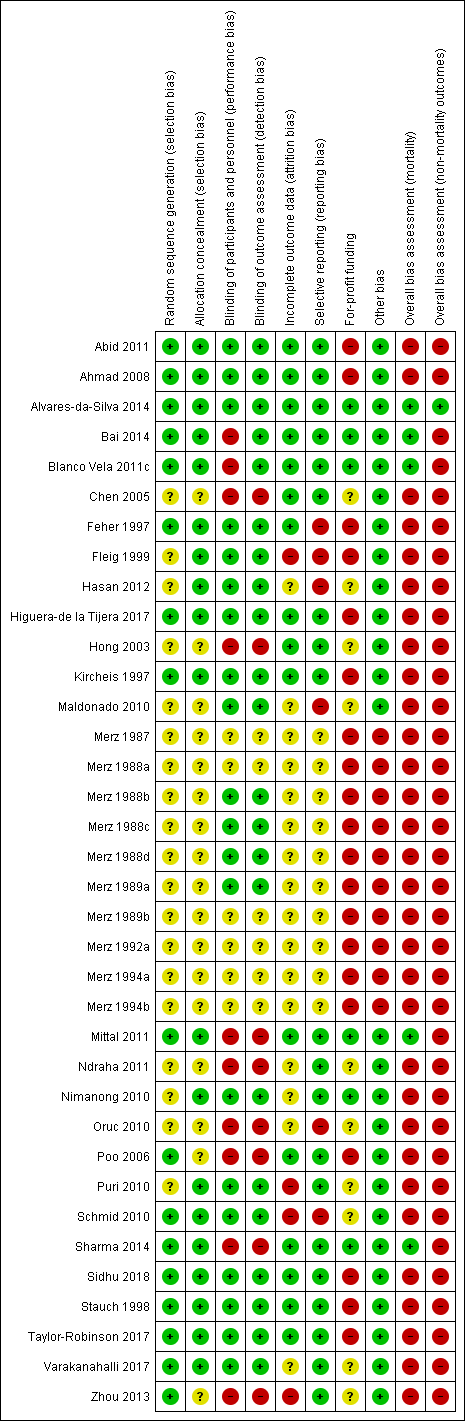
| 1 Mortality Show forest plot | 19 | 1489 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.24, 0.72] |
|
| 1.1 Low risk of bias | 4 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.58] |
| 1.2 High risk of bias | 15 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.23, 0.72] |
| 2 Mortality, by type of hepatic encephalopathy Show forest plot | 19 | 1489 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.39, 0.88] |
|
| 2.1 Acute | 6 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.40, 1.01] |
| 2.2 Chronic | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Minimal | 9 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.07, 1.94] |
| 2.4 Prevention | 5 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.18, 1.26] |
| 3 Mortality, by administration method Show forest plot | 19 | 1489 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.39, 0.88] |
|
| 3.1 Intravenous infusion | 8 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.40, 0.99] |
| 3.2 Oral | 11 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.19, 1.09] |
| 4 Mortality, by publication status Show forest plot | 19 | 1489 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.24, 0.72] |
|
| 4.1 Full paper | 14 | 1151 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.78] |
| 4.2 Abstract | 5 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.17, 1.18] |
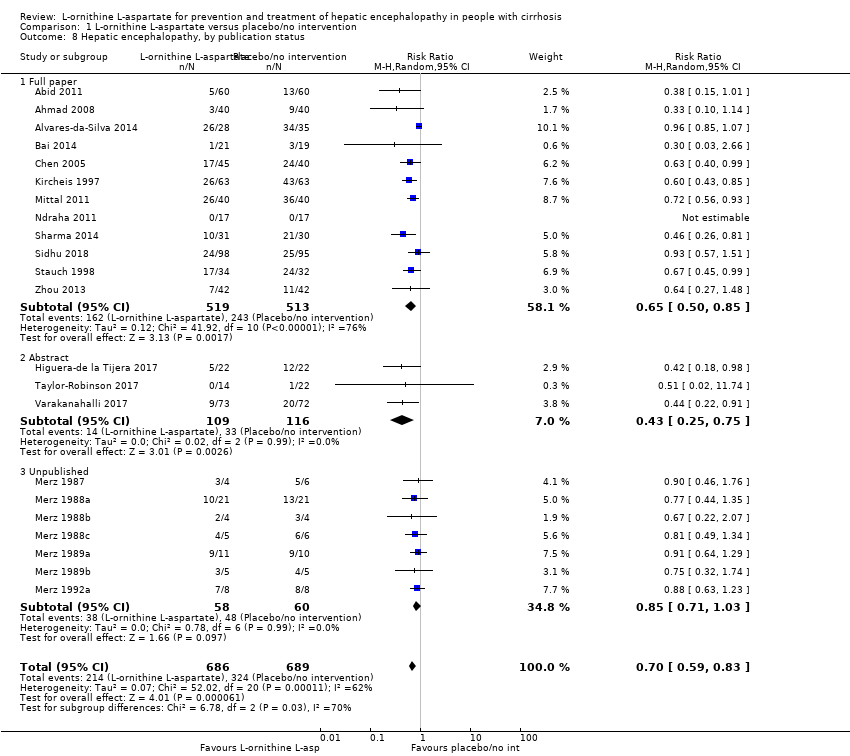
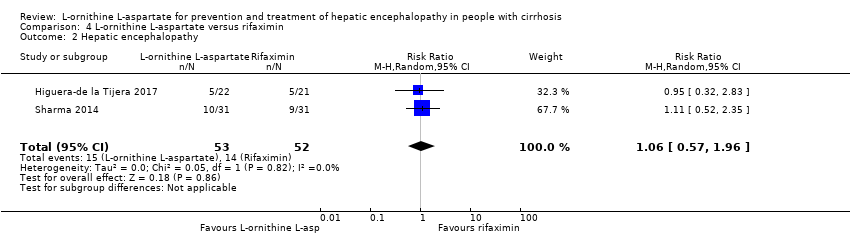
| 5 Hepatic encephalopathy Show forest plot | 22 | 1375 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.59, 0.83] |
|
| 5.1 Low risk of bias | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.07] |
| 5.2 High risk of bias | 21 | 1312 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.63, 0.79] |
| 6 Hepatic encephalopathy, by type of hepatic encephalopathy Show forest plot | 15 | 1255 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.50, 0.81] |
|
| 6.1 Acute | 5 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.91] |
| 6.2 Chronic | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.28, 0.71] |
| 6.3 Minimal | 7 | 299 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.60, 1.02] |
| 6.4 Prevention | 5 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.25, 0.72] |
| 7 Hepatic encephalopathy, by administration method Show forest plot | 22 | 1375 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.59, 0.83] |
|
| 7.1 Intravenous infusion | 11 | 784 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.88] |
| 7.2 Oral | 11 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.91] |
| 8 Hepatic encephalopathy, by publication status Show forest plot | 22 | 1375 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.59, 0.83] |
|
| 8.1 Full paper | 12 | 1032 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.50, 0.85] |
| 8.2 Abstract | 3 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.25, 0.75] |
| 8.3 Unpublished | 7 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.03] |
| 9 Hepatic encephalopathy, completeness status Show forest plot | 22 | 1375 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.59, 0.83] |
|
| 9.1 Complete data | 12 | 994 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.48, 0.83] |
| 9.2 Incomplete data | 10 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.68, 0.97] |
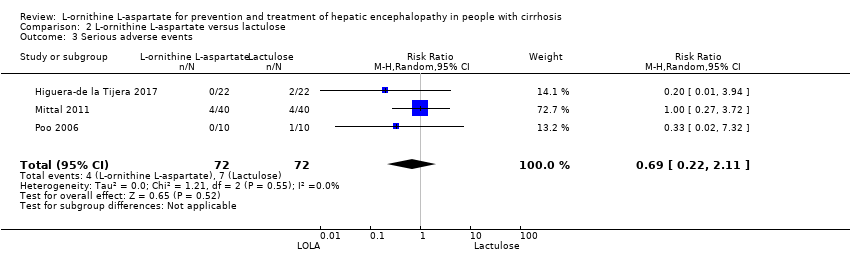
| 10 Serious adverse events Show forest plot | 19 | 1489 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.45, 0.90] |
|
| 10.1 Low risk of bias | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.15, 4.65] |
| 10.2 High risk of bias | 18 | 1426 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.44, 0.89] |
| 11 Serious adverse events, by type of hepatic encephalopathy Show forest plot | 17 | 1283 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.46, 0.97] |
|
| 11.1 Acute overt | 6 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 1.00] |
| 11.2 Chronic | 2 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 21.50] |
| 11.3 Minimal | 5 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.38] |
| 11.4 Prevention | 4 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.17, 5.47] |
| 12 Serious adverse events, by administration method Show forest plot | 17 | 1283 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.46, 0.97] |
|
| 12.1 Intravenous infusion | 8 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.46, 1.05] |
| 12.2 Oral | 9 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.23, 1.29] |
| 13 Serious adverse events, by publication status Show forest plot | 17 | 1283 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.46, 0.97] |
|
| 13.1 Full paper | 13 | 1090 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.48, 1.02] |
| 13.2 Abstract | 4 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.04, 1.34] |
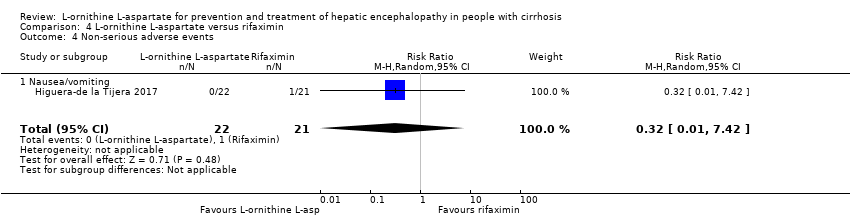
| 14 Non‐serious adverse events Show forest plot | 20 | 3158 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.91, 1.51] |
|
| 14.1 Overall | 14 | 1076 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.75, 1.77] |
| 14.2 Diarrhoea | 4 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.07, 24.18] |
| 14.3 Flatulence | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.49, 5.18] |
| 14.4 Nausea/vomiting | 10 | 639 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [1.25, 4.10] |
| 14.5 Headaches | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 7.67 [0.39, 148.82] |
| 14.6 Abdominal pain | 3 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.23, 1.69] |
| 14.7 Fever | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.12, 23.62] |
| 14.8 Gastrointestinal | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.55, 1.45] |
| 14.9 Pruritus | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.30, 1.21] |
| 14.10 Fatigue | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.58, 1.18] |
| 15 Blood ammonia concentrations Show forest plot | 21 | | Mean Difference (IV, Random, 95% CI) | Subtotals only |
|
| 15.1 End of treatment | 13 | 868 | Mean Difference (IV, Random, 95% CI) | ‐18.52 [‐33.63, ‐3.41] |
| 15.2 Change from baseline | 13 | 738 | Mean Difference (IV, Random, 95% CI) | ‐12.94 [‐20.04, ‐5.83] |