Salpingoovariectomía bilateral de reducción de riesgos para pacientes con mutaciones de los genes BRCA1 o BRCA2
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Prospective cohort study, matching design | |
| Participants | Country: multicountry: University of Vienna, Austria; Creighton University, Omaha, NE, USA; Dana‐Farber Cancer Institute, Boston, MA, USA; Fox Chase Cancer Center, Philadelphia, PA, USA; Georgetown University, Washington, DC, USA; University of Chicago, Chicago, IL, USA; University of Pennsylvania, Philadelphia, PA, USA; University of Utah, Salt Lake City, UT, USA; Netherlands Cancer Institute, Amsterdam, Netherlands; Royal Marsden Hospital, Sutton, UK; St Mary’s Hospital, Manchester, UK; University of Texas‐Southwestern, Dallas, TX, USA and Yale University, New Haven, CT, USA Enrolled: 155 surgical participants and 271 control participants Women with BRCA1 or BRCA2 mutation carriers | |
| Interventions | Arm A: RRSO Arm B: general surveillance or non‐RRSO | |
| Outcomes | Overall survival Ovarian cancer mortality Primary peritoneal cancer mortality Breast cancer mortality Ovarian cancer incidence Breast cancer incidence Primary peritoneal cancer incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 2 |
| Methods | Prospective cohort study, non‐matching design | |
| Participants | Country: multicountry: University of Vienna, Austria; Beth Israel, Boston, MA; Baylor‐Charles A. Sammons Cancer Center; City of Hope, Duarte, CA; Creighton University, Omaha, NE; Dana‐Farber Cancer Institute, Boston, MA; Duke University, Durham, NC; NorthShore University HealthSystem, Evanston, IL; Fox Chase Cancer Center, Philadelphia, PA; Guy's Hospital and St. Thomas Foundation Trust, London, UK; Georgetown University, Washington, DC; University of California, Los Angeles; Mayo Clinic College of Medicine, Rochester, MN; Netherlands Cancer Institute, Amsterdam, Netherlands; The Institute of Cancer Research & Royal Marsden NHS Foundation Trust, London & Sutton; St. Mary's Hospital, Manchester, UK; University of Texas‐Southwestern, Dallas; University of Chicago, Chicago, IL; University of Pennsylvania, Philadelphia, PA; University of Utah, Salt Lake City, UT and University of California, Irvine; Women's College Hospital, Toronto, CA and Yale University, New Haven, CT Enrolled: 465 surgical participants and 1092 control participants Women with BRCA1 or BRCA2 mutation carriers | |
| Interventions | Arm A: RRSO and RRM Arm B: general surveillance or non‐RRSO | |
| Outcomes | Overall survival Ovarian cancer mortality Breast cancer mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 2 |
| Methods | Partly retrospective and prospective cohort study, matching design | |
| Participants | Country: Netherlands Enrolled: 146 surgical participants and 576 control participants Women with BRCA1 or BRCA2 mutation carriers | |
| Interventions | Arm A: RRSO Arm B: general surveillance or non‐RRSO | |
| Outcomes | Ovarian cancer mortality Breast cancer mortality Ovarian cancer incidence Breast cancer incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 2 |
| Methods | Prospective cohort study, non‐matching design | |
| Participants | Country: UK Enrolled: 108 surgical participants and 457 control participants Women with BRCA1 or BRCA2 mutation carriers | |
| Interventions | Arm A: RRSO and RRM Arm B: general surveillance or non‐RRSO | |
| Outcomes | Overall survival Ovarian cancer mortality Breast cancer mortality Ovarian cancer incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 2 |
| Methods | Prospective cohort study, non‐matching design | |
| Participants | Country: multicountry Enrolled: 1552 surgical participants and 2170 control participants Women with BRCA1 or BRCA2 mutation carriers | |
| Interventions | Arm A: RRO Arm B: general surveillance or non‐RRSO | |
| Outcomes | Breast cancer incidence Breast cancer mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 2 |
| Methods | Prospective cohort study (non‐matched) | |
| Participants | Country: USA Enrolled: 33 surgical participants and 65 control participants Women with BRCA1 mutation carriers | |
| Interventions | Arm A: RRO Arm B: general surveillance or non‐RRSO | |
| Outcomes | Breast cancer incidence Breast cancer mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 2 |
| Methods | Prospective cohort study, non‐matching design | |
| Participants | Country: Netherlands Enrolled: 118 surgical participants and 42 control participants Women with BRCA1 or BRCA2 mutation carriers | |
| Interventions | Arm A: RRSO and RRM Arm B: general surveillance or non‐RRSO | |
| Outcomes | Quality of life (ovarian cancer risk perception) Quality of life (breast cancer risk perception) Quality of life (global health status) Quality of life (general health perception) Quality of life (mental health) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 2 |
| Methods | Prospective cohort study, matching design | |
