Interventionen bei Neugeborenen zur Vorbeugung von Cerebralparese: eine Übersicht über systematische Reviews von Cochrane
Abstract
Background
Cerebral palsy is an umbrella term that encompasses disorders of movement and posture attributed to non‐progressive disturbances occurring in the developing foetal or infant brain. As there are diverse risk factors and aetiologies, no one strategy will prevent cerebral palsy. Therefore, there is a need to systematically consider all potentially relevant interventions for prevention.
Objectives
Primary
To summarise the evidence from Cochrane Systematic Reviews regarding effects of neonatal interventions for preventing cerebral palsy (reducing cerebral palsy risk).
Secondary
To summarise the evidence from Cochrane Systematic Reviews regarding effects of neonatal interventions that may increase cerebral palsy risk.
Methods
We searched the Cochrane Database of Systematic Reviews (27 November 2016) for reviews of neonatal interventions reporting on cerebral palsy. Two review authors assessed reviews for inclusion, extracted data, and assessed review quality (using AMSTAR and ROBIS) and quality of the evidence (using the GRADE approach). Reviews were organised by topic; findings were summarised in text and were tabulated. Interventions were categorised as effective (high‐quality evidence of effectiveness); possibly effective (moderate‐quality evidence of effectiveness); ineffective (high‐quality evidence of harm); probably ineffective (moderate‐quality evidence of harm or lack of effectiveness); and no conclusions possible (low‐ to very low‐quality evidence).
Main results
Forty‐three Cochrane Reviews were included. A further 102 reviews pre‐specified the outcome cerebral palsy, but none of the included randomised controlled trials (RCTs) reported this outcome. Included reviews were generally of high quality and had low risk of bias, as determined by AMSTAR and ROBIS. These reviews involved 454 RCTs; data for cerebral palsy were available from 96 (21%) RCTs involving 15,885 children. Review authors considered interventions for neonates with perinatal asphyxia or with evidence of neonatal encephalopathy (3); interventions for neonates born preterm and/or at low or very low birthweight (33); and interventions for other specific groups of 'at risk' neonates (7). Quality of evidence (GRADE) ranged from very low to high.
Interventions for neonates with perinatal asphyxia or with evidence of neonatal encephalopathy
Effective interventions: high‐quality evidence of effectiveness
Researchers found a reduction in cerebral palsy following therapeutic hypothermia versus standard care for newborns with hypoxic ischaemic encephalopathy (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.54 to 0.82; seven trials; 881 children).
No conclusions possible: very low‐quality evidence
One review observed no clear differences in cerebral palsy following therapeutic hypothermia versus standard care.
Interventions for neonates born preterm and/or at low or very low birthweight
Possibly effective interventions: moderate‐quality evidence of effectiveness
Researchers found a reduction in cerebral palsy with prophylactic methylxanthines (caffeine) versus placebo for endotracheal extubation in preterm infants (RR 0.54, 95% CI 0.32 to 0.92; one trial; 644 children).
Probably ineffective interventions: moderate‐quality evidence of harm
Researchers reported an increase in cerebral palsy (RR 1.45, 95% CI 1.06 to 1.98; 12 trials; 1452 children) and cerebral palsy in assessed survivors (RR 1.50, 95% CI 1.13 to 2.00; 12 trials; 959 children) following early (at less than eight days of age) postnatal corticosteroids versus placebo or no treatment for preventing chronic lung disease in preterm infants.
Probably ineffective interventions: moderate‐quality evidence of lack of effectiveness
Trial results showed no clear differences in cerebral palsy following ethamsylate versus placebo for prevention of morbidity and mortality in preterm or very low birthweight infants (RR 1.13, 95% CI 0.64 to 2.00; three trials, 532 children); volume expansion versus no treatment (RR 0.76, 95% CI 0.48 to 1.20; one trial; 604 children); gelatin versus fresh frozen plasma (RR 0.94, 95% CI 0.52 to 1.69; one trial, 399 children) for prevention of morbidity and mortality in very preterm infants; prophylactic indomethacin versus placebo for preventing mortality and morbidity in preterm infants (RR 1.04, 95% CI 0.77 to 1.40; four trials; 1372 children); synthetic surfactant versus placebo for respiratory distress syndrome in preterm infants (RR 0.76, 95% CI 0.55 to 1.05; five trials; 1557 children); or prophylactic phototherapy versus standard care (starting phototherapy when serum bilirubin reached a pre‐specified level) for preventing jaundice in preterm or low birthweight infants (RR 0.96, 95% CI 0.50 to 1.85; two trials; 756 children).
No conclusions possible: low‐ to very low‐quality evidence
No clear differences in cerebral palsy were observed with interventions assessed in 21 reviews.
Interventions for other specific groups of 'at risk' neonates
No conclusions possible: low‐ to very low‐quality evidence
Review authors observed no clear differences in cerebral palsy with interventions assessed in five reviews.
Authors' conclusions
This overview summarises evidence from Cochrane Systematic Reviews regarding effects of neonatal interventions on cerebral palsy, and can be used by researchers, funding bodies, policy makers, clinicians, and consumers to aid decision‐making and evidence translation. To formally assess other benefits and/or harms of included interventions, including impact on risk factors for cerebral palsy, review of the included Reviews is recommended.
Therapeutic hypothermia versus standard care for newborns with hypoxic ischaemic encephalopathy can prevent cerebral palsy, and prophylactic methylxanthines (caffeine) versus placebo for endotracheal extubation in preterm infants may reduce cerebral palsy risk. Early (at less than eight days of age) postnatal corticosteroids versus placebo or no treatment for preventing chronic lung disease in preterm infants may increase cerebral palsy risk.
Cerebral palsy is rarely identified at birth, has diverse risk factors and aetiologies, and is diagnosed in approximately one in 500 children. To date, only a small proportion of Cochrane Systematic Reviews assessing neonatal interventions have been able to report on this outcome. There is an urgent need for long‐term follow‐up of RCTs of such interventions addressing risk factors for cerebral palsy (through strategies such as data linkage with registries) and for consideration of the use of relatively new interim assessments (including the General Movements Assessment). Such RCTs must be rigorous in their design and must aim for consistency in cerebral palsy outcome measurement and reporting to facilitate pooling of data and thus to maximise research efforts focused on prevention.
Laienverständliche Zusammenfassung
Interventionen bei Neugeborenen von der Geburt bis zu einem Lebensmonat zur Vorbeugung von Cerebralparese: eine Übersicht über systematische Reviews von Cochrane
Worum geht es?
‘Cerebralparese‘ ist ein Begriff, der eine Gruppe von Erkrankungen umfasst, die die Bewegungsfähigkeit des Menschen beeinträchtigen; es ist die häufigste körperliche Behinderung in der Kindheit. Die Cerebralparese ist in der Regel auf Ereignisse vor, während oder nach der Geburt zurückzuführen, die zu Verletzungen des sich entwickelnden Gehirns von Neugeborenen führen. Es ist keine alleinige Ursache für Cerebralparese bekannt. Bei vielen Kindern ist die Ursache der Cerebralparese unklar, aber viele Risikofaktoren sind bekannt. Der größte Risikofaktor ist die Frühgeburt (Geburt vor der 37. Schwangerschaftswoche). Weitere Risikofaktoren in der Neugeborenenperiode (von der Geburt bis zu einem Lebensmonat) sind langandauernder Sauerstoffmangel während der Geburt; Hirntrauma; Schlag‐ oder Krampfanfälle; Erkrankungen des Herzens, der Blutgefäße, der Atemwege und der Lunge; langanhaltende mechanische Unterstützung der Atmung; einige Infektionen; Gelbsucht (gelbe Verfärbung der Haut und der Augen durch erhöhtes Bilirubin im Blut); und einige Syndrome oder Anomalien der Chromosomen (Strukturen, die Gene enthalten).
Warum ist das wichtig?
Da es verschiedene Risikofaktoren und Ursachen für Cerebralparese gibt, ist es wahrscheinlich, dass unterschiedliche Maßnahmen notwendig sein dürften, um eine Cerebralparese durch Verringerung der Risikofaktoren zu verhindern. Diese Übersicht fasst die Evidenz zu Vorbeugung von Cerebralparese, die in Cochrane Reviews über Interventionen in der Neugeborenenperiode dargestellt wurde, zusammen.
Welche Evidenz haben wir gefunden?
Wir suchten am 27. November 2016 nach Evidenz und fanden 43 Cochrane Reviews, die Interventionen in der Neugeborenenperiode beurteilten und Informationen zu Zerebralparese lieferten. Diese Reviews waren alle von moderater bis hoher Qualität, aber die Qualität der Evidenz zu Cerebralparese reichte von sehr niedrig bis hoch. Drei Reviews beurteilten Interventionen für Neugeborene, die einen Sauerstoffmangel während oder um den Zeitpunkt der Geburt herum gehabt haben könnten; 33 Reviews beurteilten Interventionen bei Kindern, die zu früh oder mit niedrigem Geburtsgewicht geboren wurden; und sieben Reviews beurteilten Interventionen für andere Gruppen von Neugeborenen mit dem Risiko einer Schädigung ihres Gehirns (zum Beispiel Neugeborene mit niedrigem Blutzucker bei der Geburt).
Wir fanden, dass eine Intervention zur Vorbeugung von Cerebralparese wirksam war. Neugeborene, die einen Sauerstoffmangel während oder um den Zeitpunkt der Geburt herum gehabt haben könnten, und die mittels Hypothermie (Kühlung des Körpers oder lediglich des Gehirns) behandelt wurden, entwickelten mit geringerer Wahrscheinlichkeit eine Cerebralparese als Neugeborene, die keine Hypothermie erhielten (sieben Studien; 881 Kinder; Evidenz von hoher Qualität). Wir stellten fest, dass eine Intervention zur Vorbeugung von Cerebralparese möglicherweise wirksam war. Frühgeborene, die Methylxanthine (Coffein) erhielten, wenn die Entwöhnung von maschinengestützter Atmung (Extubation von der mechanischen Beatmung) geplant war, entwickelten weniger häufig Cerebralparese als Kinder, denen ein Placebo verabreicht wurde (eine Studie; 644 Kinder; Evidenz von moderater Qualität). Wir fanden eine Intervention, die wahrscheinlich unwirksam war und Schaden verursachen könnte: Frühgeborene, die frühzeitig (im Alter von weniger als acht Tagen) Kortikosteroide erhielten, um chronische Lungenerkrankungen zu verhindern, entwickelten mit höherer Wahrscheinlichkeit eine Cerebralparese als Kinder, die ein Placebo erhielten (12 Studien; 959 Kinder; Evidenz von moderater Qualität). Wir fanden, dass fünf andere Interventionen wahrscheinlich unwirksam waren (sie verhinderten nicht oder erhöhten die Wahrscheinlichkeit für die Entstehung einer Cerebralparese) (Evidenz von moderater Qualität). Die Review‐Autoren fanden nicht genügend Evidenz, um zu sagen, ob die anderen Interventionen eine Cerebralparese verhinderten, die Wahrscheinlichkeit dafür erhöhten oder ob sie keinen Einfluss auf Cerebralparese hatten (Evidenz von niedriger oder sehr niedriger Qualität).
Was bedeutet das?
Diese Übersicht identifizierte eine Intervention, die wirksam in der Vorbeugung einer Cerebralparese war (induzierte Hypothermie bei Neugeborenen, die einen Sauerstoffmangel gehabt haben könnten), eine, die möglicherweise wirksam in der Vorbeugung einer Cerebralparese war (Coffein bei Frühgeborenen im Rahmen der Entwöhnung von maschinengestützter Atmung), eine, bei der vermutet wird, dass sie Schaden verursacht (Gabe von Kortikosteroiden im Alter von weniger als acht Tagen bei Frühgeborenen, um chronische Lungenerkrankungen zu verhindern), und fünf, die offenbar keinen Unterschied machen. Für die anderen beurteilten Interventionen gab es nicht genügend Evidenz, um zu Schlussfolgerungen zu gelangen. Es ist wichtig, dass zusätzliche Studien von guter Qualität mit einer langen Nachbeobachtungszeit durchgeführt werden, um Interventionen zu beurteilen, die einen Einfluss auf die Risikofaktoren der Zerebralparese haben könnten. Wir identifizierten über 100 weitere Cochrane Reviews, die in der Zukunft Informationen über Interventionen in der Neugeborenenperiode zur Vorbeugung von Cerebralparese liefern könnten, falls sie eine lange Nachbeobachtungszeit einschließen.
Authors' conclusions
Background
Description of the condition
Cerebral palsy: definition and prevalence
‘Cerebral palsy’ was originally (and continues to be) defined by clinical description at a time when there was little knowledge of aetiology or pathology (Morris 2007). Today, many registries and surveillance programmes, including those in Australia, the United Kingdom, and Europe, highlight five key elements of cerebral palsy: It is an ‘umbrella term’; it is permanent but not unchanging; it involves a disorder of movement or posture or both, and of motor function; it is due to a non‐progressive interference, lesion, or abnormality; and the interference, lesion, or abnormality arose in the developing or immature brain (Cans 2000; Mutch 1992; Rosenbaum 2007; Smithers‐Sheedy 2014). As cerebral palsy is defined by clinical description, which may change over time, a longer time span for diagnosis is considered useful to confirm that the condition meets criteria for cerebral palsy and to accurately describe the motor impairment. Thus, final ascertainment for surveillance programmes across the world ranges from four to 12 years, with many considering data to be 'complete' at or near five years (Smithers‐Sheedy 2014). Although average age at diagnosis has been around 18 months, recent evidence has suggested that cerebral palsy may be reliably detected as early as three to four months' post term age via tests such as Prechtl's Qualitative Assessment of General Movements and medical resonance imaging (Bosanquet 2013; Morgan 2016).
Cerebral palsy is the most common physical disability in childhood. In a recent meta‐analysis, including 19 studies (with varying ages of ascertainment), the global pooled prevalence was 2.11 per 1000 live births (95% confidence interval (CI) 1.98 to 2.25); a cumulative meta‐analysis demonstrated stability over the past 10 years (Oskoui 2013). Similar rates have been reported in countries that have used consistent methods of ascertainment for over 20 years (such as Australia, Sweden, and England), with most published estimates in the region of 2 per 1000 (Blair 2006). In low‐ and middle‐income countries, prevalence estimates have tended to be in a similar range or higher (Blair 2006; Cans 2000). However, emerging evidence, including rates from Australia and Europe, now shows that overall rates and severity of the condition are starting to decline for the first time (Reid 2015; Sellier 2015).
Cerebral palsy: causes and risk factors
Brain injury was acquired during an event more than 28 days after birth in approximately 6% of individuals with cerebral palsy (ACPR Group 2013). In the remaining 94% of individuals, brain injury occurred during pregnancy, at birth, or over the first 28 days of life (ACPR Group 2013). Preterm birth is one of the principal risk factors for cerebral palsy and associated neurosensory disabilities (Himpens 2008; Oskoui 2013), with over 40% of individuals with cerebral palsy born preterm (ACPR Group 2013). However, more than half of all individuals with cerebral palsy are born at term (ACPR Group 2013).
Studies on antenatal, intrapartum, and neonatal risk factors for cerebral palsy are abundant. Although a great number of risk factors have been identified, their commonality is that separately, or in combination, they influence potentially preventable pathways to brain injury. Risk factors commonly reported include (i) factors before conception (e.g. low or advanced maternal age, high parity, nulliparity, a short or long interpregnancy interval, a history of stillbirth, multiple miscarriages, neonatal death or preterm birth, family history of cerebral palsy and other genetic predispositions, low socioeconomic status, pre‐existing maternal conditions (such as epilepsy or intellectual disability)); (ii) factors in early pregnancy (e.g. male sex, multiple gestation, congenital malformations or birth defects, infections (such as TORCH complex ‐ toxoplasmosis (parasite), other infections, rubella, cytomegalovirus, herpes simplex virus)); (iii) factors during pregnancy (e.g. maternal disease (such as thyroid disorders), pregnancy complications (such as pre‐eclampsia, placenta praevia, and placental abruption), intrauterine infection or inflammation and chorioamnionitis, intrauterine growth restriction, other precursors to preterm birth); and (iv) factors around the time of birth and the neonatal period (e.g. acute intrapartum hypoxic events and neonatal encephalopathy, neonatal brain injury (such as intraventricular haemorrhage, periventricular leucomalacia, and hydrocephalus), strokes or seizures, cardiovascular disorders (such as patent ductus arteriosus and hypotension), respiratory disorders, associated prolonged ventilation (such as for respiratory distress syndrome or bronchopulmonary dysplasia), infection (such as sepsis and necrotising enterocolitis), metabolic or endocrine disorders (such as hypoglycaemia and hypothyroidism), neonatal jaundice along with inborn errors of metabolism, particular syndromes or chromosomal abnormalities) (Badawi 2005; Dixon 2002; Drougia 2007; Jacobsson 2004; McIntyre 2011; McIntyre 2013; Murphy 1997; Nelson 2008; Tran 2005; Walstab 2004).
Research has shown that contrary to previous beliefs, birth asphyxia is a relatively rare cause of cerebral palsy (Blair 1988; Ellenberg 2013). A growing body of evidence suggests that genetic abnormalities contribute in some cases (MacLennan 2015; Moreno‐De‐Luca 2012; O’Callaghan 2009; Oskoui 2015). Common risk factors in the post‐neonatal period (some of which also contribute in the neonatal period) include infection (such as meningitis/encephalitis, or severe infection and subsequent severe dehydration), head injury (such as from traffic accidents, other traumatic injury, or non‐accidental injury), vascular episodes (such as post cardiac or brain surgery), and other events (such as near drowning or near sudden infant death) (Cans 2004; Germany 2013).
Cerebral palsy: consequences
Cerebral palsy, the leading cause of physical disability for children, is a condition with lifelong impact. Most individuals will survive to adulthood, and some studies suggest that life expectancy can be similar to that of the general population (Colver 2012). For known cases of antenatally or neonatally acquired cerebral palsy, the 20‐year survival rate has been estimated at 90%. However, strong associations between increasing motor impairment, severe intellectual impairment, number of severe impairments, and early mortality have been shown (Blair 2001; Hemming 2005; Reid 2012). Frequently used definitions for cerebral palsy acknowledge common co‐occurring impairments, diseases, and functional limitations (Rosenbaum 2007). A recent systematic review estimated that among children with cerebral palsy, "1 in 2 had an intellectual disability…1 in 4 could not talk; 1 in 4 had epilepsy; 1 in 4 had a behavior disorder…1 in 10 were blind…and 1 in 25 were deaf" (Novak 2012).
Economic studies have estimated lifetime costs of cerebral palsy, including healthcare, social care, and productivity costs, as EUR 860,000 for men and EUR 800,000 for women in Denmark (in 2000) (Kruse 2009), and as USD 921,000 for individuals in the United States (in 2003) (CDC 2004). In Australia, the financial cost of cerebral palsy was estimated as AUD 1.47 billion (in 2007), and the value of lost well‐being a further AUD 2.4 billion (Access Economics 2008).
The impact of cerebral palsy is considerable (Davis 2010). Accordingly, identification of primary preventive measures has been regarded as a key priority among individuals with cerebral palsy, their families, clinicians, and researchers (McIntyre 2010).
Description of the interventions
Neonatal approaches to prevention of cerebral palsy
Research efforts aimed at prevention of cerebral palsy have increasingly focused on understanding the causes of cerebral palsy. As it is now widely recognised that causes differ, for example, by gestational age (e.g. for preterm and term‐born children) and by clinical subtype of cerebral palsy, it is reasonable to consider that successful primary preventive interventions will also vary according to different aetiologies or causal factors.
In this overview, therefore, we will include a broad range of neonatal interventions (with varying primary aims or indications) that may mediate cerebral palsy risk, including (but not limited to):
-
interventions for neonates following birth asphyxia or with evidence of encephalopathy (e.g. cooling; erythropoietin; darbepoetin; allopurinol; melatonin; magnesium sulphate; anticonvulsants; xenon; naloxone; dopamine; fluid restriction; acupuncture; umbilical cord stem cells);
-
interventions for neonates with neurological disorders, such as intracranial haemorrhage or post‐haemorrhagic hydrocephalus (e.g. heparin; antithrombin; phenobarbital; diuretic therapy; erythropoietin; repeated lumbar or ventricular punctures); or those with seizures (anticonvulsants);
-
interventions for neonates requiring resuscitation (e.g. air or oxygen for positive‐pressure ventilation; lower or higher oxygen concentrations titrated to target oxygen saturations; face mask, laryngeal mask airway, nasal airway or endotracheal intubation; positive end‐expiratory pressure; respiratory function monitoring);
-
interventions for neonates with cardiovascular disorders, such as hypotension (e.g. corticosteroids; inotropes; early volume expansion; adrenaline; dopamine; dobutamine) or patent ductus arteriosus (e.g. ibuprofen; indomethacin; fluid restriction; surgical ligation);
-
interventions for neonates with respiratory disorders, such as apnoea of prematurity (e.g. kinaesthetic stimulation; methylxanthines (caffeine)); respiratory distress syndrome (e.g. early or delayed, prophylactic or selective, protein‐containing or protein‐free, animal‐derived or synthetic pulmonary surfactant; thyroid hormones; continuous distending pressure); or bronchopulmonary dysplasia (chronic lung disease) (e.g. early or late, inhaled or systemic, postnatal corticosteroids);
-
interventions for gastrointestinal tract disorders, such as necrotising enterocolitis (e.g. lactoferrin; probiotics; antibiotics; immunoglobulin; peritoneal drainage; laparotomy);
-
interventions for neonates with infection, such as for control of general infection (e.g. chlorhexidine skin or cord care; patient isolation for infection; gowning by attendants and visitors in newborn nurseries); fungal and protozoal infections (e.g. prophylactic antifungal agents; antifungal therapy for invasive fungal infection); viral infections (e.g. antiviral agents for treatment of herpes simplex virus or cytomegalovirus infection); or bacterial infections (e.g. intravenous immunoglobulin for prevention of infection, or for suspected or proven infection; antibiotics for suspected early‐ or late‐onset sepsis; intraventricular antibiotics for meningitis; prophylactic antibiotics for ventilated newborns);
-
interventions for neonates with metabolic or endocrine disorders, such as disorders of carbohydrate metabolism (e.g. oral dextrose gel for hypoglycaemia; insulin for hyperglycaemia) or thyroid disorders (postnatal thyroid hormones);
-
interventions for neonates with jaundice and liver disorders (e.g. phototherapy);
-
interventions focused on nutrition or metabolism for high‐risk neonates (i.e. preterm or low birthweight neonates, or both) including enteral nutrition interventions (e.g. high protein intake; donor breast milk; nutrient‐enriched formula; multi‐nutrient fortification of human breast milk; responsive or scheduled feeding), parenteral nutrition interventions (e.g. early or late, high or low amino acid administration), or vitamin or mineral supplementation (e.g. glutamine; arginine; iodine; vitamin E);
-
interventions for neurodevelopmental care or physical environment management (or both) for neonates (e.g. developmental care to reduce stressors in the neonatal nursery; kangaroo mother care; massage; co‐bedding in the neonatal nursery; early developmental programmes post discharge to prevent motor and cognitive impairments); and
-
interventions for all neonates at birth, such as newborn screening for inborn errors of metabolism.
We will not consider interventions in the antenatal or intrapartum period (such as magnesium sulphate for foetal neuroprotection (Doyle 2009)), as these interventions will be assessed in a separate overview (Shepherd 2016, under review).
How the intervention might work
Advances in research into several factors that modify the risk of cerebral palsy suggest many opportunities for prevention, with the main neonatal strategies focusing on protection of the immature brain through administration of neuroprotective agents or therapies.
For many individuals born at or near term who develop cerebral palsy, their neonatal course has been seemingly unremarkable, with the exception of those following perinatal asphyxia and with neonatal encephalopathy (brain injury that may be due to cerebral hypoxia and ischaemia before birth) (Badawi 2005; O’Shea 2008). For these neonates, therapeutic hypothermia, applied selectively to the head (as a ‘cooling cap’) or to the whole body, is one such intervention that can mediate cerebral palsy risk (O’Shea 2008). Beyond cooling, a range of other interventions (including those used as adjuvant therapy with cooling) may contribute to cerebral palsy prevention by protecting against secondary cell death and brain damage following hypoxic‐ischaemic insult (Robertson 2012), or by treating the underlying cause(s) of encephalopathy (such as infection or metabolic derangement).
For preterm and very low birthweight neonates, and for other groups of neonates (such as those with hypoglycaemia) who are at increased risk of brain injury, many pharmacological and non‐pharmacological interventions in the neonatal period may mediate cerebral palsy risk (O’Shea 2008). Although these interventions differ in their primary aims (such as maintaining adequate ventilation (e.g. through treatment of apnoea of prematurity with caffeine); maintaining normal metabolic status (e.g. through treatment of neonatal hypoglycaemia with dextrose gel); or controlling neonatal seizures (e.g. through use of anticonvulsants)), each may contribute to cerebral palsy prevention by reducing the likelihood or severity of brain injury, and thus of long‐term neurodevelopmental sequelae.
Why it is important to do this overview
A multitude of individual studies and Cochrane Systematic Reviews assessing a broad range of neonatal interventions (with varying primary aims or indications) acknowledge the potential for the intervention of interest to influence cerebral palsy risk. With awareness that there are many and varied risk factors for cerebral palsy, and that causes of cerebral palsy differ, there is a need to systematically consider all potentially relevant interventions for their ability to contribute to reducing cerebral palsy risk. As new data suggest possible declining rates and severity of cerebral palsy, it is important to examine the different interventions that may, together, contribute to these observations.
To our knowledge, to date, no ‘overview’ has brought together the evidence around neonatal interventions for cerebral palsy prevention from Cochrane Systematic Reviews into a single coherent document to be used by researchers, funding bodies, policy makers, clinicians, and consumers to aid decision‐making and evidence implementation.
Although the objective of this overview is to summarise the evidence from Cochrane Systematic Reviews regarding effects of neonatal interventions for preventing cerebral palsy, it is also important to consider whether such interventions may, instead, actually contribute to increasing cerebral palsy risk.
Is an overview the right approach?
We have followed the Editorial Decision Tree proposed by the Cochrane Comparing Multiple Interventions Methods Group to establish whether our review would better fit an overview format or an intervention review format, specifically:
-
we will review systematic reviews, instead of individual trials;
-
we will not compare multiple interventions with the intention of drawing inferences about the comparative effectiveness of these interventions; and
-
we intend to present a map of evidence from systematic reviews but with no attempt to rank the interventions.
On the basis of these points, the Editorial Decision Tree recommends an overview as the appropriate format for this review.
Objectives
Primary
To summarise the evidence from Cochrane Systematic Reviews regarding effects of neonatal interventions for preventing cerebral palsy (reducing cerebral palsy risk).
Secondary
To summarise the evidence from Cochrane Systematic Reviews regarding effects of neonatal interventions that may increase cerebral palsy risk.
Methods
Criteria for considering reviews for inclusion
In this overview of systematic reviews, we included only published Cochrane Systematic Reviews of neonatal interventions for which cerebral palsy was reported as a primary or secondary review outcome. We identified Cochrane protocols and titles for future inclusion and classified them as 'Ongoing reviews' (in an Appendix).
We made note of publication and search dates of the reviews; however, we did not attempt to update the individual systematic reviews.
Participants
We considered reviews that included:
-
neonates with perinatal asphyxia or with evidence of neonatal encephalopathy; and
-
neonates born preterm or at low or very low birthweight (or both preterm and low/very low birthweight neonates).
We also included reviews that included other groups of 'at risk' neonates (e.g. neonates with hypoglycaemia), so long as the intervention assessed in the Cochrane Systematic Review was recognised by the review authors as having the potential to influence cerebral palsy risk ‐ cerebral palsy had to be pre‐specified as a primary or secondary outcome in the review.
Interventions
We considered all types of interventions used in the neonatal period compared with placebo, no treatment, or an alternative intervention.
We included both pharmacological and non‐pharmacological interventions (see Description of the interventions for further description of possible interventions).
Outcomes of interest
Primary
-
Cerebral palsy (regardless of criteria used for diagnosis by review authors or trialists, and regardless of age at diagnosis; however, we have reported any variation)
Secondary
-
Cerebral palsy or death (regardless of criteria used for diagnosis by review authors or trialists, and regardless of age at diagnosis; however, we have reported any variation)
-
Severity of cerebral palsy (e.g. according to Gross Motor Function Classification System (GMFCS); Manual Ability Classification System (MACS); Communication Function Classification System (CFCS))
-
Type of cerebral palsy (e.g. according to topography (diplegia; hemiplegia; quadriplegia; monoplegia; triplegia) or motor type (spastic; dyskinetic; ataxic))
-
Motor dysfunction (regardless of criteria used for diagnosis by review authors or trialists, and regardless of age at diagnosis; however, we have reported any variation)
-
Other composite outcomes that include cerebral palsy as a component (regardless of criteria used for diagnosis by review authors or trialists, and regardless of age at diagnosis; however, we have reported any variation)
To be included, a review had to pre‐specify our overview’s primary outcome ‐ cerebral palsy (or a composite outcome that included cerebral palsy*) as a primary or secondary systematic review outcome ‐ and must have reported data for this outcome from at least one of the included trials in the review.
We listed reviews that pre‐specified cerebral palsy as a primary or secondary systematic review outcome but provided no reported data from included trials on this outcome as 'Reviews awaiting further classification', and we will reconsider these reviews in future updates of the overview.
* When possible, we extracted data related to cerebral palsy from any composite outcomes that included cerebral palsy. When it was not possible to extract only cerebral palsy data from such composite outcomes, we reported the composite outcome data; however, we reported these separately from the data for our primary outcome (i.e. as a secondary outcome).
Search methods for identification of reviews
We searched the Cochrane Database of Systematic Reviews on 27 November 2016, using the term 'cerebral palsy’. We used the search term to search 'all text', not limited to 'title, abstract, or keywords'. We did not apply any language or date restrictions. We searched no other databases. We managed citations retrieved through the search by using Covidence (Covidence 2015).
Data collection and analysis
We based our data collection and synthesis methods on Chapter 22 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When appropriate, we prepared the overview using Covidence (Covidence 2015) and Review Manager 5 software (RevMan 2014).
Selection of reviews
Two overview authors independently assessed for inclusion all potential systematic reviews identified by the search. We resolved disagreements through discussion, or, if required, we consulted a third member of the overview team.
Data extraction and management
Two overview authors independently extracted data from the reviews using a pre‐defined data extraction form. We resolved discrepancies through discussion or, if needed, through consultation with a third overview author. When information regarding review outcomes was unclear or missing, we accessed the published papers of individual studies for further details.
We extracted information on the following.
-
Review characteristics.
-
Review title and authors.
-
Date that the review was last assessed as up‐to‐date.
-
Number of included trials and numbers of participants (neonates) in the trials and their characteristics (e.g. countries in which the trials were conducted, trial inclusion criteria).
-
Quality of the included trials (as reported by the review authors; see 'Quality of studies included within reviews' below under Assessment of methodological quality of included reviews).
-
Interventions and comparisons relevant to this overview.
-
All pre‐specified outcomes relevant to this overview (their definitions, and whether they were primary or secondary outcomes in the included reviews).
-
Any other characteristics required to assess and report on review quality (see 'Quality of included reviews' under Assessment of methodological quality of included reviews).
-
-
Statistical summaries*.
-
Summary intervention effects (including pooled effects (e.g. risk ratios (RRs), odds ratios (ORs), or mean differences (MDs) as reported in the individual reviews), 95% confidence intervals (CIs), and numbers of studies and participants contributing data to each pooled effect) from comparisons and for outcomes relevant to this overview.
-
Information required to assess and report on the quality of evidence for the intervention effects extracted above (see 'Quality of evidence in included reviews' under Assessment of methodological quality of included reviews).
-
* When review authors were not able to perform meta‐analyses and therefore did not report statistical summaries, we extracted from those reviews the narrative text related to results for our overview outcomes.
Assessment of methodological quality of included reviews
Quality of included reviews
We assessed the methodological quality of each systematic review using the AMSTAR (A Measurement Tool to Assess Systematic Reviews) instrument (Shea 2009). AMSTAR evaluates the methods used in a review against 11 distinct criteria and assesses the degree to which review methods are unbiased. Each item on AMSTAR is rated as 'yes' (clearly done), 'no' (clearly not done), 'cannot answer', or 'not applicable'. These criteria were as follows:
-
Was an ‘a priori’ design provided?
-
Was there duplicate study selection and data extraction?
-
Was a comprehensive literature search performed?
-
Was status of the publication used as an inclusion criterion?
-
Was a list of studies (included and excluded) provided?
-
Were the characteristics of included studies provided?
-
Was the scientific quality of included studies assessed and documented?
-
Was the scientific quality of included studies used appropriately in formulating conclusions?
-
Were the methods used to combine the findings of studies appropriate?
-
Was the likelihood of publication bias assessed?
-
Was conflict of interest stated?
For all items except item 4, we considered a rating of 'yes' as adequate. For item 4, we considered a rating of 'no' as adequate. We considered a review that adequately met all of the 11 criteria to be a review of the highest quality (Shea 2009). For this overview, we considered reviews that achieved scores of 8 to 11 as high quality; scores of 4 to 7 as medium quality; and scores of 0 to 3 as low quality.
To further assess risk of bias of the systematic reviews, we additionally used the new ROBIS (Risk of Bias in Systematic Reviews) tool (Whiting 2015). This tool considers risk of bias across four key domains.
-
Study eligibility criteria.
-
Identification and selection of studies.
-
Data collection and study appraisal.
-
Synthesis and findings.
A series of questions within each domain elicited information about possible limitations of the systematic review, leading to a judgement about concerns within that domain (low, high, or unclear). We then considered risk of bias of the review as a whole, using signalling questions and information to support the overall judgement of risk of bias (low, high, or unclear) (Whiting 2015).
Two overview authors independently assessed the quality of included reviews using AMSTAR and ROBIS, and another overview author verified this assessment. We resolved differences through discussion or, if needed, through consultation with a third overview author.
We also noted and reported for each review the publication and search dates.
Quality of studies included within reviews
We did not reassess the quality of studies included within reviews but instead reported study quality according to review authors' assessments. We collected this information during the data extraction process.
Quality of evidence in included reviews
We assessed/reported the quality of evidence for our primary outcome (cerebral palsy) and for secondary review outcomes using the GRADE approach, as outlined in the GRADE handbook. We reported the quality of evidence as assessed by systematic review authors (who were in the best position to assess quality, given their familiarity with study‐level data) by using GRADEPro 'Summary of findings' tables from the reviews if provided (or when necessary, we constructed such tables using the GRADEpro Guideline Development Tool). The GRADE system assesses the following features for the evidence found for important outcomes.
-
Study limitations (risk of bias): internal validity of the evidence.
-
Inconsistency: heterogeneity or variability in estimates of effect across studies.
-
Indirectness: degrees of difference between populations, interventions, and comparators for the intervention and the outcome of interest.
-
Imprecision (random error): extent to which confidence in the effect estimate is adequate to support a particular decision.
-
Publication bias: degree of selective publication of studies.
The GRADE system rates the quality of evidence as follows.
-
High (further research is very unlikely to change confidence in the estimate of effect).
-
Moderate (further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate).
-
Low (further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate).
-
Very low (any estimate of effect is very uncertain).
Data synthesis
We prepared a narrative description of characteristics of the included Cochrane Reviews. We organised Review findings by groups of neonates when possible as follows: interventions for neonates with perinatal asphyxia or with evidence of neonatal encephalopathy; interventions for neonates born preterm and at low or very low birthweight; and interventions for other specific groups of 'at risk' neonates.
We summarised the main results of included reviews by categorising their findings in the following framework (as has been used within previous Cochrane and non‐Cochrane overviews, such as Farquhar 2015 and Lassi 2015).
-
Effective interventions: indicating that the review found high‐quality evidence of effectiveness for an intervention.
-
Possibly effective interventions (more evidence needed): indicating that the review found moderate‐quality evidence of effectiveness for an intervention, but more evidence is needed.
-
Ineffective interventions: indicating that the review found high‐quality evidence of lack of effectiveness for an intervention.
-
Probably ineffective interventions (more evidence needed): indicating that the review found moderate‐quality evidence suggesting lack of effectiveness for an intervention, but more evidence is needed.
-
No conclusions possible: indicating that the review found low‐ or very low‐quality evidence, or insufficient evidence to comment on the effectiveness of an intervention.
We based the choice of category on quality of evidence for the primary overview outcome (cerebral palsy). We used separate assessments for different comparisons (e.g. when one intervention was compared both with placebo (or no treatment) and with an alternative intervention). This approach to summarising the evidence was based on an earlier Cochrane overview (Jones 2012), which categorised interventions as 'What works,' 'What may work', and 'Insufficient evidence to make a judgement'.
Results
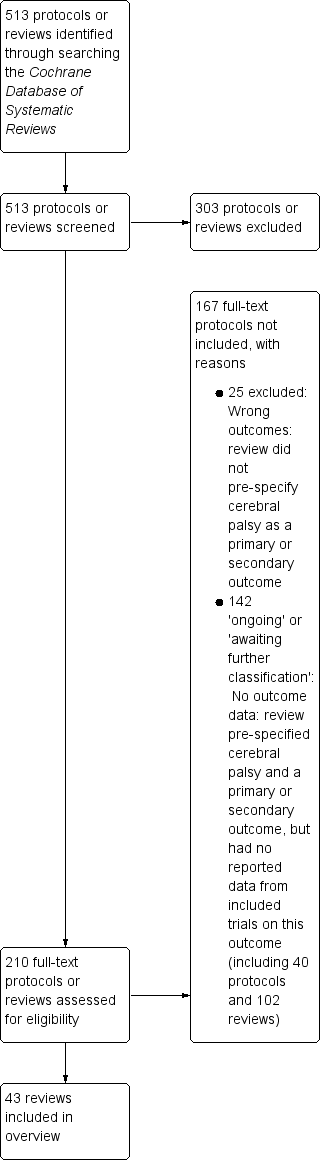
Our search of the Cochrane Database of Systematic Reviews yielded 513 protocols and reviews. Following title and abstract review, we excluded 303 protocols or reviews and assessed the full text of 210 protocols or reviews.
We excluded 25 reviews that did not pre‐specify cerebral palsy as a primary or secondary review outcome (see Table 1, 'Characteristics of excluded studies').
| Review ID and title | Reason for exclusion |
| Email for clinical communication between patients/caregivers and healthcare professionals | Wrong participants (not neonates):
|
| Parent‐infant psychotherapy for improving parental and infant mental health | Wrong participants (not neonates):
|
| Body positioning for spontaneously breathing preterm infants with apnoea | Secondary outcomes pre‐specified include the following:
No outcome data for these outcomes |
| C‐reactive protein for diagnosing late‐onset infection in newborn infants | Protocol for diagnostic test accuracy review |
| G‐CSF and GM‐CSF for treating or preventing neonatal infections | Secondary outcomes pre‐specified include:
No outcome data for cerebral palsy (single study results reported "cognition, language and social developmental performance scores were within the normal range for age and motor deficits were 'typical of high‐risk, low birth weight neonates'. However there was no comparison made between G‐CSF and control infants" |
| Intravenous dexamethasone for extubation of newborn infants | No pre‐specified outcome focused on development/disability at follow‐up |
| High‐frequency jet ventilation vs high‐frequency oscillatory ventilation for pulmonary dysfunction in preterm infants | Secondary neonatal outcomes pre‐specified include:
No outcome data for this outcome (no included trials) |
| Treatment of infantile spasms | Outcomes pre‐specified include:
No outcome data for cerebral palsy (single‐study results reported related to BSID; VABS; 'cognitive development'; Japanese Tumor Scale; DDST) |
| Antiviral therapy for symptomatic congenital cytomegalovirus infection in neonates and infants up to 3 months of age | Protocol Primary outcomes pre‐specified include:
|
| Lay health workers in primary and community health care for maternal and child health and management of infectious diseases | No pre‐specified outcome focused on development/disability at follow‐up |
| Surgical vs medical treatment with cyclo‐oxygenase inhibitors for symptomatic patent ductus arteriosus in preterm infants | Secondary outcomes pre‐specified include:
No outcome data for this outcome |
| Cycled light in the intensive care unit for preterm and low birthweight infants | Secondary outcomes pre‐specified include:
No outcome data for these outcomes |
| Transcutaneous screening for hyperbilirubinaemia in neonates | Protocol No pre‐specified outcome focused on development/disability at follow‐up |
| Granulocyte transfusions for neonates with confirmed or suspected sepsis and neutropaenia | Primary outcomes pre‐specified include:
No outcome data for this outcome |
| Molecular assays for diagnosis of sepsis in neonates | Protocol for diagnostic test accuracy review |
| Pentoxifylline for treatment of sepsis and necrotising enterocolitis in neonates | Secondary outcomes pre‐specified include:
No outcome data for this outcome |
| Hypothermia for neuroprotection in children after cardiopulmonary arrest | Primary outcomes pre‐specified include:
No outcome data for these outcomes (no included trials) |
| Intraventricular antibiotics for bacterial meningitis in neonates | Secondary outcomes pre‐specified include:
No outcome data for this outcome |
| Metalloporphyrins for treatment of unconjugated hyperbilirubinaemia in neonates | Outcomes pre‐specified include:
No outcome data for these outcomes |
| Periodic change of body position under phototherapy in term and late preterm neonates with hyperbilirubinaemia | Protocol Secondary outcomes pre‐specified include:
|
| Short‐duration vs standard‐duration antibiotic regimens for treatment of neonatal bacterial infection | Protocol Secondary outcomes pre‐specified include:
|
| Steroid therapy for meconium aspiration syndrome in newborn infants | Primary outcomes pre‐specified include:
No outcome data for this outcome |
| Diuretic therapy for newborn infants with post‐haemorrhagic ventricular dilatation | Outcomes pre‐specified include:
Data reported for these outcomes; no outcome data for cerebral palsy. "The larger trial showed that acetazolamide and furosemide treatment resulted in a borderline increase in the risk for motor impairment at one year (RR 1.27, 95% CI 1.02 ‐ 1.58; RD 0.16, 95% CI 0.02 ‐ 0.31), but did not significantly affect the risk for the combined outcome of delay, disability or motor impairment among survivors, or the risk of the combined outcome of death, delay, disability or impairment at one year" |
| Repeated lumbar or ventricular punctures in newborns with intraventricular haemorrhage | Outcomes pre‐specified include:
Data reported for these outcomes; no outcome data for cerebral palsy. "The tables and figures show that none of the trials found a significant effect of CSF tapping on a) need for shunt b) death c) major disability in survivors d) multiple disability in survivors e) death or disability. Similarly, meta‐analysis of the results of all included trials shows no significant effect of CSF tapping on any of these outcomes" |
| Permissive hypercapnia for prevention of morbidity and mortality in mechanically ventilated newborn infants | Outcomes pre‐specified include:
No outcome data for this outcome |
Abbreviations: BIND: bilirubin‐induced neurological dysfunction; BSID: Bayley Scales of Infant Development; CI: confidence interval; CSF: cerebrospinal fluid; DDST: Denver Developmental Screening Test; G‐CSF: granulocyte‐colony stimulating factor; GM‐CSF: granulocyte‐macrophage colony‐stimulating factor; RD: risk difference; RR: risk ratio; VABS: Vineland Adaptive Behavior Scales.
We listed an additional 142 protocols and reviews in the Appendices.
-
Appendix 1 ('Ongoing reviews') lists 40 Cochrane protocols that pre‐specified cerebral palsy as a primary or secondary outcome; we will consider these protocols for inclusion in future updates of the overview when they have been published as full reviews.
-
Appendix 2 ('Reviews awaiting further classification') summarises the 102 Cochrane Reviews that pre‐specified cerebral palsy as a primary or secondary outcome but reported no data from included trials on this outcome; again, we will consider these reviews for inclusion in future updates of the overview.
We therefore included 43 reviews in this overview. See Figure 1.
Study flow diagram.
Description of included reviews
Of the 43 included reviews:
-
Three reviews focused on interventions for neonates with perinatal asphyxia or with evidence of neonatal encephalopathy, categorised under the Cochrane Neonatal 'Neonatal care' topic.
-
Asphyxia: 'Allopurinol for preventing mortality and morbidity in newborn infants with hypoxic‐ischaemic encephalopathy' (Chaudhari 2012); 'Cooling for newborns with hypoxic ischaemic encephalopathy' (Jacobs 2013); 'Prophylactic barbiturate use for the prevention of morbidity and mortality following perinatal asphyxia' (Young 2016).
-
-
Thirty‐three reviews focused on interventions for neonates born preterm and/or at low or very low birthweight, categorised under the following Cochrane Neonatal 'Neonatal care' topics.
-
Haemorrhage: periventricular/intraventricular: 'Ethamsylate for the prevention of morbidity and mortality in preterm or very low birth weight infants' (Hunt 2010); 'Postnatal phenobarbital for the prevention of intraventricular haemorrhage in preterm infants' (Smit 2013).
-
Hypotension: 'The effect of inotropes on morbidity and mortality in preterm infants with low systemic or organ blood flow' (Osborn 2007b).
-
Fluid therapy: 'Early volume expansion for prevention of morbidity and mortality in very preterm infants' (Osborn 2004).
-
Patent ductus arteriosus: 'Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants' (Fowlie 2010); 'Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants' (Ohlsson 2015).
-
Blood disorders: 'Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants' (Ohlsson 2014); 'Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants' (Whyte 2011).
-
Nitric oxide: 'Inhaled nitric oxide for respiratory failure in preterm infants' (Barrington 2010).
-
Apneoa: 'Methylxanthine treatment for apnoea in preterm infants' (Henderson‐Smart 2010b); 'Prophylactic methylxanthine for prevention of apnoea in preterm infants' (Henderson‐Smart 2010c).
-
Respiratory distress syndrome: 'Inositol in preterm infants at risk for or having respiratory distress syndrome' (Howlett 2015); 'Animal derived surfactant extract for treatment of respiratory distress syndrome' (Seger 2009); 'Synthetic surfactant for respiratory distress syndrome in preterm infants' (Soll 2000); 'Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants' (Soll 2010).
-
Mechanical ventilation: 'Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants' (Cools 2015); 'Continuous distending pressure for respiratory distress in preterm infants' (Ho 2015); 'Prophylactic methylxanthines for endotracheal extubation in preterm infants' (Henderson‐Smart 2010).
-
Bronchopulmonary dysplasia: 'Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants' (Doyle 2014b); 'Moderately early (7 to 14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants' (Halliday 2003); 'Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants' (Doyle 2014); 'Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates' (Shah 2012); 'Vitamin A supplementation to prevent mortality and short‐ and long‐term morbidity in very low birth weight infants' (Darlow 2016).
-
Necrotising enterocolitis: 'Probiotics for prevention of necrotizing enterocolitis in preterm infants' (AlFaleh 2014); 'Arginine supplementation for prevention of necrotising enterocolitis in preterm infants' (Shah 2007).
-
Fungal infections: 'Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants' (Cleminson 2015).
-
Jaundice: 'Prophylactic phototherapy for preventing jaundice in preterm or low birth weight infants' (Okwundu 2012).
-
Parenteral feeding: 'Glutamine supplementation to prevent morbidity and mortality in preterm infants' (Moe‐Byrne 2016).
-
Other neonatal care (including thermal environment and developmental care): 'Thyroid hormones for preventing neurodevelopmental impairment in preterm infants' (Osborn 2001); 'Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants' (Osborn 2007); 'Sound reduction management in the neonatal intensive care unit for preterm or very low birth weight infants' (Almadhoob 2015); 'Kangaroo mother care to reduce morbidity and mortality in low birthweight infants' (Conde‐Agudelo 2016); 'Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants' (Spittle 2015).
-
-
Seven reviews focused on interventions for other specific groups of 'at risk' neonates, categorised under the following Cochrane Neonatal 'Neonatal care' topics.
-
Pulmonary hypertension: 'Endothelin receptor antagonists for persistent pulmonary hypertension in term and late preterm infants' (More 2016).
-
Resuscitation: 'Air versus oxygen for resuscitation of infants at birth' (Tan 2005).
-
Nitric oxide: 'Nitric oxide for respiratory failure in infants born at or near term' (Finer 2006).
-
Mechanical ventilation: 'Long versus short inspiratory times in neonates receiving mechanical ventilation' (Kamlin 2003); 'Volume‐targeted versus pressure‐limited ventilation in the neonate' (Wheeler 2010) (although in these reviews, relevant outcome data were from neonates born preterm and/or at low or very low birthweight only).
-
Herpes simplex: 'Antiviral agents for treatment of herpes simplex virus infection in neonates' (Jones 2009).
-
Hypoglycaemia: 'Oral dextrose gel for the treatment of hypoglycaemia in newborn infants' (Weston 2016).
-
The 43 reviews included between one ‐ as in Almadhoob 2015,Osborn 2007b, and Shah 2007 ‐ and 33 ‐ as in Ohlsson 2015 ‐ randomised controlled trials, and between 34 ‐ as in Almadhoob 2015 ‐ and 5529 ‐ as in AlFaleh 2014 ‐ infants. In total, the 43 reviews included 454 randomised trials, involving 63,977 infants.
