Intervenciones para la nefropatía crónica en pacientes con drepanocitosis
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: RCT Study grouping: parallel group | |
| Participants | Baseline characteristics Hydroxyurea
Placebo
Inclusion criteria: participants aged 9 ‐ 18 months were recruited between October 2003, and September 2007, at 13 trial centres in the USA; eligible participants had HbSS or Sβ⁰thalassaemia, and were enrolled irrespective of clinical severity. Exclusion criteria: transfusion within two months; height, weight, or head circumference less than the 5th percentile; MDI) less than 70; abnormal TCD velocity; chronic transfusion therapy; cancer; severe developmental delay (e.g. cerebral palsy or other mental retardation); grade III/IV intraventricular haemorrhage; stroke with neurological deficit; surgical splenectomy; participating in other clinical intervention trials; probable or known diagnosis of haemoglobin S‐hereditary persistence of fetal haemoglobin; known HbSβ⁺ thalassaemia (haemoglobin A present); any condition or chronic illness, which in the opinion of the principal investigator, makes participation unadvised or unsafe; inability or unwillingness to complete baseline (pre‐enrolment) studies, including blood or urine specimen collection, liver‐spleen scan, abdominal sonogram, neurological examination, neuropsychological testing, or transcranial doppler ultrasound (interpretable study not required, but confirmed velocity greater than 200 cm/second results in ineligibility); previous or current treatment with hydroxyurea or another anti‐sickling drug (additional exclusion criteria from trial registration NCT00006400). | |
| Interventions | Hydroxyurea: 20 mg/kg/day; local pharmacists reconstituted powder with syrup and water to a concentration of 100 mg/mL, and dispensed a 35‐day supply. There was no dose escalation. Placebo: hydroxyurea and placebo powders had the same appearance and packaging and the liquid formulations had the same appearance and taste. Hydroxyurea and placebo were distributed to clinical centres in encoded kits. | |
| Outcomes | Co‐primary outcomes: splenic and liver function (as measured by GFR) Secondary outcomes: investigations of the brain, lungs, hepatobiliary system, and growth and development; monitoring of height, weight, and head circumference; neuro‐development assessment (Bayley Developmental and Vineland Adaptive Behavior Scales); adverse clinical events included known complications of sickle‐cell anaemia, such as pain, dactylitis, acute chest syndrome, stroke, priapism, sepsis or bacteraemia, splenic sequestration, hospitalisation, and transfusion; SAEs. | |
| Identification | Sponsorship source: the US National Heart, Lung, and Blood Institute; and the National Institute of Child Health and Human Development Country: USA Setting: 13 medical centres Authors name: Prof W C Wang MD Institution: St Jude Children's Research Hospital, Memphis, TN, USA Email: [email protected] Address: Hematology MS 800, Room R5036 St. Jude Children's Research Hospital 262 Danny Thomas Place Memphis, TN 38105‐3678 | |
| Notes | 179 (93%) participants who completed at least 18 months of the trial and at least one exit assessment were analysed; 167 (86%) completed the full study. Hydroxyurea: 4 withdrawals: 3 lost to follow‐up; 1 incorrect diagnosis; 91 analysed. Placebo: 9 withdrawals: 4 declined further participation; 2 moved; 2 lost to follow‐up; 1 placed on chronic transfusion; 88 analysed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation sequence was pre‐decided by a randomisation schedule developed for each clinical site by the medical coordinating centre. Double‐blind randomisation was done with an automated telephone response system and the use of a random three digit kit number for each enrolled participant." |
| Allocation concealment (selection bias) | Low risk | "The kit number, which was linked to the assignment sequence, was used by the drug distribution centre to ship the appropriate study drug to the clinical site pharmacy. Hydroxycarbamide and placebo powders had the same appearance and packaging and the liquid formulations had the same appearance and taste. Hydroxyurea and placebo were distributed to clinical centres in encoded kits." |
| Blinding of participants and personnel (performance bias) | Low risk | "Hydroxyurea and placebo were distributed to clinical centres in encoded kits. Local pharmacists reconstituted powder with syrup and water to a concentration of 100 mg/mL, and dispensed a 35‐day supply. As in the HUSOFT trial, there was no dose escalation. Participants, caregivers, and medical coordinating centre staff were masked to treatment allocation." |
| Blinding of outcome assessment (detection bias) | Unclear risk | "An unmasked so‐called primary endpoint person monitored laboratory values and assisted in clinical management. Masked readings of splenic uptake on ⁹⁹ m Tc‐sulphur colloid liver‐spleen scans were categorised qualitatively as normal, decreased (but present), or absent." While the Methods state "double blind randomisation", it is not clear whether this is at the level of the outcome assessors as well as at the level of the drug administration.The statement about an unmasked primary endpoint assessor monitoring laboratory values is suggestive of a risk of bias; however; they may have only monitored for safety, whereas the splenic readings were done by someone who was blinded to the study drug allocation. It is not clear whether the assessor of the GFR was blinded or not. |