| Participants | Country: USA: Creighton University, Omaha, NE; Dana‐Farber Cancer Institute, Boston, MA; Fox Chase Cancer Center, Philadelphia, PA; University of Pennsylvania, Philadelphia, PA and University of Utah, Salt Lake City, UT). Enrolled: 43 surgical participants and 79 control participants Women with BRCA1 mutation carriers | |
| Interventions | Arm A: RRSO Arm B: general surveillance or non‐RRSO | |
| Outcomes | Breast cancer incidence Breast cancer mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 2 |
| Methods | Retrospective cohort study, matching design | |
| Participants | Country: multicountry: Creighton University, Dana–Farber Cancer Institute, Fox Chase Cancer Center, Georgetown University, University of Chicago, University of Pennsylvania, University of Utah, Netherlands Cancer Institute, St. Mary’s Hospital, Women’s College Hospital and Yale University Enrolled: 259 surgical participants and 292 control participants Women with BRCA1 or BRCA2 mutation carriers | |
| Interventions | Arm A: RRSO Arm B: general surveillance or non‐RRSO | |
| Outcomes | Ovarian cancer incidence Breast cancer incidence Primary peritoneal cancer incidence Primary peritoneal cancer mortality Breast cancer mortality Ovarian cancer mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 2 |
| Methods | Prospective cohort study, matching design | |
| Participants | Country: multicountry: Creighton University, Dana–Farber Cancer Institute, Fox Chase Cancer Center, Georgetown University, University of Chicago, University of Pennsylvania, University of Utah, Netherlands Cancer Institute, St. Mary’s Hospital, Women’s College Hospital and Yale University Enrolled: 57 surgical participants and 107 control participants Women with BRCA1 or BRCA2 mutation carriers | |
| Interventions | Arm A: RRSO and RRM Arm B: general surveillance or non‐RRSO | |
| Outcomes | Breast cancer incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 2 |
BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; RRM: risk‐reducing mastectomy; RRO: risk‐reducing oophorectomy; RRSO: risk‐reducing salpingo‐oophorectomy.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Participants in the control group did not have BRCA1/BRCA2 mutation carriers. | |
| Cohort study that assessed breast cancer risk in a large series of 1187 BRCA1 and 414 BRCA2 carriers from the International BRCA1/2 Carrier Cohort Study but included women (1 arm) with a previous or coexisting breast malignancy. | |
| Case‐control study that involved 4569 eligible women, of which 2283 women with a BRCA1/2 mutation carriers but included women (1 arm) with a previous or coexisting breast malignancy. | |
| Although the study was a cohort study that compared the frequency of peritoneal cancers among women receiving risk‐reducing surgery for ovarian cancer, not all the women enrolled were BRCA1 and BRCA2 mutation carriers and none of the included data were complete for extraction in enrolled women with known BRCA1 or BRCA2 mutation status. | |
| Cohort study that evaluated the incidence of breast cancer after RRM in healthy BRCA mutation carriers, without risk‐reducing BSO. | |
| Included women with prior history of breast cancer | |
| Controlled before‐and‐after study with no concurrent comparison groups | |
| Controlled before‐and‐after study with no concurrent comparison groups | |
| Controlled before‐and‐after study with no concurrent comparison groups | |
| Single‐arm cohort study (without comparison group) aimed at estimating the reduction in risk of ovarian, fallopian tube or peritoneal cancer in women with a BRCA1 or BRCA2 mutation after oophorectomy, by age of oophorectomy; to estimate the impact of prophylactic oophorectomy on all‐cause mortality; and to estimate 5‐year survival associated with clinically detected ovarian, occult and peritoneal cancers diagnosed in the cohort. | |
| Included women with prior history of breast cancer or ovarian cancer | |
| Cohort study that evaluated the incidence of breast cancer after RRM in healthy BRCA mutation carriers, without risk‐reducing BSO. | |
| Cohort study that evaluated the incidence of breast cancer after RRM in healthy BRCA mutation carriers, without risk‐reducing BSO. | |
| Although all the 8 women included in the study were BRCA mutation positive and received prophylactic mastectomy with BSO, there was no control or comparison group. So all women received surgical interventions. | |
| Review of cases with peritoneal cancer after PBSO and the possible aetiology of the disease as well as the possible changes in the management of such women. | |
| Retrospective cohort study of 294 women who underwent RRSO and 1228 women from the normal group aimed at evaluating the sexual pleasure and discomfort scores and frequency of sexual activity using the Sexual Activity Questionnaire. The BRCA1 or BRCA2 mutations status were not specified in any of the women included in the study. | |
| Although participants included 324 women after RRSO and 11,160 postmenopausal controls, a subsample of 950 controls had undergone BSO, whose indication for the BSO was not known and the BRCA1 or BRCA2 mutations status of the participants (study group or controls) too, were not reported. | |
