACT‐P) (Crawford 2013 (CS37)). Further studies evaluated quality of life, but we did not include their assessments as data were not relevant to this review (scale used: Anderson 2013 (CS28); Mason 2013 (CS30): International Prostate Symptom Score (IPSS); Axcrona 2012 (CS31): Benign Prostatic Hyperplasia Impact Index (BII)).
We did not include outcomes from Sawazaki 2019 because none of the reported outcomes were relevant to this review.
Funding
All studies reporting outcomes relevant to this review were sponsored by Ferring Pharmaceuticals or Astellas Pharma Inc. Conflicts of interest with pharmaceutical companies were reported in all studies. For details, see Characteristics of included studies table.
Excluded studies
We excluded 51 records after full‐text evaluation. Reasons for exclusion are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
See Figure 2; Figure 3 for details of risk of bias assessment, and Characteristics of included studies for judgments of the individual risk of bias domains.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Five studies reported an adequate method of sequence generation and were rated as at low risk of bias (Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Ozono 2018 (3550‐CL‐0010); Sayyid 2017 (DEG_PRE‐OP); Xie 2016 (PANDA)). Random sequence generation was unclear in six studies (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Mason 2013 (CS30); Sawazaki 2019; Shore 2012 (CS35)).
Allocation concealment
Three studies reported an adequate method of allocation concealment and were rated as at low risk of bias (Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Sayyid 2017 (DEG_PRE‐OP)). Allocation concealment was unclear in eight studies (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sawazaki 2019; Shore 2012 (CS35); Xie 2016 (PANDA)).
Blinding
Blinding of participants and personnel
-
Overall survival: no data were available.
-
Serious adverse events, biochemical progression, other adverse events, quality of life: all included trials were open‐label studies without blinding of participants and personnel, leading to high risk of bias.
Blinding of outcome assessment
-
Overall survival: no data were available.
-
Serious adverse events, biochemical progression, other adverse events, quality of life: two studies blinded outcome assessment, resulting in a judgment of low risk of bias (Margel 2019 (0102‐15‐RMC); Sayyid 2017 (DEG_PRE‐OP)). All other trials reported insufficient information to permit judgment.
Incomplete outcome data
We grouped outcomes with similar susceptibility to attrition bias given the reporting characteristics of the studies, as follows.
Oncological outcomes
-
Overall survival, cancer‐specific survival, clinical progression: no data were available.
-
Biochemical progression: two studies reported data for this outcome with no missing outcome data, resulting in a judgment of low risk of bias (Klotz 2008 (CS21); Xie 2016 (PANDA)). The remaining studies did not address this outcome, leading to unclear risk of bias.
Adverse events
We judged the risk of attrition bias as low for 10 included trials (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sayyid 2017 (DEG_PRE‐OP); Shore 2012 (CS35); Xie 2016 (PANDA)). The remaining included trial did not address this outcome (Sawazaki 2019).
Quality of life
We judged the risk of attrition bias as low for the three studies which provided quality of life data included in this review (Crawford 2013 (CS37); Klotz 2008 (CS21); Shore 2012 (CS35)). A further three studies reported quality of life data using scales not relevant to this review; we did not include these data in the review, leading to unclear risk of bias (Anderson 2013 (CS28); Axcrona 2012 (CS31); Mason 2013 (CS30)). The remaining included studies did not address this outcome, resulting in a judgment of unclear risk of bias.
Selective reporting
We judged two studies as at high risk of reporting bias: Margel 2019 (0102‐15‐RMC) reported no data for quality of life, although this outcome was prespecified in their protocol, and Sawazaki 2019 did not report data for adverse events when evaluation of this outcome could have been expected.
We judged the risk of reporting bias as unclear for four studies. We did not identify full‐text publications for Crawford 2013 (CS37) and Shore 2012 (CS35), or a protocol for Xie 2016 (PANDA), and information was insufficient to permit a judgment for Sayyid 2017 (DEG_PRE‐OP).
We identified the study protocols of all remaining studies, and all outcomes of interest were reported.
Other potential sources of bias
We identified no other potential sources of other bias in any of the included studies.
Effects of interventions
For details, see summary of findings Table 1; Characteristics of included studies; Data and analyses.
Degarelix versus standard androgen suppression therapy
Overall survival
No data were available for this outcome.
Serious adverse events
We included nine trials evaluating serious adverse events in 2750 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35); Xie 2016 (PANDA)). Degarelix versus standard androgen suppression therapy may result in little to no difference in serious adverse events (risk ratio (RR) 0.80, 95% confidence interval (CI) 0.62 to 1.05; I² = 9%; low‐certainty evidence). This corresponds to 23 fewer serious adverse events per 1000 participants after maximum 14 months (43 fewer to 6 more). We downgraded the certainty of evidence for study limitations and imprecision (Analysis 1.1; summary of findings Table 1; Figure 4).

