Betabloqueantes para el infarto agudo de miocardio presunto o diagnosticado
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised clinical trial, parallel design, at a single site in Denmark between March 1976 and December 1978. | |
| Participants | 480 participants with definite or suspected MI were included. Male:female = 325:155. Mean age = 62.8 years. Exclusion criteria: 1) cardiogenic shock, 2) pulmonary oedema persisting after 2 hours treatment, 3) AV‐block (Morbitz type 2 and 3rd degree AV‐block), 4) bradycardia < 40 beats/minute, 5) COPD after admission, 6) non‐resident in area, 7) treatment with a beta‐blocker at admission, 8) refusal to participate, 9) terminal or other disease, 10) contraindicated to beta‐blockade. | |
| Interventions | Experimental group: alprenolol (5 mg to 10 mg of alprenolol intravenously, followed by 200 mg orally twice daily for a year). Control group: placebo. Co‐intervention: not described. Excluded medication: other beta‐blockers and verapamil were not permitted during the study. | |
| Outcomes | Primary: mortality. Time points reported: 28 days and 12 months. | |
| Notes | No email was found on the author. The study did not inform about how it was funded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as being double‐blinded, however, no further description was given. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Low risk | All deaths, side‐effects, and dropouts were recorded and classified by an independent safety monitoring board consisting of one statistician and two cardiologists not otherwise involved in the study. |
| Incomplete outcome data (attrition bias) | Low risk | A total of 108 participants (37.5%) participants dropped out from the study: 61 from the alprenolol group and 52 from the placebo group were withdrawn. However, these were followed up on when reporting mortality. Hence, there was no risk of incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | A protocol could be obtained, but was published five years after their initial trial publication. They reported the outcomes in their trial publication stated in the protocol. The trial did not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial at an unknown place. | |
| Participants | 101 patients less than 75 years of age with a suspected AMI within 6 hours were included. Male:female = not reported. Mean age = not reported. Exclusion criteria: age > 75, previous MI, SBP < 100, HR < 50, block, COPD, LVF, high risk, beta‐blocker use. | |
| Interventions | Experimental group: timolol (5.5 mg IV + 10 mg orally twice daily for 28 days). Control group: placebo. Co‐intervention: not reported. Excluded medication: not reported. | |
| Outcomes | Primary: infarct size, chest pain, haemodynamic function, arrhythmias. Timepoints reported: 28 days. | |
| Notes | No email was found on the author. The study could not be found, so we used the data reported in the meta‐analysis ''Yusuf 1985'', who were able to get unpublished data by personal communication with the author. No information about the funding of the study was available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not mentioned if any participant was lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report on all SAEs. |
| Other bias | Unclear risk | Not able to assess if any other bias exist, as the main manuscript could not be obtained. |
| Methods | Randomised clinical trial, parallel design, at five sites in Sweden and at two sites in Australia between February 1978 and January 1980. | |
| Participants | 529 participants, of both sexes, aged up to the end of their 69th year, with the clinical diagnosis of AMI associated with electrical and/or mechanical complications 1‐21 days after the onset of symptoms were eligible to enter the study. Male:female = 439:90. Mean age = 58 years. Exclusion criteria: medical contraindications to the use of pindolol; uncontrolled heart failure; unrelated heart disease; persistent heart block of 2nd or 3rd degree, persistent bradycardia < 50 beats/minute; obstructive airways disease; uncontrollable insulin dependent diabetes; known hypersensitivity to beta‐blocking drugs; other diseases serious enough to worsen the short‐term prognosis irrespectively of the MI; pregnancy; necessity to use beta‐blocking drugs or calcium antagonists. Patients who were unable to return for regular control were also excluded. | |
| Interventions | Experimental group: pindolol (15 mg/day orally for 2 years). Could be changed to half a tablet or extra up to 20 mg/day if necessary. Control group: placebo. Co‐intervention: digitalis, diuretics, vasodilators (nitrates), antiarrhythmics and anticoagulants. Excluded medication: not described. | |
| Outcomes | Primary: death. Other: cardiac death, non‐cardiac death, sudden death, reinfarction. Time points reported: 2 years. | |
| Notes | No email was found on the author. Sandoz Ltd., Basle (a pharmaceutical company) coordinated the study and processed the data. However, the information about the funding of the study was not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | The numbers and reasons for the withdrawals and dropouts for all outcomes are clearly stated, more or less similar in both groups, and the trial handles missing data appropriately in intention‐to‐treat analysis. 76 patients from the pindolol group and 50 from the placebo group were withdrawn. However, these were followed up on regarding to mortality. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial did not report SAEs. |
| Other bias | Low risk | No other bias was found. |
| Methods | Randomised clinical trial, parallel design, at single site in France. Duration not mentioned. | |
| Participants | 26 participants with an acute anterior transmural MI less than 24 hours after the onset of pain, not complicated by clinical pump failure or persistent chest pain, were included. Male:female = not described. Mean age = 51.5 years. Exclusion criteria: patients 70 years and older and those with previous infarctions, associated cardiovascular or systemic disease, mechanical complication, persistent or recurrent ischaemic pain not relieved by opiates and nitroglycerin after 12 hours, clinical signs of coronary insufficiency present more than 3 months before the current TMI, atrioventricular or intraventricular conduction disturbances, or refractory arrhythmias were excluded from the study. Patients taking chronic drug therapy (such as beta‐blocking agents, digitalis, lidocaine or other antiarrhythmic drugs) were also excluded. | |
| Interventions | Experimental group: acebutolol (1 mg/kg intravenously for 48 hours, followed by 600 mg/day for 3 weeks.) Control group: no intervention other than co‐intervention. Co‐intervention: heparin. Excluded medication: beta‐blocking agents, digitalis, lidocaine or other antiarrhythmic drugs. | |
| Outcomes | Primary: mortality, infarct extension, myocardial function, LV angiography, myocardial ischaemia, myocardial necrosis. Time points reported: 1 month. | |
| Notes | No email was found on the author. The study did not report data on any of our outcomes. The study was supported by Delegation Generale a la Recherche Scientifique et Technique. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "The 26 patients were assigned at random to one of two groups". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial did not report on all‐cause mortality or SAEs. |
| Other bias | Low risk | No other biases was found. |
| Methods | Randomised clinical trial, parallel design, at a single site in England. Duration not mentioned. | |
| Participants | 114 participants with suspected MI within the previous 24 hours were included. Male:female = 79:35. Mean age = 59.8 years. Exclusion criteria: complete heart block, unconscious, not able to take oral medication. | |
| Interventions | Experimental group: propranolol (80 mg/day for 28 days). Control group: no intervention other than the co‐intervention. Co‐intervention: anticoagulants were given unless contraindicated, and other medication was avoided; analgesics, digitalis, diuretics, and vasopressors were given as necessary. Excluded medication: not described. | |
| Outcomes | Primary: effect of propranolol on mortality. Time points reported: 24 hours, the time point for maximum follow‐up is not described. | |
| Notes | No email was found for the author. The funding of the trial was not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not sufficiently described, however the study mentions that quote: "Random allocation was employed". |
| Allocation concealment (selection bias) | Unclear risk | Not sufficiently described, however the study mentions that quote: "Random allocation was employed". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. However the study was described as being double‐blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Five patients with proved recent myocardial infarcts were withdrawn from the trial and 6 patients were not admitted (total dropout of 9.6%). It was unclear whether the trialists had outcome data on these participants. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases was found. |
| Methods | Randomised clinical trial, parallel design, at 2 sites in Northern Ireland between December 1965 and September 1966. | |
| Participants | 107 participants with a clinical history of MI within the preceding 24 hours were included. Male:female = not described. Mean age = not described. Exclusion criteria: HR of under 60 beats per minute, whether this was due to sinus bradycardia or atrio‐ventricular block; asthma or broncho‐spasm; a SBP less than 90 mmHg. | |
| Interventions | Experimental group: propranolol (initial dose of 40 mg six‐hourly for 28 days.) Control group: placebo. Co‐intervention: not described. Excluded medication: not described. | |
| Outcomes | Primary: survival, development of heart failure, rhythm changes, cardiac pain. Timepoints reported: 4 weeks | |
| Notes | No email was found on the author. The trial was supported by Imperial Chemical Industries, Pharmaceuticals Division with advice and supplies of propranolol and placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described as being quote: "this is a restricted sequential trial with the valuation of treatments by observed preferences between patients within pairs, each member of a pair being differently treated." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Treatments were allotted at random to each pair." "...within each of the two hospitals and within each of the sub‐groups patients were allotted to two treatment groups at random in accordance with a previously prepared plan." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | High risk | Two (3.8%) from the propranolol and 6 (12.8%) from the placebo group were withdrawn and not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases found. |
| Methods | Randomised clinical trial, parallel design, at a single site in Nothern Ireland. Duration not mentioned. | |
| Participants | 298 participants with definite AMI were included. Male:female = 183:115. Mean age = 62.5 years. Exclusion criteria: not described. | |
| Interventions | Experimental group: practolol (600 mg/day for 2 years). Control group: placebo. Co‐intervention: not described. Excluded medication: not described. | |
| Outcomes | Primary: mortality, ventricular irritability, bradycardia, and heart failure. Time points reported: 3 months and 2 years. | |
| Notes | No email was found on the author. The trial was supported by Pharmaceutical Division of I.C.I. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as being a double blinded trial, however, no further description is given. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | 17 patients had stopped the tablets because of adverse side effects by 3 months, of these 3 were on placebo. However, nothing is said about whether these were lost to follow‐up or included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases was found. |
| Methods | Randomised clinical trial, parallel design, at a single site in the UK between February 1992 and September 1994. | |
| Participants | 151 patients admitted to the coronary care unit with typical signs and symptoms suggestive of AMI, i.e. chest pain, ECG changes, and serum concentration of CKe and MB isoform consistent with the diagnosis, were recruited to the study. Male:female = 123:23. Mean age = 60 years. Exclusion criteria: already on α‐ or β‐blockers and calcium antagonists or had contraindications to α‐ or β‐blockers; if they were in Killip class IV heart failure or cardiogenic shock (classes I to III were not excluded); or if they had severe bradycardia (HR < 45 bpm), hypotension (SBP < 90 mmHg), second‐ to third‐degree heart block, left bundle‐branch block, severe valvular disease, insulin‐dependent diabetes, renal failure (creatine > 159 μmol/L), known malignancy, or other severe disease or pregnancy. | |
| Interventions | Experimental group: carvedilol (2.5 mg injected intravenously followed by 12.5 mg to 25 mg orally commenced twice daily for 6 months). Control group: placebo. Co‐intervention: standard medical therapy (thrombolytic therapy, IV heparin, aspirin, and nitrates). Excluded medication: IV calcium‐channel blockers was prohibited in patients receiving esmolol. | |
| Outcomes | Cardiac death; reinfarction; unstable angina; heart failure; emergency revascularisation; ventricular arrhythmia requiring intervention; stroke; and additional cardiovascular therapy other than sublingual nitrates for angina, diuretics for hypertension, or continuation of preexisting ACE inhibitors, digitalis, or antiarrhythmics. Time points reported: 6 months follow‐up. | |
| Notes | The authors were contacted on 25 January 2017 on [email protected] and [email protected]. The trial was supported by educational grants from the NPH Cardiac Research Fund, Harrow, UK, and Boehringer Mannheim GmbH, Mannheim, Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. However, the study is described as being double‐blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | Two (2.6%) patients from the experimental group and 3 (4%) from the placebo group were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial did not report all SAEs. |
| Other bias | Low risk | No other biases was found. |
| Methods | Randomised clinical trial, parallel design, at a single site in Germany between October 2011 and February 2014. | |
| Participants | 101 participants with STEMI and successful percutaneous intervention (Killip class I and II) with less than 6 hours between symptom onset and PCI, a baseline HR > 60 bpm and a mean arterial BP > 65 mmHg were included. Male:female = 77:23. Mean age = 59.7 years. Exclusion criteria: symptomatic AV‐Block II and III; contraindication for beta blocker or esmolol; and potential pregnancy. | |
| Interventions | Experimental group: esmolol (weight‐adapted continuous plus additional bolus esmolol infusion, targeting a HR of 60 bpm for 24 hours). Control group: placebo (continuous 0.9% sodium‐chloride infusion). Co‐intervention: all patients during PCI received guideline‐directed standard medication, including aspirin and clopidogrel, prasugrel, or ticagrelor. There were no limitations on additional indicated drug therapy. All patients received for secondary prevention oral beta‐blocker, aspirin, P2Y12‐receptor antagonist, and statin. Excluded medication: not described. | |
| Outcomes | Primary: the maximum change in troponin T from baseline to 48 hours. Secondary: concentrations of creatine kinase (CK), CK isoenzyme MB (CK‐MB), and n‐terminal brain natriuretic peptide (NT‐proBNP) at 48 hours, the echocardiographic ejection fraction at 48 hours, 6 weeks, and 6 months, the 6‐minute walking test at 6 weeks and 6 months, and assessment of quality of life (EQ5D, data not shown) at 48 hours, 6 weeks, and 6 months. Time points reported: 48 hours, 6 weeks and 6 months. The safety endpoints were incidence of cardiogenic shock, symptomatic bradycardia or hypotension, re‐angina pectoris, repeated angiography and target vessel revascularisation, rehospitalisation, cerebral insult, and mortality. | |
| Notes | We had no further questions to the author, who could otherwise be contacted on fikret.er@klinikum‐guetersloh.de. The trial was funded by Baxter Healthcare Corporation, Deerfield, Illinois. It was not mentioned whether or not Baxter Healthcare Corporation was a part of conducting the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described as being randomised, however, no further description was given. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization with an allocation ratio of 1:1 was on the basis of permuted blocks of varying length and was implemented using sequentially numbered, opaque, and sealed envelopes." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Patients were blinded to the treatment. Placebo‐treated subjects received continuous 0.9% sodium‐chloride infusion." However, it is said that the study was single‐blinded. Hence, the personnel were not blinded to the treatment. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Follow‐up procedures were analysed by a blinded investigator (E.C.)." |
| Incomplete outcome data (attrition bias) | Low risk | Only one patient from the placebo group was excluded. |
| Selective reporting (reporting bias) | Low risk | The trial was registered in 2011 and a protocol was published in 2015. The outcomes called for in the protocol are reported on. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at a single site in New Zealand between August 1968 and April 1969. | |
| Participants | 172 participants who had suffered recent MI, under the age of 70, were started on the trial, of which 119 participants with diagnosed acute uncomplicated MI were included and reported on. Male:female = not described. Mean age = 55.6 years. Exclusion criteria: 1) heart block of any degree. 2) SBP of less than 90 mmHg for one hour. 3) frank pulmonary oedema, lesser degrees of LV failure not being excluded. 4) sinus bradycardia of less than 50 beats per minute. 5) bronchial asthma. 6) any patient who later did not have the diagnosis of MI proven by the usually accepted criteria was withdrawn from the trial. | |
| Interventions | Experimental group: alprenolol (400 mg/daily during hospitalisation and after discharge). Control group: placebo. Co‐intervention: not described. Excluded medication: not described. | |
| Outcomes | Primary: effects on mortality, recurrent chest pain, incidence of serious ventricular arrhythmias, cardiac failure, exertional dyspnoea. Time points reported: 3 days and 12 months. | |
| Notes | Email address not found. A total of 32 patients was lost during the follow‐up and only 87 patients were included in the 1‐year follow‐up. However, we are not informed about the proportion of participants in each group why we have used the number analysed from the first 3 days reported in Briant 1970. Furthermore, it is unclear whether the number of patients withdrawn from the trial was included in the number of patients having a major ventricular arrhythmia, why we have decided to only include data from table 3, to avoid double‐counting. The trial was supported by Astra Pharmaceuticals Ltd. who provided supplies of alprenolol and placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Bottles containing tablets of drug or placebo were prepared in random order by the hospital pharmacist who retained the key. However, it was not mentioned how the random order was generated. |
| Allocation concealment (selection bias) | Low risk | Bottles containing tablets of drug or placebo were prepared in random order by the hospital pharmacist who retained the key. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described sufficiently, however the study is described as being quote: "double‐blinded". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | High risk | At 1‐year follow‐up, a total of 41 participants were lost to follow‐up. During the first three days 7 (12.3%) participants from the placebo group and 11 (17.7%) from the experimental group were removed from the trial. Furthermore, a total of 9 patients dropped out after discharge during the one year follow‐up. A total of 172 participants were started on the trial of which 53 participants were excluded when the diagnosis of MI was not substantiated. However, we do not know how many participants were randomised to each group, hence we cannot say how many was lost from each group. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial does not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial at an unknown place. | |
| Participants | 39 patients with a suspected acute myocardial infarction were included. Male:female = not reported. Mean age = not reported. Exclusion criteria: age > 75, previous MI, SBP < 100, HR < 50, block, COPD, LVF, high risk, beta‐blocker use. | |
| Interventions | Experimental group: timolol (2 mg IV + 5 mg orally 2 hours later + 10 mg orally twice daily during hospitalisation). Control group: placebo. Co‐intervention: not reported. Excluded medication: not reported. | |
| Outcomes | Primary: in hospital mortality. Time points reported: during hospitalisation. | |
| Notes | Email not found. The study could not be found, so we used the data reported in the meta‐analysis "Yusuf 1985", who were able to get unpublished data by personal communication with the author. No information about how the study was funded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not mentioned if any participant was lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report on all SAEs. |
| Other bias | Unclear risk | Not able to assess if any other bias exist, as the main manuscript could not be obtained. |
| Methods | Multi‐centre randomised controlled trial in 67 centres in Japan between August 2010 and May 2014. | |
| Participants | Patients > 18 years old were eligible for the trial if they underwent successful primary PCI within 24 hours after the onset of STEMI and had preserved left ventricular ejection fraction (LVEF> 40%) as assessed by echocardiography. Male:female = 639:155. Mean age = 64 years old. Exclusion criteria: reduced LVEF (LVEF< 40%), prior cardioverter defibrillator implantation, or contraindications to beta‐blocker therapy such as unstable haemodynamic status, bradyarrhythmias, symptomatic HF, and severe bronchial asthma and/or chronic obstructive lung diseases. | |
| Interventions | Experimental group: carvedilol (oral, maximal dose of 20 mg). Control group: no intervention. Co‐intervention: the administration of other standard medications for STEMI patients such as aspirin, thienopyridines, statins, and inhibitors of the renin angiotensin system were also left to the physicians decision. Excluded medication: not reported. | |
| Outcomes | Primary: composite of all‐cause death, MI, hospitalisation for acute coronary syndrome (ACS), and hospitalisation for HF. Secondary outcomes: individual components of the primary endpoint as well as cardiac death, non‐cardiac death, stroke, vasospastic angina, major bleeding, definite stent thrombosis (ST), target‐lesion revascularisation (TLR), and any coronary revascularisation. 3 composite endpoints including cardiac death/MI/ACS/HF, cardiovascular death/MI/stroke, and death/MI/stroke/ACS/HF/any coronary revascularisation. Time points reported: at 3‐month, 1‐year, and final follow‐up. | |
| Notes | [email protected]‐u.ac.jp The study was supported by an educational grant from the Research Institute for Production Development (Kyoto, Japan). ClinicalTrials.gov Identifier: NCT01155635. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed centrally through the electronic data capture system with a stochastic minimization algorithm to balance treatment assignment. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) | Low risk | an independent clinical event committee being adjudicated both the primary and secondary endpoints in a fashion blinded to the assigned treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | 29 participants in total were lost to follow‐up without further description. However, this was less than 5% of the total number of included participants in the analysis. |
| Selective reporting (reporting bias) | Low risk | Registrated to clinicaltrials.gov before randomisation (https://clinicaltrials.gov/ct2/show/NCT01155635). All outcomes in the protocol are mentioned in the paper. |
| Other bias | Unclear risk | The trial was prematurely terminated due to slow enrolment which can lead to bias. |
| Methods | Randomised clinical trial, parallel design, at 163 sites in 17 countries (Australia, Belgium, Canada, Germany, Hungary, Ireland, Israel, Italy, Lithuania, Luxembourg, the Netherlands, New Zealand, Russia, Spain, UK, and USA). Duration not mentioned. | |
| Participants | 1959 participants aged 18 years or older with a stable, definite MI occurring 3–21 days before randomisation, left‐ventricular ejection fraction of 40% or less by two‐dimensional echocardiography or by radionuclide or contrast ventriculography, or wall motion‐score index of 1.3 or less; and receipt of concurrent treatment with ACE inhibitors for at least 48 hours and stable dose for more than 24 hours unless there was proven intolerance of ACE inhibitors were included. Male:female = 1440:519. Mean age = 63 years. Exclusion criteria: those patients who continued to require IV diuretics or inotropics, or who had uncontrolled heart failure, unstable angina, hypotension (SBP < 90 mmHg), uncontrolled hypertension, bradycardia (HR < 60 bpm), and unstable insulin‐dependent diabetes mellitus. Patients with a continuing indication for beta‐blockers for any clinical indication other than heart failure were excluded, as were those requiring ongoing therapy with inhaled beta2‐agonists or steroids. | |
| Interventions | Experimental group: carvedilol (initial dose of 6.25 mg twice daily, up titrated to 25 mg twice daily during 4‐6 weeks, and followed during follow‐up period). Control group: placebo. Co‐intervention: treatment for index MI (nitrates, intravenous beta‐blockers, intravenous heparin, subcutaneous heparin, intravenous diuretics, thrombolysis/primary angioplasty). Excluded medication: not described. | |
| Outcomes | Primary: all‐cause mortality or hospital admission for cardiovascular problems. Secondary and other: sudden death, hospital admission for heart failure, cardiovascular mortality, non‐fatal m‐Mi, all‐cause mortality or non‐fatalMI. Time points reported: 1.3 years (maximum follow‐up). | |
| Notes | The authors were contacted on 25 January 2017 at [email protected]. The trial was sponsored by National Heart, Lung, and Blood Institute. Roche Pharmaceuticals and Glaxo SmithKline functioned as co‐ordination. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by an automated system. |
| Allocation concealment (selection bias) | Unclear risk | It was not described how the allocation was concealed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | All the tablets were identical and the trial is described as being double‐blinded. This indicates that the participants have been blinded, however there is no indication of whether the personnel have been blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | An endpoints committee was responsible for masked adjudication of all prespecified endpoints, which were described in detail in a manual of operating procedures agreed by the steering committee. |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts were described, however carvedilol and placebo were withdrawn permanently in 192 (20%) and 174 (18%) participants. Not described if withdrawn patients were followed up on as well. |
| Selective reporting (reporting bias) | Low risk | Protocol published + reported on the predefined outcomes. |
| Other bias | Low risk | No other biases found. |
| Methods | Randomised clinical trial, parallel design, at a single site in Denmark between November 1965 and June 1966. | |
| Participants | 130 participants with suspected AMI within 24 hours of randomisation were included. Male:female = 47:19. Mean age = not described. Exclusion criteria: bronchial asthma. | |
| Interventions | Experimental group: propranolol (10 mg orally four times daily for 14 days). Control group: no intervention other than the co‐intervention. Co‐intervention: all patients, treated and non‐treated, were given other drugs (e.g. digitalis, diuretics, metaraminol, or procainamide) if necessary. Excluded medication: not described. | |
| Outcomes | Primary: mortality, incidence of arrhythmia Time points reported: 1‐7, 1‐14 and 1‐21 days. | |
| Notes | Email not found. The trial was supported by Danish League Against Heart Disease. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ”Patients were randomly treated“ no further details. |
| Allocation concealment (selection bias) | Unclear risk | The selection system was administrated by one of the secretaries in the department, and no physician concerned in the trial took any part in selection. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | Mortality data was provided according to all randomised patients. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, 2 x 2 factorial design, at 1250 sites in China between August 1999 and February 2005. | |
| Participants | 45,852 participants who presented with ST elevation, left‐bundle branch block, or ST depression within 24 hours of the onset of the symptoms of suspected AMI were potentially eligible for the study. Mean age = 61 years. Exclusion criteria: patients scheduled for primary percutaneous coronary intervention (PCI), a high risk of adverse effects (1) previous allergy to aspirin, (2) active bleeding or a history of haemostatic disorder, (3) very low BP (e.g. SBP persistently < 100 mmHg), (4) very low HR (e.g. persistently < 50 bpm), (5) third degree heart block or (6) cardiogenic shock) with one or other of the trial treatments or only a small likelihood of worthwhile benefit such as a negligibly low risk of MI death (e.g. unconvincing history and/or normal ECG). | |
| Interventions | Note: there was another comparison, aspirin plus clopidogrel vs. aspirin, as per a 2 x 2 factorial design. This comparison is not discussed in this review. Experimental group: metoprolol (initial dose of up to 15 mg given intravenously, 50 mg tablet every 6 hours during days 0‐1, after two days a 200 mg tablet was given daily for up to 4 weeks). Control group: matching placebo (same regimen as the experimental group). Co‐intervention: not described. Excluded medication: non‐study beta‐blocker (and non‐study antiplatelet) therapy was to be avoided during the scheduled treatment period unless it was believed that some strong indication had developed. | |
| Outcomes | Primary: the composite of death, reinfarction, or cardiac arrest (including ventricular fibrillation); and death from any cause during the scheduled treatment period (i.e., until first discharge or day 28). Secondary: reinfarction, ventricular fibrillation, other cardiac arrest, cardiogenic shock, and related conditions. Time points reported: first hospital discharge or 28 days, whichever came first (mean 15 days). | |
