藥物治療用於兒童及青少年肥胖
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [author‐defined order]
| Methods | Parallel randomised controlled trial, randomisation ratio 3:1 (intervention:control), superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: metformin + diet and physical activity advice Comparator: placebo + diet and physical activity advice Number of trial centres: 1 Treatment before trial: none Titration period: no | |
| Outcomes | Outcomes reported in abstract of publication: BMI, fasting insulin, 120‐min insulin levels, FGIR, HOMA‐IR, QUICKI | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: English Funding: no information given Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "To determine whether metformin treatment for 6 months is effective in reducing body weight and hyperinsulinaemia and also ameliorating insulin sensitivity indices in obese adolescents with hyperinsulinaemia" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description of randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of how allocation was concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "a 6 month, randomized, double‐blind placebo‐controlled, parallel‐group, prospective clinical trial" Comment: unsure who was blinded |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "a 6 month, randomized, double‐blind placebo‐controlled, parallel‐group, prospective clinical trial" Comment: unsure who was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "a 6 month, randomized, double‐blind placebo‐controlled, parallel‐group, prospective clinical trial." Comment: unsure who was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "a 6 month, randomized, double‐blind placebo‐controlled, parallel‐group, prospective clinical trial" Comment: unsure who was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: the trial did not report the number of dropouts, or clarify there were no dropouts |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: the trial did not report the number of dropouts, or clarify there were no dropouts |
| Selective reporting (reporting bias) | High risk | Quote: "A detailed questionnaire on food consumption was completed at the beginning and at the end of the trial medication period" Comment: no results shown for food consumption data. Also, very unclear on the number lost to follow‐up and what type of analyses were conducted |
| Other bias | High risk | Comment: there was uncertainty to whether this was a randomised controlled trial or a matched controlled trial. Concern arose over a lack of description about randomisation, blinding and allocation. No rationale for the size of intervention group and no calculation of power. They also do not declare who funded the trial |
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: behavioural programme + sibutramine Comparator: behavioural programme + placebo Number of trial centres: 1 Treatment before trial: none Titration period: in medication‐treated participants, sibutramine was increased to 10 mg/day at week 3, and to 15 mg/day at week 7 | |
| Outcomes | Outcomes reported in abstract of publication: weight (kg), BMI (kg/m2), reductions in hunger, number of participants who reduced dose or discontinued | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: English Commercial funding and noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "To increase weight loss in obese adolescents by combining a comprehensive behavioral program with pharmacotherapy" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description of the randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: trial did not describe how allocation was concealed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Participants, parents, and all study personnel were blinded to treatment condition during phase 1. Only the research pharmacist was aware of treatment status" Comment: risk of performance bias likely to be low due to blinding of participants, parents and trial personnel |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Participants, parents, and all study personnel were blinded to treatment condition during phase 1. Only the research pharmacist was aware of treatment status" Comment: risk of performance bias likely to be low due to blinding of participants, parents and trial personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Participants, parents, and all study personnel were blinded to treatment condition during phase 1. Only the research pharmacist was aware of treatment status" Comment: risk of detection bias likely to be low due to blinding of all trial personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Participants, parents, and all study personnel were blinded to treatment condition during phase 1. Only the research pharmacist was aware of treatment status" Comment: risk of detection bias likely to be low due to blinding of all trial personnel |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: whilst dropout numbers were small, a more appropriate imputation method could have been used to strengthen data analysis. Imputation method only used for primary outcome measures (weight and waist circumference, which were objectively measured) |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: whilst dropout numbers were small, a more appropriate imputation method could have been used to strengthen data analysis. Imputation method only used for primary outcome measures (weight and waist circumference, which were objectively measured) |
| Selective reporting (reporting bias) | Low risk | Comment: no differences found between clinical trial entry and publication |
| Other bias | Unclear risk | Comment: trial was partly funded by 2 pharmaceutical companies. The trial declared these companies had no involvement in the design, analysis or interpretation of the data; however, still could have influenced the reporting of results in some way |
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 3:1 (sibutramine:placebo), superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: behaviour therapy programme + sibutramine Comparator: behaviour therapy programme + placebo Number of trial centres: 33 Treatment before trial: no Titration period: no | |
| Outcomes | Outcomes reported in abstract of publication: BMI, weight, triglyceride levels, high‐density lipoprotein cholesterol levels, insulin levels, insulin sensitivity, rate of tachycardia, completion rate | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: English Commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "To see whether sibutramine reduced weight more than placebo in obese adolescents who were receiving a behavior therapy program" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization schedule was stratified by center and baseline BMI (≤37 kg/m2 or >37 kg/m2) and was computer‐generated in blocks of 4 by the sponsor. Each site was responsible for assigning sequential treatments within each stratum" Comment: an adequate randomisation method was used |
| Allocation concealment (selection bias) | Low risk | Quote: "The sponsor kept allocation codes sealed and secure until the database was locked before analysis" Comment: allocation concealment was sufficient to protect against bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Participants, their parents, and study personnel were blinded to treatment" Comment: risk of performance bias likely to be low due to blinding of participants, parents and trial personnel |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Participants, their parents, and study personnel were blinded to treatment" Comment: risk of performance bias likely to be low due to blinding of participants, parents and trial personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Participants, their parents, and study personnel were blinded to treatment" Comment: risk of performance bias likely to be low due to blinding of participants, parents and trial personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Participants, their parents, and study personnel were blinded to treatment" Comment: risk of performance bias likely to be low due to blinding of participants, parents and trial personnel |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: LOCF was only used to replace BMI missing data; other objective outcome data were expressed for completers only. Dropout rate was fairly moderate and higher in the placebo group compared to the drug group. Difficult to access level of attrition bias based on these factors |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: LOCF was only used to replace BMI missing data only |
| Selective reporting (reporting bias) | Low risk | Comment: same outcomes reported in both clinical trial register and publication |
| Other bias | Unclear risk | Quote: "the Statistics Department of Abbott Global Pharmaceutical Research and Development (including Ms. Hewkin) was responsible for data management and statistical analysis" Comment: potential influence of the funding body (Abbot Global Pharmaceuticals) |
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 2:1 (orlistat: placebo), superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: orlistat + diet + exercise + behaviour therapy Comparator: placebo + diet + exercise + behaviour therapy Number of trial centres: 32 Treatment before trial: no Titration period: no | |
| Outcomes | Outcomes reported in abstract of publication: BMI, weight, fat mass (DEXA), waist circumference, adverse events | |
| Study details | Run‐in period: placebo was given for 2 weeks before treatment began in the intervention group Trial terminated early: no | |
| Publication details | Language of publication: English Commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "To determine the efficacy and safety of orlistat in weight management of adolescents" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized centrally according to a computer‐generated randomization schedule prepared by the study’s sponsor, with stratification by body weight (<80 kg or ≥80 kg) on day 1 and by weight loss during the lead‐in period (<1 kg or ≥1 kg)" Comment: an adequate randomisation method was used |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation process was triple‐blind; the allotted treatment group was obtained through an automated telephone system" Comment: allocation concealment was sufficient to protect against bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind study" Comment: the author confirmed all participants, trial personnel and outcome assessors were blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind study" Comment: the author confirmed all participants, trial personnel and outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind study" Comment: the author confirmed all participants, trial personnel and outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind study" Comment: the author confirmed all participants, trial personnel and outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: even though an imputation method was used (LOCF), dropout rates were high. Effect on objective outcomes unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: even though an imputation method was used (LOCF), dropout rates were high. Effect on subjective outcomes unclear |
| Selective reporting (reporting bias) | Unclear risk | Comment: unable to assess if all outcomes were reported due to the trial protocol not previously been published |
| Other bias | Unclear risk | Quote: "Hoffmann‐La Roche was involved in the study design and conduct and in the analysis and interpretation of the data. All data were independently reanalyzed by an academic statistician" Comment: potential influence from the funding body (Hoffmann‐La Roche). No rationale to explain the imbalance in the number of participants in the 2 groups |
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: metformin + lifestyle intervention Comparator: lifestyle intervention Number of trial centres: 1 Treatment before trial: no Titration period: started metformin therapy at 500 mg/day, increasing by 500 mg/day every 7 days to a maximum tolerated dose of 500 mg x 3 per day | |
| Outcomes | Outcomes reported in abstract of publication: BMI, HOMA, adiponectin‐to‐leptin ratio, dyslipidaemic profiles, metabolic risk factors e.g. plasma lipids and adipocytokines | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "To access the efficacy of adding metformin to a structured lifestyle intervention in reducing BMI in obese adolescents with insulin resistance" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomized using computer random number generation to lifestyle intervention alone or lifestyle in combination with metformin" Comment: an adequate randomisation method was used |
| Allocation concealment (selection bias) | High risk | Comment: author confirmed allocation was not concealed |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "limitations to this study include the relatively small sample size and the absence of a placebo control group" Comment: the absence of a placebo in the control group meant participant and personnel blinding could not have been achieved. Author confirmed participants and personnel were not blinded |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "limitations to this study include the relatively small sample size and the absence of a placebo control group" Comment: the absence of a placebo in the control group meant participant and personnel blinding could not have been achieved. Author confirmed participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Comment: outcomes assessment was not blinded as confirmed by the author |
| Blinding of outcome assessment (detection bias) | High risk | Comment: outcomes assessment was not blinded as confirmed by the author |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: trial dropouts were fairly low; however, no imputation method was used |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: trial dropouts were fairly low; however, no imputation method was used |
| Selective reporting (reporting bias) | Unclear risk | Comment: no previously published protocol; therefore, unable to access reporting bias |
| Other bias | High risk | Quote: "limitations to this study include the relatively small sample size and the absence of a placebo control group" Comment: a power calculation was not performed, therefore likely the trial was underpowered. No placebo given to the control group. Unclear whether there were baseline differences |
| Methods | Cross‐over randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: sibutramine + dietary guidance Comparator: placebo + dietary guidance Number of trial centres: 1 Treatment before trial: all participants had to have under gone at least 6 months of lifestyle intervention prior to recruitment Titration period: none | |
| Outcomes | Outcomes reported in abstract of publication: % of participants who lost 10% of initial weight, weight, BMI | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: Portuguese Noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "The aim of this study was to evaluate the efficacy and safety of sibutramine in association with a multidisciplinary program for treatment of obesity and check its influence on metabolic laboratory changes" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From author: "patients were distributed according to a table of random numbers" Comment: randomisation process assessed as low risk |
| Allocation concealment (selection bias) | Low risk | From author: "the study was double‐blind placebo‐controlled. Patients received placebo or sibutramine for 6 months, 1 month washout and in the next six months who received placebo began receiving sibutramine and vice verse. The researchers had no knowledge who was getting the drug and who was getting the placebo" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "This study was double blinded placebo controlled cross‐over type with duration of 13 months" Comment: author confirmed participants, trial personnel and outcome assessors were all blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "This study was double blinded placebo controlled cross‐over type with duration of 13 months" Comment: author confirmed participants, trial personnel and outcome assessors were all blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "This study was double blinded placebo controlled cross‐over type with duration of 13 months" Comment: author confirmed participants, trial personnel and outcome assessors were all blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "This study was double blinded placebo controlled cross‐over type with duration of 13 months" Comment: author confirmed participants, trial personnel and outcome assessors were all blinded |
| Incomplete outcome data (attrition bias) | High risk | Quote: "of the 63 patients who initiated the study only 23 patients completed the study" Comment: high attrition rate likely to affect objective outcomes |
| Incomplete outcome data (attrition bias) | High risk | Quote: "of the 63 patients who initiated the study only 23 patients completed the study" Comment: high attrition rate likely to affect subjective outcome (i.e. adverse effects) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available so risk was unclear |
| Other bias | High risk | Comment: lacked appropriate methodological detail and the failed to present the results in a meaningful and balanced manner. The cross‐over nature of the trial added to the difficulty in deciphering the results with such a high attrition rate. No power calculation performed |
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria: ‐ Diagnostic criteria: see above | |
| Interventions | Intervention: metformin Comparator: placebo Number of trial centres: 1 Treatment before trial: none Titration period: no | |
| Outcomes | Outcomes reported in abstract of publication: BMI, serum leptin, fasting blood glucose, fasting insulin levels, insulin sensitivity, glucose effectiveness, haemoglobin A1c, serum lipids, serum lactate, adverse events | |
| Study details | Run‐in period: 48 hours' inpatient tests Trial terminated early: no | |
| Publication details | Language of publication: English Commercial funding and noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "We reasoned that drugs that increase glucose tolerance in diabetic patients might prove useful in preventing the progression to glucose intolerance in high‐risk patients" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized to the metformin and placebo groups by a research pharmacist using computer‐generated randomization tables" Comment: an appropriate randomisation method was used |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocation was made by the research pharmacist at the first medication visit. The pill bottles were coded ‐ thus the pharmacist was blinded to the medication" Comment: author confirmed allocation was concealed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "We conducted a double‐blind, placebo‐controlled study" Comment: author confirmed all participants and trial personnel were blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "We conducted a double‐blind, placebo‐controlled study" Comment: author confirmed all participants and trial personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "We conducted a double‐blind, placebo‐controlled study" Comment: author confirmed all participants and trial personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "We conducted a double‐blind, placebo‐controlled study" Comment: author confirmed all participants and trial personnel were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: a missing data method was not used; however, dropout rates were fairly low |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: a missing data method was not used; however, dropout rates were fairly low |
| Selective reporting (reporting bias) | Unclear risk | Comment: since no protocol was published before trial was completed, it is unclear whether all outcomes were reported |
| Other bias | High risk | Quote: "the study involved a small number of patients and the results must be confirmed in a larger sample" Comment: the trial did not perform a power calculation and the sample size was small. It is likely the trial was underpowered. Potential influence of a commercial funding source. Baseline differences identified and not adjusted for in the analysis |