Forest plot of comparison: 1 Degarelix 240 mg induction dose/80 mg maintenance dose versus standard androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy), outcome: 1.1 Serious adverse events.
Quality of life
We included three studies measuring quality of life (Crawford 2013 (CS37); Klotz 2008 (CS21); Shore 2012 (CS35)). Degarelix likely results in little to no clinically meaningful difference in quality of life after maximum 14 months (standardized mean difference (SMD) 0.06, 95% CI −0.05 to 0.18; I² = 39%; moderate‐certainty evidence). We downgraded the certainty of evidence for study limitations (Analysis 1.2; summary of findings Table 1).
Cancer‐specific survival
No data were available for this outcome.
Clinical progression
No data were available for this outcome.
Other adverse events
Injection site pain
We identified eight studies including 2670 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35); Xie 2016 (PANDA)). Degarelix therapy likely increases injection site pain compared to standard androgen suppression therapy (RR 15.68, 95% CI 7.41 to 33.17; I² = 63%; moderate‐certainty evidence; Analysis 1.3; Figure 5). This corresponds to 440 more injection site pains per 1000 participants after maximum 14 months (192 more to 965 more). We downgraded the certainty of evidence for study limitations (summary of findings Table 1).

Forest plot of comparison: 1 Degarelix 240 mg induction dose/80 mg maintenance dose versus standard androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy), outcome: 1.3 Injection site pain.
Cardiovascular events
Cardiovascular events were assessed in one study (80 men) that predominantly enrolled participants with pre‐existing cardiovascular morbidity (Margel 2019 (0102‐15‐RMC)). The effects of degarelix on cardiovascular events in a general population in clinical routine when compared with standard androgen suppression therapy are very uncertain (RR 0.15, 95% CI 0.04 to 0.61; very low‐certainty evidence; Analysis 1.4). This corresponds to 255 fewer cardiovascular events per 1000 participants after 12 months (288 fewer to 117 fewer). We downgraded for study limitations, imprecision, and indirectness for the patient population (summary of findings Table 1).
Back pain
We identified five studies including 2102 men (Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35)). Degarelix may reduce back pain slightly when compared with standard androgen suppression therapy (RR 0.66, 95% CI 0.46 to 0.96; I² = 0%; Analysis 1.5).
Gynecomastia
We identified one study including 25 men (Sayyid 2017 (DEG_PRE‐OP)). Degarelix may result in little to no difference in gynecomastia when compared with standard androgen suppression therapy (RR 0.31, 95% CI 0.01 to 6.94; I² = not applicable; Analysis 1.6).
Constipation
We identified four studies including 1112 men (Anderson 2013 (CS28); Crawford 2013 (CS37); Klotz 2008 (CS21); Ozono 2018 (3550‐CL‐0010)). Degarelix may result in little to no difference in constipation when compared with standard androgen suppression therapy (RR 0.75, 95% CI 0.39 to 1.46; I² = 26%; Analysis 1.7).
Diarrhea
We identified two studies including 253 men (Crawford 2013 (CS37); Sayyid 2017 (DEG_PRE‐OP)). Degarelix may result in little to no difference in diarrhea when compared with standard androgen suppression therapy (RR 1.56, 95% CI 0.47 to 5.18; I² = 0%; Analysis 1.8).
Vomiting
We identified two studies including 837 men (Crawford 2013 (CS37); Klotz 2008 (CS21)). Degarelix may result in little to no difference in vomiting when compared with standard androgen suppression therapy (RR 1.56, 95% CI 0.79 to 3.08; I² = 0%; Analysis 1.9).
Loss of sexual interest
We identified two studies including 270 men (Mason 2013 (CS30); Sayyid 2017 (DEG_PRE‐OP)). Degarelix may result in little to no difference in loss of sexual interest when compared with standard androgen suppression therapy (RR 1.06, 95% CI 0.35 to 3.17; I² = not applicable; Analysis 1.10).
Loss of sexual function
We identified two studies including 427 men (Axcrona 2012 (CS31); Mason 2013 (CS30)). Degarelix may result in little to no difference in loss of sexual interest when compared with standard androgen suppression therapy (RR 0.82, 95% CI 0.39 to 1.69; I² = 0%; Analysis 1.11).
Fatigue
We identified six studies including 1996 men (Anderson 2013 (CS28); Crawford 2013 (CS37); Klotz 2008 (CS21); Mason 2013 (CS30); Sayyid 2017 (DEG_PRE‐OP); Shore 2012 (CS35)). Degarelix likely results in little to no difference in fatigue when compared with standard androgen suppression therapy (RR 0.83, 95% CI 0.60 to 1.16; I² = 0%; Analysis 1.12).