| Notes | The authors were contacted on 25 January 2017 on [email protected] and [email protected] but no answers were received. The study was funded jointly by Sanofi‐Aventis and Bristol‐Myers Squibb (manufacturers of clopidogrel) and by AstraZeneca (manufacturers of metoprolol). However, it is said that quote: "the sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The writing committee had final responsibility for the decision to submit for publication." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation of the study treatments involved sequentially‐numbered sealed treatment packs prepared centrally. |
| Allocation concealment (selection bias) | Low risk | Random allocation of the study treatments involved sequentially‐numbered sealed treatment packs prepared centrally. |
| Blinding of participants and personnel (performance bias) | Low risk | The participants and personnel were blinded due to the use of matching placebo. It is moreover mentioned that the clinical staff who took care of the participants had no knowledge of the study treatment allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | All outcomes were reviewed and, if necessary, additional information was sought to allow adjudication (without knowledge of the study treatment allocation) by clinical staff in the co‐ordinating centres. |
| Incomplete outcome data (attrition bias) | Low risk | One from each group was lost to follow‐up. Mortality data were provided according to all randomised patients. |
| Selective reporting (reporting bias) | Low risk | Protocol found + reported on the predefined outcomes. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, in the UK between January 1976 and June 1979. | |
| Participants | 313 participants with diagnosed AMI (within 72 hours from onset of symptoms) were included. Male:female = 280:33. Mean age = 52.8 years. Exclusion criteria: radiographic evidence of left ventricular failure, all grades of heart block, dysrhythmias requiring treatment, obstructive airways disease, diabetes mellitus, other systemic illness or concurrent treatment with antidysrhythmic or beta‐blocking drugs. | |
| Interventions | Experimental group: oxprenolol (40 mg twice daily for 56 days). Control group: matching placebo. Co‐intervention: not described. Excluded medication: not described. | |
| Outcomes | Primary: reinfarction, cardiac death, trial deviations. Time points reported: 4 and 8 weeks. | |
| Notes | Email not found. The trial was supported by Ciba‐Geigy Pharmaceuticals, the Yorkshire Regional Hospital Board and the West Riding Medical Research Trust. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described as being quote: "a randomised double‐blind place controlled trial", however, no further description was given. |
| Allocation concealment (selection bias) | Unclear risk | The study was described as being quote: "a randomised double‐blind place controlled trial", however, no further description was given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study was described as being quote: "a randomised double‐blind place controlled trial", however, no further description was given. |
| Blinding of outcome assessment (detection bias) | Low risk | The critical endpoints of reinfarction and cardiac death together with trial deviations were validated blind at the end of the study by the members of the Co‐ordinating Group. |
| Incomplete outcome data (attrition bias) | Low risk | 21 (11.9%) participants from the experimental group and 17 (12.5%) from the placebo group were withdrawn from the study. However, all patients were followed up on. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found and the trial did not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, in India. Duration not mentioned. | |
| Participants | 30 patients with AMI were included. Male:female = 25:5. Mean age = 45.9 years. Exclusion criteria: any contraindication to thrombolytic therapy, any evidence of atrio‐ventricular (AV) conduction defect on ECG, patients in Killip class IV, chronic smokers (due to increased free radical activity), patients with significant airway obstruction or on any beta blockers for less than two elimination half‐lives. | |
| Interventions | Experimental group: esmolol (a loading dose of esmolol 500 μg/kg/minute for 1 minute followed by increments of 50 μg/kg up to a maximum of 300 μg/kg/ minute for 4 minutes. The infusion of maximum tolerated dose was continued for 3 hours). Control group: no intervention other than co‐intervention. Co‐intervention: thrombolysis with intravenous streptokinase (1.5 million units slow infusion over one hour). Furthermore, all patients received conventional treatment of AMI. Excluded medication: not described. | |
| Outcomes | Primary: malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GPX) were compared at 0, 2 and 24 hours. Time points reported: 24 hours. | |
| Notes | No email found for Dr. Mridul Kumar Daga. The study found no side effects of esmolol infusion. Hence, there are no useful data we can extract from this study. The funding/support was not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, other than quote: "This was a randomised double‐blind, controlled prospective clinical study". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described, other than quote: "This was a randomised double‐blind, controlled prospective clinical study". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report all‐cause mortality and SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 14 hospitals and 4 ambulance services in the Netherlands and Spain between February 2012 and November 2015. | |
| Participants | 683 STEMI patients >18 years of age presenting < 12 hours from symptom onset in Killip class I to II without atrioventricular block were included. Male:female = 511:172. Mean age = 62 years. Exclusion criteria: cardiogenic shock or signs of heart failure, Killip III (defined as severe dyspnoea, oxygen saturation < 92%, SBP <100 mmHg, HR >110 bpm). Known with asthma bronchiale. Severe hypotension SBP ≤ 100 mmHg). Severe sinus bradycardia (< 60 bpm)History of previous MI. Unable to provide informed consent. Pacemaker or ICD implantation (no MRI possible). Patient is (suspected to be) pregnant or breastfeeding | |
| Interventions | Experimental group: metoprolol (2 x 5 mg bolus IV). Control group: placebo. Co‐intervention: 500 mg of IV aspirin, 600 mg of clopidogrel or 180 mg of ticagrelor orally, and 5,000 international units of IV unfractionated heparin. All patients received oral metoprolol within 12 hours after PCI, according current guidelines during hospitalisation. At discharge, all patients received oral metoprolol at a dose recommended by their treating physician. Excluded medication: not described. | |
| Outcomes | Primary: myocardial infarct size at 30 days. Secondary: peak creatine kinase (CK), peak CK‐MB, troponin at 24 hours, the CK and CKMB AUC during the first 24 hours, residual ST‐segment deviation 1 hour after PCI/coronary angiogram, ventricular arrhythmias requiring defibrillation during transportation and hospitalisation, and major adverse cardiac event (MACE) rate, defined as cardiac death, nonfatal reinfarction, or target vessel revascularisation at 30 days. The secondary safety endpoints included symptomatic bradycardia, symptomatic hypotension, and cardiogenic shock. Time points reported: 24 hours, 30 days and 12 months follow‐up. | |
| Notes | The author Dr. Arnoud was contacted on [email protected] on 29‐03‐2017. No response was received. 8 patients from the metoprolol group and 4 patients from the placebo group in the Non‐MRI group is dead, however, it is unclear whether these were included in the cardiac mortality reported in table 4, or if we should add the cardiac mortality and these deaths when reporting all‐cause mortality. Furthermore, only 307 out of 336 and 319 out of 347 patients are analysed when reporting adverse events and CMR imaging. It is unclear what has happened with the rest of the patients. Trial funding came from a research grant of the Dutch Heart Foundation (Utrecht, the Netherlands) and an unrestricted grant by Medtronic Inc. (Heerlen, the Netherlands). Dr. Botas has been a consultant for Terumo. Dr. van 't Hof has received speakers fees from AstraZeneca, Iroko, and Daiichi‐Sankyo; has received non personal grants from Medtronic and Daiichi‐Sankyo to his research institution. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization took place without stratification and in blocks of 4.2 It was not described how the randomisation list was generated. |
| Allocation concealment (selection bias) | Low risk | Quote: "After informed consent, a blinded study medication box was opened. This box contained 2 vials of metoprolol 5 mg or matching placebo and labelled with a number that corresponded with the randomisation list." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "CMR study analyses were performed in a random manner by expert observers blinded to treatment allocation. All CMR studies were performed blinded to treatment allocation and according to a centralized protocol." The study was furthermore described as being double‐blinded and the patients in the placebo group received matching placebo. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The change occurred while the investigators were still entirely blinded to trial results and without any interim analysis performed." |
| Incomplete outcome data (attrition bias) | High risk | The CMR imaging, which was the primary endpoint, was only performed in 624 out of 684 patients. It is unclear why there were 60 patients missing. Furthermore, when reporting adverse cardiac events at 1 month follow‐up, only 307 out of 336 patients in the metoprolol group and 319 out of 347 patients in the placebo group were analysed. At 12‐months follow‐up, it was mentioned that 54 participants were lost to follow‐up (8%). |
| Selective reporting (reporting bias) | Low risk | A protocol was published before the trial was begun and the outcomes called for in the protocol are reported on. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 21 sites in the USA between September 1997 and February 1999. | |
| Participants | 108 participants examined at the hospital within 12 hours of chest pain, who met criteria for acute transmural MI (i.e., ST‐segment elevation) or unstable angina/non‐Q‐wave myocardial infarction (i.e. non‐ST‐segment elevation) and who had at least 1 relative contraindication to beta blockade, were included to the trial. Male:female = 66:42. Mean age = 59.2 years. Exclusion criteria: severe bradycardia (HR 55 bpm), hypotension (SBP 100 mmHg unresponsive to fluids), prolonged electrocardiographic PR segment (0.30 seconds), second/third‐degree AV block or junctional rhythm, acute bronchospastic episode, severe congestive heart failure, history of uncontrolled diabetes, pregnancy, atrial fibrillation/flutter, bundle branch block, pre‐excitation syndrome (i.e. Wolff‐Parkinson‐White syndrome), permanent ventricular pacemakers, and surgical revascularisation (coronary artery bypass grafting) at the time of screening. Patients with known or suspected drug or alcohol abuse, serious advanced illness, or patients who had received beta‐blockers within 24 hours of screening and intravenous calcium‐channel blockers within 48 hours of screening were not considered for participation. | |
| Interventions | Experimental group: esmolol (for 16‐30 hours), followed by metoprolol (500 µg/kg intravenous esmolol followed by 50 µg/kg/minute for 16 to 30 hours. 12.5 mg to 25 mg of oral metoprolol was given 30 minutes before discontinuation of the intravenous esmolol infusion and was up titrated to a dose of 100 mg a day for a minimum of 6 weeks). Control group: no intervention other than the co‐intervention. Co‐intervention: standard medical therapy (thrombolytic therapy, intravenous heparin, aspirin, nitrates, and narcotics). Excluded medication: intravenous calcium‐channel blockers was prohibited in patients receiving esmolol. | |
| Outcomes | Primary: composite event consisting of any of the following that occurred during the index hospitalisation: (1) death, (2) nonfatal myocardial (re)infarction, (3) recurrent ischaemia, (4) nonfatal cardiac arrest, (5) non‐fatal ventricular tachycardia or fibrillation, or (6) silent myocardial ischaemia episodes assessed by ambulatory electrocardiographic monitoring in the 24 hours after random assignment. Secondary: death, MI, rehospitalisation for cardiac causes, and classification of anginal status and congestive heart failure status as defined by the New York Heart Association (NYHA) functional capacity classification. Time points reported: 6 weeks. | |
| Notes | The authors were contacted on 25 January 2017 on [email protected], [email protected] and [email protected]. No response received. The trial was supported by Baxter Pharmaceutical Products Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were randomly assigned by means of a telephone, computer‐driven response system on a 24‐hour quote: “on‐demand” basis. |
| Allocation concealment (selection bias) | Low risk | This telephone system ensured proper sequence allocation and immediate coordinating centre confirmation of enrolment and provided security against potential randomisation bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | There were no dropouts during follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at one site in England between February 1975 and January 1976. | |
| Participants | 94 participants, under the age of 70, with their first suspected AMI were included. Male:female = 79:15. Mean age = not described. Exclusion criteria: 1) patients older than 70 years, 2) previous MI, 3) SBP less than 95 mmHg, 4) HR less than 60/minute, 5) evidence of overt left ventricular (orthopnoea, third heart sound and widespread lung crepitations), 6) second or third degree atrioventricular block or of bundle branch block. | |
| Interventions | Experimental group: practolol (initial dose of 15 mg given intravenously followed by 5 oral doses of 200 mg at 12‐hour intervals for 48 hours). Control group: no intervention. Co‐intervention: not described. Excluded medication: not described. | |
| Outcomes | Primary: mortality, major complications, the need for any anti failure therapy among patients receiving such medication, HR, SBP. Time points reported: 48 hours, 1, 4 and 7 months. | |
| Notes | Email not found. The funding/support was not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly allocated to a treated or control group according to the instructions contained in a sealed envelope opened by an observer. However, not described as opaque. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | High risk | Not described, however it appears that 4 (8.7%) patients from the experimental group and 2 (4.2%) from the control group were lost to follow‐up at 7 months, since there are no data on these. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found. The trial did not report on SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 3 sites in Sweden. Duration not mentioned. | |
| Participants | 262 participants with ongoing chest pain raising any suspicion of AMI and intensity of pain at the time of randomisation of at least 3 on a 10‐grade visual analogue scale were included in the trial. Male:female = 166:96. Mean age = 70 years. Exclusion criteria: HR (HR) < 60 bpm, SBP < 110 mmHg, second‐ or third‐degree atrioventricular (AV) block, severe congestive heart failure (defined as auscultatory rales >10 cm above the lung bases), and obstructive pulmonary disease requiring corticosteroids. | |
| Interventions | Experimental group: metoprolol (5 mg x 3 intravenously). Control group: placebo. Co‐intervention: 5 mg morphine. Excluded medication: not described. | |
| Outcomes | Primary: severity of chest pain during the first hour and before hospitalisation. Secondary: the occurrence of various complications. Time points reported: in the ambulance; 5 days (mean hospitalisation time); 1 month. | |
| Notes | The authors were contacted on 25 HJanuary 2017 on [email protected]. No response received. The trial was supported by grants from the Swedish Heart & Lung Foundation, and AB ASTRA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Patients were described as being blindly allocated, however, no further description was given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Patients were described as being blindly allocated, however, no further description was given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | The total amount of patients lost to follow‐up is not described. However, it is described how many patients were lost to follow‐up for each outcome.Three patients from the experimental group and 1 from the control group were lost to follow‐up in regarding to the outcome quote: "congestive heart failure". No follow‐up was described for all‐cause mortality. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found and the trial did not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 3 sites in Sweden between June 1976 and January 1981. | |
| Participants | 1395 participants who met the following inclusions criteria: (1) residence in the catchment area; (2) chest pain of acute onset and of 30 minutes duration, or ECG signs of AMI with estimated onset of infarction within the previous 48 hours; (3) age between 40 and 74 years, were included in the trial. Male:female = 1058:337. Mean age = 60 years. Exclusion criteria: contraindications to beta‐blockade, need for beta‐blockade, serious or multiple diseases, administrative reasons. | |
| Interventions | Experimental group: metoprolol (initial dose of 15 mg intravenouslyI followed by one half of a metoprolol tablet 15 minutes after the injections and then every 6 hours for 48 hours, and thereafter one tablet of 100 mg every 12 hours for 3 months). Control group: placebo. Co‐intervention: standard coronary care (furosemide, digitalis, lidocaine, antiarrhythmias, isoprenaline, prenalterol, atropine). Excluded medication: not described. | |
| Outcomes | Primary: mortality, reinfarction, and ventricular fibrillation. Time points reported: 3 months. | |
| Notes | The authors were contacted on 26 January 2017 on [email protected]. No response received. The trial was supported by grants from the Swedish Medical Research Council, the Swedish National Association Against Heart and Chest Diseases, the Goteborg Medical Society, and AB Hassle, subsidiary of AB Astra, Sweden. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | All data were registered on a special computer record form by the research assistant. These record forms were not available to any of the physicians managing the patient during the treatment period. However, blinding of the participants was not sufficiently described. |
| Blinding of outcome assessment (detection bias) | Low risk | All deaths within 90 days of the start of blind treatment were recorded and classified by an independent safety monitoring committee consisting of one statistician and three physicians not otherwise involved in the study. |
| Incomplete outcome data (attrition bias) | Low risk | Missing data on 11 (1.6%) from the placebo and 12 (1.7%) from the metoprolol group. Lower than 5% dropout. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases found. |
| Methods | Randomised clinical trial in Japan between January 2009 and March 2010. | |
| Participants | 96 patients with STEMI undergoing PCI within 12 hours after the onset of AMI were included. Male:female = 80:16 Mean age = 62 years. Exclusion criteria: those with Killip class 3 or 4, bradycardia < 50 bpm, hypotension with SBP < 90 mmHg, bronchospasm, or second‐ or third‐degree atrioventricular block. | |
| Interventions | Experimental intervention: landiolol (24 hours of IV 3μg/kg/minute landiolol without loading begun after successful PCI) Control intervention: no intervention other than the co‐intervention. Co‐intervention: ACE inhibitor or angiotensin‐receptor blocker, oral β‐blocker, statin, calcium‐channel blocker, diuretics, and nitrates. PCI. | |
| Outcomes | Primary: incidences of cardiovascular events such as cardiac arrhythmias and cardiac death. Secondary: effects of landiolol on HR and BP, and the development of any adverse effects. Time points reported: 14 days and 6 months. | |
| Notes | The author was contacted on 29 March 2017 on [email protected]‐u.ac.jp. No response received. No funding was received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described as being randomised by an envelope method, however, no further information was given. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Just after PCI, the patients were randomly divided into 2 groups by an envelope method." However, it was not mentioned if this envelope was sealed and opaque. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Two cardiologists who were unaware of the treatment assignment (landiolol or control) analysed the LVG results." However, it does describe whether or not the outcome assessors assessing the primary outcome 'cardiovascular events' were blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be found, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases found. |
| Methods | Randomised clinical trial, parallel design, at a single site in the UK between February 1982 and September 1983. | |
| Participants | 166 participants under 75 years of age admitted to the cardiac care unit within 6 hours of symptoms suggesting MI were included to the trial. Male:female = 137:29. Mean age = 59.5 years. Exclusion criteria: presentation more than six hours after the onset of symptoms, left ventricular failure causing alveolar oedema, persisting hypotension (SBP less than 100 mmHg) or hypertension (more than 200 mmHg), or conduction disorders (except first‐degree heart block with PR <0.22 s), tachyarrhythmias requiring treatment, haemodynamically significant valvular regurgitation, a history of bronchospasm, hepatic or renal disease judged to be severe, intercurrent disease likely to be fatal within one year, recent treatment with verapamil, or at the discretion of the physician for any other reason. | |
| Interventions | Experimental group: labetalol (initial IV dose of 62.5 µg, 125 µg or 250 µg (depending on the SBP) given by infusion for six hours, followed by 50 mg, 100 mg or 200 mg (depending on the SBP) given orally every 8 hours for 5 days). Control group: no intervention but the co‐intervention. Co‐intervention: conventional therapy, i.e. analgesics, diuretics, digoxin, atropine, other antiarrhythmics and inotropic agents. Excluded medication: other beta‐blockers. | |
| Outcomes | Primary: HR, BP. Time points reported: 7 days, 6 weeks, and 12 months. | |
| Notes | Email not found. The trial was supported by Duncan, Flockhart & Co Ltd (pharmaceutical company). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ""Patients were randomised“ no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label. Patients randomised to the control group were not given a placebo. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described. However, it appears that not all the participants were used while reporting on the different outcomes except for mortality. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found and the trial did not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial in France, Norway, Sweden, and the UK. Duration not mentioned. | |
| Participants | 144 participants of either sex, between 21‐70 years of age, and with suspected AMI (within 4 hours from onset) were included. Male:female = 123:21. Mean age = 55.5 years. Exclusion criteria: bradycardia (< 50 bpm), hypotension (SBP < 100 mmHg), clinical evidence of severe left ventricular failure, any degree of heart block, or a history of previous MI and a QRS duration longer than 0.11 seconds. Contraindication to beta‐blockade (rates >10 cm above the diaphragm), atrioventricular block I through III, bronchial obstruction, hypotension or bradycardia, or current treatment with a beta‐blocker, calcium antagonist, digitalis, or another antiarrhythmic agent. | |
| Interventions | Experimental intervention: timolol (1 mg IV for the first 24 hours followed by 10 mg orally twice a day for the duration of hospitalisation). Control intervention: matching placebo (the patients receivedIV normal saline instead of IV timolol). Co‐intervention: normal supportive measures were freely allowed, but treatment with other cardioactive drugs (digitalis, nitrates, calcium antagonists, or antiarrhythmic agents) was discouraged. If such therapy was administered, the patient was not withdrawn from the study but was considered a protocol violator for the purposes of data analysis. Intramuscular injections were not allowed. | |
| Outcomes | Outcomes: BP, pulse rate, clinical adverse events. | |
| Notes | Email not found. The trial was supported in part by a grant from Merck, Sharp and Dohme Research Laboratories, Rahway, N.J. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed within each center and was balanced in blocks of four patients." No further description was given. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | All the tablets were identical and the trial is described as being double‐blinded. This indicates that the participants have been blinded, however there is no indication of whether the personnel have been blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 245 sites in 14 countries (Australia, Belgium, Denmark, Finland, France, Germany, Norway, Sweden, England, Scotland, Wales, Northern Ireland, and Eire) between June 1981 and January 1985. | |
| Participants | 16,027 participants with suspected MI, who were thought by the responsible physician to be within 12 hours of the onset of symptoms, were included to the trial. Male:female = 12,341:3686. Mean age = 58.8 years. Exclusion criteria: beta‐blockers or verapamil, contraindication to beta‐blockade (e.g. HR persistently below 50 bpm, SBP persistently below 100 mmHg, second‐ or third‐degree heart block, severe heart failure, or bronchospasm). | |
| Interventions | Experimental group: atenolol (5 mg to 10 mg iv immediately, followed by 100 mg/day orally for 7 days). Control group: no intervention other than the co‐intervention. Co‐intervention: diuretic,IV nitrates, calcium antagonists, digitalis, antiarrhythmics, inotropic agents, antiplatelets, anticoagulants, beta‐blockers (after discharge). Excluded medication: not described. | |
| Outcomes | Primary: vascular mortality, hospital‐enzyme elevation, non‐fatal cardiac arrest, reinfarction. Time points reported: 7 days (treatment period), 12 months, and 20 months. | |
| Notes | Email not found. The entire study was financed by ICI Pharmaceuticals Ltd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised by a 24‐hour direct line telephone service. Patient identifiers, age, sex, HR, BP, hours from pain onset, diabetes, and previous MI were recorded centrally on the next available line of the computer‐generated randomisation lists (which had separate pages for each hospital). Once a complete line of patient details had been recorded properly, the random treatment allocation printed at the end of the line was issued, and the patient was irrevocably entered in the trial. |
| Allocation concealment (selection bias) | Low risk | Patients were randomised by a 24‐hour direct line telephone service. Patient identifiers, age, sex, HR, BP, hours from pain onset, diabetes, and previous MI were recorded centrally on the next available line of the computer‐generated randomisation lists (which had separate pages for each hospital). Once a complete line of patient details had been recorded properly, the random treatment allocation printed at the end of the line was issued, and the patient was irrevocably entered in the trial. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label. Quote: "No, placebo was given to the controls, partly because of the obvious effects of atenolol on the HR, but chiefly because the main aim was to study mortality, where assessment biases are non‐existent. It was hoped that the lack of placebo control would simplify patient management and so enhance recruitment. Of course, knowledge of which patients were receiving beta‐blockers modified the choice of which other treatments to give them, so the study includes both direct and indirect effects of treatment on mortality." |
| Blinding of outcome assessment (detection bias) | Unclear risk | All deaths described as quote: "definitely non‐vascular" were reviewed, blind of treatment allocation, by the chairman of the data monitoring committee. However, it was not described how the remaining outcomes were assessed. |
| Incomplete outcome data (attrition bias) | Low risk | The present report includes follow‐up to 1 January, 1985, except for 0% to 8% (69 atenolol and 60 control) who could be followed only until discharge, 0% to 8% (68 atenolol and 58 control) who could be followed only for one year, and 0% to 7% (53 atenolol and 56 control) who could not be followed at all after randomisation because erroneous identifiers were recorded at entry. A total of 190 (2,4%) participants from the experimental group and 174 (2,2%) participants from the control group were lost to follow‐up. Less than 5% dropout. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report on all SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at a single site in Norway. Duration not mentioned. | |
| Participants | 40 participants of any age were included in the study if admitted to the hospital within 6 hours after onset of symptoms of a suspected first MI and with electrocardiographic changes indicating an anterior location of the infarction. The electrocardiographic criterion for inclusion, present in at least two leads in a standard 12‐lead ECG, was new ST segment elevation of more than 1 mm in lead I and aVL or more than 2 mm in precordial leads. Male:female = not reported. Mean age = not reported. Exclusion criteria: HR below 50 bpm, SBP below 100 mmHg, clinical signs of left ventricular failure, bronchial obstruction, or any degree of heart block. Patients on concurrent treatment with digitalis, beta‐blockers, calcium antagonists, or any other cardioactive drugs were also excluded. | |
| Interventions | Experimental group: timolol maleate (IV injection of 1 mg followed by constant infusion of 0.6 mg/hour for 24 hours. Oral treatment of 10 mg twice daily was started after the infusion and continued for 10 days). Control group: placebo (IV injection of saline followed by matching placebo after the infusion). Co‐intervention: anticoagulation treatment with oral warfarin was started in all patients with a diagnosed ventricular thrombus. Excluded medication: digitalis, beta‐blockers, calcium antagonists, or any other cardioactive drugs. digitalis. Prophylactic antiarrhythmic treatment was not used.Intramuscular injections were not allowed, and pain was treated with oxygen, intravenous morphine, or oral propoxyphene (Aporex). | |
| Outcomes | Primary: left ventricular thrombi; analysis of wall motion, volumes, and ejection fractions. Time points reported: 10 days. | |
| Notes | Email not found. The funding/support was not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described sufficiently other than that the patients were randomly assigned. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The patients received matching placebo and the study was described as being double‐blinded. However, the blinding of the personnel is not described. |
| Blinding of outcome assessment (detection bias) | Low risk | The identity number of the participants were blinded for echographic registrations. |
