Beta‐blokatori za dijagnosticirani ili vjerojatni infarkt miokarda
Abstract
Background
Cardiovascular disease is the number one cause of death globally. According to the World Health Organization, 7.4 million people died from ischaemic heart diseases in 2012, constituting 15% of all deaths. Acute myocardial infarction is caused by blockage of the blood supplied to the heart muscle. Beta‐blockers are often used in patients with acute myocardial infarction. Previous meta‐analyses on the topic have shown conflicting results ranging from harms, neutral effects, to benefits. No previous systematic review using Cochrane methodology has assessed the effects of beta‐blockers for acute myocardial infarction.
Objectives
To assess the benefits and harms of beta‐blockers compared with placebo or no intervention in people with suspected or diagnosed acute myocardial infarction.
Search methods
We searched CENTRAL, MEDLINE, Embase, LILACS, Science Citation Index Expanded and BIOSIS Citation Index in June 2019. We also searched the WHO International Clinical Trials Registry Platform, ClinicalTrials.gov, Turning Research into Practice, Google Scholar, SciSearch, and the reference lists of included trials and previous reviews in August 2019.
Selection criteria
We included all randomised clinical trials assessing the effects of beta‐blockers versus placebo or no intervention in people with suspected or diagnosed acute myocardial infarction. Trials were included irrespective of trial design, setting, blinding, publication status, publication year, language, and reporting of our outcomes.
Data collection and analysis
We followed the Cochrane methodological recommendations. Four review authors independently extracted data. Our primary outcomes were all‐cause mortality, serious adverse events according to the International Conference on Harmonization ‐ Good Clinical Practice (ICH‐GCP), and major adverse cardiovascular events (composite of cardiovascular mortality and non‐fatal myocardial infarction during follow‐up). Our secondary outcomes were quality of life, angina, cardiovascular mortality, and myocardial infarction during follow‐up. Our primary time point of interest was less than three months after randomisation. We also assessed the outcomes at maximum follow‐up beyond three months. Due to risk of multiplicity, we calculated a 97.5% confidence interval (CI) for the primary outcomes and a 98% CI for the secondary outcomes. We assessed the risks of systematic errors through seven bias domains in accordance to the instructions given in the Cochrane Handbook. The quality of the body of evidence was assessed by GRADE.
Main results
We included 63 trials randomising a total of 85,550 participants (mean age 57.4 years). Only one trial was at low risk of bias. The remaining trials were at high risk of bias. The quality of the evidence according to GRADE ranged from very low to high. Fifty‐six trials commenced beta‐blockers during the acute phase of acute myocardial infarction and seven trials during the subacute phase.
At our primary time point 'less than three months follow‐up', meta‐analysis showed that beta‐blockers versus placebo or no intervention probably reduce the risk of a reinfarction during follow‐up (risk ratio (RR) 0.82, 98% confidence interval (CI) 0.73 to 0.91; 67,562 participants; 18 trials; moderate‐quality evidence) with an absolute risk reduction of 0.5% and a number needed to treat for an additional beneficial outcome (NNTB) of 196 participants. However, we found little or no effect of beta‐blockers when assessing all‐cause mortality (RR 0.94, 97.5% CI 0.90 to 1.00; 80,452 participants; 46 trials/47 comparisons; high‐quality evidence) with an absolute risk reduction of 0.4% and cardiovascular mortality (RR 0.99, 95% CI 0.91 to 1.08; 45,852 participants; 1 trial; moderate‐quality evidence) with an absolute risk reduction of 0.4%. Regarding angina, it is uncertain whether beta‐blockers have a beneficial or harmful effect (RR 0.70, 98% CI 0.25 to 1.84; 98 participants; 3 trials; very low‐quality evidence) with an absolute risk reduction of 7.1%. None of the trials specifically assessed nor reported serious adverse events according to ICH‐GCP. Only two trials specifically assessed major adverse cardiovascular events, however, no major adverse cardiovascular events occurred in either trial.
At maximum follow‐up beyond three months, meta‐analyses showed that beta‐blockers versus placebo or no intervention probably reduce the risk of all‐cause mortality (RR 0.93, 97.5% CI 0.86 to 0.99; 25,210 participants; 21 trials/22 comparisons; moderate‐quality evidence) with an absolute risk reduction of 1.1% and a NNTB of 91 participants, and cardiovascular mortality (RR 0.90, 98% CI 0.83 to 0.98; 22,457 participants; 14 trials/15 comparisons; moderate‐quality evidence) with an absolute risk reduction of 1.2% and a NNTB of 83 participants. However, it is uncertain whether beta‐blockers have a beneficial or harmful effect when assessing major adverse cardiovascular events (RR 0.81, 97.5% CI 0.40 to 1.66; 475 participants; 4 trials; very low‐quality evidence) with an absolute risk reduction of 1.7%; reinfarction (RR 0.89, 98% CI 0.75 to 1.08; 6825 participants; 14 trials; low‐quality evidence) with an absolute risk reduction of 0.9%; and angina (RR 0.64, 98% CI 0.18 to 2.0; 844 participants; 2 trials; very low‐quality evidence). None of the trials specifically assessed nor reported serious adverse events according to ICH‐GCP.
None of the trials assessed quality of life.
We identified two ongoing randomised clinical trials investigating the effect of early administration of beta‐blockers after percutaneous coronary intervention or thrombolysis to patients with an acute myocardial infarction and one ongoing trial investigating the effect of long‐term beta‐blocker therapy.
Authors' conclusions
Our present review indicates that beta‐blockers for suspected or diagnosed acute myocardial infarction probably reduce the short‐term risk of a reinfarction and the long‐term risk of all‐cause mortality and cardiovascular mortality. Nevertheless, it is most likely that beta‐blockers have little or no effect on the short‐term risk of all‐cause mortality and cardiovascular mortality. Regarding all remaining outcomes (serious adverse events according to ICH‐GCP, major adverse cardiovascular events (composite of cardiovascular mortality and non‐fatal myocardial infarction during follow‐up), the long‐term risk of a reinfarction during follow‐up, quality of life, and angina), further information is needed to confirm or reject the clinical effects of beta‐blockers on these outcomes for people with or suspected of acute myocardial infarction.
PICOs
Laički sažetak
Beta‐blokatori u usporedbi s placebom ili izostankom kontrole u pacijenata koji imaju vjerojatni ili dijagnosticirani infarkt miokarda
Dosadašnje spoznaje
Prema Svjetskoj zdravstvenoj organizaciji, 7,4 milijuna ljudi je umrlo od ishemijske bolesti srca 2012. godine, što je 15% svih smrti. Uporaba lijekova koji se nazivaju beta‐blokatori u akutnom ili subakutnom liječenju osoba kod kojih se sumnja na, ili im je dijagnosticiran infarkt miokarda, temelji se na inhibiciji beta‐receptora. Takvo djelovanje bi moglo smanjiti potrebu srca za kisikom. Inhibicija beta‐receptora možda utječe na smanjenje komplikacija srčanog udara.
Istraživačko pitanje
Cilj ovog Cochraneovog sustavnog pregleda bio je procijeniti koristi i štete uporabe beta‐blokatora u ljudi kod kojih je postavljena sumnja na ili im je dijagnosticiran infarkt miokarda (srčani udar).
Datum pretraživanja dokaza
Autori su pretražili znanstvene baze od njihovog nastanka pa do lipnja 2019. godine.
Značajke uključenih istraživanja
Autori su pronašli 63 randomizirana klinička ispitivanja u kojima su sudionici sa dijagnozom ili postavljenom sumnjom na srčani udar nasumično dodijeljeni u skupine koje primaju ili beta‐blokatore ili placebo, odnosno ništa. 63 istraživanja je uključilo 85,550 odraslih osoba prosječne dobi 57,4 godina. Samo je jedno istraživanje bilo niskog rizika pristranosti. Ostala su istraživanja bila visokog rizika pristranosti. Kvaliteta dokaza procijenjena je GRADE pristupom i kretala se od vrlo niske do visoke. U 56 istraživanja beta‐blokatori su primijenjeni u akutnoj fazi akutnog infarkta miokarda i uspoređeni s kontrolom. U sedam istraživanja beta‐blokatori primijenjeni su u subakutnoj fazi infarkta miokarda.
Izvori financiranja istraživanja
Autori su pronašli 33 istraživanja koja su djelomično ili potpuno financirana od strane farmaceutske industrije, 20 istraživanja nije izvijestilo o izvoru financiranja, a 10 istraživanja je bilo financirano iz drugih izvora.
Ključni rezultati i zaključak
Ovaj sustavni pregled pokazuje da osobe koje primaju beta‐blokatore, u usporedbi s onima koji primaju placebo ili nikakvu intervenciju, imaju niži rizik od razvoja novog srčanog udara u akutnoj fazi nakon srčanog udara. Osobe koje primaju beta‐blokatore nakon srčanog udara imaju niži rizik od smrti od bilo kojeg uzroka, kao i od smrti srčanog uzroka tijekom dugotrajnog praćenja. Unatoč tome, osobe koje primaju beta‐blokatore nemaju niži ili viši rizik od smrti od bilo kojeg uzroka ili bilo kojeg uzroka smrti povezanog sa srcem, u akutnoj fazi nakon srčanog udara. Učinci beta‐blokatora na ostale ishode (ozbiljni neželjeni učinci prema Međunarodnoj konferenciji o usklađivanju ‐ Dobra Klinička Praksa, glavni neželjeni kardiovaskularni događaji (zbirni ishod smrti od srčanog uzroka ili novi nesmrtonosni srčani udar), novi srčani udar tijekom dugotrajnog praćenja, kvaliteta života i angina) neizvjesni su zbog nedovoljno podataka.
Authors' conclusions
Summary of findings
| Beta‐blockers versus placebo or no intervention for patients with suspected or diagnosed myocardial infarction at the time point less than three months follow‐up | ||||||
| Patient or population: patients with suspected or diagnosed myocardial infarction Settings: any setting Intervention: any beta‐blocker Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect (adjusted CI) | No of Participants | Quality of the evidence | Comments | |
| Assumed risk with placebo or no intervention | Corresponding risk with beta‐blockers | |||||
| All‐cause mortality at 'less than 3 months' follow‐up. Follow‐up: mean 21.8 days (range 1 hour to 90 days). | 70 per 1000 | 67 per 1000 | RR 0.94, 97.5% CI (0.90 to 1.0) | 80,452 (46 RCTs with 47 comparisons) | ⊕⊕⊕⊕1 | Since the sensitivity analysis excluding trials at high risk of bias and the overall meta‐analysis showed similar results, we based our summary of findings and conclusion on the overall meta‐analysis. No events occurred in either group in three trials (Hanada 2012; Norris 1978; Shirotani 2010). |
| Serious adverse events at 'less than 3 months' follow‐up. No data was reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | None of the trials specifically assessed nor reported serious adverse events according to ICH‐GCP. |
| MACE (major adverse cardiovascular event) at 'less than 3 months' follow‐up. No data were reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | Only two trials specifically assessed major adverse cardiovascular events (defined as a composite of cardiovascular mortality and myocardial infarction during follow‐up). However, no major adverse cardiovascular events occurred in either trial. |
| Quality of life at 'less than 3 months' follow‐up. No data were reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | No data reported. |
| Angina at 'less than 3 months' follow‐up. Follow‐up: mean 21 days (range 12 to 30 days). | 222 per 1000 | 155 per 1000 (69 to 351) | RR 0.70, 98% CI (0.25 to 1.84) | 98 (3 RCTs) | ⊕⊝⊝⊝2,3 VERY LOW | |
| Cardiovascular mortality at 'less than 3 months' follow‐up. Follow‐up: mean 28 days. | 43 per 1000 | 42 per 1000 | RR 0.99, 95% CI (0.91 to 1.08) | 45,852 (1 RCT) | ⊕⊕⊕⊝4 | Since the sensitivity analysis excluding trials at high risk of bias differed from the overall meta‐analysis, we based our summary of findings and conclusion on the sensitivity analysis. |
| Myocardial infarction at 'less than 3 months' follow‐up. Follow‐up: mean 23.3 days (range 3 to 90 days). | 28 per 1000 | 23 per 1000 | RR 0.82, 98% CI (0.74 to 0.90) | 67,562 (18 RCTs) | ⊕⊕⊕⊝5 | Since the sensitivity analysis excluding trials at high risk of bias and the overall meta‐analysis showed similar results, we based our summary of findings and conclusion on the overall meta‐analysis. No events occurred in either group in two trials (Hanada 2012; Shirotani 2010). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its adjusted confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its adjusted CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 When assessing the risk of bias, the trial contributing most weight (COMMIT 2005, 63.4%) was assessed as at low risk of bias in all domains. The trial contributing the second highest weight (ISIS‐1 1986, 17.4%) was assessed as low risk of bias in random sequence generation, allocation concealment, and incomplete outcome data;'unclear for blinding of outcome assessors and selective reporting and at high risk for blinding of participants and personnel. Since a lack of blinding is less important for the assessment of all‐cause mortality, the overall limitations were not serious and the evidence is not downgraded for risk of bias. 2 Downgraded by one level due to serious risk of bias. All the included trials were at high risk of bias due to either unclear or high risk in several bias domains. 3 Downgraded by two levels due to very serious risk of imprecision based on the optimal information size not being reached, the very small sample size, and the absolute and relative 98% CI being very wide showing both appreciable benefit and harm. 4 Downgraded by one level due to serious risk of imprecision based on the wide absolute and relative 98% where the upper CI does not exclude the possibility of no difference between the groups. When assessing the risk of bias, the evidence was not downgraded since the result was based on the sensitivity analysis consisting of trials at low risk of bias (COMMIT 2005). 5 Downgraded by one level due to serious risk of bias. The overall limitations and specially in regard to blinding of outcome assessors were serious (around 50% of the trials were assessed at unclear risk of bias in blinding of outcome assessors). | ||||||
| Beta‐blockers compared with placebo or no intervention for patients with suspected or diagnosed myocardial infarction | ||||||
| Patient or population: patients with suspected or diagnosed myocardial infarction Settings: any setting Intervention: beta‐blockers Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk with placebo or no intervention | Corresponding risk with beta‐blockers | |||||
| All‐cause mortality at maximum follow‐up beyond 3 months. Follow‐up: mean 16.4 months (range 6 to 60 months). | 148 per 1000 | 138 per 1000 | RR 0.93, 97.5% CI (0.86 to 0.99) | 25,210 (21 RCTs with 22 comparisons) | ⊕⊕⊕⊝3 | No events occurred in either group in one trial (Hanada 2012). |
| Serious adverse events at maximum follow‐up beyond 3 months. No data were reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | None of the trials specifically assessed nor reported serious adverse events according to ICH‐GCP. |
| MACE (major adverse cardiovascular event) at maximum follow‐up beyond 3 months. Follow‐up: mean7.5 months (range 6 to 12 months). | 84 per 1000 | 68 per 1000 | RR 0.81, 97.5% CI (0.43 to 1.52) | 475 (4 RCTs) | ⊕⊝⊝⊝1, 2 | |
| Quality of life at maximum follow‐up beyond 3 months. No data were reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | No data reported. |
| Angina at maximum follow‐up beyond 3 months (mean = 6 months). | 24 per 1000 | 15 per 1000 (5 to 48) | RR 0.64, 98% CI 0.18 to 2.0 | 844 ( 2 RTCs) | ⊕⊝⊝⊝1,5 | |
| Cardiovascular mortality at maximum follow‐up beyond 3 months. Follow‐up: mean 12.9 months (range 6 to 24 months). | 124 per 1000 | 112 per 1000 | RR 0.90, 98% CI (0.83 to 0.98) | 22,457 (14 RCTs with 15 comparisons) | ⊕⊕⊕⊝1 | No events occurred in either group in one trial (Hanada 2012). |
| Myocardial infarction at maximum follow‐up beyond 3 months. Follow‐up: mean 15.5 months (range 6 to 60 months). | 92 per 1000 | 83 per 1000 | RR 0.89, 98% CI (0.75 to 1.08) | 6825 (14 RCTs) | ⊕⊕⊝⊝1, 6 Low | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to serious risk of bias. All the included trials were at high risk of bias due to either unclear or high risk in several bias domains. 2 Downgraded by two levels due to very serious risk of imprecision based on the optimal information size not being reached, the wide absolute and relative 97.5% CI showing both appreciable benefit and harm, and a small sample size. 3 Downgraded by one level due to serious risk of bias. All but one of the included trials were at high risk of bias due to either unclear or high risk in several bias domains and the sensitivity analysis excluding trials at high risk of bias showed different results than the overall analysis including trials at high risk of bias. However, the sensitivity analysis was based on only one small trial, so we have used the main analysis for the 'Summary of findings' table. 5 Downgraded by two levels due to very serious risk of imprecision based on the very small sample size included. 6 Downgraded by one level due to serious risk of imprecision based on the wide absolute and relative 98% where the upper CI does not exclude the possibility of no difference between the groups. | ||||||
Background
Description of the condition
Cardiovascular disease is the number one cause of death globally (Cooper 2000; Lloyd‐Jones 2010; Nichols 2014; Rosamond 2008; Schmidt 2012). Ischaemic heart disease accounts for almost 50% of the disease burden of the cardiovascular diseases (Nichols 2014). According to the World Health Organization (WHO), 7.4 million people died from ischaemic heart disease in 2012 (WHO 2015).
Ischaemic heart disease is caused by different underlying mechanisms: (1) atherosclerotic plaque‐related obstruction of the coronary arteries; (2) focal or diffuse spasms of normal or plaque‐diseased arteries; (3) microvascular dysfunction; and (4) left ventricular dysfunction caused by acute myocardial necrosis or ischaemic cardiomyopathy (Montalescot 2013). Ischaemic heart disease increases the risk of stable angina pectoris and acute coronary syndrome (see below).
Acute coronary syndrome is a collective term for: (1) unstable angina pectoris (chest pain during rest related to ischaemia or hypoxia of the heart muscle (Roffi 2016)); (2) acute non‐ST‐elevation myocardial infarction (NSTEMI); or (3) acute ST‐elevation myocardial infarction (STEMI) (O'Gara 2013; Steg 2012). Myocardial infarction is caused by death of cardiac myocytes (myocardial necrosis) due to ischaemia (Roffi 2016; Steg 2012). The clinical definition of myocardial infarction is elevated serum levels of cardiac biomarkers (cardiac specific troponins and CK‐MB among others) and changes of the ST‐segment on an electrocardiogram (ECG) (STEMI and NSTEMI) or symptoms of cardiac ischaemia (Roffi 2016; Steg 2012).
The diagnosis of myocardial infarction is dependent on an elevation of the serum levels of cardiac‐specific troponin I, troponin T, or the myocardial band (MB) isoenzyme of creatine kinase (CK‐MB), among others (Roffi 2016; Steg 2012). However, it will often take eight to 24 hours after the first symptoms of the myocardial infarction occur before these enzymes are detectable in serum. Beta‐blockers may accordingly be commenced as an intervention in patients suspected of myocardial infarction or may be commenced as an intervention in patients diagnosed with myocardial infarction.
The cause of myocardial infarction is generally divided in to five main classes (Thygesen 2012).
-
Type 1: spontaneous myocardial infarction related to atherosclerotic plaque rupture, ulceration, fissuring, erosion, or dissection with resulting intraluminal thrombus in one or more of the coronary arteries often caused by coronary artery disease.
