Les interventions menées par les pairs et l'entraide pour les adolescents ayant de l'asthme
Résumé scientifique
Contexte
Les adolescents ayant de l'asthme présentent un risque élevé d'observance inadéquate de leur traitement. Cette situation peut être amplifiée par les activités aggravant l'asthme, en particulier le tabagisme. Un soutien supplémentaire, allant au‐delà des soins standard a le potentiel d'encourager une bonne autogestion. Nous voulions découvrir si les séances conduites par les pairs des adolescents ou par des leaders profanes aident à réduire ces risques et à améliorer les résultats au niveau de l'asthme chez les adolescents.
Objectifs
Évaluer l'innocuité et l'efficacité des interventions menées par les pairs ainsi que de l'entraide pour les adolescents ayant de l'asthme.
Stratégie de recherche documentaire
Nous avons identifié des essais dans le registre des essais du groupe Cochrane sur les Voies Respiratoires, qui contient des rapports d'essais randomisés obtenus à partir de multiples sources électroniques et de recherches manuelles, et nous avons effectué des recherches dans les registres d'essais cliniques et dans les références bibliographiques des études primaires. Nous avons effectué les recherches les plus récentes le 25 novembre 2016.
Critères de sélection
Les études éligibles avaient randomisé des adolescents ayant de l'asthme à une intervention conduite par des personnes profanes ou par des pairs ou à un groupe témoin. Nous avons inclus les essais contrôlés randomisés d'individus en plan d'étude parallèle ainsi que ceux en grappes. Nous avons inclus les études rapportées sous forme de texte intégral, celles publiées uniquement sous forme de résumé ainsi que les données non publiées.
Recueil et analyse des données
Deux auteurs de la revue ont passé au crible les recherches, extrait les données numériques et les caractéristiques des études et évalué le risque de biais de chaque étude. Les critères de jugement principaux étaient la qualité de vie liée à l'asthme et les exacerbations nécessitant au moins un traitement à base de stéroïdes par voie orale. Nous avons classé les analyses et les preuves présentées dans un tableau « Résumé des résultats ».
Nous avons analysé les données dichotomiques en tant que rapports des cotes, et les données continues sous forme de différences moyennes (DM) ou de différences moyennes standardisées, toutes avec un modèle à effets aléatoires. Nous avons évalué l'hétérogénéité clinique, méthodologique et statistique pour la réalisation de méta‐analyses, et nous avons décrit les données asymétriques de manière narrative.
Résultats principaux
Cinq études portant sur un total de 1146 participants remplissaient les critères d'inclusion pour cette revue. Comme il est souvent le cas avec les revues systématiques d'interventions complexes, les études avaient des conceptions variées (randomisation d'individus ou de grappes), des durées (de 2,5 à 9 mois), des contextes (école, camp de jour, soins primaires) et des contenus d'intervention différents. La plupart des risques de biais et des préoccupations étaient liées à la mise en aveugle et à la documentation incomplète, ce qui a limité les méta‐analyses pouvant être effectuées. De manière générale, les études étaient bien conçues pour contrôler les biais de sélection et d'attrition.
Tous les participants avaient entre 11 et 17 ans. Le diagnostic d'asthme et la gravité variaient, tout comme la prévalence du tabagisme. Trois études utilisaient le programme triple A ; l'une de ces études portait sur l'ajout d'un engagement à ne pas fumer ; une autre a offert l'entraide et les séances de groupe par messagerie MP3 pour encourager l'observance et la troisième comparait un camp de jour mené par des pairs à un camp équivalent mené par des professionnels de la santé.
Nous avons une faible confiance dans tous les résultats en raison du risque de biais, des incohérences et des imprécisions. Les résultats d'une analyse de la qualité de vie liée à l'asthme sur la base d'un modèle à effets aléatoires prédéfini étaient imprécis et n'ont montré aucune différence (DM de 0,40, intervalle de confiance à 95 % (IC) ‐0,02 à 0,81) ; une analyse de sensibilité sur la base d'un modèle à effets fixes et d'une analyse des répondeurs a suggéré qu'un léger bénéfice pourrait être retenu pour ce critère de jugement. La plupart des autres résultats ont été résumés de manière narrative et n'ont montré aucun bénéfice important de l'intervention ; les études n'ont pas fourni de données analysables concernant les exacerbations de l'asthme ou les visites non‐planifiées (les données étaient faussées), et une étude mesurant l'observance a rapporté une baisse dans les deux groupes. Les effets sur le contrôle de l'asthme étaient en faveur de l'intervention, mais les résultats n'étaient pas statistiquement significatifs. Les résultats de deux études avec des niveaux élevés de tabagisme au commencement semblaient prometteurs quant au sentiment d'efficacité personnelle à pouvoir s'arrêter de fumer, mais, dans l'ensemble, la dépendance à la nicotine et les connaissances liées au tabagisme n'étaient pas significativement meilleures dans le groupe d'intervention. Les investigateurs n'ont pas rapporté d'événements indésirables.
Conclusions des auteurs
Bien que des preuves limitées suggèrent que l'entraide et les interventions de soutien menées par les pairs pourraient conduire à une légère amélioration de la qualité de vie liée à l'asthme pour les adolescents, les bénéfices pour le contrôle de l'asthme, les exacerbations et l'observance du traitement restent à prouver. Les preuves actuelles sont insuffisantes pour mettre en évidence si l'utilisation systématique de programmes d'entraide ou de soutien mené par les pairs sont bénéfiques pour les adolescents recevant des soins pour leur asthme.
Les recherches en cours et futures pourraient aider à identifier les populations cibles pour les interventions d'entraide et de soutien menées par les pairs ainsi que les caractéristiques des programmes fructueux.
PICO
Résumé simplifié
Le soutien par les pairs ou par des leaders non professionnels pour les adolescents ayant de l'asthme
Contexte à la question
Les adolescents peuvent avoir besoin d'une prise en charge supplémentaire pour gérer leur asthme, car ils ont un risque plus élevé de ne pas utiliser leurs inhalateurs correctement et de s'engager dans des activités qui peuvent aggraver leur asthme (par exemple, fumer). Nous voulions découvrir si les séances conduites par des pairs ou par des leaders profanes (c'est‐à‐dire des meneurs qui ne sont pas des professionnels de la santé) aident à réduire ces risques et conduisent à un meilleur contrôle de l'asthme. Nous avons inclus les études comparant cette assistance par rapport à des soins habituels ou à un autre type d'aide. Nous avons effectué les recherches les plus récentes le 25 novembre 2016.
Caractéristiques de l'étude
Nous avons trouvé cinq études incluant 1146 adolescents asthmatiques. Les études variaient en termes de conception, de durée (de 2,5 à 9 mois), de contexte (école, camp de jour, soins primaires) et dans la manière dont l'entraide ou les séances menées par les pairs étaient offertes. La gravité de l'asthme variait, de même que le nombre de fumeurs. Trois études utilisaient un programme appelé triple A (Adolescent Asthma Action), au travers duquel les adolescents plus âgés sont formés à offrir des séances à de plus jeunes élèves ; l'une de ces études portait sur l'ajout d'un engagement de s'arrêter de fumer ; une autre a offert les séances de groupe menées par des pairs ainsi que des messages inclus dans un lecteur MP3 pour encourager l'adhérence et la troisième comparait un camp de jour sur l'asthme mené par des pairs comparé à un camp mené par des infirmiers et des médecins.