One‐third (14) of the 43 reviews had conducted searches (and were considered 'up‐to‐date') in the past three years (November 2013 to November 2016) (AlFaleh 2014; Almadhoob 2015; Cleminson 2015; Conde‐Agudelo 2016; Cools 2015; Darlow 2016; Ho 2015; Howlett 2015; Moe‐Byrne 2016; More 2016; Ohlsson 2015; Spittle 2015; Weston 2016; Young 2016). The other 29 reviews had latest search end dates ranging from May 1998 ‐ in Soll 2000 ‐ to August 2013 ‐ in Doyle 2014b.
See Table 2 and Table 3 for further details of the characteristics of the 43 included reviews (including review IDs and titles, search dates and when the review was last assessed as up‐to‐date, numbers of randomised controlled trials and infants included, interventions and comparisons examined, overview outcomes reported, and summary of included trial limitations (risk of bias)).
| Review ID and title | Date of search and date assessed as up‐to‐date | No. included trials (countries and publication years) | No. participants in included trials | Inclusion criteria for 'Types of participants' | Relevant comparison interventions (no. trials and participants) | Overview outcomes for which data were reported (no. trials and participants) |
| Neonatal care: asphyxia | ||||||
| Allopurinol for preventing mortality and morbidity in newborn infants with hypoxic‐ischaemic encephalopathy | Searches: March 2012 Up‐to‐date: | 3 RCTs (Countries: Netherlands, Turkey; Published: 1990s: 1 RCT; 2000s: 2 RCTs) | 114 infants | Newborn infants (> 34 weeks' gestation) with hypoxic‐ischaemic encephalopathy defined as clinical evidence of cardiorespiratory or neurological depression (Apgar score < 7 at 5 minutes and beyond after birth) and/or evidence of severe metabolic acidosis in intrapartum foetal, umbilical arterial cord, or very early neonatal blood samples (pH < 7 or base deficit > 12 mmol/L), and/or clinical or electro‐encephalographic (multi‐channel or amplitude integrated) evidence of neonatal encephalopathy (MacLennan 1999) | Allopurinol vs control (3 RCTs, 114 neonates) | Severity of cerebral palsy ("Severe quadriplegia in surviving infants" (3 RCTs, 73 children); reported as a primary outcome) Other composite outcome that includes cerebral palsy as a component ("Death or severe neurodevelopmental disability in survivors" (3 RCTs, 110 children); reported as a primary outcome) |
| Cooling for newborns with hypoxic‐ischaemic encephalopathy | 1 May 2012 | 11 RCTs (Countries: China: 2 RCTs; New Zealand: 1 RCT; Turkey: 1 RCT; USA; 3 RCTs; international: 4 RCTs Published: 1990s: 1 RCT; 2000s: 7 RCTs; 2010s: 3 RCTs) | 1505 infants | 1. Newborn infants of 35 weeks' gestation or greater 2. Evidence of peripartum asphyxia, with each enrolled infant satisfying at least 1 of the following criteria: a. Apgar score of 5 or less at 10 minutes b. Mechanical ventilation or resuscitation at 10 minutes c. Cord pH < 7.1, or arterial pH < 7.1, or base deficit of 12 or more within 60 minutes of birth 3. Evidence of encephalopathy according to Sarnat staging (Finer 1981; Sarnat 1976): a. Stage 1 (mild): hyperalertness, hyper‐reflexia, dilated pupils, tachycardia, absence of seizures b. Stage 2 (moderate): lethargy, hyper‐reflexia, miosis, bradycardia, seizures, hypotonia with weak suck and Moro c. Stage 3 (severe): stupor, flaccidity, small to mid position pupils that react poorly to light, decreased stretch reflexes, hypothermia, and absent Moro No major congenital abnormalities recognisable at birth | Therapeutic hypothermia vs standard care (11 RCTs, 1505 neonates) | Cerebral palsy ("Cerebral palsy in survivors assessed" (7 RCTs, 881 children) and "Outcome at 6 to 7 years of age: Cerebral palsy" (1 RCT, 121 children); reported as secondary outcomes) Other composite outcomes that include cerebral palsy as a component ("Death or major disability in survivors assessed" (8 RCTs, 1344 children); reported as a primary outcome) ("Major neurodevelopmental disability" (8 RCTs, 1344 children); "Major neurodevelopmental disability in survivors assessed" (8 RCTs, 917 children); "Outcome at 6 to 7 years of age: death or moderate‐to‐severe disability" (1 RCT, 190 children); "Outcome at 6 to 7 years of age: moderate‐to‐severe disability" (1 RCT, 119 children); reported as secondary outcomes) Motor dysfunction ("Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed" (6 RCTs, 657 children); reported as a secondary outcome) |
| Prophylactic barbiturate use for the prevention of morbidity and mortality following perinatal asphyxia | 30 November 2015 | 9 RCTs (Countries: Finland: 1 RCT; India: 2 RCTs; Mexico: 1 RCT; Romania: 1 RCT; South Africa: 1 RCT; Spain: 1 RCT; USA: 2 RCTs; Published: 1980s: 2 RCTs; 1990s: 2 RCTs; 2000s: 2 RCTs; 2010s: 3 RCTs) | 456 infants |
| Barbiturates vs control (8 RCTs, 439 neonates) | Cerebral palsy ("Cerebral palsy" (2 RCTs, 69 children); reported as a secondary outcome) Other composite outcomes that include cerebral palsy as a component ("Death or major neurodevelopmental disability" (1 RCT, 31 children); reported as a primary outcome) ("Major neurodevelopmental disability" (1 RCT, 31 children); reported as a secondary outcome) |
| Neonatal care: haemorrhage: periventricular/intraventricular | ||||||
| Ethamsylate for the prevention of morbidity and mortality in preterm or very low birth weight infants | Search: 24 August 2009 Up‐to‐date: 22 September 2009 | 7 RCTs (Countries: France, Greece, UK: 1 RCT; India: 1 RCT; Switzerland: 1 RCT; Taiwan: 1 RCT; Turkey: 1 RCT; UK: 2 RCTs; Published: 1980s: 3 RCTs; 1990s: 4 RCTs) | 1410 infants | Preterm infants born before and including 34 weeks plus 6 days' completed gestation or with birthweight < 2000 g | Ethamsylate vs placebo (7 RCTs, 1410 neonates) | Cerebral palsy ("Cerebral palsy in surviving children available for follow‐up" (3 RCTs, 532 children); reported as a primary outcome) Other composite outcomes that include cerebral palsy as a component ("Neurodevelopmental disability at 2 years of age in surviving children available for follow‐up" (3 RCTs, 532 children); "Death or any disability by 2 years of age in children with known outcome at any point in time" (7 RCTs, 1334 children); reported as primary outcomes) |
| Postnatal phenobarbital for the prevention of intraventricular haemorrhage in preterm infants | Search: 31 October 2012 Up‐to‐date: 17 December 2012 | 12 RCTs (Countries: not reported; Published: 1980s: 8 RCTs; 1990s: 1 RCT: 2000s: 3 RCTs) | 982 infants | Newborn infants (less than 24 hours old) with gestational age < 34 weeks or birthweight < 1500 g. We included preterm infants with gestational age 33 to 36 weeks or birthweight up to 1750 g, if they were mechanically ventilated. We excluded infants with serious congenital malformations | Phenobarbital vs control (12 RCTs, 982 neonates) | Other composite outcomes that include cerebral palsy as a component ("Mild neurodevelopmental impairment" (1 RCT, 101 children); "Severe neurodevelopmental impairment" (1 RCT, 101 children); reported as secondary outcomes) |
| Neonatal care: hypotension | ||||||
| The effect of inotropes on morbidity and mortality in preterm infants with low systemic or organ blood flow | 19 May 2010 | 1 RCT (Country: not reported; Published: 2000s) | 42 infants | Preterm infants (< 37 weeks' gestational age) with low SBF or organ blood flow in the neonatal period. Low SBF may be determined on the basis of echocardiographically measured ventricular outputs or surrogates for SBF such as SVC flow. Low organ blood flow may be determined on the basis of techniques including ultrasound Doppler, near infrared spectroscopy, or xenon clearance techniques when evidence in the literature suggests that measurement is associated with substantial clinical outcomes and/or actual organ blood flow. The review does not include studies that include surrogates of flow such as BP, ultrasound Doppler‐measured velocities, pulsatility, or resistive indices | Dobutamine vs dopamine in preterm infants with low superior vena cava flow (1 RCT, 42 neonates) | Cerebral palsy ("Cerebral palsy at 3 years in survivors assessed" (1 RCT, 13 children); reported as a primary outcome) Other composite outcomes that include cerebral palsy as a component ("Disability at 3 years in survivors assessed" (1 RCT, 13 children); "Death or disability at 3 years" (1 RCT, 37 children); "Death or disability at latest follow‐up" (1 RCT, 41 children); reported as primary outcomes) |
| Neonatal care: fluid therapy | ||||||
| Early volume expansion for prevention of morbidity and mortality in very preterm infants | 30 July 2008 | 8 RCTs (Countries: not reported; Published: 1970s: 1 RCT; 1980s: 1 RCT; 1990s: 4 RCTs; 2000s: 2 RCTs) | 1185 infants | Very preterm infants born ≦ 32 weeks' gestation or ≦ 1500 g and enrolled and treated the first 72 hours after birth. Trials were eligible if they enrolled unselected preterm infants, preterm infants with clinically suspected poor perfusion (e.g. low BP, poor cutaneous perfusion, metabolic acidosis), or preterm infants with low blood flow (e.g. determined by Doppler ultrasound). Low BP may be defined as BP less than a specified percentile of a standard chart, mean BP ≦ 30 mmHg in any preterm infant, or mean BP ≦ 1 mmHg per week of gestation | Volume vs no treatment in very preterm infants (5 RCTs, 978 neonates) Gelatin vs fresh frozen plasma in hypotensive infants (1 RCT, 519 neonates) | Cerebral palsy ("Cerebral palsy in survivors" (1 RCT, 604 children; and 1 RCT, 399 children); reported as a primary outcome) Other composite outcomes that include cerebral palsy as a component ("Severe neurodevelopmental disability in survivors" (1 RCT, 604 children; and 1 RCT, 399 children); "Death or severe neurodevelopmental disability" (1 RCT, 776 children; and 1 RCT, 518 children); reported as primary outcomes) |
| Neonatal care: patent ductus arteriosus | ||||||
| Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants | Searches: April 2010 Up‐to‐date: | 19 RCTs (Countries: North America: 13 RCTs; Latin America, Europe, Asia: 6 RCTs; Published: 1980s: 11 RCTs; 1990s: 7 RCTs; 2000s; 1 RCT) | 2872 infants | Preterm neonates (less than 37 weeks' completed gestation) | Prophylactic IV indomethacin vs placebo or no drug (19 RCTs, 2872 neonates) | Cerebral palsy ("Neurological assessments (18‐54 months: Cerebral palsy" (4 RCTs, 1372 children); "School age neurological assessments: Cerebral palsy aged 8 years" (1 RCT, 304 children); reported as primary outcomes) Other composite outcome that includes cerebral palsy as a component ("Death or severe neurosensory impairment" (3 RCTs, 1491 children); reported as a primary outcome) |
| Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants | 7 May 2014 | 33 RCTs (Countries: Albania: 1 RCT; Belgium: 2 RCTs; Czech Republic: 1 RCT; China: 1 RCT; Egypt: 1 RCT; India: 1 RCT; Iran: 3 RCTs; Israel: 1 RCT; Italy: 6 RCTs; Poland: 1 RCT; Qatar: 1 RCT; Spain: 2 RCTs; Taiwan: 2 RCTs; Thailand: 2 RCTs; Tunisia: 1 RCT; Turkey: 3 RCTs; UK: 2 RCTs; USA: 2 RCTs; Published: 1990s: 4 RCTs; 2000s: 18 RCTs; 2010s: 11 RCTs) | 2190 infants | Preterm infants less than 37 weeks' gestational age or LBW infants (less than 2500 g) with PDA diagnosed either clinically or by echocardiographically (ECHO) guided criteria in the neonatal period (less than 28 days) | Oral ibuprofen vs IV ibuprofen (data for maximum of 4 RCTs, 304 neonates) | Cerebral palsy ("Moderate/severe cerebral palsy at 18‐24 months" (1 RCT, 57 children); reported as a secondary outcome) |
| Neonatal care: blood disorders | ||||||
| Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants | 1 July 2013 | 27 RCTs (Countries: Austria: 2 RCTs; Bangladesh: 1 RCT; Chile: 1 RCT; China: 2 RCTs; Greece: 3 RCTs; Iran: 1 RCT; Italy: 2 RCTs; Mexico: 1 RCT; New Zealand: 1 RCT; Poland: 1 RCT; Singapore: 1 RCT; South Africa: 1 RCT; Switzerland: 1 RCT; Turkey: 1 RCT; USA: 5 RCTs; Europe: 3 RCTs; Published 1990s: 12 RCTs; 2000s: 13 RCTs; 2010s: 2 RCTs) | 2209 infants | Preterm (< 37 weeks) and/or LBW (< 2500 g) neonates less than 8 days of age | Erythropoietin vs placebo or no treatment (27 RCTs, 2209 neonates) Darbepoetin alfa vs placebo or no treatment (1 RCT, 66 neonates) | Cerebral palsy ("Cerebral palsy at 18 ‐ 22 months' corrected age (in children examined)" (2 RCTs, 153 children; and 1 RCT, 51 children); reported as secondary outcomes) Other composite outcome that includes cerebral palsy as a component ("Any neurodevelopmental impairment at 18‐22 months' corrected age (in children examined)" (1 RCT< 99 children); reported as a secondary outcome) Motor dysfunction ("PDI < 70 at 18 ‐ 22 months' corrected age (in children examined)" (1 RCT, 90 children); reported as a secondary outcome) |
| Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants | Search: August 2011 Up‐to‐date: 1 September 2011 | 5 RCTs (Countries: Canada: 1 RCT; International (Canada, USA, Australia): 1 RCT; Taiwan: 1 RCT; USA: 2 RCTs; Published: 1980s: 1 RCT; 1990s: 1 RCT; 2000s: 3 RCTs) | 670 infants | VLBW infants (i.e. of birthweight less than or equal to 1500 g, or less than 32 weeks' gestational age) admitted to NICU at less than 1 week of age. We aimed specifically to include studies of infants receiving all levels of intensive care | Transfusion at a low haemoglobin or haematocrit level (restrictive) vs transfusion at a high haemoglobin or haematocrit level (liberal) (4 RCTs, 614 neonates) | Cerebral palsy ("Neurosensory impairment at 18‐21 months' follow‐up among survivors: Cerebral palsy" (1 RCT, 335 children); reported as a secondary outcome) Other composite outcomes that include cerebral palsy as a component ("Death or severe morbidity: at 18‐21 months' follow‐up with MDI < 70" (1 RCT, 421 children); "Death or severe morbidity: at 18‐21 months' follow‐up with MDI < 85" (1 RCT, 421 children); reported as primary outcomes) ("Neurosensory impairment at 18‐21 months' follow‐up among survivors: any neurosensory impairment" (1 RCT, 328 children); reported as a secondary outcome) |
| Neonatal care: pulmonary hypertension | ||||||
| Endothelin receptor antagonists for persistent pulmonary hypertension in term and late preterm infants | 28 December 2015 | 2 RCTs (Countries: Saudi Arabia: 1 RCT; unclear (multi‐centre): 1 RCT; Published: 2010s: 2 RCTs) | 68 infants | Late preterm infants (born at 34+0 to 36+6 weeks), term infants (born at 37+0 to 41+6 weeks), and post‐term infants (i.e. born after 41+6 weeks' gestation) until post‐menstrual age (PMA) up to 44 weeks with PPHN were eligible for inclusion. The diagnosis of PPHN was clinical or was based on echocardiography. Clinical diagnosis of PPHN was considered when there was hypoxaemia refractory to oxygen therapy and mechanical ventilation (Roberts 1997). The echocardiographic diagnosis of PPHN was made by demonstrating the presence of extrapulmonary right‐to‐left shunting at the ductal or atrial level, near or suprasystemic pulmonary arterial pressures, and doppler evidence of tricuspid regurgitation (Dhillon 2012; Stayer 2010) | Endothelin receptor antagonists vs placebo (1 RCT, 47 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 37 children); reported as a secondary outcome) Motor dysfunction ("Adverse neurodevelopmental outcome at 6 months" (1 RCT, 37 children); reported as a secondary outcome) |
| Neonatal care: resuscitation | ||||||
| Air versus oxygen for resuscitation of infants at birth | Search: December 2003/January 2004 Up‐to‐date: 15 February 2005 | 5 RCTs (Countries: India: 1 RCT; 6 countries: 1 RCT; not reported: 3 RCTs Published: 1990s: 2 RCTs; 2000s: 3 RCTs) | 1302 infants | Term or preterm neonates requiring IPPV at birth | Room air vs 100% oxygen (5 RCTs, 1302 neonates) | Cerebral palsy ("Long‐term neurodevelopmental outcome: cerebral palsy in those followed up at 18‐24 months" (1 RCT, 213 children); reported as a post hoc outcome) Motor dysfunction ("Long‐term neurodevelopmental outcome: not walking in those followed up at 18‐24 months" (1 RCT, 213 children); reported as a post hoc outcome) |
| Neonatal care: nitric oxide | ||||||
| Inhaled nitric oxide for respiratory failure in preterm infants | Search: June 2010 Up‐to‐date: 12 October 2010 | 14 RCTs (Countries: Europe: 3 RCTs; Taiwan: 1 RCT; USA: 1 RCT; not reported/unclear: 9 RCTs Published: 1990s: 3 RCTs; 2000s: 11 RCTs) | 3430 infants | Premature infants (less than 35 weeks' gestation) with respiratory failure after adequate treatment with surfactant | Inhaled NO compared with control; analyses conducted based on:
| Cerebral palsy ("Cerebral palsy"; reported as an outcome (2 RCTs, 209 children; 2 RCTs, 498 children; and 2 RCTs, 593 children) (not separated into primary/secondary)) Other composite outcome that includes cerebral palsy as a component ("Neurodevelopmental disability" (2 RCTs, 208 children; 2 RCTs, 498 children; and 2 RCTs, 593 children); reported as an outcome (not separated into primary/secondary)) Motor dysfunction ("Bayley MDI or PDI <‐2SD" (1 RCT, 138 children); reported as an outcome (not separated into primary/secondary)) |
| Nitric oxide for respiratory failure in infants born at or near term | Search: November 2005 Up‐to‐date: | 14 RCTs (Countries: 33 French and Belgian Units: 1 RCT; not reported: 13 RCTs Published: 1990s: 11 RCTs; 2000s: 3 RCTs) | 1715 infants | Newborn infants (< 1 month of age) with hypoxaemia suspected to be due to lung disease, pulmonary hypertension with right‐to‐left shunting, or both Only studies in term and near‐term infants (> 34 weeks' gestation) were included Efforts were made in all studies to exclude infants with intracardiac shunting due to structural congenital heart disease Infants with diaphragmatic hernia may respond differently from other near term infants (from preliminary data), and as far as possible results from infants with diaphragmatic hernias have been evaluated separately | Inhaled NO vs control (10 RCTs, 1068 infants) Inhaled NO vs control in infants with diaphragmatic hernia (2 RCTs, 84 neonates) | Cerebral palsy ("Cerebral palsy among survivors" (2 RCTs, 299 children; and 1 RCT, 22 children); reported as an outcome (not separated into primary/secondary)) Other composite outcome that includes cerebral palsy as a component ("Neurodevelopmental disability at 18 to 24 months among survivors" (2 RCTs, 301 children); reported as an outcome (not separated into primary/secondary)) Motor dysfunction ("Bayley PDI more than 2 SD below the mean" (2 RCTs, 283 children); reported as an outcome (not separated into primary/secondary)) |
| Neonatal care: apnoea | ||||||
| Methylxanthine treatment for apnoea in preterm infants | Search: Up‐to‐date: | 6 RCTs (Countries: not reported Published: 1980s: 3 RCTs; 1990s: 1 RCT; 2000s: 2 RCTs) | 959 infants | Preterm infants with recurrent apnoea. There must have been an effort to exclude specific secondary causes of apnoea | Any methylxanthine vs control (placebo or no drug therapy) (6 RCTs, 959 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 729 children); reported as a secondary outcome) Other composite outcome that includes cerebral palsy as a component ("Death or major disability by late infancy" (1 RCT, 767 children); reported as a secondary outcome) |
| Prophylactic methylxanthine for prevention of apnoea in preterm infants | Search: August 2010 Up‐to‐date: 29 September 2010 | 3 RCTs (Countries: not reported Published: 1980s: 2 RCTs; 2000s: 1 RCT) | 557 infants | Preterm infants, particularly those born at less than 34 weeks' gestation, who are at risk of developing recurrent apnoea, bradycardia, and hypoxic episodes | Prophylactic methylxanthine vs control (3 RCTs, 557 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 415 children); reported as a secondary outcome) Other composite outcome that includes cerebral palsy as a component ("Death or major disability" (1 RCT, 423 children); reported as a secondary outcome) |
| Neonatal care: respiratory distress syndrome | ||||||
| Inositol in preterm infants at risk for or having respiratory distress syndrome | 14 September 2014 | 4 RCTs (Countries: Finland: 2 RCTs; USA: 2 RCTs Published: 1980s: 1 RCT; 1990s: 2 RCTs; 2010s: 1 RCT) | 429 infants | Preterm infants (< 37 weeks' post‐menstrual age) or LBW (< 2500 g) infants | Inositol supplementation (repeat doses) vs control (3 RCTs, 355 neonates) | Other composite outcomes that include cerebral palsy as a component ("Major neural developmental impairment at one year corrected age" (1 RCT, 169 children); reported as a secondary outcome) Motor dysfunction ("Minor neural developmental impairment at one year corrected age" (1 RCT, 169 children); reported as a secondary outcome) |
| Animal derived surfactant extract for treatment of respiratory distress syndrome | Search: December 2008 Up‐to‐date: 13 February 2009 | 13 RCTs (Countries: not reported Published: 1980s: 7 RCTs; 1990s: 6 RCTs) | 1611 infants | Preterm infants (< 37 weeks' gestation) with clinical and/or radiological evidence of respiratory distress syndrome requiring assisted ventilation | Animal‐derived surfactant extract treatment of respiratory distress (all infants) (13 RCTs, 1611 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 73 children); reported as a secondary outcome) Other composite outcome that includes cerebral palsy as a component ("Major neurodevelopmental disability in survivors" (1 RCT, 73 children); reported as a secondary outcome) |
| Synthetic surfactant for respiratory distress syndrome in preterm infants | Search: Up‐to‐date: | 6 RCTs (Countries: not clearly reported; Canada/USA/both: 3 RCTs Published; 1980s: 1 RCT; 1990s: 5 RCTs) | 2358 infants | Neonates with clinical and radiological evidence of respiratory distress syndrome requiring assisted ventilation | Synthetic surfactant vs control (6 RCTs, 2358 neonates) | Cerebral palsy ("Cerebral palsy in survivors examined" (5 RCTs, 1557 children); reported as an outcome (not separated into primary/secondary)) Severity of cerebral palsy ("Moderate ‐ severe cerebral palsy in survivors examined" (5 RCTs, 1557 children); reported as an outcome (not separated into primary/secondary)) |
| Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants | Search: September 2009 Up‐to‐date: 27 October 2009 | 7 RCTs (Countries: 1: UK; 6 RCTs: not reported Published: 1980s: 3 RCTs; 1990s: 4 RCTs) | 1583 infants | Premature infants with or without evidence of surfactant deficiency | Prophylactic synthetic surfactant vs control (7 RCTs, 1583 neonates) | Cerebral palsy ("Cerebral palsy, 1‐2 years" (4 RCTs, 670 children); reported as a secondary outcome) Severity of cerebral palsy ("Cerebral palsy, moderate/severe" (4 RCTs, 670 children); reported as a secondary outcome) |
| Neonatal care: mechanical ventilation | ||||||
| Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants | 30 November 2014 | 19 RCTs (Countries: not reported Published: 1980s: 1 RCT; 1990s: 6 RCTs; 2000s: 10 RCTs; 2010s: 2 RCTs) | 4096 infants | Preterm or LBW infants with pulmonary dysfunction, mainly due to respiratory distress syndrome, who were considered to require IPPV | High‐frequency oscillatory ventilation vs conventional ventilation (19 RCTs, 4096 neonates) | Cerebral palsy (reported in text as a secondary outcome (3 RCTs)) |
| Continuous distending pressure for respiratory distress in preterm infants | 30 April 2015 | 6 RCTs (Countries: not reported Published: 1970s: 4 RCTs; 1990s: 1 RCT; 2000s: 1 RCT) | 355 infants | Preterm infants with respiratory failure | Continuous distending pressure vs standard care (6 RCTs, 355 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 36 children); reported as a secondary outcome) Other composite outcomes that include cerebral palsy as a component ("Death or severe disability" (1 RCT, 38 children); "Severe disability" (1 RCT, 37 children); "Any disability" (1 RCT, 37 children); reported as secondary outcomes) |
| Prophylactic methylxanthines for endotracheal extubation in preterm infants | Search: Up‐to‐date: 16 August 2010 | 7 RCTs (Countries: not reported Published: 1980s: 3 RCTs; 1990s: 3 RCTs; 2000s: 1 RCT) | 916 infants | Preterm or LBW infants being weaned from IPPV | Methylxanthine vs control (7 RCTs, 914 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 644 children); reported as a secondary outcome) Other composite outcome that includes cerebral palsy as a component ("Death or major disability by 18‐21 months" (1 RCT, 676 children); reported as a secondary outcome) |
| Long versus short inspiratory times in neonates receiving mechanical ventilation | Search: Up‐to‐date: | 5 RCTs (Countries: not reported Published: 1980s: 3 RCTs; 19980s; 2 RCTs) | 694 infants | Term and preterm infants at less than 28 days of age and requiring conventional mechanical ventilation. No restrictions on underlying pathophysiology were applied | Long vs short inspiratory times (5 RCTs, 694 neonates) | Cerebral palsy ("Cerebral palsy in survivors less than 33 weeks' gestation at birth" (1 RCT, 177 children); reported as a secondary outcome) |
| Volume‐targeted versus pressure‐limited ventilation in the neonate | Search: January 2010 Up‐to‐date: | 12 RCTs (Countries: not reported Published: 1990s: 2 RCTs; 2000s: 10 RCTs) | 693 infants | All intubated infants of less than 28 days' corrected age who were being mechanically ventilated with IPPV at the time of study entry. Infants of all gestational ages and both paralysed and non‐paralysed infants were eligible | Volume‐targeted vs pressure‐limited ventilation (12 RCTs, 693 neonates) | Other composite outcomes that include cerebral palsy as a component ("Severe disability (any definition)" (2 RCTs, 209 children); "Severe disability (any definition) or death" (1 RCT, 109 children; reported as outcomes from post hoc meta‐analyses) Motor dysfunction ("Gross motor developmental issue (any definition)" (1 RCT, 128 children); reported as an outcome from a post hoc meta‐analysis) |
| Neonatal care: bronchopulmonary dysplasia | ||||||
| Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants | Search: August 2013 Up‐to‐date: 18 February 2014 | 29 RCTs (Countries: not reported Published: 1970s: 1 RCT; 1990s: 17 RCTs; 2000s: 10 RCTs; 2010s: 1 RCT) | 3750 infants | Preterm infants at risk of developing chronic lung disease, including those who were ventilator dependent | Early (< 8 days) postnatal corticosteroids vs control (29 RCTs, 3750 neonates) | Cerebral palsy ("Cerebral palsy" (12 RCTs, 1452 children); "Cerebral palsy in survivors assessed" (12 RCTs, 959 children); reported as primary outcomes) Cerebral palsy or death ("Death or cerebral palsy" (12 RCTs, 1452 children); reported as a primary outcome) Other composite outcomes that include cerebral palsy as a component ("Major neurosensory disability (variable criteria ‐ see individual studies)" (7 RCTs, 1233 children); "Major neurosensory disability (variable criteria) in survivors examined" (7 RCTs, 799 children); "Death or major neurosensory disability (variable criteria)" (7 RCTs, 1233 children); reported as primary outcomes) Motor dysfunction ("Bayley Psychomotor Developmental Index (PDI) <‐2SD" (3 RCTs, 842 children); "Bayley PDI <‐2SD in tested survivors" (3 RCTs, 528 children); reported as primary outcomes) |
| Moderately early (7‐14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants | Search: October 2002 Up‐to‐date: 11 November 2008 | 7 RCTs (Countries: not reported Published: 1980s: 1 RCT; 1990s: 6 RCTs) | 669 infants | Preterm babies developing chronic lung disease including those who were ventilator dependent | Moderately early (7‐14 days) postnatal corticosteroids vs control (7 RCTs, 659 neonates) | Cerebral palsy ("Cerebral palsy" (4 RCTs, 204 children); "Cerebral palsy in survivors assessed" (4 RCTs, 130 children); reported as review outcomes (not separated into primary and secondary)) Cerebral palsy or death ("Death or cerebral palsy" (4 RCTs, 204 children); reported as a review outcome) Other composite outcomes that include cerebral palsy as a component ("Major neurosensory disability (variable criteria ‐ see individual studies)" (2 RCTs, 96 children); "Major neurosensory disability (variable criteria) in survivors assessed" (2 RCTs, 56 children); "Death or major neurosensory disability (variable criteria)" (2 RCTs, 96 children); reported as review outcomes) |
| Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants | Search: August 2013 Up‐to‐date: 18 February 2014 | 21 RCTs (Countries: Australia, Canada, New Zealand: 1 RCT; 6 countries: 1 RCT; not reported: 19 RCTs Published: 1980s: 5 RCTs; 1990s: 12 RCTs; 2000s: 3 RCTs; 2010s: 1 RCT) | 1424 infants | Preterm infants with evolving or established chronic lung disease, defined as oxygen‐dependent, ventilator‐dependent, or both, with or without radiographic changes of BPD | Late (> 7 days) postnatal corticosteroids vs control (21 RCTs, 1424 neonates) | Cerebral palsy ("Cerebral palsy: at 1 to 3 years" (14 RCTs, 876 children); "Cerebral palsy: at latest reported age" (15 RCTs, 855 children); "Cerebral palsy in survivors assessed: at 1 to 3 years" (14 RCTs, 631 children); "Cerebral palsy in survivors assessed: at latest reported age" (15 RCTs, 591 children); reported as primary outcomes) Cerebral palsy or death ("Death or cerebral palsy: at 1 to 3 years" (14 RCTs, 876 children); "Death or cerebral palsy: at latest reported age" (15 RCTs, 855 children); reported as primary outcomes) Other composite outcomes that include cerebral palsy as a component ("Major neurosensory disability (variable criteria ‐ see individual studies)" (8 RCTs, 655 children); "Major neurosensory disability (variable criteria) in survivors assessed" (8 RCTs, 480 children); "Death or major neurosensory disability (variable criteria)" (8 RCTs, 655 children); reported as primary outcomes) Motor dysfunction ("Bayley Psychomotor Developmental Index (PDI) < ‐2 SD" (1 RCT, 118 children); "Bayley PDI < ‐2 SD in survivors tested" (1 RCT, 90 children); reported as primary outcomes) |
| Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates | 29 July 2011 | 7 RCTs (Countries: Canada: 1 RCT; China: 1 RCT; Germany: 1 RCT; UK: 1 RCT; USA: 1 RCT; not reported: 2 RCTs Published: 1990s: 5 RCTs; 2000s: 2 RCTs) | 495 infants | Ventilator‐dependent preterm neonates with birthweight ≤ 1500 g and postnatal age < 2 weeks | Early inhaled steroids (< 2 weeks) vs placebo (7 RCTs, 495 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 56 children); reported as a secondary outcome) Motor dysfunction ("Mean developmental index on BSID‐II < 2 SD of the mean" (1 RCT, 56 children); reported as a secondary outcome) |
| Vitamin A supplementation to prevent mortality and short‐ and long‐term morbidity in very low birth weight infants | 1 May 2016 | 11 RCTs (Countries: Greece: 1 RCT; South Africa: 1 RCT; Thailand: 1 RCT; UK: 2 RCTs; USA: 6 RCTs Published: 1980s: 2 RCTs; 1990s: 4 RCTs; 2000s: 3 RCTs: 2010s: 2 RCTs) | 1580 infants | VLBW infants (defined as birthweight ≤ 1500 g or at less than 32 weeks' gestation) | Supplemental vitamin A vs no supplementation (10 RCTs, 1460 neonates) | Other composite outcomes that include cerebral palsy as a component ("Neurodevelopmental impairment at 18 to 22 months" (1 RCT, 538 children); "Death or neurodevelopmental impairment at 18 to 22 months" (1 RCT, 687 children); reported as secondary outcomes) |
| Neonatal care: infections: necrotising enterocolitis | ||||||
| Probiotics for prevention of necrotising enterocolitis in preterm infants | 1 October 2013 | 24 RCTs (Countries: Australia and New Zealand: 1 RCT; Brazil: 1 RCT; Colombia: 1 RCT; France: 1 RCT; Germany: 2 RCTs; Greece: 2 RCTs; India: 1 RCT; Israel: 1 RCT; Italy: 4 RCTs; Japan: 2 RCTs; Taiwan: 2 RCTs; Turkey: 1 RCT; UK: 1 RCT; USA: 1 RCT; not reported; 3 RCTs Published: 1980s: 1 RCT; 1990s: 2 RCTs; 2000s: 12 RCTs; 2010s: 9 RCTs) | 5529 infants (20 RCTs with reported outcomes) | Preterm infants at < 37 weeks and birthweight < 2500 g, or both | Probiotics vs control (20 RCTs, 5529 neonates) | Other composite outcome that includes cerebral palsy as a component ("Mental retardation and cerebral palsy" (1 RCT, 85 children); reported as a secondary outcome) |
| Arginine supplementation for prevention of necrotising enterocolitis in preterm infants | Search: August 2010 Up‐to‐date: 28 November 2010 | 1 RCT (Country: not reported Published; 2000s) | 152 infants | Preterm infants less than 37 weeks' gestation at birth | Arginine supplementation vs placebo (1 RCT, 152 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 135 children); reported as a post hoc secondary outcome) Other composite outcome that includes cerebral palsy as a component ("Major neurodevelopmental disability" (1 RCT, 132 children); reported as a post hoc secondary outcome) |
| Neonatal care: infections: fungal infections | ||||||
| Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants | Search: August 2015 Up‐to‐date: 1 September 2015 | 15 RCTs (Countries: India: 2 RCTs; Italy: 2 RCTs; Korea: 1 RCT; Saudi Arabia: 1 RCT; Turkey: 2 RCTs; USA: 7 RCTs Published: 2000s: 7 RCTs; 2010s: 8 RCTs) | 1690 infants | Very preterm or VLBW infants with or without evidence of fungal colonisation but without evidence of invasive fungal infection at study entry | Systemic antifungal agent vs placebo or no drug (10 RCTs, 1371 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 219 children); reported as a primary outcome) Other composite outcome that includes cerebral palsy as a component ("Neurodevelopmental impairment (composite)" (1 RCT, 171 children); reported as a primary outcome) |
| Neonatal care: infections: herpes simplex | ||||||
| Antiviral agents for treatment of herpes simplex virus infection in neonates | Search: November 2008 Up‐to‐date: 14 March 2009 | 2 RCTs (Countries: USA: 2 RCTs Published: 1980s: 1 RCT; 1990s: 1 RCT) | 273 infants | Hospitalised newborn infants less than 1 month of age with virologically confirmed HSV infection | Vidarabine vs placebo (1 RCT, 56 neonates) Aciclovir vs vidarabine (1 RCT, 202 neonates) | Cerebral palsy ("Cerebral palsy in CNS HSV neonatal infection up to three years by HSV serotype: HSV‐1" (1 RCT, 9 children); "Cerebral palsy in CNS HSV neonatal infection up to three years by HSV serotype: HSV‐2" (1 RCT, 14 children); reported as primary outcomes) Other composite outcomes that include cerebral palsy as a component ("Abnormal neurodevelopment at one year" (1 RCT, 56 children; and 1 RCT, 202 children); "Abnormal neurodevelopment or death at approximately one year of age" (1 RCT, 56 children; and 1 RCT, 202 children); reported as primary outcomes) |
| Neonatal care: jaundice | ||||||
| Prophylactic phototherapy for preventing jaundice in preterm or low birth weight infants | 31 March 2011 | 9 RCTs (Countries: USA: 6 RCTs; Brazil: 1 RCT; Canada: 1 RCT; India: 1 RCT Published: 1960s: 2 RCTs; 1970s: 1 RCT; 1980s: 2 RCTs; 2000s: 4 RCTs) | 3449 infants |
Originally (in the protocol), the focus of the review was narrower (to include VLBW infants; < 1500 g birthweight); however, so as not to lose valuable information, we made a post hoc decision to include any study that involved LBW (< 2500 g birthweight) or preterm infants We excluded studies of infants with a known cause that can lead to significant hyperbilirubinaemia, such as ABO incompatibility, Rh incompatibility, minor blood group incompatibility, or G‐6PD deficiency | Prophylactic phototherapy vs control (9 RCTs, 3449 neonates) | Cerebral palsy ("Cerebral palsy" (2 RCTs, 756 children); reported as a primary outcome) Other composite outcomes that include cerebral palsy as a component ("Neurodevelopmental impairment" (1 RCT, 1804 children); reported as a primary outcome) |
| Neonatal care: hypoglycaemia | ||||||
| Oral dextrose gel for the treatment of hypoglycaemia in newborn infants | 29 February 2016 | 2 RCTs (Countries: Ireland: 1 RCT; New Zealand: 1 RCT; Published: 2000s: 1 RCT; 2010s: 1 RCT) | 317 infants | We included newborn infants from birth to discharge home who were hypoglycaemic (blood glucose concentrations below the normal range, investigator defined) for any reason. We excluded infants who had received prior IV treatment for maintenance of glucose control at the time of hypoglycaemia | Dextrose gel vs control (2 RCTs, 317 neonates) | Cerebral palsy ("Cerebral palsy and severity at age 2 years or older" (1 RCT, 183 children); reported as a secondary outcome) Other composite outcomes that include cerebral palsy as a component ("Major neurosensory disability (2‐year follow‐up)" (1 RCT, 184 children); reported as a primary outcome) ("Developmental disability at age 2 years or older" (1 RCT, 184 children); reported as a secondary outcome) |
| Neonatal care: parenteral feeding | ||||||
| Glutamine supplementation to prevent morbidity and mortality in preterm infants | 18 December 2015 | 12 RCTs (Countries: China; 1 RCT; Greece: 1 RCT; Malaysia: Published: 1990s: 2 RCTs; 2000s: 6 RCTs; 2010s: 4 RCTs) | 2877 infants | We included preterm infants (gestational age < 37 weeks) admitted to neonatal intensive or special care units or comparable settings after birth. When participants in a trial included both term and preterm infants, we sought subgroup data from the report or from trial authors | Glutamine supplementation vs no supplementation (12 RCTs, 2877 neonates) | Other composite outcome that includes cerebral palsy as a component ("Neurodevelopmental impairment" (1 RCT, 72 children); reported as a primary outcome) |
| Neonatal care: other | ||||||
| Thyroid hormones for preventing neurodevelopmental impairment in preterm infants | Search: Up‐to‐date: 1 February 2009 | 5 RCTs (Countries: not reported; Published: 1980s: 2 RCTs; 1990s: 2 RCTs; 2000s; 1 RCT) | 362 infants | Studies that enrolled and treated preterm infants in the neonatal period | Thyroid hormones vs control (5 RCTs, 362 neonates) | Cerebral palsy ("Cerebral palsy in survivors" (1 RCT, 156 children); reported as a primary outcome) Cerebral palsy or death ("Death or cerebral palsy" (1 RCT, 200 children); reported as a primary outcome) |
| Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants | Search: Up‐to‐date: 12 October 2006 | 4 RCTs (Countries: not reported; Published: 1990s: 2 RCTs; 2000s: 2 RCTs) | 318 infants | Studies that enrolled preterm infants (born < 37 completed weeks' gestation) in the neonatal period. Trials that enrolled infants on the basis of results of abnormal thyroid function tests (known congenital hypothyroidism or transient hypothyroxinaemia), or with only respiratory distress syndrome, were excluded | Prophylactic thyroid hormones vs no thyroid hormones (4 RCTs, 318 neonates) | Cerebral palsy ("Cerebral palsy in survivors" (1 RCT, 156 children); reported as a primary outcome) Cerebral palsy or death ("Death or cerebral palsy" (1 RCT, 200 children); reported as a primary outcome) |
| Sound reduction management in the neonatal intensive care unit for preterm or very low birth weight infants | 18 December 2014 | 1 RCT (Country: USA; Published: 2009) | 34 infants | Preterm infants (< 32 weeks' post‐menstrual age or < 1500 g birthweight) cared for in the resuscitation area, during transport, or once admitted to an NICU or a stepdown unit | Silicone earplugs vs no earplugs (1 RCT, 34 infants) | Cerebral palsy ("Cerebral palsy at 18 to 22 months' corrected age" (1 RCT, 14 children); reported as a primary outcome) |
| Kangaroo mother care to reduce morbidity and mortality in low birthweight infants | 30 June 2016 | 21 RCTs (Countries: 13 RCTs in low‐ or middle‐income countries: Colombia: 1 RCT; Ecuador: | 3042 infants | LBW infants (defined as birthweight < 2500 g) regardless of gestational age | Kangaroo mother care vs conventional neonatal care (20 RCTs, 2969 neonates) | Cerebral palsy ("Cerebral palsy at 12 months' corrected age" (1 RCT, 588 children); reported as a primary outcome) |
| Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants | 15 August 2015 | 25 RCTs (Countries: not reported; Published: 1970s: 1 RCT; 1980s: 5 RCTs; 1990s: | 3615 infants | Preterm infants born at < 37 weeks' gestational age (according to best obstetrical estimate at the time of delivery). We excluded studies that did not report outcomes for preterm infants separately from those for infants born at term | Early developmental intervention vs standard follow‐up (25 RCTs, 3615 neonates) | Cerebral palsy ("Rate of cerebral palsy" (7 RCTs, 985 children); reported as a secondary outcome) Motor dysfunction ("Motor outcome at school age (low score on Movement ABC)" (2 RCTs, 333 children); reported as a secondary outcome) |
Abbreviations: BP: blood pressure; BPD: bronchopulmonary dysplasia; BSID: Bayley Scales of Infant Development; CNS: central nervous system; ECHO: echocardiogram; g: grams; G‐6PD: glucose‐6‐phosphate dehydrogenase; HSV: herpes simplex virus; IPPV: intermittent positive‐pressure ventilation; IV: intravenous; LBW: low birthweight; MDI: Mental Development Index; Movement‐ABC: Movement Assessment Battery for Children; NICU: neonatal intensive care unit; NO: nitric oxide; PDA: patent ductus arteriosus; PDI: Psychomotor Development Index; PMA: post‐menstrual age; PPHN: persistent pulmonary hypertension of the newborn; RCT: randomised controlled trial; Rh: Rhesus; SBF: systemic blood flow; SD: standard deviation; SVC: superior vena cava; VLBW: very low birthweight.
| Review ID and title | Summary of trial limitations (risk of bias)* |
| Neonatal care: asphyxia | |
| Allopurinol for preventing mortality and morbidity in newborn infants with hypoxic‐ischaemic encephalopathy | Random sequence generation: 2 RCTs low risk; 1 RCT unclear risk Allocation concealment: 3 RCTs low risk Blinding: 2 RCTs low risk; 1 RCT high risk Incomplete outcome data: 3 RCTs low risk Overall: "Although small, the trials were generally of good methodological quality" |
| Cooling for newborns with hypoxic‐ischaemic encephalopathy | Random sequence generation: 9 RCTs low risk; 1 RCT unclear risk; 1 RCT high risk Allocation concealment: 8 RCTs low risk; 2 RCTs unclear risk; 1 RCT high risk Blinding (participants and personnel): 11 RCTs high risk Blinding (outcome assessors): 10 RCTs low risk; 1 RCT unclear risk Incomplete outcome data: 6 RCTs low risk; 1 RCT unclear risk; 4 RCTs high risk Selective reporting: 11 RCTs low risk Overall: "Several limitations of the available evidence should be noted" |
| Prophylactic barbiturate use for the prevention of morbidity and mortality following perinatal asphyxia | Random sequence generation: 7 RCTs low risk; 2 RCTs unclear risk Allocation concealment: 4 RCTs low risk; 4 RCTs unclear risk; 1 RCT high risk Blinding: 4 RCTs unclear risk; 5 RCTs high risk Incomplete outcome data: 6 RCTs low risk; 2 RCTs unclear risk; 1 RCT high risk Selective reporting: 9 RCTs low risk |
| Neonatal care: haemorrhage: periventricular/intraventricular | |
| Ethamsylate for the prevention of morbidity and mortality in preterm or very low birth weight infants | Adequate sequence generation: 4 RCTs yes; 2 RCTs unclear; 1 RCT no Allocation concealment: 3 RCTs yes; 2 RCT unclear; 2 RCTs no Blinding: 4 RCTs yes; 3 RCTs unclear Incomplete outcome data addressed: 5 RCTs yes; 1 RCT unclear; 1 RCT no Free of selective reporting: 7 RCTs yes Free of other bias: 7 RCTs yes |
| Postnatal phenobarbital for the prevention of intraventricular haemorrhage in preterm infants | Random sequence generation: 5 RCTs low risk; 6 RCTs unclear risk; 1 RCT high risk Allocation concealment: 4 RCTs low risk; 7 RCTs unclear risk; 1 RCT high risk Blinding (participants and personnel): 2 RCTs low risk; 10 RCTs high risk Blinding (outcome assessors): 6 RCTs low risk; 6 RCTs unclear risk Incomplete outcome data: 8 RCTs low risk; 4 RCTs unclear risk Selective reporting: 2 RCTs low risk; 10 RCTs unclear risk |
| Neonatal care: hypotension | |
| The effect of inotropes on morbidity and mortality in preterm infants with low systemic or organ blood flow | Adequate sequence generation: 1 RCT yes Allocation concealment: 1 RCT yes Blinding (outcomes): 1 RCT yes Blinding (intervention): 1 RCT yes Incomplete outcome data addressed: 1 RCT yes Free of selective reporting: 1 RCT yes Free of other bias: 1 RCT yes Overall: "The study was of adequate methodology" |
| Neonatal care: fluid therapy | |
| Early volume expansion for prevention of morbidity and mortality in very preterm infants | Adequate randomisation: 7 RCTs yes; 1 RCT unclear Allocation concealment: 7 RCTs yes; 1 RCT unclear Blinding of intervention: 1 RCT yes; 7 RCTs no Blinding of measurement: 3 RCTs yes; 1 RCT unclear; 4 RCTs no Losses to follow‐up: 5 RCTs none; 1 RCT unclear; 2 RCTs yes |
| Neonatal care: patent ductus arteriosus | |
| Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants | Blinding of randomisation: 12 RCTs yes; 7 RCTs can't tell Blinding of intervention: 16 RCTs yes; 2 RCTs can't tell; 1 RCT no Blinding of outcome assessment: 16 RCTs yes; 2 RCTs can't tell; 1 RCT no Complete follow‐up (short‐term outcomes): 18 RCTs yes; 1 RCT no Overall: "Overall, the quality of the trials was good" |
| Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants | Random sequence generation: 9 RCTs low risk; 24 RCTs unclear risk Allocation concealment: 18 RCTs low risk; 14 RCTs unclear risk; 1 RCT high risk Blinding: 6 RCTs low risk; 7 RCTs unclear risk; 20 RCTs high risk Incomplete outcome data: 28 RCTs low risk; 3 RCTs unclear risk; 2 RCTs high risk Selective reporting: 5 RCTs low risk; 28 RCTs unclear risk Other: 29 RCTs low risk; 4 RCTs unclear risk Overall: "Study quality was variable…we identified concerns about bias in most individual studies and therefore for the group of studies included as well" |
| Neonatal care: blood disorders | |
| Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants | Random sequence generation: 8 RCTs low: risk; 19 RCTs unclear risk Allocation concealment: 13 RCTs low risk; 14 RCTs unclear risk Blinding: 12 RCTs low risk; 3 RCTs unclear risk; 12 RCTs high risk Incomplete outcome data: 23 RCTs low risk; 2 RCTs unclear risk; 2 RCTs high risk Selective reporting: 1 RCT low risk; 26 RCTs unclear risk Other: 26 RCTs low risk; 1 RCT unclear risk |
| Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants | Allocation concealment: 4 RCTs low risk; 1 RCT unclear risk Blinding: 1 RCT unclear risk; 4 RCTs high risk Incomplete outcome data: 3 RCTs low risk; 1 RCT unclear risk; 1 RCT high risk Selective reporting: 1 RCT low risk; 3 RCTs unclear risk; 1 RCT high risk Overall: "This review consists of five randomised controlled trials in which there appears to be no allocation bias; the overall level of evidence is high" |
| Neonatal care: pulmonary hypertension | |
| Endothelin receptor antagonists for persistent pulmonary hypertension in term and late preterm infants | Random sequence generation: 1 RCT low risk; 1 RCT unclear risk Allocation concealment: 2 RCT unclear risk Blinding (participants and personnel): 2 RCTs low risk Blinding (outcome assessors): 2 RCTs low risk Incomplete outcome data: 1 RCT low risk; 1 RCT high risk Selective reporting: 1 RCT low risk; 1 RCT unclear risk Other: 2 RCTs low risk Overall: "the quality of evidence was considered low because of the very small sample size and methodological issues in the included studies" |
| Neonatal care: resuscitation | |
| Air versus oxygen for resuscitation of infants at birth | Concealment of allocation: 2 RCTs yes; 3 RCTs no Blinding of intervention: 2 RCTs yes; 3 RCTs no Blinding of outcome assessment: 2 RCTs yes; 3 RCTs no Completeness of follow‐up (short‐term): 4 RCTs yes; 1 RCT no Completeness of follow‐up (long‐term): 3 RCTs no; 2 RCTs unclear |
| Neonatal care: nitric oxide | |
| Inhaled nitric oxide for respiratory failure in preterm infants | Allocation concealment: 12 RCTs low risk; 2 RCTs unclear risk Blinding: 7 RCTs low risk; 7 RCTs high risk Incomplete outcome data: 14 RCTs low risk Selective reporting: 8 RCTs low risk; 6 RCTs not reported Other: 3 RCTs low risk; 4 RCTs high risk; 7 RCTs not reported |
| Nitric oxide for respiratory failure in infants born at or near term | Masking of allocation: 10 RCTs yes; 4 RCTs cannot tell Masking of intervention: 6 RCTs yes; 8 RCTs no Masking of outcome assessment: 6 RCTs yes; 1 RCT can’t tell; 7 RCTs no Completeness of follow‐up: 13 RCTs yes; 1 RCT can't tell Overall: "The overall quality of these studies is quite variable" |
| Neonatal care: apnoea | |
| Methylxanthine treatment for apnoea in preterm infants | Random sequence generation: 1 RCT high risk; 5 RCTs not reported Allocation concealment: 2 RCTs low risk; 2 RCTs unclear risk; 2 RCTs high risk Blinding: 4 RCTs low risk; 2 RCTs high risk Incomplete outcome data: 3 RCTs low risk; 1 RCT unclear risk; 2 RCTs high risk Selective reporting: 2 RCTs low risk; 1 RCT unclear risk; 2 RCTs high risk; 1 RCT not reported Overall: "There was variation in trial design" |
| Prophylactic methylxanthine for prevention of apnoea in preterm infants | Allocation concealment: 3 RCTs low risk Blinding: 3 RCTs low risk Incomplete outcome data: 3 RCTs low risk Selective reporting: 2 RCTs low risk; 1 RCT not reported Overall: "Three studies are generally of high quality" |
| Neonatal care: respiratory distress syndrome | |
| Inositol in preterm infants at risk for or having respiratory distress syndrome | Random sequence generation: 1 RCT low risk; 3 RCTs unclear risk Allocation concealment: 2 RCTs low risk; 2 RCTs unclear risk Blinding: 2 RCTs low risk; 2 RCTs unclear risk Incomplete outcome data: 4 RCTs low risk Selective reporting: 3 RCTs low risk; 1 RCT unclear risk Other: 3 RCTs high risk; 1 RCT low risk Overall: "Study quality varied and interim analyses had occurred in all trials" |
| Animal derived surfactant extract for treatment of respiratory distress syndrome | Blinding of randomisation: 10 RCTs yes; 3 RCTs not described Blinding of intervention: 8 RCTs yes; 1 RCT not described; 4 RCTs no Blinding of outcome measurement: 6 RCTs yes; 4 RCTs not described; 2 RCTs no; 1 RCT not reported Complete follow‐up (short‐term): 13 RCTs yes Complete follow‐up (long‐term): 4 RCTs yes; 9 RCTs no Overall: "studies are of high methodological quality" |
| Synthetic surfactant for respiratory distress syndrome in preterm infants | Blinding of randomisation: 6 RCTs yes Blinding of intervention: 5 RCTs yes; 1 RCT no Blinding of outcome measurement: 5 RCTs yes; 1 RCT no Complete follow‐up (short term): 6 RCTs yes Complete follow‐up (long term): 80 to 100% |
| Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants | Adequate sequence generation: 6 RCTs unclear: 1 RCT not reported Allocation concealment: 7 RCTs yes Blinding of intervention: 5 RCTs yes; 1 RCT unclear; 1 RCT no Blinding of outcome measurement: 6 RCTs yes; 1 RCT no Incomplete outcome data addressed: 5 RCTs yes; 2 RCTs unclear Free of selective reporting: 7 RCTs yes Free of other bias: 7 RCTs yes |
| Neonatal care: mechanical ventilation | |
| Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants | Random sequence generation: 11 RCTs low risk; 8 RCTs unclear risk Allocation concealment: 7 RCTs low risk; 12 RCTs unclear risk Blinding of participants and personnel: 19 RCTs high risk Blinding of outcome assessment: 7 RCTs low risk; 12 RCTs unclear risk Incomplete outcome data: 19 RCTs low risk Overall: "The quality of the studies was generally high" |
| Continuous distending pressure for respiratory distress in preterm infants | Random sequence generation: 2 RCTs low risk; 3 RCTs unclear risk; 1 RCT high risk Allocation concealment: 4 RCTs low risk; 1 RCT unclear risk; 1 RCT high risk Blinding (intervention): 6 RCTs high risk Blinding (short term outcomes): 6 RCTs high risk (1 RCT low risk for long term outcomes) Incomplete outcome data (short term outcomes): 3 RCTs low risk; 3 RCTs unclear risk (1 RCT low risk for long‐term outcomes) Selective reporting: 2 RCTs low risk; 4 RCTs unclear risk Other: 6 RCTs unclear risk Overall: "These data should be interpreted with caution as in the studies reviewed, the numbers of infants were small, blinding of treatment was not possible and blinding of the outcome assessment was reported in only one study for the outcomes in childhood, thus possibly introducing bias" |
| Prophylactic methylxanthines for endotracheal extubation in preterm infants | Sequence generation: 1 RCT low risk; 6 RCTs not reported Allocation concealment: 6 RCTs low risk; 1 RCT unclear risk Blinding: 6 RCTs low risk; 1 RCT high risk Incomplete outcome data: 3 RCTs low risk; 3 RCTs high risk; 1 RCT not reported Selective reporting: 4 RCTs low risk; 2 RCTs high risk; 1 RCT not reported Other: 1 RCT low risk; 6 RCTs not reported |
| Long versus short inspiratory times in neonates receiving mechanical ventilation | Concealment of allocation: 1 RCT yes; 1 RCT cannot tell; 3 RCTs no Blinding of intervention: 5 RCTs no Blinding of outcome measurement: 3 RCTs no; 2 RCTs some Completeness of follow‐up (short term outcomes): 5 RCTs yes |
| Volume‐targeted versus pressure‐limited ventilation in the neonate | Sequence generation: 6 RCTs low risk; 6 RCTs unclear risk Allocation concealment: 11 RCTs low risk; 1 RCT unclear risk Blinding: 12 RCTs high risk Incomplete outcome data: 12 RCTs low risk Selective reporting: 10 RCTs low risk; 1 RCT unclear risk; 1 RCT high risk Other: 5 RCTs low risk; 5 RCTs unclear risk; 2 RCTs high risk Overall: "There are no major concerns about the methodology used in the twelve trials included in this review" |
| Neonatal care: bronchopulmonary dysplasia | |
| Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants | Random sequence generation: 15 RCTs low risk; 14 RCTs unclear risk Allocation concealment: 27 RCTs low risk; 2 RCTs unclear risk Blinding of participants and personnel: 23 RCTs low risk; 2 RCTs unclear risk; 4 RCTs high risk Blinding of outcome assessment: 23 RCTs low risk; 2 RCTs unclear risk; 4 RCTs high risk Incomplete outcome data: 28 RCTs low risk; 1 RCT unclear risk Overall: "Overall the risk of bias was low for most studies" |
| Moderately early (7‐14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants | Blinding of randomisation/allocation concealment: 7 RCTs yes/low risk Blinding of intervention: 5 RCTs yes; 2 RCTs no Blinding of outcome measurement: 5 RCTs yes; 1 RCT some; 1 RCT cannot tell Complete follow‐up: 6 RCTs yes/almost; 1 RCT no Overall: "the methodological quality of the studies to determine long‐term outcome is limited in some cases" |
| Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants | Random sequence generation: 12 RCTs low risk; 9 RCTs unclear risk Allocation concealment: 17 RCTs low risk; 4 RCTs unclear risk Blinding of participants and personnel: 15 RCTs low risk; 4 RCTs unclear risk; 2 RCTs high risk Blinding of outcome assessment: 16 RCTs low risk; 4 RCTs unclear risk; 1 RCT high risk Incomplete outcome data: 20 RCTs low risk; 1 RCT unclear risk Overall: "Overall the risk of bias was low for most studies" |
| Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates | Random sequence generation: 7 RCTs unclear risk Allocation concealment: 7 RCTs low risk Blinding of participants and personnel: 7 RCTs low risk Blinding of outcome assessment: 1 RCT low risk; 6 RCTs unclear risk Incomplete outcome data: 6 RCTs low risk; 1 RCT unclear risk Overall: "Overall, the studies included for this review were of high methodological quality" |
| Vitamin A supplementation to prevent mortality and short‐ and long‐term morbidity in very low birth weight infants | Random sequence generation: 9 RCTs low risk; 2 RCTs unclear risk Allocation concealment: 8 RCTs low risk; 3 RCTs unclear risk Blinding: 6 RCTs low risk; 2 RCTs unclear risk; 3 RCTs high risk Incomplete outcome data: 9 RCTs low risk; 1 RCT unclear risk; 1 RCT high risk Selective reporting: 8 RCTs low risk; 2 RCTs unclear risk; 1 RCT high risk Other: 2 RCTs low risk; 6 RCTs unclear risk; 2 RCTs high risk; 1 RCT not reported |
| Neonatal care: infections: necrotising enterocolitis | |
| Probiotics for prevention of necrotising enterocolitis in preterm infants | Random sequence generation: 15 RCTs low risk; 8 RCTs unclear risk; 1 RCT high risk Allocation concealment: 11 RCTs low risk; 12 RCTs unclear risk; 1 RCT high risk Blinding: 15 RCTs low risk; 9 RCTs unclear risk Incomplete outcome data: 21 RCTs low risk; 2 RCTs unclear risk; 1 RCT high risk Selective reporting: 17 RCTs low risk; 6 RCTs high risk; 1 RCT not reported Other: 14 RCTs low risk; 10 RCTs not reported Overall: "Eleven of our included trials were classified as high quality trials" |
| Arginine supplementation for prevention of necrotising enterocolitis in preterm infants | Masking of randomisation: 1 RCT yes Masking of intervention: 1 RCT yes Masking of outcome assessment: 1 RCT yes Completeness of follow‐up: 1 RCT yes Overall: "The methodological quality of the included study was good" |
| Neonatal care: infections: fungal infections | |
| Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants | Allocation concealment: 12 RCTs low risk; 3 RCTs unclear risk Blinding of participants and personnel: 10 RCTs low risk; 3 RCTs unclear risk; 2 RCTs high risk Blinding of outcome assessment: 10 RCTs low risk; 3 RCTs unclear risk; 2 RCTs high risk Incomplete outcome data: 15 RCTs low risk Overall: "The included trials were generally of good methodological quality" |
| Neonatal care: infections: herpes simplex | |
| Antiviral agents for treatment of herpes simplex virus infection in neonates | Allocation concealment: 1 RCT unclear; 1 RCT inadequate Overall: "The two trials... have a number of methodological flaws" |
| Neonatal care: jaundice | |
| Prophylactic phototherapy for preventing jaundice in preterm or low birth weight infants | Random sequence generation: 4 RCTs low risk; 3 RCTs unclear risk; 2 RCTs high risk Allocation concealment: 3 RCTs low risk; 4 RCTs unclear risk; 2 RCTs high risk Blinding: 1 RCT low risk; 2 RCTs unclear risk; 6 RCTs high risk Incomplete outcome data: 8 RCTs low risk; 1 RCT high risk Selective reporting: 2 RCTs low risk; 7 RCTs unclear risk Other: 7 RCTs low risk Overall: "In general, the overall methodological quality of the included studies was acceptable" |
| Neonatal care: hypoglycaemia | |
| Oral dextrose gel for the treatment of hypoglycaemia in newborn infants | Random sequence generation: 1 RCT low risk; 1 RCT unclear risk Allocation concealment: 1 RCT low risk; 1 RCT unclear risk Blinding of participants and personnel: 1 RCT low risk; 1 RCT unclear risk Blinding of outcome assessors: 1 RCT low risk; 1 RCT unclear risk Incomplete outcome data: 1 RCT low risk; 1 RCT high risk Selective reporting: 1 RCT low risk; 1 RCT unclear risk Other: 1 RCT low risk; 1 RCT unclear risk |
| Neonatal care: parenteral feeding | |
| Glutamine supplementation to prevent morbidity and mortality in preterm infants | Random sequence generation: 8 RCTs low risk; 3 RCTs unclear risk; 1 RCT high risk Allocation concealment: 8 RCTs low risk; 2 RCTs unclear risk; 2 RCTs high risk Blinding: 10 RCTs low risk; 2 RCTs unclear risk Incomplete outcome data: 8 RCTs low risk; 2 RCTs unclear risk; 2 RCTs high risk Overall: "in general the trials were of good quality" |
| Neonatal care: other | |
| Thyroid hormones for preventing neurodevelopmental impairment in preterm infants | Blinding of randomisation/allocation concealment: 4 RCTs yes; 1 RCT no Blinding of intervention: 4 RCTs yes; 1 RCT no Blinding of outcome assessment: 4 RCTs yes; 1 RCT not stated Complete follow‐up: 2 RCTs yes; 3 RCTs no Overall: "four studies... were of good methodology" |
| Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants | Allocation concealment: 4 RCTs low risk Blinding of intervention: 4 RCTs yes Blinding of outcome assessment: 3 RCTs yes; 1 RCT probably Complete follow‐up: 3 RCTs yes; 1 RCT no Overall: "All studies... were of adequate methodology" |
| Sound reduction management in the neonatal intensive care unit for preterm or very low birth weight infants | Random sequence generation: 1 RCT low risk Allocation concealment: 1 RCT low risk Blinding of participants and personnel: 1 RCT high risk Blinding of outcome assessment: 1 RCT low risk Incomplete outcome data: 1 RCT low risk Selective reporting: 1 RCT low risk Other: 1 RCT low risk Overall: "We considered the overall risk of bias to be low" |
| Kangaroo mother care to reduce morbidity and mortality in low birthweight infants | Random sequence generation: 21 RCTs low risk Allocation concealment: 10 RCTs low risk; 11 RCTs unclear risk Blinding of participants and personnel: 21 RCTs high risk Blinding of outcome assessment: 2 RCTs low risk; 15 RCTs unclear risk; 4 RCTs high risk Incomplete outcome data: 14 RCTs low risk; 3 RCTs unclear risk; 4 RCTs high risk Selective reporting: 16 RCTs low risk; 3 RCTs unclear risk; 2 RCTs high risk Other: 15 RCTs low risk; 3 RCTs unclear risk; 3 RCTs high risk Overall: "The methodological quality of the included trials was mixed" |
| Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants | Random sequence generation: 11 RCTs low risk; 8 RCTs unclear risk; 5 RCTs high risk; 1 RCT not reported Allocation concealment: 11 RCTs low risk; 9 RCTs unclear risk; 5 RCTs high risk Blinding of participants and personnel: 2 RCTs low risk; 4 RCTs unclear risk; 19 RCTs high risk Blinding of outcome assessment: 21 RCTs low risk; 3 RCTs unclear risk; 1 RCT high risk Incomplete outcome data: 12 RCTs low risk; 4 RCTs unclear risk; 9 RCT high risk Selective reporting: 3 RCTs unclear risk; 6 RCT high risk; 16 RCTs not reported Overall: "The methodological quality of included studies was variable" |
Abbreviations: RCT: randomised controlled trial.
*We have reported only the risk of bias components assessed and reported in the included reviews.
Methodological quality of included reviews
We rated the quality of included reviews using the AMSTAR and ROBIS tools (Shea 2009 and Whiting 2015, respectively).
With regards to AMSTAR criteria:
-
41/43 reviews clearly pre‐specified their design; for two reviews, this was unclear, with no reference made/access given to pre‐specified published protocols (Seger 2009; Soll 2000);
-
40/43 reviews clearly reported duplicate study selection and data extraction; for three reviews, it was unclear as to whether two independent review authors were involved in study selection and data extraction (Halliday 2003; Osborn 2001; Soll 2000);
-
42/43 reviews performed a comprehensive literature search; one review searched only one electronic database (in addition to electronic searching and handsearching of meeting abstracts) (Finer 2006);
-
all reviews considered grey literature;
-
41/43 reviews provided lists of both included and excluded studies; two reviews did not mention excluded studies and therefore provided no list (Henderson‐Smart 2010; Shah 2007);
-
all reviews provided the characteristics of included studies;
-
all reviews assessed and documented the scientific quality of included studies;
-
42/43 reviews clearly used scientific quality of included studies appropriately in formulating conclusions; one review did not clearly incorporate the quality of included studies into the conclusions (Barrington 2010);
-
35/38 reviews combined the findings of studies using appropriate methods; three reviews provided no/limited discussion and/or exploration of substantial statistical heterogeneity present in some review meta‐analyses and did not use a random‐effects model (Halliday 2003; Okwundu 2012; Soll 2000); for five reviews, review authors found this item to be 'not applicable' and conducted no meta‐analyses (Almadhoob 2015; Jones 2009; More 2016; Osborn 2007b; Shah 2007);
-
18/43 reviews assessed the likelihood of publication bias; 25 reviews did not assess publication bias likelihood and/or did not pre‐specify methods to be used if 10 or more trials were included in meta‐analyses (AlFaleh 2014; Barrington 2010; Cools 2015; Finer 2006; Fowlie 2010; Halliday 2003; Henderson‐Smart 2010; Henderson‐Smart 2010b; Henderson‐Smart 2010c; Ho 2015; Hunt 2010; Jacobs 2013; Jones 2009; Kamlin 2003; Okwundu 2012; Osborn 2001; Osborn 2004; Osborn 2007; Seger 2009; Shah 2007; Soll 2000; Soll 2010; Spittle 2015; Tan 2005; Wheeler 2010);
-
2/43 reviews clearly reported conflicts of interest/potential sources of support for both the review and the included studies (Jacobs 2013; Weston 2016); the remaining 41 reviews did not report conflicts of interests/sources of support for the included studies (AlFaleh 2014; Almadhoob 2015; Barrington 2010; Chaudhari 2012; Cleminson 2015; Conde‐Agudelo 2016; Cools 2015; Darlow 2016; Doyle 2014; Doyle 2014b; Finer 2006; Fowlie 2010; Halliday 2003; Henderson‐Smart 2010; Henderson‐Smart 2010b; Henderson‐Smart 2010c; Ho 2015; Howlett 2015; Hunt 2010; Jones 2009; Kamlin 2003; Moe‐Byrne 2016; More 2016; Ohlsson 2014; Ohlsson 2015; Okwundu 2012; Osborn 2001; Osborn 2004; Osborn 2007; Osborn 2007b; Seger 2009; Shah 2007; Shah 2012; Smit 2013; Soll 2000; Soll 2010; Spittle 2015; Tan 2005; Wheeler 2010; Whyte 2011; Young 2016).
See Table 4 for further details.
| Review ID | AMSTAR criteria | TOTAL SCORE | ||||||||||
| 'A priori' design | Duplicate selection and extraction | Comprehensive search | Grey literature considered | Included and excluded studies lists | Characteristics of included studies | Quality assessed and documented | Quality considered for conclusions | Methods for combining studies appropriate | Publication bias considered or assessed | Conflicts stated | ||
| Neonatal care: asphyxia | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | 10/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal care: haemorrhage: periventricular/intraventricular | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal care: hypotension | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✗ | 9/10 HIGH QUALITY | |
| Neonatal care: fluid therapy | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| Neonatal care: patent ductus arteriosus | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal care: blood disorders | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal care: pulmonary hypertension | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✗ | 9/10 HIGH QUALITY | |
| Neonatal care: resuscitation | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| Neonatal care: nitric oxide | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✗ | ✗ | 8/11 HIGH QUALITY | |
| ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 8/11 HIGH QUALITY | |
| Neonatal care: apnoea | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| Neonatal care: respiratory distress syndrome | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 8/11 HIGH QUALITY | |
| ? | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✗ | ✗ | 6/11 MODERATE QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| Neonatal care: mechanical ventilation | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 8/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| Neonatal care: bronchopulmonary dysplasia | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✗ | ✗ | 7/11 MODERATE QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal care: infections: necrotising enterocolitis | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ✓ | N/A | ✗ | ✗ | 7/10 HIGH QUALITY | |
| Neonatal infections: fungal infections | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal infections: herpes simplex | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✗ | ✗ | 8/10 HIGH QUALITY | |
| Neonatal care: jaundice | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✗ | ✗ | 8/11 HIGH QUALITY | |
| Neonatal care: hypoglycaemia | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 11/11 HIGH QUALITY | |
| Neonatal care: parenteral feeding | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal care: other | ||||||||||||
| ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 8/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✗ | 9/10 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
With regards to ROBIS domains:
-
40 reviews were considered to have 'low risk of bias' across study eligibility criteria, data collection and study appraisal, and synthesis and findings domains, and 39 were considered to have 'low risk of bias' for the identification and selection of studies domain;
-
three reviews were considered to have 'unclear risk of bias' for the study eligibility criteria domain; as above, two reviews provided no reference/access to pre‐specified published protocols (Seger 2009; Soll 2000); and one review made a notable protocol deviation related to the inclusion criteria (Almadhoob 2015);
-
three reviews were considered to have 'unclear risk of bias' for both the identification and selection of studies domain and the data collection and study appraisal domain because review authors did not clearly specify whether two independent review authors were involved in selection of studies, data collection, and study appraisal (Halliday 2003; Osborn 2001; Soll 2000); one further review was considered to have 'unclear risk of bias' for the identification and selection of studies domain, as above, owing to concern regarding comprehensiveness of the search (Finer 2006); and
-
finally, three reviews were considered to have 'unclear risk of bias' for the synthesis and findings domain owing to the presence of substantial statistical heterogeneity (with use of a fixed‐effect model) in some review meta‐analyses that was not clearly explained/explored (Halliday 2003; Okwundu 2012; Soll 2000).
See Table 5 for additional details.
| Review ID | ROBIS domains | OVERALL RISK OF BIAS | |||
| Study eligibility criteria | Identification and selection of studies | Data collection and study appraisal | Synthesis and findings | ||
| Neonatal care: asphyxia | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: haemorrhage: periventricular/intraventricular | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: hypotension | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: fluid therapy | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: patent ductus arteriosus | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: blood disorders | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: pulmonary hypertension | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: resuscitation | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: nitric oxide | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Unclear risk | Low risk | Low risk | UNCLEAR RISK | |
| Neonatal care: apnoea | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: respiratory distress syndrome | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Unclear risk | Low risk | Low risk | Low risk | LOW RISK | |
| Unclear risk | Unclear risk | Unclear risk | Unclear risk | UNCLEAR RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: mechanical ventilation | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: bronchopulmonary dysplasia | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Unclear risk | Unclear risk | Unclear risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal infections: necrotising enterocolitis | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal infections: fungal infections | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal infections: herpes simplex | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: jaundice | |||||
| Low risk | Low risk | Low risk | Unclear risk | LOW RISK | |
| Neonatal care: hypoglycaemia | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: parenteral feeding | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: other | |||||
| Low risk | Unclear risk | Unclear risk | Low risk | UNCLEAR RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Unclear risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
Overall, all 41 included reviews were judged to be of 'high quality' according to AMSTAR (with scores ranging from 8 to 11 out of 11, or from 7 to 9 out of 10), and two were judged to be of 'moderate quality' (with scores of 6 and 7 out of 11) (Halliday 2003; Soll 2000); according to ROBIS, 40 reviews were judged to have 'low risk of bias', and three to have 'unclear risk of bias' (Finer 2006; Osborn 2001; Soll 2000).
Effect of interventions
Below, we have summarised the main results of the included reviews by categorising their findings according to the framework discussed under Data synthesis, organised by groups of neonates and 'Neonatal care' topics.
For further details, including outcome definitions and judgements supporting the quality of the evidence for each outcome, see Table 6 (cerebral palsy); Table 7 (cerebral palsy: subgroup or sensitivity analyses); Table 8 (cerebral palsy or death); Table 9 (severity of cerebral palsy); Table 10 (other composite outcomes that include cerebral palsy); and Table 11 (motor dysfunction).
| Intervention and comparison | Outcome | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | Comments |
| Neonatal care: asphyxia | |||||||
| Therapeutic hypothermia vs standard care for newborns with hypoxic‐ischaemic encephalopathy (Jacobs 2013) | Cerebral palsy in survivors assessed at 18 to 24 months | 352 per 1000 (143/406) | 232 per 1000 (190 to 289) | RR 0.66 (0.54 to 0.82) | 881 (7 RCTs) | HIGH | Not downgraded |
| Cerebral palsy at 6 to 7 years | 288 per 1000 (15/52) | 173 per 1000 (89 to 340) | RR 0.60 (0.31 to 1.18) | 121 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size | |
| Barbiturates (phenobarbital) vs conventional therapy for prevention of morbidity and mortality following perinatal asphyxia (Young 2016) | Cerebral palsy at 3 to 6 years | 242 per 1000 (8/33) | 141 per 1000 (46 to 412) | RR 0.58 (0.19 to 1.70) | 69 (2 RCTs) | VERY LOW | Study limitations (‐1): unblinded studies; concern regarding performance bias and detection bias Imprecision (‐1): 95% CIs were wide and imprecise (GRADED by review authors themselves) |
| Neonatal care: haemorrhage: periventricular/intraventricular | |||||||
| Ethamsylate vs placebo for prevention of morbidity and mortality in preterm or very low birthweight infants (Hunt 2010) | Cerebral palsy in surviving children available for follow‐up at 2 years up to 3.5 to 4.2 years (only cerebral palsy significant enough to cause moderate or severe impairment was included) | 78 per 1000 (21/270) | 88 per 1000 (50 to 156) | RR 1.13 (0.64 to 2.00) | 532 (3 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: hypotension | |||||||
| Dobutamine vs dopamine in preterm infants with low superior vena cava flow (Osborn 2007b) | Cerebral palsy at 3 years in survivors assessed | 429 per 1000 (3/7) | 69 per 1000 (4 to 1131) | RR 0.16 (0.01 to 2.64) | 13 (1 RCT) | VERY LOW | Study limitations (‐1): 5/18 surviving infants were not assessed at 3 years of age Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with very small sample size |
| Neonatal care: fluid therapy | |||||||
| Volume vs no treatment for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | Cerebral palsy in survivors at 2 years | 132 per 1000 (27/205) | 100 per 1000 (63 to 158) | RR 0.76 (0.48 to 1.20) | 604 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| Gelatin vs fresh frozen plasma for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | 103 per 1000 (21/203) | 97 per 1000 (54 to 175) | RR 0.94 (0.52 to 1.69) | 399 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect | |
| Neonatal care: patent ductus arteriosus | |||||||
| Prophylactic indomethacin vs placebo for preventing mortality and morbidity in preterm infants (Fowlie 2010) | Cerebral palsy at 18 to 54 months | 111 per 1000 (77/694) | 115 per 1000 (85 to 155) | RR 1.04 (0.77 to 1.40) | 1372 (4 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| Cerebral palsy at 8 years | 76 per 1000 (11/145) | 94 per 1000 (45 to 199) | RR 1.24 (0.59 to 2.62) | 304 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect | |
| Oral ibuprofen vs intravenous ibuprofen for treatment of patent ductus arteriosus in preterm or low birthweight (or both) infants (Ohlsson 2015) | Moderate or severe cerebral palsy at 18 to 24 months | 74 per 1000 (2/27) | 100 per 1000 (18 to 554) | RR 1.35 (0.24 to 7.48) | 57 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection and reporting bias; high risk of performance, detection, and attrition bias Imprecision (‐2): wide CI crossing line of no effect; small sample size and few events |
| Neonatal care: blood disorders | |||||||
| Erythropoietin vs placebo for preventing red blood cell transfusion in preterm and/or low birthweight infants (Ohlsson 2014) | Cerebral palsy at 18 to 22 months' corrected age (in children examined) | 187 per 1000 (14/75) | 123 per 1000 (58 to 256) | RR 0.66 (0.31 to 1.37) | 153 (2 RCTs) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection bias and high risk of attrition bias (˜73% follow‐up) Inconsistency (‐1): I² = 72% Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Darbepoetin alfa vs placebo for preventing red blood cell transfusion in preterm and/or low birthweight infants (Ohlsson 2014) | 208 per 1000 (5/24) | 17 per 1000 (0 to 292) | RR 0.08 (0.00 to 1.40) | 51 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size and few events | |
| Transfusion at a restrictive vs a liberal haemoglobin threshold for preventing morbidity and mortality in very low birthweight infants (Whyte 2011) | Cerebral palsy at 18 to 21 months' follow‐up among survivors | 52 per 1000 (9/172) | 68 per 1000 (29 to 159) | RR 1.29 (0.55 to 3.03) | 335 (1 RCT) | LOW | Study limitations (‐1): 1 RCT at high risk of performance bias and unclear risk of reporting bias Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: pulmonary hypertension | |||||||
| Endothelin receptor antagonists vs placebo for persistent pulmonary hypertension in term and late preterm infants (More 2016) | Cerebral palsy at 6 months (delayed motor development and spasticity) | 214 per 1000 (3/14) | 19 per 1000 (0 to 345) | RR 0.09 (0.00 to 1.61) | 37 (1 RCT) | LOW | Study limitation (‐1): 8/23 infants in the placebo group were excluded from analysis Imprecision (‐1): 1 RCT; small sample size (GRADED by review authors themselves) |
| Neonatal care: resuscitation | |||||||
| Room air vs 100% oxygen for resuscitation of infants at birth (Tan 2005) | Cerebral palsy in those followed up at 18 to 24 months | 74 per 1000 (9/122) | 99 per 1000 (41 to 239) | RR 1.34 (0.55 to 3.24) | 213 (1 RCT) | VERY LOW | Study limitations (‐2): 1 qRCT with no blinding and < 70% follow‐up Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: nitric oxide | |||||||
| Inhaled NO vs placebo for respiratory failure in preterm infants (entry before 3 days based on oxygenation) (Barrington 2010) | Cerebral palsy at 18 to 22 months (moderate/severe or disabling) | 100 per 1000 (11/110) | 185 per 1000 (93 to 371) | RR 1.85 (0.93 to 3.71) | 209 (2 RCTs) | LOW | Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Inhaled NO vs placebo or no treatment for respiratory failure in preterm infants (entry after 3 days based on BPD risk) (Barrington 2010) | Cerebral palsy at 2 years' corrected age or 30 months (1 RCT all severities; 1 RCT moderate/severe or disabling) | 56 per 1000 (14/248) | 62 per 1000 (30 to 126) | RR 1.10 (0.54 to 2.23) | 498 (2 RCTs) | LOW | Study limitations (‐1): 1 RCT with no blinding of intervention or outcome measurement Imprecision (‐1): wide CI crossing line of no effect; small sample sizes |
| Inhaled NO vs placebo for respiratory failure in preterm infants (studies of routine use in intubated preterm infants) (Barrington 2010) | Cerebral palsy at 1 or 2 years' corrected age (1 RCT all severities; 1 RCT moderate/severe or disabling) | 70 per 1000 (20/286) | 66 per 1000 (36 to 119) | RR 0.94 (0.51 to 1.70) | 593 (2 RCTs) | LOW | Study limitations (‐1): 2 RCTs with 74%‐82% follow‐up Imprecision (‐1): wide CI crossing line of no effect |
| Inhaled nitric oxide vs placebo for respiratory failure in infants born at or near term (Finer 2006) | Cerebral palsy among survivors at 13 or 18 to 24 months | 89 per 1000 (16/179) | 91 per 1000 (44 to 191) | RR 1.02 (0.49 to 2.14) | 299 (2 RCTs) | LOW | Study limitations (‐1): 1 RCT masking of allocation, masking of outcomes. and completeness of follow‐up Imprecision (‐1): wide CI crossing line of no effect |
| "This group has now published follow up data, including neurodevelopmental outcomes, which were obtained by telephone interview of 60 of the 83 survivors of the original trial. The interview was conducted between one and four years of age... Although cerebral palsy [was] reported it is unclear how [it] was defined... It is not, therefore, possible to add any of these data to the meta‐analysis, but they do appear to show no evidence of neurodevelopmental impairment due to inhaled nitric oxide therapy" | NOT GRADED | ||||||
| Inhaled nitric oxide vs placebo for respiratory failure in infants with diaphragmatic hernias born at or near term (Finer 2006) | Cerebral palsy among survivors at 18 to 24 months | (0/14) | (2/8) | RR 8.33 (0.45 to 154.78) | 22 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with 76% follow‐up of survivors Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: apnoea | |||||||
| Caffeine vs placebo for treatment of apnoea in preterm infants (Henderson‐Smart 2010b) | Cerebral palsy at 18 to 21 months' corrected age | 50 per 1000 (18/361) | 30 per 1000 (14 to 62) | RR 0.60 (0.29 to 1.25) | 729 (1 RCT) | LOW | Study limitations (‐1): results based on 1 RCT subgroup from post hoc subgroup analyses (randomisation not stratified according to indication for inclusion) Imprecision (‐1): wide CI crossing line of no effect |
| Caffeine vs placebo for prevention of apnoea in preterm infants (Henderson‐Smart 2010c) | Cerebral palsy at 18 to 21 months' corrected age | 45 per 1000 (9/200) | 46 per 1000 (19 to 112) | RR 1.03 (0.43 to 2.49) | 415 (1 RCT) | LOW | Study limitations (‐1): results based on 1 RCT subgroup from post hoc subgroup analyses (randomisation not stratified according to indication for inclusion) Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: respiratory distress syndrome | |||||||
| Animal‐derived surfactant extract vs no treatment for treatment of respiratory distress syndrome (Seger 2009) | Cerebral palsy at 1 and 2 years' corrected age | 207 per 1000 (6/29) | 182 per 1000 (70 to 470) | RR 0.88 (0.34 to 2.27) | 73 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with no blinding of intervention; and blinding of outcome measurement not reported Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Synthetic surfactant vs placebo for respiratory distress syndrome in preterm infants (Soll 2000) | Cerebral palsy in survivors examined at 1 year (in 4 of the 5 RCTs) | 96 per 1000 (74/767) | 73 per 1000 (53 to 101) | RR 0.76 (0.55 to 1.05) | 1557 (5 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing the line of no effect |
| Prophylactic protein‐free synthetic surfactant vs placebo for preventing morbidity and mortality in preterm infants (Soll 2010) | Cerebral palsy at 1 or 2 years | 153 per 1000 (49/320) | 142 per 1000 (52 to 204) | RR 0.93 (0.64 to 1.33) | 670 (4 RCTs) | LOW | Study limitations (‐1): "Somewhat fewer infants who received surfactant failed to return for follow‐up evaluation (typical relative risk 0.63, 95% CI 0.48, 0.82; typical risk difference ‐0.10, 95% CI ‐0.15, ‐0.04)" Imprecision (‐1): wide CI crossing the line of no effect |
| Neonatal care: mechanical ventilation | |||||||
| Elective high‐frequency oscillatory ventilation vs conventional ventilation for acute pulmonary dysfunction in preterm infants (Cools 2015) | Cerebral palsy |
| NOT GRADED | "The age and methods of assessment varied between studies so the results were presented in the text and not included in a meta‐analysis" | |||
| Continuous distending pressure vs standard care for respiratory distress in preterm infants (Ho 2015) | Cerebral palsy at 9 to 15 years | (0/18) | (2/18) | RR 5.0 (0.26 to 97.37) | 36 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection and attrition bias and high risk of performance bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Prophylactic methylxanthines (caffeine) vs placebo for endotracheal extubation in preterm infants (Henderson‐Smart 2010) | Cerebral palsy at 18 to 21 months' corrected age | 115 per 1000 (39/339) | 62 per 1000 (37 to 106) | RR 0.54 (0.32 to 0.92) | 644 (1 RCT) | MODERATE | Study limitations (‐1): results based on 1 RCT subgroup from post hoc subgroup analyses (randomisation not stratified according to indication for inclusion) |
| Long vs short inspiratory times in neonates receiving mechanical ventilation (Kamlin 2003) | Cerebral palsy in survivors less than 33 weeks' gestation at birth at 18 months | 133 per 1000 (12/90) | 387 per 1000 (129 to 1153) | RR 2.9 (0.97 to 8.65) | 177 ( 1 RCT) | VERY LOW | Study limitations: 1 RCT at high risk of performance bias; follow‐up of subset (< 33 weeks only) Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: bronchopulmonary dysplasia | |||||||
| Early (< 8 days) postnatal corticosteroids vs placebo or no treatment for preventing chronic lung disease in preterm infants (Doyle 2014b) | Cerebral palsy at 11 months to 7 to 9 years | 88 per 1000 (63/715) | 128 per 1000 (93 to 174) | RR 1.45 (1.06 to 1.98) | 1452 (12 RCTs) | MODERATE | Study limitations (‐1): 4 RCTs at unclear risk of selection bias; 2 RCTs at high risk of performance and detection bias; 2 RCTs had 13%‐53% follow‐up overall |
| Cerebral palsy in survivors assessed at 11 months to 7 to 9 years | 134 per 1000 (63/470) | 201 per 1000 (151 to 268) | RR 1.50 (1.13 to 2.00) | 959 (12 RCTs) | MODERATE | Study limitations (‐1): 4 RCTs at unclear risk of selection bias; 2 RCTs at high risk of performance and detection bias; 2 RCTs had 13%‐53% follow‐up overall | |
| Moderately early (7‐14 days) postnatal corticosteroids vs placebo or no treatment for preventing chronic lung disease in preterm infants (Halliday 2003) | Cerebral palsy at 12 months' corrected age up to 90 months | 105 per 1000 (10/95) | 108 per 1000 (49 to 236) | RR 1.03 (0.47 to 2.24) | 204 (4 RCTs) | VERY LOW | Study limitations (‐1): 2 RCTs with 68%‐70% follow‐up; 1 RCT with unclear blinding of outcome assessment Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Cerebral palsy in survivors assessed at 12 months' corrected age up to 90 months | 175 per 1000 (10/57) | 146 per 1000 (68 to 305) | RR 0.83 (0.39 to 1.74) | 130 (4 RCTs) | VERY LOW | Study limitations (‐1): 2 RCTs with 68%‐70% follow‐up; 1 RCT with unclear blinding of outcome assessment Imprecision (‐2): wide CI crossing line of no effect; small sample sizes | |
| Late (> 7 days) postnatal corticosteroids vs placebo or no treatment for chronic lung disease in preterm infants (Doyle 2014) | Cerebral palsy at 1 to 3 years | 127 per 1000 (55/433) | 135 per 1000 (97 to 191) | RR 1.06 (0.76 to 1.50) | 876 (14 RCTs) | LOW | Study limitations (‐1): 5 RCTs unclear risk of selection bias; 5 RCTs with follow‐up from 32% to 78% Imprecision (‐1): wide CI crossing line of no effect |
| Cerebral palsy at 1 to 3 years in survivors assessed | 172 per 1000 (53/309) | 180 per 1000 (129 to 252) | RR 1.05 (0.75 to 1.47) | 631 (14 RCTs) | LOW | Study limitations (‐1): 5 RCTs unclear risk of selection bias; 5 RCTs with follow‐up between 32% and 78% Imprecision (‐1): wide CI crossing line of no effect | |
| Cerebral palsy at latest reported age (from 1 year up to 17 years) | 121 per 1000 (51/423) | 135 per 1000 (95 to 193) | RR 1.12 (0.79 to 1.60) | 855 (15 RCTs) | LOW | Study limitations (‐1): 5 RCTs unclear risk of selection bias; 7 RCTs with follow‐up between 32% and 78% Imprecision (‐1): wide CI crossing line of no effect | |
| Cerebral palsy at latest reported age in survivors assessed (from 1 year up to 17 years) | 170 per 1000 (49/289) | 190 per 1000 (134 to 268) | RR 1.12 (0.79 to 1.58) | 591 (15 RCTs) | LOW | Study limitations (‐1): 5 RCTs unclear risk of selection bias; 7 RCTs with follow‐up between 32% and 78% Imprecision (‐1): wide CI crossing line of no effect | |
| Early inhaled corticosteroids vs placebo for preventing chronic lung disease in ventilated very low birthweight preterm neonates (Shah 2012) | Cerebral palsy (age not reported in review; from trial manuscript: 3 years) | 107 per 1000 (3/28) | 143 per 1000 (35 to 581) | RR 1.33 (0.33 to 5.42) | 56 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection bias and detection bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: necrotising enterocolitis | |||||||
| Arginine supplementation vs placebo for prevention of necrotising enterocolitis in preterm infants (Shah 2007) | Cerebral palsy at 36 months' post‐menstrual age | 55 per 1000 (4/73) | 48 per 1000 (12 to 208) | RR 0.88 (0.21 to 3.80) | 135 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: fungal infections | |||||||
| Systemic antifungal agent vs placebo to prevent mortality and morbidity in very low birthweight infants (Cleminson 2015) | Cerebral palsy at 18 to 22 months post term | 112 per 1000 (12/107) | 108 per 1000 (50 to 228) | RR 0.96 (0.45 to 2.03) | 219 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: herpes simplex | |||||||
| Aciclovir vs vidarabine for treatment of herpes simplex virus infection in neonates (Jones 2009) | Cerebral palsy in CNS HSV neonatal infection up to 3 years by HSV serotype: HSV‐1 | (0/4) | (0/5) | Not estimable | 9 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with design limitations (inadequate allocation concealment) Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Cerebral palsy in CNS HSV neonatal infection up to 3 years by HSV serotype: HSV‐2 | 625 per 1000 (5/8) | 669 per 1000 (306 to 1456) (4/6) | RR 1.07 (0.49 to 2.33) | 14 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with design limitations (inadequate allocation concealment) Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT | |
| Neonatal care: jaundice | |||||||
| Prophylactic phototherapy vs standard care for preventing jaundice in preterm or low birthweight infants (Okwundu 2012) | Cerebral palsy in all infants (birthweight < 2500 g) at 1 year or 18 months | Medium risk population: 84 per 1000 (18/394) | Medium risk population: 81 per 1000 (42 to 155) | RR 0.96 (0.50 to 1.85) | 756 (2 RCTs) | MODERATE | Study limitations (‐1): "There was no mention of blinding of the outcome assessors in two of the studies" (GRADED by review authors themselves) |
| Cerebral palsy in all infants (birthweight < 1000 g) at 18 months | 250 per 1000 (4/16) | 72 per 1000 (10 to 568) | RR 0.29 (0.04 to 2.27) | 30 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with no blinding Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT | |
| Cerebral palsy at 6 years | "Secondary reports emanating from Brown 1985 at six‐year follow‐up also showed that there was no significant difference in the rate of cerebral palsy between the phototherapy and control group" | NOT GRADED | |||||
| Neonatal care: hypoglycaemia | |||||||
| Dextrose gel vs placebo for treatment of hypoglycaemia in newborn infants (Weston 2016) | Cerebral palsy at age 2 years | (0/93) | (2/90) | RR 5.16 (0.25 to 106.12) | 183 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with 78% follow‐up Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: parenteral feeding | |||||||
| Glutamine supplementation vs placebo to prevent morbidity and mortality in preterm infants (Moe‐Byrne 2016) | Cerebral palsy at 2 years | "van den Berg 2005 reported neurodevelopmental outcomes for infants aged two years post term. Outcomes assessed included... incidence of cerebral palsy... No significant differences between the glutamine and the control groups were reported for any of these individual outcomes" | NOT GRADED | ||||
| Neonatal care: other | |||||||
| Thyroid hormones vs placebo for preventing neurodevelopmental impairment in preterm infants (Osborn 2001) | Cerebral palsy at 5.7 years | 120 per 1000 (9/75) | 86 per 1000 (34 to 221) | RR 0.72 (0.28 to 1.84) | 156 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Prophylactic thyroid hormones vs placebo for prevention of morbidity and mortality in preterm infants (Osborn 2007) | |||||||
| Silicone earplugs vs no earplugs in the neonatal intensive care unit for preterm or very low birthweight infants (Almadhoob 2015) | Cerebral palsy at 18 to 22 months' corrected age | (0/7) | (1/7) | RR 3.0 (0.14 to 63.15) | 14 (1 RCT) | VERY LOW | Study limitations (‐1): "Because of funding restraints only the ELBW infants could be followed at 18 to 22 months corrected age" (14/24 survivors) Imprecision (‐1): wide CI crossing line of no effect; 1 small RCT |
| Kangaroo mother care vs conventional neonatal care to reduce morbidity and mortality in low birthweight infants (Conde‐Agudelo 2016) | Cerebral palsy at 12 months' corrected age | 25 per 1000 (7/280) | 16 per 1000 (5 to 51) | RR 0.65 (0.21 to 2.02) | 588 (1 RCT) | LOW | Study limitation (‐1): 1 RCT with unclear risk of selection bias; high risk of performance and detection bias Imprecision (‐1): wide CI crossing line of no effect |
| Early developmental intervention vs standard follow‐up post hospital discharge to prevent motor and cognitive impairment in preterm infants (Spittle 2015) | Cerebral palsy at 18 months to 6 years | 79 per 1000 (32/405) | 65 per 1000 (41 to 100) | RR 0.82 (0.52 to 1.27) | 985 (7 RCTs) | LOW | Study limitations (‐1): 7 RCTs at unclear/high risk of performance bias; 2 RCTs at unclear/high risk of selection bias and unclear/high risk of attrition bias; 1 RCT at unclear risk of detection bias Imprecision (‐1): wide CI crossing line of no effect |
Abbreviations: BPD: bronchopulmonary dysplasia; CI: confidence interval; CNS: central nervous system; CV: conventional ventilation; ELBW: extremely low birthweight; g: grams; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; HFOV: high‐frequency oscillatory ventilation; HSV: herpes simplex virus; NO: nitric oxide; OR: odds ratio; P: P value; qRCT: quasi‐randomised controlled trial; RCT: randomised controlled trial; RR: risk ratio.
| Intervention and comparison | Outcome | Subgroup or sensitivity analysis | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (trials) | Test for subgroup differences | |
| Neonatal care: asphyxia | ||||||||
| Therapeutic hypothermia vs standard care for newborns with hypoxic‐ischaemic encephalopathy (Jacobs 2013) | Cerebral palsy in survivors assessed at 18 to 24 months | Method of cooling | Selective head cooling with mild hypothermia | 338 per 1000 (49/145) | 220 per 1000 (155 to 318) | RR 0.65 (0.46 to 0.94) | 312 (3 RCTs) | Chi² = 0.01, df = 1 (P = 0.93), I² = 0.0% |
| Whole body cooling | 360 per 1000 (94/261) | 241 per 1000 (187 to 310) | RR 0.67 (0.52 to 0.86) | 569 (4 RCTs) | ||||
| Neonatal care: haemorrhage: periventricular/intraventricular | ||||||||
| Ethamsylate vs placebo for prevention of morbidity and mortality in preterm or very low birthweight infants (Hunt 2010) | Cerebral palsy in surviving children available for follow‐up at 3 years up to 3.5 to 4.2 years | Infants < 31 completed weeks or < 1500 g | 84 per 1000 (14/167) | 69 per 1000 (32 to 147) | RR 0.82 (0.38 to 1.75) | 328 (2 RCTs) | Not applicable | |
| Neonatal care: fluid therapy | ||||||||
| Volume vs no treatment for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | Cerebral palsy in survivors at 2 years | Type of volume used | Fresh frozen plasma | 132 per 1000 (27/205) | 104 per 1000 (61 to 177) | RR 0.79 (0.46 to 1.34) | 408 (1 RCT) | Not performed (as conducted as separate comparison) |
| Gelatin | 132 per 1000 (27/205) | 97 per 1000 (53 to 169) | RR 0.74 (0.42 to 1.28) | 401 (1 RCT) | ||||
| Timing of treatment | Early treatment (< 24 hours age) | 132 per 1000 (27/205) | 100 per 1000 (63 to 158) | RR 0.76 (0.48 to 1.20) | 604 (1 RCT) | Not applicable | ||
| Types of infant enrolled | Unselected preterm infants (not selected on the basis of cardiovascular compromise) | |||||||
| Methodological quality | Complete follow‐up for neurodevelopmental outcomes | |||||||
| Neonatal care: respiratory distress syndrome | ||||||||
| Animal‐derived surfactant extract vs no treatment for treatment of respiratory distress syndrome (Seger 2009) | Cerebral palsy at 1 and 2 years' corrected age | Surfactant product | Porcine surfactant extract | 207 per 1000 (6/29) | 182 per 1000 (70 to 470) | RR 0.88 (0.34 to 2.27) | 73 (1 RCT) | Not applicable |
| Prophylactic protein‐free synthetic surfactant vs placebo for preventing morbidity and mortality in preterm infants (Soll 2010) | Cerebral palsy at 1 or 2 years | Surfactant product | Exosurf Neonatal | 158 per 1000 (44/279) | 144 per 1000 (98 to 211) | RR 0.91 (0.62 to 1.34) | 591 (3 RCTs) | Not applied in review |
| DPPC/HDL | 122 per 1000 (5/41) | 132 per 1000 (41 to 420) | RR 1.08 (0.34 to 3.44) | 79 (1 RCT) | ||||
| Neonatal care: mechanical ventilation | ||||||||
| Continuous distending pressure vs standard care for respiratory distress in preterm infants (Ho 2015) | Cerebral palsy at 9 to 15 years | Type of continuous distending pressure | Continuous negative pressure | (0/18) | (2/18) | RR 5.0 (0.26 to 97.37) | 36 (1 RCT) | Not applicable |
| Neonatal care: bronchopulmonary dysplasia | ||||||||
| Early (< 8 days) postnatal corticosteroids vs placebo or no treatment for preventing chronic lung disease in preterm infants (Doyle 2014b) | Cerebral palsy at 11 months to 7 to 9 years | Type of corticosteroid used | Dexamethasone | 89 per 1000 (40/449) | 156 per 1000 (107 to 227) | RR 1.75 (1.20 to 2.55) | 921 (7 RCTs) | Chi² = 2.96, df = 1 (P = 0.09), I² = 66% |
| Hydrocortisone | 86 per 1000 (23/266) | 84 per 1000 (48 to 146) | RR 0.97 (0.55 to 1.69) | 531 (5 RCTs) | ||||
| Cerebral palsy in survivors assessed at 11 months to 7 to 9 years | Type of corticosteroid used | Dexamethasone | 139 per 1000 (40/288) | 253 per 100 (179 to 357) | RR 1.82 (1.29 to 2.57) | 586 (7 RCTs) | Chi² = 3.99, df = 1 (P = 0.05), I² = 75% | |
| Hydrocortisone | 126 per 1000 (23/182) | 120 per 1000 (71 to 206) | RR 0.95 (0.56 to 1.63) | 373 (5 RCTs) | ||||
| Neonatal care: other | ||||||||
| Prophylactic thyroid hormones vs placebo for prevention of morbidity and mortality in preterm infants (Osborn 2007) | Cerebral palsy at 5.7 years | Dosing strategy | T4 8 mcg/kg/d, day 1 to 42 | 120 per 1000 (9/75) | 86 per 1000 (34 to 221) | RR 0.72 (0.28 to 1.84) | 156 (1 RCT) | Not applicable |
| Timing | Commenced < 48 hours | |||||||
| Methodological quality | Studies with adequate methods | |||||||
| Early developmental intervention vs standard follow‐up post hospital discharge to prevent motor and cognitive impairment in preterm infants (Spittle 2015) | Cerebral palsy at 18 months to 6 years | Commencement of intervention | Inpatient | 79 per 1000 (12/152) | 74 per 1000 (36 to 152) | RR 0.94 (0.46 to 1.93) | 354 (3 RCTs) | Not applied in review |
| Post hospital discharge | 79 per 1000 (20/253) | 59 per 1000 (34 to 105) | RR 0.75 (0.43 to 1.33) | 631 (4 RCTs) | ||||
| Focus of intervention | Parent‐infant relationship and Infant development | 77 per 1000 (21/272) | 52 per 1000 (29 to 90) | RR 0.67 (0.38 to 1.17) | 716 (4 RCTs) | Not applied in review | ||
| Infant development | 83 per 1000 (11/133) | 97 per 1000 (46 to 203) | RR 1.17 (0.56 to 2.46) | 269 (3 RCTs) | ||||
| Quality of studies | Higher‐quality studies | 82 per 1000 (25/304) | 72 per 1000 (44 to 116) | RR 0.87 (0.53 to 1.41) | 776 (5 RCTs) | Not applied in review | ||
| Lower‐quality studies | 69 per 1000 (7/101) | 43 per 1000 (14 to 130) | RR 0.62 (0.20 to 1.87) | 209 (2 RCTs) | ||||
Abbreviations: CI: confidence interval; DPPC/HDL: dipalmitoylphosphatidylcholine/high‐density lipoprotein; g: grams; P: P value; RCT: randomised controlled trial; RR: risk ratio; T4: thyroxine.
| Intervention and comparison | Outcome | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | Comments |
| Neonatal care: bronchopulmonary dysplasia | |||||||
| Early (< 8 days) postnatal corticosteroids vs placebo or no treatment for preventing chronic lung disease in preterm infants (Doyle 2014b) | Cerebral palsy or death at 11 months to 7 to 9 years | 352 per 1000 (252/715) | 384 per 1000 (334 to 441) | RR 1.09 (0.95 to 1.25) | 1452 (12 RCTs) | MODERATE | Study limitations (‐1): 4 RCTs at unclear risk of selection bias; 2 RCTs at high risk of performance and detection bias; 2 RCTs had 13% to 53% follow‐up overall |
| Moderately early (7‐14 days) postnatal corticosteroids vs placebo or no treatment for preventing chronic lung disease in preterm infants (Halliday 2003) | Cerebral palsy or death at 12 months' corrected age up to 90 months | 316 per 1000 (30/95) | 262 per 1000 (174 to 388) | RR 0.83 (0.55 to 1.23) | 204 (4 RCTs) | VERY LOW | Study limitations (‐1): 2 RCTs with 68% to 70% follow‐up; 1 RCT with unclear blinding of outcome assessment Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Late (> 7 days) postnatal corticosteroids vs placebo or no treatment for chronic lung disease in preterm infants (Doyle 2014) | Cerebral palsy or death at 1 to 3 years | 328 per 1000 (142/433) | 302 per 1000 (249 to 367) | RR 0.92 (0.76 to 1.12) | 876 (14 RCTs) | LOW | Study limitations (‐1): 5 RCTs unclear risk of selection bias; 5 RCTs with follow‐up between 32% and 78% Imprecision (‐1): wide CI crossing line of no effect |
| Cerebral palsy or death at latest reported age (from 1 year up to 17 years) | 312 per 1000 (132/423) | 296 per 1000 (240 to 362) | RR 0.95 (0.77 to 1.16) | 855 (15 RCTs) | LOW | Study limitations (‐1): 5 RCTs unclear risk of selection bias; 7 RCTs with follow‐up between 32% and 78% Imprecision (‐1): wide CI crossing line of no effect | |
| Neonatal care: other | |||||||
| Thyroid hormones vs placebo for preventing neurodevelopmental impairment in preterm infants (Osborn 2001) | Cerebral palsy or death at 5.7 years | 300 per 1000 (30/100) | 210 per 1000 (129 to 342) | RR 0.70 (0.43 to 1.14) | 200 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Prophylactic thyroid hormones vs placebo for prevention of morbidity and mortality in preterm infants (Osborn 2007) | |||||||
Abbreviations: CI: confidence interval; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; RCT: randomised controlled trial; RR: risk ratio.
| Intervention and comparison | Outcome | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | Comments |
| Neonatal care: asphyxia | |||||||
| Allopurinol vs placebo or no drug for preventing mortality and morbidity in newborn infants with hypoxic‐ischaemic encephalopathy (Chaudhari 2012) | Severe quadriplegia in surviving infants (18 months and 4 to 8 years) | 343 per 1000 (12/35) | 202 per 1000 (96 to 435) | RR 0.59 (0.28 to 1.27) | 73 (3 RCTs) | VERY LOW | Study limitations (‐1): 1 RCT with unclear risk of selection bias and high risk of performance/detection bias Imprecision (‐2): wide CI crossing line of no effect; small sample sizes with few events |
| Neonatal care: respiratory distress syndrome | |||||||
| Synthetic surfactant vs placebo for respiratory distress syndrome in preterm infants (Soll 2000) | Moderate to severe cerebral palsy in survivors examined at 1 year (in 4 of the 5 RCTs) | 55 per 1000 (42/767) | 41 per 1000 (26 to 64) | RR 0.75 (0.48 to 1.16) | 1557 (5 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing the line of no effect |
| Prophylactic protein‐free synthetic surfactant vs placebo for preventing morbidity and mortality in preterm infants (Soll 2010) | Moderate/severe cerebral palsy at 1 or 2 years | 75 per 1000 (24/320) | 69 per 1000 (40 to 119) | RR 0.92 (0.53 to 1.59) | 670 (4 RCTs) | LOW | Study limitations (‐1): "Somewhat fewer infants who received surfactant failed to return for follow‐up evaluation (typical relative risk 0.63, 95% CI 0.48, 0.82; typical risk difference ‐0.10, 95% CI ‐0.15, ‐0.04)" Imprecision (‐1): wide CI crossing the line of no effect |
Abbreviations: CI: confidence interval; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; RCT: randomised controlled trial; RR: risk ratio.
| Intervention and comparison | Outcome | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | Comments |
| Neonatal care: asphyxia | |||||||
| Allopurinol vs placebo or no drug for preventing mortality and morbidity in newborn infants with hypoxic‐ischaemic encephalopathy (Chaudhari 2012) | Death or severe neurodevelopmental disability in survivors (18 months and 4 to 8 years) (defined as any 1 or combination of the following: non‐ambulant cerebral palsy, severe developmental delay assessed via validated tools, auditory and visual impairment) | 611 per 1000 (33/54) | 477 per 1000 (342 to 660) | RR 0.78 (0.56 to 1.08) | 110 (3 RCTs) | VERY LOW | Study limitations (‐1): 1 RCT with unclear risk of selection bias and high risk of performance/detection bias Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Therapeutic hypothermia vs standard care for newborns with hypoxic‐ischaemic encephalopathy (Jacobs 2013) | Death or major disability in survivors assessed at 18 to 24 months (defined as cerebral palsy, developmental delay (BSID or GMDS assessment > 2 SD below the mean) or intellectual impairment (IQ > 2 SD below mean), blindness (vision < 6/60 in both eyes), sensorineural deafness requiring amplification) | 614 per 1000 (409/666) | 461 per 1000 (418 to 510) | RR 0.75 (0.68 to 0.83) | 1344 (8 RCTs) | HIGH | Not downgraded |
| Major neurodevelopmental disability at 18 to 24 months (defined as cerebral palsy, developmental delay (BSID or GMDS assessment > 2 SD below the mean) or intellectual impairment (IQ > 2 SD below mean), blindness (vision < 6/60 in both eyes), sensorineural deafness requiring amplification) | 249 per 1000 (166/666) | 192 per 1000 (157 to 234) | RR 0.77 (0.63 to 0.94) | 1344 (8 RCTs) | HIGH | Not downgraded | |
| Major neurodevelopmental disability in survivors assessed at 18 to 24 months (defined as cerebral palsy, developmental delay (BSID or GMDS assessment > 2 SD below the mean) or intellectual impairment (IQ > 2 SD below mean), blindness (vision < 6/60 in both eyes), sensorineural deafness requiring amplification) | 393 per 1000 (166/422) | 264 per 1000 (216 to 315) | RR 0.67 (0.55 to 0.80) | 917 (8 RCTs) | HIGH | Not downgraded | |
| Death or moderate to severe disability at 6 to 7 years (defined as IQ ≥ 2 SD below the mean, a GMF level of 3 or greater, bilateral deafness (with or without amplification), bilateral blindness, or refractory epilepsy) | 645 per 1000 (60/93) | 523 per 1000 (413 to 671) | RR 0.81 (0.64 to 1.04) | 190 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size | |
| Moderate‐to‐severe disability at 6 to 7 years (defined as IQ ≥ 2 SD below the mean, a GMF level of 3 or greater, bilateral deafness (with or without amplification), bilateral blindness or refractory epilepsy) | 380 per 1000 (19/50) | 350 per 1000 (217 to 562) | RR 0.92 (0.57 to 1.48) | 119 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size | |
| Barbiturates (phenobarbital) vs conventional therapy for prevention of morbidity and mortality following perinatal asphyxia (Young 2016) | Death or major neurodevelopmental disability follow‐up: > 12 months (3 years) (defined as cerebral palsy, developmental delay (BSID or GMDS assessment > 2 SD below the mean) or intellectual impairment (IQ > 2 SD below mean), blindness (vision < 6/60 in both eyes), or sensorineural deafness requiring amplification) | 813 per 1000 (13/16) | 268 per 1000 (114 to 634) | RR 0.33 | 31 (1 RCT) | VERY LOW | Study limitations (‐1): unblinded study; concern regarding performance bias, detection bias, and incomplete follow‐up (graded by review authors themselves) |
| Major neurodevelopmental disability follow‐up: > 12 months (3 years) (defined as cerebral palsy, developmental delay (BSID or GMDS assessment > 2 SD below the mean) or intellectual impairment (IQ > 2 SD below mean), blindness (vision < 6/60 in both eyes), or sensorineural deafness requiring amplification) | 563 per 1000 (9/16) | 135 per 1000 (34 to 518) | RR 0.24 | 31 (1 RCT) | VERY LOW | Study limitations (‐1): unblinded study; concern regarding performance bias, detection bias, and incomplete follow‐up (graded by review authors themselves) | |
| Neonatal care: haemorrhage: periventricular/intraventricular | |||||||
| Ethamsylate vs placebo for prevention of morbidity and mortality in preterm or very low birthweight infants (Hunt 2010) | Neurodevelopmental disability at 2 years of age in surviving children available for follow‐up (defined as cerebral palsy on clinical examination, developmental delay > 2 SD below population mean on any standard test of development, or blindness (VA < 6/60), or deafness (any hearing impairment requiring amplification) at any time after 2 years' corrected age) | 170 per 1000 (46/270) | 135 per 1000 (90 to 199) | RR 0.79 (0.53 to 1.17) | 532 (3 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| Death or any disability by 2 years of age in children with known outcome at any point in time (not defined in review) | 338 per 1000 (233/690) | 324 per 1000 (277 to 375) | RR 0.96 (0.82 to 1.11) | 1334 (7 RCTs) | LOW | Study limitations (‐1): 4 RCTs at unclear risk of selection bias; 3 RCTs at unclear risk of bias due to lack of blinding Imprecision (‐1): wide CIs crossing line of no effect | |
| Phenobarbital vs no treatment for prevention of intraventricular haemorrhage in preterm infants (Smit 2013) | Severe neurodevelopmental impairment at 27 months (defined as clinical cerebral palsy or DQ below the range that can be measured) | 74 per 1000 (4/54) | 107 per 1000 (30 to 373) | RR 1.44 (0.41 to 5.04) | 101 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at high risk of performance bias) and unclear risk of other bias Imprecision (‐2): wide CI crossing line of no effect; small sample size, low event rate |
| Mild neurodevelopmental impairment at 27 months (defined as DQ < 80 or motor abnormality on examination) | 111 per 1000 (6/54) | 63 per 1000 (17 to 241) | RR 0.57 (0.15 to, 2.17) | 101 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at high risk of performance bias) and unclear risk of other bias Imprecision (‐2): wide CI crossing line of no effect; small sample size, low event rate | |
| Neonatal care: hypotension | |||||||
| Dobutamine vs dopamine in preterm infants with low superior vena cava flow (Osborn 2007b) | Disability at 3 years in survivors (defined as GMDS quotient ≤ 70, cerebral palsy, blind (VA < 6/60) or deaf (hearing aids)) | 714 per 1000 (5/7) | 71 per 1000 (7 to 1114) | RR 0.10 (0.01 to 1.56) | 13 (1 RCT) | VERY LOW | Study limitations (‐1): 5/18 surviving infants were not assessed at 3 years Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with very small sample size |
| Death or disability at 3 years (defined as GMDS quotient ≤ 70, cerebral palsy, blind (VA < 6/60) or deaf (hearing aids)) | 882 per 1000 (15/17) | 697 per 1000 (503 to 979) | RR 0.79 (0.57 to 1.11) | 37 (1 RCT) | VERY LOW | Study limitations (‐1): 5/18 surviving infants were not assessed at 3 years Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with very small sample size | |
| Death or disability at latest follow‐up (1 to 3 years) (defined as GMDS quotient ≤ 70, cerebral palsy, blind (VA < 6/60) or deaf (hearing aids)) | 750 per 1000 (15/20) | 713 per 1000 (495 to 1035) | RR 0.95 (0.66 to 1.38) | 41 (1 RCT) | VERY LOW | Study limitations (‐1): 5/18 surviving infants were not assessed at 3 years Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with very small sample size | |
| Neonatal care: fluid therapy | |||||||
| Volume vs no treatment for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | Severe neurodevelopmental disability in survivors at 2 years (defined as blind, dead, unable to walk, DQ > 3 SD below the mean, or another severe disability) | 141 per 1000 (29/205) | 113 per 1000 (74 to 174) | RR 0.80 (0.52 to 1.23) | 604 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| Gelatin vs fresh frozen plasma for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | 113 per 1000 (23/203) | 112 per 1000 (65 to 195) | RR 0.99 (0.57 to 1.72) | 399 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect | |
| Volume vs no treatment for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | Death or severe neurodevelopmental disability in survivors at 2 years (defined as blind, dead, unable to walk, DQ > 3 SD below the mean, or another severe disability) | 318 per 1000 (82/258) | 318 per 1000 (254 to 394) | RR 1.00 (0.80 to 1.24) | 776 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| Gelatin vs fresh frozen plasma for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | 300 per 1000 (77/257) | 333 per 1000 (258 to 428) | RR 1.11 (0.86 to 1.43) | 518 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect | |
| Neonatal care: patent ductus arteriosus | |||||||
| Prophylactic indomethacin vs placebo for preventing mortality and morbidity in preterm infants (Fowlie 2010) | Death or severe neurodevelopmental disability at 18 to 36 months (defined as any 1 or a combination of the following: non‐ambulant cerebral palsy, developmental delay (DQ < 70), auditory and visual impairment) | 400 per 1000 (299/748) | 407 per 1000 (360 to 460) | RR 1.02 (0.90 to 1.15) | 1491 (3 RCTs) | MODERATE | Study limitations (‐1): 2 RCTs at unclear risk of attrition bias (> 25% loss to follow‐up) |
| Neonatal care: blood disorders | |||||||
| Erythropoietin vs placebo for preventing red blood cell transfusion in preterm and/or low birthweight infants (Ohlsson 2014) | Any neurodevelopmental impairment at 18 to 22 months' corrected age (in children examined) (not defined in review; definition from trial manuscript: 1 of the following: MDI < 70, PDI < 70, moderate or severe cerebral palsy, blindness, or deafness) | 451 per 1000 (23/51) | 437 per 1000 (280 to 681) | RR 0.97 (0.62 to 1.51) | 99 (1 RCT) | VERY LOW | Study limitations: 1 RCT at unclear risk of selection bias and high risk of attrition bias (˜73% follow‐up) Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size |
| Transfusion at a restrictive vs a liberal haemoglobin threshold for preventing morbidity and mortality in very low birthweight infants (Whyte 2011) | Any neurosensory impairment at 18 to 21 months' follow‐up among survivors (defined as cognitive delay (MDI < 70), cerebral palsy, severe visual impairment, severe hearing impairment) | 220 per 1000 (37/168) | 289 per 1000 (198 to 418) | RR 1.31 (0.90 to 1.90) | 328 (1 RCT) | LOW | Study limitations (‐1): 1 RCT at high risk of performance bias and unclear risk of reporting bias Imprecision (‐1): wide CI crossing line of no effect |
| Death or severe morbidity at 18 to 21 months' follow‐up (defined as cognitive delay (MDI < 70), cerebral palsy, severe visual impairment, severe hearing impairment) | 385 per 1000 (82/213) | 450 per 1000 (362 to 566) | RR 1.17 (0.94 to 1.47) | 421 (1 RCT) | LOW | Study limitations (‐1): 1 RCT at high risk of performance bias and unclear risk of reporting bias Imprecision (‐1): wide CI crossing line of no effect | |
| Death or severe morbidity at 18 to 21 months' follow‐up (defined as cognitive delay (MDI < 85), cerebral palsy, severe visual impairment, severe hearing impairment) | 498 per 1000 (106/213) | 602 per 1000 (503 to 717) | RR 1.21 (1.01 to 1.44) | 421 (1 RCT) | MODERATE | Study limitations (‐1): 1 RCT at high risk of performance bias and unclear risk of reporting bias | |
| Neonatal care: nitric oxide | |||||||
| Inhaled NO vs placebo for respiratory failure in preterm infants (entry before 3 days based on oxygenation) (Barrington 2010) | Neurodevelopmental disability at 18 to 22 months (defined as moderate or severe cerebral palsy, blind, deaf, BSID MDI < 70, or PDI < 70) | 455 per 1000 (50/110) | 477 per 1000 (355 to 636) | RR 1.05 (0.78 to 1.40) | 208 (2 RCTs) | LOW | Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Inhaled NO vs placebo or no treatment for respiratory failure in preterm infants (entry after 3 days based on BPD risk) (Barrington 2010) | Neurodevelopmental disability at 2 years' corrected age or 30 months (defined as 1 RCT: moderate or severe cerebral palsy, blind, deaf, BSID MDI < 70, or PDI < 70; 1 RCT: cerebral palsy, BSID MDI or PDI < 71, or sensorineural impairment) | 480 per 1000 (119/248) | 432 per 1000 (355 to 523) | RR 0.90 (0.74 to 1.09) | 498 (2 RCTs) | LOW | Study limitations (‐1): 1 RCT with no blinding of intervention or outcome measurement Imprecision (‐1): wide CI crossing line of no effect; small sample sizes |
| Inhaled NO vs placebo for respiratory failure in preterm infants (studies of routine use in intubated preterm infants) (Barrington 2010) | Neurodevelopmental disability at 1 or 2 years' corrected age (defined as 1 RCT: cerebral palsy, blind, severe hearing loss, BSID MDI < 70, or PDI < 70; 1 RCT: cerebral palsy, bilateral blindness, bilateral hearing loss, or BSID score > 2 SD below the mean) | 364 per 100 (104/286) | 327 per 1000 (262 to 411) | RR 0.90 (0.72 to 1.13) | 593 (2 RCT) | VERY LOW | Study limitations (‐1): 2 RCTs with 74% to 82% follow‐up Imprecision (‐1): wide CI crossing line of no effect Inconsistency (‐1): substantial heterogeneity I² = 83% |
| Inhaled nitric oxide vs control for respiratory failure in infants born at or near term (Finer 2006) | Neurodevelopmental disability among survivors at 13 or 18 to 24 months (defined as 1 RCT: cerebral palsy, BSID MDI or PDI < 2 SD below the mean, blind or hearing impaired; or 1 RCT: cerebral palsy, > 2 mild (mild neurological abnormalities; mild reductions in BSID scores 1 to 2 SD below the mean), or at least 1 severe impairment) | 265 per 1000 (48/181) | 257 per 1000 (175 to 382) | RR 0.97 (0.66 to 1.44) | 301 (2 RCTs) | LOW | Study limitations (‐1): 1 RCT masking of allocation, masking of outcomes, and completeness of follow‐up 'can't tell' Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: apnoea | |||||||
| Caffeine vs placebo for apnoea in preterm infants (Henderson‐Smart 2010b) | Death or major disability at 18 to 21 months' corrected age (defined as cognitive delay, cerebral palsy, deafness, or blindness) | 417 per 1000 (153/367) | 354 per 1000 (296 to 421) | RR 0.85 (0.71 to 1.01) | 767 (1 RCT) | LOW | Study limitations (‐1): results based on 1 RCT subgroup from post hoc subgroup analyses (randomisation not stratified according to indication for inclusion) Imprecision (‐1): wide CI crossing line of no effect |
| Caffeine vs placebo for prevention of apnoea in preterm infants (Henderson‐Smart 2010c) | Death or major disability at 18 to 21 months' corrected age (not defined in review; definition from trial manuscript: cerebral palsy, cognitive delay, severe hearing loss, or bilateral blindness) | 431 per 1000 (88/204) | 431 per 1000 (345 to 535) | RR 1.00 (0.80 to 1.24) | 423 (1 RCT) | LOW | Study limitations (‐1): results based on 1 RCT subgroup from post hoc subgroup analyses (randomisation not stratified according to indication for inclusion) Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: respiratory distress syndrome | |||||||
| Inositol supplementation (repeat doses) vs placebo in preterm infants at risk for or having respiratory distress syndrome (Howlett 2015) | Major neural developmental impairment at 1 year corrected age (defined as sensory deficit, cerebral palsy, developmental delay, severe hypotonia) | 178 per 1000 (13/73) | 94 per 1000 (43 to 207) | RR 0.53 (0.24 to 1.16) | 169 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection, performance, detection, and reporting bias, and at high risk of other bias Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size |
| Animal‐derived surfactant extract vs no treatment for treatment of respiratory distress syndrome (Seger 2009) | Major developmental disability in survivors at 1 and 2 years' corrected age (defined as severe forms of cerebral palsy, blindness, deafness requiring hearing aids, or GMDS DQ < 70) | 34 per 1000 (1/29) | 114 per 1000 (5 to 923) | RR 3.30 (0.14 to 26.78) | 73 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with no blinding of intervention; and blinding of outcome measurement not reported Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: mechanical ventilation | |||||||
| Continuous distending pressure vs standard care for respiratory distress in preterm infants (Ho 2015) | Death or severe disability at 9 to 15 years (not defined in review; severe disability as defined below) | 158 per 1000 (3/19) | 210 per 1000 (54 to 816) | RR 1.33 (0.34 to 5.17) | 38 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection and attrition bias and high risk of performance bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Severe disability at 9 to 15 years (not defined in review; definition from trial manuscript: unable to undertake activity without aids or assistance most of the time, or completely dependent on carer: WASI ≤ 69; GMF level 4 to 5, arms: needs assistance to feed and dress; VA < 6/60, gross movement/light and dark only or worse; hearing loss not corrected with age; parent and teacher overall difficulties (Q26), "Yes" and impact score 6 to 10 parent and 3 to 6 teacher; or other condition needs supervision/aid constantly ‐ includes continuous home oxygen; communication severely limited) | 158 per 1000 (3/19) | 167 per 1000 (38 to 722) | RR 1.06 (0.24 to 4.57) | 37 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection and attrition bias and high risk of performance bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT | |
| Any disability at 9 to 15 years (not defined in review; definition from trial manuscript: mild: some loss of function but able to function independently; moderate: aids or assistance needed for some tasks. Moderate difficulty in doing some activities; severe: unable to undertake activity without aids or assistance most of the time, or completely dependent on carer; includes neuromotor components includes GMF levels 1 to 5) | 632 per 1000 (12/19) | 392 per 1000 (196 to 764) | RR 0.62 (0.31 to 1.21) | 37 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection and attrition bias and high risk of performance bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT | |
| Prophylactic methylxanthines (caffeine) vs placebo for endotracheal extubation in preterm infants (Henderson‐Smart 2010) | Death or major disability at 18 to 21 months' corrected age (defined as cognitive delay, cerebral palsy, deafness, or blindness) | 525 per 1000 (189/360) | 446 per 1000 (383 to 520) | RR 0.85 (0.73 to 0.99) | 676 (1 RCT) | MODERATE | Study limitations (‐1): results based on 1 RCT subgroup from post hoc subgroup analyses (randomisation not stratified according to indication for inclusion) |
| Volume‐targeted vs pressure‐limited ventilation in the neonate (Wheeler 2010) | Severe disability (any definition) at 6 to 18 months and 22 months (definitions not reported in review; definitions from trial manuscripts: 1 RCT: abnormal neurological evaluation (gross or fine motor delay) or BSID MDI < 70; 1 RCT: cerebral palsy severe enough to hamper gross motor activity, deafness needing hearing aids, registered blind or partially sighted) | 176 per 1000 (18/102) | 152 per 1000 (83 to 281) | RR 0.86 (0.47 to 1.59) | 209 (2 RCTs) | LOW | Indirectness (‐1): post hoc analysis including 2 RCTs with varied definitions Imprecision (‐1): wide CI crossing line of no effect (post hoc analysis in review) |
| Severe disability (any definition) at 22 months or death (definition not reported in review; definition from trial manuscript: cerebral palsy severe enough to hamper gross motor activity, deafness needing hearing aids, registered blind or partially sighted) | 327 per 1000 (17/52) | 177 per 1000 (88 to 347) | RR 0.54 (0.27 to 1.06) | 109 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT (post hoc analysis in review) | |
| Neonatal care: bronchopulmonary dysplasia | |||||||
| Early (< 8 days) postnatal corticosteroids vs placebo or no treatment for preventing chronic lung disease in preterm infants (Doyle 2014b) | Major neurosensory disability at 18 to 22 months to 53 months (variable criteria reported in review: 1 RCT: non‐ambulant cerebral palsy, global retardation (not specified), blindness, or deafness; 1 RCT: moderate or severe cerebral palsy, blindness, deafness, or BSID MDI or PDI < ‐2 SD; 1 RCT: cerebral palsy, BSID MDI or PDI < 71, blindness or deafness; 1 RCT: severe motor dysfunction (child non‐ambulant), or BSID MDI or PDI <‐2 SD; 2 RCTs: cerebral palsy, blindness, deafness, or developmental delay (BSID MDI < 70 (< ‐2 SD) or GMDS DQ < 70); 1 RCT: cerebral palsy, functional blindness, functional deafness, developmental delay (BSID MDI < 70 (<‐2 SD)), or motor delay (BSID PDI < 70 (<‐2 SD)) | 199 per 1000 (121/607) | 231 per 1000 (187 to 285) | RR 1.16 (0.94 to 1.43) | 1233 (7 RCTs) | LOW | Study limitations (‐1): 2 RCTs at unclear risk of selection bias; 1 RCT at high risk of performance and detection bias Imprecision (‐1): wide CI crossing line of no effect |
| Major neurosensory disability in survivors examined at 18 to 22 months to 53 months (variable criteria as above) | 307 per 1000 (121/394) | 350 per 1000 (289 to 424) | RR 1.14 (0.94 to 1.38) | 799 (7 RCTs) | LOW | Study limitations (‐1): 2 RCTs at unclear risk of selection bias; 1 RCT at high risk of performance and detection bias Imprecision (‐1): wide CI crossing line of no effect | |
| Death or major neurosensory disability at 18 to 22 months to 53 months (variable criteria as above) | 466 per 1000 (283/607) | 490 per 1000 (434 to 545) | RR 1.05 (0.93 to 1.17) | 1233 (7 RCTs) | MODERATE | Study limitations (‐1): 2 RCTs at unclear risk of selection bias; 1 RCT at high risk of performance and detection bias | |
| Moderately early (7‐14 days) postnatal corticosteroids vs placebo for preventing chronic lung disease in preterm infants (Halliday 2003) | Major neurosensory disability at 15 months' corrected age up to 90 months (variable criteria reported in review: 1 RCT: any of cerebral palsy or a BSID MDI or PDI < ‐ 1 SD; 1 RCT: not specified) | 98 per 1000 (4/41) | 123 per 1000 (44 to 340) | RR 1.26 (0.45 to 3.49) | 96 (2 RCTs) | VERY LOW | Study limitations (‐1): 1 RCT with 70% follow‐up and unclear blinding of outcome assessment Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Major neurosensory disability in survivors assessed at 15 months' corrected age up to 90 months (variable criteria reported in review as above) | 174 per 1000 (4/23) | 155 per 1000 (66 to 365) | RR 0.89 (0.38 to 2.10) | 56 (2 RCTs) | VERY LOW | Study limitations (‐1): 1 RCT with 70% follow‐up and unclear blinding of outcome assessment Imprecision (‐2): wide CI crossing line of no effect; small sample sizes | |
| Death or major neurosensory disability at 15 months' corrected age up to 90 months (variable criteria reported in review as above) | 366 per 1000 (15/41) | 373 per 1000 (241 to 571) | RR 1.02 (0.66 to 1.56) | 96 (2 RCTs) | VERY LOW | Study limitations (‐1): 1 RCT with 70% follow‐up and unclear blinding of outcome assessment Imprecision (‐2): wide CI crossing line of no effect; small sample sizes | |
| Late (> 7 days) postnatal corticosteroids vs placebo or no treatment for chronic lung disease in preterm infants (Doyle 2014) | Major neurosensory disability at 1 year corrected age up to 11 years (variable criteria reported in review: 1 RCT: non‐ambulant cerebral palsy, < 50% of age level on the Minnesota CDI, or predicted special schooling for sensory or other impairment; 1 RCT: abnormal neurological examination (i.e. cerebral palsy), cognitive delay (IQ < 71) or not in a regular classroom; 1 RCT: severe disability ‐ bilateral blindness, cerebral palsy with the child unlikely ever to walk or BSID MDI < 55 (< ‐3 SD)) or moderate disability ‐ deafness, cerebral palsy in children not walking at 2 years but expected to walk, or MDI from 55 to < 70 (‐3 SD to < ‐2 SD); 1 RCT: cerebral palsy, blindness, deafness requiring hearing aids or worse, or developmental delay (defined as BSID MDI < 70); 1 RCT: more than mild cerebral palsy, blindness, deafness, or needing extra help with schooling; 1 RCT: blindness, cerebral palsy or a BSID MDI < 70 OR cerebral palsy or mental retardation (IQ < 70 on either the DAS or the WISC‐III and a VABS composite score < 70); 1 RCT: not specified; 1 RCT moderate or severe cerebral palsy, bilateral blindness, deafness or an MDI < 2 SD | 169 per 1000 (54/320) | 197 per 1000 (143 to 270) | RR 1.17 (0.85 to 1.60) | 655 (8 RCTs) | LOW | Study limitations (‐1): 3 RCTs with unclear risk of selection bias; 3 RCTs with follow‐up rates 60% to 78% Imprecision (‐1): wide CI crossing line of no effect |
| Major neurosensory disability in survivors assessed at 1 year corrected age up to 11 years (variable criteria reported in review as above) | 231 per 1000 (54/234) | 254 per 1000 (187 to 346) | RR 1.10 (0.81 to 1.50) | 480 (8 RCTs) | LOW | Study limitations (‐1): 3 RCTs with unclear risk of selection bias; 3 RCTs with follow‐up rates 60% to 78% Imprecision (‐1): wide CI crossing line of no effect | |
| Death or major neurosensory disability at 1 year corrected age up to 11 years (variable criteria reported in review as above) | 375 per 1000 (120/320) | 390 per 1000 (323 to 473) | RR 1.04 (0.86 to 1.26) | 655 (8 RCTs) | LOW | Study limitations (‐1): 3 RCTs with unclear risk of selection bias; 3 RCTs with follow‐up rates 60% to 78% Imprecision (‐1): wide CI crossing line of no effect | |
| Supplemental vitamin A vs a sham injection to prevent mortality and short‐ and long‐term morbidity in very low birthweight infants (Darlow 2016) | Neurodevelopmental impairment at 18 to 24 months (defined as BSID‐II MDI < 70, PDI < 70, any cerebral palsy, blind in both eyes, or bilateral hearing aids ) | 481 per 1000 (128/266) | 428 per 1000 (356 to 520) | RR 0.89 (0.74 to 1.08) | 538 (1 RCT) | LOW | Imprecision (‐2): "Concerning imprecision: does not met the optimal information size criteria" (graded by review authors themselves) |
| Death or neurodevelopmental impairment at 18 to 24 months (defined as BSID‐II MDI < 70, PDI < 70, any cerebral palsy, blind in both eyes, or bilateral hearing aids) | 596 per 1000 (204/342) | 549 per 1000 (483 to 626) | RR 0.92 (0.81 to 1.05) | 687 (1 RCT) | MODERATE | Imprecision (‐1): wide CIs crossing line of no effect | |
| Neonatal care: necrotising enterocolitis | |||||||
| Probiotics vs control (distilled water) for prevention of necrotising enterocolitis in preterm infants (AlFaleh 2014) | Mental retardation and cerebral palsy at 6 years | 47 per 1000 (2/43) | 47 per 1000 (7 to 323) | RR 1.02 (0.15 to 6.94) | 85 (1 RCT) | VERY LOW | Study limitation (‐1): 1 RCT at unclear risk for selection, performance, and detection bias; and high risk of attrition and reporting bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Arginine supplementation vs placebo for prevention of necrotising enterocolitis in preterm infants (Shah 2007) | Major neurodevelopmental disability at 36 months' post‐menstrual age (definition not reported in review; definition from trial manuscript: presence of 1 or more of cerebral palsy, cognitive delay (index < 70), bilateral blindness, bilateral hearing loss requiring aids) | 127 per 1000 (9/71) | 82 per 1000 (29 to 232) | RR 0.65 (0.23 to 1.83) | 132 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: fungal infections | |||||||
| Systemic antifungal agent vs placebo to prevent mortality and morbidity in very low birthweight infants (Cleminson 2015) | Neurodevelopmental impairment (composite) at 18 to 22 months (defined as at least 1 of (i) BSID‐III cognition composite score < 70, (ii) cerebral palsy, (iii) deafness or, (iv) blindness) | 274 per 1000 (23/84) | 309 per 1000 (194 to 496) | RR 1.13 (0.71 to 1.81) | 171 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: herpes simplex | |||||||
| Vidarabine vs placebo for treatment of herpes simplex virus infection in neonates (Jones 2009) | Abnormal neurodevelopment at approximately 1 year of age (not defined in review; definition from trial manuscript: spasticity or hemiparesis only; or combinations of microcephaly, paresis, spasticity, seizures, blindness, or deafness) | 214 per 1000 (6/28) | 321 per 1000 (133 to 782) | RR 1.50 (0.62 to 3.65) | 56 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with design limitations (method of randomisation not stated) Imprecision (‐2): wide CIs crossing line of no effect; 1 small RCT |
| Abnormal neurodevelopment or death at approximately 1 year of age (not defined in review; definition from trial manuscript as above) | 750 per 1000 (21/28) | 645 per 1000 (450 to 915) | RR 0.86 (0.60 to 1.22) | 56 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with design limitations (method of randomisation not stated) Imprecision (‐2): wide CIs crossing line of no effect; 1 small RCT | |
| Aciclovir vs vidarabine for treatment of herpes simplex virus infection in neonates (Jones 2009) | Abnormal neurodevelopment at approximately 1 year of age (not defined in review; definition from trial manuscript: mild impairment: only occular sequelae; moderate neurological impairment: hemiparesis or a persistent seizure disorder and no more than a 3‐month developmental delay; severe neurological sequelae: microcephaly, spastic quadriplegia, chorioretinitis or blindness, and a serious developmental delay of > 3 months according to the DDST) | 263 per 1000 (25/95) | 216 per 1000 (132 to 353) | RR 0.82 (0.50 to 1.34) | 202 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with design limitations (inadequate allocation concealment) Imprecision (‐2): wide CIs crossing line of no effect; 1 small RCT |
| Abnormal neurodevelopment or death at approximately 1 year of age (not defined in review definition from trial manuscript as above) | 463 per 1000 (44/95) | 366 per 1000 (264 to 509) | RR 0.79 (0.57 to 1.10) | 202 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with design limitations (inadequate allocation concealment) Imprecision (‐2): wide CIs crossing line of no effect; 1 small RCT | |
| Neonatal care: jaundice | |||||||
| Prophylactic phototherapy vs standard care for preventing jaundice in preterm or low birthweight infants (Okwundu 2012) | Neurodevelopmental impairment at 18 to 22 months (defined as blindness, severe hearing loss, and moderate or severe cerebral palsy) | 305 per 1000 (275/902) | 259 per 1000 (226 to 302) | RR 0.85 (0.74 to 0.99) | 1804 (1 RCT) | MODERATE | Study limitations (‐1): 1 RCT with high risk of attrition bias |
| Neonatal care: hypoglycaemia | |||||||
| Dextrose gel vs placebo for treatment of hypoglycaemia in newborn infants (Weston 2016) | Major neurosensory disability at 2 years (defined as any of the following: legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, developmental delay/intellectual impairment (defined as DQ < 2 SD below the mean)) | 11 per 1000 (1/94) | 67 per 1000 (8 to 543) | RR 6.27 (0.77 to 51.03) | 184 (1 RCT) | VERY LOW | Study limitations (‐1): "Evidence is based on a single trial" Imprecision (‐2):"Wide confidence intervals, low event rates and small sample sizes are suggestive of imprecision: (graded by review authors themselves) |
| Developmental disability at 2 years (defined as cognitive, language, or motor score below ‐1 SD, or cerebral palsy, blindness, or deafness) | 32/94 | 34/90 | RR 1.11 (0.75 to 1.63) | 184 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with 78% follow‐up Imprecision: wide CI crossing line of no effect; 1 small RCT | |
| Neonatal care: parenteral feeding | |||||||
| Glutamine supplementation vs placebo to prevent morbidity and mortality in preterm infants (Moe‐Byrne 2016) | Neurodevelopmental impairment at 2 years post term (defined as BSID‐II MDI ≤ 85, PDI ≤ 85, cerebral palsy, blindness in 1 or both eyes, or hearing loss requiring amplification) | 375 per 1000 (12/32) | 401 per 1000 | RR 1.07 (0.59 to 1.92) | 72 (1 RCT) | LOW | Imprecision (‐2): "Total sample size = 72" (graded by review authors themselves) |
Abbreviations: BSID: Bayley Scales of Infant Development; CDI: Child Development Inventory; CI: confidence interval; DAS: Differential Ability Scales; DDST: Denver Developmental Screening Test; DQ: development quotient; GMDS: Griffiths Mental Development Scales; GMF: gross motor function; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; IQ: intelligence quotient; MDI: Mental Development Index; PDI: Psychomotor Development Index; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; VA: visual acuity; VABS: Vineland Adaptive Behaviour Scales; WASI: Wechsler Abbreviated Scale of Intelligence; WISC: Wechsler Intelligence Scale for Children.
| Intervention and comparison | Outcome | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | Comments |
| Neonatal care: asphyxia | |||||||
| Therapeutic hypothermia vs standard care for newborns with hypoxic‐ischaemic encephalopathy (Jacobs 2013) | Neuromotor delay (BSID PDI > 2 SD below mean) in survivors assessed at 18 to 24 months | 349 per 1000 (104/298) | 262 per 1000 (206 to 328) | RR 0.75 (0.59 to 0.94) | 657 (6 RCTs) | HIGH | Not downgraded |
| Neonatal care: blood disorders | |||||||
| Erythropoietin vs placebo for preventing red blood cell transfusion in preterm and/or low birthweight infants (Ohlsson 2014) | PDI < 70 at 18 to 22 months' corrected age (in children examined) | 133 per 1000 (6/45) | 311 per 1000 (131 to 737) | RR 2.33 (0.98 to 5.53) | 90 (1 RCT) | VERY LOW | Study limitations: 1 RCT at unclear risk of selection bias and high risk of attrition bias (˜73% follow‐up) Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size |
| Neonatal care: pulmonary hypertension | |||||||
| Endothelin receptor antagonists vs placebo for persistent pulmonary hypertension in term and late preterm infants (More 2016) | Adverse neurological outcomes at 6 months (defined as clinical or electrographically proven seizures, abnormal muscle tone, abnormal deep tendon reflexes, delayed motor milestones, or abnormal auditory brainstem response) | 286 per 1000 (4/14) | 20 per 1000 (0 to 343) | RR 0.07 (0.00 to 1.20) | 37 (1 RCT) | LOW | Study limitation (‐1): 8/23 infants in the placebo group were excluded from analysis Imprecision (‐1): single RCT, small sample size (graded by review authors themselves) |
| Neonatal care: resuscitation | |||||||
| Room air vs 100% oxygen for resuscitation of infants at birth (Tan 2005) | Not walking in those followed up at 18 to 24 months | 107 per 1000 (13/122) | 110 per 1000 (4 to 240) | RR 1.03 (0.04 to 2.25) | 213 (1 RCT) | VERY LOW | Study limitations (‐2): 1 qRCT with no blinding, and < 70% follow‐up Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: nitric oxide | |||||||
| Inhaled NO vs placebo for respiratory failure in preterm infants (studies of routine use in intubated preterm infants) (Barrington 2010) | BSID MDI or PDI < ‐ 2 SD at 2 years' corrected age | 412 per 1000 (28/68) | 231 per 1000 (136 to 383) | RR 0.56 (0.33 to 0.93) | 138 (1 RCT) | MODERATE | Study limitations (‐1): 1 small RCT with 82% follow‐up |
| Inhaled nitric oxide vs control for respiratory failure in infants born at or near term (Finer 2006) | BSID PDI > 2 SD below the mean at 13 or 18 to 24 months | 148 per 1000 (25/169) | 161 per 1000 (86 to 300) | RR 1.09 (0.58 to 2.03) | 283 (2 RCTs) | LOW | Study limitations (‐1): 1 RCT masking of allocation, masking of outcomes, and completeness of follow‐up 'can't tell' Inconsistency (‐1): substantial heterogeneity (I² = 77%) Note: error in review for Ninos 1996 data; intervention and control group data switched; this has been rectified in this analysis |
| Neonatal care: respiratory distress syndrome | |||||||
| Inositol supplementation (repeat doses) vs placebo in preterm infants at risk for or having respiratory distress syndrome (Howlett 2015) | Minor neural developmental impairment at 1 year corrected age (defined as sensorimotor abnormality and/or developmental delay) | 137 per 1000 (10/73) | 115 per 1000 (52 to 255) | RR 0.84 (0.38 to 1.86) | 169 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection, performance, detection, and reporting bias, and at high risk of other bias Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size |
| Neonatal care: mechanical ventilation | |||||||
| Volume‐targeted vs pressure‐limited ventilation in the neonate (Wheeler 2010) | Gross Motor Developmental Issue (any definition) at 6 to 18 months (defined as gross motor delay) | 172 per 1000 (11/64) | 172 per 1000 (81 to 368) | RR 1.00 (0.47 to 2.14) | 128 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT (post hoc analysis in review) |
| Neonatal care: bronchopulmonary dysplasia | |||||||
| Early (< 8 days) postnatal corticosteroids vs placebo for preventing chronic lung disease in preterm infants (Doyle 2014b) | BSID PDI < ‐ 2 SD at 18 to 22 months or 25 months | 146 per 1000 (61/419) | 170 per 1000 (124 to 233) | RR 1.17 (0.85 to 1.60) | 842 (3 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| BSID PDI < ‐ 2 SD in tested survivors at 18 to 22 months or 25 months | 232 per 1000 (61/263) | 271 per 1000 (202 to 364) | RR 1.17 (0.87 to 1.57) | 528 (3 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect | |
| Late (> 7 days) postnatal corticosteroids vs placebo or no treatment for chronic lung disease in preterm infants (Doyle 2014) | BSID PDI < ‐ 2 SD at 1 year corrected age | 180 per 1000 (11/61) | 141 per 1000 (61 to 325) | RR 0.78 (0.34 to 1.80) | 118 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size |
| BSID PDI < ‐ 2 SD in survivors assessed at 1 year corrected age | 256 per 1000 (11/43) | 171 per 1000 (77 to 384) | RR 0.67 (0.30 to 1.50) | 90 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size | |
| Early inhaled corticosteroids vs placebo for preventing chronic lung disease in ventilated very low birthweight preterm neonates (Shah 2012) | Mean developmental index on BSID‐II < 2 SD of the mean (age not reported in review;from trial manuscript: 3 years) | 143 per 1000 (4/28) | 179 per 1000 (53 to 596) | RR 1.25 (0.37 to 4.17) | 56 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection bias and detection bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: other | |||||||
| Early developmental intervention vs standard follow‐up post hospital discharge to prevent motor and cognitive impairment in preterm infants (Spittle 2015) | Motor outcome at school age (5 years) (defined as low score on Movement ABC) | 378 per 1000 (51/135) | 423 per 1000 (329 to 544) | RR 1.12 (0.87 to 1.44) | 333 (2 RCTs) | LOW | Study limitations (‐1): 2 RCTs at high risk of attrition bias and unclear risk of performance bias Imprecision (‐1): wide CI crossing line of no effect |
Abbreviations: BSID: Bayley Scales of Infant Development; CI: confidence interval; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; MDI: Mental Development Index; Movement ABC: Movement Assessment Battery for Children; PDI: Psychomotor Development Index; qRCT: quasi‐randomised controlled trial; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation
Interventions for neonates with perinatal asphyxia or evidence of neonatal encephalopathy
Effective interventions: high‐quality evidence of effectiveness
Neonatal care: treating asphyxia
High‐quality evidence from the Jacobs 2013 review showed a reduction in cerebral palsy among survivors assessed at 18 to 24 months following therapeutic hypothermia versus standard care for newborns with hypoxic‐ischaemic encephalopathy (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.54 to 0.82; seven trials; 881 children) (Table 6). Subgroup analysis based on method of cooling (e.g. selective head cooling with mild hypothermia, whole body cooling) showed no clear subgroup differences (Chi² = 0.01, df = 1 (P = 0.93), I² = 0.0%) (Table 7). Low‐quality evidence from Jacobs 2013 also showed no clear differences for cerebral palsy at six to seven years following therapeutic hypothermia versus standard care (RR 0.60, 95% CI 0.31 to 1.18; one trial; 121 children) (Table 6). High‐quality evidence from Jacobs 2013 showed reductions in death or major disability among survivors assessed at 18 to 24 months (RR 0.75, 95% CI 0.68 to 0.83; eight trials; 1344 children), major neurodevelopmental disability at 18 to 24 months (RR 0.77, 95% CI 0.63 to 0.94; eight trials; 1344 children), major neurodevelopmental disability among survivors assessed at 18 to 24 months (RR 0.67, 95% CI 0.55 to 0.80; eight trials; 917 children), and neuromotor delay among survivors assessed at 18 to 24 months (RR 0.75, 95% CI 0.59 to 0.94; six trials; 657 children) (Table 10; Table 11). Low‐quality evidence suggested no clear differences for death or moderate to severe disability at six to seven years (RR 0.81, 95% CI 0.64 to 1.04; one trial; 190 children) nor for moderate to severe disability at six to seven years (RR 0.92, 95% CI 0.57 to 1.48; one trial; 119 children) following therapeutic hypothermia versus standard care (Table 10).
No conclusions possible: very low‐quality evidence
Neonatal care: treating asphyxia
Very low‐quality evidence from the Young 2016 review suggested no clear differences for cerebral palsy at three to six years with barbiturates (phenobarbital) versus conventional therapy for prevention of morbidity and mortality following perinatal asphyxia (RR 0.58, 95% CI 0.19 to 1.70; two trials; 69 children) (Table 6). Very low‐quality evidence from Young 2016 also suggested a reduction in death or major neurodevelopmental disability at three years (RR 0.33, 95% CI 0.14 to 0.78; one trial; 31 children) and in major neurodevelopmental disability at three years (RR 0.24, 95% CI 0.06 to 0.92; one trial; 31 children) following barbiturates (phenobarbital) versus conventional therapy (Table 10).
Very low‐quality evidence from the Chaudhari 2012 review suggested no clear differences for severe quadriplegia among survivors at 18 months or at four to eight years following allopurinol versus placebo or no drug for preventing mortality and morbidity among newborn infants with hypoxic‐ischaemic encephalopathy (RR 0.59, 95% CI 0.28 to 1.27; three trials; 73 children) (Table 9). Very low‐quality evidence from Chaudhari 2012 also suggested no clear differences for death or severe neurodevelopmental disability among survivors at 18 months or at four to eight years following allopurinol versus placebo (RR 0.78, 95% CI 0.56 to 1.08; three trials; 110 children) (Table 10).
Interventions for neonates born preterm and/or at low or very low birthweight
Possibly effective interventions: moderate‐quality evidence of effectiveness
Neonatal care: mechanical ventilation
Moderate‐quality evidence from the Henderson‐Smart 2010 review showed a reduction in cerebral palsy at 18 to 21 months' corrected age with prophylactic methylxanthines (caffeine) versus placebo for endotracheal extubation in preterm infants (RR 0.54, 95% CI 0.32 to 0.92; one trial; 644 children) (Table 6). Moderate‐quality evidence from Henderson‐Smart 2010 also showed a reduction in death or major disability at 18 to 21 months' corrected age with prophylactic methylxanthines (caffeine) versus placebo (RR 0.85, 95% CI 0.73 to 0.99; one trial; 676 children) (Table 10).
Probably ineffective interventions: moderate‐quality evidence of harm
Neonatal care: preventing bronchopulmonary dysplasia
Moderate‐quality evidence from the Doyle 2014b review showed an increase in cerebral palsy at 11 months to seven to nine years (RR 1.45, 95% CI 1.06 to 1.98; 12 trials; 1452 children) and in cerebral palsy among survivors assessed at 11 months to seven to nine years (RR 1.50, 95% CI 1.13 to 2.00; 12 trials; 959 children) following early (less than eight days of age) postnatal corticosteroids versus placebo or no treatment for preventing chronic lung disease in preterm infants (Table 6). Subgroup analysis based on type of corticosteroid used (i.e. dexamethasone, hydrocortisone) suggested no clear subgroup differences for cerebral palsy at 11 months to seven to nine years (Chi² = 2.96, df = 1 (P = 0.09), I² = 66%); however, a possible subgroup difference was identified that was based on the type of corticosteroid used for cerebral palsy among survivors assessed at 11 months to seven to nine years (Chi² = 3.99, df = 1 (P = 0.05), I² = 75%), with an increase in risk specifically observed in the dexamethasone (not the hydrocortisone) subgroup (Table 7). Moderate‐quality evidence from Doyle 2014b also showed no clear differences for cerebral palsy or death at 11 months to seven to nine years (RR 1.09, 95% CI 0.92 to 1.25; 12 trials; 1452 children) (Table 8) nor for death or major neurosensory disability at 18 to 22 months to 53 months (RR 1.05, 95% CI 0.93 to 1.17; seven trials; 1233 children) (Table 10); Bayley Scales of Infant Development Psychomotor Developmental Index less than minus two standard deviations below the mean at 18 to 22 months or at 25 months (RR 1.17, 95% CI 0.85 to 1.60; three trials; 842 children); or Bayley Scales of Infant Development Psychomotor Developmental Index less than minus two standard deviations below the mean among tested survivors at 18 to 22 months or at 25 months (RR 1.17, 95% CI 0.87 to 1.57; three trials; 528 children) with early postnatal corticosteroids versus placebo or no treatment (Table 11). Low‐quality evidence from Doyle 2014b suggested no clear differences between major neurosensory disability at 18 to 22 months to 53 months (RR 1.16, 95% CI 0.94 to 1.43; seven trials; 1233 children) and major neurosensory disability among survivors examined at 18 to 22 months to 53 months (RR 1.14, 95% CI 0.94 to 1.38; seven trials; 799 children) with early postnatal corticosteroids versus placebo or no treatment (Table 10).
Probably ineffective interventions: moderate‐quality evidence of lack of effectiveness
Neonatal care: preventing haemorrhage: periventricular/intraventricular
Moderate‐quality evidence from the Hunt 2010 review showed no clear differences for cerebral palsy among surviving children available for follow‐up at two years up to 3.5 to 4.2 years following ethamsylate versus placebo for prevention of morbidity and mortality in preterm or very low birthweight infants (RR 1.13, 95% CI 0.64 to 2.00; three trials; 532 children) (Table 6), nor on further subgroup analysis of infants born at less than 31 completed weeks at less than 1500 grams (RR 0.82, 95% CI 0.38 to 1.75; two trials; 328 children) (Table 7). Moderate‐quality evidence from Hunt 2010 also showed no clear differences for neurodevelopmental disability at two years of age among surviving children available for follow‐up (RR 0.79, 95% CI 0.53 to 1.17; three trials; 532 children), and low‐quality evidence suggested no clear differences for death or any disability by two years of age among children with known outcome at any point in time (RR 0.96, 95% CI 0.82 to 1.11; seven trials; 1334 children) following ethamsylate versus placebo (Table 10).
Neonatal care: fluid therapy
Moderate‐quality evidence from the Osborn 2004 review showed no clear differences for cerebral palsy among survivors at two years following volume versus no treatment (RR 0.76, 95% CI 0.48 to 1.20; one trial; 604 children) and gelatin versus fresh frozen plasma (RR 0.94, 95% CI 0.52 to 1.69; one trial; 399 children) for prevention of morbidity and mortality in very preterm infants (Table 6). Formal subgroup analyses in Osborn 2004 were not applicable based on timing of treatment, types of infants enrolled, or methodological quality (with the one included trial for this outcome using early treatment (less than 24 hours of age) in unselected preterm infants (not selected on the basis of cardiovascular compromise) and providing complete follow‐up for neurodevelopmental outcomes (RR 0.76, 95% CI 0.48 to 1.20; one trial; 604 children, as in main analysis)) (Table 7). Moderate‐quality evidence from Osborn 2004 also showed no clear differences between volume versus no treatment for severe neurodevelopmental disability among survivors at two years (RR 0.80, 95% CI 0.52 to 1.23; one trial; 604 children) or for death or severe neurodevelopmental disability among survivors at two years (RR 1.00, 95% CI 0.80 to 1.24; one trial; 776 children); or between gelatin versus fresh frozen plasma for severe neurodevelopmental disability among survivors at two years (RR 0.99, 95% CI 0.57 to 1.72; one trial; 399 children) or for death or severe neurodevelopmental disability among survivors at two years (RR 1.11, 95% CI 0.86 to 1.43; one trial; 518 children) (Table 10).
Neonatal care: preventing/treating patent ductus arteriosus
Moderate‐quality evidence from the Fowlie 2010 review showed no clear differences for cerebral palsy at 18 to 54 months (RR 1.04, 95% CI 0.77 to 1.40; four trials; 1372 children) or at eight years (RR 1.24, 95% CI 0.59 to 2.62; one trial; 304 children) following prophylactic indomethacin versus placebo for preventing mortality and morbidity in preterm infants (Table 6). Moderate‐quality evidence from Fowlie 2010 also showed no clear differences for death or severe neurodevelopmental disability at 18 to 36 months following prophylactic indomethacin versus placebo (RR 1.02, 95% 0.90 to 1.15; three trials; 1491 children) (Table 10).
Neonatal care: treating respiratory distress syndrome
Moderate‐quality evidence from the Soll 2000 review showed no clear differences in cerebral palsy among survivors examined at one year (RR 0.76, 95% CI 0.55 to 1.05; five trials; 1557 children) (Table 6) nor in moderate to severe cerebral palsy among survivors examined at one year following synthetic surfactant versus placebo for respiratory distress syndrome in preterm infants (RR 0.75, 95% CI 0.48 to 1.16; five trials; 1557 children) (Table 9).
Neonatal care: preventing jaundice
Moderate‐quality evidence from the Okwundu 2012 review showed no clear differences for cerebral palsy in all infants (birthweight < 2500 grams) at one year or at 18 months following prophylactic phototherapy versus standard care (starting phototherapy when serum bilirubin reached a pre‐specified level) for preventing jaundice in preterm or low birthweight infants (RR 0.96, 95% CI 0.50 to 1.85; two trials; 756 children) (Table 6). Very low‐quality evidence suggested no clear differences for cerebral palsy among all infants (birthweight < 1000 grams) at 18 months (RR 0.29, 95% CI 0.04 to 2.27; one trial; 30 children) (Table 6). Okwundu 2012 reported in text that "Secondary reports emanating from Brown 1985 at six‐year follow‐up also showed that there was no significant difference in the rate of cerebral palsy between the phototherapy and control group" (not graded). Moderate‐quality evidence from Okwundu 2012 did however show a reduction in neurodevelopmental impairment at 18 to 22 months following prophylactic phototherapy versus standard care (RR 0.85, 95% CI 0.74 to 0.99; one trial; 1804 children) (Table 10).
No conclusions possible: low‐quality evidence
Neonatal care: preventing/treating blood disorders
Low‐quality evidence from Ohlsson 2014 suggested no clear differences for cerebral palsy at 18 to 22 months' corrected age in children examined following darbepoetin alfa versus placebo for preventing red blood cell transfusion in preterm and/or low birthweight infants (RR 0.08, 95% CI 0.00 to 1.40; one trial; 51 children) (Table 6).
Low‐quality evidence from Whyte 2011 suggested no clear differences for cerebral palsy at 18 to 21 months' follow‐up among survivors following transfusion at a restrictive (low haemoglobin) versus a liberal (high haemoglobin) threshold for preventing morbidity and mortality in very low birthweight infants (RR 1.29, 95% CI 0.55 to 3.03; one trial; 335 children) (Table 6). Low‐quality evidence from Whyte 2011 also suggested no clear differences for any neurosensory impairment at 18 to 21 months' follow‐up among survivors (RR 1.31, 95% CI 0.90 to 1.90; one trial; 328 children) nor for death or severe morbidity at 18 to 21 months' follow‐up (Mental Development Index component defined < 70) (RR 1.17, 95% CI 0.94 to 1.47; one trial; 421 children); however, moderate‐quality evidence showed a possible increase in death or severe morbidity at 18 to 21 months' follow‐up (Mental Development Index component defined < 85) (RR 1.21, 95% CI 1.01 to 1.44; one trial; 421 children) with transfusion at a restrictive (low haemoglobin) versus a liberal (high haemoglobin) threshold (Table 10).
Neonatal care: nitric oxide
Low‐quality evidence from Barrington 2010 suggested no clear differences following inhaled nitric oxide versus placebo or no treatment for respiratory failure in preterm infants for cerebral palsy at 18 to 22 months (trial entry before three days based on oxygenation) (RR 1.85, 95% CI 0.93 to 3.71; two trials; 209 children); cerebral palsy at two years' corrected age or at 30 months (trial entry after three days based on bronchopulmonary dysplasia risk) (RR 1.10, 95% CI 0.54 to 2.23; two trials; 498 children); or cerebral palsy at one or two years' corrected age (trials of routine use in intubated preterm infants) (RR 0.94, 95% CI 0.51 to 1.70; two trials; 593 children) (Table 6). Low‐ to very low‐quality evidence from Barrington 2010 also suggested no clear differences for neurodevelopmental disability at 18 to 22 months (trial entry before three days based on oxygenation) (RR 1.05, 95% CI 0.78 to 1.40; two trials; 208 children), neurodevelopmental disability at two years' corrected age or at 30 months (trial entry after three days based on bronchopulmonary dysplasia risk) (RR 0.90, 95% CI 0.74 to 1.09; two trials; 498 children), or neurodevelopmental disability at one or two years' corrected age (trials of routine use in intubated preterm infants) (RR 0.90, 95% CI 0.72 to 1.13; two trials; 593 children) following inhaled nitric oxide versus placebo or no treatment (Table 10). Moderate‐quality evidence from Barrington 2010 also showed no clear differences for Bayley Mental or Psychomotor Developmental Index less than minus two standard deviations below the mean at two years' corrected age (trials of routine use in intubated preterm infants) following inhaled nitric oxide versus placebo (RR 0.56, 95% CI 0.33 to 0.93; one trial; 138 children) (Table 11).
Neonatal care: preventing/treating apnoea
Low‐quality evidence from the Henderson‐Smart 2010b review suggested no clear differences for cerebral palsy at 18 to 21 months' corrected age following caffeine versus placebo for treatment of apnoea in preterm infants (RR 0.60, 95% CI 0.29 to 1.25; one trial; 729 children) (Table 6). Low‐quality evidence from Henderson‐Smart 2010b also suggested no clear differences in death or major disability at 18 to 21 months' corrected age following caffeine versus placebo (RR 0.85, 95% CI 0.71 to 1.01; one trial; 767 children) (Table 10).
Low‐quality evidence from the Henderson‐Smart 2010c review suggested no clear differences for cerebral palsy at 18 to 21 months' corrected age following caffeine versus placebo for prevention of apnoea in preterm infants (RR 1.03, 95% CI 0.43 to 2.49; one trial; 415 children) (Table 6). Low‐quality evidence from Henderson‐Smart 2010c also suggested no clear differences in death or major disability at 18 to 21 months' corrected age following caffeine versus placebo (RR 1.00, 95% CI 0.80 to 1.24; one trial; 423 children) (Table 10).
Neonatal care: preventing respiratory distress syndrome
Low‐quality evidence from the Soll 2010 review suggested no clear differences for cerebral palsy at one to two years following prophylactic protein‐free synthetic surfactant versus placebo for preventing morbidity and mortality in preterm infants (RR 0.93, 95% CI 0.64 to 1.33; four trials; 670 children) (Table 6). Subgroup analyses were conducted that were based on surfactant product (Exosurf Neonatal, DPPC/HDL; Burroughs Wellcome, Research Triangle Park, North Carolina, USA); however, formal tests for subgroup differences were not applied in the review (Table 7). Low‐quality evidence from Soll 2010 also suggested no clear differences for moderate or severe cerebral palsy at one or two years following prophylactic protein‐free synthetic surfactant versus placebo (RR 0.92, 95% CI 0.53 to 1.59; four trials; 670 children) (Table 9).
Neonatal care: mechanical ventilation
Low‐quality evidence from the Wheeler 2010 review suggested no clear differences for severe disability at six to 18 months and at 22 months (RR 0.86, 95% CI 0.47 to 1.59; two trials; 209 children), for severe disability at 22 months or at death (RR 0.54, 95% CI 0.27 to 1.06; one trial; 109 children) (Table 10), and for gross motor developmental issues (RR 1.00, 95% CI 0.47 to 2.14; one trial; 128 children) (Table 11) following volume‐targeted versus pressure‐limited ventilation in the neonate.
Neonatal care: preventing/treating bronchopulmonary dysplasia
Low‐quality evidence from the Doyle 2014 review suggested no clear differences for cerebral palsy at one to three years (RR 1.06, 95% CI 0.76 to 1.50; 14 trials; 876 children), cerebral palsy at one to three years among survivors assessed (RR 1.05, 95% CI 0.75 to 1.47; 14 trials; 631 children), cerebral palsy at latest age reported (one year up to 17 years) (RR 1.12, 95% CI 0.79 to 1.60; 15 trials; 855 children), or cerebral palsy at latest age reported among survivors assessed (one year up to 17 years) (RR 1.12, 95% CI 0.79 to 1.58; 15 trials; 591 children) following late (more than seven days of age) postnatal corticosteroids versus placebo or no treatment for chronic lung disease in preterm infants (Table 6). Low‐quality evidence from Doyle 2014 also suggested no clear differences for cerebral palsy or death at one to three years (RR 0.92, 95% CI 0.76 to 1.12; 14 trials; 876 children), cerebral palsy or death at latest age reported (one year up to 17 years) (RR 0.95, 95% CI 0.77 to 1.16; 15 trials; 855 children) (Table 8), major neurosensory disability at one year corrected age up to 11 years (RR 1.17, 95% CI 0.85 to 1.60; eight trials; 655 children), major neurosensory disability among survivors assessed at one year corrected age up to 11 years (RR 1.10, 95% CI 0.81 to 1.50; eight trials; 480 children), death or major neurosensory disability at one year corrected age up to 11 years (RR 1.10, 95% CI 0.81 to 1.50; eight trials; 655 children) (Table 10), Bayley Scales of Infant Development Psychomotor Development Index less than minus two standard deviations below the mean at one year corrected age (RR 0.78, 95% CI 0.34 to 1.80; one trial; 118 children), or Bayley Scales of Infant Development Psychomotor Development Index less than minus two standard deviations below the mean among survivors assessed at one year corrected age (RR 0.67, 95% CI 0.30 to 1.50; one trial; 90 children) (Table 11) with late postnatal corticosteroids versus placebo or no treatment.
Low‐quality evidence from the Darlow 2016 review suggested no clear differences for neurodevelopmental impairment at 18 to 24 months following supplemental vitamin A versus a sham injection to prevent mortality and short‐ and long‐term morbidity in very low birthweight infants (RR 0.89, 95% CI 0.74 to 1.08; one trial; 538 children) (Table 10). Moderate‐quality evidence also showed no clear differences for death or neurodevelopmental impairment at 18 to 24 months following supplemental vitamin A versus a sham injection (RR 0.92, 95% CI 0.81 to 1.05; one trial; 687 children) (Table 10).
Neonatal care: preventing necrotising enterocolitis
Low‐quality evidence from the Shah 2007 review suggested no clear differences for cerebral palsy at 36 months' post‐menstrual age following arginine supplementation versus placebo for prevention of necrotising enterocolitis in preterm infants (RR 0.88, 95% CI 0.21 to 3.80; one trial; 135 children) (Table 6). Low‐quality evidence from Shah 2007 also suggested no clear differences for major neurodevelopmental disability at 36 months' post‐menstrual age following arginine supplementation versus placebo (RR 0.65, 95% CI 0.23 to 1.83; one trial; 132 children) (Table 10).
Neonatal care: preventing/treating fungal infection
Low‐quality evidence from the Cleminson 2015 review suggested no clear differences for cerebral palsy at 18 to 22 months post term following use of a systemic antifungal agent versus placebo to prevent mortality and morbidity in very low birthweight infants (RR 0.96, 95% CI 0.45 to 2.03; one trial; 219 children) (Table 6). Low‐quality evidence from Cleminson 2015 also suggested no clear differences for neurodevelopmental impairment (composite) at 18 to 22 months following use of a systemic antifungal agent versus placebo (RR 1.13, 95% CI 0.71 to 1.81; one trial; 171 children) (Table 10).
Neonatal care: parenteral feeding
Moe‐Byrne 2016 assessed glutamine supplementation versus placebo to prevent morbidity and mortality in preterm infants and reported the following: "van den Berg 2005 reported neurodevelopmental outcomes for infants aged two years post term. Outcomes assessed included...incidence of cerebral palsy... No significant differences between the glutamine and the control groups were reported for any of these individual outcomes" (not graded) (Table 6). Low‐quality evidence from the Moe‐Byrne 2016 review also suggested no clear differences for neurodevelopmental impairment at two years post term following glutamine supplementation versus placebo (RR 1.07, 95% CI 0.59 to 1.92; one trial; 72 children) (Table 10).
Neonatal care: other
Low‐quality evidence from both the Osborn 2001 and Osborn 2007 reviews suggested no clear differences for cerebral palsy at 5.7 years following prophylactic thyroid hormones versus placebo for prevention of morbidity and mortality in preterm infants (RR 0.72, 95% CI 0.28 to 1.84; one trial; 156 children) (Table 6). In Osborn 2007, subgroup analyses based on dosing strategy, timing, and methodological quality were not possible for this outcome, with the one included trial using T4 8 mcg/kg/d, on days 1 to 42, commencing within 48 hours, and being of adequate methodological quality (Table 7). Low‐quality evidence from both Osborn 2001 and Osborn 2007 also suggested no clear differences for cerebral palsy or death at 5.7 years following prophylactic thyroid hormones versus placebo (RR 0.70, 95% CI 0.43 to 1.14; one trial; 200 children) (Table 8).
Low‐quality evidence from the Conde‐Agudelo 2016 review suggested no clear differences for cerebral palsy at 12 months' corrected age following kangaroo mother care versus conventional neonatal care to reduce morbidity and mortality among low birthweight infants (RR 0.65, 95% CI 0.21 to 2.02; one trial; 588 children) (Table 6).
Low‐quality evidence from the Spittle 2015 review suggested no clear differences for cerebral palsy at 18 months to six years following early developmental intervention versus standard follow‐up post hospital discharge to prevent motor and cognitive impairment in preterm infants (RR 0.82, 95% CI 0.52 to 1.27; seven trials; 985 children) (Table 6). Subgroup analyses based on commencement of intervention (inpatient, post hospital discharge), focus of intervention (parent‐infant relationship and infant development, infant development), and quality of studies (high‐quality studies, lower‐quality studies) were performed for this outcome; however, formal subgroup interaction tests were not applied in the review (Table 7). Low‐quality evidence from Spittle 2015 also suggested no clear differences for motor outcome at school age (five years) following early developmental intervention versus standard follow‐up (RR 1.12, 95% CI 0.87 to 1.44; two trials; 333 children) (Table 11).
No conclusions possible: very low‐quality evidence
Neonatal care: preventing haemorrhage: periventricular/intraventricular
Very low‐quality evidence from the Smit 2013 review suggested no clear differences for severe neurodevelopmental impairment at 27 months (RR 1.44, 95% CI 0.41 to 5.04; one trial; 101 children) nor for mild neurodevelopmental impairment at 27 months (RR 0.57, 95% CI 0.15 to 2.17; one trial; 101 children) following phenobarbital versus no treatment for prevention of intraventricular haemorrhage in preterm infants (Table 10).
Neonatal care: treating hypotension
Very low‐quality evidence from the Osborn 2007b review suggested no clear differences for cerebral palsy at three years among survivors assessed following dobutamine versus dopamine in preterm infants with low superior vena cava flow (RR 0.16, 95% CI 0.01 to 2.64; one trial; 13 children) (Table 6). Very low‐quality evidence from Osborn 2007b also suggested no clear differences for disability at three years among survivors (RR 0.10, 95% 0.01 to 1.56; one trial; 13 children), for death or disability at three years (RR 0.79, 0.57 to 1.11; one trial; 37 children), or for death or disability at latest follow‐up (one to three years) (RR 0.95, 95% CI 0.66 to 1.38; one trial; 41 children) following dobutamine versus dopamine (Table 10).
Neonatal care: treating patent ductus arteriosus
Very low‐quality evidence from Ohlsson 2015 suggested no clear differences for moderate or severe cerebral palsy at 18 to 24 months following oral ibuprofen versus intravenous ibuprofen for treatment of patent ductus arteriosus in preterm or low birthweight (or both) infants (RR 1.35, 95% CI 0.24 to 7.48; one trial; 57 children) (Table 6).
Neonatal care: preventing blood disorders
Very low‐quality evidence from Ohlsson 2014 suggested no clear differences for cerebral palsy at 18 to 22 months' corrected age among children examined following erythropoietin versus placebo for preventing red blood cell transfusion in preterm and/or low birthweight infants (RR 0.66, 95% CI 0.31 to 1.37; two trials; 153 children) (Table 6). Very low‐quality evidence from Ohlsson 2014 also suggested no clear differences for any neurodevelopmental impairment at 18 to 22 months' corrected age among children examined (RR 0.97, 95% CI 0.62 to 1.51; one trial; 99 children) (Table 10) nor for Psychomotor Developmental Index less than 70 at 18 to 22 months' corrected age among children examined (RR 2.33, 95% CI 0.98 to 5.53; one trial; 90 children) following erythropoietin versus placebo (Table 11).
Neonatal care: preventing/treating respiratory distress syndrome
Very low‐quality evidence from Howlett 2015 suggested no clear differences for major neural developmental impairment at one year corrected age (RR 0.53, 95% CI 0.24 to 1.16; one trial; 169 children) (Table 10) nor for minor neural developmental impairment at one year corrected age (RR 0.84, 95% CI 0.38 to 1.86; one trial; 169 children) following inositol supplementation (repeat doses) versus placebo in preterm infants at risk for or having respiratory distress syndrome (Table 11).
Very low‐quality evidence from Seger 2009 suggested no clear differences for cerebral palsy at one and two years' corrected age following animal‐derived surfactant extract versus no treatment for respiratory distress syndrome (RR 0.88, 95% CI 0.34 to 2.27; one trial; 73 children) (Table 6). Subgroup analysis based on surfactant product for this outcome was not applicable, with the one included trial using porcine surfactant extract (Table 7). Very low‐quality evidence from Seger 2009 also suggested no clear differences for major developmental disability among survivors at one and two years' corrected age following animal‐derived surfactant extract versus no treatment (RR 3.30, 95% 0.14 to 26.78; one trial; 73 children) (Table 10).
Neonatal care: mechanical ventilation
Very low‐quality evidence from the Ho 2015 review suggested no clear differences for cerebral palsy at nine to 15 years following continuous distending pressure versus standard care for respiratory distress in preterm infants (RR 5.0, 95% CI 0.26 to 97.37; one trial; 36 children) (Table 6). Subgroup analysis based on type of continuous distending pressure was not possible for this outcome, with the only included trial using continuous negative pressure (Table 7). Very low‐quality evidence from Ho 2015 also suggested no clear differences for death or severe disability at nine to 15 years (RR 1.33, 95% CI 0.34, 5.17; one trial; 38 children), for severe disability at nine to 15 years (RR 1.06, 95% CI 0.24 to 4.57; one trial; 37 children), or for any disability at nine to 15 years (RR 0.62, 95% CI 0.31 to 1.21; one trial; 37 children) following continuous distending pressure versus standard care (Table 10).
Very low‐quality evidence from the Kamlin 2003 review suggested no clear differences for cerebral palsy among survivors at less than 33 weeks' gestation, at birth, and at 18 months following long versus short inspiratory times among neonates receiving mechanical ventilation (RR 2.9, 95% CI 0.97 to 8.65; one trial; 177 children) (Table 6).
Neonatal care: preventing bronchopulmonary dysplasia
Very low‐quality evidence from the Halliday 2003 review suggested no clear differences for cerebral palsy at 12 months' corrected age up to 90 months (RR 1.03, 95% CI 0.47 to 2.24; four trials; 204 children) nor for cerebral palsy among survivors assessed at 12 months' corrected age up to 90 months (RR 0.83, 95% CI 0.39 to 1.74; four trials; 130 children) following moderately early (between seven and 14 days of age) postnatal corticosteroids versus placebo or no treatment for preventing chronic lung disease in preterm infants (Table 6). Very low‐quality evidence from Halliday 2003 also suggested no clear differences for cerebral palsy or death at 12 months' corrected age up to 90 months (RR 0.83, 95% CI 0.55 to 1.23; four trials; 204 children) (Table 8), for major neurosensory disability at 15 months' corrected age up to 90 months (RR 1.26, 95% CI 0.45 to 3.49; two trials; 96 children), for major neurosensory disability among survivors assessed at 15 months' corrected age up to 90 months (RR 0.89, 95% CI 0.38 to 2.10; two trials; 56 children), or for death or major neurosensory disability at 15 months' corrected age up to 90 months (RR 1.02, 95% CI 0.66 to 1.56; two trials; 96 children) with moderately early postnatal corticosteroids versus placebo or no treatment (Table 10).
Very low‐quality evidence from the Shah 2012 review suggested no clear differences in cerebral palsy at three years with early inhaled corticosteroids versus placebo for preventing chronic lung disease among ventilated very low birthweight preterm neonates (RR 1.33, 95% CI 0.33 to 5.42; one trial; 56 children) (Table 6). Very low‐quality evidence from Shah 2012 also suggested no clear differences for mean developmental index less than two standard deviations of the mean on the Bayley Scales of Infant Development with early inhaled corticosteroids versus placebo (RR 1.25, 95% CI 0.37 to 4.17; one trial; 56 children) (Table 11).
Neonatal care: preventing necrotising enterocolitis
Very low‐quality evidence from the AlFaleh 2014 review suggested no clear differences for mental retardation and cerebral palsy at six years following probiotics versus control (distilled water) for prevention of necrotising enterocolitis in preterm infants (RR 1.02, 95% CI 0.15 to 6.94; one trial; 85 children) (Table 10).
Neonatal care: other
Very low‐quality evidence from the Almadhoob 2015 review suggested no clear differences for cerebral palsy at 18 to 22 months' corrected age with use of silicone earplugs versus no earplugs in the neonatal intensive care unit for preterm or very low birthweight infants (RR 3.0, 95% CI 0.14 to 63.15; one trial; 14 children) (Table 6).
No conclusions possible: not graded
Neonatal care: mechanical ventilation
The Cools 2015 review assessed elective high‐frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cools 2015 did not perform a meta‐analysis for the outcome cerebral palsy, with age and methods of assessment varying between studies, and rather reported the results for three separate trials in text, as below (not graded) (Table 6).
-
"Neurodevelopmental status was assessed at 16 to 24 months' corrected age in 77% of survivors of the HIFI 1989 study (185 HFOV and 201 CV) using Bayley psychometric tests and central nervous system examinations... The rate of cerebral palsy was 11% in both groups".
-
"Moriette 2001 assessed neuromotor outcome at the corrected age of two years in 192 of 212 survivors (90%) using a physician questionnaire...the risk of spastic cerebral palsy was significantly lower for infants ventilated with HFOV (4% versus 17%; OR 0.87, 95% CI 0.79 to 0.96), even after adjustment for multiple factors. Survival without cerebral palsy was significantly more likely in the HFOV group than in the CV group (OR 1.89, 95% CI 1.04 to 3.44)".
-
"Sun 2014 assessed neurodevelopmental outcomes at 18 months' corrected age in 145 infants of the HFOV group (84% of survivors) and in 143 infants of the CV group (86% of survivors). Cerebral palsy occurred significantly less in the HFOV group (3% versus 10% in the CV group, P = 0.03)".
Interventions for other specific groups of 'at risk' neonates
No conclusions possible: low‐quality evidence
Neonatal care: treating pulmonary hypertension
Low‐quality evidence from the More 2016 review suggested no clear differences for cerebral palsy at six months following use of endothelin receptor antagonists versus placebo for persistent pulmonary hypertension in term and late preterm infants (RR 0.09, 95% CI 0.00 to 1.61; one trial; 37 children) (Table 6). Low‐quality evidence from More 2016 also suggested no clear differences for adverse neurological outcomes at six months with use of endothelin receptor antagonists versus placebo (RR 0.07, 95% CI 0.00,1.20; one trial; 37 children) (Table 11).
Neonatal care: nitric oxide
Low‐quality evidence from the Finer 2006 review suggested no clear differences for cerebral palsy among survivors at 13 or 18 to 24 months following inhaled nitric oxide versus placebo for respiratory failure in infants born at or near term (RR 1.02, 95% CI 0.49 to 2.14; two trials; 299 children) (Table 6). Finer 2006 also reported on an additional trial not included in the meta‐analysis for this outcome: "This group [Wessel 1996] has now published follow up data, including neurodevelopmental outcomes, which were obtained by telephone interview of 60 of the 83 survivors of the original trial. The interview was conducted between one and four years of age... Although cerebral palsy [was] reported it is unclear how [it] was defined... It is not, therefore, possible to add any of these data to the meta‐analysis, but they do appear to show no evidence of neurodevelopmental impairment due to inhaled nitric oxide therapy" (not graded). Low‐quality evidence from Finer 2006 also suggested no clear differences for neurodevelopmental disability among survivors at 13 or 18 to 24 months (RR 0.97, 95% CI 0.66 to 1.44; two trials; 301 children) (Table 10) nor for Bayley Psychomotor Developmental Index more than two standard deviations below the mean at 13 or 18 to 24 months (RR 1.09, 95% CI 0.58 to 2.03; two trials; 283 children) (Table 11) following inhaled nitric oxide versus placebo.
No conclusions possible: very low‐quality evidence
Neonatal care: resuscitation
Very low‐quality evidence from the Tan 2005 review suggested no clear differences for cerebral palsy among those followed up at 18 to 24 months following room air versus 100% oxygen for resuscitation of infants at birth (RR 1.34, 95% CI 0.55 to 3.24; one trial; 213 children) (Table 6). Very low‐quality evidence from Tan 2005 also suggested no clear differences in not walking among those followed up at 18 to 24 months following room air versus 100% oxygen (RR 1.03, 95% CI 0.04 to 2.25; one trial; 213 children) (Table 11).
Neonatal care: nitric oxide
Very low‐quality evidence from the Finer 2006 review suggested no clear differences for cerebral palsy among survivors at 18 to 24 months following inhaled nitric oxide versus placebo for respiratory failure among infants with diaphragmatic hernias born at or near term (RR 8.33, 95% CI 0.45 to 154.78; one trial; 22 children) (Table 6).
Neonatal care: treating herpes simplex
Very low‐quality evidence from the Jones 2009 review suggested no clear differences in cerebral palsy in central nervous system herpes simplex virus (HSV) neonatal infection up to three years by HSV serotype: HSV‐1 (no events, one trial, nine children) and HSV‐2 (RR 1.07, 95% CI 0.49 to 2.33; one trial; 14 children) following acyclovir versus vidarabine for treatment of HSV infection in neonates (Table 6). Very low‐quality evidence from Jones 2009 also suggested no clear differences for abnormal neurodevelopment at approximately one year of age (RR 1.50, 95% 0.62 to 3.65; one trial; 56 children) nor for abnormal neurodevelopment or death at approximately one year of age (RR 0.86, 95% CI 0.60 to 1.22; one trial; 56 children) following vidarabine versus placebo; and abnormal neurodevelopment at approximately one year of age (RR 0.82, 95% 0.50 to 1.34; one trial; 202 children) or abnormal neurodevelopment or death at approximately one year of age (RR 0.79, 95% CI 0.57 to 1.10; one trial; 202 children) following acyclovir versus vidarabine (Table 10).
Neonatal care: treating hypoglycaemia
Very low‐quality evidence from the Weston 2016 review suggested no clear differences in cerebral palsy at age two years following dextrose gel versus placebo for treatment of hypoglycaemia in newborn infants (RR 5.16, 95% CI 0.25 to 106.12; one trial; 183 children) (Table 6). Very low‐quality evidence from Weston 2016 also suggested no clear differences in major neurosensory disability at two years (RR 6.27, 95% CI 0.77 to 51.03; one trial; 184 children) nor in any developmental disability at two years (RR 1.11, 95% CI 0.75 to 1.63; one trial; 184 children) following dextrose gel versus placebo (Table 10).
Discussion
Summary of main results
This review included 43 Cochrane Reviews with outcome data for cerebral palsy available from meta‐analyses of data from 96 randomised controlled trials (RCTs) involving 15,885 children.
Interventions for neonates with perinatal asphyxia or with evidence of neonatal encephalopathy
-
Effective interventions (high‐quality evidence of effectiveness): High‐quality evidence showed a reduction in cerebral palsy following therapeutic hypothermia versus standard care for newborns with hypoxic ischaemic encephalopathy.
-
No conclusions possible: very low‐quality evidence: Very low‐quality evidence suggested no clear differences in cerebral palsy following barbiturates (phenobarbital) versus conventional therapy for prevention of morbidity and mortality following perinatal asphyxia.
Interventions for neonates born preterm and/or at low or very low birthweight
-
Possibly effective interventions (moderate‐quality evidence of effectiveness): Moderate‐quality evidence showed a reduction in cerebral palsy with prophylactic methylxanthines (caffeine) versus placebo for endotracheal extubation in preterm infants.
-
Probably ineffective interventions (moderate‐quality evidence of harm): Moderate‐quality evidence showed an increase in cerebral palsy and cerebral palsy among survivors assessed following early (less than eight days) postnatal corticosteroids versus control for preventing chronic lung disease in preterm infants.
-
Probably ineffective interventions (moderate‐quality evidence of lack of effectiveness): Moderate‐quality evidence showed no clear differences in cerebral palsy following ethamsylate versus placebo for prevention of morbidity and mortality in preterm or very low birthweight infants; volume versus no treatment and gelatin versus fresh frozen plasma for prevention of morbidity and mortality in very preterm infants; prophylactic indomethacin versus placebo or no drug for preventing mortality and morbidity in preterm infants; synthetic surfactant versus placebo for respiratory distress syndrome in preterm infants; or prophylactic phototherapy versus standard care (starting phototherapy when serum bilirubin reached a pre‐specified level) for preventing jaundice in preterm or low birthweight infants.
-
No conclusions possible (low‐ to very low‐quality evidence): Low‐ to very low‐quality evidence suggested no clear differences for cerebral palsy following dobutamine versus dopamine in preterm infants with low superior vena cava flow; oral ibuprofen versus intravenous ibuprofen for treatment of patent ductus arteriosus in preterm or low birthweight (or both) infants; darbepoetin alfa versus placebo and erythropoietin versus placebo for preventing red blood cell transfusion in preterm and/or low birthweight infants; transfusion at a restrictive (low haemoglobin) versus a liberal (high haemoglobin) threshold for preventing morbidity and mortality in very low birthweight infants; inhaled nitric oxide versus placebo or no treatment for respiratory failure in preterm infants; caffeine versus placebo for treatment of apnoea in preterm infants; caffeine versus placebo for prevention of apnoea in preterm infants; animal‐derived surfactant extract versus no treatment for treatment of respiratory distress syndrome; prophylactic protein‐free synthetic surfactant versus placebo for preventing morbidity and mortality in preterm infants; continuous distending pressure versus standard care for respiratory distress in preterm infants; long versus short inspiratory times in neonates receiving mechanical ventilation; moderately early (between seven and 14 days of age) postnatal corticosteroids versus placebo or no treatment for preventing chronic lung disease in preterm infants; late (more than seven days of age) postnatal corticosteroids versus placebo or no treatment for chronic lung disease in preterm infants; early inhaled corticosteroids versus placebo for preventing chronic lung disease in ventilated very low birthweight preterm neonates; arginine supplementation versus placebo for prevention of necrotising enterocolitis in preterm infants; systemic antifungal agents versus placebo for prevention of mortality and morbidity in very low birthweight infants; prophylactic thyroid hormones versus placebo for prevention of morbidity and mortality in preterm infants; use of silicone earplugs versus no earplugs in the neonatal intensive care unit for preterm or very low birthweight infants; kangaroo mother care versus conventional neonatal care to reduce morbidity and mortality in low birthweight infants; and early developmental intervention versus standard follow‐up post hospital discharge to prevent motor and cognitive impairment in preterm infants.
Interventions for other specific groups of 'at risk' neonates
-
No conclusions possible (low‐ to very low‐quality evidence): Low‐ to very low‐quality evidence suggested no clear differences for cerebral palsy following endothelin receptor antagonists versus placebo for persistent pulmonary hypertension in term and late preterm infants; inhaled nitric oxide versus placebo for respiratory failure in infants born at or near term; room air versus 100% oxygen for resuscitation of infants at birth; acyclovir versus vidarabine for treatment of HSV infection in neonates; and dextrose gel versus placebo for treatment of hypoglycaemia in newborn infants.
Overall completeness and applicability of evidence
This overview summarises published Cochrane Systematic Reviews assessing neonatal interventions reporting on cerebral palsy and does not consider interventions in the antenatal or intrapartum period, which is the focus of a companion overview (Shepherd 2016).
We were able to include only 43 reviews (representing less than 13% of the 343 Neonatal reviews in the Cochrane Database of Systematic Reviews). We identified an additional 40 protocols that have pre‐specified cerebral palsy as a primary or secondary outcome and will be considered for inclusion in future updates of the overview when they have been published as full reviews. These protocols plan to assess a variety of interventions (see Appendix 1: 'Ongoing reviews'). We were not able to include an additional 102 reviews assessing a wide range of neonatal interventions, although we recognised the potential impact of the intervention of interest on cerebral palsy (through pre‐specifying cerebral palsy as a review outcome); none of the included trials within these reviews reported on this outcome. We summarised the main conclusions of these reviews in Appendix 2 ('Reviews awaiting further classification') and will again consider them for inclusion in future updates of the overview. In total, the 43 reviews included 454 RCTs involving infants.
Although the 43 reviews in this overview included 454 randomised trials involving over 63,977 infants, the body of evidence for our review was substantially reduced by the fact that the included reviews (and trials) did not report on our overview outcomes. For our primary outcome ‐ cerebral palsy ‐ we included data from meta‐analyses of 35 reviews involving 96 randomised trials, or only 21% of the trials within the included reviews.
The body of evidence for our secondary outcomes was further reduced for the composite outcome including cerebral palsy (30 reviews), motor dysfunction (12 reviews), cerebral palsy or death (five reviews), and severity of cerebral palsy (three reviews). None of our included reviews reported specifically on type of cerebral palsy. For most of our outcomes, reviews reported data from only one or two trials, up to a maximum of 15 trials. Thus, review authors often presented too few data to permit firm conclusions on effects on cerebral palsy and on our secondary outcomes. For most of the included reviews, data related to cerebral palsy were commonly short term (reported at one to three years of age), and longer‐term follow‐up was less commonly reported (although follow‐up to 17 years was reported). Included reviews often did not report information regarding definitions nor criteria for cerebral palsy diagnosis and assessment methods.
We did not attempt to make indirect comparisons to address questions concerning the relative performance of different neonatal interventions. Rather we aimed to systematically consider all potentially relevant interventions for their ability to contribute to prevention of cerebral palsy. Within this overview, we did not attempt to duplicate details of participants and interventions (and control conditions) in individual trials. The reader may refer to these individual reviews and trials for more information on these factors. Further, the scope of this overview was limited to effects of interventions on cerebral palsy (and a restricted number of pre‐specified secondary review outcomes). To assess effects (benefits or harms) of the included interventions on other outcomes, readers are encouraged to refer to the included Cochrane Reviews themselves. For example, although low‐quality evidence presented in this overview suggested no clear differences in cerebral palsy following kangaroo mother care, the Conde‐Agudelo 2016 review reported moderate‐quality evidence of benefit for outcomes including mortality, severe infection/sepsis, hypothermia, weight gain, and breastfeeding, and thus supports the use of kangaroo mother care for low birthweight infants as an alternative to conventional neonatal care (mainly in resource‐limited settings). Similarly, although very low‐quality evidence in this overview suggested no clear differences in cerebral palsy following dextrose gel for treatment of hypoglycaemia, the Weston 2016 review found moderate‐quality evidence of benefit for outcomes including mother‐infant separation and breastfeeding, and thus concluded that oral dextrose therapy should be considered first‐line treatment for neonates with hypoglycaemia.
Although our overview could demonstrate high‐quality evidence of a reduction in cerebral palsy following therapeutic hypothermia for newborns with hypoxic‐ischaemic encephalopathy (Jacobs 2013), the incidence of death and disability, including cerebral palsy, remains high despite therapy. Thus, optimisation of hypothermia strategies or adjuvant therapies is urgently needed to further improve outcomes. A range of possible agents such as antiepileptic drugs (including topiramate), xenon, erythropoietin, melatonin, magnesium sulphate, and cord blood continue to be under investigation (AAP 2014; Robertson 2012).
Quality of the evidence
We assessed almost all of the included reviews to be of high quality and to have low risk of bias using the AMSTAR and ROBIS tools (see Table 4: AMSTAR assessments for included reviews; and Table 5: ROBIS assessments for included reviews). Although these two tools differ in their approaches to assessing review quality or risk of bias, findings of these assessments were similar. All of the included reviews assessed risk of bias of included randomised trials (most used current guidance as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)), and the quality of randomised trials was variable within and between reviews (see Table 3: Risk of bias assessments from included reviews). Six of the 43 reviews used the GRADE approach to assess the quality of evidence for overview outcomes (Darlow 2016; Moe‐Byrne 2016; More 2016; Okwundu 2012; Weston 2016; Young 2016). For the other reviews, we used the GRADE system to rate the quality of evidence and incorporated assessments of study limitations (risk of bias) as reported by the review authors. For our primary review outcome ‐ cerebral palsy ‐ evidence ranged from very low to high quality, and for our secondary review outcomes, quality of evidence varied similarly. Downgrading of quality was most commonly due to study limitations (risk of bias) and imprecision (small sample sizes, low numbers of events, and wide confidence intervals). Findings regarding the quality of evidence for each outcome are set out in Table 6: Cerebral palsy; Table 8: Cerebral palsy or death; Table 9: Severity of cerebral palsy; Table 10: Other composite outcomes that include cerebral palsy as a component; and Table 11: Motor dysfunction.
Potential biases in the overview process
We were aware of risks of introducing bias at all stages of the overview process, and we took several steps to minimise this, including developing a Cochrane overview protocol. At least two overview authors independently assessed reviews for inclusion, carried out data extraction and quality assessment, and assessed the quality of evidence using the GRADE approach. A potential source of bias is related to the fact that one overview author (Nadia Badawi) is an author of one of the included reviews (Jones 2009). As pre‐specified in our protocol, two other overview authors, who were not authors of this review, carried out data extraction and quality assessment for this review.
We undertook a comprehensive search of the Cochrane Database of Systematic Reviews without applying language or date restrictions, and we identified published reviews, as well as planned/ongoing reviews (protocols). We did not search other databases; thus it is possible that non‐Cochrane systematic reviews assessing neonatal interventions and reporting on cerebral palsy have been conducted but were not identified. It is also the case that Cochrane Reviews assessing interventions that could have the potential to impact cerebral palsy risk (see Description of the interventions for further discussion of various interventions) may not have acknowledged this through inclusion of cerebral palsy as a review outcome. Thus, data from relevant randomised trials assessing these interventions will not have been identified and included in this overview. Based on our search strategy, even Cochrane Reviews that pre‐specified outcomes such as 'long‐term growth and neurodevelopment' (Cools 2015) but subsequently reported specifically on 'cerebral palsy' were captured in our search and were included in this overview. However, reviews that have reported on long‐term neurodevelopmental outcomes without any mention of 'cerebral palsy' will not have been identified; this highlights the need for all Cochrane Reviews to provide clear definitions accompanying any reported outcome measures.
Although we judged almost all of our included reviews to be of high quality and to have low risk of bias, we did not consider all as 'up‐to‐date', with only approximately one‐third conducting searches in the past three years; similarly, not all of the 'Reviews awaiting further classification' were 'up‐to‐date'. Thus, it is possible that additional trials assessing neonatal interventions and reporting on cerebral palsy have been published but have not yet been included in relevant Cochrane Reviews; it is also possible that additional trials have been conducted but have not yet been published. If/when such trials are included in relevant Cochrane Reviews, we will incorporate them into an update of this overview.
Agreements and disagreements with other studies or reviews
We have not identified any other overviews or systematic reviews specifically designed to assess neonatal interventions for preventing cerebral palsy.
McIntyre 2013 conducted a systematic review of cohort and case‐control studies that focused on identifying risk factors for cerebral palsy in children born at term and aimed to assess whether the potential for prevention of these risk factors has been adequately explored. Intrapartum and neonatal risk factors identified included birth asphyxia, neonatal seizures, respiratory distress syndrome, hypoglycaemia, jaundice, and infections including meningitis and sepsis. It is recognised that a strategy for prevention of cerebral palsy currently exists for only one of these risk factors ‐ hypothermia for birth asphyxia ‐ as was identified in this overview. McIntyre 2013 highlighted that prevention strategies are urgently required.
A further recent systematic review ‐ Hadders‐Algra 2016 ‐ focused on early interventions in infants younger than 12 months' corrected age with or at very high risk for cerebral palsy (such as on the basis of a lesion of the brain ‐ periventricular leucomalacia or intraventricular haemorrhage, or definitely abnormal general movements). This review included seven studies of moderate to high quality assessing interventions such as neurodevelopmental treatment only, multi‐sensory stimulation, developmental stimulation, and multi‐faceted interventions combining developmental stimulation, support of parent‐infant interaction, and neurodevelopmental treatment (Hadders‐Algra 2016). Hadders‐Algra 2016 concluded that although two suggestions emerged (dosing may be critical for effectiveness; multi‐faceted interventions may offer the best opportunities), current evidence is limited.
