Jumlah suapan enteral yang standard vs tinggi untuk menggalakkan pertumbuhan bayi pramatang atau berat badan rendah
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT | |
| Participants | 64 newborn VLBW infants were enrolled when they achieved 200 mL/kg/d enteral feeds. Both appropriate‐for‐gestational‐age and small‐for‐gestational‐age infants were included. Only birth weight (not gestational age) criteria were used for enrolment | |
| Interventions | Intervention arm (N = 32): *Feeds were graded up by 20 mL/kg/d up to 300 mL/kg/d Control arm (N = 32): *Feeds were continued at 200 mL/kg/d Babies in both intervention and control arms were given expressed breast milk along with individual micronutrient supplements for calcium, iron, and vitamins. Multi‐nutrient milk fortifiers, which supplement calories and proteins, were not used. Feeds were given by nasogastric tube at 2‐ to 3‐hourly intervals | |
| Outcomes | Primary outcome: weight gain (g/kg/d) from enrolment until baby reached weight of 1700 grams Secondary outcomes: feed intolerance, tachypnoea, NEC (stage 2a or greater), bacteraemia or fungaemia, biochemical abnormalities | |
| Notes | Setting: Neonatology Unit, Christian Medical College Hospital, Vellore (a tertiary care teaching hospital in South India) *Twelve infants in the high‐volume group did not achieve the targeted 300 mL/kg/d (although all achieved feed volumes > 250 mL/kg/d), and 6 infants in the standard‐volume group received higher volumes than targeted (up to 215 mL/kg/d), but analyses were done by "intention‐to‐treat" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal communication: 'computer‐generated random sequence' |
| Allocation concealment (selection bias) | Low risk | Personal communication: 'sealed opaque envelopes opened by the principal investigator only at the time of allocation' |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded |
| Incomplete outcome data (attrition bias) | Low risk | Three (of 64) randomised infants were removed from the study by parents, did not complete the intervention, and were not included in analyses |
| Selective reporting (reporting bias) | Low risk | Personal communication: 'all proposed outcomes reported' |
| Other bias | Low risk | Nil |
NEC: necrotising enterocolitis.
RCT: randomised controlled trial.
VLBW: very low birth weight.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This study compared 2 enteral feed volumes: 150 mL/kg/d and 200 mL/kg/d (both "standard" volumes) | |
| This is a retrospective study of 87 low birth weight infants fed 250 mL/kg/d; not a randomised controlled trial | |
| This is a cohort study comparing 2 feed volumes: 180 mL/kg/d and 230 mL/kg/d; not a randomised controlled trial | |
| This study compared 2 enteral feed volumes: 170 mL/kg/d and 200 mL/kg/d (both "standard" volumes); rate of advancement of feeds was different between groups (in 170‐mL groups, feeds were started at 60 mL/kg/d on day 1 and were advanced to full feeds on day 9; in 200‐mL group, feeds were started at 100 mL/kg/d on day 1 and were advanced to full feeds on day 4) |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weight gain (g/kg/d) Show forest plot | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [2.71, 9.69] |
| Analysis 1.1  Comparison 1 High‐volume vs standard‐volume feeds, Outcome 1 Weight gain (g/kg/d). | ||||
| 2 Feed intolerance Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.89, 3.67] |
| Analysis 1.2 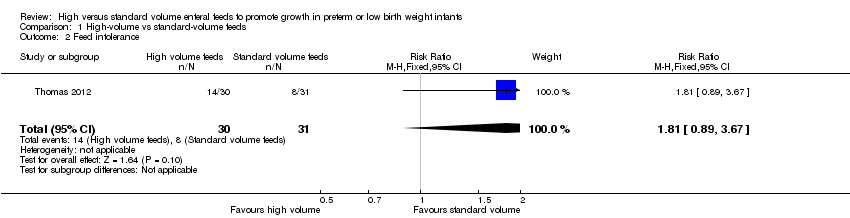 Comparison 1 High‐volume vs standard‐volume feeds, Outcome 2 Feed intolerance. | ||||
| 3 Necrotising enterocolitis Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.78] |
| Analysis 1.3 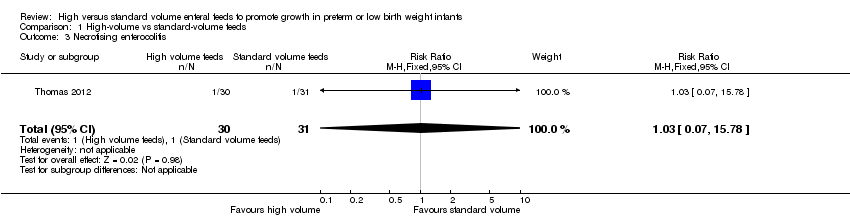 Comparison 1 High‐volume vs standard‐volume feeds, Outcome 3 Necrotising enterocolitis. | ||||

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for the included study.

Forest plot of comparison: 1 High‐volume vs standard‐volume feeds, outcome: 1.1 Weight gain (g/kg/d).

Forest plot of comparison: 1 High‐volume vs standard‐volume feeds, outcome: 1.2 Feed intolerance.

Forest plot of comparison: 1 High‐volume vs standard‐volume feeds, outcome: 1.3 Necrotising enterocolitis.
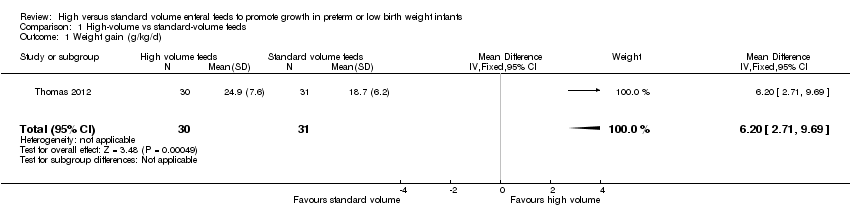
Comparison 1 High‐volume vs standard‐volume feeds, Outcome 1 Weight gain (g/kg/d).

Comparison 1 High‐volume vs standard‐volume feeds, Outcome 2 Feed intolerance.

Comparison 1 High‐volume vs standard‐volume feeds, Outcome 3 Necrotising enterocolitis.
| High‐volume feeds vs standard‐volume feeds for preterm or low birth weight infants | |||||
| Patient or population: preterm or low birth weight infants | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with standard‐volume feeds | Risk with high‐volume feeds | ||||
| Weight gain (g/kg/d) | Mean weight gain was 18.7 g/kg/d | Mean weight gain was 6.2 g/kg/d higher | ‐ | 61 | ⊕⊕⊝⊝ |
| Feed intolerance | Study population | RR 1.81 | 61 | ⊕⊕⊝⊝ | |
| 258 per 1000 | 467 per 1000 | ||||
| Necrotising enterocolitis | Study population | RR 1.03 | 61 | ⊕⊝⊝⊝ | |
| 32 per 1000 | 33 per 1000 | ||||
| *Risk in the intervention group (and its 95% CI) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). | |||||
| aDowngraded for risk of bias (lack of blinding). bDowngraded for imprecision. cDowngraded (by 2) for serious imprecision. | |||||
| per 100 mL | Expressed breast milk (EBM) | EBM + Fortifier | Term formula | Preterm formula |
| Energy (kCal) | 67 | 74 to 80 | 67 | 80 |
| Protein (g) | 1.2 to 1.7 | 2.0 to 2.5 | 1.5 | 2.4 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weight gain (g/kg/d) Show forest plot | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [2.71, 9.69] |
| 2 Feed intolerance Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.89, 3.67] |
| 3 Necrotising enterocolitis Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.78] |