Hot flushes
We identified eight studies including 2412 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sayyid 2017 (DEG_PRE‐OP); Shore 2012 (CS35)). Degarelix likely results in little to no difference in hot flushes when compared with standard androgen suppression therapy (RR 0.99, 95% CI 0.86 to 1.14; I² = 21%; Analysis 1.13).
Anemia
We identified five studies including 1914 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Klotz 2008 (CS21); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35)). Degarelix likely reduces the occurrence of anemia when compared with standard androgen suppression therapy (RR 0.31, 95% CI 0.13 to 0.74; I² = 0%; Analysis 1.14).
Hepatic enzyme increase (alanine aminotransferase)
We identified four studies including 1014 men (Klotz 2008 (CS21); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sayyid 2017 (DEG_PRE‐OP)). Degarelix likely increases the occurrence of hepatic enzyme increase (measured: alanine aminotransferase) when compared with standard androgen suppression therapy (RR 2.15, 95% CI 1.26 to 3.66; I² = 0%; Analysis 1.15).
Dyspnea
We identified one study including 182 men (Axcrona 2012 (CS31)). Degarelix may result in little to no difference in dyspnea when compared with standard androgen suppression therapy (RR 0.39, 95% CI 0.02 to 9.41; I² = not applicable; Analysis 1.16).
Urinary tract infection
We identified five studies including 1908 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Shore 2012 (CS35)). Degarelix likely reduces the occurrence of urinary tract infection when compared with standard androgen suppression therapy (RR 0.47, 95% CI 0.25 to 0.87; I² = 0%; Analysis 1.17).
Hematuria
We identified two studies including 636 men (Crawford 2013 (CS37); Klotz 2008 (CS21)). Degarelix may result in little to no difference in hematuria when compared with standard androgen suppression therapy (RR 1.69, 95% CI 0.58 to 4.94; I² = 0%; Analysis 1.18).
Urinary retention
We identified five studies including 1925 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Klotz 2008 (CS21); Mason 2013 (CS30); Shore 2012 (CS35)). Degarelix may result in little to no difference in urinary retention when compared with standard androgen suppression therapy (RR 0.43, 95% CI 0.13 to 1.40; I² = 0%; Analysis 1.19).
Mortality during study conduction (post hoc)
We added this outcome post hoc. We identified four studies including 1821 men (Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Shore 2012 (CS35); Xie 2016 (PANDA)). Degarelix probably reduces mortality during study conduction slightly when compared with standard androgen suppression therapy (RR 0.45, 95% CI 0.21 to 0.97; I² = 0%; Analysis 1.20).
Discontinuation due to adverse events (post hoc)
We added this outcome post hoc. We identified eight studies including 2666 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35); Xie 2016 (PANDA)). Degarelix may result in little to no difference in discontinuation due to adverse events when compared with standard androgen suppression therapy (RR 1.11, 95% CI 0.79 to 1.56; I² = 0%; Analysis 1.21).
Total non‐serious adverse events (post hoc)
We added this outcome post hoc. We identified eight studies including 2412 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sayyid 2017 (DEG_PRE‐OP); Shore 2012 (CS35)). Degarelix likely increases total non‐serious adverse events slightly when compared with standard androgen suppression therapy (RR 1.08, 95% CI 1.01 to 1.15; I² = 49%; Analysis 1.22).
Other
No data were available for the following outcomes: rash, pruritus, hemorrhage, nocturia, urinary frequency, edema, anorexia, and gastrointestinal disorders.
Biochemical progression
Two studies assessed biochemical progression (Klotz 2008 (CS21); Xie 2016 (PANDA)). The effects of degarelix on biochemical progression when compared with standard androgen suppression therapy are very uncertain (RR 0.61, 95% CI 0.43 to 0.87; I² = 0%; low‐certainty evidence). This corresponds to 75 fewer biochemical progressions per 1000 participants after 12 months (110 fewer to 25 fewer). We downgraded the certainty of evidence for study limitations and imprecision. We additionally downgraded by one level for indirectness because the percentage of men with locally advanced or metastatic prostate cancer was < 80% (Analysis 1.23; Figure 6).