| Incomplete outcome data (attrition bias) | Unclear risk | States that all participants randomly assigned to a treatment group were analysed for the co‐primary endpoints ‐ but also 'Total number of participants assessed for each endpoint. N differs from the number reported in table one because only entry values that are paired with exit values from the same participants are included. Both co‐primary endpoints are per protocol analysis and only include participants with paired entry and exit values. There were approximately 25% of participants with no entry/exit GFR values. 'All other outcomes reported as intention to treat'. |
| Selective reporting (reporting bias) | Low risk | All of the outcomes stated in the methods were reported in the results. |
| Other bias | Low risk | No other sources of bias were detected from the Baby Hug trial |
| Methods | Study design: RCT Study grouping: parallel group | |
| Participants | Baseline characteristics ACEI (captopril)
Placebo
Inclusion criteria: homozygous for haemoglobin SS; 18 years of age or older; diagnosis of sickle cell anaemia based on clinical and biological data including haemoglobin electrophoresis;urinary albumin excretion between 30 and 300 mg per 24 hours on three separate occasions during the 6‐month period preceding the study. Exclusion criteria: non‐HbSS genotype; age < 18 years; hypertension (blood pressure > 140/90 mm Hg); evidence of heart, kidney, liver, or systemic disease; pregnant; taking anti‐inflammatory or antihypertensive medications. Pre‐treatment: no statistical differences. | |
| Interventions | Intervention characteristics ACEI (captopril):
Placebo:
| |
| Outcomes | Outcomes: efficacy of ACE inhibitors in the progression of albuminuria and their effects on blood pressure in people with sickle cell anemia. | |
| Identification | Sponsorship source: supported by grants from the Programme Hospitalier de Recherche Clinique (PHRC), France. Country: France (Guadeloupe) Setting: outpatients in one hospital Comments: Centre Hospitalo Universitaire (CHU) of Pointe‐a`‐Pitre in Guadeloupe in 1996 Authors name: Lydia Foucan Institution: University Hospital, Pointe‐a‐Pitre, Guadeloupe; the Sickle Cell Center of Guadeloupe Email: lydia.foucan@chu‐guadeloupe.fr Address: Departement d’Information Medicale et Sante Publique, Centre Hospitalier Universitaire de Pointe‐a‐Pitre 97159, Guadeloupe, French West Indies. | |
| Notes | Blood pressure was measured by the automated oscillometric method (Dynamap) after 5 minutes of rest in a half‐sitting position. Systolic pressure, diastolic pressure, and mean arterial pressure (mBP) were measured as the average of three measurements taken at 5‐minute intervals. We contacted the lead author of Foucan 1998 for additional data on creatinine clearance and also to confirm the actual number of participants that are included in the proteinuria analysis, at the time of review publication we had not received a response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: no description of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no description of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Patients were randomly assigned to two groups, and received captopril or an indistinguishable placebo". Judgement comment: participants may have been blinded to treatment ‐ but not clear if dosing was done similarly in both arms. No description or statement regarding blinding of personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: no description if outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | "All patients were included in an intention‐to‐treat analysis." Judgement comment: 1 in the captopril group had an unusual pain in the shoulder and discontinued treatment on the sixth day, and 1 in the placebo group was unavailable for follow‐up after the first month. These 2 participants were included in the results for as long as they participated.Does not appear that they were included in 6‐month analysis even though state intention‐to‐treat. Very small sample size so all results should be included. |
| Selective reporting (reporting bias) | High risk | "Creatinine clearance was calculated by the modified Cockroft and Gault formula (10,11). All measurements were repeated at baseline and at 1, 3, and 6 months." "creatinine concentrations and creatinine clearance remained constant throughout the study in both groups (data not shown)." Judgement comment: creatinine clearance important marker of kidney progression but data not shown. |
| Other bias | High risk | Judgement comment: this is a small sample size and likely not powered to detect any differences. Also follow‐up is too short to assess longer‐term AEs or actual effects on kidney disease progression. |