| Incomplete outcome data (attrition bias) | High risk | Two patients was lost to follow‐up (one in each group) (5%). |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, in India. Duration not mentioned. | |
| Participants | 50 participants with uncomplicated AMI within 24 hours of symptom onset were included. Male:female = 41:9. Mean age = 48.6 years. Exclusion criteria: (1) patients with previous MI. (2) Patients with contraindications to beta‐blockers: hypotension (SBP less than 100 mmHg); bradycardia (HR less than 45/minute); significant heart failure (rales present more than 10 cm above lung bases, poor peripheral circulation, shocks); 2nd or 3rd degree atrioventricular block; bronchial asthma, chronic obstructive airway disease; peripheral vascular disease. (3) patients with serious or multiple diseases. (4) patients more than 70 years of age. | |
| Interventions | Experimental group: propranolol (0.1 mg/kg intravenously in 3 divided doses at 2‐minute intervals followed by doses of 40 mg every 6 hours). Control group: matching placebo. Co‐intervention: not reported. Excluded medication: not reported. | |
| Outcomes | Primary: (1) all cardiac deaths; (2) non‐fatal reinfarction; and (3) post‐infarction angina. Time points reported: 3‐9 months (mean 6 months). | |
| Notes | Email not found. No funding/support was described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described other than quote:"'A total of 50 patients satisfying the above criteria were randomly allocated to blind treatment.." |
| Allocation concealment (selection bias) | Unclear risk | Not described other than quote: "A total of 50 patients satisfying the above criteria were randomly allocated to blind treatment.." |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study was described as being double‐blinded and the control group were said to receive matching placebo. However, no detailed description of blinding was described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | The trial stated that there were no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained and not all SAEs were reported. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial at a single site in Russia. | |
| Participants | 41 participants with verified diagnosis of AMI were included. Male:female = 23:18. Mean age = 57 years. Exclusion criteria: chronic infectious diseases. | |
| Interventions | Experimental group: propranolol (80 mg per day until discharge). Control group: no intervention other than co‐intervention. Co‐intervention: traditional therapy (anticoagulants, hypotensive drugs (not including beta‐blockers and calcium antagonists), antiarrhythmic drugs, diuretics and nitrates). Excluded medication: not described. | |
| Outcomes | Primary: level of blood ceruloplasmin. Time points reported: 28 days. | |
| Notes | Email not found. The funding/support was not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, other than that quote: "the patients were randomised into two groups.." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not mentioned if any participant was lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report on all SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at a single site in Scotland. Duration not mentioned. | |
| Participants | 40 participants between 40‐80 years of age who were admitted to hospital within 48 hours of the onset of chest pain of over 50 minutes' duration were included to the trial. Male:female = 40:0. Mean age = 60.4 years. Exclusion criteria: systolic pressures below 100 mmHg or electrocardiographic evidence of impaired A‐V conduction. | |
| Interventions | Experimental group: propranolol (20 mg three times daily (group 1) or 30 mg four times daily (group 2) for 7 days). Control group: placebo. Co‐intervention: none described. Excluded medication: none described. | |
| Outcomes | Primary: the number of ectopic beats, mortality. Time points reported: 7 days. | |
| Notes | Email not found. The trial was supported by Imperial Chemical Industries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, other than that the study was described as being double‐blinded. |
| Allocation concealment (selection bias) | Unclear risk | Not described, other than quote: "The patients received propranolol or identical placebo tablets for seven days according to a random scheme." |
| Blinding of participants and personnel (performance bias) | Low risk | The control patients received identical placebo tablets. The tablets were made up into individual seven‐day courses and labelled with a code letter by the hospital pharmacist, who kept a record of the code. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | Only one patient from the experimental group were withdrawn from the study. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial did not report all SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 2 sites in South Africa. Duration not mentioned. | |
| Participants | 30 participants with AMI with less than 12 hours from onset were included to the study. Male:female = 22:8. Mean age = 55.2 years. Exclusion criteria: already receiving beta‐blockers, calcium antagonists or anti‐arrhythmic agents, older than 70 years, persistent hypotension (< 90 mmHg SBP), bradycardia (< 50/minute), pulmonary capillary wedge pressure (PCWP) greater than 24 mmHg, 2nd‐ or 3rd‐degree AV‐block, ventricular fibrillation, severe chronic obstructive airways disease or diabetic ketosis. | |
| Interventions | Experimental group: sotalol (40 mg x 3 intravenously, followed by the maximum dosage of 120 mg every 6 hours for 72 hours). Control group: placebo. Co‐intervention: not described. Excluded medication: beta blockers (other than the intervention); calcium antagonists; anti‐arrhythmic agents. | |
| Outcomes | Primary: completion of 72 hours from admission, persistent hypotension (< 90 mmHg SBP), the occurrence of AV‐block greater than first degree, ventricular arrhythmias, a need for other antiarrhythmic agents, or any side‐effect attributable to the drug. Time points reported: 72 hours. | |
| Notes | Email not found. No funding described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, other than quote: "30 patients with clinical syndrome of AMI of less than 12 hours' duration were prospectively randomised into a control group and a treatment group..." |
| Allocation concealment (selection bias) | Unclear risk | Not described, other than quote" "'30 patients with clinical syndrome of AMI of less than 12 hours' duration were prospectively randomised into a control group and a treatment group..." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | High risk | One patient (6.7 %) from each group were withdrawn from the study. The reason for dropout was described. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found. The trial did not adequately report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 2 sites in the UK. Duration not mentioned. | |
| Participants | 51 participants admitted to the Coronary Care Unit, under the care of the participating physicians, with a diagnosis of MI within 12 hours of onset were considered for enrolment in the trial. Male:female = 35:16. Mean age = 58.5 years. Exclusion criteria: (a) chest pain for longer than 12 hours prior to admission; (b) age greater than 80 years; (c) women capable of bearing children; (d) patients with chronic obstructive airways disease, insulin‐treated diabetes mellitus or significant renal, endocrine, hepatic or haemopoietic disease; (e) concurrent treatment with a beta‐blocker or verapamil; (f) patients with severe heart failure (defined as New York Heart Association Class IV or a diuretic requirement of more than 80 mg of frusemide or equivalent); (g) SBP of less than 90 mmHg; (h) second or third‐degree atrioventricular block; (i) persistent sinus bradycardia (i.e. less than 50 bpm lasting beyond day 2 of admission); (j) requirement for a pacemaker: (k) patients with atrial fibrillation; (l) recurrent ventricular tachycardia or ventricular fibrillation despite treatment. | |
| Interventions | Experimental group: xamoterol (200 mg twice daily for 7 days). Control group: placebo. Co‐intervention: diuretics, antianginal therapy, or oral antiarrhythmic therapy. Excluded medication: not described. | |
| Outcomes | Primary: pulse rate and BP, 24‐hour ECG recording, additional medication, serum potassium. Time points reported: 10 days. | |
| Notes | The author was contacted on 26 January 2017 on [email protected]. No response received. The trial was supported by ICI Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described as being double‐blinded and randomised, no further information was given. |
| Allocation concealment (selection bias) | Unclear risk | The study was described as being double‐blinded and randomised, no further information was given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Low risk | All analyses were performed blind to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | 2 (7.7%) participants from the experimental group and 1 (4%) from the placebo group were withdrawn from the study. All patients, including the dropouts, were followed up on. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found. The trial did not report on all‐cause mortality and SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 7 sites in Spain between November 2010 and October 2012. | |
| Participants | 270 participants between 18 to 80 years of age with symptoms consistent with STEMI for > 30 minutes and ST elevation ≥2 mm in ≥2 contiguous leads in V1 through V5 with an anticipated time of symptom onset to reperfusion of ≤ 6 hours (within 4.5 hours from symptom onset) were included to the trial. Male:female = 233:37. Mean age = 58.4 years. Exclusion criteria: Killip class III to IV AMI, SBP persistently < 120 mmHg, PR interval >240 milliseconds (or type II–III atrioventricular block), HR persistently < 60 bpm, or active treatment with any β‐blocker agent. | |
| Interventions | Experimental group: metoprolol (5 mg x 3 boluses of metoprolol tartrate 2 minutes apart intravenously). Control group: placebo. Co‐intervention: oral metoprolol (25 mg to 100 mg/12 hours) 12 to 24 hours post STEMI. Thrombus aspiration and glycoprotein IIb/IIIa during PCI. Excluded medication: not described. | |
| Outcomes | Primary: infarct size on MRI (extent of myocardial necrosis quantified by delayed gadolinium enhancement). Secondary: the extent of myocardial salvage on MRI, infarct size quantified by MRI in the subgroup of patients with a pre‐PCI Thrombolysis in Myocardial Infarction (TIMI) grade 0 to 1 flow, and infarct size estimated by peak and (AUC; 72 hours) release of creatine kinase (CK), the incidence of major adverse cardiac events, defined as a composite of death, malignant ventricular arrhythmias, advanced atrioventricular block, cardiogenic shock, and reinfarction during the first 24 hours after STEMI. Time points reported: 24 hourS, 7 days, 6 month, 12 month and 24 months. | |
| Notes | The authors were contacted on 26 January 2017 on [email protected]. No response received. The METOCARD‐CNIC trial was a noncommercial trial independent to the pharmaceutical industry; the main sponsor was the CNIC through competitive CNIC translational grant 01‐2009. We also had an independent research grant from the Spanish National Ministry of Health and Social Policy (EC10‐042), a Mutua Madrileña Foundation grant (AP8695‐2011), and a master research agreement between Philips Healthcare and CNIC. Dr Ibanez is recipient of the ISCIII grant “Fondo de Investigación Sanitaria PI10/02268,” which relates to the topic of this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | When a researcher calls the randomisation centre, trained nurses ask for the classification code that relates the participant with a particular stratum and enter this code into a computer program designed ad hoc that reads the allocation treatment group from any of the 16 lists according to the stratum where the participant has been assigned; subsequently the nurses communicate the treatment allocation to the recruiting researcher. |
| Allocation concealment (selection bias) | Low risk | When a researcher calls the randomisation centre, trained nurses ask for the classification code that relates the participant with a particular stratum and enter this code into a computer program designed ad hoc that reads the allocation treatment group from any of the 16 lists according to the stratum where the participant has been assigned; subsequently the nurses communicate the treatment allocation to the recruiting researcher. |
| Blinding of participants and personnel (performance bias) | High risk | Single‐blinded (only blinded to outcome assessment). |
| Blinding of outcome assessment (detection bias) | Low risk | MRI and angiography were evaluated at independent core laboratories; endpoint events were adjudicated by an independent clinical events committee. All were blinded to treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | 33 (23.7%) from the experimental group and 17 (13%) from the placebo group were not included in the MRI performed‐primary endpoint analysis. However, when reporting the adverse cardiac events including death, only 6 patients were lost to follow‐up (2.22%). |
| Selective reporting (reporting bias) | Low risk | Protocol published + reported on the predefined outcomes. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 104 sites in 17 countries (Australia, Austria, Belgium, Canada, Denmark, Finland, Holland, Hong Kong, Italy, Malaysia, New Zealand, Norway, Philippines, Singapore, Sweden, UK, and West Germany) between December 1982 and March 1984. | |
| Participants | 5778 participants with definite or suspected AMI, within 24 hours of the onset of symptoms. The patients had to be less than 75 years of age; chest pain of acute onset and of at least 15‐minute duration with a suspicion of MI; or ECG signs and symptoms of AMI. Male:female = 4484:1294. Mean age = 60 years. Exclusion criteria: current treatment with beta‐blockers (within 48 hours); current treatment with calcium channel blockers (within 48 hours); HR ≤ 65 bpm; SBP ≤ 105 mmHg; left ventricular failure (basal rates > 10 cm); poor peripheral circulation; AV‐conduction disturbances (PQ > 0.24 s); severe COPD; implanted pacemaker; resuscitation outside hospital; other serious disease; Previous MIAMI participation; participation in other randomised trials; unwilling or unable to give informed consent; reason unknown. | |
| Interventions | Experimental intervention: metoprolol (intravenous (15 mg) rapid injection of 5 mL every 2 minutes. Then oral treatment (200 mg daily) was continued for 15 days). Control intervention: placebo (saline). Co‐intervention: not described other than quote: "the general management of patients was according to local practice". | |
| Outcomes | Primary: all‐cause mortality. Secondary: incidence of definite AMI; serum enzyme activity; ventricular fibrillation; supraventricular tachyarrhythmias; the use of cardiac glycosides and other antiarrhythmics; the need for pain‐relieving treatment. Adverse events. Infarct development and infarct size; chest pain; tolerance. | |
| Notes | The author was contacted on 13 February 2017 on [email protected] and [email protected]. No answer received. No funding was described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation code was computer‐generated on a microcomputer by a member of the Safety Monitoring Committee, independently of all other organizing committees and the sponsor of the trial." |
| Allocation concealment (selection bias) | Low risk | Quote: "Only 4 sets of the randomisation code were generated, 1 for the Chairman and 1 for the statistician of the Safety Monitoring Committee, 1 for the Pharmacy Department at the Rostra University Hospital whose members were responsible for packing the clinical trial material and 1 for emergency use. This last code was provided sealed in separate envelopes for individual patients at each trial center." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Packaging and labelling according to the randomisation code were done in the Department of Pharmacy of the Ijstra University Hospital in Goteborg. The stock was packaged in sets of 50, together with the individual code sealed in 50 separate envelopes and with the BCRFs, and then distributed to the trial centers. The procedure was performed in this way so that the study could be conducted blindly by the organizing committees, the investigators, the patients and the sponsor." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The Independent Data Audit Committee was a statistician, located in Norway, who obtained continuous notification of randomised patients and deaths, directly from each trial centre.' 'The data file was then transferred from the Data Management Center to the University of Goteborg where, for the first time, the code was entered and analyses begun." |
| Incomplete outcome data (attrition bias) | Low risk | Only two patients (0,07%) in the placebo group were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | A protocol was made Quote: "The important elements of the design of the trial were contained in the final project protocol (dated November 16,1982), which is available from the authors upon request", but could not be obtained. However, a protocol published at the time of study publication describing the different methods used in the trial was found. The trial reported all‐cause mortality, but not all SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 5 sites in the USA between August 1978 and February 1983. | |
| Participants | 269 (3697) participants under the age of 76 who had had pain typical of myocardial ischaemia for at least 30 minutes, and had electrocardiographic changes indicative of acute ischaemia or infarction (new Q waves, or 0.1 mV ST‐segment elevation or depression or both) or left bundle‐branch block or idioventricular rhythm, within 18 hours were included in the trial. Male:female = 197:72. Mean age = 54.8 years. Exclusion criteria: more than 18 hours since the onset of symptoms; pregnancy; in cardiogenic shock; concurrent serious illnesses or pacemakers; undergone major surgery, had had an infarction within the previous two weeks; were receiving therapy with nitrates ir beta‐blockers that could not be discontinued for 72 hours; worse than Killip Class I‐II. Patients >75 years old, if qualifying ischaemic symptoms had an indistinct onset or began >18 hours before screening, terminal diseases or major organ system failure, cardiomyopathy or acute cerebrovascular disease; they are also excluded if they are participating in conflicting protocols, if they have previously participated in MILIS, or if geographic, physical or psychological factors will interfere with follow‐up. | |
| Interventions | Experimental intervention: propranolol (intravenously upon randomisation (0.1 mg/kg body weight) administered in three portions over six minutes. Three hours after IV maintenance dose, treatment with oral propranolol was initiated in a dose of 20 mg and subsequently in doses to keep the HR between 45 bpm and 60 bpm and the systolic arterial pressure above 90 mmHg. Patients, unable to take oral medication, were treated with 0.025 mg/kg intravenously every six hours. This method was used for seven days; the dose was tapered to one half during the 8th and 9th days and discontinued on the 10th day). Control intervention: placebo. Co‐intervention: patients received routine treatment for MI that followed guidelines established by MILIS to avoid the use of other therapies intended specifically to limit infarct size. | |
| Outcomes | Primary: left ventricular ejection fraction; HR; cardiac failure; cardiac rhythm disturbances; ventricular tachycardia; heart block; infarct size. Secondary: mortality. Time point reported: follow‐up was at three and six months for all patients enrolled. Subsequently, the vital status of all patients was ascertained at six‐month intervals by a questionnaire administered by phone. | |
| Notes | The author was contacted on 26 January 2017 on [email protected]. The email could not be sent to the email address and no other email address could be found. The total, in‐hospital or 1‐month, deaths for both groups was 12 [NEJM1984; 311: p.223]. If 4 deaths occurred in the propranolol group (Am J Cardio The trial was supported by the Cardiac Diseases Branch, Division of Heart and Vascular Diseases, National Heart, Lung and Blood Institute, National Institutes of health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, other than quote: "patients randomized to receive..." |
| Allocation concealment (selection bias) | Unclear risk | Not described, other than quote: "patients randomized to receive..." |
| Blinding of participants and personnel (performance bias) | High risk | Patients randomise to receive hyaluronidase or placebo were given blinded treatment for 48 hours after randomisation, whereas propranolol was administered intravenously in a loading dose, followed by a single IV maintenance dose, and then oral doses until the tenth day after randomisation. Single‐blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Demographic data from the clinical units and endpoint data from core laboratories are stored at a data co‐ordinating centre (Research Triangle Institute, Research Triangle Park, North Carolina), where results are collated. Endpoint data analyses for the different treatment groups are available only to an independent data and policy monitoring board, which supervises this ongoing study. |
| Incomplete outcome data (attrition bias) | Low risk | 1 patient was lost to follow‐up. Mortality data were provided according to all randomised patients |
| Selective reporting (reporting bias) | Unclear risk | A protocol was described quote: "MILIS Study Group: National Heart, Lung, and Blood Institute Multicenter Investigation of the Limitation of Infarct Size (MILIS): design and methods of the clinical trial. Number 100, 1984: American Heart Association, Dallas, Texas." but could not be obtained, and the trial did not report SAEs. Nevertheless, the trial was post‐registered 28 October, 1999 on clinicaltrials.gov (NCT00000493). |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at a single site in the USA. Duration not mentioned. | |
| Participants | 70 participants who met the following inclusion criteria: (a) suspected or definite AMI, as evidenced by a characteristic history, acute ischaemic changes in the ECG, and, if possible, by plasma creatine kinase MB (CKMB) elevations; (b) functional (Killip) classes I and II; (c) SBP > 95 mm Hg; (d) HR > 55 bpm; (e) age < 75 years, were included to the trial. Male:female = 59:11. Mean age = 56.5 years. Exclusion criteria: no electrocardiographic evidence of an old transmural MI (Q‐waves); absence of acute bundle branch block, of acute or old second‐ or third‐degree atrioventricular block; absence of insulin‐dependent diabetes (>20 U/day); absence of spastic lung disease. | |
| Interventions | Experimental group: propranolol (0.1 mg/kg IV followed by an oral dose of 40 mg increased to 80 mg for 3 days). Control group: placebo. Co‐intervention: not described. Excluded medication: not described. | |
| Outcomes | Outcome: mortality, levels of plasma 1‐norepinephrine and epinephrine, haemodynamic data. Time points reported: 24, 48, and 72 hours. | |
| Notes | Email not found. The randomisation schedule was provided by Ayerst Laboratories. The study was supported by the contract "Clinical Investigation of Techniques to Protect Ischemic Myocardium and Minimize Infarct Size," N01‐62960, National Institute of Heart, Lung, and Blood. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described sufficiently, other than quote: "propranolol or placebo was administered randomly....' and ...'informed consent, indicating the randomized double‐blind character...'. 'The randomization schedule was provided by Ayerst Laboratories, New York." |
| Allocation concealment (selection bias) | Unclear risk | Not described sufficiently, other than quote: "propranolol or placebo was administered randomly....' and ...'informed consent, indicating the randomized double‐blind character...'. 'The randomization schedule was provided by Ayerst Laboratories, New York." |
| Blinding of participants and personnel (performance bias) | Unclear risk | The trial was describes as double‐blinded, however no detailed description of blinding was described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The trial was describes as double‐blinded, however no detailed description of blinding was described. |
| Incomplete outcome data (attrition bias) | Low risk | There were no dropouts during follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found and the trial did not report on all‐cause mortality or SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 10 sites in Scotland, England, and Ireland. Duration not mentioned. | |
| Participants | 195 participants with a history of AMI within the preceding 24 hours (later extended to 48 hours) were included in the trial. Male:female = 155:40. Mean age = 58 years. Exclusion criteria: the diagnostic criteria were not fulfilled; there was evidence of bronchospasm or a clinical history of bronchial asthma; the heart‐rate was less than 60 bpm persisting throughout a 24‐hour period; or the SBP was less than 80 mmHg after admission. | |
| Interventions | Experimental group: propranolol (20 mg orally dose 6‐hourly for 28 days). Control group: placebo. Co‐intervention: digitalis, quinidine, procainamide, diuretics, and pain‐relieving drugs were given when indicated. Excluded medication: not described. | |
| Outcomes | Outcomes: pulse‐rate (4‐hourly); temperature (twice daily); blood‐pressure (daily); Hb, erythrocyte‐sedimentation rate (within 24 hours of admission); serum‐enzymes (between 24 and 48 hours after admission); ECG (on admission, twice in first week, and thereafter at weekly intervals, more frequent records if necessary); and chest X‐ray (if thought necessary). Time points reported: 24 hours and 28 days. | |
| Notes | Email not found. The trial was coordinated by Dr. SA. Stephen from ICI Research Laboratories. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described sufficiently, other than quote: "Propranolol (’Inderal’) 20 mg. or an identical placebo was given 6‐hourly by mouth for 28 days and the study was double‐blind with random allocation of patients to the placebo or treated group.." |
| Allocation concealment (selection bias) | Unclear risk | Not described sufficiently, other than quote: "Propranolol (’Inderal’) 20 mg. or an identical placebo was given 6‐hourly by mouth for 28 days and the study was double‐blind with random allocation of patients to the placebo or treated group.." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not mentioned if any participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be found, and the trial did not report on all SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial in Denmark. | |
| Participants | 130 participants with definite or suspected MI were included. Male:female = 90:40. Mean age = not reported. Exclusion criteria: patients with complicated asthma. | |
| Interventions | Experimental group: propranolol (10 mg orally 4 times daily, or 5 mg IV. instead of 10 mg orally for 14 days). Control group: no intervention other than co‐intervention. Co‐intervention: digitalis, diuretics, metaradrine, and procainamide. Excluded medication: not described. | |
| Outcomes | Primary: rhythm complications and death. Time points reported: 28 days. | |
| Notes | Email not found. Supported by 'Foreningen til hjertesygdommenes bekæmpelse'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "Each of the 8 groups were consecutively numbered and corresponding to each number a sealed envelope, which stated whether the patient should be treated with pro‐propranolol or not." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "This system, which was developed by one of the department secretaries in the start of the study, ruled that the investigating personnel had no bearing on whether treatment should be given or not." However, the control group did not receive any matching placebo, which indicates that the study was not double‐blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be found, and the trial did not adequately report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 3 sites in New Zealand between March 1966 and March 1967. | |
| Participants | 536 participants who on clinical and ECG evidence were thought to have had a MI within the previous three days were included in the trial. Male:female = not described. Mean age = not described. Exclusion criteria: shock (BP below 90 mmHg), sinus bradycardia (HR below 50 bpm), heart failure, or acute pulmonary oedema, heart block. | |
| Interventions | Experimental group: propranolol (20 mg four times daily for 21 days). Control group: placebo. Co‐intervention: not described. Excluded medication: not described. | |
| Outcomes | Outcomes: mortality and morbidity. Time points reported: not reported. | |
| Notes | The author was contacted on 26 January 2017 on [email protected]. We defined the endpoint of cardiovascular death according to table V consisting of arrhythmia or sudden death, cardiogenic shock and heart failure. However we might have missed out on cardiac rupture as this was noted as a composite outcome with pulmonary embolism and other causes. Supported by Imperical Chemical Industries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described sufficiently, other than quote: "Patients admitted to the trial were allocated three weeks' supply of propranolol 20 mg. q.i.d. or placebo tablets according to a random code known only by the hospital pharmacy." |
| Blinding of participants and personnel (performance bias) | Low risk | The study was described as being double‐blinded, and the allocation was performed quote: "'according to a random code known only by the hospital pharmacy.." and since the control group received placebo, we assume that both the personnel and participants were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | 536 patients were admitted to the trial. Only 454 remained and received treatment, and 21 participants from the treatment group and 15 from the control group were removed from the study, however, these were included in the follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be found, and the trial did not adequately report on SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at two sites in New Zealand over a 15‐month period. | |
| Participants | 43 participants admitted to the coronary‐care unit at Green Lane Hospital or Middlemore Hospital within 4 hours of the onset of characteristic myocardial ischaemic pain lasting 30 minutes or more were included to the trial. Male:female = 32:11. Mean age = 52.5 years. Exclusion criteria: established unstable angina i.e. repeated attacks of pain over the last days or weeks so that the presenting attack could be considered as an extension of previous symptoms, contraindications to propranolol therapy (history of cardiac failure or bronchial asthma, HR less than 60 bpm), those who had taken a beta‐adrenoceptor‐blocking drug in the 72 hours before admission, and those in whom serum‐CK levels might have been elevated because of electrical defibrillation or multiple intramuscular injections. | |
