Opioids for chronic non‐cancer pain in children and adolescents
Abstract
Background
Pain is a common feature of childhood and adolescence around the world, and for many young people, that pain is chronic. The World Health Organization guidelines for pharmacological treatments for children's persisting pain acknowledge that pain in children is a major public health concern of high significance in most parts of the world. While in the past, pain was largely dismissed and was frequently left untreated, views on children's pain have changed over time, and relief of pain is now seen as important.
We designed a suite of seven reviews on chronic non‐cancer pain and cancer pain (looking at antidepressants, antiepileptic drugs, non‐steroidal anti‐inflammatory drugs, opioids, and paracetamol as priority areas) in order to review the evidence for children's pain utilising pharmacological interventions in children and adolescents.
As the leading cause of morbidity in children and adolescents in the world today, chronic disease (and its associated pain) is a major health concern. Chronic pain (lasting three months or longer) can arise in the paediatric population in a variety of pathophysiological classifications: nociceptive, neuropathic, idiopathic, visceral, nerve damage pain, chronic musculoskeletal pain, and chronic abdominal pain, and other unknown reasons.
Opioids are used worldwide for the treatment of pain. They bind to opioid receptors in the central nervous system (mu, kappa, delta, and sigma) and can be agonists, antagonists, mixed agonist‐antagonists, or partial agonists. Opioids are generally available in healthcare settings across most high‐income countries, but access may be restricted in low‐ and middle‐income countries. For example, opioids currently available in the UK include: buprenorphine, codeine, fentanyl, hydromorphone, methadone, morphine, oxycodone, and tramadol. Opioids are used in varying doses (generally based on body weight for paediatric patients) by means of parenteral, transmucosal, transdermal, or oral administration (immediate release or modified release). To achieve adequate pain relief in children using opioids, with an acceptable grade of adverse effects, the recommended method is a lower dose gradually titrated to effect in the child.
Objectives
To assess the analgesic efficacy and adverse events of opioids used to treat chronic non‐cancer pain in children and adolescents aged between birth and 17 years, in any setting.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Library, MEDLINE via Ovid, and Embase via Ovid from inception to 6 September 2016. We also searched the reference lists of retrieved studies and reviews, and searched online clinical trial registries.
Selection criteria
Randomised controlled trials, with or without blinding, of any dose and any route, treating chronic non‐cancer pain in children and adolescents, comparing opioids with placebo or an active comparator.
Data collection and analysis
Two review authors independently assessed studies for eligibility. We planned to use dichotomous data to calculate risk ratio and number needed to treat, using standard methods. We planned to assess GRADE (Grading of Recommendations Assessment, Development and Evaluation) and planned to create a 'Summary of findings' table.
Main results
No studies were eligible for inclusion in this review.
There is no evidence to support or refute the use of opioids for treating chronic non‐cancer pain in children and adolescents.
Authors' conclusions
We are unable to comment about efficacy or harm from the use of opioids to treat chronic non‐cancer pain in children and adolescents.
We know from adult randomised controlled trials that some opioids, such as morphine and codeine, can be effective in certain chronic pain conditions.
PICO
Plain language summary
Opioids for chronic non‐cancer pain in children and adolescents
Bottom line
There is no evidence from randomised controlled trials to support or refute the suggestion that opioids in any dose will provide pain relief for children or adolescents with chronic non‐cancer pain.
Background
Children can experience chronic or recurrent pain related to genetic conditions, damaged nerves, chronic musculoskeletal pain, and chronic abdominal pain, as well as for other unknown reasons. Chronic pain is pain that lasts three months or longer and is commonly accompanied by changes in lifestyle and functional abilities, as well as by signs and symptoms of depression and anxiety.
Opioids are used worldwide for the treatment of pain. Opioids are generally available in healthcare settings across most high‐income countries, but access may be restricted in low‐ and middle‐income countries. For example, opioids currently available in the UK include: buprenorphine, codeine, fentanyl, hydromorphone, methadone, morphine, oxycodone, and tramadol. Opioids are used in varying doses and are commonly administered via injection or oral tablets.
Key results
In September 2016 we searched for clinical trials where any opioids were used to treat chronic pain (potentially from either nerve pain, musculoskeletal problems, menstrual cramps, or abdominal discomfort).
We found no studies that met the requirements for this review. Several studies tested opioids in adults with chronic pain, but none in participants from birth to 17 years old.
Quality of the evidence
We planned to rate the quality of the evidence from studies using four levels: very low, low, moderate, or high. Very low quality evidence means that we are very uncertain about the results. High quality evidence means that we are very confident in the results.
We were unable to rate the quality of the evidence as there was no evidence from randomised controlled trials to support or refute the suggestion that opioids in any dose will reduce chronic non‐cancer pain in children or adolescents.
Authors' conclusions
Background
Pain is a common feature of childhood and adolescence around the world, and for many young people, that pain is chronic. The World Health Organization guidelines for pharmacological treatments for persisting pain in children acknowledge that pain in children is a major public health concern of high significance in most parts of the world (WHO 2012). While in the past, pain was largely dismissed and was frequently left untreated, views on children's pain have changed over time, and relief of pain is now seen as important. Since the 1970s, studies comparing child and adult pain management have revealed a variety of responses to pain, fuelling the need for a more in‐depth focus on paediatric pain (Caes 2016).
Infants (zero to 12 months), children (1 to 9 years), and adolescents (10 to 18 years), WHO 2012, account for 27% (1.9 billion) of the world's population (United Nations 2015); the proportion of those aged 14 years and under ranges from 12% (in Hong Kong) to 50% (in Niger) (World Bank 2014). However, little is known about the pain management needs of this population. For example, in the Cochrane Library, approximately 12 reviews produced by the Cochrane Pain, Palliative and Supportive Care Review Group in the past 18 years have been specifically concerned with children and adolescents, compared to over 100 reviews specific to adults. Additional motivating factors for investigating children's pain include the vast amount of unmanaged pain in the paediatric population and the development of new technologies and treatments. We convened an international group of leaders in paediatric pain to design a suite of seven reviews in chronic pain and cancer pain (looking at antidepressants, antiepileptic drugs, non‐steroidal anti‐inflammatory drugs, opioids, and paracetamol as priority areas) in order to review the evidence under a programme grant for children's pain utilising pharmacological interventions in children and adolescents (Appendix 1).
This review is based on a template for reviews of pharmacotherapies used to relieve pain in infants, children, and adolescents. The aim is for all reviews to use the same methods, based on new criteria for what constitutes reliable evidence (Appendix 2) (Moore 2010a; Moore 2012). This review focused on opioids to treat chronic non‐cancer pain.
Description of the condition
This review focused on chronic non‐cancer pain experienced by children and adolescents as a result of any type of chronic disease that occurs throughout the global paediatric population. Children's level of pain can be mild, moderate, or severe, and pain management is an essential element of patient management during all care stages of chronic disease.
As the leading cause of morbidity in children and adolescents in the world today, chronic disease (and its associated pain) is a major health concern. Chronic pain can arise in the paediatric population in a variety of pathophysiological classifications: nociceptive, neuropathic, idiopathic, or visceral. Chronic pain is pain that lasts three months or longer and is commonly accompanied by changes in lifestyle and functional abilities, as well as by signs and symptoms of depression and anxiety (Ripamonti 2008).
Whilst diagnostic and perioperative procedures performed to treat chronic diseases are a known common cause of pain in these patients, this review did not cover perioperative pain or adverse effects of treatments such as mucositis.
Description of the intervention
Opioids are used worldwide for the treatment of pain. They bind to opioid receptors in the central nervous system (mu, kappa, delta, and sigma) and can be agonists, antagonists, mixed agonist‐antagonists, or partial agonists. Opioids are generally available in healthcare settings across most high‐income countries, but access may be restricted in low‐ and middle‐income countries. For example, opioids currently available in the UK include: buprenorphine, codeine, fentanyl, hydromorphone, methadone, morphine, oxycodone, and tramadol.
Opioids are used in varying doses (generally based on body weight for paediatric patients) by means of parenteral or oral administration (immediate release or modified release). However, receiving injections or swallowing tablets can sometimes prove challenging in paediatric patients, in which case tablets can be crushed. Alternatively, buccal, transdermal, nasal, or rectal administration may also be adopted (Verghese 2010). To achieve adequate pain relief in children using opioids, with an acceptable grade of adverse effects, the recommended method is a lower dose gradually titrated to effect in the child (WHO 2012).
