Self‐management for bronchiectasis
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: prospective RCT (single blind, parallel group; trial identifier: ISRCTN05557928) Total duration of study: 12 months 'Run in' period: none Number of study centres and location: 2 centres; UK Study setting: an acute cardiorespiratory unit in a teaching hospital and an acute medical unit in an affiliated teaching district general hospital Date of study: January 2010‐September 2012 | |
| Participants | Note: This study recruited participants with any chronic respiratory condition; Dr Greening kindly provided disaggregated data for the participants with bronchiectasis reported here. Number randomised: 20 participants with bronchiectasis randomised (early rehabilitation: n = 8; usual care: n = 12) Number of withdrawals: none Number analysed: variable per outcome Mean age (SD), years: early rehabilitation: 78 (7.8); usual care: 68.8 (11.5) Gender, n (%) male: early rehabilitation: NA; usual care: NA Severity of condition Mean (SD) baseline MRC dyspnoea grade on admission: early rehabilitation: 4.9 (0.35); usual care: 4.4 (0.67) Mean (SD) stable state MRC dyspnoea grade: early rehabilitation: 4.3 (0.46); usual care: 3.5 (0.67) Mean (SD) stable state FEV1 (L): early rehabilitation: 0.84 (0.28); usual care: 1.18 (0.49) Diagnostic criteria: MRC dyspnoea score; spirometry was measured to British Thoracic Society standards. Baseline lung function: see severity of condition Smoking history: smoker, n yes/no/ex‐smoker/missing: early rehabilitation: 1/4/2/1 ; usual care: 1/5/6/0 Inclusion criteria: the study recruited patients with a diagnosis of chronic respiratory disease, including COPD; chronic asthma, bronchiectasis or interstitial lung disease); we have considered only the subset of participants with bronchiectasis. Participants had to be aged ≥ 40 years with self‐reported breathlessness on exertion when stable (MRC dyspnoea grade 3 or worse). Exclusion criteria: inability to provide informed consent; concomitant acute cardiac event; presence of musculoskeletal, neurological, or psychiatric comorbidities that would prevent the delivery of the rehabilitation intervention; and > 4 emergency admissions to hospital for any cause in the previous 12 months. | |
| Interventions | Intervention: early rehabilitation (6 weeks' duration), comprising usual care plus daily, supervised volitional (strength and aerobic training) and non‐volitional (neuromuscular electrical stimulation) techniques. This complex intervention also included a self‐management programme, described by the study authors as: "The intervention team delivered education using the SPACE (Self management programme of Activity, Coping and Education) manual for chronic obstructive pulmonary disease, a structured programme of exercise, education, and psychosocial support. Motivational interviewing techniques were used to introduce patients to the manual and to familiarise them with the content. The manual was used throughout the participants’ inpatient stay and in the subsequent discussions during telephone calls." As this component of the complex intervention was substantial we deemed the trial to be eligible for inclusion in this review; however, we are mindful that because SPACE was included alongside other components the findings from this trial in relation to the predefined inclusion criteria for this review