Agents antiplaquettaires et anticoagulants pour la prévention primaire de la thrombose chez les individus ayant des anticorps antiphospholipidiques
Appendices
Appendix 1. CENTRAL search strategy 15 February 2017
| Search run on Wed Feb 15 2017 | Hits retrieved | |
| #1 | MESH DESCRIPTOR Antiphospholipid Syndrome | 52 |
| #2 | MESH DESCRIPTOR Antibodies, Antiphospholipid EXPLODE ALL TREES | 90 |
| #3 | (aPL antibodies):TI,AB,KY | 3 |
| #4 | APS :TI,AB,KY | 191 |
| #5 | (Hughes syndrome):TI,AB,KY | 0 |
| #6 | (antiphospholipid near2 syndrome):TI,AB,KY | 135 |
| #7 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 | 341 |
| #8 | MESH DESCRIPTOR Thrombosis | 1238 |
| #9 | MESH DESCRIPTOR Thromboembolism | 899 |
| #10 | MESH DESCRIPTOR Venous Thromboembolism | 242 |
| #11 | MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES | 2005 |
| #12 | (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*):TI,AB,KY | 17951 |
| #13 | MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES | 735 |
| #14 | (PE or DVT or VTE):TI,AB,KY | 4704 |
| #15 | ((vein* or ven*) near thromb*):TI,AB,KY | 6380 |
| #16 | (blood near3 clot*):TI,AB,KY | 2761 |
| #17 | (pulmonary near3 clot*):TI,AB,KY | 5 |
| #18 | (lung near3 clot*):TI,AB,KY | 4 |
| #19 | #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 | 23298 |
| #20 | MESH DESCRIPTOR Anticoagulants EXPLODE ALL TREES | 8078 |
| #21 | (anticoagul* or anti‐coagu*):TI,AB,KY | 7680 |
| #22 | MESH DESCRIPTOR Heparin EXPLODE ALL TREES | 3826 |
| #23 | *parin*:TI,AB,KY | 54717 |
| #24 | UFH:TI,AB,KY | 447 |
| #25 | LMWH:TI,AB,KY | 809 |
| #26 | LMH:TI,AB,KY | 6 |
| #27 | (Ariven or Arteven or Calcilean or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Pabyrin or Pularin or Thromboliquine or Vetren ):TI,AB,KY | 9 |
| #28 | (Clexane or klexane or lovenox ):TI,AB,KY | 42 |
| #29 | (Fragmin):TI,AB,KY | 178 |
| #30 | (Innohep):TI,AB,KY | 11 |
| #31 | clivarin* :TI,AB,KY | 20 |
| #32 | (danaproid or danaparoid):TI,AB,KY | 37 |
| #33 | (antixarin):TI,AB,KY | 2 |
| #34 | (Zibor or cy 222 or embolex or monoembolex):TI,AB,KY | 38 |
| #35 | (rd 11885 or RD1185):TI,AB,KY | 0 |
| #36 | (Kabi‐2165 or Kabi 2165):TI,AB,KY | 39 |
| #37 | (emt‐966 or emt966 or emt‐967 or emt977 or pk‐10169 or pk10169):TI,AB,KY | 8 |
| #38 | (fr‐860 or fr860 or cy‐216 or cy216):TI,AB,KY | 51 |
| #39 | (kb101 or lomoparan or orgaran ):TI,AB,KY | 28 |
| #40 | (fluxum or lohepa or lowhepa):TI,AB,KY | 11 |
| #41 | (op 2123 or op2123):TI,AB,KY | 1 |
| #42 | (ave 5026 or ave5026 ):TI,AB,KY | 2 |
| #43 | (M118 or RO‐1):TI,AB,KY | 9 |
| #44 | *coumar*:TI,AB,KY | 616 |
| #45 | (*warfarin or (vitamin near/3 antagonist*)):TI,AB,KY | 2855 |
| #46 | (VKA or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcoumar or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin):TI,AB,KY | 389 |
| #47 | MESH DESCRIPTOR Antithrombins EXPLODE ALL TREES | 1235 |
| #48 | MESH DESCRIPTOR Hirudin Therapy | 75 |
| #49 | (thrombin near3 inhib*):TI,AB,KY | 506 |
| #50 | hirudin*:TI,AB,KY | 364 |
| #51 | (dabigatran or Pradaxa or Rendix):TI,AB,KY | 393 |
| #52 | (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048):TI,AB,KY | 9 |
| #53 | (ximelagatran or Exanta or Exarta or melagatran):TI,AB,KY | 150 |
| #54 | (AZD0837 or AZD‐0837):TI,AB,KY | 14 |
| #55 | (S35972 or S‐35972):TI,AB,KY | 0 |
| #56 | MESH DESCRIPTOR Factor Xa Inhibitors | 295 |
| #57 | (Factor X* near4 (antag* or inhib* or block*)):TI,AB,KY | 584 |
| #58 | (FX* near4 (antag* or inhib* or block*)):TI,AB,KY | 48 |
| #59 | (10* near4 (antag* or inhib* or block*) ):TI,AB,KY | 1073 |
| #60 | (rivaroxaban or Xarelto):TI,AB,KY | 526 |
| #61 | (Bay‐597939 or Bay597939):TI,AB,KY | 0 |
| #62 | (betrixaban or PRT054021):TI,AB,KY | 19 |
| #63 | apixaban:TI,AB,KY | 300 |
| #64 | (BMS‐562247 or BMS‐562247 or ELIQUIS):TI,AB,KY | 0 |
| #65 | (DU‐176b or DU176b):TI,AB,KY | 11 |
| #66 | (PRT‐054021 or PRT054021):TI,AB,KY | 1 |
| #67 | (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*):TI,AB,KY | 39 |
| #68 | (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893):TI,AB,KY | 3 |
