Антиагреганты и антикоагулянты для первичной профилактики тромбозов у лиц с антифосфолипидными антителами
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study type: RCT Location: Iraq Time frame: September 2007 to July 2010 Setting: maternity teaching hospital, Erbil city, Kurdistan region, North of Iraq Number of centres: 1 Follow‐up: NR | |
| Participants | Total number of participants: 141 Recruitment method: maternity teaching hospital patients Informed consent: yes Inclusion criteria: aged 18 to 42 years at the time of interview; a history of at least 2 unexplained consecutive pregnancy losses before 20 weeks gestation; persistent presence of aCL antibodies or LA or both on 2 occasions 8 weeks apart Exclusion criteria: SLE, known peptic ulcer disease, sensitivity to ASA or heparin depending on patient's history report, previous venous thromboembolic disease requiring ongoing anticoagulant therapy, and failure to consent to participate Age mean (SD): control: 30.61 (6.325), intervention: 31.44 (5.8) Female: control 61 (100%), intervention 80 (100%) SLE: NR Cardiovascular risk factors: NR Antibodies present: LA, aCL, anti‐β2GPI (details NR) | |
| Interventions | Treatment groups: control: ASA 100 mg coated tablets, intervention: bemiparin 2500 IU anti‐Xa/0.2 mL solution for injection in prefilled syringes Duration of interventions: up to 36 weeks of gestation Concomitant treatment: yes ‐ folic acid | |
| Outcomes | Primary outcomes: live birth rate Secondary outcomes: obstetric complications, foetal and maternal adverse events Other outcomes: side effects | |
| Notes | Funding: NR Originally planned sample size: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation | High risk | Quote: "1st case attained the hospital complaining from RM and proved to have APS, was randomized to LDA group, the second case attained the hospital and filled the inclusion criteria; LMWH was prescribed to her, sometimes two cases of Heparin followed one case of LDA. All the cases received the drug randomly." Comment: women referred to the hospital were consecutively given LDA, then LMWH, sometimes in a 2:1 ratio. |
| Allocation concealment | High risk | Quote: "the 1st case attained the hospital complaining from RM and proved to have APS, was randomized to LDA group, the second case attained the hospital and filled the inclusion criteria" Comment: no information about concealment |
| Blinding of participants and personnel | Unclear risk | Comment: no blinding, no clear definitions or methods of measurements of outcomes, not possible to assess if lack of blinding could have influenced the outcome |
| Blinding of outcome assessment | Unclear risk | Comment: no blinding, no clear definitions or methods of measurements of outcomes, not possible to assess if lack of blinding could have influenced the outcome |
| Incomplete outcome data | Low risk | Comment: no missing outcome data |
| Selective reporting | High risk | Comment: no protocol available, side effects described in methods section not completely reported |
| Other sources of bias | High risk | Comment: 1. Low‐dose aspirin given before pregnancy in the LDA group. Bemiparin group received treatment after pregnancy was diagnosed. No details on the structure of the groups in terms of diabetes. 2. No sample size calculation. |
| Methods | Study type: RCT, minimisation Location: UK, other countries in Europe, Mexico Time frame: February 2001 to June 2006 Setting: 5 tertiary referral centres in the UK, 8 tertiary referral centres and 1 district hospital in other European countries, 1 tertiary referral centre in Mexico Number of centres: 15 Follow‐up: control group: 37.2 months (median); intervention group: 32.4 months (median) | |
| Participants | Total number of participants: 232 Recruitment method: locally in 14 centres Informed consent: yes Inclusion criteria: (i) presence of aPLs (medium or high titres of aCL defined as IgG > 20 GPL and/or IgM > 20 MPL and/or LA positive) on at least 2 occasions, with an interval of 6 weeks, during the year previous to inclusion in the study; (ii) SLE patients meeting 4 or more ACR criteria for the classification of SLE and/or patients with a history of pregnancy morbidity as defined in the APS Sapporo classification criteria; and (iii) age between 18 and 65 years Exclusion criteria: positive for aPLs but without SLE or obstetric APS, previous thrombotic events, uncontrolled hypertension, active gastric or duodenal ulceration, platelets < 50,000/mm3, hepatic failure, severe illness, allergy to ASA, warfarin, current pregnancy Age mean (SD): control: 37.8 (10.7), intervention 37.8 (106) Female: control 80 (96%), intervention 80 (95%) SLE: control 62 (75%), intervention 62 (73%) Cardiovascular risk factors: Control: hypertension 9 (11%), hypercholesterolaemia 8 (10%), hypertriglyceridaemia 0 (0%), smoking 23 (29%), obesity 6 (7%) Intervention: hypertension 10 (12%), hypercholesterolaemia 12 (14%), hypertriglyceridaemia 4 (5%), smoking 24 (31%), obesity 12 (14%) Antibodies present: Control: LA 47/68 (69%), aCL IgG 31/67 (46%) IgM 11/66 (16%), anti‐β2GPI: IgG 10/57 (17.5%) IgM 4/57 (7%) Intervention: LA 53/64 (83%), aCL IgG 23/62 (37%) IgM 13/61 (21%), anti‐β2GPI: IgG 13/58 (22.4%) IgM 6/58 (10%) | |
| Interventions | Treatment groups: control: ASA 75 to 125 mg, intervention: ASA 75 to 125 mg and warfarin (target INR = 1.5, range 1.3 to 1.7) (ASA dose depending on the preparation available in the participant country) Duration of interventions: 37.2 (median months) Concomitant treatment: no | |