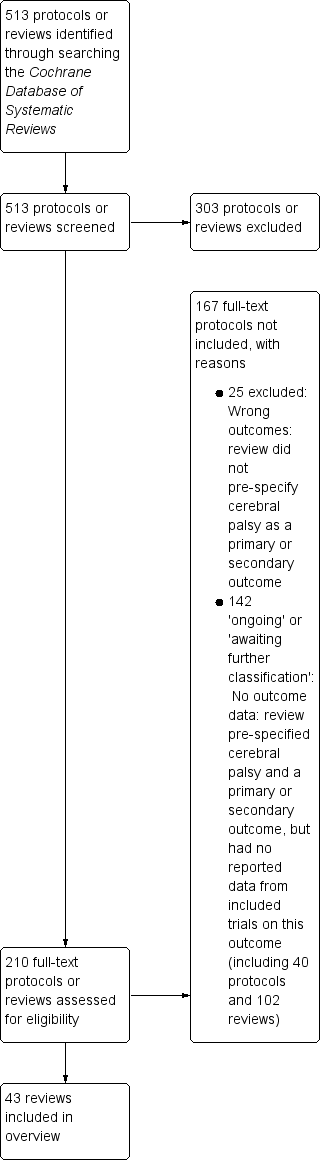
Study flow diagram.
| Review ID and title | Reason for exclusion |
| Email for clinical communication between patients/caregivers and healthcare professionals | Wrong participants (not neonates):
|
| Parent‐infant psychotherapy for improving parental and infant mental health | Wrong participants (not neonates):
|
| Body positioning for spontaneously breathing preterm infants with apnoea | Secondary outcomes pre‐specified include the following:
No outcome data for these outcomes |
| C‐reactive protein for diagnosing late‐onset infection in newborn infants | Protocol for diagnostic test accuracy review |
| G‐CSF and GM‐CSF for treating or preventing neonatal infections | Secondary outcomes pre‐specified include:
No outcome data for cerebral palsy (single study results reported "cognition, language and social developmental performance scores were within the normal range for age and motor deficits were 'typical of high‐risk, low birth weight neonates'. However there was no comparison made between G‐CSF and control infants" |
| Intravenous dexamethasone for extubation of newborn infants | No pre‐specified outcome focused on development/disability at follow‐up |
| High‐frequency jet ventilation vs high‐frequency oscillatory ventilation for pulmonary dysfunction in preterm infants | Secondary neonatal outcomes pre‐specified include:
No outcome data for this outcome (no included trials) |
| Treatment of infantile spasms | Outcomes pre‐specified include:
No outcome data for cerebral palsy (single‐study results reported related to BSID; VABS; 'cognitive development'; Japanese Tumor Scale; DDST) |
| Antiviral therapy for symptomatic congenital cytomegalovirus infection in neonates and infants up to 3 months of age | Protocol Primary outcomes pre‐specified include:
|
| Lay health workers in primary and community health care for maternal and child health and management of infectious diseases | No pre‐specified outcome focused on development/disability at follow‐up |
| Surgical vs medical treatment with cyclo‐oxygenase inhibitors for symptomatic patent ductus arteriosus in preterm infants | Secondary outcomes pre‐specified include:
No outcome data for this outcome |
| Cycled light in the intensive care unit for preterm and low birthweight infants | Secondary outcomes pre‐specified include:
No outcome data for these outcomes |
| Transcutaneous screening for hyperbilirubinaemia in neonates | Protocol No pre‐specified outcome focused on development/disability at follow‐up |
| Granulocyte transfusions for neonates with confirmed or suspected sepsis and neutropaenia | Primary outcomes pre‐specified include:
No outcome data for this outcome |
| Molecular assays for diagnosis of sepsis in neonates | Protocol for diagnostic test accuracy review |
| Pentoxifylline for treatment of sepsis and necrotising enterocolitis in neonates | Secondary outcomes pre‐specified include:
No outcome data for this outcome |
| Hypothermia for neuroprotection in children after cardiopulmonary arrest | Primary outcomes pre‐specified include:
No outcome data for these outcomes (no included trials) |
| Intraventricular antibiotics for bacterial meningitis in neonates | Secondary outcomes pre‐specified include:
No outcome data for this outcome |
| Metalloporphyrins for treatment of unconjugated hyperbilirubinaemia in neonates | Outcomes pre‐specified include:
No outcome data for these outcomes |
| Periodic change of body position under phototherapy in term and late preterm neonates with hyperbilirubinaemia | Protocol Secondary outcomes pre‐specified include:
|
| Short‐duration vs standard‐duration antibiotic regimens for treatment of neonatal bacterial infection | Protocol Secondary outcomes pre‐specified include:
|
| Steroid therapy for meconium aspiration syndrome in newborn infants | Primary outcomes pre‐specified include:
No outcome data for this outcome |
| Diuretic therapy for newborn infants with post‐haemorrhagic ventricular dilatation | Outcomes pre‐specified include:
Data reported for these outcomes; no outcome data for cerebral palsy. "The larger trial showed that acetazolamide and furosemide treatment resulted in a borderline increase in the risk for motor impairment at one year (RR 1.27, 95% CI 1.02 ‐ 1.58; RD 0.16, 95% CI 0.02 ‐ 0.31), but did not significantly affect the risk for the combined outcome of delay, disability or motor impairment among survivors, or the risk of the combined outcome of death, delay, disability or impairment at one year" |
| Repeated lumbar or ventricular punctures in newborns with intraventricular haemorrhage | Outcomes pre‐specified include:
Data reported for these outcomes; no outcome data for cerebral palsy. "The tables and figures show that none of the trials found a significant effect of CSF tapping on a) need for shunt b) death c) major disability in survivors d) multiple disability in survivors e) death or disability. Similarly, meta‐analysis of the results of all included trials shows no significant effect of CSF tapping on any of these outcomes" |
| Permissive hypercapnia for prevention of morbidity and mortality in mechanically ventilated newborn infants | Outcomes pre‐specified include:
No outcome data for this outcome |
| Abbreviations: BIND: bilirubin‐induced neurological dysfunction; BSID: Bayley Scales of Infant Development; CI: confidence interval; CSF: cerebrospinal fluid; DDST: Denver Developmental Screening Test; G‐CSF: granulocyte‐colony stimulating factor; GM‐CSF: granulocyte‐macrophage colony‐stimulating factor; RD: risk difference; RR: risk ratio; VABS: Vineland Adaptive Behavior Scales. | |
| Review ID and title | Date of search and date assessed as up‐to‐date | No. included trials (countries and publication years) | No. participants in included trials | Inclusion criteria for 'Types of participants' | Relevant comparison interventions (no. trials and participants) | Overview outcomes for which data were reported (no. trials and participants) |
| Neonatal care: asphyxia | ||||||
| Allopurinol for preventing mortality and morbidity in newborn infants with hypoxic‐ischaemic encephalopathy | Searches: March 2012 Up‐to‐date: | 3 RCTs (Countries: Netherlands, Turkey; Published: 1990s: 1 RCT; 2000s: 2 RCTs) | 114 infants | Newborn infants (> 34 weeks' gestation) with hypoxic‐ischaemic encephalopathy defined as clinical evidence of cardiorespiratory or neurological depression (Apgar score < 7 at 5 minutes and beyond after birth) and/or evidence of severe metabolic acidosis in intrapartum foetal, umbilical arterial cord, or very early neonatal blood samples (pH < 7 or base deficit > 12 mmol/L), and/or clinical or electro‐encephalographic (multi‐channel or amplitude integrated) evidence of neonatal encephalopathy (MacLennan 1999) | Allopurinol vs control (3 RCTs, 114 neonates) | Severity of cerebral palsy ("Severe quadriplegia in surviving infants" (3 RCTs, 73 children); reported as a primary outcome) Other composite outcome that includes cerebral palsy as a component ("Death or severe neurodevelopmental disability in survivors" (3 RCTs, 110 children); reported as a primary outcome) |
| Cooling for newborns with hypoxic‐ischaemic encephalopathy | 1 May 2012 | 11 RCTs (Countries: China: 2 RCTs; New Zealand: 1 RCT; Turkey: 1 RCT; USA; 3 RCTs; international: 4 RCTs Published: 1990s: 1 RCT; 2000s: 7 RCTs; 2010s: 3 RCTs) | 1505 infants | 1. Newborn infants of 35 weeks' gestation or greater 2. Evidence of peripartum asphyxia, with each enrolled infant satisfying at least 1 of the following criteria: a. Apgar score of 5 or less at 10 minutes b. Mechanical ventilation or resuscitation at 10 minutes c. Cord pH < 7.1, or arterial pH < 7.1, or base deficit of 12 or more within 60 minutes of birth 3. Evidence of encephalopathy according to Sarnat staging (Finer 1981; Sarnat 1976): a. Stage 1 (mild): hyperalertness, hyper‐reflexia, dilated pupils, tachycardia, absence of seizures b. Stage 2 (moderate): lethargy, hyper‐reflexia, miosis, bradycardia, seizures, hypotonia with weak suck and Moro c. Stage 3 (severe): stupor, flaccidity, small to mid position pupils that react poorly to light, decreased stretch reflexes, hypothermia, and absent Moro No major congenital abnormalities recognisable at birth | Therapeutic hypothermia vs standard care (11 RCTs, 1505 neonates) | Cerebral palsy ("Cerebral palsy in survivors assessed" (7 RCTs, 881 children) and "Outcome at 6 to 7 years of age: Cerebral palsy" (1 RCT, 121 children); reported as secondary outcomes) Other composite outcomes that include cerebral palsy as a component ("Death or major disability in survivors assessed" (8 RCTs, 1344 children); reported as a primary outcome) ("Major neurodevelopmental disability" (8 RCTs, 1344 children); "Major neurodevelopmental disability in survivors assessed" (8 RCTs, 917 children); "Outcome at 6 to 7 years of age: death or moderate‐to‐severe disability" (1 RCT, 190 children); "Outcome at 6 to 7 years of age: moderate‐to‐severe disability" (1 RCT, 119 children); reported as secondary outcomes) Motor dysfunction ("Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed" (6 RCTs, 657 children); reported as a secondary outcome) |
| Prophylactic barbiturate use for the prevention of morbidity and mortality following perinatal asphyxia | 30 November 2015 | 9 RCTs (Countries: Finland: 1 RCT; India: 2 RCTs; Mexico: 1 RCT; Romania: 1 RCT; South Africa: 1 RCT; Spain: 1 RCT; USA: 2 RCTs; Published: 1980s: 2 RCTs; 1990s: 2 RCTs; 2000s: 2 RCTs; 2010s: 3 RCTs) | 456 infants |
| Barbiturates vs control (8 RCTs, 439 neonates) | Cerebral palsy ("Cerebral palsy" (2 RCTs, 69 children); reported as a secondary outcome) Other composite outcomes that include cerebral palsy as a component ("Death or major neurodevelopmental disability" (1 RCT, 31 children); reported as a primary outcome) ("Major neurodevelopmental disability" (1 RCT, 31 children); reported as a secondary outcome) |
| Neonatal care: haemorrhage: periventricular/intraventricular | ||||||
| Ethamsylate for the prevention of morbidity and mortality in preterm or very low birth weight infants | Search: 24 August 2009 Up‐to‐date: 22 September 2009 | 7 RCTs (Countries: France, Greece, UK: 1 RCT; India: 1 RCT; Switzerland: 1 RCT; Taiwan: 1 RCT; Turkey: 1 RCT; UK: 2 RCTs; Published: 1980s: 3 RCTs; 1990s: 4 RCTs) | 1410 infants | Preterm infants born before and including 34 weeks plus 6 days' completed gestation or with birthweight < 2000 g | Ethamsylate vs placebo (7 RCTs, 1410 neonates) | Cerebral palsy ("Cerebral palsy in surviving children available for follow‐up" (3 RCTs, 532 children); reported as a primary outcome) Other composite outcomes that include cerebral palsy as a component ("Neurodevelopmental disability at 2 years of age in surviving children available for follow‐up" (3 RCTs, 532 children); "Death or any disability by 2 years of age in children with known outcome at any point in time" (7 RCTs, 1334 children); reported as primary outcomes) |
| Postnatal phenobarbital for the prevention of intraventricular haemorrhage in preterm infants | Search: 31 October 2012 Up‐to‐date: 17 December 2012 | 12 RCTs (Countries: not reported; Published: 1980s: 8 RCTs; 1990s: 1 RCT: 2000s: 3 RCTs) | 982 infants | Newborn infants (less than 24 hours old) with gestational age < 34 weeks or birthweight < 1500 g. We included preterm infants with gestational age 33 to 36 weeks or birthweight up to 1750 g, if they were mechanically ventilated. We excluded infants with serious congenital malformations | Phenobarbital vs control (12 RCTs, 982 neonates) | Other composite outcomes that include cerebral palsy as a component ("Mild neurodevelopmental impairment" (1 RCT, 101 children); "Severe neurodevelopmental impairment" (1 RCT, 101 children); reported as secondary outcomes) |
| Neonatal care: hypotension | ||||||
| The effect of inotropes on morbidity and mortality in preterm infants with low systemic or organ blood flow | 19 May 2010 | 1 RCT (Country: not reported; Published: 2000s) | 42 infants | Preterm infants (< 37 weeks' gestational age) with low SBF or organ blood flow in the neonatal period. Low SBF may be determined on the basis of echocardiographically measured ventricular outputs or surrogates for SBF such as SVC flow. Low organ blood flow may be determined on the basis of techniques including ultrasound Doppler, near infrared spectroscopy, or xenon clearance techniques when evidence in the literature suggests that measurement is associated with substantial clinical outcomes and/or actual organ blood flow. The review does not include studies that include surrogates of flow such as BP, ultrasound Doppler‐measured velocities, pulsatility, or resistive indices | Dobutamine vs dopamine in preterm infants with low superior vena cava flow (1 RCT, 42 neonates) | Cerebral palsy ("Cerebral palsy at 3 years in survivors assessed" (1 RCT, 13 children); reported as a primary outcome) Other composite outcomes that include cerebral palsy as a component ("Disability at 3 years in survivors assessed" (1 RCT, 13 children); "Death or disability at 3 years" (1 RCT, 37 children); "Death or disability at latest follow‐up" (1 RCT, 41 children); reported as primary outcomes) |
| Neonatal care: fluid therapy | ||||||
| Early volume expansion for prevention of morbidity and mortality in very preterm infants | 30 July 2008 | 8 RCTs (Countries: not reported; Published: 1970s: 1 RCT; 1980s: 1 RCT; 1990s: 4 RCTs; 2000s: 2 RCTs) | 1185 infants | Very preterm infants born ≦ 32 weeks' gestation or ≦ 1500 g and enrolled and treated the first 72 hours after birth. Trials were eligible if they enrolled unselected preterm infants, preterm infants with clinically suspected poor perfusion (e.g. low BP, poor cutaneous perfusion, metabolic acidosis), or preterm infants with low blood flow (e.g. determined by Doppler ultrasound). Low BP may be defined as BP less than a specified percentile of a standard chart, mean BP ≦ 30 mmHg in any preterm infant, or mean BP ≦ 1 mmHg per week of gestation | Volume vs no treatment in very preterm infants (5 RCTs, 978 neonates) Gelatin vs fresh frozen plasma in hypotensive infants (1 RCT, 519 neonates) | Cerebral palsy ("Cerebral palsy in survivors" (1 RCT, 604 children; and 1 RCT, 399 children); reported as a primary outcome) Other composite outcomes that include cerebral palsy as a component ("Severe neurodevelopmental disability in survivors" (1 RCT, 604 children; and 1 RCT, 399 children); "Death or severe neurodevelopmental disability" (1 RCT, 776 children; and 1 RCT, 518 children); reported as primary outcomes) |
| Neonatal care: patent ductus arteriosus | ||||||
| Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants | Searches: April 2010 Up‐to‐date: | 19 RCTs (Countries: North America: 13 RCTs; Latin America, Europe, Asia: 6 RCTs; Published: 1980s: 11 RCTs; 1990s: 7 RCTs; 2000s; 1 RCT) | 2872 infants | Preterm neonates (less than 37 weeks' completed gestation) | Prophylactic IV indomethacin vs placebo or no drug (19 RCTs, 2872 neonates) | Cerebral palsy ("Neurological assessments (18‐54 months: Cerebral palsy" (4 RCTs, 1372 children); "School age neurological assessments: Cerebral palsy aged 8 years" (1 RCT, 304 children); reported as primary outcomes) Other composite outcome that includes cerebral palsy as a component ("Death or severe neurosensory impairment" (3 RCTs, 1491 children); reported as a primary outcome) |
| Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants | 7 May 2014 | 33 RCTs (Countries: Albania: 1 RCT; Belgium: 2 RCTs; Czech Republic: 1 RCT; China: 1 RCT; Egypt: 1 RCT; India: 1 RCT; Iran: 3 RCTs; Israel: 1 RCT; Italy: 6 RCTs; Poland: 1 RCT; Qatar: 1 RCT; Spain: 2 RCTs; Taiwan: 2 RCTs; Thailand: 2 RCTs; Tunisia: 1 RCT; Turkey: 3 RCTs; UK: 2 RCTs; USA: 2 RCTs; Published: 1990s: 4 RCTs; 2000s: 18 RCTs; 2010s: 11 RCTs) | 2190 infants | Preterm infants less than 37 weeks' gestational age or LBW infants (less than 2500 g) with PDA diagnosed either clinically or by echocardiographically (ECHO) guided criteria in the neonatal period (less than 28 days) | Oral ibuprofen vs IV ibuprofen (data for maximum of 4 RCTs, 304 neonates) | Cerebral palsy ("Moderate/severe cerebral palsy at 18‐24 months" (1 RCT, 57 children); reported as a secondary outcome) |
| Neonatal care: blood disorders | ||||||
| Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants | 1 July 2013 | 27 RCTs (Countries: Austria: 2 RCTs; Bangladesh: 1 RCT; Chile: 1 RCT; China: 2 RCTs; Greece: 3 RCTs; Iran: 1 RCT; Italy: 2 RCTs; Mexico: 1 RCT; New Zealand: 1 RCT; Poland: 1 RCT; Singapore: 1 RCT; South Africa: 1 RCT; Switzerland: 1 RCT; Turkey: 1 RCT; USA: 5 RCTs; Europe: 3 RCTs; Published 1990s: 12 RCTs; 2000s: 13 RCTs; 2010s: 2 RCTs) | 2209 infants | Preterm (< 37 weeks) and/or LBW (< 2500 g) neonates less than 8 days of age | Erythropoietin vs placebo or no treatment (27 RCTs, 2209 neonates) Darbepoetin alfa vs placebo or no treatment (1 RCT, 66 neonates) | Cerebral palsy ("Cerebral palsy at 18 ‐ 22 months' corrected age (in children examined)" (2 RCTs, 153 children; and 1 RCT, 51 children); reported as secondary outcomes) Other composite outcome that includes cerebral palsy as a component ("Any neurodevelopmental impairment at 18‐22 months' corrected age (in children examined)" (1 RCT< 99 children); reported as a secondary outcome) Motor dysfunction ("PDI < 70 at 18 ‐ 22 months' corrected age (in children examined)" (1 RCT, 90 children); reported as a secondary outcome) |
| Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants | Search: August 2011 Up‐to‐date: 1 September 2011 | 5 RCTs (Countries: Canada: 1 RCT; International (Canada, USA, Australia): 1 RCT; Taiwan: 1 RCT; USA: 2 RCTs; Published: 1980s: 1 RCT; 1990s: 1 RCT; 2000s: 3 RCTs) | 670 infants | VLBW infants (i.e. of birthweight less than or equal to 1500 g, or less than 32 weeks' gestational age) admitted to NICU at less than 1 week of age. We aimed specifically to include studies of infants receiving all levels of intensive care | Transfusion at a low haemoglobin or haematocrit level (restrictive) vs transfusion at a high haemoglobin or haematocrit level (liberal) (4 RCTs, 614 neonates) | Cerebral palsy ("Neurosensory impairment at 18‐21 months' follow‐up among survivors: Cerebral palsy" (1 RCT, 335 children); reported as a secondary outcome) Other composite outcomes that include cerebral palsy as a component ("Death or severe morbidity: at 18‐21 months' follow‐up with MDI < 70" (1 RCT, 421 children); "Death or severe morbidity: at 18‐21 months' follow‐up with MDI < 85" (1 RCT, 421 children); reported as primary outcomes) ("Neurosensory impairment at 18‐21 months' follow‐up among survivors: any neurosensory impairment" (1 RCT, 328 children); reported as a secondary outcome) |
| Neonatal care: pulmonary hypertension | ||||||
| Endothelin receptor antagonists for persistent pulmonary hypertension in term and late preterm infants | 28 December 2015 | 2 RCTs (Countries: Saudi Arabia: 1 RCT; unclear (multi‐centre): 1 RCT; Published: 2010s: 2 RCTs) | 68 infants | Late preterm infants (born at 34+0 to 36+6 weeks), term infants (born at 37+0 to 41+6 weeks), and post‐term infants (i.e. born after 41+6 weeks' gestation) until post‐menstrual age (PMA) up to 44 weeks with PPHN were eligible for inclusion. The diagnosis of PPHN was clinical or was based on echocardiography. Clinical diagnosis of PPHN was considered when there was hypoxaemia refractory to oxygen therapy and mechanical ventilation (Roberts 1997). The echocardiographic diagnosis of PPHN was made by demonstrating the presence of extrapulmonary right‐to‐left shunting at the ductal or atrial level, near or suprasystemic pulmonary arterial pressures, and doppler evidence of tricuspid regurgitation (Dhillon 2012; Stayer 2010) | Endothelin receptor antagonists vs placebo (1 RCT, 47 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 37 children); reported as a secondary outcome) Motor dysfunction ("Adverse neurodevelopmental outcome at 6 months" (1 RCT, 37 children); reported as a secondary outcome) |
| Neonatal care: resuscitation | ||||||
| Air versus oxygen for resuscitation of infants at birth | Search: December 2003/January 2004 Up‐to‐date: 15 February 2005 | 5 RCTs (Countries: India: 1 RCT; 6 countries: 1 RCT; not reported: 3 RCTs Published: 1990s: 2 RCTs; 2000s: 3 RCTs) | 1302 infants | Term or preterm neonates requiring IPPV at birth | Room air vs 100% oxygen (5 RCTs, 1302 neonates) | Cerebral palsy ("Long‐term neurodevelopmental outcome: cerebral palsy in those followed up at 18‐24 months" (1 RCT, 213 children); reported as a post hoc outcome) Motor dysfunction ("Long‐term neurodevelopmental outcome: not walking in those followed up at 18‐24 months" (1 RCT, 213 children); reported as a post hoc outcome) |
| Neonatal care: nitric oxide | ||||||
| Inhaled nitric oxide for respiratory failure in preterm infants | Search: June 2010 Up‐to‐date: 12 October 2010 | 14 RCTs (Countries: Europe: 3 RCTs; Taiwan: 1 RCT; USA: 1 RCT; not reported/unclear: 9 RCTs Published: 1990s: 3 RCTs; 2000s: 11 RCTs) | 3430 infants | Premature infants (less than 35 weeks' gestation) with respiratory failure after adequate treatment with surfactant | Inhaled NO compared with control; analyses conducted based on:
| Cerebral palsy ("Cerebral palsy"; reported as an outcome (2 RCTs, 209 children; 2 RCTs, 498 children; and 2 RCTs, 593 children) (not separated into primary/secondary)) Other composite outcome that includes cerebral palsy as a component ("Neurodevelopmental disability" (2 RCTs, 208 children; 2 RCTs, 498 children; and 2 RCTs, 593 children); reported as an outcome (not separated into primary/secondary)) Motor dysfunction ("Bayley MDI or PDI <‐2SD" (1 RCT, 138 children); reported as an outcome (not separated into primary/secondary)) |
| Nitric oxide for respiratory failure in infants born at or near term | Search: November 2005 Up‐to‐date: | 14 RCTs (Countries: 33 French and Belgian Units: 1 RCT; not reported: 13 RCTs Published: 1990s: 11 RCTs; 2000s: 3 RCTs) | 1715 infants | Newborn infants (< 1 month of age) with hypoxaemia suspected to be due to lung disease, pulmonary hypertension with right‐to‐left shunting, or both Only studies in term and near‐term infants (> 34 weeks' gestation) were included Efforts were made in all studies to exclude infants with intracardiac shunting due to structural congenital heart disease Infants with diaphragmatic hernia may respond differently from other near term infants (from preliminary data), and as far as possible results from infants with diaphragmatic hernias have been evaluated separately | Inhaled NO vs control (10 RCTs, 1068 infants) Inhaled NO vs control in infants with diaphragmatic hernia (2 RCTs, 84 neonates) | Cerebral palsy ("Cerebral palsy among survivors" (2 RCTs, 299 children; and 1 RCT, 22 children); reported as an outcome (not separated into primary/secondary)) Other composite outcome that includes cerebral palsy as a component ("Neurodevelopmental disability at 18 to 24 months among survivors" (2 RCTs, 301 children); reported as an outcome (not separated into primary/secondary)) Motor dysfunction ("Bayley PDI more than 2 SD below the mean" (2 RCTs, 283 children); reported as an outcome (not separated into primary/secondary)) |
| Neonatal care: apnoea | ||||||
| Methylxanthine treatment for apnoea in preterm infants | Search: Up‐to‐date: | 6 RCTs (Countries: not reported Published: 1980s: 3 RCTs; 1990s: 1 RCT; 2000s: 2 RCTs) | 959 infants | Preterm infants with recurrent apnoea. There must have been an effort to exclude specific secondary causes of apnoea | Any methylxanthine vs control (placebo or no drug therapy) (6 RCTs, 959 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 729 children); reported as a secondary outcome) Other composite outcome that includes cerebral palsy as a component ("Death or major disability by late infancy" (1 RCT, 767 children); reported as a secondary outcome) |
| Prophylactic methylxanthine for prevention of apnoea in preterm infants | Search: August 2010 Up‐to‐date: 29 September 2010 | 3 RCTs (Countries: not reported Published: 1980s: 2 RCTs; 2000s: 1 RCT) | 557 infants | Preterm infants, particularly those born at less than 34 weeks' gestation, who are at risk of developing recurrent apnoea, bradycardia, and hypoxic episodes | Prophylactic methylxanthine vs control (3 RCTs, 557 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 415 children); reported as a secondary outcome) Other composite outcome that includes cerebral palsy as a component ("Death or major disability" (1 RCT, 423 children); reported as a secondary outcome) |
| Neonatal care: respiratory distress syndrome | ||||||
| Inositol in preterm infants at risk for or having respiratory distress syndrome | 14 September 2014 | 4 RCTs (Countries: Finland: 2 RCTs; USA: 2 RCTs Published: 1980s: 1 RCT; 1990s: 2 RCTs; 2010s: 1 RCT) | 429 infants | Preterm infants (< 37 weeks' post‐menstrual age) or LBW (< 2500 g) infants | Inositol supplementation (repeat doses) vs control (3 RCTs, 355 neonates) | Other composite outcomes that include cerebral palsy as a component ("Major neural developmental impairment at one year corrected age" (1 RCT, 169 children); reported as a secondary outcome) Motor dysfunction ("Minor neural developmental impairment at one year corrected age" (1 RCT, 169 children); reported as a secondary outcome) |
| Animal derived surfactant extract for treatment of respiratory distress syndrome | Search: December 2008 Up‐to‐date: 13 February 2009 | 13 RCTs (Countries: not reported Published: 1980s: 7 RCTs; 1990s: 6 RCTs) | 1611 infants | Preterm infants (< 37 weeks' gestation) with clinical and/or radiological evidence of respiratory distress syndrome requiring assisted ventilation | Animal‐derived surfactant extract treatment of respiratory distress (all infants) (13 RCTs, 1611 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 73 children); reported as a secondary outcome) Other composite outcome that includes cerebral palsy as a component ("Major neurodevelopmental disability in survivors" (1 RCT, 73 children); reported as a secondary outcome) |
| Synthetic surfactant for respiratory distress syndrome in preterm infants | Search: Up‐to‐date: | 6 RCTs (Countries: not clearly reported; Canada/USA/both: 3 RCTs Published; 1980s: 1 RCT; 1990s: 5 RCTs) | 2358 infants | Neonates with clinical and radiological evidence of respiratory distress syndrome requiring assisted ventilation | Synthetic surfactant vs control (6 RCTs, 2358 neonates) | Cerebral palsy ("Cerebral palsy in survivors examined" (5 RCTs, 1557 children); reported as an outcome (not separated into primary/secondary)) Severity of cerebral palsy ("Moderate ‐ severe cerebral palsy in survivors examined" (5 RCTs, 1557 children); reported as an outcome (not separated into primary/secondary)) |
| Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants | Search: September 2009 Up‐to‐date: 27 October 2009 | 7 RCTs (Countries: 1: UK; 6 RCTs: not reported Published: 1980s: 3 RCTs; 1990s: 4 RCTs) | 1583 infants | Premature infants with or without evidence of surfactant deficiency | Prophylactic synthetic surfactant vs control (7 RCTs, 1583 neonates) | Cerebral palsy ("Cerebral palsy, 1‐2 years" (4 RCTs, 670 children); reported as a secondary outcome) Severity of cerebral palsy ("Cerebral palsy, moderate/severe" (4 RCTs, 670 children); reported as a secondary outcome) |
| Neonatal care: mechanical ventilation | ||||||
| Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants | 30 November 2014 | 19 RCTs (Countries: not reported Published: 1980s: 1 RCT; 1990s: 6 RCTs; 2000s: 10 RCTs; 2010s: 2 RCTs) | 4096 infants | Preterm or LBW infants with pulmonary dysfunction, mainly due to respiratory distress syndrome, who were considered to require IPPV | High‐frequency oscillatory ventilation vs conventional ventilation (19 RCTs, 4096 neonates) | Cerebral palsy (reported in text as a secondary outcome (3 RCTs)) |
| Continuous distending pressure for respiratory distress in preterm infants | 30 April 2015 | 6 RCTs (Countries: not reported Published: 1970s: 4 RCTs; 1990s: 1 RCT; 2000s: 1 RCT) | 355 infants | Preterm infants with respiratory failure | Continuous distending pressure vs standard care (6 RCTs, 355 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 36 children); reported as a secondary outcome) Other composite outcomes that include cerebral palsy as a component ("Death or severe disability" (1 RCT, 38 children); "Severe disability" (1 RCT, 37 children); "Any disability" (1 RCT, 37 children); reported as secondary outcomes) |
| Prophylactic methylxanthines for endotracheal extubation in preterm infants | Search: Up‐to‐date: 16 August 2010 | 7 RCTs (Countries: not reported Published: 1980s: 3 RCTs; 1990s: 3 RCTs; 2000s: 1 RCT) | 916 infants | Preterm or LBW infants being weaned from IPPV | Methylxanthine vs control (7 RCTs, 914 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 644 children); reported as a secondary outcome) Other composite outcome that includes cerebral palsy as a component ("Death or major disability by 18‐21 months" (1 RCT, 676 children); reported as a secondary outcome) |
| Long versus short inspiratory times in neonates receiving mechanical ventilation | Search: Up‐to‐date: | 5 RCTs (Countries: not reported Published: 1980s: 3 RCTs; 19980s; 2 RCTs) | 694 infants | Term and preterm infants at less than 28 days of age and requiring conventional mechanical ventilation. No restrictions on underlying pathophysiology were applied | Long vs short inspiratory times (5 RCTs, 694 neonates) | Cerebral palsy ("Cerebral palsy in survivors less than 33 weeks' gestation at birth" (1 RCT, 177 children); reported as a secondary outcome) |
| Volume‐targeted versus pressure‐limited ventilation in the neonate | Search: January 2010 Up‐to‐date: | 12 RCTs (Countries: not reported Published: 1990s: 2 RCTs; 2000s: 10 RCTs) | 693 infants | All intubated infants of less than 28 days' corrected age who were being mechanically ventilated with IPPV at the time of study entry. Infants of all gestational ages and both paralysed and non‐paralysed infants were eligible | Volume‐targeted vs pressure‐limited ventilation (12 RCTs, 693 neonates) | Other composite outcomes that include cerebral palsy as a component ("Severe disability (any definition)" (2 RCTs, 209 children); "Severe disability (any definition) or death" (1 RCT, 109 children; reported as outcomes from post hoc meta‐analyses) Motor dysfunction ("Gross motor developmental issue (any definition)" (1 RCT, 128 children); reported as an outcome from a post hoc meta‐analysis) |
| Neonatal care: bronchopulmonary dysplasia | ||||||
| Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants | Search: August 2013 Up‐to‐date: 18 February 2014 | 29 RCTs (Countries: not reported Published: 1970s: 1 RCT; 1990s: 17 RCTs; 2000s: 10 RCTs; 2010s: 1 RCT) | 3750 infants | Preterm infants at risk of developing chronic lung disease, including those who were ventilator dependent | Early (< 8 days) postnatal corticosteroids vs control (29 RCTs, 3750 neonates) | Cerebral palsy ("Cerebral palsy" (12 RCTs, 1452 children); "Cerebral palsy in survivors assessed" (12 RCTs, 959 children); reported as primary outcomes) Cerebral palsy or death ("Death or cerebral palsy" (12 RCTs, 1452 children); reported as a primary outcome) Other composite outcomes that include cerebral palsy as a component ("Major neurosensory disability (variable criteria ‐ see individual studies)" (7 RCTs, 1233 children); "Major neurosensory disability (variable criteria) in survivors examined" (7 RCTs, 799 children); "Death or major neurosensory disability (variable criteria)" (7 RCTs, 1233 children); reported as primary outcomes) Motor dysfunction ("Bayley Psychomotor Developmental Index (PDI) <‐2SD" (3 RCTs, 842 children); "Bayley PDI <‐2SD in tested survivors" (3 RCTs, 528 children); reported as primary outcomes) |
| Moderately early (7‐14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants | Search: October 2002 Up‐to‐date: 11 November 2008 | 7 RCTs (Countries: not reported Published: 1980s: 1 RCT; 1990s: 6 RCTs) | 669 infants | Preterm babies developing chronic lung disease including those who were ventilator dependent | Moderately early (7‐14 days) postnatal corticosteroids vs control (7 RCTs, 659 neonates) | Cerebral palsy ("Cerebral palsy" (4 RCTs, 204 children); "Cerebral palsy in survivors assessed" (4 RCTs, 130 children); reported as review outcomes (not separated into primary and secondary)) Cerebral palsy or death ("Death or cerebral palsy" (4 RCTs, 204 children); reported as a review outcome) Other composite outcomes that include cerebral palsy as a component ("Major neurosensory disability (variable criteria ‐ see individual studies)" (2 RCTs, 96 children); "Major neurosensory disability (variable criteria) in survivors assessed" (2 RCTs, 56 children); "Death or major neurosensory disability (variable criteria)" (2 RCTs, 96 children); reported as review outcomes) |
| Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants | Search: August 2013 Up‐to‐date: 18 February 2014 | 21 RCTs (Countries: Australia, Canada, New Zealand: 1 RCT; 6 countries: 1 RCT; not reported: 19 RCTs Published: 1980s: 5 RCTs; 1990s: 12 RCTs; 2000s: 3 RCTs; 2010s: 1 RCT) | 1424 infants | Preterm infants with evolving or established chronic lung disease, defined as oxygen‐dependent, ventilator‐dependent, or both, with or without radiographic changes of BPD | Late (> 7 days) postnatal corticosteroids vs control (21 RCTs, 1424 neonates) | Cerebral palsy ("Cerebral palsy: at 1 to 3 years" (14 RCTs, 876 children); "Cerebral palsy: at latest reported age" (15 RCTs, 855 children); "Cerebral palsy in survivors assessed: at 1 to 3 years" (14 RCTs, 631 children); "Cerebral palsy in survivors assessed: at latest reported age" (15 RCTs, 591 children); reported as primary outcomes) Cerebral palsy or death ("Death or cerebral palsy: at 1 to 3 years" (14 RCTs, 876 children); "Death or cerebral palsy: at latest reported age" (15 RCTs, 855 children); reported as primary outcomes) Other composite outcomes that include cerebral palsy as a component ("Major neurosensory disability (variable criteria ‐ see individual studies)" (8 RCTs, 655 children); "Major neurosensory disability (variable criteria) in survivors assessed" (8 RCTs, 480 children); "Death or major neurosensory disability (variable criteria)" (8 RCTs, 655 children); reported as primary outcomes) Motor dysfunction ("Bayley Psychomotor Developmental Index (PDI) < ‐2 SD" (1 RCT, 118 children); "Bayley PDI < ‐2 SD in survivors tested" (1 RCT, 90 children); reported as primary outcomes) |
| Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates | 29 July 2011 | 7 RCTs (Countries: Canada: 1 RCT; China: 1 RCT; Germany: 1 RCT; UK: 1 RCT; USA: 1 RCT; not reported: 2 RCTs Published: 1990s: 5 RCTs; 2000s: 2 RCTs) | 495 infants | Ventilator‐dependent preterm neonates with birthweight ≤ 1500 g and postnatal age < 2 weeks | Early inhaled steroids (< 2 weeks) vs placebo (7 RCTs, 495 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 56 children); reported as a secondary outcome) Motor dysfunction ("Mean developmental index on BSID‐II < 2 SD of the mean" (1 RCT, 56 children); reported as a secondary outcome) |
| Vitamin A supplementation to prevent mortality and short‐ and long‐term morbidity in very low birth weight infants | 1 May 2016 | 11 RCTs (Countries: Greece: 1 RCT; South Africa: 1 RCT; Thailand: 1 RCT; UK: 2 RCTs; USA: 6 RCTs Published: 1980s: 2 RCTs; 1990s: 4 RCTs; 2000s: 3 RCTs: 2010s: 2 RCTs) | 1580 infants | VLBW infants (defined as birthweight ≤ 1500 g or at less than 32 weeks' gestation) | Supplemental vitamin A vs no supplementation (10 RCTs, 1460 neonates) | Other composite outcomes that include cerebral palsy as a component ("Neurodevelopmental impairment at 18 to 22 months" (1 RCT, 538 children); "Death or neurodevelopmental impairment at 18 to 22 months" (1 RCT, 687 children); reported as secondary outcomes) |
| Neonatal care: infections: necrotising enterocolitis | ||||||
| Probiotics for prevention of necrotising enterocolitis in preterm infants | 1 October 2013 | 24 RCTs (Countries: Australia and New Zealand: 1 RCT; Brazil: 1 RCT; Colombia: 1 RCT; France: 1 RCT; Germany: 2 RCTs; Greece: 2 RCTs; India: 1 RCT; Israel: 1 RCT; Italy: 4 RCTs; Japan: 2 RCTs; Taiwan: 2 RCTs; Turkey: 1 RCT; UK: 1 RCT; USA: 1 RCT; not reported; 3 RCTs Published: 1980s: 1 RCT; 1990s: 2 RCTs; 2000s: 12 RCTs; 2010s: 9 RCTs) | 5529 infants (20 RCTs with reported outcomes) | Preterm infants at < 37 weeks and birthweight < 2500 g, or both | Probiotics vs control (20 RCTs, 5529 neonates) | Other composite outcome that includes cerebral palsy as a component ("Mental retardation and cerebral palsy" (1 RCT, 85 children); reported as a secondary outcome) |
| Arginine supplementation for prevention of necrotising enterocolitis in preterm infants | Search: August 2010 Up‐to‐date: 28 November 2010 | 1 RCT (Country: not reported Published; 2000s) | 152 infants | Preterm infants less than 37 weeks' gestation at birth | Arginine supplementation vs placebo (1 RCT, 152 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 135 children); reported as a post hoc secondary outcome) Other composite outcome that includes cerebral palsy as a component ("Major neurodevelopmental disability" (1 RCT, 132 children); reported as a post hoc secondary outcome) |
| Neonatal care: infections: fungal infections | ||||||
| Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants | Search: August 2015 Up‐to‐date: 1 September 2015 | 15 RCTs (Countries: India: 2 RCTs; Italy: 2 RCTs; Korea: 1 RCT; Saudi Arabia: 1 RCT; Turkey: 2 RCTs; USA: 7 RCTs Published: 2000s: 7 RCTs; 2010s: 8 RCTs) | 1690 infants | Very preterm or VLBW infants with or without evidence of fungal colonisation but without evidence of invasive fungal infection at study entry | Systemic antifungal agent vs placebo or no drug (10 RCTs, 1371 neonates) | Cerebral palsy ("Cerebral palsy" (1 RCT, 219 children); reported as a primary outcome) Other composite outcome that includes cerebral palsy as a component ("Neurodevelopmental impairment (composite)" (1 RCT, 171 children); reported as a primary outcome) |
| Neonatal care: infections: herpes simplex | ||||||
| Antiviral agents for treatment of herpes simplex virus infection in neonates | Search: November 2008 Up‐to‐date: 14 March 2009 | 2 RCTs (Countries: USA: 2 RCTs Published: 1980s: 1 RCT; 1990s: 1 RCT) | 273 infants | Hospitalised newborn infants less than 1 month of age with virologically confirmed HSV infection | Vidarabine vs placebo (1 RCT, 56 neonates) Aciclovir vs vidarabine (1 RCT, 202 neonates) | Cerebral palsy ("Cerebral palsy in CNS HSV neonatal infection up to three years by HSV serotype: HSV‐1" (1 RCT, 9 children); "Cerebral palsy in CNS HSV neonatal infection up to three years by HSV serotype: HSV‐2" (1 RCT, 14 children); reported as primary outcomes) Other composite outcomes that include cerebral palsy as a component ("Abnormal neurodevelopment at one year" (1 RCT, 56 children; and 1 RCT, 202 children); "Abnormal neurodevelopment or death at approximately one year of age" (1 RCT, 56 children; and 1 RCT, 202 children); reported as primary outcomes) |
| Neonatal care: jaundice | ||||||
| Prophylactic phototherapy for preventing jaundice in preterm or low birth weight infants | 31 March 2011 | 9 RCTs (Countries: USA: 6 RCTs; Brazil: 1 RCT; Canada: 1 RCT; India: 1 RCT Published: 1960s: 2 RCTs; 1970s: 1 RCT; 1980s: 2 RCTs; 2000s: 4 RCTs) | 3449 infants |
Originally (in the protocol), the focus of the review was narrower (to include VLBW infants; < 1500 g birthweight); however, so as not to lose valuable information, we made a post hoc decision to include any study that involved LBW (< 2500 g birthweight) or preterm infants We excluded studies of infants with a known cause that can lead to significant hyperbilirubinaemia, such as ABO incompatibility, Rh incompatibility, minor blood group incompatibility, or G‐6PD deficiency | Prophylactic phototherapy vs control (9 RCTs, 3449 neonates) | Cerebral palsy ("Cerebral palsy" (2 RCTs, 756 children); reported as a primary outcome) Other composite outcomes that include cerebral palsy as a component ("Neurodevelopmental impairment" (1 RCT, 1804 children); reported as a primary outcome) |
| Neonatal care: hypoglycaemia | ||||||
| Oral dextrose gel for the treatment of hypoglycaemia in newborn infants | 29 February 2016 | 2 RCTs (Countries: Ireland: 1 RCT; New Zealand: 1 RCT; Published: 2000s: 1 RCT; 2010s: 1 RCT) | 317 infants | We included newborn infants from birth to discharge home who were hypoglycaemic (blood glucose concentrations below the normal range, investigator defined) for any reason. We excluded infants who had received prior IV treatment for maintenance of glucose control at the time of hypoglycaemia | Dextrose gel vs control (2 RCTs, 317 neonates) | Cerebral palsy ("Cerebral palsy and severity at age 2 years or older" (1 RCT, 183 children); reported as a secondary outcome) Other composite outcomes that include cerebral palsy as a component ("Major neurosensory disability (2‐year follow‐up)" (1 RCT, 184 children); reported as a primary outcome) ("Developmental disability at age 2 years or older" (1 RCT, 184 children); reported as a secondary outcome) |
| Neonatal care: parenteral feeding | ||||||
| Glutamine supplementation to prevent morbidity and mortality in preterm infants | 18 December 2015 | 12 RCTs (Countries: China; 1 RCT; Greece: 1 RCT; Malaysia: Published: 1990s: 2 RCTs; 2000s: 6 RCTs; 2010s: 4 RCTs) | 2877 infants | We included preterm infants (gestational age < 37 weeks) admitted to neonatal intensive or special care units or comparable settings after birth. When participants in a trial included both term and preterm infants, we sought subgroup data from the report or from trial authors | Glutamine supplementation vs no supplementation (12 RCTs, 2877 neonates) | Other composite outcome that includes cerebral palsy as a component ("Neurodevelopmental impairment" (1 RCT, 72 children); reported as a primary outcome) |
| Neonatal care: other | ||||||
| Thyroid hormones for preventing neurodevelopmental impairment in preterm infants | Search: Up‐to‐date: 1 February 2009 | 5 RCTs (Countries: not reported; Published: 1980s: 2 RCTs; 1990s: 2 RCTs; 2000s; 1 RCT) | 362 infants | Studies that enrolled and treated preterm infants in the neonatal period | Thyroid hormones vs control (5 RCTs, 362 neonates) | Cerebral palsy ("Cerebral palsy in survivors" (1 RCT, 156 children); reported as a primary outcome) Cerebral palsy or death ("Death or cerebral palsy" (1 RCT, 200 children); reported as a primary outcome) |
| Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants | Search: Up‐to‐date: 12 October 2006 | 4 RCTs (Countries: not reported; Published: 1990s: 2 RCTs; 2000s: 2 RCTs) | 318 infants | Studies that enrolled preterm infants (born < 37 completed weeks' gestation) in the neonatal period. Trials that enrolled infants on the basis of results of abnormal thyroid function tests (known congenital hypothyroidism or transient hypothyroxinaemia), or with only respiratory distress syndrome, were excluded | Prophylactic thyroid hormones vs no thyroid hormones (4 RCTs, 318 neonates) | Cerebral palsy ("Cerebral palsy in survivors" (1 RCT, 156 children); reported as a primary outcome) Cerebral palsy or death ("Death or cerebral palsy" (1 RCT, 200 children); reported as a primary outcome) |
| Sound reduction management in the neonatal intensive care unit for preterm or very low birth weight infants | 18 December 2014 | 1 RCT (Country: USA; Published: 2009) | 34 infants | Preterm infants (< 32 weeks' post‐menstrual age or < 1500 g birthweight) cared for in the resuscitation area, during transport, or once admitted to an NICU or a stepdown unit | Silicone earplugs vs no earplugs (1 RCT, 34 infants) | Cerebral palsy ("Cerebral palsy at 18 to 22 months' corrected age" (1 RCT, 14 children); reported as a primary outcome) |
| Kangaroo mother care to reduce morbidity and mortality in low birthweight infants | 30 June 2016 | 21 RCTs (Countries: 13 RCTs in low‐ or middle‐income countries: Colombia: 1 RCT; Ecuador: | 3042 infants | LBW infants (defined as birthweight < 2500 g) regardless of gestational age | Kangaroo mother care vs conventional neonatal care (20 RCTs, 2969 neonates) | Cerebral palsy ("Cerebral palsy at 12 months' corrected age" (1 RCT, 588 children); reported as a primary outcome) |
| Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants | 15 August 2015 | 25 RCTs (Countries: not reported; Published: 1970s: 1 RCT; 1980s: 5 RCTs; 1990s: | 3615 infants | Preterm infants born at < 37 weeks' gestational age (according to best obstetrical estimate at the time of delivery). We excluded studies that did not report outcomes for preterm infants separately from those for infants born at term | Early developmental intervention vs standard follow‐up (25 RCTs, 3615 neonates) | Cerebral palsy ("Rate of cerebral palsy" (7 RCTs, 985 children); reported as a secondary outcome) Motor dysfunction ("Motor outcome at school age (low score on Movement ABC)" (2 RCTs, 333 children); reported as a secondary outcome) |
| Abbreviations: BP: blood pressure; BPD: bronchopulmonary dysplasia; BSID: Bayley Scales of Infant Development; CNS: central nervous system; ECHO: echocardiogram; g: grams; G‐6PD: glucose‐6‐phosphate dehydrogenase; HSV: herpes simplex virus; IPPV: intermittent positive‐pressure ventilation; IV: intravenous; LBW: low birthweight; MDI: Mental Development Index; Movement‐ABC: Movement Assessment Battery for Children; NICU: neonatal intensive care unit; NO: nitric oxide; PDA: patent ductus arteriosus; PDI: Psychomotor Development Index; PMA: post‐menstrual age; PPHN: persistent pulmonary hypertension of the newborn; RCT: randomised controlled trial; Rh: Rhesus; SBF: systemic blood flow; SD: standard deviation; SVC: superior vena cava; VLBW: very low birthweight. | ||||||
| Review ID and title | Summary of trial limitations (risk of bias)* |
| Neonatal care: asphyxia | |
| Allopurinol for preventing mortality and morbidity in newborn infants with hypoxic‐ischaemic encephalopathy | Random sequence generation: 2 RCTs low risk; 1 RCT unclear risk Allocation concealment: 3 RCTs low risk Blinding: 2 RCTs low risk; 1 RCT high risk Incomplete outcome data: 3 RCTs low risk Overall: "Although small, the trials were generally of good methodological quality" |
| Cooling for newborns with hypoxic‐ischaemic encephalopathy | Random sequence generation: 9 RCTs low risk; 1 RCT unclear risk; 1 RCT high risk Allocation concealment: 8 RCTs low risk; 2 RCTs unclear risk; 1 RCT high risk Blinding (participants and personnel): 11 RCTs high risk Blinding (outcome assessors): 10 RCTs low risk; 1 RCT unclear risk Incomplete outcome data: 6 RCTs low risk; 1 RCT unclear risk; 4 RCTs high risk Selective reporting: 11 RCTs low risk Overall: "Several limitations of the available evidence should be noted" |
| Prophylactic barbiturate use for the prevention of morbidity and mortality following perinatal asphyxia | Random sequence generation: 7 RCTs low risk; 2 RCTs unclear risk Allocation concealment: 4 RCTs low risk; 4 RCTs unclear risk; 1 RCT high risk Blinding: 4 RCTs unclear risk; 5 RCTs high risk Incomplete outcome data: 6 RCTs low risk; 2 RCTs unclear risk; 1 RCT high risk Selective reporting: 9 RCTs low risk |
| Neonatal care: haemorrhage: periventricular/intraventricular | |
| Ethamsylate for the prevention of morbidity and mortality in preterm or very low birth weight infants | Adequate sequence generation: 4 RCTs yes; 2 RCTs unclear; 1 RCT no Allocation concealment: 3 RCTs yes; 2 RCT unclear; 2 RCTs no Blinding: 4 RCTs yes; 3 RCTs unclear Incomplete outcome data addressed: 5 RCTs yes; 1 RCT unclear; 1 RCT no Free of selective reporting: 7 RCTs yes Free of other bias: 7 RCTs yes |
| Postnatal phenobarbital for the prevention of intraventricular haemorrhage in preterm infants | Random sequence generation: 5 RCTs low risk; 6 RCTs unclear risk; 1 RCT high risk Allocation concealment: 4 RCTs low risk; 7 RCTs unclear risk; 1 RCT high risk Blinding (participants and personnel): 2 RCTs low risk; 10 RCTs high risk Blinding (outcome assessors): 6 RCTs low risk; 6 RCTs unclear risk Incomplete outcome data: 8 RCTs low risk; 4 RCTs unclear risk Selective reporting: 2 RCTs low risk; 10 RCTs unclear risk |
| Neonatal care: hypotension | |
| The effect of inotropes on morbidity and mortality in preterm infants with low systemic or organ blood flow | Adequate sequence generation: 1 RCT yes Allocation concealment: 1 RCT yes Blinding (outcomes): 1 RCT yes Blinding (intervention): 1 RCT yes Incomplete outcome data addressed: 1 RCT yes Free of selective reporting: 1 RCT yes Free of other bias: 1 RCT yes Overall: "The study was of adequate methodology" |
| Neonatal care: fluid therapy | |
| Early volume expansion for prevention of morbidity and mortality in very preterm infants | Adequate randomisation: 7 RCTs yes; 1 RCT unclear Allocation concealment: 7 RCTs yes; 1 RCT unclear Blinding of intervention: 1 RCT yes; 7 RCTs no Blinding of measurement: 3 RCTs yes; 1 RCT unclear; 4 RCTs no Losses to follow‐up: 5 RCTs none; 1 RCT unclear; 2 RCTs yes |
| Neonatal care: patent ductus arteriosus | |
| Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants | Blinding of randomisation: 12 RCTs yes; 7 RCTs can't tell Blinding of intervention: 16 RCTs yes; 2 RCTs can't tell; 1 RCT no Blinding of outcome assessment: 16 RCTs yes; 2 RCTs can't tell; 1 RCT no Complete follow‐up (short‐term outcomes): 18 RCTs yes; 1 RCT no Overall: "Overall, the quality of the trials was good" |
| Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants | Random sequence generation: 9 RCTs low risk; 24 RCTs unclear risk Allocation concealment: 18 RCTs low risk; 14 RCTs unclear risk; 1 RCT high risk Blinding: 6 RCTs low risk; 7 RCTs unclear risk; 20 RCTs high risk Incomplete outcome data: 28 RCTs low risk; 3 RCTs unclear risk; 2 RCTs high risk Selective reporting: 5 RCTs low risk; 28 RCTs unclear risk Other: 29 RCTs low risk; 4 RCTs unclear risk Overall: "Study quality was variable…we identified concerns about bias in most individual studies and therefore for the group of studies included as well" |
| Neonatal care: blood disorders | |
| Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants | Random sequence generation: 8 RCTs low: risk; 19 RCTs unclear risk Allocation concealment: 13 RCTs low risk; 14 RCTs unclear risk Blinding: 12 RCTs low risk; 3 RCTs unclear risk; 12 RCTs high risk Incomplete outcome data: 23 RCTs low risk; 2 RCTs unclear risk; 2 RCTs high risk Selective reporting: 1 RCT low risk; 26 RCTs unclear risk Other: 26 RCTs low risk; 1 RCT unclear risk |
| Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants | Allocation concealment: 4 RCTs low risk; 1 RCT unclear risk Blinding: 1 RCT unclear risk; 4 RCTs high risk Incomplete outcome data: 3 RCTs low risk; 1 RCT unclear risk; 1 RCT high risk Selective reporting: 1 RCT low risk; 3 RCTs unclear risk; 1 RCT high risk Overall: "This review consists of five randomised controlled trials in which there appears to be no allocation bias; the overall level of evidence is high" |
| Neonatal care: pulmonary hypertension | |
| Endothelin receptor antagonists for persistent pulmonary hypertension in term and late preterm infants | Random sequence generation: 1 RCT low risk; 1 RCT unclear risk Allocation concealment: 2 RCT unclear risk Blinding (participants and personnel): 2 RCTs low risk Blinding (outcome assessors): 2 RCTs low risk Incomplete outcome data: 1 RCT low risk; 1 RCT high risk Selective reporting: 1 RCT low risk; 1 RCT unclear risk Other: 2 RCTs low risk Overall: "the quality of evidence was considered low because of the very small sample size and methodological issues in the included studies" |
| Neonatal care: resuscitation | |
| Air versus oxygen for resuscitation of infants at birth | Concealment of allocation: 2 RCTs yes; 3 RCTs no Blinding of intervention: 2 RCTs yes; 3 RCTs no Blinding of outcome assessment: 2 RCTs yes; 3 RCTs no Completeness of follow‐up (short‐term): 4 RCTs yes; 1 RCT no Completeness of follow‐up (long‐term): 3 RCTs no; 2 RCTs unclear |
| Neonatal care: nitric oxide | |
| Inhaled nitric oxide for respiratory failure in preterm infants | Allocation concealment: 12 RCTs low risk; 2 RCTs unclear risk Blinding: 7 RCTs low risk; 7 RCTs high risk Incomplete outcome data: 14 RCTs low risk Selective reporting: 8 RCTs low risk; 6 RCTs not reported Other: 3 RCTs low risk; 4 RCTs high risk; 7 RCTs not reported |
| Nitric oxide for respiratory failure in infants born at or near term | Masking of allocation: 10 RCTs yes; 4 RCTs cannot tell Masking of intervention: 6 RCTs yes; 8 RCTs no Masking of outcome assessment: 6 RCTs yes; 1 RCT can’t tell; 7 RCTs no Completeness of follow‐up: 13 RCTs yes; 1 RCT can't tell Overall: "The overall quality of these studies is quite variable" |
| Neonatal care: apnoea | |
| Methylxanthine treatment for apnoea in preterm infants | Random sequence generation: 1 RCT high risk; 5 RCTs not reported Allocation concealment: 2 RCTs low risk; 2 RCTs unclear risk; 2 RCTs high risk Blinding: 4 RCTs low risk; 2 RCTs high risk Incomplete outcome data: 3 RCTs low risk; 1 RCT unclear risk; 2 RCTs high risk Selective reporting: 2 RCTs low risk; 1 RCT unclear risk; 2 RCTs high risk; 1 RCT not reported Overall: "There was variation in trial design" |
| Prophylactic methylxanthine for prevention of apnoea in preterm infants | Allocation concealment: 3 RCTs low risk Blinding: 3 RCTs low risk Incomplete outcome data: 3 RCTs low risk Selective reporting: 2 RCTs low risk; 1 RCT not reported Overall: "Three studies are generally of high quality" |
| Neonatal care: respiratory distress syndrome | |
| Inositol in preterm infants at risk for or having respiratory distress syndrome | Random sequence generation: 1 RCT low risk; 3 RCTs unclear risk Allocation concealment: 2 RCTs low risk; 2 RCTs unclear risk Blinding: 2 RCTs low risk; 2 RCTs unclear risk Incomplete outcome data: 4 RCTs low risk Selective reporting: 3 RCTs low risk; 1 RCT unclear risk Other: 3 RCTs high risk; 1 RCT low risk Overall: "Study quality varied and interim analyses had occurred in all trials" |
| Animal derived surfactant extract for treatment of respiratory distress syndrome | Blinding of randomisation: 10 RCTs yes; 3 RCTs not described Blinding of intervention: 8 RCTs yes; 1 RCT not described; 4 RCTs no Blinding of outcome measurement: 6 RCTs yes; 4 RCTs not described; 2 RCTs no; 1 RCT not reported Complete follow‐up (short‐term): 13 RCTs yes Complete follow‐up (long‐term): 4 RCTs yes; 9 RCTs no Overall: "studies are of high methodological quality" |
| Synthetic surfactant for respiratory distress syndrome in preterm infants | Blinding of randomisation: 6 RCTs yes Blinding of intervention: 5 RCTs yes; 1 RCT no Blinding of outcome measurement: 5 RCTs yes; 1 RCT no Complete follow‐up (short term): 6 RCTs yes Complete follow‐up (long term): 80 to 100% |
| Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants | Adequate sequence generation: 6 RCTs unclear: 1 RCT not reported Allocation concealment: 7 RCTs yes Blinding of intervention: 5 RCTs yes; 1 RCT unclear; 1 RCT no Blinding of outcome measurement: 6 RCTs yes; 1 RCT no Incomplete outcome data addressed: 5 RCTs yes; 2 RCTs unclear Free of selective reporting: 7 RCTs yes Free of other bias: 7 RCTs yes |
| Neonatal care: mechanical ventilation | |
| Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants | Random sequence generation: 11 RCTs low risk; 8 RCTs unclear risk Allocation concealment: 7 RCTs low risk; 12 RCTs unclear risk Blinding of participants and personnel: 19 RCTs high risk Blinding of outcome assessment: 7 RCTs low risk; 12 RCTs unclear risk Incomplete outcome data: 19 RCTs low risk Overall: "The quality of the studies was generally high" |
| Continuous distending pressure for respiratory distress in preterm infants | Random sequence generation: 2 RCTs low risk; 3 RCTs unclear risk; 1 RCT high risk Allocation concealment: 4 RCTs low risk; 1 RCT unclear risk; 1 RCT high risk Blinding (intervention): 6 RCTs high risk Blinding (short term outcomes): 6 RCTs high risk (1 RCT low risk for long term outcomes) Incomplete outcome data (short term outcomes): 3 RCTs low risk; 3 RCTs unclear risk (1 RCT low risk for long‐term outcomes) Selective reporting: 2 RCTs low risk; 4 RCTs unclear risk Other: 6 RCTs unclear risk Overall: "These data should be interpreted with caution as in the studies reviewed, the numbers of infants were small, blinding of treatment was not possible and blinding of the outcome assessment was reported in only one study for the outcomes in childhood, thus possibly introducing bias" |
| Prophylactic methylxanthines for endotracheal extubation in preterm infants | Sequence generation: 1 RCT low risk; 6 RCTs not reported Allocation concealment: 6 RCTs low risk; 1 RCT unclear risk Blinding: 6 RCTs low risk; 1 RCT high risk Incomplete outcome data: 3 RCTs low risk; 3 RCTs high risk; 1 RCT not reported Selective reporting: 4 RCTs low risk; 2 RCTs high risk; 1 RCT not reported Other: 1 RCT low risk; 6 RCTs not reported |
| Long versus short inspiratory times in neonates receiving mechanical ventilation | Concealment of allocation: 1 RCT yes; 1 RCT cannot tell; 3 RCTs no Blinding of intervention: 5 RCTs no Blinding of outcome measurement: 3 RCTs no; 2 RCTs some Completeness of follow‐up (short term outcomes): 5 RCTs yes |
| Volume‐targeted versus pressure‐limited ventilation in the neonate | Sequence generation: 6 RCTs low risk; 6 RCTs unclear risk Allocation concealment: 11 RCTs low risk; 1 RCT unclear risk Blinding: 12 RCTs high risk Incomplete outcome data: 12 RCTs low risk Selective reporting: 10 RCTs low risk; 1 RCT unclear risk; 1 RCT high risk Other: 5 RCTs low risk; 5 RCTs unclear risk; 2 RCTs high risk Overall: "There are no major concerns about the methodology used in the twelve trials included in this review" |
| Neonatal care: bronchopulmonary dysplasia | |
| Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants | Random sequence generation: 15 RCTs low risk; 14 RCTs unclear risk Allocation concealment: 27 RCTs low risk; 2 RCTs unclear risk Blinding of participants and personnel: 23 RCTs low risk; 2 RCTs unclear risk; 4 RCTs high risk Blinding of outcome assessment: 23 RCTs low risk; 2 RCTs unclear risk; 4 RCTs high risk Incomplete outcome data: 28 RCTs low risk; 1 RCT unclear risk Overall: "Overall the risk of bias was low for most studies" |
| Moderately early (7‐14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants | Blinding of randomisation/allocation concealment: 7 RCTs yes/low risk Blinding of intervention: 5 RCTs yes; 2 RCTs no Blinding of outcome measurement: 5 RCTs yes; 1 RCT some; 1 RCT cannot tell Complete follow‐up: 6 RCTs yes/almost; 1 RCT no Overall: "the methodological quality of the studies to determine long‐term outcome is limited in some cases" |
| Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants | Random sequence generation: 12 RCTs low risk; 9 RCTs unclear risk Allocation concealment: 17 RCTs low risk; 4 RCTs unclear risk Blinding of participants and personnel: 15 RCTs low risk; 4 RCTs unclear risk; 2 RCTs high risk Blinding of outcome assessment: 16 RCTs low risk; 4 RCTs unclear risk; 1 RCT high risk Incomplete outcome data: 20 RCTs low risk; 1 RCT unclear risk Overall: "Overall the risk of bias was low for most studies" |
| Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates | Random sequence generation: 7 RCTs unclear risk Allocation concealment: 7 RCTs low risk Blinding of participants and personnel: 7 RCTs low risk Blinding of outcome assessment: 1 RCT low risk; 6 RCTs unclear risk Incomplete outcome data: 6 RCTs low risk; 1 RCT unclear risk Overall: "Overall, the studies included for this review were of high methodological quality" |
| Vitamin A supplementation to prevent mortality and short‐ and long‐term morbidity in very low birth weight infants | Random sequence generation: 9 RCTs low risk; 2 RCTs unclear risk Allocation concealment: 8 RCTs low risk; 3 RCTs unclear risk Blinding: 6 RCTs low risk; 2 RCTs unclear risk; 3 RCTs high risk Incomplete outcome data: 9 RCTs low risk; 1 RCT unclear risk; 1 RCT high risk Selective reporting: 8 RCTs low risk; 2 RCTs unclear risk; 1 RCT high risk Other: 2 RCTs low risk; 6 RCTs unclear risk; 2 RCTs high risk; 1 RCT not reported |
| Neonatal care: infections: necrotising enterocolitis | |
| Probiotics for prevention of necrotising enterocolitis in preterm infants | Random sequence generation: 15 RCTs low risk; 8 RCTs unclear risk; 1 RCT high risk Allocation concealment: 11 RCTs low risk; 12 RCTs unclear risk; 1 RCT high risk Blinding: 15 RCTs low risk; 9 RCTs unclear risk Incomplete outcome data: 21 RCTs low risk; 2 RCTs unclear risk; 1 RCT high risk Selective reporting: 17 RCTs low risk; 6 RCTs high risk; 1 RCT not reported Other: 14 RCTs low risk; 10 RCTs not reported Overall: "Eleven of our included trials were classified as high quality trials" |
| Arginine supplementation for prevention of necrotising enterocolitis in preterm infants | Masking of randomisation: 1 RCT yes Masking of intervention: 1 RCT yes Masking of outcome assessment: 1 RCT yes Completeness of follow‐up: 1 RCT yes Overall: "The methodological quality of the included study was good" |
| Neonatal care: infections: fungal infections | |
| Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants | Allocation concealment: 12 RCTs low risk; 3 RCTs unclear risk Blinding of participants and personnel: 10 RCTs low risk; 3 RCTs unclear risk; 2 RCTs high risk Blinding of outcome assessment: 10 RCTs low risk; 3 RCTs unclear risk; 2 RCTs high risk Incomplete outcome data: 15 RCTs low risk Overall: "The included trials were generally of good methodological quality" |
| Neonatal care: infections: herpes simplex | |
| Antiviral agents for treatment of herpes simplex virus infection in neonates | Allocation concealment: 1 RCT unclear; 1 RCT inadequate Overall: "The two trials... have a number of methodological flaws" |
| Neonatal care: jaundice | |
| Prophylactic phototherapy for preventing jaundice in preterm or low birth weight infants | Random sequence generation: 4 RCTs low risk; 3 RCTs unclear risk; 2 RCTs high risk Allocation concealment: 3 RCTs low risk; 4 RCTs unclear risk; 2 RCTs high risk Blinding: 1 RCT low risk; 2 RCTs unclear risk; 6 RCTs high risk Incomplete outcome data: 8 RCTs low risk; 1 RCT high risk Selective reporting: 2 RCTs low risk; 7 RCTs unclear risk Other: 7 RCTs low risk Overall: "In general, the overall methodological quality of the included studies was acceptable" |
| Neonatal care: hypoglycaemia | |
| Oral dextrose gel for the treatment of hypoglycaemia in newborn infants | Random sequence generation: 1 RCT low risk; 1 RCT unclear risk Allocation concealment: 1 RCT low risk; 1 RCT unclear risk Blinding of participants and personnel: 1 RCT low risk; 1 RCT unclear risk Blinding of outcome assessors: 1 RCT low risk; 1 RCT unclear risk Incomplete outcome data: 1 RCT low risk; 1 RCT high risk Selective reporting: 1 RCT low risk; 1 RCT unclear risk Other: 1 RCT low risk; 1 RCT unclear risk |
| Neonatal care: parenteral feeding | |
| Glutamine supplementation to prevent morbidity and mortality in preterm infants | Random sequence generation: 8 RCTs low risk; 3 RCTs unclear risk; 1 RCT high risk Allocation concealment: 8 RCTs low risk; 2 RCTs unclear risk; 2 RCTs high risk Blinding: 10 RCTs low risk; 2 RCTs unclear risk Incomplete outcome data: 8 RCTs low risk; 2 RCTs unclear risk; 2 RCTs high risk Overall: "in general the trials were of good quality" |
| Neonatal care: other | |
| Thyroid hormones for preventing neurodevelopmental impairment in preterm infants | Blinding of randomisation/allocation concealment: 4 RCTs yes; 1 RCT no Blinding of intervention: 4 RCTs yes; 1 RCT no Blinding of outcome assessment: 4 RCTs yes; 1 RCT not stated Complete follow‐up: 2 RCTs yes; 3 RCTs no Overall: "four studies... were of good methodology" |
| Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants | Allocation concealment: 4 RCTs low risk Blinding of intervention: 4 RCTs yes Blinding of outcome assessment: 3 RCTs yes; 1 RCT probably Complete follow‐up: 3 RCTs yes; 1 RCT no Overall: "All studies... were of adequate methodology" |
| Sound reduction management in the neonatal intensive care unit for preterm or very low birth weight infants | Random sequence generation: 1 RCT low risk Allocation concealment: 1 RCT low risk Blinding of participants and personnel: 1 RCT high risk Blinding of outcome assessment: 1 RCT low risk Incomplete outcome data: 1 RCT low risk Selective reporting: 1 RCT low risk Other: 1 RCT low risk Overall: "We considered the overall risk of bias to be low" |
| Kangaroo mother care to reduce morbidity and mortality in low birthweight infants | Random sequence generation: 21 RCTs low risk Allocation concealment: 10 RCTs low risk; 11 RCTs unclear risk Blinding of participants and personnel: 21 RCTs high risk Blinding of outcome assessment: 2 RCTs low risk; 15 RCTs unclear risk; 4 RCTs high risk Incomplete outcome data: 14 RCTs low risk; 3 RCTs unclear risk; 4 RCTs high risk Selective reporting: 16 RCTs low risk; 3 RCTs unclear risk; 2 RCTs high risk Other: 15 RCTs low risk; 3 RCTs unclear risk; 3 RCTs high risk Overall: "The methodological quality of the included trials was mixed" |
| Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants | Random sequence generation: 11 RCTs low risk; 8 RCTs unclear risk; 5 RCTs high risk; 1 RCT not reported Allocation concealment: 11 RCTs low risk; 9 RCTs unclear risk; 5 RCTs high risk Blinding of participants and personnel: 2 RCTs low risk; 4 RCTs unclear risk; 19 RCTs high risk Blinding of outcome assessment: 21 RCTs low risk; 3 RCTs unclear risk; 1 RCT high risk Incomplete outcome data: 12 RCTs low risk; 4 RCTs unclear risk; 9 RCT high risk Selective reporting: 3 RCTs unclear risk; 6 RCT high risk; 16 RCTs not reported Overall: "The methodological quality of included studies was variable" |
| Abbreviations: RCT: randomised controlled trial. | |
| Review ID | AMSTAR criteria | TOTAL SCORE | ||||||||||
| 'A priori' design | Duplicate selection and extraction | Comprehensive search | Grey literature considered | Included and excluded studies lists | Characteristics of included studies | Quality assessed and documented | Quality considered for conclusions | Methods for combining studies appropriate | Publication bias considered or assessed | Conflicts stated | ||
| Neonatal care: asphyxia | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | 10/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal care: haemorrhage: periventricular/intraventricular | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal care: hypotension | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✗ | 9/10 HIGH QUALITY | |
| Neonatal care: fluid therapy | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| Neonatal care: patent ductus arteriosus | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal care: blood disorders | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal care: pulmonary hypertension | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✗ | 9/10 HIGH QUALITY | |
| Neonatal care: resuscitation | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| Neonatal care: nitric oxide | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✗ | ✗ | 8/11 HIGH QUALITY | |
| ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 8/11 HIGH QUALITY | |
| Neonatal care: apnoea | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| Neonatal care: respiratory distress syndrome | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 8/11 HIGH QUALITY | |
| ? | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✗ | ✗ | 6/11 MODERATE QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| Neonatal care: mechanical ventilation | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 8/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| Neonatal care: bronchopulmonary dysplasia | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✗ | ✗ | 7/11 MODERATE QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal care: infections: necrotising enterocolitis | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ✓ | N/A | ✗ | ✗ | 7/10 HIGH QUALITY | |
| Neonatal infections: fungal infections | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal infections: herpes simplex | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✗ | ✗ | 8/10 HIGH QUALITY | |
| Neonatal care: jaundice | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✗ | ✗ | 8/11 HIGH QUALITY | |
| Neonatal care: hypoglycaemia | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 11/11 HIGH QUALITY | |
| Neonatal care: parenteral feeding | ||||||||||||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| Neonatal care: other | ||||||||||||
| ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 8/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✗ | 9/10 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | 10/11 HIGH QUALITY | |
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 9/11 HIGH QUALITY | |
| Review ID | ROBIS domains | OVERALL RISK OF BIAS | |||
| Study eligibility criteria | Identification and selection of studies | Data collection and study appraisal | Synthesis and findings | ||
| Neonatal care: asphyxia | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: haemorrhage: periventricular/intraventricular | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: hypotension | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: fluid therapy | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: patent ductus arteriosus | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: blood disorders | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: pulmonary hypertension | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: resuscitation | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: nitric oxide | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Unclear risk | Low risk | Low risk | UNCLEAR RISK | |
| Neonatal care: apnoea | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: respiratory distress syndrome | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Unclear risk | Low risk | Low risk | Low risk | LOW RISK | |
| Unclear risk | Unclear risk | Unclear risk | Unclear risk | UNCLEAR RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: mechanical ventilation | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: bronchopulmonary dysplasia | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Unclear risk | Unclear risk | Unclear risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal infections: necrotising enterocolitis | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal infections: fungal infections | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal infections: herpes simplex | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: jaundice | |||||
| Low risk | Low risk | Low risk | Unclear risk | LOW RISK | |
| Neonatal care: hypoglycaemia | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: parenteral feeding | |||||
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Neonatal care: other | |||||
| Low risk | Unclear risk | Unclear risk | Low risk | UNCLEAR RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Unclear risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Low risk | Low risk | Low risk | Low risk | LOW RISK | |
| Intervention and comparison | Outcome | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | Comments |
| Neonatal care: asphyxia | |||||||
| Therapeutic hypothermia vs standard care for newborns with hypoxic‐ischaemic encephalopathy (Jacobs 2013) | Cerebral palsy in survivors assessed at 18 to 24 months | 352 per 1000 (143/406) | 232 per 1000 (190 to 289) | RR 0.66 (0.54 to 0.82) | 881 (7 RCTs) | HIGH | Not downgraded |
| Cerebral palsy at 6 to 7 years | 288 per 1000 (15/52) | 173 per 1000 (89 to 340) | RR 0.60 (0.31 to 1.18) | 121 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size | |
| Barbiturates (phenobarbital) vs conventional therapy for prevention of morbidity and mortality following perinatal asphyxia (Young 2016) | Cerebral palsy at 3 to 6 years | 242 per 1000 (8/33) | 141 per 1000 (46 to 412) | RR 0.58 (0.19 to 1.70) | 69 (2 RCTs) | VERY LOW | Study limitations (‐1): unblinded studies; concern regarding performance bias and detection bias Imprecision (‐1): 95% CIs were wide and imprecise (GRADED by review authors themselves) |
| Neonatal care: haemorrhage: periventricular/intraventricular | |||||||
| Ethamsylate vs placebo for prevention of morbidity and mortality in preterm or very low birthweight infants (Hunt 2010) | Cerebral palsy in surviving children available for follow‐up at 2 years up to 3.5 to 4.2 years (only cerebral palsy significant enough to cause moderate or severe impairment was included) | 78 per 1000 (21/270) | 88 per 1000 (50 to 156) | RR 1.13 (0.64 to 2.00) | 532 (3 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: hypotension | |||||||
| Dobutamine vs dopamine in preterm infants with low superior vena cava flow (Osborn 2007b) | Cerebral palsy at 3 years in survivors assessed | 429 per 1000 (3/7) | 69 per 1000 (4 to 1131) | RR 0.16 (0.01 to 2.64) | 13 (1 RCT) | VERY LOW | Study limitations (‐1): 5/18 surviving infants were not assessed at 3 years of age Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with very small sample size |
| Neonatal care: fluid therapy | |||||||
| Volume vs no treatment for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | Cerebral palsy in survivors at 2 years | 132 per 1000 (27/205) | 100 per 1000 (63 to 158) | RR 0.76 (0.48 to 1.20) | 604 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| Gelatin vs fresh frozen plasma for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | 103 per 1000 (21/203) | 97 per 1000 (54 to 175) | RR 0.94 (0.52 to 1.69) | 399 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect | |
| Neonatal care: patent ductus arteriosus | |||||||
| Prophylactic indomethacin vs placebo for preventing mortality and morbidity in preterm infants (Fowlie 2010) | Cerebral palsy at 18 to 54 months | 111 per 1000 (77/694) | 115 per 1000 (85 to 155) | RR 1.04 (0.77 to 1.40) | 1372 (4 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| Cerebral palsy at 8 years | 76 per 1000 (11/145) | 94 per 1000 (45 to 199) | RR 1.24 (0.59 to 2.62) | 304 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect | |
| Oral ibuprofen vs intravenous ibuprofen for treatment of patent ductus arteriosus in preterm or low birthweight (or both) infants (Ohlsson 2015) | Moderate or severe cerebral palsy at 18 to 24 months | 74 per 1000 (2/27) | 100 per 1000 (18 to 554) | RR 1.35 (0.24 to 7.48) | 57 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection and reporting bias; high risk of performance, detection, and attrition bias Imprecision (‐2): wide CI crossing line of no effect; small sample size and few events |
| Neonatal care: blood disorders | |||||||
| Erythropoietin vs placebo for preventing red blood cell transfusion in preterm and/or low birthweight infants (Ohlsson 2014) | Cerebral palsy at 18 to 22 months' corrected age (in children examined) | 187 per 1000 (14/75) | 123 per 1000 (58 to 256) | RR 0.66 (0.31 to 1.37) | 153 (2 RCTs) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection bias and high risk of attrition bias (˜73% follow‐up) Inconsistency (‐1): I² = 72% Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Darbepoetin alfa vs placebo for preventing red blood cell transfusion in preterm and/or low birthweight infants (Ohlsson 2014) | 208 per 1000 (5/24) | 17 per 1000 (0 to 292) | RR 0.08 (0.00 to 1.40) | 51 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size and few events | |
| Transfusion at a restrictive vs a liberal haemoglobin threshold for preventing morbidity and mortality in very low birthweight infants (Whyte 2011) | Cerebral palsy at 18 to 21 months' follow‐up among survivors | 52 per 1000 (9/172) | 68 per 1000 (29 to 159) | RR 1.29 (0.55 to 3.03) | 335 (1 RCT) | LOW | Study limitations (‐1): 1 RCT at high risk of performance bias and unclear risk of reporting bias Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: pulmonary hypertension | |||||||
| Endothelin receptor antagonists vs placebo for persistent pulmonary hypertension in term and late preterm infants (More 2016) | Cerebral palsy at 6 months (delayed motor development and spasticity) | 214 per 1000 (3/14) | 19 per 1000 (0 to 345) | RR 0.09 (0.00 to 1.61) | 37 (1 RCT) | LOW | Study limitation (‐1): 8/23 infants in the placebo group were excluded from analysis Imprecision (‐1): 1 RCT; small sample size (GRADED by review authors themselves) |
| Neonatal care: resuscitation | |||||||
| Room air vs 100% oxygen for resuscitation of infants at birth (Tan 2005) | Cerebral palsy in those followed up at 18 to 24 months | 74 per 1000 (9/122) | 99 per 1000 (41 to 239) | RR 1.34 (0.55 to 3.24) | 213 (1 RCT) | VERY LOW | Study limitations (‐2): 1 qRCT with no blinding and < 70% follow‐up Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: nitric oxide | |||||||
| Inhaled NO vs placebo for respiratory failure in preterm infants (entry before 3 days based on oxygenation) (Barrington 2010) | Cerebral palsy at 18 to 22 months (moderate/severe or disabling) | 100 per 1000 (11/110) | 185 per 1000 (93 to 371) | RR 1.85 (0.93 to 3.71) | 209 (2 RCTs) | LOW | Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Inhaled NO vs placebo or no treatment for respiratory failure in preterm infants (entry after 3 days based on BPD risk) (Barrington 2010) | Cerebral palsy at 2 years' corrected age or 30 months (1 RCT all severities; 1 RCT moderate/severe or disabling) | 56 per 1000 (14/248) | 62 per 1000 (30 to 126) | RR 1.10 (0.54 to 2.23) | 498 (2 RCTs) | LOW | Study limitations (‐1): 1 RCT with no blinding of intervention or outcome measurement Imprecision (‐1): wide CI crossing line of no effect; small sample sizes |
| Inhaled NO vs placebo for respiratory failure in preterm infants (studies of routine use in intubated preterm infants) (Barrington 2010) | Cerebral palsy at 1 or 2 years' corrected age (1 RCT all severities; 1 RCT moderate/severe or disabling) | 70 per 1000 (20/286) | 66 per 1000 (36 to 119) | RR 0.94 (0.51 to 1.70) | 593 (2 RCTs) | LOW | Study limitations (‐1): 2 RCTs with 74%‐82% follow‐up Imprecision (‐1): wide CI crossing line of no effect |
| Inhaled nitric oxide vs placebo for respiratory failure in infants born at or near term (Finer 2006) | Cerebral palsy among survivors at 13 or 18 to 24 months | 89 per 1000 (16/179) | 91 per 1000 (44 to 191) | RR 1.02 (0.49 to 2.14) | 299 (2 RCTs) | LOW | Study limitations (‐1): 1 RCT masking of allocation, masking of outcomes. and completeness of follow‐up Imprecision (‐1): wide CI crossing line of no effect |
| "This group has now published follow up data, including neurodevelopmental outcomes, which were obtained by telephone interview of 60 of the 83 survivors of the original trial. The interview was conducted between one and four years of age... Although cerebral palsy [was] reported it is unclear how [it] was defined... It is not, therefore, possible to add any of these data to the meta‐analysis, but they do appear to show no evidence of neurodevelopmental impairment due to inhaled nitric oxide therapy" | NOT GRADED | ||||||
| Inhaled nitric oxide vs placebo for respiratory failure in infants with diaphragmatic hernias born at or near term (Finer 2006) | Cerebral palsy among survivors at 18 to 24 months | (0/14) | (2/8) | RR 8.33 (0.45 to 154.78) | 22 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with 76% follow‐up of survivors Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: apnoea | |||||||
| Caffeine vs placebo for treatment of apnoea in preterm infants (Henderson‐Smart 2010b) | Cerebral palsy at 18 to 21 months' corrected age | 50 per 1000 (18/361) | 30 per 1000 (14 to 62) | RR 0.60 (0.29 to 1.25) | 729 (1 RCT) | LOW | Study limitations (‐1): results based on 1 RCT subgroup from post hoc subgroup analyses (randomisation not stratified according to indication for inclusion) Imprecision (‐1): wide CI crossing line of no effect |
| Caffeine vs placebo for prevention of apnoea in preterm infants (Henderson‐Smart 2010c) | Cerebral palsy at 18 to 21 months' corrected age | 45 per 1000 (9/200) | 46 per 1000 (19 to 112) | RR 1.03 (0.43 to 2.49) | 415 (1 RCT) | LOW | Study limitations (‐1): results based on 1 RCT subgroup from post hoc subgroup analyses (randomisation not stratified according to indication for inclusion) Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: respiratory distress syndrome | |||||||
| Animal‐derived surfactant extract vs no treatment for treatment of respiratory distress syndrome (Seger 2009) | Cerebral palsy at 1 and 2 years' corrected age | 207 per 1000 (6/29) | 182 per 1000 (70 to 470) | RR 0.88 (0.34 to 2.27) | 73 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with no blinding of intervention; and blinding of outcome measurement not reported Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Synthetic surfactant vs placebo for respiratory distress syndrome in preterm infants (Soll 2000) | Cerebral palsy in survivors examined at 1 year (in 4 of the 5 RCTs) | 96 per 1000 (74/767) | 73 per 1000 (53 to 101) | RR 0.76 (0.55 to 1.05) | 1557 (5 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing the line of no effect |
| Prophylactic protein‐free synthetic surfactant vs placebo for preventing morbidity and mortality in preterm infants (Soll 2010) | Cerebral palsy at 1 or 2 years | 153 per 1000 (49/320) | 142 per 1000 (52 to 204) | RR 0.93 (0.64 to 1.33) | 670 (4 RCTs) | LOW | Study limitations (‐1): "Somewhat fewer infants who received surfactant failed to return for follow‐up evaluation (typical relative risk 0.63, 95% CI 0.48, 0.82; typical risk difference ‐0.10, 95% CI ‐0.15, ‐0.04)" Imprecision (‐1): wide CI crossing the line of no effect |
| Neonatal care: mechanical ventilation | |||||||
| Elective high‐frequency oscillatory ventilation vs conventional ventilation for acute pulmonary dysfunction in preterm infants (Cools 2015) | Cerebral palsy |
| NOT GRADED | "The age and methods of assessment varied between studies so the results were presented in the text and not included in a meta‐analysis" | |||
| Continuous distending pressure vs standard care for respiratory distress in preterm infants (Ho 2015) | Cerebral palsy at 9 to 15 years | (0/18) | (2/18) | RR 5.0 (0.26 to 97.37) | 36 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection and attrition bias and high risk of performance bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Prophylactic methylxanthines (caffeine) vs placebo for endotracheal extubation in preterm infants (Henderson‐Smart 2010) | Cerebral palsy at 18 to 21 months' corrected age | 115 per 1000 (39/339) | 62 per 1000 (37 to 106) | RR 0.54 (0.32 to 0.92) | 644 (1 RCT) | MODERATE | Study limitations (‐1): results based on 1 RCT subgroup from post hoc subgroup analyses (randomisation not stratified according to indication for inclusion) |
| Long vs short inspiratory times in neonates receiving mechanical ventilation (Kamlin 2003) | Cerebral palsy in survivors less than 33 weeks' gestation at birth at 18 months | 133 per 1000 (12/90) | 387 per 1000 (129 to 1153) | RR 2.9 (0.97 to 8.65) | 177 ( 1 RCT) | VERY LOW | Study limitations: 1 RCT at high risk of performance bias; follow‐up of subset (< 33 weeks only) Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: bronchopulmonary dysplasia | |||||||
| Early (< 8 days) postnatal corticosteroids vs placebo or no treatment for preventing chronic lung disease in preterm infants (Doyle 2014b) | Cerebral palsy at 11 months to 7 to 9 years | 88 per 1000 (63/715) | 128 per 1000 (93 to 174) | RR 1.45 (1.06 to 1.98) | 1452 (12 RCTs) | MODERATE | Study limitations (‐1): 4 RCTs at unclear risk of selection bias; 2 RCTs at high risk of performance and detection bias; 2 RCTs had 13%‐53% follow‐up overall |
| Cerebral palsy in survivors assessed at 11 months to 7 to 9 years | 134 per 1000 (63/470) | 201 per 1000 (151 to 268) | RR 1.50 (1.13 to 2.00) | 959 (12 RCTs) | MODERATE | Study limitations (‐1): 4 RCTs at unclear risk of selection bias; 2 RCTs at high risk of performance and detection bias; 2 RCTs had 13%‐53% follow‐up overall | |
| Moderately early (7‐14 days) postnatal corticosteroids vs placebo or no treatment for preventing chronic lung disease in preterm infants (Halliday 2003) | Cerebral palsy at 12 months' corrected age up to 90 months | 105 per 1000 (10/95) | 108 per 1000 (49 to 236) | RR 1.03 (0.47 to 2.24) | 204 (4 RCTs) | VERY LOW | Study limitations (‐1): 2 RCTs with 68%‐70% follow‐up; 1 RCT with unclear blinding of outcome assessment Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Cerebral palsy in survivors assessed at 12 months' corrected age up to 90 months | 175 per 1000 (10/57) | 146 per 1000 (68 to 305) | RR 0.83 (0.39 to 1.74) | 130 (4 RCTs) | VERY LOW | Study limitations (‐1): 2 RCTs with 68%‐70% follow‐up; 1 RCT with unclear blinding of outcome assessment Imprecision (‐2): wide CI crossing line of no effect; small sample sizes | |
| Late (> 7 days) postnatal corticosteroids vs placebo or no treatment for chronic lung disease in preterm infants (Doyle 2014) | Cerebral palsy at 1 to 3 years | 127 per 1000 (55/433) | 135 per 1000 (97 to 191) | RR 1.06 (0.76 to 1.50) | 876 (14 RCTs) | LOW | Study limitations (‐1): 5 RCTs unclear risk of selection bias; 5 RCTs with follow‐up from 32% to 78% Imprecision (‐1): wide CI crossing line of no effect |
| Cerebral palsy at 1 to 3 years in survivors assessed | 172 per 1000 (53/309) | 180 per 1000 (129 to 252) | RR 1.05 (0.75 to 1.47) | 631 (14 RCTs) | LOW | Study limitations (‐1): 5 RCTs unclear risk of selection bias; 5 RCTs with follow‐up between 32% and 78% Imprecision (‐1): wide CI crossing line of no effect | |
| Cerebral palsy at latest reported age (from 1 year up to 17 years) | 121 per 1000 (51/423) | 135 per 1000 (95 to 193) | RR 1.12 (0.79 to 1.60) | 855 (15 RCTs) | LOW | Study limitations (‐1): 5 RCTs unclear risk of selection bias; 7 RCTs with follow‐up between 32% and 78% Imprecision (‐1): wide CI crossing line of no effect | |
| Cerebral palsy at latest reported age in survivors assessed (from 1 year up to 17 years) | 170 per 1000 (49/289) | 190 per 1000 (134 to 268) | RR 1.12 (0.79 to 1.58) | 591 (15 RCTs) | LOW | Study limitations (‐1): 5 RCTs unclear risk of selection bias; 7 RCTs with follow‐up between 32% and 78% Imprecision (‐1): wide CI crossing line of no effect | |
| Early inhaled corticosteroids vs placebo for preventing chronic lung disease in ventilated very low birthweight preterm neonates (Shah 2012) | Cerebral palsy (age not reported in review; from trial manuscript: 3 years) | 107 per 1000 (3/28) | 143 per 1000 (35 to 581) | RR 1.33 (0.33 to 5.42) | 56 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection bias and detection bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: necrotising enterocolitis | |||||||
| Arginine supplementation vs placebo for prevention of necrotising enterocolitis in preterm infants (Shah 2007) | Cerebral palsy at 36 months' post‐menstrual age | 55 per 1000 (4/73) | 48 per 1000 (12 to 208) | RR 0.88 (0.21 to 3.80) | 135 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: fungal infections | |||||||
| Systemic antifungal agent vs placebo to prevent mortality and morbidity in very low birthweight infants (Cleminson 2015) | Cerebral palsy at 18 to 22 months post term | 112 per 1000 (12/107) | 108 per 1000 (50 to 228) | RR 0.96 (0.45 to 2.03) | 219 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: herpes simplex | |||||||
| Aciclovir vs vidarabine for treatment of herpes simplex virus infection in neonates (Jones 2009) | Cerebral palsy in CNS HSV neonatal infection up to 3 years by HSV serotype: HSV‐1 | (0/4) | (0/5) | Not estimable | 9 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with design limitations (inadequate allocation concealment) Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Cerebral palsy in CNS HSV neonatal infection up to 3 years by HSV serotype: HSV‐2 | 625 per 1000 (5/8) | 669 per 1000 (306 to 1456) (4/6) | RR 1.07 (0.49 to 2.33) | 14 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with design limitations (inadequate allocation concealment) Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT | |
| Neonatal care: jaundice | |||||||
| Prophylactic phototherapy vs standard care for preventing jaundice in preterm or low birthweight infants (Okwundu 2012) | Cerebral palsy in all infants (birthweight < 2500 g) at 1 year or 18 months | Medium risk population: 84 per 1000 (18/394) | Medium risk population: 81 per 1000 (42 to 155) | RR 0.96 (0.50 to 1.85) | 756 (2 RCTs) | MODERATE | Study limitations (‐1): "There was no mention of blinding of the outcome assessors in two of the studies" (GRADED by review authors themselves) |
| Cerebral palsy in all infants (birthweight < 1000 g) at 18 months | 250 per 1000 (4/16) | 72 per 1000 (10 to 568) | RR 0.29 (0.04 to 2.27) | 30 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with no blinding Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT | |
| Cerebral palsy at 6 years | "Secondary reports emanating from Brown 1985 at six‐year follow‐up also showed that there was no significant difference in the rate of cerebral palsy between the phototherapy and control group" | NOT GRADED | |||||
| Neonatal care: hypoglycaemia | |||||||
| Dextrose gel vs placebo for treatment of hypoglycaemia in newborn infants (Weston 2016) | Cerebral palsy at age 2 years | (0/93) | (2/90) | RR 5.16 (0.25 to 106.12) | 183 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with 78% follow‐up Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: parenteral feeding | |||||||
| Glutamine supplementation vs placebo to prevent morbidity and mortality in preterm infants (Moe‐Byrne 2016) | Cerebral palsy at 2 years | "van den Berg 2005 reported neurodevelopmental outcomes for infants aged two years post term. Outcomes assessed included... incidence of cerebral palsy... No significant differences between the glutamine and the control groups were reported for any of these individual outcomes" | NOT GRADED | ||||
| Neonatal care: other | |||||||
| Thyroid hormones vs placebo for preventing neurodevelopmental impairment in preterm infants (Osborn 2001) | Cerebral palsy at 5.7 years | 120 per 1000 (9/75) | 86 per 1000 (34 to 221) | RR 0.72 (0.28 to 1.84) | 156 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Prophylactic thyroid hormones vs placebo for prevention of morbidity and mortality in preterm infants (Osborn 2007) | |||||||
| Silicone earplugs vs no earplugs in the neonatal intensive care unit for preterm or very low birthweight infants (Almadhoob 2015) | Cerebral palsy at 18 to 22 months' corrected age | (0/7) | (1/7) | RR 3.0 (0.14 to 63.15) | 14 (1 RCT) | VERY LOW | Study limitations (‐1): "Because of funding restraints only the ELBW infants could be followed at 18 to 22 months corrected age" (14/24 survivors) Imprecision (‐1): wide CI crossing line of no effect; 1 small RCT |
| Kangaroo mother care vs conventional neonatal care to reduce morbidity and mortality in low birthweight infants (Conde‐Agudelo 2016) | Cerebral palsy at 12 months' corrected age | 25 per 1000 (7/280) | 16 per 1000 (5 to 51) | RR 0.65 (0.21 to 2.02) | 588 (1 RCT) | LOW | Study limitation (‐1): 1 RCT with unclear risk of selection bias; high risk of performance and detection bias Imprecision (‐1): wide CI crossing line of no effect |
| Early developmental intervention vs standard follow‐up post hospital discharge to prevent motor and cognitive impairment in preterm infants (Spittle 2015) | Cerebral palsy at 18 months to 6 years | 79 per 1000 (32/405) | 65 per 1000 (41 to 100) | RR 0.82 (0.52 to 1.27) | 985 (7 RCTs) | LOW | Study limitations (‐1): 7 RCTs at unclear/high risk of performance bias; 2 RCTs at unclear/high risk of selection bias and unclear/high risk of attrition bias; 1 RCT at unclear risk of detection bias Imprecision (‐1): wide CI crossing line of no effect |
| Abbreviations: BPD: bronchopulmonary dysplasia; CI: confidence interval; CNS: central nervous system; CV: conventional ventilation; ELBW: extremely low birthweight; g: grams; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; HFOV: high‐frequency oscillatory ventilation; HSV: herpes simplex virus; NO: nitric oxide; OR: odds ratio; P: P value; qRCT: quasi‐randomised controlled trial; RCT: randomised controlled trial; RR: risk ratio. | |||||||
| Intervention and comparison | Outcome | Subgroup or sensitivity analysis | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (trials) | Test for subgroup differences | |
| Neonatal care: asphyxia | ||||||||
| Therapeutic hypothermia vs standard care for newborns with hypoxic‐ischaemic encephalopathy (Jacobs 2013) | Cerebral palsy in survivors assessed at 18 to 24 months | Method of cooling | Selective head cooling with mild hypothermia | 338 per 1000 (49/145) | 220 per 1000 (155 to 318) | RR 0.65 (0.46 to 0.94) | 312 (3 RCTs) | Chi² = 0.01, df = 1 (P = 0.93), I² = 0.0% |
| Whole body cooling | 360 per 1000 (94/261) | 241 per 1000 (187 to 310) | RR 0.67 (0.52 to 0.86) | 569 (4 RCTs) | ||||
| Neonatal care: haemorrhage: periventricular/intraventricular | ||||||||
| Ethamsylate vs placebo for prevention of morbidity and mortality in preterm or very low birthweight infants (Hunt 2010) | Cerebral palsy in surviving children available for follow‐up at 3 years up to 3.5 to 4.2 years | Infants < 31 completed weeks or < 1500 g | 84 per 1000 (14/167) | 69 per 1000 (32 to 147) | RR 0.82 (0.38 to 1.75) | 328 (2 RCTs) | Not applicable | |
| Neonatal care: fluid therapy | ||||||||
| Volume vs no treatment for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | Cerebral palsy in survivors at 2 years | Type of volume used | Fresh frozen plasma | 132 per 1000 (27/205) | 104 per 1000 (61 to 177) | RR 0.79 (0.46 to 1.34) | 408 (1 RCT) | Not performed (as conducted as separate comparison) |
| Gelatin | 132 per 1000 (27/205) | 97 per 1000 (53 to 169) | RR 0.74 (0.42 to 1.28) | 401 (1 RCT) | ||||
| Timing of treatment | Early treatment (< 24 hours age) | 132 per 1000 (27/205) | 100 per 1000 (63 to 158) | RR 0.76 (0.48 to 1.20) | 604 (1 RCT) | Not applicable | ||
