青少年哮喘患者的外行主导和同伴支持干预措施
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: 3‐month open‐label cluster RCT Setting: 4 high schools in Irbid, Jordan | |
| Participants | Population: 4 schools including 261 included students; 2 schools took part in the Triple A programme (132 students) and 2 schools provided no intervention (129 students) Age range: approximately 14 to 16 years based on included school years Baseline characteristics Triple A students: 72.7% had asthma; 63.6% male; 20.5% took ICS (23.5% took a reliever); 25% student smokers; 72% family member smokes Control students: 68.2% had asthma; 49% male; 23.3% took ICS (43.3% took a reliever); 30% student smokers; 71.3% family member smokes Inclusion criteria: Students from years 8, 9 and 10 were eligible for participation in the study if they had reported wheezing in the past 12 months as identified by the Arabic version of the International Study for Asthma and Allergy Committee (ISAAC) written questionnaire; were physically and cognitively capable of completing the survey; were able to read and converse in both Arabic and English; attended regular school classes; were free of any other major diseases that could affect quality of life measures; and were not concurrently involved in another health‐related study Exclusion criteria: not regularly attending in year 8, 9 or 10; did not experience wheezing in the past 12 months; had other chronic conditions | |
| Interventions | Intervention: Bilingual Jordanian health workers delivered the content of the peer leader training programme in both English and Arabic. Health workers trained 11 peer leaders from year 11 at each of the intervention schools to deliver the 3 Triple A lessons to year 10 students Control: standard care ‐ no intervention | |
| Outcomes | The main study outcomes (health‐related quality of life (Arabic PAQLQ), self‐efficacy to resist smoking (subscale of the Self‐Administered Nicotine Dependence Scale) and knowledge of self‐management of asthma (Arabic Asthma Knowledge Consumer Questionnaire)) were collected at baseline and at 3 months after the intervention "Baseline data included demographic characteristics, smoking habits of students and their families, presence of asthma diagnosis by health professionals, and medications using a self‐reporting checklist developed by the researchers. Data on asthma symptoms and severity were collected using the Arabic version of the ISAAC written questionnaire (8 questions about symptoms, diagnosis and severity over past 12 months)" | |
| Notes | Trial registration: ISRCTN63833842 Funding: The study was supported by Jordan University of Science and Technology, Irbid, Jordan. We also thank the Nursing Council in Jordan for financial support provided throughout the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Four high schools in the Irbid region in northern Jordan were selected using a closed‐envelope technique, from a total of 54 public schools that included Year 8 through Year 11, by an individual independent of the research team. Two schools were randomly selected from all the eligible high schools for girls, and the other 2 schools were randomly selected from all the eligible high schools for boys. Schools were stratified according gender to ensure a balanced sample. The selected schools, which were all single gender (2 for boys and 2 for girls), agreed to participate in the study" "Allocation to groups occurred by using the cluster method of randomization at the school level and the closed‐envelope technique stratified for the gender at the school (2 each)" |
| Allocation concealment (selection bias) | Low risk | Most of the information given relates to blinding of sampling rather than to group allocation |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel could not be kept blind to group allocation |
| Blinding of outcome assessment (detection bias) | High risk | All outcomes were self‐reported by participants who could not be blinded to treatment assignment |
| Incomplete outcome data (attrition bias) | Low risk | Six students from the intervention group (4.6%) and 11 (8.5%) from the control group did not complete the trial because they were absent from school on the day of outcome data collection. As school absence due to asthma was one of the outcomes the intervention was intended to impact, it is possible that exclusion of these children from the analysis |
| Selective reporting (reporting bias) | Low risk | Trial was retrospectively (ISRCTN63833842), not prospectively, registered. Named outcomes are reported in full, but the study was not prospectively registered. Study authors responded to contact and confirmed that they possess no additional data relevant to our analyses |
| Other bias | Unclear risk | School selection is described in detail but may have introduced a selection bias before randomisation |