Principaux résultats
Les adolescents ayant reçu l'entraide avaient une meilleure qualité de vie que ceux du groupe de contrôle, bien que cela variait selon la manière dont les résultats avaient été analysés, nous sommes ainsi incertains concernant cet effet. La plupart des autres résultats n'ont montré aucun bénéfice important de l'intervention. Ces études fournissaient très peu d'informations sur les crises d'asthme ou les visites non‐planifiées chez un professionnel de la santé durant les études, et nous n'avons pas pu établir avec certitude si l'intervention était bénéfique en termes de contrôle de l'asthme. Les résultats de deux études dans lesquelles une grande quantité des adolescents fumaient étaient prometteurs et indiquaient que les adolescents se sentaient capables d'arrêter, mais dans l'ensemble la dépendance à la nicotine et les connaissances liées au tabagisme n'étaient que peu différentes par rapport au groupe témoin. Les études n'ont pas rapporté d'informations quant aux événements indésirables.
Qualité des preuves
Nous ne pouvons pas être sûrs des résultats car la plupart avaient été évalués par des personnes sachant le groupe dans lequel les adolescents avaient été assignés, et cela peut affecter la manière dont les personnes se comportent ainsi que leur manière de répondre aux questions. Certaines études n'ont pas rapporté tous les éléments qu'elles ont indiqué mesurer, ou n'ont pas rapporté des informations que nous pouvions analyser. Parfois, les résultats des études étaient en désaccord avec ceux d'autres études et nous n'avons que rarement pu déterminer avec certitude si les adolescents avaient obtenu un bénéfice. Pour ces raisons, nous avons faiblement confiance dans tous les résultats de l'étude.
Authors' conclusions
Summary of findings
| Lay‐led and peer support interventions compared with usual care for adolescents with asthma | |||||
| Patient or population: adolescents with asthma Settings: school, day camp or primary care Intervention: lay‐led and peer support interventions Comparison: usual care/no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| Usual care/no intervention | Lay‐led or peer support intervention | ||||
| Asthma‐related quality of life (PAQLQ) 1 to 7 scale; higher = better 3 to 9 months | Mean change in control groups was 0.05 | Mean change in intervention groups was | 578 | ⊕⊕⊝⊝ | |
| Asthma‐related quality of life (MCID) 8 months | 123 per 1000 | 248 per 1000 | 251 | ⊕⊕⊝⊝ | |
| Asthma control Scale (range, score) ACT (5‐23) and ACQ (4‐16) 4 to 9 months | Not pooled. Two studies reported 2 different measures. Both effects favoured peer support, but neither result was statistically significant | 166 | ⊕⊕⊝⊝ | ||
| Unscheduled visits 9 months | Somewhat fewer mean visits per person in the intervention group than in the control group, but the data are skewed and are difficult to interpret | 84 | Not graded | ||
| Medication adherence 2.5 months | Skewed data reported non‐parametrically. Low baseline adherence (˜ 26%), which dropped further in both groups after the intervention, although it was less in the intervention group | 68 (1 RCT) | Not graded | Adherence to ICS was measured objectively with a dose counter | |
| Smoking 3 to 4 months | Mean self‐efficacy to stop smoking score in control group was 6.9 | Mean score in intervention groups was 4.63 better (3.04 to 6.22 better) | 244 | ⊕⊕⊝⊝ | SANDS subscale Range 0 to 16 |
| Mean smoking‐related knowledge score in control group was 10.1 | Mean score in intervention groups was 0.62 better (‐0.17 worse to 1.41 better) | 103 | Modified Tar‐Wars scale Range 0 to 13 | ||
| Mean nicotine dependence score in control group was 23.3 | Mean score in intervention groups was 1.88 better (‐0.49 worse to 4.25 better) | 33 | SANDS total Range 0 to 32 | ||
| Adverse events | No reports of adverse events, although only specifically mentioned in 1 study | ‐ | Not graded | ||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to the estimate of effect. | |||||
| aDowngraded for risk of bias. Outcome measured on a self‐rated scale. Likely to be affected by both performance and detection biases. bDowngraded for inconsistency (I2 = 71%). Random‐effects analysis used as planned, resulting in wide confidence intervals that just cross the line of no effect. Sensitivy analysis with a fixed‐effect model showed much tighter CIs around a mean difference of 0.16 (0.06 to 0.26). Not downgraded for imprecision. cConfidence intervals favour the intervention, but the effect is based on one study of 251 people (downgraded for imprecision). dTwo other studies reported the measure but did not plan a responder analysis (not downgraded for publication bias). eDowngraded for imprecision. Point estimates favoured the intervention, but lower confidence limits do not rule out possible harm. | |||||
Background
Description of the condition
Asthma is a long‐term respiratory disease that is characterised by reversible breathing difficulties due to narrowing of the airways, thickening of the airway walls and increased mucus production (GINA 2016). Common symptoms include wheezing, shortness of breath, chest tightness and cough, and diagnosis is established on the basis of medical history and investigations such as spirometry, peak flow diaries, reversibility, biomarkers or methacholine challenge (GINA 2016). Asthma is a prevalent disease that affects more than 334 million people worldwide, with direct treatment costs and indirect costs to society among the highest for non‐communicable diseases (Global Asthma Network 2014). Incidence varies according to many factors, including age, country, sex and smoking exposure, but has been estimated recently at 10.2 per 1000 person‐years among Canadian adolescents (Lawson 2014). Asthma is a significant cause of avoidable morbidity and mortality for patients and families in developed countries, and asthma accounts for many lost working days (GINA 2016; Global Asthma Network 2014; NRAD 2014), especially in low‐ and middle‐income countries, where the condition often is undiagnosed and untreated (Global Asthma Network 2014).
Adults and adolescents generally are considered similar in terms of diagnosis and pharmacological treatment for asthma, but symptom type, frequency and severity vary significantly over time and between people (BTS/SIGN 2016). Adolescents may have different needs and preferences compared with children and adults; the clinician must consider this when providing care (Koster 2015). Adolescents are more likely to have anxiety and depression and to engage in smoking and recreational drug use, both of which may exacerbate their asthma (Bender 2006). Furthermore, adolescents with asthma are more likely to under‐report symptoms and to exhibit poor adherence to maintenance inhalers (Bender 2006).
Description of the intervention
Peer support is a general term that may apply to many types of interventions for which the common factor is participation of a person or people similar to those for whom the intervention is provided. One concept analysis fully defined peer support as "the provision of emotional, appraisal and informational assistance by a created social network member who possesses experiential knowledge of a specific behaviour or stressor and similar characteristics as the target population" (Dennis 2003). Peer support interventions may be aimed at individuals or conducted in groups, with goal of improving well‐being and enhancing disease management by sharing experiences and information with those who have been through similar experiences.