Adverse effects of analgesic opioids in children in the short term include: constipation, hives, nausea, pruritus, respiratory depression, and vomiting. The long‐term potential for addiction and withdrawals is lessened due to controlled administration (Rosenblum 2008), and even fewer cases result in opioid tolerance, overdose, and more rarely, death.
How the intervention might work
Opioids act on opioid receptors. The four opioid receptors (mu, kappa, delta, and ORL‐1) are distributed throughout the body in different densities, especially in nervous tissues. The peptides and receptors contribute to various physiological functions including the modulation of pain, the immune system, and hormones (PCF 2014). Opioid receptors are G‐protein‐coupled receptors and are located primarily in the central nervous system. Once agonistic opioids have bound to the opioid receptor, they produce intracellular effects throughout the coupled G‐protein that result in an inhibition of the nociceptive transmission. Activation results in neural inhibition by decreasing the release of excitatory neurotransmitters from the presynaptic terminals (Verghese 2010). The clinically important opioid analgesics act as agonists at the mu‐receptor, with some potential significant effects on delta‐opioid receptors (e.g. methadone) and kappa‐opioid receptors (e.g. oxycodone). Some opioids are mixed agonist‐antagonists (e.g. buprenorphine). Some opioids also possess non‐opioid activity (e.g. methadone, tapentadol, and tramadol) (PCF 2014).
Why it is important to do this review
The paediatric population is at risk of inadequate management of pain (AMA 2013). Some conditions that would be aggressively treated in adult patients are being managed with insufficient analgesia in younger populations (AMA 2013). Although there have been repeated calls for best evidence to treat children's pain, such as Eccleston 2003, there are no easily available summaries of the most effective paediatric pain relief.
This review formed part of a Programme Grant addressing the unmet needs of people with chronic pain, commissioned by the National Institute for Health Research (NIHR) in the UK. This topic was identified in June 2015 during consultation with experts in paediatric pain. Please see Appendix 1 for full details of the meeting. The standards used to assess evidence in chronic pain trials have changed substantially in recent years, with particular attention being paid to trial duration, withdrawals, and statistical imputation following withdrawal, all of which can substantially alter estimates of efficacy. The most important change was to encourage a move from using average pain scores, or average change in pain scores, to the number of people who have a large decrease in pain (by at least 50%). Pain intensity reduction of 50% or more has been shown to correlate with improvements in comorbid symptoms, function, and quality of life (Moore 2011a). These standards are set out in the reference guide for pain studies (AUREF 2012).
Objectives
To assess the analgesic efficacy and adverse events of opioids used to treat chronic non‐cancer pain in children and adolescents aged between birth and 17 years, in any setting.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials, with or without blinding, and participant‐ or observer‐reported outcomes.
Full journal publication was required, with the exception of online clinical trial results, summaries of otherwise unpublished clinical trials, and abstracts with sufficient data for analysis. We planned to include studies published in any language. We planned to exclude abstracts (usually meeting reports) or unpublished data, non‐randomised studies, studies of experimental pain, case reports, and clinical observations.
Types of participants
We planned to include studies of infants, children, and adolescents, aged from birth to 17 years old, with chronic or recurrent pain (lasting for three months or longer), arising from genetic conditions, neuropathy, or other conditions. These included but were not limited to chronic musculoskeletal pain and chronic abdominal pain.
We planned to exclude studies of perioperative pain, acute pain, cancer pain, headache, migraine, and pain associated with primary disease or its treatment. These topics are covered in their own respective reviews.
We planned to include studies of participants with more than one type of chronic pain, in which case we would analyse results according to the primary condition.
Types of interventions
We planned to include studies reporting interventions prescribing any opioid (alone or in combination) for the relief of chronic non‐cancer pain; by any route, in any dose, with comparison to a placebo or any active comparator.
Types of outcome measures
In order to be eligible for inclusion in this review, studies had to report pain assessment, as well as meeting the other selection criteria.