| Interventions | Experimental group: propranolol (0.1 mg/kg intravenously over 10 minutes followed by a total of 320 mg propranolol orally over the next 27 hours). Control group: no intervention. Co‐intervention: not described. Excluded medication: not described. | |
| Outcomes | Correlation between enzyme measurements and changes in serial ECG'S. Time points reported: during hospital stay. | |
| Notes | The author was contacted on 26 Januart 2017 on [email protected]. No response received. The trial was supported by the Medical Research Council and the National Heart Foundation of New Zealand. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described other than quote: "Patients were allocated at random by the envelope method within each of these two groups for treatment with propranolol or no specific treatment.'' |
| Allocation concealment (selection bias) | Unclear risk | Envelope. However, not described as opaque. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | The trial did not describe if any participants were dropped out. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be found, and the trial did not sufficiently report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at two sites in New Zealand between March 1977 and March 1979. | |
| Participants | 62 participants < 65 years of age, admitted within 4 h of the onset of uncomplicated MI were included. Male:female = 58:4. Mean age = 51 years. Exclusion criteria: history of bronchial asthma, SBP above 100 mmHg, HR greater than 60 bpm, and without breathlessness or basal rales. Patients who had had DC cardioversion were excluded. | |
| Interventions | Experimental group: propranolol (0.1 mg/kg intravenously over 10 minutes followed by a total of 320 mg propranolol orally over the next 27 hours). Control group: no intervention other than co‐intervention. Co‐intervention: lignocaine was used as necessary in both treated and control patients for the control of ventricular arrhythmias and in a few cases intravenous or oral frusemide was given for left ventricular failure. Excluded medication: not described. | |
| Outcomes | Peak CK activity levels, changes in ECG's and clinical evaluation and exercise testing. Time points reported: during hospital stay and 1 months follow‐up on exercise testing. | |
| Notes | The author was contacted on 26 January 2017 on [email protected]. No response received. The trial was supported by the Medical Research Council and the National Heart Foundation of New Zealand. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as being randomised, however, no further description is given. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Immediately on entry to the trial patients were randomised by the envelope method for treatment with propranolol or no specific treatment." However, it was not mentioned if the envelope was opaque. |
| Blinding of participants and personnel (performance bias) | High risk | The control group did not receive any intervention beside the co‐intervention. Hence, both participants and personnel were aware of whom received the experimental intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | The trial did not describe if any participants dropped out. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be found, and the trial did not adequately report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 3 sites in New Zealand between May 1981 and March 1984. | |
| Participants | 735 participants under 70 years old who complained of chest pain, judged to be ischaemic, which had continued for more than 30 minutes with onset not more than 4 hours previously were included in the trial. Male:female = 588:147. Mean age = 54.5 years. Exclusion criteria: Contraindications such as: a history of asthma or bronchitis requiring bronchodilators, current treatment for cardiac failure, the presence of dyspnoea or widespread chest rales on examination, SBP below 110 mmHg, or HR below 60 bpm. | |
| Interventions | Experimental group: propranolol (5 mg for those weighing less than 55 kg, 6 mg for 55 to 65 kg, 7 mg for 65 to 75 kg, and 8 mg for >75 kg. Subsequent doses of propranolol 40 mg were given orally over the next 27 hours). Control group: no intervention other than the co‐intervention. Co‐intervention: morphine, diuretics, inotropic drugs, or lignocaine. Excluded medication: not described. | |
| Outcomes | Outcomes: mortality, major arrhythmias and conduction disturbances. Time points reported: 48 hours and 3 weeks. | |
| Notes | The author was contacted on 26 Januart 2017 on [email protected].. No response received. We defined the endpoint of cardiovascular death according to table III consisting of cardiogenic shock/failure, cardiac rupture, ventricular fibrillation, ventricular septal rupture, cardiac death (mechanism not clear) and post‐cardiac surgery. ICI Pharmaceuticals, UK, funded the medicine. Supported by the NZ Medical Research Council and the National Heart Foundation of New Zealand. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described as being randomised, however, no further information was given. |
| Allocation concealment (selection bias) | Low risk | Numbered envelopes. Quote: "The nurse in charge of the unit, after asking some standard questions to confirm the patient’s eligibility for the trial and opening a numbered envelope, advised the practitioner whether the patient was randomised to receive propranolol or no intervention." |
| Blinding of participants and personnel (performance bias) | High risk | The control group did not receive any intervention beside the co‐intervention. Hence, both participants and personnel were aware of whom received the experimental intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts described after the randomisation. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be found, and the trial did not adequately report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at 12 sites in Norway between December 1977 and July 1980. | |
| Participants | 560 high‐risk survivors, 35‐70 years of age, were included 4‐6 days after their AMI. Male:female = 476:84. Mean age = 58.4 years. Exclusion criteria: severe heart failure (cardiogenic shock or pulmonary oedema); persistent signs of heart failure; good‐risk patients; need for beta‐blockade; diabetes mellitus; AV block II‐III or SA block; hypotension; need for antiarrhythmics. | |
| Interventions | Experimental intervention: propranolol (160 mg/day, 40 mg four times daily for 12 months). Control intervention: placebo (identical tablets). Co‐intervention: none mentioned. | |
| Outcomes | Primary: sudden cardiac death; total death; fatal and nonfatal reinfarction; total number of cardiac events. Time point reported: 12 months follow‐up. | |
| Notes | The author was contacted on 26 January2017 on [email protected]. However, this email did not belong to Dr. Hansteen. No email found. The trial was supported by Bio‐Science Laboratories. Grants from the Norwegian Council for Cardiovascular Diseases and the National Centre for Medical Products Control. Imperial Chemical Industries Ltd, who provided the test tablets for the study, and ICI‐Pharma. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The two risk groups were randomised separately at each participating centre in balanced blocks of 10, using a double‐blind design. No further information was given. |
| Allocation concealment (selection bias) | Unclear risk | The two risk groups were randomised separately at each participating centre in balanced blocks of 10, using a double‐blind design. No further information was given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | All the tablets were identical and the trial is described as being double‐blinded. This indicates that the participants have been blinded, however there is no indication of whether the personnel have been blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts described. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained. The trial did not report all SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at a single site in Australia over a four‐year period. | |
| Participants | 100 participants who were admitted to the coronary care unit and suspected of having an evolving AMI, within 12 hours of onset, were considered for entry. Selection criteria included: 1) age under 72 years, previously healthy and active; 2) typical symptoms of evolving MI with onset of worst symptoms within 12 hours of trial entry; 3) ECG evidence of evolving infarction (ST elevation > 1 mm in at least one lead or evolving pathological Q waves in at least one lead serial tracings. Male:female = 80:20. Mean age = 54.9 years. Exclusion criteria: already receiving beta‐blockers, contraindications to beta blockade (history of asthma, bradycardia with HR < 60 bpm, any degree of AV‐block, or any clinical or radiographic evidence of cardiac failure). | |
| Interventions | The study divides the control and experimental group into those whose symptoms began four hours or less before trial entry or those whose symptoms began between four and 12 hours before entry. Experimental group: pindolol (initially 3 mg intravenously every 8 hours for three doses followed by 5 mg orally every 8 hours for six doses). Control group: no intervention other than the co‐intervention. Co‐intervention: routine use of oxygen‐enriched air for 24 hours and anticoagulation with IV heparin for five days. Narcotic analgesics, sedatives, nitrate preparations, diuretic, and antiarrhythmic agents. Excluded medication: not described. | |
| Outcomes | Outcomes: effect on SBP and HR, estimated infarct size, and incidence of in‐hospital complications. Time points reported: 72 hours. | |
| Notes | The author was contacted on 26 January 2017 on M.O'[email protected]. No response received. The funding was not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described as being a randomised controlled trial with quote: ”Random allocation“, ”Stratified according to timing of entrance (< 4 and > 4 hours)“. However, it was not mentioned how the participants were allocated. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope. However, not described as opaque. |
| Blinding of participants and personnel (performance bias) | High risk | The control group did not receive any intervention beside the co‐intervention. Hence, both participants and personnel were aware of whom received the experimental intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | The trial had no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial did not adequately report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at a single site in New Zealand. Duration not mentioned. | |
| Participants | 95 participants 1)who were seen within 12 hours of onset of typical prolonged chest pain, 2) who had ECG evidence of either epicardial injury (more than 2 mm of ST‐segment elevation in the anterior chest leads or more than 1 mm in leads II, III and aVF) or pathological Q waves, but 3) who had neither potential contraindications to propranolol therapy nor potential interferences with measurement of blood CPK levels were included in the trial. Male:female = 77:18. Mean age = 53.8 years. Exclusion criteria: patients over 65 years of age, interstitial oedema, pulmonary oedema, HR below 60 bpm, atrioventricular block of more severe than first‐degree block, ingestion of beta‐blocking drugs within the previous 72 hours, a history of asthma, or patients who had had DC shock for ventricular arrhythmias. | |
| Interventions | Experimental group: propranolol (0.1 mg/kg intravenously over 10 minutes followed by a total of 320 mg propranolol orally over the next 27 hours). Control group: no intervention other than the co‐intervention. Co‐intervention: furosemide, lidocaine. Excluded medication: not described. | |
| Outcomes | Serum creatine phosphokinase, death, reinfarction, cardiac failure. | |
| Notes | The author was contacted on 26 January 2017 on [email protected].. However, the email address did not exist. Supported by the Medical Research Council and the National Heart Foundation of New Zealand. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ”Patients were randomised“ no further details. |
| Allocation concealment (selection bias) | Unclear risk | Using an envelope. However, not described as opaque. |
| Blinding of participants and personnel (performance bias) | High risk | Not described, but since the control group did not receive any intervention, the study must have been open‐labelled. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | There were no dropouts in the trial. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found. The trial did not report on all SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial at a single site in Norway. | |
| Participants | 41 participants with definite AMI within 48 hours were included. Male:female = 33:8. Mean age = not reported (between 43 and 88 years). Exclusion criteria: cardiogenic shock, heart failure, and patients with contraindications to the drug. | |
| Interventions | Experimental group: propranolol (40 mg every 8 hours for three weeks). Control group: placebo. Co‐intervention: standard therapy (oxygen, analgetica, anticoagulants, digitalis, and quinidine sulphate) Excluded medication: not described. | |
| Outcomes | Primary: death. Time points reported: 3 weeks. | |
| Notes | Email not found. Funding was not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described sufficiently other than quote: "The patients were randomised according to a list of randomisation". |
| Allocation concealment (selection bias) | Unclear risk | Not described sufficiently other than quote: "The patients were randomised according to a list of randomisation". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described sufficiently other than quote: "the study was double‐blinded". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be found. The trial did not report all SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at two sites in the UK. Duration not mentioned. | |
| Participants | 18 participants with chest pain and ECG evidence of AMI with within 12 hours of onset of chest pain, no analgesics received within 1 hour of presentation, no treatment with beta‐blockers within the past 24 hours, SBP > 100 mm Hg, HR > 45 bpm, absence of clinical or radiologic features of LV failure (dyspnoea, loud triple rhythm, or pulmonary oedema on chest roentgenogram), absence of second‐or third‐degree AV block, and absence of severe airway obstruction were included. Male:female = 15:3. Mean age = 58.5 years. Exclusion criteria: patients with severe pain needing opiates, AV block, severe airway obstruction, clinical or radiologic features of LV failure, treatment with beta‐blockers within the past 24 hours. | |
| Interventions | Experimental group: atenolol (atenolol(0.5 mg/mL) given at 2 mL/minute over 5 minutes, followed by 50 mg atenolol orally 10 minutes later). Control group: placebo (10 mL 0.9% saline intravenously, given at 2 mL/minutes over 5 minutes, followed by 50 mg matching placebo orally 10 minutes later). Co‐intervention: opiates. Excluded medication: other beta‐blockers and verapamil were not permitted during the study. | |
| Outcomes | Primary: HR, BP, and subjective and objective measurements of pain. Time points reported: 60 minutes? | |
| Notes | Email not found. D. R. Ramsdale, M.R.C.P., Regional Cardiac Centre, Wythenshawe Hospital, Southmoor Rd., Manchester M23 9LT, England. No funding described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described as being randomised double‐blind trial, however, no further description was given. |
| Allocation concealment (selection bias) | Unclear risk | Not described other than quote: "18 AMI patients were randomly allocated immediately after admission to treatment.." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described, however the study is described as being 'double‐blinded'. |
| Blinding of outcome assessment (detection bias) | Low risk | 'Quote: "the physician administering the drug and recording the pain scores was unaware of the BP and HR responses which were recorded either by a nurse or by another physician." |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found. All‐cause mortality and SAEs were not reported. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, in Canada. Duration not mentioned. | |
| Participants | 94 participants with suspected AMI within 24 hours of the onset of symptoms were included in the study. The inclusion criteria required the presence of acute‐phase MI documented by a history strongly suggestive of AMI, including prolonged (at least 30 minutes) cardiac‐type chest pain and conventional 12‐lead ECG changes compatible with acute infarction, including diagnostic Q waves, ST depression or elevation or 0.1 mV or more, symmetrical T wave inversion. Male:female = 85:9. Mean age = 55.7 years. Exclusion criteria: enzyme‐negative patients; pregnancy; clinically significant conduction disturbances, including second‐degree AV block, complete AV block, and sinoatrial block; Killip class III or IV; pulse rate below 40 bpm; known congenital or significant valvular heart disease; known bronchospasm or clinically significant COPD; known past history of severe impairment of liver or renal function; patients currently receiving beta‐blocker medication, verapamil, digitalis, or other antiarrhythmic agents (patients receiving nifedipine were eligible for the study only if it could be safely discontinued, as judged by the attending physician at the time of entry into the study); patients in whom timolol was contraindicated as per the investigator's brochure; patients under the age of legal consent or over 75 years of age. | |
| Interventions | The trial had two phases; in phase 1, from day 1 to day 28, the study design was a parallel randomised trial placebo‐controlled trial. In phase 2, from day 29 to day 84, the trial was not placebo‐controlled. However, the trial investigated early versus late initiation of timolol therapy. We will therefore only focus and extract data from phase 1, as according to our protocol. Experimental intervention: intravenous timolol (initial 1.0 mg bolus injection followed by 1.5 mg doses at 10 minutes, 1 hour, and 2 hours. All injections were administered over a 2‐minute period. The oral dosage of 10 mg twice daily was initiated 2 hours after the last intravenous dose and was continued until day 28). Control intervention: placebo (administration as above). Co‐intervention: normal supportive measures such as oxygen, narcotic analgesics, stool softeners, benzodiazepines, and early in‐hospital treatment with diuretics or lidocaine or other non beta‐blocking antiarrhythmic medications. Nifedepine and digitalis, only if specifically required. Excluded medication: verapamil and diltiazem. Other antiarrhythmic drugs after the in‐hospital stage. | |
| Outcomes | Outcomes: mean and peak hourly ventricular premature complex rates ventricular premature complex couplets, or runs. Frequency distribution of QRS duration. Adverse effects. | |
| Notes | The authors were contacted on 26 January 20177 on [email protected] and [email protected]. No response received. The trial does not present the result from the different phases separately in regard to mortality etc.However, we have only used data from within 48 hours to be sure, that we only include data from the first phase of the study. The trial was supported in part by the Nova Scotia Heart Foundation, by Medical Research Council Program Grant PG‐30 (Dr. Rautaharju), and by Merck Frosst Canada, Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described sufficiently other than quote: "according to the study protocol; these patients were randomly assigned to either the group receiving timolol maleate (early‐timolol group) or the group receiving placebo." |
| Allocation concealment (selection bias) | Unclear risk | Not described sufficiently other than quote: "according to the study protocol; these patients were randomly assigned to either the group receiving timolol maleate (early‐timolol group) or the group receiving placebo." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described other than that the study was described as being double‐blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | 2 participants were lost to follow‐up in the placebo group (4.08%). |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report SAEs. |
| Other bias | Unclear risk | This was a cross‐over/ double‐phase study where the placebo group later received the active drug and the results were not presented separately from these two phases. |
| Methods | Randomised clinical trial in Italy. | |
| Participants | 250 participants, 75 years or less, admitted to the coronary care units less than 24 hours from the onset of symptoms, typical chest pain lasting more than 30 minutes not relieved by sublingual nitrates, plasma CK rise to at least twice the upper limit of local normal values, ST‐segment elevation > 0.2 mV in V1‐V4 and 0.1 mV or more in V5‐V6, I, II, III, aVL, aVF in at least two contiguous leads. The presence of at least two of the three criteria was considered necessary. Male:female = 203:47. Mean age = 58 years. Exclusion criteria: previous MI, Killip class III or more, severe hypertension (>180 mmHg in systole, >120 mmHg in diastole, irrespective of pharmacological therapy), hypotension (<100 mmHg in systole) lasting more than 3 hours during the observation period, hypovolaemia, cardiomyopathics and severe valvular heart disease, diabetes uncontrolled by therapy, preexisting or new left bundle branch block, all contraindications to ACE‐inhibition or to beta‐blocker treatment, based on physician's decision, presence of a concomitant life‐threatening disease, and inadequate thoracic acoustic window. | |
| Interventions | The patients were randomly divided into three study groups 1) captopril, 2) metoprolol and 3) captopril + metoprolol. We only used data from study group 1 and 3, where we classified captopril as being a co‐intervention given to both groups so that the experimental group only received metoprolol. Experimental group: metoprolol (orally, titrated up to 200 mg for at least 3 months). Control group: no intervention other than the co‐intervention. Co‐intervention: captopril up titrated to 75 mg. Excluded medication: all cardioactive drugs were allowed in the study period, with the exclusion of beta blockers. | |
| Outcomes | Outcomes: cardiac death and non‐fatal MI; cardiac death, non‐fatal MI, unstable angina requiring hospitalisation, and congestive heart failure (transitory increase of at least two NYHA classes, or stable increase of at least one NYHA class). Time points: 15 days, 3, and 6 months. | |
| Notes | Dr. Claudia Coletta. No email found. The study initially included 250 patients but did not report how many patients there were in each group. Later, there were only data on 236 patients. It is, therefore, unclear how many patients has been lost in each group. The funding/support of the trial was not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described other than quote: "250 consecutive patients were randomly allocated to receive an open‐labelled treatment..." |
| Allocation concealment (selection bias) | Unclear risk | Not described other than quote: "250 consecutive patients were randomly allocated to receive an open‐labelled treatment..." |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) | High risk | 24 participants were lost to follow‐up (9.6%). It is, however, not described to which group they belonged. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained. The trial did not report all SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised trial in Italy. | |
| Participants | 42 patients with AMI within 6 hours were included. Male:female = 39:3. Mean age = 52 years. Exclusion criteria: other cardiac diseases (hypertensive heart disease, valvular heart disease, cardiopathy). No patient had suffered from previous MI. | |
| Interventions | Experimental intervention: propranolol (2 mg IV bolus followed by 0.1 mg/kg/day for the next 48 hours). Control intervention: no intervention. Co‐intervention: not described. | |
| Outcomes | Outcomes: cumulated activity, peak plasma value, rate of release and total duration of release of MB‐CK. Time points reported: 72 hours. | |
| Notes | Email not found. Prof, GIUSEPPE BOTTI Divisione di Cardiologia Ospedale Maggiore Via Gramsci, 14. Funding was not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described sufficiently, other than that they were quote: "randomly assigned... |
| Allocation concealment (selection bias) | Unclear risk | Not described sufficiently, other than that they were quote: "randomly assigned..." |
| Blinding of participants and personnel (performance bias) | High risk | Not described. However, the control group did not receive any intervention, hence it could not be blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropout described. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be found. The trial did not report all‐cause mortality and all SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at one site in Northern Ireland between June 1979 and January 1981. | |
| Participants | 800 participants with suspected AMI. Male:female = 572:228. Mean age = not described. Exclusion criteria: delay from onset of pain exceeded 6 hours; initial rhythm ventricular fibrillation; initial rhythm agonal; SBP less than 90 mmHg associated with HR greater than 100 bpm; clinical pulmonary oedema or congestive heart failure; sinus or junctional bradycardia (60 bpm) with SBP less than 90 mmHg and not responding to elevation of the patient's legs; if the patient had received a beta‐adrenergic blocking drug or a type I anti‐arrhythmic drug in the previous 48 hours; AVheart block greater than first degree. | |
| Interventions | Experimental intervention: metoprolol (15 mg IV over 5 minutes followed by oral administration 50 mg every 6 hours for 48 hours and then 100 mg every 12 hours for one year). Control intervention: matching placebo injection and tablets. Co‐intervention: none mentioned. | |
| Outcomes | Outcomes: total mortality, cardiac mortality and sudden death, (within 1 hour of symptoms), ventricular fibrillation, adverse reactions. Time points reported: in hospital, at 3 months, and 12 months. | |
| Notes | Email not found. Astra Pharmaceuticals supplied drug and placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described sufficiently other than quote: "Patients were randomly allocated to treatment in two separate blocks, one for administration by the Mobile Coronary Care team and the second for other admissions." |
| Allocation concealment (selection bias) | Unclear risk | Not described sufficiently other than quote: "Patients were randomly allocated to treatment in two separate blocks, one for administration by the Mobile Coronary Care team and the second for other admissions." |
| Blinding of participants and personnel (performance bias) | Unclear risk | All the tablets were identical and the trial is described as being double‐blinded. This indicates that the participants have been blinded, however there is no indication of whether the personnel have been blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | Data from 1 patient in the metoprolol group were missing at three‐month follow‐up. Data from 3 patients in the metoprolol group were missing at one‐year follow‐up. No patient was missing in the placebo group at three‐month follow‐up. Data from 1 patient were missing in the placebo group at 1‐year follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Controlled clinical trial, parallel design, at a single site in Japan. Duration not mentioned. | |
| Participants | 69 participants with AMI who underwent emergency primary percutaneous coronary intervention (PCI) for an infarct‐related artery (IRA) were recruited within 12 hours of pain onset were included. Male:female = 55:14. Mean age = 62 years. Exclusion criteria: atenolol allergy, bradycardia (HR 50 bpm), advanced heart block, Forrester subset ≥2 by right heart catheterisation including cardiogenic shock, chronic obstructive lung disease, or arteriosclerosis obliterans. | |
| Interventions | Experimental group: atenolol (50 mg tablet once daily after successful PCI). Control group: no intervention other than the co‐intervention. Co‐intervention: PCI. Excluded medication: calcium antagonists were not allowed in either group. | |
| Outcomes | Primary: incidence of coronary vasospasm by ergonovine provocation test, defined as N90% constriction. Secondary: death, any AMI, target vessel revascularisation, HF, anginal attack, stroke, and ventricular arrhythmia. Time points reported: 1 month. | |
| Notes | Email not found. D. R. Ramsdale, M.R.C.P., Regional Cardiac Centre, Wythenshawe Hospital, Southmoor Rd., Manchester M23 9LT, England. The funding or support of the trial was not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The patients were randomised alternatively (simple randomisation). It was not mentioned if this was done by a independent adjudicator. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eligible patients were assigned alternatively.." No further information was provided. |
| Blinding of participants and personnel (performance bias) | High risk | Not described, however, since the control group did not receive any matching placebo other than the co‐intervention (PCI), we assume that the trial has not been blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Qualitative and quantitative assessments were performed by two independent cardiologists unaware of treatment assignment. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not described if any participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report all SAEs. |
| Other bias | Unclear risk | It is mentioned that the trial might not haven been strictly randomised which can therefore affect the validity of the trial results. |
| Methods | Randomised clinical trial at a single site in Russia. | |
| Participants | 40 participants with definite or suspected AMI within 24 hours and Killip class I‐II were included. Male:female = 22:18. Mean age = 60 years. Exclusion criteria: bradycardia < 50 per minute, AV‐block II or III‐grade, congestive heart failure. | |
| Interventions | Experimental group: esmolol (25 mcg/kg/min given IV (average 37 mcg/kg/min) and up titrated for 30 minutes followed by metoprolol orally for 1 month). Control group: no intervention other than co‐intervention. Co‐intervention: metoprolol every 3‐4 hours (for the experimental group this was started after the initial treatment with esmolol), standard therapy (narcotics analgesics, nitrates,thrombolytics, anticoagulator, antiaggregant). Excluded medication: not described. | |
| Outcomes | Primary: haemodynamics, post‐infarction angina, arrhthymias, progressive congestive heart failure, and death. Time points reported: 30 days. | |
| Notes | Email not found. Funding not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described other than that quote: "The patients were randomly assigned.." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not mentioned if any participant was lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report on all SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial at an unknown place. | |
| Participants | 97 patients with a suspected AMI were included. Male:female = not reported. Mean age = not reported. Exclusion criteria: age > 75, previous MI, SBP < 100, HR < 50 bpm, block, COPD, LVF, high risk, beta‐blocker use. | |
| Interventions | Experimental group: practolol (100 mg/2 hours for 5 days). Control group: placebo. Co‐intervention: not reported. Excluded medication: not reported. | |
| Outcomes | Primary: infarct size. Time points reported: 5 days. | |
| Notes | Email not found. The study could not be found, so we used the data reported in the meta‐analysis ''Yusuf 1985'', who were able to get unpublished data by personal communication with the author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not mentioned if any participant was lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report on all SAEs. |
| Other bias | Unclear risk | Not able to assess if any other bias exist, as the main manuscript could not be obtained. |
| Methods | Randomised clinical trial, parallel design, at 11 sites in Argentina between November 1982 and March 1985. | |
| Participants | 200 participants with AMI of less than 6 hours of evolution based on the following criteria: 1) history of pain that strongly suggested AMI with a duration of 30 minutes or more and an onset of less than 6 hours previously; 2) elevation of serum enzymes during the 24 hours after onset of chest pain (total CK activity of more than 100 IU/L and plasma CK‐MB activity of more than 10 IU/L, corresponding to at least 6% of the CK) were included. Male:female = 175:25. Mean age = 52.5 years. Exclusion criteria: later than 6 hours of onset; drug treatment at entry (beta blockers, amiodarone, calcium‐channel blockers, or digitalis); left ventricular failure; insulin‐dependent diabetes; bradycardia; hypotension; bronchospasm; severe concomitant disease; non‐ischaemic heart disease; intermittent claudication; previous cardiac surgery. | |
| Interventions | Experimental intervention: timolol (5.5 mg in total given intravenously followed by 10 mg orally twice daily for 1 month). Control intervention: matched placebo. Co‐intervention: none mentioned. | |
| Outcomes | Outcomes: infarct size; ventricular tachycardia; mortality. Time point reported: 2 years maximal follow‐up. | |
| Notes | Email not found. Supported by a grant from Merck Sharp and Dohme Argentina. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described other than quote: "patients were assigned randomly either to...." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | All the tablets were identical and the trial is described as being double‐blinded. This indicates that the participants have been blinded, however there is no indication of whether the personnel have been blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts were described. However treatment was discontinued in 17 patients from the placebo group and 13 patients in the timolol group but according to the trialist these were considered in the final analysis according to the intention‐to‐treat approach. It was unknown if they had data on these participants after their withdrawal. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at a single site in Australia. Duration not mentioned. | |
| Participants | 88 participants, presented within 24 hours of onset of symptoms which were thought to be referable to a first myocardial infarction, and subsequently confirmed by accepted enzyme and electrocardiographic changes were included, Male:female = 72:16. Mean age = not described. Exclusion criteria: already receiving beta blocking agents, or with the following contraindications to these agents: sinus bradycardia, less than 45 bpm; SBP, lower than 13.3 kPa (100 mmHg); P‐R interval, greater than 0.22 s; second‐ or third‐degree AV block; moderate or severe heart failure; and obstructive airways disease. Patients were withdrawn from study if they subsequently developed any of these contraindications | |
| Interventions | Experimental intervention: timolol (10 mg orally twice daily for 7 days). Control intervention: placebo (one tablet twice a day for 7 days). Co‐intervention: routine way, e.g. antiarrhythmic drug and anti failure therapy. | |
| Outcomes | Outcomes: all‐cause mortality; infarct size; incidence of arrhythmias; significant LV failure Time point reported: follow‐up at three and 12 months. | |
| Notes | The author was contacted on 26 January 2017 on [email protected]. No response received. The trial was supported by Merck, Sharp and Dohme Laboratories. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described other than quote: "...were administered orally in a randomized double‐blind manner..." |
| Allocation concealment (selection bias) | Unclear risk | Not described other than quote: "...were administered orally in a randomized double‐blind manner..." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described sufficiently other than the study was described as being double‐blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts described. However, 36 patients were withdrawn from the study and whether these are included in the final analysis or not is unclear. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, multi‐arm, parallel design, at 20 sites in Belgium between June 1988 and December 1990. | |
| Participants | 292 patients with AMI of less or equal to 5 hours of duration were included if they met the following inclusion criteria: 1) less than 71 years of age; 2) chest pain suggestive of AMI lasting more than or equal to 30 minutes; 3) ST segment elevation of 0.2 mV in two or more limb leads or leads Vs and V6 or 0.3 mV in two or more precordial leads (V1 to V4 ) or ST elevation of 0.1 mV in two leads (II, III, aVF or Vs and V6) associated with ST depression of 0.2 mV in two precordial leads, suggesting posterior wall infarction; 4) onset of chest pain < 5 hours before initiation of therapy; 5) no previous history of angioplasty or bypass surgery; 6) no contraindications to thrombolytic therapy; 7) no intake of a beta‐blocker or calcium antagonist in the week preceding the onset of infarction; and 8) no contraindications for acute intravenous administration of beta‐blockers. Male:female = 246:46. Mean age = 58 years. Exclusion criteria: HR persistently <50 bpm, SBP persistently < 90 mmHg, clinical signs of overt heart failure or cardiogenic shock, second‐ or third‐degree AV block and history of bronchospasm or sick sinus syndrome. | |
| Interventions | Three‐armed intervention with two experimental interventions. Experimental intervention: atenolol (5 mg to 10 mg intravenously followed by oral treatment of 25 mg to 50 mg every 12 hours for 10‐14 days). Control intervention: placebo (one or two IV injections) Co‐intervention: all patients received 100 mg alteplase over 3 hours and full IV heparinisation (5000 IU bolus injection followed by continuous infusion of 1000 IU/hour until angiography). Excluded medication: aspirin. | |
| Outcomes | Primary: global and regional LVF and infarct size. Secondary: exercise capacity; incidence of arrhythmias, and coronary artery patency. Time point reported: 14th day. | |
| Notes | The author was contacted on 26 January 2017 on [email protected]. No response received. The study was supported by the Belgium National Fund for Scientific Research, ICI Pharma, Belgium and Boehringer Ingelherim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described sufficiently, however, it is said that quote: "with the use of a double‐dummy technique, eligible patients were randomised..". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described sufficiently, other than the study is described as being double‐blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not mentioned if any participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at two sites one in Germany and one in Switzerland. Duration not mentioned. | |
| Participants | 51 participants (at 38 to 83 years of age) and with acute suspected MI within 24 hours were included. Male:female = 45:6. Mean age = 59.5 years. Exclusion criteria: high‐grade AV‐blockages, SA‐blockages, QRS broadening of 100 ms and (beta‐block intake during the last 24 hours before clinical admission.) | |
| Interventions | Experimental group: metoprolol (0.1 mg/kg given intravenously for 20 minutes followed by 100 mg orally twice daily for 14 days). Control group: placebo. Co‐intervention: not described. Excluded medication: other beta‐blockers and verapamil were not permitted during the study. | |
| Outcomes | Primary: infarct extension; reinfarctions, infarction size, Time points reported: maximum follow‐up of 14 days. | |
| Notes | Email not found. Supported by Ciba‐Geigy, a former pharmaceutical company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients allocated according to a randomisation list“, however, it was not mentioned how the randomisation list was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described, however, the study was described as being quote: "double‐blinded". |
| Blinding of outcome assessment (detection bias) | Low risk | The ECGs were evaluated centrally without knowledge of the clinical data by an independent investigator using a semi‐automatic evaluation system. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not mentioned if any participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found, and the trial did not adequately report on SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, multi‐arm, parallel design, in Sweden. Duration not mentioned. | |
| Participants | 43 patients with clearcut ECG evidence of AMI who met the following inclusion criteria: still suffering from chest pain of moderate to severe degree; no treatment with beta‐blockers within the last 24 hours; no clinical signs of left ventricular backward failure such as bilateral lung rales and/or severe dyspnoea; SBP above 100 mmHg; no signs of poor peripheral circulation with coldness and pallor regardless of BP; HR more than 45 bpm; no AV‐block I, II or III were included in the trial. Male:female = not mentioned. Mean age = 62 years. Exclusion criteria: none mentioned. | |
| Interventions | A 4‐armed trial with 3 different beta‐blockers. Experimental intervention 1: practolol (initial dose of 5 mL (total of 15 mL) intravenously). Experimental intervention 2: H 87/07 (initial dose of 5 mL (total of 15 mL) intravenously). Experimental intervention 3: metoprolol (initial dose of 5 mL (total of 15 mL) intravenously). Control intervention: placebo (15 mL saline). Co‐intervention: Patients with more than 5 multifocal ventricular extra beats (VEB) per min and ventricular tachycardia (VT) more than 3 beats were given lidocaine 50‐100 mg intravenously as a bolus and 2‐4 mg/minute as infusion. | |
| Outcomes | Outcomes: chest pain; the product of HR and SBP; left ventricular backward failure; shock; bradycardia. | |
| Notes | The author was contacted on 26 January 2017 on [email protected]. No response received. Supported by grants from Swedish Medical Research Council, the Swedish National Association against Heart and Chest Disease, and ICI‐Pharma AB. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described sufficiently other than the study was described as being double‐blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report all‐cause mortality or SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, multi‐arm, parallel design, at two sites in the UK. Duration not mentioned. | |
| Participants | 388 participants with a clinical diagnosis of suspected MI within the past 24 hours were included in the trial. Male:female = 328:60. Mean age = not mentioned. Exclusion criteria: already taking a beta‐blocker; severe heart failure (as defined by breathlessness, elevated jugular venous pressure, and basal crepitations), sinus bradycardia of under 40 bpm, second‐ or third‐degree heart block; SBP of under 90 mmHg, a history of asthma or diabetes mellitus, not a resident of Nottingham, in another study. | |
| Interventions | Three‐armed intervention with two experimental interventions. Experimental intervention 1: propranolol (40 mg three times daily for 6 weeks followed by only twice daily (80 mg per day) for 12 months). Experimental intervention 2: atenolol (50 mg twice daily plus midday placebo for six weeks followed by only once daily (50 mg/day) and one placebo Control intervention: matching placebo (three times daily for 6 weeks followed by only twice daily). Co‐intervention: none mentioned. | |
| Outcomes | Outcomes: all‐cause mortality at six weeks and one year; cardiac enzyme activities; pulse rate; BP. Time point reported: 12 months. | |
| Notes | Email not found. This study was made possible by a generous grant from Imperial Chemical Industries Limited. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described other than the study is described as being double‐blinded and randomised. |
| Allocation concealment (selection bias) | Unclear risk | Not described other than the study is described as being double‐blinded and randomised. |
| Blinding of participants and personnel (performance bias) | Unclear risk | All the tablets were identical and the trial is described as being double‐blinded. This indicates that the participants have been blinded, however there is no indication of whether the personnel have been blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not mentioned if any participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at a single site in Belgium. Duration not mentioned. | |
| Participants | 32 participants with definite clinical ECG and enzymatic signs of a first transmural AMI, admitted to a coronary care unit 48‐72 hours after onset of AMI and thereafter to a post‐coronary care area, were included. Male:female = 26:6. Mean age = 58.9 years. Exclusion criteria: sinus rhythm slower than 55 bpm, SBP lower than 90 mmHg, AV conduction disturbances of the 2nd or 3rd degree, a history of bronchospasm, treatment with beta‐blocking agents before or after admission, definite heart failure or cardiogenic shock during the first 48 to 72 hours. | |
| Interventions | Experimental group: betaxolol (20 mg once daily for 12 days). Control group: placebo. Co‐intervention: not described. Excluded medication: not described. | |
| Outcomes | Outcomes: effects on HR, ventricular and supraventricular arrhythmias. Time points reported: 12th day. | |
| Notes | Email not found. The funding was not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described sufficiently other than quote: "patients were randomized in a double‐blind fashion in a placebo and betaxolol group..." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Identical tablets without active drug were administered at the same time and for the same duration to the placebo group", this indicates that the participants have been blinded, however there is no indication of whether the personnel have been blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not mentioned if any participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found. The trial did not report on all‐cause mortality or all SAEs. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at two sites in the UK between October 1978 and August 1981. | |
| Participants | 477 participants with a clinical history strongly suggestive of MI within the previous 12 hours. Male:female = 404:73. Mean age = 56 years. Exclusion criteria: HR less than 40 bpm; SBP less than 90 mmHg; or heart failure requiring digoxin or more than 80 mg of frusemide; history of asthma or bronchospasm; 2nd or 3rd degree heart block Patients already on a beta‐blocker at entry or requiring immediate beta‐blockade were also excluded. | |
| Interventions | Experimental intervention: atenolol (5 mg intravenously followed by an oral dose of 50 mg and 12 hours later, with 100 mg once daily thereafter for 10 days). Control intervention: no intervention other than the co‐intervention (were allowed beta blockade if it was clearly indicated). Co‐intervention: routine coronary care. | |
| Outcomes | Outcomes: infarct size (cumulative enzyme release and ECG changes); ventricular arrhythmias; mortality; morbidity. Time point reported: 10 days and 24 months. | |
| Notes | The authors were contacted on 26 January 2017 on [email protected]. The author replied back on 26 January 2017. Unfortunately Dr. Yusuf did not have any of the materials from the study but answered our questions out of his memory. This study was supported by the British Heart Foundation and I.C.I. Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not described in the study, but according to the author quote: " The randomisation was a central computer generated system with opaque envelopes." |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed envelopes were used, and according to the author it was quote: "opaque envelopes". |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This study was deliberately open in design, as our preliminary experience using placebo suggested that the decrease in heart rate and systolic blood pressure after i.v. atenolol was so marked that "true blindness" would not be achieved by placebo injections or tablets in the majority of patients. To avoid bias, we analysed the data "blind" whenever possible, and the investigators were not directly involved in the management of the majority of patients" |
| Blinding of outcome assessment (detection bias) | Low risk | Not described in the study, but according to the author quote: "The main endpoints were various measures of infarct size that was assessed using ECG and enzymes which were analysed without knowledge of the allocation. Clinical events were abstracted from the charts." |
| Incomplete outcome data (attrition bias) | Low risk | One atenolol patient and two control patients had missing data on the outcomes on clinical course and morbidity. All data were available for mortality. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report SAEs. However, according to the author they did have a protocol before the study was started, which he is no longer in the possession of. |
| Other bias | Low risk | No other biases were found. |
| Methods | Randomised clinical trial, parallel design, at a single site in Sweden. Duration not mentioned. | |
| Participants | 20 participants with AMI with onset of symptoms within 24 hours and ventricular arrhythmias (> 5 ventricular ectopic beats (VEB) min‐1, paired VEBs, R‐on‐T VEBs and short runs of ventricular tachycardia), were included. Male:female = not described. Mean age = 60.5 years. Exclusion criteria: over 74 years of age; physical signs of more than slight cardiac decompensation (basal rales ≤ 10 cm; atrial fibrillation or flutter; complete bundle branch block or AV‐block II or IIII; SBP < 110 mmHg; HR below 65 bpm; history of bronchial asthma or chronic obstructive pulmonary disease; treatment with a calcium blocker or an antiarrhythmic drug during the last 24 hours and treatment with a beta‐blocking agent or digitalis during the last month; renal failure; significant demand for treatment with beta‐blocking agents, calcium blockers and anti‐arrhythmic drugs. | |
| Interventions | Experimental intervention: sotalol (Intravenously using a continuous infusion, first a bolus infusion of 0.5 mg/kg for 15 minutes followed by a loading infusion of 0.6 mg/kg for 1 hour and then a maintenance infusion of 0.2 mg/kg per hour up to 12 hours). Control intervention: placebo (saline for 12 hours). Co‐intervention: 2‐5 mL of lidocaine, 10 mg/mL. | |
| Outcomes | Outcomes: haemodynamic effects of sotalol; sinus cycle length; sinus node recovery time; AV nodal effective refractory period; myocardial repolarization; atrial effective refractory period; QT interval. | |
| Notes | Email not found. The study was supported by Bristol Myers International Corporation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described sufficiently other than quote: "the patients were randomised for double‐blind treatment with sotalol or placebo (saline)". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described sufficiently, however the study is described as being quote: "double‐blind treatment with sotalol or placebo (saline)". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not mentioned if any participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report all‐cause mortality or SAEs. |
| Other bias | Low risk | No other biases were found. |
ACE: angiotensin‐converting enzyme;AMI: acute myocardial infarction; AUC: area under the curve;AV: atrioventricular;aVL: lead V;BP: blood pressure; bpm: beats per minute;CK: creatine kinase; CK‐MB: myocardial band isoenzyme of creatine kinase; CMR: cardiac magnetic resonance;COPD: chronic obstructive pulmonary disease; CPK: creatine phosphokinase; DC: direct current;ECG: electrocardiogram;EQ5D: EuroQoL Group Quality of Life Questionnaire based on five dimensions; GPX: glutathione peroxidase; Hb: haemoglobin; HR: heart rate; ICD: implantable cardioverter defibrillator; IRA: infarct‐related artery IV: intravenous;LV: left ventricular; LVF: left ventricle function;MACE: major adverse cardiac event; MDA: malondialdehyde; MI: myocardial infarction; MILIS: Multicenter Investigation of the Limitation of Infarct Size;MRI: magnetic resonance imaging; NT‐proBNP: n‐terminal brain natriuretic peptide; NYHA: New York Heart Association; PCI: percutaneous coronary intervention;PCWP: pulmonary capillary wedge pressure;SAE: serious adverse event(s); SBP: systolic blood pressure; SOD: superoxide dismutase; STEMI: ST‐elevation myocardial infarction;TIMI: thrombolysis in myocardial infarction;VEB: ventricular extra beat.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomised controlled trial. | |
| The trial was not randomised. | |
| Did not include patients in the acute/subacute phase of a myocardial infarction. | |
| The trial had no placebo/control group. | |
| It is unclear whether or not they were randomised to metoprolol in addition to captopril and how this intervention was given. | |
| Not a randomised controlled trial. | |
| The trial was not randomised. | |
| The patients were not randomised. | |
| The trial has no placebo/control group. | |
| The trial did not include participants with suspected of or diagnosed with an acute myocardial infarction. | |
| No indication of the trial being randomised. | |
| The trial had no placebo/control group. | |
| The trial is quasi‐randomised. | |
| The trial had no placebo/control group. | |
| The trial had no placebo/control group. | |
| Did not include patients with acute or recent myocardial infarction. | |
| Included patients with ischaemic heart diseases and not only patients with acute myocardial infarction. | |
| A multicentre prospective cohort study and not a randomised clinical trial. | |
| Did not include patients with acute or recent myocardial infarction. | |
| The control group did not receive either placebo or no intervention. | |
| The trial was not randomised. | |
| The trial was not randomised. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | BEtablocker Treatment after Acute Myocardial Infarction in patients without reduced left ventricular systolic function (BETAMI) |
| Methods | Randomised open‐label clinical trial. |
| Participants | Patients older than 18 diagnosed with an acute myocardial infarction and treated with PCI or thrombolysis during current hospitalisation within the past 1‐8 days. |
| Interventions | Control group: no betablocker will be administered. Any other treatment or management is to be given as per usual care. Experimental group: metoprolol succinate up to a total dose of 200 mg daily. Bisoprolol up to a total dose of 10 mg daily. Carvedilol up to a total dose of 50 mg daily. Any other treatment or management is to be given as per usual care. |
| Outcomes | Primary outcome: the composite of death of any cause and non‐fatal MI. Secondary outcomes:
|
| Starting date | 23 August, 2018 |
| Contact information | John Munkhaugen, MD PhD, [email protected] Vidar Ruddox, MD PhD, [email protected] |
| Notes | Estimated Study Completion: 1 October, 2023. ClinicalTrials.gov Identifier: NCT03646357. |
| Trial name or title | Effect of intravenous metoprolol combining RIPC on myocardial protection in anterior ST‐segment elevation myocardial infarction patients undergoing primary PCI. |
| Methods | A prospective, multicentre, randomised, open‐label trial. |
| Participants | Patients ages 18 to 80 years, presenting within 6 hours of symptoms onset, with anterior STEMI, de novo occlusion of LAD (TIMI flow grade 0 to 1), and planned pPCI were eligible. Anterior STEMI was defined as the occurrence of > 20 minutes of chest pain and ST‐segment elevation (> 2 mm) in at least 2 contiguous precordial leads. |
| Interventions |
|
| Outcomes | Primary outcome: MI size. Secondary outcomes:
|
| Starting date | 17 May, 2018. |
| Contact information | Yu Bo, Harbin Medical University. No email found. |
| Notes | Estimated Study Completion: 31 December, 2020. ClinicalTrials.gov Identifier: NCT03579914 |
| Trial name or title | Evaluation of decreased usage of betablockers after myocardial infarction in the SWEDEHEART Registry (REDUCE‐SWEDEHEART) |
| Methods | A registry‐based, randomised, parallel, open‐label, multicentre trial. |
| Participants | Patients > 18 years with MI within 1‐7 days and preserved left ventricular systolic ejection fraction who have undergone coronary angiography during hospitalisation. |
| Interventions | Experimental: oral beta‐blocker treatment (metoprolol succinate, bisoprolol). No Intervention: no beta‐blocker treatment. |
| Outcomes | Primary outcome: composite of death of any cause or MI Secondary outcomes:
|
| Starting date | 11 September, 2017 |
| Contact information | Eva Jacobsson, PhD, [email protected] Troels Yndigegn, MD, |
| Notes | Estimated Primary Completion Date 31 August, 2020. NCT number: NCT03278509 |
COPD: chronic obstructive pulmonary disease; LAD: left anterior descending ; MACCE: major adverse cardiovascular and cerebrovascular event; MI: myocardial infarction; MRI: magnetic resonance imaging; PCI: percutaneous coronary intervention; pPCI: primary percutaneous coronary intervention; STEMI: ST‐elevation myocardial infarction; TIMI: thrombolysis in myocardial infarction.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| Analysis 1.1  Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 All‐cause mortality. | ||||
| 2 All‐cause mortality ‐ Acute/subacute phase Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| Analysis 1.2  Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 2 All‐cause mortality ‐ Acute/subacute phase. | ||||
| 2.1 Acute phase | 42 | 76857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.00] |
| 2.2 Subacute phase | 4 | 3595 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.50, 1.25] |
| 3 All‐cause mortality ‐ Reperfusion/no reperfusion Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| Analysis 1.3  Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 3 All‐cause mortality ‐ Reperfusion/no reperfusion. | ||||
| 3.1 No reperfusion | 40 | 78206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 6 | 2246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.43, 1.50] |
| 4 All‐cause mortality ‐ Type of beta‐blocker Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| Analysis 1.4  Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 4 All‐cause mortality ‐ Type of beta‐blocker. | ||||
| 4.1 Alprenolol | 2 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.77, 1.66] |
| 4.2 Atenolol | 4 | 16890 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.74, 0.94] |
| 4.3 Esmolol | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.21, 3.62] |
| 4.4 Labetolol | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.60, 41.88] |
| 4.5 Metoprolol | 8 | 55034 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.03] |
| 4.6 Oxprenolol | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.47, 4.03] |
| 4.7 Pindolol | 2 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.49, 2.08] |
| 4.8 Practolol | 3 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.65, 1.60] |
| 4.9 Propranolol | 14 | 2630 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.18] |
| 4.10 Timolol | 7 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.33, 1.31] |
| 4.11 Carvedilol | 2 | 2753 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.25, 1.04] |
| 4.12 Mixed | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.18, 20.63] |
| 5 All‐cause mortality ‐ Intravenously/orally commenced Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| Analysis 1.5  Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 5 All‐cause mortality ‐ Intravenously/orally commenced. | ||||
| 5.1 Intravenously commenced | 4 | 1036 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.46, 2.04] |
| 5.2 Orally commenced | 16 | 5658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.10] |
| 5.3 Mixed | 26 | 73758 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.00] |
| 6 All‐cause mortality ‐ Above/below 75 years of age Show forest plot | 39 | 79161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| Analysis 1.6  Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 6 All‐cause mortality ‐ Above/below 75 years of age. | ||||
| 6.1 Below 75 years | 24 | 12602 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.74, 0.99] |
| 6.2 Mixed | 15 | 66559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.90, 1.01] |
| 7 All‐cause mortality ‐ NSTEMI/STEMI/UAP Show forest plot | 24 | 53337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.92, 1.03] |
| Analysis 1.7  Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 7 All‐cause mortality ‐ NSTEMI/STEMI/UAP. | ||||
| 7.1 STEMI | 5 | 1828 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.48, 2.10] |
| 7.2 Mixed | 19 | 51509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.92, 1.03] |
| 8 All‐cause mortality ‐ Registration status Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| Analysis 1.8  Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 8 All‐cause mortality ‐ Registration status. | ||||
| 8.1 Pre‐registration | 5 | 47642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| 8.2 Post‐registration | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.16, 1.63] |
| 8.3 No registration | 40 | 32541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.80, 0.95] |
| 9 All‐cause mortality ‐ Funding Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| Analysis 1.9  Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 9 All‐cause mortality ‐ Funding. | ||||
| 9.1 Industry funded or unknown funded trials | 39 | 78702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.00] |
| 9.2 Non‐industry funded trials | 7 | 1750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.20] |
| 10 All‐cause mortality ‐ Trials at low risk of bias Show forest plot | 2 | 46122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| Analysis 1.10  Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 10 All‐cause mortality ‐ Trials at low risk of bias. | ||||
| 11 All‐cause mortality ‐ 'Best‐worst case scenario' Show forest plot | 46 | 80522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.89, 0.98] |
| Analysis 1.11  Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 11 All‐cause mortality ‐ 'Best‐worst case scenario'. | ||||