should be interpreted with caution. It should also be noted that SPACE was primarily designed for COPD patients and is not specifically targeted for bronchiectasis. The pulmonary rehabilitation team, consisting of physiotherapists and nurses, delivered the intervention. The exercise programme was individually prescribed and progressed. Early rehabilitation was performed on the acute medical ward and by the participants’ bedside. After discharge, participants underwent an unsupervised home based programme, supported by telephone consultations. Those who were readmitted after the 6‐week intervention period did not receive a further early rehabilitation intervention. Comparison: usual care (standard care from the ward clinical physiotherapy team as directed by the responsible clinical team) including physiotherapist‐delivered techniques for airway clearance, assessment and supervision of mobility, and advice on smoking cessation. Nutritional status was assessed using the malnutrition universal screening tool score in all participants, and referral for dietetic advice and nutritional support was carried out if appropriate. No supervised or progressive exercise programme was provided during the admission or immediately after discharge, but outpatient pulmonary rehabilitation was offered to all participants 3 months after discharge as part of standard care. Concomitant medications: not stated Excluded medications: not stated | |
| Outcomes | Primary outcomes (pre‐specified): readmission rate at 12 months Secondary outcomes (pre‐specified): number of hospital days, mortality, physical performance and HRQoL. Secondary functional measures were recorded at baseline, at discharge from hospital, 6 weeks, 3 months and 12 months. Timepoints: 12 months Outcomes collected: 1ll specified outcomes were collected | |
| Notes | Funding: not stated Notable author conflicts of interest: none stated/identified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The clinical trials unit at the University of Leicester coordinated randomisation by an automated internet based service (www.sealedenvelope.com)" |
| Allocation concealment (selection bias) | Low risk | Quote: "The clinical trials unit at the University of Leicester coordinated randomisation by an automated internet based service (www.sealedenvelope.com)" |
| Blinding of participants and personnel (performance bias) | High risk | It is not feasible to blind participants to the intervention. Knowledge of treatment group could influence self‐reporting of subjective outcomes by participants. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Single blind". It is not feasible to blind participants to the treatment group. However, knowledge of treatment group would be unlikely to influence objective outcomes. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “..all investigators performing the outcome measures were blinded to treatment allocation..” |
| Incomplete outcome data (attrition bias) | Low risk | During trial: 14/196 withdrew from intervention group; 10/193 withdrew from control group. Missing data imputed |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | High risk | Baseline imbalances in age and disease severity in the subset of participants with bronchiectasis; participants in the control group appeared to have less severe disease. Higher mortality with intervention in bronchiectasis subgroup (intervention: 4/8; control: 2/12) |
| Methods | Study design: proof‐of‐concept RCT (NCT01117493). Total duration of study: 6 months 'Run in' period: none Number of study centres and location: single regional respiratory centre, Belfast, Northern Ireland, UK. Study setting: Tertiary care Date of study: September 2006‐October 2007 | |
| Participants | Number randomised: 64 (32/32) Number of withdrawals: 4 (2/2) Number analysed: 60 (30/30) Mean age (SD), years: intervention: 60 (9); control: 60 (8) Gender, % m/f: intervention: 44/56; control: 47/53 Diagnostic criteria: assessment by respiratory clinician Baseline lung function ‐ mean (SD) % predicted FEV1: intervention: 59 (20); control: 65 (23) Smoking history: not stated Inclusion criteria: adults (aged ≥ 18 years) with a primary diagnosis of bronchiectasis based on a respiratory physician’s assessment, including a CT scan Exclusion criteria: primary diagnosis of CF, MRSA infection, or a condition that would have an impact on the assessment procedures (e.g. sensory impairment, pregnancy, language barrier, or a factor that would prevent adherence to the self‐management programme) | |
| Interventions | Intervention: usual care plus EPP: disease‐specific EPP was delivered in a group format during 1 session/week (2.5 h) for 8 weeks in total (2 weeks of disease‐specific education; 6 weeks of standardised EPP). The disease‐specific component included causes of bronchiectasis, disease process, medical investigations, dealing with symptoms, airway clearance techniques, exacerbations, health promotion and support available. The format of the disease‐specific EPP was delivered by a physiotherapist and a nurse with specialist expertise in the management of bronchiectasis who were trained and followed a scripted manual to standardise delivery. The format of the EPP was developed and piloted before this RCT. Topics included general health education, education on self‐management treatment strategies, action planning, and problem solving. Comparison: usual care: reviews at a specialist respiratory clinic on a 3‐monthly basis to monitor spirometry results, inflammatory blood marker levels, and sputum microbiologic assessment. Inhaled therapy and antibiotics were prescribed if required, and treatment was adjusted to the needs of the participant as necessary, including hospital admission. Concomitant medications: not stated Excluded medications: not stated | |
| Outcomes | Primary outcomes (pre‐specified): CDSS ‐ comprises 10 scales as follows: exercise, disease information, obtaining help, communication with physician, managing disease, doing cores, social activity, managing symptoms, managing breathlessness, managing depression. Responses are measured using a 1‐10 Likert scale where higher scores indicate greater self‐efficacy. There is no Minimum Clinically Important Difference value for these scales. Secondary outcomes (pre‐specified): revised Illness Perception Questionnaire, SGRQ, FEV1, frequency of oral and/or intravenous antibiotic therapy prescribed at respiratory clinics Timepoints: baseline, post‐intervention (8 weeks), 3 and 6 months postintervention Outcomes collected: all specified outcomes were collected | |
| Notes | Funding: supported by the Health and Social Care Research and Development Division, Public Health Agency, Belfast, Northern Ireland Notable author conflicts of interest: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “…computer generated concealed randomisation process conducted by an independent person...” |
| Allocation concealment (selection bias) | Low risk | Quote “..an envelope corresponding to the patient’s number was opened to reveal if they were assigned to the intervention or control group.” |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded and lack of blinding may have influenced outcome (1 withdrew who ‘wanted intervention’) |
| Blinding of participants and personnel (performance bias) | Low risk | It is not feasible to blind participants or personnel to the intervention. However, knowledge of treatment group would be unlikely to influence objective outcomes. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Data were collected by trained health professionals who were blinded to participants’ groups and had no other contact with the participants. Study participants were requested not to disclose their group assignment.” |
| Incomplete outcome data (attrition bias) | Low risk | 6 months: 2/32 missing from intervention group (1 illness, 1 did not complete outcome measures); 2/32 missing from control group (1 wanted intervention, 1 disliked questionnaire). Even balance between groups |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported. Subjective outcomes were well‐reported but objective outcomes poorly reported. |
| Other bias | High risk | This was a proof‐of‐concept study so there was no power calculation. There is therefore a risk that negative findings were influenced by an inadequate sample size |
CDSS: Chronic Disease Self‐Efficacy Scale; CF: cystic fibrosis; COPD: chronic obstructive pulmonary disease; CT: computed tomographic; EPP: expert patient programme; FEV1: forced expiratory volume in one second; HRQoL: health‐related quality of life; MRC: medical research council; MRSA: methicillin‐resistant Staphylococcus aureus ;NA: not applicable; RCT: randomised controlled trial; SGRQ: St Georges Respiratory Questionnaire; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The intervention was not self‐management | |
| Inspiratory muscle training was the sole intervention | |
| Information resource was the sole intervention | |
| Exercise programme plus airway clearance therapy (ACT) advice at baseline versus ACT advice at baseline alone. Therefore, the only intervention component was exercise and does not meet our criteria of at least 2 components in the intervention group alone | |
| Inspiratory muscle training was the sole intervention | |
| Pulmonary rehabilitation plus chest physiotherapy plus education plus self‐management plan versus chest physiotherapy plus education plus self‐management. Therefore, the only component in the intervention group alone is pulmonary rehabilitation and does not meet our criteria of at least 2 components in the intervention group alone | |
| The study included 40 participants with a range of respiratory conditions, including 2 with bronchiectasis. We contacted the study authors for data on the bronchiectasis participants alone but did not receive a reply. | |
| Inspiratory muscle training plus pulmonary rehabilitation plus education versus pulmonary rehabilitation plus education. Therefore, the only component in the intervention group alone is inspiratory muscle training and does not meet our criteria of at least 2 components in the intervention group alone |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Tayside Rehabilitation in Bronchiectasis Exacerbations (TRIBE) : a randomized controlled trial (TRIBE) |