We planned to include trials measuring pain intensity and pain relief assessed using validated tools such as numerical rating scale (NRS), visual analogue scale (VAS), Faces Pain Scale ‐ Revised (FPS‐R), Colour Analogue Scale (CAS), or any other validated numerical rating scale.
We were particularly interested in Pediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (PedIMMPACT) definitions for moderate and substantial benefit in chronic pain studies (PedIMMPACT 2008). These were defined as: at least 30% pain relief over baseline (moderate); at least 50% pain relief over baseline (substantial); much or very much improved on Patient Global Impression of Change scale (PGIC) (moderate); very much improved on PGIC (substantial).
These outcomes differ from those used in most earlier reviews, concentrating as they do on dichotomous outcomes where pain responses do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50% pain intensity reduction, and ideally having no worse than mild pain (Moore 2013a; O'Brien 2010).
We planned to record any reported adverse events. We planned to report the timing of outcome assessments.
Primary outcomes
-
Participant‐reported pain relief of 30% or greater
-
Participant‐reported pain relief of 50% or greater
-
PGIC much or very much improved
In the absence of self reported pain, we planned to consider the use of 'other‐reported' pain, typically by an observer such as a parent, carer, or healthcare professional (Stinson 2006; von Baeyer 2007).
Secondary outcomes
We identified the following with reference to the PedIMMPACT recommendations, which suggest core outcome domains and measures for consideration in paediatric acute and chronic/recurrent pain clinical trials (PedIMMPACT 2008).
-
Carer Global Impression of Change
-
Requirement for rescue analgesia
-
Sleep duration and quality
-
Acceptability of treatment
-
Physical functioning as defined by validated scales
-
Quality of life as defined by validated scales
-
Any adverse events
-
Withdrawals due to adverse events
-
Any serious adverse event. Serious adverse events typically include any untoward medical occurrence or effect that at any dose results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the patient, or may require an intervention to prevent one of the above characteristics or consequences.
Search methods for identification of studies
We developed the search strategy based on previous strategies used by the Cochrane Pain, Palliative and Supportive Care Review Group and carried out the searches.
Electronic searches
We searched the following databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL) (via the Cochrane Library) searched 6 September 2016;
-
MEDLINE (via Ovid) 1947 to September week 2 2016;Embase (via Ovid) 1974 to 2016 week 38.
We used medical subject headings (MeSH) or equivalent and text word terms. We restricted our search to randomised controlled trials and clinical trials. There were no language or date restrictions. We restricted our search to published papers only and excluded conference abstracts or meeting reports. The focus of the key words in our search terms was on chronic pain and opioids. We tailored searches to individual databases. The search strategies for MEDLINE, Embase, and CENTRAL are in Appendix 3, Appendix 4, and Appendix 5, respectively.
Searching other resources
We searched ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/) for ongoing trials in June 2017. In addition, we checked reference lists of reviews and retrieved articles for additional studies, and performed citation searches on key articles. We planned to contact experts in the field for unpublished and ongoing trials. We planned to contact study authors for additional information where necessary.
Data collection and analysis
We planned to perform separate analyses according to particular chronic pain conditions. We planned to combine different chronic pain conditions in analyses for exploratory purposes only.
Selection of studies
Two review authors independently determined study eligibility by reading the abstract of each study identified by the search. Review authors independently eliminated studies that clearly did not satisfy the inclusion criteria, and obtained full copies of the remaining studies. Two review authors independently read these studies to select those that met the inclusion criteria, a third review author adjudicating in the event of disagreement. We did not anonymise the studies in any way before assessment. We included a PRISMA flow chart (Figure 1) to illustrate the results of the search and the process of screening and selecting studies for inclusion in the review (Moher 2009), as recommended in section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to include studies in the review irrespective of whether measured outcome data were reported in a ‘usable’ way.