| Prospective cohort study that compared the effect of RRSO with that of surveillance for ovarian cancer on the incidence of subsequent breast cancer and BRCA related gynaecological cancers in women with BRCA mutations but included women with prior history of breast cancer, with 70% of the salpingo‐oophorectomy group and 62% of the surveillance group having prior history of breast cancer. | |
| Prospective cohort study that compared the effect of RRSO with that of surveillance for ovarian cancer on the incidence of subsequent breast cancer and BRCA related gynaecological cancers in women with BRCA mutations but the study included women with prior history of breast cancer in both the RRSO group and the surveillance group. | |
| The 2 different comparison groups received bilateral salpingectomy alone or bilateral salpingectomy with delayed oophorectomy. It did not include a control or comparison group of women who carry BRCA1 or BRCA2 mutations and did not receive prophylactic salpingo‐oophorectomy. So all groups received surgical interventions. | |
| Retrospective study of 89 BRCA1/BRCA2 mutation carriers who underwent BSO. It did not include a control or comparison group of women who carried BRCA1 or BRCA2 mutations and did not receive prophylactic salpingo‐oophorectomy. So all groups received surgical interventions. | |
| Although the study determined the quality of life effects of PBSO versus gynaecologic screening, only 368/846 included women had known BRCA1/2 mutation carriers (265 (72%) women opted for PBSO, and 103 (28%) women, opted for gynaecological screening. Analysis was not based on BRCA1/2 status. | |
| Prospective cohort single‐arm study (without comparison or surveillance group) of women from high‐risk families whose mutation status was unknown, in addition to women who were confirmed BRCA1 or BRCA2 mutation carriers. | |
| Cohort study that evaluated the incidence of breast cancer after RRM in healthy BRCA mutation carriers, without RRSO. | |
| Cohort study of 195 women who were carriers of 1 of 3 mutations in BRCA1 gene most commonly occurring | |
| Cohort study included 390 women with a family history of stage I or II breast cancer who were carriers of BRCA1 and BRCA2 mutations and initially treated with unilateral or bilateral mastectomy, without bilateral RRSO. | |
| Retrospective observational cohort study of 70 women that assessed the potential role of peritoneal and omental biopsies in women undergoing RRSO for prophylactic management of hereditary breast/ovarian cancer syndromes. There is a single arm study without a comparison group. | |
| Although all 6 women included in the study received prophylactic mastectomy with BSO (4 women had BRCA‐1 mutations, 1 woman had a BRCA‐2 mutation and 1 woman had a family inheritance pattern with no mutations), there was no control or comparison group. So all groups received surgical interventions. | |
| Single arm study (without comparison group) of 111 women who were carriers of BRCA mutations and had RRSO in order to identify risk factors associated with finding an occult malignancy at RRSO using a rigorous surgical‐pathological protocol. | |
| Matched population‐based cohort study that investigated the survival patterns of 2390 women who had received an oophorectomy compared with 2390 women who had not received an oophorectomy but the BRCA1 or BRCA2 mutation status of the participants were not reported. | |
| Cohort study that assessed the level and persistence of reduction of ovarian (including peritoneal) cancer risk after gynaecological surgeries for women who carried BRCA1/2 mutations but were not selected from high‐risk clinics but not all women enrolled in the study have known BRCA1/2 mutation status. | |
| Included women with a personal history of breast cancer. | |
| Cohort study that evaluated the incidence of breast cancer after RRM in healthy BRCA mutation carriers, without risk‐reducing BSO. | |
| Prospective multicentre cohort study that determined the incidence of postoophorectomy carcinomatosis and quantified the effectiveness of preventive surgery, none of the enrolled women had known BRCA1 or BRCA2 mutation status. | |
| Cohort study included women with a family history or personal history of breast cancer who were carriers of BRCA1 and BRCA2 mutations and initially treated with unilateral or bilateral mastectomy, but without bilateral RRSO. | |
| Systematic review of implications of premenopausal RRSO on quality of life, endocrine symptoms, sexual function, osteoporosis, cardiovascular health, metabolic syndrome, cognitive impairment and safety of hormone replacement therapy. |
BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; BSO: bilateral salpingo‐oophorectomy; PBSO: prophylactic bilateral salpingo‐oophorectomy; RRM: risk‐reducing mastectomy; RRSO: risk‐reducing salpingo‐oophorectomy.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 1 Overall survival. | ||||
| 1.1 BRCA1 or BRCA2 | 3 | Hazard Ratio (Random, 95% CI) | 0.32 [0.19, 0.54] | |
| 2 High‐grade serous cancer (HGSC) mortality Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.06 [0.02, 0.17] | |
| Analysis 1.2  Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 2 High‐grade serous cancer (HGSC) mortality. | ||||
| 2.1 BRCA1 or BRCA2 | 3 | Hazard Ratio (Random, 95% CI) | 0.06 [0.02, 0.17] | |
| 3 Breast cancer mortality Show forest plot | 7 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 3 Breast cancer mortality. | ||||
| 3.1 BRCA1 or BRCA | 7 | Hazard Ratio (Random, 95% CI) | 0.58 [0.39, 0.88] | |
| 4 HGSC incidence Show forest plot | 4 | 3328 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.04, 0.75] |
| Analysis 1.4  Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 4 HGSC incidence. | ||||
| 4.1 BRCA1 or BRCA2 | 4 | 3328 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.04, 0.75] |
| 5 Breast cancer incidence Show forest plot | 7 | 5595 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.96] |
| Analysis 1.5  Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 5 Breast cancer incidence. | ||||
| 5.1 BRCA1 or BRCA2 | 7 | 5595 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.96] |
| 6 Quality of life (ovarian cancer risk perception) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 6 Quality of life (ovarian cancer risk perception). | ||||
| 6.1 BRCA1 or BRCA2 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of life (global health status) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 7 Quality of life (global health status). | ||||
| 8 Quality of life (general health perception) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 8 Quality of life (general health perception). | ||||
| 9 Quality of life (mental health) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.9  Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 9 Quality of life (mental health). | ||||
| 10 Quality of life (breast cancer risk perception) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 10 Quality of life (breast cancer risk perception). | ||||
| 10.1 BRCA1 or BRCA2 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.35 [0.25, 0.50] | |
| Analysis 2.1  Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 1 Overall survival. | ||||
| 1.1 BRCA1 only | 3 | Hazard Ratio (Random, 95% CI) | 0.30 [0.17, 0.52] | |
| 1.2 BRCA2 only | 2 | Hazard Ratio (Random, 95% CI) | 0.44 [0.23, 0.85] | |
| 2 High‐grade serous cancer (HGCS) mortality Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 0.10 [0.02, 0.41] | |
| Analysis 2.2  Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 2 High‐grade serous cancer (HGCS) mortality. | ||||
| 2.1 BRCA1 only | 2 | Hazard Ratio (Random, 95% CI) | 0.10 [0.02, 0.41] | |
| 2.2 BRCA2 only | 2 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Breast cancer mortality Show forest plot | 6 | Hazard Ratio (Random, 95% CI) | 0.59 [0.35, 1.00] | |
| Analysis 2.3  Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 3 Breast cancer mortality. | ||||
| 3.1 BRCA1 only | 4 | Hazard Ratio (Random, 95% CI) | 0.45 [0.30, 0.67] | |
| 3.2 BRCA2 only | 3 | Hazard Ratio (Random, 95% CI) | 0.88 [0.42, 1.87] | |
| 4 Quality of life (ovarian cancer risk perception) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 4 Quality of life (ovarian cancer risk perception). | ||||
| 4.1 BRCA1 only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 BRCA2 only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery, Outcome 1 Overall survival. | ||||
| 1.1 RRSO and risk‐reducing mastectomy (RRM) versus no RRSO | 1 | Hazard Ratio (Random, 95% CI) | 0.14 [0.02, 0.98] | |
| 2 Breast cancer mortality Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery, Outcome 2 Breast cancer mortality. | ||||
| 2.1 RRSO and RRM versus no RRSO | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast cancer mortality Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.85 [0.64, 1.11] | |
| Analysis 4.1  Comparison 4 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 mutation carriers according to age at RRSO, Outcome 1 Breast cancer mortality. | ||||
| 1.1 50 years or less | 3 | Hazard Ratio (Random, 95% CI) | 0.78 [0.55, 1.09] | |
| 1.2 Above 50 years | 3 | Hazard Ratio (Random, 95% CI) | 1.27 [0.67, 2.38] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast cancer mortality Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 0.88 [0.42, 1.87] | |
| Analysis 5.1  Comparison 5 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA2 mutation carriers according to age at RRSO, Outcome 1 Breast cancer mortality. | ||||
| 1.1 50 years or less | 2 | Hazard Ratio (Random, 95% CI) | 0.49 [0.08, 2.90] | |
| 1.2 Above 50 years | 2 | Hazard Ratio (Random, 95% CI) | 1.36 [0.68, 2.75] | |
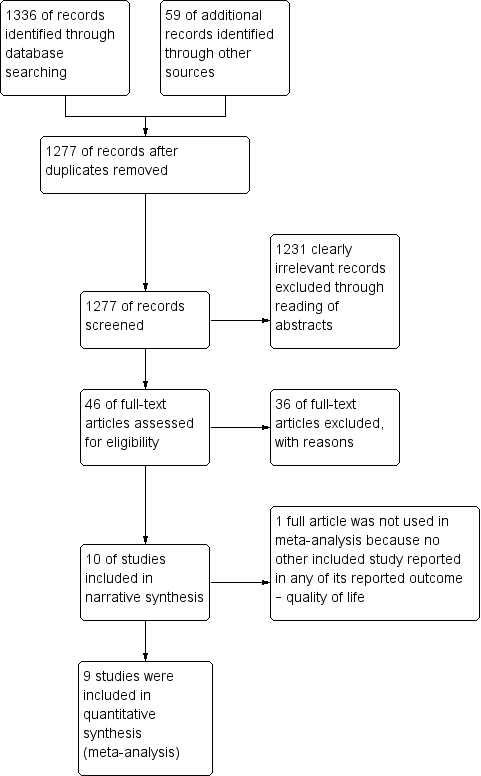
Study flow diagram for searches on risk‐reducing salpingo‐oophorectomy in women with BRCA1 or BRCA2 mutation carriers.

Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 1 Overall survival.
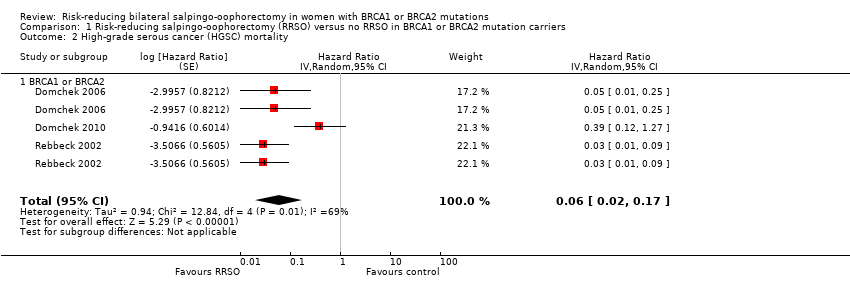
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 2 High‐grade serous cancer (HGSC) mortality.

Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 3 Breast cancer mortality.

Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 4 HGSC incidence.
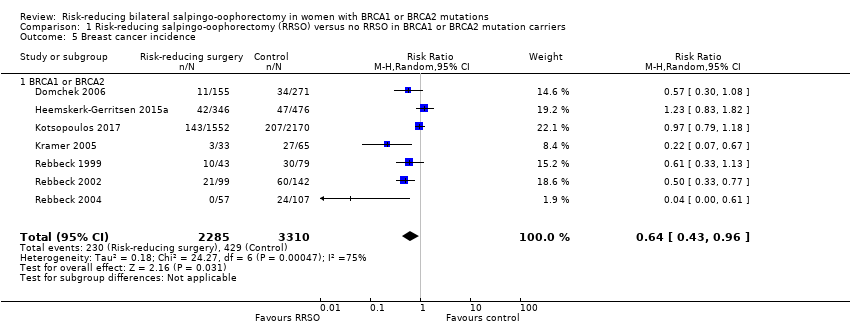
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 5 Breast cancer incidence.

Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 6 Quality of life (ovarian cancer risk perception).
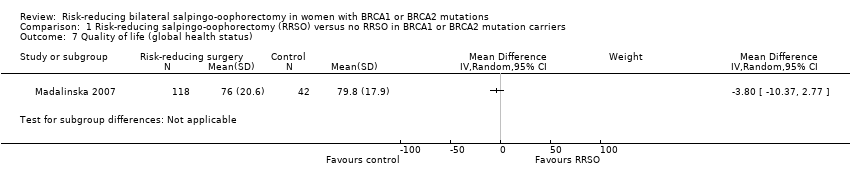
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 7 Quality of life (global health status).

Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 8 Quality of life (general health perception).
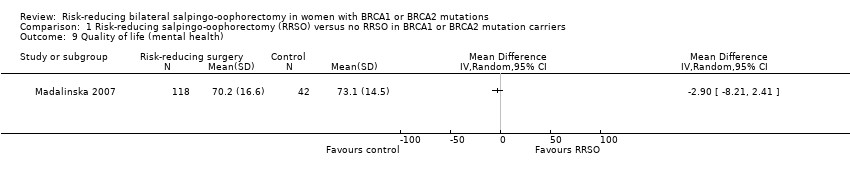
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 9 Quality of life (mental health).

Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 10 Quality of life (breast cancer risk perception).

Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 1 Overall survival.
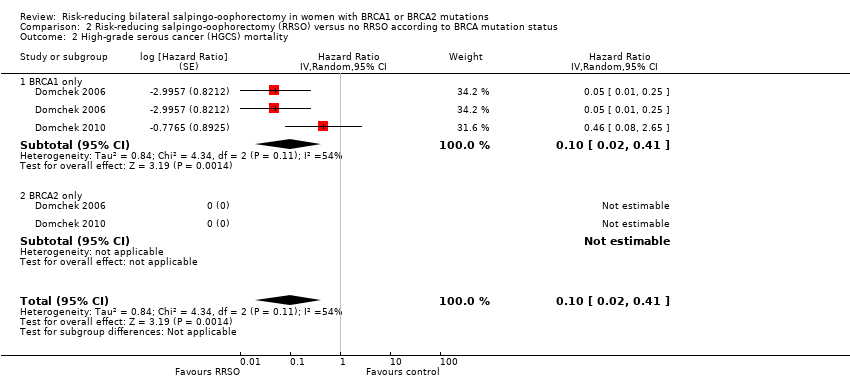
Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 2 High‐grade serous cancer (HGCS) mortality.

Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 3 Breast cancer mortality.

Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 4 Quality of life (ovarian cancer risk perception).

Comparison 3 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery, Outcome 1 Overall survival.

Comparison 3 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery, Outcome 2 Breast cancer mortality.

Comparison 4 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 mutation carriers according to age at RRSO, Outcome 1 Breast cancer mortality.

Comparison 5 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA2 mutation carriers according to age at RRSO, Outcome 1 Breast cancer mortality.
| RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and USA Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers | |||||
| Overall survival: BRCA1 or BRCA2 | Study population | HR 0.32 | 2548 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| HGSC mortality: BRCA1 or BRCA2 | Study population | HR 0.06 | 2534 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: BRCA1 or BRCA | Study population | HR 0.58 | 7198 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Bone fracture incidence | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Quality of life (ovarian cancer risk perception): BRCA1 or BRCA2 | See comment | See comment | Not estimable | 200 | ⊕⊝⊝⊝ | Unable to perform meta‐analysis as only 1 study reported the outcome. |
| Quality of life (breast cancer risk perception): BRCA1 or BRCA2 | See comment | See comment | Not estimable | 200 | ⊕⊝⊝⊝ | Unable to perform meta‐analysis as only 1 study reported the outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies. | ||||||
| RRSO vs no RRSO according to BRCA mutation status | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and USA Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO according to BRCA mutation status | |||||
| Overall survival: BRCA1 only | Study population | HR 0.30 | 2548 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Overall survival: BRCA2 only | Study population | HR 0.44 | 2122 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| HGSC mortality: BRCA1 only | Study population | HR 0.1 | 1983 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| HGSC mortality: BRCA2 only | See commentb | See commentb | Not estimable See commentc | 1983 | ⊕⊝⊝⊝ | bAs a result of the way HRs were calculated, assumed and corresponding risks were not estimated. cUnable to perform meta‐analysis as no mortality events were recorded in any study and HRs could not be estimated. |
| Breast cancer mortality: BRCA1 only | Study population | HR 0.45 | 2203 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: BRCA2 only | Study population | HR 0.88 | 5882 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Quality of life (ovarian cancer risk perception): BRCA1 only | See comment | See comment | Not estimable | 98 | ⊕⊝⊝⊝ | Unable to perform meta‐analysis as only 1 study reported the outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies. | ||||||
| RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and USA Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery | |||||
| Overall survival: RRSO alone vs RRSO and RRM | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Overall survival: RRSO and RRM vs no RRSO | Study population | HR 0.14 | 261 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: RRSO alone vs RRSO and RRM | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Breast cancer mortality: RRSO and RRM vs no RRSO | See comment | See comment | Not estimable | 722 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. |
| Bone fracture incidence | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to confounding and bias due in selection of participants in the study). | ||||||
| RRSO vs no RRSO in BRCA1 mutation carriers according to age at RRSO | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and America Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA1 mutation carriers according to age at RRSO | |||||
| Overall survival | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| HGSC mortality | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Breast cancer mortality: ≤ 50 years | Study population | HR 0.78 | 4566 | ⊕⊝⊝⊝ Very lowa,b | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: > 50 years | Study population | HR 1.27 | 4566 | ⊕⊝⊝⊝v Very lowa,b | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Bone fracture incidence | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies. bDowngraded by one level for serious imprecision: the confidence intervals overlapped 1 and either 0.75 or 1.25 or both (i.e. wide confidence intervals in all included studies, which crossed the line of unity). | ||||||
| RRSO versus no RRSO in BRCA2 mutation carriers according to age at RRSO | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and America Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA2 mutation carriers according to age at RRSO | |||||
| Overall survival | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| HGSC mortality | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Breast cancer mortality: ≤ 50 years | Study population | HR 0.49 | 444 | ⊕⊝⊝⊝ Very lowa,b | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: > 50 years | Study population | HR 1.36 | 444 | ⊕⊝⊝⊝ Very lowa,b | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Bone fracture incidence | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; CI: confidence interval; HGSC: high‐grade serous cancer; HR: hazard ratio; RRSO: risk‐reducing salpingo‐oophorectomy. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies. bDowngraded by one level for serious imprecision: the confidence intervals overlapped 1 and either 0.75 or 1.25 or both (i.e. wide confidence intervals in all included studies, which cross the line of unity). | ||||||
| Judgement | Within each domain | Across domains | Criterion |
| Low risk of bias | The study is comparable to a well‐performed randomised trial with regard to this domain. | The study is comparable to a well‐performed randomised trial. | The study is judged to be at low risk of bias for all domains. |
| Moderate risk of bias | The study is sound for a non‐randomised study with regard to this domain but cannot be considered comparable to a well‐performed randomised trial. | The study provides sound evidence for a non‐randomised study but cannot be considered comparable to a well‐performed randomised trial. | The study is judged to be atlow or moderate risk of bias for all domains. |
| Serious risk of bias | The study has some important problems in this domain. | The study has some important problems. | The study is judged to be at serious risk of bias in at least 1 domain, but not at critical risk of bias in any domain. |
| Critical risk of bias | The study is too problematic in this domain to provide any useful evidence on the effects of intervention. | The study is too problematic to provide any useful evidence and should not be included in any synthesis. | The study is judged to be at critical risk of bias in at least 1 domain. |
| No information | No information on which to base a judgement about risk of bias for this domain | No information on which to base a judgement about risk of bias. | There is no clear indication that the study is at serious or critical risk of bias and there is a lack of information in 1 or more key domains of bias (a judgement is required for this). |
| ROBINS‐I: Risk Of Bias In Non‐randomised Studies‐of Interventions. | |||
| Study ID | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias |
| Low | Low | Low | Moderate | |
| Support for judgement | Quote: "Questionnaires were administered at every centre and were self‐administered or completed with the help of clinical‐research staff." All missing data were analysed (intention‐to‐treat). | Quote: "For our primary analysis, we undertook a matched design that selected controls who had not undergone BPSO at any time during follow‐up, and who were matched within 5 years of age to the corresponding one.” | Quote: "Follow‐up data for BPSO, cancer diagnoses, and deaths were verified by review of medical records, and surgical notes, pathology reports, or both." | At least 1 of the domain was moderate. |
| Moderate | Low | Low | Moderate | |
| Support for judgement | Quote: "When missing data were encountered, the individual was dropped from the analysis that involved the missing data point, but the individual was included in other analyses where complete data were available; in fact, because many of the data items were required for enrolment missing data was only applicable to ovarian cancer endpoints, with missing OCP data." | Quote: "For BC endpoints, women were excluded if they underwent RRM prior to ascertainment. Women who had RRM after ascertainment but before RRSO were considered unexposed and were censored at RRM. Women were followed until BC or were censored at OC, RRM, death, or last contact." | Quote: "A robust variance‐covariance estimation method was used to correct for non‐independence of observations among participants from the same family or within centers... Adjustment for year of birth was undertaken in all analyses using Cox regression. Oral contraceptive use was adjusted for when OC was the outcome. Adjustment for center of ascertainment was undertaken by stratifying analyses by center to avoid imposing linear constraints in the model." | At least 1 of the domain was moderate. |
| Moderate | Low | Low | Moderate | |
| Support for judgement | Quote: "Eventually, parity was not considered as a potential confounder because of the large proportion (41.0%) of missing data on this variable." | Quote: "We performed sensitivity analyses to estimate the effect of RRSO on BC risk in different settings. First, to investigate the effect of excluding the BC‐free time before RRM, we estimated BC risk reduction after RRSO for participants who never underwent RRM." | Quote: "We adjusted our analyses for differences in age by using chronological age as the time variable." | At least 1 of the domain was moderate. |
| Low | Moderate | Low | Moderate | |
| Support for judgement | Quote: "Women were censored at either date of last follow‐up (date of last contact with the genetics department or other NHS service) or date of death (obtained from NWCIS or death certification).” No evidence of missing data. | Quote: "Date of breast cancer was confirmed in the family files or from records at the North West Cancer Intelligence Service (NWCIS)." Also, possible testing bias of women who developed cancer was made. | Quote: "The proportional hazards assumption was checked in all analyses by looking at log–log plots and Schoenfeld residuals." | At least 1 of the domain was moderate. |
| Moderate | Low | Moderate | Moderate | |
| Support for judgement | Quote: "Women with both a BRCA1 and BRCA2 mutation were coded as missing." | Quote: "We also performed analyses stratified by BRCA mutation type, estrogen receptor status of the tumor, and excluding women with an oophorectomy at or prior to the baseline questionnaire, as well as analyses censoring at different ages." | Quote: "this finding was based on a post hoc analysis." | At least 1 of the domain was moderate. |
| Low | Moderate | Low | Serious | |
| Support for judgement | There was no evidence of missing data. | Quote: "A competing risks model (with death as the competing risk) was then used to estimate the 10‐year cumulative incidence of breast cancer in the two groups of BRCA1 mutation carriers (ie, those with and without ovaries)." | Quote: "To provide estimates of the absolute risk of breast cancer by age in mutation carriers, landmark analyses were performed in which oophorectomy was treated as a time‐fixed covariate, as defined at the beginning of a given age interval. Follow‐up time was divided into 10‐year intervals, with mutation carriers divided into two groups based on oophorectomy status at the beginning of that interval (and conditional on the participant being alive and breast cancer free at that time)." | At least 1 of the domain was serious. |
| Low | Low | Low | Moderate | |
| Support for judgement | Quote: "These records were complete, and in cases where there was any uncertainty, contact was sought with the responsible gynecologist." "Non respondents did not differ significantly from respondents regarding age or choice of preventive measure." | Quote: "All raw scale scores were linearly converted to a 0 to100 scale, with higher scores indicating better perceived health, mental health, and quality of life. The internal consistency reliability of the two Short Form‐36 scales was high (α = 0.81 and 0.85)." | Quote: "Because of restrictions by the medical ethics committees, no other clinical data on the nonrespondents were available (eg, DNA status)." | At least 1 of the domain was moderate. |
| Low | Low | Moderate | Moderate | |
| Support for judgement | Quote: "However, only BRCA1 mutation carriers were studied here, and no OCCR region has been identified in BRCA1." No evidence of missing data. | Quote: "Because most women were followed only until the time of censoring or until the diagnosis of breast cancer, the incidences reported here do not represent lifetime breast cancer risks in BRCA1 mutation carriers." | Quote: "Furthermore, the inferences from both the robust and nonrobust analyses were identical. Therefore, only the standard model results are presented." | At least 1 of the domain was moderate. |
| Moderate | Low | Low | Moderate | |
| Support for judgement | Quote: "Bias that arises when later follow‐up is missing for individuals initially included and followed." | Quote: "on vital status and the occurrence of cancer was obtained from medical records, telephone interviews, self‐administered questionnaires, or a combination of these. For women who had died since their entry into the study, we reviewed medical records and family‐history reports to establish the presence or absence of cancer and to verify that they had died." | Quote: "For women who had died since their entry into the study, we reviewed medical records and family‐history reports to establish the presence or absence of cancer and to verify that they had died." | At least 1 of the domain is moderate. |
| Moderate | Low | Low | Moderate | |
| Support for judgement | Quote: "Percentages calculated using nonmissing data." | Quote: "Survival analyses were adjusted to account for duration of endogenous ovarian hormone exposure as measured by the time from age at menarche to age at bilateral prophylactic | Quote: "Subjects were censored at the date they developed ovarian cancer, or died, or at the date of last contact. Diagnosis of invasive breast cancer or ductal carcinoma‐in‐situ was considered the primary event of interest." | At least 1 of the domain is moderate. |
| BC: Breast Cancer; BPSO: Bilateral prophylactic salpingo‐oophorectomy; BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; NHS: National Health Service; NWCI: North West Cancer Intelligence Service; SOC: Site of Care; OCP: Oral Contraceptive Pill; ROBIS‐I: Risk Of Bias In Non‐randomised Studies‐of Interventions; RRM: risk‐reducing bilateral mastectomy; RRSO: risk‐reducing bilateral salpingo‐oophorectomy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 BRCA1 or BRCA2 | 3 | Hazard Ratio (Random, 95% CI) | 0.32 [0.19, 0.54] | |
| 2 High‐grade serous cancer (HGSC) mortality Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.06 [0.02, 0.17] | |
| 2.1 BRCA1 or BRCA2 | 3 | Hazard Ratio (Random, 95% CI) | 0.06 [0.02, 0.17] | |
| 3 Breast cancer mortality Show forest plot | 7 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 BRCA1 or BRCA | 7 | Hazard Ratio (Random, 95% CI) | 0.58 [0.39, 0.88] | |
| 4 HGSC incidence Show forest plot | 4 | 3328 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.04, 0.75] |
| 4.1 BRCA1 or BRCA2 | 4 | 3328 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.04, 0.75] |
| 5 Breast cancer incidence Show forest plot | 7 | 5595 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.96] |
| 5.1 BRCA1 or BRCA2 | 7 | 5595 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.96] |
| 6 Quality of life (ovarian cancer risk perception) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 BRCA1 or BRCA2 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of life (global health status) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8 Quality of life (general health perception) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9 Quality of life (mental health) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Quality of life (breast cancer risk perception) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 BRCA1 or BRCA2 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.35 [0.25, 0.50] | |
| 1.1 BRCA1 only | 3 | Hazard Ratio (Random, 95% CI) | 0.30 [0.17, 0.52] | |
| 1.2 BRCA2 only | 2 | Hazard Ratio (Random, 95% CI) | 0.44 [0.23, 0.85] | |
| 2 High‐grade serous cancer (HGCS) mortality Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 0.10 [0.02, 0.41] | |
| 2.1 BRCA1 only | 2 | Hazard Ratio (Random, 95% CI) | 0.10 [0.02, 0.41] | |
| 2.2 BRCA2 only | 2 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Breast cancer mortality Show forest plot | 6 | Hazard Ratio (Random, 95% CI) | 0.59 [0.35, 1.00] | |
| 3.1 BRCA1 only | 4 | Hazard Ratio (Random, 95% CI) | 0.45 [0.30, 0.67] | |
| 3.2 BRCA2 only | 3 | Hazard Ratio (Random, 95% CI) | 0.88 [0.42, 1.87] | |
| 4 Quality of life (ovarian cancer risk perception) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 BRCA1 only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 BRCA2 only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 RRSO and risk‐reducing mastectomy (RRM) versus no RRSO | 1 | Hazard Ratio (Random, 95% CI) | 0.14 [0.02, 0.98] | |
| 2 Breast cancer mortality Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 2.1 RRSO and RRM versus no RRSO | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast cancer mortality Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.85 [0.64, 1.11] | |
| 1.1 50 years or less | 3 | Hazard Ratio (Random, 95% CI) | 0.78 [0.55, 1.09] | |
| 1.2 Above 50 years | 3 | Hazard Ratio (Random, 95% CI) | 1.27 [0.67, 2.38] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast cancer mortality Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 0.88 [0.42, 1.87] | |
| 1.1 50 years or less | 2 | Hazard Ratio (Random, 95% CI) | 0.49 [0.08, 2.90] | |
| 1.2 Above 50 years | 2 | Hazard Ratio (Random, 95% CI) | 1.36 [0.68, 2.75] | |