| 12 All‐cause mortality ‐ 'Worst‐best case scenario' Show forest plot | 46 | 80522 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.02] |
| Analysis 1.12  Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 12 All‐cause mortality ‐ 'Worst‐best case scenario'. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.98] |
| Analysis 2.1  Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 All‐cause mortality. | ||||
| 2 All‐cause mortality ‐ Acute/subacute phase Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 2.2  Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 2 All‐cause mortality ‐ Acute/subacute phase. | ||||
| 2.1 Acute phase | 17 | 21368 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.89, 1.01] |
| 2.2 Subacute phase | 4 | 3842 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.68, 0.96] |
| 3 All‐cause mortality ‐ Reperfusion/no reperfusion Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 2.3  Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 3 All‐cause mortality ‐ Reperfusion/no reperfusion. | ||||
| 3.1 No reperfusion | 16 | 23768 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 5 | 1442 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 4 All‐cause mortality ‐ Type of beta‐blocker Show forest plot | 21 | 25098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 2.4  Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 4 All‐cause mortality ‐ Type of beta‐blocker. | ||||
| 4.1 Alprenolol | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.74, 1.31] |
| 4.2 Atenolol | 3 | 16696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.88, 1.02] |
| 4.3 Carvedilol | 3 | 2899 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.64, 0.96] |
| 4.4 Labetolol | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.71, 4.14] |
| 4.5 Metoprolol | 4 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.09] |
| 4.6 Pindolol | 1 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.67, 1.40] |
| 4.7 Practolol | 2 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.68, 1.33] |
| 4.8 Propranolol | 4 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 4.9 Timolol | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.96] |
| 4.10 Esmolol | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 5 All‐cause mortality ‐ Above/below 75 years of age Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.98] |
| Analysis 2.5  Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 5 All‐cause mortality ‐ Above/below 75 years of age. | ||||
| 5.1 Below 75 years | 16 | 5862 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.04] |
| 5.2 Mixed | 5 | 19348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 0.99] |
| 6 All‐cause mortality ‐ Intravenously/orally commenced Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 2.6  Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 6 All‐cause mortality ‐ Intravenously/orally commenced. | ||||
| 6.1 Intravenously commenced | 2 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.30, 2.39] |
| 6.2 Orally commenced | 7 | 4614 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.72, 0.96] |
| 6.3 Mixed | 12 | 20226 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.89, 1.01] |
| 7 All‐cause mortality ‐ Registration status Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 2.7  Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 7 All‐cause mortality ‐ Registration status. | ||||
| 7.1 Pre‐registration | 3 | 1164 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.40] |
| 7.2 Post‐registration | 1 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.70, 2.08] |
| 7.3 No registration | 17 | 23777 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.98] |
| 8 All‐cause mortality ‐ NSTEMI/STEMI/UAP Show forest plot | 7 | 2128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.70, 1.18] |
| Analysis 2.8  Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 8 All‐cause mortality ‐ NSTEMI/STEMI/UAP. | ||||
| 8.1 STEMI | 3 | 1164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.51, 1.39] |
| 8.2 Mixed | 4 | 964 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.27] |
| 9 All‐cause mortality ‐ Length of intervention period Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 2.9  Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 9 All‐cause mortality ‐ Length of intervention period. | ||||
| 9.1 0 to 7 days length of intervention | 5 | 16651 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.89, 1.03] |
| 9.2 7 to 30 days length of intervention | 3 | 946 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.60, 1.24] |
| 9.3 1 month and more length of intervention | 13 | 7613 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.80, 0.98] |
| 10 All‐cause mortality ‐ Funding Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.98] |
| Analysis 2.10  Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 10 All‐cause mortality ‐ Funding. | ||||
| 10.1 Industry funded or unknown funded trials | 18 | 23877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.98] |
| 10.2 Non‐industry funded trials | 3 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.69, 1.45] |
| 11 All‐cause mortality ‐ Trials at low risk of bias Show forest plot | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.31, 2.85] |
| Analysis 2.11  Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 11 All‐cause mortality ‐ Trials at low risk of bias. | ||||
| 12 All‐cause mortality ‐ 'Best‐worst case scenario' Show forest plot | 21 | 25283 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.97] |
| Analysis 2.12  Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 12 All‐cause mortality ‐ 'Best‐worst case scenario'. | ||||
| 13 All‐cause mortality ‐ 'Worst‐best case scenario' Show forest plot | 21 | 25283 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.06] |
| Analysis 2.13  Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 13 All‐cause mortality ‐ 'Worst‐best case scenario'. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MACE (Composite of cardiovascular death and non‐fatal myocardial infarction) Show forest plot | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.1  Comparison 3 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 MACE (Composite of cardiovascular death and non‐fatal myocardial infarction). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MACE (Composite of cardiovascular death and non‐fatal myocardial infarction) Show forest plot | 4 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.43, 1.52] |
| Analysis 4.1  Comparison 4 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 MACE (Composite of cardiovascular death and non‐fatal myocardial infarction). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 5.1  Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 Cardiovascular mortality. | ||||
| 2 Cardiovascular mortality ‐ Acute/subacute phase Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 5.2  Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 2 Cardiovascular mortality ‐ Acute/subacute phase. | ||||
| 2.1 Acute phase | 17 | 72309 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.99] |
| 2.2 Subacute phase | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.41, 3.67] |
| 3 Cardiovascular mortality ‐ Reperfusion/no reperfusion Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 5.3  Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 3 Cardiovascular mortality ‐ Reperfusion/no reperfusion. | ||||
| 3.1 No reperfusion | 15 | 71702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 3 | 920 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.29, 1.62] |
| 4 Cardiovascular mortality ‐ Type of beta‐blocker Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 5.4  Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 4 Cardiovascular mortality ‐ Type of beta‐blocker. | ||||
| 4.1 Esmolol | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.21, 3.62] |
| 4.2 Atenolol | 2 | 16221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.96] |
| 4.3 Metoprolol | 6 | 54501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.03] |
| 4.4 Oxprenolol | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.41, 3.67] |
| 4.5 Propranolol | 5 | 1103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.65, 1.73] |
| 4.6 Timolol | 2 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.20, 1.39] |
| 5 Cardiovascular mortality ‐ Above/below 75 years of age Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 5.5  Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 5 Cardiovascular mortality ‐ Above/below 75 years of age. | ||||
| 5.1 Below 75 years | 14 | 56308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.88, 1.03] |
| 5.2 Mixed | 4 | 16314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.97] |
| 6 Cardiovascular mortality ‐ Intravenously/orally commenced Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 5.6  Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 6 Cardiovascular mortality ‐ Intravenously/orally commenced. | ||||
| 6.1 Intravenously commenced | 3 | 766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.42, 2.26] |
| 6.2 Orally commenced | 3 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.54, 1.82] |
| 6.3 Mixed | 12 | 71307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.99] |
| 7 Cardiovascular mortality ‐ NSTEMI/STEMI/UAP Show forest plot | 9 | 49303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.05] |
| Analysis 5.7  Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 7 Cardiovascular mortality ‐ NSTEMI/STEMI/UAP. | ||||
| 7.1 STEMI | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.35, 2.41] |
| 7.2 NSTEMI | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Unstable angina pectoris | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Mixed | 7 | 48577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.05] |
| 8 Cardiovascular mortality ‐ Registration status Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 5.8  Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 8 Cardiovascular mortality ‐ Registration status. | ||||
| 8.1 Pre‐registration | 3 | 46578 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.08] |
| 8.2 Post‐registration | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 No registration | 15 | 26044 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.76, 0.93] |
| 9 Cardiovascular mortality ‐ Funding Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| Analysis 5.9  Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 9 Cardiovascular mortality ‐ Funding. | ||||
| 9.1 Industry funded or unknown funded trials | 17 | 72560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.99] |
| 9.2 Non‐industry funded trials | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.11, 62.56] |
| 10 Cardiovascular mortality ‐ Trials with low risk of bias Show forest plot | 1 | 45852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.08] |
| Analysis 5.10  Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 10 Cardiovascular mortality ‐ Trials with low risk of bias. | ||||
| 11 Cardiovascular mortality ‐ 'Best‐worst case scenario' Show forest plot | 18 | 72681 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.72, 0.97] |
| Analysis 5.11  Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 11 Cardiovascular mortality ‐ 'Best‐worst case scenario'. | ||||
| 12 Cardiovascular mortality ‐ 'Worst‐best case scenario' Show forest plot | 18 | 72681 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.79, 1.10] |
| Analysis 5.12  Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 12 Cardiovascular mortality ‐ 'Worst‐best case scenario'. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.97] |
| Analysis 6.1  Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 Cardiovascular mortality. | ||||
| 2 Cardiovascular mortality ‐ Acute/subacute phase Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| Analysis 6.2  Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 2 Cardiovascular mortality ‐ Acute/subacute phase. | ||||
| 2.1 Acute phase | 10 | 18615 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.00] |
| 2.2 Subacute phase | 4 | 3842 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.95] |
| 3 Cardiovascular mortality ‐ Reperfusion/no reperfusion Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| Analysis 6.3  Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 3 Cardiovascular mortality ‐ Reperfusion/no reperfusion. | ||||
| 3.1 No reperfusion | 8 | 20386 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.97] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 6 | 2071 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.51] |
| 4 Cardiovascular mortality ‐ Type of beta‐blocker Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| Analysis 6.4  Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 4 Cardiovascular mortality ‐ Type of beta‐blocker. | ||||
| 4.1 Esmolol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.99] |
| 4.2 Alprenolol | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.22, 4.77] |
| 4.3 Atenolol | 2 | 16219 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 4.4 Carvedilol | 3 | 2899 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.97] |
| 4.5 Metoprolol | 4 | 1827 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.18] |
| 4.6 Pindolol | 1 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.63, 1.40] |
| 4.7 Propranolol | 3 | 806 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.06] |
| 5 Cardiovascular mortality ‐ Above/below 75 years of age Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| Analysis 6.5  Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 5 Cardiovascular mortality ‐ Above/below 75 years of age. | ||||
| 5.1 Below 75 years | 11 | 5366 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.68, 0.94] |
| 5.2 Mixed | 3 | 17091 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 6 Cardiovascular mortality ‐ Intravenously/orally commenced Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| Analysis 6.6  Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 6 Cardiovascular mortality ‐ Intravenously/orally commenced. | ||||
| 6.1 Intravenously commenced | 3 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.33, 1.60] |
| 6.2 Orally commenced | 6 | 4307 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.67, 0.95] |
| 6.3 Mixed | 5 | 17151 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 7 Cardiovascular mortality ‐ NSTEMI/STEMI/UAP Show forest plot | 6 | 2002 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.49, 1.59] |
| Analysis 6.7  Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 7 Cardiovascular mortality ‐ NSTEMI/STEMI/UAP. | ||||
| 7.1 STEMI | 4 | 1793 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.44, 1.64] |
| 7.2 Mixed | 2 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.27, 3.93] |
| 8 Cardiovascular mortality ‐ Registration status Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| Analysis 6.8  Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 8 Cardiovascular mortality ‐ Registration status. | ||||
| 8.1 Pre‐registration | 4 | 1793 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.44, 1.64] |
| 8.2 No registration | 10 | 20664 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.97] |
| 9 Cardiovascular mortality ‐ Length of intervention period Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| Analysis 6.9  Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 9 Cardiovascular mortality ‐ Length of intervention period. | ||||
| 9.1 0 to 7 days length of intervention | 4 | 17026 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 9.2 1 month and more length of intervention | 10 | 5431 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.69, 0.94] |
| 10 Cardiovascular mortality ‐ Funding Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| Analysis 6.10  Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 10 Cardiovascular mortality ‐ Funding. | ||||
| 10.1 Industry funded or unknown funded trials | 12 | 21393 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 10.2 Non‐industry funded trials | 2 | 1064 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.36, 2.20] |
| 11 Cardiovascular mortality ‐ 'Best‐worst case scenario' Show forest plot | 14 | 22587 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.53, 0.86] |
| Analysis 6.11  Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 11 Cardiovascular mortality ‐ 'Best‐worst case scenario'. | ||||
| 12 Cardiovascular mortality ‐ 'Worst‐best case scenario' Show forest plot | 14 | 22587 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.83, 1.36] |
| Analysis 6.12  Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 12 Cardiovascular mortality ‐ 'Worst‐best case scenario'. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarction Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| Analysis 7.1  Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 Myocardial infarction. | ||||
| 2 Myocardial infarction ‐ Acute/subacute phase Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| Analysis 7.2  Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 2 Myocardial infarction ‐ Acute/subacute phase. | ||||
| 2.1 Acute phase | 17 | 67249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.75, 0.90] |
| 2.2 Subacute phase | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.09, 1.25] |
| 3 Myocardial infarction ‐ Reperfusion/no reperfusion Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| Analysis 7.3  Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 3 Myocardial infarction ‐ Reperfusion/no reperfusion. | ||||
| 3.1 No reperfusion | 14 | 66372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 4 | 1190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.44, 1.82] |
| 4 Myocardial infarction ‐ Type of beta‐blocker Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| Analysis 7.4  Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 4 Myocardial infarction ‐ Type of beta‐blocker. | ||||
| 4.1 Esmolol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.06] |
| 4.2 Atenolol | 3 | 12312 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.42] |
| 4.3 Metoprolol | 5 | 53921 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.72, 0.89] |
| 4.4 Oxprenolol | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.09, 1.25] |
| 4.5 Pindolol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.82] |
| 4.6 Propranolol | 2 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.08, 14.01] |
| 4.7 Timolol | 3 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.26, 2.08] |
| 4.8 Xamoterol | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [0.13, 73.06] |
| 4.9 Mixed | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.37, 10.08] |
| 5 Myocardial infarction ‐ Above/below 75 years of age Show forest plot | 17 | 67432 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| Analysis 7.5  Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 5 Myocardial infarction ‐ Above/below 75 years of age. | ||||
| 5.1 Below 75 years | 13 | 9561 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.89] |
| 5.2 Mixed | 4 | 57871 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.76, 0.94] |
| 6 Myocardial infarction ‐ Intravenously/orally commenced Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| Analysis 7.6  Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 6 Myocardial infarction ‐ Intravenously/orally commenced. | ||||
| 6.1 Intravenously commenced | 3 | 996 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.28, 4.51] |
| 6.2 Orally commenced | 3 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.16, 4.47] |
| 6.3 Mixed | 12 | 66114 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 7 Myocardial infarction ‐ NSTEMI/STEMI/UAP Show forest plot | 11 | 49361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.71, 0.90] |
| Analysis 7.7  Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 7 Myocardial infarction ‐ NSTEMI/STEMI/UAP. | ||||
| 7.1 STEMI | 3 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.29, 3.46] |
| 7.2 NSTEMI | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Unstable angina pectoris | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Mixed | 8 | 48365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.71, 0.89] |
| 8 Myocardial infarction ‐ Registration status Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| Analysis 7.8  Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 8 Myocardial infarction ‐ Registration status. | ||||
| 8.1 Pre‐registration | 4 | 46848 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 8.2 Post‐registration | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 No registration | 14 | 20714 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| 9 Myocardial infarction ‐ Funding Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| Analysis 7.9  Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 9 Myocardial infarction ‐ Funding. | ||||
| 9.1 Industry funded or unknown funded trials | 15 | 67067 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 9.2 Non‐industry funded trials | 3 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.22, 6.77] |
| 10 Myocardial infarction ‐ Trials with low risk of bias Show forest plot | 1 | 45852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.92] |
| Analysis 7.10  Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 10 Myocardial infarction ‐ Trials with low risk of bias. | ||||
| 11 Myocardial infarction ‐ 'Best‐worst case scenario' Show forest plot | 18 | 67620 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.61, 0.92] |
| Analysis 7.11  Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 11 Myocardial infarction ‐ 'Best‐worst case scenario'. | ||||
| 12 Myocardial infarction ‐ 'Worst‐best case scenario' Show forest plot | 18 | 67620 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.07] |
| Analysis 7.12  Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 12 Myocardial infarction ‐ 'Worst‐best case scenario'. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarction Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| Analysis 8.1  Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 Myocardial infarction. | ||||
| 2 Myocardial infarction ‐ Acute/subacute phase Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| Analysis 8.2  Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 2 Myocardial infarction ‐ Acute/subacute phase. | ||||
| 2.1 Acute phase | 10 | 2983 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.72, 1.33] |
| 2.2 Subacute phase | 4 | 3842 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 1.01] |
| 3 Myocardial infarction ‐ Reperfusion/no reperfusion Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| Analysis 8.3  Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 3 Myocardial infarction ‐ Reperfusion/no reperfusion. | ||||
| 3.1 No reperfusion | 7 | 4658 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.15] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 7 | 2167 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.46, 1.66] |
| 4 Myocardial infarction ‐ Type of beta‐blocker Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| Analysis 8.4  Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 4 Myocardial infarction ‐ Type of beta‐blocker. | ||||
| 4.1 Esmolol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.06] |
| 4.2 Alprenolol | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.28, 3.81] |
| 4.3 Landiolol | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.01, 8.32] |
| 4.4 Carvedilol | 3 | 2899 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.91] |
| 4.5 Metoprolol | 4 | 2426 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.84, 1.24] |
| 4.6 Pindolol | 1 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.61, 1.38] |
| 4.7 Propranolol | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.08, 3.15] |
| 4.8 Timolol | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 6.57 [0.82, 52.35] |
| 5 Myocardial infarction ‐ Above/below 75 years of age Show forest plot | 13 | 6729 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.72, 1.09] |
| Analysis 8.5  Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 5 Myocardial infarction ‐ Above/below 75 years of age. | ||||
| 5.1 Below 75 years | 11 | 5665 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.10] |
| 5.2 Mixed | 2 | 1064 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.45, 4.07] |
| 6 Myocardial infarction ‐ Intravenously/orally commenced Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| Analysis 8.6  Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 6 Myocardial infarction ‐ Intravenously/orally commenced. | ||||
| 6.1 Intravenously commenced | 4 | 1095 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.26, 3.29] |
| 6.2 Orally commenced | 6 | 4007 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.17] |
| 6.3 Mixed | 4 | 1723 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.50] |
| 7 Myocardial infarction ‐ NSTEMI/STEMI/UAP Show forest plot | 7 | 2098 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.57, 2.18] |
| Analysis 8.7  Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 7 Myocardial infarction ‐ NSTEMI/STEMI/UAP. | ||||
| 7.1 STEMI | 5 | 1889 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.45, 2.54] |
| 7.2 Mixed | 2 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.42, 3.40] |
| 8 Myocardial infarction ‐ Registration status Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| Analysis 8.8  Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 8 Myocardial infarction ‐ Registration status. | ||||
| 8.1 Pre‐registration | 4 | 1793 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.48, 2.88] |
| 8.2 No registration | 10 | 5032 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.68, 1.09] |
| 9 Myocardial infarction ‐ Length of intervention period Show forest plot | 14 | 6913 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.71, 1.13] |
| Analysis 8.9  Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 9 Myocardial infarction ‐ Length of intervention period. | ||||
| 9.1 0 to 7 days length of intervention | 5 | 1183 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.46, 4.96] |
| 9.2 7 to 30 days length of intervention | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 6.57 [0.82, 52.35] |
| 9.3 1 month and more length of intervention | 9 | 5642 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.74, 1.06] |
| 10 Myocardial infarction ‐ Funding Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.04] |
| Analysis 8.10  Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 10 Myocardial infarction ‐ Funding. | ||||
| 10.1 Industry funded or unknown funded trials | 11 | 5665 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.04] |
| 10.2 Non‐industry funded trials | 3 | 1160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.31, 2.04] |
| 11 Myocardial infarction ‐ 'Best‐worst case scenario' Show forest plot | 14 | 6951 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.85] |
| Analysis 8.11  Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 11 Myocardial infarction ‐ 'Best‐worst case scenario'. | ||||
| 12 Myocardial infarction ‐ 'Worst‐best case scenario' Show forest plot | 14 | 6951 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.95, 1.26] |
| Analysis 8.12  Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 12 Myocardial infarction ‐ 'Worst‐best case scenario'. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Angina on a dichotomous scale Show forest plot | 3 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.31, 1.58] |
| Analysis 9.1  Comparison 9 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 Angina on a dichotomous scale. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Angina on a dichotomous scale Show forest plot | 2 | 844 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.18, 2.30] |
| Analysis 10.1  Comparison 10 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 Angina on a dichotomous scale. | ||||

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Funnel plot of comparison: 1 Beta‐blockers versus placebo or no intervention at 'less than 3 months' follow‐up, outcome: 1.1 All‐cause mortality.

Funnel plot of comparison: 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, outcome: 2.1 All‐cause mortality.

Funnel plot of comparison: 5 Beta‐blockers versus placebo or no intervention at 'less than 3 months' follow‐up beyond 3 months, outcome: 5.1 Cardiovascular mortality.

Funnel plot of comparison: 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, outcome: 6.1 Cardiovascular mortality.

Funnel plot of comparison: 7 Beta‐blockers versus placebo or no intervention at 'less than 3 months' follow‐up, outcome: 7.1 Myocardial infarction.
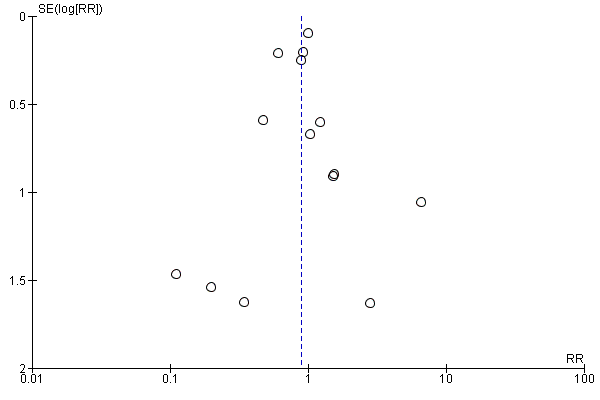
Funnel plot of comparison: 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, outcome: 8.1 Myocardial infarction.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 All‐cause mortality.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 2 All‐cause mortality ‐ Acute/subacute phase.
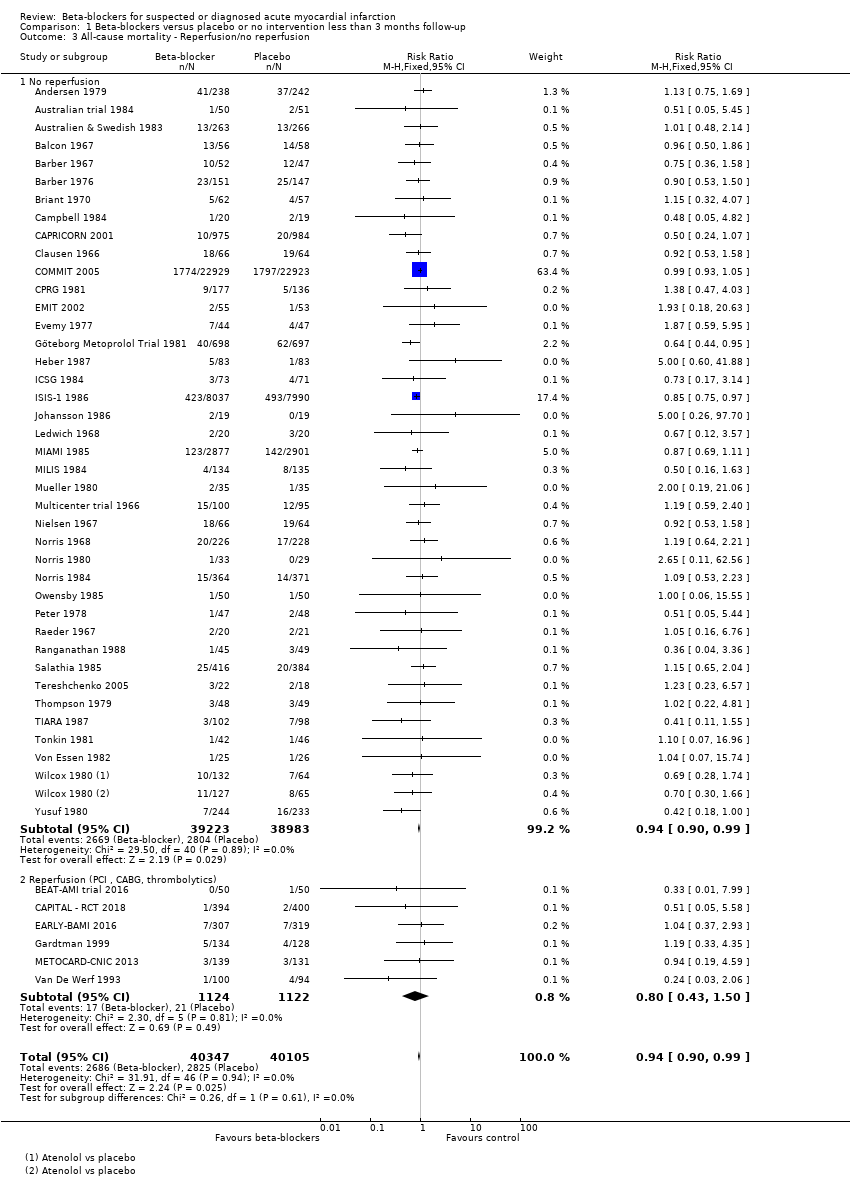
Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 3 All‐cause mortality ‐ Reperfusion/no reperfusion.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 4 All‐cause mortality ‐ Type of beta‐blocker.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 5 All‐cause mortality ‐ Intravenously/orally commenced.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 6 All‐cause mortality ‐ Above/below 75 years of age.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 7 All‐cause mortality ‐ NSTEMI/STEMI/UAP.
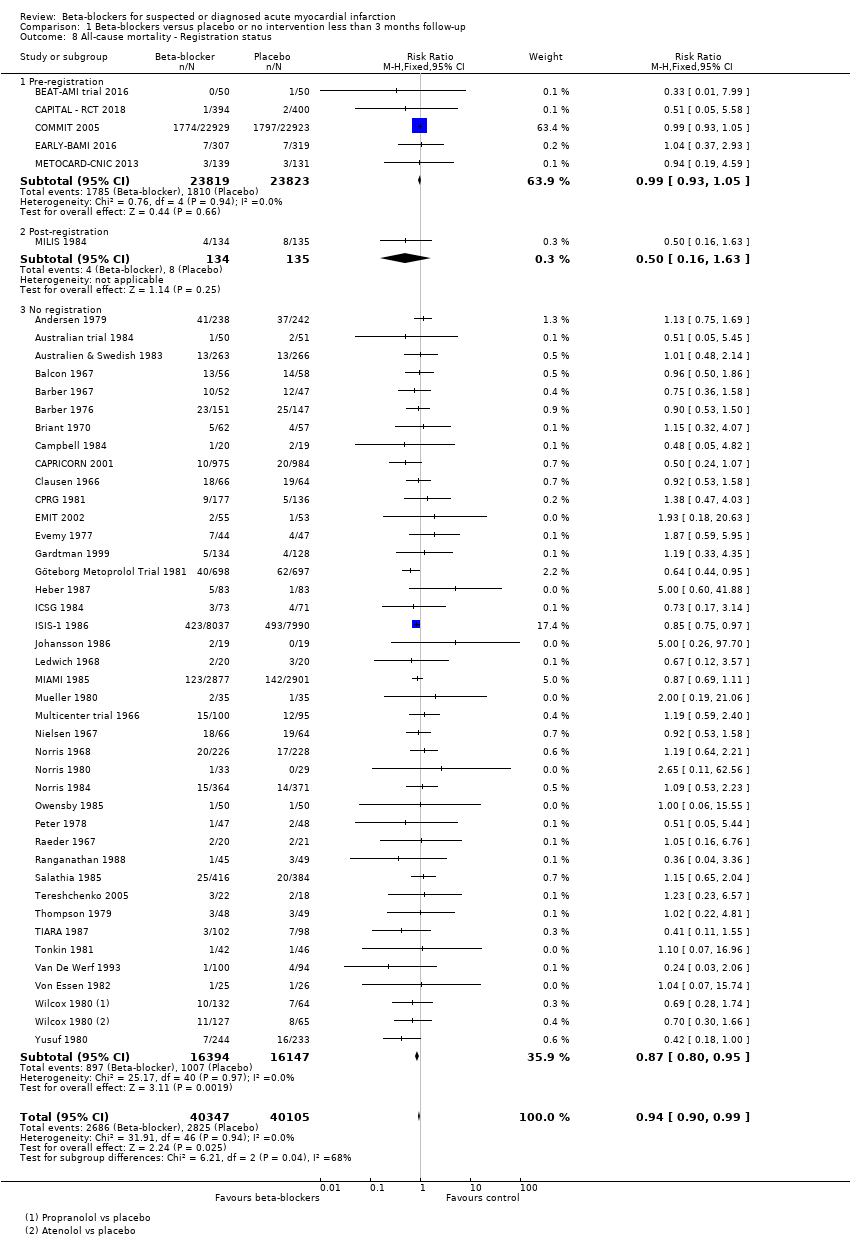
Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 8 All‐cause mortality ‐ Registration status.
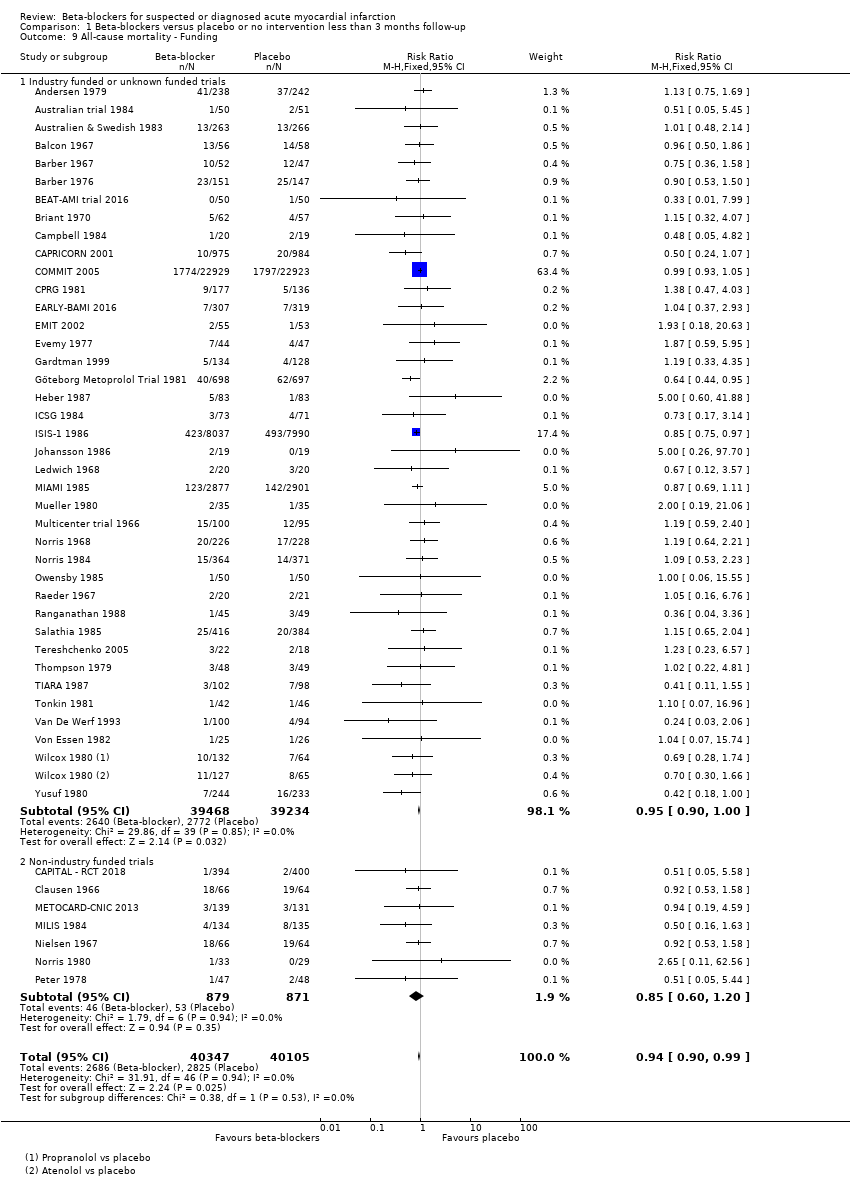
Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 9 All‐cause mortality ‐ Funding.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 10 All‐cause mortality ‐ Trials at low risk of bias.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 11 All‐cause mortality ‐ 'Best‐worst case scenario'.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 12 All‐cause mortality ‐ 'Worst‐best case scenario'.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 All‐cause mortality.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 2 All‐cause mortality ‐ Acute/subacute phase.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 3 All‐cause mortality ‐ Reperfusion/no reperfusion.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 4 All‐cause mortality ‐ Type of beta‐blocker.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 5 All‐cause mortality ‐ Above/below 75 years of age.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 6 All‐cause mortality ‐ Intravenously/orally commenced.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 7 All‐cause mortality ‐ Registration status.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 8 All‐cause mortality ‐ NSTEMI/STEMI/UAP.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 9 All‐cause mortality ‐ Length of intervention period.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 10 All‐cause mortality ‐ Funding.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 11 All‐cause mortality ‐ Trials at low risk of bias.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 12 All‐cause mortality ‐ 'Best‐worst case scenario'.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 13 All‐cause mortality ‐ 'Worst‐best case scenario'.

Comparison 3 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 MACE (Composite of cardiovascular death and non‐fatal myocardial infarction).

Comparison 4 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 MACE (Composite of cardiovascular death and non‐fatal myocardial infarction).
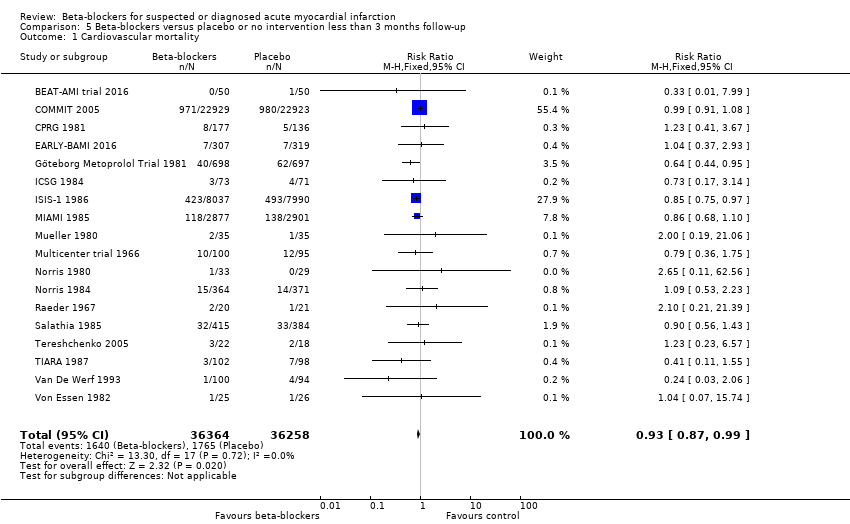
Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 Cardiovascular mortality.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 2 Cardiovascular mortality ‐ Acute/subacute phase.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 3 Cardiovascular mortality ‐ Reperfusion/no reperfusion.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 4 Cardiovascular mortality ‐ Type of beta‐blocker.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 5 Cardiovascular mortality ‐ Above/below 75 years of age.
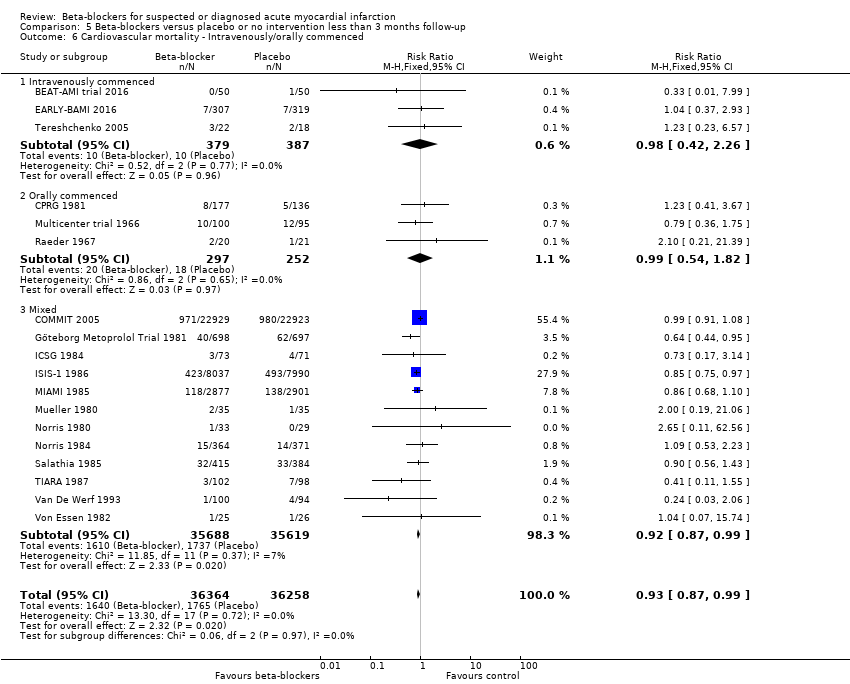
Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 6 Cardiovascular mortality ‐ Intravenously/orally commenced.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 7 Cardiovascular mortality ‐ NSTEMI/STEMI/UAP.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 8 Cardiovascular mortality ‐ Registration status.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 9 Cardiovascular mortality ‐ Funding.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 10 Cardiovascular mortality ‐ Trials with low risk of bias.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 11 Cardiovascular mortality ‐ 'Best‐worst case scenario'.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 12 Cardiovascular mortality ‐ 'Worst‐best case scenario'.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 Cardiovascular mortality.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 2 Cardiovascular mortality ‐ Acute/subacute phase.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 3 Cardiovascular mortality ‐ Reperfusion/no reperfusion.
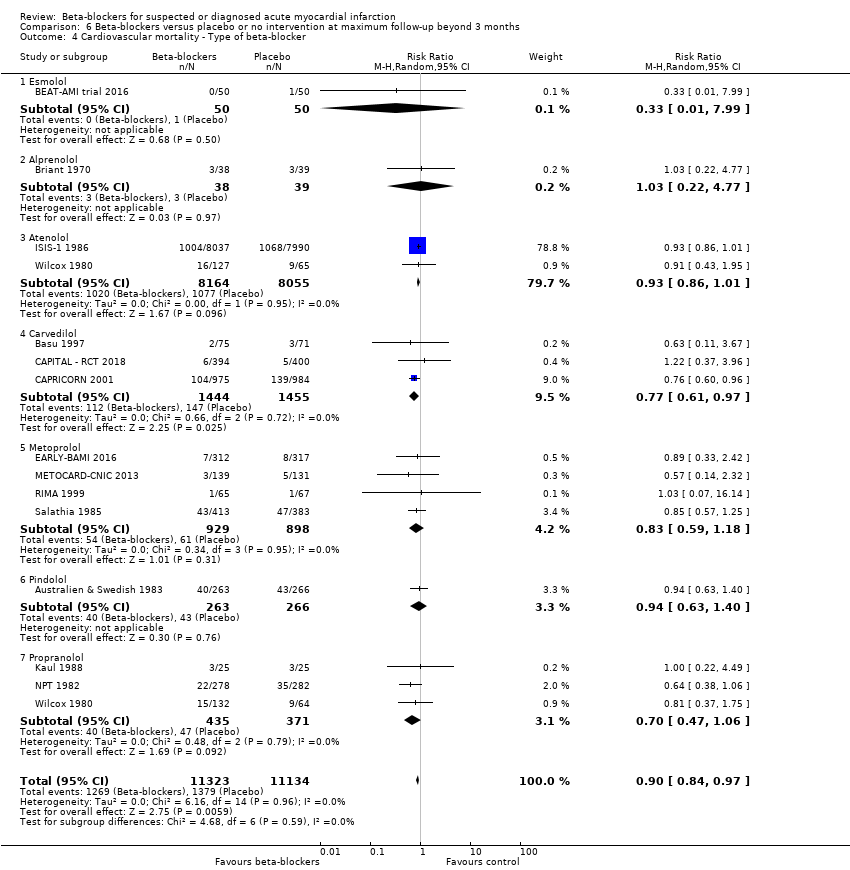
Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 4 Cardiovascular mortality ‐ Type of beta‐blocker.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 5 Cardiovascular mortality ‐ Above/below 75 years of age.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 6 Cardiovascular mortality ‐ Intravenously/orally commenced.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 7 Cardiovascular mortality ‐ NSTEMI/STEMI/UAP.
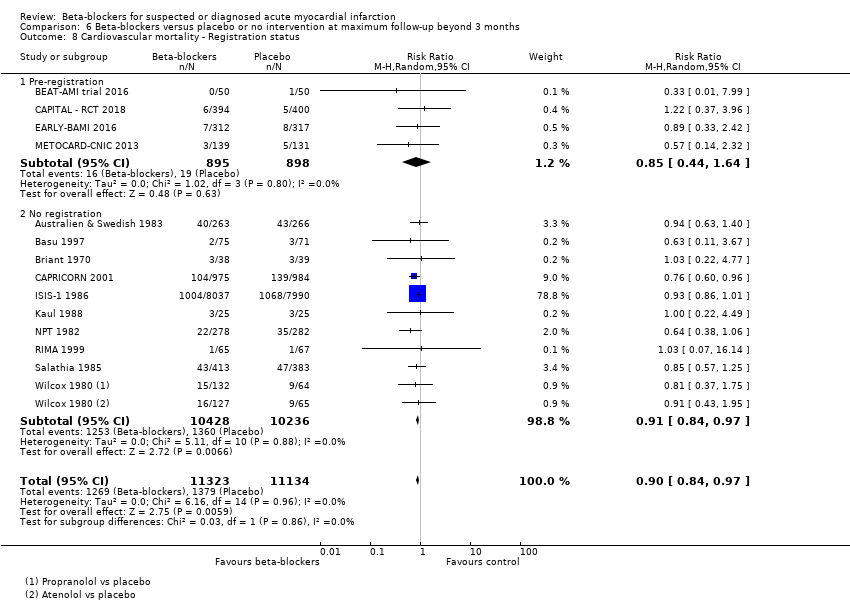
Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 8 Cardiovascular mortality ‐ Registration status.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 9 Cardiovascular mortality ‐ Length of intervention period.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 10 Cardiovascular mortality ‐ Funding.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 11 Cardiovascular mortality ‐ 'Best‐worst case scenario'.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 12 Cardiovascular mortality ‐ 'Worst‐best case scenario'.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 Myocardial infarction.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 2 Myocardial infarction ‐ Acute/subacute phase.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 3 Myocardial infarction ‐ Reperfusion/no reperfusion.
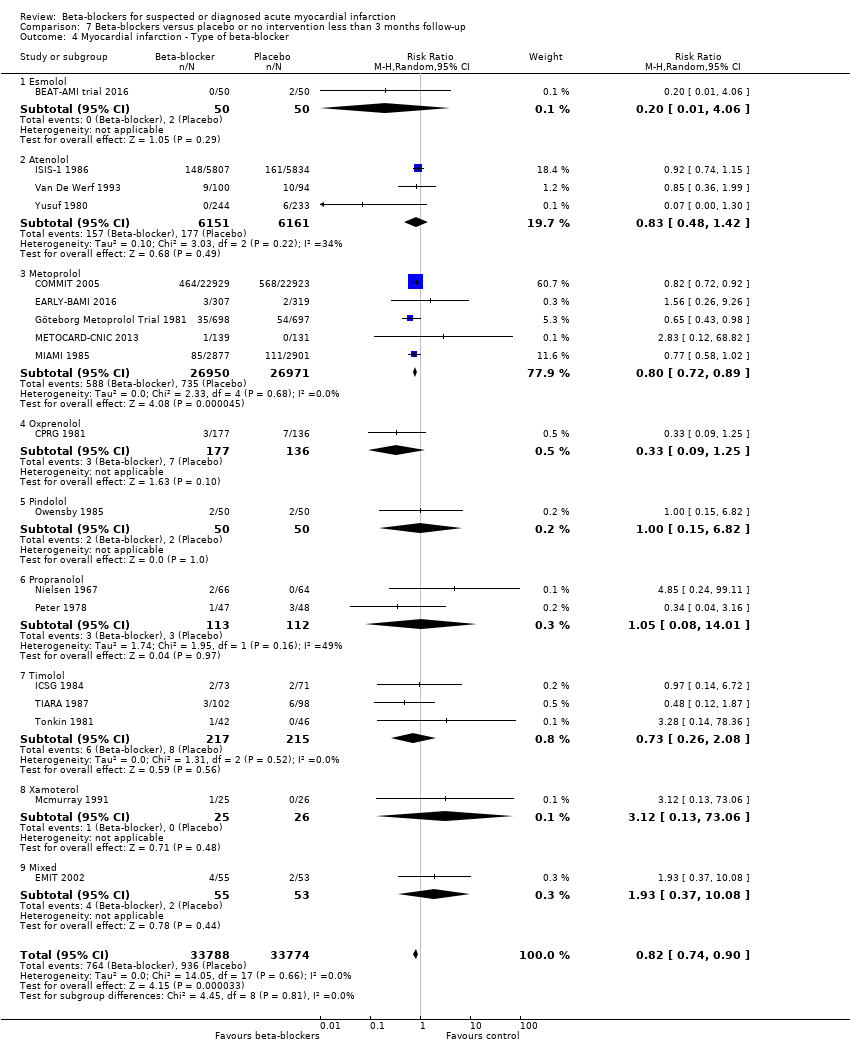
Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 4 Myocardial infarction ‐ Type of beta‐blocker.
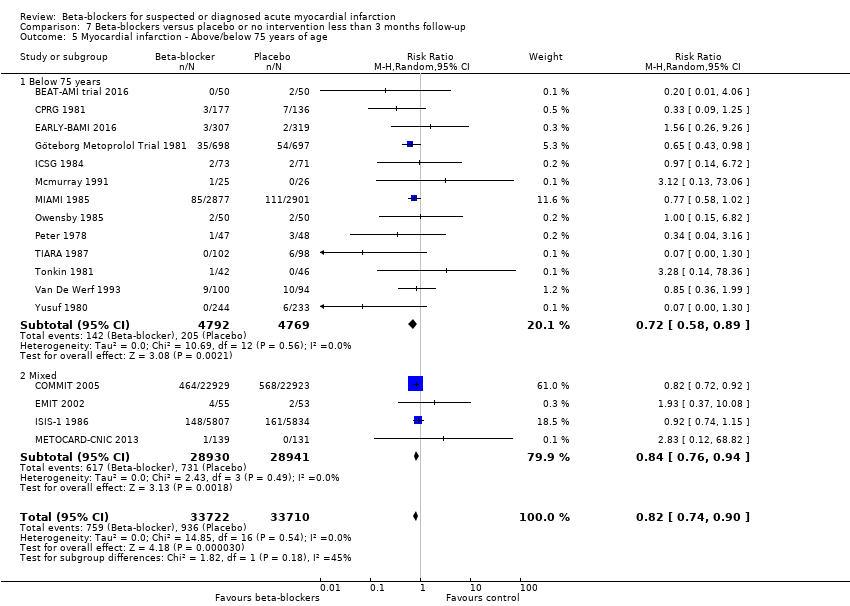
Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 5 Myocardial infarction ‐ Above/below 75 years of age.
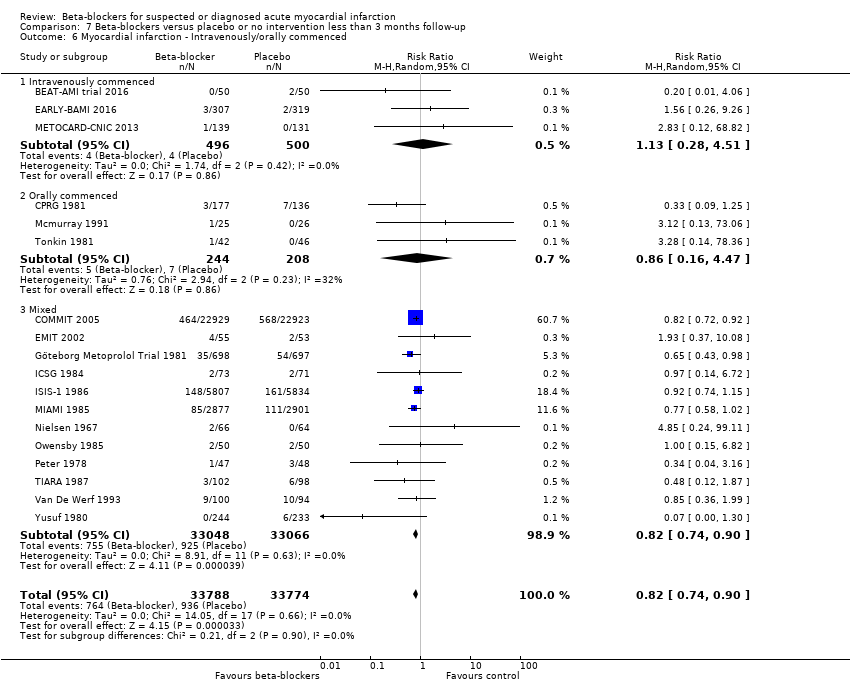
Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 6 Myocardial infarction ‐ Intravenously/orally commenced.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 7 Myocardial infarction ‐ NSTEMI/STEMI/UAP.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 8 Myocardial infarction ‐ Registration status.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 9 Myocardial infarction ‐ Funding.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 10 Myocardial infarction ‐ Trials with low risk of bias.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 11 Myocardial infarction ‐ 'Best‐worst case scenario'.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 12 Myocardial infarction ‐ 'Worst‐best case scenario'.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 Myocardial infarction.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 2 Myocardial infarction ‐ Acute/subacute phase.
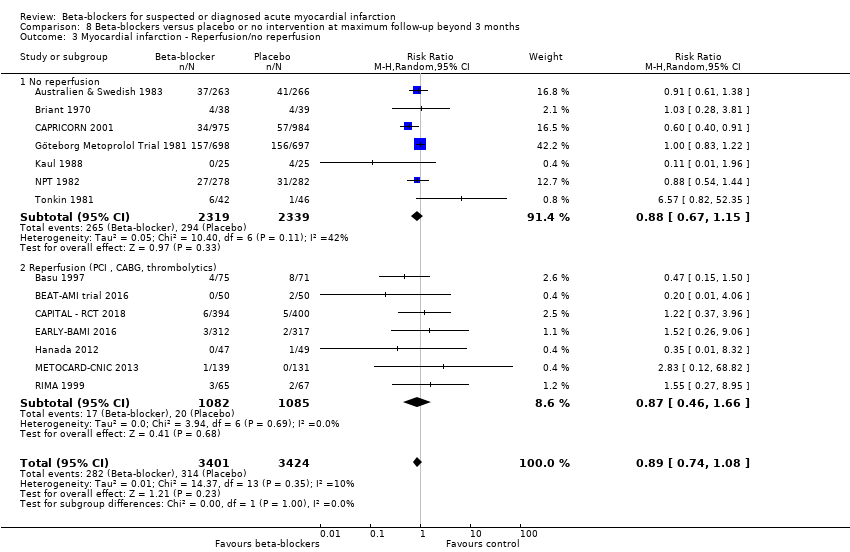
Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 3 Myocardial infarction ‐ Reperfusion/no reperfusion.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 4 Myocardial infarction ‐ Type of beta‐blocker.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 5 Myocardial infarction ‐ Above/below 75 years of age.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 6 Myocardial infarction ‐ Intravenously/orally commenced.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 7 Myocardial infarction ‐ NSTEMI/STEMI/UAP.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 8 Myocardial infarction ‐ Registration status.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 9 Myocardial infarction ‐ Length of intervention period.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 10 Myocardial infarction ‐ Funding.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 11 Myocardial infarction ‐ 'Best‐worst case scenario'.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 12 Myocardial infarction ‐ 'Worst‐best case scenario'.

Comparison 9 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 Angina on a dichotomous scale.

Comparison 10 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 Angina on a dichotomous scale.
| Beta‐blockers versus placebo or no intervention for patients with suspected or diagnosed myocardial infarction at the time point less than three months follow‐up | ||||||
| Patient or population: patients with suspected or diagnosed myocardial infarction Settings: any setting Intervention: any beta‐blocker Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect (adjusted CI) | No of Participants | Quality of the evidence | Comments | |
| Assumed risk with placebo or no intervention | Corresponding risk with beta‐blockers | |||||
| All‐cause mortality at 'less than 3 months' follow‐up. Follow‐up: mean 21.8 days (range 1 hour to 90 days). | 70 per 1000 | 67 per 1000 | RR 0.94, 97.5% CI (0.90 to 1.0) | 80,452 (46 RCTs with 47 comparisons) | ⊕⊕⊕⊕1 | Since the sensitivity analysis excluding trials at high risk of bias and the overall meta‐analysis showed similar results, we based our summary of findings and conclusion on the overall meta‐analysis. No events occurred in either group in three trials (Hanada 2012; Norris 1978; Shirotani 2010). |
| Serious adverse events at 'less than 3 months' follow‐up. No data was reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | None of the trials specifically assessed nor reported serious adverse events according to ICH‐GCP. |
| MACE (major adverse cardiovascular event) at 'less than 3 months' follow‐up. No data were reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | Only two trials specifically assessed major adverse cardiovascular events (defined as a composite of cardiovascular mortality and myocardial infarction during follow‐up). However, no major adverse cardiovascular events occurred in either trial. |
| Quality of life at 'less than 3 months' follow‐up. No data were reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | No data reported. |
| Angina at 'less than 3 months' follow‐up. Follow‐up: mean 21 days (range 12 to 30 days). | 222 per 1000 | 155 per 1000 (69 to 351) | RR 0.70, 98% CI (0.25 to 1.84) | 98 (3 RCTs) | ⊕⊝⊝⊝2,3 VERY LOW | |
| Cardiovascular mortality at 'less than 3 months' follow‐up. Follow‐up: mean 28 days. | 43 per 1000 | 42 per 1000 | RR 0.99, 95% CI (0.91 to 1.08) | 45,852 (1 RCT) | ⊕⊕⊕⊝4 | Since the sensitivity analysis excluding trials at high risk of bias differed from the overall meta‐analysis, we based our summary of findings and conclusion on the sensitivity analysis. |
| Myocardial infarction at 'less than 3 months' follow‐up. Follow‐up: mean 23.3 days (range 3 to 90 days). | 28 per 1000 | 23 per 1000 | RR 0.82, 98% CI (0.74 to 0.90) | 67,562 (18 RCTs) | ⊕⊕⊕⊝5 | Since the sensitivity analysis excluding trials at high risk of bias and the overall meta‐analysis showed similar results, we based our summary of findings and conclusion on the overall meta‐analysis. No events occurred in either group in two trials (Hanada 2012; Shirotani 2010). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its adjusted confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its adjusted CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 When assessing the risk of bias, the trial contributing most weight (COMMIT 2005, 63.4%) was assessed as at low risk of bias in all domains. The trial contributing the second highest weight (ISIS‐1 1986, 17.4%) was assessed as low risk of bias in random sequence generation, allocation concealment, and incomplete outcome data;'unclear for blinding of outcome assessors and selective reporting and at high risk for blinding of participants and personnel. Since a lack of blinding is less important for the assessment of all‐cause mortality, the overall limitations were not serious and the evidence is not downgraded for risk of bias. 2 Downgraded by one level due to serious risk of bias. All the included trials were at high risk of bias due to either unclear or high risk in several bias domains. 3 Downgraded by two levels due to very serious risk of imprecision based on the optimal information size not being reached, the very small sample size, and the absolute and relative 98% CI being very wide showing both appreciable benefit and harm. 4 Downgraded by one level due to serious risk of imprecision based on the wide absolute and relative 98% where the upper CI does not exclude the possibility of no difference between the groups. When assessing the risk of bias, the evidence was not downgraded since the result was based on the sensitivity analysis consisting of trials at low risk of bias (COMMIT 2005). 5 Downgraded by one level due to serious risk of bias. The overall limitations and specially in regard to blinding of outcome assessors were serious (around 50% of the trials were assessed at unclear risk of bias in blinding of outcome assessors). | ||||||
| Beta‐blockers compared with placebo or no intervention for patients with suspected or diagnosed myocardial infarction | ||||||
| Patient or population: patients with suspected or diagnosed myocardial infarction Settings: any setting Intervention: beta‐blockers Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk with placebo or no intervention | Corresponding risk with beta‐blockers | |||||
| All‐cause mortality at maximum follow‐up beyond 3 months. Follow‐up: mean 16.4 months (range 6 to 60 months). | 148 per 1000 | 138 per 1000 | RR 0.93, 97.5% CI (0.86 to 0.99) | 25,210 (21 RCTs with 22 comparisons) | ⊕⊕⊕⊝3 | No events occurred in either group in one trial (Hanada 2012). |
| Serious adverse events at maximum follow‐up beyond 3 months. No data were reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | None of the trials specifically assessed nor reported serious adverse events according to ICH‐GCP. |
| MACE (major adverse cardiovascular event) at maximum follow‐up beyond 3 months. Follow‐up: mean7.5 months (range 6 to 12 months). | 84 per 1000 | 68 per 1000 | RR 0.81, 97.5% CI (0.43 to 1.52) | 475 (4 RCTs) | ⊕⊝⊝⊝1, 2 | |
| Quality of life at maximum follow‐up beyond 3 months. No data were reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | No data reported. |
| Angina at maximum follow‐up beyond 3 months (mean = 6 months). | 24 per 1000 | 15 per 1000 (5 to 48) | RR 0.64, 98% CI 0.18 to 2.0 | 844 ( 2 RTCs) | ⊕⊝⊝⊝1,5 | |
| Cardiovascular mortality at maximum follow‐up beyond 3 months. Follow‐up: mean 12.9 months (range 6 to 24 months). | 124 per 1000 | 112 per 1000 | RR 0.90, 98% CI (0.83 to 0.98) | 22,457 (14 RCTs with 15 comparisons) | ⊕⊕⊕⊝1 | No events occurred in either group in one trial (Hanada 2012). |
| Myocardial infarction at maximum follow‐up beyond 3 months. Follow‐up: mean 15.5 months (range 6 to 60 months). | 92 per 1000 | 83 per 1000 | RR 0.89, 98% CI (0.75 to 1.08) | 6825 (14 RCTs) | ⊕⊕⊝⊝1, 6 Low | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to serious risk of bias. All the included trials were at high risk of bias due to either unclear or high risk in several bias domains. 2 Downgraded by two levels due to very serious risk of imprecision based on the optimal information size not being reached, the wide absolute and relative 97.5% CI showing both appreciable benefit and harm, and a small sample size. 3 Downgraded by one level due to serious risk of bias. All but one of the included trials were at high risk of bias due to either unclear or high risk in several bias domains and the sensitivity analysis excluding trials at high risk of bias showed different results than the overall analysis including trials at high risk of bias. However, the sensitivity analysis was based on only one small trial, so we have used the main analysis for the 'Summary of findings' table. 5 Downgraded by two levels due to very serious risk of imprecision based on the very small sample size included. 6 Downgraded by one level due to serious risk of imprecision based on the wide absolute and relative 98% where the upper CI does not exclude the possibility of no difference between the groups. | ||||||
| Trial | Year | All‐cause mortality | Major adverse cardiovascular events | Cardiovascular mortality | Myocardial infarction during follow‐up |
| Andersen | 1979 | 28 days | NR | NR | NR |
| Åström | 1986 | NR | NR | NR | NR |
| Australian | 1984 | 28 days | NR | NR | NR |
| Australia & Swedish | 1983 | 28 days | NR | NR | NR |
| Balcon | 1967 | 28 days | NR | NR | NR |
| Barbar | 1967 | 28 days | NR | NR | NR |
| Barber | 1976 | 90 days | NR | NR | NR |
| BEAT‐AMI trial | 2016 | During hospitalisation (no mean time) | NR | During hospitalisation (no mean time) | During hospitalisation (no mean time) |
| Briant | 1970 | 3 days | NR | NR | NR |
| Campbell | 1984 | 7 days | NR | NR | NR |
| CAPRICORN | 2001 | 30 days | NR | NR | NR |
| Clausen | 1966 | 28 days | NR | NR | NR |
| COMMIT | 2005 | 28 days | NR | 28 days | 28 days |
| CPRG | 1980 | 60 days | NR | 56 days | 56 days |
| EARLY‐BAMI | 2016 | 30 days | NR | 30 days | 30 days |
| EMIT | 2002 | 42 days | NR | NR | 42 days |
| Evemy | 1977 | 30 days | NR | NR | NR |
| Gardtman | 1999 | 30 days | NR | NR | NR |
| Göteborg Metoprolol Trial | 1981 | 90 days | NR | 90 days | 90 days |
| Hanada | 2012 | During hospitalisation (no mean time) (no events) | During hospitalisation (no mean time) (no events) | During hospitalisation (no mean time) (no events) | During hospitalisation (no mean time) (no events) |
| Heber | 1986 | 5 days | NR | NR | NR |
| ICSG | 1984 | During hospitalisation (no mean time) | NR | During hospitalisation (no mean time) | During hospitalisation (no mean time) |
| ISIS‐1 | 1986 | 14 days | NR | 7 days | 7 days |
| Johannessen | 1987 | 10 days | NR | NR | NR |
| Ledwich | 1968 | 7 days | NR | NR | NR |
| McMurray | 1991 | NR | NR | NR | 10 days |
| METOCARD‐CNIC | 2013 | 7 days | NR | NR | 7 days |
| MIAMI | 1985 | 15 days | NR | 15 days | 15 days |
| MILIS | 1984 | 30 days | NR | NR | NR |
| Mueller | 1980 | 3 days | NR | 10 days | NR |
| Multicenter trial | 1966 | 30 days | NR | 30 days | NR |
| Nielsen | 1967 | 28 days | NR | NR | 28 days |
| Norris | 1968 | 21 days | NR | NR | NR |
| Norris | 1978 | 8.5 days (no events) | NR | 8.5 days (no events) | NR |
| Norris | 1980 | During hospitalisation (no mean time) | NR | During hospitalisation (no mean time) | NR |
| Norris | 1984 | 21 days | NR | 21 days | NR |
| Owensby | 1985 | 3 days | NR | NR | 3 days |
| Peter | 1978 | 3 days | NR | NR | 3 days |
| Raeder | 1967 | 21 days | NR | 21 days | NR |
| Ranganathan | 1988 | 2 days | NR | NR | NR |
| Rolli | 1980 | NR | NR | NR | NR |
| Salathia | 1985 | During hospitalisation (no mean time) | NR | 90 days | NR |
| Shirotani | 2010 | 30 days (no events) | 30 days (no events) | 30 days (no events) | 30 days (no events) |
| Tereschenko | 2005 | 30 days | NR | 30 days | NR |
| Thompson | 1979 | 5 days | NR | NR | NR |
| TIARA | 1987 | 30 days | NR | 30 days | 30 days |
| Tonkin | 1981 | 7 days | NR | NR | 7 days |
| Van De Werf | 1993 | 14 days | NR | 14 days | 14 days |
| Von Essen | 1982 | 14 days | NR | 14 days | NR |
| Wilcox | 1980 | 42 days | NR | NR | NR |
| Yang | 1984 | NR | NR | NR | NR |
| Yusuf | 1980 | 10 days | NR | NR | During hospitalisation (no mean time) |
| Trial | Year | All‐cause mortality | Major adverse cardiovascular events | Cardiovascular mortality | Myocardial infarction |
| Andersen | 1979 | 12 months | NR | NR | NR |
| Australia & Swedish | 1983 | 24 months | NR | 24 months | 24 months |
| Barber | 1976 | 24 months | NR | NR | NR |
| Basu | 1997 | 6 months | 6 months | 6 months | 6 months |
| BEAT‐AMI trial | 2016 | 6 months | NR | 6 months | 6 months |
| Briant | 1970 | 12 months | 12 months | 12 months | 12 months |
| CAPRICORN | 2001 | 15.6 months | NR | 15.6 months | 15.6 months |
| EARLY‐BAMI | 2016 | 1 month (not included) | NR | 12 months | 12 months |
| Evemy | 1977 | 7 months | NR | NR | NR |
| Göteborg Metoprolol Trial | 1981 | 60 months | NR | 3 months | 60 months |
| Hanada | 2012 | 6 months (no events) | 6 months | 6 months (no events) | 6 months |
| Heber | 1986 | 12 months | NR | NR | NR |
| ISIS‐1 | 1986 | 20 months | NR | 20 months | 0.23 months (not included) |
| Kaul | 1988 | 6 months | NR | 6 months | 6 months |
| METOCARD‐CNIC | 2013 | 24 months | NR | 24 months | 24 months |
| MILIS | 1984 | 36 months | NR | NR | NR |
| NPT | 1982 | 12 months | NR | 12 months | 12 months |
| RIMA | 1999 | 6 months | 6 months | 6 months | 6 months |
| Salathia | 1985 | 12 months | NR | 12 months | NR |
| TIARA | 1987 | 24 months | NR | 1 month (not included) | 1 month (not included) |
| Tonkin | 1981 | 0.23 months (not included) | NR | NR | 12 months |
| Wilcox | 1980 | 12 months | NR | 12 months | NR |
| Yusuf | 1980 | 24 months | NR | NR | During hospitalisation (no mean time) (not included) |
| Trial | Year | Type and number of serious adverse events (beta‐blocker group) | Type and number of serious adverse events (control group) |
| Andersen | 1979 |
|
|
| Åstrøm | 1986 | None |
|
| Australian trial | 1984 |
|
|
| Australia & Swedish | 1983 |
|
|
| Balcon | 1967 |
|
|
| Barbar | 1967 |
|
|
| Barber | 1976 |
|
|
| BEAT‐AMI | 2016 |
|
|
| Briant | 1970 |
|
|
| Campbell | 1984 |
|
|
| CAPRICORN | 2001 |
|
|
| Clausen | 1966 |
|
|
| COMMIT | 2005 |
|
|
| CPRG | 1980 |
|
|
| EARLY‐BAMI | 2016 |
|
|
| EMIT | 2002 |
|
|
| Evemy | 1977 |
|
|
| Gardtman | 1999 |
|
|
| Göteborg | 1981 |
|
|
| Heber | 1986 |
|
|
| ICSG | 1984 |
|
|
| ISIS‐1 | 1986 |
|
|
| Johannessen | 1987 |
|
|
| Ledwich | 1968 |
|
|
| Lloyd | 1988 |
|
|
| Mcmurray | 1991 |
|
|
| METOCARD‐CNIC | 2013 |
|
|
| Miami | 1985 |
|
|
| MILIS | 1984 |
|
|
| Mueller | 1980 |
|
|
| Multicenter trial | 1966 |
|
|
| Nielsen | 1967 |
|
|
| Norris | 1968 |
|
|
| Norris | 1978 |
|
|
| Norris | 1980 |
|
|
| Norris | 1984 |
|
|
| Owensby | 1985 |
|
|
| Peter | 1978 |
|
|
| Raeder | 1967 |
|
|
| Ramsdale | 1982 | None |
|
| Ranganathan | 1988 |
|
|
| Rolli | 1980 |
|
|
| Salathia | 1985 |
|
|
| Tereschenko | 2005 |
|
|
| Thompson | 1979 |
|
|
| TIARA | 1987 |
|
|
| Tonkin | 1981 |
|
|
| Van De Werf | 1993 |
|
|
| Von Essen | 1982 |
|
|
| Wilcox (atenolol) | 1980 |
|
|
| Wilcox (propranolol) | 1980 |
|
|
| Yang | 1984 |
|
|
| Yusuf | 1980 |
|
|
| Trial | Year | Type and number of serious adverse events (beta‐blocker group) | Type and number of serious adverse events (control group) |
| Andersen | 1979 |
|
|
| Åstrøm | 1986 | None |
|
| Australian trial | 1984 |
|
|
| Australia & Swedish | 1983 |
|
|
| Balcon | 1967 |
|
|
| Barbar | 1967 |
|
|
| Barber | 1976 |
|
|
| Basu | 1997 |
|
|
| BEAT‐AMI | 2016 |
|
|
| Briant | 1970 |
|
|
| Campbell | 1984 |
|
|
| CAPRICORN | 2001 |
|
|
| Clausen | 1966 |
|
|
| COMMIT | 2005 |
|
|
| CPRG | 1980 |
|
|
| EARLY‐BAMI | 2016 |
|
|
| EMIT | 2002 |
|
|
| Evemy | 1977 |
|
|
| Gardtman | 1999 |
|
|
| Göteborg | 1981 |
|
|
| Hanada | 2012 | None |
|
| Heber | 1986 |
|
|
| ICSG | 1984 |
|
|
| ISIS‐1 | 1986 |
|
|
| Johannessen | 1987 |
|
|
| Kaul | 1988 |
|
|
| Ledwich | 1968 |
|
|
| Lloyd | 1988 |
|
|
| Mcmurray | 1991 |
|
|
| METOCARD‐CNIC | 2013 |
|
|
| Miami | 1985 |
|
|
| MILIS | 1984 |
|
|
| Mueller | 1980 |
|
|
| Multicenter trial | 1966 |
|
|
| Nielsen | 1967 |
|
|
| Norris | 1968 |
|
|
| Norris | 1978 |
|
|
| Norris | 1980 |
|
|
| Norris | 1984 |
|
|
| NPT | 1982 |
|
|
| Owensby | 1985 |
|
|
| Peter | 1978 |
|
|
| Raeder | 1967 |
|
|
| Ramsdale | 1982 | None |
|
| Ranganathan | 1988 |
|
|
| RIMA | 1999 |
|
|
| Rolli | 1980 |
|
|
| Salathia | 1985 |
|
|
| Tereschenko | 2005 |
|
|
| Thompson | 1979 |
|
|
| TIARA | 1987 |
|
|
| Tonkin | 1981 |
|
|
| Van De Werf | 1993 |
|
|
| Von Essen | 1982 |
|
|
| Wilcox (atenolol) | 1980 |
|
|
| Wilcox (propranolol) | 1980 |
|
|
| Yang | 1984 |
|
|
| Yusuf | 1980 |
|
|
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 2 All‐cause mortality ‐ Acute/subacute phase Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 2.1 Acute phase | 42 | 76857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.00] |
| 2.2 Subacute phase | 4 | 3595 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.50, 1.25] |
| 3 All‐cause mortality ‐ Reperfusion/no reperfusion Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 3.1 No reperfusion | 40 | 78206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 6 | 2246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.43, 1.50] |
| 4 All‐cause mortality ‐ Type of beta‐blocker Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 4.1 Alprenolol | 2 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.77, 1.66] |
| 4.2 Atenolol | 4 | 16890 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.74, 0.94] |
| 4.3 Esmolol | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.21, 3.62] |
| 4.4 Labetolol | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.60, 41.88] |
| 4.5 Metoprolol | 8 | 55034 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.03] |
| 4.6 Oxprenolol | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.47, 4.03] |
| 4.7 Pindolol | 2 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.49, 2.08] |
| 4.8 Practolol | 3 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.65, 1.60] |
| 4.9 Propranolol | 14 | 2630 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.18] |
| 4.10 Timolol | 7 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.33, 1.31] |
| 4.11 Carvedilol | 2 | 2753 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.25, 1.04] |
| 4.12 Mixed | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.18, 20.63] |
| 5 All‐cause mortality ‐ Intravenously/orally commenced Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 5.1 Intravenously commenced | 4 | 1036 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.46, 2.04] |
| 5.2 Orally commenced | 16 | 5658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.10] |
| 5.3 Mixed | 26 | 73758 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.00] |
| 6 All‐cause mortality ‐ Above/below 75 years of age Show forest plot | 39 | 79161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 6.1 Below 75 years | 24 | 12602 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.74, 0.99] |
| 6.2 Mixed | 15 | 66559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.90, 1.01] |
| 7 All‐cause mortality ‐ NSTEMI/STEMI/UAP Show forest plot | 24 | 53337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.92, 1.03] |
| 7.1 STEMI | 5 | 1828 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.48, 2.10] |
| 7.2 Mixed | 19 | 51509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.92, 1.03] |
| 8 All‐cause mortality ‐ Registration status Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 8.1 Pre‐registration | 5 | 47642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| 8.2 Post‐registration | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.16, 1.63] |
| 8.3 No registration | 40 | 32541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.80, 0.95] |
| 9 All‐cause mortality ‐ Funding Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 9.1 Industry funded or unknown funded trials | 39 | 78702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.00] |
| 9.2 Non‐industry funded trials | 7 | 1750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.20] |
| 10 All‐cause mortality ‐ Trials at low risk of bias Show forest plot | 2 | 46122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| 11 All‐cause mortality ‐ 'Best‐worst case scenario' Show forest plot | 46 | 80522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.89, 0.98] |
| 12 All‐cause mortality ‐ 'Worst‐best case scenario' Show forest plot | 46 | 80522 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.98] |
| 2 All‐cause mortality ‐ Acute/subacute phase Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 2.1 Acute phase | 17 | 21368 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.89, 1.01] |
| 2.2 Subacute phase | 4 | 3842 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.68, 0.96] |
| 3 All‐cause mortality ‐ Reperfusion/no reperfusion Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 3.1 No reperfusion | 16 | 23768 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 5 | 1442 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 4 All‐cause mortality ‐ Type of beta‐blocker Show forest plot | 21 | 25098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 4.1 Alprenolol | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.74, 1.31] |
| 4.2 Atenolol | 3 | 16696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.88, 1.02] |
| 4.3 Carvedilol | 3 | 2899 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.64, 0.96] |
| 4.4 Labetolol | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.71, 4.14] |
| 4.5 Metoprolol | 4 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.09] |
| 4.6 Pindolol | 1 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.67, 1.40] |
| 4.7 Practolol | 2 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.68, 1.33] |
| 4.8 Propranolol | 4 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 4.9 Timolol | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.96] |
| 4.10 Esmolol | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 5 All‐cause mortality ‐ Above/below 75 years of age Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.98] |
| 5.1 Below 75 years | 16 | 5862 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.04] |
| 5.2 Mixed | 5 | 19348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 0.99] |
| 6 All‐cause mortality ‐ Intravenously/orally commenced Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 6.1 Intravenously commenced | 2 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.30, 2.39] |
| 6.2 Orally commenced | 7 | 4614 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.72, 0.96] |
| 6.3 Mixed | 12 | 20226 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.89, 1.01] |
| 7 All‐cause mortality ‐ Registration status Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 7.1 Pre‐registration | 3 | 1164 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.40] |
| 7.2 Post‐registration | 1 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.70, 2.08] |
| 7.3 No registration | 17 | 23777 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.98] |
| 8 All‐cause mortality ‐ NSTEMI/STEMI/UAP Show forest plot | 7 | 2128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.70, 1.18] |
| 8.1 STEMI | 3 | 1164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.51, 1.39] |
| 8.2 Mixed | 4 | 964 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.27] |
| 9 All‐cause mortality ‐ Length of intervention period Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 9.1 0 to 7 days length of intervention | 5 | 16651 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.89, 1.03] |
| 9.2 7 to 30 days length of intervention | 3 | 946 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.60, 1.24] |
| 9.3 1 month and more length of intervention | 13 | 7613 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.80, 0.98] |
| 10 All‐cause mortality ‐ Funding Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.98] |
| 10.1 Industry funded or unknown funded trials | 18 | 23877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.98] |
| 10.2 Non‐industry funded trials | 3 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.69, 1.45] |
| 11 All‐cause mortality ‐ Trials at low risk of bias Show forest plot | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.31, 2.85] |
| 12 All‐cause mortality ‐ 'Best‐worst case scenario' Show forest plot | 21 | 25283 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.97] |
| 13 All‐cause mortality ‐ 'Worst‐best case scenario' Show forest plot | 21 | 25283 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MACE (Composite of cardiovascular death and non‐fatal myocardial infarction) Show forest plot | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MACE (Composite of cardiovascular death and non‐fatal myocardial infarction) Show forest plot | 4 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.43, 1.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 2 Cardiovascular mortality ‐ Acute/subacute phase Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 2.1 Acute phase | 17 | 72309 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.99] |
| 2.2 Subacute phase | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.41, 3.67] |
| 3 Cardiovascular mortality ‐ Reperfusion/no reperfusion Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 3.1 No reperfusion | 15 | 71702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 3 | 920 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.29, 1.62] |
| 4 Cardiovascular mortality ‐ Type of beta‐blocker Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 4.1 Esmolol | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.21, 3.62] |
| 4.2 Atenolol | 2 | 16221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.96] |
| 4.3 Metoprolol | 6 | 54501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.03] |
| 4.4 Oxprenolol | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.41, 3.67] |
| 4.5 Propranolol | 5 | 1103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.65, 1.73] |
| 4.6 Timolol | 2 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.20, 1.39] |
| 5 Cardiovascular mortality ‐ Above/below 75 years of age Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 5.1 Below 75 years | 14 | 56308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.88, 1.03] |
| 5.2 Mixed | 4 | 16314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.97] |
| 6 Cardiovascular mortality ‐ Intravenously/orally commenced Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 6.1 Intravenously commenced | 3 | 766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.42, 2.26] |
| 6.2 Orally commenced | 3 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.54, 1.82] |
| 6.3 Mixed | 12 | 71307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.99] |
| 7 Cardiovascular mortality ‐ NSTEMI/STEMI/UAP Show forest plot | 9 | 49303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.05] |
| 7.1 STEMI | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.35, 2.41] |
| 7.2 NSTEMI | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Unstable angina pectoris | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Mixed | 7 | 48577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.05] |
| 8 Cardiovascular mortality ‐ Registration status Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 8.1 Pre‐registration | 3 | 46578 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.08] |
| 8.2 Post‐registration | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 No registration | 15 | 26044 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.76, 0.93] |
| 9 Cardiovascular mortality ‐ Funding Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 9.1 Industry funded or unknown funded trials | 17 | 72560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.99] |
| 9.2 Non‐industry funded trials | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.11, 62.56] |
| 10 Cardiovascular mortality ‐ Trials with low risk of bias Show forest plot | 1 | 45852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.08] |
| 11 Cardiovascular mortality ‐ 'Best‐worst case scenario' Show forest plot | 18 | 72681 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.72, 0.97] |
| 12 Cardiovascular mortality ‐ 'Worst‐best case scenario' Show forest plot | 18 | 72681 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.79, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.97] |
| 2 Cardiovascular mortality ‐ Acute/subacute phase Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 2.1 Acute phase | 10 | 18615 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.00] |
| 2.2 Subacute phase | 4 | 3842 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.95] |
| 3 Cardiovascular mortality ‐ Reperfusion/no reperfusion Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 3.1 No reperfusion | 8 | 20386 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.97] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 6 | 2071 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.51] |
| 4 Cardiovascular mortality ‐ Type of beta‐blocker Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 4.1 Esmolol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.99] |
| 4.2 Alprenolol | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.22, 4.77] |
| 4.3 Atenolol | 2 | 16219 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 4.4 Carvedilol | 3 | 2899 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.97] |
| 4.5 Metoprolol | 4 | 1827 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.18] |
| 4.6 Pindolol | 1 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.63, 1.40] |
| 4.7 Propranolol | 3 | 806 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.06] |
| 5 Cardiovascular mortality ‐ Above/below 75 years of age Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 5.1 Below 75 years | 11 | 5366 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.68, 0.94] |
| 5.2 Mixed | 3 | 17091 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 6 Cardiovascular mortality ‐ Intravenously/orally commenced Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 6.1 Intravenously commenced | 3 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.33, 1.60] |
| 6.2 Orally commenced | 6 | 4307 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.67, 0.95] |
| 6.3 Mixed | 5 | 17151 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 7 Cardiovascular mortality ‐ NSTEMI/STEMI/UAP Show forest plot | 6 | 2002 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.49, 1.59] |
| 7.1 STEMI | 4 | 1793 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.44, 1.64] |
| 7.2 Mixed | 2 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.27, 3.93] |
| 8 Cardiovascular mortality ‐ Registration status Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 8.1 Pre‐registration | 4 | 1793 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.44, 1.64] |
| 8.2 No registration | 10 | 20664 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.97] |
| 9 Cardiovascular mortality ‐ Length of intervention period Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 9.1 0 to 7 days length of intervention | 4 | 17026 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 9.2 1 month and more length of intervention | 10 | 5431 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.69, 0.94] |
| 10 Cardiovascular mortality ‐ Funding Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 10.1 Industry funded or unknown funded trials | 12 | 21393 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 10.2 Non‐industry funded trials | 2 | 1064 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.36, 2.20] |
| 11 Cardiovascular mortality ‐ 'Best‐worst case scenario' Show forest plot | 14 | 22587 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.53, 0.86] |
| 12 Cardiovascular mortality ‐ 'Worst‐best case scenario' Show forest plot | 14 | 22587 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.83, 1.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarction Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| 2 Myocardial infarction ‐ Acute/subacute phase Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| 2.1 Acute phase | 17 | 67249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.75, 0.90] |
| 2.2 Subacute phase | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.09, 1.25] |
| 3 Myocardial infarction ‐ Reperfusion/no reperfusion Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| 3.1 No reperfusion | 14 | 66372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 4 | 1190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.44, 1.82] |
| 4 Myocardial infarction ‐ Type of beta‐blocker Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 4.1 Esmolol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.06] |
| 4.2 Atenolol | 3 | 12312 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.42] |
| 4.3 Metoprolol | 5 | 53921 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.72, 0.89] |
| 4.4 Oxprenolol | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.09, 1.25] |
| 4.5 Pindolol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.82] |
| 4.6 Propranolol | 2 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.08, 14.01] |
| 4.7 Timolol | 3 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.26, 2.08] |
| 4.8 Xamoterol | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [0.13, 73.06] |
| 4.9 Mixed | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.37, 10.08] |
| 5 Myocardial infarction ‐ Above/below 75 years of age Show forest plot | 17 | 67432 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 5.1 Below 75 years | 13 | 9561 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.89] |
| 5.2 Mixed | 4 | 57871 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.76, 0.94] |
| 6 Myocardial infarction ‐ Intravenously/orally commenced Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 6.1 Intravenously commenced | 3 | 996 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.28, 4.51] |
| 6.2 Orally commenced | 3 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.16, 4.47] |
| 6.3 Mixed | 12 | 66114 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 7 Myocardial infarction ‐ NSTEMI/STEMI/UAP Show forest plot | 11 | 49361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.71, 0.90] |
| 7.1 STEMI | 3 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.29, 3.46] |
| 7.2 NSTEMI | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Unstable angina pectoris | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Mixed | 8 | 48365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.71, 0.89] |
| 8 Myocardial infarction ‐ Registration status Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 8.1 Pre‐registration | 4 | 46848 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 8.2 Post‐registration | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 No registration | 14 | 20714 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| 9 Myocardial infarction ‐ Funding Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 9.1 Industry funded or unknown funded trials | 15 | 67067 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 9.2 Non‐industry funded trials | 3 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.22, 6.77] |
| 10 Myocardial infarction ‐ Trials with low risk of bias Show forest plot | 1 | 45852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.92] |
| 11 Myocardial infarction ‐ 'Best‐worst case scenario' Show forest plot | 18 | 67620 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.61, 0.92] |
| 12 Myocardial infarction ‐ 'Worst‐best case scenario' Show forest plot | 18 | 67620 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarction Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 2 Myocardial infarction ‐ Acute/subacute phase Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 2.1 Acute phase | 10 | 2983 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.72, 1.33] |
| 2.2 Subacute phase | 4 | 3842 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 1.01] |
| 3 Myocardial infarction ‐ Reperfusion/no reperfusion Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 3.1 No reperfusion | 7 | 4658 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.15] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 7 | 2167 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.46, 1.66] |
| 4 Myocardial infarction ‐ Type of beta‐blocker Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 4.1 Esmolol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.06] |
| 4.2 Alprenolol | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.28, 3.81] |
| 4.3 Landiolol | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.01, 8.32] |
| 4.4 Carvedilol | 3 | 2899 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.91] |
| 4.5 Metoprolol | 4 | 2426 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.84, 1.24] |
| 4.6 Pindolol | 1 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.61, 1.38] |
| 4.7 Propranolol | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.08, 3.15] |
| 4.8 Timolol | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 6.57 [0.82, 52.35] |
| 5 Myocardial infarction ‐ Above/below 75 years of age Show forest plot | 13 | 6729 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.72, 1.09] |
| 5.1 Below 75 years | 11 | 5665 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.10] |
| 5.2 Mixed | 2 | 1064 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.45, 4.07] |
| 6 Myocardial infarction ‐ Intravenously/orally commenced Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 6.1 Intravenously commenced | 4 | 1095 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.26, 3.29] |
| 6.2 Orally commenced | 6 | 4007 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.17] |
| 6.3 Mixed | 4 | 1723 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.50] |
| 7 Myocardial infarction ‐ NSTEMI/STEMI/UAP Show forest plot | 7 | 2098 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.57, 2.18] |
| 7.1 STEMI | 5 | 1889 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.45, 2.54] |
| 7.2 Mixed | 2 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.42, 3.40] |
| 8 Myocardial infarction ‐ Registration status Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 8.1 Pre‐registration | 4 | 1793 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.48, 2.88] |
| 8.2 No registration | 10 | 5032 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.68, 1.09] |
| 9 Myocardial infarction ‐ Length of intervention period Show forest plot | 14 | 6913 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.71, 1.13] |
| 9.1 0 to 7 days length of intervention | 5 | 1183 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.46, 4.96] |
| 9.2 7 to 30 days length of intervention | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 6.57 [0.82, 52.35] |
| 9.3 1 month and more length of intervention | 9 | 5642 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.74, 1.06] |
| 10 Myocardial infarction ‐ Funding Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.04] |
| 10.1 Industry funded or unknown funded trials | 11 | 5665 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.04] |
| 10.2 Non‐industry funded trials | 3 | 1160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.31, 2.04] |
| 11 Myocardial infarction ‐ 'Best‐worst case scenario' Show forest plot | 14 | 6951 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.85] |
| 12 Myocardial infarction ‐ 'Worst‐best case scenario' Show forest plot | 14 | 6951 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.95, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Angina on a dichotomous scale Show forest plot | 3 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.31, 1.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Angina on a dichotomous scale Show forest plot | 2 | 844 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.18, 2.30] |