| Outcomes | Primary outcomes: thrombosis (objectively verified thrombotic events; the following investigations were performed in order to document the event: ultrasonography or venography for deep vein thrombosis, spiral CT scan or radionuclide lung scan or angiography for pulmonary embolism, MRI or angiography for thrombosis in intracerebral vessels, ophthalmological examination and fluorescein angiography (where possible) for retinal thrombosis, and arteriography for peripheral or mesenteric arterial thrombosis; for the diagnosis of myocardial infarction the World Health Organization (WHO) classification was followed, where two‐thirds of the following criteria were required: (i) ECG characteristic changes (2 mm ST increase in ECG leads V4‐V6 or 1 mm in I, II and aVF); (ii) ischaemic chest pain lasting > 30 min; and (iii) increase in cardiac enzymes (at least twice their normal value), amaurosis fugax was defined as sudden monocular blindness lasting < 24 h and transient ischaemic attack as neurological symptoms or signs lasting < 24 h) Secondary outcomes: clinical and serological risk factors for thrombosis, side effects of medications, death of any cause Other outcomes: NR | |
| Notes | Funding: Arthritis Research UK (clinical trial grant 15600) Originally planned sample size: 1000 We attempted to contact Dr Cuadrado for results clarification but were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation | Low risk | Comment: randomisation carried out using minimisation protocol |
| Allocation concealment | Low risk | Comment: allocation by independent individual, explicit statement that treatment allocation was concealed |
| Blinding of participants and personnel | Low risk | Comment: no blinding, outcome not likely to be influenced |
| Blinding of participants and personnel | Unclear risk | Comment: no blinding, unclear outcome definition |
| Blinding of participants and personnel | Low risk | Comment: no blinding, however outcome verified by objective methods, not likely to be influenced |
| Blinding of participants and personnel | High risk | Comment: no blinding, outcome is not objectively verified and likely to be influenced |
| Blinding of outcome assessment | Low risk | Comment: no blinding, however outcome verified by objective methods, not likely to be influenced |
| Blinding of outcome assessment | Low risk | Comment: no blinding, outcome not likely to be influenced |
| Blinding of outcome assessment | Unclear risk | Comment: no blinding, unclear outcome definition |
| Blinding of outcome assessment | High risk | Comment: no blinding, outcome is not objectively verified and likely to be influenced |
| Incomplete outcome data | High risk | Comment: approximately 10% more participants in the experimental group than in the control group have follow‐up shorter than 1 year |
| Selective reporting | Low risk | Comment: all outcomes reported |
| Other sources of bias | High risk | Comment: low recruitment rate, sample size not achieved |
| Methods | Study type: RCT Location: Greece Time frame: March 2002 to March 2006 Setting: university department Number of centres: 1 Follow‐up: up to 2 months postpartum | |
| Participants | Total number of participants: 85 Recruitment method: patients referred to the authors' department Informed consent: yes Inclusion criteria: age 18 to 39 years; >= 3 consecutive spontaneous abortions before 10 weeks of gestation, and positive aPL antibodies (aCL antibody of IgG and/or IgM isotype in blood, present in medium or high titres, or on 2 or more occasions at least 6 weeks apart; and LA present in plasma, on 2 or more occasions at least 6 weeks apart) Exclusion criteria: SLE; ASA allergy or sensitivity to ASA; a chromosomal or anatomic abnormality or a luteal phase defect; confirmed peptic ulcer; previous thromboembolism, hypertension, or current treatment with antihypertensive drugs; previous prednisone therapy; an abnormal chest radiograph result; or a positive result of a tuberculin skin test Age mean (SD): intervention 31.1 (1), control 32 (0.8) Female: intervention 40 (100%), control 38 (100%) SLE: NR Cardiovascular risk factors: NR Antibodies present: LA, aPL, anti‐β2GPI (details NR) | |
| Interventions | Treatment groups: Control: IVIG 400 mg/kg as soon as positive pregnancy test every 28 days until 32 weeks of gestation Intervention: ASA 75 mg/d and 4500 IU of heparin (Innohep) as soon as woman had a positive pregnancy test result (LMWH dose adapted so that factor Xa levels were within the recommended prophylactic range); ASA was discontinued at 32 weeks of gestation or at the time of an abortion, and heparin at 38 weeks of gestation or at the time of an abortion Duration of interventions: until 32/38 weeks of gestation or abortion Concomitant treatment: control group: calcium 500 mg/day | |
| Outcomes | Primary outcomes: live birth Secondary outcomes: maternal adverse effects during pregnancy and postdelivery (haemorrhages, pregnancy‐associated hypertension, fractures during pregnancy or up to 2 months postpartum, reduced maternal bone mineral density, and death) Other outcomes: NR | |
| Notes | Funding: NR Originally planned sample size: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation | Low risk | Comment: randomisation was performed in blocks of 8 and 6 using random number tables |
| Allocation concealment | Unclear risk | Comment: no information about concealment |
| Blinding of participants and personnel | Low risk | Comment: no information, outcome not likely to be influenced by lack of blinding |
| Blinding of participants and personnel | Unclear risk | Comment: no information about blinding, not clear how the outcome was assessed |
| Blinding of participants and personnel | Unclear risk | Comment: no information about blinding, not clear how the outcomes were assessed, therefore not possible to assess the influence of lack of blinding |
| Blinding of outcome assessment | Unclear risk | Comment: no information about blinding, not clear how the outcome was assessed |
| Blinding of outcome assessment | Low risk | Comment: no blinding, but lack of blinding probably did not influence the outcome |
| Blinding of outcome assessment | Unclear risk | Comment: no information about blinding, no information about the assessment of outcomes or their definition, therefore not possible to assess if lack of blinding could have influenced outcome |
| Incomplete outcome data | High risk | Comment: participants excluded from the analysis after randomisation due to non‐compliance or discontinuations. Intention‐to‐treat analysis cited for primary outcome, but not explained. |
| Selective reporting | Unclear risk | Comment: no protocol available, all outcomes described in methods section were reported |
| Other sources of bias | Low risk | Comment: no other bias identified |
| Methods | Study type: RCT, double‐blind Location: NR Time frame: June 2001 to April 2005 Setting: NR Number of centres: 3 Follow‐up: 2.30 +/‐ 0.95 years | |
| Participants | Total number of participants: 98 Recruitment method: patients from clinic and collaborative centres Informed consent: yes Inclusion criteria: individuals who were >= 18 years of age, with or without systemic autoimmune diseases, who fulfilled at least 1 of the following criteria: a positive LA test result, as defined by the International Society on Thrombosis and Haemostasis on >= 2 occasions, at least 6 weeks apart, and/or positive aCL antibodies (IgG/IgM/IgA) >= 20 units on >= 2 occasions at least 6 weeks apart Exclusion criteria: diagnosis of APS based on the original Sapporo classification criteria; a history of thrombosis, pulmonary embolism, or transient ischaemic attack; use of regular‐dose warfarin or an antiplatelet agent (including ASA); ASA allergy; history of bleeding within the last 5 years that required hospitalisation or blood transfusion or both; severe thrombocytopenia, active gastric/duodenal ulcer; active malignancy, chronic viral infection with HIV or hepatitis C; pregnancy Age mean (SD): intervention 43.1 (12.8), control 42.7 (14) Female: intervention 44 (92%), control 44 (88%) SLE: intervention 30 (63%), control 34 (68%) Cardiovascular risk factors: Intervention: hypertension 12 (25%), diabetes 4 (8%), hyperlipidaemia/dyslipidaemia 3 (6%), smoking 10 (21%) Control: hypertension 10 (20%), diabetes 2 (4%), hyperlipidaemia/dyslipidaemia 2 (4%), smoking 4 (8%) Antibodies present: information on high risk/low‐risk antibodies only (the low‐risk aPL profile was defined as IgG, IgM, or IgA 20 to 39 units; the high‐risk aPL profile was defined as IgG, IgM, or IgA 40 units and/or positive LA test results) | |
| Interventions | Treatment groups: intervention: ASA 81 mg daily, control: placebo Duration of interventions: 2.27 +/‐ 0.91 (ASA) vs 2.33 +/‐ 0.99 (control) Concomitant treatment: HCQ, steroids, NSAID (57%, 33%, 40% respectively) | |
| Outcomes | Primary outcomes: acute thrombosis (stroke confirmed by neuroimaging, deep vein thrombosis by Doppler ultrasonography, pulmonary embolism by CT scan (ventilation/perfusion or spiral)), acute MI confirmed by ECG and increased cardiac enzymes Secondary outcomes: TIA (transient focal neurologic abnormalities with negative neuroimaging study) Other outcomes: ASA‐related adverse events (abdominal pain, nausea, vomiting, anorexia, heartburn, dyspepsia, any bleeding) | |
| Notes | Funding: New York Chapter of the Arthiritis Foundation and New York Community Trust (Bayer Pharmaceutical provided ASA and placebo) Originally planned sample size: 220 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation | Low risk | Comment: computer‐generated stratified randomisation |
| Allocation concealment | Unclear risk | Comment: details not provided |
| Blinding of participants and personnel | Low risk | Comment: investigators, co‐ordinators, participants blinded |
| Blinding of outcome assessment | Low risk | Comment: outcomes clearly defined and confirmed objectively or assessors blinded (participants) |
| Incomplete outcome data | Unclear risk | Comment: insufficient information about analysis of data for participants lost to follow‐up |
| Selective reporting | Unclear risk | Comment: no protocol available |
| Other sources of bias | High risk | Comment: Bayer was the sponsor of the study ‐ potential risk of favouring the ASA group. Initially planned sample size was 110 per group, after interim analysis it was estimated as 30,363 in each arm; the study included a total of 98 participants. |
| Methods | Study type: RCT Location: UK Time frame: January 1997 to January 2000 Setting: miscarriage clinic Number of centres: 1 Follow‐up: NR | |
| Participants | Total number of participants: 98 Recruitment method: miscarriage clinic patients Informed consent: NR Inclusion criteria: women, 18 to 41 years, at least 3 consecutive pregnancy losses or 2 consecutive losses with proven foetal death after 10 weeks’ gestation; 2 positive tests for aPL antibody more than 6 weeks apart (with positive levels defined as > 9 U/mL for IgG and > 5 U/mL for IgM) Exclusion criteria: parental chromosomal abnormality, uterine anomaly, previous arterial or venous thrombosis, use of steroids during pregnancy, systemic lupus erythematosus requiring medication or complicated by nephritis, and other thrombophilia such as activated protein C resistance or protein C/S deficiency Age mean (SD): control 33 (4.9), intervention 33 (4.8) Female: control 47 (100), intervention 51 (100) SLE: NR Cardiovascular risk factors: NR Antibodies present: Control: LA 18 (38%), aCL 7 (15%) Intervention: LA 23 (45%), aCL 9 (18%) | |
| Interventions | Treatment groups: control: ASA 75 mg/d, intervention: ASA 75 mg/d + LMWH 5000 U sc Duration of interventions: from before 12 weeks' gestation until delivery Concomitant treatment: NR | |
| Outcomes | Primary outcomes: live birth Secondary outcomes: other pregnancy‐related outcomes Other outcomes: maternal thrombosis | |
| Notes | Funding: LUPUS UK and NHS R&D (NWEST) Originally planned sample size: 220 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation | Low risk | Comment: computer‐generated randomisation, blocks of 20 |
| Allocation concealment | Low risk | Comment: telephone randomisation to the Trials Unit at the Centre for Cancer Epidemiology at the University of Manchester |
| Blinding of participants and personnel | Unclear risk | Comment: no information about blinding, not clear how the outcome was assessed |
| Blinding of outcome assessment | Unclear risk | Comment: no information about blinding, not clear how the outcome was assessed |
| Incomplete outcome data | Low risk | Comment: no missing data, ITT analysis, 24 not adherent but included in the analysis |
| Selective reporting | Unclear risk | Comment: no protocol available |
| Other sources of bias | High risk | Comment: sample size calculated by the authors (n = 220) not achieved; interim analysis mentioned as planned, but not reported |
| Methods | Study type: RCT Location: Egypt Time frame: December 2008 to May 2010 Setting: Obstetrics and Gynecology Department, Cairo University Hospital, Egypt Number of centres: 1 Follow‐up: NR | |
| Participants | Total number of participants: 60 Recruitment method: clinic patients Informed consent: yes Inclusion criteria: minimum of 3 consecutive pregnancy losses before 10 weeks’ gestation and positive LA and/or aCL antibodies (IgG and IgM) on at least 2 occasions at least 12 weeks apart Exclusion criteria: paternal chromosomal abnormalities or uterine abnormalities (detected by hysterosalpingography, saline infusion sonography or office hysteroscopy), luteal phase defect, abnormal thyroid function tests, hyperprolactinaemia, polycystic ovary syndrome, SLE, previous thromboembolism, peptic ulcer, age < 19 years or > 37 years, BMI < 19 or > 30, or sensitivity to ASA or heparin Age mean (SD): intervention 27.13 (3.67), control 28.93 (4.18) Female: intervention 30 (100%), control 30 (100%) SLE: NR Cardiovascular risk factors: NR Antibodies present: Intervention: LA 10 (33%), aCL IgG 8 (27%) IgM 4 (20%) Control: LA 9 (30%), aCL IgG 6 (20%) IgM 5 (17%) | |
| Interventions | Treatment groups: intervention: enoxaparin 40 mg/day + ASA 75 mg/day, control: enoxaparin 20 mg/day + ASA 75 mg/day Duration of interventions: whole pregnancy Concomitant treatment: prenatal vitamins, oral calcium (600 mg twice daily), vitamin D | |
| Outcomes | Primary outcomes: live birth rate Secondary outcomes: excessive bleeding, thrombocytopenia, IUGR; spontaneous osteoporotic fractures; preterm delivery, pre‐eclampsia; intrauterine foetal death; neonatal bleeding; congenital anomalies, thrombotic event Other outcomes: NR | |
| Notes | Funding: NR Originally planned sample size: calculated 410, reduced to 60 due to time constraints | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation | Low risk | Comment: computer‐generated random numbers |
| Allocation concealment | Low risk | Comment: concealed in opaque envelopes |
| Blinding of participants and personnel | Unclear risk | Comment: no blinding, insufficient definition |
| Blinding of participants and personnel | Unclear risk | Comment: no blinding, no definition of thrombotic event is given |
| Blinding of participants and personnel | Unclear risk | Comment: no blinding, insufficient definition |
| Blinding of outcome assessment | Unclear risk | Comment: insufficient information to judge (no information about outcome assessment) |
| Blinding of outcome assessment | Unclear risk | Comment: no blinding, insufficient definition |
| Blinding of outcome assessment | Unclear risk | Comment: no blinding, insufficient definition |
| Incomplete outcome data | Low risk | Comment: no missing outcome data |
| Selective reporting | Unclear risk | Comment: no protocol available, most of the prespecified outcomes were reported |
| Other sources of bias | High risk | Comment: low recruitment rate, sample size not achieved |
| Methods | Study type: RCT Location: Egypt Time frame: June 2006 to December 2009 Setting: Cairo University Number of centres: 2 Follow‐up: NR | |
| Participants | Total number of participants: 60 Recruitment method: hospital patients Informed consent: yes Inclusion criteria: a history of 3 or more consecutive spontaneous abortions before 10 weeks of gestation, and positive LA and/or aCL antibodies (IgG and IgM) on 2 or more occasions at least 12 weeks apart; age between 18 and 37 years; and BMI between 19 and 29 Exclusion criteria: paternal chromosomal abnormalities; uterine malformation detected by hysterosalpingography or office hysteroscopy; cervical incompetence; luteal phase defect; abnormal thyroid function tests; hyperprolactinaemia; polycystic ovary syndrome; hereditary thrombophilia; SLE; previous venous or arterial thrombotic episodes; diabetes mellitus; kidney or liver disease; gastric ulcer; and sensitivity to ASA, UFH, or enoxaparin Age mean (SD): intervention 27.47 (SD 3.2), control 28.57 (SD 3.48) Female: intervention 30 (100%), control 30 (100%) SLE: NR Cardiovascular risk factors: NR Antibodies present: Intervention: LA 12 (40%), aCL IgG 7 (23%) IgM 5 (17%) Control: LA 10 (33%), aCL IgG 5 (17%) IgM 8 (27%) | |
| Interventions | Treatment groups: intervention: enoxaparin 40 mg/day sc + ASA 75 mg/day, control: heparin calcium 5000 IU sc, twice daily + ASA 75 mg/day Duration of interventions: whole pregnancy Concomitant treatment: prenatal vitamins, 600 mg oral calcium twice daily, and 400 IU vitamin D3 twice daily | |
| Outcomes | Primary outcomes: live birth rate Secondary outcomes: excessive haemorrhage, thrombocytopenia, IUGR, intrauterine foetal death, preterm delivery, neonatal bleeding, congenital anomalies, pre‐eclampsia, spontaneous osteoporotic fractures Other outcomes: NR | |
| Notes | Funding: NR Originally planned sample size: calculated 1447, reduced to 60 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation | Low risk | Comment: computer‐generated randomisation list |
| Allocation concealment | Low risk | Comment: opaque, sealed envelopes |
| Blinding of participants and personnel | Low risk | Comment: open‐label study, but outcome clearly defined and objectively confirmed, so not likely to be influenced by lack of blinding |
| Blinding of participants and personnel | Unclear risk | Comment: open‐label study, no clear definition of the outcome and assessment |
| Blinding of participants and personnel | Unclear risk | Comment: open‐label study, no clear definition of the outcome and assessment |
| Blinding of outcome assessment | Unclear risk | Comment: open‐label study, no clear definition of the outcome and assessment |
| Blinding of outcome assessment | Low risk | Comment: open‐label study, but outcome clearly defined and objectively confirmed, so not likely to be influenced by lack of blinding |
| Blinding of outcome assessment | Unclear risk | Comment: open‐label study, no clear definition of the outcome and assessment |
| Incomplete outcome data | Low risk | Comment: no missing outcome data |
| Selective reporting | Unclear risk | Comment: no protocol, all outcomes reported |
| Other sources of bias | High risk | Comment: low recruitment rate (estimated: 1447; recruited: 60) |
| Methods | Study type: RCT Location: Egypt Time frame: January 2011 to June 2013 Setting: Women's Health Hospital, Assiut University, Egypt Number of centres: 1 Follow‐up: to 6 weeks after delivery | |
| Participants | Total number of participants: 180 Recruitment method: gynaecology outpatient clinic patients Informed consent: yes Inclusion criteria: diagnosis of APS was made on the basis of 2 positive test results, at least 12 weeks apart, for the presence of either LA or aCL antibodies and a history of 3 or more consecutive first‐trimester (≤ 13 weeks) spontaneous abortions, or 2 or more second‐trimester spontaneous abortions (13 to 24 weeks) with the same partner Exclusion criteria: history of thromboembolic events, bleeding tendencies, hypersensitivity to ASA or enoxaparin, congenital anomalies of the uterus, cervical insufficiency, uncontrolled diabetes mellitus, or chromosomal anomalies affecting either participants or their partners, pregnancy at study enrolment following the use of assisted reproductive techniques Age mean (SD): intervention 25.5 (4.7), control 27.6 (4.8) Female: intervention 90 (100%), control 90 (100%) SLE: NR Cardiovascular risk factors: NR Antibodies present: NR | |
| Interventions | Treatment groups: intervention: 40 mg of enoxaparin sc daily + 81 mg of ASA daily, control: placebo Duration of interventions: from preconception to 6 weeks after delivery Concomitant treatment: folic acid, calcium | |
| Outcomes | Primary outcomes: rate of live birth after 24 weeks of pregnancy, clinical pregnancy rate at 0 to 6 months, clinical pregnancy rate at 6 to 12 months Secondary outcomes: rate of first‐ and second‐trimester spontaneous abortion, vaginal bleeding during pregnancy, pre‐eclampsia, pregnancy‐induced hypertension, abruptio placentae, preterm delivery, IUGR Other outcomes: complications of enoxaparin use (maternal or neonatal bleeding or both, heparin‐induced thrombocytopenia, pain and bruising at injection sites, hypersensitivity to heparin, teratogenicity), and thromboembolic events | |
| Notes | Funding: NR Originally planned sample size: 180 We obtained additional information and clarification of results from Dr Ismail. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation | Low risk | Quote: "Using a computer‐generated sequence, participants were randomly allocated in a 1:1 ratio to receive enoxaparin plus aspirin or placebo.(...) A minimization procedure employing a computer‐based algorithm was applied to avoid chance imbalances in important stratification variables" |
| Allocation concealment | Low risk | Quote: "To ensure allocation concealment, an independent secretary stored all the sealed envelopes containing each participant’s group assignment; all envelopes were kept closed until data analysis was completed. Additionally, a central telephone system was used whereby an independent secretary spoke with patients to arrange all follow‐up visits." Comment: allocation concealment properly implemented |
| Blinding of participants and personnel | Low risk | Quote: "Study drugs and placebo were prepared by the pharmacy department of the study institution, with all placebo treatments manufactured to be identical to study medications. Study drugs and placebo were stored in identical ampoules and as tablets of identical size, shape, and colour; study treatments were distributed to participants by study institution staff without informing them of their treatment assignment. Clinicians, investigators, and data analysts were masked to the group assignments." Comment: blinding implemented appropriately |
| Blinding of outcome assessment | Unclear risk | Comment: no clear definition of outcome and assessment, no information about blinding of outcome assessors |
| Blinding of outcome assessment | Low risk | Comment: no blinding but lack of blinding would not have influenced the outcome, as clear definition of outcome provided |
| Blinding of outcome assessment | Low risk | Comment: participants, clinicians, and investigators were reported to be blinded |
| Incomplete outcome data | Low risk | Comment: similar percentage of participants lost to follow up, ITT analysis |
| Selective reporting | Unclear risk | Comment: no protocol, no information about thrombotic events |
| Other sources of bias | Low risk | Comment: no other sources of bias |
| Methods | Study type: RCT Location: UK Time frame: April 1993 to July 1995 Setting: recurrent miscarriage clinic at St Mary's Hospital in London Number of centres: 1 Follow‐up: NR | |
| Participants | Total number of participants: 90 Recruitment method: recurrent miscarriage clinic patients Informed consent: yes Inclusion criteria: a history of 3 or more consecutive miscarriages and positive results for aPL antibodies on at least 2 occasions more than 8 weeks apart before becoming pregnant Exclusion criteria: previous thromboembolism, SLE, a uterine abnormality, hypersecretion of luteinising hormone, and multiple pregnancy, abnormal karyotype Age mean (range): control 34 (22 to 44), intervention 32 (23 to 40) Female: control 45 (100%), intervention 45 (100%) SLE: NR Cardiovascular risk factors: NR Antibodies present: Control: LA 34 (76%), aCL 5 (11%) Intervention: LA 40 (89%), aCL 3 (7%) | |
| Interventions | Treatment groups: control: ASA 75 mg/day, intervention: ASA 75 mg/day + calcium heparin 5000 IU sc daily Duration of interventions: up to 34 weeks gestation Concomitant treatment: NR | |
| Outcomes | Primary outcomes: live births Secondary outcomes: premature delivery (before 37 weeks of gestation) Other outcomes: safety, adverse events | |
| Notes | Funding: Arthritis and Rheumatism Council Originally planned sample size: 80 to 90 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation | Low risk | Quote: "Patients were randomly assigned in equal proportion to the two treatment groups by means of a computer generated random number list (Systat 5.2.1; Macintosh)." Comment: computer‐generated random number list |
| Allocation concealment | Low risk | Quote: "The randomisation list was kept by an independent member of staff not involved in the trial." Comment: only a person outside the study had access |
| Blinding of participants and personnel | Unclear risk | Comment: lack of blinding, definition of outcome unclear, therefore not possible to judge if lack of blinding could have influenced the outcomes |
| Blinding of outcome assessment | Unclear risk | Comment: lack of blinding, definition of outcome unclear, therefore not possible to judge if lack of blinding could have influenced the outcomes |
| Incomplete outcome data | Low risk | Comment: no missing data |
| Selective reporting | Unclear risk | Comment: no protocol available |
| Other sources of bias | Low risk | Comment: no other sources of bias |
aCL: anticardiolipin
ACR: American College of Rheumatology
anti‐β2GPI: anti‐beta2‐glycoprotein I
aPL: antiphospholipid
APS: antiphospholipid syndrome
ASA: acetylsalicylic acid
BMI: body mass index
CT: computed tomography
ECG: electrocardiography
GPI: glycoprotein I
GPL: G phospholipids
HCQ: hydroxychloroquine
IgA: immunoglobulin A
IgG: immunoglobulin G
IgM: immunoglobulin M
INR: international normalised ratio
ITT: intention‐to‐treat
IUGR: intrauterine growth restriction
LA: lupus anticoagulant
LDA: low‐dose aspirin
LMWH: low molecular weight heparin
MI: myocardial infarction
MPL: M phospholipids
MRI: magnetic resonance imaging
NR: not reported
NSAID: non‐steroidal anti‐inflammatory drugs
RCT: randomised controlled trial
RM: recurrent miscarriage
sc: subcutaneously
SD: standard deviation
SLE: systemic lupus erythematosus
TIA: transient ischaemic attack
UFH: unfractionated heparin
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Wrong patient population: aPL antibodies not confirmed | |
| Wrong patient population: secondary prevention | |
| Wrong outcomes: obstetric failure only | |
| Wrong patient population: secondary prevention | |
| Wrong outcomes: only obstetric outcomes, thromboembolism not assessed | |
| Wrong outcomes: obstetric failure only | |
| Wrong patient population: secondary prevention | |
| Wrong outcomes: prothrombin fragment 1 + 2 level (F1 + 2); thromboembolic events/bleeding not assessed | |
| Wrong patient population: secondary prevention | |
| Wrong study design: non‐RCT | |
| Wrong patient population: secondary prevention | |
| Wrong study design: non‐RCT | |
| Wrong intervention: no anticoagulant/antiplatelet intervention | |
| Wrong study design: non‐randomised comparative study | |
| Wrong study design: non‐RCT | |
| Wrong patient population: secondary prevention | |
| Wrong patient population: secondary prevention | |
| Wrong study design: non‐randomised trial | |
| Wrong patient population: secondary prevention | |
| Wrong outcomes: obstetric failure only | |
| Wrong patient population: secondary prevention | |
| Wrong patient population: study group consisted of aPL‐positive patients or people with other thrombophilia | |
| Wrong outcomes: only obstetric outcomes, thromboembolism not assessed | |
| Wrong outcomes: obstetric failure only | |
| Wrong outcomes: only effectiveness of implantation assessed | |
| Wrong intervention: no antiplatelet/anticoagulant intervention | |
| Wrong patient population: secondary prevention | |
| Wrong patient population: not primary prevention |
aPL: antiphospholipid
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A pilot study assessing the feasibility of a randomized controlled trial evaluating aspirin versus low‐molecular‐weight heparin (LMWH) and aspirin in women with antiphospholipid syndrome and pregnancy loss |
| Methods | Study type: multicentre, open‐label, randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome: the primary feasibility outcome of the pilot trial is the mean recruitment rate per centre per month. Secondary outcomes:
|
| Starting date | 6 November 2017 |
| Contact information | Contact: Leslie Skeith, MD, Veronica Whitham, BSc 613‐737‐8899 ext 71068 Ottawa Hospital Research Institute |
| Notes | Funding: Ottawa Hospital Research Institute NCT03100123 |
APS: antiphospholipid syndrome
ASA: acetylsalicylic acid
LMWH: low molecular weight heparin
sc: subcutaneously
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.25, 3.77] |
| Analysis 1.1 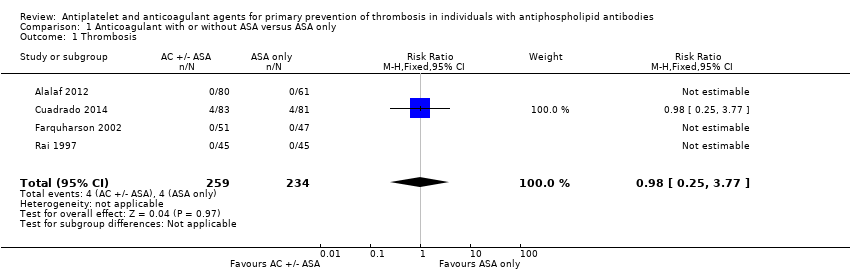 Comparison 1 Anticoagulant with or without ASA versus ASA only, Outcome 1 Thrombosis. | ||||
| 2 Minor bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Anticoagulant with or without ASA versus ASA only, Outcome 2 Minor bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 ASA only versus placebo, Outcome 1 Thrombosis. | ||||
| 2 Bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 ASA only versus placebo, Outcome 2 Bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 ASA with LMWH versus placebo or IVIG, Outcome 1 Thrombosis. | ||||
| 2 Bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 ASA with LMWH versus placebo or IVIG, Outcome 2 Bleeding. | ||||
| 2.1 Bleeding requiring transfusion | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.49, 164.76] |
| 2.2 Bleeding during first trimester | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.50, 1.61] |
| 2.3 Postpartum haemorrhage | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.60, 2.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.1  Comparison 4 ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH, Outcome 1 Thrombosis. | ||||
| 2 Major/excessive bleeding Show forest plot | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.2  Comparison 4 ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH, Outcome 2 Major/excessive bleeding. | ||||
| 3 Subcutaneous bruises Show forest plot | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.93] |
| Analysis 4.3 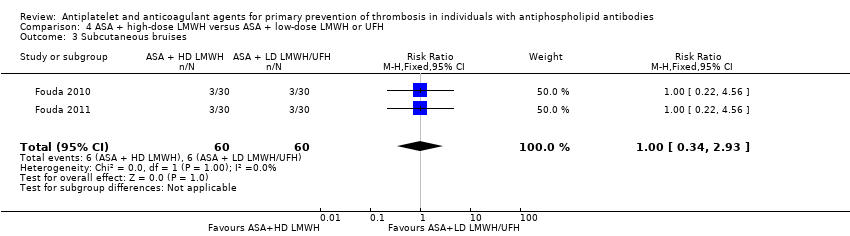 Comparison 4 ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH, Outcome 3 Subcutaneous bruises. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis best‐worst scenario Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.13, 1.19] |
| Analysis 5.1  Comparison 5 Sensitivity analyses anticoagulant with or without ASA versus ASA only, Outcome 1 Thrombosis best‐worst scenario. | ||||
| 2 Thrombosis worst‐best scenario Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.39 [1.55, 12.42] |
| Analysis 5.2  Comparison 5 Sensitivity analyses anticoagulant with or without ASA versus ASA only, Outcome 2 Thrombosis worst‐best scenario. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis best‐worst scenario Show forest plot | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.51] |
| Analysis 6.1  Comparison 6 Sensitivity analyses ASA with LMWH versus placebo or IVIG, Outcome 1 Thrombosis best‐worst scenario. | ||||
| 2 Thrombosis worst‐best scenario Show forest plot | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.4 [0.47, 151.34] |
| Analysis 6.2  Comparison 6 Sensitivity analyses ASA with LMWH versus placebo or IVIG, Outcome 2 Thrombosis worst‐best scenario. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Anticoagulant with or without ASA versus ASA only, Outcome 1 Thrombosis.

Comparison 1 Anticoagulant with or without ASA versus ASA only, Outcome 2 Minor bleeding.

Comparison 2 ASA only versus placebo, Outcome 1 Thrombosis.

Comparison 2 ASA only versus placebo, Outcome 2 Bleeding.

Comparison 3 ASA with LMWH versus placebo or IVIG, Outcome 1 Thrombosis.

Comparison 3 ASA with LMWH versus placebo or IVIG, Outcome 2 Bleeding.

Comparison 4 ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH, Outcome 1 Thrombosis.
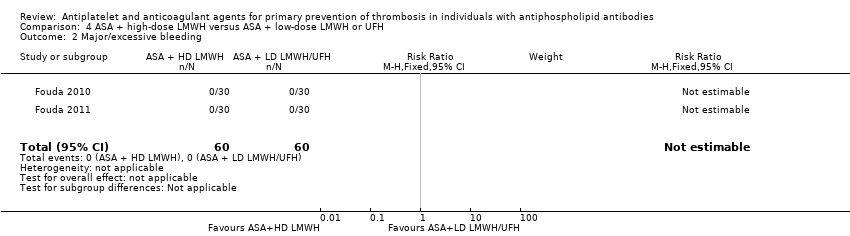
Comparison 4 ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH, Outcome 2 Major/excessive bleeding.

Comparison 4 ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH, Outcome 3 Subcutaneous bruises.
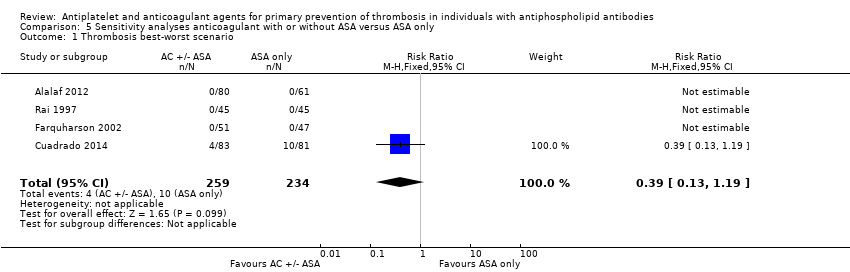
Comparison 5 Sensitivity analyses anticoagulant with or without ASA versus ASA only, Outcome 1 Thrombosis best‐worst scenario.

Comparison 5 Sensitivity analyses anticoagulant with or without ASA versus ASA only, Outcome 2 Thrombosis worst‐best scenario.

Comparison 6 Sensitivity analyses ASA with LMWH versus placebo or IVIG, Outcome 1 Thrombosis best‐worst scenario.

Comparison 6 Sensitivity analyses ASA with LMWH versus placebo or IVIG, Outcome 2 Thrombosis worst‐best scenario.