Forest plot of comparison: 1 Degarelix 240 mg induction dose/80 mg maintenance dose versus standard androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy), outcome: 1.2 Biochemical progression.
Subgroup analysis
We attempted to perform subgroup analyses for the main outcomes included in the summary of findings table, as follows.
Overall survival
We were not able to perform a subgroup analysis for this outcome.
Serious adverse events
The risk of suffering serious adverse events was RR 0.66, 95% CI 0.39 to 1.14 with degarelix 240 mg induction dose/80 mg maintenance dose monthly s.c.; RR 0.85, 95% CI 0.51 to 1.42 with degarelix 240 mg induction dose/160 mg maintenance dose monthly s.c.; and RR 0.90, 95% CI 0.64 to 1.26 with degarelix 240 mg induction dose/480 mg maintenance dose every 3 months s.c. The test for interaction was not significant (P = 0.65; I² = 0%; Analysis 2.1).
Quality of life
The SMD for participants receiving degarelix 240 mg induction dose/80 mg maintenance dose monthly s.c. was −0.03, 95% CI ‐0.33 to 0.28; the SMD for participants receiving degarelix 240 mg induction dose/480 mg maintenance dose every 3 months s.c. was 0.10, 95% CI −0.04 to 0.24. The test for interaction was not significant (P = 0.46; I² = 0%; Analysis 2.2).
Injection site pain
The risk of suffering injection site pain was RR 14.94, 95% CI 4.48 to 49.81 with 240 mg induction dose/80 mg maintenance dose monthly s.c.; RR 61.20, 95% CI 3.82 to 979.36 with degarelix 240 mg induction dose/160 mg maintenance dose monthly s.c.; and RR 15.24, 95% CI 8.50 to 27.31 with degarelix 240 mg induction dose/480 mg maintenance dose every 3 months s.c. The test for interaction was not significant (P = 0.63; I² = 0%; Analysis 2.3).
Cardiovascular events
We were not able to perform a subgroup analysis for this outcome.
Sensitivity analysis
Because of substantial heterogeneity, we performed a sensitivity analysis for the outcome injection site pain by excluding the following trials: Anderson 2013 (CS28), Mason 2013 (CS30), Axcrona 2012 (CS31), and Crawford 2013 (CS37) (see Sensitivity analysis). The effect estimate remained stable favoring standard androgen suppression therapy (RR 44.28, 95% CI 10.99 to 178.38; I² = 0%; not shown).
Discussion
Summary of main results
We identified 11 randomized controlled trials and included data from 10 studies in meta‐analyses. We additionally identified five ongoing trials.
No data were available for the outcomes overall survival, cancer‐specific survival, and clinical progression. Degarelix likely results in no clinically meaningful difference in quality of life compared to standard androgen suppression therapy, and the two treatment groups may be similar in terms of serious adverse events. Degarelix likely increases the occurrence of injection site pain. The effects of degarelix on cardiovascular events are very uncertain.
Overall completeness and applicability of evidence
Several limitations to this review deserve consideration by the reader.
-
We did not find data on patient‐relevant oncological outcomes because no study prospectively planned to assess outcomes such as 'overall survival,' 'cancer‐specific survival,' or 'clinical progression.'
-
Participants enrolled in the included trials differed substantially from our predefined patient characteristics, as the percentage of participants with locally advanced or metastatic prostate cancer was less than 80% in most trials.
-
We were unable to evaluate long‐term oncological outcomes (i.e. survival) because none of the included studies had a follow‐up greater than 365 days. While some studies reported mortality during study conduction, we considered this as an adverse event outcome because with a short‐term follow‐up of less than one year, no survival/mortality data could be mature.
-
Data were insufficient to conduct all of the intended subgroup analyses, so we are uncertain whether the different standard androgen suppression therapies (surgical castration versus medical castration versus antiandrogen monotherapy versus combination of medical castration and antiandrogen therapy) or the different stages of advanced hormone‐sensitive prostate cancer (non‐metastatic versus metastatic disease) impacts the effectiveness of degarelix.
-
All of the included trials reporting outcomes relevant to this review were funded by Ferring Pharmaceuticals or Astellas Pharma Inc, and many study authors had industry relationships.
Quality of the evidence
We rated the certainty of the evidence as moderate, low, or very low for the reasons described below.
-
We consistently downgraded the evidence for study limitations for at least one of the following reasons:
-
performance bias, as none of the included trials blinded participants or personnel. This might have impacted the intensity of follow‐up and the type of care men received;
-
detection bias, as most of the included trials did not blind outcome assessors (or this was not reported). This might have impacted information relating to whether the intervention or control treatment was effective;
-
reporting bias, as one study did not report quality of life data although this outcome was prespecified in their protocol (Margel 2019 (0102‐15‐RMC)). Another study did not report data for adverse events when evaluation of this outcome could have been expected (Sawazaki 2019);
-
we furthermore had concerns about insufficient reporting resulting in an unclear risk of reporting and attrition bias.
-
-
We downgraded the evidence for imprecision in the setting of wide confidence intervals and low numbers of events.
