Neuromonitorización intraoperatoria versus identificación nerviosa visual para la prevención de la lesión del nervio laríngeo recurrente en pacientes adultos sometidos a cirugía tiroidea
Appendices
Appendix 1. Search strategies
| MEDLINE (OvidSP) |
| 1. Recurrent Laryngeal Nerve Injuries/ 2. Vocal Cord Paralysis/ 3. Recurrent Laryngeal Nerve/ 4. Intraoperative Complications/ 5. ((vocal or laryngeal) adj3 (nerve? or pals* or paralys* or injur*)).tw. 6. rln.tw. 7. or/1‐6 8. exp Monitoring, Intraoperative/ 9. Electromyography/ 10. monitor*.tw. 11. neuromonitor*.tw. 12. (ionm or rlnm).tw. 13. electromyogra*.tw. 14. or/8‐13 15. Thyroidectomy/ 16. Thyroid Diseases/su 17. exp Thyroid Neoplasms/su 18. Thyroid Gland/su 19. ((parathyroid or thyroid) adj3 (surg* or dissect* or resect* or cancer or neoplasm? or operat* or malign*)).tw. 20. thyroidectom*.tw. 21. or/15‐20 22. 7 and 14 and 21 [23‐33: Cochrane Handbook 2008 RCT filter ‐ sensitivity maximizing version] 23. randomized controlled trial.pt. 24. controlled clinical trial.pt. 25. randomi?ed.ab. 26. placebo.ab. 27. drug therapy.fs. 28. randomly.ab. 29. trial.ab. 30. groups.ab. 31. or/23‐30 32. exp animals/ not humans/ 33. 31 not 32 34. 22 and 33 [35:Wong 2006a– systematic reviews filter – SensSpec version] 35. meta analysis.mp,pt. or review.pt. or search*.tw. 36. 22 and 35 37. 34 or 36 |
| Embase (Ovid SP) |
| 1. recurrent laryngeal nerve injury/ 2. recurrent laryngeal nerve palsy/ 3. recurrent laryngeal nerve/ 4. vocal cord paralysis/ 5. peroperative complication/ 6. ((vocal or laryngeal) adj3 (nerve? or pals* or paralys* or injur*)).tw. 7. rln.tw. 8. or/1‐7 9. neurophysiological monitoring/ 10. neuromonitoring/ 11. electromyography/ 12. monitor*.tw. 13. neuromonitor*.tw. 14. (ionm or rlnm).tw. 15. electromyogra*.tw. 16. or/9‐15 17. exp thyroid surgery/ 18. ((parathyroid or thyroid) adj3 (surg* or dissect* or resect* or cancer or neoplasm? or operat* or malign*)).tw. 19. thyroidectom*.tw. 20. or/17‐19 21. 8 and 16 and 20 [22:Wong 2006b"sound treatment studies" filter – BS version] 22. random*.tw. or clinical trial*.mp. or exp health care quality/ 23. 21 and 22 |
| Cochrane Central Register of Controlled Trials (Cochrane Register of Studies Online) |
| 1. MESH DESCRIPTOR Recurrent Laryngeal Nerve Injuries 2. MESH DESCRIPTOR Vocal Cord Paralysis 3. MESH DESCRIPTOR Recurrent Laryngeal Nerve 4. MESH DESCRIPTOR Intraoperative Complications 5. ((vocal or laryngeal) ADJ3 (nerve? or pals* or paralys* or injur*)):TI,AB,KY 6. rln:TI,AB,KY 7. #1 OR #2 OR #3 OR #4 OR #5 OR #6 8. MESH DESCRIPTOR Monitoring, Intraoperative EXPLODE ALL TREES 9. MESH DESCRIPTOR Electromyography 10. monitor*:TI,AB,KY 11. neuromonitor*:TI,AB,KY 12. (ionm or rlnm):TI,AB,KY 13. electromyogra*:TI,AB,KY 14. #8 OR #9 OR #10 OR #11 OR #12 OR #13 15. MESH DESCRIPTOR Thyroidectomy 16. MESH DESCRIPTOR Thyroid Diseases WITH QUALIFIERS SU 17. MESH DESCRIPTOR Thyroid Neoplasms EXPLODE ALL TREES WITH QUALIFIERS SU 18. MESH DESCRIPTOR Thyroid Gland WITH QUALIFIERS SU 19. ((parathyroid or thyroid) ADJ3 (surg* or dissect* or resect* or cancer or neoplasm? or operat* or malign*)):TI,AB,KY 20. thyroidectom*:TI,AB,KY 21. #15 OR #16 OR #17 OR #18 OR #19 OR #20 22. #7 AND #14 AND #21 |
| WHO ICTRP Search Portal (Standard search) |
| laryin* AND neuromonitor* OR vocal AND neuromonitor* OR rln AND neuromonitor* OR laryin* AND monitor* OR vocal AND monitor* OR rln AND monitor* OR laryin* AND electromyograph* OR vocal AND electromyograph* OR rln AND electromyograph* OR ionm OR rlnm |
| ClinicalTrials.gov (Basic search) |
| (laryngeal OR vocal OR RLN OR complication OR complications) AND (monitor OR neuromonitor OR monitoring OR neuromonitoring OR electromyography OR electromyographic OR IONM OR RLNM) AND (thyroid OR parathyroid OR thyroidectomy) |
Appendix 2. Assessment of risk of bias
| 'Risk of bias' domains |
| Random sequence generation (selection bias due to inadequate generation of a randomised sequence) For each included trial, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Allocation concealment (selection bias due to inadequate concealment of allocation prior to assignment) We described for each included trial the method used to conceal allocation to interventions prior to assignment and we assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We also evaluated trial baseline data to incorporate assessment of baseline imbalance into the 'Risk of bias' judgment for selection bias (Corbett 2014; Egbewale 2014; Riley 2013). Chance imbalances may also affect judgments on the risk of attrition bias. In the case of unadjusted analyses, we distinguished between trials that we rated as being at low risk of bias on the basis of both randomisation methods and baseline similarity, and trials that we judged as being at low risk of bias on the basis of baseline similarity alone (Corbett 2014). We will reclassify judgements of unclear, low or high risk of selection bias as specified in Appendix 5. Blinding of participants and study personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the trial) We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed or adjudicated outcome measures (see below).
Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessment We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed or adjudicated outcome measures (see below).
Incomplete outcome data (attrition bias due to amount, nature or handling of incomplete outcome data) For each included trial and/or each outcome, we described the completeness of data, including attrition and exclusions from the analyses. We stated whether the trial reported attrition and exclusions, and report the number of participants included in the analysis at each stage (compared with the number of randomised participants per intervention/comparator groups). We also noted if the trial reported the reasons for attrition or exclusion and whether missing data were balanced across groups or were related to outcomes. We considered the implications of missing outcome data per outcome such as high dropout rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between trial arms).
Selective reporting (reporting bias due to selective outcome reporting) We assessed outcome reporting bias by integrating the results of the appendix 'Matrix of trial endpoints (publications and trial documents)' (Boutron 2014; Jones 2015; Mathieu 2009), with those of the appendix 'High risk of outcome reporting bias according to the Outcome Reporting Bias In Trials (ORBIT) classification' (Kirkham 2010). This analysis formed the basis for the judgement of selective reporting.
Other bias
|
Appendix 3. Selection bias decisions
| Selection bias decisions for trials reporting unadjusted analyses: comparison of results obtained using method details alone with results using method details and trial baseline informationa | |||
| Reported randomisation and allocation concealment methods | 'Risk of bias' judgement using methods reporting | Information gained from study characteristics data | 'Risk of bias' judgement using baseline information and methods reporting |
| Unclear methods | Unclear risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited or no baseline details | Unclear risk | ||
| Would generate a truly random sample, with robust allocation concealment | Low risk | Baseline imbalances present for important prognostic variable(s) | Unclear riskc |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesb | Low risk | ||
| No baseline details | Unclear risk | ||
| Sequence is not truly random, or allocation concealment is inadequate | High risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesb | Unclear risk | ||
| No baseline details | High risk | ||
| aTaken from Corbett 2014; judgements highlighted in bold indicate situations in which the addition of baseline assessments would change the judgement about risk of selection bias, compared with using methods reporting alone. | |||
Appendix 4. Description of interventions
| Trial ID | Intervention | Comparator |
| RILN visualisation + IONM | RILN visualisation | |
| RILN visualisation + IONM | RILN visualisation | |
| RILN visualisation + IONM | RILN visualisation | |
| RILN visualisation + IONM | RILN visualisation | |
| RILN visualisation + IONM | RILN visualisation | |
| IONM: intraoperative nerve monitoring; RILN: recurrent inferior laryngeal nerve | ||
Appendix 5. Baseline characteristics (I)
| Trial ID | Intervention and comparator | Duration of follow‐up | Description of participants | Trial period | Country | Setting | Ethnic groups | Duration of thyroid disease |
| I: RILN visualisation + IONM | 6 months | Participants with thyroid neoplastic (papillary, follicular and medullary carcinoma) (77%) or nontoxic nodular goitre recurrence (23%) after thyroidectomy | January 2012 to August 2014 | China | Inpatient | Asian (100) | ‐ | |
| C: RILN visualisation | Asian (100) | ‐ | ||||||
| I: RILN visualisation + IONM | 12 months | Participants with papillary thyroid carcinoma (100%) | March 2011 to September 2012 | Korea | Inpatient | Asian (100) | ‐ | |
| C: RILN visualisation | Asian (100) | ‐ | ||||||
| I: RILN visualisation + IONM | 6 months | Participants with Graves’ disease (5%), thyroid carcinoma (12%), toxic (15%) and nontoxic (68%) nodular goitre | September 2009 to June 2010 | Poland | Inpatient | White (100) | ‐ | |
| C: RILN visualisation | White (100) | ‐ | ||||||
| I: RILN visualisation + IONM | 12 months | Participants with Graves’ disease (9%), toxic adenoma (6%), solitary adenoma (14%), thyroid carcinoma (17%), toxic (14%) and nontoxic (39%) multinodular goitre | September 2007 to September 2009 | Turkey | Inpatient | White (100) | ‐ | |
| C: RILN visualisation | White (100) | ‐ | ||||||
| I: RILN visualisation + IONM | 12 months | Participants with Graves’ disease (6%), thyroid carcinoma (12%), thyroiditis (2%), toxic (10%) and nontoxic nodular goitre (70%) | January 2006 to June 2007 | Poland | Inpatient | White (100) | ‐ | |
| C: RILN visualisation | White (100) | ‐ | ||||||
| ‐ denotes not reported C: comparator; I: intervention; IONM: intraoperative nerve monitoring; RILN: recurrent inferior laryngeal nerve | ||||||||
Appendix 6. Baseline characteristics (II)
| Trial ID | Intervention and comparator | Sex | Age | BMI | Type of thyroidectomy | Experience in thyroid surgery | Comedications/co‐interventions | Comorbidities |
| I: RILN visualisation + IONM | 70 | 48.3 (9.1) | ‐ | Thyroid reoperation (100) | "1 experienced thyroid surgeon who had more than 20 years’ experience with thyroidectomy" | Extended central neck compartment dissection (94) | ‐ | |
| C: RILN visualisation | 84 | 46.8 (10.6) | ‐ | Thyroid reoperation (100) | Extended central neck compartment dissection (94) | ‐ | ||
| I: RILN visualisation + IONM | 92 | 44.2 (11.9) | ‐ | Robotic thyroidectomy using the bilateral axillo‐breast approach (100) | "The same surgeon with more than 10 years of experience in robotic surgery" | ‐ | ‐ | |
| C: RILN visualisation | 76 | 41.7 (9.0) | ‐ | Robotic thyroidectomy using the bilateral axillo‐breast approach (100) | ‐ | ‐ | ||
| I: RILN visualisation + IONM | 100 | 50.3 (15.3) | ‐ | Total thyroidectomy (100) | "Three experienced endocrine surgeons" | Central neck compartment dissection (13) | ‐ | |
| C: RILN visualisation | 100 | 49.7 (14.1) | ‐ | Total thyroidectomy (100) | Central neck compartment dissection (13) | ‐ | ||
| I: RILN visualisation + IONM | 85 | 47.2 (14) | 26.9 (3) | Total thyroidectomy (19), lobectomy (81) | "The same surgeons in all patients" | ‐ | ‐ | |
| C: RILN visualisation | 80 | 48.3 (12) | 27.3 (3) | Total thyroidectomy (23), lobectomy (77) | ‐ | . | ||
| I: RILN visualisation + IONM | 76 | 51.3 (14.4) | ‐ | Total thyroidectomy (76), Dunhill operation (19), bilateral subtotal thyroidectomy (5) | "Three experienced endocrine surgeons" | Central neck compartment dissection (12), lateral neck dissection (3) | ‐ | |
| C: RILN visualisation | 76 | 51.9 (14.7) | ‐ | Total thyroidectomy (74), Dunhill operation (20), bilateral subtotal thyroidectomy (6) | Central neck compartment dissection (12), lateral neck dissection (2) | ‐ | ||
| ‐ denotes not reported C: comparator; Dunhill operation: unilateral thyroid lobectomy and contralateral subtotal thyroid resection; I: intervention; IONM: intraoperative nerve monitoring; RILN: recurrent inferior laryngeal nerve; SD: standard deviation | ||||||||
Appendix 7. Matrix of trial endpoints (publications and trial documents)
| Trial ID | Endpoints quoted in trial document(s) | Trial results available in trial register | Endpoints quoted in publication(s)b,c | Endpoints quoted in abstract of publication(s)b,c |
| N/T | Primary outcome measures: temporary and permanent RILN paralysis | Primary outcome measures: temporary and permanent RILN paralysis | ||
| Secondary outcome measure: ‐ | Secondary outcome measure: ‐ | |||
| Other outcome measure: ‐ | Other outcome measure: ‐ | |||
| N/T | Primary outcome measures: transient or permanent laryngeal nerve lesions; the Voice Handicap Index and the Voice Range Profile | Primary outcome measures: transient or permanent laryngeal nerve lesions, the Voice Handicap Index and the Voice Range Profile | ||
| Secondary outcome measure: ‐ | Secondary outcome measure: ‐ | |||
| Other outcome measure: ‐ | Other outcome measure: ‐ | |||
| Source:NCT01395134 Primary outcome measure: identification rate of the EBSLN | Yes (last verified: 1 March 2017) | Primary outcome measure: identification rate of the EBSLN | Primary outcome measure: identification rate of the EBSLN | |
| Secondary outcome measures: anatomical variability of the external branch of the superior laryngeal nerve according to Cernea classification, incidence of EBSLN and RILN injuries assessed by videostrobolaryngoscopy (transient and permanent), changes in postoperative voice performance (pre‐ and postoperative assessment): analysis of maximum phonation time, voice level, fundamental frequency and voice quality rating on GRBAS scale | Secondary outcome measure: incidence of EBSLN and RILN injuries assessed by videostrobolaryngoscopy (transient, permanent and overall); changes in postoperative voice performance (pre‐ and postoperative assessment): analysis of maximum phonation time, voice level, fundamental frequency and voice quality rating on GRBAS scale | Secondary outcome measures: transient RILN injuries; changes in postoperative voice performance: analysis of maximum phonation time, voice level, fundamental frequency and voice quality rating on GRBAS scale | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measure: ‐ | ||
| History of changes: 2 documented changes; last change 14 July 2011 | ||||
| N/T | Primary outcome measures: identification time of RILN, operating time, persistent and transient RILN, persistent and transient hypoparathyroidism | Primary outcome measures: identification time of RILN, operating time, postoperative complications | ||
| Secondary outcome measure: ‐ | Secondary outcome measure: ‐ | |||
| Other outcome measure: ‐ | Other outcome measure: ‐ | |||
| Source:NCT00661024 Primary outcome measure: incidence of the recurrent laryngeal nerve injury (evaluated on 2nd postoperative day and at 1, 2, 4, 6 and 12 months postoperatively, if paresis was noted on first examination) | Yes (last verified: 1 March 2017) | Primary outcome measure: transient and permanent RILN injuries | Primary outcome measure: transient and permanent RILN injuries | |
| Secondary outcome measures: IONM‐added value to RILN identification, the value of IONM in prediction of postoperative vocal cords function (intraoperative data compared with observation of vocal cords function postoperatively on the 2nd postoperative day) | Secondary outcome measures: IONM‐added value to RILN identification, value of IONM in prediction of postoperative vocal cords function | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measures: technical problems and intraoperative complications related to IONM | Other outcome measure: ‐ | ||
| History of changes: 2 documented changes; last change 17 April 2008 | ||||
| ‐ denotes not reported aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trials registers). EMA: European Medicines Agency; EBSLN: external branch of the superior laryngeal nerve; FDA: Food and Drug Administration (US); GRBAS: grade, roughness, breathiness, asthenia, strain; IONM: intraoperative nerve monitoring; N/T: no trial document available; RILN: recurrent inferior laryngeal nerve | ||||
Appendix 8. High risk of outcome reporting bias according to ORBIT classification
| Trial ID | Outcome | High risk of bias | High risk of bias | High risk of bias | High risk of bias |
| N/A | |||||
| N/A | |||||
| N/A | |||||
| N/A | |||||
| N/A | |||||
| aClear that outcome was measured and analysed; trial report states that outcome was analysed but reports only that result was not significant (Classification 'A', table 2, Kirkham 2010). N/A: not applicable; ORBIT: Outcome Reporting Bias In Trials | |||||
Appendix 9. Definition of endpoint measurement (I)a
| Trial ID | All‐cause mortality | Operative time | Transient RILN palsy | Health‐related quality of life | Permanent RILN palsy | Socioeconomic effects |
| N/R | N/R | "If RILN paralysis occurred, laryngoscopy was carried out routinely at 1, 3, and 6 months after operation and at the time that the patients felt that their voice obviously improved" (IO) | N/R | Dysfunction was defined as no recovery of function during the first 6 months after thyroid reoperation (IO) | N/R | |
| N/R | N/D | "VHI, VRP, and laryngoscopy were used to test voice function before surgery and at 2 weeks, 3 months, and 6 months after the operation" (IO) | N/R | RILN palsy was considered permanent if it persisted for 12 months (IO) | N/R | |
| N/D | The time from skin incision to skin closure"(IO) | "VSL was performed on day 1 postoperatively; in case of abnormal findings, reevaluation was done at 3 and 6 months postoperatively" (IO) | N/R | Vocal cord paresis for 6 months or more following the operation was regarded as permanent palsy (IO) | N/R | |
| N/D | "The time from skin preparation to closure of the skin incisions" (IO) | "In cases of dysphonia with vocal cord injury, indirect laryngoscopy was also performed 1 and 6 months later" (IO) | N/R | "Persistent nerve palsy was defined as persistent dysfunction and clinical dysphonia that lasted for 12 months postoperatively" (IO) | N/R | |
| N/D | "The time from skin incision to skin closure" (IO) | "Indirect laryngoscopy by a throat specialist was mandatory before surgery and on day 2 after surgery. In patients with RILN paresis, an additional examination was scheduled at 2 weeks and 1, 2, 4, 6 and 12 months after surgery, or until the vocal cord function recovered" (IO) | N/R | "Vocal cord paresis for more than 12 months after the operation was regarded as permanent palsy" (IO) | N/R | |
| aIn addition to definition of endpoint measurement, description of who measured the outcome (AO: adjudicated outcome measurement; IO: investigator‐assessed outcome measurement; SO: self‐reported outcome measurement) N/D: not defined; N/R: not reported; RILN: recurrent inferior laryngeal nerve; VHI: Voice Handicap Index; VRP: Voice Range Profile; VSL: videostrobolaryngoscopy | ||||||
Appendix 10. Definition of endpoint measurement (II)a
| Trial ID | Adverse events other than permanent or transient RILN palsy | Severe/serious |
| Transient hypoparathyroidism: N/R Permanent hypoparathyroidism: N/R | N/R | |
| Transient hypoparathyroidism: N/D (IO) Permanent hypoparathyroidism: N/D (IO) | N/R | |
| Transient hypoparathyroidism: N/R Permanent hypoparathyroidism: N/R | N/R | |
| Transient hypoparathyroidism: N/D (IO) Permanent hypoparathyroidism: N/D (IO) | N/R | |
| Transient hypoparathyroidism: N/R Permanent hypoparathyroidism: N/R | N/R | |
| aIn addition to definition of endpoint measurement, description who measured the outcome (AO: adjudicated outcome measurement; IO: investigator‐assessed outcome measurement; SO: self‐reported outcome measurement) N/D: not defined; N/R: not reported; RILN: recurrent inferior laryngeal nerve | ||
Appendix 11. Adverse events (I)
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis | Deaths | Deaths | Participants with at least one adverse event | Participants with at least one adverse event | Participants with at least one severe/serious adverse event | Participants with at least one severe/serious adverse event |
| I: RILN visualisation + IONM | 33 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C: RILN visualisation | 37 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| I: RILN visualisation + IONM | 25 | ‐ | ‐ | 9 | 36 | 0 | 0 | |
| C: RILN visualisation | 25 | ‐ | ‐ | 4 | 12 | 0 | 0 | |
| I: RILN visualisation + IONM | 100 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| C: RILN visualisation | 101 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| I: RILN visualisation + IONM | 122 | 0 | 0 | 11 | 11.1 | ‐ | ‐ | |
| C: RILN visualisation | 114 | 0 | 0 | 13 | 8.8 | ‐ | ‐ | |
| I: RILN visualisation + IONM | 500 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| C: RILN visualisation | 500 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| ‐ denotes not reported C: comparator; I: intervention; IONM: intraoperative nerve monitoring; N: number of participants; RILN: recurrent inferior laryngeal nerve | ||||||||
Appendix 12. Adverse events (II)
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis | Participants discontinuing trial due to an adverse event | Participants discontinuing trial due to an adverse event |
| I: RILN visualisation + IONM | 33 | 0 | 0 | |
| C: RILN visualisation | 37 | 0 | 0 | |
| I: RILN visualisation + IONM | 25 | 0 | 0 | |
| C: RILN visualisation | 25 | 0 | 0 | |
| I: RILN visualisation + IONM | 100 | 0 | 0 | |
| C: RILN visualisation | 101 | 0 | 0 | |
| I: RILN visualisation + IONM | 122 | 0 | 0 | |
| C: RILN visualisation | 114 | 0 | 0 | |
| I: RILN visualisation + IONM | 500 | 0 | 0 | |
| C: RILN visualisation | 500 | 0 | 0 | |
| ‐ denotes not reported C: comparator; I: intervention; IONM: intraoperative nerve monitoring; RILN: recurrent inferior laryngeal nerve | ||||
Appendix 13. Adverse events (III)
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis | Participants with a specific adverse event | Participants with at least one specific adverse events | Participants with at least one specific adverse event |
| I: RILN visualisation + IONM | 33 | (1) Permanent RILN palsy (2) Transient RILN palsy | (1) 0 (2) 5 | (1) 0 (2) 15.2 | |
| C: RILN visualisation | 37 | (1) Permanent RILN palsy (2) Transient RILN palsy | (1) 0 (2) 3 | (1) 0 (2) 8.1 | |
| I: RILN visualisation + IONM | 25 | (1) Permanent RILN palsy (2) Transient RILN palsy (3) Permanent hypoparathyroidism (4) Transient hypoparathyroidism | (1) 0 (2) 0 (3) 0 (4) 9 | (1) 0 (2) 0 (3) 0 (4) 36 | |
| C: RILN visualisation | 25 | (1) Permanent RILN palsy (2) Transient RILN palsy (3) Permanent hypoparathyroidism (4) Transient hypoparathyroidism | (1) 0 (2) 0 (3) 0 (4) 4 | (1) 0 (2) 0 (3) 0 (4) 12 | |
| I: RILN visualisation + IONM | 100 | (1) Permanent RILN palsy (2) Transient RILN palsy | (1) 0 (2) 3 | (1) 0 (2) 3 | |
| C: RILN visualisation | 101 | (1) Permanent RILN palsy (2) Transient RILN palsy | (1) 0 (2) 8 | (1) 0 (2) 7.9 | |
| I: RILN visualisation + IONM | 122 | (1) Permanent RILN palsy (2) Transient RILN palsy (3) Permanent hypoparathyroidism (4) Transient hypoparathyroidism | (1) 0 (2) 3 (3) 0 (4) 11 | (1) 0 (2) 2.5 (3) 0 (4) 11.1 | |
| C: RILN visualisation | 114 | (1) Permanent RILN palsy (2) Transient RILN palsy (3) Permanent hypoparathyroidism (4) Transient hypoparathyroidism | (1) 0 (2) 3 (3) 0 (4) 13 | (1) 0 (2) 2.6 (3) 0 (4) 8.8 | |
| I: RILN visualisation + IONM | 500 | (1) Permanent RILN palsy (2) Transient RILN palsy | (1) 0 (2) 19 | (1) 0 (2) 3.8 | |
| C: RILN visualisation | 500 | (1) Permanent RILN palsy (2) Transient RILN palsy | (1) 0 (2) 38 | (1) 0 (2) 7.6 | |
| ‐ denotes not reported C: comparator; I: intervention; IONM: intraoperative nerve monitoring; RILN: recurrent inferior laryngeal nerve | |||||
Appendix 14. Survey of trial investigators providing information on included trials
| Trial ID | Date trial author contacted | Date trial author replied |
| 14 June 2017 and 18 January 2018 | No answer | |
| 14 June 2017 and 18 January 2018 | No answer | |
| Co‐author of this review | ||
| 14 June 2017 and 18 January 2018 | No answer | |
| Co‐author of this review | ||
Appendix 15. Checklist to aid consistency and reproducibility of GRADE assessments
| (1) Permanent RILN palsy | (2) Transient RILN palsy | (3) Health‐related quality of life | (4) Adverse events other than permanent or transient RILN palsy | (5) Operative time | (6) All‐cause mortality | (7) Socioeconoic effects | ||
| Trial limitations | Was random sequence generation used (i.e. no potential for selection bias)? | Yes | Yes | N/A | Yes | Yes | Yes | N/A |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Unclear | |||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | No (↓) | No (↓) | Unclear | No (↓) | Yes | |||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Unclear | Unclear | Unclear | Yes | |||
| Was an objective outcome used? | Yes | Yes | Yes | Yes | Yes | |||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Yes | Yes | Yes | |||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | Yes | Yes | Yes | |||
| No other biases reported (i.e. no potential of other bias)? | Unclear | Unclear | Unclear | Unclear | Unclear | |||
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | Yes | |||
| Inconsistencyb | Point estimates did not vary widely? | Yes | Yes | Yes | Yes | N/A | ||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Substantial | Substantial | Substantial | Substantial | N/A | |||
| Was the direction of effect consistent? | No (↓) | Yes | No (↓) | No (↓) | N/A | |||
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40%‐60%), high I² > 60%)? | Low | Low | High (↓) | High (↓) | N/A | |||
| Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | Not statistically significant | Not statistically significant | Statistically significant (↓) | N/A | |||
| Indirectness | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | ||
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | |||
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | Yes | Yes | |||
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | |||
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes | |||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | No (↓) | No (↓) | No (↓) | No (↓) | N/A | ||
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100‐300 participants, low: < 100 participants)?e | Intermediate | Intermediate | Intermediate | Intermediate | Intermediate | |||
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5‐10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | |||
| Was the outcome a common event (e.g. occurs more than 1/100)? | No (↓) | Yes | Yes | N/A | N/A | |||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | ||
| Was grey literature searched? | Yes | Yes | Yes | Yes | Yes | |||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | |||
| There was no industry influence on studies included in the review? | Yes | Yes | Yes | Yes | Yes | |||
| There was no evidence of funnel plot asymmetry? | N/A | N/A | N/A | N/A | N/A | |||
| There was no discrepancy in findings between published and unpublished trials? | N/A | N/A | N/A | N/A | N/A | |||
| aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials. cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); N/A: not applicable | ||||||||
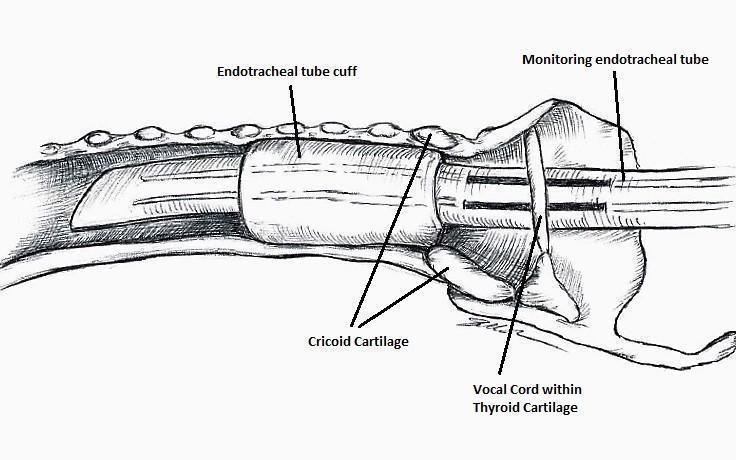
Nerve integrity monitoring endotracheal tube for electromyography signals of a patient's laryngeal muscles (drawn by Silvia Marola)
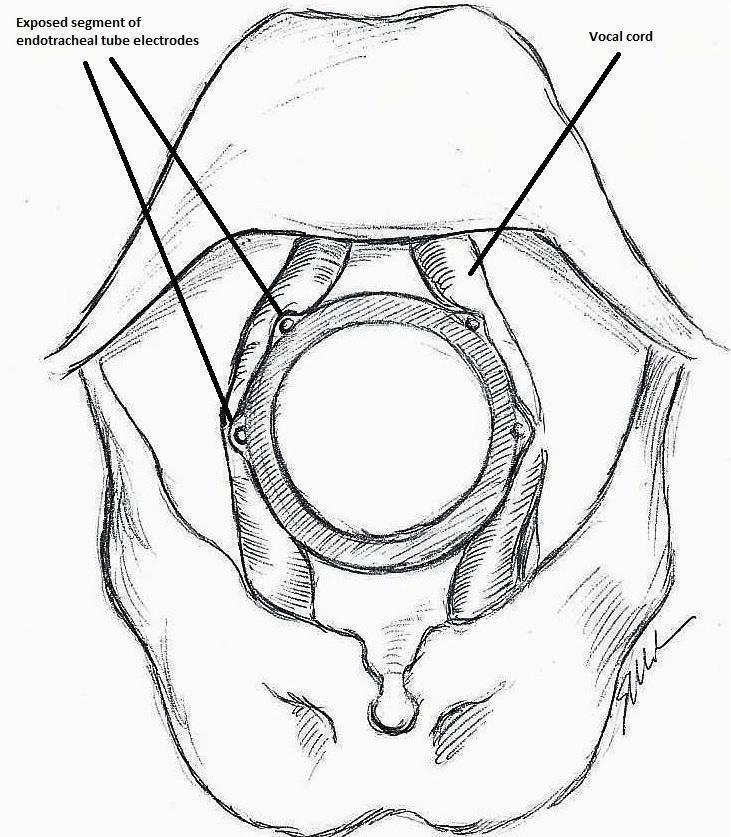
Monitoring endotracheal tube in position positioned at the patient's vocal folds (drawn by Silvia Marola)

Basic monitoring equipment setup (drawn by Silvia Marola)
EMG: electromyography;ET: endotracheal tube; GND: ground electrodes; REC: recording electrodes

Trial flow diagram
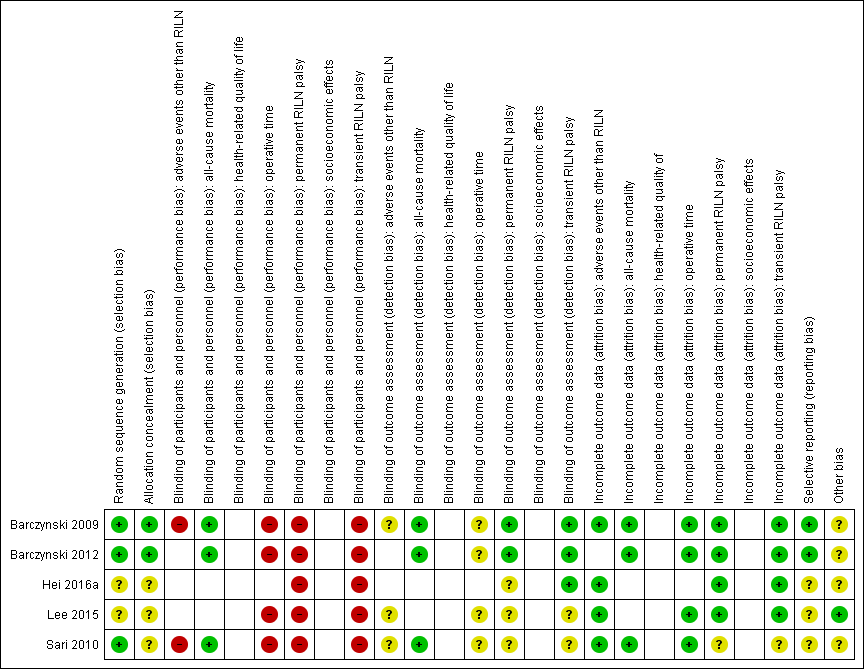
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included trial ((blank cells indicate that the particular outcome was not measured in the associated trial)

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included trials (blank cells indicate that the particular outcome was not measured in some or all trials)

Comparison 1 Intraoperative neuromonitoring versus visual nerve identification only, Outcome 1 Permanent RILN palsy.

Comparison 1 Intraoperative neuromonitoring versus visual nerve identification only, Outcome 2 Transient RILN palsy.

Comparison 1 Intraoperative neuromonitoring versus visual nerve identification only, Outcome 3 Adverse events other than RILN palsy.

Comparison 1 Intraoperative neuromonitoring versus visual nerve identification only, Outcome 4 Operative time.

Comparison 1 Intraoperative neuromonitoring versus visual nerve identification only, Outcome 5 All‐cause mortality.
| Intraoperative neuromonitoring compared to visual nerve identification only | ||||||
| Patients: adults undergoing thyroid surgery | ||||||
| Outcomes | Risk with visual nerve identification only | Risk with intraoperative neuromonitoring | Relative effect (95% CI) | № of participants/nerves at risk (trials) | Certainty of the evidence (GRADE) | Comments |
| Permanent RILN palsy (nerves) Definition: injury detected clinically, by laryngoscopy or both, in which the motility of the vocal cords did not recover within 6 months after surgery Follow‐up: 6‐12 months | 9 per 1000 | 7 per 1000 (3 to 16) | RR 0.77 | 2895 (4) | ⊕⊝⊝⊝ | Numbers refer to 'nerves at risk'; CI probably wider because of clustered data The 95% prediction interval ranged between 0.12 and 4.79 |
| Transient RILN palsy (nerves) Definition: injury detected clinically, by laryngoscopy or both, in which the motility of the vocal cords recovered within 6 months after surgery Follow‐up: 6‐12 months | 36 per 1000 | 22 per 1000 (13 to 39) | RR 0.62 | 2895 (4) | ⊕⊝⊝⊝ | Numbers refer to 'nerves at risk'; CI probably wider because of clustered data The 95% prediction interval ranged between 0.12 and 3.11 |
| Health‐related quality of life | Not reported | |||||
| Adverse events other than RILN palsy (participants) Definition: transient hypoparathyroidism Follow‐up: 6‐12 months | 122 per 1000 | 153 per 1000 (55 to 424) | RR 1.25 | 286 (2) | ⊕⊝⊝⊝ | ‐ |
| Operative time (min) Definition: time from the first skin incision to skin closure Follow‐up: 6‐12 months | The mean operative time ranged across control groups from 82.4 min to 274.2 min | The mean operative time in the intervention groups was 5.5 min longer (0.7 min shorter to 11.8 min longer) | ‐ | 1251 (3) | ⊕⊝⊝⊝ | The 95% prediction interval ranged between −60.6 min and 71.7 min |
| All‐cause mortality (nerves) | See comment | 1438 (3) | ⊕⊕⊕⊝ | 3 of 5 trials provided data on all‐cause mortality, no deaths were reported | ||
| Socioeconomic effects | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level because of performance bias, by one level because of inconsistency (no consistent direction of effects) and by one level because of imprecision (CI consistent with both benefit and harm, small number of trials, not a common event, i.e. ≤ 1/100) ‐ see Appendix 15 | ||||||
| Trial ID | Intervention(s) and comparator(s) | Description of power and sample size calculation | Screened/eligible | Randomised | Analysed | Finishing trial | Randomised finishing trial | Follow‐up |
| (parallel RCT) | I: RILN visualisation with neuromonitoring | "Our study had some limitations. First, this was a small sample study. To draw more persuasive conclusions, at least 434 RLNs in each group are needed to evaluate RLN injury reduction from 10% to 5% with a power of 80% and P = .05" | ‐ | 33 | 33 | 33 | 100 | 6 months |
| C: RILN visualisation alone | 37 | 37 | 37 | 100 | ||||
| total: | 70 | 70 | 70 | 100 | ||||
| (parallel RCT) | I: RILN visualisation with neuromonitoring | "The sample size was estimated based on the principle of detecting a difference of ‐10 units for VRP and of ‐5 for VHI between the mean of the IONM and non‐IONM groups with a 90% probability at P < .05, using power curve and sample size tools for one‐way analysis of variance" | ‐ | 25 | 25 | 25 | 100 | 12 months |
| C: RILN visualisation alone | 25 | 25 | 25 | 100 | ||||
| total: | 50 | 50 | 50 | 100 | ||||
| (parallel RCT) | I: RILN visualisation with neuromonitoring | "The sample size was estimated based on the principle of detecting a 5 % difference in the incidence of primary or secondary outcome measures with a 90 % probability at P < 0.05" | 517 | 105 | 105 | 100 | 95.2 | 6 months |
| C: RILN visualisation alone | 105 | 105 | 101 | 96.2 | ||||
| total: | 210 | 210 | 201 | 95.7 | ||||
| (parallel RCT) | I: RILN visualisation with neuromonitoring | ‐ | 254 | 123 | ‐a | ‐a | ‐a | 12 months |
| C: RILN visualisation alone | 114 | ‐a | ‐a | ‐a | ||||
| total: | 236 | ‐a | ‐a | ‐a | ||||
| (parallel RCT) | I: RILN visualisation with neuromonitoring | "The sample size was estimated based on the principle of detecting a 2 per cent difference in the incidence of transient RLN injury with a 90 per cent probability at P < 0·050" | 1488 | 500 | 500 | 500 | 100 | 12 months |
| C: RILN visualisation alone | 500 | 500 | 500 | 100 | ||||
| total: | 1000 | |||||||
| Grand total | All interventions | 781 | ||||||
| All comparators | 777 | |||||||
| All interventions and comparators | 1558 | |||||||
| ‐ denotes not reported. aTrial authors did not report the number of participants but the number of nerves C: comparator; I: intervention; RCT: randomised controlled trial; RILN: recurrent inferior laryngeal nerve | ||||||||
| Author and year of publication | Number of trials included | RCTs included in current review and other published meta‐analyses | Quasi‐RCTs included in other published meta‐analyses | Number of nerves at risk | Permanent RILN palsy | Transient RILN palsy |
| 34 | 58,247 | RD −0.0026 (95% CI −0.0039 to −0.0012) | RR 0.71 (95% CI 0.57 to 0.88) | |||
| 24 | 17,203 | OR 0.78 (95% CI 0.55 to 1.09) | OR 0.76 (95% CI 0.61 to 0.94) | |||
| 10a | None | None | 10,615 | OR 1.33 (95% CI 0.94 to 1.88) | OR 1.47 (95% CI 1.07 to 2.00) | |
| 9b | None | None | 2436 | RR 0.426 (95% CI 0.196 to 0.925) | RR 0.607 (95% CI 0.270 to 1.366) | |
| 4c | 1465d | RD 0.00 (95% CI 0.01 to 0.00) | NR | |||
| 10e | None | None | 38,820 | RR 0.79 (95% CI 0.61to 1.01) | NR | |
| 17 | None | 44,575 | NR | NR | ||
| 8 | 5257 | RR 0.73 (95% CI 0.44 to 1.23) | RR 0.73 (95% CI 0.54 to 0.98) | |||
| 20 | 35,513 | OR 0.884 (95% CI 0.687 to 1.136) | OR 0.946 (95% CI 0.817 to 1.096) | |||
| 6 | 3064 | RD 0 (95% CI ‐1 to 0) | RD −2 (95% CI −5.1 to 1) | |||
| 14 | None | 36,487 | OR 0.74 (95% CI 0.59 to 0.92) | OR 0.80 (95% CI 0.65 to 0.99) | ||
| aHigh‐risk thyroidectomy (reoperation, thyroidectomy for malignancy, thyrotoxicosis or retrosternal goitre) CI: confidence interval; EBSLN: external branch of superior laryngeal; N: number; NAR: nerves at risk; NR: not reported; OR: odds ratio; RCT: randomized controlled trial; RD: risk difference; RILN: recurrent inferior laryngeal nerve; RR: risk ratio | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Permanent RILN palsy Show forest plot | 4 | 2895 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.33, 1.77] |
| 2 Transient RILN palsy Show forest plot | 4 | 2895 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.35, 1.08] |
| 3 Adverse events other than RILN palsy Show forest plot | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.45, 3.47] |
| 4 Operative time Show forest plot | 4 | 1488 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐11.22, 9.62] |
| 5 All‐cause mortality Show forest plot | 3 | 1438 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