ACE: angiotensin converting enzyme
AEs: adverse events
GFR: glomerular filtration rate
MDI: mental developmental index
RCT: randomised controlled trial
SAEs: serious adverse events
SD: standard deviation
TCD: transcranial doppler ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Participants did not have chronic kidney disease. Trial addressed outcomes that are not relevant to this review (effect on vaso‐occlusive crises, blood transfusions and hospitalizations). | |
| Wrong study design; not randomised. | |
| Wrong study design; not randomised. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The Effect of Atorvastatin on Endothelial Dysfunction and Albuminuria in Sickle Cell Disease (in the Grant Entitled: Endothelial Dysfunction in the Pathogenesis of Sickle Cell Nephropathy) |
| Methods | Phase 2 randomised cross‐over assignment; double‐blind (participant, care provider, investigator, outcomes assessor) trial |
| Participants | Inclusion criteria:
Exclusion criteria:
Patients will also be encouraged to avoid grape fruit juice and red yeast rice for the duration of the study. Atorvastatin is contraindicated during pregnancy and breast‐feeding. |
| Interventions | Atorvastatin 40 mg tablet once daily for 6 weeks Placebo (for atorvastatin) 1 tablet once daily for 6 weeks |
| Outcomes | Primary: change from baseline in endothelial function at 6 weeks Secondary: change from baseline in plasma markers of endothelial activation; change from baseline in heme oxygenase activity; change from baseline in plasma levels of sFLT‐1; change from baseline in monocyte activation;change from baseline in renal function; occurrence of AEs; change from baseline in rho/rho kinase activity; change from baseline in plasma levels of VEGF; change from baseline in absolute cell counts; change from baseline in TF expression; change from baseline in TF‐mediated sFLT release from monocytes; change from baseline to week 6 in TR jet |
| Starting date | September 2013 |
| Contact information | Kenneth Ataga, MD, University of North Carolina, Chapel Hill |
| Notes | Completion date December 2017; R01HL111659 ( US NIH Grant/Contract Award Number ) |
ACE: angiotensin converting enzyme
AEs: adverse events
ALT: alanine aminotransferase
ARB: angiotensin blockers
GGT: gamma glutamyl transferase
INR: International Normalized Ratio
LDL: low‐density lipoprotein
PT: prothrombin time
PTT: partial thromboplastin time
sFLT‐1: sSoluble fms‐like tyrosine kinase‐1
TF: tissue factor
TR: tricuspid regurgitant
VEGF: vascular endothelial growth factor
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Slower progression or improvement in GFR (mL per min per 1·73 m²) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Hydroxyurea vs placebo, Outcome 1 Slower progression or improvement in GFR (mL per min per 1·73 m²). | ||||
| 1.1 at 18 to 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improvement in ability to concentrate urine (mOsm/kg) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Hydroxyurea vs placebo, Outcome 2 Improvement in ability to concentrate urine (mOsm/kg). | ||||
| 2.1 at 18 to 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 SAEs assessed with acute chest syndrome Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Hydroxyurea vs placebo, Outcome 3 SAEs assessed with acute chest syndrome. | ||||
| 4 SAEs assessed with painful crisis Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| Analysis 1.4 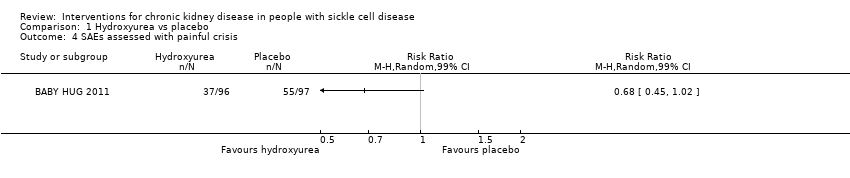 Comparison 1 Hydroxyurea vs placebo, Outcome 4 SAEs assessed with painful crisis. | ||||
| 5 SAEs assessed with hospitalisations Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| Analysis 1.5 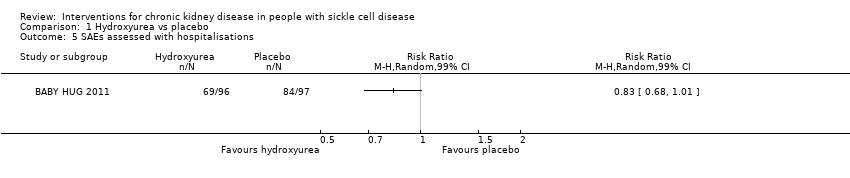 Comparison 1 Hydroxyurea vs placebo, Outcome 5 SAEs assessed with hospitalisations. | ||||
| 6 SAEs assessed with stroke Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 99% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Hydroxyurea vs placebo, Outcome 6 SAEs assessed with stroke. | ||||
| 7 AEs assessed with neutropenia Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| Analysis 1.7 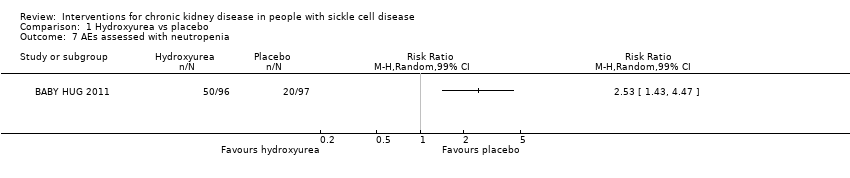 Comparison 1 Hydroxyurea vs placebo, Outcome 7 AEs assessed with neutropenia. | ||||
| 8 AEs assessed with thrombocytopenia Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Hydroxyurea vs placebo, Outcome 8 AEs assessed with thrombocytopenia. | ||||
| 9 Number of participants transfused Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.9  Comparison 1 Hydroxyurea vs placebo, Outcome 9 Number of participants transfused. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Slower progression or reduction in proteinuria (mg/day) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 ACEI (captopril) vs placebo, Outcome 1 Slower progression or reduction in proteinuria (mg/day). | ||||
| 1.1 at 6 months follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Other drug‐related adverse events (dry cough) Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 ACEI (captopril) vs placebo, Outcome 2 Other drug‐related adverse events (dry cough). | ||||

