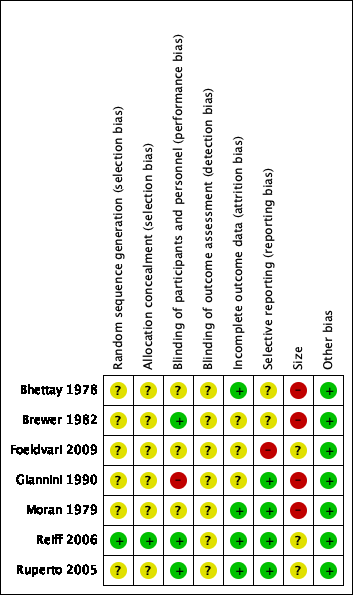
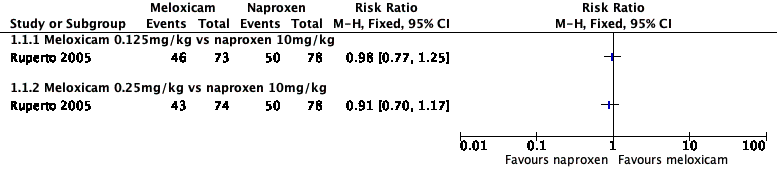
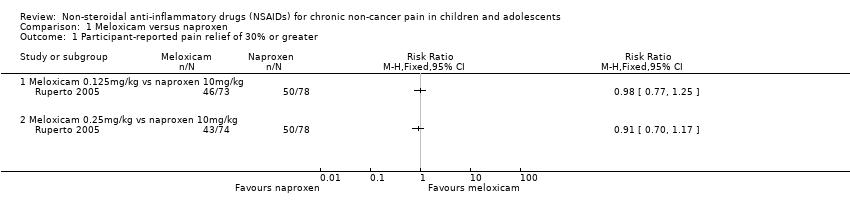
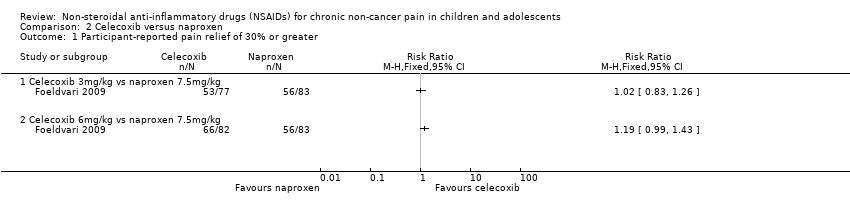
Contenido relacionado
Revisiones y protocolos relacionados
Tess E Cooper, Lauren C Heathcote, Brian Anderson, Marie‐Claude Grégoire, Gustaf Ljungman, Christopher Eccleston | 24 julio 2017
Tess E Cooper, Philip J Wiffen, Lauren C Heathcote, Jacqui Clinch, Richard Howard, Elliot Krane, Susan M Lord, Navil Sethna, Neil Schechter, Chantal Wood | 5 agosto 2017
Joseph F Standing, Imogen Savage, Deborah Pritchard, Marina Waddington | 2 julio 2015
Tess E Cooper, Lauren C Heathcote, Jacqui Clinch, Jeffrey I. Gold, Richard Howard, Susan M Lord, Neil Schechter, Chantal Wood, Philip J Wiffen | 5 agosto 2017
Ewan D McNicol, Emily Rowe, Tess E Cooper | 7 julio 2018
McKenzie C Ferguson, Roman Schumann, Sean Gallagher, Ewan D McNicol | 9 septiembre 2021
Sarah E Garner, Dogan Fidan, Ruth R Frankish, Maria Judd, Tanveer Towheed, Peter Tugwell, George A Wells | 24 enero 2005
Sheena Derry, Philip Conaghan, José António P Da Silva, Philip J Wiffen, R Andrew Moore | 22 abril 2016
Darin Jaturapatporn, Mokhtar Gad El Kareem Nasr Isaac, Jenny McCleery, Naji Tabet | 15 febrero 2012
Simon Bulley, Sheena Derry, R Andrew Moore, Henry J McQuay | 7 octubre 2009