Propofol w poprawie jakości snu u dorosłych na oddziałach intensywnej terapii
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Critical Care] explode all trees
#2 MeSH descriptor: [Critical Illness] explode all trees
#3 MeSH descriptor: [Intensive Care Units] explode all trees
#4 MeSH descriptor: [Respiration, Artificial] explode all trees
#5 ((intensive or critical) near/3 (care or unit*)) or (critical* near/3 ill*)
#6 (mechanical* near/3 ventilat*) or (artificial* near/3 respiration*)
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 propofol or sleep*
#9 MeSH descriptor: [Propofol] explode all trees
#10 MeSH descriptor: [Hypnotics and Sedatives] explode all trees
#11 MeSH descriptor: [Sleep] explode all trees
#12 #8 or #9 or #10 or #11
#13 #7 and #12
#14 #13 in Trials
Appendix 2. MEDLINE Ovid search strategy
-
Critical Illness/ or Critical Care/ or exp Intensive Care Units/ or (ICU or ((intensive or critical) adj3 (care or unit*)) or (critical* adj3 ill*)).mp. or Respiration, Artificial/ or (mechanical* adj3 ventilat*).mp. or (artificial* adj3 respiration*).mp.
-
exp "Hypnotics and Sedatives"/ or Propofol/ or sleep/ or (sleep* or hypnotic* or sedat* or propofol).mp.
-
((randomized controlled trial or controlled clinical trial).pt. or randomi*.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh.
-
1 and 2 and 3
Appendix 3. Embase Ovid search strategy
-
critical illness/ or exp intensive care unit/ or exp intensive care/ or (ICU or ((intensive or critical) adj3 (care or unit*)) or (critical* adj3 ill*)).mp. or artificial ventilation/ or (mechanical* adj3 ventil*).mp. or (artificial* adj3 respiration*).mp.
-
hypnotic sedative agent/ or propofol/ or sleep/ or (sleep* or hypnot* or sedat* or propofol).mp.
-
((crossover procedure or double blind procedure or single blind procedure).sh. or (crossover* or cross over*).ti,ab. or placebo*.ti,ab,sh. or (doubl* adj blind*).ti,ab. or (controlled adj3 (study or design or trial)).ti,ab. or allocat*.ti,ab. or trial*.ti,ab. or randomized controlled trial.sh. or random*.ti,ab.) not ((exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.))
-
1 and 2 and 3
Appendix 4. CINAHL EBSCO search strategy
-
(MM "Critical Illness")
-
(MM "Critically Ill Patients")
-
(MH "Critical Care+")
-
(MH "Intensive Care Units+")
-
(intensive or critical) N3 (care or unit*) OR critical* N3 ill*
-
(MH "Respiration, Artificial+")
-
mechanical* N3 ventilat* OR artificial* N3 respiration*
-
(S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7)
-
(MH "Hypnotics and Sedatives+")
-
(MM "Propofol")
-
(MH "Sleep+")
-
sleep* OR hynotic* OR sedat* OR propofol*
-
(S9 OR S10 OR S11 OR S12)
-
TX allocat* random*
-
(MH "Quantitative Studies")
-
(MH "Placebos")
-
TX placebo*
-
TX random* allocat*
-
(MH "Random Assignment")
-
TX randomi* control* trial*
-
TX ((singl* n1 blind*) or (singl* n1 mask*)) or TX ((doubl* n1 blind*) or (doubl* n1 mask*)) or TX ((tripl* n1 blind*) or (tripl* n1 mask*)) or TX ((trebl* n1 blind*) or (trebl* n1 mask*))
-
TX clinic* n1 trial*
-
PT Clinical trial
-
(MH "Clinical Trials+")
-
S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24
-
S8 AND S13 AND S25
Appendix 5. PsycINFO EBSCO search strategy
-
MM "Intensive Care"
-
MM "Artificial Respiration"
-
(intensive or critical) N3 (care or unit*) OR critical* N3 ill*
-
mechanical* N3 ventilat* OR artificial* N3 respiration*
-
(S1 OR S2 OR S3 OR S4)
-
MM "Propofol"
-
MM "Sedatives" OR MM "Hypnotic Drugs"
-
DE "Sleep" OR DE "Napping" OR DE "NREM Sleep" OR DE "REM Sleep"
-
sleep* OR hynotic* OR sedat* OR propofol*
-
(S6 OR S7 OR S8 OR S9)
-
DE ("Treatment Effectiveness Evaluation")
-
DE ("Treatment Outcomes")
-
DE ("Placebo")
-
DE ("Followup Studies")
-
placebo* OR random* OR "comparative stud*" OR clinical N3 trial* OR research N3 design OR evaluat* N3 stud* OR prospectiv* N3 stud* OR (singl* OR doubl* OR trebl* OR tripl*) N3 (blind* OR mask*)
-
S11 OR S12 OR S13 OR S14 OR S15
-
S5 AND S10 AND S16
Appendix 6. Data extraction form template
Data Collection Form
| Study ID |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
1. General Information
| Date form completed (dd/mm/yyyy) | |
| Report title (title of paper/ abstract/ report that data extracted from) | |
| Reference details | |
| Report author contact details | |
| Publication type (e.g. full report, abstract, letter) | |
| Study funding sources (including role of funders) | |
| Possible conflicts of interest (for study authors) |
2. Study Eligibility
| Study Characteristics | Eligibility criteria | Yes No Unclear | Location in text (pg /fig / table) |
| Type of study | Randomized controlled trial Quasi‐randomized controlled trial | ||
| Participants | Adult >16yrs. Intensive Care Unit admission | ||
| Intervention | Propofol | ||
| Comparisons | No agent; or A different dose of propofol; or Another agent, specifically administered for the promotion of sleep | ||
| INCLUDE EXCLUDE | |||
| Reason for exclusion | |||
3. Population and setting
| Description (include comparative information for each group (i.e. intervention and controls) if available | Location in text (pg /fig / table) | ||
| Population and description (from which study participants are drawn) | |||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method/s of recruitment of participants | |||
| Informed consent obtained | Yes/No/Unclear | ||
4. Methods
| Descriptions as stated in report/paper | Location in text (pg /fig / table | ||
| Aim of study | |||
| Design(e.g. parallel, crossover, cluster) | |||
| Unit of allocation (by individuals, cluster /groups or body parts) | |||
| Ethical approval needed/obtained for study | Yes/No/Unclear | ||
| Registered with clinical trial registry | Yes/No | Clinical trials ID number: | |
5. Risk of Bias assessment
| Domain | Risk of bias Low/ High/ Unclear | Support for judgement | Location in text (pg /fig / table |
| Sequence generation (selection bias) | |||
| Allocation concealment (selection bias) | |||
| Baseline Imbalances | |||
| Blinding of participants and personnel (performance bias) | |||
| Blinding of outcome assessment (detection bias) | |||
| Incomplete outcome data (attrition bias) | |||
| Selective outcome reporting (reporting bias) | |||
| Other bias |
6. Participants
| Description as stated in report/paper | Location in text(pg /fig / table | ||
| Total no. randomized | |||
| Withdrawals and exclusions (if not provided below by outcome) | |||
| Age age range (mean) | Intervention | Comparison | |
| Sex Male/Female | Intervention | Comparison | |
| Other baseline characteristics | |||
| Post‐operative admission or emergency admission | |||
| Co‐morbidities | |||
| Time in ICU prior to intervention | |||
7.1 Intervention group
| Description as stated in report/paper | Location in text (pg /fig / table | |
| Intervention | Propofol | |
| No. randomized to group | ||
| Description (dose, method of administration) | ||
| Duration of treatment period | ||
| Time of administration | ||
| Concomitant agents (type, dose, method of administration etc) |
7.2 Comparison groups – repeated as required
| Description as stated in report/paper | Location in text (pg /fig / table | |
| Comparison group type (placebo, no treatment, different drug) | ||
| No. randomized to group | ||
| Description (type, dose, method of administration) | ||
| Duration of treatment period | ||
| Time of administration | ||
| Concomitant agents (type, dose, method of administration etc) |
8.1 Outcomes (repeat for each outcome)
| Description as stated in report/paper | Location in text (pg /fig / table | |
| Outcome name | ||
| Time points measured | ||
| Time points reported | ||
| Outcome definition | ||
| Person measuring/reporting | ||
| Unit of measurement | ||
| Scales: upper and lower limits (indicate whether high or low score is good) | ||
| Is outcome tool validated? | Yes/No/Unclear | |
| Imputation of missing data (e.g. assumptions made for ITT analysis) | ||
| Assumed risk estimate (e.g. baseline or population risk noted in Background) | ||
| Power |
9.1 Results (repeat for each outcome)
| Description as stated in report/paper | Location in text (pg & ¶ /fig / table | |||
| Comparison | ||||
| Outcome | ||||
| Results | Intervention | Comparison | ||
| No. missing participants and reasons | ||||
| Any other results reported | ||||
| Unit of analysis | ||||
| Statistical methods used & appropriateness of these methods | ||||
10. Applicability
| Have important population groups been excluded from the study? | Yes No Unclear | |
| Does the study directly address the review question? (any issues of partial or indirect applicability) | Yes No Unclear | |
| Are there any limitations in the design of the study? | Yes No Unclear |
11. Other information
| Description as stated in report/paper | Location in text (pg & ¶ /fig / table | |
| Key conclusion of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information (from whom, what and when) |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
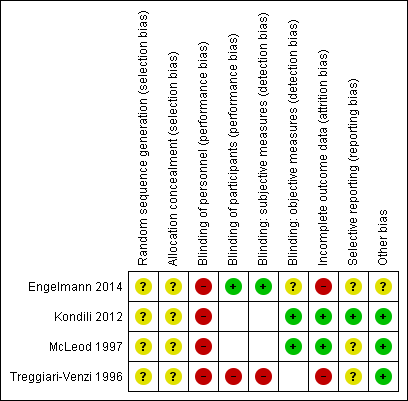
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Note: blank spaces indicate outcome measures that are not reported by study authors
| Propofol for the promotion of sleep in the intensive care unit versus no agent | ||||
| Patient or population: critically ill adults in the intensive care unit Settings: intensive care unit, Greece Intervention: propofol given to promote overnight sleep Comparison: no agent | ||||
| Outcomes | Impacts | No of Participants | Quality of the evidence | Comments |
| Quantity and quality of sleep as measured through reports of participants or family members or by personnel assessments Data collected at end of study follow‐up | Not reported. | ‐ | ‐ | ‐ |
| Quantity and quality of sleep as measured by PSG, actigraphy, BIS or EEG Data collected at end of study follow‐up | Outcome measured by PSG. Study authors reported no evidence of a difference in duration of sleep or sleep efficiency, and reported disruption to usual REM sleep with propofol. | 13 (1 study) | ⊕⊝⊝⊝ | We identified only 1 study and could not conduct a meta‐analysis. |
| Anxiety or depression, or both, as measured using validated tools Data collected at end of study follow‐up | Not reported. | ‐ | ‐ | ‐ |
| Adverse events (such as cardiovascular events, respiratory events or illness resulting from immune deficiency) | Not reported. | ‐ | ‐ | ‐ |
| BIS: Bispectral Index; EEG: electroencephalogram; PSG: polysomnography; REM: rapid eye movement. | ||||
| GRADE Working Group grades of evidence | ||||
| 1High level of performance bias; downgraded by one level. Data from single study with few participants; downgraded two levels for imprecision. | ||||
| Propofol for the promotion of sleep in the intensive care unit versus propofol at a different rate or dose | ||||
| Patient or population: critically ill adults in the intensive care unit Settings: intensive care unit, UK Intervention: propofol given to promote overnight sleep Comparison: propofol at a different rate or dose | ||||
| Outcomes | Impacts | No of Participants | Quality of the evidence | Comments |
| Quantity and quality of sleep as measured through reports of participants or family members or by personnel assessments Data collected at end of study follow‐up | Outcome measured using Ramsay Sedation Scale. Study authors reported that more participants who were given a higher dose of propofol had a successful diurnal rhythm, and achieved a greater sedation rhythmicity. | 30 (1 study) | ⊕⊝⊝⊝ | We identified only 1 study and could not conduct meta‐analysis. |
| Quantity and quality of sleep as measured by PSG, actigraphy, BIS or EEG Data collected at end of study follow‐up | Not reported. | ‐ | ‐ | ‐ |
| Anxiety or depression, or both, as measured using validated tools Data collected at end of study follow‐up | Not reported. | ‐ | ‐ | ‐ |
| Adverse events (such as cardiovascular events, respiratory events or illness resulting from immune deficiency) | Not reported. | ‐ | ‐ | |
| BIS: Bispectral Index; EEG: electroencephalogram; PSG: polysomnography. | ||||
| GRADE Working Group grades of evidence | ||||
| 1High level of performance bias; downgraded by one level. Concern about use of a sedation scale rather than a sleep scale; downgraded by one level for indirectness. One study, with few participants; downgraded one level for imprecision. | ||||
| Propofol for the promotion of sleep in the intensive care unit versus another agent specifically administered to promote sleep in the intensive care unit | ||||
| Patient or population: critically ill adults in the intensive care unit Settings: intensive care units in Germany and Switzerland Intervention: propofol given to promote overnight sleep Comparison: another agent specifically administered to promote overnight sleep | ||||
| Outcomes | Impacts | No of Participants | Quality of the evidence | Comments |
| Quantity and quality of sleep as measured through reports of participants or family members or by personnel assessments Data collected at end of study follow‐up | Outcome measured by different methods in each study (Pittsburgh Sleep Diary; Hospital Anxiety and Depression Scale). 1 study reported that participants given propofol had fewer awakenings, reduced duration of awakenings and similar total sleep duration. 1 study reported no evidence of a difference in sleep quality. | 106 (2 studies) | ⊕⊝⊝⊝ | We did not conduct a meta‐analysis because studies differed in types of measurement tools. |
| Quantity and quality of sleep as measured by PSG, actigraphy, BIS or EEG Data collected at end of study follow‐up | Outcome measured by BIS. 1 study reported that participants given propofol had longer time in deep sleep, with fewer arousals from sleep. | 66 (1 study) | ⊕⊝⊝⊝ | We identified only 1 study and could not conduct a meta‐analysis. |
| Anxiety or depression, or both, as measured using validated tools Data collected at end of study follow‐up | Study authors reported high levels of anxiety and depression in both participant groups, and no evidence of a difference with those given propofol. | 40 | ⊕⊝⊝⊝ | We identified only 1 study and could not conduct a meta‐analysis. |
| Adverse events (such as cardiovascular events, respiratory events or illness resulting from immune deficiency) | Not reported. | ‐ | ‐ | ‐ |
| BIS: Bispectral Index; EEG: electroencephalogram; PSG: polysomnography. | ||||
| GRADE Working Group grades of evidence | ||||
| 1High level of performance bias in studies; downgraded by one level. Outcome data were not consistent between studies; downgraded one level for inconsistency. Concern about validity of measurement tool in Treggiari‐Venzi 1996; downgraded by one level for indirectness. Limited number of studies, with few participants; downgraded one level for imprecision. 2High level of performance bias; downgraded by one level. Data from single study with few participants; downgraded by two levels for imprecision. Use of BIS to measure quality and quantity may not be appropriate, and may not provide a direct measurement, for this outcome; downgraded by one level for indirectness. 3High level of performance bias in studies; downgraded by one level. Data from single study with few participants; downgraded two levels for imprecision. | ||||
| Outcome: quantity and quality of sleep as measured by PSG, actigraphy, BIS or EEG | ||||
| Study ID | Interventions | Measurement tool | Narrative results as reported by study authors | Data; median (IQR) |
| Cross‐over design over 2 nights. Propofol vs no propofol | PSG | No statistically significant difference in sleep time. | Total sleep time (minutes) Propofol: 260 (113‐417) No propofol: 214 (40‐285) | |
| No statistically significant difference in sleep efficiency (note: study authors do not give a definition of sleep efficiency). | Total sleep time. Sleep efficiency (%) Propofol: 76.3 (28.4‐96.9) No propofol: 62.6 (13.1‐85.9) P = 0.37 | |||
| No statistically significant difference in Stage 1 sleep. | Total sleep time (%) Propofol: 20.8 (5.6‐80.6) No propofol: 30.7 (4.6‐66.7) P = 1.00 | |||
| No statistically significant difference in Stage 2 sleep. | Total sleep time (%) Propofol: 48.9 (4.8‐84.0) No propofol: 46.1 (3.0‐80.4) P = 0.66 | |||
| No statistically significant difference in SWS. | Total sleep time (%) Propofol: 0 (0 – 5.8) No propofol: 0 (0 – 0) P = 0.75 | |||
| Statistically less REM sleep in propofol group. | Total sleep time (%) Propofol: 0 (0‐0) No propofol: 1.4 (0‐13.0) P = 0.04 | |||
| BIS: Bispectral Index; EEG: electroencephalogram; HADS: Hospital Anxiety and Depression Scale; IQR: interquartile range; PSG: polysomnography; REM: rapid eye movement; SWS: slow wave sleep. | ||||
| Outcome: quantity and quality of sleep as measured through reports of participants or family members or by personnel assessments | ||||
| Study ID | Interventions | Measurement tool | Narrative results as reported by study authors | Data |
| Propofol ANS vs propofol CLS | Ramsay Sedation Scale | Greater rhythmicity of sedation in the intervention group. | Median (range) r% ANS: 27 (6‐35) CLS: 8 (0‐56) | |
| Achievement of diurnal rhythm. | ANS: 9/15 CLS: 3/14 | |||
| ANS: additional night sedation; CLS: constant light sedation; r%: percentage fit to a normal 24‐hour sleep rhythm (≥ 40% is indicative of normal 24‐hour rhythm); SD: standard deviation. | ||||
| Outcome: quantity and quality of sleep as measured through reports of participants or family members or by personnel assessments | ||||
| Study ID | Interventions | Measurement tool | Narrative results as reported by study authors | Data |
| Propofol vs flunitrazepam | Pittsburgh Sleep Diary | Fewer awakenings in the propofol group. | Maximum number of awakenings per participant Propofol: 6 Flunitrazepam: 30 | |
| Shorter duration of awakenings in the propofol group. | Maximum duration of awakenings Propofol: 45 minutes Flunitrazepam: 390 minutes | |||
| Total sleep duration similar between groups. | Total sleep duration Propofol: 6 hours Flunitrazepam: 5 hours P = 0.623 | |||
| Sleep quality significantly better in propofol group. | Median score for sleep quality Propofol: 2.0 Flunitrazepam: 3.0 P < 0.0001 | |||
| Regeneration and refreshment significantly better in propofol group. | Results not reported by authors | |||
| Quality of falling asleep did not differ between groups. | Median score for falling asleep Propofol: 2.0 Flunitrazepam: 2.0 P = 0.341 | |||
| Propofol vs midazolam | HADS | Sleep quality improved during 5‐day study. No significant differences in sleep quality between 2 groups* (*unclear how HADS measured this outcome) | Mean (± SD) HADS Propofol: day 1: 6.5 (± 3.3) day 3: 6.6 (± 2.9) day 5: 7.2 (± 2.3) Midazolam: day 1: 6.3 (± 3.4) day 3: 6.3 (± 3.2) day 5: 7.2 (± 2.9) | |
| Outcome: quantity and quality of sleep as measured by PSG, actigraphy, BIS or EEG | ||||
| Study ID | Interventions | Measurement tool | Narrative results as reported by study authors | Data as median (IQR) |
| Propofol vs flunitrazepam | BIS | Significantly lower median BIS values in propofol group. | Propofol: 74.05 Flunitrazepam: 78.70 P = 0.016 | |
| Flunitrazepam reduced sedative effect over time. | Flunitrazepam at 1st hour: 72.05 Flunitrazepam at 5th hour: 81.00 | |||
| Longer time in deep sleep in propofol group. | Time in deep sleep (hours:minutes:seconds) Propofol: 2:23:30 Flunitrazepam: 1:23:30 | |||
| Longer time in light and REM sleep in flunitrazepam group. | Time in light and REM sleep (hours:minutes:seconds) Propofol: 1:44:00 Flunitrazepam: 2:34:00 | |||
| Outcome: anxiety or depression, or both, as measured using validated tools | ||||
| Study ID | Interventions | Measurement tool | Narrative results as reported by study authors | Data as mean (± SD) HAD score |
| Propofol vs midazolam | HADS | High levels of anxiety in both groups. No significant differences in mean scores between groups. | Propofol: day 1: 6.7 (± 3.9) day 3: 6.8 (± 3.1) day 5: 5.7 (± 4.1) Midazolam: day 1: 6.7 (± 4.7) day 3: 6.5 (± 4.5) day 5: 7.5 (± 5.2) | |
| High levels of depression in both groups. No significant differences in mean scores between groups. | Propofol: day 1: 5.9 (± 4.0) day 3: 6.0 (± 3.0) day 5: 5.5 (± 3.9) Midazolam: day 1: 7.5 (± 5.5) day 3: 6.8 (± 4.8) day 5: 7.2 (± 5.1) | |||
| ANS: additional night sedation; BIS: Bispectral Index; CLS: constant light sedation; EEG: electroencephalogram; HADS: Hospital Anxiety and Depression Scale; IQR: interquartile range; min: minute; PSG: polysomnography; REM: rapid eye movement; SD: standard deviation. | ||||