-
We downgraded the evidence for indirectness when the participant population of the included studies did not correspond to our predefined study population.
Potential biases in the review process
We employed a comprehensive search strategy of multiple data sources to search for randomized controlled trials without any publication or language restrictions. However, there remains the possibility that we may have missed studies published in a language other than English, those published in non‐indexed journals, or studies that were not published at all, resulting in potential publication bias. We contacted the authors of all of the included trials to seek further information and data, but only received a response from the authors of two studies.
Agreements and disagreements with other studies or reviews
We are very uncertain as to the effect of degarelix on cardiovascular events in a general population in clinical routine. However, there is considerable evidence available from observational studies including a large number of participants for evaluation of cardiovascular events in patients receiving GnRH antagonists (Cardwell 2020; Davey 2020; George 2020; Perrone 2020). Both degarelix and GnRH agonists increase the risks of cardiovascular disease in prostate cancer patients (Cardwell 2020; George 2020). George 2020 evaluated data from five countries including 48,757 men receiving GnRH agonists and 2144 men receiving GnRH antagonists. Study authors found no difference between groups in risk of any cardiovascular disease, but there may be an increased risk of acute myocardial infarction and arrhythmia in men receiving GnRH antagonists. Cardwell 2020 identified 20,216 prostate cancer patients followed for 73,570 person‐years from the Scottish Cancer Registry. GnRH antagonists and agonists were associated with a 30% increase in cardiovascular events. Data from the UK primary care setting suggest there is a decreased risk of experiencing cardiac events with degarelix. However, patients that received degarelix switched treatment more frequently to a GnRH agonist than the other way round (Davey 2020). It has been suggested that patients receiving androgen suppression therapy in any form should be stratified based on level of cardiovascular disease and monitored accordingly (Davey 2020). Whether degarelix offers any benefit to the subset of individuals at increased risk, as suggested by one included trial (Margel 2019 (0102‐15‐RMC)), remains to be seen.
The results of this Cochrane Review are largely consistent with those of other previously published reviews. Kunath 2015 performed a very similar rigorous systematic review evaluating how GnRH antagonists compared with standard androgen suppression therapy. However, we were able to provide an updated search and include additional trial data. Other reviews did not use a rigorous methodology (i.e. predefined methodology, published protocol, comprehensive search strategy, risk of bias assessment, evaluation of evidence certainty using GRADE) or even consider risk of bias assessments in their conclusions (Abufaraj 2020; Cui 2014; Hosseini 2016; Klotz 2014; Kunath 2015; Sciarra 2016). This Cochrane Review includes data that were not previously included in systematic reviews and is therefore the most up‐to‐date.
The current guideline of the American Urological Association does not make a distinction between the different types of androgen suppression therapy in advanced hormone‐sensitive prostate cancer, but recommends that the use of non‐steroidal antiandrogens (i.e. bicalutamide) should be restricted to testosterone flare protection only (AUA 2020). Also, the guideline of the European Association of Urology determines that there is no high‐level evidence available favoring one specific type of androgen suppression therapy (EAU 2020). The guideline recommends that GnRH antagonist and bilateral surgical castration are the preferred treatment options for men with impending spinal cord compression (EAU 2020).
The National Institute for Health and Care Excellence (NICE) invited Ferring Pharmaceuticals to submit evidence for the clinical and cost‐effectiveness (Uttley 2017). Uttley 2017 published a review of the evidence contained within the company's submission to NICE. They identified that the GnRH antagonist degarelix was non‐inferior to standard androgen suppression therapy regarding the reduction of testosterone levels, but achieved a more rapid suppression of PSA. Degarelix also decreased the incidence of testosterone flare that is typically associated with GnRH agonists (Uttley 2017). However, there was no testosterone flare protection in the control groups of the included trials, and Uttley 2017 stated that this was not in accordance with current UK clinical practice. This evaluation on behalf of NICE suggested that degarelix was not cost‐effective for the subgroup with metastatic disease, but could be cost‐effective for the subgroup with spinal metastases (Uttley 2017). However, it should be considered that the recommendation for degarelix in patients with impending spinal cord compression is based on the results of small (post hoc defined) subgroup analyses and on reflection that a rapid androgen suppression with prevention of testosterone flare might be clinically useful. Most participants included in randomized controlled trials had a non‐advanced disease stage, and the studies were not predefined to evaluate degarelix for this purpose.