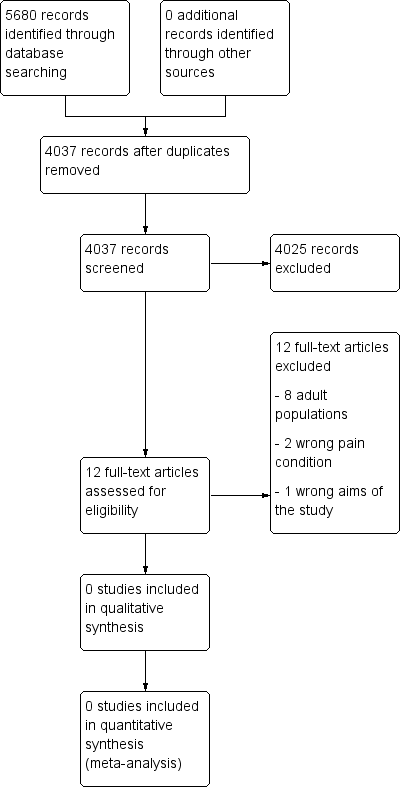
Study flow diagram.
Data extraction and management
We planned to obtain full copies of the studies with two review authors independently carrying out data extraction. Where available, we would have extracted information about the pain condition, number of participants treated, drug and dosing regimen, study design (placebo or active control), study duration and follow‐up, analgesic outcome measures and results, withdrawals, and adverse events (participants experiencing any adverse event or serious adverse event). We planned to collate multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We planned to collect characteristics of the included studies in sufficient detail to populate a ‘Characteristics of included studies’ table.
We planned to use a template data extraction form and check for agreement before entry into Cochrane's statistical software Review Manager 5 (RevMan 2014).
If a study had more than two intervention arms, we planned to only include the intervention and control groups that met the eligibility criteria. If multi‐arm studies were included, we planned to analyse multiple intervention groups in an appropriate way that avoided arbitrary omission of relevant groups and double‐counting of participants.
Assessment of risk of bias in included studies
We planned for two review authors to independently assess risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We planned to complete a 'Risk of bias' table for each included study using the Cochrane 'Risk of bias' tool in Review Manager 5 (RevMan 2014).
We planned to assess the following for each study. Any disagreements would have been resolved by discussion between review authors or by consulting a third review author when necessary.
-
Random sequence generation (checking for possible selection bias). We planned to assess the method used to generate the allocation sequence as: low risk of bias (i.e. any truly random process, e.g. random number table; computer random number generator); or unclear risk of bias (when the method used to generate the sequence was not clearly stated). We planned to exclude studies that used a non‐random process and were therefore at high risk of bias (e.g. odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We planned to assess the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); or unclear risk of bias (when the method was not clearly stated). We planned to exclude studies that did not conceal allocation and were therefore at a high risk of bias (e.g. open list).
-
Blinding of participants and personnel (checking for possible performance bias). We planned to assess any methods used to blind the participants and personnel from knowledge of which intervention a participant received. We planned to assess the methods as: low risk of bias (study states that the participants and personnel involved were blinded to treatment groups); unclear risk of bias (study does not state whether or not participants and personnel were blinded to treatment groups); or high risk of bias (participants or personnel were not blinded) (as stated in Types of studies, we planned to include trials with or without blinding, and participant‐ or observer‐reported outcomes).
-
Blinding of outcome assessment (checking for possible detection bias). We planned to assess any methods used to blind the outcome assessors from knowledge of which intervention a participant received. We planned to assess the methods as: low risk of bias (e.g. study states that it was single‐blinded and describes the method used to achieve blinding of the outcome assessor); unclear risk of bias (study states that outcome assessors were blinded but does not provide an adequate description of how this was achieved); or high risk of bias (outcome assessors were not blinded) (as stated in Types of studies, we planned to include trials with or without blinding, and participant‐ or observer‐reported outcomes).
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We planned to assess the methods used to deal with incomplete data as: low risk of bias (i.e. less than 10% of participants did not complete the study or 'baseline observation carried forward' (BOCF) analysis was used, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); or high risk of bias (used 'completer' analysis).
-
Selective reporting (checking for possible reporting bias). We planned to assess the methods used to report the outcomes of the study as: low risk of bias (if all planned outcomes in the protocol or methods were reported in the results); unclear risk of bias (if there was not a clear distinction between planned outcomes and reported outcomes); high risk of bias (if some planned outcomes from the protocol or methods were clearly not reported in the results).
-
Size of study (checking for possible biases confounded by small size). We planned to assess studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); or high risk of bias (fewer than 50 participants per treatment arm).
-
Other bias. We planned to assess studies for any additional sources of bias as low, unclear, or high risk of bias, and provide rationale.
Measures of treatment effect
Where dichotomous data were available, we planned to calculate a risk ratio (RR) with 95% confidence interval (CI) and meta‐analyse the data as appropriate. We planned to calculate number needed to treat for an additional beneficial outcome (NNTB) where appropriate (McQuay 1998); for unwanted effects the NNTB becomes the number needed to treat for an additional harmful outcome (NNTH) and is calculated in the same manner. Where continuous data were reported, we planned to use appropriate methods to combine these data in the meta‐analysis.
Unit of analysis issues
We planned to accept randomisation to the individual participant only. We planned to split the control treatment arm between active treatment arms in a single study if the active treatment arms were not combined for analysis. We would only accept studies with minimum 10 participants per treatment arm.
Dealing with missing data
We planned to use intention‐to‐treat analysis where the intention‐to‐treat population consisted of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one post baseline assessment. We would have assigned missing participants zero improvement wherever possible.
Assessment of heterogeneity
We planned to identify and measure heterogeneity as recommended in Chapter 9 of theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to deal with clinical heterogeneity by combining studies that examined similar conditions. We planned to undertake and present a meta‐analysis only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar to ensure a clinically meaningful answer. We planned to assess statistical heterogeneity visually and by using the I² statistic (L'Abbé 1987). When I² was greater than 50%, we planned to consider the possible reasons.
Assessment of reporting biases
We planned to assess the risk of reporting bias, as recommended in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The aim of this review was to use dichotomous outcomes of known utility and of value to patients (Hoffman 2010; Moore 2010b; Moore 2010c; Moore 2010d; Moore 2013a). The review did not depend on what the authors of the original studies chose to report or not, though clearly difficulties would arise in studies failing to report any dichotomous results. We planned to extract and use continuous data, which probably reflected efficacy and utility poorly, and is useful for illustrative purposes only.
We planned to assess publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean a number needed to treat (NNT) of 10 or higher) (Moore 2008).
Data synthesis
We planned to use a fixed‐effect model for meta‐analysis. We planned to use a random‐effects model for meta‐analysis if there is significant clinical heterogeneity and we considered it appropriate to combine studies. We planned to conduct our analysis using the primary outcomes of pain and adverse events, and to calculate the NNTHs for adverse events. We planned to use the Cochrane software program Review Manager 5 (RevMan 2014).
Quality of the evidence
To analyse data, two review authors independently rated the quality of each outcome. We used the GRADE approach to assess the quality of the body of evidence related to each of the key outcomes. We planned to report our judgement in a 'Summary of findings' table per Chapter 12 of the Cochrane Handbook (Appendix 6) (Higgins 2011).
In addition, there may be circumstances where the overall rating for a particular outcome would need to be adjusted per GRADE guidelines (Guyatt 2013a). For example, if there were so few data that the results were highly susceptible to the random play of chance, or if studies used LOCF imputation in circumstances where there were substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by three levels, to very low quality. In circumstances where no data were reported for an outcome, we planned to report that there was no evidence to support or refute (Guyatt 2013b).
'Summary of findings' table
We planned to include a 'Summary of findings' table as set out in the Cochrane Pain, Palliative and Supportive Care Review Group’s author guide (AUREF 2012), and recommended in section 4.6.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to justify and document all assessments of the quality of the body of evidence.
In an attempt to interpret reliability of the findings for this systematic review, we planned to assess the summarised data using the GRADE guidelines (Appendix 6) to rate the quality of the body of evidence of each of the key outcomes listed in Types of outcome measures per Chapter 12 of the Cochrane Handbook (Guyatt 2011; Higgins 2011), as appropriate. Utilising the explicit criteria against study design, risk of bias, imprecision, inconsistency, indirectness, and magnitude of effect, we planned to summarise the evidence in an informative, transparent, and succinct 'Summary of findings' table or 'Evidence profile' table (Guyatt 2011).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses where a minimum number of data were available (at least 200 participants per treatment arm). We planned to analyse according to age group; type of drug; geographical location or country; type of control group; baseline measures; frequency, dose, and duration of drugs; and nature of drug.
We planned to investigate whether the results of subgroups were significantly different by inspecting the overlap of confidence intervals and by performing the test for subgroup differences available in Review Manager 5.