Sickle cell nephropathy pathophysiology in sickle cell disease: Adapted fromOkafor 2013andNath 2015
RBC: red blood cells; FSGS: focal segmental glomerulosclerosis; ESRD: end‐stage renal disease

Structure of the kidney. From: Wikispaces. Human Physiology. 12. Urology.https://humanphysiology2011.wikispaces.com/12.+Urology

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
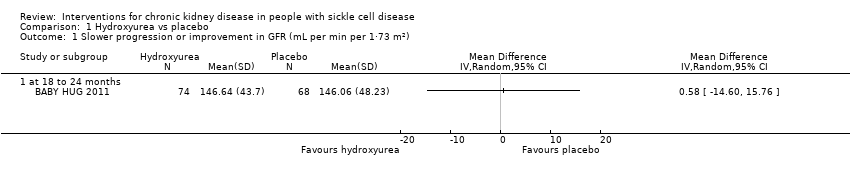
Comparison 1 Hydroxyurea vs placebo, Outcome 1 Slower progression or improvement in GFR (mL per min per 1·73 m²).

Comparison 1 Hydroxyurea vs placebo, Outcome 2 Improvement in ability to concentrate urine (mOsm/kg).
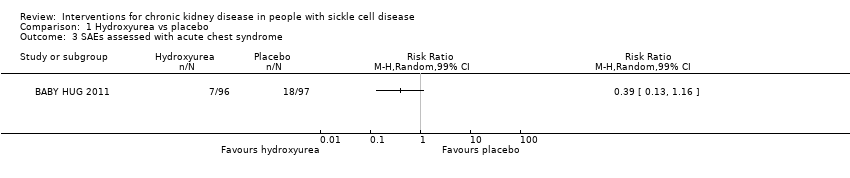
Comparison 1 Hydroxyurea vs placebo, Outcome 3 SAEs assessed with acute chest syndrome.

Comparison 1 Hydroxyurea vs placebo, Outcome 4 SAEs assessed with painful crisis.
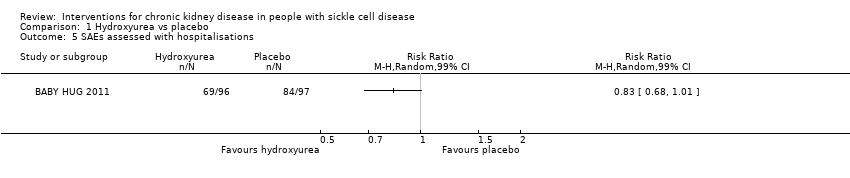
Comparison 1 Hydroxyurea vs placebo, Outcome 5 SAEs assessed with hospitalisations.

Comparison 1 Hydroxyurea vs placebo, Outcome 6 SAEs assessed with stroke.

Comparison 1 Hydroxyurea vs placebo, Outcome 7 AEs assessed with neutropenia.

Comparison 1 Hydroxyurea vs placebo, Outcome 8 AEs assessed with thrombocytopenia.