| Methods | Parallel randomised controlled clinical trial, randomisation ratio: 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: sibutramine + diet + exercise Comparator: placebo + diet + exercise Number of trial centres: 1 Treatment before trial: participants received dietetic advice 15 days before the beginning of the medications. In addition, clinical control visits also occurred before the start of the trial Titration period: no | |
| Outcomes | Outcomes reported in abstract of publication: mean weight loss, net weight loss, waist circumference, % BMI loss, SBP, DBP, heart rate, adverse events | |
| Study details | Run‐in period: yes ‐ dietetic advice Trial terminated early: no | |
| Publication details | Language of publication: English Commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "The goal of this article was to assess the efficacy and safety of sibutramine in obese Mexican adolescents" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were block‐randomized by using a computer generated list" Comment: an appropriate randomisation method was used |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were block‐randomized by using a computer generated list. All the materials for a patient were identified by the patient number. The placebo and drug capsules were identical in appearance and smell. The trial medications were prepared by one author (A.B.), who did not know the identity of the patients. Another author (L.M.G.‐M.) received the trial materials without any knowledge of the procedures or order in the random number list" Comment: allocation was appropriately concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "This was a 6 month, randomized, double blind, placebo‐controlled, prospective clinical trial of sibutramine QD" Comment: unclear who was blinded |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "This was a 6 month, randomized, double blind, placebo‐controlled, prospective clinical trial of sibutramine QD" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "This was a 6 month, randomized, double blind, placebo‐controlled, prospective clinical trial of sibutramine QD" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "This was a 6 month, randomized, double blind, placebo‐controlled, prospective clinical trial of sibutramine QD" Comment: unclear who was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "the last observation replaced the missing values" Comment: LOCF and modified intention‐to‐treat analysis was used to replace missing data for the primary outcomes. However, the 5 participants who dropped out before the first month were not included |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: baseline and follow‐up data for subjective outcomes were not reported in the publication |
| Selective reporting (reporting bias) | High risk | Quote: "A detailed questionnaire on food consumption was completed at the beginning and end of the trial" Comment: data on food consumption were not provided in the publication |
| Other bias | Unclear risk | Quote: "This trial was supported by Abbott Laboratories de Mexico, S.A. de C.V., Mexico City, D.F, Mexico. Dr. Berber was the medical manager of sibutramine in Mexico from 1995 to April 2004. The protocol was designed by all the authors; the study was conducted by the non industry authors; and analysis and publication formalities were performed by Drs. Garcia‐Morales, Del‐Rio‐Navarro, and Berber. The non industry authors had access to all the data generated" Comment: the trial sponsor (Abbot Laboratories) may have influenced the trial's results |
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: sibutramine + hypocaloric diet + exercise Comparator: placebo + hypocaloric diet + exercise Number of trial centres: 1 Treatment before trial: during the run‐in period all participants received dietary counselling to achieve an energy deficit of 500 kcal/day. They also all received placebo capsules Titration period: no | |
| Outcomes | Outcomes reported in abstract of publication: weight loss, mean BMI reduction, adverse events | |
| Study details | Run‐in period: a single‐blind, 4‐week, placebo run‐in period Trial terminated early: no | |
| Publication details | Language of publication: English Commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "The aim of this study was to determine the efficacy and safety of sibutramine in obese adolescents" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were allocated in a random block fashion to placebo or sibutramine" Comment: details of the randomisation process was provided by the author ‐ process seems adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "By means of a sealed envelope with a coded number. A container with boxes for each patient displaying the code number were provided. Each box had blisters for each visit with 40 capsules (similar for placebo or active drug). Patients were supplied in each visit with a new box. Adherence was judged by counting used capsules" Comment: allocation was concealed as confirmed by the author |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "a randomised, double‐blind, placebo‐controlled trial" Comment: author confirmed all participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "a randomised, double‐blind, placebo‐controlled trial" Comment: author confirmed all participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "a randomised, double‐blind, placebo‐controlled trial" Comment: author confirmed all participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "a randomised, double‐blind, placebo‐controlled trial" Comment: author confirmed all participants and personnel were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: dropout fairly low; however, was higher in the placebo group. Only completers results shown |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: dropout fairly low; however, was higher in the placebo group. Only completers results shown |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol was not published before the trial was completed, therefore it is unclear whether all outcomes were reported |
| Other bias | High risk | Quote: "this work was supported by a grant from Abbott Laboratories" Comment: the trial did not highlight how involved Abbott Laboratories were the trial design, analysis and interpretation of the results Quote: "Conclusions regarding treatment group differences are somewhat limited by the small sample size" Comment: the trial did not perform a power calculation. Likely the trial was underpowered |
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: metformin + healthy lifestyle advice Comparator: placebo + healthy lifestyle advice Number of trial centres: 6 Treatment before trial: all participants were provided with standardised healthy lifestyle advice at the start in a 1‐to‐1 session, including a healthy diet advice sheet and increased levels of exercise (available upon request) Titration period: participants were instructed to gradually increase the dose by taking 1 pill with breakfast for 1 week and then 1 pill with breakfast and the evening meal the next week and then 2 pills with breakfast and 1 pill with the evening meal thereafter (1.5 g/day) | |
| Outcomes | Outcomes reported in abstract of publication: BMI‐SDS, fasting glucose, ALT, ALR | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "The objective of the study was to assess the effect of metformin on body mass index SD score (BMI‐SDS), metabolic risk factors, and adipokines" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Independent pharmacists dispensed either metformin or placebo according to a computer‐generated randomization list for each stratification group" Comment: an appropriate randomisation method was used |
| Allocation concealment (selection bias) | Low risk | Quote: "The third party, concealed allocation process ensured that participants and all investigators were unaware of the allocated treatment" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "This was a prospective, randomized, double‐blind, placebo‐controlled trial" Comment: unclear who was blinded |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "This was a prospective, randomized, double‐blind, placebo‐controlled trial" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "This was a prospective, randomized, double‐blind, placebo‐controlled trial" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "This was a prospective, randomized, double‐blind, placebo‐controlled trial" Comment: unclear who was blinded |
| Incomplete outcome data (attrition bias) | High risk | Quote: "There were a number of limitations to the MOCA [Metformin in Obese Children and Adolescents] trial including the dropout rate" Comment: dropout rate was high and no imputation method was used to replace missing data |
| Incomplete outcome data (attrition bias) | High risk | Quote: "There were a number of limitations to the MOCA trial including the dropout rate" Comment: dropout rate was high and no imputation method was used to replace missing data |
| Selective reporting (reporting bias) | Unclear risk | Quote: "In the MOCA trial, three previously validated questionnaires (food frequency, diet and eating behavior, and physical activity) were completed by each child at the start and end of the trial. This amounted to a large amount of data, and resources were unfortunately insufficient to allow analysis of these data for inclusion in this paper" Comment: behaviour change results were not reported; however, the publication did give a valid reason to why |
| Other bias | Unclear risk | Comment: insufficient information to assess whether an important risk of bias exists |
| Methods | Parallel randomised controlled clinical trial, randomisation ratio: 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: orlistat + diet and exercise therapy Control: placebo + diet and exercise therapy Number of trial centres: 1 Treatment before trial: none Titration period: no | |
| Outcomes | Outcomes reported in abstract of publication: BMI reduction, adverse effects, laboratory measurements | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "To evaluate the efficacy of orlistat to enhance weight loss in obese adolescents" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The GCRC [General Clinical Research Center] statistician generated the randomization sequence before the start of the study" "Two sets of subjects (a sister‐sister pair and a girlfriend‐boyfriend pair) were assigned to the same cohort, as determined by the order of entry of the first member of the pair; the next paired subject was blocked into the same cohort and given the next available number in that cohort" Comment: not all participants were randomised |
| Allocation concealment (selection bias) | Low risk | Quote: "The list of randomization assignments was sealed and sent to the study pharmacist, who had no contact with study subjects" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Only the research pharmacist was aware of treatment status" Comment: participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Only the research pharmacist was aware of treatment status" Comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Only the research pharmacist was aware of treatment status" Comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Only the research pharmacist was aware of treatment status" Comment: participants and personnel were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: an imputation method was not used to replace missing data; however, dropout was fairly low |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: unable to access effect on subjective outcomes as quality of life results were not reported |
| Selective reporting (reporting bias) | High risk | Comment: results from the quality of life questionnaires were not reported |
| Other bias | Unclear risk | Comment: unclear if any other bias exists |
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: metformin + diet/exercise intervention Comparator: diet/exercise intervention Number of trial centres: 1 Treatment before trial: no Titration period: metformin was started at 250 mg orally, twice daily, before meals titrating up to 500 mg twice daily in children < 12 years old and 1000 mg twice daily as tolerated in older children | |
| Outcomes | Outcomes reported in abstract of publication: weight loss, hsCRP, fibrinogen, intrahepatic fat | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "To determine if metformin improves markers of inflammation, thrombosis, and intrahepatic fat contents in children with uncomplicated obesity" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from the author): "randomisation assignments were balanced for pubertal status. We used sealed envelopes with equal amount of labels organized at random for pubertal and pre‐pubertal kids to choose from at their CRC visit (baseline)" Comment: adequate randomisation process |
| Allocation concealment (selection bias) | Low risk | Comment: the author of the trial confirmed allocation was concealed via the sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no placebo was given to the control group, therefore the participants would not have been blinded |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no placebo was given to the control group, therefore the participants would not have been blinded |
| Blinding of outcome assessment (detection bias) | High risk | Comment: author confirmed the outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Comment: author confirmed the outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | High risk | Comment: there was a high number of dropouts and no imputation method was used to replace missing data |
| Incomplete outcome data (attrition bias) | High risk | Comment: there was a high number of dropouts and no imputation method was used to replace missing data |
| Selective reporting (reporting bias) | Low risk | Quote: "The study was registered at http://www.clinicaltrials.gov (NCT00139477)" Comment: all outcomes reported on the clinical trial register page were reported in the publication |
| Other bias | Unclear risk | Comment: unable to access if any other bias were present |
| Methods | Type of trial: interventional, randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: double blind (participant, carer, investigator, outcomes assessor) Primary purpose: treatment | |
| Participants | Condition:
Enrolment: 200 Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Intervention: orlistat Comparator: placebo | |
| Outcomes | Primary outcome: change in BMI SDS (baseline to 6 months) Secondary outcomes:
| |
| Study details | NCT number: NCT00001723 Other trial ID numbers: 980111, 98‐CH‐0111 | |
| Publication details | "Safety and Efficacy of Orlistat (Xenical, Hoffmann LaRoche) in African American and Caucasian Children and Adolescents with Obesity‐Related Comorbid Conditions" | |
| Stated aim for study | Quote: "Researchers propose to determine the safety, tolerability, and efficacy of Xenical [orlistat] in 12‐17 year old severely obese African American and Caucasian children and adolescents who have one or more obesity‐related disease (hypertension, hyperlipidemia, sleep apnea, hepatic steatosis, insulin resistance, impaired glucose tolerance, or Type 2 diabetes)" | |
| Notes | The trial was completed when identified. Trial collaborators:
Results presented on the clinicaltrials.gov website and in a conference abstract. Results from ClinicalTrials.gov Results Database: change in BMI SDS orlistat: ‐0.12 ± 0.02 and placebo: ‐0.06 ± 0.02. ANCOVA differences between groups P value = 0.007. Change in bodyweight orlistat: ‐2.9 ± 0.7 and placebo: ‐0.6 ± 0.7. No statistical analysis provided. Change in BMI orlistat: ‐1.44 ± 0.26 and placebo: ‐0.50 ± 0.20. No statistical analysis provided. 95/100 participants in orlistat and 94/100 in placebo group experienced adverse events with the most common being gastrointestinal disorders. No serious adverse events in orlistat group. In placebo group, 1 participant had hypoglycaemia and 1 participant had left lower quadrant pain and vomiting, and was admitted to hospital overnight | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from author (via email): "We randomized participants in a 1:1 fashion to orlistat 120 mg or identical appearing placebo thrice daily with meals plus a daily multivitamin (Centrum, Whitehall‐Robins Healthcare, Madison, NJ) containing 5000 IU vitamin A (80% as retinol, 20% as beta carotene), 400 IU vitamin D as ergocalciferol, 30 IU vitamin E (as di‐α tocopheryl acetate), and 25 mcg vitamin K (as phytonadione). Investigators assigned consecutive code numbers to participants from pre‐specified lists that were stratified by race (Caucasian versus African American), sex (Male, Female), and degree of pubertal development (3 strata for boys: testes <15ml, testes 15‐20mL, and testes >20mL; for girls: Breast Tanner stage I‐III; Tanner stage IV, and Tanner stage V). The NIH CRC Pharmaceutical Development Section used permuted blocks with stratification to generate allocations that translated code numbers into trial group assignments by using a pseudo‐random number program" Comment: randomisation process described |
| Allocation concealment (selection bias) | Low risk | Quote from author (via email): "Pharmacy personnel not involved with the conduct of the study, dispensed identical‐appearing study capsules in containers that differed only by participant code number. During the trial, no participant, investigator, or other medical or nursing staff interacting with participants was aware of study group assignments" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" Comment: participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" Comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" Comment: assessors were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" Comment: assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Comment: according to ClinicalTrials.gov, 87% of orlistat participants completed the trial, 84% completed placebo arm |
| Incomplete outcome data (attrition bias) | Low risk | Comment: according to ClinicalTrials.gov, 87% of orlistat participants completed the trial, 84% completed placebo arm |
| Selective reporting (reporting bias) | High risk | Comment: there are differences in the results reported on the ClinicalTrial.gov website and in the conference abstract |
| Other bias | Unclear risk | Comment: unclear as limited information available |
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria: ‐ Diagnostic criteria: see above | |
| Interventions | Intervention: conventional treatment + orlistat Control: conventional treatment Number of trial centres: 1 Treatment before trial: no Titration period: no | |
| Outcomes | Outcomes reported in abstract of publication: adverse effects, bodyweight loss, % bodyweight lost, BMI | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "To investigate the efficacy and tolerability of orlistat in obese adolescents, a prospective, open‐label, randomised, controlled pilot trial was performed" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Randomisation was done by alternation of successive patients, who met the inclusion criteria, to receive conventional treatment alone or orlistat in addition to conventional treatment" Comment: an inappropriate randomisation method was used |
| Allocation concealment (selection bias) | High risk | Comment: allocation was likely not concealed due to the randomisation method used |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "the true benefit of orlistat versus conventional therapy remains to be determined in a larger placebo‐controlled study" Comment: the control group did not receive a placebo therefore could not have been blinded |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "the true benefit of orlistat versus conventional therapy remains to be determined in a larger placebo‐controlled study" Comment: the control group did not receive a placebo therefore could not have been blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear if outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | High risk | Comment: an imputation method to replace missing data were not performed, and dropout rate was moderate |