Lay‐led interventions may overlap significantly with what is considered peer support, but they may not necessarily be led by people with asthma or other chronic conditions. Interventions may be led by 'patient experts' to be condition‐specific, such as the Arthritis Self‐Management Program (Lorig 1986), or may be designed to be applicable to various chronic conditions, such as the Chronic Disease Self‐Management Program (Lorig 2001) and the Expert Patients Programme (Department of Health 2001). The person who delivers the intervention might be considered a 'lay' person or a peer, or both, depending on the person's age, profession and health status in relation to the target population. For example, if adolescents with asthma are the target population, an adolescent with or without asthma and an adult with asthma might be considered peers, as might individuals of any age with a different long‐term condition. In all of these cases, leaders do not receive formal medical training and can be considered 'lay' people as well, whereas an adult without asthma or another health condition might only fit into the category of a lay leader. Delivery of asthma self‐management education through lay leaders, regardless of their own health status and similarity to the target population, can be as effective as that delivered by practice nurses (Partridge 2008). Peer and lay leaders may often fall under the term 'community health workers' ‐ a role that is increasingly adopted as a way of improving outreach and promoting healthy behaviour, particularly among high‐risk populations in lower‐resource settings (Butz 1994; Haines 2007).
Peer support and lay‐led interventions may vary substantially regarding the number and content of sessions, the degree of structure within the intervention, locations at which sessions are conducted and individuals presenting the sessions. Recent studies have capitalised on adolescents' familiarity with communication technologies and social networking as a way of delivering peer support interventions (NCT01169883; Stewart 2013). Asthma treatment guidelines now recognise peer‐led and peer support education as ways to complement the usual clinician‐based care to address poor adherence among adolescents (BTS/SIGN 2016; GINA 2016), although they do not elaborate on the content or method of delivery of such interventions.
How the intervention might work
Dennis 2003 describes three types of support that are common to most peer support interventions and the ways they are likely to improve the lives of people in the context of health care. The first ‐ emotional support ‐ is thought to enhance self‐esteem and self‐efficacy by exchanging personal difficulties, empathy and reassurance with people in similar situations. The second ‐ informational support ‐ provides relevant factual information and advice that may help people engage in more effective self‐management, which is increasingly emphasised as a key factor in maintaining asthma control (NRAD 2014). The third ‐ appraisal or "affirmational" support ‐ helps people generate a positive outlook by discussing and receiving encouragement that one's thoughts and behaviours are normal and appropriate, with a view toward reducing the stigma of a long‐term health condition.
Interventions led by 'patient experts' and lay people rather than by healthcare professionals may improve the rapport between leader and patient by removing the formality of traditional medical contacts, and by helping to engage people who normally would not visit their family physician or nurse. It is hoped that establishment of self‐management and lay‐led programmes in health care might "allow people with chronic diseases to have access to opportunities to develop the confidence, knowledge and skills to manage their conditions better, and thereby gain a greater measure of control and independence to enhance their quality of life" (Department of Health 2001). Increased social support for those living with asthma, specifically from parents or peers, has been associated with maintenance of a healthy lifestyle among adolescents, which may serve to reduce their exposure to unhealthy behaviours likely to exacerbate their symptoms (Yang 2010). However, interventions that are not led by trained medical professionals may have the opposite effect, for example, by sharing unsafe asthma management behaviours, or by advising about treatments without appropriate knowledge of their harms and benefits. Use of community health workers has highlighted the need for "focused tasks, adequate remuneration, training, supervision [and] evaluation" to document potential cost‐effectiveness and "to elucidate factors associated with success and sustainability" (Haines 2007).
Why it is important to do this review
The burden of asthma disability and mortality is greatest in the elderly and in adolescents between 10 and 14 years of age (Global Asthma Network 2014). Given higher engagement in risk‐taking behaviours and the tendency to be reliant on and influenced by peers, it is important that health workers target adolescents by providing interventions that might improve adherence or reduce the likelihood of engaging in activities that will make their asthma worse (Bender 2006). It is also an important time to minimise school absence while establishing good self‐management behaviours to be taken into adulthood.
The prevalence of mental health problems among adolescents with asthma has been associated with the increased burden of asthma symptoms and inability to cope with the disease (Richardson 2006) and makes adolescence an important time for optimal asthma care. The National Review of Asthma Deaths in the UK identified one or more avoidable factors that contributed to 17 of 18 deaths that occurred in individuals 10 to 19 years old during the year studied (NRAD 2014). Of these 18 deaths, poor adherence to medical advice was a contributing factor in 13 cases, psychosocial factors in four cases and smoke exposure in seven. Peer support or lay‐led interventions may be provided to educate and motivate young people to avoid these factors, increase social support and reduce the stigma of asthma among adolescents.
Objectives
To assess the safety and efficacy of lay‐led and peer support interventions for adolescents with asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel randomised controlled trials (RCTs). We included studies that used individual or cluster randomisation, but we excluded cross‐over studies owing to the likelihood of carry‐over effects. We included studies reported as full text, those published as abstract only and unpublished data.
Types of participants
We included adolescents with a diagnosis of asthma. We included studies that described inclusion criteria for asthma, such as confirmation by a physician or via spirometry, to exclude people with wheeze not associated with obstructive airways disease. For the purposes of this review, we defined adolescents as those between 10 and 19 years of age, in keeping with the definition of the World Health Organization (WHO 2016). If a study had an unclear age range, included a subset of the age group of interest (e.g. younger adolescents between 10 and 14 years of age) or included participants outside our predefined age criteria (e.g. university students 18 to 21 years of age), we included the study if the mean age of participants was between 10 and 19 years. We excluded studies that enrolled adolescents with other long‐term conditions, such as cystic fibrosis, unless the study authors presented results for participants with asthma separately.
Types of interventions
We included studies that assessed an intervention delivered by peers or by lay people to adolescents with asthma. We defined peers as people who are not medically trained but are similar to the target population in terms of age, presence of an asthma diagnosis or diagnosis of a different long‐term condition. These interventions may also be considered lay led, but other eligible interventions that meet the criteria for a lay‐led intervention may not be considered to include peer support (e.g. those delivered by adult community health workers). We undertook meta‐analyses only when interventions were similar enough for pooling to make sense, and we presented intervention characteristics in a summary table in the review. We explored differences in the characteristics of those who deliver the interventions, when possible, using subgroup analysis.
We analysed studies that compared the intervention versus usual care or a minimal control intervention separately from those that compared the intervention against another active intervention. We excluded studies that used basic peer support itself as a minimal control for a more intensive intervention. We included interventions delivered to individuals or groups of adolescents with asthma, irrespective of the mode of delivery (face‐to‐face or via technology). We excluded studies of interventions that involved multiple components other than the peer support or lay‐led intervention unless the control group also received them.
We included studies regardless of the aim of the intervention (e.g. improving self‐esteem, improving medication adherence, providing asthma education).
Types of outcome measures
Primary outcomes
-
Asthma‐related quality of life (measured on a validated scale, e.g. Asthma Quality of Life Questionnaire (AQLQ))
-
Asthma exacerbations requiring at least a course of oral steroids
Secondary outcomes
-
Asthma control (measured on a validated scale e.g. Asthma Control Questionnaire (ACQ) or Asthma Control Test (ACT))
-
Unscheduled contacts with health services for asthma
-
Medication adherence
-
Smoking
-
Adverse events
Smoking is a behaviour that is commonly taken up in adolescence and is particularly risky for those with asthma. It was unclear in advance whether or how studies might measure this outcome (e.g. as mean frequency of cigarettes or people per group smoking by the end of the trial), but we summarised available information as a reduction in smoking may be an important benefit of peer support or lay‐led interventions.
Asthma exacerbations and unscheduled contacts with health services may occur as unintended adverse events of interventions not delivered by a healthcare professional, but higher rates of contact with health services may represent better preventative care. We were mindful of this when we interpreted these data.
Interventions may lead to other adverse outcomes, for example, from sharing of unsafe management behaviours or provision of incorrect advice. This may be reflected in the direction and magnitude of effect for several of the outcomes listed, but we analysed additional information about adverse events and safety issues when reported by study authors. We meta‐analysed this information when possible, or we described it narratively, depending on the nature of the data.
We presented details about cost and resource implications of these interventions when available, but we did not conduct formal cost analyses.
Reporting one or more of the outcomes listed here in the study was not an inclusion criterion for this review. We used completion of study measurement as the main time point of interest, and we extracted and presented longer‐term follow‐up data when available.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. The Cochrane Airways Group Trials Register contains studies identified from several sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org).
-
Weekly searches of MEDLINE Ovid SP (1946 to date).
-
Weekly searches of Embase Ovid SP (1974 to date).
-
Monthly searches of PsycINFO Ovid SP.
-
Monthly searches of the Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO.
-
Monthly searches of the Allied and Complementary Medicine Database (AMED) EBSCO.
-
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of the Cochrane Airways Group. We have presented details of these strategies, as well as a list of handsearched conference proceedings, in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We also conducted searches of the clinical trials registries ClinicalTrials.gov (www.ClinicalTrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/en/), using appropriately adapted search terms. We searched all databases from their inception to the present, and we imposed no restriction on language of publication.
We conducted the most recent searches on 25 November 2016.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references.
On 2 December 2016, we searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (KK and RC or IC) independently screened titles and abstracts of all studies identified for possible inclusion as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved full‐text study reports/publications of articles that we coded as 'retrieve'. Two review authors (KK and RC or IC) independently screened these full‐text articles and identified studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion. We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram and a Characteristics of excluded studies table (Moher 2009).
Data extraction and management
To record study characteristics and outcome data, we used a data collection form that we had piloted on at least one included study in the review. One review author (KK) extracted the following study characteristics from the included studies.
-
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals and date of study.
-
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications, excluded medications, costs and resources involved.
-
Outcomes: primary and secondary outcomes specified and collected and time points reported.
-
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (KK and RC or IC) independently extracted outcome data from the included studies. We noted in the Characteristics of included studies table if study authors did not report outcome data in a useable way. We resolved disagreements by consensus. One review author (KK) transferred data into the Review Manager (RevMan) file (RevMan 2014). We double‐checked that data were entered correctly by comparing data presented in the systematic review against the study reports. A second review author (RC) spot‐checked study characteristics for accuracy against the study reports.
Assessment of risk of bias in included studies
Two review authors (KK and RC or IC) independently assessed the risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion and assessed risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised 'Risk of bias' judgements across studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a trial author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to these outcomes.
Assessment of bias in conducting the systematic review
We conducted the review according to this published protocol and reported deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios (OR) and continuous data as mean differences (MD) or standardised mean differences (SMD). We entered data presented as a scale with a consistent direction of effect.
We undertook meta‐analysis only when this was meaningful (i.e. if treatments, participants and the underlying clinical question were similar enough for pooling to make sense).
We narratively described skewed data reported as medians and interquartile ranges.
When study authors reported multiple trial arms in a single trial, we included only the relevant arms. If we combined two comparisons (e.g. two types of peer support vs a minimal control intervention) in the same meta‐analysis, we halved the control group to avoid double‐counting.
If adjusted analyses of variance or co‐variance (ANOVA or ANCOVA) were available, we used these in our meta‐analyses. If both change from baseline and endpoint scores were available for continuous data, we used change from baseline unless most studies reported endpoint scores. If a study reported outcomes at multiple time points, we used the end of study measurement.
When both an analysis including only participants who completed the trial and an analysis that imputed data for participants who were randomly assigned but did not provide endpoint data (e.g. last observation carried forward) were available, we used the latter.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (i.e. number of children admitted to hospital, rather than number of admissions per child). We meta‐analysed data from cluster RCTs only if available data had been adjusted (or could be adjusted) to account for the clustering.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when we identified a study as an abstract only). When this was not possible, and we considered the missing data to have introduced serious bias, we explored the impact in the Grading of Recommendations Assessment, Development and Evaluation (GRADE) rating for that outcome.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the studies in each analysis. If we identified substantial heterogeneity, we reported this and explored possible causes by conducting prespecified subgroup analysis.
Assessment of reporting biases
We were not able to pool more than 10 studies, so we could not create and examine a funnel plot to explore possible small study and publication biases.
Data synthesis
We used a random‐effects model and performed a sensitivity analysis based on a fixed‐effect model.
'Summary of findings' table
We created summary of findings Table for the main comparison using all outcomes listed in this protocol. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data to the meta‐analyses for prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we used the GRADEpro Guideline Development Tool (GDT) software (GRADEpro GDT). We justified all decisions to downgrade or upgrade the quality of the evidence using footnotes, and we made comments to aid the reader's understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses for primary outcomes.
-
Group versus one‐to‐one interventions.
-
Person delivering the intervention (e.g. peer supported by an adolescent vs lay led by an adult).
-
Face‐to‐face versus remotely delivered interventions (e.g. over the Internet, by telephone).
We used the formal test for subgroup interactions provided in RevMan (RevMan 2014).
We did not intend to conduct a formal subgroup analysis on the basis of age unless studies used age criteria that do not overlap (e.g. 10 to 14 years of age and 15 to 19 years of age). Studies may recruit adolescents from a range of ages that would not fit predefined arbitrary categories.
We presented key characteristics of study populations and interventions in an additional table to display other potential sources of heterogeneity that could not be easily assessed in subgroups (Table 1), and we described key characteristics in the review text (e.g. mean age, healthcare setting, measures of asthma severity, frequency and duration of sessions, baseline social support).
| Study ID | Design | Observation | Age range, years | N | Intervention | Comparison | Country |
| Cluster OL | 3 months | 14 to 16 | 261 (4 clusters) | Triple A programme | No intervention | Jordan | |
| Cluster OL | 4 months | 12 to 13 | 433 (4 clusters) | Triple A programme + smoking pledge | Triple A programme alone | Jordan | |
| Individual OL | 2.5 months | 11 to 16 | 68 | Peer support + mp3 messaging | Attention control | USA | |
| Individual SB | 9 months | 13 to 17 | 112 | Peer‐led asthma camp | Adult‐led asthma camp | USA | |
| Cluster OL | 8 months | 12 to 16 | 272 (6 clusters) | Triple A programme | No intervention | Australia |
OL = open‐label; SB: single‐blind.
Other details such as mean age, healthcare setting, measures of asthma severity, frequency and duration of sessions and baseline social support are described in the text (Included studies).
Sensitivity analysis
We planned the following sensitivity analyses by removing the following from the primary analyses.
-
Studies at high risk of selection bias (judgement of high risk for either of the selection bias domains).
-
Unpublished data (provided by study authors or derived from non‐peer‐reviewed sources such as conference abstracts).
-
Studies that include a subset of ineligible participants (e.g. those younger or older than the predefined population).
Results
Description of studies
Results of the search
We identified 126 records through electronic database searches and an additional nine through other resources. We removed five duplicates and sifted the remaining 130 unique records. We excluded 89 by reviewing titles and abstracts alone because it was obvious they were not relevant to the research question. We obtained full texts for the remaining 41 and excluded 20 because they did not meet the inclusion criteria. One study met the inclusion criteria but is ongoing (NCT02293499). Sixteen records met all inclusion criteria and could be collated into five included studies (Al‐sheyab 2012; NCT01938976; NCT01169883; NCT01161225; Shah 2001). Figure 1 illustrates the process of study selection.
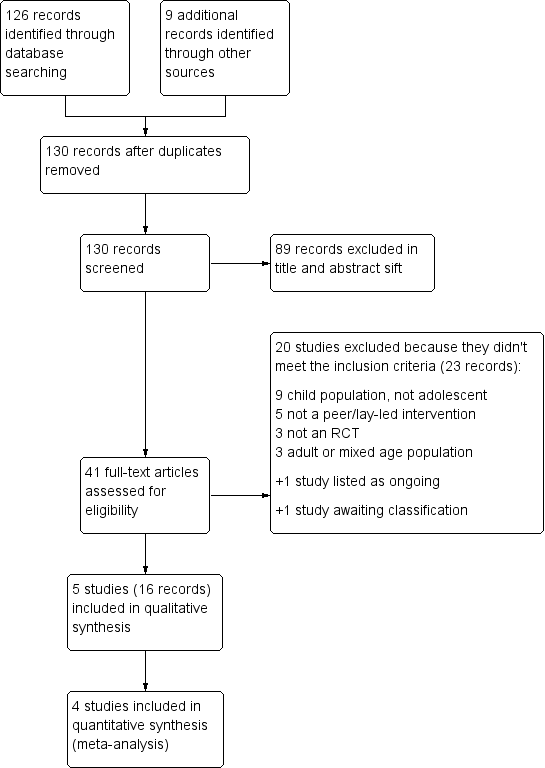
Study flow diagram.
Although one study identified through clinicaltrials.gov met the inclusion criteria, we have listed it as awaiting classification because we found no results or publications posted (NCT00217776). We tried to make contact with the study team, but the lead investigator is now deceased. This 12‐month study, which is registered in the USA, is described as double‐blind and aimed to recruit 1292 younger adolescents between 11 and 12 years of age. Investigators designed this study to test addition of peer asthma education to the Open Airways programme.
Included studies
Five studies, including a total of 1146 participants, met the inclusion criteria for this review (Al‐sheyab 2012; NCT01938976; NCT01169883; NCT01161225; Shah 2001). We have provided a summary of study, participant and intervention characteristics in Table 1, and have given additional details about each individual study and risk of bias in the Characteristics of included studies tables. Four studies contributed to at least one meta‐analysis, and one was described narratively (NCT01169883).
Three studies had a cluster randomised design (Al‐sheyab 2012; NCT01938976; Shah 2001), and two studies randomised individuals to receive intervention or control (NCT01169883; NCT01161225). The number of adolescents included within a study ranged from 68 to 433 (median 261). The three cluster trials randomised four (Al‐sheyab 2012; NCT01938976) or six schools (Shah 2001). Two studies took place in Jordan (Al‐sheyab 2012; NCT01938976), two in the USA (NCT01169883; NCT01161225) and one in Australia (Shah 2001). Three studies provided interventions through schools (Al‐sheyab 2012; NCT01938976; Shah 2001), one at a day camp (NCT01161225) and one through primary care (NCT01169883). Studies lasted between 2.5 and 9 months.
Population characteristics and inclusion criteria
Lower age limits ranged from 11 to 14 and upper limits from 13 to 17 (Table 1). Al‐sheyab 2012 and NCT01938976 did not report mean age, but means in other studies ranged from 12.5 to 15.5 years. Four studies recruited roughly equal proportions of males and females (42.8% to 56.3%), and one study specifically recruited from boys' schools (NCT01938976). Studies offered very little information about ethnicity; NCT01169883 specifically recruited adolescents who self‐identified as African American or Hispanic, and around 45% of participants in NCT01161225 were white. Studies excluded adolescents who could not read or write or were not capable of participating in the intervention, along with anyone with another major disease that would affect their questionnaire responses.
In three studies, all recruited participants had a diagnosis of asthma or reported asthma symptoms (NCT01169883; NCT01161225; Shah 2001), and two studies recruited participants for whom some outcomes were measured in the subset with a diagnosis of asthma (Al‐sheyab 2012; NCT01938976). Severity of asthma and how it was described varied; less than a third of participants in Al‐sheyab 2012 and Shah 2001 were taking a daily inhaled corticosteroid (ICS), as was everyone in NCT01169883 and 71% of participants in NCT01161225. NCT01169883 required that participants have a current ICS prescription and excluded those with adherence above 48% (measured objectively over 14 days at baseline with an electronic dose counter), as the intervention was aimed at improving adherence. This study recruited a population with relatively severe persistent asthma compared with participants in the other studies; around 80% had uncontrolled asthma and around half had one or more emergency department (ED) or hospital visits over the past year.
Smoking varied across studies; around a quarter of participants in Al‐sheyab 2012 were current smokers, and nearly three‐quarters had a family member who smoked. Smoking was much less prevalent in NCT01169883 at less than 5% of participants and less than 10% of family members, and 37% of the population in NCT01938976 were 'ever smokers'.
Interventions and comparisons
Three studies used the Triple A programme, which stands for "Adolescent Asthma Action" (Al‐sheyab 2012; NCT01938976; Shah 2001). Triple A is a three‐step programme that teaches older students to educate and empower their peers about asthma and its management. Teaching tools include games, videos, worksheets, discussions and role‐plays. In step 1 volunteers take part in a one day workshop and are trained to become Triple A Peer Leaders. In step 2, small teams of peer leaders conduct health lessons in schools, and in step 3, participants develop and present key messages to other students. In Al‐sheyab 2012, bilingual (English and Arabic) Jordanian health workers delivered the programme. Peers were year 11 students, and participants were year 10 students. The control group received no intervention. NCT01938976 tested the addition of a smoke‐free pledge to the Triple A programme; peers in that study were in grade 10, and participants were in grades 7 and 8. Shah 2001 trained year 11 peers, and both intervention and control groups received various input from school staff and local doctors.
NCT01169883 delivered an intervention that consisted of peer support group sessions and mp3 messaging. Social workers trained to use motivational interviewing led the sessions. During each session, participants developed and recorded messages to be played between music tracks to encourage ICS adherence. The attention control condition included weekly individual sessions with a research assistant and adherence promotion messages recorded by an asthma physician.
The intervention group in NCT01161225 attended a one‐day camp, with group activities facilitated by 12 peer leaders. Peers were 16 to 20 years old, attended three‐week intensive structured training sessions and facilitated activities in small groups of 6 to 10 campers, overseen by adults. Three 45‐ to 60‐minute sessions based on the Power Breathing™ program covered basic asthma education, psychosocial issues and asthma self‐management. Group activities involved discussion, strategic thinking, knowledge‐testing games and role playing. At completion of the camp, peer leaders conducted monthly phone follow‐ups to provide continuous peer support and encouragement using a checklist. The control group attended a day camp, during which healthcare practitioners presented asthma education at the same camp site on different days.
Excluded studies
After viewing the full texts, we excluded 20 studies because they did not meet the inclusion criteria. Reasons for exclusion were child rather than adolescent population (Bryant‐Stephens 2008; Chernoff 2002; Flores 2009; Horner 2008; Krieger 2009; NCT02747706; Pulgaron 2010; Rice 2015; Valery 2010); intervention not peer supported or lay led (Bruzzese 2008; Duncan 2013; Martin 2015; Srof 2012; Wallis 2015); not an RCT (Gibson 1998; JPRN‐UMIN000018186; Mosnaim 2010); and adult or mixed age study population (NCT00214669; NCT01725815; Partridge 2008).
Risk of bias in included studies
Most of our concerns for the five included studies were related to blinding and reporting biases. Studies generally controlled well for selection and attrition biases. We have summarised risk of bias judgements across studies in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All five studies used appropriate methods of generating the random sequence to allocate participants to groups; thus we rated them as having low risk of bias. Three studies also described methods of allocation concealment at the point of randomisation (Al‐sheyab 2012; NCT01938976; Shah 2001), but the other two studies did not describe these methods in adequate detail, so we rated them as having unclear risk (NCT01169883; NCT01161225).
Blinding
We considered all studies to have high risk of performance bias because the interventions were behavioural and could not be blinded. Knowledge of group allocation, regardless of outcomes measured, may have inadvertently affected how study investigators or participants in each group behaved, which may have biased the results. However, this may have been less of an issue in studies in which the control group also received an intervention, such as the adult‐led day camp in NCT01161225, the active control in NCT01938976 and healthcare professional input in Shah 2001.
It was possible to blind outcome assessors in all studies, but not for outcomes rated by the individual or by people who were aware of group allocation. NCT01169883 specifically described measures to blind outcome assessors, but the other studies did not. For this reason, we rated risk as high in all studies except NCT01169883 owing to the types of outcomes reported, but we considered this separately for each outcome when applying the GRADE framework.
Incomplete outcome data
We rated four studies as having low risk of attrition bias because of the extent of dropout and imbalance between groups (Al‐sheyab 2012; NCT01938976; NCT01169883; NCT01161225). We rated Shah 2001 as high risk because the study reported only the number of participants who had matched data among those with baseline measurements, rather than among the total number of participants with asthma in the randomised schools. Study authors state that missing data were due to misclassification, a change in students' schools or absence on the day of testing or failure to complete the questionnaire, but it is unclear how these reasons compared between groups.
Selective reporting
We rated two studies as having high risk of bias because some researchers reported some results relevant to this review in insufficient detail for inclusion in the meta‐analysis (NCT01169883; Shah 2001). This usually occurred when results were reported narratively as non‐significant or graphically without the numerical data required for pooling with other studies, or when outcomes mentioned in the methods were not reported in the results. We rated NCT01161225 as low risk because the trial was prospectively registered and publications reported all prespecified outcomes. We also rated Al‐sheyab 2012 and NCT01938976 as low risk; although these studies were not prospectively registered, study authors responded to our contact and confirmed that they possessed no additional data that were relevant to our analyses.
Other potential sources of bias
Al‐sheyab 2012 described school selection in detail but may have introduced a selection bias before randomisation. NCT01938976 used the split‐plot design to adjust outcome analyses for clustering effects as well as for baseline differences between groups.
Effects of interventions
See: Summary of findings for the main comparison
We have summarised all results and have displayed GRADE quality ratings in summary of findings Table for the main comparison. Table footnotes list factors that decreased our confidence in study findings; we have explained these in greater detail in the discussion (Quality of the evidence).
Primary outcomes
Asthma‐related quality of life
Three studies reported mean change in asthma‐related quality of life using the paediatric version of the Asthma Quality of Life Questionnaire (PAQLQ) (Al‐sheyab 2012; NCT01161225; Shah 2001). The mean difference (MD) observed with the prespecified random‐effects model was 0.40 (95% confidence interval (CI) ‐0.02 to 0.81). Results revealed significant statistical heterogeneity because the effect in Shah 2001 was much smaller than that described in the other two studies. When we used a fixed‐effect model, the effect size was much smaller and the confidence intervals tighter (MD 0.16, 95% CI 0.06 to 0.26). We used the longest time point available for each study, and sensitivity analyses based on the shorter time points available in NCT01161225 did not change our conclusions.
Shah 2001 also reported a responder analysis showing that more participants in the intervention group had an improvement of at least 0.5 points in their quality of life score (the minimal clinically important difference on the PAQLQ); 25% of those who received peer support responded compared with 12% of those in the control group (odds ratio (OR) 2.34, 95% CI 1.21 to 4.55; 251 participants; one study).
Asthma exacerbations requiring at least a course of oral steroids
None of the data on asthma exacerbations were suitable for meta‐analysis. Shah 2001 reported exacerbations narratively as follows: "The proportion of students reporting asthma attacks at school in year 10 increased in the control group (21.2% v 34.8%). No change was found in the intervention group (24.2 % v 25.8%). The intervention had no effect on school absenteeism and asthma attacks in year 7 students". Al‐sheyab 2012 and NCT01938976 confirmed that investigators received no reports of asthma exacerbation during these studies.
Secondary outcomes
Asthma control
Two studies used a measure of asthma control; NCT01938976 used the Asthma Control Test (ACT, range of scores 5 to 23), and NCT01161225 used the Asthma Control Questionnaire (ACQ, range of scores 4 to 16).
We did not pool results because comparisons made in these studies were not similar; NCT01938976 tested a smoking pledge added to the Triple A programme versus Triple A alone, and NCT01161225 compared a peer‐led intervention versus one delivered by adults. NCT01938976 found a mean difference on the ACT of 0.50 favouring the smoking pledge (95% CI ‐0.61 to 1.61), and NCT01161225 found a mean difference on the ACQ of 0.65 favouring peer‐led over adult‐led interventions (95% CI ‐0.14 to 1.44). Neither effect was statistically significant.
Unscheduled contacts with health services for asthma
NCT01161225 was the only study that reported the effect of a peer‐led intervention on the need for unscheduled visits to a healthcare provider. Investigators measured this outcome at nine months as the mean number per participant over the previous three months, but the data were skewed, so we did not calculate a mean difference. The 43 adolescents in the intervention group had a mean of 0.53 exacerbations each (standard deviation (SD) 1.12), and the 41 adolescents in the control group had a mean of 0.78 exacerbations each (SD 1.27). Al‐sheyab 2012 and NCT01938976 confirmed to us that no one needed urgent care during these studies.
Medication adherence
NCT01169883 was specifically aimed at improving adherence and was the only study to report this as an outcome. Researchers measured adherence to inhaled steroids objectively using an electronic dose counter as average daily adherence over 14 days at the 10‐week endpoint.
We did not present the data on a forest plot as they were skewed and were analysed by study authors as medians and interquartile ranges (IQRs). Baseline adherence to inhaled steroids was very low at 27.4% in the peer support group (IQR 14.3 to 35.0) and 25.9% in the control group (IQR 14.0 to 37.5). After 10 weeks, median adherence had dropped even lower in both the peer support group (median 7.1%; IQR 0.9 to 21.4) and the control group (median 14.3%; IQR 5.4 to 21.4).
Smoking
Two studies reported smoking outcomes (Al‐sheyab 2012; NCT01938976); one of these specifically tested the effect of a smoking pledge added to a peer intervention (NCT01938976). Results are presented on one forest plot but have not been pooled. Al‐sheyab 2012 reported self‐efficacy to stop smoking on a subscale of the Self‐Administered Nicotine Dependence Scale (SANDS; Alanasari 2004; Davis 1994). NCT01938976 reported the total nicotine dependence score on the SANDS, as well as a measure of smoking‐related knowledge (Cain 2006). All point estimates favoured the intervention, but only results on the self‐efficacy scale in Al‐sheyab 2012 showed a statistically significant result over those in the control group (MD 4.63, 95% CI 3.04 to 6.22; 0 to 16 subscale, higher scores better); results for asthma‐related knowledge (MD 0.62, 95% CI ‐0.17 to 1.41; 0 to 13 scale; higher scores better) and nicotine dependence measured on the SANDS (MD 1.88, 95% CI ‐0.49 to 4.25; 0 to 32 scale; higher scores better) favoured peer support, but confidence intervals did not rule out the possibility of no difference.
Adverse events
NCT01161225 was the only study that reported adverse events, stating that no serious or non‐serious adverse events occurred in either group.
Subgroup and sensitivity analyses
It was not possible to conduct any planned subgroup analyses owing to the small number of studies reporting the outcomes of interest and the differences between comparisons made. Therefore, we were not able to test for possible moderating effects of interventions delivered in groups versus one‐on‐one, the person delivering the intervention or face‐to‐face versus remotely delivered interventions.
We rated no studies as having high risk of bias for either of the selection bias domains, so no sensitivity analysis was needed. Neither did we include unpublished data or studies that included a subset of ineligible participants, so these sensitivity analyses also were not necessary.
Discussion
Summary of main results
Five studies including a total of 1146 participants met the inclusion criteria for this review. As ever with systematic reviews of complex interventions, studies varied by design (cluster and individually randomised), duration (2.5 to 9 months), setting (school, day camp, primary care) and intervention content. Most risk of bias concerns were related to blinding and incomplete reporting, which limited the meta‐analyses that could be performed. Studies generally controlled well for selection and attrition biases.
All participants were between 11 and 17 years of age. Asthma diagnosis and severity varied, as did smoking prevalence. Three studies used the Triple A programme (Al‐sheyab 2012; NCT01938976; Shah 2001), one of which tested the addition of a smoke‐free pledge. NCT01169883 delivered peer support group sessions and mp3 messaging to encourage adherence, and NCT01161225 compared a peer‐led asthma day camp versus an equivalent session led by healthcare practitioners.
We had low confidence in all study findings owing to risk of bias, inconsistency and imprecision. Results from an analysis of asthma‐related quality of life conducted through the prespecified random‐effects model were imprecise and showed no difference (mean difference (MD) 0.40, 95% confidence interval (CI) ‐0.02 to 0.81); a sensitivity analysis based on a fixed‐effect model and a responder analysis in Shah 2001 suggested possible benefit. Most other results were summarised narratively and generally did not show an important benefit of the intervention; studies yielded no analysable data on asthma exacerbations or unscheduled visits (which were skewed), and one study measuring adherence noted a drop in both groups. Effects on asthma control favoured the intervention but were not statistically significant. Results from two studies with high levels of baseline smoking showed promising results for self‐efficacy to stop smoking, but overall nicotine dependence and smoking‐related knowledge were not significantly better in the intervention group. Investigators reported no adverse events.
Overall completeness and applicability of evidence
We designed this review to focus on adolescents as a high‐risk group, and although this allowed us to be more specific and results to be more applicable, it means that the evidence base for younger children has not been considered (see Agreements and disagreements with other studies or reviews). This focus means led to identification of only five relevant studies, although we are aware of two more in the pipeline. One, listed as an ongoing study (NCT02293499), is testing a peer‐led programme for asthma self‐management in adolescents, and is aiming to recruit 420 adolescents. The second was flagged by the author of NCT01161225, a larger replication and extension study in inner‐city schools. We will include both of these studies in a future update of this review.
It is possible that these interventions may best target higher‐risk populations, in terms of the background of the adolescents or the severity of their asthma, but it is difficult to tease out these moderating factors from the current evidence base. It has been suggested that boys, non‐white adolescents and those from lower socioeconomic backgrounds may benefit more from these interventions (Al‐Sheyab 2012, commentary on NCT01161225), but this review cannot substantiate these claims without receiving individual patient data. Both NCT01169883 and NCT01161225 looked into these issues; results are helpful for informing where future research should be directed, but associations are usually observational and may be tied to the specific contexts in which these studies were conducted. Non‐randomised and feasibility studies may supplement the randomised evidence base to inform whether peer support and lay‐led interventions are likely to be cost‐effective, and for whom.
As is often the case with reviews of complex interventions, variation in the characteristics of interventions evaluated makes it difficult to assess their general applicability, or to pick out particularly successful aspects of interventions to aid implementation. At present, evidence is insufficient to conduct subgroup analyses that would tell us whether a group format is more effective than a one‐on‐one approach, whether it matters who delivers the intervention and whether interventions delivered remotely (e.g. over the phone, by Internet) are as successful as face‐to‐face support.
Quality of the evidence
When we were able to apply the GRADE framework, we rated evidence as low quality, meaning that our confidence in the effect estimates is limited. We did not apply GRADE to unscheduled visits, medication adherence or adverse events because results were not pooled for these outcomes and were primarily described narratively. Nonetheless, our confidence in these results is very limited because they were not well reported or were skewed and were difficult to interpret as analysed in the published reports.
When we were able to grade estimates (quality of life, asthma control and smoking), we downgraded quality across the board for risk of bias. The most serious risk of bias for all graded outcomes involved lack of blinding, which, as previously described, may have influenced how participants and study personnel behaved or responded to questionnaires during the study. All graded outcomes were self‐reported, which further increases the risk of bias because those filling in the questionnaires were aware of their treatment allocation and may have responded more or less favourably as a result. This may have been less of an issue in studies in which the control group received more than usual care, such as an alternative intervention or an attention control.
Our confidence in the estimates was also decreased by imprecision, which was related to the numbers of included studies and participants. For both asthma control and smoking, point estimates and most confidence intervals strongly favoured the intervention, but we could not rule out the possibility of no difference, or indeed that the control group saw greater benefit.
The pooled estimate for quality of life was very different depending on whether a random‐effects or a fixed‐effect model was used because statistical heterogeneity between study effects was great. All study point estimates favoured the intervention, so we were fairly confident in the direction of the effect, but we downgraded owing to inconsistency in the size of the effect; we did not downgrade for imprecision even though confidence intervals were wide with the random‐effects model.
Potential biases in the review process
We prespecified the methods of this review in the published protocol (Kew 2016), and we carried out the review in accordance with this plan. In some instances, primarily owing to insufficient data, we were unable to carry out planned analyses; we have detailed these deviations in the section titled Differences between protocol and review. We minimised biases by carrying out study selection, data extraction and risk of bias assessments in duplicate; however, reflecting a change to the published protocol, this duplication was done by someone who was not part of the review team owing to time constraints.
Electronic and additional searches were broad and were repeated close to the time of publication of this review, so we feel confident that we have prepared a complete and up‐to‐date review of the relevant literature. We attempted to contact study authors for additional outcome data and to resolve uncertainties related to risk of bias. We received replies related to three studies (Al‐sheyab 2012; NCT01938976; NCT01161225), which increased our confidence that we had not missed any relevant data measured in those studies.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review that brings together randomised evidence about lay‐led and peer support interventions for adolescents with asthma. A fair amount of research has been conducted to look into the role of lay and peer support in some fields, particularly in mental health and cancer, and among prison populations (Bagnall 2015; Hoey 2008; Pfeiffer 2010), and for other specific purposes such as to support breastfeeding or to increase the uptake of immunisations (Glenton 2011; Kaunonen 2012). Researchers have placed less focus on the possible benefit of lay and peer workers for individuals with a chronic physical condition.
Raphael 2013 brought together evidence from 17 studies on the role of lay health workers in supporting children (0 to 18 years) with chronic conditions. Although the review looked for studies of children and adolescents with any chronic condition, review findings are broadly in agreement with our own. Synthesising evidence of complex interventions is difficult, and even more so when children with a range of conditions and varying needs are considered. Similar to our review, Raphael 2013 acknowledges the heterogeneity of interventions and provides a fairly narrative synthesis, concluding that the interventions "may lead to modest improvements in urgent care use, symptoms, and parental psychosocial outcomes". The Raphael review considers some studies excluded by our own that were conducted in younger populations (Bryant‐Stephens 2008; Flores 2009; Krieger 2009). We came across several additional studies conducted in child rather than adolescent asthma populations (Chernoff 2002; Horner 2008; NCT02747706; Pulgaron 2010; Rice 2015; Valery 2010), suggesting that this has been more of a focus for researchers, but to date, no systematic review has examined this evidence.
We found some non‐randomised evidence in the search, including a small feasibility study of children between 9 and 12 years of age that has not yet been published (JPRN‐UMIN000018186), an impact evaluation of a peer‐led asthma programme for adolescents (Gibson 1998) and an evaluation of the "Fight Asthma Now (FAN)" programme for 8‐ to 13‐year‐olds (Mosnaim 2010). These evaluations are useful for testing the feasibility of implementing programmes, and generally showed improvement in asthma knowledge and attitudes; effects on clinical outcomes and on quality of life are less certain, which is consistent with our findings.
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Peer‐led vs control, Outcome 1 Change in asthma‐related quality of life (PAQLQ).

Comparison 1 Peer‐led vs control, Outcome 2 Asthma‐related quality of life (MCID).

Comparison 1 Peer‐led vs control, Outcome 3 Asthma control.
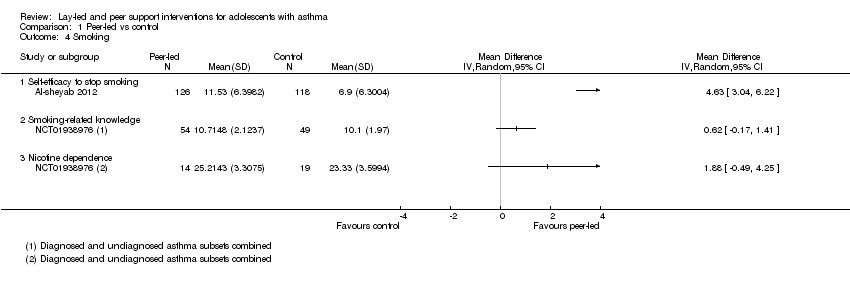
Comparison 1 Peer‐led vs control, Outcome 4 Smoking.
| Lay‐led and peer support interventions compared with usual care for adolescents with asthma | |||||
| Patient or population: adolescents with asthma Settings: school, day camp or primary care Intervention: lay‐led and peer support interventions Comparison: usual care/no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| Usual care/no intervention | Lay‐led or peer support intervention | ||||
| Asthma‐related quality of life (PAQLQ) 1 to 7 scale; higher = better 3 to 9 months | Mean change in control groups was 0.05 | Mean change in intervention groups was | 578 | ⊕⊕⊝⊝ | |
| Asthma‐related quality of life (MCID) 8 months | 123 per 1000 | 248 per 1000 | 251 | ⊕⊕⊝⊝ | |
| Asthma control Scale (range, score) ACT (5‐23) and ACQ (4‐16) 4 to 9 months | Not pooled. Two studies reported 2 different measures. Both effects favoured peer support, but neither result was statistically significant | 166 | ⊕⊕⊝⊝ | ||
| Unscheduled visits 9 months | Somewhat fewer mean visits per person in the intervention group than in the control group, but the data are skewed and are difficult to interpret | 84 | Not graded | ||
| Medication adherence 2.5 months | Skewed data reported non‐parametrically. Low baseline adherence (˜ 26%), which dropped further in both groups after the intervention, although it was less in the intervention group | 68 (1 RCT) | Not graded | Adherence to ICS was measured objectively with a dose counter | |
| Smoking 3 to 4 months | Mean self‐efficacy to stop smoking score in control group was 6.9 | Mean score in intervention groups was 4.63 better (3.04 to 6.22 better) | 244 | ⊕⊕⊝⊝ | SANDS subscale Range 0 to 16 |
| Mean smoking‐related knowledge score in control group was 10.1 | Mean score in intervention groups was 0.62 better (‐0.17 worse to 1.41 better) | 103 | Modified Tar‐Wars scale Range 0 to 13 | ||
| Mean nicotine dependence score in control group was 23.3 | Mean score in intervention groups was 1.88 better (‐0.49 worse to 4.25 better) | 33 | SANDS total Range 0 to 32 | ||
| Adverse events | No reports of adverse events, although only specifically mentioned in 1 study | ‐ | Not graded | ||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to the estimate of effect. | |||||
| aDowngraded for risk of bias. Outcome measured on a self‐rated scale. Likely to be affected by both performance and detection biases. bDowngraded for inconsistency (I2 = 71%). Random‐effects analysis used as planned, resulting in wide confidence intervals that just cross the line of no effect. Sensitivy analysis with a fixed‐effect model showed much tighter CIs around a mean difference of 0.16 (0.06 to 0.26). Not downgraded for imprecision. cConfidence intervals favour the intervention, but the effect is based on one study of 251 people (downgraded for imprecision). dTwo other studies reported the measure but did not plan a responder analysis (not downgraded for publication bias). eDowngraded for imprecision. Point estimates favoured the intervention, but lower confidence limits do not rule out possible harm. | |||||
| Study ID | Design | Observation | Age range, years | N | Intervention | Comparison | Country |
| Cluster OL | 3 months | 14 to 16 | 261 (4 clusters) | Triple A programme | No intervention | Jordan | |
| Cluster OL | 4 months | 12 to 13 | 433 (4 clusters) | Triple A programme + smoking pledge | Triple A programme alone | Jordan | |
| Individual OL | 2.5 months | 11 to 16 | 68 | Peer support + mp3 messaging | Attention control | USA | |
| Individual SB | 9 months | 13 to 17 | 112 | Peer‐led asthma camp | Adult‐led asthma camp | USA | |
| Cluster OL | 8 months | 12 to 16 | 272 (6 clusters) | Triple A programme | No intervention | Australia | |
| OL = open‐label; SB: single‐blind. Other details such as mean age, healthcare setting, measures of asthma severity, frequency and duration of sessions and baseline social support are described in the text (Included studies). | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in asthma‐related quality of life (PAQLQ) Show forest plot | 3 | Mean Difference (Random, 95% CI) | 0.40 [‐0.02, 0.81] | |
| 2 Asthma‐related quality of life (MCID) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Asthma control Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Smoking Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Self‐efficacy to stop smoking | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Smoking‐related knowledge | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Nicotine dependence | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |

