对接受外科服务的老年人进行老年综合评估
Abstract
研究背景
老年人群术后并发症的风险持续增加。为从手术中恢复的老年人提供新的照护方法,可能会减少手术相关并发症。已有文献证实老年综合评估(Comprehensive geriatric assessment, CGA)可改善内科患者的一些结局指标,例如使他们能够继续住在家里,这种方法对外科患者也产生了积极影响。CGA是一个协调的、多学科的合作,它评估了老年人在医疗、社会心理和功能方面的能力和局限性,目的是建立一个治疗和长期随访计划。
研究目的
评估CGA干预与标准照护相比,对外科住院老年人术后结局的有效性。
检索策略
我们检索了以下数据库:Cochrane 对照试验中心注册库(Cochrane Central Registerof Controlled Trials, CENTRAL)、MEDLINE、Embase、PsycINFO、CINAHL(Cumulative Index to Nursing and Allied Health Literature) 和两个临床试验注册中心,检索时限截止到2017年1月13日。我们还检索了灰色文献。
标准/纳入排除标准
纳入标准:随机对照试验;接受手术的受试者年龄在65岁及以上;试验比较了CGA与常规手术护理,并报告了主要结局指标(死亡率和出院后接受高水平护理)和次要结局指标(住院时间、再入院率、总费用和术后并发症)。我们排除了以下研究:受试者没有接受完整的CGA;受试者没有接受手术;研究招募的受试者年龄小于65岁或来自非急性护理医院。
数据收集与分析
两位作者独立筛选文献,对所纳入文献进行偏倚风险评估,提取数据,并评估证据的确定程度。我们用风险比(RR),95%置信区间表示二分类数据,连续性数据用均数差(MD)表示。
主要结果
我们纳入了8项在北美和欧洲进行的随机试验,其中7项试验招募了髋部骨折恢复中的受试者(N=1583),另一项试验招募了肿瘤择期手术的受试者(N=260)。2项试验中的CGA被应用于术前,其余试验中CGA则被应用于术后。6项试验进行了充分的随机化,5项试验的实施偏倚风险较低,4项试验的测量偏倚风险较低。试验中对受试者实施盲法是不可能的。所有8项试验的失访率都很低,7项试验报告了所有的预期结局指标。
CGA可能会降低髋部骨折老年人的死亡率(RR=0.85, 95% CI [0.68, 1.05];5项试验,1316名受试者,I² = 0%;中质量证据)。干预减少了出院后接受更高水平护理的风险(RR=0.71, 95% CI [0.55, 0.92];5项试验,941名受试者,I² = 0%;高质量证据)。
住院时间具有很高的异质性,两组间住院时间的均差在‐12.8到8.3天之间。CGA可能导致住院时间略微缩短(4项试验,841名受试者,中质量证据)。干预可能对再入院率几乎没有影响(RR=1.00, 95%CI [0.76, 1.32];3项试验,741名受试者,I²=37%;中质量证据)。
CGA可能会略微降低总成本(1项试验,397名受试者,中质量证据)。干预可能对主要术后并发症(2项试验,579名受试者,低质量证据)和谵妄发生率(RR=0.75, 95%CI [0.60, 0.94],3项试验,705名受试者,I²=0%;低质量证据)几乎没有影响。
作者结论
有证据表明CGA可以改善髋部骨折患者的预后。目前还没有足够的研究来确定CGA在外科干预方面何时最有效,或者CGA是否对髋部骨折以外的外科患者有效。
PICO
Plain language summary
对接受手术的老年人进行特殊评估是否改善了他们手术后的恢复?
本系统综述的目的是什么
我们的目的是找出一种被称为CGA的评估方法,是否改善了65岁及以上的人手术后的情况。CGA涉及多名医疗保健专业人员,并解决了医疗疾病、身体衰退和社会因素导致的恢复缓慢的问题。
关键信息
我们发现接受CGA的髋部骨折的老年人死亡的可能性较小,而回家的可能性更大。在其他组患者中,没有足够的高质量研究来确定CGA是否对他们有用。
本综述的研究内容是什么?
世界人口正在老龄化;超过65岁的人越来越多,而且在手术后发生并发症的风险在增加,包括感染、心脏病发作、甚至死亡。众所周知,CGA可以降低住院老年人的并发症,但没有专门针对术后老年人做过综述。本系统综述是为了弥补这一差距。我们比较了在手术前(2项研究)或手术后(6项研究)接受CGA的人与接受外科医生传统术后护理的人。
本综述的主要结果是什么?
我们纳入了在北美和欧洲进行的8项研究。7项研究招募了髋部骨折的人(1583名受试者),1项研究涉及肿瘤切除的人(260名受试者)。
我们发现接受CGA的老年人可能死亡风险较低,并且在出院后,更有可能回到他们入院前居住的地方。接受干预的老年人可能住院天数较少,但由于研究结果的差异太大,我们无法确定住院时间具体有多长。接受干预的人和没有接受干预的人再入院的次数很相近。当老年人接受CGA时,他们花费的护理费用可能会少一点。至于术后并发症,不同研究的结果差异很大,所以我们不能确定CGA是否会导致更多的并发症。
这篇综述的时效性如何?
我们检索了截止2017年1月13日前的研究。
Authors' conclusions
Summary of findings
| Comprehensive geriatric assessment for older people admitted to a surgical service | ||||||
| Patient or population: Improving outcomes in older adult people admitted to a surgical service. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with control | Risk with geriatric care | |||||
| Mortality | 214 per 1000 | 182 per 1000 | RR 0.85 | 1316 | ⊕⊕⊕◯ 1 | Hip fracture studies. |
| Discharge to an increased level of care | 247 per 1000 | 176 per 1000 | RR 0.71 | 941 | ⊕⊕⊕⊕ | Hip fracture studies. |
| Length of stay | Meta‐analysis was not performed due to high heterogeneity (Analysis 1.3) | MD in studies ranged from ‐12.8 days to 8.3 days | ‐ | 841 | ⊕⊕⊕⊝ | Hip fracture studies ‐ length of stay until final discharge from hospital (including rehabilitation hospital). Meta‐analysis was not retained due to high heterogeneity (I² = 88%, P < 0.00001). |
| Re‐admission | 316 per 1000 | 316 per 1000 | RR 1.00 | 741 | ⊕⊕⊕⊝ | All studies included; removing elective surgical oncology study doesn't change effect. |
| Total cost | The mean total cost was EUR 59,486 | MD EUR 5154 lower | 397 | ⊕⊕⊕⊝ | 1 study reported cost. | |
| Major complication | Meta‐analysis was not performed due to high heterogeneity (Analysis 1.5) | Two studies reported this outcome with RRs of 0.74 and 1.16 | 579 | ⊕⊕⊝⊝ | Hempenius 2013 defined major as 2 or more complications. Vidán 2005 defined major as delirium, congestive heart failure, pneumonia, DVT, PE, pressure ulcer, arrhythmia and myocardial infarction. Meta‐analysis was not retained due to high heterogeneity (I² = 77%, P = 0.04). | |
| Major complication ‐ delirium | 327 per 1000 | 245 per 1000 | RR 0.75 | 705 | ⊕⊕⊝⊝ | Delirium assessed by Delirium Observation Scale (Hempenius 2013) or confusion assessment method (Marcantonio 2001; Vidán 2005) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded due to imprecision because there were wide confidence intervals that include both no effect and a high risk of benefit or harm. 2 We downgraded due to inconsistency because there was significant variability among studies. 3 We downgraded due to other considerations because costing was calculated in an imprecise manner (costs are presented as the total cost over one year, however the admission cost did not include rehabilitation hospital costs despite the authors identifying a higher proportion of control patients being transferred to rehabilitation centres before discharge). 4 We downgraded due to the high risk of bias. | ||||||
Background
This review assesses the effects of comprehensive geriatric assessment (CGA) on postoperative outcomes of older people admitted to hospital with a surgical problem.