| #69 | edoxaban or lixiana:TI,AB,KY | 157 |
| #70 | *ixaban:TI,AB,KY | 326 |
| #71 | *axaban:TI,AB,KY | 6 |
| #72 | *exaban:TI,AB,KY | 11 |
| #73 | etexilate:TI,AB,KY | 126 |
| #74 | agatroban:TI,AB,KY | 1 |
| #75 | *parinux:TI,AB,KY | 303 |
| #76 | MESH DESCRIPTOR Platelet Aggregation Inhibitors EXPLODE ALL TREES | 8396 |
| #77 | MESH DESCRIPTOR Phosphodiesterase Inhibitors EXPLODE ALL TREES | 5495 |
| #78 | MESH DESCRIPTOR Tetrazoles | 1823 |
| #79 | (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*):TI,AB,KY | 3253 |
| #80 | (((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) near3 (antagonist or inhibitor))):TI,AB,KY | 2349 |
| #81 | ((gp* or glycoprotein* or protease or P2Y12 or TXA2) near3 inhibit*):TI,AB,KY | 3199 |
| #82 | thienopyridine:TI,AB,KY | 266 |
| #83 | (ticlopidine or Ticlid):TI,AB,KY | 1748 |
| #84 | (clopidogrel or Plavix):TI,AB,KY | 3014 |
| #85 | (Prasugrel or Effient or Efient or Prasita):TI,AB,KY | 501 |
| #86 | (ticagrelor or AZD6140 or Brilinta):TI,AB,KY | 436 |
| #87 | (elinogrel or PRT060128 or PRT‐060128):TI,AB,KY | 8 |
| #88 | (cangrelor or AR‐C6993* or ARC6993*):TI,AB,KY | 50 |
| #89 | (SCH530348 or SCH‐530348):TI,AB,KY | 19 |
| #90 | E5555:TI,AB,KY | 5 |
| #91 | (terutroban or Triplion):TI,AB,KY | 14 |
| #92 | (aspirin* or nitroaspirin or ASA):TI,AB,KY | 17036 |
| #93 | (acetylsalicylic acid):TI,AB,KY | 4935 |
| #94 | (acetyl salicylic acid*):TI,AB,KY | 109 |
| #95 | (triflusal or disgren):TI,AB,KY | 97 |
| #96 | (Cilostazol or Pletal or Pletaal):TI,AB,KY | 464 |
| #97 | (dipyridamol* or Persantine):TI,AB,KY | 1129 |
| #98 | (OPC‐13013 or OPC13013):TI,AB,KY | 5 |
| #99 | (picotamide or picotinamide):TI,AB,KY | 41 |
| #100 | satigrel:TI,AB,KY | 3 |
| #101 | vorapaxar:TI,AB,KY | 84 |
| #102 | indobufen:TI,AB,KY | 82 |
| #103 | #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 OR #91 OR #92 OR #93 OR #94 OR #95 OR #96 OR #97 OR #98 OR #99 OR #100 OR #101 OR #102 | 92582 |
| #104 | #7 AND #19 AND #103 | 72 |
Appendix 2. Trials registries searches 15 February 2017
ClinicalTrials.gov
73 studies found for: antiphospholipid
World Health Organization International Clinical Trials Registry Platform
65 records for 57 trials found for: antiphospholipid
ISRCTN Register
21 results for: antiphospholipid
Appendix 3. Follow‐up search strategies November/December 2017
| Source | Search strategy | Hits retrieved |
| 1. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) (2017 ONLY) Searched 4 December 2017 | 1 Antiphospholipid Syndrome/ 8228 2 exp Antibodies, Antiphospholipid/ 9033 3 aPL antibodies.ti,ab. 321 4 APS.ti,ab. 10942 5 "Hughes syndrome".ti,ab. 180 6 "antiphospholipid adj2 syndrome".ti,ab. 0 7 or/1‐6 21671 8 Thrombosis/ 69315 9 Thromboembolism/ 23814 10 Venous Thromboembolism/ 8453 11 exp Venous Thrombosis/ 54084 12 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*).ti,ab. 316286 13 exp Pulmonary Embolism/ 38164 14 (PE or DVT or VTE).ti,ab. 47775 15 ((vein* or ven*) adj3 thromb*).ti,ab. 72239 16 (blood adj3 clot*).ti,ab. 10458 17 (pulmonary adj3 clot*).ti,ab. 198 18 (lung adj3 clot*).ti,ab. 48 19 or/8‐18 408490 20 exp Anticoagulants/ 214555 21 (anticoagul* or anti‐coagu*).ti,ab. 86806 22 exp Heparin/ 65466 23 heparin*.ti,ab. 85147 24 UFH.ti,ab. 2032 25 LMWH.ti,ab. 4479 26 LMH.ti,ab. 434 27 (Ariven or Arteven or Calcilean or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Pabyrin or Pularin or Thromboliquine or Vetren).ti,ab. 55 28 (Clexane or klexane or lovenox).ti,ab. 241 29 Fragmin.ti,ab. 384 30 Innohep.ti,ab. 21 31 clivarin*.ti,ab. 28 32 (danaproid or danaparoid).ti,ab. 449 33 antixarin.ti,ab. 1 34 (Zibor or cy 222 or embolex or monoembolex).ti,ab. 59 35 (rd 11885 or RD1185).ti,ab. 1 36 (Kabi‐2165 or Kabi 2165).ti,ab. 41 37 (emt‐966 or emt966 or emt‐967 or emt977 or pk‐10169 or pk10169).ti,ab. 43 38 (fr‐860 or fr860 or cy‐216 or cy216).ti,ab. 99 39 (kb101 or lomoparan or orgaran).ti,ab. 113 40 (fluxum or lohepa or lowhepa).ti,ab. 12 41 (op 2123 or op2123).ti,ab. 3 42 (ave 5026 or ave5026).ti,ab. 8 43 (M118 or RO‐1).ti,ab. 140 44 coumar*.ti,ab. 16405 45 (warfarin or (vitamin adj3 antagonist*)).ti,ab. 26213 46 (VKA or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcoumar or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).ti,ab. 2709 47 exp Antithrombins/ 20598 48 Hirudin Therapy/ 684 49 (thrombin adj3 inhib*).ti,ab. 10229 50 hirudin*.ti,ab. 3471 51 (dabigatran or Pradaxa or Rendix).ti,ab. 3811 52 (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048).ti,ab. 14 53 (ximelagatran or Exanta or Exarta or melagatran).ti,ab. 652 54 (AZD0837 or AZD‐0837).ti,ab. 31 55 (S35972 or S‐35972).ti,ab. 2 56 Factor Xa Inhibitors.ti,ab. 1086 57 (Factor X* adj4 (antag* or inhib* or block*)).ti,ab. 4546 58 (FX* adj4 (antag* or inhib* or block*)).ti,ab. 1654 59 (10* adj4 (antag* or inhib* or block*)).ti,ab. 81139 60 (rivaroxaban or Xarelto).ti,ab. 3487 61 (Bay‐597939 or Bay597939).ti,ab. 2 62 apixaban.ti,ab. 2183 63 (BMS‐562247 or BMS‐562247 or ELIQUIS).ti,ab. 47 64 (DU‐176b or DU176b).ti,ab. 26 65 (PRT‐054021 or PRT054021).ti,ab. 4 66 (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*).ti,ab. 67 67 (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893).ti,ab. 16 68 (edoxaban or lixiana).ti,ab. 892 69 etexilate.ti,ab. 723 70 agatroban.ti,ab. 1 71 exp Platelet Aggregation Inhibitors/ 107579 72 exp Phosphodiesterase Inhibitors/ 85845 73 Tetrazoles/ 11647 74 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*).ti,ab. 31484 75 ((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) adj3 (antagonist or inhibitor)).ti,ab. 20364 76 ((gp* or glycoprotein* or protease or P2Y12 or TXA2) adj3 inhibit*).ti,ab. 49431 77 thienopyridine.ti,ab. 983 78 (ticlopidine or Ticlid).ti,ab. 2653 79 (clopidogrel or Plavix).ti,ab. 11473 80 (Prasugrel or Effient or Efient or Prasita).ti,ab. 1828 81 (ticagrelor or AZD6140 or Brilinta).ti,ab. 1801 82 (elinogrel or PRT060128 or PRT‐060128).ti,ab. 52 83 (cangrelor or AR‐C6993* or ARC6993*).ti,ab. 456 84 (SCH530348 or SCH‐530348).ti,ab. 68 85 E5555.ti,ab. 20 86 (terutroban or Triplion).ti,ab. 59 87 (aspirin* or nitroaspirin or ASA).ti,ab. 70039 88 acetylsalicylic acid.ti,ab. 8940 89 acetyl salicylic acid*.ti,ab. 802 90 (triflusal or disgren).ti,ab. 183 91 (Cilostazol or Pletal or Pletaal).ti,ab. 1589 92 (dipyridamol* or Persantine).ti,ab. 8584 93 (OPC‐13013 or OPC13013).ti,ab. 31 94 (picotamide or picotinamide).ti,ab. 111 95 satigrel.ti,ab. 4 96 vorapaxar.ti,ab. 230 97 indobufen.ti,ab. 174 98 or/20‐97 654251 99 7 and 19 and 98 4435 100 randomized controlled trial.pt. 505457 101 controlled clinical trial.pt. 100426 102 randomized.ab. 442085 103 placebo.ab. 205331 104 drug therapy.fs. 2147127 105 randomly.ab. 305213 106 trial.ab. 465590 107 groups.ab. 1884917 108 or/100‐107 4448119 109 exp animals/ not humans.sh. 4743197 110 108 not 109 3846919 111 99 and 110 1424 112 2017*.ed. 954730 113 111 and 112 62 114 from 113 keep 1‐62 62 | 62 |
| 2. EMBASE 1974 to (2017 ONLY) Searched 4 December 2017 | 1 Antiphospholipid Syndrome/ 13340 2 exp Antibodies, Antiphospholipid/ 10672 3 aPL antibodies.ti,ab. 450 4 APS.ti,ab. 13550 5 "Hughes syndrome".ti,ab. 174 6 "antiphospholipid adj2 syndrome".ti,ab. 0 7 or/1‐6 27751 8 Thrombosis/ 92570 9 Thromboembolism/ 48884 10 Venous Thromboembolism/ 29859 11 exp Venous Thrombosis/ 93279 12 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*).ti,ab. 334991 13 exp Pulmonary Embolism/ 66110 14 (PE or DVT or VTE).ti,ab. 65140 15 ((vein* or ven*) adj3 thromb*).ti,ab. 82886 16 (blood adj3 clot*).ti,ab. 9074 17 (pulmonary adj3 clot*).ti,ab. 218 18 (lung adj3 clot*).ti,ab. 55 19 or/8‐18 474200 20 exp Anticoagulants/ 457874 21 (anticoagul* or anti‐coagu*).ti,ab. 99680 22 exp Heparin/ 92647 23 heparin*.ti,ab. 70674 24 UFH.ti,ab. 3144 25 LMWH.ti,ab. 7352 26 LMH.ti,ab. 505 27 (Ariven or Arteven or Calcilean or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Pabyrin or Pularin or Thromboliquine or Vetren).ti,ab. 27 28 (Clexane or klexane or lovenox).ti,ab. 542 29 Fragmin.ti,ab. 279 30 Innohep.ti,ab. 38 31 clivarin*.ti,ab. 24 32 (danaproid or danaparoid).ti,ab. 616 33 antixarin.ti,ab. 0 34 (Zibor or cy 222 or embolex or monoembolex).ti,ab. 12 35 (rd 11885 or RD1185).ti,ab. 0 36 (Kabi‐2165 or Kabi 2165).ti,ab. 0 37 (emt‐966 or emt966 or emt‐967 or emt977 or pk‐10169 or pk10169).ti,ab. 0 38 (fr‐860 or fr860 or cy‐216 or cy216).ti,ab. 4 39 (kb101 or lomoparan or orgaran).ti,ab. 107 40 (fluxum or lohepa or lowhepa).ti,ab. 11 41 (op 2123 or op2123).ti,ab. 2 42 (ave 5026 or ave5026).ti,ab. 13 43 (M118 or RO‐1).ti,ab. 95 44 coumar*.ti,ab. 16168 45 (warfarin or (vitamin adj3 antagonist*)).ti,ab. 34403 46 (VKA or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcoumar or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).ti,ab. 3830 47 exp Antithrombins/ 7897 48 Hirudin Therapy/ 42908 49 (thrombin adj3 inhib*).ti,ab. 10057 50 hirudin*.ti,ab. 2574 51 (dabigatran or Pradaxa or Rendix).ti,ab. 6972 52 (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048).ti,ab. 18 53 (ximelagatran or Exanta or Exarta or melagatran).ti,ab. 804 54 (AZD0837 or AZD‐0837).ti,ab. 43 55 (S35972 or S‐35972).ti,ab. 6 56 Factor Xa Inhibitors.ti,ab. 1374 57 (Factor X* adj4 (antag* or inhib* or block*)).ti,ab. 5202 58 (FX* adj4 (antag* or inhib* or block*)).ti,ab. 2626 59 (10* adj4 (antag* or inhib* or block*)).ti,ab. 67594 60 (rivaroxaban or Xarelto).ti,ab. 6586 61 (Bay‐597939 or Bay597939).ti,ab. 1 62 apixaban.ti,ab. 3945 63 (BMS‐562247 or BMS‐562247 or ELIQUIS).ti,ab. 90 64 (DU‐176b or DU176b).ti,ab. 54 65 (PRT‐054021 or PRT054021).ti,ab. 4 66 (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*).ti,ab. 119 67 (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893).ti,ab. 34 68 (edoxaban or lixiana).ti,ab. 1313 69 etexilate.ti,ab. 1267 70 agatroban.ti,ab. 1 71 exp Platelet Aggregation Inhibitors/ 239121 72 exp Phosphodiesterase Inhibitors/ 53488 73 Tetrazoles/ 2414 74 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*).ti,ab. 41493 75 ((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) adj3 (antagonist or inhibitor)).ti,ab. 15191 76 ((gp* or glycoprotein* or protease or P2Y12 or TXA2) adj3 inhibit*).ti,ab. 50010 77 thienopyridine.ti,ab. 1504 78 (ticlopidine or Ticlid).ti,ab. 2396 79 (clopidogrel or Plavix).ti,ab. 20219 80 (Prasugrel or Effient or Efient or Prasita).ti,ab. 3255 81 (ticagrelor or AZD6140 or Brilinta).ti,ab. 3110 82 (elinogrel or PRT060128 or PRT‐060128).ti,ab. 86 83 (cangrelor or AR‐C6993* or ARC6993*).ti,ab. 643 84 (SCH530348 or SCH‐530348).ti,ab. 94 85 E5555.ti,ab. 28 86 (terutroban or Triplion).ti,ab. 81 87 (aspirin* or nitroaspirin or ASA).ti,ab. 83196 88 acetylsalicylic acid.ti,ab. 6791 89 acetyl salicylic acid*.ti,ab. 771 90 (triflusal or disgren).ti,ab. 175 91 (Cilostazol or Pletal or Pletaal).ti,ab. 2322 92 (dipyridamol* or Persantine).ti,ab. 5207 93 (OPC‐13013 or OPC13013).ti,ab. 5 94 (picotamide or picotinamide).ti,ab. 74 95 satigrel.ti,ab. 20 96 vorapaxar.ti,ab. 333 97 indobufen.ti,ab. 59 98 or/20‐97 710273 99 7 and 19 and 98 7447 100 randomized controlled trial/ 437650 101 controlled clinical trial/ 408481 102 random$.ti,ab. 1132164 103 randomization/ 68186 104 intermethod comparison/ 223714 105 placebo.ti,ab. 215213 106 (compare or compared or comparison).ti. 326736 107 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1562487 108 (open adj label).ti,ab. 60147 109 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 153458 110 double blind procedure/ 119366 111 parallel group$1.ti,ab. 18989 112 (crossover or cross over).ti,ab. 70151 113 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 241228 114 (assigned or allocated).ti,ab. 282341 115 (controlled adj7 (study or design or trial)).ti,ab. 252973 116 (volunteer or volunteers).ti,ab. 168202 117 trial.ti. 206175 118 or/100‐117 3373917 119 2017*.dc. 1694109 120 99 and 118 and 119 63 | 63 |
| 3. CINAHL (EBSCOhost) (2017 ONLY) Searched 4 December 2017 | S107 S98 AND S105 AND S106 6 S106 EM 2017 182,744 S105 S99 OR S100 OR S101 OR S102 OR S103 OR S104 952,284 S104 TX randomly 41,763 S103 TX "treatment as usual" 709 S102 TX "double‐blind*" 755,656 S101 TX "single‐blind*" 8,678 S100 TX trial 236,802 S99 MH "Clinical Trials" 90,899 S98 S7 AND S19 AND S97 231 S97 S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76 OR S77 OR S78 OR S79 OR S80 OR S81 OR S82 OR S83 OR S84 OR S85 OR S86 OR S87 OR S88 OR S89 OR S90 OR S91 ... 51,427 S96 TX indobufen 6 S95 TX vorapaxar 48 S94 TX satigrel 0 S93 TX picotamide or picotinamide 8 S92 TX OPC‐13013 or OPC13013 0 S91 TX Cilostazol or Pletal or Pletaal 204 S90 TX triflusal or disgren 23 S89 TX acetylsalicylic acid 408 S88 TX terutroban or Triplion 12 S87 TX E5555 4 S86 TX SCH530348 or SCH‐530348 12 S85 TX cangrelor or AR‐C6993* or ARC6993* 60 S84 TX elinogrel or PRT060128 or PRT‐060128 2 S83 TX ticagrelor or AZD6140 or Brilinta 391 S82 TX Prasugrel or Effient or Efient or Prasita 360 S81 TX clopidogrel or Plavix 2,595 S80 TX ticlopidine or Ticlid 1,020 S79 TX thienopyridine 193 S78 TX gp* or glycoprotein* or protease or P2Y12 or TXA2) n3 inhibit*): 4,288 S77 TX platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) n3 (antagonist or inhibitor 6,893 S76 TX antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg* 3,535 S75 (MH "Phosphodiesterase Inhibitors+") 1,834 S74 (MH "Platelet Aggregation Inhibitors+") 11,567 S73 *parinux 0 S72 argatroban 195 S71 etexilate 325 S70 *exaban 0 S69 *axaban 0 S68 *ixaban 0 S67 edoxaban or lixiana 136 S66 GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893 24 S65 GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893 0 S64 YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176* 5 S63 PRT‐054021 or PRT054021 0 S62 DU‐176b or DU176b 3 S61 BMS‐562247 or BMS‐562247 or ELIQUIS 15 S60 apixaban 387 S59 betrixaban or PRT054021 15 S58 Bay‐597939 or Bay597939 0 S57 rivaroxaban or Xarelto 693 S56 (10* n4 (antag* or inhib* or block*) ) 1,798 S55 (FX* n4 (antag* or inhib* or block*)) 43 S54 (Factor X* n4 (antag* or inhib* or block*)) 9,042 S53 TX S35972 or S‐35972 0 S52 TX AZD0837 or AZD‐0837 0 S51 TX ximelagatran or Exanta or Exarta or melagatran 89 S50 TX BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048 2 S49 TX dabigatran or Pradaxa or Rendix 883 S48 TX hirudin* 283 S47 TX thrombin n3 inhib* 656 S46 (MH "Hirudin") 237 S45 TX VKA or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcoumar or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin 233 S44 TX (*warfarin or (vitamin n3 antagonist*)) 6,298 S43 TX M118 or RO‐1 4,530 S42 TX ave 5026 or ave5026 0 S41 TX op 2123 or op2123 0 S40 TX fluxum or lohepa or lowhepa 1 S39 TX kb101 or lomoparan or orgaran 2 S38 TX fr‐860 or fr860 or cy‐216 or cy216 0 S37 TX emt‐966 or emt966 or emt‐967 or emt977 or pk‐10169 or pk10169 0 S36 TX Kabi‐2165 or Kabi 2165 0 S35 TX rd 11885 or RD1185 0 S34 TX Zibor or cy 222 or embolex or monoembolex 3 S33 TX antixarin 0 S32 TX danaproid or danaparoid 34 S31 TX clivarin* 1 S30 TX Innohep 3 S29 TX Fragmin 19 S28 TX Clexane or klexane or lovenox 28 S27 TX Ariven or Arteven or Calcilean or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Pabyrin or Pularin or Thromboliquine or Vetren 1 S26 TX LMH 55 S25 TX LMWH 463 S24 TX UFH 235 S23 TX *parin* 270 S22 (MH "Heparin+") 5,527 S21 TX anticoagul* or anti‐coagu* 13,627 S20 (MH "Anticoagulants+") 15,828 S19 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 44,186 S18 lung n3 clot* 11 S17 pulmonary n3 clot* 19 S16 (blood n3 clot*) 699 S15 TX ((vein* or ven*) n thromb*) 140 S14 TX PE or DVT or VTE 11,499 S13 (MH "Pulmonary Embolism") 4,591 S12 TX thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*): 35,081 S11 (MH "Venous Thrombosis+") 6,154 S10 (MH "Venous Thromboembolism") 2,904 S9 (MH "Thromboembolism") 3,174 S8 (MH "Thrombosis") 4,493 S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 2,647 S6 TX antiphospholipid n2 syndrome 865 S5 TX "Hughes syndrome" 11 S4 TX APS 1,946 S3 TX aPL antibodies 105 S2 (MH "Antiphospholipid Syndrome") 699 S1 (MH "Antiphospholipid Syndrome") 699 | 6 |
| 4. AMED (2017 ONLY) Searched 4 December 2017 | 1 APS.ti,ab. 31 2 Thrombosis/ 195 3 Thromboembolism/ 71 4 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*).ti,ab. 640 5 exp Pulmonary Embolism/ 53 6 (PE or DVT or VTE).ti,ab. 242 7 ((vein* or ven*) adj3 thromb*).ti,ab. 318 8 (blood adj3 clot*).ti,ab. 34 9 exp Anticoagulants/ 148 10 (anticoagul* or anti‐coagu*).ti,ab. 189 11 exp Heparin/ 26 12 heparin*.ti,ab. 93 13 UFH.ti,ab. 1 14 LMWH.ti,ab. 12 15 LMH.ti,ab. 1 16 coumar*.ti,ab. 347 17 (warfarin or (vitamin adj3 antagonist*)).ti,ab. 84 18 (VKA or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcoumar or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).ti,ab. 1 19 (thrombin adj3 inhib*).ti,ab. 14 20 hirudin*.ti,ab. 4 21 (dabigatran or Pradaxa or Rendix).ti,ab. 1 22 (ximelagatran or Exanta or Exarta or melagatran).ti,ab. 4 23 (Factor X* adj4 (antag* or inhib* or block*)).ti,ab. 2 24 (FX* adj4 (antag* or inhib* or block*)).ti,ab. 3 25 (10* adj4 (antag* or inhib* or block*)).ti,ab. 574 26 (rivaroxaban or Xarelto).ti,ab. 1 27 exp Platelet Aggregation Inhibitors/ 8 28 Tetrazoles/ 0 29 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*).ti,ab. 150 30 ((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) adj3 (antagonist or inhibitor)).ti,ab. 55 31 ((gp* or glycoprotein* or protease or P2Y12 or TXA2) adj3 inhibit*).ti,ab. 107 32 (ticlopidine or Ticlid).ti,ab. 4 33 (clopidogrel or Plavix).ti,ab. 11 34 (aspirin* or nitroaspirin or ASA).ti,ab. 261 35 acetylsalicylic acid.ti,ab. 39 36 acetyl salicylic acid*.ti,ab. 5 37 (Cilostazol or Pletal or Pletaal).ti,ab. 4 38 (dipyridamol* or Persantine).ti,ab. 12 39 Antiphospholipid.mp. [mp=abstract, heading words, title] 9 40 1 or 39 40 41 or/2‐8 862 42 or/9‐38 1758 43 40 and 41 and 42 2 44 2017*.up. 6951 45 43 and 44 0 46 39 and 44 0 | 0 |
| 5. VASCULAR SPECIALISED REGISTER Searched 4 December 2017 | #1 thrombosis AND INREGISTER # 2 2017 AND INREGISTER #3 1 AND 2 | 6 |
| 6. CENTRAL Searched 29 November 2017 | #1 MESH DESCRIPTOR Antiphospholipid Syndrome 54 #2 MESH DESCRIPTOR Antibodies, Antiphospholipid EXPLODE ALL TREES 90 #3 (aPL antibodies):TI,AB,KY 5 #4 APS :TI,AB,KY 258 #5 (Hughes syndrome):TI,AB,KY 0 #6 (antiphospholipid near2 syndrome):TI,AB,KY 193 #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 443 #8 MESH DESCRIPTOR Thrombosis 1303 #9 MESH DESCRIPTOR Thromboembolism 943 #10 MESH DESCRIPTOR Venous Thromboembolism 294 #11 MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES 2085 #12 ((thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*)):TI,AB,KY 21115 #13 MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES 769 #14 (PE or DVT or VTE):TI,AB,KY 5562 #15 (((vein* or ven*) near thromb*)):TI,AB,KY 7332 #16 ((blood near3 clot*)):TI,AB,KY 3497 #17 (pulmonary near3 clot*):TI,AB,KY 6 #18 (lung near3 clot*):TI,AB,KY 5 #19 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 27424 #20 MESH DESCRIPTOR Anticoagulants EXPLODE ALL TREES 8429 #21 (anticoagul* or anti‐coagu*):TI,AB,KY 8976 #22 MESH DESCRIPTOR Heparin EXPLODE ALL TREES 3943 #23 *parin*:TI,AB,KY 62652 #24 UFH:TI,AB,KY 487 #25 LMWH:TI,AB,KY 902 #26 LMH:TI,AB,KY 8 #27 (Ariven or Arteven or Calcilean or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Pabyrin or Pularin or Thromboliquine or Vetren ):TI,AB,KY 9 #28 (Clexane or klexane or lovenox ):TI,AB,KY 46 #29 Fragmin:TI,AB,KY 182 #30 Innohep:TI,AB,KY 12 #31 clivarin*:TI,AB,KY 20 #32 (danaproid or danaparoid):TI,AB,KY 46 #33 antixarin:TI,AB,KY 2 #34 (Zibor or cy 222 or embolex or monoembolex):TI,AB,KY 38 #35 (rd 11885 or RD1185):TI,AB,KY 0 #36 (Kabi‐2165 or Kabi 2165):TI,AB,KY 39 #37 (emt‐966 or emt966 or emt‐967 or emt977 or pk‐10169 or pk10169):TI,AB,KY 8 #38 (fr‐860 or fr860 or cy‐216 or cy216):TI,AB,KY 51 #39 (kb101 or lomoparan or orgaran ):TI,AB,KY 28 #40 (fluxum or lohepa or lowhepa):TI,AB,KY 11 #41 (op 2123 or op2123):TI,AB,KY 1 #42 (ave 5026 or ave5026 ):TI,AB,KY 2 #43 (M118 or RO‐1):TI,AB,KY 9 #44 *coumar*:TI,AB,KY 664 #45 (*warfarin or (vitamin near/3 antagonist*)):TI,AB,KY 3285 #46 (VKA or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcoumar or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin):TI,AB,KY 476 #47 MESH DESCRIPTOR Antithrombins 277 #48 MESH DESCRIPTOR Antithrombins EXPLODE ALL TREES 1354 #49 MESH DESCRIPTOR Hirudin Therapy 75 #50 (thrombin near3 inhib*):TI,AB,KY 559 #51 hirudin*:TI,AB,KY 383 #52 (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048):TI,AB,KY 9 #53 (ximelagatran or Exanta or Exarta or melagatran):TI,AB,KY 154 #54 (AZD0837 or AZD‐0837):TI,AB,KY 14 #55 (S35972 or S‐35972):TI,AB,KY 0 #56 MESH DESCRIPTOR Factor Xa Inhibitors 358 #57 (Factor X* near4 (antag* or inhib* or block*)):TI,AB,KY 708 #58 (FX* near4 (antag* or inhib* or block*)):TI,AB,KY 56 #59 (10* near4 (antag* or inhib* or block*) ):TI,AB,KY 1226 #60 (rivaroxaban or Xarelto):TI,AB,KY 767 #61 (Bay‐597939 or Bay597939):TI,AB,KY 0 #62 (betrixaban or PRT054021):TI,AB,KY 38 #63 apixaban:TI,AB,KY 457 #64 (BMS‐562247 or BMS‐562247 or ELIQUIS):TI,AB,KY 0 #65 (DU‐176b or DU176b):TI,AB,KY 12 #66 (PRT‐054021 or PRT054021):TI,AB,KY 1 #67 (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*):TI,AB,KY 44 #68 (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893):TI,AB,KY 3 #69 (edoxaban or lixiana):TI,AB,KY 254 #70 *ixaban:TI,AB,KY 495 #71 *axaban:TI,AB,KY 8 #72 *exaban:TI,AB,KY 17 #73 etexilate:TI,AB,KY 162 #74 agatroban:TI,AB,KY 1 #75 *parinux:TI,AB,KY 340 #76 MESH DESCRIPTOR Platelet Aggregation Inhibitors EXPLODE ALL TREES 8757 #77 MESH DESCRIPTOR Phosphodiesterase Inhibitors EXPLODE ALL TREES 5673 #78 MESH DESCRIPTOR Tetrazoles EXPLODE ALL TREES 2866 #79 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*):TI,AB,KY 3916 #80 (((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) near3 (antagonist or inhibitor))):TI,AB,KY 2589 #81 (((gp* or glycoprotein* or protease or P2Y12 or TXA2) near3 inhibit*)):TI,AB,KY 3512 #82 thienopyridine:TI,AB,KY 309 #83 (ticlopidine or Ticlid):TI,AB,KY 1909 #84 (clopidogrel or Plavix):TI,AB,KY 3552 #85 (Prasugrel or Effient or Efient or Prasita):TI,AB,KY 648 #86 (ticagrelor or AZD6140 or Brilinta):TI,AB,KY 647 #87 (elinogrel or PRT060128 or PRT‐060128):TI,AB,KY 8 #88 (cangrelor or AR‐C6993* or ARC6993*):TI,AB,KY 66 #89 (SCH530348 or SCH‐530348):TI,AB,KY 24 #90 E5555:TI,AB,KY 6 #91 (terutroban or Triplion):TI,AB,KY 17 #92 (aspirin* or nitroaspirin or ASA):TI,AB,KY 18368 #93 (acetylsalicylic acid):TI,AB,KY 5734 #94 (acetyl salicylic acid*):TI,AB,KY 117 #95 (triflusal or disgren):TI,AB,KY 103 #96 (Cilostazol or Pletal or Pletaal):TI,AB,KY 520 #97 (dipyridamol* or Persantine):TI,AB,KY 1165 #98 (OPC‐13013 or OPC13013):TI,AB,KY 5 #99 (picotamide or picotinamide):TI,AB,KY 41 #100 satigrel:TI,AB,KY 3 #101 vorapaxar:TI,AB,KY 103 #102 indobufen:TI,AB,KY 82 #103 #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 OR #91 OR #92 OR #93 OR #94 OR #95 OR #96 OR #97 OR #98 OR #99 OR #100 OR #101 OR #102 104894 #104 #7 AND #19 AND #103 100 #105 01/01/2017 TO 29/11/2017:CD 109501 #106 #104 AND #105 28 | 28 |
| 7. ClinicalTrials.gov (www.clinicaltrials.gov) Searched 29 November 2017 | antiphospholipid | 10 |
| 8. ICTRP Searched 29 November 2017 | antiphospholipid | 1 |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Anticoagulant with or without ASA versus ASA only, Outcome 1 Thrombosis.

Comparison 1 Anticoagulant with or without ASA versus ASA only, Outcome 2 Minor bleeding.

Comparison 2 ASA only versus placebo, Outcome 1 Thrombosis.

Comparison 2 ASA only versus placebo, Outcome 2 Bleeding.

Comparison 3 ASA with LMWH versus placebo or IVIG, Outcome 1 Thrombosis.

Comparison 3 ASA with LMWH versus placebo or IVIG, Outcome 2 Bleeding.

Comparison 4 ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH, Outcome 1 Thrombosis.
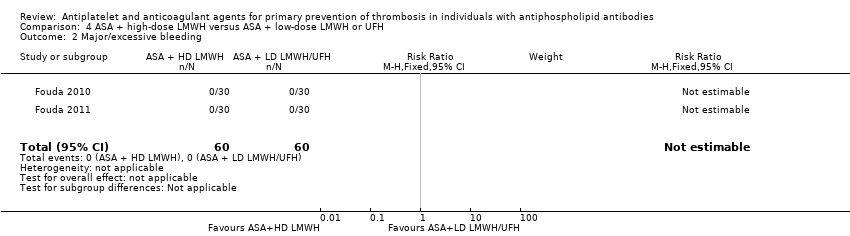
Comparison 4 ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH, Outcome 2 Major/excessive bleeding.

Comparison 4 ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH, Outcome 3 Subcutaneous bruises.
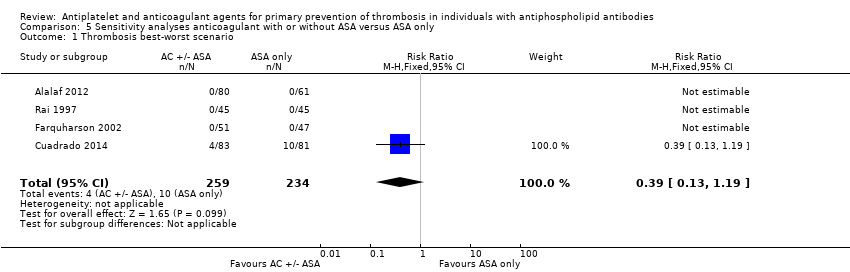
Comparison 5 Sensitivity analyses anticoagulant with or without ASA versus ASA only, Outcome 1 Thrombosis best‐worst scenario.

Comparison 5 Sensitivity analyses anticoagulant with or without ASA versus ASA only, Outcome 2 Thrombosis worst‐best scenario.

Comparison 6 Sensitivity analyses ASA with LMWH versus placebo or IVIG, Outcome 1 Thrombosis best‐worst scenario.

Comparison 6 Sensitivity analyses ASA with LMWH versus placebo or IVIG, Outcome 2 Thrombosis worst‐best scenario.
| Anticoagulant with or without ASA versus ASA only | |||||
| Patient or population: people with antiphospholipid antibodies and no history of thrombosis Settings: specialist centres Intervention: anticoagulant with or without ASA Comparison: ASA only | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| ASA | AC or AC + ASA | ||||
| Thrombosis Median follow‐up reported in 1 study: 37.2 months (ASA group) 32.4 (AC or AC + ASA group), no IQR given | 17 per 1000 | 16 per 1000 | RR 0.98 (0.25, 3.77) | 493 (4 RCTs) | ⊕⊕⊝⊝ |
| Bleeding ‐ major | No events reported. | No events reported. | Not estimable | 493 (4 RCTs) | ‐ |
| Bleeding ‐ minor (not requiring hospital admission) Median follow‐up reported in 1 study: 37.2 months (ASA group) 32.4 (AC or AC + ASA group), no IQR given | 0 per 1000 | 133 per 1000 (8 to 2294) | RR 22.45 (1.34, 374.81) | 164 (1 RCT) | ⊕⊕⊝⊝ |
| Mortality | Not reported | Not reported | Not reported | Not reported | ‐ |
| Quality of life | Not reported | Not reported | Not reported | Not reported | ‐ |
| Adverse event other than bleeding | In Cuadrado 2014, 4 cases of mild gastrointestinal symptoms (2 severe constipations and 2 stomach upsets, not clear if the same or different participants) in the ASA group and 1 case of an allergic reaction in the combination therapy group were reported. Farquharson 2002 and Alalaf 2012 reported no information about adverse events not related to bleeding or obstetric failure, while Rai 1997 reported that in both groups interventions were well tolerated and that in the heparin group there were no cases of thrombocytopenia or vertebral fractures. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AC: anticoagulant; ASA: acetylsalicylic acid; CI: confidence interval; IQR: interquartile range; RCT: randomised clinical trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to unclear or high risk of bias in more than one domain in all studies and one level due to wide 95% CI for both benefit and harm. | |||||
| ASA versus placebo | |||||
| Patient or population: people with antiphospholipid antibodies and no history of thrombosis Settings: university department and 3 unspecified centres Intervention: ASA Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo | ASA | ||||
| Thrombosis Mean follow‐up 2.3 +/‐ 0.95 years | 20 per 1000 | 105 per 1000 | RR 5.21 (0.63, 42.97) | 98 (1 RCT) | ⊕⊕⊝⊝ |
| Bleeding ‐ major | No events reported. | No events reported. | Not estimable | 98 (1 RCT) | ‐ |
| Bleeding ‐ minor Mean follow‐up 2.3 +/‐ 0.95 years | 20 per 1000 | 63 per 1000 | RR 3.13 (0.34, 29.01) | 98 (1 RCT) | ⊕⊕⊝⊝ |
| Mortality | Not reported | Not reported | Not reported | Not reported | ‐ |
| Quality of life | Not reported | Not reported | Not reported | Not reported | ‐ |
| Adverse event other than bleeding | Minor gastrointestinal disturbances occurred in 5 participants who received ASA compared to 1 participant in the placebo group. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASA: acetylsalicylic acid; CI: confidence interval; RCT: randomised clinical trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to risk of bias (unclear risk of bias for allocation concealment, selective reporting, and incomplete outcome data, and high risk of other bias) and one level because of single study and imprecision (wide 95% CI). | |||||
| ASA with LMWH versus placebo or IVIG | |||||
| Patient or population: people with antiphospholipid antibodies and no history of thrombosis Settings: university department and women's health hospital Intervention: ASA with LMWH Comparison: placebo or IVIG | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo or IVIG | ASA + LMWH | ||||
| Thrombosis Up to 2 months postpartum in Dendrinos 2009, not specified in Ismail 2016 | 0 participants developed thrombosis in both studies. | Not estimable | 258 (2 RCTs) | ‐ | |
| Bleeding ‐ major (bleeding requiring transfusion) Length of follow‐up not specified | 0 per 1000 | 45 per 1000 | RR 9.00 (0.49, 164.76) | 180 (1 RCT) | ⊕⊕⊕⊝ |
| Bleeding ‐ other (bleeding during first trimester) Length of follow‐up not specified | 212 per 1000 | 189 per 1000 | RR 0.89 (0.50, 1.61) | 180 (1 RCT) | ⊕⊕⊕⊝ |
| Bleeding ‐ other (postpartum haemorrhage) Length of follow‐up not specified | 112 per 1000 | 146 per 1000 | RR 1.30 (0.60, 2.81) | 180 (1 RCT) | ⊕⊕⊕⊝ |
| Mortality | Not reported | Not reported | Not reported | Not reported | ‐ |
| Quality of life | Not reported | Not reported | Not reported | Not reported | ‐ |
| Adverse events other than bleeding Follow‐up: up to 2 months postpartum | In 1 study examining pregnant women, 3 cases of nausea, hypotension, and tachycardia were reported in the IVIG (control) group, as compared to none in the intervention group. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASA: acetylsalicylic acid; CI: confidence interval; IVIG: intravenous immunoglobulin; LMWH: low molecular weight heparin; RCT: randomised clinical trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level because of single study and imprecision (wide 95% CI). | |||||
| ASA + high‐dose LMWH versus ASA + low‐dose LMWH or UFH | |||||
| Patient or population: people with antiphospholipid antibodies and no history of thrombosis Settings: specialist centres Intervention: ASA + high‐dose LMWH Comparison: ASA + low‐dose LMWH or UFH | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| ASA + low‐dose LMWH or UFH | ASA + high‐dose LMWH | ||||
| Thrombosis | 0 participants developed thrombosis. | Not estimable | 120 (2 RCTs) | ‐ | |
| Bleeding ‐ major/excessive | 0 participants developed major/excessive bleeding episodes. | Not estimable | 120 (2 RCTs) | ‐ | |
| Subcutaneous bruises Follow‐up: entire pregnancy | 100 per 1000 | 100 per 1000 | RR 1.00 (0.34 to 2.93) | 120 (2 RCTs) | ⊕⊕⊝⊝ |
| Mortality | Not reported | Not reported | Not reported | Not reported | ‐ |
| Quality of life | Not reported | Not reported | Not reported | Not reported | ‐ |
| Adverse events other than bleeding | 1 case of skin allergy was reported in the ASA + UFH group. | ||||
| The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASA: acetylsalicylic acid; CI: confidence interval; LMWH: low molecular weight heparin; RCT: randomised clinical trial; RR: risk ratio; UFH: unfractionated heparin | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to risk of bias (unclear risk of bias for blinding and selective reporting, high risk for other bias) and one level due to small selected population (pregnant women) and imprecision (wide 95% CI). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.25, 3.77] |
| 2 Minor bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Bleeding requiring transfusion | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.49, 164.76] |
| 2.2 Bleeding during first trimester | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.50, 1.61] |
| 2.3 Postpartum haemorrhage | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.60, 2.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis Show forest plot | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Major/excessive bleeding Show forest plot | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Subcutaneous bruises Show forest plot | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis best‐worst scenario Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.13, 1.19] |
| 2 Thrombosis worst‐best scenario Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.39 [1.55, 12.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Thrombosis best‐worst scenario Show forest plot | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.51] |
| 2 Thrombosis worst‐best scenario Show forest plot | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.4 [0.47, 151.34] |