| Incomplete outcome data (attrition bias) | High risk | Comment: an imputation method to replace missing data were not performed, and dropout rate was moderate |
| Selective reporting (reporting bias) | Unclear risk | Comment: BMI was reported in different formats; median BMI at baseline and mean BMI at follow‐up. No protocol published |
| Other bias | High risk | Comment: there were significant differences in baseline BMI between groups which were not accounted for. A power calculation was not performed, therefore trial may have been underpowered |
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: obesity defined as BMI > 95th percentile for age and sex. Risk factors for type 2 diabetes include first‐ or second‐degree relative with a history of type 2 diabetes, or alteration in the results of the following examinations within the past 6 months: glycaemia fasting ≥ 100 mg/dL, postload glucose ≥ 140 mg/dL or HOMA > 3.0 | |
| Interventions | Intervention: metformin + nutritional guide + exercise programme Comparator: placebo + nutritional guide + exercise programme Number of trial centres: 1 Treatment before trial: none Titration period: no | |
| Outcomes | Outcomes reported in abstract of publication: weight, BMI, metabolic risk profile | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: Spanish Noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "To analyze the anthropometric and metabolic impact of metformin in obese adolescents at risk for type 2 diabetes" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Recruited adolescents were randomly assigned into two groups (A and B) through a sequence computational randomization" Comment: an appropriate randomisation method was used |
| Allocation concealment (selection bias) | Low risk | Quote: "An external laboratory was in charge of packing and labelling bottles, keeping content knowledge in confidence until the study ended" Comment: there was allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: author confirmed participants and trial personnel were blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: author confirmed participants and trial personnel were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear if outcome assessment was blinded and if this would have results in detection bias for the objective outcomes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear if outcome assessment was blinded and if this would have results in detection bias for the subjective outcomes |
| Incomplete outcome data (attrition bias) | High risk | Comment: there was no imputation method to replace missing data and dropout rates were fairly high |
| Incomplete outcome data (attrition bias) | High risk | Comment: there was no imputation method to replace missing data and dropout rates were fairly high |
| Selective reporting (reporting bias) | Unclear risk | Comment: do not give follow‐up data for some outcomes such as blood pressure |
| Other bias | Unclear risk | Comment: unable to make an assessment on other bias due to lack of information |
| Methods | Parallel randomised controlled trial, randomisation ratio: 1:1:1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention 1: metformin + healthy eating + physical activity advice Intervention 2: fluoxetine + healthy eating + physical activity advice Intervention 3: metformin + fluoxetine + healthy eating + physical activity advice Comparator: placebo + healthy eating + physical activity advice Number of trial centres: 1 Treatment before trial: 3 months of nonpharmacological treatment (by lifestyle modification advised in study author's clinic) Titration period: metformin dosage increased weekly from 500 mg/day to 1500 mg/day. Fluoxetine dosage of 10 mg/day increased to 20 mg/day after 3 weeks | |
| Outcomes | Outcomes reported in abstract of publication: BMI, waist circumference, adverse effects | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "We aimed to compare the effects of three types of drug regimens and placebo on generalized and abdominal obesity among obese children and adolescents who did not succeed to lose weight 3 months after lifestyle modification (diet and exercise)" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Sequence was generated by computer generated random number table" Comment: randomisation was an adequate method |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "triple‐masked randomized clinical trial" Comment: participants and personnel would have been blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "triple‐masked randomized clinical trial" Comment: participants and personnel would have been blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "triple‐masked randomized clinical trial" Comment: outcomes assessors would have been blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "triple‐masked randomized clinical trial" Comment: outcomes assessors would have been blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: an imputation method was not used to replace missing data; however, dropout rate was fairly low |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: an imputation method was not used to replace missing data; however, dropout rate was fairly low |
| Selective reporting (reporting bias) | Unclear risk | Comment: unable to assess if all outcomes were reported due to the unavailability of a protocol |
| Other bias | Unclear risk | Comment: unable to access if any other bias was present |
| Methods | Cross‐over randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: metformin + "standardised information on healthy eating and exercise" Comparator: placebo + "standardised information on healthy eating and exercise" Number of trial centres: 1 Treatment before trial: no Titration period: both metformin and placebo doses were gradually built up over a 3‐week period to a final dose of 1 g twice daily | |
| Outcomes | Outcomes reported in abstract of publication: mean age, median BMI z score, weight, BMI, waist circumference, subcutaneous abdominal adipose tissue, fasting insulin | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "We assessed the effect of metformin on body composition and insulin sensitivity in pediatric subjects with exogenous obesity" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomization (blocks of four) with stratification by pubertal stage (Tanner 1‐2 or Tanner 3‐5) was performed by computer generated random number allocation" Comment: an adequate randomisation method was used |
| Allocation concealment (selection bias) | Low risk | Quote (from the author): "randomisation was performed in the hospital pharmacy by random number generation and only revealed for data analysis" Comment: allocation was likely concealed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "All participants and investigators were blinded to the intervention" Comment: participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "All participants and investigators were blinded to the intervention" Comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All participants and investigators were blinded to the intervention" Comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All participants and investigators were blinded to the intervention" Comment: participants and personnel were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: an imputation method was not used to replace missing data; however, dropout rates were fairly low |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: an imputation method was not used to replace missing data; however, dropout rates were fairly low |
| Selective reporting (reporting bias) | Unclear risk | Comment: the publication did not report raw data for some of the outcomes, but a clinical trial entry was available and there were no differences |
| Other bias | Unclear risk | Comment: no power calculation was performed; therefore, the trial may have been underpowered |
| Methods | Parallel controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: sibutramine + energy‐restricted diet and exercise plan Comparator: placebo + energy‐restricted diet and exercise plan Number of trial centres: 1 Treatment before trial: no Titration period: 5 mg placebo or sibutramine, taken once daily in the morning. After 2 weeks, the dose was increased to 10 mg/day | |
| Outcomes | Outcomes reported in abstract of publication: BMI‐SDS, BMI, % fat mass, BMRadj, total energy expenditure | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: English Commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "The objective of this trial was to examine the effect of treatment with sibutramine (10 mg) on body composition and energy expenditure in obese adolescents" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was performed by Knoll Pharmaceuticals. Boxes with medication for each visit were numbered for each subject. Subjects received their number and the boxes with medication that belonged to that number. The numbers/medication was handed out in order of inclusion in the study" Comment: author clarified randomisation process; however, it was unclear if the process would have introduced selection bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Knoll Pharmaceuticals BV [currently Abbott Laboratories (Hoofddorp, The Netherlands)], manufactured and provided code‐numbered placebo and sibutramine capsules. Subjects received their trial and medication code according to order of entrance into the study, without stratification" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: author confirmed participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: author confirmed participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: author confirmed outcome assessment was blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: author confirmed outcome assessment was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: an imputation method was used; however, results only shown for completers. Dropout rates fairly low |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: an imputation method was used; however, results only shown for completers. Dropout rates fairly low |
| Selective reporting (reporting bias) | Unclear risk | Comment: unclear whether all outcomes were reported due to no previously published protocol |
| Other bias | Unclear risk | Quote: "E.G.A.H.V.M. was previously employed by Maastricht University, partly on a research grant from Knoll, currently Abbott Pharmaceuticals, The Netherlands" Comment: potential influence of funding source |
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: obesity (not defined) | |
| Interventions | Intervention: metformin + multiprofessional lifestyle intervention Comparator: placebo + multiprofessional lifestyle intervention Number of trial centres: 2 Treatment before trial: 6‐month multiprofessional lifestyle intervention Titration period: no | |
| Outcomes | Outcomes reported in abstract of publication: BMI, HOMA‐IR, fasting insulin, insulin sensitivity index, metabolic syndrome | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: English Commercial and noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "To study whether metformin reduces obesity, homeostasis model assessment for insulin resistance index (HOMA‐IR), and the metabolic syndrome (MtS) in obese European adolescents in addition to previous unsuccessful lifestyle intervention" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description of the randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "we performed a double‐blind, randomized controlled clinical trial" Comment: unclear who was blinded |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "we performed a double‐blind, randomized controlled clinical trial" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "we performed a double‐blind, randomized controlled clinical trial" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "we performed a double‐blind, randomized controlled clinical trial" Comment: unclear who was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: no imputation method was used to replace missing data; however, dropout was fairly low |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: no imputation method was used to replace missing data; however, dropout was fairly low |
| Selective reporting (reporting bias) | Unclear risk | Comment: unable to find the clinical trial entry; hence, it is unclear whether selective reporting occurred |
| Other bias | High risk | Quote: "The study was supported in part by BMBF Research grant 01 GS 0825 and by MERCK SANTE S.A.S, Lyon, France (10’000,‐ Euro)" Comment: trial was partly funded by a pharmaceutical company. The authors do not declare their involvement in the design, analysis and interpretation of the results |
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: metformin + lifestyle intervention programme Comparator: placebo + lifestyle intervention programme Number of trial centres: 6 Treatment before trial: 4‐week placebo run‐in phase, during which participants were required to attend at least 2 of 3 scheduled lifestyle modification sessions and demonstrate 80% compliance with daily placebo treatment (pill count) for subsequent randomisation Titration period: participants either given metformin XR or identical placebo tablets and instructed to take 1 tablet/day (metformin hydrochloride XR 500 mg or placebo) orally before dinner for 2 weeks, then 2 tablets/day for 2 weeks, then 4 tablets/day from week 8 to week 52 | |
| Outcomes | Outcomes reported in abstract of publication: mean adjusted BMI, body compositions, abdominal fat, insulin indices | |
| Study details | Run‐in period: 4‐week placebo run‐in phase (see above) Trial terminated early: no | |
| Publication details | Language of publication: English Noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "to test the hypothesis that 48 weeks of daily metformin hydrochloride extended release (EX) will reduce body mass index in obese adolescents, as compared with placebo" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects who successfully completed the run‐in period were randomized to metformin XR or placebo treatment according to random sequences constructed at the Data Coordinating Center. To ensure balance across major factors, the randomization was stratified by site and sex" "To ensure nonpredictability of assignment, the randomization sequence was grouped in randomly permuted blocks of 2 and 4, and assignments were randomly permuted within block" Comment: an adequate randomisation method was used |
| Allocation concealment (selection bias) | Low risk | Quote: "Subjects who successfully completed the run‐in period were randomized to metformin XR or placebo treatment according to random sequences constructed at the Data Coordinating Center" Comment: adequate allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Subjects and study personnel were blinded to assignment throughout the entire study" Comment: performance bias likely to be reduced by blinding participants and trial personnel |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Subjects and study personnel were blinded to assignment throughout the entire study" "Unblinded data were seen only by the Data and Safety Monitoring Board and study statistician" Comment: performance bias likely to be reduced by blinding participants and trial personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Subjects and study personnel were blinded to assignment throughout the entire study" "Unblinded data were seen only by the Data and Safety Monitoring Board and study statistician" Comment: outcomes assessors blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Subjects and trial personnel were blinded to assignment throughout the entire study" "Unblinded data were seen only by the Data and Safety Monitoring Board and study statistician" Comment: outcomes assessors blinded |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Ninety‐two subjects were screened and 77 were randomized, 39 to metformin XR, 38 to placebo; 27 and 19 in each group were measured at weeks 52 and 100, respectively" Comment: dropout fairly high in each group and no imputation method was performed to replace missing data |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Ninety‐two subjects were screened and 77 were randomized, 39 to metformin XR, 38 to placebo; 27 and 19 in each group were measured at weeks 52 and 100, respectively" Comment: dropout fairly high in each group and no imputation method was performed to replace missing data |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | Unclear risk | Comment: baseline means seemed to be adjusted |
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1, superiority design | |
| Participants | Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: see above | |
| Interventions | Intervention: metformin + dietitian‐administered weight‐reduction programme Comparator: placebo + dietitian‐administered weight‐reduction programme Number of trial centres: 1 Treatment before trial: no Titration period: once baseline assessments were completed, participant's trial medication dose was progressively increased according to a prespecified algorithm over a 3‐week period, starting with 500 mg twice daily and increasing to a maximum dose of 1000 mg twice daily | |
| Outcomes | Outcomes reported in abstract of publication: BMI, bodyweight, BMI z score, fat mass, fasting plasma glucose, HOMA‐IR, adverse events | |
| Study details | Run‐in period: no Trial terminated early: no | |
| Publication details | Language of publication: English Commercial and noncommercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "To determine whether metformin treatment causes weight loss and improves obesity related comorbidities in obese children, who are insulin resistant" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomly assigned participants in a 1:1 randomization ratio to receive metformin hydrochloride or placebo, twice daily with meals. Investigators assigned consecutive code numbers to participants from prespecified lists stratified by race/ethnicity, sex, and degree of pubertal development" Comment: an adequate randomisation method was used |
| Allocation concealment (selection bias) | Low risk | Quote: "The CRC Pharmaceutical Development Section used permuted blocks with stratification to generate allocations that translated code numbers into study group assignments by using a pseudo‐random number program and prepared identically appearing placebo and metformin capsules" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "No participant, investigator, or other medical or nursing staff interacting with participants was aware of study group assignments during the trial" Comment: both the participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "No participant, investigator, or other medical or nursing staff interacting with participants was aware of study group assignments during the trial" Comment: both the participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "No participant, investigator, or other medical or nursing staff interacting with participants was aware of study group assignments during the trial" Comment: both the participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "No participant, investigator, or other medical or nursing staff interacting with participants was aware of study group assignments during the trial" Comment: both the participants and personnel were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "We assessed efficacy in the intention‐to‐treat sample of all randomly assigned participants using a multiple imputation model for missing data under a missing‐at‐random assumption" Comment: low risk of attrition bias for objective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "We assessed efficacy in the intention‐to‐treat sample of all randomly assigned participants using a multiple imputation model for missing data under a missing‐at‐random assumption" Comment: low risk of attrition bias for subjective outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported from protocol |
| Other bias | Unclear risk | Comment: unclear if any other bias was present |
"‐" denotes not reported.
ALR: adiponectin‐to‐leptin ratio; ALT: alanine transaminase; AST: aspartate transaminase; BMI: body mass index; BMIadj: adjusted body mass index: BMI‐SDS: body mass index standardised score; BMRadj: adjusted basal metabolic rate; CDC: Centers for Disease Control and Prevention; DBP: diastolic blood pressure; DEXA: dual energy X‐ray absorptiometry; FGIR: fasting glucose insulin ratio; HbA1c: glycosylated haemoglobin A1c; HOMA‐IR: homeostasis model assessment for insulin resistance index; hsCRP: highly sensitive C‐reactive protein; LOCF: last observation carried forward; min: minute; OGTT: oral glucose tolerance test; QUICKI: quantitative insulin check index; SBP: systolic blood pressure; SD: standard deviation; SDS: standard deviation score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Duration of treatment only 11 weeks | |
| Duration of drug treatment only 2 months | |
| Treatment of schizophrenia or schizoaffective disorder | |
| Duration of treatment only 2 months | |
| Adults | |
| Duration of follow‐up < 6 months | |
| Duration of follow‐up only 12 weeks | |
| Adults | |
| Children had neurogenic or myogenic motor deficit | |
| Not an RCT | |
| Duration of follow‐up < 6 months | |
| Aim was to treat hypothalamic obesity | |
| Duration of follow‐up < 6 months | |
| Duration of drug treatment only 6 weeks | |
| Adults | |
| Not an RCT | |
| Study aim, not all obese at baseline | |
| Not an RCT | |
| A dietary therapy, intervention not relevant for this review | |
| Duration of treatment only 6 weeks | |
| Adults | |
| Adults | |
| Adults | |
| Duration of follow‐up < 6 months | |
| Not an RCT | |
| Not an RCT | |
| Adults | |
| Adults | |
| Not a pharmacological intervention | |
| Study aim not to treat to obesity | |
| Adults | |
| Duration of treatment only 90 days | |
| Adults | |
| Adults | |
| Adults | |
| Adults | |
| Adults | |
| Adults | |
| Adults | |
| Duration of follow‐up in the placebo group only 13 weeks | |
| Adults | |
| Duration of follow‐up only 3 months | |
| Adults | |
| Adults | |
| Not an RCT | |
| Adults | |
| Adults | |
| Adults | |
| Aim of trial to treat fatty liver disease | |
| Not an RCT | |
| Duration of follow‐up only 4 months | |
| Adults | |
| Not an RCT | |
| Duration of treatment only 10 weeks | |
| 1 arm of the cross‐over trial was only followed up for 3 months after receiving the drug | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Duration of follow‐up < 6 months | |
| Adults | |
| Not an RCT | |
| Adults | |
| Duration of treatment only 8 weeks | |
| Adults | |
| Not an RCT | |
| Adults | |
| Adults | |
| Participants had type 1 diabetes ‐ secondary cause of obesity | |
| Adults | |
| Adults | |
| Duration of treatment only 4 weeks | |
| The aim of the study was to treat insulin resistance, not all participants were obese | |
| Adults | |
| Duration of follow‐up only 12 weeks | |
| Not an RCT | |
| Not an RCT | |
| Duration of follow‐up only 20 weeks | |
| Adults | |
| Participants had type 1 diabetes ‐ secondary cause of obesity | |
| A description paper of 2 trials which do not meet the inclusion criteria of this review | |
| Aim of trial to treat hypothalamic obesity | |
| Not a pharmacological intervention | |
| Withdrawn prior to enrolment ‐ inadequate enrolment | |
| Withdrawn prior to enrolment ‐ no reason provided | |
| Not a pharmacological RCT ‐ all participants were given drugs then randomised to lifestyle intervention or control | |
| Aim to treat hypothalamic obesity | |
| Withdrawn prior to enrolment ‐ poor recruitment to the study | |
| Intervention was a growth hormone therapy | |
| Aim of the study: not treatment of obesity | |
| Not a pharmacological intervention | |
| Not an RCT | |
| Duration of follow‐up only 3 months | |
| Not a pharmacological intervention | |
| Not an RCT | |
| Not an RCT | |
| Duration of follow‐up only 4 months | |
| Not an RCT | |
| Surgery intervention | |
| No control group | |
| Duration of follow‐up < 6 months | |
| Participants had bipolar disorder and were critically ill. They were all treated with anti‐psychotics which can cause obesity (potential secondary cause of obesity) | |
| Participants had type 1 diabetes ‐ secondary cause of obesity | |
| Adults | |
| Not an RCT | |
| Not an RCT | |
| Adults | |
| Adults | |
| Adults | |
| Adults | |
| Adults | |
| Duration of follow‐up only 12 weeks | |
| Adults | |
| Adults | |
| Not an RCT | |
| Adults | |
| Adults | |
| Adults | |
| Mainly adults | |
| Not an RCT | |
| Duration of treatment only 6 weeks | |
| Not an RCT | |
| Duration of treatment only 4 weeks | |
| Duration of treatment only 4 weeks | |
| Adults | |
| Duration of follow‐up only 16 weeks | |
| Not an RCT | |
| The aim of the study was to treat diabetes, not obesity | |
| Adults | |
| Adults | |
| Not an RCT | |
| Adults | |
| Not an RCT | |
| Adults | |
| Not an RCT | |
| Duration of drug treatment only 10 weeks |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study identifier | |
| Official title | |
| Stated purpose of study | |
| Notes | Unable to source |
| Methods | Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: not reported Primary purpose: treatment |
| Participants | Condition: adolescent obesity Enrolment: target 48 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: metformin + lifestyle intervention Comparator: placebo + lifestyle intervention |
| Outcomes | Primary outcome:
Secondary outcomes:
Other outcomes: not reported |
| Study identifier | ISRCTN number: ISRCTN08063839 Trial start date: 1 July 2010 Trial completion date: 30 June 2014 |
| Official title | Investigating the use of pharmacotherapy in adolescents for weight loss maintenance: the role of appetite: a randomised, placebo controlled trial |
| Stated purpose of study | Quote: "Eat Smart is a novel research study in which 2 dietary approaches to treat childhood obesity are being tested." |
| Notes | Trial completed in 2014, no publication available and page not found on website Trial sponsor: Royal Children's Hospital (Australia) Ethics approved by the Human Research Ethics Committee (HREC) of the Royal Children's Hospital (ref: HREC/10/QRCH/53) Sources of funding are:
Further information obtained from trial website: www2.som.uq.edu.au/som/Research/ResearchCentres/cnrc/Pages/CNRCHome.aspx |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study identifier | |
| Official title | |
| Stated purpose of study | |
| Notes | Unable to source |
| Methods | Type of trial: interventional; randomised controlled trial Allocation: participants are randomised to metformin medication or placebo, and then randomised to engage in a moderate or vigorous intensity exercise programme for the first 12 weeks of the 2‐year programme Intervention model: parallel assignment Masking: single blind (participant) Primary purpose: prevention |
| Participants | Condition: obesity, type 2 diabetes Enrolment: estimated 72 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Interventions:
Comparators:
|
| Outcomes | Primary outcome:
Secondary outcomes:
Other outcomes: not reported |
| Study identifier | NCT number: NCT00934570 Other trial ID numbers: R‐08‐259, 15590 Trial start date: April 2009 Trial completion date: May 2012 |
| Official title | Reduction of Adolescent Risk Factors for Type 2 Diabetes and Cardiovascular Disease |
| Stated purpose of study | Quote: "Assess the sustainability of a two‐year intervention aimed at improving body mass index (BMI) and metabolic and vascular health in obese youth." |
| Notes | No full publication Results were presented in a poster (Clarson et al 2013) ‐ "In the MXR [metformin] group, there were significant differences in BMI z score at baseline (2.22 ± 037) and 6 months (2.08 ± 0.48, P < .001), 12 months (2.05 ± 0.49, P = .002) and 24 months (2.10 ± 0.46, P = 0.04)" Author asked for additional results but none were provided Sponsored by: Lawson Health Research Institute and Canadian Institutes of Health Research (CIHR) Protocol: Wilson et al 2009 Further trial details are provided by Lawson Health Research Institute The health authority associated with this trial: "Canada: Health Canada" |
| Methods | Type of trial: interventional; randomised control trial Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Condition: obesity Enrolment: 60 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: orlistat (Xenical) + diet and exercise programme Comparator: diet and exercise programme |
| Outcomes | Primary outcome:
Secondary outcomes:
Other outcomes: not reported |
| Study identifier | NCT number: NCT00940628 Other trial ID number: ML19569 Trial start date: April 2008 Trial completion date: September 2010 |
| Official title | Open‐label Comparative Randomized Study of the Efficacy and Safety of Orlistat (Xenical) in Complex Therapy of Obesity and Metabolic Disorders in Adolescents |
| Stated purpose of study | Quote: "This 2 arm study will assess the effect of Xenical on body mass index (BMI) in obese or overweight adolescents" |
| Notes | Trial was completed in 2010, no publication is available The health authority associated with this trial is "Russia: Federal Service on Surveillance in Healthcare and Social Development of RF" |
| Methods | Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: double blind (participant, carer, investigator) Primary purpose: treatment |
| Participants | Condition: obesity; insulin resistance Enrolment: 127 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: metformin + lifestyle intervention Comparator: placebo + lifestyle intervention |
| Outcomes | Primary outcomes:
Secondary outcomes:
Other outcomes: not reported |
| Study identifier | NCT number: NCT01487993 Other trial ID numbers: metformin 2011‐6, 2010‐023980‐17 |
| Official title | An Efficacy, Safety and Pharmacokinetic Study on the Short‐term and Long‐term Use of Metformin in Obese Children and Adolescents |
| Stated purpose of study | Quote: "The purpose of this study is to determine whether metformin is effective in reducing BMI and insulin resistance in obese children and adolescents" |
| Notes | The trial was sponsored by St. Antonius Hospital; Jeroen Bosch Ziekenhuis is a collaborator on the trial; the health authority associated to this trial is "Netherlands: The Central Committee on Research Involving Human Subjects (CCMO)" Results of trial are now published (see Van der Aa 2016 ‐ NCT01487993): 62 participants randomised (32 metformin, 30 placebo), 42 analysed (23 metformin, 19 placebo); 18 months' intervention; median change in BMI was +0.2 kg/m2 (95% CI ‐2.9 to 1.3) (metformin) versus +1.2 kg/m2 (95% CI ‐0.3 to 2.4) kg/m2 (placebo) (P = 0.02). No serious adverse events reported. 2 out of 9 participants lost to follow‐up in the metformin group discontinued treatment because of adverse events. No placebo participants dropped out due to adverse events (13 participants lost to follow‐up) |
| Methods | Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: unclear Primary purpose: treatment |
| Participants | Condition: overweight and obesity in children and adolescents Enrolment: 145 Inclusion criteria:
Exclusion criteria: ‐ |
| Interventions | Interventions:
Comparators:
|
| Outcomes | Primary outcomes:
Secondary outcomes: none given Other outcomes: none given |
| Study identifier | ‐ |
| Official title | ‐ |
| Stated purpose of study | "To assess the efficacy and safety of Metformin use in combination with lifestyle changes or alone for weight management in OW and OB children and adolescents" |
| Notes | Project supported by Research Council of Lithuania (grant Nr MIP‐039/2013) and Research Foundation of Lithuanian University of Health Sciences (grants 2012 and 2013) Results: reduction in BMI, waist circumference and waist circumference SDS adjusted by sex and puberty stages was significantly greater in the metformin + lifestyle changes group compared to the controls no treatment group. Change in BMI after 12 months' intervention: controls = +0.18 kg/m2, lifestyle changes only = +0.43 kg/m2, metformin only = ‐0.59 kg/m2, metformin + lifestyle changes = ‐1.07 kg/m2. Change in waist circumference after 12 months' intervention: controls = ‐1.8 cm, lifestyle changes only = ‐2.8 cm, metformin only = ‐2.3 cm, metformin + lifestyle changes = ‐4.5 cm. Change in waist circumference SDS after 12 months' intervention: controls = ‐0.38, lifestyle changes only = ‐0.58, metformin only = ‐0.49, metformin + lifestyle changes = ‐0.85. Initially, there were mild adverse effects with metformin (nausea, diarrhoea) in 21.6% of participants from metformin only group and metformin + lifestyle changes group, which disappeared within 1 week of metformin administration. Adjusted by sex and puberty status, lean mass was significantly increased in lifestyle only group compared to controls no treatment and metformin only groups. Change in lean mass after 12 months' intervention: controls = +1.6 kg, lifestyle changes only = +3.98 kg, metformin only = ‐0.36 kg, metformin + lifestyle changes = ‐0.37 kg. 12 months' metformin treatment with lifestyle modification was effective and safe method reducing BMI and waist circumference in overweight/obese children and adolescents, superior to that of lifestyle changes alone Correspondence with author: the results presented in the poster are only partial results of a larger trial, where these data are currently being analysed. They aim to publish the results in a publication and as part of a PhD thesis |
"‐" denotes not reported.
ACE: angiotensin converting enzyme; ALT: alanine transaminase; AST: aspartate transaminase; BMI: body mass index; GFR: glomerular filtration rate; HbA1c: haemoglobin A1c; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; HOMA‐IR: homeostasis model assessment for insulin resistance; IOTF: International Obesity Task Force; min: minute; SD: standard deviation; SDS: standard deviation score.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Efectos de la metformina en la obesidad infantil: "Effects of metformin on childhood obesity" |
| Methods | Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: double blind Primary purpose: treatment |
| Participants | Condition: obesity in prepubertal and pubertal children Enrolment: target 160 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: metformin Comparator: placebo |
| Outcomes | Primary outcome:
Secondary outcomes:
Other outcomes: not given |
| Starting date | Trial start date: not given Trial completion date: not given |
| Contact information | Trial sponsor: Ramón Cañete Estrada Name of organisation: Instituto de Salud Carlos III Country: Spain Contact details: Avda Menendez Pidal s/n, Córdoba, 14004, Spain. Tel: 34957011227. Email: [email protected] |
| Study identifier | EU clinical trials register number: EUCTR2010‐023061‐21 |
| Official title | Original title: Ensayo clínico sobre efectos de la metformina en la obesidad pediátrica: efectos en el peso corporal, perfil de biomarcadores inflamatorios y de riesgo cardiovascular, e impacto en factores relacionados con el síndrome metabólico English title: Clinical trial on the effect of metformin in pediatric obesity: effects on bodyweight, profile and inflammatory biomarkers of cardiovascular risk, and impact on factors related to metabolic syndrome |
| Stated purpose of study | To study the clinical and biochemical impact of metformin along with changing lifestyle (diet and exercise) in obese children |
| Notes | Majority of this online entry is in Spanish; sponsor status: noncommercial; trial was ongoing when identified |
| Trial name or title | A study with lifestyle intervention and study medication once weekly or lifestyle intervention and placebo in adolescents with obesity to explore differences between groups with regard to change in BMI |
| Methods | Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: double blind Primary purpose: treatment |
| Participants | Condition: obesity in adolescents Enrolment: 44 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: exenatide + lifestyle intervention Comparator: placebo + lifestyle intervention |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | Trial start date: not given Trial completion date: not given |
| Contact information | Responsible party/principal investigator: Peter Bergsten, Department of Medical Cell Biology Uppsala University |
| Study identifier | EudraCT Number: 2015‐001628‐45 |
| Official title | A parallel, double‐blinded, randomized, 6 months, two arms trial with lifestyle intervention and exenatide 2 mg once weekly or lifestyle intervention and placebo in adolescents with obesity to explore differences between groups with regard to change in BMI SDS (according to WHO) |
| Stated purpose of study | Quote: "To compare the change from baseline to the 6 months visit at the end of treatment, between lifestyle intervention + exenatide 2 mg once weekly and lifestyle intervention + placebo, in BMI SDS (according to WHO) for adolescents with obesity" |
| Notes | Trial registered on 27 July 2015. Trial status: ongoing (when identified). Trial sponsor: Department of Medical Cell Biology Uppsala University. Monetary or material support provided by: European Commission's Seventh Framework Programme (FP7) project Beta_JUDO (grant 279153). Country: Sweden |
| Trial name or title | Effect of exercise or metformin on nocturnal blood pressure and other risk factors for CVD among obese adolescents |
| Methods | Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: factorial assignment Masking: open label Primary purpose: treatment |
| Participants | Condition: CVDs Enrolment: 100 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: metformin Comparator: exercise |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | Trial start date: February 2009 Trial completion date: December 2012 (estimated) |
| Contact information | Responsible party/principal investigator: Professor Claude Marcus, Karolinska Institutet, Karolinska Institute |
| Study identifier | NCT number: NCT00889876 Other trial ID numbers: 2008‐000461‐28 |
| Official title | Effect of Exercise or Metformin on Nocturnal Blood Pressure and Other Risk Factors for Cardiovascular Disease (CVD) Among Obese Adolescents |
| Stated purpose of study | Quote: "The objective is to, among obese adolescents, study impact of regular physical activity or metformin therapy on nocturnal blood pressure and related cardiovascular disease risk factors" |
| Notes | This trial has not been verified on the clinicaltrials.gov website since February 2011. We have attempted to contact the principal investigator via email; however, have not received a response. Trial sponsor: Karolinska Institutet |
| Trial name or title | Obesity in children and adolescents: associated risks and early intervention (OCA) |
| Methods | Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Condition: obesity Enrolment: 400 (estimated) Inclusion criteria:
Exclusion criteria:
|
| Interventions | Interventions:
Comparators:
|
| Outcomes | Primary outcome:
Secondary outcomes:
Other outcomes:
|
| Starting date | Trial start date: May 2013 Trial completion date: December 2015 |
| Contact information | Responsible party/principal investigator: Rasa Verkauskiene, Lithuanian University of Health Sciences. [email protected]. 00370‐37‐327097 |
| Study identifier | NCT number: NCT01677923 Other trial ID numbers: BE‐2‐1 |
| Official title | Phase 3: Effect of Diet, Physical Activity and Insulin Sensitizer Metformin on Obesity and Associated Risks in Children and Adolescents |
| Stated purpose of study | Quote: "The investigators hypothesize that Metformin decreases weight, normalizes lipid profile and increases insulin sensitivity; the study team hope to get better effect of weight decrease and metabolic processes repair in the intensive treatment group with intervention of physical activity, diet correction and Metformin use" |
| Notes | The health authorities associated with this trial are Lithuania: Bioethics Committee and Lithuania: State Medicine Control Agency ‐ Ministry of Health; the trial is sponsored by Lithuanian University of Health Sciences; this trial was recruiting participants when identified |
| Trial name or title | Topiramate in Adolescents with Severe Obesity |
| Methods | Type of trial: interventional; randomised controlled trial Allocation: randomised Intervention model: parallel assignment Masking: double blind (participant, carer, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Condition: obesity, morbid obesity, weight loss Enrolment: estimated 36 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: topiramate Comparator: placebo |
| Outcomes | Primary outcome:
Secondary outcomes:
Other outcomes: not reported |
| Starting date | Trial start date: June 2013 Trial completion date: December 2015 |
| Contact information | Responsible party/principal investigator: Aaron S Kelly, PhD University of Minnesota ‐ Clinical and Translational Science Institute. Tel: 612‐626‐3492. Email: [email protected] |
| Study identifier | NCT number: NCT01859013 Other trial ID number: 1304M31241 |
| Official title | BMI Reduction with Meal Replacements + Topiramate in Adolescents with Severe Obesity |
| Stated purpose of study | Quote: "the goal of this pilot study is to evaluate the safety and efficacy of 24 weeks of topiramate therapy with a 4‐week run‐in of meal replacement therapy in adolescents with severe obesity" |
| Notes | The health authority associated with this trial: "United States: Institutional Review Board"; the trial is sponsored by University of Minnesota ‐ Clinical and Translational Science Institute; the trial was recruiting participants when identified; publication identified for retrospective analysis of participants who received topiramate: Fox et al 2015 |
| Trial name or title | Topiramate and Severe Obesity (TOBI) |
| Methods | Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: double blind (participant, carer, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Condition: obese children and adolescents Enrolment: estimated 160 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: topiramate Comparator: placebo |
| Outcomes | Primary outcome:
Secondary outcomes:
Other outcomes: none given |
| Starting date | Trial start date: June 2015 Trial completion date: December 2020 |
| Contact information | Responsible party: Assistance Publique ‐ Hôpitaux de Paris Principal investigator: Marie‐Laure Frelut, MD |
| Study identifier | NCT number: NCT02273804 |
| Official title | Topiramate and Severe Obesity in Children and Adolescents |
| Stated purpose of study | The purpose of this trial is to evaluate the efficacy of topiramate on the decrease of BMI compared to placebo at 9 months |
| Notes | The trial is sponsored by Assistance Publique ‐ Hôpitaux de Paris; recruitment status when identified: not yet recruiting |
| Trial name or title | Use of Metformin in Treatment of Childhood Obesity |
| Methods | Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: double blind (participant, carer, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Condition: paediatric obesity Enrolment: estimated 120 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: metformin Comparator: placebo |
| Outcomes | Primary outcomes:
Secondary outcome:
Other outcomes: none given |
| Starting date | Trial start date: July 2014 Trial completion date: February 2016 |
| Contact information | Responsible party/principal investigator: Pujitha Wickramasinghe, University of Colombo |