| Anticoagulant with or without ASA versus ASA only | |||||
| Patient or population: people with antiphospholipid antibodies and no history of thrombosis Settings: specialist centres Intervention: anticoagulant with or without ASA Comparison: ASA only | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| ASA | AC or AC + ASA | ||||
| Thrombosis Median follow‐up reported in 1 study: 37.2 months (ASA group) 32.4 (AC or AC + ASA group), no IQR given | 17 per 1000 | 16 per 1000 | RR 0.98 (0.25, 3.77) | 493 (4 RCTs) | ⊕⊕⊝⊝ |
| Bleeding ‐ major | No events reported. | No events reported. | Not estimable | 493 (4 RCTs) | ‐ |
| Bleeding ‐ minor (not requiring hospital admission) Median follow‐up reported in 1 study: 37.2 months (ASA group) 32.4 (AC or AC + ASA group), no IQR given | 0 per 1000 | 133 per 1000 (8 to 2294) | RR 22.45 (1.34, 374.81) | 164 (1 RCT) | ⊕⊕⊝⊝ |
| Mortality | Not reported | Not reported | Not reported | Not reported | ‐ |
| Quality of life | Not reported | Not reported | Not reported | Not reported | ‐ |
| Adverse event other than bleeding | In Cuadrado 2014, 4 cases of mild gastrointestinal symptoms (2 severe constipations and 2 stomach upsets, not clear if the same or different participants) in the ASA group and 1 case of an allergic reaction in the combination therapy group were reported. Farquharson 2002 and Alalaf 2012 reported no information about adverse events not related to bleeding or obstetric failure, while Rai 1997 reported that in both groups interventions were well tolerated and that in the heparin group there were no cases of thrombocytopenia or vertebral fractures. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AC: anticoagulant; ASA: acetylsalicylic acid; CI: confidence interval; IQR: interquartile range; RCT: randomised clinical trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to unclear or high risk of bias in more than one domain in all studies and one level due to wide 95% CI for both benefit and harm. | |||||
| ASA versus placebo | |||||
| Patient or population: people with antiphospholipid antibodies and no history of thrombosis Settings: university department and 3 unspecified centres Intervention: ASA Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo | ASA | ||||
| Thrombosis Mean follow‐up 2.3 +/‐ 0.95 years | 20 per 1000 | 105 per 1000 | RR 5.21 (0.63, 42.97) | 98 (1 RCT) | ⊕⊕⊝⊝ |
| Bleeding ‐ major | No events reported. | No events reported. | Not estimable | 98 (1 RCT) | ‐ |
| Bleeding ‐ minor Mean follow‐up 2.3 +/‐ 0.95 years | 20 per 1000 | 63 per 1000 | RR 3.13 (0.34, 29.01) | 98 (1 RCT) | ⊕⊕⊝⊝ |
| Mortality | Not reported | Not reported | Not reported | Not reported | ‐ |
| Quality of life | Not reported | Not reported | Not reported | Not reported | ‐ |
| Adverse event other than bleeding | Minor gastrointestinal disturbances occurred in 5 participants who received ASA compared to 1 participant in the placebo group. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASA: acetylsalicylic acid; CI: confidence interval; RCT: randomised clinical trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to risk of bias (unclear risk of bias for allocation concealment, selective reporting, and incomplete outcome data, and high risk of other bias) and one level because of single study and imprecision (wide 95% CI). | |||||
| ASA with LMWH versus placebo or IVIG | |||||
| Patient or population: people with antiphospholipid antibodies and no history of thrombosis Settings: university department and women's health hospital Intervention: ASA with LMWH Comparison: placebo or IVIG | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo or IVIG | ASA + LMWH | ||||
| Thrombosis Up to 2 months postpartum in Dendrinos 2009, not specified in Ismail 2016 | 0 participants developed thrombosis in both studies. | Not estimable | 258 (2 RCTs) | ‐ | |
| Bleeding ‐ major (bleeding requiring transfusion) Length of follow‐up not specified | 0 per 1000 | 45 per 1000 | RR 9.00 (0.49, 164.76) | 180 (1 RCT) | ⊕⊕⊕⊝ |
| Bleeding ‐ other (bleeding during first trimester) Length of follow‐up not specified | 212 per 1000 | 189 per 1000 | RR 0.89 (0.50, 1.61) | 180 (1 RCT) | ⊕⊕⊕⊝ |
| Bleeding ‐ other (postpartum haemorrhage) Length of follow‐up not specified | 112 per 1000 | 146 per 1000 | RR 1.30 (0.60, 2.81) | 180 (1 RCT) | ⊕⊕⊕⊝ |
| Mortality | Not reported | Not reported | Not reported | Not reported | ‐ |
| Quality of life | Not reported | Not reported | Not reported | Not reported | ‐ |
| Adverse events other than bleeding Follow‐up: up to 2 months postpartum | In 1 study examining pregnant women, 3 cases of nausea, hypotension, and tachycardia were reported in the IVIG (control) group, as compared to none in the intervention group. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASA: acetylsalicylic acid; CI: confidence interval; IVIG: intravenous immunoglobulin; LMWH: low molecular weight heparin; RCT: randomised clinical trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level because of single study and imprecision (wide 95% CI). | |||||
| ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH | |||||
| Patient or population: people with antiphospholipid antibodies and no history of thrombosis Settings: specialist centres Intervention: ASA + high‐dose LMWH Comparison: ASA + low‐dose LMWH or UFH | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| ASA + low‐dose LMWH or UFH | ASA + high‐dose LMWH | ||||
| Thrombosis | 0 participants developed thrombosis. | Not estimable | 120 (2 RCTs) | ‐ | |
| Bleeding ‐ major/excessive | 0 participants developed major/excessive bleeding episodes. | Not estimable | 120 (2 RCTs) | ‐ | |
| Subcutaneous bruises Follow‐up: entire pregnancy | 100 per 1000 | 100 per 1000 | RR 1.00 (0.34 to 2.93) | 120 (2 RCTs) | ⊕⊕⊝⊝ |
| Mortality | Not reported | Not reported | Not reported | Not reported | ‐ |
| Quality of life | Not reported | Not reported | Not reported | Not reported | ‐ |
| Adverse events other than bleeding | 1 case of skin allergy was reported in the ASA + UFH group. | ||||
| The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASA: acetylsalicylic acid; CI: confidence interval; LMWH: low molecular weight heparin; RCT: randomised clinical trial; RR: risk ratio; UFH: unfractionated heparin | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to risk of bias (unclear risk of bias for blinding and selective reporting, high risk for other bias) and one level due to small selected population (pregnant women) and imprecision (wide 95% CI). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.25, 3.77] |
| 2 Minor bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Bleeding requiring transfusion | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.49, 164.76] |
| 2.2 Bleeding during first trimester | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.50, 1.61] |
| 2.3 Postpartum haemorrhage | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.60, 2.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Major/excessive bleeding Show forest plot | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Subcutaneous bruises Show forest plot | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis best‐worst scenario Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.13, 1.19] |
| 2 Thrombosis worst‐best scenario Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.39 [1.55, 12.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis best‐worst scenario Show forest plot | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.51] |
| 2 Thrombosis worst‐best scenario Show forest plot | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.4 [0.47, 151.34] |