Sensitivity analysis
We did not plan to carry out any sensitivity analysis because the evidence base is known to be too small to allow reliable analysis; we did not plan to pool results from chronic pain of different origins in the primary analyses. We planned to examine details of dose escalation schedules in the unlikely circumstance that this could provide some basis for a sensitivity analysis.
Results
Description of studies
Results of the search
A PRISMA flow diagram of the search results is shown in Figure 1.
Searches of the three main databases revealed 5680 records of titles and abstracts, of which 1643 duplicates were removed. Our searches of ClinicalTrials.gov and the WHO ICTRP yielded no additional eligible studies.
We screened the remaining 4037 titles and abstracts for eligibility, removing 4025 as ineligible studies.
We read the full texts of the remaining 12 studies, of which all 12 were found to be ineligible and excluded. We identified no ongoing studies. No studies fulfilled the inclusion criteria or were eligible to be entered into a quantitative analysis.
Included studies
No studies met our inclusion criteria for this review.
Excluded studies
See Characteristics of excluded studies.
We excluded 12 studies in this review. Eight were of adult populations (Argoff 2015; Berry 1975; Coutinho 1975; Daniel 1971; Gilbert 1978; Lapane 2013; Mehl‐Madrona 1999; Middleton 1985), three evaluated acute pain (either abdominal or fracture injury) (Frohna 2005; Poonai 2014; Rose 2003), and one study did not investigate opioids (Valkenburg 2015).
Risk of bias in included studies
No studies were eligible for inclusion in this review, therefore we did not perform a 'Risk of bias' assessment.
Effects of interventions
No studies were eligible for inclusion in this review, therefore we were not able to comment on the efficacy or harm from the use of opioids to treat chronic non‐cancer pain in children and adolescents.
Due to the lack of evidence in this field, we were unable to judge the quality of evidence. There is no evidence to support or refute the use of opioids for treating chronic non‐cancer pain in children and adolescents.
Discussion
Summary of main results
We were unable to find any randomised controlled trials for inclusion in this review and so could not assess the efficacy or adverse events of opioid interventions to treat chronic non‐cancer pain in children and adolescents. There are known negative issues (as mentioned previously) related to the use of opioids for chronic non‐cancer pain in adults that may well apply to children, so caution is advised (Stannard 2016).
Overall completeness and applicability of evidence
As we could identify no randomised controlled trials, we are unable to comment about efficacy or harm from the use of opioids to treat chronic non‐cancer pain in children and adolescents. Similarly, we cannot comment on our remaining secondary outcomes: Carer Global Impression of Change; requirement for rescue analgesia; sleep duration and quality; acceptability of treatment; physical functioning; and quality of life.
The suite of reviews
This review is part of a suite of reviews on pharmacological interventions for chronic pain and cancer‐related pain in children and adolescents (Appendix 1). Taking a broader view on this suite of reviews, some pharmacotherapies (investigated in our other reviews) are likely to provide more data than others. The results were thus as expected considering that randomised controlled trials in children are known to be limited. The results have the potential to inform policymaking decisions for funding future clinical trials into opioid treatment of child and adolescent pain, therefore any results (large or small) are important in order to capture a snapshot of the current evidence for opioids.
Quality of the evidence
Due to the lack of evidence in this field, we were unable to judge the quality of evidence. There is no evidence to support or refute the use of opioids for treating chronic non‐cancer pain in children and adolescents.
This review shows that there is an absence of evidence from randomised controlled trials that opioids are effective in the management of chronic non‐cancer pain in children. While it may be the case that this absence of evidence reflects the inadequacy of opioids for this purpose and that their use as monotherapy analgesics is more likely to cause harm than benefit, the opposite may also pertain, as the data are lacking. It is difficult to conduct long‐term randomised controlled trials in children with chronic non‐cancer conditions, and few observational/clinical data have been published.
Potential biases in the review process
We carried out extensive searches of major databases using broad search criteria, and also searched two large clinical trial registries. We consider it to be unlikely that we have missed relevant studies.
Agreements and disagreements with other studies or reviews
We were not able to identify any published systematic reviews on this topic.

Study flow diagram.