| Study identifier | NCT number: NCT02274948 |
| Official title | Effects of Metformin on Body Weight, Composition and Metabolic Derangements in Obese Children. A Randomized Clinical Trial |
| Stated purpose of study | This study expects to evaluate the use of metformin in the management of obese children. Insulin resistance among obese Sri Lankan children (south Asian origin) is high, which had been shown in the investigators previous work. This study will look at the effect of metformin on changes in insulin resistance, fatty liver state, body fat content, BMI and other metabolic derangement |
| Notes | Trial sponsored by University of Colombo; recruitment status when identified: this trial is currently recruiting participants; location: Sri Lanka |
| Trial name or title | Enhancing Weight Loss Maintenance With GLP‐1RA (BYDUREON™) in Adolescents with Severe Obesity |
| Methods | Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: double blind (participant, carer, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Condition: severe obesity Enrolment: estimated 100 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: exenatide extended‐release for injectable suspension (BYDUREON™) Comparator: placebo |
| Outcomes | Primary outcomes:
Secondary outcomes:
Other outcomes: none given |
| Starting date | Trial start date: December 2015 Trial completion date: July 2020 |
| Contact information | Responsible party/principal investigator: University of Minnesota ‐ Clinical and Translational Science Institute |
| Study identifier | NCT number: NCT02496611 |
| Official title | Enhancing Weight Loss Maintenance with GLP‐1RA (BYDUREON™) in Adolescents with Severe Obesity |
| Stated purpose of study | Primary objective: evaluate the effect of GLP‐1RA treatment on the maintenance of weight loss and durability of cardiometabolic risk factor improvements among adolescents with severe obesity following a meal replacement induction period Secondary objectives: investigate the mechanisms by which glucagon‐like peptide‐1 receptor agonists treatment facilitates weight loss maintenance and identify predictors of response to treatment |
| Notes | Trial sponsored by University of Minnesota ‐ Clinical and Translational Science Institute; recruitment status when identified: this trial is currently recruiting participants; location: USA |
ACE: angiotensin‐converting enzyme; ALT: alanine transaminase; AST: aspartate transaminase; BMI: body mass index; CVD: cardiovascular disease; HbA1c: glycated haemoglobin; HOMA‐IR: homeostasis model assessment‐insulin resistance; IOTF: International Obesity Task Force; SD: standard deviation; GFR: glomerular filtration rate; min: minute; MRI: magnetic resonance imaging; PK: pharmacokinetics; PCOS: polycystic ovary syndrome; SDS: standard deviation score; WHO: World Health Organization.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in BMI (all trials) Show forest plot | 16 | 1884 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| Analysis 1.1  Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 1 Change in BMI (all trials). | ||||
| 2 Change in BMI (drug type) Show forest plot | 16 | 1884 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| Analysis 1.2  Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 2 Change in BMI (drug type). | ||||
| 2.1 Metformin | 8 | 543 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [0.00, ‐0.69] |
| 2.2 Orlistat | 3 | 773 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.08, ‐0.51] |
| 2.3 Sibutramine | 5 | 568 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐2.89, ‐0.51] |
| 3 Change in BMI (dropout rate) Show forest plot | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| Analysis 1.3  Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 3 Change in BMI (dropout rate). | ||||
| 3.1 Dropouts < 20% | 9 | 597 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.78, ‐0.44] |
| 3.2 Dropouts ≥ 20% | 6 | 1145 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐2.34, ‐0.50] |
| 3.3 Unclear dropout rate | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐2.73 [‐3.74, ‐1.72] |
| 4 Change in BMI (intention‐to‐treat (ITT) analysis) Show forest plot | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| Analysis 1.4 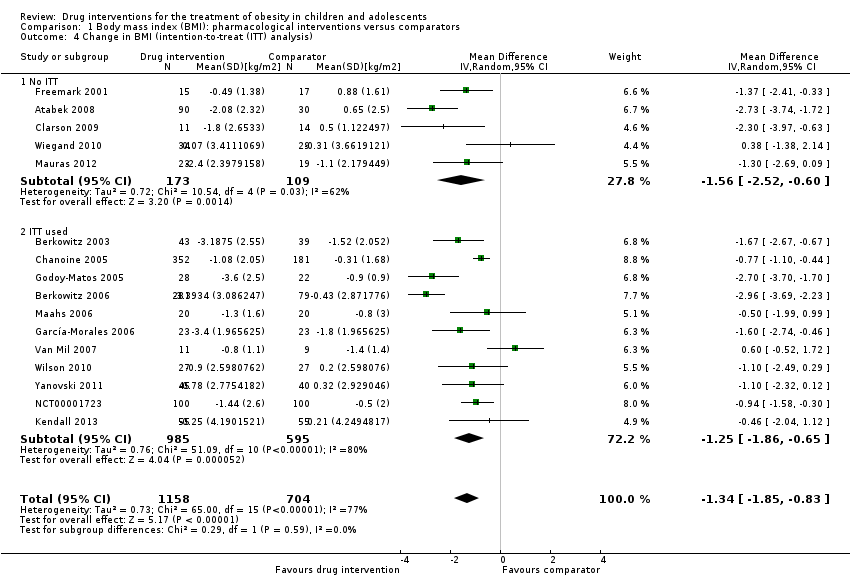 Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 4 Change in BMI (intention‐to‐treat (ITT) analysis). | ||||
| 4.1 No ITT | 5 | 282 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐2.52, ‐0.60] |
| 4.2 ITT used | 11 | 1580 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.86, ‐0.65] |
| 5 Change in BMI (funding) Show forest plot | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| Analysis 1.5 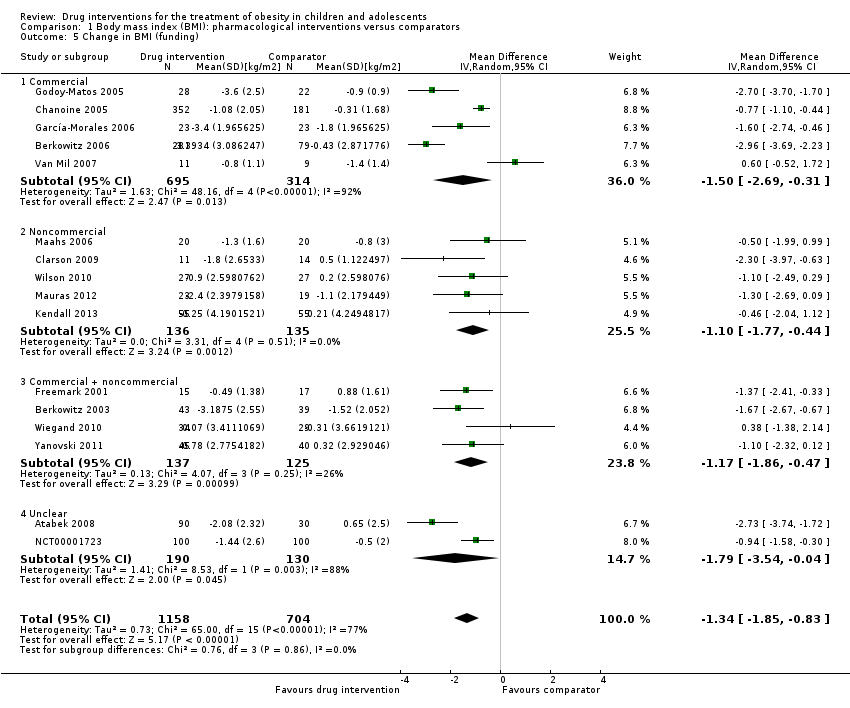 Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 5 Change in BMI (funding). | ||||
| 5.1 Commercial | 5 | 1009 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.69, ‐0.31] |
| 5.2 Noncommercial | 5 | 271 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.77, ‐0.44] |
| 5.3 Commercial + noncommercial | 4 | 262 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐1.86, ‐0.47] |
| 5.4 Unclear | 2 | 320 | Mean Difference (IV, Random, 95% CI) | ‐1.79 [‐3.54, ‐0.04] |
| 6 Change in BMI (publication date) Show forest plot | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| Analysis 1.6  Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 6 Change in BMI (publication date). | ||||
| 6.1 2007 or before | 8 | 1163 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐2.21, ‐0.60] |
| 6.2 After 2007 | 8 | 699 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐1.90, ‐0.62] |
| 7 Change in BMI (quality of trial) Show forest plot | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| Analysis 1.7  Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 7 Change in BMI (quality of trial). | ||||
| 7.1 Low | 6 | 322 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐2.28, ‐0.52] |
| 7.2 Moderate | 10 | 1540 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐1.95, ‐0.67] |
| 8 Change in BMI (country) Show forest plot | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| Analysis 1.8  Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 8 Change in BMI (country). | ||||
| 8.1 Middle income | 3 | 216 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐3.08, ‐1.69] |
| 8.2 High income | 13 | 1646 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.62, ‐0.56] |
| 9 Change in BMI (mean age) Show forest plot | 16 | 1884 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| Analysis 1.9  Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 9 Change in BMI (mean age). | ||||
| 9.1 Mean age < 12 years | 2 | 220 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.53, ‐0.34] |
| 9.2 Mean age ≥ 12 years | 14 | 1664 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.79, ‐0.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in weight (all trials) Show forest plot | 11 | 1180 | Mean Difference (IV, Random, 95% CI) | ‐3.90 [‐5.86, ‐1.94] |
| Analysis 2.1  Comparison 2 Weight: pharmacological interventions versus comparators, Outcome 1 Change in weight (all trials). | ||||
| 2 Change in weight (drug type) Show forest plot | 11 | 1180 | Mean Difference (IV, Random, 95% CI) | ‐3.90 [‐5.86, ‐1.94] |
| Analysis 2.2 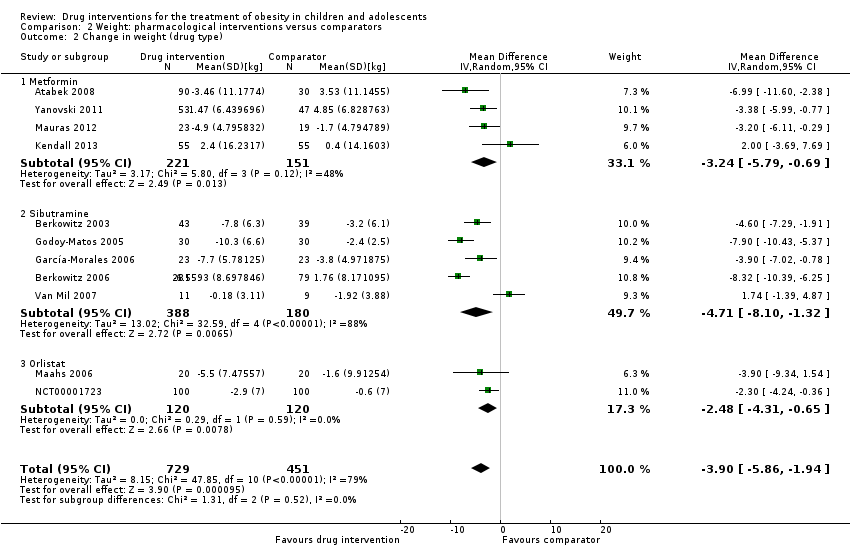 Comparison 2 Weight: pharmacological interventions versus comparators, Outcome 2 Change in weight (drug type). | ||||
| 2.1 Metformin | 4 | 372 | Mean Difference (IV, Random, 95% CI) | ‐3.24 [‐5.79, ‐0.69] |
| 2.2 Sibutramine | 5 | 568 | Mean Difference (IV, Random, 95% CI) | ‐4.71 [‐8.10, ‐1.32] |
| 2.3 Orlistat | 2 | 240 | Mean Difference (IV, Random, 95% CI) | ‐2.48 [‐4.31, ‐0.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serious adverse events Show forest plot | 5 | 1347 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.63, 3.25] |
| Analysis 3.1  Comparison 3 Adverse effects: pharmacological interventions versus comparator, Outcome 1 Serious adverse events. | ||||
| 1.1 Metformin | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 100.80] |
| 1.2 Orlistat | 3 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.41, 2.67] |
| 1.3 Sibutramine | 1 | 498 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.46, 27.33] |
| 2 Discontinued trial because of adverse events Show forest plot | 10 | 1664 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.83, 2.52] |
| Analysis 3.2 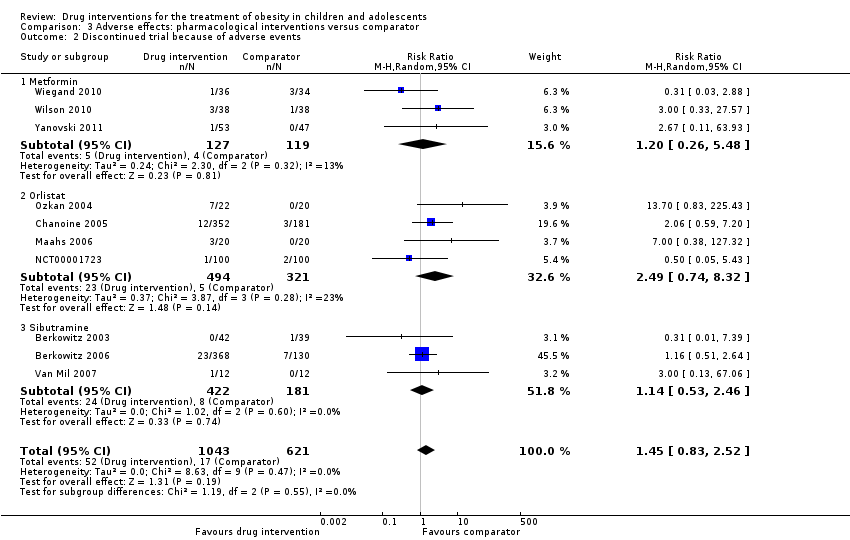 Comparison 3 Adverse effects: pharmacological interventions versus comparator, Outcome 2 Discontinued trial because of adverse events. | ||||
| 2.1 Metformin | 3 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.26, 5.48] |
| 2.2 Orlistat | 4 | 815 | Risk Ratio (M‐H, Random, 95% CI) | 2.49 [0.74, 8.32] |
| 2.3 Sibutramine | 3 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.53, 2.46] |

Trial flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Funnel plot of comparison: 1 Body mass index (BMI): pharmacological interventions versus comparators, outcome: 1.1 Change in BMI (all trials) (kg/m2).
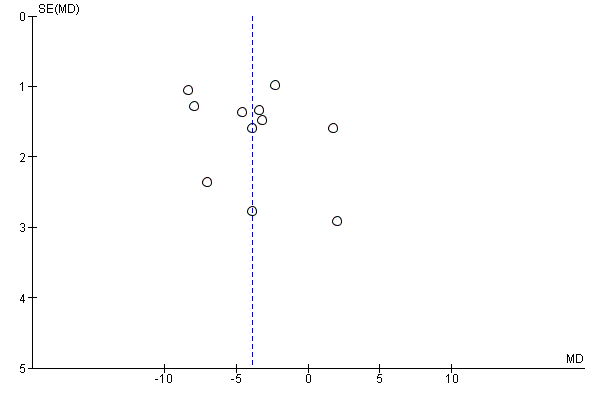
Funnel plot of comparison: 2 Weight: pharmacological interventions versus comparators, outcome: 2.1 Change in weight (all trials) (kg).

Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 1 Change in BMI (all trials).

Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 2 Change in BMI (drug type).

Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 3 Change in BMI (dropout rate).

Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 4 Change in BMI (intention‐to‐treat (ITT) analysis).

Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 5 Change in BMI (funding).

Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 6 Change in BMI (publication date).
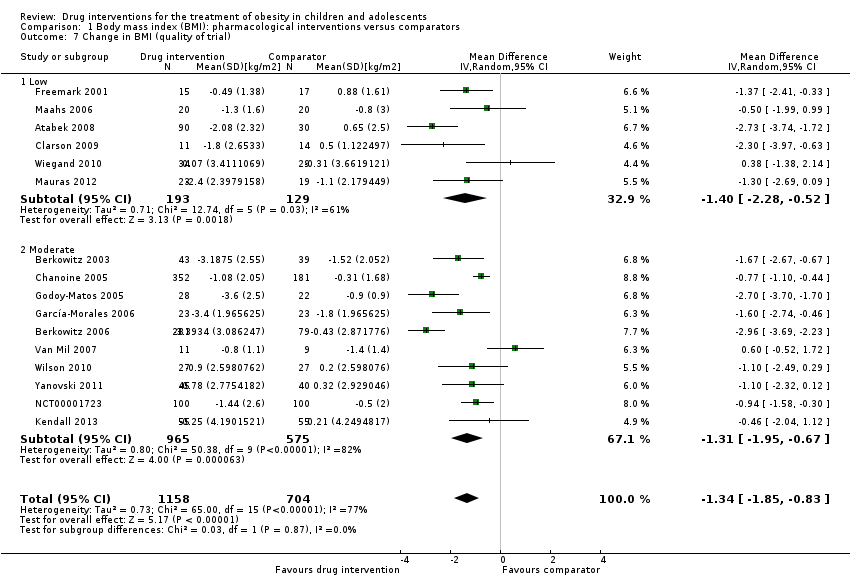
Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 7 Change in BMI (quality of trial).
