Agentes antiplaquetarios y anticoagulantes para la prevención primaria de la trombosis en pacientes con anticuerpos antifosfolípidos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012534.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 13 julio 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vascular
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
MMB developed the concept of the study, contributed to the preparation of this review and agreed upon this final version.
EP developed the concept of the study, contributed to the preparation of this review and agreed upon this final version.
WL contributed to the preparation of this review and agreed upon this final version.
DWK contributed to the preparation of this review and agreed upon this final version.
KJ contributed to the preparation of this review and agreed upon this final version.
AU developed the concept of the study, contributed to the preparation of this review and agreed upon this final version.
Sources of support
Internal sources
-
Jagiellonian University Medical College, Poland.
External sources
-
Ministry of Science and Higher Education, Poland.
K/ZDS/007162; Core funding for statutory R&D activities
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
MMB receives honoraria as freelancer from a systematic review company that also works for pharmaceutical companies (Kleijnen Systematic Reviews Ltd). I am not aware of any direct conflict of interest.
EP: Abbott Vascular paid for her registration fee for the 20th International Congress of the Polish Cardiac Society.
WL has no known conflicts of interest.
DWK is an investigator in a clinical trial on drug‐resistant epilepsy conducted by UCB Pharma.
KJ has no known conflicts of interest.
AU received honoraria for lectures relating to anticoagulant therapy in Poland and had travel expenses covered by Bayer, Boehringer Ingelheim, Pfizer, and Sanofi‐Aventis.
Acknowledgements
We thank Mr Mateusz Swierz for help in preparing the Background section of the protocol of this review, Dr Karsten Juhl Jørgensen for comments on the draft protocol, and Ms Anna Witkowska for help in managing the references and obtaining full‐text articles. We thank Prof Pier L Meroni, Prof Munther A Khamashta, Prof Vittorio Pengo, Prof Phillippe de Moerloose, and representatives of Eli Lilly, Boehringer Ingelheim International, and Aspen Pharma for help with checking for additional studies.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Jul 13 | Antiplatelet and anticoagulant agents for primary prevention of thrombosis in individuals with antiphospholipid antibodies | Review | Malgorzata M Bala, Elżbieta Paszek, Wiktoria Lesniak, Dorota Wloch‐Kopec, Katarzyna Jasinska, Anetta Undas | |
| 2017 Feb 02 | Antiplatelet and anticoagulant agents for primary prevention of thrombosis in individuals with antiphospholipid antibodies | Protocol | Malgorzata M Bala, Elzbieta M Paszek, Dorota Wloch‐Kopec, Wiktoria Lesniak, Anetta Undas | |
Differences between protocol and review
In our protocol we planned to analyse obstetric failure among thrombotic events, but as this outcome was thoroughly addressed by other Cochrane Reviews, de Jong 2014; Empson 2005, we did not analyse it in this review.
Notes
We have based parts of the Methods section on a standard template established by Cochrane Vascular.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Anticoagulant with or without ASA versus ASA only, Outcome 1 Thrombosis.

Comparison 1 Anticoagulant with or without ASA versus ASA only, Outcome 2 Minor bleeding.

Comparison 2 ASA only versus placebo, Outcome 1 Thrombosis.

Comparison 2 ASA only versus placebo, Outcome 2 Bleeding.

Comparison 3 ASA with LMWH versus placebo or IVIG, Outcome 1 Thrombosis.

Comparison 3 ASA with LMWH versus placebo or IVIG, Outcome 2 Bleeding.

Comparison 4 ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH, Outcome 1 Thrombosis.
Comparison 4 ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH, Outcome 2 Major/excessive bleeding.

Comparison 4 ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH, Outcome 3 Subcutaneous bruises.
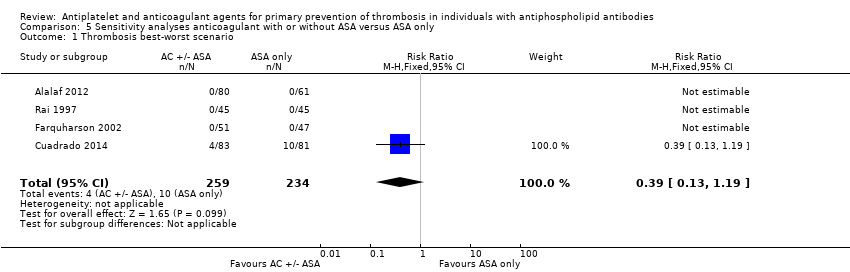
Comparison 5 Sensitivity analyses anticoagulant with or without ASA versus ASA only, Outcome 1 Thrombosis best‐worst scenario.

Comparison 5 Sensitivity analyses anticoagulant with or without ASA versus ASA only, Outcome 2 Thrombosis worst‐best scenario.

Comparison 6 Sensitivity analyses ASA with LMWH versus placebo or IVIG, Outcome 1 Thrombosis best‐worst scenario.

Comparison 6 Sensitivity analyses ASA with LMWH versus placebo or IVIG, Outcome 2 Thrombosis worst‐best scenario.
| Anticoagulant with or without ASA versus ASA only | |||||
| Patient or population: people with antiphospholipid antibodies and no history of thrombosis Settings: specialist centres Intervention: anticoagulant with or without ASA Comparison: ASA only | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| ASA | AC or AC + ASA | ||||
| Thrombosis Median follow‐up reported in 1 study: 37.2 months (ASA group) 32.4 (AC or AC + ASA group), no IQR given | 17 per 1000 | 16 per 1000 | RR 0.98 (0.25, 3.77) | 493 (4 RCTs) | ⊕⊕⊝⊝ |
| Bleeding ‐ major | No events reported. | No events reported. | Not estimable | 493 (4 RCTs) | ‐ |
| Bleeding ‐ minor (not requiring hospital admission) Median follow‐up reported in 1 study: 37.2 months (ASA group) 32.4 (AC or AC + ASA group), no IQR given | 0 per 1000 | 133 per 1000 (8 to 2294) | RR 22.45 (1.34, 374.81) | 164 (1 RCT) | ⊕⊕⊝⊝ |
| Mortality | Not reported | Not reported | Not reported | Not reported | ‐ |
| Quality of life | Not reported | Not reported | Not reported | Not reported | ‐ |
| Adverse event other than bleeding | In Cuadrado 2014, 4 cases of mild gastrointestinal symptoms (2 severe constipations and 2 stomach upsets, not clear if the same or different participants) in the ASA group and 1 case of an allergic reaction in the combination therapy group were reported. Farquharson 2002 and Alalaf 2012 reported no information about adverse events not related to bleeding or obstetric failure, while Rai 1997 reported that in both groups interventions were well tolerated and that in the heparin group there were no cases of thrombocytopenia or vertebral fractures. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AC: anticoagulant; ASA: acetylsalicylic acid; CI: confidence interval; IQR: interquartile range; RCT: randomised clinical trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to unclear or high risk of bias in more than one domain in all studies and one level due to wide 95% CI for both benefit and harm. | |||||
| ASA versus placebo | |||||
| Patient or population: people with antiphospholipid antibodies and no history of thrombosis Settings: university department and 3 unspecified centres Intervention: ASA Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo | ASA | ||||
| Thrombosis Mean follow‐up 2.3 +/‐ 0.95 years | 20 per 1000 | 105 per 1000 | RR 5.21 (0.63, 42.97) | 98 (1 RCT) | ⊕⊕⊝⊝ |
| Bleeding ‐ major | No events reported. | No events reported. | Not estimable | 98 (1 RCT) | ‐ |
| Bleeding ‐ minor Mean follow‐up 2.3 +/‐ 0.95 years | 20 per 1000 | 63 per 1000 | RR 3.13 (0.34, 29.01) | 98 (1 RCT) | ⊕⊕⊝⊝ |
| Mortality | Not reported | Not reported | Not reported | Not reported | ‐ |
| Quality of life | Not reported | Not reported | Not reported | Not reported | ‐ |
| Adverse event other than bleeding | Minor gastrointestinal disturbances occurred in 5 participants who received ASA compared to 1 participant in the placebo group. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASA: acetylsalicylic acid; CI: confidence interval; RCT: randomised clinical trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to risk of bias (unclear risk of bias for allocation concealment, selective reporting, and incomplete outcome data, and high risk of other bias) and one level because of single study and imprecision (wide 95% CI). | |||||
| ASA with LMWH versus placebo or IVIG | |||||
| Patient or population: people with antiphospholipid antibodies and no history of thrombosis Settings: university department and women's health hospital Intervention: ASA with LMWH Comparison: placebo or IVIG | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo or IVIG | ASA + LMWH | ||||
| Thrombosis Up to 2 months postpartum in Dendrinos 2009, not specified in Ismail 2016 | 0 participants developed thrombosis in both studies. | Not estimable | 258 (2 RCTs) | ‐ | |
| Bleeding ‐ major (bleeding requiring transfusion) Length of follow‐up not specified | 0 per 1000 | 45 per 1000 | RR 9.00 (0.49, 164.76) | 180 (1 RCT) | ⊕⊕⊕⊝ |
| Bleeding ‐ other (bleeding during first trimester) Length of follow‐up not specified | 212 per 1000 | 189 per 1000 | RR 0.89 (0.50, 1.61) | 180 (1 RCT) | ⊕⊕⊕⊝ |
| Bleeding ‐ other (postpartum haemorrhage) Length of follow‐up not specified | 112 per 1000 | 146 per 1000 | RR 1.30 (0.60, 2.81) | 180 (1 RCT) | ⊕⊕⊕⊝ |
| Mortality | Not reported | Not reported | Not reported | Not reported | ‐ |
| Quality of life | Not reported | Not reported | Not reported | Not reported | ‐ |
| Adverse events other than bleeding Follow‐up: up to 2 months postpartum | In 1 study examining pregnant women, 3 cases of nausea, hypotension, and tachycardia were reported in the IVIG (control) group, as compared to none in the intervention group. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASA: acetylsalicylic acid; CI: confidence interval; IVIG: intravenous immunoglobulin; LMWH: low molecular weight heparin; RCT: randomised clinical trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level because of single study and imprecision (wide 95% CI). | |||||
| ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH | |||||
| Patient or population: people with antiphospholipid antibodies and no history of thrombosis Settings: specialist centres Intervention: ASA + high‐dose LMWH Comparison: ASA + low‐dose LMWH or UFH | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| ASA + low‐dose LMWH or UFH | ASA + high‐dose LMWH | ||||
| Thrombosis | 0 participants developed thrombosis. | Not estimable | 120 (2 RCTs) | ‐ | |
| Bleeding ‐ major/excessive | 0 participants developed major/excessive bleeding episodes. | Not estimable | 120 (2 RCTs) | ‐ | |
| Subcutaneous bruises Follow‐up: entire pregnancy | 100 per 1000 | 100 per 1000 | RR 1.00 (0.34 to 2.93) | 120 (2 RCTs) | ⊕⊕⊝⊝ |
| Mortality | Not reported | Not reported | Not reported | Not reported | ‐ |
| Quality of life | Not reported | Not reported | Not reported | Not reported | ‐ |
| Adverse events other than bleeding | 1 case of skin allergy was reported in the ASA + UFH group. | ||||
| The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASA: acetylsalicylic acid; CI: confidence interval; LMWH: low molecular weight heparin; RCT: randomised clinical trial; RR: risk ratio; UFH: unfractionated heparin | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to risk of bias (unclear risk of bias for blinding and selective reporting, high risk for other bias) and one level due to small selected population (pregnant women) and imprecision (wide 95% CI). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.25, 3.77] |
| 2 Minor bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Bleeding requiring transfusion | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.49, 164.76] |
| 2.2 Bleeding during first trimester | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.50, 1.61] |
| 2.3 Postpartum haemorrhage | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.60, 2.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Major/excessive bleeding Show forest plot | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Subcutaneous bruises Show forest plot | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis best‐worst scenario Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.13, 1.19] |
| 2 Thrombosis worst‐best scenario Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.39 [1.55, 12.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis best‐worst scenario Show forest plot | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.51] |
| 2 Thrombosis worst‐best scenario Show forest plot | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.4 [0.47, 151.34] |