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Degarelix 240 mg induction dose/80 mg maintenance dose versus standard androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy), outcome: 1.1 Serious adverse events.

Forest plot of comparison: 1 Degarelix 240 mg induction dose/80 mg maintenance dose versus standard androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy), outcome: 1.3 Injection site pain.

Forest plot of comparison: 1 Degarelix 240 mg induction dose/80 mg maintenance dose versus standard androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy), outcome: 1.2 Biochemical progression.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 1: Serious adverse events

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 2: Quality of life

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 3: Injection site pain

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 4: Cardiovascular events

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 5: Back pain

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 6: Gynecomastia

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 7: Constipation

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 8: Diarrhea

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 9: Vomiting

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 10: Loss of sexual interest

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 11: Loss of sexual function

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 12: Fatigue

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 13: Hot flushes

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 14: Anemia

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 15: Hepatic enzyme increase (alanine aminotransferase)

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 16: Dyspnea

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 17: Urinary tract infection

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 18: Hematuria

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 19: Urinary retention

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 20: Mortality during study conduction (post hoc)

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 21: Discontinuation due to adverse events (post hoc)

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 22: Total non‐serious adverse events (post hoc)

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 23: Biochemical progression

Comparison 2: Degarelix versus androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy): subgroup analysis based on different doses, Outcome 1: Serious adverse events

Comparison 2: Degarelix versus androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy): subgroup analysis based on different doses, Outcome 2: Quality of life