| Methods | Study design: 9‐month single‐blind parallel RCT Setting: an urban city and adjacent suburbs in upstate New York | |
| Participants | Population: 112 adolescents were randomised to an asthma programme led by peers (n = 59) or by adults (n = 53) Age range: 13 to 17 years Baseline characteristics 71% on at least 1 controller medicine Peer‐led: 44.1% male; mean age 14.9 years (SD 1.4); 45.8% white Adult‐led: 41.5% male; mean age 14.5 years (SD 1.3); 47.2% white Inclusion criteria: (1) age 13 to 17 years; (2) mild, moderate or severe persistent asthma specified by NHLBI guidelines; (3) asthma diagnosis ≥ 1 year; (4) no other chronic/emotional health conditions (e.g. diabetes, cystic fibrosis, major depression); and (5) ability to understand spoken and written English Exclusion criteria: "Adolescents with learning disabilities based on reports from parents, teachers or clinicians were excluded, because this could influence the implementation and outcomes of the intervention program" | |
| Interventions | Intervention: The Intervention group attended a 1‐day camp in which group activities were facilitated by 12 peer leaders. Peers (10 females, 2 males) were 16 to 20 years old, were nominated by school teachers/nurses or clinicians and attended 3‐week intensive structured training sessions (5 hours/wk). Paired peer leaders facilitated learning activities in small groups of 6 to 10 campers, overseen by adults. Younger leaders (16 to 17 years of age) led younger groups (13 to 14 years of age); older leaders (18 to 20 years of age) led older groups (15 to 17 years of age). Three 45‐ to 60‐minute sessions based on Power Breathing™ programme covered basic asthma education, psychosocial issues and asthma self‐management skills. Group activities involved discussion, strategic thinking, knowledge‐testing games and role playing. At completion of camp, peer leaders conducted monthly phone follow‐ups to provide continuous peer support and encouragement using a checklist. Approximately 49% were successfully reached each month, and average length of the interaction was 2 to 5 minutes for each contact Control: The control group attended an adult‐led day camp that was held at the same camp site on a different day. Two nurse practitioners and a physician offered the campers didactic asthma education. The length of the day camp and the content of the asthma programme were comparable with those of the intervention group | |
| Outcomes | The Children's Attitude toward Asthma Scale and the Pediatric Asthma‐related Quality of Life Questionnaire were administered at baseline, and immediately and 3, 6 and 9 months post intervention. Spirometry was conducted twice ‐ before the intervention and 9 months after the intervention | |
| Notes | Trial registration: NCT01161225 Funding: supported by a grant from the NIH/NINR (R21 NR009837), awarded to Hyekyun Rhee | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A total of 112 adolescents were randomly assigned using a computer generated random table to either the intervention (peer‐led camp) or control (adult‐led camp) group" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | Study states, "Participants were blind to their group assignment", and is described as "single blind (subject)" on clinicaltrials.gov. Study personnel's knowledge of group assignment may have introduced bias |
| Blinding of outcome assessment (detection bias) | High risk | Most outcomes were rated by participants, who were unaware of their group assignment |
| Incomplete outcome data (attrition bias) | Low risk | A fair quantity of data were missing by the 9‐month measurement (27.1% and 22.6% in intervention and control groups) but the quantity was less at earlier time points and "analyses were performed using all available data (i.e., intent to treat), including participants who subsequently dropped out". Risk of bias is likely to be different depending on the outcome and the time point of interest, which will be considered in the Grade rating for each outcome individually |
| Selective reporting (reporting bias) | Low risk | Study was prospectively registered (NCT01161225), and as planned, all named outcomes were reported in published papers or on clinicaltrials.gov |
| Other bias | Low risk | None noted |
| Methods | Study design: 10‐week open‐label parallel RCT Setting: 3 primary care practices at Rush University Medical Center in Chicago, Illinois | |
| Participants | Population: 68 adolescents were randomised to a peer support and messaging intervention (n = 34) or to an attention control group (n = 34) Age range: 11 to 16 years Baseline characteristics Peer support:: 50% male; mean age 13.3 (range 11 to 16) years; 85% had uncontrolled asthma; 26.5% had 2 or more OCS courses in past 12 months and 57.6 had 1 or more ED visit or hospital admission in past 12 months; 5.9% current smokers; 8.8% family member smokes Attention control: 47.1% male; mean age 13.6 (range 11 to 16) years; 76.5% had uncontrolled asthma (76.5%); 29.4% had 2 or more OCS courses in past 12 months and 44.1% had 1 or more ED visit or hospital admission in past 12 months; 0 current smokers; 5.9% family member smokes Inclusion criteria: 11 to 16 years of age and self‐identified as African American or Hispanic, diagnosis of persistent asthma and possessing an active prescription for a daily ICS for asthma. Persistent asthma was defined as asthma symptoms (e.g. cough, wheeze, shortness of breath, chest tightness) more than 2 days per week or night‐time awakening more than twice a month; or being on a prescribed daily ICS for asthma. The latter requirement was met when the adolescent within the past 12 months had (1) an outpatient visit to Rush University Medical Center with asthma listed as a diagnosis code for that visit; and (2) a prescription for ICS Exclusion criteria: caregiver or child unable to speak English, comorbidities that could interfere with study participation, or ≥ 48% adherence over 2 weeks during the run‐in period. Participants with ≥ 48% adherence were excluded, as the aim of the study was to target children with poor adherence (i.e. who could benefit most from this behavioural intervention) | |
| Interventions | Intervention: peer support and mp3 messaging. Those in the intervention group received music tracks and attended coping peer group sessions led by social workers during weeks 1 to 4 and 6 to 9. Session leaders were trained to use a motivational interviewing approach and to follow the study guide. During the session, participants developed and recorded 2 to 4 messages from the discussion to encourage daily use of ICS, to be played at random between music tracks Control: attention control. All participants received medical supervision, peak flow meters and an iPod during the run‐in. Those in the attention control group attended weekly individual sessions with a research assistant who did not promote adherence. They received the same number of iPod messages as those in the active intervention group with content promoting adherence to ICS, also played at random between music tracks, but recorded by an asthma doctor rather than by peers | |
| Outcomes | The primary study outcome was ICS adherence (average daily adherence over the previous 14 days) measured with the electronic medication monitor for ICS, measured at baseline and at 5 and 10 weeks. Secondary outcomes were asthma knowledge (ZAP Caregiver Asthma Knowledge Instrument), ICS knowledge, ICS self‐efficacy, social support and asthma exacerbations. Asthma exacerbations included self‐reported missed schooldays; oral prednisone bursts; unscheduled urgent visits to the doctor’s office; emergency department visits; hospitalisations; intensive care unit admissions; and intubations | |
| Notes | Trial registration: NCT01169883 Funding: National Heart Lung and Blood Institute grants K23 HL092292 and R21 HL098812. Support in the form of study drug was provided by a grant from GlaxoSmithKline (FLV114794) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Blocked group randomization, using a computer‐generated allocation schedule" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | It was not possible to blind participants, although adherence, the only outcome reported that is of interest in this review, was measured objectively. However, awareness of the intervention group and of monitoring may have affected adherence behaviour beyond the effect intended by the intervention |
| Blinding of outcome assessment (detection bias) | Low risk | "Outcomes data were collected at baseline and at 5 and 10 weeks post‐randomization (during the active treatment phase) by research assistants blinded to the participants’ group assignment" |
| Incomplete outcome data (attrition bias) | Low risk | More than 80% in both arms attended at least 1 follow‐up visit (at 5 or 10 weeks) and were included in the analysis; reasons for dropping out were similar between groups |
| Selective reporting (reporting bias) | High risk | Prospectively registered trial (NCT01169883) and outcomes listed on trial register clearly reported (although medians and IQRs used, so unable to include in meta‐analysis). Several outcomes of interest in this review were listed as measured in the methods section of the published report but were not reported in the results (e.g. unscheduled visits, exacerbations) |
| Other bias | Low risk | None noted |
| Methods | Study design: 4‐month open‐label cluster RCT Setting: 4 male schools in Irbid, Jordan | |
| Participants | Population: 4 schools, 2 randomised to Triple A + smoking pledge, 2 randomised to Triple A alone. 53 peer leaders and 433 students (215 Triple A + smoking pledge, 218 Triple A alone) included Age range: 12 to 13 years Baseline characteristics Whole population: all male, 13% with diagnosed asthma and a further 13% with reported recent wheezing; 37% were “ever” smokers Inclusion criteria: Students from grades 7 and 8 (ages 12 to 13) were eligible to participate if they were capable of completing the survey, were able to read and write in the Arabic language and were free of any major disease that could affect their responses Exclusion criteria: Students who were concurrently involved in another health‐related study were excluded | |
| Interventions | Intervention: The Triple A programme uses a 3‐step cascade process plus a smoke‐free pledge. Trained health educators trained senior students from grade 10 to be peer leaders (n = 53) who deliver 3 sessions to peers in grades 7 and 8 (n = 433). Sessions focused on asthma knowledge, smoking refusal and resistance skills, empowerment and leadership. Resources of the Triple A programme included standardised training manuals for educators and leaders, DVDs about asthma management and smoking and an asthma first aid kit. Motivational strategies included interactive teaching, role‐playing, group discussion and a quiz show. Volunteer peers then developed asthma and smoking messages to be presented to the school community as creative performances. Peer leaders also implemented the smoke‐free pledge for peers who voluntarily signed the pledge for 4 months. The class, facilitated by peer leaders, monitored adherence on a fortnightly basis for 4 months Control: The comparison group received the same intervention components as the intervention group, minus the smoke‐free pledge | |
| Outcomes | Smoking‐related knowledge and perceptions (for all selected students), smoking behaviour (for all selected students), level of nicotine dependence (for selected students who reported ever cigarette smoking), screening questionnaire for asthma and recent wheezing; students from this sample who had an asthma diagnosis or recent wheezing in the past 12 months also completed the questionnaire on level of asthma control | |
| Notes | Trial registration: not reported Funding: The Deanship of Scientific Research at Jordan University of Science and Technology, Irbid, Jordan (96/2012) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The four selected schools were randomly assigned to either the TAJ or the TAJ‐Plus using an opaque envelope technique to ensure allocation was blinded (Hedges, 2007)." Students were then allocated using "simple random assignment allocation using opaque envelope technique". Of all those allocated (815), a random selection was chosen by "every second student in the alphabetical class list" |
| Allocation concealment (selection bias) | Low risk | "The four selected schools were randomly assigned to either the TAJ or the TAJ‐Plus using an opaque envelope technique to ensure allocation was blinded (Hedges, 2007). The opaque envelope technique is a method used to blind the personnel who were (1) selecting the schools to be approached to join the study and (2) allocating the schools to the experimental and control groups. For the allocation to group, an independent researcher undertook the creation of four allocations (two experimental and two control) and sealed them in opaque envelopes. Once the school principal agreed that the school would participate in the study, an envelope was opened by the independent researcher and the result recorded and the chief investigator advised" "The random sampling and allocation technique was conducted by a trained, independent researcher" |
| Blinding of participants and personnel (performance bias) | High risk | "The cluster design made it possible to blind students to the intervention type as all participating students within the same school received the same intervention" However, it would not be possible to blind personnel to group allocation, and this may have introduced performance bias |
| Blinding of outcome assessment (detection bias) | High risk | All questionnaires were self‐report and hence were subject to detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Overall, the trial is at low risk of attrition bias, as 195/215 (9.3% dropout) students in the 2 intervention schools and 202/218 (7.3% dropout) students in the 2 control schools were included in the analyses, but lower numbers were available depending on the outcome, as "Only students who provided both baseline and follow‐up data were analyzed" |
| Selective reporting (reporting bias) | Low risk | Named outcomes were reported appropriately but no prospective protocol was available. Study authors responded to contact and confirmed that they possess no additional data relevant to our analyses |
| Other bias | Unclear risk | "The outcome analyses were adjusted for clustering effects as well as any baseline differences between the two groups using the split‐plot design" "All baseline differences between the study groups were adjusted for in the analyses" |
| Methods | Study design: 8‐month cluster RCT (baseline measures taken in February 1998 and follow‐up in October 1998) Setting: 6 high schools in rural Australia | |
| Participants | Population: 272 adolescents were randomised to the Triple A programme (n = 124) or to a control group (n = 148) Age range: year 7 (12 to 13 years of age) and year 10 (15 to 16 years of age) students Baseline characteristics Triple A: mean age 12.5 for year 7's, 15.5 for year 10's; 34.5% male; 40.7% taking ICS Control: mean age 12.5 for year 7's, 15.5 for year 10's; 54.3% male; 32.6% taking ICS Inclusion criteria: A video questionnaire from the International Study of Asthma and Allergies in Childhood was administered to all students in years 7 and 10 who were present on the test day (1379 students) at each school in February 1998. Consenting students reporting recent wheeze (272 students) underwent baseline spirometry and completed questionnaires on asthma quality of life and asthma symptoms Exclusion criteria: not reported | |
| Interventions | Intervention: The Triple A programme involved a 3‐step approach to educating and empowering students with asthma. In step 1, year 11 student volunteers were trained as asthma peer leaders during a 6‐hour workshop conducted by the study team. Students learnt how to educate their peers about asthma and its management using games, videos, worksheets and discussions as teaching tools. In step 2, teams of 3 to 4 peer leaders conducted three 45‐minute health lessons for each year 10 class in their school. In step 3, year 10 students developed and presented key messages learnt in lessons to the year 7 students. Presentations by year 10 students included short acts, dramas and songs, with titles such as “don't smoke,” “asthma can kill” and “visit your doctor” Control: Before the study, all schools received first aid kits for asthma and asthma workshops for school staff. All students known to have asthma were issued a record card to be completed by their doctor. In addition, a workshop on adolescent asthma was held for local doctors, and regular reports of the study appeared in local print and electronic media | |
| Outcomes | Quality of life, school absenteeism, asthma attacks and lung function | |
| Notes | Trial registration: not reported Funding: The Commonwealth Department of Health and Aged Care and Asthma New South Wales | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Concealed random allocation was performed...using a random number generator" |
| Allocation concealment (selection bias) | Low risk | "Concealed random allocation was performed by PGG (who was not involved with the administration of the study), using...the closed envelope technique" |
| Blinding of participants and personnel (performance bias) | High risk | No description of any blinding procedures. Participants and personnel would have been aware of group assignment |
| Blinding of outcome assessment (detection bias) | High risk | No description of any blinding procedures. Participants and personnel would have been aware of group assignment, and outcomes were self‐reported |
| Incomplete outcome data (attrition bias) | High risk | 10/148 who had baseline measurements in the intervention group (6.8%) and 11/124 in the control group (8.9%) had matched data at the end of the trial. It is not clear at which point randomisation took place. "Overall 1379 (91%) students completed the asthma screening questionnaire; 325 reported recent wheeze and 272 (83.7%) participated in baseline testing (fig 1). Matched data at both baseline and after the intervention were available for 251 students. Missing data occurred owing to misclassification, students moving schools or being absent on the day of testing, or failure to complete the questionnaire. These students were similar to the participants in terms of quality of life and related morbidity measures" |
| Selective reporting (reporting bias) | High risk | Exacerbations are not reported for year 7 pupils in the population, just that "The intervention had no effect on school absenteeism and asthma attacks in year 7 students" Other outcomes are reported, but no associated trial protocol was provided |
| Other bias | Low risk | None noted |
ED: emergency department; ICS: inhaled corticosteroid; ISAAC: International Study for Asthma and Allergy Committee; NHLBI: National Heart, Lung, and Blood Institute; OCS: oral corticosteroid; PAQLQ: Paediatric Asthma Quality of Life Questionnaire; RCT: randomised controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Intervention does not match inclusion criteria. Peers and lay leaders not involved | |
| Population does not match inclusion criteria. Children between 2 and 16 years included, with mean age of 6 (i.e. not adolescents) | |
| Population does not match inclusion criteria. Children between 7 and 11 years included (i.e. not adolescents), with a range of chronic illnesses | |
| Intervention does not match inclusion criteria. Peers and lay leaders not involved; interventions were education and parent/child teamwork | |
| Population does not match inclusion criteria. Intervention aimed at parents of children 2 to 18 years of age; mean age was around 7 (i.e. not adolescents) | |
| Design does not match inclusion criteria. "One school received the peer‐led Triple A (Adolescent Asthma Action) program, and the second school served as a comparison school". | |
| Population does not match inclusion criteria. Children in grades 2 to 6 (USA and Canada), with mean age of 8.8 (SD 1.2) (i.e. not adolescents) | |
| Design does not match inclusion criteria. Single‐arm/uncontrolled | |
| Population does not match inclusion criteria ‐ children were between 3 and 13 years of age (mean age 8) | |
| Intervention does not match inclusion criteria ‐ home visit community health worker educational intervention for families | |
| Design does not match inclusion criteria ‐ non‐randomly allocated to groups | |
| Population does not match inclusion criteria ‐ people of all ages (3 to 65), not just adolescents | |
| Population does not match inclusion criteria ‐ adults only (18+), and eligible participants could have any of a range of chronic diseases (hypertension, arthritis, coronary artery disease, hepatitis, diabetes, asthma, hyperlipidaemia, HIV) | |
| Population does not match inclusion criteria ‐ children between 3 and 12 and intervention aimed at mentoring parents | |
| Population does not match inclusion criteria ‐ adults only (18+) | |
| Population does not match inclusion criteria. Mean age was 10.4, which is just at the lower limit of adolescence, but study included children as young as 7 and as old as 14. Judged to not be an adolescent population | |
| Population does not match inclusion criteria. Children 2 to 17 years of age, with mean age of 6.9 (SD 3.9) (i.e. not adolescents) | |
| Intervention does not match inclusion criteria ‐ not peer supported or lay led | |
| Population does not match inclusion criteria. Children 1 to 17 years of age, with mean age of 6.9 (SD 4.0) (i.e. not adolescents) | |
| Intervention does not match inclusion criteria ‐ aimed at adolescents and young adults 19 years of age and older to help transition into adult services |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Study design: 12‐month double‐blind parallel randomised controlled trial (RCT) Setting: 19 public middle schools in Detroit, Michigan |
| Participants | Population: 1292 early adolescents planned ‐ unclear whether recruitment started Age range: 11 to 12 years Baseline characteristics None. Unclear whether this study was ever completed Inclusion criteria: 6th grade students (11 to 12 years of age) enrolled in 19 public middle schools in Detroit, Michigan, who meet the following criteria: attend a participating school; based on National Asthma Education and Prevention Program (NAEPP) guidelines, (1) have a diagnosis of asthma and have active asthma symptoms and/or have received a prescription for asthma medications in the past year, or (2) report the presence of 3 of 5 non‐exercise‐related asthma symptoms in the past year on 5 or more occasions, or (3) report 2 or more exercise‐related asthma symptoms in the past year on 5 or more occasions or (4) have a severity classification of persistent disease (mild, moderate, severe) based on night‐time questions Exclusion criteria: not reported |
| Interventions | Intervention: "Peer Asthma Action Educational Intervention". Children in this arm will receive BOTH the Open Airways asthma education programme and the Peer Asthma Action education programme. A 6‐week asthma educational self‐management programme is provided for middle school students plus a Peer Asthma Action Educational Intervention, which is a peer‐led training programme for children in multiple grades provided to teach them about asthma and asthma management Control: "Open Airways Educational Intervention". Children in this arm will receive the Open Airways educational programme, which is an evidence‐based asthma educational programme for children, developed by the investigator. A 6‐week asthma educational self‐management programme is provided for middle school students |
| Outcomes | Use of healthcare services will be self‐reported in telephone interviews with parents at baseline, 12 months and 24 months. Additional primary outcomes listed in the protocol include asthma‐related quality of life, disease management behaviour and academic performance. Secondary outcomes include peer support, school attendance, physical activity, use of healthcare services and smoking behaviour |
| Notes | *No results reported or publications listed. Principal investigator deceased (2013). Contacted University of Michigan on 12/10/2016* Trial registration: NCT00217776 Funding: University of Michigan and National Heart, Lung, and Blood Institute (NHLBI) |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Peer Led Asthma Self Management for Adolescents: PLASMA (PLASMA) |
| Methods | 15‐month open‐label parallel randomised controlled trial (RCT) |
| Participants | Estimated enrolment: 420 Inclusion criteria: adolescent (camp) participants 12 to 17 years of age; physician‐diagnosed asthma that has required use of healthcare services within 12 months; persistent asthma determined by current use of a control medication or > 2 days/wk of daytime symptoms, >3 to 4 times of night‐time awakening, >2 days/wk of short‐acting beta‐agonist (SABA) use or any interference with normal activities due to asthma. Investigators will include those with chronic health conditions, except those with conditions affecting respiratory system, heart disease, pneumonia, etc., and those with moderate to severe cognitive impairment; primary residence located in participating inner cities based on zip codes; and ability to understand spoken and written English Eligibility criteria for peer leaders include age 16 to 20 years; nomination from school teachers/nurses or healthcare providers for candidates' exemplary asthma self‐management, leadership and emotional intelligence; and fulfilment of eligibility criteria prescribed for adolescent participants Exclusion criteria: adolescents who are pregnant or incarcerated at enrolment; have learning disabilities based on reports from teachers or clinicians due to possible confounding of results; or have serious health (other than asthma) and emotional preconditions (e.g. severe depression, anxiety disorders, schizophrenia) |
| Interventions | Intervention: peer‐led asthma self‐management for adolescents: PLASMA will be implemented in small groups at a camp setting where paired peer leaders will facilitate learning activities. Paired peer leaders will share and coordinate the responsibilities of facilitating group activities. Training content includes the following: Day 1: asthma basics and prevention; Day 2: asthma monitoring and management; Day 3: communication/ psychosocial issue management/leadership training/hands‐on practice in simulated peer‐led group settings (role‐play) Control: Adult‐led asthma self‐management will take place within 2 weeks of the peer‐led camp to minimise the history effect. Two healthcare professionals will attend peer leader training sessions to become familiar with programme content, then will lead instructional activities. As in PLASMA, adult leaders will base their instruction on the programme manual to ensure comparable programme content. Adult leaders will adopt mainly a didactic format and skill demonstration |
| Outcomes | Primary outcome measures: Pediatric Asthma Quality of Life Questionnaire (PAQLQ) |
| Starting date | November 2014. Final data collection estimated by May 2018. Estimated completion November 2019 |
| Contact information | Hyekyun Rhee, PhD; [email protected] |
| Notes | Currently recruiting participants. Sponsored by University of Rochester. Collaborators listed as Johns Hopkins University and University of Tennessee |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in asthma‐related quality of life (PAQLQ) Show forest plot | 3 | Mean Difference (Random, 95% CI) | 0.40 [‐0.02, 0.81] | |
| Analysis 1.1  Comparison 1 Peer‐led vs control, Outcome 1 Change in asthma‐related quality of life (PAQLQ). | ||||
| 2 Asthma‐related quality of life (MCID) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Peer‐led vs control, Outcome 2 Asthma‐related quality of life (MCID). | ||||
| 3 Asthma control Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Peer‐led vs control, Outcome 3 Asthma control. | ||||
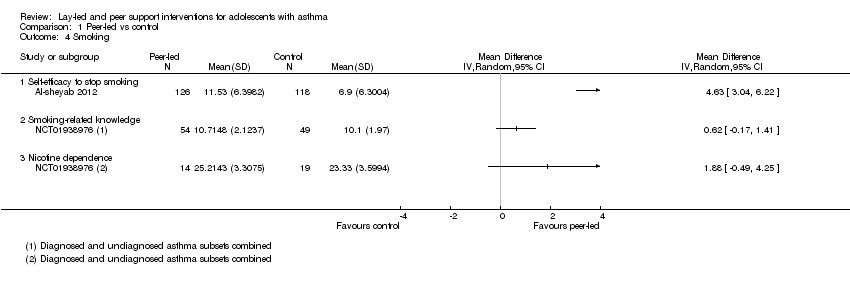
| 4 Smoking Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Peer‐led vs control, Outcome 4 Smoking. | ||||
| 4.1 Self‐efficacy to stop smoking | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Smoking‐related knowledge | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Nicotine dependence | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
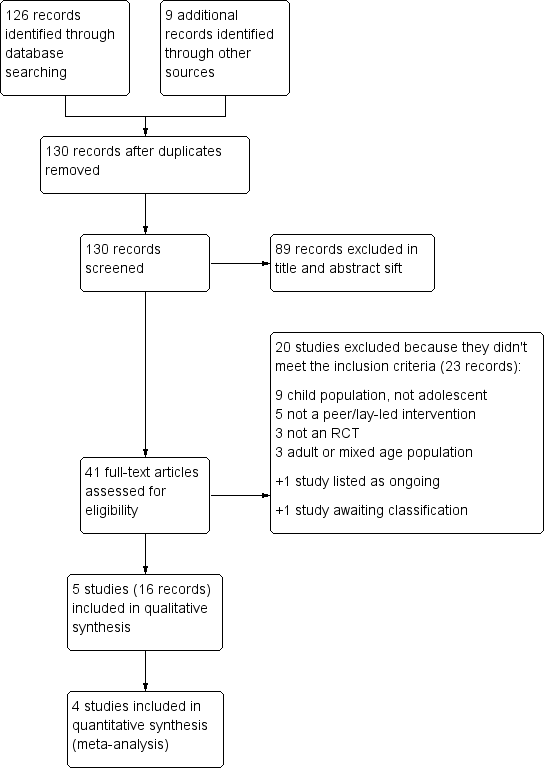
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Peer‐led vs control, Outcome 1 Change in asthma‐related quality of life (PAQLQ).

Comparison 1 Peer‐led vs control, Outcome 2 Asthma‐related quality of life (MCID).

Comparison 1 Peer‐led vs control, Outcome 3 Asthma control.
Comparison 1 Peer‐led vs control, Outcome 4 Smoking.
| Lay‐led and peer support interventions compared with usual care for adolescents with asthma | |||||
| Patient or population: adolescents with asthma Settings: school, day camp or primary care Intervention: lay‐led and peer support interventions Comparison: usual care/no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| Usual care/no intervention | Lay‐led or peer support intervention | ||||
| Asthma‐related quality of life (PAQLQ) 1 to 7 scale; higher = better 3 to 9 months | Mean change in control groups was 0.05 | Mean change in intervention groups was | 578 | ⊕⊕⊝⊝ | |
| Asthma‐related quality of life (MCID) 8 months | 123 per 1000 | 248 per 1000 | 251 | ⊕⊕⊝⊝ | |
| Asthma control Scale (range, score) ACT (5‐23) and ACQ (4‐16) 4 to 9 months | Not pooled. Two studies reported 2 different measures. Both effects favoured peer support, but neither result was statistically significant | 166 | ⊕⊕⊝⊝ | ||
| Unscheduled visits 9 months | Somewhat fewer mean visits per person in the intervention group than in the control group, but the data are skewed and are difficult to interpret | 84 | Not graded | ||
| Medication adherence 2.5 months | Skewed data reported non‐parametrically. Low baseline adherence (˜ 26%), which dropped further in both groups after the intervention, although it was less in the intervention group | 68 (1 RCT) | Not graded | Adherence to ICS was measured objectively with a dose counter | |
| Smoking 3 to 4 months | Mean self‐efficacy to stop smoking score in control group was 6.9 | Mean score in intervention groups was 4.63 better (3.04 to 6.22 better) | 244 | ⊕⊕⊝⊝ | SANDS subscale Range 0 to 16 |
| Mean smoking‐related knowledge score in control group was 10.1 | Mean score in intervention groups was 0.62 better (‐0.17 worse to 1.41 better) | 103 | Modified Tar‐Wars scale Range 0 to 13 | ||
| Mean nicotine dependence score in control group was 23.3 | Mean score in intervention groups was 1.88 better (‐0.49 worse to 4.25 better) | 33 | SANDS total Range 0 to 32 | ||
| Adverse events | No reports of adverse events, although only specifically mentioned in 1 study | ‐ | Not graded | ||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to the estimate of effect. | |||||
| aDowngraded for risk of bias. Outcome measured on a self‐rated scale. Likely to be affected by both performance and detection biases. bDowngraded for inconsistency (I2 = 71%). Random‐effects analysis used as planned, resulting in wide confidence intervals that just cross the line of no effect. Sensitivy analysis with a fixed‐effect model showed much tighter CIs around a mean difference of 0.16 (0.06 to 0.26). Not downgraded for imprecision. cConfidence intervals favour the intervention, but the effect is based on one study of 251 people (downgraded for imprecision). dTwo other studies reported the measure but did not plan a responder analysis (not downgraded for publication bias). eDowngraded for imprecision. Point estimates favoured the intervention, but lower confidence limits do not rule out possible harm. | |||||
| Study ID | Design | Observation | Age range, years | N | Intervention | Comparison | Country |
| Cluster OL | 3 months | 14 to 16 | 261 (4 clusters) | Triple A programme | No intervention | Jordan | |
| Cluster OL | 4 months | 12 to 13 | 433 (4 clusters) | Triple A programme + smoking pledge | Triple A programme alone | Jordan | |
| Individual OL | 2.5 months | 11 to 16 | 68 | Peer support + mp3 messaging | Attention control | USA | |
| Individual SB | 9 months | 13 to 17 | 112 | Peer‐led asthma camp | Adult‐led asthma camp | USA | |
| Cluster OL | 8 months | 12 to 16 | 272 (6 clusters) | Triple A programme | No intervention | Australia | |
| OL = open‐label; SB: single‐blind. Other details such as mean age, healthcare setting, measures of asthma severity, frequency and duration of sessions and baseline social support are described in the text (Included studies). | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in asthma‐related quality of life (PAQLQ) Show forest plot | 3 | Mean Difference (Random, 95% CI) | 0.40 [‐0.02, 0.81] | |
| 2 Asthma‐related quality of life (MCID) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Asthma control Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Smoking Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Self‐efficacy to stop smoking | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Smoking‐related knowledge | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Nicotine dependence | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |

