Salpingoovariectomía bilateral de reducción de riesgos para pacientes con mutaciones de los genes BRCA1 o BRCA2
Resumen
Antecedentes
La presencia de mutaciones nocivas del gen 1 del cáncer de mama (BRCA1) o del gen 2 del cáncer de mama (BRCA2) aumenta de forma significativa el riesgo de desarrollar algunos cánceres, como cáncer de mama y seroso de grado alto (CSGA) de origen ovárico, tubárico y peritoneal. Por lo general a las portadoras de mutaciones de los genes BRCA1 o BRCA2 se les recomienda la salpingoovariectomía de reducción de riesgos (SORR) después de la finalización de la maternidad. A pesar de las revisiones sistemáticas y los metanálisis previos sobre la función de la SORR para reducir la mortalidad y la incidencia de cáncer de mama, CSGA y otros cánceres, todavía la SORR suscita debate y no está claro si su efectividad difiere según el tipo de mutación.
Objetivos
Evaluar los efectos beneficiosos y perjudiciales de la SORR en las pacientes con mutaciones de los genes BRCA1 o BRCA2.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; 2017, número 7) en The Cochrane Library, MEDLINE Ovid, Embase Ovid y en registros de ensayos, sin restricciones de idioma hasta julio de 2017. Se realizaron búsquedas manuales de resúmenes de congresos científicos y otras publicaciones relevantes.
Criterios de selección
Se incluyeron los ensayos no aleatorios, los estudios de cohortes prospectivos y retrospectivos y las series de casos clínicos que utilizaron un ajuste estadístico para la mezcla de casos iniciales mediante análisis de múltiples variables que compararon SORR versus ninguna SORR en pacientes sin neoplasias malignas anteriores o coexistentes de mama, ovárica o de las trompas de Falopio, en pacientes con o sin histerectomía y en pacientes con una mastectomía de reducción de riesgos (MRR) anterior, con o después de la SORR.
Obtención y análisis de los datos
Se extrajeron los datos y se realizaron metanálisis de los cocientes de riesgos instantáneos (CRI) para las variables de tiempo hasta el evento y los cocientes de riesgos (CR) para los resultados dicotómicos, con los intervalos de confianza (IC) del 95%. Para evaluar el sesgo en los estudios se utilizó la herramienta ROBINS‐I "Riesgo de sesgo". La inconsistencia entre los estudios se calificó al calcular la estadística I2. Se utilizaron los modelos de efectos aleatorios para calcular las estimaciones agrupadas del efecto.
Resultados principales
Se incluyeron diez estudios de cohortes con 8087 participantes (2936 [36%] participantes quirúrgicas y 5151 [64%] participantes control que eran portadoras de mutaciones de los genes BRCA1 o BRCA2. Todos los estudios compararon la SORR con o sin MRR versus ninguna SORR (vigilancia). La certeza de la evidencia con el uso de la evaluación GRADE fue muy baja debido al alto riesgo de sesgo. En los metanálisis se incluyeron nueve estudios con 7927 pacientes. La mediana del período de seguimiento varió de 0,5 a 27,4 años.
Resultados principales: la supervivencia general fue más prolongada con la SORR en comparación con ninguna SORR (CRI 0,32; IC del 95%: 0,19 a 0,54; P < 0,001; tres estudios, 2548 mujeres; evidencia de certeza muy baja). La mortalidad por CSGA (CRI 0,06; IC del 95%: 0,02 a 0,17; I² = 69%; P < 0,0001; tres estudios, 2534 mujeres; evidencia de certeza muy baja) y la mortalidad por cáncer de mama (CRI 0,58; IC del 95%: 0,39 a 0,88; I² = 65%; P = 0,009; siete estudios, 7198 mujeres; evidencia de certeza muy baja) fueron inferiores con la SORR en comparación con ninguna SORR. Ninguno de los estudios informó la incidencia de fracturas óseas. Hubo una diferencia a favor de la SORR en comparación con ninguna SORR en cuanto a la calidad de vida para la percepción del riesgo de cáncer de ovario (DM 15,40; IC del 95%: 8,76 a 22,04; P < 0,00001; un estudio; evidencia de certeza muy baja). Ninguno de los estudios informó eventos adversos.
Análisis de subgrupos de los resultados principales: el metanálisis mostró un aumento de la supervivencia general entre las pacientes sometidas a SORR versus las pacientes con ninguna SORR que eran portadoras de mutaciones del gen BRCA1 (CRI 0,30; IC del 95%: 0,17 a 0,52; P < 0001; I² = 23%; tres estudios; evidencia de certeza muy baja) y las portadoras de mutaciones del gen BRCA2 (CRI 0,44; IC del 95%: 0,23 a 0,85; P = 0,01; I² = 0%; dos estudios; evidencia de certeza muy baja). El metanálisis mostró una disminución en la mortalidad por CSGA entre las pacientes con SORR versus ninguna SORR que eran portadoras de mutaciones del gen BRCA1 (CRI 0,10; IC del 95%: 0,02 a 0,41; I² = 54%; P = 0,001; dos estudios; evidencia de certeza muy baja), pero no estuvo claro en las portadoras de mutaciones del gen BRCA2, debido a la baja frecuencia de muertes por CSGA en las portadoras de mutaciones del gen BRCA2. Hubo una disminución en la mortalidad por cáncer de mama entre las pacientes con SORR versus ninguna SORR que eran portadoras de mutaciones del gen BRCA1 (CRI 0,45; IC del 95%: 0,30 a 0,67; I² = 0%; P < 0,0001; cuatro estudios; evidencia de certeza muy baja), pero no en las portadoras de mutaciones del gen BRCA2 (CRI 0,88; IC del 95%: 0,42 a 1,87; I² = 63%; P = 0,75; tres estudios; evidencia de certeza muy baja). Un estudio mostró una diferencia a favor de la SORR versus ninguna SORR para mejorar la calidad de vida para la percepción del riesgo de cáncer de ovario en las pacientes que eran portadoras de mutaciones del gen BRCA1 (DM 10,70; IC del 95%: 2,45 a 18,95; P = 0,01; 98 mujeres; evidencia de certeza muy baja) y las portadoras de mutaciones del gen BRCA2 (DM 13,00; IC del 95%: 3,59 a 22,41; P = 0,007; evidencia de certeza muy baja). Los datos de un estudio mostraron una diferencia a favor de la SORR y la MRR versus ninguna SORR en el aumento de la supervivencia general (CRI 0,14; IC del 95%: 0,02 a 0,98; P = 0,0001; I² = 0%; evidencia de certeza baja), pero ninguna diferencia para la mortalidad por cáncer de mama (CRI 0,78; IC del 95%: 0,51 a 1,19; P = 0,25; evidencia de certeza muy baja). Las estimaciones del riesgo para la mortalidad por cáncer de mama según la edad en el momento de la SORR (50 años de edad o menos versus más de 50 años) no indicaron protección y no difirieron en las portadoras de mutaciones de los genes BRCA1 (CRI 0,85; IC del 95%: 0,64 a 1,11; I² = 16%; P = 0,23; evidencia de certeza muy baja) y BRCA2 (CRI 0,88; IC del 95%: 0,42 a 1,87; I² = 63%; P = 0,75; evidencia de certeza muy baja).
Conclusiones de los autores
Hay evidencia de certeza muy baja de que la SORR puede aumentar la supervivencia general y reducir la mortalidad por CSGA y cáncer de mama en las portadoras de mutaciones de los genes BRCA1 y BRCA2. Evidencia de certeza muy baja indica que la SORR reduce el riesgo de muerte por CSGA y cáncer de mama en las pacientes con mutaciones del gen BRCA1. La evidencia del efecto de la SORR sobre el CSGA y el cáncer de mama en las portadoras de mutaciones del gen BRCA2 no estuvo clara debido a que los números fueron escasos. Estos resultados se deben interpretar con cuidado debido a los diseños cuestionables de los estudios, los perfiles de riesgo de sesgo y la evidencia de certeza muy baja. No fue posible establecer conclusiones con respecto a la incidencia de fracturas óseas, la calidad de vida o los eventos adversos graves de la SORR, ni de los efectos de la SORR según el tipo de cirugía de reducción de riesgos y la edad a la que se realiza. Se justifica la realización de estudios de investigación adicionales sobre estos resultados para explorar los efectos diferenciales de las mutaciones de los genes BRCA1 o BRCA2.
PICO
Resumen en términos sencillos
Extracción quirúrgica de las trompas de Falopio y de los ovarios para la reducción de los riesgos en las pacientes con mutaciones de los genes BRCA1 o BRCA2
Antecedentes
Las mutaciones del gen 1 del cáncer de mama (BRCA1) o del gen 2 del cáncer de mama (BRCA2) aumentan el riesgo de desarrollar algunos cánceres, incluidos los cánceres de mama, ováricos, tubáricos y peritoneales. La salpingoovariectomía de reducción de riesgos (SORR) (extracción de las trompas de Falopio y los ovarios) se ofrece por lo general a las pacientes con mutaciones de los genes BRCA1, BRCA2 o ambos, después que han finalizado la maternidad. Sin embargo, todavía no está claro cuánta reducción del riesgo de cáncer de mama y seroso de grado alto (CSGA) de las trompas de Falopio, ovárico y de origen peritoneal primario ofrece la SORR ni cuál es el efecto sobre otros resultados de salud, ni tampoco si la efectividad de la SORR difiere según el tipo de mutación.
Pregunta de la revisión
¿La SORR en las pacientes con mutaciones de los genes BRCA1 o BRCA2 reduce el riesgo de desarrollar cáncer de mama y CSGA, y qué efecto tiene en el riesgo de muerte (supervivencia general) y la calidad de vida?
Características de los estudios
En esta revisión se analizaron los datos de diez estudios no aleatorios (cohorte; un estudio en el cual un grupo definido de personas [la cohorte] se sigue durante un tiempo para examinar las asociaciones entre diferentes tratamientos recibidos y los resultados posteriores). Todos los estudios compararon la SORR con o sin mastectomía de reducción de riesgos (MRR; extracción quirúrgica de las mamas) versus ninguna SORR (vigilancia). La evidencia está actualizada hasta julio de 2017.
Principales hallazgos
Al incluir los datos de las portadoras de las mutaciones de los genes BRCA1 y BRCA2, este análisis encontró que la SORR puede mejorar la supervivencia general y reducir las muertes por CSGA y cáncer de mama. Cuando se analizó por gen mutado, hubo evidencia de una reducción en el riesgo de CSGA y cáncer de mama en las pacientes con mutaciones del gen BRCA1, pero puede o no haber un efecto sobre las pacientes con mutaciones del gen BRCA2 debido al escaso número de pacientes con estas mutaciones en los estudios. Ninguno de los estudios informó sobre fracturas óseas o efectos secundarios graves. La SORR y la MRR pueden haber mejorado la supervivencia general, pero no redujeron las muertes por cáncer de mama. No hubo protección ni diferencias en la mortalidad por cáncer de mama en dependencia de la edad en el momento de la SORR (50 años de edad o menos versus más de 50 años) en las portadoras de mutaciones de los genes BRCA1 o BRCA2. La SORR puede haber mejorado la calidad de vida con respecto a la percepción de riesgo de cáncer de ovario.
Fiabilidad de la evidencia
La fiabilidad de la evidencia fue baja a muy baja debido al escaso número de participantes y la calidad metodológica baja de los estudios incluidos.
¿Cuáles son las conclusiones?
La SORR en las pacientes con mutaciones de los genes BRCA1 o BRCA2 puede mejorar la supervivencia general y reducir el número de muertes por CSGA y cáncer de mama cuando se combinaron las pacientes con mutaciones en ambos genes. La SORR puede reducir el riesgo de muerte por CSGA y cáncer de mama en las pacientes con mutaciones del gen BRCA1, pero puede reducir o no el riesgo en las portadoras de mutaciones del gen BRCA2. Estos resultados se deben interpretar con cuidado debido a la calidad baja de los diseños de los estudios y los perfiles de riesgo de sesgo. No fue posible establecer conclusiones con respecto al número de fracturas óseas, la calidad de vida general, los efectos secundarios graves de la SORR ni los efectos de la SORR según el tipo de cirugía de reducción de riesgos y la edad en el momento de la SORR. Sin embargo, se encontró que la fiabilidad de la evidencia fue muy baja, por lo que todavía se necesitan estudios grandes de alta calidad que consideren específicamente estos resultados para las diferencias en las portadoras de mutaciones de los genes BRCA1 o BRCA2.
Conclusiones de los autores
Summary of findings
| RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and USA Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers | |||||
| Overall survival: BRCA1 or BRCA2 | Study population | HR 0.32 | 2548 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| HGSC mortality: BRCA1 or BRCA2 | Study population | HR 0.06 | 2534 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: BRCA1 or BRCA | Study population | HR 0.58 | 7198 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Bone fracture incidence | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Quality of life (ovarian cancer risk perception): BRCA1 or BRCA2 | See comment | See comment | Not estimable | 200 | ⊕⊝⊝⊝ | Unable to perform meta‐analysis as only 1 study reported the outcome. |
| Quality of life (breast cancer risk perception): BRCA1 or BRCA2 | See comment | See comment | Not estimable | 200 | ⊕⊝⊝⊝ | Unable to perform meta‐analysis as only 1 study reported the outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies. | ||||||
| RRSO vs no RRSO according to BRCA mutation status | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and USA Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO according to BRCA mutation status | |||||
| Overall survival: BRCA1 only | Study population | HR 0.30 | 2548 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Overall survival: BRCA2 only | Study population | HR 0.44 | 2122 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| HGSC mortality: BRCA1 only | Study population | HR 0.1 | 1983 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| HGSC mortality: BRCA2 only | See commentb | See commentb | Not estimable See commentc | 1983 | ⊕⊝⊝⊝ | bAs a result of the way HRs were calculated, assumed and corresponding risks were not estimated. cUnable to perform meta‐analysis as no mortality events were recorded in any study and HRs could not be estimated. |
| Breast cancer mortality: BRCA1 only | Study population | HR 0.45 | 2203 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: BRCA2 only | Study population | HR 0.88 | 5882 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Quality of life (ovarian cancer risk perception): BRCA1 only | See comment | See comment | Not estimable | 98 | ⊕⊝⊝⊝ | Unable to perform meta‐analysis as only 1 study reported the outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies. | ||||||
| RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and USA Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery | |||||
| Overall survival: RRSO alone vs RRSO and RRM | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Overall survival: RRSO and RRM vs no RRSO | Study population | HR 0.14 | 261 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: RRSO alone vs RRSO and RRM | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Breast cancer mortality: RRSO and RRM vs no RRSO | See comment | See comment | Not estimable | 722 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. |
| Bone fracture incidence | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to confounding and bias due in selection of participants in the study). | ||||||
| RRSO vs no RRSO in BRCA1 mutation carriers according to age at RRSO | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and America Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA1 mutation carriers according to age at RRSO | |||||
| Overall survival | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| HGSC mortality | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Breast cancer mortality: ≤ 50 years | Study population | HR 0.78 | 4566 | ⊕⊝⊝⊝ Very lowa,b | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: > 50 years | Study population | HR 1.27 | 4566 | ⊕⊝⊝⊝v Very lowa,b | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Bone fracture incidence | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies. bDowngraded by one level for serious imprecision: the confidence intervals overlapped 1 and either 0.75 or 1.25 or both (i.e. wide confidence intervals in all included studies, which crossed the line of unity). | ||||||
| RRSO versus no RRSO in BRCA2 mutation carriers according to age at RRSO | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and America Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA2 mutation carriers according to age at RRSO | |||||
| Overall survival | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| HGSC mortality | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Breast cancer mortality: ≤ 50 years | Study population | HR 0.49 | 444 | ⊕⊝⊝⊝ Very lowa,b | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: > 50 years | Study population | HR 1.36 | 444 | ⊕⊝⊝⊝ Very lowa,b | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Bone fracture incidence | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; CI: confidence interval; HGSC: high‐grade serous cancer; HR: hazard ratio; RRSO: risk‐reducing salpingo‐oophorectomy. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies. bDowngraded by one level for serious imprecision: the confidence intervals overlapped 1 and either 0.75 or 1.25 or both (i.e. wide confidence intervals in all included studies, which cross the line of unity). | ||||||
Antecedentes
Descripción de la afección
El cáncer de ovario es el quinto tipo más común de cáncer y la cuarta causa más común de mortalidad por cáncer en las mujeres (ESMO 2013; Gottschau 2016). A nivel global a aproximadamente 204 000 mujeres se les diagnostica cáncer de ovario cada año, de las que alrededor de 115 000 mueren debido a la enfermedad, con una tasa de incidencia de 6,1/100 000 y una tasa de mortalidad de 3,8/100 000(IARC 2012; Ozols 2006). La estimación del riesgo para toda la vida de que una mujer desarrolle cáncer de ovario es alrededor de 1/54 (ESMO 2013). La incidencia de cáncer de ovario aumenta con la edad y es muy prevalente en las mujeres posmenopáusicas, con una mediana de edad de 63 años en el momento del diagnóstico (McGuire 2016; NCCN 2014). Las pacientes con enfermedad en estadio inicial presentan pocos síntomas o síntomas vagos, que pueden contribuir a que acudan tarde a consulta (Ang 2011; NCCN 2014). Más del 70% de las pacientes consultan con enfermedad avanzada y menos del 40% de las pacientes con cáncer de ovario en los EE.UU. sobreviven más de cinco años después del diagnóstico (NCCN 2014), pero en las poblaciones europeas sobrevive más del 40% (Gottschau 2016).
Los estudios han mostrado que la presencia de mutaciones nocivas del gen 1 del cáncer de mama (BRCA1) o del gen 2 del cáncer de mama (BRCA2) aumenta el riesgo de desarrollar diversos cánceres que incluyen el cáncer de mama y el cáncer seroso de grado alto (CSGA) (Eccles 2016; Guidozzi 2016; Iavazzo 2016). El BRCA1 y el BRCA2 son genes separados que se localizan en dos cromosomas diferentes, 17q21 y 13q12,3; respectivamente (Girolimetti 2014; Staples 2013). Tienen secuencias primarias diferenciadas, aunque la interrupción de cualquiera de los genes BRCA da lugar a efectos fisiopatológicos equivalentes, además de espectros similares de cáncer. El BRCA1 y el BRCA2 son genes supresores de tumores para la reparación del ADN. Además, y como parte de las funciones como genes supresores de tumores, el BRCA1 y el BRCA2 participan en la reparación del ADN homólogo, la estabilidad genómica, la regulación transcripcional, la ubiquitinación de proteínas, la remodelación de la cromatina y el control del ciclo celular (Iodice 2010; Tutt 2002; Venkitaraman 2014). La pérdida de la función del BRCA da lugar al desarrollo de inestabilidad cromosómica (Tutt 2002; Venkitaraman 2014).
Las mutaciones de los genes BRCA solo representan una fracción pequeña de los cánceres generales de mama y ováricos. Aproximadamente 1/300 a 1/800 mujeres portan las mutaciones en la población general (ACOG 2009). En un estudio de cohortes prospectivo más reciente que incluye principalmente a participantes de estudios nacionales grandes realizados en el Reino Unido, los Países Bajos y Francia, se informaron incidencias de cáncer de mama u ovario del 44% en las portadoras de mutaciones del gen BRCA1, y del 17% en las portadoras de mutaciones del gen BRCA2 (Kuchenbaecker 2017). Las frecuencias generales de las mutaciones de los genes BRCA1 y BRCA2 fueron del 10,2% en las pacientes árabes con cáncer de mama y del 30,7% en las pacientes árabes con cáncer de ovario (Alhuqail 2018). Dos estudios encontraron que las mutaciones de los genes BRCA1 y BRCA2 representan del 10% al 15% de todos los cánceres ováricos(Pal 2005; Risch 2001).
Las mutaciones específicas en los genes BRCA1 o BRCA2 ocurren con mayor frecuencia en ciertas poblaciones, que incluyen los judíos Ashkenazis, los francocanadienses y los islandeses (Hartge 1999; Lynch 2013). El riesgo para toda la vida de cáncer de ovario en una mujer es del 39% al 46% con una mutación en el BRCA1 y del 12% al 20% con una mutación en el BRCA2, y el riesgo de cáncer de mama en una mujer con una mutación de los genes BRCA1 o BRCA2 es del 65% al 74% (Girolimetti 2014; Meaney‐Delman 2013).
En las mujeres con mutaciones del gen BRCA1, menos del 2% al 3% de las portadoras desarrollan cáncer de ovario a los 40 años de edad. Esta cifra aumenta del 10% al 21% a los 50 años de edad. En las mujeres con mutaciones del gen BRCA2, menos del 3% de las portadoras desarrollan cáncer de ovario a los 50 años de edad. Sin embargo, del 26% al 34% de estas mujeres parecen desarrollar cáncer de mama a los 50 años de edad (Ford 1998; King 2003; Rebbeck 2002; Satagopan 2002; Struewing 1997). Por lo tanto, se ha recomendado que a las mujeres con mutaciones del gen BRCA1/2 se les debe ofrecer salpingoovariectomía de reducción de riesgos (SORR) a los 40 años de edad o cuando se ha completado la maternidad (ACOG 2009). Las estimaciones de la frecuencia de cáncer de las trompas de Falopio en las portadoras de mutaciones del gen BRCA son limitadas por la falta de precisión en la asignación del sitio de origen de los carcinomas serosos de grado alto metastásicos en la consulta inicial (Lengyel 2013).
Las pacientes positivas para las mutaciones de los genes BRCA con cáncer de ovario tienen un mejor pronóstico que los controles (mujeres que son negativas para las mutaciones de los genes BRCA1 o BRCA2) en cuanto a la supervivencia general, debido a la mayor quimiosensibilidad de los tumores BRCA positivos (Biglia 2016). En la histopatología de los cánceres ováricos asociados con las mutaciones BRCA1 y BRCA2 predominan los carcinomas serosos de grado altos y endometrioides, en lugar de en los tumores mucinosos y dudosos (ACOG 2009). El cáncer peritoneal primario es una neoplasia maligna agresiva que, debido a la falta de una prueba de detección específica, no se puede diagnosticar en los estadios iniciales (Iavazzo 2016). Los estudios han indicado que muchos cánceres peritoneales ováricos y primarios pueden ser de origen tubárico y, por lo tanto, parte del espectro de la enfermedad asociada con estas mutaciones se conoce de forma colectiva como CSGA (Callahan 2007; NCCN 2014). Se necesitan esfuerzos comunes para crear guías internacionales alrededor de las pruebas del BRCA1 y el BRCA2 en el cáncer de ovario y otros cánceres y asegurar prácticas consistentes de cribado (Arts‐de Jong 2016; Karakasis 2016; Lheureux 2016).
Descripción de la intervención
La SORR profiláctica se refiere a la extracción quirúrgica de las trompas de Falopio y los ovarios en pacientes en las que se piensa que no tienen cáncer antes del procedimiento quirúrgico, pero que tienen un riesgo alto para toda la vida (Rebbeck 2009; Shu 2016). El protocolo específico para la SORR en las pacientes de alto riesgo incluye la exploración de los órganos pelvianos en busca de cualquier evidencia de cáncer, la realización de un lavado peritoneal (la pelvis se baña en solución salina y se recoge el líquido para buscar cualquier célula cancerosa que pueda estar libre en la cavidad abdominal) y la extracción de los ovarios y las trompas de Falopio por completo. El protocolo "intensivo" de la SORR incluye: salpingoovariectomía bilateral y extracción completa de las trompas de Falopio, el examen citológico de los lavados peritoneales y biopsias aleatorias peritoneales y omentales (Powell 2014; Ready 2011). Si hay adherencias entre el peritoneo de la pared lateral de la pelvis y el ovario, se debe tener cuidado en no fracturarlas (Dowdy 2004). Se recomienda que las adherencias se resequen junto con el ovario con el uso de un enfoque retroperitoneal (Dowdy 2004). Lo anterior es necesario para prevenir el síndrome de ovárico remanente (Dowdy 2004). Más importante, cualquier célula ovárica residual tiene una probabilidad alta de experimentar una transformación maligna.
Es posible que después de la SORR se identifique el cáncer microscópico (oculto) de ovario o de las trompas de Falopio, y proporcionalmente se han detectado más cánceres de las trompas de Falopio que cánceres ováricos después de la cirugía profiláctica (Powell 2005). Un estudio en 122 mujeres positivas para las mutaciones de los genes BRCA sometidas a SORR detectó cánceres ocultos en el 6% en el momento de la cirugía, todos originados dentro de las trompas de Falopio (Callahan 2007). Este estudio indica que muchos de los cánceres "ováricos" en las portadoras de BRCA pueden comenzar en las trompas de Falopio. Por lo tanto, es importante retirar las trompas de las portadoras de mutaciones de los genes BRCA y realizar "secciones seriadas" de las trompas de Falopio para extirpar los cánceres ocultos o los carcinomas tubáricos intraepiteliales serosos (CTIS). En el protocolo SEE‐FIM (Sectioning and Extensively Examining of the Fimbriated end), se examina histológicamente la mayor superficie de la trompa, sobre la base de la indicación de que se deben examinar múltiples secciones más profundas, si las secciones iniciales con hematoxilina y eosina (H&E) son negativas. En un estudio, la sección H&E única, en comparación con el enfoque SEE‐FIM, detecta solo el 75% (intervalo de confianza [IC] del 95%: 51% a 90%) de los CTIS (Mahe 2013). El protocolo SEE‐FIM se debe considerar en especial en los casos del carcinoma de endometrio, los cánceres serosos pelvianos no uterinos o los tumores ováricos serosos dudosos (Crum 2007; Koc 2018; Leonhardt 2011).
La laparoscopía es el método preferido para realizar una SORR (Blok 2016), debido a que tiene una menor morbilidad que la laparotomía. Aunque la histerectomía no es una parte de la cirugía de reducción de riesgos por las mutaciones de los genes BRCA1/2, teóricamente podría reducir el riesgo de cáncer en las trompas de Falopio cornuales (Karlan 2004). La histerectomía se puede considerar para otras indicaciones médicas potenciales o en las pacientes que toman tamoxifeno para reducir el riesgo de cáncer endometrial (ACOG 2009). Sin embargo, la mayoría de los médicos consideran polémica la función de la histerectomía síncrona (Lee 2017a; Saule 2018; Segev 2013; Shu 2016), ya que el riesgo de cáncer endometrial en las pacientes con cáncer hereditario de mama y ovario no es significativamente elevado (Lee 2017a; Segev 2013), aunque los autores de Shu 2016 informaron un mayor riesgo de carcinoma seroso de endometrio.
Los efectos adversos potenciales de la SORR son la morbilidad quirúrgica asociada y la menopausia prematura en las pacientes más jóvenes (Bober 2015). Aparte de los síntomas menopáusicos significativos, la SORR podría dar lugar a un aumento en el riesgo de pérdida mineral ósea (osteopenia y osteoporosis) y disfunción cognitiva (Guidozzi 2016). El riesgo de enfermedades cardiovasculares también aumenta si el procedimiento se realiza en mujeres menores de 50 años de edad (Guidozzi 2016). Es importante que las pacientes sometidas a menopausia quirúrgica o que consideran someterse a SORR, analicen los síntomas menopáusicos y su tratamiento con el equipo de asistencia sanitaria. Los estudios han encontrado que la terapia de reemplazo hormonal (TRH) a corto plazo no anula el efecto protector de la salpingoovariectomía sobre el riesgo de cáncer de mama posterior en las portadoras de mutaciones de los genes BRCA1/2 hasta el momento de la menopausia natural, esperada alrededor de los 50 años de edad (Armstrong 2004; Rebbeck 2005).
En las pacientes a las que tampoco se les realiza mastectomía de reducción de riesgos, hay inquietud creciente con respecto al posible efecto adverso sobre el riesgo de cáncer de mama asociado con la administración de una combinación de estrógeno y progesterona, en especial entre las pacientes más jóvenes que utilizarían los agentes por más de diez años. Debido al aumento teórico del riesgo de cáncer de mama asociado con el tratamiento combinado con TRH con estrógeno y progesterona (en comparación con la TRH con estrógeno solo), la Society of Gynecologic Oncology indica el uso de un dispositivo intrauterino que contiene progestina para acompañar el reemplazo con estrógeno y, por lo tanto, evitar la administración de tratamiento sistémico con progestina (Hartmann 2015; Hartmann 2016; Walker 2015). Sin embargo, la realización de la mastectomía bilateral de reducción de riesgos (MBRR) puede dar lugar a una reducción muy significativa en el riesgo de cáncer de mama en las portadoras de mutaciones de los genes BRCA1 y BRCA2 (De Felice 2015). La reducción en el riesgo de cáncer de mama se calcula entre el 94% y el 95% cuando se realiza la MBRR, casi del 89% en las pacientes que reciben MBRR más SORR, y del 46% cuando se realiza la SORR sola, lo que indica que la SORR sola no puede reemplazar la repercusión beneficiosa de la MBRR en la aparición de cáncer de mama (De Felice 2015). Esta información puede permitir a los médicos tratar todas las opciones disponibles con las pacientes para diseñar las estrategias de tratamiento individuales.
De qué manera podría funcionar la intervención
La SORR puede reducir el riesgo de cánceres ováricos y de las trompas de Falopio del 85% al 90% y de cáncer de mama del 40% al 70% en las pacientes con mutaciones conocidas de los genes BRCA1/2 (ACOG 2009; Finch 2014). Además, se ha mostrado que las estrategias de reducción del riesgo se asocian con un aumento en la esperanza de vida en las portadoras de mutaciones de los genes BRCA1/2 (Salhab 2010). Previamente se consideraba que los cánceres ováricos se desarrollaban en el recubrimiento del ovario, como resultado del proceso constante de rotura y reparación durante la ovulación. Los estudios más recientes indican que muchos cánceres ováricos en las portadoras de mutaciones de los genes BRCA se originan en la zona distal (parte de la trompa más cercana al ovario) de las trompas de Falopio, lo que hace que los investigadores se cuestionen si la salpingectomía sola (extracción de las trompas de Falopio) podría reducir el riesgo de cáncer de ovario. Un precursor candidato del carcinoma intraepitelial tubárico, llamado "firma p53", indica que los eventos moleculares asociados con el cáncer seroso (mutaciones p53) se pueden detectar en la mucosa benigna (Crum 2007; Leonhardt 2011).
Las guías especializadas actuales recomiendan que a las pacientes con mutaciones de los genes BRCA se les debe ofrecer la SORR entre los 35 y 40 años de edad, o después que hayan completado la maternidad. Los ovarios secretan hormonas que controlan el ciclo reproductivo. La extracción quirúrgica de los ovarios reducirá de manera significativa los niveles de las hormonas estrógeno y progesterona que circulan en el cuerpo (Metcalfe 2015; Olivier 2004). La salpingoovariectomía bilateral puede detener o enlentecer el crecimiento de los cánceres de mama que necesitan estas hormonas para crecer (van Verschuer 2014). Algunos estudios han indicado que el nivel de reducción del riesgo de cáncer de mama puede diferir entre las portadoras de mutaciones de los genes BRCA1 y BRCA2 que eligen la SORR (Powell 2011; Powell 2014; van Verschuer 2014). Kauff 2008 informó a partir de un estudio multicéntrico que las pacientes con mutaciones del gen BRCA2 sometidas a SORR redujeron su riesgo de cáncer de mama en el 72%. La reducción del riesgo fue menor (alrededor del 29%) en las pacientes con mutaciones del gen BRCA1. Kauff 2008 indicó que la ovariectomía puede ser más protectora para las pacientes con mutaciones del gen BRCA2, ya que es más probable que los cánceres de mama sean positivos al receptor hormonal, mientras que en las portadoras de mutaciones del gen BRCA1 por lo general los cánceres de mama son negativos al receptor hormonal (van Verschuer 2014; Veronesi 2005). En general, el riesgo de muerte por cáncer de mama se reduce en el 56% en las portadoras de mutaciones de los genes BRCA1/2 a las que se les realizó ovariectomía (Domchek 2010). Debido a que los tumores de mama en gran parte están estimulados por los estrógenos, se ha indicado que el bloqueo hormonal mediante ovariectomía inhibe su desarrollo (Narod 2001). Por lo tanto, la ovariectomía profiláctica puede tener la ventaja de reducir el riesgo de cáncer de mama, así como de cáncer de ovario (Mitrunen 2003). La reducción en el riesgo de cáncer de mama de las portadoras de mutaciones de los genes BRCA sometidas a SORR puede ser mayor en las pacientes menores de 50 años de edad (la media de la edad de la menopausia), pero algunos estudios han indicado un beneficio en la reducción del riesgo de cáncer de mama de las pacientes sometidas a SORR después de la menopausia. Barlin 2013 informó que 199 pacientes posmenopáusicas portadoras de mutaciones de los genes BRCA sometidas a SORR después de la menopausia tuvieron una reducción del 57% en el riesgo de cáncer de mama. Barlin 2013 formuló la hipótesis de que, aunque los ovarios dejan de producir estrógeno y progesterona después de la menopausia natural, siguen produciendo algunas hormonas, incluida la testosterona, lo que quizás explique por qué la SORR después de la menopausia todavía tiene efectos protectores contra el cáncer de mama.
Por qué es importante realizar esta revisión
En las pacientes con mayor riesgo, debido a antecedentes familiares o mutaciones confirmadas en genes de alta penetrancia como los genes BRCA1/2, el cribado anual con CA125 con el uso de un valor de corte y la ecografía transvaginal no detectaron cánceres en estadio inicial (Hermsen 2007; Stirling 2005). Este hecho se volvió a confirmar mediante el UK Familial Ovarian Cancer Screening Study (UKFOCSS) (Rosenthal 2013a; Rosenthal 2013b). De igual manera, un ensayo aleatorio grande (el Prostate, Lung, Colorectal and Ovarian [PLCO] Cancer Screening Trial) encontró que el cribado no disminuyó la mortalidad por cáncer de ovario (Pinsky 2013). Aunque los resultados del estudio de fase II fueron alentadores, en la actualidad el cribado no se puede considerar una alternativa segura a la SORR. Como la vigilancia para el cáncer de ovario, peritoneal y de las trompas de Falopio no ha resultado efectiva, la SORR se ha adoptado ampliamente como un componente clave para la reducción del riesgo de cáncer de mama y ginecológico en las pacientes con mutaciones de los genes BRCA1 o BRCA2 (Girolimetti 2014). El riesgo de cáncer de mama se puede reducir con ovariectomía o mastectomía de reducción de riesgos, o ambas (Maeshima 2016). Aunque algunos autores han indicado que las trompas de Falopio pueden ser la causa de muchos cánceres ginecológicos en las portadoras de mutaciones, los investigadores advierten que no hay evidencia suficiente para indicar que todos los casos de cáncer de ovario comienzan en las trompas de Falopio (Kramer 2013). Además, no es probable que la extracción de las trompas de Falopio solo disminuya el riesgo de cáncer de mama. Se necesitan más estudios de investigación para comprender completamente la función de las trompas de Falopio en el desarrollo de estos cánceres. Aunque se han publicado revisiones no sistemáticas (Calderon‐Margalit 2004; Domchek 2007; Dowdy 2004; Oliver 2015; Olopade 2004; Salhab 2010; Schenberg 2014), revisiones sistemáticas (Ludwig 2016; Marchetti 2014; Tschernichovsky 2017), y metanálisis (Rebbeck 2009) previos, o ambos (Li 2016), sobre el beneficio de la SORR en las pacientes con mutaciones de los genes BRCA1 o BRCA2, su función en la reducción de la incidencia de cáncer de mama, ovárico, de las trompas de Falopio y otros cánceres, incluidos otros resultados de salud, no está clara (De Felice 2017; Fakkert 2015; Heemskerk‐Gerritsen 2015a). Se necesita una revisión sistemática Cochrane para evaluar la eficacia y los efectos adversos de la SORR en las pacientes con mutaciones de los genes BRCA1 o BRCA2.
Objetivos
Evaluar los efectos beneficiosos y perjudiciales de la SORR en las pacientes con mutaciones de los genes BRCA1 o BRCA2.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Los ensayos controlados aleatorios (ECA) y cuasialeatorios (estudios donde la asignación o el reclutamiento de los participantes son propensos a sesgo/errores sistemáticos, ya que todos los participantes no tienen iguales probabilidades de estar en un grupo o en el otro) fueron poco probables o no fueron posibles debido a razones éticas. Por lo tanto, se examinaron los siguientes tipos de estudios.
-
Estudios no aleatorios (ENA), estudios de cohortes prospectivos y retrospectivos y series de casos clínicos (todos con grupos de comparación concurrentes).
Para disminuir el sesgo de selección, solo se incluyeron los estudios que utilizaron el ajuste estadístico para la mezcla de casos iniciales mediante análisis de múltiples variables.
Se excluyeron los estudios de casos y controles y los estudios observacionales no controlados. También se excluyeron los estudios controlados tipo antes y después (un estudio en el que las observaciones se hacen antes y después de la implementación de una intervención, en el grupo que recibe la intervención y en el grupo control que no la recibe) porque no hubo grupos de comparación concurrentes.
Tipos de participantes
Mujeres, 18 años o más, con mutaciones conocidas de los genes BRCA1 o BRCA2. Se incluyeron las pacientes sin una neoplasia maligna anterior o coexistente de mama, ovárica o de las trompas de Falopio y las pacientes con o sin histerectomía concomitante. Se incluyeron las pacientes con una mastectomía antes, concomitante con, o después de la SORR, aunque la mastectomía es el foco de otra revisión Cochrane (Lostumbo 2010). Se excluyeron las pacientes con una neoplasia maligna anterior o coexistente de la mama y las pacientes con ovariectomía o salpingectomía unilateral o salpingoovariectomía (ambas). Además, se excluyeron las pacientes con salpingectomía profiláctica con ovariectomía retardada o conservación ovárica (Harmsen 2015; Harmsen 2016; Tschernichovsky 2017).
Tipos de intervenciones
SORR (cirugía para retirar las trompas de Falopio y los ovarios como una opción para las pacientes con mutaciones de los genes BRCA1 o BRCA2 en las que se piensa que no tienen cáncer antes del procedimiento quirúrgico, pero que tienen un riesgo alto para toda la vida de presentar cáncer de ovario, cáncer de las trompas de Falopio o cáncer de mama) versus ninguna SORR.
Tipos de medida de resultado
Resultados primarios
-
Supervivencia general: supervivencia hasta la muerte por todas las causas. La supervivencia se evaluó desde el momento en que las pacientes ingresaron al estudio.
-
Mortalidad por CSGA (cáncer de las trompas de Falopio, ovárico y peritoneal primario).
-
Mortalidad por cáncer de mama.
Resultados secundarios
-
Incidencia de CSGA (cáncer de las trompas de Falopio, carcinoma intraepitelial seroso tubárico, ovárico y peritoneal primario) (todos los casos de cáncer peritoneal seroso diagnosticados después de la salpingoovariectomía profiláctica que se consideraron cáncer peritoneal primario).
-
Incidencia de cáncer de mama.
-
Incidencia de fracturas óseas.
-
Supervivencia libre de enfermedad: tiempo transcurrido desde el procedimiento quirúrgico hasta el diagnóstico de cáncer.
-
Morbilidad:
-
morbilidad quirúrgica directa;
-
morbilidad sistémica relacionada con la cirugía (p.ej., infección torácica/de la herida/urinaria, tromboembolia venosa, menopausia prematura, etc.).
-
-
Recuperación, reingreso.
-
Calidad de vida, medida con una escala validada a través del informe de normas en una publicación revisada por pares (Roila 2001; Spitzer 1981).
-
Eventos adversos; se intentó categorizar la gravedad de los siguientes eventos adversos según los Common Terminology Criteria for Adverse Events (CTCAE 2010): complicaciones relacionadas con la cirugía medidas como la proporción de pacientes que desarrollaron uno o más de los ítems siguientes (según la definición de los estudios) en el transcurso de 12 semanas. Las complicaciones se clasificaron en intraoperatorias y posoperatorias:
-
complicaciones intraoperatorias:
-
hemorragia;
-
lesión uretérica;
-
complicaciones cardíacas o respiratorias;
-
anafilaxia;
-
-
las complicaciones posoperatorias se clasificaron en tempranas (antes del alta hospitalaria o en el transcurso de los siete días desde la cirugía), tardías (desde los siete días hasta el seguimiento: en el transcurso de las 12 semanas desde la cirugía) o totales (tempranas y tardías):
-
apertura espontánea de la herida;
-
embolia pulmonar;
-
trombosis venosa profunda;
-
problema psiquiátrico/psicosexual.
-
-
Métodos de búsqueda para la identificación de los estudios
We searched for papers in all languages and translated them as necessary.
Búsquedas electrónicas
We searched the following electronic databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 7) in The Cochrane Library (Appendix 1).
-
MEDLINE Ovid (January 1946 to July week 2 2017) (Appendix 2).
-
Embase (January 1980 to 2017 week 30) (Appendix 3).
We identified all relevant articles on PubMed and using the 'related articles' feature we performed a further search for newly published articles.
Búsqueda de otros recursos
Unpublished and grey literature
We searched the following for ongoing studies:
-
metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com/rct).
-
Physicians Data Query (www.nci.nih.gov).
-
USA National Institutes of Health (clinicaltrials.gov/ct).
-
USA National Cancer Institute (www.cancer.gov/clinicaltrials).
-
ISRCTN registry (www.isrctn.com/).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/).
If ongoing studies that have not been published were identified through these searches, we approached the principal investigators, and major co‐operative groups active in this area, to ask for relevant data.
Handsearching
We handsearched the citation lists of included studies, key textbooks and previous systematic reviews and contacted experts in the field to identify further reports of studies. We also handsearched the reports of conferences in the following sources.
-
Gynecologic Oncology (Annual Meeting of the American Society of Gynecologic Oncologist).
-
International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society).
-
British Journal of Cancer.
-
British Cancer Research Meeting.
-
Annual Meeting of European Society of Medical Oncology (ESMO).
-
Annual Meeting of the American Society of Clinical Oncology (ASCO).
We handsearched the following breast cancer journals:
Obtención y análisis de los datos
Selección de los estudios
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database (EndNote X7), and removed duplicates. Two review authors (GE and IE) examined the remaining references independently. We excluded those studies that clearly did not meet the inclusion criteria and we obtained full‐text copies of potentially relevant references. Two review authors (GE and IE) independently assessed the eligibility of the retrieved reports/publications. We resolved any disagreement through discussion or, if required, we consulted a third review author (AC). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Liberati 2009).
Extracción y manejo de los datos
For included studies, we extracted the following data.
-
Author, year of publication and journal citation (including language).
-
Country.
-
Setting.
-
Ethnicity.
-
Inclusion and exclusion criteria.
-
Study design, methodology.
-
Study population:
-
total number enrolled;
-
participant characteristics (e.g. BRCA1, BRCA2 or both);
-
age;
-
comorbidities;
-
other baseline characteristics.
-
-
Intervention details:
-
type of surgery;
-
occult cancer;
-
type of screening test;
-
period of screening test;
-
type of chemoprevention;
-
dose of chemoprevention;
-
course of chemoprevention;
-
type of histology protocol adopted (e.g. the SEE‐FIM protocol) as documented in Blok 2016 and Mahe 2013;
-
use of peritoneal washing cytology (Blok 2016);
-
use of oral contraceptives.
-
-
Comparison: we compared the outcomes for women with adnexa‐preserving.
-
Risk of bias in study (Assessment of risk of bias in included studies).
-
Duration of follow‐up.
-
Outcomes: for each outcome, we extracted the outcome definition and unit of measurement (if relevant). For adjusted estimates, we recorded variables adjusted for in analyses.
-
Results: we extracted the number of participants allocated to each intervention group, the total number analysed for each outcome and the missing participants.
We extracted the results as follows.
-
For time‐to‐event data (overall survival and disease‐specific survival), we extracted the log of the hazard ratio (log(HR)) and its standard error from trial reports. If these were not reported, we estimated the log(HR) and its standard error using the methods described by Parmar 1998.
-
For dichotomous outcomes (e.g. adverse events or deaths, if it is not possible to use a HR), we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint, in order to estimate a risk ratio (RR).
-
For continuous outcomes (e.g. quality of life measures), we extracted the final value and standard deviation (SD) of the outcome of interest and the number of participants assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference (MD) between treatment arms and its standard error.
If reported, we extracted both unadjusted and adjusted statistics. Where possible, all data extracted were those relevant to an intention‐to‐treat analysis, in which we analysed participants in groups to which they were assigned.
When possible, we noted the time points at which outcomes were collected and reported.
Two review authors (GE and IE) extracted data independently onto a data abstraction form specially designed for the review. We resolved differences between review authors by discussion or by appeal to a third review author (AE) if necessary. We approached the principal investigators of included studies to ask for any missing relevant unpublished data.
Evaluación del riesgo de sesgo de los estudios incluidos
As detailed in Results of the search, we identified no RCTs or quasi‐randomised studies were identified, therefore we assessed the risk of bias for NRS using the ROBINS‐I (Risk Of Bias In Non‐randomised Studies‐of Interventions). A new tool for evaluating risk of bias in estimates of the comparative effectiveness (harm or benefit) of interventions from studies that did not use randomisation to allocate units (individuals or clusters of individuals) to comparison groups (Sterne 2016).
We achieved consensus on seven domains through which bias might be introduced into an NRS:
-
confounding;
-
selection of participants into the study;
-
classification of interventions;
-
deviations from intended interventions;
-
missing data;
-
measurement of outcomes;
-
selection of the reported result.
The first two domains, covering confounding and selection of participants into the study, addressed issues before the start of the interventions that were compared ('baseline'). The third domain addressed classification of the interventions themselves. The other four domains addressed issues arising after the start of interventions: biases due to deviations from intended interventions, missing data, measurement of outcomes and selection of the reported result (Sterne 2016).
The assessment of each NRSI included in the review involved following the six steps below. Steps 3 to 6 were repeated for each key outcome of interest: 1. specifying the research question through consideration of a target trial; 2. specifying the outcome and result being assessed; 3. for the specified result, examining how the confounders and cointerventions were addressed; 4. answering signalling questions for the seven bias domains; 5. formulating risk of bias judgements for each of the seven bias domains, informed by answers to the signalling questions; 6. formulating an overall judgement on risk of bias for the outcome and result being assessed (Sterne 2016).
Examination of confounders and cointerventions involves determining whether the important confounders and cointerventions were measured or administered in the study at hand, and whether additional confounders and cointerventions were identified (Sterne 2016). The following were the potential confounding variables: coexisting or history of breast or ovarian cancer, type of mutation, race, year of birth, parity, socioeconomic status, breastfeeding, oral contraceptive use, oestrogen therapy, ovarian stimulation and type of surgery (oophorectomy without concomitant salpingectomy or risk‐reducing mastectomy, or both). We assessed whether study authors had employed methods to control for selection bias at the design stage (e.g. matching or restriction to particular subgroups) and in their methods of analysis (e.g. the use of stratification or regression modelling). The focus was on whether a solution to a bias concern in a study was adequate.
The full tool with the signalling questions were addressed within each bias domain. The response options were: 'yes;' 'probably yes;' 'probably no;' 'no;' and 'no information.' Some questions were answered only if the response to a previous question was 'yes' or 'probably yes' (or 'no' or 'probably no'). Responses of 'yes' were intended to have similar implications to responses of 'probably yes' (and similarly for 'no' and 'probably no'), but allowed for a distinction between something that was known and something that was likely to be the case. Free text was used to provide support for each answer, using direct quotations from the text of the study where possible. Responses to signalling questions provided the basis for domain‐level judgements about risk of bias, which then provided the basis for an overall risk of bias judgement for a particular outcome (Sterne 2016).
The categories for risk of bias judgements were 'low risk,' 'moderate risk,' 'serious risk' and 'critical risk' of bias. Importantly, 'low risk' corresponded to the risk of bias in a high‐quality randomised trial (Sterne 2016).
Two review authors (GE and IE) independently applied the new ROBINS‐I 'Risk of bias' assessment tool and resolved any differences in opinion by discussion or by appeal to a third review author (AE). We summarised results in both a 'Risk of bias' table and a 'Risk of bias' summary. We interpreted the results of meta‐analyses in light of the findings with respect to risk of bias.
We listed the individual 'Risk of bias' items that we adapted for our review in Appendix 2. Table 1 depicts the interpretation of domain‐level and overall risk of bias judgements in ROBINS‐I (Sterne 2016).
Medidas del efecto del tratamiento
We used the following measures of the effect of treatment.
-
For time to event data, we used the HR, if possible.
-
For dichotomous outcomes, we used the RR.
-
For continuous outcomes, we used the MD between treatment arms.
Cuestiones relativas a la unidad de análisis
We did not anticipate unit of analysis issues.
Manejo de los datos faltantes
We did not impute missing outcome data for the primary or secondary outcomes. If data were missing or the included studies only reported imputed data, we contacted study authors to request data on the outcomes only among participants who were assessed.
Evaluación de la heterogeneidad
We assessed heterogeneity between studies by visual inspection of forest plots, by estimation of the percentage heterogeneity between studies that could not be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001), and, if possible, by subgroup analyses. If there was evidence of substantial heterogeneity, we investigated and reported the possible reasons for this.
Evaluación de los sesgos de notificación
When we suspected or there was direct evidence of selective outcome reporting, we asked the study authors for additional information. We examined funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small‐study effects, such as publication bias, if we identified a sufficient number of studies.
Síntesis de los datos
If sufficient, clinically similar studies were available, we pooled their results in meta‐analyses using Review Manager 2014 (RevMan 5).
-
For time‐to‐event data, we pooled HRs using the generic inverse variance facility of RevMan 5 (Review Manager 2014).
-
For any dichotomous outcomes, we calculated the RRs for each study and we then pool these values.
-
For continuous outcomes, we pooled the MDs between the treatment arms at the end of follow‐up if all studies measured the outcome on the same scale, otherwise we pooled standardised MD values.
We used the random‐effects model with inverse variance weighting for all meta‐analyses (DerSimonian 1986).
'Summary of findings' table
We assessed the certainty of evidence using the GRADE system, used GRADEpro software and presented the review results in ’Summary of findings’ tables. A 'Summary of findings' table consists of three parts: information about the review, a summary of the statistical results and the grade of the certainty of evidence (Appendix 3). Appendix 3 displays a draft 'Summary of findings' table, which were prepared to summarise the results of the meta‐analysis based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We presented the results of the meta‐analysis for the following outcomes as outlined in the Types of outcome measures section.
-
Overall survival.
-
HGSC mortality.
-
Breast cancer mortality.
-
Bone fracture incidence.
-
Quality of life.
-
Severe adverse events.
We presented the overall certainty of the evidence for each outcome according to the GRADE approach, which took into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity such as directness of results (Langendam 2013). The five factors were used to judge whether the quality of the collected evidence should be decreased if we were dealing with RCTs or increased if we were dealing with observational studies. We created a 'Summary of findings' table based on the methods described by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and using GRADEpro Guideline Development Tool (GDT) (GRADEpro GDT 2014). We used the GRADE checklist and GRADE Working Group certainty of evidence definitions (Meader 2014). We downgraded the evidence from 'high' certainty by one level for serious (or by two for very serious) limitations.
-
High‐certainty: we were very confident that the true effect lay close to that of the estimate of the effect.
-
Moderate‐certainty: we were moderately confident in the effect estimate: the true effect was likely to be close to the estimate of the effect, but there was a possibility that it was substantially different.
-
Low‐certainty: our confidence in the effect estimate was limited: the true effect may have been substantially different from the estimate of the effect.
-
Very low‐certainty: we had very little confidence in the effect estimate: the true effect was likely to be substantially different from the estimate of effect.
If meta‐analysis was not possible, we could have presented results in a narrative ‘Summary of findings’ table format, such as that used by Chan 2011.
Análisis de subgrupos e investigación de la heterogeneidad
We subgrouped by BRCA mutation (BRCA1, BRCA2 or both) and the type of surgery (RRSO alone versus RRSO and mastectomy, or RRSO and mastectomy versus no RRSO). When reported by any of the included studies, we considered factors such as age at RRSO, obesity, race, reproductive history, ovarian stimulation, menstrual history, use of the oral contraceptives, breastfeeding, oestrogens therapy, pelvic inflammatory disease, length of follow‐up and risk of bias status in our interpretation of any heterogeneity. We also considered women who were BRCA mutation carriers receiving bilateral prophylactic risk‐reducing oophorectomy without concomitant breast malignancy, with or without concomitant hysterectomy, and with or without concomitant mastectomy. Where possible, we assessed the difference between subgroups by interaction tests.
Análisis de sensibilidad
We could not perform sensitivity analyses for each type of effect measure, as there were insufficient numbers of studies as well as the fact that the overall survival and mortality outcomes (which were main outcomes reported) were analysed appropriately using HRs which took into account all points in time and allowed for censoring. Similarly, sensitivity analyses based on the risk of bias assessment, although planned, were not carried out because of the moderate risk of bias in all (except Kramer 2005 which was serious risk of bias) of included studies.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies tables.
Results of the search
The search identified 1395 bibliographic references, 1336 through database searching and 59 through other sources. We excluded 118 duplicates and screened the 1277 remaining references and excluded 1231 records as clearly irrelevant. We obtained full‐text articles of 46 records, and two review authors (GUE and IUE) independently assessed them for eligibility. After careful scrutiny, we excluded 36 references as they did not fulfil the inclusion criteria. We present reasons for exclusion in the Characteristics of excluded studies table. Subsequently, 10 references describing 10 non‐randomised prospective or retrospective cohort studies met the inclusion criteria for this systematic review (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Madalinska 2007; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004), and all but one study were included in the meta‐analysis (Madalinska 2007). We outlined the study selection in the PRISMA flow diagram shown in Figure 1.
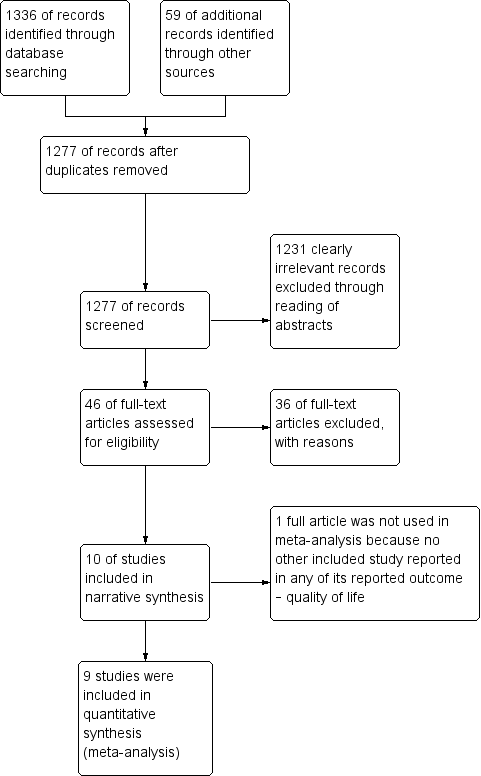
Study flow diagram for searches on risk‐reducing salpingo‐oophorectomy in women with BRCA1 or BRCA2 mutation carriers.
Included studies
Setting
The year of publication for the included studies ranged from 1999 to 2017 and all were published in English (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Madalinska 2007; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004).
The country of origin for the included studies were two USA (Kramer 2005; Rebbeck 1999), two Netherlands (Heemskerk‐Gerritsen 2015a; Madalinska 2007), one UK (Ingham 2013), and five multiple countries (Domchek 2006; Domchek 2010; Kotsopoulos 2017; Rebbeck 2002; Rebbeck 2004).
All 10 included studies had different settings:
Domchek 2006 took place in 13 US and European centres that comprised the PROSE (PRevention and Observation of Surgical Endpoints) consortium.
Domchek 2010 took place in 22 centres who were part of the PROSE consortium.
Heemskerk‐Gerritsen 2015a took place in the Netherlands as part of the Hereditary Breast and Ovarian Cancer in the Netherlands (HEBON) study. Data on participant and tumour characteristics and on preventive strategies were retrospectively as well as prospectively retrieved and updated through medical files and questionnaires, and through linkages to the Netherlands Cancer Registry and the Dutch Pathology Database.
Ingham 2013 study data were from the Genetic Medicine Database (Manchester Regional Genetics Service, UK), patient records and from records at the North West Cancer Intelligence Service (NWCIS), UK.
Kotsopoulos 2017 identified deleterious BRCA1 and BRCA2 mutation carriers from 78 participating centres in 12 countries worldwide.
Kramer 2005 took place at the National Cancer Institute, USA.
Madalinska 2007 was conducted at gynaecology departments of eight hospitals in the Netherlands that had a clinical genetics centre.
Rebbeck 1999 obtained study data from the registry databases of five institutions in USA, while Rebbeck 2002 and Rebbeck 2004 studies identified women from 11 North American and European registries.
Ethnicity
None of the studies reported ethnicity.
Inclusion criteria
Eight studies included women with either BRCA1 or BRCA2 mutations (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Madalinska 2007; Rebbeck 2002; Rebbeck 2004). Two studies included only women with BRCA1 mutations (Kramer 2005; Rebbeck 1999). None of the studies reported or recruited women with both BRCA1 and BRCA2 mutations or only BRCA2 mutations.
Domchek 2006 included women who had undergone RRSO and control participants who were cancer free (i.e. had never had a cancer diagnosis) at enrolment and did not have a cancer diagnosis within six months after enrolment and had not had any previous prophylactic surgery, including mastectomy and oophorectomy.
Heemskerk‐Gerritsen 2015a selected women with BRCA1 or BRCA2 mutation from the HEBON cohort: 1. no history of cancer at the date of DNA test result, 2. both breasts and ovaries in situ at the date of DNA test result and 3. no cancer diagnosis within the first six months of the study.
Ingham 2013 included women if they were alive at the date of family ascertainment (i.e. the date when all incident tumours in a family registry's surveillance population were captured in the registry's database) and did not have a diagnosis of breast or ovarian cancer (this translated to inclusion of a small number of women who had already undergone RRSO).
Kotsopoulos 2017 included women who consented and completed at least one follow‐up questionnaire on family information and personal history of cancer, and reproductive and medical histories, including preventive oophorectomy and mastectomy. However, women with unilateral oophorectomy were included in the no‐oophorectomy group.
Kramer 2005 eligibility criteria were: women; bloodline family member (siblings, parents, grandparents); no history of breast cancer before ascertainment; no history of bilateral mastectomy and under 20 years of age by the study closing date. A diagnosis of malignancy other than breast cancer did not affect eligibility.
Madalinska 2007 included women aged between 30 and 70 years and completed childbearing, hereditary breast/ovarian cancer (HBOC) in the family, and referral to the gynaecology clinic by a clinical geneticist specifically for the purpose of discussing the prevention of ovarian cancer.
Rebbeck 1999 included women if they had undergone bilateral oophorectomy prior to or at the time of enrolment or if they reported having had this procedure during follow‐up by the collaborating institutions. Surgical participants were also included if their surgery was not performed to treat ovarian or related peritoneal cancers. Potential control participants were eligible if they had the BRCA1 mutation, were alive and had both ovaries (i.e. no history of oophorectomy), had no history of breast or ovarian cancer, and had no history of prophylactic mastectomy at or before the time of the surgical participant’s surgery. Control participants were matched to surgical participants on year of birth (within five years) and on the collaborative institution from which they were ascertained.
Rebbeck 2002 selected one or more controls for inclusion if they could be matched to a participant who had undergone prophylactic oophorectomy according to type of mutation (BRCA1 or BRCA2 ), treatment centre and year of birth (within five years). The authors also included women to determine the risk of ovarian cancer only if their surgery was not performed to treat ovarian cancer, and a control participant was eligible if she had BRCA1 or BRCA2 mutation, was alive with both ovaries intact at the time the woman with whom she was matched underwent prophylactic oophorectomy and had no history of ovarian cancer at the time of the matched participant’s prophylactic oophorectomy.
Rebbeck 2004 included a subset of women from the total sample who had undergone bilateral prophylactic mastectomy but had not undergone bilateral prophylactic oophorectomy before this procedure. Control participants were eligible if they had not undergone bilateral prophylactic oophorectomy and were alive and cancer‐free with both breasts intact at the time of the matched participant’s bilateral prophylactic mastectomy. The analysis was performed on the subset of women who had not had bilateral prophylactic mastectomy at the time of their centre ascertainment and controls were excluded if they had a diagnosis of breast or ovarian cancer at or before the time of the matched surgical participant’s bilateral prophylactic mastectomy. Surgical participants and matched control participants were included regardless of their history of bilateral prophylactic oophorectomy and included 57 bilateral prophylactic mastectomy participants and 107 control participants.
Exclusion criteria
Madalinska 2007 excluded women with prior oophorectomy performed as treatment for breast cancer or for any pathology in the ovaries and metastatic cancer or any other severe comorbidity.
Domchek 2006 excluded women with BRCA1 or BRCA2 variants of unknown functional importance, and women who underwent bilateral prophylactic mastectomy – either before enrolment or during follow‐up period.
Domchek 2010 excluded women if they had a cancer diagnosis within the first six months of follow‐up to avoid including cancers that would have been minimally influenced by RRSO or RRM as well as women who had both BRCA1 and BRCA2 mutations, women who underwent RRSO before ascertainment date, or women diagnosed with ovarian cancer before ascertainment date, or women with diagnosis of cancer within the first six months of follow‐up, or incident cases.
Kotsopoulos 2017 excluded women with prior diagnosis of breast cancer, ovarian cancer, other cancers or completion of follow‐up questionnaire prior to receipt of their genetic test results.
Kramer 2005 excluded families with variants of uncertain significance.
Rebbeck 1999 excluded women if they had only unilateral oophorectomies, if they had undergone mastectomy prior to their oophorectomy, or if they had a personal history of breast or ovarian cancer at or before the time of their oophorectomy. Women with BRCA2 mutations carriers were excluded because of relatively small numbers of BRCA2 mutation carriers available in their study population and because the risk of breast and ovarian cancers (and possibly patterns of surgery use) may have differed from BRCA1 mutation carriers.
Rebbeck 2002 excluded women with BRCA1 or BRCA2 variants of unknown functional importance as well as women who had undergone unilateral oophorectomy or had a history of ovarian cancer (including borderline tumours or tumours of low malignant potential) before undergoing prophylactic oophorectomy risk, except that women who had undergone prophylactic oophorectomy were excluded if they had previously undergone mastectomy or had a history of breast cancer (including carcinoma in situ) at the time of the prophylactic oophorectomy. Control women were excluded if they had undergone prophylactic oophorectomy or had a history of breast cancer at the time of the matched participant’s prophylactic oophorectomy.
Rebbeck 2004 excluded women with BRCA1 or BRCA2 variants of unknown functional significance as well as study participants who had prior or concurrent breast cancer at time of surgery.
Heemskerk‐Gerritsen 2015a and Ingham 2013 presented no exclusion criteria.
Study design and methodology
The 10 articles included in this review were very heterogeneous. None of the studies was case series. Three studies included in this review were prospective cohort studies with matching design (Domchek 2006; Rebbeck 1999; Rebbeck 2004); one was a retrospective cohort study with matching design (Rebbeck 2002); five studies were prospective cohort studies with unmatching design (Domchek 2010; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Madalinska 2007), while one study was partly retrospective and partly prospective cohort study with matching design (Heemskerk‐Gerritsen 2015a). Control participants were matched to surgical participants on year of birth (within five years) (Domchek 2006; Rebbeck 1999), and on the collaborative institution from which they were ascertained in Rebbeck 1999 study. Although Kramer 2005 reported women who were both BRCA1‐positive and BRCA1‐negative mutation carriers, we used only the data of women who were BRCA1‐positive mutation carriers among women who had RRSO and no RRSO (surveillance).
Heemskerk‐Gerritsen 2015a replicated the analyses of four previous studies, performed by Domchek 2006; Domchek 2010; Eisen 2005; and Kauff 2008, within a Dutch cohort, first to examine if their study cohort was comparable with the cohorts used in the previous studies and second to estimate the effect of RRSO on breast cancer risk in the Dutch cohort using a specified design and analyses in order to minimise bias.
Nine studies used survival analysis (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004), and one study used a validated scale for quality of life assessment (Madalinska 2007). All the nine studies that used survival analysis reportedly used adjusted or corrected models (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004). Seven studies adjusted for age or date of birth (within five years) (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Rebbeck 2002); four studies adjusted for centre (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Rebbeck 2002); five studies adjusted for type of BRCA mutation (Domchek 2006; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Rebbeck 2002); three adjusted for age at menarche in order to account for duration of endogenous ovarian hormone exposure (Kotsopoulos 2017; Rebbeck 1999; Rebbeck 2004); two adjusted for oral contraceptive use (Domchek 2010; Kotsopoulos 2017); and one adjusted for family history of breast cancer, country of residence, parity, breastfeeding and oestrogen receptor status of the breast cancer (Kotsopoulos 2017).
The analytic techniques employed in the studies included Kaplan‐Meier survival analysis in two studies (Ingham 2013; Kramer 2005), and Cox proportional hazards regression in nine studies (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004). Madalinska 2007 assessed the overall quality of life using the single quality of life item of the EORTC Quality of Life Questionnaire. Time origin for survival analysis was generally the time of DNA testing, except in the case of treatment or surgical cohorts where the time of origin was the beginning of treatment or the date of surgery.
Heemskerk‐Gerritsen 2015a used the Simon and Makuch method for survival analysis (Simon 1984), with chronological age as the time variable. This method takes into account the change in an person’s covariate status over time per 1000 person‐years of observation. Variables that were considered as potential confounders were type of mutation, year of birth and centre but they did not meet the criteria for incorporation in a multivariable Cox model. They used a robust variance‐covariance estimation method to correct for non‐independence of observations in women from the same family. The study also performed sensitivity analyses to estimate the effect of RRSO on breast cancer risk in different settings. The study estimated breast cancer risk reduction after RRSO for participants who never underwent RRM in order to investigate the effect of excluding the breast cancer‐free time before RRM, and the authors explored the effect of RRSO on breast cancer risk when the time before RRSO was excluded from the analysis. The authors also examined the effect on breast cancer risk when RRSO was performed in women under the age of 51 years (mean age of postmenopausal status in the Netherlands), and at the age of 51 years and above.
Study population
Total number enrolled
The review included a total of 8087 women (2936 (36%) surgical participants and 5151 (64%) control participants). The number of women in the included studies were 155 surgical participants and 271 control participants (Domchek 2006), 465 surgical participants and 1092 control participants (Domchek 2010), 146 surgical participants and 576 control participants (Heemskerk‐Gerritsen 2015a), 108 surgical participants (RRSO or bilateral prophylactic mastectomy (or both) participants) and 457 control participants (Ingham 2013), 1552 surgical participants and 2170 control participants (Kotsopoulos 2017), 33 surgical participants and 65 control participants (Kramer 2005), 118 surgical participants and 42 control participants (Madalinska 2007), 43 surgical participants and 79 control participants (Rebbeck 1999), 259 surgical participants and 292 control participants (Rebbeck 2002), 57 surgical participants (bilateral prophylactic mastectomy participants plus RRSO) and 107 control participants (Rebbeck 2004),
Participant characteristics (e.g. BRCA1, BRCA2 or both)
All participants included were either BRCA1 or BRCA2 mutation carriers. Participants with both BRCA1 and BRCA2 mutation status were either not reported or excluded. Domchek 2010 excluded 12 participants because they had both BRCA1 and BRCA2 mutations. Heemskerk‐Gerritsen 2015a reported 46 BRCA1 and 100 BRCA2 mutation carriers in RRSO participants. Ingham 2013 reported 56 BRCA1 and 52 BRCA2 mutation carriers in RRSO participants and 219 BRCA1 and 238 BRCA2 mutation carriers in control participants. Kotsopoulos 2017 reported 1187 BRCA1 and 355 BRCA2 mutation carriers in RRSO participants and 1782 BRCA1 and 370 BRCA2 mutation carriers in control participants. Kramer 2005 reported only 98 BRCA1 mutation carriers while Rebbeck 1999 reported 122 BRCA1 mutation carriers.
Age
Reporting of age varied widely across studies. The mean ages were 39.4 (range: 22 to 63) years in surgical participants and 35.3 (range: 17 to 65) years in control participants (Rebbeck 1999), 44.8 (SD 8.5) years in surgical participants and 42.6 (SD 10.0) years in control participants (Domchek 2006), 43.2 (range: 20.5 to 79.0) years in surgical participants and 36.7 (range: 18.1 to 90.4) years in control participants (Domchek 2010), 44 years in surgical participants and 33 years in control participants (Heemskerk‐Gerritsen 2015a), 46.2 (SD 6.35) (range: 21 to 88) years in surgical participants and 33.4 (SD 5.45) (range: 13 to 85) years in control participants (Kotsopoulos 2017), 48.3 (SD 8.4) years in surgical participants and 45.3 (SD 8.1) years in control participants (Madalinska 2007), 42.0 (range: 21.2 to 74.8) years in surgical participants and 40.9 (range: 19.6 to 79.1) years in control participants (Rebbeck 2002).
Ingham 2013 did not report the mean age of participants in either the surgical and control groups, but they only reported median age of ascertainment of 34.4 (range: 2 to 87) years for BRCA1 mutation carriers and 37.4 (range: 5 to 85) years for BRCA2 mutation carriers. Kramer 2005 did not report the mean or median age of the participants.
Comorbidities
Only Madalinska 2007 reported comorbidities, which were asthma and other chronic respiratory diseases; and cardiovascular, renal, rheumatic diseases, hypertension and diabetes.
Other baseline characteristics
Baseline characteristics of the women were not comparable between the two groups in all studies.
Intervention details
-
Type of surgery
Three studies performed bilateral oophorectomy (Kotsopoulos 2017; Kramer 2005; Rebbeck 2002), while in other seven studies performed bilateral salpingo‐oophorectomy (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Madalinska 2007; Rebbeck 1999; Rebbeck 2004).
Four trials performed concurrent risk‐reducing mastectomy (Domchek 2010; Ingham 2013; Madalinska 2007; Rebbeck 2004), four studies excluded concurrent risk‐reducing mastectomy (Domchek 2006; Kotsopoulos 2017; Kramer 2005; Rebbeck 2002), and three trials did not report concurrent risk‐reducing mastectomy (Heemskerk‐Gerritsen 2015a; Rebbeck 1999).
None of the trials reported concurrent hysterectomy.
-
Route of surgery
Only Ingham 2013 described the route of surgery, which used both laparoscopy and open surgical techniques.
-
Occult cancer
Only two studies reported occult cancers (cancer of unknown primary origin) (Domchek 2010; Ingham 2013). Domchek 2010 reported nine cases of occult cancers (seven cases in BRCA1 mutation carriers and two in BRCA2 mutation carriers), but women were excluded if they were diagnosed with an occult ovarian cancer at RRSO. Ingham 2013 reported six cases of occult cancer (three in BRCA1 and three in BRCA2 mutation carriers).
-
Type of screening test
All studies confirmed the BRCA1/2 mutation status of all participants by direct mutation or DNA testing.
-
Period of screening test
None of the studies reported the period of screening, except that the screening for BRCA1 or BRCA2 mutation status was prior to enrolment in all the included studies.
-
Type of chemoprevention
None of the studies reported on type of chemoprevention.
-
Dose of chemoprevention
None of the studies stated the dose of chemoprevention except that Ingham 2013 reported that chemoprevention agents such as tamoxifen and raloxifene could not be used in any of the recruited participants because they were not licensed for chemoprevention in the UK and most European countries.
-
Course of chemoprevention
None of the studies reported the course of chemoprevention.
-
Type of histology protocol adopted
None of the studies reported the type of histology protocol adopted (e.g. the SEE‐FIM protocol) as documented in Blok 2016 and Mahe 2013.
-
Use of peritoneal washing cytology
None of the studies reported the use of peritoneal washing cytology.
-
Use of oral contraceptives
Only Domchek 2006 reported the use of oral contraceptives. Domchek 2006; Kotsopoulos 2017; and Rebbeck 2002 also reported on HRT.
Duration of follow‐up
The reporting follow‐up time varied widely across studies. Postsurgery, follow‐up duration was 3.1 (SD 2.4) years in surgical participants and 2.1 (SD 2.0) years in controls participants (Domchek 2006), 6.8 years in surgical participants and 3.1 years in control participants (Heemskerk‐Gerritsen 2015a), 5.6 years (Kotsopoulos 2017), 35 years (Kramer 2005); 1 year in both surgical and control participants (Madalinska 2007), 9.6 years in surgical participants and 8.1 years in control participants (Rebbeck 1999), 5.5 years in surgical participants and 6.7 years in control participants (Rebbeck 2004).
Domchek 2010 and Ingham 2013 did not report the mean duration of follow‐up. Domchek 2010 reported the median duration was 3.65 (range: 0.52 to 27.4) years in participants who underwent RRSO and 4.29 (range: 0.5 to 27.9) years in control participants who did not undergo surgery. Ingham 2013 reported the median duration of follow‐up (from ascertainment to death or loss to follow‐up) was 13.3 years.
Studies followed up surgical and control participants from the date of the participant's RRSO (Rebbeck 2002), or date of ascertainment (i.e. date of genetic testing or date of baseline questionnaire, whichever was later) (Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017), or time of centre ascertainment to censoring or death due to: any cause, breast or HGSC (Domchek 2006; Ingham 2013; Kramer 2005). Domchek 2010 followed surgical participants from date of RRSO or RRM and non‐surgical participants or controls from date of ascertainment.
Outcome
-
Three studies assessed and reported overall survival until death from all causes (Domchek 2006; Domchek 2010; Ingham 2013).
-
Three studies assessed and reported HGSC mortality (Domchek 2006; Domchek 2010; Rebbeck 2002).
-
Seven studies assessed and reported breast cancer mortality (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002).
-
Four studies assessed and reported HGSC incidence (Domchek 2006; Heemskerk‐Gerritsen 2015a; Ingham 2013; Rebbeck 2002). (All cases of serous peritoneal cancer diagnosed after prophylactic salpingo‐oophorectomy were considered primary peritoneal cancer.)
-
Six studies assessed and reported breast cancer incidence (Domchek 2006; Heemskerk‐Gerritsen 2015a; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004).
-
One study evaluated and reported on quality of life (Madalinska 2007), measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication (Roila 2001; Spitzer 1981).
Excluded studies
We excluded 36 studies (Benshushan 2009; Chang‐Claude 2007; Eisen 2005; Evans 2009; Evans 2013; Finch 2006; Finch 2009; Finch 2011; Finch 2013; Finch 2014; Finkelman 2012; Heemskerk‐Gerritsen 2013; Heemskerk‐Gerritsen 2015b; Hunsinger 2016; Iavazzo 2016; Johansen 2016; Johansen 2017; Kauff 2002; Kauff 2008; Kwon 2013; Laki 2007; Madalinska 2005; Manchanda 2011; Meijers‐Heijboer 2001; Menkiszak 2016; Metcalfe 2014; Miller 2017; Perabo 2014; Powell 2011; Rocca 2006; Rutter 2003; Schmeler 2006; Skytte 2011; Struewing 1995; van Sprundel 2005; Vermeulen 2017).
-
Seven studies included women with a previous or coexisting breast malignancy (Chang‐Claude 2007; Eisen 2005; Finch 2006; Finkelman 2012; Kauff 2002; Kauff 2008; Schmeler 2006).
-
Four studies did not report the BRCA1 or BRCA2 mutations status of the participants (study group or controls), even though the participants consisted of two groups either receiving RRSO or no RRSO (Benshushan 2009; Johansen 2016; Johansen 2017; Rocca 2006).
-
Four studies included some women with unknown BRCA1 or BRCA2 mutation carrier status (Evans 2009; Madalinska 2005; Rutter 2003; Struewing 1995).
-
Three studies were controlled before‐and‐after studies (Finch 2009; Finch 2011; Finch 2013).
-
Eight studies were single arm studies without comparison groups (Finch 2014; Hunsinger 2016; Laki 2007; Manchanda 2011; Menkiszak 2016; Miller 2017; Perabo 2014; Powell 2011).
-
Seven studies included women with or without a family history or personal history of breast cancer who were carriers of BRCA1 and BRCA2 mutations and initially treated with unilateral or bilateral mastectomy, but without bilateral RRSO (Evans 2013; Heemskerk‐Gerritsen 2013; Heemskerk‐Gerritsen 2015b; Meijers‐Heijboer 2001; Metcalfe 2014; Skytte 2011; van Sprundel 2005).
-
One study had two comparison groups that received surgical interventions (Kwon 2013).
-
Two studies were review articles (Iavazzo 2016; Vermeulen 2017).
None of the studies was excluded because they used unadjusted analysis.
We did not identify any ongoing studies.
Risk of bias in included studies
Risk of bias of included studies is presented in Table 2. No studies were at low risk for bias, primarily because none of the studies was an RCT.
| Study ID | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias |
| Low | Low | Low | Moderate | |
| Support for judgement | Quote: "Questionnaires were administered at every centre and were self‐administered or completed with the help of clinical‐research staff." All missing data were analysed (intention‐to‐treat). | Quote: "For our primary analysis, we undertook a matched design that selected controls who had not undergone BPSO at any time during follow‐up, and who were matched within 5 years of age to the corresponding one.” | Quote: "Follow‐up data for BPSO, cancer diagnoses, and deaths were verified by review of medical records, and surgical notes, pathology reports, or both." | At least 1 of the domain was moderate. |
| Moderate | Low | Low | Moderate | |
| Support for judgement | Quote: "When missing data were encountered, the individual was dropped from the analysis that involved the missing data point, but the individual was included in other analyses where complete data were available; in fact, because many of the data items were required for enrolment missing data was only applicable to ovarian cancer endpoints, with missing OCP data." | Quote: "For BC endpoints, women were excluded if they underwent RRM prior to ascertainment. Women who had RRM after ascertainment but before RRSO were considered unexposed and were censored at RRM. Women were followed until BC or were censored at OC, RRM, death, or last contact." | Quote: "A robust variance‐covariance estimation method was used to correct for non‐independence of observations among participants from the same family or within centers... Adjustment for year of birth was undertaken in all analyses using Cox regression. Oral contraceptive use was adjusted for when OC was the outcome. Adjustment for center of ascertainment was undertaken by stratifying analyses by center to avoid imposing linear constraints in the model." | At least 1 of the domain was moderate. |
| Moderate | Low | Low | Moderate | |
| Support for judgement | Quote: "Eventually, parity was not considered as a potential confounder because of the large proportion (41.0%) of missing data on this variable." | Quote: "We performed sensitivity analyses to estimate the effect of RRSO on BC risk in different settings. First, to investigate the effect of excluding the BC‐free time before RRM, we estimated BC risk reduction after RRSO for participants who never underwent RRM." | Quote: "We adjusted our analyses for differences in age by using chronological age as the time variable." | At least 1 of the domain was moderate. |
| Low | Moderate | Low | Moderate | |
| Support for judgement | Quote: "Women were censored at either date of last follow‐up (date of last contact with the genetics department or other NHS service) or date of death (obtained from NWCIS or death certification).” No evidence of missing data. | Quote: "Date of breast cancer was confirmed in the family files or from records at the North West Cancer Intelligence Service (NWCIS)." Also, possible testing bias of women who developed cancer was made. | Quote: "The proportional hazards assumption was checked in all analyses by looking at log–log plots and Schoenfeld residuals." | At least 1 of the domain was moderate. |
| Moderate | Low | Moderate | Moderate | |
| Support for judgement | Quote: "Women with both a BRCA1 and BRCA2 mutation were coded as missing." | Quote: "We also performed analyses stratified by BRCA mutation type, estrogen receptor status of the tumor, and excluding women with an oophorectomy at or prior to the baseline questionnaire, as well as analyses censoring at different ages." | Quote: "this finding was based on a post hoc analysis." | At least 1 of the domain was moderate. |
| Low | Moderate | Low | Serious | |
| Support for judgement | There was no evidence of missing data. | Quote: "A competing risks model (with death as the competing risk) was then used to estimate the 10‐year cumulative incidence of breast cancer in the two groups of BRCA1 mutation carriers (ie, those with and without ovaries)." | Quote: "To provide estimates of the absolute risk of breast cancer by age in mutation carriers, landmark analyses were performed in which oophorectomy was treated as a time‐fixed covariate, as defined at the beginning of a given age interval. Follow‐up time was divided into 10‐year intervals, with mutation carriers divided into two groups based on oophorectomy status at the beginning of that interval (and conditional on the participant being alive and breast cancer free at that time)." | At least 1 of the domain was serious. |
| Low | Low | Low | Moderate | |
| Support for judgement | Quote: "These records were complete, and in cases where there was any uncertainty, contact was sought with the responsible gynecologist." "Non respondents did not differ significantly from respondents regarding age or choice of preventive measure." | Quote: "All raw scale scores were linearly converted to a 0 to100 scale, with higher scores indicating better perceived health, mental health, and quality of life. The internal consistency reliability of the two Short Form‐36 scales was high (α = 0.81 and 0.85)." | Quote: "Because of restrictions by the medical ethics committees, no other clinical data on the nonrespondents were available (eg, DNA status)." | At least 1 of the domain was moderate. |
| Low | Low | Moderate | Moderate | |
| Support for judgement | Quote: "However, only BRCA1 mutation carriers were studied here, and no OCCR region has been identified in BRCA1." No evidence of missing data. | Quote: "Because most women were followed only until the time of censoring or until the diagnosis of breast cancer, the incidences reported here do not represent lifetime breast cancer risks in BRCA1 mutation carriers." | Quote: "Furthermore, the inferences from both the robust and nonrobust analyses were identical. Therefore, only the standard model results are presented." | At least 1 of the domain was moderate. |
| Moderate | Low | Low | Moderate | |
| Support for judgement | Quote: "Bias that arises when later follow‐up is missing for individuals initially included and followed." | Quote: "on vital status and the occurrence of cancer was obtained from medical records, telephone interviews, self‐administered questionnaires, or a combination of these. For women who had died since their entry into the study, we reviewed medical records and family‐history reports to establish the presence or absence of cancer and to verify that they had died." | Quote: "For women who had died since their entry into the study, we reviewed medical records and family‐history reports to establish the presence or absence of cancer and to verify that they had died." | At least 1 of the domain is moderate. |
| Moderate | Low | Low | Moderate | |
| Support for judgement | Quote: "Percentages calculated using nonmissing data." | Quote: "Survival analyses were adjusted to account for duration of endogenous ovarian hormone exposure as measured by the time from age at menarche to age at bilateral prophylactic | Quote: "Subjects were censored at the date they developed ovarian cancer, or died, or at the date of last contact. Diagnosis of invasive breast cancer or ductal carcinoma‐in‐situ was considered the primary event of interest." | At least 1 of the domain is moderate. |
BC: Breast Cancer; BPSO: Bilateral prophylactic salpingo‐oophorectomy; BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; NHS: National Health Service; NWCI: North West Cancer Intelligence Service; SOC: Site of Care; OCP: Oral Contraceptive Pill; ROBIS‐I: Risk Of Bias In Non‐randomised Studies‐of Interventions; RRM: risk‐reducing bilateral mastectomy; RRSO: risk‐reducing bilateral salpingo‐oophorectomy.
Bias due to confounding
In all cohort studies included, women in the non‐surgical or surveillance group were drawn from the same population as the surgical cohort. Additionally, all eligible studies containing the population cohort excluded women who had a history of ovarian and breast cancer at the beginning of follow‐up. Seven studies had low risk of bias because the studies excluded women with prior oophorectomy performed as treatment for breast cancer or for any pathology in the ovaries and metastatic cancer or any other severe comorbidity (Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Madalinska 2007; Rebbeck 2002; Rebbeck 2004). Two studies had moderate risk of bias because although potential confounders of BRCA1 versus BRCA2 mutation, age, and centre were adjusted for in a multivariate Cox‐regression model in Domchek 2006, not all analyses were stratified by centre, while Rebbeck 1999 had bias of confounding by indication and familial‐event bias. One study had serious risk of bias due to confounding because the authors stated that the diagnosis of malignancy other than breast cancer did not affect eligibility for their analysis (Kramer 2005) (see Table 2).
Bias in selection of participants into the study
Five studies selected population cohorts matched for age and centre (Domchek 2006; Heemskerk‐Gerritsen 2015a; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004). The remaining five cohort studies constituted women with unmatched design (enrolment of controls without regard to the number or characteristics of the cases) (Domchek 2010; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Madalinska 2007). All studies confirmed mutation status by genetic testing. Five studies had low risk of bias because the cohorts constituted women with matched design (Domchek 2006; Heemskerk‐Gerritsen 2015a; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004), and five studies had moderate risk of bias due to selection of participants because the cohorts constituted women with unmatched design (Domchek 2010; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Madalinska 2007) (Table 2)
Bias in classification of interventions
Eight studies had low risk of bias because the criteria for entry, data collection and follow‐up were undertaken at each collaborating centre without regard to surgical status (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004), and two studies had moderate risk of bias due to classification of interventions because the authors stated that women with unilateral oophorectomy were included in the no‐oophorectomy group (Kotsopoulos 2017), or the authors stated that the study was not exclusive of a larger prospective investigation focusing on the psychosocial impact of ovarian cancer prevention (Madalinska 2007) (Table 2).
Bias due to deviations from intended interventions
Eight studies had low risk of bias because the criteria for choosing participants and matched or unmatched controls for the group studied to determine breast‐cancer risk were identical to those for the group studied to determine ovarian‐cancer in those studies (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kramer 2005; Madalinska 2007; Rebbeck 2002; Rebbeck 2004). Two studies had moderate risk of bias due to deviations from intended interventions because in Kotsopoulos 2017 study, bilateral oophorectomy was coded as a time‐dependent variable but If a woman had a bilateral oophorectomy after the completion of the baseline questionnaire (or at any point in the follow‐up), the exposure of interest was changed while in Rebbeck 1999 study, the authors excluded women with BRCA2 mutations because of relatively small numbers BRCA2 available during the study and because the authors perceived that the risk of breast and ovarian cancers (and possibly patterns of surgery use) in BRCA2 might differ from BRCA1 mutation carriers (Table 2).
Bias due to missing data
Five studies had low risk of bias as either there was no evidence of missing data or all missing data were analysed in an intention‐to‐treat basis (Domchek 2006; Ingham 2013; Kramer 2005; Madalinska 2007; Rebbeck 1999). Five studies had moderate risk of bias due to missing data because the authors of these studies reported the existence of bias that arose when later follow‐up is missing for participants initially included and followed up in their studies (Domchek 2010; Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Rebbeck 2002; Rebbeck 2004) (Table 2).
Bias in measurement of outcomes
Eight studies had low risk of bias because there was no evidence of bias in the measurement of any of the outcomes assessed in these studies (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Madalinska 2007; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004). Two studies had moderate risk of bias in measurement of outcomes because the authors reported that there were possible testing bias of women who developed cancer (Ingham 2013), or a competing risks model (instead of actual report) was used to estimate the 10‐year cumulative incidence of breast cancer in the two groups of BRCA1 mutation carriers (Kramer 2005) (Table 2).
Bias in selection of the reported result
Eight studies had low risk of bias because the medical records and family‐history reports were verified to establish the presence or absence of cancer or deaths or other outcomes (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kramer 2005; Madalinska 2007; Rebbeck 2002; Rebbeck 2004). Two studies had moderate risk of bias in selection of the reported result because the authors either reported some of their findings based on a post hoc analysis (Kotsopoulos 2017), or reported and presented only standard model results (Rebbeck 1999) (Table 2).
Effects of interventions
See: Summary of findings for the main comparison Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers; Summary of findings 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status; Summary of findings 3 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery; Summary of findings 4 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 mutation carriers according to age at RRSO; Summary of findings 5 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA2 mutation carriers according to age at RRSO
All meta‐analyses pooled data from at least two of these nine studies (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004). Meta‐analyses of survival were based on HRs that were adjusted for prognostic variables.
1. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers
Primary outcomes
Overall survival: survival until death from all causes
We assessed survival from the time when women were enrolled in the study. Meta‐analysis of three studies assessing 2548 participants showed very low‐certainty evidence and found that there may be an increase in the overall survival among women who were BRCA1 or BRCA2 mutation carriers who had RRSO compared to women with no RRSO, after adjustment for important prognostic factors including age and BRCA mutation status (HR 0.32, 95% CI 0.19 to 0.54; P < 0.001; Analysis 1.1; summary of findings Table for the main comparison) (Domchek 2006; Domchek 2010; Ingham 2013). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was not important (I2 = 0%).
High‐grade serous cancer mortality
Meta‐analysis of three studies assessing 2534 participants showed very low‐certainty evidence and found a difference in favour of RRSO versus no RRSO in HGSC (fallopian tube, ovarian and primary peritoneal cancer) mortality, after adjustment for important prognostic factors including age and BRCA mutation status (HR 0.06, 95% CI 0.02 to 0.17; P < 0.0001; Analysis 1.2; summary of findings Table for the main comparison) (Domchek 2006; Domchek 2010; Rebbeck 2002). The percentage of the variability in effect estimates that was due to heterogeneity rather than to chance may have represented substantial heterogeneity (I² = 69%).
Breast cancer mortality
Meta‐analysis of seven studies assessing 7198 participants showed very low‐certainty evidence and found a difference in favour of RRSO versus no RRSO in breast cancer mortality, after adjustment for important prognostic factors including age and BRCA mutation status (HR 0.58, 95% CI 0.39 to 0.88; P = 0.009; Analysis 1.3; summary of findings Table for the main comparison) (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002). The percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance) may have represented substantial heterogeneity (I² = 65%).
Secondary outcomes
High‐grade serous cancer incidence
Four studies with 1269 participants in the RRSO groups and 2059 participants in the control groups reported HGSC incidence in women who were BRCA1 or BRCA2 mutation carriers (Domchek 2006; Heemskerk‐Gerritsen 2015a; Ingham 2013; Rebbeck 2002). A total of 14/1269 (1%) participants with RRSO versus 194/2059 (9%) participants with no RRSO developed HGSC. The meta‐analysis showed very low‐certainty evidence that RRSO versus no RRSO may have reduced HGSC incidence (RR 0.17, 95% CI 0.04 to 0.75; P = 0.02; Analysis 1.4). The percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) may have represented substantial heterogeneity (I² = 84%).
Breast cancer incidence
Seven studies with 2285 participants in the RRSO groups and 3310 participants in the control groups reported breast cancer incidence in women with BRCA1 or BRCA2 mutations (Domchek 2006; Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004). A total of 230/2285 (10%) participants who had had RRSO versus 429/3310 (13%) participants with no RRSO developed breast cancer. The meta‐analysis showed very low‐certainty evidence in favour of RRSO versus no RRSO in reducing breast cancer incidence (RR 0.64, 95% CI 0.43 to 0.96; P = 0.03; Analysis 1.5). The percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) may represent substantial heterogeneity (I² = 75%).
Bone fracture incidence
None of the studies reported bone fracture incidence.
Disease‐free survival (time from surgical procedure to cancer diagnosis)
None of the studies reported disease‐free survival.
Morbidity: direct surgical morbidity and surgically related systemic morbidity
None of the studies reported on direct surgical morbidity or surgically related systemic morbidity (e.g. chest/wound/urine infection, venous thromboembolism, premature menopause, etc.).
Recovery and readmission
None of the studies reported on recovery or readmission.
Quality of life
Quality of life measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication (Roila 2001; Spitzer 1981). Only the Madalinska 2007 study assessed quality of life as an outcome measure and, therefore, no meta‐analysis was performed. Data from one study showed that women who had had RRSO experienced more quality of life disruption, which was higher, when compared to those who did not have RRSO in terms of general health perception (MD (SD): 70.9 (SD 20.5) with RRSO versus 82.0 (SD 13.3) with no RRSO; P < 0.0001; Analysis 1.8), but it was not different in terms of global health status quality of life (MD (SD): 76.0 (SD 20.6) with RRSO versus 79.8 (SD 17.9) with no RRSO; P = 0.26; Analysis 1.7) and mental health quality of life (MD (SD): 70.2 (SD 16.6) with RRSO versus 73.1 (SD 14.5) with no RRSO; P = 0.28; Analysis 1.9). However, there was a difference in favour of RRSO compared with no RRSO in terms of ovarian cancer risk perception quality of life (MD 15.40, 95% CI 8.76 to 22.04; P < 0.00001; very low‐certainty evidence; Analysis 1.6), and breast cancer risk perception quality of life (MD 8.20, 95% CI 0.85 to 15.55; P = 0.03; very low‐certainty evidence; Analysis 1.10).
Severe adverse events, classified according to CTCAE 2010
None of the studies reported on severe adverse events.
2. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status: subgroup analyses
Primary outcomes
Overall survival: survival until death from all causes
Three studies reported overall survival among participants with BRCA1 mutations (Domchek 2006; Domchek 2010; Ingham 2013). Meta‐analysis showed a lower risk of death among women with BRCA1 mutations who had had RRSO than in women who had not had RRSO (HR 0.30, 95% CI 0.17 to 0.52; P < 0001; very low‐certainty evidence; Analysis 2.1; summary of findings Table 2). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was not important (I² = 23%).
Two studies reported overall survival among participants who were BRCA2 mutation carriers (Domchek 2010; Ingham 2013). Meta‐analysis showed a lower risk of death among women who were BRCA2 mutation carriers who received RRSO than in women with no RRSO (HR 0.44, 95% CI 0.23 to 0.85; P = 0.01; very low‐certainty evidence; Analysis 2.1; summary of findings Table 2). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was not important (I² = 0%).
Tests for subgroup differences showed no significant difference in effect between studies assessing RRSO versus no RRSO on overall survival according to the mutation status: BRCA1 and BRCA2 (P = 0.38).
High‐grade serous cancer mortality
Two studies reported HGSC deaths among participants who were BRCA1 mutation carriers (Domchek 2006; Domchek 2010). Meta‐analysis from the two studies showed a difference in favour of RRSO than no RRSO for HGSC deaths among women who were BRCA1 mutation carriers (HR 0.10, 95% CI 0.02 to 0.41; P = 0.001; very low‐certainty evidence; Analysis 2.2; summary of findings Table 2). The percentage of the variability in effect estimates that was due to heterogeneity rather than to chance may have represented moderate heterogeneity (I² = 54%).
Two studies reported HGSC deaths among participants who were BRCA2 mutation carriers (Domchek 2006; Domchek 2010). Data from two studies showed no HGSC deaths among women who were BRCA2 mutation carriers who received RRSO versus no RRSO (HR not applicable; I² = not applicable; Analysis 2.2). No tests for subgroup differences could be performed as HGSC mortality was only reported in women who were BRCA1 only mutation carriers with no reported event in BRCA2 mutation carriers.
Breast cancer mortality
Four studies reported breast cancer mortality among participants who were BRCA1 mutation carriers (Domchek 2006; Domchek 2010; Kramer 2005, Rebbeck 1999). Meta‐analysis from the four studies showed a difference in favour of RRSO compared with no RRSO for breast cancer mortality among women who were BRCA1 only mutation carriers (HR 0.45, 95% CI 0.30 to 0.67; P < 0.0001; very low‐certainty evidence; Analysis 2.3). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was not important (I² = 0%).
Three studies reported breast cancer mortality among participants who were BRCA2 mutation carriers (Domchek 2010; Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017). Meta‐analysis from the two studies showed no difference in favour of RRSO compared with no RRSO for breast cancer mortality among women who were BRCA2 mutation carriers (HR 0.88, 95% CI 0.42 to 1.87; P = 0.75; very low‐certainty evidence; Analysis 2.3; summary of findings Table 2) (Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017). Data from one study showed no breast cancer deaths among women who were BRCA2 mutation carriers who received RRSO versus no RRSO (HR not applicable) (Domchek 2010). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance may represent substantial heterogeneity (I² = 63%).
Tests for subgroup differences showed no difference in effect between studies assessing RRSO versus no RRSO on breast cancer mortality according to the mutation status: BRCA1 and BRCA2 (P = 0.12).
Secondary outcomes
High‐grade serous cancer incidence
None of the studies reported HGSC incidence according to BRCA mutation carrier status: BRCA1 and BRCA2.
Breast cancer incidence
None of the studies reported breast cancer incidence according to BRCA mutation carrier status: BRCA1 and BRCA2.
Bone fracture incidence
None of the studies reported bone fracture incidence according to BRCA mutation carrier status: BRCA1 and BRCA2.
Disease‐free survival (time from surgical procedure to cancer diagnosis)
None of the studies reported disease‐free survival according to BRCA mutation carrier status: BRCA1 and BRCA2.
Morbidity: direct surgical morbidity and surgically related systemic morbidity
None of the studies reported morbidity according to BRCA mutation carrier status: BRCA1 and BRCA2.
Recovery and readmission
None of the studies reported recovery and readmission according to BRCA mutation carrier status: BRCA1 and BRCA2.
Quality of life (ovarian cancer risk perception)
One study with 58 participants in the RRSO groups and 40 participants in the control groups reported quality of life for ovarian cancer risk perception in women who were BRCA1 mutation carriers (Madalinska 2007). Data from one study showed a difference in favour of RRSO compared with no RRSO in improving the quality of life for ovarian cancer risk perception in women who were BRCA1 mutation carriers (MD 10.70, 95% CI 2.45 to 18.95; P = 0.01; very low‐certainty of evidence; Analysis 2.4).
One study with 42 participants in the RRSO groups and 60 participants in the control groups reported quality of life for ovarian cancer risk perception in women who were BRCA2 mutation carriers (Madalinska 2007). Data from one study showed a difference in favour of RRSO compared with no RRSO in improving the quality of life for ovarian cancer risk perception in women who were BRCA2 mutation carriers (MD 13.00, 95% CI 3.59 to 22.41; P = 0.007; very low‐certainty of evidence; Analysis 2.4). Tests for subgroup differences showed no significant difference in effect between studies comparing RRSO versus no RRSO on quality of life for ovarian cancer risk perception according to the mutation status: BRCA1 and BRCA2 (P = 0.72).
Severe adverse events, classified according to CTCAE 2010
None of the studies reported on severe adverse events according to BRCA mutation status.
3. BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery: subgroup analyses
Primary outcomes
Overall survival: survival until death from all causes
RRSO alone versus RRSO and risk‐reducing mastectomy (RRM)
None of the studies reported overall survival among participants who were BRCA1 or BRCA2 mutation carriers who received RRSO alone versus RRSO and RRM.
RRSO and RRM versus no RRSO
One study reported overall survival among participants with BRCA1 or BRCA2 mutations who received RRSO and mastectomy versus no RRSO (Ingham 2013). Data from one study showed an increase in overall survival among women who were BRCA1 or BRCA2 mutation carriers who received RRSO and RRM compared to women with no RRSO (HR 0.14, 95% CI 0.02 to 0.98; P = 0.0001; very low‐certainty evidence; Analysis 3.1; summary of findings Table 3).
Since only subgroup analysis among participants with BRCA1 or BRCA2 mutations who received RRSO and mastectomy versus no RRSO was possible for overall survival, tests for subgroup differences could not be performed for this outcome.
High‐grade serous cancer mortality
None of the studies reported HGSC mortality among participants who were BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery.
Breast cancer mortality
RRSO alone versus RRSO and risk‐reducing mastectomy (RRM)
None of the studies reported breast cancer mortality among participants who were BRCA1 or BRCA2 mutation carriers who received RRSO alone versus RRSO and RRM.
RRSO and RRM versus no RRSO
One study reported cancer mortality among participants who received bilateral salpingo‐oophorectomy and bilateral mastectomy (Heemskerk‐Gerritsen 2015a). There was no difference between bilateral salpingo‐oophorectomy plus bilateral mastectomy and no RRSO for breast cancer mortality among women who received bilateral salpingo‐oophorectomy plus bilateral mastectomy (HR 0.78, 95% CI 0.51 to 1.19; P = 0.25; very low‐certainty evidence; Analysis 3.2; summary of findings Table 3). Since only one subgroup analysis was possible for breast cancer mortality, tests for subgroup differences could not be performed for this outcome.
Secondary outcomes
High‐grade serous cancer incidence
None of the studies reported on HGSC incidence according to type of risk‐reducing surgery.
Breast cancer incidence
None of the studies reported on breast cancer incidence according to type of risk‐reducing surgery.
Bone fracture incidence
None of the studies reported on bone fracture incidence according to type of risk‐reducing surgery.
Disease‐free survival (time from surgical procedure to cancer diagnosis)
None of the studies reported on disease‐free survival according to type of risk‐reducing surgery.
Morbidity: direct surgical morbidity and surgically related systemic morbidity
None of the studies reported on morbidity according to type of risk‐reducing surgery.
Recovery and readmission
None of the studies reported on recovery and readmission according to type of risk‐reducing surgery.
Quality of life
None of the studies reported on quality of life according to type of risk‐reducing surgery.
Severe adverse events, classified according to CTCAE 2010
None of the studies reported on severe adverse events according to type of risk‐reducing surgery.
4. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO for BRCA1 mutation carriers according to age at time of RRSO: subgroup analyses
Primary outcomes
Overall survival: survival until death from all causes
None of the studies reported on overall survival according to age at time of RRSO.
High‐grade serous cancer mortality
None of the studies reported on HGSC mortality according to age at time of RRSO.
Breast cancer mortality
Three studies reported breast cancer mortality among participants who were BRCA1 mutation carriers who received RRSO at 50 years of age or less (Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Rebbeck 1999). Meta‐analysis from the three studies showed no difference between RRSO and no RRSO for breast cancer mortality among women who were BRCA1 mutation carriers who received RRSO at 50 years of age or less (HR 0.78, 95% CI 0.55 to 1.09; P = 0.15; very low‐certainty evidence; Analysis 4.1; summary of findings Table 4). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was moderate heterogeneity (I² = 42%).
Three studies reported breast cancer mortality among participants who were BRCA1 mutation carriers who received RRSO at more than 50 years of age (Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Rebbeck 1999). Meta‐analysis from the three studies showed no difference between RRSO and no RRSO for breast cancer mortality among women who were BRCA1 mutation carriers who received RRSO at more than 50 years of age (HR 1.27, 95% CI 0.67 to 2.38; P = 0.46; very low‐certainty evidence; Analysis 4.1; summary of findings Table 4). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was not important (I² = 0%).
Tests for subgroup differences showed no difference in studies that reported breast cancer mortality according to age at surgery: 50 years of age or less and more than 50 years of age (P = 0.18; I² = 43.8%).
Secondary outcomes
High‐grade serous cancer incidence
None of the studies reported on HGSC incidence according to age at time of RRSO.
Breast cancer incidence
None of the studies reported on breast cancer incidence according to age at time of RRSO.
Bone fracture incidence
None of the studies reported on bone fracture incidence according to age at time of RRSO.
Disease‐free survival (time from surgical procedure to cancer diagnosis)
None of the studies reported on disease‐free survival according to age at time of RRSO.
Morbidity: direct surgical morbidity and surgically related systemic morbidity
None of the studies reported on morbidity according to age at time of RRSO.
Recovery and readmission
None of the studies reported on recovery and readmission according to age at time of RRSO.
Quality of life
None of the studies reported on quality of life according to age at time of RRSO.
Severe adverse events, classified according to CTCAE 2010
None of the studies reported on severe adverse events according to age at time of RRSO.
5. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO for BRCA2 mutation carriers according to age at time of RRSO: subgroup analyses
Primary outcomes
Overall survival: survival until death from all causes
None of the studies reported on overall survival according to age at time of RRSO.
High‐grade serous cancer mortality
None of the studies reported on HGSC mortality according to age at time of RRSO.
Breast cancer mortality
Two studies reported breast cancer deaths among participants who were BRCA2 mutation carriers who received RRSO at 50 years of age or less (Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017). Meta‐analysis from the two studies showed no difference between RRSO and no RRSO for breast cancer mortality among women who were BRCA2 mutation carriers who received RRSO at 50 years of age or less (HR 0.49, 95% CI 0.08 to 2.90; P = 0.43; very low‐certainty evidence; Analysis 5.1; summary of findings Table 5). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance may represent moderate heterogeneity (I² = 85%).
Two studies reported disease‐free survival for breast cancer deaths among participants who were BRCA2 mutation carriers who received RRSO at more than 50 years of age (Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017). Meta‐analysis from the two studies showed no difference between RRSO and no RRSO for breast cancer mortality among women who were BRCA2 mutation carriers who received RRSO at more than 50 years of age (HR 1.36, 95% CI 0.68 to 2.75; P = 0.39; very low‐certainty evidence; Analysis 5.1; summary of findings Table 5). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was unimportant (I² = 0%).
Tests for subgroup differences showed no difference in studies that reported breast cancer mortality among participants who were BRCA2 mutation carriers according to age at surgery: 50 years of age or less and more than 50 years of age (P = 0.29; I² = 8.9%).
Secondary outcomes
High‐grade serous cancer incidence
None of the studies reported on HGSC incidence according to age at time of RRSO.
Breast cancer incidence
None of the studies reported on breast cancer incidence according to age at time of RRSO.
Bone fracture incidence
None of the studies reported on bone cancer incidence according to age at time of RRSO.
Disease‐free survival (time from surgical procedure to cancer diagnosis)
None of the studies reported on disease‐free survival according to age at time of RRSO.
Morbidity: direct surgical morbidity and surgically related systemic morbidity
None of the studies reported on morbidity according to age at time of RRSO.
Recovery and readmission
None of the studies reported on recovery and remission according to age at time of RRSO.
Quality of life
None of the studies reported on quality of life according to age at time of RRSO.
Severe adverse events, classified according to CTCAE 2010
None of the studies reported on severe adverse events according to age at time of RRSO.
Discusión
Resumen de los resultados principales
Evidencia limitada indica que la SORR puede haber aumentado la supervivencia general y reducido la mortalidad por CSGA en las portadoras de mutaciones de los genes BRCA1 y BRCA2. Además, evidencia muy limitada indica que la mortalidad por cáncer de mama se puede haber reducido en las portadoras de mutaciones del gen BRCA1 después de la SORR, pero puede no haberse reducido en las pacientes portadoras de mutaciones del gen BRCA2. La SORR también puede haber reducido el riesgo de muerte por CSGA y cáncer de mama en las pacientes portadoras de mutaciones del gen BRCA1, pero la evidencia del efecto sobre el cáncer de mama no estuvo clara en las portadoras de mutaciones del gen BRCA2 debido al escaso número de casos informados. No hubo evidencia de que la SORR afectara la incidencia de fracturas óseas, la calidad de vida o los eventos adversos graves, ni sobre los efectos de la SORR según el tipo de cirugía de reducción de riesgos y la edad en el momento de la SORR. Sin embargo, estos resultados se deben analizar con precaución, ya que todos los estudios incluidos en esta Revisión Cochrane fueron estudios observacionales no aleatorios, con la posibilidad de introducir varias formas de sesgo (factores de confusión por indicación, sesgo de detección, sesgo de prueba inducido por el cáncer, sesgo de tiempo‐persona inmortal, sesgo de evaluación, sesgo de eventos familiares y sesgo de censura informativa), y todos tuvieron períodos de seguimiento de las participantes del estudio relativamente cortos en relación con la incidencia máxima de HGSC. Sin embargo, los resultados deben ser muy importantes para un número cada vez mayor de pacientes con mutaciones de los genes BRCA1/2 y que necesitan tomar decisiones acerca de la cirugía. A pesar de la falta de ensayos aleatorios (que son éticamente imposibles), esta revisión Cochrane excluyó a las pacientes con una neoplasia maligna anterior o coexistente de mama, y algunos estudios excluyeron a las pacientes con ovariectomía o salpingectomía o salpingoovariectomía unilaterales (Rebbeck 1999; Rebbeck 2002). Además, los diez estudios incluidos utilizaron un ajuste estadístico o una escala validada para la evaluación de la calidad de vida en los análisis y todos realizaron ajustes para variables relevantes. Éstas son las principales fortalezas de esta revisión. La tabla 1 "Resumen de los hallazgos" resume los resultados principales.
Compleción y aplicabilidad general de las pruebas
No se encontraron estudios que evaluaran los eventos adversos, la morbilidad ni la mortalidad quirúrgica. Aunque se especificó la calidad de vida como un resultado de interés y un estudio la informó, la calidad de vida después del tratamiento quirúrgico con SORR es un resultado muy importante, ya que es muy frecuente que la morbilidad relacionada con el tratamiento reduzca la calidad de vida de las pacientes sometidas a SORR para profilaxis por ser portadoras de mutaciones de los genes BRCA1 o BRCA2. Además, ninguno de los estudios informó haber examinado los especímenes de biopsias de los tejidos eliminados en conformidad con el protocolo Sectioning and Extensively Examining the Fimbria (SEE/FIM).
Un problema importante en la interpretación de esta revisión Cochrane fue la tasa de neoplasia maligna oculta en las pacientes sometidas a SORR, ya que solo dos estudios informaron las tasas de carcinoma oculto (Domchek 2010; Ingham 2013). Por lo tanto, si en el tejido retirado durante la cirugía profiláctica se encontrara un tumor clínicamente no detectado, la cirugía en último término no previno la aparición tumoral, aunque puede haber aumentado la esperanza de vida. Es debatible cómo se debe incorporar en el análisis este posible efecto favorable de la cirugía (Klaren 2003). Al contar el evento en el grupo de cirugía, el riesgo de cáncer se sobrestima y la eficacia se subestima (Klaren 2003), y al excluir el evento, la eficacia se puede sobrestimar.
La contribución principal a la evidencia fue la inclusión de la ovariectomía como una variable dependiente del tiempo en Kotsopoulos 2017. Otro factor importante es el momento de la SORR, ya que las pacientes que se sometieron a cirugía a los 40 años de edad tendieron a tener una prevalencia mayor de lesiones precursoras, en especial las portadoras de mutaciones del gen BRCA1 (Lee 2017). El carcinoma oculto se observó en el 5,4% de las portadoras asintomáticas de mutaciones de los genes BRCA1/2 y el 86% de estos carcinomas fueron de origen tubárico (Zakhour 2016). Se detectaron carcinomas ocultos en alrededor del 10% de las portadoras de mutaciones de los genes BRCA1/2 y en el 19% de las portadoras de mutaciones de los genes BRCA1/2 mayores de 45 años(Domchek 2007). A diferencia de en un ECA, que compara la incidencia de la enfermedad después que las participantes se asignan al azar a recibir la intervención o ninguna intervención, en un estudio de cohortes clínico observacional y no aleatorio, retrospectivo o prospectivo, el período de seguimiento y la evaluación de los casos comienza de forma apropiada cuando la paciente es atendida por primera vez en el consultorio. Por lo tanto, los diagnósticos y el sesgo de tiempo‐persona que se producen antes de la intervención se consideran no expuestos, mientras que los diagnósticos y el sesgo de tiempo‐persona que se producen después de la intervención se consideran expuestos (Wacholder 2004).
Los estudios también compararon la SORR versus ninguna SORR en la reducción del riesgo de cánceres de mama en el análisis de subgrupos en pacientes premenopáusicas de menos de 50 años de edad y 50 años de edad y más. Se ha indicado que la alteración en la expresión de los receptores de estrógenos en las células de las glándulas mamarias desempeña una función importante durante la génesis tumoral del cáncer de mama (Hussein 2008; Leygue 1998). Debido a que los cánceres de mama asociados con mutaciones en el gen BRCA2 son principalmente positivos al receptor de estrógeno, mientras que la mayoría de los cánceres de mama asociados con mutaciones en el gen BRCA1 son negativos al receptor de estrógeno (Loman 1998), se puede esperar un efecto de reducción de riesgos de la SORR en el cáncer de mama en las portadoras de mutaciones del gen BRCA2, pero no en las portadoras de mutaciones del gen BRCA1. Desafortunadamente, en la cohorte actual, los números de portadoras de mutaciones del gen BRCA2, y en especial los números de eventos en ese grupo específico, fueron demasiado pequeños para realizar análisis concluyentes estratificados por gen con el diseño y el método analítico propuestos.
Sin embargo, debido a que el objetivo principal del estudio Domchek 2010 fue analizar la repercusión de la SORR en las portadoras de mutaciones de los genes BRCA1 y BRCA2 de forma independiente, Domchek 2010 excluyó a 12 participantes porque tuvieron mutaciones de los genes BRCA1 y BRCA2. Es interesante señalar que ninguna de las participantes incluidas en esta revisión sistemática Cochrane tenía un estado de portadora de mutaciones de los genes BRCA1 y BRCA2.
Calidad de la evidencia
El grupo de evidencia a partir de estudios observacionales o no aleatorios por lo general comienza con una confianza baja y luego aumenta si demuestran características que aumentarían la confianza de los autores de la revisión en los resultados de estos diseños, como una magnitud grande del efecto, una relación dosis‐respuesta o factores de confusión verosímiles que de otro modo habrían debilitado la estimación del efecto (Agoritsas 2013). Cuando un estudio observacional metodológicamente fuerte produce estimaciones grandes o muy grandes y consistentes de la magnitud de un tratamiento o efecto de la exposición, es posible estar seguros acerca de los resultados. En estas situaciones, un diseño deficiente del estudio tiene pocas probabilidades de explicar todo el efecto beneficioso o el daño evidente, incluso aunque es probable que los estudios observacionales proporcionen una sobrestimación del efecto verdadero. En términos generales, la certeza de la evidencia se obtuvo de estudios no aleatorios y se consideró de calidad muy baja según la metodología GRADE para la comparación principal, principalmente debido a los estudios que tuvieron riesgo moderado de sesgo (Resumen de los hallazgos, tabla 1; Resumen de los hallazgos, tabla 2; Resumen de los hallazgos, tabla 3; Resumen de los hallazgos, tabla 4; Resumen de los hallazgos, tabla 5). Diversas limitaciones en los estudios individuales pueden haber interferido de manera adicional con la certeza de la evidencia. Aunque se realizaron ajustes de los factores de confusión significativos, de manera específica hubo períodos de seguimiento relativamente cortos de los participantes en los estudios con relación a la incidencia máxima del cáncer de ovario o el cáncer peritoneal primario. Aparte de Kramer 2005 que tuvo alto riesgo de sesgo, todos los otros estudios incluidos tuvieron riesgo moderado de sesgo. Solo uno de los diez estudios de cohortes incluidos excluyó a las pacientes si tuvieron un diagnóstico de cáncer en el transcurso de los primeros seis meses de seguimiento para evitar incluir los cánceres que habrían podido estar influenciados por la SORR o la MRR (Domchek 2010). La heterogeneidad entre los estudios se podría haber debido, por ejemplo, a las diferencias en el momento de la realización de los estudios, ya que los estudios más recientes (en un plazo de cinco años) parecieron ser muy diferentes de los estudios anteriores con respecto a los resultados. Para considerar esta inquietud con la heterogeneidad, todos los análisis se realizaron mediante el análisis de efectos fijos y el análisis de efectos aleatorios, pero los resultados de los dos modelos no difirieron, por lo que se informó el análisis de efectos aleatorios. El metanálisis de los estudios que informaron sobre la mortalidad por CSGA y la mortalidad por cáncer de mama, y según las portadoras de mutaciones del gen BRCA2 y la edad en el momento de la SORR en portadoras de mutaciones del gen BRCA2, así como la incidencia de CSGA y la incidencia de cáncer de mama, mostró heterogeneidad moderada a significativa. Sin embargo, todos los análisis de supervivencia y los análisis de mortalidad se evaluaron mediante el CRI, que es la mejor estadística para resumir las diferencias en el riesgo entre dos grupos de tratamiento durante un estudio cuando el tiempo hasta la muerte o la progresión de la enfermedad es "censurado" o desconocido en algunas mujeres, ya que aún estaban vivas (o libres de la enfermedad) al final del estudio.
Sesgos potenciales en el proceso de revisión
Cuando se evalúa la eficacia y la seguridad de la cirugía de reducción de riesgos, el objetivo es identificar a dos grupos de pacientes que difieren en la exposición de interés, a saber la cirugía profiláctica, pero que son, o en el análisis pueden ser, similares con respecto a otros factores asociados con el resultado de la enfermedad. En condiciones ideales, lo anterior se podría realizar en un ensayo clínico aleatorio, pero la asignación al azar para cirugía profiláctica de las portadoras de mutaciones de los genes BRCA1 o BRCA2 obviamente es poco ético, ya que no se puede limitar el tratamiento quirúrgico de reducción de riesgos a las pacientes que lo requieran de forma exclusiva por la investigación. Esta revisión Cochrane se limitó a los estudios observacionales no aleatorios, que tienen la posibilidad de introducir varias formas de sesgo (factores de confusión por indicación, sesgo de detección, sesgo de prueba inducido por el cáncer, sesgo de tiempo‐persona inmortal, sesgo de evaluación, sesgo de eventos familiares y sesgo de censura informativa) (Klaren 2003; Wacholder 2004). El sesgo de evaluación se podría deber a que las pacientes con un riesgo mayor de cáncer tienen mayores probabilidades de solicitar y ser reclutadas en estudios que las pacientes con menor riesgo de cáncer. Este efecto se ha documentado en varios estudios previos, en especial los que se basan en la agregación familiar del cáncer en familias con alto riesgo (Begg 2002).
Además, los factores utilizados en muchos estudios incluidos para el pareamiento (es decir, edad, centro o tipo de mutación) pueden favorecer la selección de familiares en el grupo de ninguna cirugía. Esta selección puede aumentar de forma no intencional el sesgo. Por ejemplo, podría haber un fenómeno de dependencia potencial entre el diagnóstico de cáncer dentro de la familia y las decisiones individuales de los familiares sometidos a pruebas de ADN, el método de cribado del cáncer, la cirugía de reducción de riesgos o una combinación de estos. Si se supone que estos eventos son independientes en los estudios que incluyen a varios miembros de la misma familia, pueden surgir sesgos como el de confusión por indicación y el sesgo de evento familiar, como se observó en Rebbeck 1999. Por ejemplo, un estudio informó que el 59% de las pacientes estaban relacionadas con al menos otra participante del estudio y el 32% estaban relacionadas con al menos otras cuatro participantes del estudio (Rebbeck 1999). A pesar de reconocerse la relación entre los participantes del estudio, los autores no consideraron la dependencia entre los eventos dentro de una familia. Si se selecciona el período apropiado durante el cual los miembros de una familia están en riesgo, se puede prevenir el sesgo.
Para evitar el sesgo de evento familiar, la mejor elección para comenzar el seguimiento es la edad a la cual se le realizó la prueba al propio control, o la edad del control en la fecha de la cirugía profiláctica de su familiar, cualquiera que haya sido la última (Klaren 2003; Wacholder 2004). Lo anterior se debe a que las pacientes del grupo de comparación deben estar libres de cáncer en el momento de la evaluación.
Esta revisión Cochrane empleó los metanálisis que utilizaron un enfoque basado en la publicación y no fue posible obtener los datos individuales de las participantes de cada estudio. Por lo tanto, se adoptaron definiciones claras de exposiciones y resultados mediante ROBINS‐I y se cumplieron los procedimientos que redujeran al mínimo el sesgo de extracción, registro y recuperación, al buscar de forma cuidadosa en la bibliografía "gris". Además, no se aplicaron restricciones de idioma. No hubo evidencia de sesgo de publicación documentado, ya que el metanálisis no incluyó más de diez estudios.
Algunos estudios incluidos en esta revisión Cochrane emplearon cuestionarios para la extracción de datos sobre información relacionada con los antecedentes reproductivos, antecedentes quirúrgicos (incluidas ovariectomía y mastectomía preventivas) y la administración de hormonas. Dichos cuestionarios no pueden distinguir entre la ovariectomía y la salpingoovariectomía, aunque un estudio sueco reciente mostró que los médicos y los consejeros genéticos pueden depender del autoinforme con respecto al cáncer de mama y el cáncer de ovario en las portadoras de mutaciones de los genes BRCA1 o BRCA2 (Augustinsson 2018).
Se ha indicado que las alteraciones en la expresión de los receptores de estrógenos en las células de las glándulas mamarias desempeñan una función importante durante la génesis tumoral del cáncer de mama (Archey 2017). Debido a que los cánceres de mama asociados con mutaciones del gen BRCA2 son principalmente positivos al receptor de estrógeno, mientras que la mayoría de los cánceres de mama asociados con mutaciones en el gen BRCA1 son negativos al receptor de estrógeno (Archey 2017; Loman 1998), se puede esperar un efecto de reducción de riesgos de la SORR en el cáncer de mama en las portadoras de mutaciones del gen BRCA2, pero no en las portadoras de mutaciones del gen BRCA1. Desafortunadamente, en la revisión actual los números de portadoras de mutaciones del gen BRCA2, y en especial los números de eventos en ese grupo específico, fueron demasiado pequeños para realizar análisis concluyentes estratificados por genes en los análisis de subgrupos.
Algunos estudios de esta revisión utilizaron controles que necesariamente no se sometieron a vigilancia (Rebbeck 2002) y otros estudios utilizaron controles que se siguieron de manera prospectiva en un programa de vigilancia anual activo. Además, en algunos estudios no se realizó el pareamiento directo de los casos (Domchek 2010; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Madalinska 2007). Es posible que los efectos biológicos de otras variables demográficas puedan haber sido diferentes entre la SORR y el grupo de vigilancia o control, como la edad al ingreso al estudio, el número de partos y los antecedentes de TRH, pero quizás no se hayan corregido completamente mediante el tratamiento de las covariables en el análisis.
Lo que es más importante, se excluyeron las pacientes con una neoplasia maligna de mama anterior o coexistente y algunas estudios excluyeron a las pacientes con ovariectomía o salpingectomía unilateral o salpingoovariectomía (Rebbeck 1999; Rebbeck 2002). Además, se excluyeron las pacientes con salpingectomía profiláctica con ovariectomía retardada o conservación ovárica (Harmsen 2015; Harmsen 2016; Nebgen 2018; Tschernichovsky 2017). Éstas son las principales fortalezas de esta revisión. Si se hubieran incluido los antecedentes personales de cáncer de mama en el momento del ingreso a los estudios, se habría introducido un sesgo potencial en el análisis. Por ejemplo, la reducción de la mortalidad calculada para las pacientes con y sin antecedentes previos de cáncer de mama puede diferir. Por lo tanto, limitar los análisis a las participantes sin antecedentes personales de cáncer de mama en el momento del ingreso a los estudios o de la SORR, le confiere una mayor magnitud a la evaluación de protección.
Se utilizó una nueva herramienta ROBINS‐I para la evaluación del riesgo de sesgo en los estudios observacionales. Esta herramienta incluye un enfoque estructurado para la evaluación del riesgo de sesgo debido a factores de confusión, que comienza en el estadio de protocolo de la revisión y permite hacer evaluaciones integrales del riesgo de sesgos que son aplicables a una variedad amplia de diseños y análisis de estudio. La herramienta ROBINS‐I se enfoca específicamente en el sesgo y no aborda los problemas relacionados con la imprecisión de los resultados.
El cociente 1:2 de las participantes control entre todas las pacientes que fueron portadoras de mutaciones de los genes BRCA1 o BRCA2 reclutadas en esta revisión Cochrane coincidió con las recomendaciones de Klaren 2003 y Wacholder 2004, que pretenden abordar los posibles sesgos de muestreo y de información en los estudios de cirugías de reducción de riesgos con cohortes multicéntricas. Los autores de Klaren 2003 y Wacholder 2004 recomendaron la selección de entre uno y cuatro controles por comparación para cada participante con SORR, que fue el resultado de esta revisión Cochrane. Sin embargo, las conclusiones estuvieron limitadas por la falta de ensayos controlados aleatorios (Finch 2011). La ausencia de muertes por CSGA en los estudios que informaron sobre el CSGA en portadoras de mutaciones del gen BRCA2 se puede deber al número más pequeño de muestras de portadoras de mutaciones del gen BRCA2 en comparación con las portadoras de mutaciones del gen BRCA1 en los estudios incluidos. A falta de eventos de cáncer, no se pueden calcular los CRI.
Acuerdos y desacuerdos con otros estudios o revisiones
Se han publicado revisiones sistemáticas (Ludwig 2016; Marchetti 2014; Tschernichovsky 2017), y metanálisis (Rebbeck 2009) previos, o ambos (Li 2016), sobre el beneficio de la SORR en las pacientes con mutaciones de los genes BRCA1 o BRCA2. Algunos estudios incluidos en estas revisiones o metanálisis sistemáticos previos no se incluyeron en el presente metanálisis (Chang‐Claude 2007; Eisen 2005; Evans 2009; Finch 2006; Finch 2014; Finkelman 2012; Kauff 2002; Kauff 2008; Rutter 2003; Schmeler 2006). Lo anterior fue necesario porque en una revisión sistemática en la cual el CSGA o el cáncer de mama es la variable principal de evaluación, los estudios que incluyeran pacientes con cáncer de ovario o cáncer de mama previos (o ambos) se deben excluir para evitar sesgos que favorecerían al grupo quirúrgico o al no quirúrgico.
Los autores de Ludwig 2016 concluyeron que la reducción en el riesgo de cáncer de mama y de ovario con el uso de la SORR mejora la supervivencia y el tratamiento clínico de las pacientes con mayor riesgo de cáncer de mama, pero requiere que se consideren el riesgo y la calidad de vida. Excluyeron publicaciones no inglesas. Cinco de los seis estudios incluidos se excluyeron en esta revisión Cochrane (Evans 2009; Finch 2014; Kauff 2002; Kauff 2008; Schmeler 2006). Evans 2009 se excluyó porque no se conocía el estado de portadora de mutaciones de los genes BRCA1 o BRCA2 de todas las pacientes incluidas; Finch 2014 fue un estudio de brazo único sin grupo de comparación; y Kauff 2002, Kauff 2008 y Schmeler 2006 incluyeron a pacientes con una neoplasia maligna de mama anterior o coexistente.
Los autores de Marchetti 2014 concluyeron que se justificaba recomendar la SORR para reducir el riesgo de cáncer de ovario y la mortalidad por todas las causas en las pacientes con una mutación de los genes BRCA1 o BRCA2. Dos estudios de los tres incluidos se excluyeron de esta revisión (Finch 2014; Kauff 2008). Finch 2014 se excluyó porque fue un estudio de brazo único sin grupo de comparación y Kauff 2008 incluyó pacientes con una neoplasia maligna de mama anterior o coexistente.
Los autores de la revisión sistemática Tschernichovsky 2017 concluyeron que hasta que haya más datos disponibles, la SORR y las píldoras anticonceptivas orales siguen siendo las únicas medidas preventivas recomendadas en las portadoras de mutaciones de los genes BRCA1 o BRCA2 para reducir de manera significativa el riesgo de cáncer de ovario. Solo un estudio (Domchek 2010) de los cuatro (Domchek 2010; Finch 2014; Marchetti 2014; Rebbeck 2009) incluidos por los autores de la revisión sistemática Tschernichovsky 2017 se incluyó en la presente revisión. Finch 2014 se excluyó porque fue un estudio de brazo único sin grupo de comparación y Marchetti 2014 y Rebbeck 2009 fueron artículos de revisión.
El metanálisis Rebbeck 2009 concluyó que la SORR se asoció con firmeza con reducciones en el riesgo de cáncer de mama y CSGA y se debe proporcionar asesoramiento a las pacientes al planificar las estrategias de reducción de riesgos del cáncer. Seis de diez estudios se excluyeron de esta revisión (Chang‐Claude 2007; Eisen 2005; Finch 2006; Kauff 2002; Kauff 2008; Rutter 2003). Rutter 2003 se excluyó porque no se conocía el estado de portadoras de mutaciones de los genes BRCA1 o BRCA2 de todas las pacientes incluidas; Chang‐Claude 2007, Eisen 2005, Finch 2006, Kauff 2002 y Kauff 2008 incluyeron pacientes con una neoplasia maligna de mama anterior o coexistente.
Li 2016 concluyó que las portadoras de mutaciones de los genes BRCA1 o BRCA2 tratadas con SORR tienen una reducción significativa en la incidencia y la mortalidad por cáncer de mama. De los 15 estudios, 11 estudios se excluyeron de esta revisión Cochrane (Chang‐Claude 2007; Eisen 2005; Evans 2013; Finkelman 2012; Heemskerk‐Gerritsen 2013; Heemskerk‐Gerritsen 2015b; Kauff 2008; Meijers‐Heijboer 2001; Metcalfe 2014; Skytte 2011; van Sprundel 2005). Además de los que ya se señalaron, se excluyeron siete estudios porque incluyeron a pacientes con o sin antecedentes familiares o personales de cáncer de mama que eran portadoras de mutaciones de los genes BRCA1 y BRCA2 e inicialmente habían sido tratadas con MRR unilateral o MBRR, pero sin SORR (Evans 2013; Heemskerk‐Gerritsen 2013; Heemskerk‐Gerritsen 2015b; Meijers‐Heijboer 2001; Metcalfe 2014; Skytte 2011; van Sprundel 2005). Finkelman 2012 incluyó pacientes con una neoplasia maligna de mama anterior o coexistente.
Una revisión sistemática no Cochrane describió las implicaciones de la SORR premenopáusica sobre la calidad de vida, los síntomas endocrinos, la función sexual, la osteoporosis, la salud cardiovascular, el síndrome metabólico, la deficiencia cognitiva y seguridad de la TRH (Vermeulen 2017). Los resultados de la revisión revelaron que la menopausia quirúrgica provoca más molestias menopáusicas y disfunción sexual que la menopausia natural, pero que la cirugía no afecta la calidad de vida general. Los autores no encontraron evidencia de que la SORR provocara más osteopenia en comparación con la menopausia natural a una edad temprana, pero revelaron que los estudios de cohortes mostraron un deterioro leve en la salud cardiovascular y disminuciones de la función cognoscitiva en etapas posteriores de la vida en las pacientes premenopáusicas sometidas a ovariectomía. Los autores también concluyeron que la TRH a corto plazo pareció reducir las molestias posmenopáusicas y no pareció aumentar el riesgo de carcinoma de mama en las portadoras de mutaciones sin antecedentes personales de carcinoma de mama (Vermeulen 2017).
Otra revisión no Cochrane determinó la repercusión de la SORR sobre la calidad de vida y la salud en las pacientes que portan una mutación de los genes BRCA y mostró estudios preliminares enfocados en los efectos a corto plazo como la calidad de vida general, que fue similar ante y después de la cirugía (Finch 2011). Sin embargo, los síntomas vasomotores relacionados con la menopausia quirúrgica y los cambios en el funcionamiento sexual fueron frecuentes. La TRH pareció mitigar algunos síntomas, pero no todos. Por lo tanto, un tratamiento de corta duración con la TRH puede no estar contraindicado en las portadoras de mutaciones del gen BRCA1 que han presentado menopausia y que no tienen antecedentes personales de cáncer (Kotsopoulos 2016). Las pacientes informaron niveles altos de satisfacción con la decisión de someterse a cirugía a pesar de la repercusión de la SORR.
Study flow diagram for searches on risk‐reducing salpingo‐oophorectomy in women with BRCA1 or BRCA2 mutation carriers.

Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 1 Overall survival.
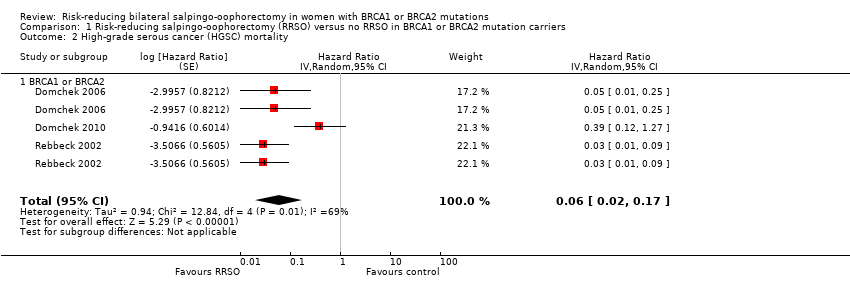
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 2 High‐grade serous cancer (HGSC) mortality.

Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 3 Breast cancer mortality.

Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 4 HGSC incidence.
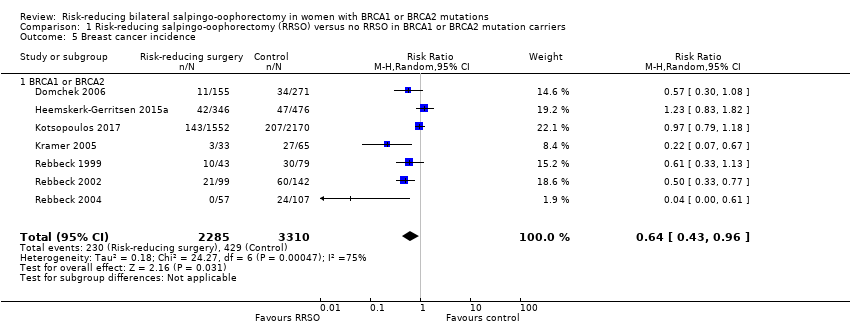
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 5 Breast cancer incidence.

Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 6 Quality of life (ovarian cancer risk perception).
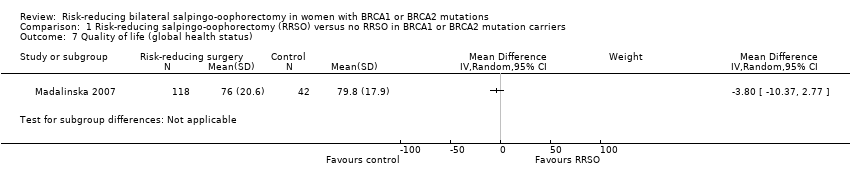
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 7 Quality of life (global health status).

Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 8 Quality of life (general health perception).
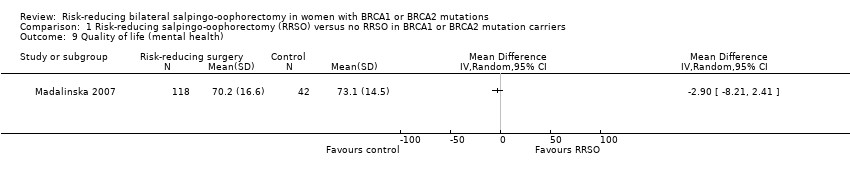
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 9 Quality of life (mental health).

Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 10 Quality of life (breast cancer risk perception).

Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 1 Overall survival.
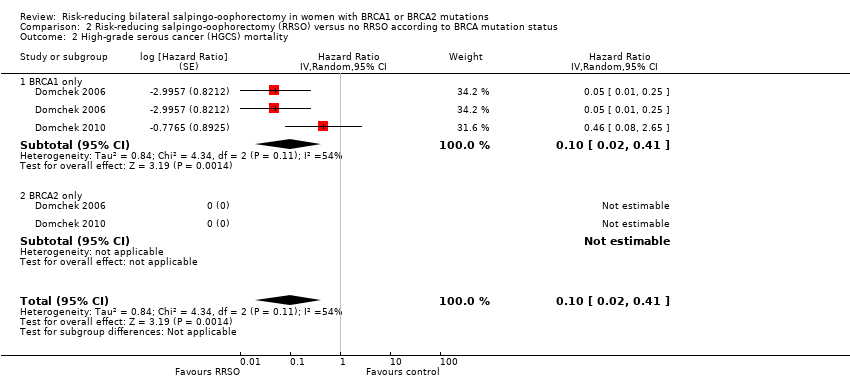
Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 2 High‐grade serous cancer (HGCS) mortality.

Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 3 Breast cancer mortality.

Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 4 Quality of life (ovarian cancer risk perception).

Comparison 3 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery, Outcome 1 Overall survival.

Comparison 3 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery, Outcome 2 Breast cancer mortality.

Comparison 4 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 mutation carriers according to age at RRSO, Outcome 1 Breast cancer mortality.

Comparison 5 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA2 mutation carriers according to age at RRSO, Outcome 1 Breast cancer mortality.
| RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and USA Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers | |||||
| Overall survival: BRCA1 or BRCA2 | Study population | HR 0.32 | 2548 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| HGSC mortality: BRCA1 or BRCA2 | Study population | HR 0.06 | 2534 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: BRCA1 or BRCA | Study population | HR 0.58 | 7198 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Bone fracture incidence | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Quality of life (ovarian cancer risk perception): BRCA1 or BRCA2 | See comment | See comment | Not estimable | 200 | ⊕⊝⊝⊝ | Unable to perform meta‐analysis as only 1 study reported the outcome. |
| Quality of life (breast cancer risk perception): BRCA1 or BRCA2 | See comment | See comment | Not estimable | 200 | ⊕⊝⊝⊝ | Unable to perform meta‐analysis as only 1 study reported the outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies. | ||||||
| RRSO vs no RRSO according to BRCA mutation status | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and USA Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO according to BRCA mutation status | |||||
| Overall survival: BRCA1 only | Study population | HR 0.30 | 2548 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Overall survival: BRCA2 only | Study population | HR 0.44 | 2122 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| HGSC mortality: BRCA1 only | Study population | HR 0.1 | 1983 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| HGSC mortality: BRCA2 only | See commentb | See commentb | Not estimable See commentc | 1983 | ⊕⊝⊝⊝ | bAs a result of the way HRs were calculated, assumed and corresponding risks were not estimated. cUnable to perform meta‐analysis as no mortality events were recorded in any study and HRs could not be estimated. |
| Breast cancer mortality: BRCA1 only | Study population | HR 0.45 | 2203 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: BRCA2 only | Study population | HR 0.88 | 5882 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Quality of life (ovarian cancer risk perception): BRCA1 only | See comment | See comment | Not estimable | 98 | ⊕⊝⊝⊝ | Unable to perform meta‐analysis as only 1 study reported the outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies. | ||||||
| RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and USA Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery | |||||
| Overall survival: RRSO alone vs RRSO and RRM | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Overall survival: RRSO and RRM vs no RRSO | Study population | HR 0.14 | 261 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: RRSO alone vs RRSO and RRM | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Breast cancer mortality: RRSO and RRM vs no RRSO | See comment | See comment | Not estimable | 722 | ⊕⊝⊝⊝ | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. |
| Bone fracture incidence | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to confounding and bias due in selection of participants in the study). | ||||||
| RRSO vs no RRSO in BRCA1 mutation carriers according to age at RRSO | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and America Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA1 mutation carriers according to age at RRSO | |||||
| Overall survival | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| HGSC mortality | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Breast cancer mortality: ≤ 50 years | Study population | HR 0.78 | 4566 | ⊕⊝⊝⊝ Very lowa,b | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: > 50 years | Study population | HR 1.27 | 4566 | ⊕⊝⊝⊝v Very lowa,b | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Bone fracture incidence | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies. bDowngraded by one level for serious imprecision: the confidence intervals overlapped 1 and either 0.75 or 1.25 or both (i.e. wide confidence intervals in all included studies, which crossed the line of unity). | ||||||
| RRSO versus no RRSO in BRCA2 mutation carriers according to age at RRSO | ||||||
| Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and America Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA2 mutation carriers according to age at RRSO | |||||
| Overall survival | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| HGSC mortality | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Breast cancer mortality: ≤ 50 years | Study population | HR 0.49 | 444 | ⊕⊝⊝⊝ Very lowa,b | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: > 50 years | Study population | HR 1.36 | 444 | ⊕⊝⊝⊝ Very lowa,b | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Bone fracture incidence | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; CI: confidence interval; HGSC: high‐grade serous cancer; HR: hazard ratio; RRSO: risk‐reducing salpingo‐oophorectomy. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies. bDowngraded by one level for serious imprecision: the confidence intervals overlapped 1 and either 0.75 or 1.25 or both (i.e. wide confidence intervals in all included studies, which cross the line of unity). | ||||||
| Judgement | Within each domain | Across domains | Criterion |
| Low risk of bias | The study is comparable to a well‐performed randomised trial with regard to this domain. | The study is comparable to a well‐performed randomised trial. | The study is judged to be at low risk of bias for all domains. |
| Moderate risk of bias | The study is sound for a non‐randomised study with regard to this domain but cannot be considered comparable to a well‐performed randomised trial. | The study provides sound evidence for a non‐randomised study but cannot be considered comparable to a well‐performed randomised trial. | The study is judged to be atlow or moderate risk of bias for all domains. |
| Serious risk of bias | The study has some important problems in this domain. | The study has some important problems. | The study is judged to be at serious risk of bias in at least 1 domain, but not at critical risk of bias in any domain. |
| Critical risk of bias | The study is too problematic in this domain to provide any useful evidence on the effects of intervention. | The study is too problematic to provide any useful evidence and should not be included in any synthesis. | The study is judged to be at critical risk of bias in at least 1 domain. |
| No information | No information on which to base a judgement about risk of bias for this domain | No information on which to base a judgement about risk of bias. | There is no clear indication that the study is at serious or critical risk of bias and there is a lack of information in 1 or more key domains of bias (a judgement is required for this). |
| ROBINS‐I: Risk Of Bias In Non‐randomised Studies‐of Interventions. | |||
| Study ID | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias |
| Low | Low | Low | Moderate | |
| Support for judgement | Quote: "Questionnaires were administered at every centre and were self‐administered or completed with the help of clinical‐research staff." All missing data were analysed (intention‐to‐treat). | Quote: "For our primary analysis, we undertook a matched design that selected controls who had not undergone BPSO at any time during follow‐up, and who were matched within 5 years of age to the corresponding one.” | Quote: "Follow‐up data for BPSO, cancer diagnoses, and deaths were verified by review of medical records, and surgical notes, pathology reports, or both." | At least 1 of the domain was moderate. |
| Moderate | Low | Low | Moderate | |
| Support for judgement | Quote: "When missing data were encountered, the individual was dropped from the analysis that involved the missing data point, but the individual was included in other analyses where complete data were available; in fact, because many of the data items were required for enrolment missing data was only applicable to ovarian cancer endpoints, with missing OCP data." | Quote: "For BC endpoints, women were excluded if they underwent RRM prior to ascertainment. Women who had RRM after ascertainment but before RRSO were considered unexposed and were censored at RRM. Women were followed until BC or were censored at OC, RRM, death, or last contact." | Quote: "A robust variance‐covariance estimation method was used to correct for non‐independence of observations among participants from the same family or within centers... Adjustment for year of birth was undertaken in all analyses using Cox regression. Oral contraceptive use was adjusted for when OC was the outcome. Adjustment for center of ascertainment was undertaken by stratifying analyses by center to avoid imposing linear constraints in the model." | At least 1 of the domain was moderate. |
| Moderate | Low | Low | Moderate | |
| Support for judgement | Quote: "Eventually, parity was not considered as a potential confounder because of the large proportion (41.0%) of missing data on this variable." | Quote: "We performed sensitivity analyses to estimate the effect of RRSO on BC risk in different settings. First, to investigate the effect of excluding the BC‐free time before RRM, we estimated BC risk reduction after RRSO for participants who never underwent RRM." | Quote: "We adjusted our analyses for differences in age by using chronological age as the time variable." | At least 1 of the domain was moderate. |
| Low | Moderate | Low | Moderate | |
| Support for judgement | Quote: "Women were censored at either date of last follow‐up (date of last contact with the genetics department or other NHS service) or date of death (obtained from NWCIS or death certification).” No evidence of missing data. | Quote: "Date of breast cancer was confirmed in the family files or from records at the North West Cancer Intelligence Service (NWCIS)." Also, possible testing bias of women who developed cancer was made. | Quote: "The proportional hazards assumption was checked in all analyses by looking at log–log plots and Schoenfeld residuals." | At least 1 of the domain was moderate. |
| Moderate | Low | Moderate | Moderate | |
| Support for judgement | Quote: "Women with both a BRCA1 and BRCA2 mutation were coded as missing." | Quote: "We also performed analyses stratified by BRCA mutation type, estrogen receptor status of the tumor, and excluding women with an oophorectomy at or prior to the baseline questionnaire, as well as analyses censoring at different ages." | Quote: "this finding was based on a post hoc analysis." | At least 1 of the domain was moderate. |
| Low | Moderate | Low | Serious | |
| Support for judgement | There was no evidence of missing data. | Quote: "A competing risks model (with death as the competing risk) was then used to estimate the 10‐year cumulative incidence of breast cancer in the two groups of BRCA1 mutation carriers (ie, those with and without ovaries)." | Quote: "To provide estimates of the absolute risk of breast cancer by age in mutation carriers, landmark analyses were performed in which oophorectomy was treated as a time‐fixed covariate, as defined at the beginning of a given age interval. Follow‐up time was divided into 10‐year intervals, with mutation carriers divided into two groups based on oophorectomy status at the beginning of that interval (and conditional on the participant being alive and breast cancer free at that time)." | At least 1 of the domain was serious. |
| Low | Low | Low | Moderate | |
| Support for judgement | Quote: "These records were complete, and in cases where there was any uncertainty, contact was sought with the responsible gynecologist." "Non respondents did not differ significantly from respondents regarding age or choice of preventive measure." | Quote: "All raw scale scores were linearly converted to a 0 to100 scale, with higher scores indicating better perceived health, mental health, and quality of life. The internal consistency reliability of the two Short Form‐36 scales was high (α = 0.81 and 0.85)." | Quote: "Because of restrictions by the medical ethics committees, no other clinical data on the nonrespondents were available (eg, DNA status)." | At least 1 of the domain was moderate. |
| Low | Low | Moderate | Moderate | |
| Support for judgement | Quote: "However, only BRCA1 mutation carriers were studied here, and no OCCR region has been identified in BRCA1." No evidence of missing data. | Quote: "Because most women were followed only until the time of censoring or until the diagnosis of breast cancer, the incidences reported here do not represent lifetime breast cancer risks in BRCA1 mutation carriers." | Quote: "Furthermore, the inferences from both the robust and nonrobust analyses were identical. Therefore, only the standard model results are presented." | At least 1 of the domain was moderate. |
| Moderate | Low | Low | Moderate | |
| Support for judgement | Quote: "Bias that arises when later follow‐up is missing for individuals initially included and followed." | Quote: "on vital status and the occurrence of cancer was obtained from medical records, telephone interviews, self‐administered questionnaires, or a combination of these. For women who had died since their entry into the study, we reviewed medical records and family‐history reports to establish the presence or absence of cancer and to verify that they had died." | Quote: "For women who had died since their entry into the study, we reviewed medical records and family‐history reports to establish the presence or absence of cancer and to verify that they had died." | At least 1 of the domain is moderate. |
| Moderate | Low | Low | Moderate | |
| Support for judgement | Quote: "Percentages calculated using nonmissing data." | Quote: "Survival analyses were adjusted to account for duration of endogenous ovarian hormone exposure as measured by the time from age at menarche to age at bilateral prophylactic | Quote: "Subjects were censored at the date they developed ovarian cancer, or died, or at the date of last contact. Diagnosis of invasive breast cancer or ductal carcinoma‐in‐situ was considered the primary event of interest." | At least 1 of the domain is moderate. |
| BC: Breast Cancer; BPSO: Bilateral prophylactic salpingo‐oophorectomy; BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; NHS: National Health Service; NWCI: North West Cancer Intelligence Service; SOC: Site of Care; OCP: Oral Contraceptive Pill; ROBIS‐I: Risk Of Bias In Non‐randomised Studies‐of Interventions; RRM: risk‐reducing bilateral mastectomy; RRSO: risk‐reducing bilateral salpingo‐oophorectomy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 BRCA1 or BRCA2 | 3 | Hazard Ratio (Random, 95% CI) | 0.32 [0.19, 0.54] | |
| 2 High‐grade serous cancer (HGSC) mortality Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.06 [0.02, 0.17] | |
| 2.1 BRCA1 or BRCA2 | 3 | Hazard Ratio (Random, 95% CI) | 0.06 [0.02, 0.17] | |
| 3 Breast cancer mortality Show forest plot | 7 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 BRCA1 or BRCA | 7 | Hazard Ratio (Random, 95% CI) | 0.58 [0.39, 0.88] | |
| 4 HGSC incidence Show forest plot | 4 | 3328 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.04, 0.75] |
| 4.1 BRCA1 or BRCA2 | 4 | 3328 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.04, 0.75] |
| 5 Breast cancer incidence Show forest plot | 7 | 5595 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.96] |
| 5.1 BRCA1 or BRCA2 | 7 | 5595 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.96] |
| 6 Quality of life (ovarian cancer risk perception) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 BRCA1 or BRCA2 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of life (global health status) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8 Quality of life (general health perception) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9 Quality of life (mental health) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Quality of life (breast cancer risk perception) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 BRCA1 or BRCA2 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.35 [0.25, 0.50] | |
| 1.1 BRCA1 only | 3 | Hazard Ratio (Random, 95% CI) | 0.30 [0.17, 0.52] | |
| 1.2 BRCA2 only | 2 | Hazard Ratio (Random, 95% CI) | 0.44 [0.23, 0.85] | |
| 2 High‐grade serous cancer (HGCS) mortality Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 0.10 [0.02, 0.41] | |
| 2.1 BRCA1 only | 2 | Hazard Ratio (Random, 95% CI) | 0.10 [0.02, 0.41] | |
| 2.2 BRCA2 only | 2 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Breast cancer mortality Show forest plot | 6 | Hazard Ratio (Random, 95% CI) | 0.59 [0.35, 1.00] | |
| 3.1 BRCA1 only | 4 | Hazard Ratio (Random, 95% CI) | 0.45 [0.30, 0.67] | |
| 3.2 BRCA2 only | 3 | Hazard Ratio (Random, 95% CI) | 0.88 [0.42, 1.87] | |
| 4 Quality of life (ovarian cancer risk perception) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 BRCA1 only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 BRCA2 only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 RRSO and risk‐reducing mastectomy (RRM) versus no RRSO | 1 | Hazard Ratio (Random, 95% CI) | 0.14 [0.02, 0.98] | |
| 2 Breast cancer mortality Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 2.1 RRSO and RRM versus no RRSO | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast cancer mortality Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.85 [0.64, 1.11] | |
| 1.1 50 years or less | 3 | Hazard Ratio (Random, 95% CI) | 0.78 [0.55, 1.09] | |
| 1.2 Above 50 years | 3 | Hazard Ratio (Random, 95% CI) | 1.27 [0.67, 2.38] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast cancer mortality Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 0.88 [0.42, 1.87] | |
| 1.1 50 years or less | 2 | Hazard Ratio (Random, 95% CI) | 0.49 [0.08, 2.90] | |
| 1.2 Above 50 years | 2 | Hazard Ratio (Random, 95% CI) | 1.36 [0.68, 2.75] | |