Description of the condition
As the world's population ages, the demand for surgery among older people is increasing (Etzioni 2003). It is estimated that over half of all surgical operations are performed on people aged over 65 years (Geriatric Review Syllabus 2006). Compared to their younger counterparts, older people experience higher rates of postoperative complications, have a longer length of stay in hospital, and are more likely to require institutionalization after discharge (Lidsky 2012; Turrentine 2006). The increased costs and health resource use associated with older surgical patients place an additional strain on the healthcare system, highlighting the need for evidence‐based interventions that can improve the outcomes of this patient population (Etzioni 2011).
Description of the intervention
CGA is a "multidisciplinary diagnostic process intended to determine a frail older person's medical, psychosocial, and functional capabilities and limitations in order to develop an overall plan for treatment and long‐term follow‐up" (Rubenstein 1991). CGA is not any one intervention in isolation, but rather a coordinated, multidisciplinary collaboration. This has already been successfully demonstrated on medical and orthogeriatric units (Ellis 2017; Frondini 2010; Prestmo 2015). Aspects of CGA are organized into three categories (medical, psychosocial, and functional) and may include a combination of the following factors (Rubenstein 1989).
Medical
-
Primary diagnosis resulting in admission.
-
Geriatrician following every eligible patient during their admission.
-
Minimising the use of medications prone to causing delirium and adjusting dosing for geriatric syndromes.
-
Comprehensive medication review by pharmacist.
Psychosocial
-
Environmental cues to orient patient.
-
Regular comfort rounds by nursing staff.
-
Early discharge planning to anticipate and manage potential challenges.
Functional
-
Fall risk assessment and mitigation.
-
Physiotherapist intervention to prevent neuromuscular deconditioning.
-
Occupational therapist to identify and manage barriers to independence.
-
Physical environment modifications to reduce confusion, falls, delirium.
These interventions are conducted within a multidisciplinary collaboration to develop a unified plan of care for older people and were compared with usual care in a standard inpatient ward. CGA can be delivered at any point in a patient's care for elective surgical interventions and can be delivered postoperatively for emergency procedures. It is unclear if geriatric interventions before and after surgery are equally effective or if the interventions produce different effects in elective versus emergency surgery.
How the intervention might work
Older surgical patients have complex healthcare needs: frailty, multi‐morbidity, and polypharmacy are common in this patient population (Bettelli 2011). However, most hospitals are structured to care for patients with a single, acute illness and are often ill‐equipped to meet the needs of older people, leading to poor surgical outcomes. By performing a CGA, healthcare providers can identify and optimize medical and social issues associated with surgical complications before they have a negative impact on the health of the patient, which could improve outcomes.
Why it is important to do this review
Previous studies, notably a recent Cochrane Review that examined the effect of CGA on medical patient outcomes (Ellis 2017), have reported that if older people receive CGA on admission to hospital they are more likely to be alive and in their own homes at follow‐up. However, most studies have focused on people admitted to hospital with general internal medicine issues, and to date there have not been any systematic reviews of CGA interventions focusing on surgical patients. There has also been no attempt to evaluate the role of timing of CGA and surgery on the effectiveness of the intervention.
Objectives
To assess the effectiveness of CGA interventions compared to standard care on the postoperative outcomes of older people admitted to hospital for surgical care.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised trials of postoperative participants. These could be from any surgical specialty, including emergency and elective surgery. The intervention groups received comprehensive geriatric assessment (CGA), compared to a control group receiving standard care. To reduce the likelihood of publication bias, we did not limit articles to the English language. We screened studies found in trial databases and the grey literature for eligibility.
Types of participants
The focus of this review was people aged 65 years or over in hospital under the care of an inpatient surgical ward. Although there is not a standard numerical criterion to define old age, 65 years is widely accepted as the chronological age to be considered an older person.
People admitted to hospital for elective or emergency surgery, or for an acute medical condition or injury requiring close observation and expectant management by a surgical team, were eligible for inclusion in the analysis.
Studies containing a subset of surgical patients aged over 65 years were eligible for inclusion; we included study data pertaining to our population of interest in the meta‐analysis
Types of interventions
We included studies in which a geriatrician, internist, geriatric nurse or another physician trained in geriatric assessment performed a multi‐component geriatric assessment in hospital. Studies included participants receiving the intervention compared with participants receiving standard postoperative care. The CGA had to be performed by a clinician trained in geriatric assessment. CGA is typically performed as part of a mobile, multidisciplinary team consulted to provide patient management recommendations, or as part of a specialized ward dedicated to providing multidisciplinary care to geriatric surgical patients. The CGA intervention may be carried out pre‐operatively, postoperatively, or throughout the patient's stay in hospital.
We excluded studies in which CGA was used exclusively as a tool to predict adverse postoperative events. We also excluded studies examining one aspect of the CGA instead of employing a multidimensional assessment, and we also excluded cross‐over studies. We excluded enhanced recovery after surgery programmes because CGA is not a routine component of these programmes. Studies that did not report any of our predefined outcomes were also excluded.
Types of outcome measures
Primary outcomes
The primary outcomes were mortality and discharge to an increased level of care.
We measured mortality to the end of follow‐up after treatment. We measured discharge to an increased level of care reported as participants being discharged to a setting where they would receive an increased level of care such as an assisted‐living or long‐term care facility, as opposed to returning to their pre‐admission place of residence.
Secondary outcomes
Secondary outcomes included length of stay, re‐admission rate, total cost and postoperative complications.
We measured length of stay as a continuous outcome reported as the number of days spent in hospital after surgery. Re‐admission was measured as a dichotomous outcome representing the number of participants who were re‐admitted in a given time period. Cost was recorded in euros (EUR) for 2016 after converting using Purchasing Power Parity (PPP) and the Gross Domestic Product (GDP) inflator as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), but was not combined due to cross‐jurisdictional differences in cost reporting and variation in data sources.
Postoperative complications included any of the following events in hospital after surgery: intensive care unit admission, vascular complications (e.g. myocardial infarction, stroke, deep venous thrombosis, and pulmonary embolism), serious infection, and delirium. For studies that did not report major complication categories, we recorded complication frequency by organ system (e.g. cardiovascular, respiratory, gastrointestinal, neurologic, etc.) We reported all complications as a dichotomous outcomes. Complications not prone to detection bias, such as stroke and myocardial infarction, and those detected in studies with appropriate blinding of complication assessment, were more strongly weighted in the discussion. Delirium is particularly prone to detection bias due to the CGA intervention being more likely to detect delirium; we assessed how each study controlled for this aspect.
Search methods for identification of studies
Electronic searches
We used a sensitive search strategy to retrieve studies from electronic databases. We searched the following databases, with publication dates ranging from inception to 13 January 2017.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1), including the Cochrane Effective Practice and Organisation of Care (EPOC) Group Specialized Register, part of the Cochrane Library (www.cochranelibrary.com);
-
MEDLINE In‐Process & Other Non‐Indexed Citations, OvidSP (1946 to 13 January 2017);
-
Embase, OvidSP (1974 to 13 January 2017);
-
PsycINFO, OvidSP (1987 to 13 January 2017); and
-
CINAHL (Cumulative Index to Nursing and Allied Health Literature), EBSCO (1980 to 13 January 2017).
The search terms combined Medical Subject Headings (MeSH) and free text words as shown in the search strategies in Appendix 1. We placed no restrictions on language, publication type, or publication year.
Searching other resources
We conducted a grey literature search to identify non‐indexed studies not appearing in the databases listed above. Sources included:
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en/); and
-
USA National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov).
We used Science Citation Index to search the cited and citing articles of included studies.
Data collection and analysis
Selection of studies
Two review authors (GE and QD) screened titles and abstracts to identify potentially eligible articles for full‐text review. We assessed potential eligibility based on design, participants, intervention, and outcomes as described, and excluded studies that did not meet the inclusion criteria at this stage. Two review authors (GE and AT or SC) independently carried out full‐text review. We resolved conflicts between review authors at all stages of article screening and data extraction by discussion and consensus. We reported the number of excluded studies and the reason for exclusion as per Section 7.2.5 of the Cochrane Handbook (Higgins 2011).
Data extraction and management
Two review authors (GE and AT or SC) independently extracted data onto web‐based electronic data collection forms (Covidence), resolving disagreements between review authors by discussion and consensus. Data were exported to Review Manager 5 for analysis (Review Manager 2014).
During data extraction, we took note of the study source, eligibility, methods, participants, interventions, outcomes of interest, results, and other information as defined in Table 7.3.a of the Cochrane Handbook, in Higgins 2011, and the EPOC good‐practice data extraction form (EPOC 2017a). All costs were reported in Euros.
Assessment of risk of bias in included studies
Two independent review authors used Cochrane's 'Risk of bias' tool (Higgins 2011) modified based on the EPOC guidance for risk of bias criteria (EPOC 2017b) to assess each study. Each study was evaluated based on the following criteria: low risk, high risk, or uncertain risk.
-
Random sequence generation ‐ was the allocation sequence adequately generated?
-
Allocation concealment ‐ was allocation concealment adequate?
-
Baseline demographics between groups ‐ were baseline outcomes measured before the intervention and were they similar between groups?
-
Incomplete data ‐ were loss to follow‐up or dropouts low enough to limit risk of bias?
-
Blinding of participants and personnel ‐ were participants and personnel blind to the intervention?
-
Blinding of outcome assessment ‐ were outcome assessors blind to the intervention?
-
Protection from cross‐contamination ‐ were there safeguards to cross‐contamination of the control group?
-
Selective reporting ‐ were all outcomes in the methods reported in the results?
-
Other risks of bias ‐ were any additional risks noted during bias assessment?
Measures of treatment effect
We reported dichotomous outcome data, such as the effect of CGA on patient mortality and discharge to an increased level of care, as risk ratios with 95% confidence intervals. We reported continuous outcome data such as the effect of CGA on length of stay using the mean difference between the CGA intervention and standard care with a 95% confidence interval. For all continuous‐variable outcomes, we reported the mean and standard deviations or standard error of the outcome measurements in each intervention group, as well as the number of participants on which the outcome was measured. Due to the differences in delivery of CGA between studies we elected to use the random effects model for meta‐analysis. We expected to find both study‐to‐study variability and within study variability resulting in differing true effects between studies. We used the fixed‐effect model as a form of sensitivity analysis.
Unit of analysis issues
We performed analyses at the participant level to avoid unit of analysis errors. If we had identified cluster randomised trials, we would have used a ratio estimator approach to reduce the size of each cluster trial to its effective sample size (Rao 1992), which is its original sample size divided by design effect. The design effect is 1 + (M ‐ 1) ICC, where M is the average cluster size and ICC is the intra‐cluster correlation coefficient. For dichotomous data, the number of participants and the number of events would have been divided by the design effect. For continuous data, the sample size would have been divided by the design effect. Missing ICCs would have been selected from other cluster randomised trials included in the review or obtained from similar external studies. We would have conducted sensitivity analyses to investigate whether removing clustered trials affects the conclusions.
If the results of a study could not be adjusted for the unit of analysis error, we would have excluded it from the pooled analysis. We assessed length of stay based on time since admission to discharge and end of follow‐up as predefined outcome measurement points but were unable to pool it due to high heterogeneity.
Dealing with missing data
Where feasible, we attempted to obtain missing data from study authors. We investigated attrition rates (e.g. dropouts, losses to follow‐up, and withdrawals), and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward). Where standard deviations for outcomes were not reported, we imputed these values by assuming the standard deviation of the missing outcome to be the average of the standard deviations from those studies where this information was reported. We investigated the impact of imputation on meta‐analyses by means of sensitivity analysis.
Assessment of heterogeneity
Where we considered studies similar enough based on population, study design, and setting to allow pooling of data using meta‐analysis, we assessed the degree of heterogeneity by visual inspection of forest plots and by examining the Chi² test for heterogeneity. We quantified heterogeneity between studies using the I² test. An I² of less than 40% was considered unimportant; 40% to 60% may indicate moderate heterogeneity; 60% to 75% may indicate substantial heterogeneity; and 75% to 100% indicates considerable heterogeneity. Where we detected substantial clinical, methodological, or statistical heterogeneity across included studies, we did not retain the pooled results from meta‐analysis but instead used a narrative approach to data synthesis.
Assessment of reporting biases
We assessed publication bias by searching trial registries and searching for grey literature through citation chaining. For studies published after 1 July 2005, we noted lack of registration of the trial protocol with the WHO ICTRP in the 'Risk of bias' table. We also noted selective reporting of predefined outcomes.
Data synthesis
We compared random‐effects and fixed‐effect models to assess if smaller studies affect the results. Given the complex and multidimensional nature of CGA, variation is expected in measured outcomes due to sampling error and differing patterns of implementation of CGA. If there was a difference between fixed‐effect and random‐effects models, we assessed the impact of small studies on the estimate of effect before deciding which model to use.
Summary of findings
We summarized the findings of the main intervention comparison for the most important outcomes included in the review. We graded our primary outcomes (mortality and discharge to an increased level of care) and secondary outcomes (length of stay, re‐admission rate, cost and postoperative complication rates) as a means to assess the certainty of the evidence. Two review authors (GE and AT) independently assessed the certainty of the evidence (high, moderate, low, and very low) using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook (Higgins 2011), the EPOC worksheets (EPOC 2017c), and the GRADE Working Group guidelines (Guyatt 2008), and the GRADEpro software was used to grade each outcome (GRADEpro GDT 2015). We resolved disagreements on certainty ratings by discussion. Justification for decisions to either downgrade or upgrade the ratings are available as footnotes in summary of findings Table for the main comparison and the full GRADE evidence profile is available as Appendix 2.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analysis for the a priori defined variables listed below.
-
Orthopedic versus other surgical specialties.
-
CGA timing ‐ is the CGA conducted pre‐operatively, postoperatively, or throughout an admission?
-
Emergency versus elective surgery.
We analyzed these subgroups at discharge and at end of follow‐up. We determined if the subgroups differ by inspecting the overlap of confidence intervals and testing for subgroup differences using Review Manager 5 (Review Manager 2014).
Timing of the CGA in relation to surgery could affect patient outcomes because the potential benefits of CGA intervention could arise from optimizing patient medical and social issues before surgery; by providing a better level of care following surgery; or both pre‐ and postoperative intervention may be necessary to see benefits. Most studies of CGA in surgical patients have been performed in orthopedic trauma (hip fracture); the effect of CGA may play an important role in recuperation from hip surgery but not in other surgical interventions or populations. Finally, elective versus emergency surgery can give rise to different risk profiles. Determining if there is a benefit in one population versus another is important.
Sensitivity analysis
We were unable to perform sensitivity analysis to explore changes in effect size after removing studies with a high risk of bias due to the small number of studies identified. We compared the use of a fixed‐effect and random‐effects models.
Results
Description of studies
Results of the search
A literature search conducted by a trained librarian on 13 January 2017 identified 14,874 citations for title screening. The citations were from CENTRAL (666 citations), MEDLINE (5663 citations), Embase (7823 citations), PsycINFO (446 citations) and CINAHL (3229 citations). We identified three additional citations through reference screening. During title and abstract screening we identified and removed 655 additional duplicated citations leaving 14,222 records to screen; 363 citations underwent full text screening (Figure 1). We included eight randomised trials (Hempenius 2013; Hempsall 1990; Kennie 1988; Marcantonio 2001; Naglie 2002; Prestmo 2015; Stenvall 2007; Vidán 2005). All hip fracture studies excluded pathologic fractures and participants who were entirely dependent on others for care before their fracture.

Study flow diagram.
Included studies
We included eight randomised trials with a total of 1843 participants enrolled. Three studies enrolled participants aged 70 years and over (Naglie 2002; Prestmo 2015; Stenvall 2007) while the remaining five enrolled participants who were aged 65 years and over (Hempenius 2013; Hempsall 1990; Kennie 1988; Marcantonio 2001; Vidán 2005). All but one study (Hempenius 2013) were conducted at a single site. Seven studies recruited participants with hip fracture (Hempsall 1990; Kennie 1988; Marcantonio 2001; Naglie 2002; Prestmo 2015; Stenvall 2007; Vidán 2005) while the remaining study recruited participants admitted for elective surgical oncology (Hempenius 2013). Six studies randomised participants to comprehensive geriatric assessment (CGA) versus standard care pre‐operatively (Hempenius 2013; Marcantonio 2001; Naglie 2002; Prestmo 2015; Stenvall 2007; Vidán 2005) and two studies randomised postoperatively (Hempsall 1990; Kennie 1988). CGA and geriatric care were delivered during acute postoperative recovery in six studies (Hempenius 2016; Marcantonio 2001; Naglie 2002; Prestmo 2015; Stenvall 2007; Vidán 2005) and in a rehabilitation setting in two trials (Hempsall 1990; Kennie 1988). Additionally, two studies included a pre‐operative assessment (Hempenius 2013; Prestmo 2015). All studies were published in the English language. There two ongoing studies (Baroni 2016, Brugel 2014) and no studies are awaiting classification.
Hempenius 2013 enrolled participants undergoing elective surgery for a solid tumour. The intervention included a pre‐operative CGA, development of a individualized care plan and daily visits postoperatively by a geriatric liaison nurse who provided advice on any problems encountered. Funding was from the Netherlands Organization for Health Research and Development.
Hempsall 1990 enrolled participants presenting with neck of femur fracture, who were randomised based on their geographic setting to geriatric assessment and rehabilitation at a dedicated geriatric facility or standard orthopedic rehabilitation. The funding source was not disclosed.
Kennie 1988 enrolled participants presenting with a hip fracture, who were randomised to a dedicated orthogeriatric rehabilitation ward at a separate hospital or to remain on the orthopedic ward for rehabilitation. Funding was provided by the Forth Valley Health Board.
Marcantonio 2001 also enrolled participants with hip fractures, who were randomised to usual surgical care or proactive geriatric consultation pre‐operatively or within 24 hours of surgery. The consultant geriatrician provided daily assessments and advice to the surgical team. Funding was provided by the Older Americans Independence Center, the Charles Farnsworth Trust, the National Institute on Aging and the Medical Foundation: Charles A King Trust.
Participants in Naglie 2002 also had hip fracture and were randomised to postoperative interdisciplinary care or usual surgical care. Interdisciplinary care consisted of routine assessment by a geriatrician, physiotherapy, occupational therapy, social worker and clinical nurse specialists. Funding was provided by Canadian research and governmental entities.
The participants in Prestmo 2015, also with hip fracture, were randomised to comprehensive geriatric care versus usual surgical care. Geriatric care consisted of primary pre‐ and postoperative care from a geriatrician on a dedicated geriatric ward without regular input from orthopedic surgeons. The study was funded by Norwegian research, educational, and governmental entities.
Stenvall 2007 enrolled and randomised participants with hip fracture, who then received care in either a geriatric or an orthopedic ward. The geriatric ward provided comprehensive geriatric assessments and rehabilitation that included early mobilization. Funding was provided by Swedish research, educational, and governmental entities.
Participants in Vidán 2005 also had hip fracture, and were randomised to either usual care or to postoperative care from a dedicated geriatric team that included a geriatrician, a rehabilitation specialist and a geriatric social worker. Funding was provided by the Spanish governmental entities.
Intervention
All identified studies used CGA to assess participants in the experimental arm, defined as a biopsychosocial approach to care for the elderly that incorporates a multidisciplinary team to address patients' medical illness, physical decline and social factors that slow recovery.
The model for delivery of CGA was quite varied; the physician responsible for care was a surgeon in three studies (Hempenius 2013; Marcantonio 2001; Naglie 2002), a geriatrician in three (Prestmo 2015; Stenvall 2007; Vidán 2005), a general practitioner in one (Kennie 1988) and was unclear in one study (Hempsall 1990). Trials with a non‐orthopedic primary physician all had consultation from the orthopedic surgeon available as needed.
The interventions varied among studies, but all included a comprehensive geriatric assessment. One study developed a geriatric treatment plan pre‐operatively that was monitored by a geriatric nurse postoperatively; postoperative consultation with a geriatrician was performed as needed (Hempenius 2013). Three studies performed geriatric rounds as a consultation service, two conducted rounds on a daily basis (Marcantonio 2001; Naglie 2002) and one conducted rounds twice a week (Kennie 1988). One study included only female participants (Kennie 1988).
Outcomes
Our primary outcomes were mortality and discharge to an increased level of care. Six studies reported mortality (Hempenius 2013; Hempsall 1990; Naglie 2002; Prestmo 2015; Stenvall 2007; Vidán 2005) and six studies reported discharge to an increased level of care (Hempenius 2013; Kennie 1988; Naglie 2002; Prestmo 2015; Stenvall 2007; Vidán 2005).
Our secondary outcomes were length of stay, re‐admission, cost and complications. Five studies reported length of stay (Hempsall 1990; Naglie 2002; Prestmo 2015; Stenvall 2007; Vidán 2005), three studies reported re‐admission (Hempenius 2013; Prestmo 2015; Stenvall 2007), one study reported cost (Prestmo 2015) and three studies reported complications (Hempenius 2013; Marcantonio 2001; Vidán 2005). Complications were presented in different manners among studies, limiting the ability to pool results.
Setting
The eight included trials were conducted in seven countries. Two studies were conducted in North America (USA and Canada) (Marcantonio 2001; Naglie 2002) and six studies were conducted in Europe (Spain, UK, Netherlands, Norway and Sweden) (Hempenius 2013; Hempsall 1990; Kennie 1988; Prestmo 2015; Stenvall 2007; Vidán 2005).
Excluded studies
We assessed 331 studies as irrelevant and excluded 22 studies with reasons, most commonly due to having the wrong patient population and ineligible outcomes. (See Figure 1 and Characteristics of excluded studies).
Ongoing studies
Two studies are ongoing (Baroni 2016; Brugel 2014).
Risk of bias in included studies
Allocation
Six studies used adequate methods to generate random sequencing (Hempenius 2013; Kennie 1988; Marcantonio 2001; Naglie 2002; Prestmo 2015; Stenvall 2007) and five studies appropriately concealed allocation (Naglie 2002; Hempenius 2013; Marcantonio 2001; Prestmo 2015; Stenvall 2007). One study was unclear about randomisation technique (Vidán 2005), one did not adequately perform allocation and did not conceal allocation (Hempsall 1990) and two studies did not adequately describe allocation concealment methods to permit judgement (Kennie 1988; Vidán 2005).
Blinding
Blinding of participants was not possible because of the nature of the intervention; many of the studies included in our review measured outcomes, such as mortality or length of stay, that are objective and less prone to performance or detection bias. Consequently, where we felt the outcome being assessed was not prone to bias and the study design was adequately described, we assessed the risk of bias for blinding of participants as low. Overall, five studies were deemed to have a low risk of performance bias (Hempsall 1990; Kennie 1988; Marcantonio 2001; Naglie 2002; Stenvall 2007) and four studies had a low risk of detection bias (Hempsall 1990; Marcantonio 2001; Naglie 2002; Stenvall 2007). Two studies did not adequately explain how they blinded participants (Prestmo 2015; Vidán 2005) and four did not explain the how they blinded their outcome assessors (Hempenius 2013; Kennie 1988; Prestmo 2015; Vidán 2005). One study had a high risk of performance bias (Hempenius 2013); the primary outcome was delirium and we cannot be sure that lack of blinding did not influence the results.
Incomplete outcome data
Seven of eight studies reported low attrition rates (Hempenius 2013; Hempsall 1990; Kennie 1988; Marcantonio 2001; Naglie 2002; Prestmo 2015; Stenvall 2007) while one study provided insufficient data to assess attrition (Vidán 2005).
Selective reporting
Seven studies reported all outcomes that were expected and were therefore judged to be at low risk of reporting bias (Hempenius 2013; Kennie 1988; Marcantonio 2001; Naglie 2002; Prestmo 2015; Stenvall 2007; Vidán 2005). One study did not report all expected outcomes and consequently was deemed to have a high risk of reporting bias (Hempsall 1990). Two studies were published during or after 2005 and did not register their trial. Vidán 2005 collected data in 1997 and Stenvall 2007 collected data between 2000 and 2002, consequently we did not downgrade the risk of bias assessment for these trials for being unregistered.
Other potential sources of bias
Studies typically excluded participants who would likely not benefit from the CGA intervention. These participants were identified using different criteria including whether the patient previously resided in a long‐term care facility (Naglie 2002; Prestmo 2015), had fewer than six months expected lifespan or had a pathologic fracture (Kennie 1988; Marcantonio 2001; Naglie 2002; Prestmo 2015; Stenvall 2007; Vidán 2005) or were unable to ambulate before hip fracture (Naglie 2002; Prestmo 2015; Stenvall 2007; Vidán 2005). This would result in a healthier population being included in the studies than those typically presenting for emergent surgical intervention but may accurately represent older people presenting for elective procedures. For this reason, we did not deem the exclusion criteria to be a potential source of bias. No studies were assessed to have an increased risk of bias. For further details see the Characteristics of included studies tables and Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See the summary of findings Table for the main comparison for summarized results and certainty of evidence.
We identified eight trials, representing 1843 participants. Seven trials, with 1583 participants, were in people with hip fracture while one study (Hempenius 2013), with 260 patients, was in elective surgical oncology participants. Pooled analysis conducted both with and without the elective surgical oncology trial are presented.
Comprehensive geriatric assessment versus usual care for older people admitted to a surgical service
Primary outcomes
Mortality
Five orthopedic trials with 1316 participants reported mortality outcomes. Using a random‐effects model, CGA probably reduces mortality in older people with hip fracture (risk ratio (RR) 0.85, 95% confidence interval (CI) 0.68 to 1.05, 5 trials, 1316 participants, Analysis 1.1, moderate‐certainty evidence). No heterogeneity was identified between the trials reporting mortality (I² = 0%). Using a fixed‐effect model did not change the outcome of the analysis. When the elective surgical oncology trial was included in the analysis, heterogeneity increased (I² = 26%) and the risk ratio moved closer to 1 (RR 0.90, 95% CI 0.73 to 1.10, 6 trials, 1576 participants).
Discharge to an increased level of care
Five orthopedic trials reported discharge to an increased level of care from hospital for 941 participants. Discharge to an increased level of care was defined as participants being discharged to a higher level of care than they required before admission (e.g. discharged to an assisted living residence instead of returning to independent living). Using a random‐effects model, the intervention reduces discharge to an increased level of care (RR 0.71, 95% CI 0.55 to 0.92, 5 trials, 941 participants, Analysis 1.2, high‐certainty evidence). There was no heterogeneity between the orthopedic studies (I² = 0%). Using a fixed‐effect model did not change the results. One study reported discharge from hospital to a "nursing home [or] rehab hospital" (Marcantonio 2001). They did not distinguish between nursing home admission and rehabilitation hospital stay and did not report discharge destination following rehabilitation. Consequently, this study was excluded from the assessment. Inclusion of the elective surgical oncology trial profoundly increased heterogeneity (I² = 61%) and resulted in CGA having little or no effect on discharge destination (RR 0.86, 95% CI 0.69 to 1.07, 6 trials, 1164 participants).
Secondary outcomes
Length of stay
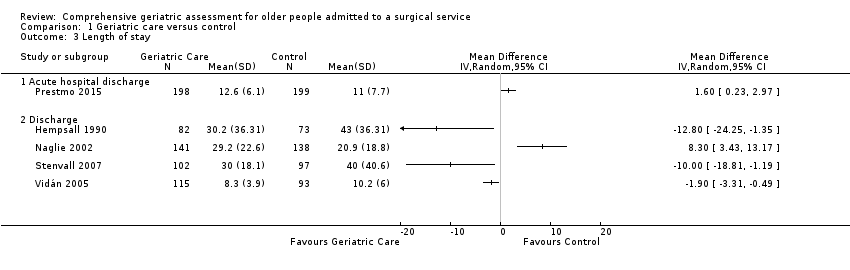
Five trials reported length of stay. There was considerable heterogeneity among studies when using either fixed‐effect (I² = 88%) or random‐effects models (I² = 88%). Due to the high heterogeneity, we examined the outcomes individually. All studies that reported length of stay recruited participants with hip fracture. The mean difference between participants allocated to the intervention and the control groups ranged between ‐12.8 and 8.3 days (Analysis 1.3). Three studies reported a reduction in length of stay (Hempsall 1990; Stenvall 2007; Vidán 2005) while two reported an increased length of stay (Naglie 2002; Prestmo 2015).
Prestmo 2015 reported the length of time participants spent in the acute hospital; they reported fewer participants in the CGA arm required admission to a rehabilitation hospital but did not report a cumulative length of stay including rehabilitation.
Naglie 2002 reported that fewer participants in the CGA arm were transferred from the acute hospital to a nursing home but more were transferred to a rehabilitation hospital. Overall, at six months follow‐up, results were similar for the mean number of "days spent in institutions (including acute hospitals, rehabilitation hospitals and nursing homes) over 6 months".
Four of the five studies reporting length of stay found decreased length of stay or decreased transfer to a rehabilitation hospital while one found little or no difference in "institution" use (mean 111 versus 110 days, P = 0.84) at six months follow‐up. The intervention probably leads to slightly reduced length of stay (4 trials, 841 participants, moderate‐certainty evidence).
Re‐admission
Re‐admission was reported by three trials: two orthopedic and one surgical oncology. Pooled results were limited by the small number of studies reporting re‐admission. The intervention probably makes little or no difference in re‐admission rates (RR 1.00, 95% CI 0.76 to 1.32, 3 studies, 741 participants, Analysis 1.4, moderate‐certainty evidence). There was moderate heterogeneity (I² = 37%) among the three studies. Removing the elective surgical oncology study (Hempenius 2013) increased heterogeneity (I² = 53%) but did not change the pooled result.
Cost
Cost was reported by one study (Prestmo 2015). The mean total cost at one year follow‐up was EUR 59,486 in the control arm and EUR 54,332 in the CGA arm (MD EUR 5154, 95% CI ‐13,288 to 2980, 1 trial, 397 participants, moderate‐certainty evidence). Prestmo 2015 found that the incremental cost effectiveness ratio was EUR 71,751, suggesting CGA probably slightly reduced total cost (1 trial, 397 participants, moderate‐certainty evidence).
Postoperative complications
Three studies reported postoperative complications, but we were unable to pool results due to the manner in which they were reported. Two studies, representing 579 participants, reported major complications (Hempenius 2013; Vidán 2005). There was considerable heterogeneity between the studies making pooled meta‐analysis inappropriate. Hempenius 2013 defined a major complication as two or more pulmonary, neurologic (excluding delirium), cardiovascular or thromboembolic complications. Vidán 2005 defined major complications as delirium, congestive heart failure, pneumonia, deep venous thrombosis, pulmonary embolus, pressure ulcer, arrhythmia and myocardial infarction. Most significantly, Vidán 2005 included delirium as a major complication and Hempenius 2013 did not. CGA may make little or no difference for major postoperative complications (2 trials, 579 participants, low‐certainty evidence).
Three studies reported delirium as an independent outcome (Hempenius 2013; Marcantonio 2001; Vidán 2005), representing 705 participants. Using a random‐effects model, CGA may make litte or no difference for delirium (RR 0.75, 95% CI 0.60 to 0.94, 3 trials, 705 participants, Analysis 1.6, I² = 0%, low‐certainty evidence). Using a fixed‐effect model did not change the results of pooled analysis.
Sensitivity analysis by trial quality
There were too few studies in the low risk of bias subgroups to permit sensitivity analysis by trial quality.
Subgroup analysis
Analysis of orthopedic versus non‐orthopedic results was conducted by removing the only non‐orthopedic trial from pooled analysis. The results are reported above. The non‐orthopedic trial was also the only elective trial identified by our search.