Comparison 2: Degarelix versus androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy): subgroup analysis based on different doses, Outcome 3: Injection site pain
| Degarelix compared to standard androgen suppression therapy for treating advanced hormone‐sensitive prostate cancer | ||||||
| Patient or population: hormone‐sensitive prostate cancer | ||||||
| Outcomes | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) |
What happens | |
|---|---|---|---|---|---|---|
| Risk with standard androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy) | Risk difference with degarelix | |||||
| Overall survival | ‐ | ‐ | ‐ | ‐ | N/A | We do not know the effect of degarelix on overall survival. |
| Serious adverse events | 2750 | ⊕⊕⊝⊝ | RR 0.80 | Study population | Degarelix may have little to no effect on serious adverse events. | |
| 114 per 1000 | 23 fewer per 1000 | |||||
| Quality of life | 2887 | ⊕⊕⊕⊝ | ‐ | The mean quality of life was 0. | SMD 0.06 higher | Degarelix probably has little to no effect on quality of life. |
| Cancer‐specific survival | ‐ | ‐ | ‐ | ‐ | N/A | We do not know the effect of degarelix on cancer‐specific survival. |
| Cardiovascular events | 80 | ⊕⊕⊝⊝ | RR 0.15 | General population4 | The effect of degarelix on cardiovascular events is very uncertain. | |
| 300 per 1000 | 255 fewer per 1000 | |||||
| Injection site pain | 2670 | ⊕⊕⊕⊝ | RR 15.68 | Study population | Degarelix probably increases the occurrence of injection site pain. | |
| 30 per 1000 | 440 more per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level for study limitations (performance or detection bias, or both). | ||||||
| Study name | Intervention(s) and comparators (s) | Follow‐up | Number of participants | Study dates | Stage of disease |
|---|---|---|---|---|---|
| Degarelix 240/80 mg1 | 12 weeks | 27 | 2009 to 2010 | Localized/locally advanced: 9 (22.5%) Metastatic: 14 (35%) Unclear: 17 (42.5%) | |
| GnRH agonist with flare protection (goserelin 3.6 mg s.c. every 28 days with bicalutamide 50 mg orally per day for 14 days) | 13 | ||||
| Degarelix 240/80 mg1 | 12 weeks | 82 | 2009 to 2011 | Localized: 56 (31%) Advanced: 106 (59%) Unclear: 17 (9%) | |
| GnRH agonist with flare protection (goserelin 3.6 mg s.c. every 28 days with bicalutamide 50 mg orally per day for 28 days) | 97 | ||||
| Degarelix 240/80 mg2 (intermittent; data not included) | 14 months | 175 | 2009 to 2012 | Unclear (not reported) | |
| Degarelix 240/80 mg1 | 50 | ||||
| GnRH agonist with flare protection (leuprolide 7.5 mg i.m. monthly, maintenance dose 22.5 mg i.m. 3‐monthly with bicalutamide 50 mg orally per day for 28 days on Investigator's discretion) | 178 | ||||
| Degarelix 240/160 mg (degarelix starting dose of 240 mg s.c. with maintenance doses of 80 mg s.c. every 28 days) | 364 days | 202 | 2006 to 2007 | Localized: 191 (31%) Locally advanced: 178 (29%) Metastatic: 125 (20%) Not classifiable: 116 (19%) | |
| Degarelix 240/80 mg1 | 207 | ||||
| GnRH agonist (leuprolide 7.5 mg i.m. monthly) | 201 | ||||
| Degarelix 240/80 mg s.c.1 | 12 months | 41 | 2015 to 2019 | Localized: 59 (74%) Metastatic: 21 (26%) | |
| GnRH agonist 3‐monthly (at the discretion of the treating urologist/oncologist) | 28 | ||||
| Degarelix 240/80 mg1 | 12 weeks | 180 | 2009 to 2011 | Localized: 152 (62%) Advanced: 83 (34%) Unclear: 9 (4%) | |
| GnRH agonist with flare protection (goserelin 3.6 mg s.c. every 28 days with bicalutamide 50 mg orally per day for 14 days) | 64 | ||||
| Degarelix 240/480 mg (starting dose of 240 mg s.c. with maintenance doses of 480 mg s.c. every 84 days) | 12 months | 117 | 2013 to 2016 | Localized: 124 (53%) Locally advanced: 63 (27%) Metastatic: 44 (19%) Unclear: 3 (1%) | |
| GnRH agonist (goserelin 3.6 mg s.c. with maintenance dose 10.8 mg s.c. every 84 days) | 117 | ||||
| Degarelix 240/80 mg1 | 6 months | 50 | 2016 to 2018 | Localized: 76 (76%) Locally advanced and/or metastatic: 24 (24%) | |
| GnRH agonist (leuprolide 3.75 mg every 28 days) | 50 | ||||
| Degarelix 240/80 mg1 | 12 weeks | 13 | 2012 to 2015 | Localized: 10 (26%) Locally advanced: 15 (60%) Node positive: 6 (24%) PSA failure (> 0.2 ng/mL) or use of adjuvant androgen suppression/radiotherapy: 8 (21%)3 | |
| Degarelix 240/80 mg s.c. 2‐monthly + bicalutamide 50 mg orally per day (data not included) | 14 | ||||
| GnRH agonist + bicalutamide (leuprorelin 22.5 mg, leuprolide 22.5 mg, or goserelin acetate 10.8 mg 3‐monthly and bicalutamide 50 mg orally per day) | 12 | ||||
| Degarelix 240/480 mg (starting dose of 240 mg s.c. with maintenance doses of 480 mg s.c. every 3 months) | 13 months | 565 | 2009 to 2011 | Unclear (not reported) | |
| GnRH agonist (goserelin 3.6 mg s.c. with maintenance doses of 10.8 mg s.c. 3‐monthly) | 283 | ||||
| Degarelix 240/80 mg1 | 364 days | 143 | 2013 to 2015 | Unclear (not reported) | |
| GnRH agonist (goserelin 3.6 mg s.c. monthly) | 142 | ||||
| Abbreviations: GnRH: gonadotropin‐releasing hormone; i.m.: intramuscular; PSA: prostate‐specific antigen; s.c.: subcutaneous 1Degarelix starting dose of 240 mg s.c. with maintenance doses of 80 mg s.c. every 28 days. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Serious adverse events Show forest plot | 9 | 2750 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.05] |
| 1.2 Quality of life Show forest plot | 3 | 2887 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.05, 0.18] |
| 1.3 Injection site pain Show forest plot | 8 | 2670 | Risk Ratio (M‐H, Random, 95% CI) | 15.68 [7.41, 33.17] |
| 1.4 Cardiovascular events Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.04, 0.61] |
| 1.5 Back pain Show forest plot | 5 | 2102 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.96] |
| 1.6 Gynecomastia Show forest plot | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 6.94] |
| 1.7 Constipation Show forest plot | 4 | 1112 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.46] |
| 1.8 Diarrhea Show forest plot | 2 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.47, 5.18] |
| 1.9 Vomiting Show forest plot | 2 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.79, 3.08] |
| 1.10 Loss of sexual interest Show forest plot | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.35, 3.17] |
| 1.11 Loss of sexual function Show forest plot | 2 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.39, 1.69] |
| 1.12 Fatigue Show forest plot | 6 | 1996 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.60, 1.16] |
| 1.13 Hot flushes Show forest plot | 8 | 2412 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.14] |
| 1.14 Anemia Show forest plot | 5 | 1914 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.13, 0.74] |
| 1.15 Hepatic enzyme increase (alanine aminotransferase) Show forest plot | 4 | 1014 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.26, 3.66] |
| 1.16 Dyspnea Show forest plot | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.02, 9.41] |
| 1.17 Urinary tract infection Show forest plot | 5 | 1908 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.25, 0.87] |
| 1.18 Hematuria Show forest plot | 2 | 636 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.58, 4.94] |
| 1.19 Urinary retention Show forest plot | 5 | 1925 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.13, 1.40] |
| 1.20 Mortality during study conduction (post hoc) Show forest plot | 4 | 1821 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.21, 0.97] |
| 1.21 Discontinuation due to adverse events (post hoc) Show forest plot | 8 | 2666 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.79, 1.56] |
| 1.22 Total non‐serious adverse events (post hoc) Show forest plot | 8 | 2412 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.01, 1.15] |
| 1.23 Biochemical progression Show forest plot | 2 | 691 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.43, 0.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Serious adverse events Show forest plot | 9 | 2951 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.03] |
| 2.1.1 Degarelix 240 mg/80 mg | 7 | 1466 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.39, 1.14] |
| 2.1.2 Degarelix 240 mg/160 mg | 1 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.42] |
| 2.1.3 Degarelix 240 mg/480 mg | 2 | 1082 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.26] |
| 2.2 Quality of life Show forest plot | 3 | 2887 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.05, 0.18] |
| 2.2.1 Degarelix 240 mg/80 mg | 2 | 2040 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.33, 0.28] |
| 2.2.2 Degarelix 240 mg/480 mg | 1 | 847 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.04, 0.24] |
| 2.3 Injection site pain Show forest plot | 8 | 2670 | Risk Ratio (M‐H, Random, 95% CI) | 15.68 [7.41, 33.17] |
| 2.3.1 Degarelix 240 mg/80 mg | 6 | 1286 | Risk Ratio (M‐H, Random, 95% CI) | 14.94 [4.48, 49.81] |
| 2.3.2 Degarelix 240 mg/160 mg | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 61.20 [3.82, 979.36] |
| 2.3.3 Degarelix 240 mg/480 mg | 2 | 1082 | Risk Ratio (M‐H, Random, 95% CI) | 15.24 [8.50, 27.31] |