| Methods | RCT of pulmonary rehabilitation after exacerbations of bronchiectasis |
| Participants | Inclusion Criteria
Exclusion Criteria
|
| Interventions |
|
| Outcomes | Primary Outcome Measures
Secondary Outcome Measures
|
| Starting date | June 2014 |
| Contact information | James D Chalmers, University of Dundee |
| Notes |
CF: cystic fibrosis; COPD: chronic obstructive pulmonary disorder; CT: computed tomographic; RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SGRQ Total: mean difference Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Early rehab versus usual care, Outcome 1 SGRQ Total: mean difference. | ||||
| 1.1 6 weeks | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐12.70 [‐30.39, 4.99] |
| 1.2 3 months | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐9.15 [‐28.08, 9.78] |
| 1.3 12 months | 1 | 7 | Mean Difference (IV, Fixed, 95% CI) | ‐10.27 [‐45.15, 24.61] |
| 2 FEV1 L: mean difference Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 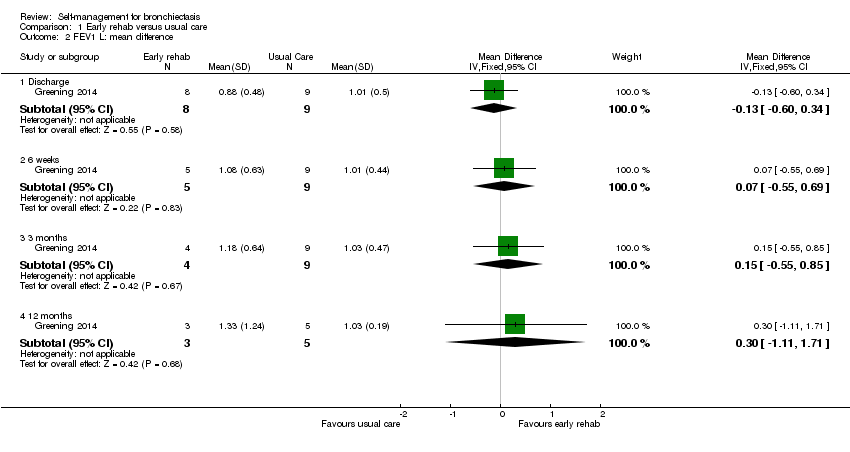 Comparison 1 Early rehab versus usual care, Outcome 2 FEV1 L: mean difference. | ||||
| 2.1 Discharge | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.60, 0.34] |
| 2.2 6 weeks | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.55, 0.69] |
| 2.3 3 months | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.55, 0.85] |
| 2.4 12 months | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.11, 1.71] |
| 3 Mortality Show forest plot | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.64, 39.06] |
| Analysis 1.3  Comparison 1 Early rehab versus usual care, Outcome 3 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SGRQ Total: mean difference Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Expert patient programme versus usual care, Outcome 1 SGRQ Total: mean difference. | ||||
| 1.1 Post‐intervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐6.5 [‐16.59, 3.59] |
| 1.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐12.97, 7.77] |
| 1.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐6.64, 13.04] |
| 2 Self‐efficacy: Exercise Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2 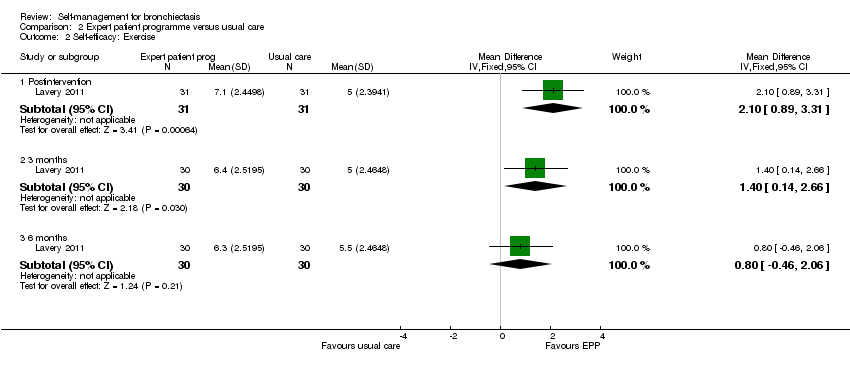 Comparison 2 Expert patient programme versus usual care, Outcome 2 Self‐efficacy: Exercise. | ||||
| 2.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [0.89, 3.31] |
| 2.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [0.14, 2.66] |
| 2.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.46, 2.06] |
| 3 Self‐efficacy: Disease info Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3 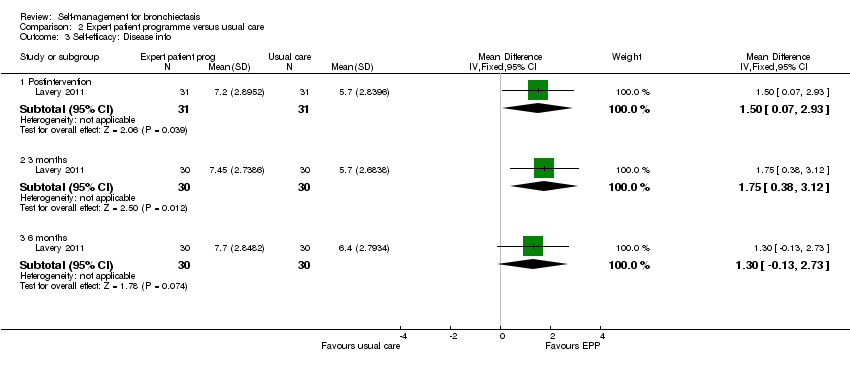 Comparison 2 Expert patient programme versus usual care, Outcome 3 Self‐efficacy: Disease info. | ||||
| 3.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [0.07, 2.93] |
| 3.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.75 [0.38, 3.12] |
| 3.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐0.13, 2.73] |
| 4 Self‐efficacy: Obtain help Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4 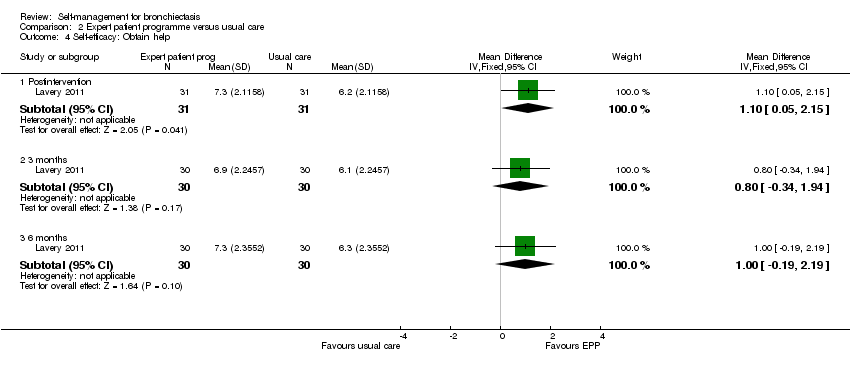 Comparison 2 Expert patient programme versus usual care, Outcome 4 Self‐efficacy: Obtain help. | ||||
| 4.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.05, 2.15] |
| 4.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.34, 1.94] |
| 4.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.19, 2.19] |
| 5 Self‐efficacy: Communication Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Expert patient programme versus usual care, Outcome 5 Self‐efficacy: Communication. | ||||
| 5.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.14, 2.06] |
| 5.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.04, 1.76] |
| 5.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.07, 1.87] |
| 6 Self‐efficacy: Manage disease Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Expert patient programme versus usual care, Outcome 6 Self‐efficacy: Manage disease. | ||||
| 6.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.17, 2.03] |
| 6.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.27, 1.93] |
| 6.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.27, 1.67] |
| 7 Self‐efficacy: Do chores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.7 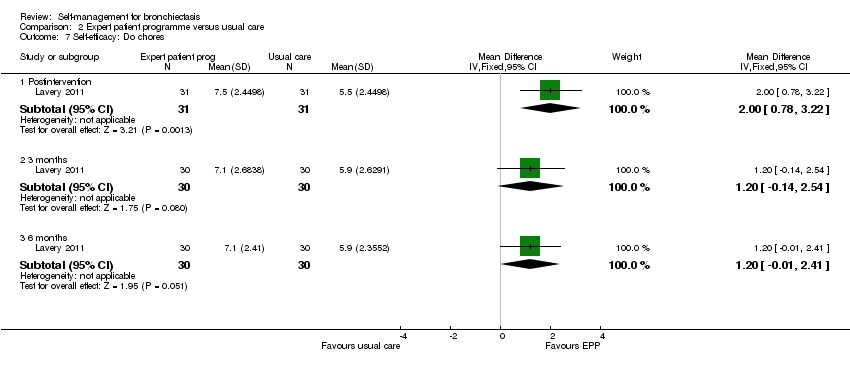 Comparison 2 Expert patient programme versus usual care, Outcome 7 Self‐efficacy: Do chores. | ||||
| 7.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.78, 3.22] |
| 7.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.14, 2.54] |
| 7.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.01, 2.41] |
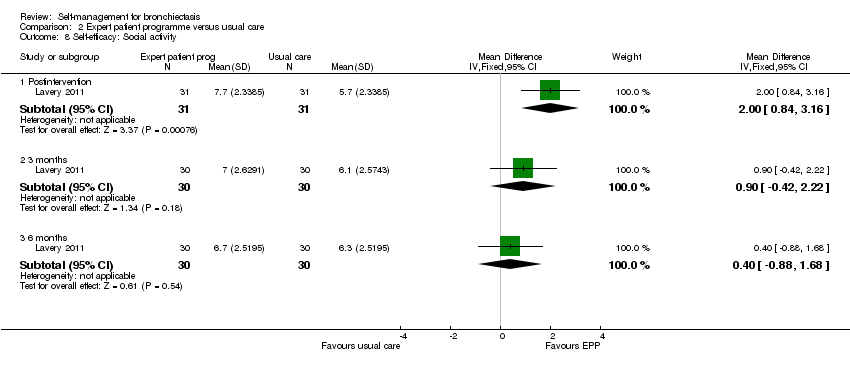
| 8 Self‐efficacy: Social activity Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 Expert patient programme versus usual care, Outcome 8 Self‐efficacy: Social activity. | ||||
| 8.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.84, 3.16] |
| 8.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.42, 2.22] |
| 8.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.88, 1.68] |
| 9 Self‐efficacy: Manage symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.9  Comparison 2 Expert patient programme versus usual care, Outcome 9 Self‐efficacy: Manage symptoms. | ||||
| 9.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [0.78, 3.02] |
| 9.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.13, 2.27] |
| 9.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.41, 1.81] |
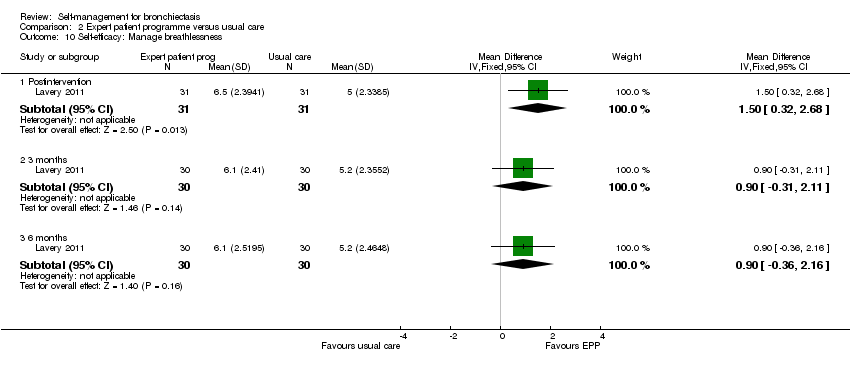
| 10 Self‐efficacy: Manage breathlessness Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.10  Comparison 2 Expert patient programme versus usual care, Outcome 10 Self‐efficacy: Manage breathlessness. | ||||
| 10.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [0.32, 2.68] |
| 10.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.31, 2.11] |
| 10.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.36, 2.16] |
| 11 Self‐efficacy: Manage depression Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.11 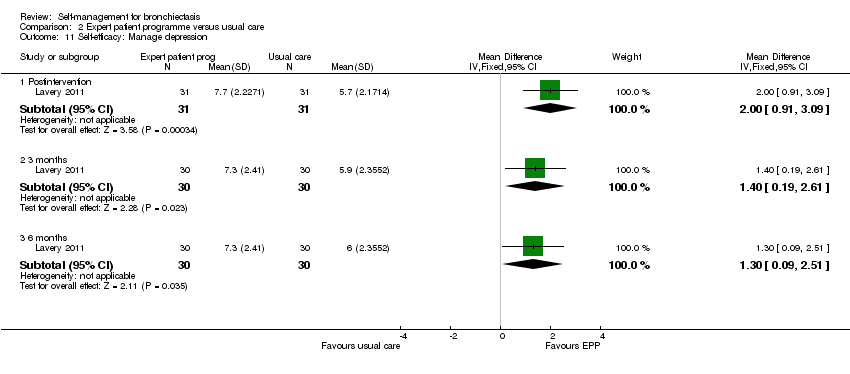 Comparison 2 Expert patient programme versus usual care, Outcome 11 Self‐efficacy: Manage depression. | ||||
| 11.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.91, 3.09] |
| 11.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [0.19, 2.61] |
| 11.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.09, 2.51] |

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
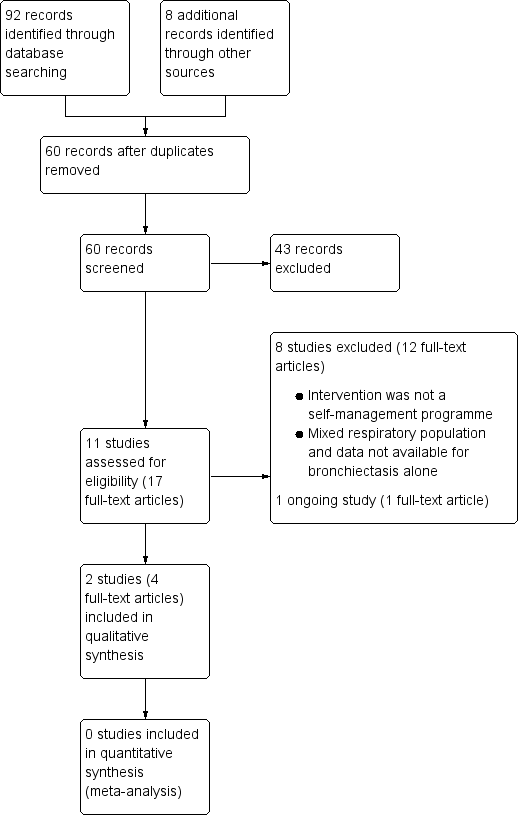
Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
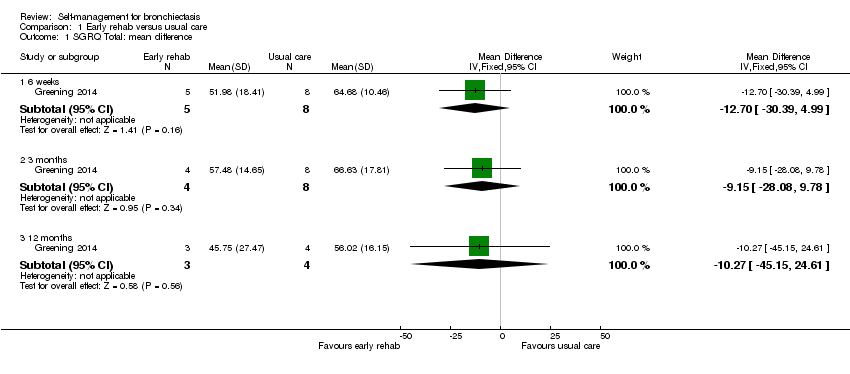
Comparison 1 Early rehab versus usual care, Outcome 1 SGRQ Total: mean difference.

Comparison 1 Early rehab versus usual care, Outcome 2 FEV1 L: mean difference.
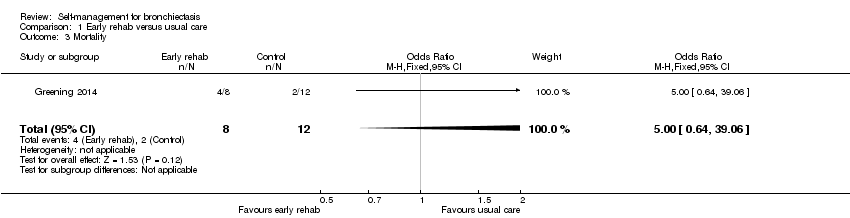
Comparison 1 Early rehab versus usual care, Outcome 3 Mortality.
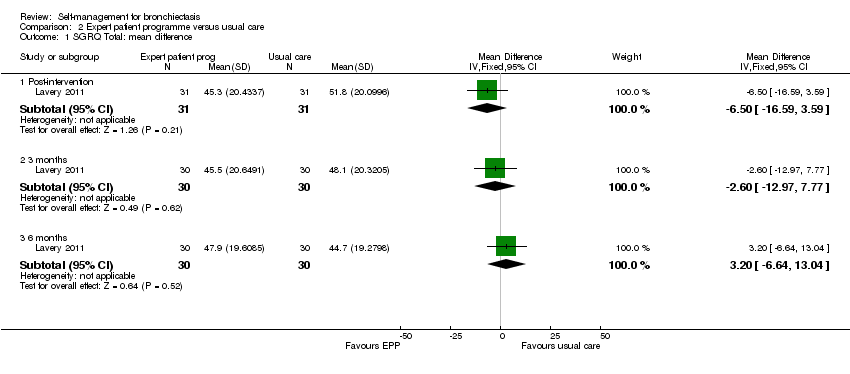
Comparison 2 Expert patient programme versus usual care, Outcome 1 SGRQ Total: mean difference.

Comparison 2 Expert patient programme versus usual care, Outcome 2 Self‐efficacy: Exercise.
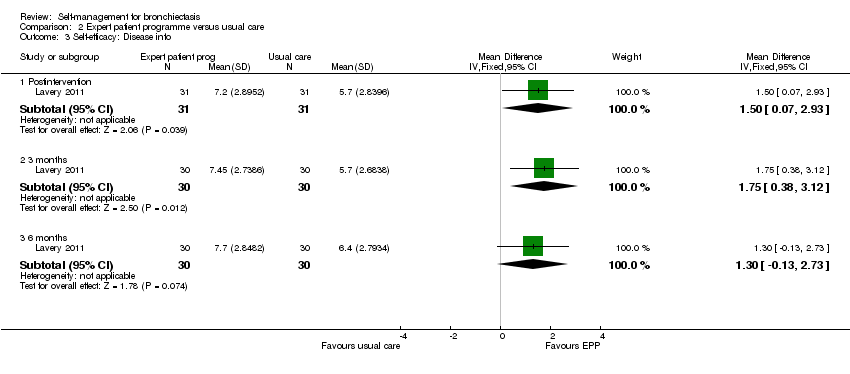
Comparison 2 Expert patient programme versus usual care, Outcome 3 Self‐efficacy: Disease info.

Comparison 2 Expert patient programme versus usual care, Outcome 4 Self‐efficacy: Obtain help.
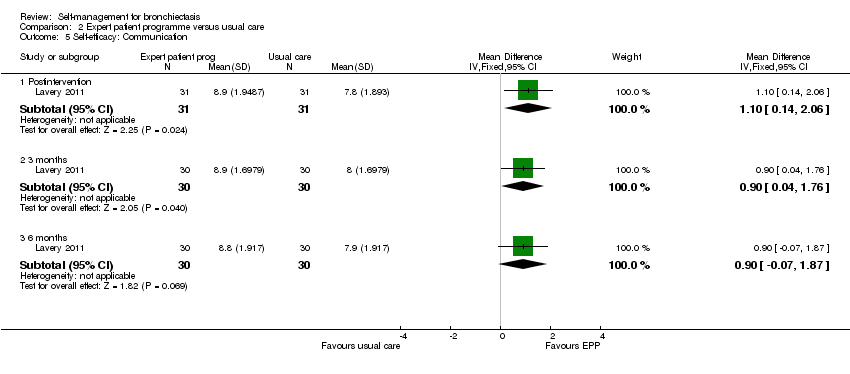
Comparison 2 Expert patient programme versus usual care, Outcome 5 Self‐efficacy: Communication.

Comparison 2 Expert patient programme versus usual care, Outcome 6 Self‐efficacy: Manage disease.

Comparison 2 Expert patient programme versus usual care, Outcome 7 Self‐efficacy: Do chores.
Comparison 2 Expert patient programme versus usual care, Outcome 8 Self‐efficacy: Social activity.

Comparison 2 Expert patient programme versus usual care, Outcome 9 Self‐efficacy: Manage symptoms.
Comparison 2 Expert patient programme versus usual care, Outcome 10 Self‐efficacy: Manage breathlessness.

Comparison 2 Expert patient programme versus usual care, Outcome 11 Self‐efficacy: Manage depression.
| Self‐management compared to usual care for bronchiectasis | ||||||
| Patient or population: people with non‐cystic fibrosis bronchiectasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with usual care | Risk with self‐management | |||||
| Health‐related quality of life | The mean health‐related quality of life was 56.02 points | MD 10.27 lower | ‐ | 20 | ⊕⊝⊝⊝ | No clear benefit or harm from self‐management (very low‐quality evidence) |
| Health‐related quality of life Follow up: range post‐intervention to 6 months | The mean health‐related quality of life was 44.7 points | MD 3.2 higher | ‐ | 60 | ⊕⊕⊝⊝ | No clear benefit or harm from self‐management |
| Exacerbations requiring antibiotics | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Serious adverse events: mortality | ‐ | ‐ | not estimable | 20 | ||
| Hospital admissions (number admitted at least once) | ‐ | ‐ | not estimable | 20 | ‐ | |
| Lung function assessed with: FEV1 L | The mean FEV1 was 1.03 L | MD 0.3 higher | ‐ | 20 | ⊕⊝⊝⊝ | No clear benefit or harm from self‐management |
| Self‐efficacy assessed with: CDSS Follow‐up: postintervention to 6 months | ‐ | ‐ | not estimable | 60 | ⊕⊕⊝⊝ | Six out of ten scales showed significant improvements over time with the intervention. We elected not to include all 10 scales in the table but graded the evidence based on overall quality of the study |
| Economic costs | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1One point deducted for the unblinded nature of the comparison. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SGRQ Total: mean difference Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 weeks | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐12.70 [‐30.39, 4.99] |
| 1.2 3 months | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐9.15 [‐28.08, 9.78] |
| 1.3 12 months | 1 | 7 | Mean Difference (IV, Fixed, 95% CI) | ‐10.27 [‐45.15, 24.61] |
| 2 FEV1 L: mean difference Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Discharge | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.60, 0.34] |
| 2.2 6 weeks | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.55, 0.69] |
| 2.3 3 months | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.55, 0.85] |
| 2.4 12 months | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.11, 1.71] |
| 3 Mortality Show forest plot | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.64, 39.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SGRQ Total: mean difference Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Post‐intervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐6.5 [‐16.59, 3.59] |
| 1.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐12.97, 7.77] |
| 1.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐6.64, 13.04] |
| 2 Self‐efficacy: Exercise Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [0.89, 3.31] |
| 2.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [0.14, 2.66] |
| 2.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.46, 2.06] |
| 3 Self‐efficacy: Disease info Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [0.07, 2.93] |
| 3.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.75 [0.38, 3.12] |
| 3.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐0.13, 2.73] |
| 4 Self‐efficacy: Obtain help Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.05, 2.15] |
| 4.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.34, 1.94] |
| 4.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.19, 2.19] |
| 5 Self‐efficacy: Communication Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.14, 2.06] |
| 5.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.04, 1.76] |
| 5.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.07, 1.87] |
| 6 Self‐efficacy: Manage disease Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.17, 2.03] |
| 6.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.27, 1.93] |
| 6.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.27, 1.67] |
| 7 Self‐efficacy: Do chores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.78, 3.22] |
| 7.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.14, 2.54] |
| 7.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.01, 2.41] |
| 8 Self‐efficacy: Social activity Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.84, 3.16] |
| 8.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.42, 2.22] |
| 8.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.88, 1.68] |
| 9 Self‐efficacy: Manage symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [0.78, 3.02] |
| 9.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.13, 2.27] |
| 9.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.41, 1.81] |
| 10 Self‐efficacy: Manage breathlessness Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [0.32, 2.68] |
| 10.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.31, 2.11] |
| 10.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.36, 2.16] |
| 11 Self‐efficacy: Manage depression Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.91, 3.09] |
| 11.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [0.19, 2.61] |
| 11.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.09, 2.51] |