Subgroup analysis of trials where CGA was conducted postoperatively was performed by excluding studies where CGA was conducted before surgery. We assessed the primary outcomes after removing Hempenius 2013 and Prestmo 2015; CGA probably reduces mortality if it is performed after surgery (RR 0.87, 95% CI 0.68 to 1.11, 938 participants, 4 studies, random‐effects model, I² = 0%, moderate‐certainty evidence) and probably reduces discharge to an increased level of care (RR 0.71, 95% CI 0.49 to 1.02, 612 participants, 4 studies, random‐effects model, I² = 25%, moderate‐certainty evidence) although the confidence interval was slightly wider and crossed 1.
We also assessed secondary outcomes. Length of stay was highly heterogeneous, three of five studies found decreased length of stay, one found increased length of stay but a lower transfer rate to rehabilitation hospitals and one did not find any difference at six month follow‐up. The two studies reporting delirium rates after performing postoperative CGA (Marcantonio 2001; Vidán 2005) indicate that the intervention may make little or no difference to delirium (RR 0.75, 95% CI 0.56 to 1.01, 445 participants, 2 studies, I² = 24%, low‐certainty evidence). After removing both studies that performed pre‐operative assessment, one trial reported major complications (Vidán 2005), one study reported re‐admission (Stenvall 2007) and no trials reported cost.
Meta‐regression could not be performed due to the small number of included studies.
Discussion
Summary of main results
We included eight randomised trials (N = 1843). Seven recruited participants who were recovering from a hip fracture (1583 participants) and one was in elective surgical oncology (260 participants). Pooled analysis of five studies of hip fracture in older people indicated that CGA probably reduces mortality (moderate‐certainty evidence). The intervention reduced discharge to an increased level of care in older adults with hip fracture (high‐certainty evidence).
Heterogeneity was considerable for length of stay, preventing data pooling. The intervention probably leads to slightly reduced length of stay (moderate‐certainty evidence). CGA probably makes little or no difference to re‐admission rates (moderate‐certainty evidence). One study reported cost, bootstrap analysis suggests CGA probably slightly reduces total cost (moderate‐certainty evidence). Major complications were highly heterogenous and were defined differently by the two studies reporting this outcome. CGA may make little or no difference for major postoperative complications and delirium (low‐certainty evidence).
Overall completeness and applicability of evidence
All studies recruited surgical participants who were admitted to an acute care hospital in Western Europe or North America. We identified one high quality trial in non‐orthopedic surgical populations (Baroni 2016) and two ongoing studies from non‐orthopedic surgical older people that have not yet reported results (Baroni 2016; Brugel 2014).
All geriatric assessments were supervised by a geriatrician, however the physician responsible for care varied among studies. The responsible physician in the experimental arm was a geriatrician in four studies (Naglie 2002; Prestmo 2015; Stenvall 2007; Vidán 2005) and the surgeon in two (Hempenius 2013; Marcantonio 2001). Additionally, two studies did not perform CGA until the patient was ready for transfer to a rehabilitation ward (Hempsall 1990; Kennie 1988).
The total number of participants identified (N = 1843) is sufficiently large that we feel we can confirm effectiveness and safety for outcomes reported by most trials. Outcomes reported by a small number of trials included fewer participants and may not reliably represent the true safety and efficacy of the intervention. These outcomes include cost (N = 397), complications (N = 579) and re‐admission (N = 516). The time of follow‐up was also quite varied among studies; some participants were followed until discharge while others as long as one year. This aspect may limit the reliability of these results due to smaller numbers of participants in the pooled results.
We included two studies that did not perform geriatric assessment until the patient was ready for transfer to a rehabilitation ward (Hempsall 1990; Kennie 1988). Neither study reported postoperative complications or delirium. The participants in these studies were cared for by an orthopedic surgeon until they were transferred to a rehabilitation facility that provided specialized geriatric rehabilitation with CGA compared to usual orthopedic care. The physician primarily responsible for patients' care during rehabilitation was a general practitioner (Kennie 1988) or it was unclear who cared for them (Hempsall 1990). Hempsall 1990 reported three outcomes that are included in our review: mortality, length of stay and discharge to an increased level of care. Initial geriatric assessment was conducted between postoperative day 3 and 7. The authors felt that events that occurred before postoperative day 8 could not have been affected by geriatric assessment; these events were censored from their results (Hempsall 1990). We feel that, given the censored data, these results are an accurate reflection of the effects of CGA (Hempsall 1990). Overall, the variability in implementation of CGA between studies introduces considerable heterogeneity and may limit comparability of the outcomes in the pooled results.
The potential to improve the care of older surgical patients is particularly relevant; aging populations are increasingly requiring surgical intervention and are prone to increased postoperative morbidity and mortality. The included studies support the implementation of CGA to decrease discharge to an increased level of care and complications for older people with hip fracture, but we cannot extend this recommendation for other surgical populations due to lack of high quality studies.
Certainty of evidence
We assessed certainty of evidence using the GRADE method and classified the certainty of evidence for our primary outcomes as moderate for mortality and high for discharge to an increased level of care for orthopedic studies. Individual studies had varied risk of bias, which partially depended on what outcome was being examined. Despite an overall elevated risk of bias in some of the included studies, the nature of our primary outcomes (mortality and discharge to an increased level of care) reduces the risk that these results are not representative of the overall population because these outcomes are not prone to detection or performance bias. The included studies had low dropout rates and most study authors responded when contacted. One study did not report mortality and the study authors were unable to provide data due to the time elapsed since the study was conducted. We noted no heterogeneity for mortality or discharge to an increased level of care when orthopedic studies were pooled; however, mortality was downgraded due to a wide confidence interval that crossed 1.
Secondary outcomes had lower certainty of evidence. Length of stay was downgraded due to high variability and heterogeneity, re‐admission was downgraded due to imprecision and cost was downgraded due to indirectness; all were graded as moderate‐certainty evidence. Evidence related to postoperative delirium was downgraded due to high risk of bias and imprecision. The evidence for the outcome of major complication was graded as low‐certainty due to indirectness and imprecision of the measure.
Potential biases in the review process
We used the standard review methods of the Cochrane EPOC group to conduct this review. The use of an inclusive search strategy will have included all relevant studies.
Agreements and disagreements with other studies or reviews
Several other published reviews have examined the effect of CGA on outcomes. All identified reviews were for orthopedic trauma patients (hip fracture) and included both randomised trials and lower quality studies. Sabharwal 2015 identified five articles, comprising two prospective randomised and three retrospective cohort or observational trials, that all reported lower mortality in the CGA arm. Sabharwal 2015 identified multiple, predominantly retrospective, studies that identified reduced postoperative complications and reported that length of stay was lower with CGA in four of the five included studies. Three studies, one retrospective and two prospective randomised trials, identified improved functional outcomes in the intervention arms. Grigoryan 2014, in a systematic review of 18 studies, reported that geriatric consultation services but not shared care reduced short‐ and long‐term mortality, and that shared care but not geriatric consultations services reduced length of stay. Buecking 2013 included five trials, with high heterogeneity, and found little or no effect on length of stay and short‐ or long‐term mortality. Deschodt 2013 performed a systematic review and meta‐analysis of people receiving care on dedicated geriatric wards versus usual care. Deschodt 2013 included both medical (n = 9) and surgical (n = 3) studies. Deschodt 2013 reported no effect on functional status with CGA in 11 trials (including all 3 surgical trials), no effect on length of stay in 10 trials (including all 3 surgical trials) and no effect on re‐admission in eight trials (including 2 surgical trials). Deschodt 2013 identified discordant results for mortality, which was reduced at six and eight months follow‐up but not at one, three or 12 months follow‐up (mortality was reported by all 3 surgical trials). Finally, Eamer 2017b, also reported a systematic review and meta‐analysis of eight cost analysis or economic evaluation studies of CGA versus usual care. Seven of the eight studies were retrospective and of lower quality; however, all eight studies found improved outcomes at lower cost to the healthcare system with CGA.
Prestmo 2015 conducted a systematic review as part of their publication searching for orthogeriatric care models, but did not summarize results. Kammerlander 2010 performed a systematic literature review of enhanced orthopedic care for people with hip fracture and summarized findings classified by geriatric intervention type. Kammerlander 2010 performed a narrative analysis but did not pool data from the included studies. Several different models of care delivery including proactively consulted geriatrician (daily or less frequent consultations), geriatric ward postoperatively with orthopedic consultation and interdisciplinary care with integrated geriatric and orthopedic care teams were identified. Most studies, no matter the intervention type, did not identify any difference in mortality. There was wide variability in length of stay depending on the model used, but no clear reason was given to fully explain these differences. Complications were examined, but the high variability in how complications were defined made comparison between studies difficult (Kammerlander 2010).
No reviews identified adverse events related to the introduction of CGA into the model of care and most identified improvement in at least one outcome. Sabharwal 2015 and Grigoryan 2014 reported reduced mortality with CGA; Buecking 2013 did not find reduction in mortality, and Deschodt 2013 reported discordant results. Sabharwal 2015 reported decreased discharge to an increased level of care but Deschodt 2013 did not find a difference. No reviews reported cost. Only Sabharwal 2015 reported complications, which were decreased with CGA.
All identified reviews included lower‐quality study designs, including retrospective chart reviews and historically‐controlled trials. All reviews concluded that CGA showed benefits. However, Deschodt 2013 was unable to identify a clear added benefit from an integrated geriatric consultation service.

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Geriatric care versus control, Outcome 1 Mortality.

Comparison 1 Geriatric care versus control, Outcome 2 Discharge to an increased level of care.
Comparison 1 Geriatric care versus control, Outcome 3 Length of stay.

Comparison 1 Geriatric care versus control, Outcome 4 Re‐admission.

Comparison 1 Geriatric care versus control, Outcome 5 Major complication.

Comparison 1 Geriatric care versus control, Outcome 6 Major complication ‐ delirium.
| Comprehensive geriatric assessment for older people admitted to a surgical service | ||||||
| Patient or population: Improving outcomes in older adult people admitted to a surgical service. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with control | Risk with geriatric care | |||||
| Mortality | 214 per 1000 | 182 per 1000 | RR 0.85 | 1316 | ⊕⊕⊕◯ 1 | Hip fracture studies. |
| Discharge to an increased level of care | 247 per 1000 | 176 per 1000 | RR 0.71 | 941 | ⊕⊕⊕⊕ | Hip fracture studies. |
| Length of stay | Meta‐analysis was not performed due to high heterogeneity (Analysis 1.3) | MD in studies ranged from ‐12.8 days to 8.3 days | ‐ | 841 | ⊕⊕⊕⊝ | Hip fracture studies ‐ length of stay until final discharge from hospital (including rehabilitation hospital). Meta‐analysis was not retained due to high heterogeneity (I² = 88%, P < 0.00001). |
| Re‐admission | 316 per 1000 | 316 per 1000 | RR 1.00 | 741 | ⊕⊕⊕⊝ | All studies included; removing elective surgical oncology study doesn't change effect. |
| Total cost | The mean total cost was EUR 59,486 | MD EUR 5154 lower | 397 | ⊕⊕⊕⊝ | 1 study reported cost. | |
| Major complication | Meta‐analysis was not performed due to high heterogeneity (Analysis 1.5) | Two studies reported this outcome with RRs of 0.74 and 1.16 | 579 | ⊕⊕⊝⊝ | Hempenius 2013 defined major as 2 or more complications. Vidán 2005 defined major as delirium, congestive heart failure, pneumonia, DVT, PE, pressure ulcer, arrhythmia and myocardial infarction. Meta‐analysis was not retained due to high heterogeneity (I² = 77%, P = 0.04). | |
| Major complication ‐ delirium | 327 per 1000 | 245 per 1000 | RR 0.75 | 705 | ⊕⊕⊝⊝ | Delirium assessed by Delirium Observation Scale (Hempenius 2013) or confusion assessment method (Marcantonio 2001; Vidán 2005) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded due to imprecision because there were wide confidence intervals that include both no effect and a high risk of benefit or harm. 2 We downgraded due to inconsistency because there was significant variability among studies. 3 We downgraded due to other considerations because costing was calculated in an imprecise manner (costs are presented as the total cost over one year, however the admission cost did not include rehabilitation hospital costs despite the authors identifying a higher proportion of control patients being transferred to rehabilitation centres before discharge). 4 We downgraded due to the high risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 5 | 1316 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.68, 1.05] |
| 1.1 4 to 6 months | 2 | 476 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.46, 1.20] |
| 1.2 1 year | 3 | 840 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.69, 1.11] |
| 2 Discharge to an increased level of care Show forest plot | 5 | 941 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.55, 0.92] |
| 2.1 Discharge | 1 | 108 | Risk Ratio (IV, Random, 95% CI) | 0.31 [0.12, 0.79] |
| 2.2 4 to 6 months | 2 | 344 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.54, 1.21] |
| 2.3 1 year | 2 | 489 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 3 Length of stay Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Acute hospital discharge | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Discharge | 4 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Re‐admission Show forest plot | 3 | 741 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.76, 1.32] |
| 4.1 1 to 3 months | 1 | 225 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.74, 2.09] |
| 4.2 1 year | 2 | 516 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.67, 1.33] |
| 5 Major complication Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Discharge | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Major complication ‐ delirium Show forest plot | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Discharge | 3 | 705 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.60, 0.94] |