Comparison 1 Hydroxyurea vs placebo, Outcome 9 Number of participants transfused.
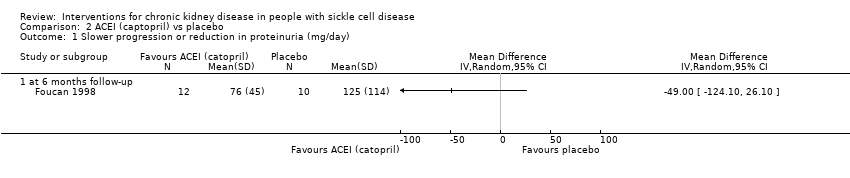
Comparison 2 ACEI (captopril) vs placebo, Outcome 1 Slower progression or reduction in proteinuria (mg/day).

Comparison 2 ACEI (captopril) vs placebo, Outcome 2 Other drug‐related adverse events (dry cough).
| Hydroxyurea compared to placebo for preventing or reducing kidney complications in people with sickle cell disease | ||||||
| Patient or population: people with sickle cell disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with hydroxyurea | |||||
| Slower progression or improvement in GFR mL per min per 1.73 m² (measured at 18 to 24 months) | The mean slower progression or improvement in GFR mL per min per 1.73 m² (measured at 18 to 24 months) was 146.64 (43.7) | MD 0.58 higher | ‐ | 142 | ⊕⊝⊝⊝ | |
| Improvement in ability to concentrate urine mOsm/kg (measured at 18 to 24 months) | The mean improvement in ability to concentrate urine mOsm/kg (measured at 18 to 24 months) was 494.57 (110.07) | MD 42.23 higher | ‐ | 178 | ⊕⊕⊝⊝ | |
| SAEs assessed with acute chest syndrome | Study population | RR 0.39 | 193 | ⊕⊕⊝⊝ | ||
| 186 per 1000 | 72 per 1000 | |||||
| SAEs assessed with painful crisis | Study population | RR 0.68 | 193 | ⊕⊕⊝⊝ | ||
| 567 per 1000 | 386 per 1000 | |||||
| SAEs assessed with hospitalisations | Study population | RR 0.83 | 193 | ⊕⊕⊝⊝ | ||
| 866 per 1000 | 719 per 1000 | |||||
| Mortality due to any cause | No deaths reported in either group | not estimable | 193 | ⊕⊕⊝⊝ | ||
| Quality of life | Not reported | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of evidence by one due to unclear risk of attrition bias. | ||||||
| ACEI compared to placebo in preventing or reducing kidney complications in people with sickle cell disease | ||||||
| Patient or population: people with sickle cell disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with ACEI | |||||
| Slower progression or reduction in proteinuria (mg/day 6 months follow‐up) | The mean slower progression or reduction in proteinuria (mg/day 6 months follow‐up) was 76 (45) | MD 49.00 lower | ‐ | 22 | ⊕⊝⊝⊝ | |
| Improvement in ability to concentrate urine mOsm/kg | Not reported | ‐ | ‐ | ‐ | ||
| SAEs assessed with acute chest syndrome | Not reported | ‐ | ‐ | ‐ | ||
| SAEs assessed with painful crisis | Not reported | ‐ | ‐ | ‐ | ||
| SAEs assessed with hospitalisations | Not reported | ‐ | ‐ | ‐ | ||
| Mortality due to any cause | Not reported | ‐ | ‐ | ‐ | ||
| Quality of life | Not reported | ‐ | ‐ | ‐ | ||
| ACEI: angiotensin converting enzyme inhibitor; CI: confidence interval; MD: mean difference; SAEs: serious adverse events | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of evidence by two due to unclear or high risk of bias in all domains. | ||||||
| Unadjusted HRs reported in BABY HUG 2011 | ||
| Outcome | HR | 95% CI |
| Acute chest syndrome | 0.36 | 0.15 to 0.87 |
| Painful crisis | 0.54 | 0.36 to 0.83 |
| Hospitalisations | 0.73 | 0.53 to 1.00 |
| Neutropenia | 3.0 | 1.7 to 5.1 |
| Thrombocytopenia | 1.6 | 0.6 to 4.1 |
| Transfusions | 0.55 | 0.32 to 0.96 |
| CI: confidence interval | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Slower progression or improvement in GFR (mL per min per 1·73 m²) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 at 18 to 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improvement in ability to concentrate urine (mOsm/kg) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 at 18 to 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 SAEs assessed with acute chest syndrome Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 4 SAEs assessed with painful crisis Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 5 SAEs assessed with hospitalisations Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 6 SAEs assessed with stroke Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 99% CI) | Totals not selected | |
| 7 AEs assessed with neutropenia Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 8 AEs assessed with thrombocytopenia Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 9 Number of participants transfused Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Slower progression or reduction in proteinuria (mg/day) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 at 6 months follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Other drug‐related adverse events (dry cough) Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |