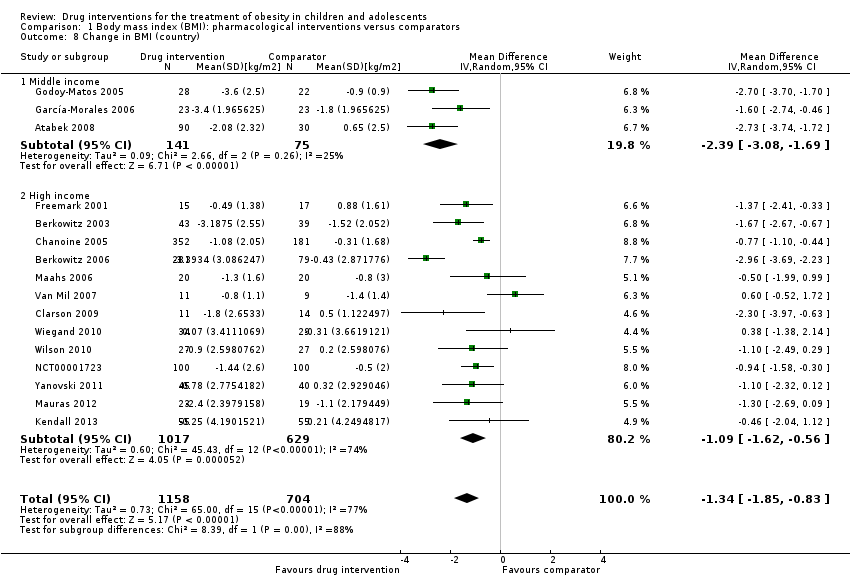
Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 8 Change in BMI (country).

Comparison 1 Body mass index (BMI): pharmacological interventions versus comparators, Outcome 9 Change in BMI (mean age).
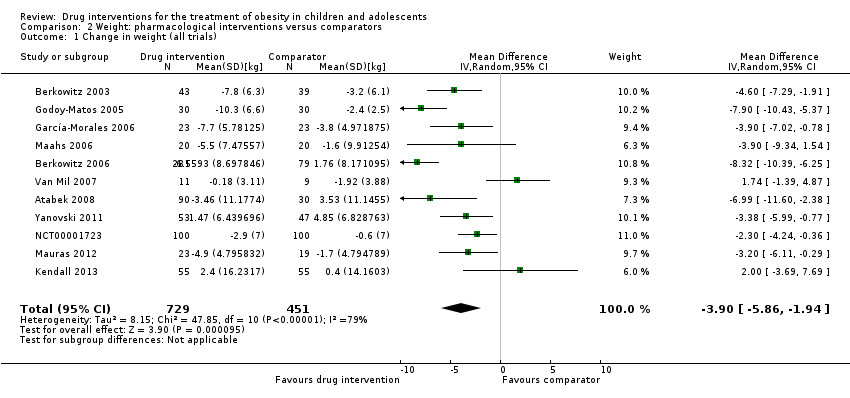
Comparison 2 Weight: pharmacological interventions versus comparators, Outcome 1 Change in weight (all trials).
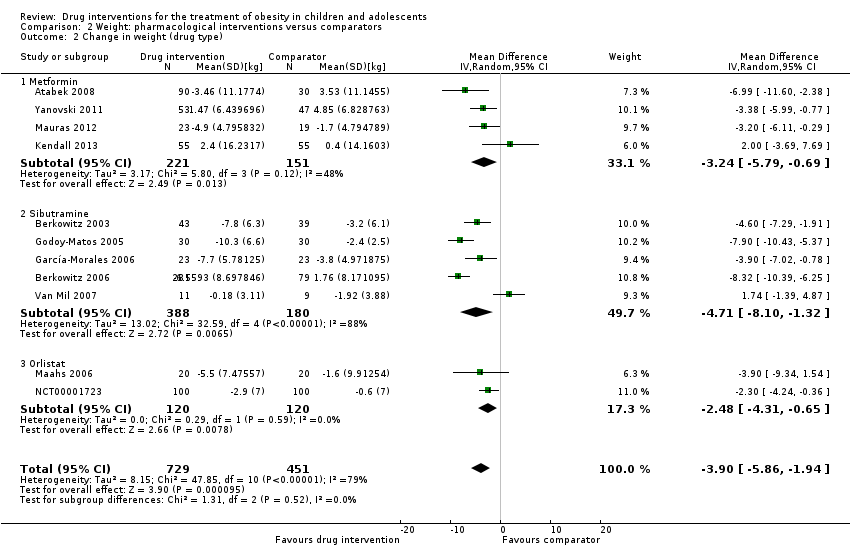
Comparison 2 Weight: pharmacological interventions versus comparators, Outcome 2 Change in weight (drug type).

Comparison 3 Adverse effects: pharmacological interventions versus comparator, Outcome 1 Serious adverse events.
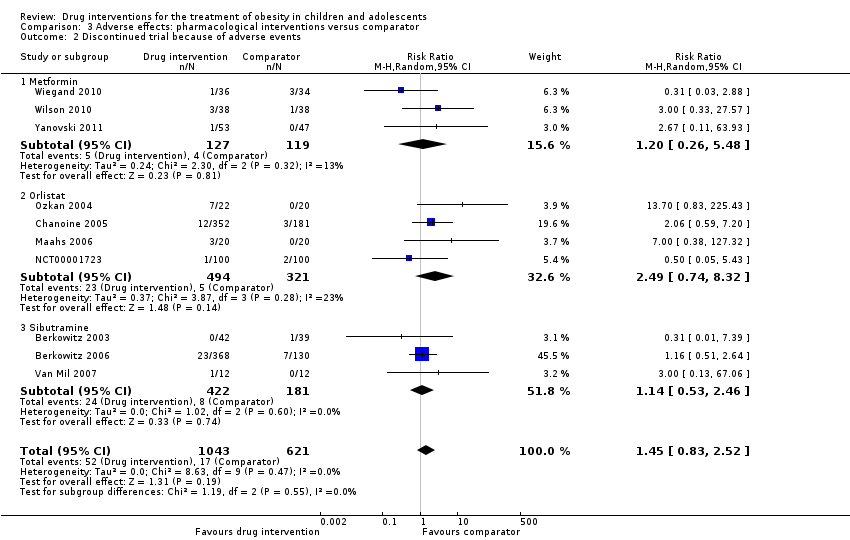
Comparison 3 Adverse effects: pharmacological interventions versus comparator, Outcome 2 Discontinued trial because of adverse events.
| Drug interventions for the treatment of obesity in children and adolescents | ||||||
| Population: obese children and adolescents Settings: mainly outpatient settings Intervention: metformin, orlistat, sibutramine usually combined with behaviour changing interventions Comparison: placebo or no placebo usually with behaviour changing interventions | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Comparator | Pharmacological intervention | |||||
| a. BMI (kg/m2) b. Body weight (kg) Follow‐up: 6 months (10 trials) ‐ 12 months (1 trial) | a. The mean reduction in BMI ranged across control groups from ‐1.8 to +0.9 b. The mean reduction in weight ranged across control groups from ‐3.8 kg to +4.9 kg | a. The mean reduction in BMI in the intervention groups was ‐1.3 higher (‐1.9 to ‐0.8 higher) b. The mean reduction in weight in the intervention groups was ‐3.9 kg higher (‐5.9 kg to ‐1.9 kg higher) | ‐ | a. 1884 (16) b. 1180 (11) | a. ⊕⊕⊝⊝ b. ⊕⊕⊝⊝ | ‐ |
| Adverse events a. Serious adverse events b. Discontinuation of trial because of adverse events Follow‐up: mostly 6 months, maximum 100 weeks (1 trial) | a. 17 per 1000 b. 27 per 1000 | a. 24 per 1000 (11 to 55) b. 40 per 1000 (23 to 69) | a.RR 1.43 (0.63 to 3.25) b.RR 1.45 (0.83 to 2.52) | a. 1347 (5) b. 1664 (10) | a. ⊕⊕⊕⊝ L owb b. ⊕⊕⊕⊝ Lowb | All trials reported if adverse events occurred; however, only 7/20 trials reported the number of participants who experienced at least 1 adverse event |
| Health‐related quality of life 3 questionnaires (1 trial) and SF‐36 (1 trial) Follow‐up: 6 months | See comment | See comment | See comment | 86 (2) | ⊕⊝⊝⊝ V ery lowc | Results were only reported for SF‐36 (1 trial on sibutramine, 46 children), there were no marked differences between intervention and comparator groups |
| All‐cause mortality Follow‐up: mostly 6 months, maximum 100 weeks (1 trial) | See comment | See comment | See comment | 2176 (20) | ⊕⊕⊕⊝ L owd | 1 suicide in the orlistat intervention group |
| Morbidity | See comment | See comment | See comment | 533 (1) | ⊕⊝⊝⊝ V ery lowe | Only 1 trial investigated morbidity defined as illness or harm associated with the intervention (Chanoine 2005). In the orlistat group 6/352 (1.7%) participants developed new gallstones compared with 1/181 (0.6%) in the placebo group |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| *Assumed risk was derived from the event rates in the comparator groups. aDowngraded by two levels because of potential other risk of bias, inconsistency and imprecision (see Appendix 13). | ||||||
| Trial | Intervention(s) and comparator(s) | Description of power and sample size calculation | Screened/eligible | Randomised | Safety | ITT | Finishing trial | Randomised finishing trial | Follow‐up timea |
| Atabek 2008b | I: metformin + diet and physical activity advice | ‐ | ‐ | 90 | 90 | ‐ | 90 | 100 | 6 months |
| C: placebo + diet and physical activity advice | 30 | 30 | ‐ | 30 | 100 | ||||
| total: | 120 | 120 | ‐ | 120 | 100 | ||||
| Berkowitz 2003 | I: behavioural programme + sibutramine | Powered to detect a 4% difference in % change in BMI between the 2 treatment groups with an SD of 5% (α = 0.05, β = 93%)c | 146 | 43 | 43 | 43 | 40 | 93.0 | 6 months (not including the 6‐month open‐label period where all participants received sibutramine) |
| C: behavioural programme + placebo | 39 | 39 | 39 | 34 | 87.2 | ||||
| total: | 82 | 82 | 82 | 62 | 75.6 | ||||
| Berkowitz 2006 | I: behavioural programme + sibutramine | "Planned sample size was approximately 400 participants with a 3:1 randomization ratio of sibutramine to placebo. On the basis of previous 12‐month adult trials, we determined that 300 participants in the sibutramine group would be adequate to assess safety and exposure, allowing an overall dropout rate of approximately 50% and a probability that approximately 50% of participants receiving 10 mg of sibutramine would lose 10% or more of initial BMI at 6 months" "Although the protocol did not document a formal sample size calculation for efficacy, approximately 132 adolescents (99 in the sibutramine group and 33 in the placebo group) would allow a between‐group difference in BMI of 2 kg/m2, with 90% power (2‐sided level of 0.05) to be statistically significant, assuming a common SD of 3 kg/m2)"d | ‐ | 368 | 368 | ‐ | 281 | 76.4 | 12 months |
| C: behavioural programme + placebo | 130 | 130 | ‐ | 80 | 61.5 | ||||
| total: | 498 | 498 | ‐ | 361 | 72.5 | ||||
| Chanoine 2005 | I: orlistat + diet + exercise + behaviour therapy | "We planned to enroll at least 450 individuals to provide more than 80% power to detect a difference of 1 BMI unit, assuming a 30% dropout rate" | 588 | 357 | 352 | 348 | 232 | 65.0 | 54 weeks |
| C: placebo + diet + exercise + behaviour therapy | 182 | 181 | 180 | 117 | 64.3 | ||||
| total: | 539 | 533 | 528 | 349 | 64.7 | ||||
| Clarson 2009 | I: metformin + lifestyle intervention | ‐ | 65 | 14 | ‐ | ‐ | 11 | 78.6 | 6 months |
| C: lifestyle intervention only | 17 | ‐ | ‐ | 14 | 82.4 | ||||
| total: | 31 | ‐ | ‐ | 25 | 80.6 | ||||
| Franco 2014 (cross‐over trial) | I: sibutramine + dietary guidance | ‐ | 73 | ‐ | ‐ | ‐ | ‐ | ‐ | 13 months |
| C: placebo + dietary guidance | ‐ | ‐ | ‐ | ‐ | ‐ | ||||
| total: | 63 | 63 | ‐ | 23 | 36.5 | ||||
| Freemark 2001 | I: metformin | ‐ | ‐ | 15 | ‐ | ‐ | 14 | 93.3 | 6 months |
| C: placebo | 17 | ‐ | ‐ | 15 | 88.2 | ||||
| total: | 32 | ‐ | ‐ | 29 | 90.6 | ||||
| Garcia‐Morales 2006 | I: sibutramine + diet + exercise | 13 participants per group (expectations: mean loss of 7.5 kg (SD 5.3) in the sibutramine group vs 3.6 kg (SD 4.5) in the placebo group)e | 70 | 26 | 26 | 23 | 21 | 80.8 | 6 months |
| C: placebo + diet + exercise | 25 | 25 | 23 | 19 | 76.0 | ||||
| total: | 51 | 51 | 46 | 40 | 78.4 | ||||
| Godoy‐Matos 2005 | I: sibutramine + hypocaloric diet + exercise | ‐ | ‐ | 30 | 30 | 30 | 28 | 93.3 | 24 weeks |
| C: placebo + hypocaloric diet + exercise | 30 | 30 | 30 | 22 | 73.3 | ||||
| total: | 60 | 60 | 60 | 50 | 83.3 | ||||
| Kendall 2013 | I: metformin + healthy lifestyle advice | "The target recruitment was 140 patients, based on a power calculation using the results of a previous study. A standard power calculation was used to detect a reduction in BMI of 0.15 kg/m2 (SD 0.3). Sixty‐four participants in each group give a statistical power of 80% for a t test at the 5% significance level. This was rounded up to allow for some loss to follow‐up but recognizing that adjustment using multifactorial analysis would likely enhance the trial power by an unpredictable amount"f | 234 | ‐ | 74 | 74 | 55 | ‐ | 6 months |
| C: placebo + healthy lifestyle advice | ‐ | 77 | 77 | 55 | ‐ | ||||
| total: | 155 | 151 | 151 | 110 | 71.0 | ||||
| Maahs 2006 | I: orlistat + diet and exercise therapy | "We determined that a clinically important mean difference in decrease in BMI between the orlistat and placebo groups would be 2.0 kg/m2 at 6 months and used an SD of 1.8. On the basis of this approach, a sample size of 15 subjects per group would be adequate to detect a 2.0 kg/m2 difference in Student’s t test with 80% power and alpha = 0.05. In order to allow for a 25% dropout rate, 20 subjects were randomized to each group"g | 43 | 20 | ‐ | 20 | 18 | 90.0 | 6 months |
| C: placebo + diet and exercise therapy | 20 | ‐ | 20 | 16 | 80.0 | ||||
| total: | 40 | ‐ | 40 | 34 | 85.0 | ||||
| Mauras 2012 | I: metformin + diet/exercise intervention | "Differences in hsCRP and fibrinogen concentrations at 6 months were the primary outcomes. An n = 42 completed subjects provided > 90 % power to detect significant changes" | ‐ | 35 | 35 | ‐ | 23 | 65.7 | 6 months |
| C: diet/exercise intervention | 31 | 31 | ‐ | 19 | 61.3 | ||||
| total: | 66 | 66 | ‐ | 42 | 63.6 | ||||
| NCT00001723 | I: orlistat + behavioural weight loss programme | ‐ | ‐ | 100 | 100 | 100 | 87 | 87.0 | 6 months |
| C: placebo + behavioural weight loss programme | 100 | 100 | 100 | 84 | 84.0 | ||||
| 200 | 100 | 100 | 171 | 85.5 | |||||
| Ozkan 2004 | I: conventional treatment (nutritional and lifestyle modification programmes) + orlistat | ‐ | ‐ | 22 | ‐ | ‐ | 15 | 68.2 | 5 to 15 months |
| C: conventional treatment: nutritional and lifestyle modification programmes | 20 | ‐ | ‐ | 15 | 75.0 | ||||
| total: | 42 | ‐ | ‐ | 30 | 71.4 | ||||
| Prado 2012 | I: metformin + nutritional guide and exercise programme | 8 participants were required per intervention group (SD 0.4; difference of 0.6, P < 0.05, power = 90%) | 41/26 | ‐ | 9 | ‐ | 7 | ‐ | 6 months |
| C: placebo + nutritional guide and exercise programme | ‐ | 10 | ‐ | 6 | ‐ | ||||
| total: | 26 | 19 | ‐ | 13 | 50 | ||||
| Rezvanian 2010 | I1: metformin + diet and physical activity advice | "By considering alpha = 0.05 and a power level of 0.8, the sample size was calculated as 160, and by considering the attrition during the follow‐up, we increased it to 180" | 180 | 45 | ‐ | ‐ | 41 | 91.1 | 24 weeks |
| I2: fluoxetine + diet and physical activity advice | 45 | ‐ | ‐ | 40 | 88.9 | ||||
| I3: metformin and fluoxetine + diet and physical activity advice | 45 | ‐ | ‐ | 41 | 91.1 | ||||
| C: placebo + diet and physical activity advice | 45 | ‐ | ‐ | 42 | 93.3 | ||||
| total: | 180 | ‐ | ‐ | 164 | 91.1 | ||||
| Srinivasan 2006 (cross‐over trial) | I: metformin + "standardised information on healthy eating and exercise" | ‐ | 34 | ‐ | ‐ | ‐ | ‐ | ‐ | 12 months |
| C: placebo + "standardised information on healthy eating and exercise" | ‐ | ‐ | ‐ | ‐ | ‐ | ||||
| total: | 28 | ‐ | ‐ | 22 | 78.6 | ||||
| Van Mil 2007 | I: sibutramine + energy‐restricted diet and exercise plan | "The number of patients required per treatment group to detect a difference between treatment groups in mean change in BMI at endpoint intervention of 1.0 kg/m2, based on an estimate of variance (sd) of 0.65, an overall significance level of 5%, and a power of 90%, was nine. Allowing a drop‐out rate of 25%, the number of patients needed in each group was 12"h | ‐ | 12 | 12 | 12 | 11 | 91.7 | 24 weeks |
| C: placebo + energy‐restricted diet and exercise plan | 12 | 12 | 12 | 9 | 75.0 | ||||
| total: | 24 | 24 | 24 | 20 | 83.3 | ||||
| Wiegand 2010 | I: metformin + lifestyle intervention | "Since a clinically significant effect was defined as a decrease in HOMA‐IR by ‐1, two groups of 37 patients had to be included in the study to achieve a power of 0.9 with a α value of 0.05" | 278 | 36 | ‐ | ‐ | 34 | 94.4 | 6 months |
| C: placebo + lifestyle intervention | 34 | ‐ | ‐ | 29 | 85.3 | ||||
| total: | 70 | ‐ | ‐ | 63 | 90 | ||||
| Wilson 2010 | I: metformin + lifestyle intervention | "Assuming an SD of 1.9 for BMI change, an enrolled sample of 72 provided 80% power to detect a differential of 1.46 between treatment arms or between sexes and 1.75 between white subjects and others"i | 92 | 39 | 39 | 39 | 19 | 48.7 | 100 weeks |
| C: placebo + lifestyle intervention | 38 | 38 | 38 | 19 | 50.0 | ||||
| total: | 77 | 76 | 76 | 38 | 49.4 | ||||
| Yanovski 2011 | I: metformin + dietitian‐administered weight‐reduction programme | "A total sample size of 60 participants would detect a between‐group difference of 0.09 BMI SD score units (approximately equivalent to a 2 kg/m2 difference) with 80% power. Participant accrual was set at 100 participants to allow as much as 40% loss to follow‐up"j | 278 | 53 | ‐ | 53 | 45 | 84.9 | 6 months (not including the 6‐month open‐label phase) |
| C: placebo + dietitian‐administered weight‐reduction programme | 47 | ‐ | 47 | 40 | 85.1 | ||||
| total: | 100 | ‐ | 100 | 85 | 85.0 | ||||
| Grand total | All interventionsk | 1395 | 1153 | ||||||
| All comparatorsk | 817 | 665 | |||||||
| All interventions and comparatorsk | 2484 | 1851 | |||||||
| aDuration of intervention and follow‐up under randomised conditions until end of trial. "‐" denotes not reported. BMI: body mass index; C: comparator; hsCRP: high sensitivity C‐reactive protein; HOMA‐IR: homeostasis model assessment for insulin resistance index; I: intervention; ITT: intention‐to‐treat; n: number of participants; SD: standard deviation. | |||||||||
| Trials with data on mean change only | |
| Number of trials | 14 |
| Point estimate (95% CI) (kg/m2) | ‐ 1.5 (‐2.0 to ‐0.9) favouring drug intervention |
| Trials with concealment of allocation | |
| Number of trials | 12 |
| Point estimate (95% CI) (kg/m2) | ‐1.3 (‐1.8 to ‐0.7) favouring drug interventions |
| Trials with blinding of participants/personnel | |
| Number of trials | 10 |
| Point estimate (95% CI) (kg/m2) | ‐1.3 (‐1.9 to ‐0.7) favouring drug interventions |
| Trials with blinding of outcome assessors | |
| Number of trials | 10 |
| Point estimate (95% CI) (kg/m2) | ‐1.3 (‐1.9 to ‐0.7) favouring drug interventions |
| Trials without large sample size trials | |
| Number of trials | 14 |
| Point estimate (95% CI) (kg/m2) | ‐1.3 (‐1.8 to ‐0.7) favouring drug interventions |
| Trials with trials with 6 months' follow‐up only | |
| Number of trials | 14 |
| Point estimate (95% CI) (kg/m2) | ‐1.2 (‐1.7 to ‐0.7) favouring drug interventions |
| Trials without trials with higher drug dose | |
| Number of trials | 14 |
| Point estimate (95% CI) (kg/m2) | ‐1.2 (‐1.7 to ‐0.7) favouring drug interventions |
| Trials with trials with a high dose/active lifestyle intervention | |
| Number of trials | 10 |
| Point estimate (95% CI) (kg/m2) | ‐1.3 (‐1.9 to ‐0.7) favouring drug interventions |
| Trials without trials with high attrition | |
| Number of trials | 13 |
| Point estimate (95% CI) (kg/m2) | ‐1.4 (‐2.0 to ‐0.8) favouring drug interventions |
| BMI: body mass index; CI: confidence interval. | |
| Trials with data on mean change only | |
| Number of trials | 8 |
| Point estimate (95% CI) (kg) | ‐ 4.1 (‐6.3 to ‐1.8) favouring drug intervention |
| Trials with concealment of allocation | |
| Number of trials | 9 |
| Point estimate (95% CI) (kg) | ‐3.5 (‐5.8 to ‐1.2) favouring drug interventions |
| Trials with blinding of participants/personnel | |
| Number of trials | 7 |
| Point estimate (95% CI) (kg) | ‐4.2 (‐6.8 to ‐1.5) favouring drug interventions |
| Trials with blinding of outcome assessors | |
| Number of trials | 7 |
| Point estimate (95% CI) (kg) | ‐4.2 (‐6.8 to ‐1.5) favouring drug interventions |
| Trials without large sample size trials | |
| Number of trials | 10 |
| Point estimate (95% CI) (kg) | ‐3.4 (‐5.2 to ‐1.6) favouring drug interventions |
| Trials with 6 months' follow‐up only | |
| Number of trials | 9 |
| Point estimate (95% CI) (kg) | ‐3.5 (‐5.6 to ‐1.4) favouring drug interventions |
| Trials without trials with higher drug dose | |
| Number of trials | 10 |
| Point estimate (95% CI) (kg) | ‐3.4 (‐5.2 to ‐1.6) favouring drug interventions |
| Trials with trials with a high dose/active lifestyle intervention | |
| Number of trials | 6 |
| Point estimate (95% CI) (kg) | ‐4.3 (‐6.5 to ‐2.2) favouring drug interventions |
| Trials without trials with high attrition | |
| Number of trials | 9 |
| Point estimate (95% CI) (kg) | ‐4.4 (‐6.6 to ‐2.2) favouring drug interventions |
| CI: confidence interval. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in BMI (all trials) Show forest plot | 16 | 1884 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 2 Change in BMI (drug type) Show forest plot | 16 | 1884 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 2.1 Metformin | 8 | 543 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [0.00, ‐0.69] |
| 2.2 Orlistat | 3 | 773 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.08, ‐0.51] |
| 2.3 Sibutramine | 5 | 568 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐2.89, ‐0.51] |
| 3 Change in BMI (dropout rate) Show forest plot | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 3.1 Dropouts < 20% | 9 | 597 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.78, ‐0.44] |
| 3.2 Dropouts ≥ 20% | 6 | 1145 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐2.34, ‐0.50] |
| 3.3 Unclear dropout rate | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐2.73 [‐3.74, ‐1.72] |
| 4 Change in BMI (intention‐to‐treat (ITT) analysis) Show forest plot | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 4.1 No ITT | 5 | 282 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐2.52, ‐0.60] |
| 4.2 ITT used | 11 | 1580 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.86, ‐0.65] |
| 5 Change in BMI (funding) Show forest plot | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 5.1 Commercial | 5 | 1009 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.69, ‐0.31] |
| 5.2 Noncommercial | 5 | 271 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.77, ‐0.44] |
| 5.3 Commercial + noncommercial | 4 | 262 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐1.86, ‐0.47] |
| 5.4 Unclear | 2 | 320 | Mean Difference (IV, Random, 95% CI) | ‐1.79 [‐3.54, ‐0.04] |
| 6 Change in BMI (publication date) Show forest plot | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 6.1 2007 or before | 8 | 1163 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐2.21, ‐0.60] |
| 6.2 After 2007 | 8 | 699 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐1.90, ‐0.62] |
| 7 Change in BMI (quality of trial) Show forest plot | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 7.1 Low | 6 | 322 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐2.28, ‐0.52] |
| 7.2 Moderate | 10 | 1540 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐1.95, ‐0.67] |
| 8 Change in BMI (country) Show forest plot | 16 | 1862 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 8.1 Middle income | 3 | 216 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐3.08, ‐1.69] |
| 8.2 High income | 13 | 1646 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.62, ‐0.56] |
| 9 Change in BMI (mean age) Show forest plot | 16 | 1884 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.85, ‐0.83] |
| 9.1 Mean age < 12 years | 2 | 220 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.53, ‐0.34] |
| 9.2 Mean age ≥ 12 years | 14 | 1664 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.79, ‐0.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in weight (all trials) Show forest plot | 11 | 1180 | Mean Difference (IV, Random, 95% CI) | ‐3.90 [‐5.86, ‐1.94] |
| 2 Change in weight (drug type) Show forest plot | 11 | 1180 | Mean Difference (IV, Random, 95% CI) | ‐3.90 [‐5.86, ‐1.94] |
| 2.1 Metformin | 4 | 372 | Mean Difference (IV, Random, 95% CI) | ‐3.24 [‐5.79, ‐0.69] |
| 2.2 Sibutramine | 5 | 568 | Mean Difference (IV, Random, 95% CI) | ‐4.71 [‐8.10, ‐1.32] |
| 2.3 Orlistat | 2 | 240 | Mean Difference (IV, Random, 95% CI) | ‐2.48 [‐4.31, ‐0.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serious adverse events Show forest plot | 5 | 1347 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.63, 3.25] |
| 1.1 Metformin | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 100.80] |
| 1.2 Orlistat | 3 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.41, 2.67] |
| 1.3 Sibutramine | 1 | 498 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.46, 27.33] |
| 2 Discontinued trial because of adverse events Show forest plot | 10 | 1664 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.83, 2.52] |
| 2.1 Metformin | 3 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.26, 5.48] |
| 2.2 Orlistat | 4 | 815 | Risk Ratio (M‐H, Random, 95% CI) | 2.49 [0.74, 8.32] |
| 2.3 Sibutramine | 3 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.53, 2.46] |