-
Type 2: myocardial infarction secondary to an ischaemic imbalance such as coronary artery spasm, coronary embolism, anaemia, arrhythmias, hypertension, or hypotension.
-
Type 3: myocardial infarction with symptoms suggestive of myocardial ischaemia and resulting in sudden unexpected cardiac death when biomarker values are unavailable or could not be obtained before death.
-
Type 4a: myocardial infarction associated with percutaneous coronary intervention (PCI).
-
Type 4b: myocardial infarction associated with stent thrombosis as documented by angiography or at autopsy.
-
Type 5: myocardial infarction associated with coronary artery bypass graft (CABG).
Major complications associated with myocardial infarction
-
Life‐threatening ventricular arrhythmias caused by changes in the electrophysiologic characteristics of the myocyte, electrolyte imbalance, continuous ischaemia, and variations in heart rate all due to obstruction and hence, reduced flow to the myocardium and myocardial necrosis (Brieger 2009; Stevenson 1989).
-
Mechanical complications caused by necrosis of the myocardium such as ventricular wall rupture, septum rupture, and papillary muscle rupture (Brieger 2009; Pohjola‐Sintonen 1989; Stevenson 1989).
-
Cardiogenic shock caused by failure of the ventricle to pump adequate amount of blood leading to a systemic hypotension (Brieger 2009; Stevenson 1989).
-
Acute decompensated heart failure caused by impairment in systolic and diastolic function due to myocardial ischaemia (Brieger 2009).
-
Depression (Thombs 2006).
Description of the intervention
The discovery of the difference between adrenergic receptors by Raymond Ahlquist in 1948 led Sir James Black to develop the first clinically useful beta‐receptor blocker (propranolol) in 1964 (Ahlquist 1948; Black 1964). This discovery was awarded the Nobel Prize in 1988 (Quirke 2006). Beta‐blockers are classified as non‐selective beta‐blockers or selective beta‐blockers according to their selectivity for one of the three subtypes of beta‐receptors.
-
The beta1‐receptor is mainly located in: (1) the heart, where it induces positive chronotropic effects (increases heart rate) and positive inotropic effects (increases contractility of the myocardium); and (2) in the kidneys where activation of the beta1‐receptor results in an increased release of renin which in turn increases blood pressure, among other effects (Golan 2011; Marlin 1975; Singh 1975).
-
The beta2‐receptor is mainly located in smooth muscle cells where it promotes relaxation, in skeletal muscle cells where it promotes tremor and increased glycogenolysis, and in the liver, where it increases glycogenolysis (Golan 2011).
-
The beta3‐receptor is mainly located in adipose tissue where it primarily induces lipolysis (Golan 2011).
Beta‐blockers may be administered both intravenously and orally. Three different classes of beta‐blockers exist: (1) the first generation non‐selective beta‐blockers (e.g. propranolol, oxprenolol, sotalol, timolol) affecting all beta‐receptors; (2) the second generation selective beta1‐blockers (e.g. metoprolol, bisoprolol, acebutolol, atenolol, esmolol) mainly affecting the heart; and (3) the third generation beta‐blockers which have combined non‐selective beta‐blocking effects and alpha‐blocking effects (e.g. carvedilol) affecting all beta‐receptors plus alpha‐receptors in the vessels lowering the blood pressure.
Several beta‐blockers have been used in the management of patients with myocardial infarction. The first beta‐blockers used were the non‐selective beta‐blockers (e.g. propranolol) (Clausen 1966b; Friedman 1986). Today, the most frequently used beta‐blockers for managing myocardial infarction are the cardiac‐specific beta1‐blockers (Chen 2005; Roffi 2016; Steg 2012).
How the intervention might work
The beta‐receptor is an adrenergic heterodimeric G‐protein‐coupled receptor (G protein‐coupled receptors are transmembrane proteins that act as key gatekeepers between external signals and cellular responses), located throughout the body. Beta‐receptors are stimulated by the sympathetic nervous system with catecholamines epinephrine (adrenaline) and norepinephrine (noradrenaline) as their primary endogenous agonists. The role of acute treatment or subacute treatment with beta‐blockers in patients suspected of or diagnosed with myocardial infarction, rests on their inhibition of the chronotropic and inotropic effects of the beta‐receptor. This may result in a reduction in heart rate, heart contractility, and blood pressure, thereby decreasing the oxygen demand of the heart (Lopez‐Sendon 2004). Hence, the inhibition of the beta‐receptor is thought to decrease ischaemia and might decrease the risk of life‐threatening ventricular arrhythmias and other complications associated with myocardial infarction (Roffi 2016; Steg 2012).
Why it is important to do this review
The prevalence of ischaemic heart disease is considerable. According to the WHO, 7.4 million people died from ischaemic heart disease in 2012 (Lloyd‐Jones 2010; Nichols 2014; Rosamond 2008; WHO 2015). A considerable reduction in disease burden and healthcare cost may therefore be alleviated by effective treatment. However, as demonstrated below, previous meta‐analyses and guidelines show contrasting findings and recommendations.
Evidence on the effects of beta‐blockers for suspected or diagnosed acute myocardial infarction
Outcomes assessed at hospital discharge
Five studies have compared the effects of beta‐blockers versus placebo, standard medical therapy, or late administration of beta‐blockers in participants with suspected or diagnosed myocardial infarction on outcomes reported at hospital discharge (Al‐Reesi 2008; Brandler 2010; Chatterjee 2013; Freemantle 1999; Yusuf 1985). While Chatterjee 2013 only assessed intravenously assessed beta‐blockers and showed a beneficial effect of early beta‐blockers on mortality, Al‐Reesi 2008, Brandler 2010, and Freemantle 1999 assessed any type of beta‐blockers and could not demonstrate a beneficial effect of beta‐blockers on mortality. Yusuf 1985 assessed the effects of beta‐blockers on the size of myocardial infarction and showed a beneficial effect when compared with no beta‐blockers. One of the meta‐analyses showed a beneficial effect of beta‐blockers on the risk of myocardial reinfarction and ventricular arrhythmia, while no beneficial or harmful effects were found on cardiogenic shock (Chatterjee 2013). Al‐Reesi 2008, Brandler 2010, and Freemantle 1999 did not assess the effects of beta‐blockers on the risk of myocardial reinfarction, ventricular arrhythmias, or cardiogenic shock.
Long‐term outcomes
Three studies compared the effects of beta‐blockers versus no beta‐blockers in participants with suspected or diagnosed myocardial infarction on long‐term outcomes (Bangalore 2014; Freemantle 1999; Yusuf 1985). While Freemantle 1999 and Yusuf 1985 showed a beneficial effect of beta‐blockers on mortality, Bangalore 2014 only found a beneficial effect on mortality in trials where the participants did not receive reperfusion in the form of revascularisation (percutaneous coronary intervention or coronary artery bypass graft) or thrombolytics (e.g. streptokinase). Bangalore 2014 found a beneficial effect of beta‐blockers on symptoms of angina and risk of recurrent myocardial infarction regardless of whether the participants received intervention for reperfusion (revascularisation or thrombolytics) or not. However, Bangalore 2014 also showed that beta‐blockers seem to increase the severity of heart failure in participants receiving intervention for reperfusion (revascularisation or thrombolytics). It must be noted that Bangalore 2014 included a larger number of trials than Freemantle 1999 and Yusuf 1985, and only Bangalore 2014 included trials after the introduction of reperfusion strategies around 1990s.
No newer studies assessing beta‐blocker treatment for patients with suspected or diagnosed myocardial infarction consisting of randomised clinical trials including different types of beta‐blocker interventions and types of myocardial infarctions have been conducted since 2014. Hoedemaker 2019 and Elgendy 2016 each included four randomised clinical trials assessing only intravenously‐assessed beta‐blocker treatment in STEMI patients. Two other studies, Dahl 2019 and Misumida 2016, only included cohort studies and observational studies, respectively.
Despite these studies, the question of whether or not beta‐blockers should be administered in the acute phase of an acute myocardial infarction has not yet been sufficiently answered. The above‐mentioned studies show conflicting results and suggest that more randomised clinical trials are needed to determine the effects of beta‐blockers. Hence, this review is of uttermost importance and is the first to take fully account of all existing randomised clinical trials assessing the effects and harms of beta‐blockers for acute myocardial infarction.
Beta‐blockers for other conditions
The role of beta‐blockers for other conditions than myocardial infarction is still debated. Beta‐blockers used to be contraindicated in patients with congestive heart failure. Beta‐blockers and non‐selective combined alpha‐ and beta‐blockers are now a part of standard treatment of congestive heart failure (Chatterjee 2013a; Yancy 2013).
Beta‐blockers are also considered an option in the treatment of hypertension, but are rarely used as first‐line treatment (Mancia 2013). A recent Cochrane Review found that beta‐blockers were inferior when compared with other antihypertensive drugs (Wiysonge 2012). Non‐selective beta‐blockers are used in the treatment of anxiety due to their effect on decreasing tremor and tachycardia (Turner 1994).
The adverse effects of beta‐blockers include both cardiac adverse effects and non‐cardiac adverse effects. Among the most serious cardiac adverse effects is exacerbation of heart failure in patients with acute decompensated heart failure, due to the need of sympathetic activity to maintain the cardiac output (Taylor 1982). In addition, beta‐blocker withdrawal has also been shown to cause exacerbation of ischaemic symptoms and precipitate acute myocardial infarction in patients with ischaemic heart disease (Houston 1981).
Perioperative beta‐blockade for major non‐cardiac surgery in patients with risk factors for ischaemic heart disease has been tested in several trials (Bangalore 2008; Devereaux 2008; Juul 2006), and seems to increase 30‐day all‐cause mortality, increase the risk of stroke, although the risk of non‐fatal myocardial infarction seems to be reduced (Bangalore 2008).
Studies of individual patients have suggested that depression, fatigue, and sexual dysfunction are among the beta‐blocker induced non‐cardiac adverse effects (Greenblatt 1974; Waal 1967; Warren 1977). However, a meta‐analysis comparing beta‐blockers versus placebo showed no difference on depressive symptoms and only a minor increase in sexual dysfunction and fatigue in patients randomised to beta‐blockers compared with placebo (Ko 2002).
Current guidelines for using beta‐blockers in patients with suspected or diagnosed myocardial infarction
The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guideline recommends acute intravenous beta‐blockers in patients suspected of STEMI who are hypertensive or have ongoing ischaemia, unless there are contraindications to beta‐blockers (allergy towards beta‐blockers, signs of acute decompensated heart failure, increased risk of cardiogenic shock, atrio‐ventricular block, asthma, or chronic obstructive lung disease) (O'Gara 2013). The guideline recommends oral beta‐blockers within the first 24 hours in patients with a STEMI and no contraindications (see above) (O'Gara 2013).
The ACCF/AHA guideline does not recommend acute intravenous beta‐blockers in patients suspected of acute NSTEMI and advise against intravenous beta‐blockers in acute patients with risk factors for cardiogenic shock (Amsterdam 2014). However, the guideline recommends oral beta‐blockers commenced within the first 24 hours in patients with a NSTEMI or unstable angina pectoris and no contraindications (see above) (Amsterdam 2014).
Former meta‐analyses have shown conflicting results and no former reviews have used Cochrane methodology to assess the effects of beta‐blockers as an acute intervention in patients suspected or diagnosed with myocardial infarction. The present systematic review has been the first to use the GRADE approach to assess the quality of a body of evidence associated with each of the outcomes, to assess the risk of systematic errors ('bias'), design errors, and risks of random errors ('play of chance'), and to include trials irrespective of outcome, follow‐up duration, number of participants, language, and publication status (Guyatt 2008; Higgins 2011; Schünemann 2013).
Objectives
We assessed the benefits and harms of beta‐blockers compared with placebo or no intervention in people with suspected or diagnosed acute myocardial infarction.
The present review is based on our peer‐reviewed, published protocol (Nielsen 2016) with amendments during the review process (see Differences between protocol and review).
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised clinical trials (RCTs) irrespective of trial design, setting, blinding, publication status, publication year, language, and reported outcomes.
Types of participants
We included any participant (irrespective of age and sex) with suspected or diagnosed acute myocardial infarction (as defined by trialists).
Types of interventions
We included three types of comparisons:
-
beta‐blockers compared with placebo;
-
beta‐blockers compared with no intervention (including no placebo tablet); and
-
beta‐blockers added to a co‐intervention compared with a similar co‐intervention.
We accepted any type of beta‐blocker (intravenous therapy or oral administration) commenced in the acute or subacute phase of acute myocardial infarction (non‐selective beta‐blockers (propranolol, oxprenolol, sotalol, timolol); selective beta1‐blockers (metoprolol, bisoprolol, acebutolol, atenolol, esmolol); and beta‐blockers which are combined alpha‐ and non‐selective beta‐blockers (carvedilol)) as the experimental intervention, irrespective of dose, route of administration, and duration.
We accepted any type of co‐intervention (medical therapy as well as revascularisation strategies) provided they were intended to be delivered similarly to the experimental and the control groups.
Types of outcome measures
We assessed all outcomes at two time points:
-
less than three months after randomisation (this was the time point of primary interest). If multiple time points were reported at less than three months, we chose the one closest to one‐month follow‐up.
-
maximum follow‐up beyond three months.
We chose 'less than three months follow‐up' as our primary follow‐up time point because the possible effects of beta‐blockers need some time to show, and the follow‐up period is not too long so other factors unrelated to the given trial affecting the outcomes might decrease the statistical power, i.e. the results are 'diluted' by events (e.g. traffic accidents) unrelated to the trial.
Primary outcomes
-
All‐cause mortality.
-
Serious adverse events. We defined a serious adverse event as any untoward medical occurrence that: resulted in death; was life‐threatening; required hospitalisation or prolongation of existing hospitalisation; resulted in persistent or significant disability; or jeopardised the participant according to the International Conference on Harmonization ‐ Good Clinical Practice (ICH‐GCP Guidelines) (ICH‐GCP 1997). None of the trials specifically assessed serious adverse events according to the definition by ICH‐GCP. Instead, the trials either reported composites of several specific serious adverse events or one specific serious adverse event.
-
Major adverse cardiovascular events (MACE), defined as a composite outcome consisting of cardiovascular mortality (defined by trialists) and non‐fatal myocardial infarction during follow‐up (defined by trialists). Additionally, we assessed cardiovascular mortality and myocardial infarction during follow‐up separately as secondary outcomes (see below).
Secondary outcomes
-
Quality of life measured on any valid continuous scale, such as the Short‐Form (36) Health Survey (SF‐36) (Ware 1992). None of the trials adequately assessed quality of life.
-
Angina measured on any valid scale, such as the Canadian Cardiovascular Angina Score (CCS) (Campeau 1976).
-
Cardiovascular mortality.
-
Myocardial infarction during follow‐up (i.e. reinfarction in the participants who were diagnosed with myocardial infarction at, or shortly after, randomisation, and first myocardial infarction in the participants who were suspected of myocardial infarction at randomisation, but where the suspicion was later rejected).
Search methods for identification of studies
Electronic searches
We searched the following databases on 18 June 2019 to identify reports of relevant randomised clinical trials (Royle 2003).
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 6) in the Cochrane Library
-
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 14 June 2019)
-
Embase (Ovid, 1974 to 17 June 2019)
-
LILACS (Latin American and Caribbean Health Science Information Database) (Bireme, 1982 to 18 June 2019)
-
Science Citation Index Expanded on the Web of Science (Clarivate Analytics, 1900 to 18 June 2019)
-
BIOSIS Citation Index on the Web of Science (Clarivate Analytics, 1926 to 18 June 2019)
We adapted the preliminary search strategy for MEDLINE (Ovid) for use in these databases. We applied the Cochrane sensitivity‐maximising filter for randomised clinical trials (Lefebvre 2011) to MEDLINE Ovid and adaptations of it to the other databases, except CENTRAL. The search strategy can be found in Appendix 1.
We searched all databases from their inception to the present and we imposed no restriction on language of publication or publication status. We assessed non‐English language papers by asking individuals that speak the language fluently for help. This is acknowledged in the Acknowledgements section.
Searching other resources
We searched the reference lists of included randomised clinical trials, previous systematic reviews, and other kinds of reviews for any unidentified randomised clinical trials.
Furthermore, we searched for ongoing and unidentified randomised clinical trials on 14 August 2019:
-
the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch);
-
ClinicalTrials.gov (www.clinicaltrials.gov);
-
Turning Research Into Practice (TRIP) (http://www.tripdatabase.com/);
-
Google Scholar (http://scholar.google.dk/); and
-
Scisearch (http://ipscience.thomsonreuters.com/).
We also examined relevant retraction statements and errata for included trials.
Data collection and analysis
We performed this systematic review following the recommendations of Cochrane (Higgins 2011). The analyses were performed using Review Manager 5.3 (RevMan 2014).
Selection of studies
Two review authors (Sanam Safi (SS) and Naqash J Sethi (NJS)) independently screened titles and abstracts for inclusion of all the potentially eligible trials. We coded all these studies as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. If there was any disagreements, a third author were asked to arbitrate (Janus C Jakobsen (JCJ)). We retrieved the full‐text study reports/publications and four review authors (SS, NJS, Emil Eik Nielsen (EEN), and Joshua Feinberg (JF)) independently screened the full‐text reports and identified trials for inclusion. Reasons for exclusion of the ineligible studies were reported (Excluded studies). We resolved any disagreement through discussion or, if required, we consulted a third author (JCJ). We identified and excluded duplicated and collated multiple reports of the same trial so that each trial rather than each report is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009) Figure 1 and 'Characteristics of excluded studies' table.