| Types of infant enrolled | Unselected preterm infants (not selected on the basis of cardiovascular compromise) | |||||||
| Methodological quality | Complete follow‐up for neurodevelopmental outcomes | |||||||
| Neonatal care: respiratory distress syndrome | ||||||||
| Animal‐derived surfactant extract vs no treatment for treatment of respiratory distress syndrome (Seger 2009) | Cerebral palsy at 1 and 2 years' corrected age | Surfactant product | Porcine surfactant extract | 207 per 1000 (6/29) | 182 per 1000 (70 to 470) | RR 0.88 (0.34 to 2.27) | 73 (1 RCT) | Not applicable |
| Prophylactic protein‐free synthetic surfactant vs placebo for preventing morbidity and mortality in preterm infants (Soll 2010) | Cerebral palsy at 1 or 2 years | Surfactant product | Exosurf Neonatal | 158 per 1000 (44/279) | 144 per 1000 (98 to 211) | RR 0.91 (0.62 to 1.34) | 591 (3 RCTs) | Not applied in review |
| DPPC/HDL | 122 per 1000 (5/41) | 132 per 1000 (41 to 420) | RR 1.08 (0.34 to 3.44) | 79 (1 RCT) | ||||
| Neonatal care: mechanical ventilation | ||||||||
| Continuous distending pressure vs standard care for respiratory distress in preterm infants (Ho 2015) | Cerebral palsy at 9 to 15 years | Type of continuous distending pressure | Continuous negative pressure | (0/18) | (2/18) | RR 5.0 (0.26 to 97.37) | 36 (1 RCT) | Not applicable |
| Neonatal care: bronchopulmonary dysplasia | ||||||||
| Early (< 8 days) postnatal corticosteroids vs placebo or no treatment for preventing chronic lung disease in preterm infants (Doyle 2014b) | Cerebral palsy at 11 months to 7 to 9 years | Type of corticosteroid used | Dexamethasone | 89 per 1000 (40/449) | 156 per 1000 (107 to 227) | RR 1.75 (1.20 to 2.55) | 921 (7 RCTs) | Chi² = 2.96, df = 1 (P = 0.09), I² = 66% |
| Hydrocortisone | 86 per 1000 (23/266) | 84 per 1000 (48 to 146) | RR 0.97 (0.55 to 1.69) | 531 (5 RCTs) | ||||
| Cerebral palsy in survivors assessed at 11 months to 7 to 9 years | Type of corticosteroid used | Dexamethasone | 139 per 1000 (40/288) | 253 per 100 (179 to 357) | RR 1.82 (1.29 to 2.57) | 586 (7 RCTs) | Chi² = 3.99, df = 1 (P = 0.05), I² = 75% | |
| Hydrocortisone | 126 per 1000 (23/182) | 120 per 1000 (71 to 206) | RR 0.95 (0.56 to 1.63) | 373 (5 RCTs) | ||||
| Neonatal care: other | ||||||||
| Prophylactic thyroid hormones vs placebo for prevention of morbidity and mortality in preterm infants (Osborn 2007) | Cerebral palsy at 5.7 years | Dosing strategy | T4 8 mcg/kg/d, day 1 to 42 | 120 per 1000 (9/75) | 86 per 1000 (34 to 221) | RR 0.72 (0.28 to 1.84) | 156 (1 RCT) | Not applicable |
| Timing | Commenced < 48 hours | |||||||
| Methodological quality | Studies with adequate methods | |||||||
| Early developmental intervention vs standard follow‐up post hospital discharge to prevent motor and cognitive impairment in preterm infants (Spittle 2015) | Cerebral palsy at 18 months to 6 years | Commencement of intervention | Inpatient | 79 per 1000 (12/152) | 74 per 1000 (36 to 152) | RR 0.94 (0.46 to 1.93) | 354 (3 RCTs) | Not applied in review |
| Post hospital discharge | 79 per 1000 (20/253) | 59 per 1000 (34 to 105) | RR 0.75 (0.43 to 1.33) | 631 (4 RCTs) | ||||
| Focus of intervention | Parent‐infant relationship and Infant development | 77 per 1000 (21/272) | 52 per 1000 (29 to 90) | RR 0.67 (0.38 to 1.17) | 716 (4 RCTs) | Not applied in review | ||
| Infant development | 83 per 1000 (11/133) | 97 per 1000 (46 to 203) | RR 1.17 (0.56 to 2.46) | 269 (3 RCTs) | ||||
| Quality of studies | Higher‐quality studies | 82 per 1000 (25/304) | 72 per 1000 (44 to 116) | RR 0.87 (0.53 to 1.41) | 776 (5 RCTs) | Not applied in review | ||
| Lower‐quality studies | 69 per 1000 (7/101) | 43 per 1000 (14 to 130) | RR 0.62 (0.20 to 1.87) | 209 (2 RCTs) | ||||
| Abbreviations: CI: confidence interval; DPPC/HDL: dipalmitoylphosphatidylcholine/high‐density lipoprotein; g: grams; P: P value; RCT: randomised controlled trial; RR: risk ratio; T4: thyroxine. | ||||||||
| Intervention and comparison | Outcome | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | Comments |
| Neonatal care: bronchopulmonary dysplasia | |||||||
| Early (< 8 days) postnatal corticosteroids vs placebo or no treatment for preventing chronic lung disease in preterm infants (Doyle 2014b) | Cerebral palsy or death at 11 months to 7 to 9 years | 352 per 1000 (252/715) | 384 per 1000 (334 to 441) | RR 1.09 (0.95 to 1.25) | 1452 (12 RCTs) | MODERATE | Study limitations (‐1): 4 RCTs at unclear risk of selection bias; 2 RCTs at high risk of performance and detection bias; 2 RCTs had 13% to 53% follow‐up overall |
| Moderately early (7‐14 days) postnatal corticosteroids vs placebo or no treatment for preventing chronic lung disease in preterm infants (Halliday 2003) | Cerebral palsy or death at 12 months' corrected age up to 90 months | 316 per 1000 (30/95) | 262 per 1000 (174 to 388) | RR 0.83 (0.55 to 1.23) | 204 (4 RCTs) | VERY LOW | Study limitations (‐1): 2 RCTs with 68% to 70% follow‐up; 1 RCT with unclear blinding of outcome assessment Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Late (> 7 days) postnatal corticosteroids vs placebo or no treatment for chronic lung disease in preterm infants (Doyle 2014) | Cerebral palsy or death at 1 to 3 years | 328 per 1000 (142/433) | 302 per 1000 (249 to 367) | RR 0.92 (0.76 to 1.12) | 876 (14 RCTs) | LOW | Study limitations (‐1): 5 RCTs unclear risk of selection bias; 5 RCTs with follow‐up between 32% and 78% Imprecision (‐1): wide CI crossing line of no effect |
| Cerebral palsy or death at latest reported age (from 1 year up to 17 years) | 312 per 1000 (132/423) | 296 per 1000 (240 to 362) | RR 0.95 (0.77 to 1.16) | 855 (15 RCTs) | LOW | Study limitations (‐1): 5 RCTs unclear risk of selection bias; 7 RCTs with follow‐up between 32% and 78% Imprecision (‐1): wide CI crossing line of no effect | |
| Neonatal care: other | |||||||
| Thyroid hormones vs placebo for preventing neurodevelopmental impairment in preterm infants (Osborn 2001) | Cerebral palsy or death at 5.7 years | 300 per 1000 (30/100) | 210 per 1000 (129 to 342) | RR 0.70 (0.43 to 1.14) | 200 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Prophylactic thyroid hormones vs placebo for prevention of morbidity and mortality in preterm infants (Osborn 2007) | |||||||
| Abbreviations: CI: confidence interval; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; RCT: randomised controlled trial; RR: risk ratio. | |||||||
| Intervention and comparison | Outcome | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | Comments |
| Neonatal care: asphyxia | |||||||
| Allopurinol vs placebo or no drug for preventing mortality and morbidity in newborn infants with hypoxic‐ischaemic encephalopathy (Chaudhari 2012) | Severe quadriplegia in surviving infants (18 months and 4 to 8 years) | 343 per 1000 (12/35) | 202 per 1000 (96 to 435) | RR 0.59 (0.28 to 1.27) | 73 (3 RCTs) | VERY LOW | Study limitations (‐1): 1 RCT with unclear risk of selection bias and high risk of performance/detection bias Imprecision (‐2): wide CI crossing line of no effect; small sample sizes with few events |
| Neonatal care: respiratory distress syndrome | |||||||
| Synthetic surfactant vs placebo for respiratory distress syndrome in preterm infants (Soll 2000) | Moderate to severe cerebral palsy in survivors examined at 1 year (in 4 of the 5 RCTs) | 55 per 1000 (42/767) | 41 per 1000 (26 to 64) | RR 0.75 (0.48 to 1.16) | 1557 (5 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing the line of no effect |
| Prophylactic protein‐free synthetic surfactant vs placebo for preventing morbidity and mortality in preterm infants (Soll 2010) | Moderate/severe cerebral palsy at 1 or 2 years | 75 per 1000 (24/320) | 69 per 1000 (40 to 119) | RR 0.92 (0.53 to 1.59) | 670 (4 RCTs) | LOW | Study limitations (‐1): "Somewhat fewer infants who received surfactant failed to return for follow‐up evaluation (typical relative risk 0.63, 95% CI 0.48, 0.82; typical risk difference ‐0.10, 95% CI ‐0.15, ‐0.04)" Imprecision (‐1): wide CI crossing the line of no effect |
| Abbreviations: CI: confidence interval; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; RCT: randomised controlled trial; RR: risk ratio. | |||||||
| Intervention and comparison | Outcome | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | Comments |
| Neonatal care: asphyxia | |||||||
| Allopurinol vs placebo or no drug for preventing mortality and morbidity in newborn infants with hypoxic‐ischaemic encephalopathy (Chaudhari 2012) | Death or severe neurodevelopmental disability in survivors (18 months and 4 to 8 years) (defined as any 1 or combination of the following: non‐ambulant cerebral palsy, severe developmental delay assessed via validated tools, auditory and visual impairment) | 611 per 1000 (33/54) | 477 per 1000 (342 to 660) | RR 0.78 (0.56 to 1.08) | 110 (3 RCTs) | VERY LOW | Study limitations (‐1): 1 RCT with unclear risk of selection bias and high risk of performance/detection bias Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Therapeutic hypothermia vs standard care for newborns with hypoxic‐ischaemic encephalopathy (Jacobs 2013) | Death or major disability in survivors assessed at 18 to 24 months (defined as cerebral palsy, developmental delay (BSID or GMDS assessment > 2 SD below the mean) or intellectual impairment (IQ > 2 SD below mean), blindness (vision < 6/60 in both eyes), sensorineural deafness requiring amplification) | 614 per 1000 (409/666) | 461 per 1000 (418 to 510) | RR 0.75 (0.68 to 0.83) | 1344 (8 RCTs) | HIGH | Not downgraded |
| Major neurodevelopmental disability at 18 to 24 months (defined as cerebral palsy, developmental delay (BSID or GMDS assessment > 2 SD below the mean) or intellectual impairment (IQ > 2 SD below mean), blindness (vision < 6/60 in both eyes), sensorineural deafness requiring amplification) | 249 per 1000 (166/666) | 192 per 1000 (157 to 234) | RR 0.77 (0.63 to 0.94) | 1344 (8 RCTs) | HIGH | Not downgraded | |
| Major neurodevelopmental disability in survivors assessed at 18 to 24 months (defined as cerebral palsy, developmental delay (BSID or GMDS assessment > 2 SD below the mean) or intellectual impairment (IQ > 2 SD below mean), blindness (vision < 6/60 in both eyes), sensorineural deafness requiring amplification) | 393 per 1000 (166/422) | 264 per 1000 (216 to 315) | RR 0.67 (0.55 to 0.80) | 917 (8 RCTs) | HIGH | Not downgraded | |
| Death or moderate to severe disability at 6 to 7 years (defined as IQ ≥ 2 SD below the mean, a GMF level of 3 or greater, bilateral deafness (with or without amplification), bilateral blindness, or refractory epilepsy) | 645 per 1000 (60/93) | 523 per 1000 (413 to 671) | RR 0.81 (0.64 to 1.04) | 190 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size | |
| Moderate‐to‐severe disability at 6 to 7 years (defined as IQ ≥ 2 SD below the mean, a GMF level of 3 or greater, bilateral deafness (with or without amplification), bilateral blindness or refractory epilepsy) | 380 per 1000 (19/50) | 350 per 1000 (217 to 562) | RR 0.92 (0.57 to 1.48) | 119 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size | |
| Barbiturates (phenobarbital) vs conventional therapy for prevention of morbidity and mortality following perinatal asphyxia (Young 2016) | Death or major neurodevelopmental disability follow‐up: > 12 months (3 years) (defined as cerebral palsy, developmental delay (BSID or GMDS assessment > 2 SD below the mean) or intellectual impairment (IQ > 2 SD below mean), blindness (vision < 6/60 in both eyes), or sensorineural deafness requiring amplification) | 813 per 1000 (13/16) | 268 per 1000 (114 to 634) | RR 0.33 | 31 (1 RCT) | VERY LOW | Study limitations (‐1): unblinded study; concern regarding performance bias, detection bias, and incomplete follow‐up (graded by review authors themselves) |
| Major neurodevelopmental disability follow‐up: > 12 months (3 years) (defined as cerebral palsy, developmental delay (BSID or GMDS assessment > 2 SD below the mean) or intellectual impairment (IQ > 2 SD below mean), blindness (vision < 6/60 in both eyes), or sensorineural deafness requiring amplification) | 563 per 1000 (9/16) | 135 per 1000 (34 to 518) | RR 0.24 | 31 (1 RCT) | VERY LOW | Study limitations (‐1): unblinded study; concern regarding performance bias, detection bias, and incomplete follow‐up (graded by review authors themselves) | |
| Neonatal care: haemorrhage: periventricular/intraventricular | |||||||
| Ethamsylate vs placebo for prevention of morbidity and mortality in preterm or very low birthweight infants (Hunt 2010) | Neurodevelopmental disability at 2 years of age in surviving children available for follow‐up (defined as cerebral palsy on clinical examination, developmental delay > 2 SD below population mean on any standard test of development, or blindness (VA < 6/60), or deafness (any hearing impairment requiring amplification) at any time after 2 years' corrected age) | 170 per 1000 (46/270) | 135 per 1000 (90 to 199) | RR 0.79 (0.53 to 1.17) | 532 (3 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| Death or any disability by 2 years of age in children with known outcome at any point in time (not defined in review) | 338 per 1000 (233/690) | 324 per 1000 (277 to 375) | RR 0.96 (0.82 to 1.11) | 1334 (7 RCTs) | LOW | Study limitations (‐1): 4 RCTs at unclear risk of selection bias; 3 RCTs at unclear risk of bias due to lack of blinding Imprecision (‐1): wide CIs crossing line of no effect | |
| Phenobarbital vs no treatment for prevention of intraventricular haemorrhage in preterm infants (Smit 2013) | Severe neurodevelopmental impairment at 27 months (defined as clinical cerebral palsy or DQ below the range that can be measured) | 74 per 1000 (4/54) | 107 per 1000 (30 to 373) | RR 1.44 (0.41 to 5.04) | 101 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at high risk of performance bias) and unclear risk of other bias Imprecision (‐2): wide CI crossing line of no effect; small sample size, low event rate |
| Mild neurodevelopmental impairment at 27 months (defined as DQ < 80 or motor abnormality on examination) | 111 per 1000 (6/54) | 63 per 1000 (17 to 241) | RR 0.57 (0.15 to, 2.17) | 101 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at high risk of performance bias) and unclear risk of other bias Imprecision (‐2): wide CI crossing line of no effect; small sample size, low event rate | |
| Neonatal care: hypotension | |||||||
| Dobutamine vs dopamine in preterm infants with low superior vena cava flow (Osborn 2007b) | Disability at 3 years in survivors (defined as GMDS quotient ≤ 70, cerebral palsy, blind (VA < 6/60) or deaf (hearing aids)) | 714 per 1000 (5/7) | 71 per 1000 (7 to 1114) | RR 0.10 (0.01 to 1.56) | 13 (1 RCT) | VERY LOW | Study limitations (‐1): 5/18 surviving infants were not assessed at 3 years Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with very small sample size |
| Death or disability at 3 years (defined as GMDS quotient ≤ 70, cerebral palsy, blind (VA < 6/60) or deaf (hearing aids)) | 882 per 1000 (15/17) | 697 per 1000 (503 to 979) | RR 0.79 (0.57 to 1.11) | 37 (1 RCT) | VERY LOW | Study limitations (‐1): 5/18 surviving infants were not assessed at 3 years Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with very small sample size | |
| Death or disability at latest follow‐up (1 to 3 years) (defined as GMDS quotient ≤ 70, cerebral palsy, blind (VA < 6/60) or deaf (hearing aids)) | 750 per 1000 (15/20) | 713 per 1000 (495 to 1035) | RR 0.95 (0.66 to 1.38) | 41 (1 RCT) | VERY LOW | Study limitations (‐1): 5/18 surviving infants were not assessed at 3 years Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with very small sample size | |
| Neonatal care: fluid therapy | |||||||
| Volume vs no treatment for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | Severe neurodevelopmental disability in survivors at 2 years (defined as blind, dead, unable to walk, DQ > 3 SD below the mean, or another severe disability) | 141 per 1000 (29/205) | 113 per 1000 (74 to 174) | RR 0.80 (0.52 to 1.23) | 604 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| Gelatin vs fresh frozen plasma for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | 113 per 1000 (23/203) | 112 per 1000 (65 to 195) | RR 0.99 (0.57 to 1.72) | 399 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect | |
| Volume vs no treatment for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | Death or severe neurodevelopmental disability in survivors at 2 years (defined as blind, dead, unable to walk, DQ > 3 SD below the mean, or another severe disability) | 318 per 1000 (82/258) | 318 per 1000 (254 to 394) | RR 1.00 (0.80 to 1.24) | 776 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| Gelatin vs fresh frozen plasma for prevention of morbidity and mortality in very preterm infants (Osborn 2004) | 300 per 1000 (77/257) | 333 per 1000 (258 to 428) | RR 1.11 (0.86 to 1.43) | 518 (1 RCT) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect | |
| Neonatal care: patent ductus arteriosus | |||||||
| Prophylactic indomethacin vs placebo for preventing mortality and morbidity in preterm infants (Fowlie 2010) | Death or severe neurodevelopmental disability at 18 to 36 months (defined as any 1 or a combination of the following: non‐ambulant cerebral palsy, developmental delay (DQ < 70), auditory and visual impairment) | 400 per 1000 (299/748) | 407 per 1000 (360 to 460) | RR 1.02 (0.90 to 1.15) | 1491 (3 RCTs) | MODERATE | Study limitations (‐1): 2 RCTs at unclear risk of attrition bias (> 25% loss to follow‐up) |
| Neonatal care: blood disorders | |||||||
| Erythropoietin vs placebo for preventing red blood cell transfusion in preterm and/or low birthweight infants (Ohlsson 2014) | Any neurodevelopmental impairment at 18 to 22 months' corrected age (in children examined) (not defined in review; definition from trial manuscript: 1 of the following: MDI < 70, PDI < 70, moderate or severe cerebral palsy, blindness, or deafness) | 451 per 1000 (23/51) | 437 per 1000 (280 to 681) | RR 0.97 (0.62 to 1.51) | 99 (1 RCT) | VERY LOW | Study limitations: 1 RCT at unclear risk of selection bias and high risk of attrition bias (˜73% follow‐up) Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size |
| Transfusion at a restrictive vs a liberal haemoglobin threshold for preventing morbidity and mortality in very low birthweight infants (Whyte 2011) | Any neurosensory impairment at 18 to 21 months' follow‐up among survivors (defined as cognitive delay (MDI < 70), cerebral palsy, severe visual impairment, severe hearing impairment) | 220 per 1000 (37/168) | 289 per 1000 (198 to 418) | RR 1.31 (0.90 to 1.90) | 328 (1 RCT) | LOW | Study limitations (‐1): 1 RCT at high risk of performance bias and unclear risk of reporting bias Imprecision (‐1): wide CI crossing line of no effect |
| Death or severe morbidity at 18 to 21 months' follow‐up (defined as cognitive delay (MDI < 70), cerebral palsy, severe visual impairment, severe hearing impairment) | 385 per 1000 (82/213) | 450 per 1000 (362 to 566) | RR 1.17 (0.94 to 1.47) | 421 (1 RCT) | LOW | Study limitations (‐1): 1 RCT at high risk of performance bias and unclear risk of reporting bias Imprecision (‐1): wide CI crossing line of no effect | |
| Death or severe morbidity at 18 to 21 months' follow‐up (defined as cognitive delay (MDI < 85), cerebral palsy, severe visual impairment, severe hearing impairment) | 498 per 1000 (106/213) | 602 per 1000 (503 to 717) | RR 1.21 (1.01 to 1.44) | 421 (1 RCT) | MODERATE | Study limitations (‐1): 1 RCT at high risk of performance bias and unclear risk of reporting bias | |
| Neonatal care: nitric oxide | |||||||
| Inhaled NO vs placebo for respiratory failure in preterm infants (entry before 3 days based on oxygenation) (Barrington 2010) | Neurodevelopmental disability at 18 to 22 months (defined as moderate or severe cerebral palsy, blind, deaf, BSID MDI < 70, or PDI < 70) | 455 per 1000 (50/110) | 477 per 1000 (355 to 636) | RR 1.05 (0.78 to 1.40) | 208 (2 RCTs) | LOW | Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Inhaled NO vs placebo or no treatment for respiratory failure in preterm infants (entry after 3 days based on BPD risk) (Barrington 2010) | Neurodevelopmental disability at 2 years' corrected age or 30 months (defined as 1 RCT: moderate or severe cerebral palsy, blind, deaf, BSID MDI < 70, or PDI < 70; 1 RCT: cerebral palsy, BSID MDI or PDI < 71, or sensorineural impairment) | 480 per 1000 (119/248) | 432 per 1000 (355 to 523) | RR 0.90 (0.74 to 1.09) | 498 (2 RCTs) | LOW | Study limitations (‐1): 1 RCT with no blinding of intervention or outcome measurement Imprecision (‐1): wide CI crossing line of no effect; small sample sizes |
| Inhaled NO vs placebo for respiratory failure in preterm infants (studies of routine use in intubated preterm infants) (Barrington 2010) | Neurodevelopmental disability at 1 or 2 years' corrected age (defined as 1 RCT: cerebral palsy, blind, severe hearing loss, BSID MDI < 70, or PDI < 70; 1 RCT: cerebral palsy, bilateral blindness, bilateral hearing loss, or BSID score > 2 SD below the mean) | 364 per 100 (104/286) | 327 per 1000 (262 to 411) | RR 0.90 (0.72 to 1.13) | 593 (2 RCT) | VERY LOW | Study limitations (‐1): 2 RCTs with 74% to 82% follow‐up Imprecision (‐1): wide CI crossing line of no effect Inconsistency (‐1): substantial heterogeneity I² = 83% |
| Inhaled nitric oxide vs control for respiratory failure in infants born at or near term (Finer 2006) | Neurodevelopmental disability among survivors at 13 or 18 to 24 months (defined as 1 RCT: cerebral palsy, BSID MDI or PDI < 2 SD below the mean, blind or hearing impaired; or 1 RCT: cerebral palsy, > 2 mild (mild neurological abnormalities; mild reductions in BSID scores 1 to 2 SD below the mean), or at least 1 severe impairment) | 265 per 1000 (48/181) | 257 per 1000 (175 to 382) | RR 0.97 (0.66 to 1.44) | 301 (2 RCTs) | LOW | Study limitations (‐1): 1 RCT masking of allocation, masking of outcomes, and completeness of follow‐up 'can't tell' Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: apnoea | |||||||
| Caffeine vs placebo for apnoea in preterm infants (Henderson‐Smart 2010b) | Death or major disability at 18 to 21 months' corrected age (defined as cognitive delay, cerebral palsy, deafness, or blindness) | 417 per 1000 (153/367) | 354 per 1000 (296 to 421) | RR 0.85 (0.71 to 1.01) | 767 (1 RCT) | LOW | Study limitations (‐1): results based on 1 RCT subgroup from post hoc subgroup analyses (randomisation not stratified according to indication for inclusion) Imprecision (‐1): wide CI crossing line of no effect |
| Caffeine vs placebo for prevention of apnoea in preterm infants (Henderson‐Smart 2010c) | Death or major disability at 18 to 21 months' corrected age (not defined in review; definition from trial manuscript: cerebral palsy, cognitive delay, severe hearing loss, or bilateral blindness) | 431 per 1000 (88/204) | 431 per 1000 (345 to 535) | RR 1.00 (0.80 to 1.24) | 423 (1 RCT) | LOW | Study limitations (‐1): results based on 1 RCT subgroup from post hoc subgroup analyses (randomisation not stratified according to indication for inclusion) Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: respiratory distress syndrome | |||||||
| Inositol supplementation (repeat doses) vs placebo in preterm infants at risk for or having respiratory distress syndrome (Howlett 2015) | Major neural developmental impairment at 1 year corrected age (defined as sensory deficit, cerebral palsy, developmental delay, severe hypotonia) | 178 per 1000 (13/73) | 94 per 1000 (43 to 207) | RR 0.53 (0.24 to 1.16) | 169 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection, performance, detection, and reporting bias, and at high risk of other bias Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size |
| Animal‐derived surfactant extract vs no treatment for treatment of respiratory distress syndrome (Seger 2009) | Major developmental disability in survivors at 1 and 2 years' corrected age (defined as severe forms of cerebral palsy, blindness, deafness requiring hearing aids, or GMDS DQ < 70) | 34 per 1000 (1/29) | 114 per 1000 (5 to 923) | RR 3.30 (0.14 to 26.78) | 73 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with no blinding of intervention; and blinding of outcome measurement not reported Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: mechanical ventilation | |||||||
| Continuous distending pressure vs standard care for respiratory distress in preterm infants (Ho 2015) | Death or severe disability at 9 to 15 years (not defined in review; severe disability as defined below) | 158 per 1000 (3/19) | 210 per 1000 (54 to 816) | RR 1.33 (0.34 to 5.17) | 38 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection and attrition bias and high risk of performance bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Severe disability at 9 to 15 years (not defined in review; definition from trial manuscript: unable to undertake activity without aids or assistance most of the time, or completely dependent on carer: WASI ≤ 69; GMF level 4 to 5, arms: needs assistance to feed and dress; VA < 6/60, gross movement/light and dark only or worse; hearing loss not corrected with age; parent and teacher overall difficulties (Q26), "Yes" and impact score 6 to 10 parent and 3 to 6 teacher; or other condition needs supervision/aid constantly ‐ includes continuous home oxygen; communication severely limited) | 158 per 1000 (3/19) | 167 per 1000 (38 to 722) | RR 1.06 (0.24 to 4.57) | 37 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection and attrition bias and high risk of performance bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT | |
| Any disability at 9 to 15 years (not defined in review; definition from trial manuscript: mild: some loss of function but able to function independently; moderate: aids or assistance needed for some tasks. Moderate difficulty in doing some activities; severe: unable to undertake activity without aids or assistance most of the time, or completely dependent on carer; includes neuromotor components includes GMF levels 1 to 5) | 632 per 1000 (12/19) | 392 per 1000 (196 to 764) | RR 0.62 (0.31 to 1.21) | 37 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection and attrition bias and high risk of performance bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT | |
| Prophylactic methylxanthines (caffeine) vs placebo for endotracheal extubation in preterm infants (Henderson‐Smart 2010) | Death or major disability at 18 to 21 months' corrected age (defined as cognitive delay, cerebral palsy, deafness, or blindness) | 525 per 1000 (189/360) | 446 per 1000 (383 to 520) | RR 0.85 (0.73 to 0.99) | 676 (1 RCT) | MODERATE | Study limitations (‐1): results based on 1 RCT subgroup from post hoc subgroup analyses (randomisation not stratified according to indication for inclusion) |
| Volume‐targeted vs pressure‐limited ventilation in the neonate (Wheeler 2010) | Severe disability (any definition) at 6 to 18 months and 22 months (definitions not reported in review; definitions from trial manuscripts: 1 RCT: abnormal neurological evaluation (gross or fine motor delay) or BSID MDI < 70; 1 RCT: cerebral palsy severe enough to hamper gross motor activity, deafness needing hearing aids, registered blind or partially sighted) | 176 per 1000 (18/102) | 152 per 1000 (83 to 281) | RR 0.86 (0.47 to 1.59) | 209 (2 RCTs) | LOW | Indirectness (‐1): post hoc analysis including 2 RCTs with varied definitions Imprecision (‐1): wide CI crossing line of no effect (post hoc analysis in review) |
| Severe disability (any definition) at 22 months or death (definition not reported in review; definition from trial manuscript: cerebral palsy severe enough to hamper gross motor activity, deafness needing hearing aids, registered blind or partially sighted) | 327 per 1000 (17/52) | 177 per 1000 (88 to 347) | RR 0.54 (0.27 to 1.06) | 109 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT (post hoc analysis in review) | |
| Neonatal care: bronchopulmonary dysplasia | |||||||
| Early (< 8 days) postnatal corticosteroids vs placebo or no treatment for preventing chronic lung disease in preterm infants (Doyle 2014b) | Major neurosensory disability at 18 to 22 months to 53 months (variable criteria reported in review: 1 RCT: non‐ambulant cerebral palsy, global retardation (not specified), blindness, or deafness; 1 RCT: moderate or severe cerebral palsy, blindness, deafness, or BSID MDI or PDI < ‐2 SD; 1 RCT: cerebral palsy, BSID MDI or PDI < 71, blindness or deafness; 1 RCT: severe motor dysfunction (child non‐ambulant), or BSID MDI or PDI <‐2 SD; 2 RCTs: cerebral palsy, blindness, deafness, or developmental delay (BSID MDI < 70 (< ‐2 SD) or GMDS DQ < 70); 1 RCT: cerebral palsy, functional blindness, functional deafness, developmental delay (BSID MDI < 70 (<‐2 SD)), or motor delay (BSID PDI < 70 (<‐2 SD)) | 199 per 1000 (121/607) | 231 per 1000 (187 to 285) | RR 1.16 (0.94 to 1.43) | 1233 (7 RCTs) | LOW | Study limitations (‐1): 2 RCTs at unclear risk of selection bias; 1 RCT at high risk of performance and detection bias Imprecision (‐1): wide CI crossing line of no effect |
| Major neurosensory disability in survivors examined at 18 to 22 months to 53 months (variable criteria as above) | 307 per 1000 (121/394) | 350 per 1000 (289 to 424) | RR 1.14 (0.94 to 1.38) | 799 (7 RCTs) | LOW | Study limitations (‐1): 2 RCTs at unclear risk of selection bias; 1 RCT at high risk of performance and detection bias Imprecision (‐1): wide CI crossing line of no effect | |
| Death or major neurosensory disability at 18 to 22 months to 53 months (variable criteria as above) | 466 per 1000 (283/607) | 490 per 1000 (434 to 545) | RR 1.05 (0.93 to 1.17) | 1233 (7 RCTs) | MODERATE | Study limitations (‐1): 2 RCTs at unclear risk of selection bias; 1 RCT at high risk of performance and detection bias | |
| Moderately early (7‐14 days) postnatal corticosteroids vs placebo for preventing chronic lung disease in preterm infants (Halliday 2003) | Major neurosensory disability at 15 months' corrected age up to 90 months (variable criteria reported in review: 1 RCT: any of cerebral palsy or a BSID MDI or PDI < ‐ 1 SD; 1 RCT: not specified) | 98 per 1000 (4/41) | 123 per 1000 (44 to 340) | RR 1.26 (0.45 to 3.49) | 96 (2 RCTs) | VERY LOW | Study limitations (‐1): 1 RCT with 70% follow‐up and unclear blinding of outcome assessment Imprecision (‐2): wide CI crossing line of no effect; small sample sizes |
| Major neurosensory disability in survivors assessed at 15 months' corrected age up to 90 months (variable criteria reported in review as above) | 174 per 1000 (4/23) | 155 per 1000 (66 to 365) | RR 0.89 (0.38 to 2.10) | 56 (2 RCTs) | VERY LOW | Study limitations (‐1): 1 RCT with 70% follow‐up and unclear blinding of outcome assessment Imprecision (‐2): wide CI crossing line of no effect; small sample sizes | |
| Death or major neurosensory disability at 15 months' corrected age up to 90 months (variable criteria reported in review as above) | 366 per 1000 (15/41) | 373 per 1000 (241 to 571) | RR 1.02 (0.66 to 1.56) | 96 (2 RCTs) | VERY LOW | Study limitations (‐1): 1 RCT with 70% follow‐up and unclear blinding of outcome assessment Imprecision (‐2): wide CI crossing line of no effect; small sample sizes | |
| Late (> 7 days) postnatal corticosteroids vs placebo or no treatment for chronic lung disease in preterm infants (Doyle 2014) | Major neurosensory disability at 1 year corrected age up to 11 years (variable criteria reported in review: 1 RCT: non‐ambulant cerebral palsy, < 50% of age level on the Minnesota CDI, or predicted special schooling for sensory or other impairment; 1 RCT: abnormal neurological examination (i.e. cerebral palsy), cognitive delay (IQ < 71) or not in a regular classroom; 1 RCT: severe disability ‐ bilateral blindness, cerebral palsy with the child unlikely ever to walk or BSID MDI < 55 (< ‐3 SD)) or moderate disability ‐ deafness, cerebral palsy in children not walking at 2 years but expected to walk, or MDI from 55 to < 70 (‐3 SD to < ‐2 SD); 1 RCT: cerebral palsy, blindness, deafness requiring hearing aids or worse, or developmental delay (defined as BSID MDI < 70); 1 RCT: more than mild cerebral palsy, blindness, deafness, or needing extra help with schooling; 1 RCT: blindness, cerebral palsy or a BSID MDI < 70 OR cerebral palsy or mental retardation (IQ < 70 on either the DAS or the WISC‐III and a VABS composite score < 70); 1 RCT: not specified; 1 RCT moderate or severe cerebral palsy, bilateral blindness, deafness or an MDI < 2 SD | 169 per 1000 (54/320) | 197 per 1000 (143 to 270) | RR 1.17 (0.85 to 1.60) | 655 (8 RCTs) | LOW | Study limitations (‐1): 3 RCTs with unclear risk of selection bias; 3 RCTs with follow‐up rates 60% to 78% Imprecision (‐1): wide CI crossing line of no effect |
| Major neurosensory disability in survivors assessed at 1 year corrected age up to 11 years (variable criteria reported in review as above) | 231 per 1000 (54/234) | 254 per 1000 (187 to 346) | RR 1.10 (0.81 to 1.50) | 480 (8 RCTs) | LOW | Study limitations (‐1): 3 RCTs with unclear risk of selection bias; 3 RCTs with follow‐up rates 60% to 78% Imprecision (‐1): wide CI crossing line of no effect | |
| Death or major neurosensory disability at 1 year corrected age up to 11 years (variable criteria reported in review as above) | 375 per 1000 (120/320) | 390 per 1000 (323 to 473) | RR 1.04 (0.86 to 1.26) | 655 (8 RCTs) | LOW | Study limitations (‐1): 3 RCTs with unclear risk of selection bias; 3 RCTs with follow‐up rates 60% to 78% Imprecision (‐1): wide CI crossing line of no effect | |
| Supplemental vitamin A vs a sham injection to prevent mortality and short‐ and long‐term morbidity in very low birthweight infants (Darlow 2016) | Neurodevelopmental impairment at 18 to 24 months (defined as BSID‐II MDI < 70, PDI < 70, any cerebral palsy, blind in both eyes, or bilateral hearing aids ) | 481 per 1000 (128/266) | 428 per 1000 (356 to 520) | RR 0.89 (0.74 to 1.08) | 538 (1 RCT) | LOW | Imprecision (‐2): "Concerning imprecision: does not met the optimal information size criteria" (graded by review authors themselves) |
| Death or neurodevelopmental impairment at 18 to 24 months (defined as BSID‐II MDI < 70, PDI < 70, any cerebral palsy, blind in both eyes, or bilateral hearing aids) | 596 per 1000 (204/342) | 549 per 1000 (483 to 626) | RR 0.92 (0.81 to 1.05) | 687 (1 RCT) | MODERATE | Imprecision (‐1): wide CIs crossing line of no effect | |
| Neonatal care: necrotising enterocolitis | |||||||
| Probiotics vs control (distilled water) for prevention of necrotising enterocolitis in preterm infants (AlFaleh 2014) | Mental retardation and cerebral palsy at 6 years | 47 per 1000 (2/43) | 47 per 1000 (7 to 323) | RR 1.02 (0.15 to 6.94) | 85 (1 RCT) | VERY LOW | Study limitation (‐1): 1 RCT at unclear risk for selection, performance, and detection bias; and high risk of attrition and reporting bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Arginine supplementation vs placebo for prevention of necrotising enterocolitis in preterm infants (Shah 2007) | Major neurodevelopmental disability at 36 months' post‐menstrual age (definition not reported in review; definition from trial manuscript: presence of 1 or more of cerebral palsy, cognitive delay (index < 70), bilateral blindness, bilateral hearing loss requiring aids) | 127 per 1000 (9/71) | 82 per 1000 (29 to 232) | RR 0.65 (0.23 to 1.83) | 132 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: fungal infections | |||||||
| Systemic antifungal agent vs placebo to prevent mortality and morbidity in very low birthweight infants (Cleminson 2015) | Neurodevelopmental impairment (composite) at 18 to 22 months (defined as at least 1 of (i) BSID‐III cognition composite score < 70, (ii) cerebral palsy, (iii) deafness or, (iv) blindness) | 274 per 1000 (23/84) | 309 per 1000 (194 to 496) | RR 1.13 (0.71 to 1.81) | 171 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: herpes simplex | |||||||
| Vidarabine vs placebo for treatment of herpes simplex virus infection in neonates (Jones 2009) | Abnormal neurodevelopment at approximately 1 year of age (not defined in review; definition from trial manuscript: spasticity or hemiparesis only; or combinations of microcephaly, paresis, spasticity, seizures, blindness, or deafness) | 214 per 1000 (6/28) | 321 per 1000 (133 to 782) | RR 1.50 (0.62 to 3.65) | 56 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with design limitations (method of randomisation not stated) Imprecision (‐2): wide CIs crossing line of no effect; 1 small RCT |
| Abnormal neurodevelopment or death at approximately 1 year of age (not defined in review; definition from trial manuscript as above) | 750 per 1000 (21/28) | 645 per 1000 (450 to 915) | RR 0.86 (0.60 to 1.22) | 56 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with design limitations (method of randomisation not stated) Imprecision (‐2): wide CIs crossing line of no effect; 1 small RCT | |
| Aciclovir vs vidarabine for treatment of herpes simplex virus infection in neonates (Jones 2009) | Abnormal neurodevelopment at approximately 1 year of age (not defined in review; definition from trial manuscript: mild impairment: only occular sequelae; moderate neurological impairment: hemiparesis or a persistent seizure disorder and no more than a 3‐month developmental delay; severe neurological sequelae: microcephaly, spastic quadriplegia, chorioretinitis or blindness, and a serious developmental delay of > 3 months according to the DDST) | 263 per 1000 (25/95) | 216 per 1000 (132 to 353) | RR 0.82 (0.50 to 1.34) | 202 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with design limitations (inadequate allocation concealment) Imprecision (‐2): wide CIs crossing line of no effect; 1 small RCT |
| Abnormal neurodevelopment or death at approximately 1 year of age (not defined in review definition from trial manuscript as above) | 463 per 1000 (44/95) | 366 per 1000 (264 to 509) | RR 0.79 (0.57 to 1.10) | 202 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with design limitations (inadequate allocation concealment) Imprecision (‐2): wide CIs crossing line of no effect; 1 small RCT | |
| Neonatal care: jaundice | |||||||
| Prophylactic phototherapy vs standard care for preventing jaundice in preterm or low birthweight infants (Okwundu 2012) | Neurodevelopmental impairment at 18 to 22 months (defined as blindness, severe hearing loss, and moderate or severe cerebral palsy) | 305 per 1000 (275/902) | 259 per 1000 (226 to 302) | RR 0.85 (0.74 to 0.99) | 1804 (1 RCT) | MODERATE | Study limitations (‐1): 1 RCT with high risk of attrition bias |
| Neonatal care: hypoglycaemia | |||||||
| Dextrose gel vs placebo for treatment of hypoglycaemia in newborn infants (Weston 2016) | Major neurosensory disability at 2 years (defined as any of the following: legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, developmental delay/intellectual impairment (defined as DQ < 2 SD below the mean)) | 11 per 1000 (1/94) | 67 per 1000 (8 to 543) | RR 6.27 (0.77 to 51.03) | 184 (1 RCT) | VERY LOW | Study limitations (‐1): "Evidence is based on a single trial" Imprecision (‐2):"Wide confidence intervals, low event rates and small sample sizes are suggestive of imprecision: (graded by review authors themselves) |
| Developmental disability at 2 years (defined as cognitive, language, or motor score below ‐1 SD, or cerebral palsy, blindness, or deafness) | 32/94 | 34/90 | RR 1.11 (0.75 to 1.63) | 184 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT with 78% follow‐up Imprecision: wide CI crossing line of no effect; 1 small RCT | |
| Neonatal care: parenteral feeding | |||||||
| Glutamine supplementation vs placebo to prevent morbidity and mortality in preterm infants (Moe‐Byrne 2016) | Neurodevelopmental impairment at 2 years post term (defined as BSID‐II MDI ≤ 85, PDI ≤ 85, cerebral palsy, blindness in 1 or both eyes, or hearing loss requiring amplification) | 375 per 1000 (12/32) | 401 per 1000 | RR 1.07 (0.59 to 1.92) | 72 (1 RCT) | LOW | Imprecision (‐2): "Total sample size = 72" (graded by review authors themselves) |
| Abbreviations: BSID: Bayley Scales of Infant Development; CDI: Child Development Inventory; CI: confidence interval; DAS: Differential Ability Scales; DDST: Denver Developmental Screening Test; DQ: development quotient; GMDS: Griffiths Mental Development Scales; GMF: gross motor function; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; IQ: intelligence quotient; MDI: Mental Development Index; PDI: Psychomotor Development Index; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; VA: visual acuity; VABS: Vineland Adaptive Behaviour Scales; WASI: Wechsler Abbreviated Scale of Intelligence; WISC: Wechsler Intelligence Scale for Children. | |||||||
| Intervention and comparison | Outcome | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | Comments |
| Neonatal care: asphyxia | |||||||
| Therapeutic hypothermia vs standard care for newborns with hypoxic‐ischaemic encephalopathy (Jacobs 2013) | Neuromotor delay (BSID PDI > 2 SD below mean) in survivors assessed at 18 to 24 months | 349 per 1000 (104/298) | 262 per 1000 (206 to 328) | RR 0.75 (0.59 to 0.94) | 657 (6 RCTs) | HIGH | Not downgraded |
| Neonatal care: blood disorders | |||||||
| Erythropoietin vs placebo for preventing red blood cell transfusion in preterm and/or low birthweight infants (Ohlsson 2014) | PDI < 70 at 18 to 22 months' corrected age (in children examined) | 133 per 1000 (6/45) | 311 per 1000 (131 to 737) | RR 2.33 (0.98 to 5.53) | 90 (1 RCT) | VERY LOW | Study limitations: 1 RCT at unclear risk of selection bias and high risk of attrition bias (˜73% follow‐up) Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size |
| Neonatal care: pulmonary hypertension | |||||||
| Endothelin receptor antagonists vs placebo for persistent pulmonary hypertension in term and late preterm infants (More 2016) | Adverse neurological outcomes at 6 months (defined as clinical or electrographically proven seizures, abnormal muscle tone, abnormal deep tendon reflexes, delayed motor milestones, or abnormal auditory brainstem response) | 286 per 1000 (4/14) | 20 per 1000 (0 to 343) | RR 0.07 (0.00 to 1.20) | 37 (1 RCT) | LOW | Study limitation (‐1): 8/23 infants in the placebo group were excluded from analysis Imprecision (‐1): single RCT, small sample size (graded by review authors themselves) |
| Neonatal care: resuscitation | |||||||
| Room air vs 100% oxygen for resuscitation of infants at birth (Tan 2005) | Not walking in those followed up at 18 to 24 months | 107 per 1000 (13/122) | 110 per 1000 (4 to 240) | RR 1.03 (0.04 to 2.25) | 213 (1 RCT) | VERY LOW | Study limitations (‐2): 1 qRCT with no blinding, and < 70% follow‐up Imprecision (‐1): wide CI crossing line of no effect |
| Neonatal care: nitric oxide | |||||||
| Inhaled NO vs placebo for respiratory failure in preterm infants (studies of routine use in intubated preterm infants) (Barrington 2010) | BSID MDI or PDI < ‐ 2 SD at 2 years' corrected age | 412 per 1000 (28/68) | 231 per 1000 (136 to 383) | RR 0.56 (0.33 to 0.93) | 138 (1 RCT) | MODERATE | Study limitations (‐1): 1 small RCT with 82% follow‐up |
| Inhaled nitric oxide vs control for respiratory failure in infants born at or near term (Finer 2006) | BSID PDI > 2 SD below the mean at 13 or 18 to 24 months | 148 per 1000 (25/169) | 161 per 1000 (86 to 300) | RR 1.09 (0.58 to 2.03) | 283 (2 RCTs) | LOW | Study limitations (‐1): 1 RCT masking of allocation, masking of outcomes, and completeness of follow‐up 'can't tell' Inconsistency (‐1): substantial heterogeneity (I² = 77%) Note: error in review for Ninos 1996 data; intervention and control group data switched; this has been rectified in this analysis |
| Neonatal care: respiratory distress syndrome | |||||||
| Inositol supplementation (repeat doses) vs placebo in preterm infants at risk for or having respiratory distress syndrome (Howlett 2015) | Minor neural developmental impairment at 1 year corrected age (defined as sensorimotor abnormality and/or developmental delay) | 137 per 1000 (10/73) | 115 per 1000 (52 to 255) | RR 0.84 (0.38 to 1.86) | 169 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection, performance, detection, and reporting bias, and at high risk of other bias Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size |
| Neonatal care: mechanical ventilation | |||||||
| Volume‐targeted vs pressure‐limited ventilation in the neonate (Wheeler 2010) | Gross Motor Developmental Issue (any definition) at 6 to 18 months (defined as gross motor delay) | 172 per 1000 (11/64) | 172 per 1000 (81 to 368) | RR 1.00 (0.47 to 2.14) | 128 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT (post hoc analysis in review) |
| Neonatal care: bronchopulmonary dysplasia | |||||||
| Early (< 8 days) postnatal corticosteroids vs placebo for preventing chronic lung disease in preterm infants (Doyle 2014b) | BSID PDI < ‐ 2 SD at 18 to 22 months or 25 months | 146 per 1000 (61/419) | 170 per 1000 (124 to 233) | RR 1.17 (0.85 to 1.60) | 842 (3 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect |
| BSID PDI < ‐ 2 SD in tested survivors at 18 to 22 months or 25 months | 232 per 1000 (61/263) | 271 per 1000 (202 to 364) | RR 1.17 (0.87 to 1.57) | 528 (3 RCTs) | MODERATE | Imprecision (‐1): wide CI crossing line of no effect | |
| Late (> 7 days) postnatal corticosteroids vs placebo or no treatment for chronic lung disease in preterm infants (Doyle 2014) | BSID PDI < ‐ 2 SD at 1 year corrected age | 180 per 1000 (11/61) | 141 per 1000 (61 to 325) | RR 0.78 (0.34 to 1.80) | 118 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size |
| BSID PDI < ‐ 2 SD in survivors assessed at 1 year corrected age | 256 per 1000 (11/43) | 171 per 1000 (77 to 384) | RR 0.67 (0.30 to 1.50) | 90 (1 RCT) | LOW | Imprecision (‐2): wide CI crossing line of no effect; 1 RCT with small sample size | |
| Early inhaled corticosteroids vs placebo for preventing chronic lung disease in ventilated very low birthweight preterm neonates (Shah 2012) | Mean developmental index on BSID‐II < 2 SD of the mean (age not reported in review;from trial manuscript: 3 years) | 143 per 1000 (4/28) | 179 per 1000 (53 to 596) | RR 1.25 (0.37 to 4.17) | 56 (1 RCT) | VERY LOW | Study limitations (‐1): 1 RCT at unclear risk of selection bias and detection bias Imprecision (‐2): wide CI crossing line of no effect; 1 small RCT |
| Neonatal care: other | |||||||
| Early developmental intervention vs standard follow‐up post hospital discharge to prevent motor and cognitive impairment in preterm infants (Spittle 2015) | Motor outcome at school age (5 years) (defined as low score on Movement ABC) | 378 per 1000 (51/135) | 423 per 1000 (329 to 544) | RR 1.12 (0.87 to 1.44) | 333 (2 RCTs) | LOW | Study limitations (‐1): 2 RCTs at high risk of attrition bias and unclear risk of performance bias Imprecision (‐1): wide CI crossing line of no effect |
| Abbreviations: BSID: Bayley Scales of Infant Development; CI: confidence interval; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; MDI: Mental Development Index; Movement ABC: Movement Assessment Battery for Children; PDI: Psychomotor Development Index; qRCT: quasi‐randomised controlled trial; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation | |||||||