Study flow diagram.
Data extraction and management
Four review authors (SS, NJS, EEN, JF) extracted and validated data independently from included trials. Any disagreement concerning the extracted data were discussed between the two authors. If no agreement could be reached, a third author (JCJ) resolved the issue. In case of relevant data not being available, we contacted the trial authors.
We used a data collection form for trial data and outcome data which was piloted on at least one trial in the review.
We extracted the following data mentioned below.
-
Trial characteristics: trial design (parallel, factorial, or cross‐over); number of intervention arms; duration of the trial; details of any 'run‐in' period; date of publication; inclusion and exclusion criteria; and 'Risk of bias' components (as defined below).
-
Participants characteristics: number of participants randomised; number of participants analysed; number of participants lost to follow‐up; mean age, and sex ratio.
-
Intervention characteristics: type of beta‐blocker; dose of beta‐blocker; duration of beta‐blocker therapy; and mode of administration.
-
Control characteristics: placebo or no intervention.
-
Co‐intervention characteristics: type of co‐intervention; dose of co‐intervention; duration of co‐intervention; and mode of administration.
-
Outcomes: primary, secondary, and exploratory outcomes specified and collected, and time points reported.
-
Notes: funding for trial, and notable conflicts of interest of trial authors.
Assessment of risk of bias in included studies
We used the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions in our evaluation of the methodology and the risk of bias of the included trials (Higgins 2017). Four review authors (SS, NJS, EEN, and JF) assessed the included trials independently. We evaluated the risk of bias in the following 'Risk of bias' domains:
-
random sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessment;
-
incomplete outcome data;
-
selective outcome reporting; and
-
other risks of bias.
This was done because these domains enable classification of randomised clinical trials at low risk of bias and at high risk of bias. The latter trials overestimate positive intervention effects (benefits) and underestimate negative effects (harms) (Gluud 2006; Kjaergard 2001; Lundh 2017; Moher 1998; Savovic 2012; Savovic 2012a; Schulz 1995; Wood 2008). For additional details on how the risk of bias was assessed, please see Appendix 2.
We graded each potential source of bias as high, low, or unclear and provided evidence from the study report together with a justification for our judgement in the 'Risk of bias' table. We have summarised the 'Risk of bias' judgments across different trials for each of the domains listed (see below).
Overall risk of bias
-
Low risk of bias: the outcome result was classified as at overall low risk of bias only if all of the bias domains described in the above paragraphs were classified as at low risk of bias.
-
High risk of bias: the outcome result was classified as at overall high risk of bias if any of the bias risk domains described above were classified as at unclear or high risk of bias.
Measures of treatment effect
We calculated risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes. We planned to calculate mean differences (MD) with 95% CI for continuous outcomes. However, none of the included trials adequately reported quality of life (our only continuous outcome).
Unit of analysis issues
We only included randomised clinical trials. For trials using cross‐over design, we planned to only include data from the first period (Elbourne 2002; Deeks 2017). For trials where multiple trial intervention groups were reported, we included only the relevant groups. If two comparisons were combined in the same meta‐analysis, we halved the control group to avoid double‐counting (Deeks 2017).
Dealing with missing data
We contacted trial authors to obtain missing data (i.e. for data extraction and for assessment of risk of bias, as specified above). However, not all trial authors responded (see Characteristics of included studies).
Dichotomous outcomes
If included trials used rigorous methodology (i.e. reporting on outcomes for all participants or multiple imputation to deal with missing data), we used these data in our primary analysis (Sterne 2009). We did not impute missing values for any outcomes in our primary analysis. In two of our sensitivity analyses ('best‐worst' and 'worst‐best'), we imputed data; see below.
Continuous outcomes
If included trials used rigorous methodology (i.e. reporting on outcomes for all participants or multiple imputation to deal with missing data), we planned to use these data in our primary analysis (Sterne 2009). We did not impute missing values for any outcomes in our primary analysis. If standard deviations (SDs) were not reported, we planned to calculate the SDs using data from the trial if possible. In two of our sensitivity analyses outcomes ('best‐worst' and 'worst‐best'), we planned to impute data, see below. However, none of the included trials adequately reported quality of life (our only continuous outcome).
Best‐worst and worst‐best case scenarios
To assess the potential impact of the missing data for dichotomous outcomes, we performed the following two sensitivity analyses when assessing each dichotomous outcome (all‐cause mortality, cardiovascular mortality, and myocardial infarction during follow‐up). We were not able to perform the following sensitivity analyses on 'MACE' as limited data were available and on serious adverse events as no data were available.
-
'Best‐worst' case scenario: we assumed that all participants lost to follow‐up in the experimental group survived and had no cardiovascular event; and all those participants with missing outcomes in the control group did not survive and had a cardiovascular event.
-
'Worst‐best' case scenario: we assumed that all participants lost to follow‐up in the experimental group did not survive and had a cardiovascular event; and all those participants with missing outcomes in the control group survived and had no cardiovascular event.
Results from both scenarios are presented in our review.
We planned that when analysing quality of life (our only continuous outcome), a ‘beneficial outcome’ would have been the group mean plus two SDs (we would then have used one SD in another sensitivity analysis) of the group mean, and a ‘harmful outcome’ would have been the group mean minus two SDs (we would then have used one SD in another sensitivity analysis) of the group mean (Jakobsen 2014).
To assess the potential impact of missing SDs for continuous outcomes, we performed the following sensitivity analysis.
-
Where SDs were missing and not possible to calculate, we planned to impute SDs from trials with similar populations and low risk of bias. If no such trials could be found, we planned to impute SDs from trials with a similar population. As the final option, we planned to impute SDs from all trials.
We planned to present results of this scenario in our review. However, none of the included trials adequately reported quality of life (our only continuous outcome).
Assessment of heterogeneity
Initially, we investigated forest plots to visually assess any sign of heterogeneity. We secondly assessed the presence of statistical heterogeneity by Chi2 test (threshold P < 0.10) and measured the quantities of heterogeneity by the I2 statistic (Higgins 2002; Higgins 2003).
We followed the recommendations for threshold by the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017):
-
0% to 40%: might not be important;
-
30% to 60%: may represent moderate heterogeneity;
-
50% to 90%: may represent substantial heterogeneity;
-
75% to 100%: may represent considerable heterogeneity.
We investigated possible heterogeneity through subgroup analyses. We had to decide whether or not a meta‐analysis might have to be avoided (Higgins 2011). Ultimately however, none of the planned meta‐analyses were avoided.
Assessment of reporting biases
We used a funnel plot to assess reporting bias in the meta‐analyses including 10 or more trials. We visually inspected the funnel plots to assess the risk of bias. For dichotomous outcomes, we tested asymmetry with the Harbord test (Harbord 2006) if tau2 < 0.1 and with the Rücker test (Rücker 2008) if tau2 > 0.1. For continuous outcomes, we planned to use the regression asymmetry test (Egger 1997). However, none of the included trials adequately reported quality of life (our only continuous outcome).
Data synthesis
Meta‐analysis
We undertook this systematic review according to the recommendations stated in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017; Higgins 2011) for better validation of meta‐analytic results in systematic reviews. We used the statistical software Review Manager 5.3 (RevMan 2014) provided by Cochrane to meta‐analyse data.
Assessment of significance
We assessed our intervention effects with both random‐effects model meta‐analyses (DerSimonian 1986) and fixed‐effect model meta‐analyses (DeMets 1987). We used the more conservative result of the two as out primary result (Jakobsen 2014). The more conservative result was the result with the highest P value and the widest confidence interval (CI). If there was a substantial discrepancy between the results of the two models, we reported both and discussed the results (Amrhein 2019; Jakobsen 2014). We used three primary outcomes and due to the risk of multiplicity we calculated a P value less than P ≤ 0.025 and a 97.5% CI for the primary outcomes (Jakobsen 2014). We used four secondary outcomes and we, therefore, calculated a P value less than P ≤ 0.020 and a 98% CI for the secondary outcomes (Jakobsen 2014). We used an online calculator for the 97.5% and 98% CI (/www.omnicalculator.com/statistics/relative‐risk).
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses when assessing each outcome (all‐cause mortality, cardiovascular mortality, and myocardial infarction during follow‐up) at both our time points. We were not able to perform subgroup analyses on 'MACE' and angina as limited data were available and on serious adverse events according to ICH‐GCP and quality of life as no data were available.
A: Comparison of the effects between trials where the participants commenced beta‐blockers at different time points.
-
Acute phase ‐ suspected of myocardial infarction.
-
Subacute phase ‐ diagnosed with myocardial infarction.
B: Comparison of the effects between trials where the participants received intervention for reperfusion (coronary artery bypass graft, percutaneous coronary intervention or thrombolytics) to that in trials where the participants did not receive intervention for reperfusion.
C: Comparison of the effects between trials where the experimental group received different types of beta‐blockers.
D: Comparison of the effects between trials with different age of participants.
-
Age 0 to 18 years.
-
Age 19 to 75 years.
-
Age 76 years or above.
E: Comparision of the effects between trials with different clinical trial registration status.
-
Pre‐registration.
-
Post‐registration.
-
No registration.
F: Comparison of the effects between trials including different types of acute myocardial infarction.
-
NSTEMI.
-
STEMI.
-
Unstable angina pectoris.
-
Mixed.
Post hoc subgroup analysis
After the publication of the protocol, we added two subgroups.
G: Comparison of the effects between trials with different lengths of intervention period. This subgroup analysis was only performed at maximum follow‐up.
-
0 to 7 days length of intervention,
-
7 to 30 days length of intervention,
-
1 month or more length of intervention.
H: Comparison of the effects between trials with different funding.
-
Industry funded trials or unknown funding,
-
Non‐industry funded trials.
We used the formal test for subgroup differences in RevMan 5.3 (RevMan 2014).
Sensitivity analysis
To assess the potential impact of bias, we performed a sensitivity analysis in which we excluded trials at overall high risk of bias.
To assess the potential impact of the missing data for dichotomous outcomes, we performed best‐worst and worst‐best case scenarios (see Dealing with missing data).
'Summary of findings' tables
We used the GRADE system (Guyatt 2008; https://gdt.gradepro.org/app/handbook/handbook.html) to assess the quality of the body of evidence associated with each of the primary outcomes (all‐cause mortality, serious adverse events according to ICH‐GCP, and major adverse cardiovascular events); and secondary outcomes (quality of life, angina, cardiovascular mortality, and myocardial infarction during follow‐up) at both our time points constructing 'Summary of Findings' tables using the GRADEpro GDT software (ims.cochrane.org/revman/other‐resources/gradepro). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed (Schünemann 2003; Guyatt 2008; Guyatt 2011). We assessed the GRADE levels of evidence as high, moderate, low, and very low and downgraded the evidence by one or two levels depending on the following quality measures: within‐study risk of bias, the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias (Schünemann 2003; Guyatt 2008; Guyatt 2011). We used the methods and recommendations described in Chapter 8 (section 8.5) (Higgins 2017) and chapter 12 (Schünemann 2017) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the GRADE handbook (https://gdt.gradepro.org/app/handbook/handbook.html). We justified all decisions to downgrade the quality of trials using footnotes and we made comments to aid the reader's understanding of the review where necessary.
We included all trials in our analyses, and conducted a sensitivity analysis excluding trials at high risk of bias. If the results were similar, we based our 'Summary of findings' tables and conclusions on the overall analysis. If they differed, we based our 'Summary of findings' tables and conclusions on trials at low risk of bias.
We found one low risk of bias trial and reported its findings. For cardiovascular mortality, the results on all trials and on trials at low risk of bias differed significantly. For all other outcomes, the results did not differ significantly. Consequently, we based our 'Summary of findings' tables and conclusions on the results of trials at low risk of bias when assessing cardiovascular mortality and on the results of all trials when assessing all other outcomes (summary of findings Table for the main comparison (less than three months follow‐up) and summary of findings Table 2 (maximum follow‐up beyond three months)).
Results
Description of studies
We assessed all trials according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), and the protocol for this review (Nielsen 2016). Characteristics of each trial can be found in 'Characteristics of included studies' and 'Characteristics of excluded studies'. We identified three eligible ongoing studies (Characteristics of ongoing studies).
Results of the search
We identified a total of 18,450 potentially relevant references through searching CENTRAL (the Cochrane Library) (n = 2749), MEDLINE (n = 2412), Embase (n = 5798), Science Citation Index Expanded (n = 4862), BIOSIS (n = 2537), LILACS (n = 92), and four additional records were identified through other sources. The search strategies are presented in Appendix 1. After removing duplicates, 12,315 records were screened, and 11,842 references were excluded based on titles and abstracts. Four hundred and seventy‐three full‐text articles were assessed for eligibility and we excluded 50 reports reporting on 22 trials according to our inclusion criteria and exclusion criteria (only the main references are listed for these trials). Reasons for exclusion are listed in the table 'Characteristics of excluded studies'. We therefore included 423 publications reporting results from 66 trials. Accordingly, 63 trials could be included in our analyses while three trials were still on‐going. The study flow chart can be seen in Figure 1.
Included studies
We included 417 publications reporting on 63 trials comparing beta‐blockers versus placebo or no intervention in patients with suspected or diagnosed acute myocardial infarction (Figure 1). The trials were conducted between 1966 and 2018. The trials (often conducted in more than one country) were conducted at sites in 31 different countries: 15 from the UK; eight each from Australia and New Zealand; seven each from Ireland, Norway, and Sweden; five each from Belgium, Denmark, and Germany; four each from Italy and the USA; three each from Canada, Japan, Scotland, France, Russia, and Spain; two each from Finland, India, and the Netherlands; one each from Argentina, Austria, China, Hungary, Israel, Lithuania, Luxemborg, Malaysia,the Phillippines, Singapore, South Africa, and Switzerland.
We included 56 trials where the beta‐blockers were commenced in the acute phase of a myocardial infarction (48 trials enrolled patients within 24 hours of the onset of symptoms, three trials within 48 hours of the onset of symptoms, and five trials did not report the specific time point of enrolment). The remaining seven trials were included where the beta‐blockers were commenced in the subacute phase of a myocardial infarction (timing from the initial symptoms to randomisation varied from three to 21 days after a myocardial infarction).
Twenty‐four trials received the intervention for zero to seven days; 23 trials received the intervention for seven to 30 days; and the remaining 16 trials received the intervention for at least one month or more.
Seven trials specifically randomised participants suspected of or diagnosed with ST‐elevation myocardial infarction, 20 trials randomised a mixed group of participants (ST‐myocardial infarction, non‐ST myocardial infarction, unstable angina), and the remaining 36 trials did not report data on the different kinds of acute coronary syndrome included.
Two trials were multi‐arm trials with more than one comparison (Waagstein 1975; Wilcox 1980).
Four trials did not report data on any of our outcomes (Azancot 1982; Daga 2003; Korochkin 1991; Waagstein 1975). Forty‐nine out of the 63 included trials reported data on all‐cause mortality at less than three months follow‐up and 22 trials reported data at maximum follow‐up beyond three months. None of the trials specifically assessed serious adverse events according to ICH‐GCP. Only two trials at the time point 'less than three months' follow‐up (with no events reported) and four trials at maximum follow‐up specifically assessed major adverse cardiovascular events according to our definition (composite of cardiovascular mortality and non‐fatal myocardial infarction during follow‐up).
Thirty‐three trials were fully or partly funded by the industry, 20 trials did not report how they were funded, and 10 trials were funded by other sources than the industry.
For further details on included studies and baseline characteristics of included participants, see 'Characteristics of included studies'.
Participants
A total of 85,550 participants with suspected or diagnosed acute myocardial infarction were randomised in the 63 included trials. The number of participants in each trial ranged from 18 participants to 45,852 participants. The mean age was 57.4 years (mean range 45.9 to 70.0 years) (13 out of the 63 trials did not report the mean age among the participants). Fifteen trials included participants older than 75 years. The mean proportion of women was 25.5% (10 out of the 63 trials did not report the sex distribution). The mean proportion of participants with a myocardial infarction at the time of randomisation was 80.2%. The mean proportion of participants with a former myocardial infarction was 11.8%. The majority of the trials based their inclusion criteria only on signs and symptoms suggestive of myocardial infarction. Hence, the majority of the trials included participants with suspected myocardial infarction, while a few trials only included participants with diagnosed myocardial infarction.
Experimental intervention
The included trials used 16 different types of beta‐blockers as their experimental intervention: 12 trials used propranolol, eight trials used metoprolol, five trials used timolol, four trials used atenolol, four trials used carvedilol, three trials used practolol, two trials used alprenolol, two trials used pindolol, two trials used sotalol, one trial used acebutolol, one trial used betaxolol, one trial used H 87/07, one trial used labetalol, one trial used oxprenolol, one trial used xamoterol, one trial used esmolol, one trial used landiolol, and one trial used mixed beta‐blockers (esmolol + metoprolol).
Control intervention
We included 41 trials where the control group received placebo. In the remaining 22 trials, the control group either received only the co‐intervention (in 18 trials) or no intervention (in four trials).
Co‐interventions
We included 40 trials where the participants received a co‐intervention. In 27 trials, the co‐interventions consisted of digitalis, diuretics, nitrates, antiarrhythmics, anticoagulants, and aspirin; in six trials, the co‐intervention consisted of either percutaneous coronary intervention or thrombolysis; in two trials, the co‐intervention consisted of only heparin; in two trials, the co‐intervention consisted of only morphine; in two trials, the co‐intervention consisted of only lidocaine; and in one trial, the co‐intervention consisted of only captopril. In the remaining 23 trials,any use of co‐interventions was not mentioned. For further details, see 'Characteristics of included studies'.
Excluded studies
We excluded 22 studies after full‐text assessment based on our inclusion and exclusion criteria: eight studies were not randomised, six studies did not use a control or placebo group, three studies did not assess acute or subacute patients with myocardial infarction, two studies where either the control group or the experimental group did not meet our criteria, one study assessed participants with Ischaemic heart disease, one study was a cohort study, and one study was quasi‐randomised. For further details, see 'Characteristics of excluded studies'.
Risk of bias in included studies
Based on information that we collected from published reports and from study authors, we considered one trial to be at low risk of bias (COMMIT 2005) and the remaining 62 trials to be at high risk of bias. We judged many trials to be at unclear risk of bias in several domains and could not obtain additional information from study authors when we contacted them. We have provided additional information in the 'Risk of bias' summary (Figure 2), the 'Risk of bias' graph (Figure 3), and the Characteristics of included studies table.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The generation of the random sequence was at low risk of bias in eight trials. The remaining 55 trials were described as being randomised, but the method used for sequence generation was either not described or insufficiently described and were therefore judged to be of unclear risk of bias.
The method used to conceal allocation was at low risk of bias in 11 trials. The remaining 52 trials were described as being randomised, but the method used for allocation concealment was either not described or insufficiently described and were judged to be of unclear risk of bias.
Blinding
The blinding of participants and personnel was performed and adequately described in five trials and were judged to be at low risk of bias. Sixteen trials were either open‐label or single‐blinded and were judged to be at high risk of bias. In the remaining 42 trials, the method for blinding of participants and personnel was either not described or insufficiently described and were judged to be of unclear risk of bias.
The blinding of outcome assessors was performed and adequately described in 17 trials and were judged to be at low risk of bias. In one trial, the outcome assessors were not blinded and were judged to be at high risk of bias (RIMA 1999). In the remaining 45 trials, the method for blinding of outcome assessors were either not described or insufficiently described and were judged to be at unclear risk of bias.
Incomplete outcome data
Incomplete outcome data were addressed adequately in 25 trials and were judged to be at low risk of bias. Seven trials did not properly deal with incomplete outcome data and were judged to be at high risk of bias. In the remaining 31 trials, incomplete outcome data was either not described or insufficiently described and were judged to be at unclear risk of bias.
Selective reporting
Six trials reported the results of the outcomes stated in their respective protocols, or reported our primary outcomes, resulting in low risk of bias according to our predefined bias risk assessment. In the remaining 57 trials, no protocol could be obtained and the trial did not adequately report on our primary outcomes and were judged to be of unclear risk of bias.
Other potential sources of bias
Fifty‐seven trials had no other biases resulting in low risk of bias. The remaining six trials reported insufficient information to assess whether an important risk of bias exists or terminated the trial prematurely and were judged to be at 'unclear risk of bias'.
Effects of interventions
See: Summary of findings for the main comparison Beta‐blockers versus placebo or no intervention for suspected or diagnosed acute myocardial infarction at the time point less than three months follow‐up; Summary of findings 2 Beta‐blockers versus placebo or no intervention for suspected or diagnosed acute myocardial infarction at maximum follow‐up beyond three months
Primary outcomes
All‐cause mortality
Time point at less than three months follow‐up
In total 46/63 trials involving 80,452 participants and a median follow‐up of 21.8 days (range one hour to 90 days) reported all‐cause mortality at the time point 'less than three months follow‐up'. The specific assessment time points in each trial are presented in Table 1. No events occurred in either group in three trials (Hanada 2012; Norris 1978; Shirotani 2010). A total of 2686/40,347 (6.66%) participants receiving beta‐blockers died versus 2825/40,105 (7.04%) control participants. Fixed‐effect meta‐analysis showed no sufficient evidence of a difference (risk ratio (RR) 0.94, 97.5% confidence interval (CI) 0.90 to 1.00; I2 = 0%; 80,452 participants; 46 trials/47 comparisons; high‐quality evidence; Analysis 1.1). Hence, the absolute risk for mortality at less than three months follow‐up corresponds to 67 out of 1000 participants receiving beta‐blockers dying of any reason compared with 71 out of 1000 participants receiving placebo or no intervention. The optimal information size according to the GRADE Handbook using a proportion of 7.04% in the control group, a relative risk reduction (RRR) of 10%, an alpha of 2.5%, and a beta of 10% was estimated to be 63,246 participants and we included 80,452 participants (see summary of findings Table for the main comparison).
| Trial | Year | All‐cause mortality | Major adverse cardiovascular events | Cardiovascular mortality | Myocardial infarction during follow‐up |
| Andersen | 1979 | 28 days | NR | NR | NR |
| Åström | 1986 | NR | NR | NR | NR |
| Australian | 1984 | 28 days | NR | NR | NR |
| Australia & Swedish | 1983 | 28 days | NR | NR | NR |
| Balcon | 1967 | 28 days | NR | NR | NR |
| Barbar | 1967 | 28 days | NR | NR | NR |
| Barber | 1976 | 90 days | NR | NR | NR |
| BEAT‐AMI trial | 2016 | During hospitalisation (no mean time) | NR | During hospitalisation (no mean time) | During hospitalisation (no mean time) |
| Briant | 1970 | 3 days | NR | NR | NR |
| Campbell | 1984 | 7 days | NR | NR | NR |
| CAPRICORN | 2001 | 30 days | NR | NR | NR |
| Clausen | 1966 | 28 days | NR | NR | NR |
| COMMIT | 2005 | 28 days | NR | 28 days | 28 days |
| CPRG | 1980 | 60 days | NR | 56 days | 56 days |
| EARLY‐BAMI | 2016 | 30 days | NR | 30 days | 30 days |
| EMIT | 2002 | 42 days | NR | NR | 42 days |
| Evemy | 1977 | 30 days | NR | NR | NR |
| Gardtman | 1999 | 30 days | NR | NR | NR |
| Göteborg Metoprolol Trial | 1981 | 90 days | NR | 90 days | 90 days |
| Hanada | 2012 | During hospitalisation (no mean time) (no events) | During hospitalisation (no mean time) (no events) | During hospitalisation (no mean time) (no events) | During hospitalisation (no mean time) (no events) |
| Heber | 1986 | 5 days | NR | NR | NR |
| ICSG | 1984 | During hospitalisation (no mean time) | NR | During hospitalisation (no mean time) | During hospitalisation (no mean time) |
| ISIS‐1 | 1986 | 14 days | NR | 7 days | 7 days |
| Johannessen | 1987 | 10 days | NR | NR | NR |
| Ledwich | 1968 | 7 days | NR | NR | NR |
| McMurray | 1991 | NR | NR | NR | 10 days |
| METOCARD‐CNIC | 2013 | 7 days | NR | NR | 7 days |
| MIAMI | 1985 | 15 days | NR | 15 days | 15 days |
| MILIS | 1984 | 30 days | NR | NR | NR |
| Mueller | 1980 | 3 days | NR | 10 days | NR |
| Multicenter trial | 1966 | 30 days | NR | 30 days | NR |
| Nielsen | 1967 | 28 days | NR | NR | 28 days |
| Norris | 1968 | 21 days | NR | NR | NR |
| Norris | 1978 | 8.5 days (no events) | NR | 8.5 days (no events) | NR |
| Norris | 1980 | During hospitalisation (no mean time) | NR | During hospitalisation (no mean time) | NR |
| Norris | 1984 | 21 days | NR | 21 days | NR |
| Owensby | 1985 | 3 days | NR | NR | 3 days |
| Peter | 1978 | 3 days | NR | NR | 3 days |
| Raeder | 1967 | 21 days | NR | 21 days | NR |
| Ranganathan | 1988 | 2 days | NR | NR | NR |
| Rolli | 1980 | NR | NR | NR | NR |
| Salathia | 1985 | During hospitalisation (no mean time) | NR | 90 days | NR |
| Shirotani | 2010 | 30 days (no events) | 30 days (no events) | 30 days (no events) | 30 days (no events) |
| Tereschenko | 2005 | 30 days | NR | 30 days | NR |
| Thompson | 1979 | 5 days | NR | NR | NR |
| TIARA | 1987 | 30 days | NR | 30 days | 30 days |
| Tonkin | 1981 | 7 days | NR | NR | 7 days |
| Van De Werf | 1993 | 14 days | NR | 14 days | 14 days |
| Von Essen | 1982 | 14 days | NR | 14 days | NR |
| Wilcox | 1980 | 42 days | NR | NR | NR |
| Yang | 1984 | NR | NR | NR | NR |
| Yusuf | 1980 | 10 days | NR | NR | During hospitalisation (no mean time) |
Heterogeneity
The visual inspection of the forest plot and the tests for statistical heterogeneity (I2 = 0%; P = 0.94) indicated no signs of heterogeneity.
Risk of bias and sensitivity analyses
One trial was assessed at low risk of bias in all domains (COMMIT 2005). One trial was assessed at low risk of bias in all but one domain and since the blinding of participants and personnel was not considered of key importance for an objective outcome like all‐cause mortality, the study was assessed at overall low risk of bias (METOCARD‐CNIC 2013). Hence, the most weighted trials of the meta‐analysis were either at overall low risk of bias or had few domains of key importance that were not at low risk of bias and the risk of bias of the outcome result was assessed as low risk of bias.
The sensitivity analysis excluding trials at high risk of bias showed no evidence of a difference (RR 0.99, 95% CI 0.93 to 1.05; 46,122 participants; 2 trials; high‐quality evidence; Analysis 1.10). Since the sensitivity analysis and the overall meta‐analysis showed similar results, we based our summary of findings and conclusion on the overall meta‐analysis.
The sensitivity analysis on incomplete outcome data showed that incomplete outcome data bias alone had the potential to influence the results in the best‐worst sensitivity analysis, but not in the worst‐best sensitivity analysis: best‐worst fixed‐effect meta‐analysis (RR 0.93, 95% CI 0.89 to 0.98; I2 = 1%; 80,522 participants; 45 trials/46 comparisons; Analysis 1.11); worst‐best random‐effects meta‐analysis (RR 0.93, 95% CI 0.86 to 1.02; I2 = 9%; 80,522 participants; 45 trials/46 comparisons; Analysis 1.12). Data were imputed for 5 trials.
Visual inspection of the funnel plot showed no signs of asymmetry (Figure 4). Based on the visual inspection of the funnel plot, we assessed the risk of publication bias as low.

Funnel plot of comparison: 1 Beta‐blockers versus placebo or no intervention at 'less than 3 months' follow‐up, outcome: 1.1 All‐cause mortality.
Subgroup analyses
Tests for subgroup differences showed evidence of a difference when comparing trials according to the clinical trial registration status (I2 = 67.8%; P = 0.04; Analysis 1.8). The unregistered trials showed evidence of a beneficial effect of beta‐blockers versus placebo or no intervention on all‐cause mortality (RR 0.87, 95% CI 0.80 to 0.95; I2 = 0%; 32,541 participants; 40 trials; Analysis 1.8), while the pre‐registered trials (RR 0.99, 95% CI 0.93 to 1.05; I2 = 0%; 47,642 participants, 5 trials; Analysis 1.8) and the post‐registered trial (RR 0.50, 95% CI 0.16 to 1.63; 269 participants, 1 trial; Analysis 1.8) showed no evidence of a difference on all‐cause mortality.
All remaining tests for subgroup differences showed no evidence of a difference in subgroup analyses according to the acute and subacute phase of commencing beta‐blockers (Analysis 1.2); reperfusion compared to no reperfusion (Analysis 1.3); types of beta‐blockers (Analysis 1.4); intravenously compared to orally commenced beta‐blockers (Analysis 1.5); age either below compared to a mixture of above/below 75 years (40/45 trials reported the age of the participants) (Analysis 1.6); different types of acute myocardial infarction (NSTEMI, STEMI, UAP, or mixed) (24/45 trials reported data on the different types of acute myocardial infarction) (Analysis 1.7), and funding (Analysis 1.9).
Maximum follow‐up beyond three months
A total of 21/63 trials involving 25,210 participants and a median follow‐up of 17.7 months (range 6 to 60 months) reported all‐cause mortality at maximum follow‐up beyond three months. The specific assessment time points in each trial are presented in Table 2. No events occurred in either group in one trial (Hanada 2012). A total of 1742/12,708 (13.7%) participants receiving beta‐blockers died versus 1850/12,502 (14.8%) control participants. Fixed‐effect meta‐analysis showed evidence of a beneficial effect of beta‐blockers versus placebo or no intervention (RR 0.93, 97.5% CI 0.86 to 0.99; I2 = 0%; 25,210 participants; 21 trials/22 comparisons; moderate‐quality evidence, Analysis 2.1). Hence, the absolute risk for mortality at maximum follow‐up corresponds to 140 out of 1000 participants receiving beta‐blockers dying of any reason compared with 151 out of 1000 participants receiving placebo or no intervention and a number needed to treat for an additional beneficial outcome (NNTB) of 91 participants. The optimal information size according to the GRADE Handbook using a proportion of 14.8% in the control group, a RRR of 10%, an alpha of 2.5%, and a beta of 10% was estimated to be 27,387 participants and we included 25,210 participants.
| Trial | Year | All‐cause mortality | Major adverse cardiovascular events | Cardiovascular mortality | Myocardial infarction |
| Andersen | 1979 | 12 months | NR | NR | NR |
| Australia & Swedish | 1983 | 24 months | NR | 24 months | 24 months |
| Barber | 1976 | 24 months | NR | NR | NR |
| Basu | 1997 | 6 months | 6 months | 6 months | 6 months |
| BEAT‐AMI trial | 2016 | 6 months | NR | 6 months | 6 months |
| Briant | 1970 | 12 months | 12 months | 12 months | 12 months |
| CAPRICORN | 2001 | 15.6 months | NR | 15.6 months | 15.6 months |
| EARLY‐BAMI | 2016 | 1 month (not included) | NR | 12 months | 12 months |
| Evemy | 1977 | 7 months | NR | NR | NR |
| Göteborg Metoprolol Trial | 1981 | 60 months | NR | 3 months | 60 months |
| Hanada | 2012 | 6 months (no events) | 6 months | 6 months (no events) | 6 months |
| Heber | 1986 | 12 months | NR | NR | NR |
| ISIS‐1 | 1986 | 20 months | NR | 20 months | 0.23 months (not included) |
| Kaul | 1988 | 6 months | NR | 6 months | 6 months |
| METOCARD‐CNIC | 2013 | 24 months | NR | 24 months | 24 months |
| MILIS | 1984 | 36 months | NR | NR | NR |
| NPT | 1982 | 12 months | NR | 12 months | 12 months |
| RIMA | 1999 | 6 months | 6 months | 6 months | 6 months |
| Salathia | 1985 | 12 months | NR | 12 months | NR |
| TIARA | 1987 | 24 months | NR | 1 month (not included) | 1 month (not included) |
| Tonkin | 1981 | 0.23 months (not included) | NR | NR | 12 months |
| Wilcox | 1980 | 12 months | NR | 12 months | NR |
| Yusuf | 1980 | 24 months | NR | NR | During hospitalisation (no mean time) (not included) |
Heterogeneity
The visual inspection of the forest plot and the tests for statistical heterogeneity (I2 = 0%; P = 0.95) indicated no sign of heterogeneity.
Risk of bias and sensitivity analyses
One trial was assessed at low risk of bias in all but one domain (blinding) and since the blinding of participants and personnel was not considered of key importance for an objective outcome like all‐cause mortality, the study was assessed at low risk of bias (METOCARD‐CNIC 2013). All other trials were at high risk of bias mainly due to domains being at unclear risk of bias. Overall, the risk of bias of the outcome result was assessed as high risk of bias.
The sensitivity analysis excluding trials at high risk of bias showed, however, no evidence of a difference (RR 0.94, 95% CI 0.31 to 2.85; 270 participants; 1 trial; low‐quality evidence; Analysis 2.11). Nevertheless, only one small trial (METOCARD‐CNIC 2013) with a low event rate in the control group (4.56%) was included in this sensitivity analysis. Hence, the result from this sensitivity analysis might be assessed at low risk of bias, but has other very serious problems such as imprecision and indirectness. As a consequence of these serious limitations, we have not based our summary of findings and conclusions on this sensitivity analysis. Instead, we have based our summary of findings and conclusion on the overall meta‐analysis.
The sensitivity analysis on incomplete outcome data showed that incomplete outcome data bias alone had the potential to influence the results in the best‐worst sensitivity analysis, but not in the worst‐best sensitivity analysis: best‐worst random‐effects meta‐analysis (RR 0.89, 95% CI 0.81 to 0.97; I2 = 15%; 25,283 participants; 21 trials/22 comparisons; Analysis 2.12); worst‐best random‐effects meta‐analysis (RR 0.95, 95% CI 0.85 to 1.06; I2 = 33%; 25,283 participants; 21 trials/22 comparisons; Analysis 2.13). Data were imputed for five trials.
Visual inspection of the funnel plot showed no signs of asymmetry (Figure 5). Based on the visual inspection of the funnel plot, we assessed the risk of publication bias as low.
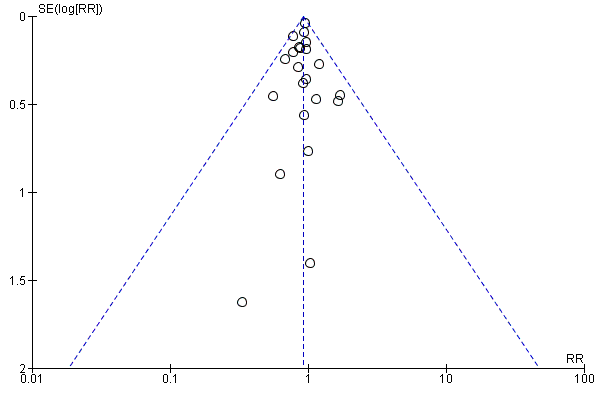
Funnel plot of comparison: 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, outcome: 2.1 All‐cause mortality.
Subgroup analyses
Tests for subgroup differences showed no evidence of a difference in subgroup analyses according to the acute and subacute phase of commencing beta‐blockers (Analysis 2.2); reperfusion compared to no reperfusion (Analysis 2.3); types of beta‐blockers (Analysis 2.4); age either below compared to a mixture of above/below 75 years (Analysis 2.5); intravenously compared to orally commenced (Analysis 2.6); different types of acute myocardial infarction (NSTEMI, STEMI, UAP, or mixed) (7/20 trials reported data on the different types of acute myocardial infarction) (Analysis 2.8); clinical trial registration status (Analysis 2.7); length of the intervention period (Analysis 2.9); and funding (Analysis 2.10).
Serious adverse events
Time point less than three months follow‐up
None of the trials specifically assessed nor reported serious adverse events according to ICH‐GCP. Instead, the trials either reported composites of several specific serious adverse events or one specific serious adverse event.
We reported narratively the individual types of serious adverse events in each trial at the time point less than three months follow‐up in Table 3.
| Trial | Year | Type and number of serious adverse events (beta‐blocker group) | Type and number of serious adverse events (control group) |
| Andersen | 1979 |
|
|
| Åstrøm | 1986 | None |
|
| Australian trial | 1984 |
|
|
| Australia & Swedish | 1983 |
|
|
| Balcon | 1967 |
|
|
| Barbar | 1967 |
|
|
| Barber | 1976 |
|
|
| BEAT‐AMI | 2016 |
|
|
| Briant | 1970 |
|
|
| Campbell | 1984 |
|
|
| CAPRICORN | 2001 |
|
|
| Clausen | 1966 |
|
|
| COMMIT | 2005 |
|
|
| CPRG | 1980 |
|
|
| EARLY‐BAMI | 2016 |
|
|
| EMIT | 2002 |
|
|
| Evemy | 1977 |
|
|
| Gardtman | 1999 |
|
|
| Göteborg | 1981 |
|
|
| Heber | 1986 |
|
|
| ICSG | 1984 |
|
|
| ISIS‐1 | 1986 |
|
|
| Johannessen | 1987 |
|
|
| Ledwich | 1968 |
|
|
| Lloyd | 1988 |
|
|
| Mcmurray | 1991 |
|
|
| METOCARD‐CNIC | 2013 |
|
|
| Miami | 1985 |
|
|
| MILIS | 1984 |
|
|
| Mueller | 1980 |
|
|
| Multicenter trial | 1966 |
|
|
| Nielsen | 1967 |
|
|
| Norris | 1968 |
|
|
| Norris | 1978 |
|
|
| Norris | 1980 |
|
|
| Norris | 1984 |
|
|
| Owensby | 1985 |
|
|
| Peter | 1978 |
|
|
| Raeder | 1967 |
|
|
| Ramsdale | 1982 | None |
|
| Ranganathan | 1988 |
|
|
| Rolli | 1980 |
|
|
| Salathia | 1985 |
|
|
| Tereschenko | 2005 |
|
|
| Thompson | 1979 |
|
|
| TIARA | 1987 |
|
|
| Tonkin | 1981 |
|
|
| Van De Werf | 1993 |
|
|
| Von Essen | 1982 |
|
|
| Wilcox (atenolol) | 1980 |
|
|
| Wilcox (propranolol) | 1980 |
|
|
| Yang | 1984 |
|
|
| Yusuf | 1980 |
|
|
Maximum follow‐up beyond three months
None of the trials specifically assessed nor reported serious adverse events according to ICH‐GCP. Instead, the trials either reported composites of several specific serious adverse events or one specific serious adverse event.
We reported narratively the individual types of serious adverse events in each trial at maximum follow‐up beyond three months in Table 4.
| Trial | Year | Type and number of serious adverse events (beta‐blocker group) | Type and number of serious adverse events (control group) |
| Andersen | 1979 |
|
|
| Åstrøm | 1986 | None |
|
| Australian trial | 1984 |
|
|
| Australia & Swedish | 1983 |
|
|
| Balcon | 1967 |
|
|
| Barbar | 1967 |
|
|
| Barber | 1976 |
|
|
| Basu | 1997 |
|
|
| BEAT‐AMI | 2016 |
|
|
| Briant | 1970 |
|
|
| Campbell | 1984 |
|
|
| CAPRICORN | 2001 |
|
|
| Clausen | 1966 |
|
|
| COMMIT | 2005 |
|
|
| CPRG | 1980 |
|
|
| EARLY‐BAMI | 2016 |
|
|
| EMIT | 2002 |
|
|
| Evemy | 1977 |
|
|
| Gardtman | 1999 |
|
|
| Göteborg | 1981 |
|
|
| Hanada | 2012 | None |
|
| Heber | 1986 |
|
|
| ICSG | 1984 |
|
|
| ISIS‐1 | 1986 |
|
|
| Johannessen | 1987 |
|
|
| Kaul | 1988 |
|
|
| Ledwich | 1968 |
|
|
| Lloyd | 1988 |
|
|
| Mcmurray | 1991 |
|
|
| METOCARD‐CNIC | 2013 |
|
|
| Miami | 1985 |
|
|
| MILIS | 1984 |
|
|
| Mueller | 1980 |
|
|
| Multicenter trial | 1966 |
|
|
| Nielsen | 1967 |
|
|
| Norris | 1968 |
|
|
| Norris | 1978 |
|
|
| Norris | 1980 |
|
|
| Norris | 1984 |
|
|
| NPT | 1982 |
|
|
| Owensby | 1985 |
|
|
| Peter | 1978 |
|
|
| Raeder | 1967 |
|
|
| Ramsdale | 1982 | None |
|
| Ranganathan | 1988 |
|
|
| RIMA | 1999 |
|
|
| Rolli | 1980 |
|
|
| Salathia | 1985 |
|
|
| Tereschenko | 2005 |
|
|
| Thompson | 1979 |
|
|
| TIARA | 1987 |
|
|
| Tonkin | 1981 |
|
|
| Van De Werf | 1993 |
|
|
| Von Essen | 1982 |
|
|
| Wilcox (atenolol) | 1980 |
|
|
| Wilcox (propranolol) | 1980 |
|
|
| Yang | 1984 |
|
|
| Yusuf | 1980 |
|
|
Major adverse cardiovascular events
Time point less than three months follow‐up
Only two trials specifically assessed major adverse cardiovascular events (defined as a composite of cardiovascular mortality and non‐fatal myocardial infarction during follow‐up) at the time point less than three months follow‐up. Nevertheless, no major adverse cardiovascular events occurred in either trial (Analysis 3.1). The specific assessment time points in each trial are presented in Table 1.
The rest of the trials reported either cardiovascular mortality or myocardial infarction during follow‐up.
Maximum follow‐up beyond three months
In total 4/62 trials involving 475 participants with a median follow‐up of 7.5 months (range 6 to 12 months) reported major adverse cardiovascular events (defined as a composite of cardiovascular mortality and non‐fatal myocardial infarction during follow‐up) at maximum follow‐up beyond three months. The specific assessment time points in each trial are presented in Table 2. A total of 16/237 (6.74%) participants receiving beta‐blockers had a major adverse cardiovascular event versus 20/238 (8.40%) control participants. Random‐effects meta‐analysis showed no evidence of a difference of beta‐blockers versus placebo or no intervention on major adverse cardiovascular events (RR 0.81, 97.5% CI 0.40 to 1.66; I2 = 0%; 475 participants; 4 trials; very low‐quality evidence; Analysis 4.1). Hence, the absolute risk for a major adverse cardiovascular event at maximum follow‐up corresponds to 68 out of 1000 participants receiving beta‐blockers having a major adverse cardiovascular event compared with 84 out of 1000 participants receiving placebo or no intervention. The optimal information size according to the GRADE Handbook using a proportion of 8.40% in the control group, a RRR of 10%, an alpha of 2.5%, and a beta of 10% was estimated to be 51,666 participants and we only included 475 participants.
No further analyses were conducted due to sparse data.
Secondary outcomes
Cardiovascular mortality
Time point less than three months follow‐up
In total 18/62 trials involving 72,622 participants and a median follow‐up of 27.0 days (range 7 to 90 days) reported cardiovascular mortality at the time point less than three months follow‐up. The specific assessment time points in each trial are presented in Table 1. No events occurred in either group in three trials (Hanada 2012; Norris 1978; Shirotani 2010). A total of 1640/36,364 (4.51%) participants receiving beta‐blockers died because of a cardiovascular event versus 1765/36,258 (4.87%) control participants. Fixed‐effect meta‐analysis showed no sufficient evidence of a difference when assessing beta‐blockers versus placebo or no intervention on cardiovascular mortality (RR 0.93, 98% CI 0.86 to 1.00; I2 = 0%; 72,622 participants; 18 trials; low‐quality evidence; Analysis 5.1). Hence, the absolute risk for a cardiovascular death at less than three months follow‐up corresponds to 45 out of 1000 participants receiving beta‐blockers dying of a cardiovascular event compared with 49 out of 1000 participants receiving placebo or no intervention and a NNTB of 278 participants.The optimal information size according to the GRADE Handbook using a proportion of 4.85% in the control group, a RRR of 10%, an alpha of 2.0%, and a beta of 10% was estimated to be 95,569 participants and we included 72,622 participants.
Heterogeneity
The visual inspection of the forest plot and the tests for statistical heterogeneity (I2 = 0 %; P = 0.72) indicated no signs of heterogeneity.
Risk of bias and sensitivity analyses
One trial was assessed at low risk of bias (COMMIT 2005) contributing to 55.4% of the weight to the analysis. All other trials were at high risk of bias mainly due to domains being at unclear risk of bias. Overall, the risk of bias of the outcome result was assessed as high risk of bias.
The sensitivity analysis excluding trials at high risk of bias showed, however, no evidence of a difference (RR 0.99, 95% CI 0.91 to 1.08; 45,852 participants; 1 trial; moderate‐quality evidence; Analysis 5.10). Hence, the absolute risk for a cardiovascular death at less than three months follow‐up corresponds to 42 out of 1000 participants receiving beta‐blockers dying of a cardiovascular event compared with 43 out of 1000 participants receiving placebo or no intervention. The optimal information size according to the GRADE Handbook using a proportion of 4.28% in the control group, a RRR of 10%, an alpha of 2.0%, and a beta of 10% was estimated to be 109,817 participants and we included 45,852 participants (see summary of findings Table for the main comparison). Since the sensitivity analysis and the overall meta‐analysis showed different results, we based our summary of findings and conclusions on the sensitivity meta‐analysis only including trials at low risk of bias.
The sensitivity analyses on incomplete outcome data showed that incomplete outcome data bias alone had the potential to influence the results in the best‐worst sensitivity analysis, but not in the worst‐best sensitivity analysis: best‐worst random‐effects meta‐analysis (RR 0.84, 95% CI 0.72 to 0.97; I2 = 38%; 72,681 participants; 18 trials; Analysis 5.11); worst‐best random‐effects meta‐analysis (RR 0.93, 95% CI 0.79 to 1.10; I2 = 46%; 72,681 participants; 18 trials; Analysis 5.12). Data were imputed for three trials.
Visual inspection of the funnel plots showed no signs of asymmetry (Figure 6). Based on the visual inspection of the funnel plot, we assessed the risk of publication bias as low.
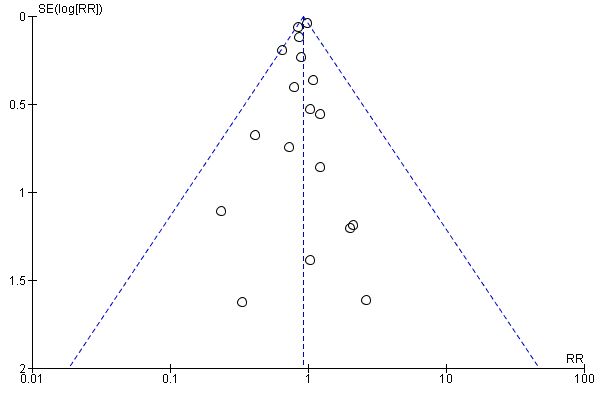
Funnel plot of comparison: 5 Beta‐blockers versus placebo or no intervention at 'less than 3 months' follow‐up beyond 3 months, outcome: 5.1 Cardiovascular mortality.
Subgroup analyses
Tests for subgroup differences showed evidence of a difference when comparing trials according to the clinical trial registration status (I2 = 81.8%; P = 0.02; Analysis 5.8). The unregistered trials showed evidence of a beneficial effect of beta‐blockers versus placebo or no intervention on cardiovascular mortality (RR 0.84, 95% CI 0.76 to 0.93; I2 = 0%; 26,044 participants; 15 trials; Analysis 5.8), while the pre‐registered trials showed no evidence of a difference on cardiovascular mortality (RR 0.99, 95% CI 0.91 to 1.08; I2 = 0%; 46,578 participants; 3 trials; Analysis 5.8).
All remaining tests for subgroup differences showed no evidence of a difference in subgroup analyses according to the acute and subacute phase of commencing beta‐blockers (Analysis 5.2); reperfusion compared to no reperfusion (Analysis 5.3); types of beta‐blockers (Analysis 5.4); age either below compared to a mixture of above/below 75 years (Analysis 5.5); intravenous compared to oral administration of beta‐blockers (Analysis 5.6); different types of acute myocardial infarction (NSTEMI, STEMI, UAP or mixed) (9/18 trials reported data on the different types of acute myocardial infarction) (Analysis 5.7); and funding (Analysis 5.9).
Maximum follow‐up beyond three months
In total, 14/63 trials involving 22,457 participants and a median follow‐up of 12.9 months (range 6 to 24 months) reported cardiovascular mortality at maximum follow‐up beyond three months. The specific assessment time points in each trial are presented in Table 2. No events occurred in either group in one trial (Hanada 2012). A total of 1269/11.323 (11.2%) participants receiving beta‐blockers died because of a cardiovascular event versus 1379/11,134 (12.4%) control participants. Fixed‐effect meta‐analysis showed evidence of a beneficial effect of beta‐blockers versus placebo or no intervention on cardiovascular mortality (RR 0.90, 98% CI 0.83 to 0.98; I2 = 0%; 22,457 participants; 14 trials/15 comparisons; moderate‐quality evidence; Analysis 6.1). Hence, the absolute risk for a cardiovascular death at maximum follow‐up corresponds to 116 out of 1000 participants receiving beta‐blockers dying of a cardiovascular event compared with 128 out of 1000 participants receiving placebo or no intervention and a NNTB of 83 participants (see summary of findings Table 2). The optimal information size according to the GRADE Handbook using a proportion of 12.4% in the control group, a RRR of 10%, an alpha of 2.0%, and a beta of 10% was estimated to be 35,192 participants and we included 22,457 participants.
Heterogeneity
The visual inspection of the forest plot and the tests for statistical heterogeneity (I2 = 0 %; P = 0.96) indicated no signs of heterogeneity.
Risk of bias and sensitivity analyses
All trials were at high risk of bias mainly due to domains being at unclear risk of bias. Overall, the risk of bias of the outcome result was assessed as high risk of bias.
The sensitivity analyses on incomplete outcome data showed that incomplete outcome data bias alone had the potential to influence the results in the best‐worst sensitivity analysis, but not in the worst‐best sensitivity analysis: best‐worst random‐effects meta‐analysis (RR 0.67, 95% CI 0.53 to 0.86; I2 = 65%; 22,587 participants; 14 trials/15 comparisons; Analysis 6.11); worst‐best random‐effects meta‐analysis (RR 1.06, 95% CI 0.83 to 1.36; I2 = 67%; 22,587 participants; 14 trials/15 comparisons; Analysis 6.12). Data were imputed for five trials.
Visual inspection of the funnel plots showed no signs of asymmetry (Figure 7). Based on the visual inspection of the funnel plot, we assessed the risk of publication bias as low.
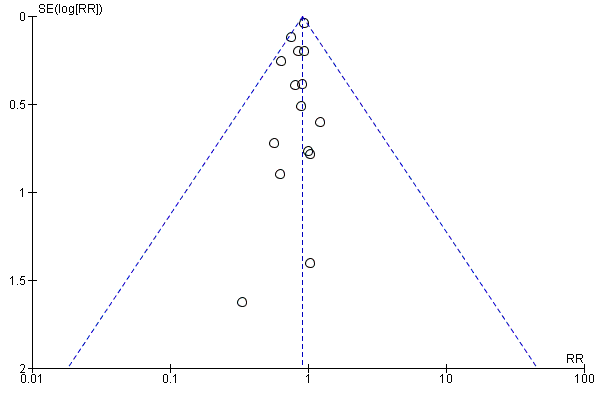
Funnel plot of comparison: 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, outcome: 6.1 Cardiovascular mortality.
Subgroup analyses
All remaining tests for subgroup differences showed no evidence of a difference in subgroup analyses according to the acute and subacute phase of commencing beta‐blockers (Analysis 6.2); reperfusion compared to no reperfusion (Analysis 6.3); types of beta‐blockers (Analysis 6.4); age either below compared to a mixture of above/below 75 years (Analysis 6.5); intravenous compared to oral administration of beta‐blockers (Analysis 6.6); different types of acute myocardial infarction (NSTEMI, STEMI, UAP or mixed) (6/14 trials reported data on the different types of acute myocardial infarction) (Analysis 6.7); clinical trial registration status (Analysis 6.8); different lengths of the intervention period (Analysis 6.9); and funding (Analysis 6.10).
Myocardial infarction during follow‐up
Time point less than three months follow up
In total, 18/62 trials involving 67,562 participants and a median follow‐up of 20.6 days (range 3 to 90 days) reported myocardial infarction during follow‐up at the time point less than three months follow‐up. The specific assessment time points in each trial are presented in Table 1. No events occurred in either group in two trials (Hanada 2012; Shirotani 2010). A total of 764/33,788 (2.26%) participants receiving beta‐blockers had a reinfarction versus 936/33,744 (2.77%) control participants. Fixed‐effect meta‐analysis showed evidence of a beneficial effect of beta‐blockers versus placebo or no intervention on myocardial infarction during follow‐up (RR 0.82, 98% CI 0.73 to 0.91; I2 = 0%; 67,562 participants; 18 trials; moderate‐quality evidence; Analysis 7.1). Hence, the absolute risk for a reinfarction at less than three months follow‐up corresponds to 23 out of 1000 participants receiving beta‐blockers having a reinfarction compared with 28 out of 1000 participants receiving placebo or no intervention and a NNTB of 196 participants (see summary of findings Table for the main comparison). The optimal information size according to the GRADE Handbook using a proportion of 2.77% in the control group, a RRR of 10%, an alpha of 2.0%, and a beta of 10% was estimated to be 170,073 participants and we only included 67,562 participants.
Heterogeneity
The visual inspection of the forest plot and the tests for statistical heterogeneity (I2 = 0 %; P = 0.66) indicated no signs of heterogeneity.
Risk of bias and sensitivity analyses
One trial was assessed at overall low risk of bias (COMMIT 2005). All other trials were at high risk of bias mainly due to domains being at unclear risk of bias. Overall, the risk of bias of the outcome result was assessed as high risk of bias.
The sensitivity analysis excluding trials at high risk of bias showed evidence of a beneficial effect of beta‐blockers versus placebo or no intervention on myocardial infarction during follow‐up at the time point less than three months follow‐up (RR 0.82, 95% CI 0.72 to 0.92; 45,852 participants; 1 trial; high‐quality evidence; Analysis 7.10). Since the sensitivity analysis and the overall meta‐analysis showed similar results, we based our'Ssummary of findings' tables and conclusion on the overall meta‐analysis.
The sensitivity analyses on incomplete outcome data showed that incomplete outcome data bias alone had the potential to influence the results in the best‐worst sensitivity analysis, but not in the worst‐best sensitivity analysis: (best‐worst random‐effects meta‐analysis: RR 0.75, 95% CI 0.61 to 0.92; I2 = 34%; 67,620 participants; 18 trials; Analysis 7.11); worst‐best random‐effects meta‐analysis: RR 0.85, 95% CI 0.67 to 1.07; I2 = 45%; 67,620 participants; 18 trials; Analysis 7.12). Data were imputed for two trials.
Visual inspection of the funnel plots showed no signs of asymmetry (Figure 8). Based on the visual inspection of the funnel plot, we assessed the risk of publication bias as low.

Funnel plot of comparison: 7 Beta‐blockers versus placebo or no intervention at 'less than 3 months' follow‐up, outcome: 7.1 Myocardial infarction.
Subgroup analyses
Tests for subgroup differences showed no evidence of a difference in subgroup analyses according to the acute and subacute phase of commencing beta‐blockers (Analysis 7.2); reperfusion compared to no reperfusion (Analysis 7.3); types of beta‐blockers (Analysis 7.4); age either below compared to a mixture of above/below 75 years (Analysis 7.5); intravenous compared to oral administration of beta‐blockers (Analysis 7.6); different types of acute myocardial infarction (NSTEMI, STEMI, UAP, or mixed) (11/18 trials reported data on the different types of acute myocardial infarction) (Analysis 7.7); clinical trial registration status (Analysis 7.8); and funding (Analysis 7.9).
Maximum follow‐up beyond three months
In total, 14/63 trials involving 6825 participants and a median follow‐up of 15.5 months (range 6 to 60 months) reported myocardial infarction during follow‐up at maximum follow‐up beyond three months. The specific assessment time points in each trial are presented in Table 2. A total of 282/3401 (8.3%) participants receiving beta‐blockers had a reinfarction versus 314/3424 (9.2%) control participants. Random‐effects meta‐analysis showed no evidence of a difference on myocardial infarction during follow‐up at maximum follow‐up beyond three months (RR 0.89, 98% CI 0.75 to 1.08; I2 = 10%; 6825 participants; 14 trials; low‐quality evidence; Analysis 8.1). (see summary of findings Table for the main comparison). Hence, the absolute risk for a reinfarction at maximum follow‐up corresponds to 89 out of 1000 participants receiving beta‐blockers having a reinfarction compared with 102 out of 1000 participants receiving placebo or no intervention. The optimal information size according to the GRADE Handbook using a proportion of 9.2% in the control group, a RRR of 10%, an alpha of 2.0%, and a beta of 10% was estimated to be 49,069 participants and we only included 6825 participants.
Heterogeneity
The visual inspection of the forest plot and the tests for statistical heterogeneity (I2 = 10%; P = 0.35) indicated no signs of heterogeneity.
Risk of bias and sensitivity analyses
All trials were at high risk of bias mainly due to domains being at unclear risk of bias. Overall, the risk of bias of the outcome result was assessed as high risk of bias.
The sensitivity analyses on incomplete outcome data showed that incomplete outcome data bias alone had the potential to influence the results in the best‐worst sensitivity analysis, but not in the worst‐best sensitivity analysis: best‐worst random‐effects meta‐analysis (RR 0.56, 95% CI 0.37 to 0.85; I2 = 71%; 6951 participants; 14 trials; Analysis 8.11); worst‐best random‐effects meta‐analysis: RR 1.10, 95% CI 0.95 to 1.26; I2 = 74%; 6951 participants; 14 trials; Analysis 8.12). Data were imputed for four trials.
Visual inspection of the funnel plots showed no signs of asymmetry (Figure 9). Based on the visual inspection of the funnel plot, we assessed the risk of publication bias as low.
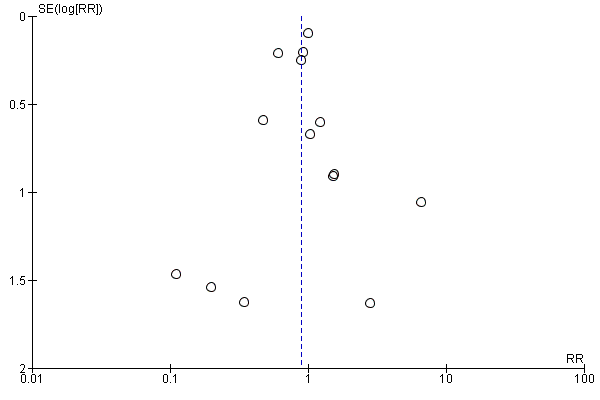
Funnel plot of comparison: 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, outcome: 8.1 Myocardial infarction.
Subgroup analyses
Tests for subgroup differences showed no evidence of a difference in subgroup analyses according to the acute and subacute phase of commencing beta‐blockers (Analysis 8.2); reperfusion compared to no reperfusion (Analysis 8.3); types of beta‐blockers (Analysis 8.4); age either below compared to a mixture of above/below 75 years (12/13 trials reported the age of the participants) (Analysis 8.5); intravenous compared to oral administration of beta‐blockers (Analysis 8.6); different types of acute myocardial infarction (NSTEMI, STEMI, UAP, or mixed) (6/13 trials reported data on the different types of acute myocardial infarction) (Analysis 8.7); clinical trial registration status (Analysis 8.8); different lengths of the intervention periods (Analysis 8.9); and funding (Analysis 8.10).
Quality of life
None of the included trials reported any data on quality of life on a continuous or any other scale at any time point.
Angina
Time point less than three months follow‐up
Four trials reported angina with a median follow‐up of 29 days (range 12 days to 42 days) at the time point less than three months follow‐up (EMIT 2002; Norris 1980; Tereshchenko 2005; and Yang 1986). Different definitions and ways of measuring angina were used: Norris 1980 and Yang 1986 reported angina on exercise testing, Tereshchenko 2005 reported post‐infarction angina without defining it, and EMIT 2002 reported angina on an ordinal scale using anginal status defined by the NYHA functional capacity classification.
In total, 3/62 trials involving 98 participants reported on the proportion of participants with angina (Norris 1980; Tereshchenko 2005; Yang 1986). A total of 8/53 (15.1%) participants receiving beta‐blockers had angina compared with 10/45 (22.2%) control participants. Random‐effects meta‐analysis showed no evidence of a difference of beta‐blockers versus placebo or no intervention on angina (RR 0.70, 98% CI 0.25 to 1.84; I2 = 0%; 98 participants; 3 trials; very low‐quality evidence; Analysis 9.1). The optimal information size according to the GRADE Handbook using a proportion of 22.2% in the control group, a RRR of 10%, an alpha of 2.0%, and a beta of 10% was estimated to be 17,583 participants and we only included 98 participants.
No further analyses were conducted due to sparse data.
Narrative description of the remaining trials reporting angina
EMIT 2002 reported angina on an ordinal scale using anginal status defined by the NYHA functional capacity classification. This classification reported four categories of angina: class I, II, III, and IV. In the experimental group, 34 patients were classified with class I, four with class II, two with class III, and two with class IV. In the control group, 34 patients were classified with class I, two with class II, two with class III, and one with class IV.
Maximum follow‐up beyond three months
Two trials reported angina with a total of 844 participants reported on the proportion of participants with angina at maximum follow‐up beyond three months (average months) (Kaul 1988; CAPITAL ‐ RCT 2018). Kaul 1988 reported post‐infarction angina without further definition, and CAPITAL ‐ RCT 2018 reported vasospastic angina. A total of 6/419 (1.43%) participants receiving beta‐blockers had angina compared with 10/425 (2.35%%) control participants. Random‐effects meta‐analysis showed no evidence of a difference of beta‐blockers versus placebo or no intervention on angina (RR 0.64, 98% CI 0.18 to 2.00; I2 = 24%; 844 participants; 2 trials; very low‐quality evidence; Analysis 10.1). The optimal information size according to the GRADE Handbook using a proportion of 2.35% in the control group, a RRR of 10%, an alpha of 2.0%, and a beta of 10% was estimated to be 215,068 participants and we only included 844 participants.
No further analyses were conducted due to sparse data.
'Summary of findings' tables
Our main results (i.e. primary and secondary outcomes) are summarised in the summary of findings Table for the main comparison (time point less than three months follow‐up) and summary of findings Table 2 (maximum follow‐up beyond three months).
Discussion
Summary of main results
We were able to include 63 trials randomising a total of 85,550 participants (mean age 57.4 years; mean range 45.9 to 70.0 years). One trial (COMMIT 2005) was considered to be at low risk of bias for all outcomes at the time point 'less than three months' follow‐up. One trial was assessed at low risk of bias in all but one domain (blinding) but since the blinding of participants and personnel was not considered of key importance for an objective outcome like all‐cause mortality, the study was assessed at low risk of bias for all‐cause mortality (METOCARD‐CNIC 2013). The remaining trials and all our meta‐analysed outcome results were considered to be at high risk of bias, mainly due to domains being at unclear risk of bias. The quality of the evidence according to GRADE ranged from very low to high at the time point 'less than three months' follow‐up and very low to moderate at maximum follow‐up beyond three months. The mean proportion of participants with a diagnosis of acute myocardial infarction at the time of randomisation was 80.3%. eight out of 63 trials specifically randomised participants suspected of or diagnosed with ST‐elevation myocardial infarction; 20 out of 63 trials randomised a mixed group of participants (ST‐elevation myocardial infarction, non‐ST‐elevation myocardial infarction, or unstable angina); and the remaining 35 trials did not report data on the different kinds of acute myocardial infarction included. 24 trials received the trial interventions for zero to seven days, 23 trials received the trial interventions for seven to 30 days, and the remaining 16 trials received the trial interventions for at least one month or more.
At the time point 'less than three months' follow‐up, meta‐analysis with a moderate‐quality of evidence showed that beta‐blockers versus placebo or no intervention probably reduce the risk of a new myocardial infarction during follow‐up. The effects of beta‐blockers versus placebo or no intervention on the risks of all‐cause mortality and cardiovascular mortality were with a high‐ to moderate‐quality evidence less convincing and showed little to no evidence of a difference. Regarding angina, very low‐quality evidence suggests that it is uncertain whether beta‐blockers have a beneficial, neutral, or harmful effect versus placebo or no intervention. The optimal information size was reached for all‐cause mortality but not for cardiovascular mortality, myocardial infarction, and angina. Accordingly, all‐cause mortality was not downgraded for imprecision. However, due to the large sample sizes and narrow 98% confidence intervals (CIs) not showing any appreciable benefit nor harm, cardiovascular mortality and myocardial infarction during follow‐up were not downgraded for the risk of imprecision. Nervertheless, when assessing angina, we downgraded two levels for imprecision due to the optimal information size not being reached, the very small sample size included, and the wide 98% CIs showing both appreciable benefit and harm.
At maximum follow‐up beyond three months, meta‐analysis with moderate‐quality evidence showed that beta‐blockers versus placebo or no intervention probably reduce the risk of all‐cause mortality and cardiovascular mortality. The effects of beta‐blockers versus placebo or no intervention on the risk of myocardial infarction during follow‐up were with low quality of evidence uncertain, as the risk ratio (RR) and lower CI suggested a trend towards a beneficial effect but the upper CI did not exclude the possibility of no difference between the two groups. When assessing major adverse cardiovascular events and angina, very low‐quality evidence suggests that it is uncertain whether beta‐blockers have a beneficial, neutral, or harmful effect. The optimal information size was not reached for any of the outcomes at maximum follow‐up beyond three months. However, due to the large sample sizes and narrow 97.5% and 98% CIs only showing benefit, all‐cause mortality and cardiovascular mortality were not downgraded for risk of imprecision. Nevertheless, when assessing myocardial infarction during follow‐up, we downgraded one level for imprecision due to the wide 98% CI where the upper CI did not exclude the possibility of no difference between the two groups. When assessing major adverse cardiovascular events and angina, we downgraded two levels for imprecision due to the optimal information size not being reached, the very small sample size included and the wide 97.5% CI showing both appreciable benefit and harm.
None of the trials specifically assessed serious adverse events according to International Conference on Harmonization ‐ Good Clinical Practice Guidelines (ICH‐GCP; ICH‐GCP 1997). Instead, the trials either reported composites of several specific serious adverse events or one specific serious adverse event.
Two trials at the time point 'less than three months' follow‐up (no events reported) and four trials at maximum follow‐up beyond three months specifically assessed major adverse cardiovascular events according to our definition (composite of cardiovascular mortality and non‐fatal myocardial infarction during follow‐up). Instead, the trials reported either cardiovascular mortality or myocardial infarction. Due to sparse data, no further analyses were conducted.
No data were provided on quality of life.
Overall completeness and applicability of evidence
This review provides the most comprehensive and contemporary appraisal of the evidence on beta‐blockers for suspected or diagnosed myocardial infarction to date. We searched for published and unpublished trials irrespective of trial design, setting, blinding, publication status, publication year, language, and reporting of our outcomes. None of our funnel plots indicated any significant signs of publication bias. Otherwise, all trials but one (COMMIT 2005) were at high risk of bias which suggests that our results might overestimate benefits and underestimate harms (Gluud 2006; Kjaergard 2001; Lundh 2017; Moher 1998; Savovic 2012; Savovic 2012a; Schulz 1995; Wood 2008). However, our positive meta‐analytic findings were supported by the findings of COMMIT 2005 for all outcomes beside cardiovascular mortality at the time point 'less than three months' follow‐up.
We included all participants with either suspected or diagnosed acute myocardial infarction irrespective of age, sex, severity of disease, type of beta‐blocker used, and type of control group intervention (placebo or no intervention). Nevertheless, we found limited signs of statistical heterogeneity which indicates that the pooling of these diverse participants and interventions was appropriate.
There were no data on the effects of beta‐blockers versus placebo or no intervention on serious adverse events according to the definition of ICH‐GCP and quality of life, and only very limited data on major adverse cardiovascular events according to our definition (composite of cardiovascular mortality or non‐fatal myocardial infarction during follow‐up) and angina. We were, therefore, not able to adequately assess the harmful effects and the overall safety of beta‐blockers. A balanced assessment of interventions requires analysis of both benefits and harms (Zorzela 2014). Hence, without adequate reporting of both harms and benefits, it is more difficult to estimate if an intervention is safe (Ioannidis 2009; Zorzela 2014). Poorly reported data on adverse events in randomised trials (especially in small randomised trials lacking the power to study major but uncommon harms) has in many years led to systematic reviews and meta‐analyses providing inadequate information concerning safety data of the drug (Cornelius 2009; Papanikolaou 2004; Zorzela 2014). However, besides reporting on all‐cause mortality, cardiovascular mortality, and myocardial reinfarction, the trials also reported other adverse events that were described as serious according to the trialists. These serious adverse events reported were mainly cardiovascular for all trials. We reported narratively the individual types of serious adverse events in each trial in Table 3 (less than three months follow‐up) and Table 4 (maximum follow‐up beyond three months).
Quality of the evidence
Risk of systematic error (bias)
Our 'Risk of bias' assessment showed that only one trial was at low risk of bias in all domains (COMMIT 2005). One trial was assessed at 'ow risk of bias in all but one domain and since the blinding of participants and personnel was not considered of key importance for an objective outcome like all‐cause mortality, the study was assessed at low risk of bias (METOCARD‐CNIC 2013). However, recent data show that even all‐cause mortality may be biased due to lack of blinding (Savovic 2018). All other trials were at high risk of bias (mainly due to domains being assessed at unclear risk of bias) (see Risk of bias in included studies). There is, therefore, a risk of our results overestimating the beneficial effects and underestimating the harmful effects of beta‐blockers (Gluud 2006; Kjaergard 2001; Lundh 2017; Moher 1998; Savovic 2012; Savovic 2012a; Schulz 1995; Wood 2008).
When assessing all‐cause mortality at the time point 'less than three months' follow‐up, the trial contributing most weight (COMMIT 2005, 63.4%) was assessed at low risk of bias in all domains. The trials contributing the second highest weight (ISIS‐1 1986, 17.4%) and third highest weight (MIAMI 1985, 5.0%) were assessed as low risk of bias in most key domains. Hence, since the overall limitations of ISIS‐1 1986 and MIAMI 1985 were not of key importance for an objective outcome like all‐cause mortality, the evidence was not downgraded for risk of bias. However, when assessing all‐cause mortality at maximum follow‐up beyond three months, the overall analysis including trials at high risk of bias due to either unclear or high risk in several bias domains showed different results compared to the sensitivity analysis excluding trials at high risk of bias. Hence, the evidence was downgraded by one level for risk of bias.
When assessing cardiovascular mortality at the time point 'less than three months' follow‐up, the result was based on the sensitivity analysis excluding trials at high risk of bias and the evidence was not downgraded for risk of bias. However, when assessing cardiovascular mortality at maximum follow‐up beyond three months, the evidence was downgraded by one level since all the included trials were at high risk of bias due to either unclear or high risk in several bias domains.
When assessing myocardial infarction at the time point 'less than three months' follow‐up, the trial contributing most weight (COMMIT 2005, 60,4%) was assessed at low risk of bias in all domains. The trial contributing the second highest weight (ISIS‐1 1986, 17,1%) was assessed at low risk of bias in random sequence generation, allocation concealment, and incomplete outcome data; unclear for blinding of outcome assessors and selective reporting and at high risk for blinding of participants and personnel. The third highest contributing trial (MIAMI 1985, 11,5%) was assessed at unclear risk of bias in selective reporting and at low risk of bias in all the other domains. Since the overall limitations and especially in regard to blinding of outcome assessors were serious (approximately 50% of the trials contributing with more than 30% of the total weight were assessed as unclear risk of bias in blinding of outcome assessors), the evidence was downgraded by one level for risk of bias. The evidence was also downgraded by one level at maximum follow‐up beyond three months since all the included trials were at high risk of bias due to either unclear or high risk in several bias domains.
When assessing major adverse cardiovascular events and angina, the evidence was downgraded by one level since all the included trials were at high risk of bias due to either unclear or high risk in several bias domains at both time points.
None of the funnel plots showed any clear signs of asymmetry. Hence, there is not a strong suspicion of small‐study bias or publication bias.
Risk of random errors ‐ imprecision ('play of chance')
When assessing the GRADE optimal information sizes, the optimal information size was only reached for all‐cause mortality at less than three months follow‐up. Accordingly, all‐cause mortality was not downgraded for imprecision. Regarding all‐cause mortality at maximum follow‐up beyond three months, the overall‐analysis was not downgraded due to the large sample size and the narrow absolute 97.5% CI showing only appreciable benefit and no harm, while the sensitivity analysis excluding trials at high risk of bias was downgraded by two levels due to the very small sample size included.
When assessing cardiovascular mortality at maximum follow‐up beyond three months and myocardial infarction during follow‐up at 'less than three months' follow‐up, the evidence was not downgraded for imprecision due to a large sample size and a narrow 98% CI not showing any appreciable benefit or harm. Nevertheless, when assessing cardiovascular mortality at 'less than three months' follow‐up and myocardial infarction during follow‐up at maximum follow‐up beyond three months, we downgraded by one level for imprecision, due to the wide absolute 98% CI where the upper CI did not exclude the possibility of no difference between the groups.
When assessing major adverse cardiovascular events at maximum follow‐up beyond three months and angina at both time points, the evidence was downgraded by two levels due to the very small sample sizes included, the optimal information sizes not being reached, and the wide 97.5% and 98% CIs, respectively, showing both appreciable benefit and harm.
Heterogeneity ‐ inconsistency
We assessed the statistical heterogeneity in the planned analyses of our primary and secondary outcomes as low. The limited signs of statistical heterogeneity increases the validity of our results. We have therefore not downgraded any outcomes for risk of inconsistency.
Indirectness of evidence
We assessed the risk of indirectness in the planned analyses and found that the sensitivity analysis excluding trials at high risk of bias when assessing all‐cause mortality beyond three months had serious risk of indirectness. This was due to the only trial included in the sensitivity analysis having several specific inclusion and exclusion criteria, which were not comparable to our pragmatic objective and inclusion and exclusion criteria. Regarding all the other analyses of our primary and secondary outcomes, we found no, or a low risk of indirectness due to the included trials directly comparing the intervention which we were interested in, delivered to the population in which we were interested, and most of the times measured the outcomes important to patients.
Publication bias
We assessed the risk of publication bias in the planned analyses of our primary and secondary outcomes as low due to none of the funnel plots showing asymmetry.
Funding
We found no effect of funding from industry in our subgroup analyses.
GRADE
We have assessed the quality of evidence of each outcome results using GRADE both at the time point 'less than three months' follow‐up (summary of findings Table for the main comparison) and maximum follow‐up beyond three months (summary of findings Table 2). The GRADE assessment showed that the quality of the evidence was very low to high at the time point 'less than three months' follow‐up and very low to moderate at maximum follow‐up beyond three months. Reasons for the GRADE assessment are given in the footnotes of the table (summary of findings Table for the main comparison (time point 'less than three months' follow‐up) and summary of findings Table 2 (maximum follow‐up beyond three months)).
Potential biases in the review process
Strengths
Our review has several strengths. We are the first to conduct a systematic review using Cochrane methodology comparing beta‐blockers versus placebo or no intervention in patients with suspected or diagnosed myocardial infarction. We followed our peer‐reviewed protocol, which was published before the literature search began regarding Cochrane methodology (Nielsen 2016), and we conducted the review using the methods recommended by Cochrane (Higgins 2011). We included trials regardless of language of publication and whether they reported data on the outcomes we had planned to assess. We contacted all relevant authors if additional information was needed. We included more trials and more participants than any previous systematic review or meta‐analysis which gives us increased power and precision to detect any evidence of a difference between the intervention and control group. Data were double‐extracted by independent review authors minimising the risk of inaccurate data‐extraction, and we assessed the risk of bias in all trials according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used GRADE to assess the quality of the body of evidence (Guyatt 2008; Guyatt 2011; Schünemann 2003; Schünemann 2017), and sensitivity analyses (best‐worst and worst‐best) to test the potential impact of incomplete outcome data bias. Hence, this systematic review considered both risks of random errors and risks of systematic errors which adds further robustness to our results and conclusions.
Our meta‐analyses had little statistical heterogeneity strengthening the validity of our results.
Limitations
Our systematic review has several limitations. Our findings, interpretations, and conclusions are affected by the quality, quantity, and outcome reporting of the included trials. Some limitations have already been discussed in the above section Overall completeness and applicability of evidence).
'Risk of bias' assessment
Our 'Risk of bias' assessment showed that only one trial was at low risk of bias in all domains (COMMIT 2005). We conducted sensitivity analyses excluding trials at high risk of bias to assess if the results differed compared to the overall analyses. We based our primary analyses and primary conclusions on trials at low risk of bias if their results differed compared to the overall analyses, since meta‐epidemiological studies have shown that trials at high risk of bias overestimate benefits and underestimate harms (Hrobjartsson 2012; Hrobjartsson 2013; Hrobjartsson 2014; Hrobjartsson 2014a; Jakobsen 2014; Lundh 2017; Savovic 2012; Wood 2008). For cardiovascular mortality at the time point 'less than three months' follow‐up, we based our primary analyses and primary conclusions on trials at low risk of bias. For all the other outcomes, we based our primary analyses and primary conclusions on the overall analyses.
Incomplete outcome data
We assessed 38/62 trials as either at unclear or high risk of bias on the incomplete outcome data bias domain. Our 'best‐worst' and 'worst‐best' case analyses confirmed that there is a high risk of incomplete outcome data bias influencing our primary analyses for all our outcomes. In 31/62 trials, it was not reported in sufficient detail if any participant was lost to follow‐up. It was often only reported that a certain number of participants died or experienced a type of serious adverse event without reporting if any of the participants were lost to follow‐up, etc. If insufficient data were reported by the trialists, we tried to contact the authors, but often the authors did not reply. Hence, the extent of the incomplete outcome data was often unclear. Our 'best‐worst' and 'worst‐best' case analyses might underestimate the potential impact of missing data because only the data on the reported population were used if no other information was available. Incomplete outcome data might potentially have an even greater bias impact than our 'best‐worst' and 'worst‐best' case analyses show, i.e. the 'true' differences between the actually observed cases and the intention‐to‐treat population might be larger than our data suggest.
Assessed time points
We predefined the time point 'less than three months' as our primary assessment time point. However, only a few studies reported data at this time point (Table 1). We chose 'less than three months' as the possible effects of beta‐blockers would have some time to show and the follow‐up period would be short enough to assess any short term effect of beta‐blockers without other factors (e.g. non‐cardiac‐related deaths) diluting the intervention effect. Nevertheless, we also assessed all outcomes at maximum follow‐up beyond three months. The meta‐analyses at maximum follow‐up showed different results in regard to all‐cause mortality, cardiovascular mortality, and myocardial infarction during follow‐up when compared to the results at the time point 'less than 3' months follow‐up.
Continous outcomes
We included quality of life and angina as continuous outcomes. However, no trials reported quality of life and angina on a continuous scale.
Clinical heterogeneity
The beta‐blockers used in the experimental group, the control group and the co‐interventions used in the different trials differed. Furthermore, the length of intervention period, the dosage of the beta‐blockers, and the follow‐up period differed between the trials. Even though our results showed limited sign of statistical heterogeneity, this is a limitation of our review because the subsequent transferability into a specific clinical context may be impaired.
Serious adverse events
The trials included in this review reported harms inadequately. None of the trials specifically assessed nor reported serious adverse events as planned according to ICH‐GCP (ICH‐GCP 1997). Instead, the trials either reported composites of several specific serious adverse events or one specific serious adverse event. We could therefore not conduct a meta‐analysis as planned on all serious adverse events. Hence, it was not possible to assess the overall safety of beta‐blockers in patients with suspected or diagnosed myocardial infarction. This is problematic, as the reporting of harm is as important as the reporting of efficacy. Without adequate reporting of both harms and benefits, it is not possible to estimate if an intervention is useful or not (Ioannidis 2009). Because of the utmost importance of assessing harmful effects and to not lose important data reported by the trialists, one could conduct an exploratory outcome assessing either the composite of several specific adverse events described as 'serious adverse event' by the trialists (without referring to ICH‐GCP), or one single reported serious adverse event that had the largest number of events when it was unclear if participants had experienced more than one serious adverse event to avoid double‐counting. In addition, an exploratory meta‐analysis could be conducted according to different types of serious adverse events assessed. These exploratory outcomes were not presented in this present review in accordance with the Cochrane Heart Group's methods.
Major adverse cardiovascular events
Only two trials at the time point 'less than three months' follow‐up (with no events reported) and four trials at maximum follow‐up beyond three months specifically assesseD major adverse cardiovascular events according to our definition (composite of cardiovascular mortality and non‐fatal myocardial infarction during follow‐up). The effects of beta‐blockers on major adverse cardiovascular events are therefore uncertain due to lack of data. Instead, the trials reported either cardiovascular mortality or myocardial infarction during follow‐up. Because of the utmost importance of assessing harmful effects and not to lose important data reported by the trialists, one could conduct exploratory meta‐analyses assessing the composite of either cardiovascular mortality or myocardial infarction with the largest number of events to avoid double‐counting. In addition. an exploratory meta‐analysis could be conducted according to different types of major adverse cardiovascular events assessed. These exploratory outcomes were not presented in this present review in accordance with the Cochrane Heart Group's methods.
Multiplicity
We used broad inclusion criteria to fully assess the effects of beta‐blockers in patients with suspected or diagnosed myocardial infarction. Therefore, we included a large number of outcomes, time points, subgroup analyses, and sensitivity analyses. We are aware that this increases the risk of type 1 error due to multiplicity. To minimise the potential risk, we decided on a more conservative alpha of 2.5% with a 97.5% CI for our primary outcomes and an alpha of 2.0% with a 98% CI for our secondary outcomes (Jakobsen 2014; Jakobsen 2016).
Disease‐related outcomes
The validity of the results of this meta‐analysis in regard to our disease‐related outcomes (major adverse cardiovascular events, cardiovascular mortality, and myocardial infarction) might be questionable as there are numerous methodological limitations of the use of disease‐related mortality and other outcomes (Heneghan 2017; Higgins 2011).
-
When assessing disease‐related outcomes, the ’true’ causality of an outcome (e.g. disease‐specific death) will often be unclear and the validity of results on disease‐related mortality might therefore also be unclear. As a consequence, the results on disease‐related events might not reflect the ‘true’ effect due to the risk of erroneous classifications of certain causes of events.
-
Clinical events that are apparently unrelated to a disease might actually be caused by the disease or the intervention for the disease. For example, if a trial participant suddenly dies in a traffic accident, the underlying cause might be nausea or dizziness caused by the disease or it might be an adverse reaction of an intervention for the disease. Hence, the impact of possible unknown adverse reactions might be overlooked in the results.
-
As the trials included in this review used different definitions of cardiovascular mortality and reinfarction, the validity of such meta‐analyses result might be questionable. Even if the definitions of the disease‐related mortality such as cardiovascular mortality are described similarly in each trial, the subjective assessment of whether or not an event is disease‐related might still differ substantially between trials. This is, for obvious reasons, particularly a problem if the outcome assessors in the trials are not adequately blinded to treatment allocation of the patients. The vast majority of the included trials did not adequately blind the personnel and outcome assessors, one should, therefore, interpret outcomes such as major adverse cardiovascular events, cardiovascular mortality, and myocardial infarction with even greater caution.
The most valid outcome is considered to be all‐cause mortality assessed at low risk of bias. When the intervention effect estimates differ between disease‐related mortality and all‐cause mortality, the results should especially be interpreted with caution. Our apparent beneficial effect of beta‐blockers on myocardial infarction during follow‐up at the time point 'less than three months' follow‐up does not comply with our findings in regard to all‐cause mortality at the time point 'less than three months' follow‐up showing little to no benefit. One should, therefore, interpret this apparent beneficial effect on myocardial infarction with some caution. Nevertheless, at maximum follow‐up beyond three months, cardiovascular mortality and the overall analysis when assessing all‐cause mortality showed a beneficial effect of beta‐blockers on these outcomes.
Agreements and disagreements with other studies or reviews
We have identified multiple reviews assessing the effects of beta‐blockers versus placebo or no intervention. However, only one of the reviews systematically assessed the risks of random errors and employed adequate assessments of risks of bias using some of the Cochrane domains (Bangalore 2014), while the rest of them had either a very limited bias risk assessment (Al‐Reesi 2008; Freemantle 1999; Brandler 2010; Chatterjee 2013) or no bias risk assessment at all (Lewis 1982; Yusuf 1985). None of the reviews used the GRADE system to assess the quality of the body of evidence associated with the outcome results reported.
Al‐Reesi 2008 assessed patients randomised within the first 72 hours following an acute myocardial infarction receiving any type of beta‐blockers commenced either intravenously or orally. The review found no evidence of a beneficial effect on all‐cause mortality at six weeks follow‐up (odds ratio (OR) 0.95, 95% CI 0.90 to 1.01). The same was true when assessing the subgroup of only high‐quality studies (studies were considered high‐quality if they scored 2 on the Jadad score and had adequate allocation concealment (A), or if they scored 3 or more on the Jadad score with an allocation concealment score of at least B) (OR 0.96, 95% CI 0.91 to 1.02). The study limited their search to only English studies.
Freemantle 1999 assessed patients who had had a myocardial infarction and where treatment with any type of beta‐blockers commenced either intravenously or orally started at any stage before or after the myocardial infarction. The review found no evidence of a beneficial effect on mortality at short‐term follow‐up (OR 0.96, 95% CI 0.85 to 1.08). However, when assessing long‐term treatment effect, evidence of a beneficial effect was found (OR 0.77, 95% CI 0.69 to 0.85).
Brandler 2010 assessed patients with an acute or suspected myocardial infarction within 24 hours of onset of chest pain treated within eight hours with any type of beta‐blockers commenced either intravenously or orally. The review found no evidence of a beneficial effect on in‐hospital mortality (RR 0.95, 95% CI 0.90 to 1.01).
Perez 2009 assessed patients with an acute cardiovascular event (e.g. myocardial infarction) treated with different anti‐hypertensive drugs (e.g. beta‐blockers) within 24 hours of the onset of symptoms. The review found 20 trials where patients were treated with beta‐blockers commenced either intravenously or orally and showed no evidence of a difference on all‐cause mortality at 10 days follow‐up (RR 0.96, 95% CI 0.91 to 1.02, P = 0.21, I2=0%), nor at weighted average of 12 months of follow‐up (RR 0.91, 95% CI 0.84 to 0.99, P = 0.03, I2 = 0%).
Chatterjee 2013 assessed patients with an acute or suspected myocardial infarction within 48 hours of onset of chest pain treated with intravenously commenced beta‐blockers within 12 hours. The review only included 16 trials and showed a beneficial effect of early beta‐blockers on mortality (RR 0.92, 95% CI 0.86 to 1; P = 0.04) and myocardial reinfarction rates (RR 0.73, 95% CI 0.59 to 0.91, P = 0.004).
Lewis 1982 assessed patients with myocardial infarction treated with either intravenously or orally commenced beta‐blockers and distinguished between early (within 48 hours) and late intervention. The review showed evidence of a beneficial effect when pooling both late and early intervention (RR 0.79, 95% CI 0.72 to 0.87). However, when assessing only the early‐entry trials, it found evidence of a small beneficial effect (RR 0.92). The review only included 17 trials compared to our 62 trials.
Yusuf 1985 assessed the effects of beta‐blockers on all‐cause mortality at short‐term and long‐term treatment with beta‐blockers commenced either intravenously or orally and showed evidence of a beneficial effect at long‐term treatment while no effect was seen in the short‐term treatment with beta‐blockers. Furthermore, a reduction in reinfarction was found at long‐term treatment with beta‐blockers.
Bangalore 2014 assessed participants with myocardial infarction treated with beta‐blockers commenced either intravenously or orally and distinguished between the pre‐reperfusion and reperfusion period (if > 50% of patients received reperfusion either with thrombolytics or with revascularisation or aspirin/statin). In the pre‐reperfusion period, beta‐blockers were associated with a statistically significant reduction in mortality (IRR 0.86, 95% CI 0.79 to 0.94), cardiovascular mortality, and myocardial infarction at both short‐term follow up (30 days) and long‐term follow‐up (12 months). In the reperfusion period, beta‐blockers were associated with no beneficial effect at both time points except for myocardial infarction and angina at 30 days follow‐up. However, a significant increase in heart failure, cardiogenic shock, and drug discontinuation was found in the reperfusion period. Furthermore, a reduction in all‐cause mortality, myocardial infarction, and angina pectoris was seen in the pre‐reperfusion period when observing trials where early intravenous beta‐blockers were administered. In the reperfusion period the beneficial effect was only associated with myocardial infarction and angina and not all‐cause mortality when observing trials where early intravenous beta‐blockers were administered.
Our present review result, when analysing the short‐term effect of beta‐blockers on all‐cause mortality, is in agreement with Al‐Reesi 2008, Freemantle 1999, Brandler 2010, Perez 2009, and Yusuf 1985, where the conclusions are that beta‐blockers do not seem to have any short‐term effect on the risk of all‐cause mortality. The same is seen in Bangalore 2014, when assessing all‐cause mortality for the reperfusion period, while the pre‐reperfusion period shows evidence of a beneficial effect on all‐cause mortality. However, our present review's results are not in agreement with Chatterjee 2013 and Lewis 1982, as these reviews showed a beneficial short‐term effect of beta‐blockers on all‐cause mortality. This disagreement may potentially be due to different inclusion criteria as Chatterjee 2013 included only trials where participants were treated with intravenous beta‐blockers within 12 hours and not oral beta‐blockers, which differs from our review where we have included both oral and intravenous beta‐blocker treatment as the reviews mentioned above. This beneficial effect on mortality observed in Chatterjee 2013 may reflect the superior effect of intravenous beta‐blocker administration, which is also seen in Bangalore 2014 in the pre‐reperfusion period, however, this has not been observed with our subgroup analysis differentiating between orally and intravenously commenced beta‐blockers. Furthermore, both Chatterjee 2013 and Lewis 1982 only included respectively, 16 and 17 trials each which differs from our review and the majority of the reviews mentioned above.
When assessing the long‐term effect of beta‐blockers on all‐cause mortality, our present review result is in agreement with Freemantle 1999 and Yusuf 1985, but not with Perez 2009 which differs from our review and the other reviews mentioned above as this review included only five trials in the assessment of the long‐term follow‐up effect of beta‐blockers.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Funnel plot of comparison: 1 Beta‐blockers versus placebo or no intervention at 'less than 3 months' follow‐up, outcome: 1.1 All‐cause mortality.

Funnel plot of comparison: 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, outcome: 2.1 All‐cause mortality.

Funnel plot of comparison: 5 Beta‐blockers versus placebo or no intervention at 'less than 3 months' follow‐up beyond 3 months, outcome: 5.1 Cardiovascular mortality.

Funnel plot of comparison: 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, outcome: 6.1 Cardiovascular mortality.

Funnel plot of comparison: 7 Beta‐blockers versus placebo or no intervention at 'less than 3 months' follow‐up, outcome: 7.1 Myocardial infarction.
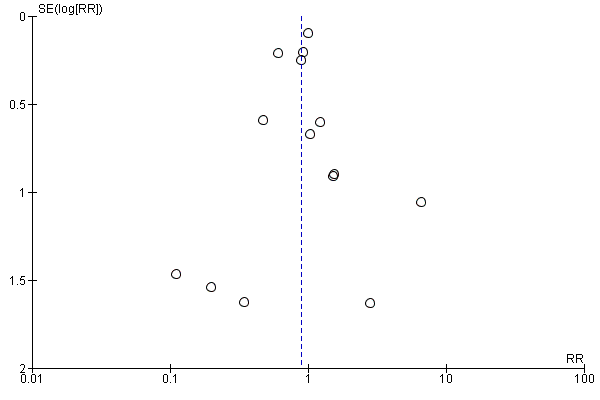
Funnel plot of comparison: 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, outcome: 8.1 Myocardial infarction.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 All‐cause mortality.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 2 All‐cause mortality ‐ Acute/subacute phase.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 3 All‐cause mortality ‐ Reperfusion/no reperfusion.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 4 All‐cause mortality ‐ Type of beta‐blocker.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 5 All‐cause mortality ‐ Intravenously/orally commenced.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 6 All‐cause mortality ‐ Above/below 75 years of age.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 7 All‐cause mortality ‐ NSTEMI/STEMI/UAP.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 8 All‐cause mortality ‐ Registration status.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 9 All‐cause mortality ‐ Funding.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 10 All‐cause mortality ‐ Trials at low risk of bias.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 11 All‐cause mortality ‐ 'Best‐worst case scenario'.

Comparison 1 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 12 All‐cause mortality ‐ 'Worst‐best case scenario'.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 All‐cause mortality.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 2 All‐cause mortality ‐ Acute/subacute phase.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 3 All‐cause mortality ‐ Reperfusion/no reperfusion.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 4 All‐cause mortality ‐ Type of beta‐blocker.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 5 All‐cause mortality ‐ Above/below 75 years of age.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 6 All‐cause mortality ‐ Intravenously/orally commenced.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 7 All‐cause mortality ‐ Registration status.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 8 All‐cause mortality ‐ NSTEMI/STEMI/UAP.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 9 All‐cause mortality ‐ Length of intervention period.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 10 All‐cause mortality ‐ Funding.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 11 All‐cause mortality ‐ Trials at low risk of bias.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 12 All‐cause mortality ‐ 'Best‐worst case scenario'.

Comparison 2 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 13 All‐cause mortality ‐ 'Worst‐best case scenario'.

Comparison 3 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 MACE (Composite of cardiovascular death and non‐fatal myocardial infarction).

Comparison 4 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 MACE (Composite of cardiovascular death and non‐fatal myocardial infarction).

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 Cardiovascular mortality.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 2 Cardiovascular mortality ‐ Acute/subacute phase.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 3 Cardiovascular mortality ‐ Reperfusion/no reperfusion.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 4 Cardiovascular mortality ‐ Type of beta‐blocker.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 5 Cardiovascular mortality ‐ Above/below 75 years of age.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 6 Cardiovascular mortality ‐ Intravenously/orally commenced.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 7 Cardiovascular mortality ‐ NSTEMI/STEMI/UAP.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 8 Cardiovascular mortality ‐ Registration status.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 9 Cardiovascular mortality ‐ Funding.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 10 Cardiovascular mortality ‐ Trials with low risk of bias.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 11 Cardiovascular mortality ‐ 'Best‐worst case scenario'.

Comparison 5 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 12 Cardiovascular mortality ‐ 'Worst‐best case scenario'.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 Cardiovascular mortality.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 2 Cardiovascular mortality ‐ Acute/subacute phase.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 3 Cardiovascular mortality ‐ Reperfusion/no reperfusion.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 4 Cardiovascular mortality ‐ Type of beta‐blocker.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 5 Cardiovascular mortality ‐ Above/below 75 years of age.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 6 Cardiovascular mortality ‐ Intravenously/orally commenced.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 7 Cardiovascular mortality ‐ NSTEMI/STEMI/UAP.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 8 Cardiovascular mortality ‐ Registration status.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 9 Cardiovascular mortality ‐ Length of intervention period.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 10 Cardiovascular mortality ‐ Funding.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 11 Cardiovascular mortality ‐ 'Best‐worst case scenario'.

Comparison 6 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 12 Cardiovascular mortality ‐ 'Worst‐best case scenario'.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 Myocardial infarction.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 2 Myocardial infarction ‐ Acute/subacute phase.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 3 Myocardial infarction ‐ Reperfusion/no reperfusion.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 4 Myocardial infarction ‐ Type of beta‐blocker.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 5 Myocardial infarction ‐ Above/below 75 years of age.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 6 Myocardial infarction ‐ Intravenously/orally commenced.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 7 Myocardial infarction ‐ NSTEMI/STEMI/UAP.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 8 Myocardial infarction ‐ Registration status.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 9 Myocardial infarction ‐ Funding.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 10 Myocardial infarction ‐ Trials with low risk of bias.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 11 Myocardial infarction ‐ 'Best‐worst case scenario'.

Comparison 7 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 12 Myocardial infarction ‐ 'Worst‐best case scenario'.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 Myocardial infarction.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 2 Myocardial infarction ‐ Acute/subacute phase.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 3 Myocardial infarction ‐ Reperfusion/no reperfusion.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 4 Myocardial infarction ‐ Type of beta‐blocker.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 5 Myocardial infarction ‐ Above/below 75 years of age.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 6 Myocardial infarction ‐ Intravenously/orally commenced.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 7 Myocardial infarction ‐ NSTEMI/STEMI/UAP.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 8 Myocardial infarction ‐ Registration status.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 9 Myocardial infarction ‐ Length of intervention period.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 10 Myocardial infarction ‐ Funding.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 11 Myocardial infarction ‐ 'Best‐worst case scenario'.

Comparison 8 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 12 Myocardial infarction ‐ 'Worst‐best case scenario'.

Comparison 9 Beta‐blockers versus placebo or no intervention less than 3 months follow‐up, Outcome 1 Angina on a dichotomous scale.

Comparison 10 Beta‐blockers versus placebo or no intervention at maximum follow‐up beyond 3 months, Outcome 1 Angina on a dichotomous scale.
| Beta‐blockers versus placebo or no intervention for patients with suspected or diagnosed myocardial infarction at the time point less than three months follow‐up | ||||||
| Patient or population: patients with suspected or diagnosed myocardial infarction Settings: any setting Intervention: any beta‐blocker Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect (adjusted CI) | No of Participants | Quality of the evidence | Comments | |
| Assumed risk with placebo or no intervention | Corresponding risk with beta‐blockers | |||||
| All‐cause mortality at 'less than 3 months' follow‐up. Follow‐up: mean 21.8 days (range 1 hour to 90 days). | 70 per 1000 | 67 per 1000 | RR 0.94, 97.5% CI (0.90 to 1.0) | 80,452 (46 RCTs with 47 comparisons) | ⊕⊕⊕⊕1 | Since the sensitivity analysis excluding trials at high risk of bias and the overall meta‐analysis showed similar results, we based our summary of findings and conclusion on the overall meta‐analysis. No events occurred in either group in three trials (Hanada 2012; Norris 1978; Shirotani 2010). |
| Serious adverse events at 'less than 3 months' follow‐up. No data was reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | None of the trials specifically assessed nor reported serious adverse events according to ICH‐GCP. |
| MACE (major adverse cardiovascular event) at 'less than 3 months' follow‐up. No data were reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | Only two trials specifically assessed major adverse cardiovascular events (defined as a composite of cardiovascular mortality and myocardial infarction during follow‐up). However, no major adverse cardiovascular events occurred in either trial. |
| Quality of life at 'less than 3 months' follow‐up. No data were reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | No data reported. |
| Angina at 'less than 3 months' follow‐up. Follow‐up: mean 21 days (range 12 to 30 days). | 222 per 1000 | 155 per 1000 (69 to 351) | RR 0.70, 98% CI (0.25 to 1.84) | 98 (3 RCTs) | ⊕⊝⊝⊝2,3 VERY LOW | |
| Cardiovascular mortality at 'less than 3 months' follow‐up. Follow‐up: mean 28 days. | 43 per 1000 | 42 per 1000 | RR 0.99, 95% CI (0.91 to 1.08) | 45,852 (1 RCT) | ⊕⊕⊕⊝4 | Since the sensitivity analysis excluding trials at high risk of bias differed from the overall meta‐analysis, we based our summary of findings and conclusion on the sensitivity analysis. |
| Myocardial infarction at 'less than 3 months' follow‐up. Follow‐up: mean 23.3 days (range 3 to 90 days). | 28 per 1000 | 23 per 1000 | RR 0.82, 98% CI (0.74 to 0.90) | 67,562 (18 RCTs) | ⊕⊕⊕⊝5 | Since the sensitivity analysis excluding trials at high risk of bias and the overall meta‐analysis showed similar results, we based our summary of findings and conclusion on the overall meta‐analysis. No events occurred in either group in two trials (Hanada 2012; Shirotani 2010). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its adjusted confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its adjusted CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 When assessing the risk of bias, the trial contributing most weight (COMMIT 2005, 63.4%) was assessed as at low risk of bias in all domains. The trial contributing the second highest weight (ISIS‐1 1986, 17.4%) was assessed as low risk of bias in random sequence generation, allocation concealment, and incomplete outcome data;'unclear for blinding of outcome assessors and selective reporting and at high risk for blinding of participants and personnel. Since a lack of blinding is less important for the assessment of all‐cause mortality, the overall limitations were not serious and the evidence is not downgraded for risk of bias. 2 Downgraded by one level due to serious risk of bias. All the included trials were at high risk of bias due to either unclear or high risk in several bias domains. 3 Downgraded by two levels due to very serious risk of imprecision based on the optimal information size not being reached, the very small sample size, and the absolute and relative 98% CI being very wide showing both appreciable benefit and harm. 4 Downgraded by one level due to serious risk of imprecision based on the wide absolute and relative 98% where the upper CI does not exclude the possibility of no difference between the groups. When assessing the risk of bias, the evidence was not downgraded since the result was based on the sensitivity analysis consisting of trials at low risk of bias (COMMIT 2005). 5 Downgraded by one level due to serious risk of bias. The overall limitations and specially in regard to blinding of outcome assessors were serious (around 50% of the trials were assessed at unclear risk of bias in blinding of outcome assessors). | ||||||
| Beta‐blockers compared with placebo or no intervention for patients with suspected or diagnosed myocardial infarction | ||||||
| Patient or population: patients with suspected or diagnosed myocardial infarction Settings: any setting Intervention: beta‐blockers Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk with placebo or no intervention | Corresponding risk with beta‐blockers | |||||
| All‐cause mortality at maximum follow‐up beyond 3 months. Follow‐up: mean 16.4 months (range 6 to 60 months). | 148 per 1000 | 138 per 1000 | RR 0.93, 97.5% CI (0.86 to 0.99) | 25,210 (21 RCTs with 22 comparisons) | ⊕⊕⊕⊝3 | No events occurred in either group in one trial (Hanada 2012). |
| Serious adverse events at maximum follow‐up beyond 3 months. No data were reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | None of the trials specifically assessed nor reported serious adverse events according to ICH‐GCP. |
| MACE (major adverse cardiovascular event) at maximum follow‐up beyond 3 months. Follow‐up: mean7.5 months (range 6 to 12 months). | 84 per 1000 | 68 per 1000 | RR 0.81, 97.5% CI (0.43 to 1.52) | 475 (4 RCTs) | ⊕⊝⊝⊝1, 2 | |
| Quality of life at maximum follow‐up beyond 3 months. No data were reported in the included trials. | ‐ | ‐ | ‐ | ‐ | ‐ | No data reported. |
| Angina at maximum follow‐up beyond 3 months (mean = 6 months). | 24 per 1000 | 15 per 1000 (5 to 48) | RR 0.64, 98% CI 0.18 to 2.0 | 844 ( 2 RTCs) | ⊕⊝⊝⊝1,5 | |
| Cardiovascular mortality at maximum follow‐up beyond 3 months. Follow‐up: mean 12.9 months (range 6 to 24 months). | 124 per 1000 | 112 per 1000 | RR 0.90, 98% CI (0.83 to 0.98) | 22,457 (14 RCTs with 15 comparisons) | ⊕⊕⊕⊝1 | No events occurred in either group in one trial (Hanada 2012). |
| Myocardial infarction at maximum follow‐up beyond 3 months. Follow‐up: mean 15.5 months (range 6 to 60 months). | 92 per 1000 | 83 per 1000 | RR 0.89, 98% CI (0.75 to 1.08) | 6825 (14 RCTs) | ⊕⊕⊝⊝1, 6 Low | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to serious risk of bias. All the included trials were at high risk of bias due to either unclear or high risk in several bias domains. 2 Downgraded by two levels due to very serious risk of imprecision based on the optimal information size not being reached, the wide absolute and relative 97.5% CI showing both appreciable benefit and harm, and a small sample size. 3 Downgraded by one level due to serious risk of bias. All but one of the included trials were at high risk of bias due to either unclear or high risk in several bias domains and the sensitivity analysis excluding trials at high risk of bias showed different results than the overall analysis including trials at high risk of bias. However, the sensitivity analysis was based on only one small trial, so we have used the main analysis for the 'Summary of findings' table. 5 Downgraded by two levels due to very serious risk of imprecision based on the very small sample size included. 6 Downgraded by one level due to serious risk of imprecision based on the wide absolute and relative 98% where the upper CI does not exclude the possibility of no difference between the groups. | ||||||
| Trial | Year | All‐cause mortality | Major adverse cardiovascular events | Cardiovascular mortality | Myocardial infarction during follow‐up |
| Andersen | 1979 | 28 days | NR | NR | NR |
| Åström | 1986 | NR | NR | NR | NR |
| Australian | 1984 | 28 days | NR | NR | NR |
| Australia & Swedish | 1983 | 28 days | NR | NR | NR |
| Balcon | 1967 | 28 days | NR | NR | NR |
| Barbar | 1967 | 28 days | NR | NR | NR |
| Barber | 1976 | 90 days | NR | NR | NR |
| BEAT‐AMI trial | 2016 | During hospitalisation (no mean time) | NR | During hospitalisation (no mean time) | During hospitalisation (no mean time) |
| Briant | 1970 | 3 days | NR | NR | NR |
| Campbell | 1984 | 7 days | NR | NR | NR |
| CAPRICORN | 2001 | 30 days | NR | NR | NR |
| Clausen | 1966 | 28 days | NR | NR | NR |
| COMMIT | 2005 | 28 days | NR | 28 days | 28 days |
| CPRG | 1980 | 60 days | NR | 56 days | 56 days |
| EARLY‐BAMI | 2016 | 30 days | NR | 30 days | 30 days |
| EMIT | 2002 | 42 days | NR | NR | 42 days |
| Evemy | 1977 | 30 days | NR | NR | NR |
| Gardtman | 1999 | 30 days | NR | NR | NR |
| Göteborg Metoprolol Trial | 1981 | 90 days | NR | 90 days | 90 days |
| Hanada | 2012 | During hospitalisation (no mean time) (no events) | During hospitalisation (no mean time) (no events) | During hospitalisation (no mean time) (no events) | During hospitalisation (no mean time) (no events) |
| Heber | 1986 | 5 days | NR | NR | NR |
| ICSG | 1984 | During hospitalisation (no mean time) | NR | During hospitalisation (no mean time) | During hospitalisation (no mean time) |
| ISIS‐1 | 1986 | 14 days | NR | 7 days | 7 days |
| Johannessen | 1987 | 10 days | NR | NR | NR |
| Ledwich | 1968 | 7 days | NR | NR | NR |
| McMurray | 1991 | NR | NR | NR | 10 days |
| METOCARD‐CNIC | 2013 | 7 days | NR | NR | 7 days |
| MIAMI | 1985 | 15 days | NR | 15 days | 15 days |
| MILIS | 1984 | 30 days | NR | NR | NR |
| Mueller | 1980 | 3 days | NR | 10 days | NR |
| Multicenter trial | 1966 | 30 days | NR | 30 days | NR |
| Nielsen | 1967 | 28 days | NR | NR | 28 days |
| Norris | 1968 | 21 days | NR | NR | NR |
| Norris | 1978 | 8.5 days (no events) | NR | 8.5 days (no events) | NR |
| Norris | 1980 | During hospitalisation (no mean time) | NR | During hospitalisation (no mean time) | NR |
| Norris | 1984 | 21 days | NR | 21 days | NR |
| Owensby | 1985 | 3 days | NR | NR | 3 days |
| Peter | 1978 | 3 days | NR | NR | 3 days |
| Raeder | 1967 | 21 days | NR | 21 days | NR |
| Ranganathan | 1988 | 2 days | NR | NR | NR |
| Rolli | 1980 | NR | NR | NR | NR |
| Salathia | 1985 | During hospitalisation (no mean time) | NR | 90 days | NR |
| Shirotani | 2010 | 30 days (no events) | 30 days (no events) | 30 days (no events) | 30 days (no events) |
| Tereschenko | 2005 | 30 days | NR | 30 days | NR |
| Thompson | 1979 | 5 days | NR | NR | NR |
| TIARA | 1987 | 30 days | NR | 30 days | 30 days |
| Tonkin | 1981 | 7 days | NR | NR | 7 days |
| Van De Werf | 1993 | 14 days | NR | 14 days | 14 days |
| Von Essen | 1982 | 14 days | NR | 14 days | NR |
| Wilcox | 1980 | 42 days | NR | NR | NR |
| Yang | 1984 | NR | NR | NR | NR |
| Yusuf | 1980 | 10 days | NR | NR | During hospitalisation (no mean time) |
| Trial | Year | All‐cause mortality | Major adverse cardiovascular events | Cardiovascular mortality | Myocardial infarction |
| Andersen | 1979 | 12 months | NR | NR | NR |
| Australia & Swedish | 1983 | 24 months | NR | 24 months | 24 months |
| Barber | 1976 | 24 months | NR | NR | NR |
| Basu | 1997 | 6 months | 6 months | 6 months | 6 months |
| BEAT‐AMI trial | 2016 | 6 months | NR | 6 months | 6 months |
| Briant | 1970 | 12 months | 12 months | 12 months | 12 months |
| CAPRICORN | 2001 | 15.6 months | NR | 15.6 months | 15.6 months |
| EARLY‐BAMI | 2016 | 1 month (not included) | NR | 12 months | 12 months |
| Evemy | 1977 | 7 months | NR | NR | NR |
| Göteborg Metoprolol Trial | 1981 | 60 months | NR | 3 months | 60 months |
| Hanada | 2012 | 6 months (no events) | 6 months | 6 months (no events) | 6 months |
| Heber | 1986 | 12 months | NR | NR | NR |
| ISIS‐1 | 1986 | 20 months | NR | 20 months | 0.23 months (not included) |
| Kaul | 1988 | 6 months | NR | 6 months | 6 months |
| METOCARD‐CNIC | 2013 | 24 months | NR | 24 months | 24 months |
| MILIS | 1984 | 36 months | NR | NR | NR |
| NPT | 1982 | 12 months | NR | 12 months | 12 months |
| RIMA | 1999 | 6 months | 6 months | 6 months | 6 months |
| Salathia | 1985 | 12 months | NR | 12 months | NR |
| TIARA | 1987 | 24 months | NR | 1 month (not included) | 1 month (not included) |
| Tonkin | 1981 | 0.23 months (not included) | NR | NR | 12 months |
| Wilcox | 1980 | 12 months | NR | 12 months | NR |
| Yusuf | 1980 | 24 months | NR | NR | During hospitalisation (no mean time) (not included) |
| Trial | Year | Type and number of serious adverse events (beta‐blocker group) | Type and number of serious adverse events (control group) |
| Andersen | 1979 |
|
|
| Åstrøm | 1986 | None |
|
| Australian trial | 1984 |
|
|
| Australia & Swedish | 1983 |
|
|
| Balcon | 1967 |
|
|
| Barbar | 1967 |
|
|
| Barber | 1976 |
|
|
| BEAT‐AMI | 2016 |
|
|
| Briant | 1970 |
|
|
| Campbell | 1984 |
|
|
| CAPRICORN | 2001 |
|
|
| Clausen | 1966 |
|
|
| COMMIT | 2005 |
|
|
| CPRG | 1980 |
|
|
| EARLY‐BAMI | 2016 |
|
|
| EMIT | 2002 |
|
|
| Evemy | 1977 |
|
|
| Gardtman | 1999 |
|
|
| Göteborg | 1981 |
|
|
| Heber | 1986 |
|
|
| ICSG | 1984 |
|
|
| ISIS‐1 | 1986 |
|
|
| Johannessen | 1987 |
|
|
| Ledwich | 1968 |
|
|
| Lloyd | 1988 |
|
|
| Mcmurray | 1991 |
|
|
| METOCARD‐CNIC | 2013 |
|
|
| Miami | 1985 |
|
|
| MILIS | 1984 |
|
|
| Mueller | 1980 |
|
|
| Multicenter trial | 1966 |
|
|
| Nielsen | 1967 |
|
|
| Norris | 1968 |
|
|
| Norris | 1978 |
|
|
| Norris | 1980 |
|
|
| Norris | 1984 |
|
|
| Owensby | 1985 |
|
|
| Peter | 1978 |
|
|
| Raeder | 1967 |
|
|
| Ramsdale | 1982 | None |
|
| Ranganathan | 1988 |
|
|
| Rolli | 1980 |
|
|
| Salathia | 1985 |
|
|
| Tereschenko | 2005 |
|
|
| Thompson | 1979 |
|
|
| TIARA | 1987 |
|
|
| Tonkin | 1981 |
|
|
| Van De Werf | 1993 |
|
|
| Von Essen | 1982 |
|
|
| Wilcox (atenolol) | 1980 |
|
|
| Wilcox (propranolol) | 1980 |
|
|
| Yang | 1984 |
|
|
| Yusuf | 1980 |
|
|
| Trial | Year | Type and number of serious adverse events (beta‐blocker group) | Type and number of serious adverse events (control group) |
| Andersen | 1979 |
|
|
| Åstrøm | 1986 | None |
|
| Australian trial | 1984 |
|
|
| Australia & Swedish | 1983 |
|
|
| Balcon | 1967 |
|
|
| Barbar | 1967 |
|
|
| Barber | 1976 |
|
|
| Basu | 1997 |
|
|
| BEAT‐AMI | 2016 |
|
|
| Briant | 1970 |
|
|
| Campbell | 1984 |
|
|
| CAPRICORN | 2001 |
|
|
| Clausen | 1966 |
|
|
| COMMIT | 2005 |
|
|
| CPRG | 1980 |
|
|
| EARLY‐BAMI | 2016 |
|
|
| EMIT | 2002 |
|
|
| Evemy | 1977 |
|
|
| Gardtman | 1999 |
|
|
| Göteborg | 1981 |
|
|
| Hanada | 2012 | None |
|
| Heber | 1986 |
|
|
| ICSG | 1984 |
|
|
| ISIS‐1 | 1986 |
|
|
| Johannessen | 1987 |
|
|
| Kaul | 1988 |
|
|
| Ledwich | 1968 |
|
|
| Lloyd | 1988 |
|
|
| Mcmurray | 1991 |
|
|
| METOCARD‐CNIC | 2013 |
|
|
| Miami | 1985 |
|
|
| MILIS | 1984 |
|
|
| Mueller | 1980 |
|
|
| Multicenter trial | 1966 |
|
|
| Nielsen | 1967 |
|
|
| Norris | 1968 |
|
|
| Norris | 1978 |
|
|
| Norris | 1980 |
|
|
| Norris | 1984 |
|
|
| NPT | 1982 |
|
|
| Owensby | 1985 |
|
|
| Peter | 1978 |
|
|
| Raeder | 1967 |
|
|
| Ramsdale | 1982 | None |
|
| Ranganathan | 1988 |
|
|
| RIMA | 1999 |
|
|
| Rolli | 1980 |
|
|
| Salathia | 1985 |
|
|
| Tereschenko | 2005 |
|
|
| Thompson | 1979 |
|
|
| TIARA | 1987 |
|
|
| Tonkin | 1981 |
|
|
| Van De Werf | 1993 |
|
|
| Von Essen | 1982 |
|
|
| Wilcox (atenolol) | 1980 |
|
|
| Wilcox (propranolol) | 1980 |
|
|
| Yang | 1984 |
|
|
| Yusuf | 1980 |
|
|
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 2 All‐cause mortality ‐ Acute/subacute phase Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 2.1 Acute phase | 42 | 76857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.00] |
| 2.2 Subacute phase | 4 | 3595 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.50, 1.25] |
| 3 All‐cause mortality ‐ Reperfusion/no reperfusion Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 3.1 No reperfusion | 40 | 78206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 6 | 2246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.43, 1.50] |
| 4 All‐cause mortality ‐ Type of beta‐blocker Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 4.1 Alprenolol | 2 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.77, 1.66] |
| 4.2 Atenolol | 4 | 16890 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.74, 0.94] |
| 4.3 Esmolol | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.21, 3.62] |
| 4.4 Labetolol | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.60, 41.88] |
| 4.5 Metoprolol | 8 | 55034 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.03] |
| 4.6 Oxprenolol | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.47, 4.03] |
| 4.7 Pindolol | 2 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.49, 2.08] |
| 4.8 Practolol | 3 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.65, 1.60] |
| 4.9 Propranolol | 14 | 2630 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.18] |
| 4.10 Timolol | 7 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.33, 1.31] |
| 4.11 Carvedilol | 2 | 2753 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.25, 1.04] |
| 4.12 Mixed | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.18, 20.63] |
| 5 All‐cause mortality ‐ Intravenously/orally commenced Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 5.1 Intravenously commenced | 4 | 1036 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.46, 2.04] |
| 5.2 Orally commenced | 16 | 5658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.10] |
| 5.3 Mixed | 26 | 73758 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.00] |
| 6 All‐cause mortality ‐ Above/below 75 years of age Show forest plot | 39 | 79161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 6.1 Below 75 years | 24 | 12602 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.74, 0.99] |
| 6.2 Mixed | 15 | 66559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.90, 1.01] |
| 7 All‐cause mortality ‐ NSTEMI/STEMI/UAP Show forest plot | 24 | 53337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.92, 1.03] |
| 7.1 STEMI | 5 | 1828 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.48, 2.10] |
| 7.2 Mixed | 19 | 51509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.92, 1.03] |
| 8 All‐cause mortality ‐ Registration status Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 8.1 Pre‐registration | 5 | 47642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| 8.2 Post‐registration | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.16, 1.63] |
| 8.3 No registration | 40 | 32541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.80, 0.95] |
| 9 All‐cause mortality ‐ Funding Show forest plot | 46 | 80452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 9.1 Industry funded or unknown funded trials | 39 | 78702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.00] |
| 9.2 Non‐industry funded trials | 7 | 1750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.20] |
| 10 All‐cause mortality ‐ Trials at low risk of bias Show forest plot | 2 | 46122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| 11 All‐cause mortality ‐ 'Best‐worst case scenario' Show forest plot | 46 | 80522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.89, 0.98] |
| 12 All‐cause mortality ‐ 'Worst‐best case scenario' Show forest plot | 46 | 80522 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.98] |
| 2 All‐cause mortality ‐ Acute/subacute phase Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 2.1 Acute phase | 17 | 21368 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.89, 1.01] |
| 2.2 Subacute phase | 4 | 3842 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.68, 0.96] |
| 3 All‐cause mortality ‐ Reperfusion/no reperfusion Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 3.1 No reperfusion | 16 | 23768 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 5 | 1442 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 4 All‐cause mortality ‐ Type of beta‐blocker Show forest plot | 21 | 25098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 4.1 Alprenolol | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.74, 1.31] |
| 4.2 Atenolol | 3 | 16696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.88, 1.02] |
| 4.3 Carvedilol | 3 | 2899 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.64, 0.96] |
| 4.4 Labetolol | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.71, 4.14] |
| 4.5 Metoprolol | 4 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.09] |
| 4.6 Pindolol | 1 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.67, 1.40] |
| 4.7 Practolol | 2 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.68, 1.33] |
| 4.8 Propranolol | 4 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 4.9 Timolol | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.96] |
| 4.10 Esmolol | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 5 All‐cause mortality ‐ Above/below 75 years of age Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.98] |
| 5.1 Below 75 years | 16 | 5862 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.04] |
| 5.2 Mixed | 5 | 19348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 0.99] |
| 6 All‐cause mortality ‐ Intravenously/orally commenced Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 6.1 Intravenously commenced | 2 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.30, 2.39] |
| 6.2 Orally commenced | 7 | 4614 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.72, 0.96] |
| 6.3 Mixed | 12 | 20226 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.89, 1.01] |
| 7 All‐cause mortality ‐ Registration status Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 7.1 Pre‐registration | 3 | 1164 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.40] |
| 7.2 Post‐registration | 1 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.70, 2.08] |
| 7.3 No registration | 17 | 23777 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.98] |
| 8 All‐cause mortality ‐ NSTEMI/STEMI/UAP Show forest plot | 7 | 2128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.70, 1.18] |
| 8.1 STEMI | 3 | 1164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.51, 1.39] |
| 8.2 Mixed | 4 | 964 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.27] |
| 9 All‐cause mortality ‐ Length of intervention period Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 9.1 0 to 7 days length of intervention | 5 | 16651 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.89, 1.03] |
| 9.2 7 to 30 days length of intervention | 3 | 946 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.60, 1.24] |
| 9.3 1 month and more length of intervention | 13 | 7613 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.80, 0.98] |
| 10 All‐cause mortality ‐ Funding Show forest plot | 21 | 25210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.98] |
| 10.1 Industry funded or unknown funded trials | 18 | 23877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.98] |
| 10.2 Non‐industry funded trials | 3 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.69, 1.45] |
| 11 All‐cause mortality ‐ Trials at low risk of bias Show forest plot | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.31, 2.85] |
| 12 All‐cause mortality ‐ 'Best‐worst case scenario' Show forest plot | 21 | 25283 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.97] |
| 13 All‐cause mortality ‐ 'Worst‐best case scenario' Show forest plot | 21 | 25283 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MACE (Composite of cardiovascular death and non‐fatal myocardial infarction) Show forest plot | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MACE (Composite of cardiovascular death and non‐fatal myocardial infarction) Show forest plot | 4 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.43, 1.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 2 Cardiovascular mortality ‐ Acute/subacute phase Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 2.1 Acute phase | 17 | 72309 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.99] |
| 2.2 Subacute phase | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.41, 3.67] |
| 3 Cardiovascular mortality ‐ Reperfusion/no reperfusion Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 3.1 No reperfusion | 15 | 71702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 3 | 920 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.29, 1.62] |
| 4 Cardiovascular mortality ‐ Type of beta‐blocker Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 4.1 Esmolol | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.21, 3.62] |
| 4.2 Atenolol | 2 | 16221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.96] |
| 4.3 Metoprolol | 6 | 54501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.03] |
| 4.4 Oxprenolol | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.41, 3.67] |
| 4.5 Propranolol | 5 | 1103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.65, 1.73] |
| 4.6 Timolol | 2 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.20, 1.39] |
| 5 Cardiovascular mortality ‐ Above/below 75 years of age Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 5.1 Below 75 years | 14 | 56308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.88, 1.03] |
| 5.2 Mixed | 4 | 16314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.97] |
| 6 Cardiovascular mortality ‐ Intravenously/orally commenced Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 6.1 Intravenously commenced | 3 | 766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.42, 2.26] |
| 6.2 Orally commenced | 3 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.54, 1.82] |
| 6.3 Mixed | 12 | 71307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.99] |
| 7 Cardiovascular mortality ‐ NSTEMI/STEMI/UAP Show forest plot | 9 | 49303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.05] |
| 7.1 STEMI | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.35, 2.41] |
| 7.2 NSTEMI | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Unstable angina pectoris | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Mixed | 7 | 48577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.05] |
| 8 Cardiovascular mortality ‐ Registration status Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 8.1 Pre‐registration | 3 | 46578 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.08] |
| 8.2 Post‐registration | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 No registration | 15 | 26044 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.76, 0.93] |
| 9 Cardiovascular mortality ‐ Funding Show forest plot | 18 | 72622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 0.99] |
| 9.1 Industry funded or unknown funded trials | 17 | 72560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.99] |
| 9.2 Non‐industry funded trials | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.11, 62.56] |
| 10 Cardiovascular mortality ‐ Trials with low risk of bias Show forest plot | 1 | 45852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.08] |
| 11 Cardiovascular mortality ‐ 'Best‐worst case scenario' Show forest plot | 18 | 72681 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.72, 0.97] |
| 12 Cardiovascular mortality ‐ 'Worst‐best case scenario' Show forest plot | 18 | 72681 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.79, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.97] |
| 2 Cardiovascular mortality ‐ Acute/subacute phase Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 2.1 Acute phase | 10 | 18615 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.00] |
| 2.2 Subacute phase | 4 | 3842 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.95] |
| 3 Cardiovascular mortality ‐ Reperfusion/no reperfusion Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 3.1 No reperfusion | 8 | 20386 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.97] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 6 | 2071 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.51] |
| 4 Cardiovascular mortality ‐ Type of beta‐blocker Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 4.1 Esmolol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.99] |
| 4.2 Alprenolol | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.22, 4.77] |
| 4.3 Atenolol | 2 | 16219 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 4.4 Carvedilol | 3 | 2899 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.97] |
| 4.5 Metoprolol | 4 | 1827 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.18] |
| 4.6 Pindolol | 1 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.63, 1.40] |
| 4.7 Propranolol | 3 | 806 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.06] |
| 5 Cardiovascular mortality ‐ Above/below 75 years of age Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 5.1 Below 75 years | 11 | 5366 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.68, 0.94] |
| 5.2 Mixed | 3 | 17091 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 6 Cardiovascular mortality ‐ Intravenously/orally commenced Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 6.1 Intravenously commenced | 3 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.33, 1.60] |
| 6.2 Orally commenced | 6 | 4307 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.67, 0.95] |
| 6.3 Mixed | 5 | 17151 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 7 Cardiovascular mortality ‐ NSTEMI/STEMI/UAP Show forest plot | 6 | 2002 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.49, 1.59] |
| 7.1 STEMI | 4 | 1793 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.44, 1.64] |
| 7.2 Mixed | 2 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.27, 3.93] |
| 8 Cardiovascular mortality ‐ Registration status Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 8.1 Pre‐registration | 4 | 1793 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.44, 1.64] |
| 8.2 No registration | 10 | 20664 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.97] |
| 9 Cardiovascular mortality ‐ Length of intervention period Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 9.1 0 to 7 days length of intervention | 4 | 17026 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 9.2 1 month and more length of intervention | 10 | 5431 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.69, 0.94] |
| 10 Cardiovascular mortality ‐ Funding Show forest plot | 14 | 22457 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 10.1 Industry funded or unknown funded trials | 12 | 21393 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 10.2 Non‐industry funded trials | 2 | 1064 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.36, 2.20] |
| 11 Cardiovascular mortality ‐ 'Best‐worst case scenario' Show forest plot | 14 | 22587 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.53, 0.86] |
| 12 Cardiovascular mortality ‐ 'Worst‐best case scenario' Show forest plot | 14 | 22587 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.83, 1.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarction Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| 2 Myocardial infarction ‐ Acute/subacute phase Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| 2.1 Acute phase | 17 | 67249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.75, 0.90] |
| 2.2 Subacute phase | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.09, 1.25] |
| 3 Myocardial infarction ‐ Reperfusion/no reperfusion Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| 3.1 No reperfusion | 14 | 66372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 4 | 1190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.44, 1.82] |
| 4 Myocardial infarction ‐ Type of beta‐blocker Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 4.1 Esmolol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.06] |
| 4.2 Atenolol | 3 | 12312 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.42] |
| 4.3 Metoprolol | 5 | 53921 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.72, 0.89] |
| 4.4 Oxprenolol | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.09, 1.25] |
| 4.5 Pindolol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.82] |
| 4.6 Propranolol | 2 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.08, 14.01] |
| 4.7 Timolol | 3 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.26, 2.08] |
| 4.8 Xamoterol | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [0.13, 73.06] |
| 4.9 Mixed | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.37, 10.08] |
| 5 Myocardial infarction ‐ Above/below 75 years of age Show forest plot | 17 | 67432 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 5.1 Below 75 years | 13 | 9561 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.89] |
| 5.2 Mixed | 4 | 57871 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.76, 0.94] |
| 6 Myocardial infarction ‐ Intravenously/orally commenced Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 6.1 Intravenously commenced | 3 | 996 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.28, 4.51] |
| 6.2 Orally commenced | 3 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.16, 4.47] |
| 6.3 Mixed | 12 | 66114 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 7 Myocardial infarction ‐ NSTEMI/STEMI/UAP Show forest plot | 11 | 49361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.71, 0.90] |
| 7.1 STEMI | 3 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.29, 3.46] |
| 7.2 NSTEMI | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Unstable angina pectoris | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Mixed | 8 | 48365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.71, 0.89] |
| 8 Myocardial infarction ‐ Registration status Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 8.1 Pre‐registration | 4 | 46848 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 8.2 Post‐registration | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 No registration | 14 | 20714 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| 9 Myocardial infarction ‐ Funding Show forest plot | 18 | 67562 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 9.1 Industry funded or unknown funded trials | 15 | 67067 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 9.2 Non‐industry funded trials | 3 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.22, 6.77] |
| 10 Myocardial infarction ‐ Trials with low risk of bias Show forest plot | 1 | 45852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.92] |
| 11 Myocardial infarction ‐ 'Best‐worst case scenario' Show forest plot | 18 | 67620 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.61, 0.92] |
| 12 Myocardial infarction ‐ 'Worst‐best case scenario' Show forest plot | 18 | 67620 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarction Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 2 Myocardial infarction ‐ Acute/subacute phase Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 2.1 Acute phase | 10 | 2983 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.72, 1.33] |
| 2.2 Subacute phase | 4 | 3842 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 1.01] |
| 3 Myocardial infarction ‐ Reperfusion/no reperfusion Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 3.1 No reperfusion | 7 | 4658 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.15] |
| 3.2 Reperfusion (PCI , CABG, thrombolytics) | 7 | 2167 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.46, 1.66] |
| 4 Myocardial infarction ‐ Type of beta‐blocker Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 4.1 Esmolol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.06] |
| 4.2 Alprenolol | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.28, 3.81] |
| 4.3 Landiolol | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.01, 8.32] |
| 4.4 Carvedilol | 3 | 2899 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.91] |
| 4.5 Metoprolol | 4 | 2426 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.84, 1.24] |
| 4.6 Pindolol | 1 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.61, 1.38] |
| 4.7 Propranolol | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.08, 3.15] |
| 4.8 Timolol | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 6.57 [0.82, 52.35] |
| 5 Myocardial infarction ‐ Above/below 75 years of age Show forest plot | 13 | 6729 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.72, 1.09] |
| 5.1 Below 75 years | 11 | 5665 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.10] |
| 5.2 Mixed | 2 | 1064 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.45, 4.07] |
| 6 Myocardial infarction ‐ Intravenously/orally commenced Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 6.1 Intravenously commenced | 4 | 1095 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.26, 3.29] |
| 6.2 Orally commenced | 6 | 4007 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.17] |
| 6.3 Mixed | 4 | 1723 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.50] |
| 7 Myocardial infarction ‐ NSTEMI/STEMI/UAP Show forest plot | 7 | 2098 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.57, 2.18] |
| 7.1 STEMI | 5 | 1889 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.45, 2.54] |
| 7.2 Mixed | 2 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.42, 3.40] |
| 8 Myocardial infarction ‐ Registration status Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 8.1 Pre‐registration | 4 | 1793 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.48, 2.88] |
| 8.2 No registration | 10 | 5032 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.68, 1.09] |
| 9 Myocardial infarction ‐ Length of intervention period Show forest plot | 14 | 6913 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.71, 1.13] |
| 9.1 0 to 7 days length of intervention | 5 | 1183 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.46, 4.96] |
| 9.2 7 to 30 days length of intervention | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 6.57 [0.82, 52.35] |
| 9.3 1 month and more length of intervention | 9 | 5642 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.74, 1.06] |
| 10 Myocardial infarction ‐ Funding Show forest plot | 14 | 6825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.04] |
| 10.1 Industry funded or unknown funded trials | 11 | 5665 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.04] |
| 10.2 Non‐industry funded trials | 3 | 1160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.31, 2.04] |
| 11 Myocardial infarction ‐ 'Best‐worst case scenario' Show forest plot | 14 | 6951 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.85] |
| 12 Myocardial infarction ‐ 'Worst‐best case scenario' Show forest plot | 14 | 6951 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.95, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Angina on a dichotomous scale Show forest plot | 3 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.31, 1.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Angina on a dichotomous scale Show forest plot | 2 | 844 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.18, 2.30] |

