Les interventions visant à améliorer la technique d'inhalation pour les personnes ayant de l'asthme
Résumé scientifique
Contexte
L'asthme est une maladie chronique courante dans le monde entier. Les inhalateurs sont souvent prescrits pour aider à contrôler les symptômes de l'asthme, améliorer la qualité de vie et pour réduire le risque d'exacerbations ou de crises. Cependant, les preuves suggèrent que de nombreuses personnes asthmatiques n'utilisent pas leur inhalateur correctement. Il est donc important d'évaluer si les interventions visant spécifiquement à améliorer cette technique sont efficaces et sûres, et si l'utilisation de ces interventions se traduit par une amélioration des résultats cliniques.
Objectifs
Évaluer l'impact des interventions visant à améliorer la technique d'inhalation sur les résultats cliniques ainsi que son innocuité chez les adultes et les enfants asthmatiques.
Stratégie de recherche documentaire
Nous avons consulté le registre Cochrane des essais sur les voies respiratoires, qui contient des dossiers élaborés à partir de plusieurs bases de données électroniques et provenant de recherches manuelles. Nous avons également effectué des recherches dans des registres d'essais cliniques et dans les références bibliographiques des études primaires. La recherche la plus récente a été effectuée le 23 novembre 2016.
Critères de sélection
Nous avons inclus les études comparant un groupe d'adultes ou d'enfants asthmatiques recevant une intervention quant à une technique d'inhalation par rapport à un groupe témoin ou un groupe recevant une intervention alternative. Nous avons inclus des essais randomisés en groupes parallèles, de n'importe quelle durée, conduits dans tout contexte, et nous avions prévu d'inclure uniquement la première phase de tous les essais croisés identifiés. Nous avons inclus les études rapportées en tant qu'articles complets, celles publiés uniquement sous forme de résumés et les données non publiées.
Recueil et analyse des données
Deux auteurs de la revue ont passé au crible les résultats des recherches pour identifier les études éligibles. Nous avons extrait les données de résultats, évalué le risque de biais en double et résolu les divergences en impliquant un autre auteur de la revue. Nous avons regroupé les études rapportant des comparaisons similaires par consensus (par ex. toutes celles comparant l'éducation améliorée quant à la technique d'inhalation à des soins habituels), et nous avons réalisé des méta‐analyses uniquement si les traitements, les participants et la question clinique sous‐jacente étaient suffisamment semblables pour que le regroupement paraisse pertinent. Nous avons analysé les données dichotomiques en tant que rapports des cotes, et les données continues sous forme de différences moyennes ou de différences moyennes standardisées, toujours avec des modèles à effets aléatoires. Nous avons décrit les données asymétriques de manière narrative. Nous avons classé les résultats et les preuves présentées dans des tableaux « Résumé des résultats » pour chaque comparaison. Les critères de jugement principaux étaient la technique d'inhalation, le contrôle de l'asthme et les exacerbations nécessitant au moins des corticoïdes oraux (CO).
Résultats principaux
Cette revue inclut 29 essais contrôlés randomisés en parallèle (ECR) (n = 2210), mais certains n'ont pas rapporté de données pertinentes ou utilisables. Tous les participants présentaient de l'asthme et la durée du suivi allait de 2 à 26 semaines. La plupart des études étaient à risque faible ou incertain de biais de sélection et de biais d'attrition et à risque élevé de biais associés à la mise en aveugle. Nous avons considéré la plupart des preuves comme étant de faible qualité en raison de ces biais et de l'imprécision dans les estimations de l'effet.
Nous avons classé les études en trois comparaisons : séance(s) améliorée(s) d'enseignement en face à face, entraînement offert au travers d'un moyen multimédia sur l'inhalateur (par ex. DVD, application informatique ou jeu) et les dispositifs offrant un feedback quant à la technique. Les différences entre les interventions, populations et les critères de jugement ont limité les analyses quantitatives, en particulier pour les exacerbations, les événements indésirables, les visites non‐planifiées chez un professionnel de la santé et l'absentéisme au travail ou à l'école.
L'éducation améliorée sur la technique d'inhalation et l'enseignement multimédia amélioraient la technique dans la plupart des études immédiatement après l'intervention et lors du suivi, bien que la gamme de listes à cocher utilisées impliquent qu'il était difficile d'évaluer cela de manière fiable. A la fois pour les adultes et pour les enfants, comment et à quel moment la technique d'inhalation a été évaluée a semblé affecter si la technique d'inhalation serait améliorée et à quel point.
Les analyses du nombre de personnes ayant démontré une technique correcte ou « suffisamment bonne » étaient généralement plus utiles que les scores des listes à cocher. Les études portant sur l'éducation améliorée chez des adultes ont montré un bénéfice lorsque cette mesure était utilisée de 2 à 26 semaines de suivi (rapport des cotes (RC) 5,00, intervalle de confiance à 95 % (IC) 1,83 à 13,65 ; 258 participants ; trois études ; 31 pour 100 avaient une technique correcte dans le groupe témoin par rapport à 69 (IC à 95 % de 45 à 86) dans le groupe éducatif ; preuves de qualité moyenne). Un résultat similaire était observé dans les études examinant des dispositifs offrant un feedback au bout de quatre semaines de suivi (RC 4,80, IC à 95 % 1,87 à 12,33 ; 97 participants ; une étude ; 51 pour 100 avaient une technique correcte dans le groupe témoin par rapport à 83 (IC à 95 % de 66 à 93) dans le groupe recevant un feedback ; preuves de faible qualité). Toutefois, le bénéfice de l'entraînement multimédia pour les adultes même immédiatement après l'intervention était incertain (RC 2,15, IC à 95 % 0,84 à 5,50 ; 164 participants ; deux études ; I² = 49 % ; 30 pour 100 dans le groupe témoin avaient une technique correcte comparés à 47 (IC à 95 % 26 à 70) dans le groupe multimédia ; preuves de qualité moyenne). Les preuves avaient tendance à être moins claires concernant les enfants, en général parce que les résultats étaient basés sur moins d'études et de petite taille.
Certaines études n'ont pas rapporté les exacerbations d'une manière permettant une méta‐analyse ; d'autres ont fourni des résultats non concluants. Les interventions quant à la technique d'inhalation ont montré un certain bénéfice quant au contrôle de l'asthme et sur la qualité de vie, mais n'ont que rarement conduit à des bénéfices cliniques importants et cohérents pour les adultes et les enfants. Les intervalles de confiance comprenaient l'absence de différence ou n'atteignaient pas un seuil pouvant être considéré comme cliniquement important. Les analyses de réponse montraient parfois une amélioration chez davantage de personnes dans les groupes d'intervention, même si la différence moyenne entre les groupes était de petite taille. Nous n'avons trouvé aucune preuve concernant les effets délétères.
Conclusions des auteurs
Bien que les interventions visant à améliorer la technique d'inhalation puissent fonctionner dans certains cas, la variété des interventions et des méthodes de mesure ont entravé notre capacité à effectuer des méta‐analyses et conduit à une confiance faible à modérée dans nos résultats. La plupart des études incluses ne rapportaient pas d'amélioration importante dans les résultats cliniques. Les directives recommandent fréquemment que les cliniciens vérifient régulièrement la technique d'inhalation de leurs patients ; la manière la plus efficace d'intervenir si les cliniciens trouvent la technique d'un patient comme étant inadéquate et si ces interventions auront un impact perceptible sur les résultats cliniques n'est pas clair.
PICO
Résumé simplifié
Les stratégies visant à aider les personnes asthmatiques à utiliser leur inhalateur correctement
Contexte
De nombreux médicaments pour l'asthme sont pris au travers d'un inhalateur, qui dépose les médicaments directement dans les poumons. Il est important que l'inhalateur soit utilisé correctement, de sorte que le patient en obtienne le plus grand bénéfice. Lorsqu'ils sont pris correctement, les médicaments peuvent améliorer les symptômes de l'asthme et réduire le nombre de crises.
De nombreuses personnes n'utilisent pas leurs dispositifs correctement. Cela signifie que le médicament n'est pas correctement envoyé dans les poumons, et en conséquence, l'asthme peut ne pas être aussi bien contrôlé qu'il devrait l'être. Certaines personnes disent également qu'elles peuvent avoir plus d'un type d'inhalateur, de sorte qu'il peut être difficile de savoir comment s'y prendre.
Nous voulions découvrir si enseigner aux personnes souffrant d'asthme comment utiliser leurs inhalateurs est efficace, et si cela conduit à un meilleur contrôle des symptômes et à moins de crises. Cela peut sembler évident, mais il est important que les médecins et les infirmiers sachent la meilleure manière d'aider les personnes ayant de l'asthme.
Caractéristiques de l'étude
Nous avons trouvé 29 études portant sur 2210 personnes ayant de l'asthme. Les études ont duré entre 2 et 26 semaines. Les études ont rapporté la technique d'inhalation selon un éventail de différentes listes à cocher.
Nous avons regroupé les études en trois types : les études testant une ou des séance(s) améliorée(s) d'entraînement en face à face, les études utilisant un moyen multimédia pour enseigner l'utilisation d'un inhalateur (par exemple, une vidéo, une application informatique ou un jeu) et les études testant des appareils qui offrent un feedback visuel ou audio aux personnes à propos de leur technique.
Les études portaient sur différents types de formations et utilisaient différentes mesures pour évaluer la réussite, ce qui signifie que nous n'avons pas pu combiner les données. Cette observation était particulièrement marquée lorsque nous avons tenté d'évaluer les effets sur les crises d'asthme, les événements indésirables, les visites chez un professionnel de la santé et l'absentéisme au travail ou à l'école.
Résultats principaux
A la fois les entraînements en face à face et avec des moyens multimédia ont amélioré la technique d'inhalation dans la plupart des études, bien que les résultats variaient selon la manière dont chaque technique avait été évaluée et le moment de l'évaluation.
Certaines études ont rapporté le nombre de personnes ayant une technique correcte ou « suffisamment bonne ». Davantage de personnes avaient une technique correcte ou « suffisamment bonne » après un entraînement en face à face et avec des dispositifs offrant un feedback. Mais le bénéfice de l'entraînement multimédia pour les adultes était incertain.
Les interventions fournissant un enseignement sur les inhalateurs peuvent apporter un certain bénéfice au niveau de la qualité de vie et du contrôle de l'asthme chez les adultes et les enfants, mais les résultats étaient variés et les études étaient de petite taille.
Les enfants peuvent en obtenir un certain bénéfice, mais les résultats tendaient à être moins clairs pour les enfants car il y avait moins d'études ayant inclus des enfants comme participants et celles‐ci étaient plus petites.
Qualité des preuves
Pour ce genre d'études, il n'est pas possible de masquer aux participants le groupe auquel ils sont assignés. Cela peut modifier la manière dont les gens se comportent ou comment ils répondent aux questionnaires, ce qui réduit notre confiance dans les résultats. Nous étions incertains à propos d'autres résultats car les études n'ont pas fourni suffisamment de données pour démontrer un bénéfice clair.
Conclusions
Nous ne pouvons pas affirmer avec certitude quelle est la meilleure manière d'aider les personnes à apprendre à utiliser leur inhalateur correctement. Il est important que les patients comprennent comment leur inhalateur fonctionne, ils devraient donc demander de l'aide à leur médecin ou à un infirmier.
Les revues Cochrane permettent également d'émettre des suggestions pour les futures recherches. Nous proposons que les futures études devraient durer plus de six mois et devraient rapporter des informations quant à l'adhérence. L'information rapportée la plus utile était le nombre de personnes ayant une technique d'inhalation « suffisamment bonne », de sorte que nous conseillons aux futures études de la rapporter également.
Authors' conclusions
Summary of findings
| Enhanced education compared with control/usual care for people with asthma | |||||||
| Patient or population: adults and children with asthma | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | ||
|---|---|---|---|---|---|---|---|
| Risk with control/usual care | Risk with enhanced education | ||||||
| Correct inhaler technique Follow‐up: 2 to 26 weeks (adults) 12 to 26 weeks (children) | Adults | 31 per 100 | 69 per 100 | OR 5.00 (1.83 to 13.65) | 258 | ⊕⊕⊕⊝ MODERATEa | Additional results from technique rating scales could not be combined (Analysis 1.2) |
| Children | 49 per 100 | 55 per 1,00 | OR 1.29 | 175 | ⊕⊕⊝⊝ LOWa,b | One study measured PIF as a marker for inhaler technique and showed benefit (Analysis 2.2; Analysis 2.3) | |
| Asthma control score 4 to 26 weeks | Adults | ‐ | Score in the intervention group was 0.48 SDs higher than in the control group | ‐ | 247 | ⊕⊝⊝⊝ VERY LOWa,b,c | We were not able to calculate a control risk, as the outcome was measured on different scales |
| Asthma control responders 8 to 12 weeks | Adults | 42 per 100 | 70 per 100 | OR 3.18 | 134 | ⊕⊕⊝⊝ LOWd | |
| Exacerbations requiring at least OCS 26 weeks | Adults | 10 per 100 | 13 per 100 (5 to 28) | OR 1.32 (0.49 to 3.55) | 158 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | The same study also reported exacerbations requiring ED/hospitalisation. Events were rare and results imprecise |
| Quality of life 26 weeks | Adults | ‐ | Score in the intervention group was 0.52 SDs higher than in the control group | ‐ | 247 | ⊕⊕⊝⊝ LOWa,c,e | We were not able to calculate a control risk as the outcome was measured on different scales |
| Other outcomes | No results could be analysed for adverse events, unscheduled visits to a healthcare provider or school/work absences | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | |||||||
| GRADE Working Group grades of evidence | |||||||
| aStudies contributing to this outcome were at high risk of bias in several domains (including performance and detection bias). Downgraded once bWide confidence intervals including possible harm and benefit of intervention. Downgraded once cHigh level of statistical inconsistency detected. Downgraded once dThe two small studies contributing to this outcome were identified as abstracts only; it is therefore difficult to assess methodological quality. Studies were considered at high or unclear risk of bias in multiple domains (including selection, performance, detection and reporting biases). Downgraded twice eConfidence interval includes no difference with random‐effects model, driven by statistical heterogeneity. Fixed‐effect sensitivity analysis yields more precise result. Not downgraded | |||||||
| Multimedia training compared with control/usual care for people with asthma | |||||||
| Patient or population: adults and children with asthma | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | ||
|---|---|---|---|---|---|---|---|
| Risk with control/usual care | Risk with multi‐media training | ||||||
| Correct inhaler technique Immediately after intervention | Adults | 30 per 100 | 47 per 100 | OR 2.15 | 164 | ⊕⊕⊕⊝ MODERATEa,b | |
| Correct inhaler technique 2‐Week follow‐up | Adults | 43 per 100 | 57 per 100 | OR 1.78 | 28 | ⊕⊕⊝⊝ LOWa,c | |
| Inhaler technique score | Adults | Not pooled; narrative summary of evidence provided in data table (Analysis 3.3) | ‐ | 136 (2 RCTs) | Not graded | Suggests benefit of multi‐media training over control | |
| Children | Not pooled; narrative summary of evidence provided in data table (Analysis 4.1) | ‐ | 127 (2 RCTs) | Not graded | Suggests benefit of multi‐media training over control | ||
| Asthma control (ACT) 4 weeks | Children | Mean change in control group was 1.2 | Mean change was 0.73 better (‐0.99 worse to 2.45 better) | ‐ | 91 (1 RCT) | ⊕⊕⊝⊝ LOWa,c | |
| Other outcomes | No results could be analysed for quality of life, exacerbations, adverse events, unscheduled visits to a healthcare provider or school/work absences | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | |||||||
| GRADE Working Group grades of evidence | |||||||
| aConfidence intervals include no difference. Downgraded once bAlthough participants in both studies were unblinded, inhaler technique was rated by a blinded assessor, and both groups received an intervention. Not downgraded cHigh risk of bias for performance and detection. Downgraded once | |||||||
| Feedback device compared with control/usual care for people with asthma | |||||||
| Patient or population: adults and children with asthma | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | ||
|---|---|---|---|---|---|---|---|
| Risk with control/usual care | Risk with feedback device | ||||||
| Correct inhaler technique 4‐Week follow‐up | Adults | 51 per 100 | 83 per 100 (66 to 93) | OR 4.80 (1.87 to 12.33) | 97 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | Additional results from technique rating scales could not be combined (Analysis 5.3) |
| Inhaler technique (PIF) Follow‐up: 6 weeks (adults) 6 to 12 weeks (children) | Adults | 66 per 100 | 97 per 100 | OR 18.26 | 71 | ⊕⊕⊝⊝ LOWa,b | |
| Children | Mean PIF was 51.2 litres/min | Mean PIF was 9.22 litres/min better (33.71 better to 15.27 worse) | ‐ | 98 | ⊕⊕⊝⊝ LOWa,c | ||
| Asthma control (ACQ) 6 to 12 weeks | Adults | Mean ACQ score was 1.4 | Mean score in the intervention group was 0.1 better (0.46 better to 0.26 worse) | ‐ | 97 (1 RCT) | ⊕⊕⊝⊝ LOWa,c | |
| Children | Mean ACQ score was 0.7 | Mean score in the intervention group was 0.02 worse | ‐ | 98 | ⊕⊕⊕⊝ MODERATEa | ||
| Quality of life Follow‐up: 6 weeks (adults) 6 to 12 weeks (children) | Adults | Mean score on the mini‐AQLQ was 4.2 | Mean score in the intervention group was 0.38 better | ‐ | 100 | ⊕⊕⊝⊝ LOWa,d | |
| Children | Mean change in quality of life was 0.07 | Mean change was 0.25 better | ‐ | 91 | ⊕⊕⊝⊝ LOWa,d | One study reported endpoint quality of life (Analysis 6.4) | |
| Quality of life (responders) 6 weeks | Adults | 49 per 100 | 83 per 100 | OR 5.29 | 71 | ⊕⊕⊕⊝ MODERATEa | |
| Other outcomes | No results could be analysed for exacerbations, adverse events, unscheduled visits to a healthcare provider or school/work absences | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | |||||||
| GRADE Working Group grades of evidence | |||||||
| aHigh risk of performance and detection bias. Downgraded once bVery wide confidence intervals based on one study. Downgraded once cConfidence intervals include possible harm and benefit of the intervention. Downgraded once dConfidence interval does not exclude no difference, and upper limit exceeds the MCID of 0.5 units. Downgraded once | |||||||
Background
Description of the condition
Asthma is one of the most common chronic diseases in the world. It affects more than 300 million adults and children, and its prevalence is rising. By 2025, it is estimated that a further 100 million people may be affected by asthma. Asthma is thought to be responsible for approximately 1% of the disability‐adjusted life‐years lost globally, and for one in 250 deaths, which makes effective treatment an international priority (Masoli 2004). Although previously asthma was thought to be a disease primarily of high‐income countries, it is now recognised that much of the global asthma burden affects low‐ and middle‐income countries (Asher 2006). Asthma is estimated to be the 14th most important disease in the world in terms of extent and duration of disability (Global Asthma Network 2014). Asthma is characterised by chronic inflammation of the airways, leading to symptoms that include cough, wheeze, chest tightness and shortness of breath, which typically vary over time. People with asthma may experience a short‐term worsening of symptoms, known as a ‘flare‐up’, an ‘attack’ or an ‘exacerbation’, which may be life‐threatening (GINA 2016).
Inhalation therapy is the most effective treatment for almost all people with asthma (Dekhuijzen 2007). More than 50 years has passed since the first inhalers for asthma were introduced for routine clinical use (Crompton 2006a). The major advantage of the inhaled route is that the drug is delivered directly to the airways, where it has a rapid onset of action with a minimal dose, thus limiting systemic side effects. Inhalers are also portable and compact, which makes them suitable for ambulatory therapy (McFadden 1995).
Today, commonly used inhaled therapies include short‐acting beta‐agonists (SABAs, e.g. salbutamol); short‐acting muscarinic antagonists (SAMAs, e.g. ipratropium); long‐acting beta‐agonists (LABAs, e.g. salmeterol); long‐acting muscarinic antagonists (LAMAs, e.g. tiotropium); and inhaled corticosteroids (ICSs, e.g. beclomethasone) (BNF 2016). These medications can be used as required to relieve acute symptoms of asthma (e.g. SABAs, SAMAs) or for daily maintenance to prevent worsening of symptoms (i.e. ICS with LABA or LAMA as an add‐on) (BTS/SIGN 2014). Sustained use of ICSs reduces airway inflammation, improves symptoms and reduces asthma‐related morbidity and mortality (Barnes 2003; Bårnes 2015).
The first inhalation devices for asthma were pressurised metered dose inhalers (pMDIs), which were introduced in the 1950s; today, many devices are available and different techniques are required for their proper use (Crompton 2006a; GINA 2016). Inhaler devices can be divided into two main groups: pMDIs and dry powder inhalers (DPIs). pMDIs require the patient to co‐ordinate pressing down on the inhaler canister whilst initiating a slow and deep inhalation, and DPIs require a rapid and forcible inhalation. Both inhaler types require a full exhalation before use, and breath‐holding is recommended after use (Haughney 2010). For those who find the co‐ordination required to use a pMDI difficult, a modified 'breath‐actuated' pMDI can be prescribed or an add‐on device can be used with the inhaler, such as a ‘spacer’ or a ‘holding chamber’. Spacers provide the added advantage of improving delivery of the drug to the appropriate portion of the airway while preventing oropharyngeal deposition, which is particularly important for ICSs (GINA 2016). Some experts recommend that a spacer device should be used universally for children five years of age or younger (Sanchis 2013; GINA 2016). The best choice of inhaler depends on patient factors such as age, co‐ordination and lung function, as well as on patient preference and local availability and cost (Haughney 2010; GINA 2016).
Description of the intervention
An intervention to improve inhaler technique may take many forms, but these interventions broadly fall into three categories: technological adaptations or interventions; education of the healthcare provider; and education of the patient or caregiver (Inhaler Error Steering Committee 2013). However, in reality, any given intervention is unlikely to fall neatly into any single category; for example, a device that provides feedback on inhaler technique may have the dual effect of providing education to the patient and healthcare provider. Interventions may promote self‐management by the patient, monitoring by the healthcare provider or both. They may be delivered face‐to‐face, in writing or through the use of visual or technological aids.
Several currently available inhaler ‘training tools’ could fall under the ‘technological’ umbrella. These devices have various purposes, which include giving feedback to the patient or healthcare provider about inhaler technique and allowing the patient to ‘practise’ the correct technique; helping to identify the best type of inhaler for that patient; or testing the inhalation capacity and co‐ordination of the patient (Lavorini 2010).
In addition, inhalers themselves may be developed to be easier to use; of note, the newer DPIs may be associated with better adherence (Roy 2011), which may be a result of ease of use, and breath‐actuated pMDIs should be considered for those who struggle with co‐ordination (Levy 2013). Simple technological devices such as ‘spacers’ may also improve technique and drug delivery for some patients (McFadden 1995). However, the literature does not suggest that one type of inhaler is consistently associated with a better technique than any other (van Beerendonk 1998). A 2001 Health Technology Assessment report concluded that no consistent advantage is offered by one type of inhaler over another after proper training, and so the cheapest option should be selected in most cases (Brocklebank 2001). More recently, a 'real‐world' cost‐effectiveness analysis based on routine observational data compared outcomes for patients initiated on ICS via a pMDI, a breath‐actuated inhaler pMDI or a DPI. Findings suggest that the real‐world effectiveness of ICS inhalers varies (which may be attributed in part to technique) and that both subsequent healthcare usage costs and the cost of the inhaler should be taken into account when prescribing (Kemp 2010).
Education of both healthcare professionals and patients about correct technique may be delivered in several ways. Multi‐media training tools may comprise Internet‐based or DVD video clips that demonstrate correct inhaler use, as well as interactive components such as games or quizzes (Navarre 2007; Lavorini 2010; Poureslami 2012). Indeed, it has been demonstrated that education delivered via the Internet can be as effective, at least in the short term, as face‐to‐face training for healthcare professionals (Erikson 2003; Toumas 2009).
Face‐to‐face education or coaching is another commonly used strategy for improving inhaler technique. This may be done as part of a comprehensive asthma management package and can be delivered to, or by, a variety of healthcare professionals, including nurses, pharmacists and physicians (Verver 1996; Basheti 2005; Basheti 2008; Armour 2013). Studies have shown that even brief interventions can substantially improve medical professionals’ knowledge about correct technique (Basheti 2009; Kim 2009). Trials of telephone interventions aimed at patients have yielded mixed results (Bynum 2001; Nelson 2011).
How the intervention might work
The breadth of interventions available to improve inhaler technique means that no single mechanism has been identified by which clinical outcomes might benefit. Some technological interventions may allow optimisation of the choice of inhaler for a patient by characterising the patient's breathing pattern, thus improving technique, or by allowing a patient to hone his or her technique at home or with the assistance of a healthcare professional (Lavorini 2010). Attempts to minimise the number of different types of inhalers prescribed for one patient may also improve technique; studies suggest that both adults and children who are prescribed more than one type of inhaler, especially a mixture of pMDIs and DPIs, are more likely to make inhaler errors (van der Palen 1999; Alotabi 2011).
Other interventions may ensure that a patient is following the correct sequence of steps for proper use; physical demonstration appears superior to verbal or written instructions alone (Basheti 2005; Bosnic‐Anticevich 2010), and a multi‐media presentation may be superior to written information alone (Savage 2003). This suggests that being able to watch and copy as the correct technique is demonstrated in person or via a video clip or the Internet is an important requirement for the intervention in many cases.
Children present a unique challenge, and evidence suggests that even after training, their inhaler technique may remain inadequate for effective drug delivery (Kamps 2000). Some studies suggest that repeated training with reinforcement is important (Deerojanawong 2009), that audiovisual training alone is insufficient and that children show the greatest improvement when they receive individual training with follow‐up at home (Agertoft 1998).
Knowledge of how a technique is assessed and recorded is important for determining whether or not an intervention has worked. As anticipated, we found that not all studies used a validated measurement instrument (Rootmensen 2010). We considered how each trial that contributed to this Cochrane Review assessed technique and how this impacted our confidence in this outcome.
Why it is important to do this review
As many as 70% to 80% of people with asthma are unable to use their inhaler device correctly (GINA 2016); poor technique has been associated with age, sex, educational level and emotional problems (Rootmensen 2010). Studies suggest that up to 67% of clinicians cannot describe the steps involved or cannot demonstrate correct inhaler use. Furthermore, of the approximate USD 50 billion spent annually on inhalers (for all respiratory conditions) in the USA, USD 7 to 15 billion is wasted owing to improper use (Fink 2005). Mistakes in inhaler use have been associated with poor clinical outcomes in asthma, including more frequent emergency department (ED) visits; hospitalisations; prescriptions of oral steroids and antimicrobials; and poorer asthma control as assessed by the Asthma Control Questionnaire (ACQ), the Asthma Instability Score (AIS) and the Asthma Therapy Assessment Questionnaire (ATAQ) (Giraud 2001; Melani 2011; Al‐Jahdali 2013; Westerik 2016). A similar association is seen in children (Capanoglu 2015). Evidence suggests that even after a successful intervention, many patients revert to incorrect use within a short time (Price 2002; Crompton 2006b).
International and national guidelines highlight that assessment of inhaler technique is an essential component of asthma care, especially for patients with inadequate control (BTS/SIGN 2014; GINA 2016). Recently, the Aerosol Drug Management Improvement Team (ADMIT) published a series of papers that focus on the need to improve inhalation technique in Europe (ADMIT 2016). In summary, the ADMIT reports suggest that specific education for patients and clinicians on correct technique for inhaler use is needed and should be repeated frequently; devices that provide ‘feedback’ about appropriate technique are useful; information about the correct technique for each device should be easily accessible; and standardised prescribing throughout Europe is preferable (Crompton 2006b; Dekhuijzen 2007). The Global Allergy and Asthma European Network (GA²LEN) initiative, which focusses on problematic severe asthma in children, calls for repeated checking of an individual's inhaler technique (Hedlin 2010). This inevitably raises the question of what is the most effective way to correct and improve improper technique.
Lewis 2016 developed a model to estimate the impact of poor inhalation technique on the economic burden of asthma and chronic obstructive pulmonary disease (COPD) in three European countries (Spain, Sweden and the United Kingdom). Study authors attributed 2.2% to 2.7% of direct asthma and COPD costs to poor inhalation technique, totaling 105 million euros across the three countries. Inclusion of lost productivity costs in additional analyses revealed that the annual expenditure was increased to 3.3 billion euros in the UK and 6.4 billion euros across all three countries. These figures further highlight the need for effective interventions to improve inhaler technique.
Although the literature provides compelling evidence of the need for good inhaler technique in maintaining asthma control, the most effective ways to improve inhaler technique and thus improve clinical outcomes remain unclear. This Cochrane Review examined the evidence for this question for both adults and children.
Objectives
To assess the impact of interventions to improve inhaler technique on clinical outcomes and safety in adults and children with asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel and cluster‐randomised controlled trials (RCTs) of any duration conducted in any setting. If we identified cross‐over trials, we included only data from the first part of the study because of the potential for carry‐over effects from the intervention.
We included studies reported as full‐text articles, published as abstracts only and described in unpublished data.
Types of participants
We included both adults and children with asthma, diagnosed according to national or international guidelines or by a healthcare professional. We excluded participants with other respiratory comorbidities, including COPD and bronchiectasis. If we identified a study in which only a subset of participants had asthma, we included these participants if we were able to obtain disaggregated data.
We intended to analyse studies aimed solely at children (younger than 12 years) separately from those aimed at adults and adolescents (12 years of age and older). If within each of these comparisons we found trials focused on a more narrow age range (e.g. children younger than five years), we explored this potential source of heterogeneity. If a study recruited both adults and children and did not provide disaggregated data, we were guided by the average age of participants to determine in which comparison we should include the study.
Types of interventions
We included studies that compared an intervention primarily aimed at improving inhaler technique versus any of the following.
-
Usual care/No additional intervention.
-
An alternative intervention that did not primarily aim to improve inhaler technique (e.g. asthma education only vs asthma education plus an inhaler technique demonstration).
-
An alternative intervention of a different type or intensity, also aimed at improving inhaler technique (e.g. written instructions only vs written instructions plus physical demonstration).
It is likely that the effect of the intervention will be systematically different depending on which of these three types of comparison groups each study used, so we meta‐analysed results separately unless we had a justification for pooling data (i.e. if treatments, participants and the underlying clinical question were similar enough for pooling to make sense).
Trial interventions may be delivered to healthcare professionals (e.g. pharmacists, healthcare assistants, nurses, physicians) or directly to patients or their parents/caregivers. Interventions may involve education delivered face‐to‐face or remotely, and may include written, verbal or multi‐media methods of delivery. Interventions may also involve a device or a piece of technology designed to improve inhaler technique.
'Usual care' comprises the treatment that a patient with asthma in this setting would normally receive according to local or national guidelines, or according to the judgement of their healthcare provider. This may include routine advice about inhaler technique but not about the specific intervention that is being studied.
Types of outcome measures
Primary outcomes
-
Inhaler technique (as assessed/rated by triallist; change from baseline scores preferred if available)
-
Asthma control (preferably measured on a validated scale, e.g. Asthma Control Questionnaire (ACQ))
-
Asthma exacerbations requiring at least oral corticosteroids (OCSs)
Secondary outcomes
-
Quality of life (preferably measured on a validated scale, e.g. Asthma Quality of Life Questionnaire (AQLQ))
-
Adverse events (including local drug side effects, which can be associated with improper inhaler technique)
-
Unscheduled visits to healthcare provider (e.g. emergency department (ED), primary care)
-
Absenteeism from work or school
Reporting of one or more of the outcomes listed here within a study was not an inclusion criterion for the review.
We chose these outcomes to reflect the main aim of the intervention (improved inhaler technique) but also patient‐important clinical outcomes, such as exacerbations and quality of life. Adverse events may be associated with improper inhaler use (e.g. oropharyngeal deposition of ICS) and may decrease post intervention. Alternatively, some participants may have noted an increase in medication side effects as they were not receiving a therapeutic dose of their inhaler previously. We did not anticipate many serious adverse events linked to the intervention and so chose to capture all adverse events.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register includes studies identified from several sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org).
-
Weekly searches of MEDLINE Ovid SP 1946 to date.
-
Weekly searches of Embase Ovid SP 1974 to date.
-
Monthly searches of PsycINFO Ovid SP.
-
Monthly searches of the Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO.
-
Monthly searches of Allied and Complementary Medicine (AMED) EBSCO.
-
Handsearches of the proceedings of major respiratory conferences.
Studies included in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. We have provided details of these strategies, as well as a list of handsearched conference proceedings, in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We searched the following trials registries.
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov).
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch).
We searched the Cochrane Airways Trials Register and additional sources from inception to the present, with no restriction on language of publication. We conducted the most recent search on 23 November 2016.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information.
On 24 November 2016, we searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (KK and RN or AM) screened titles and abstracts independently of all studies identified for potential inclusion as a result of the search, and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved full‐text study reports/publications; two review authors (RN and KK) independently screened the full‐text reports, identified studies for inclusion and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion, or, if required, we consulted a third review author. We identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram and a Characteristics of excluded studies table (Moher 2009).
Data extraction and management
We piloted a data collection form on at least one study included in the review and used it to document study characteristics and outcome data. One review author (RN, KK or AM) extracted the following study characteristics from the included studies.
-
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study settings, withdrawals, dates of study.
-
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, exclusion criteria.
-
Interventions: intervention, comparison, allowed medications and concomitant interventions, excluded medications and interventions.
-
Outcomes: primary and secondary outcomes specified and collected, time points reported.
-
Notes: funding for trial, notable conflicts of interest of trial authors.
Two review authors (RN and KK or AM) independently extracted outcome data from the included studies. We noted in the Characteristics of included studies table if the included trials did not report outcome data in a useable way. We resolved disagreements by reaching consensus or by consulting a third review author. One review author (RN) transferred data into Review Manager (RevMan) (RevMan 2014). We double‐checked that the review author had entered data correctly by comparing data presented in the systematic review versus the study reports. A second review author (KK or RN) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (RN and KK or AM) independently assessed the risk of bias of each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by consultation with a third review author. We assessed risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report and a justification for our judgement in the 'Risk of bias' table. We summarised 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different from that determined for a patient‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a triallist, we noted this in the 'Risk of bias' table.
When we considered treatment effects, we took into account the risk of bias for studies that contributed to those outcomes.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data using Mantel‐Haenzsel odds ratios (ORs) with a random‐effects model and 95% confidence intervals (CIs). If we had encountered outcomes with very rare events, we planned to use Peto ORs. If the included trials had reported data as rates or time‐to‐event (e.g. exacerbations), we planned to analyse these as time‐to‐event or rate ratios. We planned to transform reported rate ratios into log rate ratios and to analyse them using a random‐effects model and generic inverse variance (GIV) in RevMan (RevMan 2014). We entered data presented as a scale with a consistent direction of effect.
We analysed continuous outcomes (e.g. ACQ, AQLQ) as mean difference (MD) or standardised mean difference (SMD) values using a random‐effects model and 95% CIs. We used change from baseline scores when available.
We undertook meta‐analyses only where this was meaningful i.e. if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
We narratively described skewed data reported as medians and interquartile ranges.
When a single trial reported multiple trial arms, we included only the relevant trial arms. If we combined two comparisons (e.g. intervention A vs usual care and intervention B vs usual care) in one meta‐analysis, we halved the control group to avoid double‐counting.
Unit of analysis issues
We analysed dichotomous data by using participants (rather than events) as the unit of analysis. However, if the included trials had reported exacerbations as rate ratios, we planned to analyse them on this basis. We meta‐analysed data from cluster RCTs only if we could adjust available data to account for clustering.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when we identified a study as an abstract only). When this was not possible, and we thought that the missing data introduced serious bias, we took this into consideration in the Grading of Recommendations Assessment, Development and Evaluation (GRADE) rating for affected outcomes.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity amongst the trials in each analysis. If we identified substantial heterogeneity, we reported this and explored possible causes by performing prespecified subgroup analysis.
Assessment of reporting biases
When we were able to pool more than 10 trials, we created and examined a funnel plot to explore possible small study and publication biases.
Data synthesis
We used a random‐effects model and performed a sensitivity analysis with a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses.
-
To whom the intervention is delivered: healthcare provider versus patient/caregiver.
-
Duration of intervention: one‐off session versus repeated sessions.
We did not pool studies that included children with studies that included adults. If within each of these two populations we found trials that focused on a more narrow age range (e.g. children younger than five years of age), we planned to explore this potential source of heterogeneity.
We suspected that interventions would differ from one another in various ways (e.g. inhaler type, physical demonstration vs no demonstration, remote vs face‐to‐face), which may make exploration through multiple formal subgroup analyses misleading. Instead we summarised intervention characteristics in additional tables (Table 1; Table 2; Table 3).
| Study ID | Total n | Study duration (weeks) | Age | Country | Intervention | Control | Inhaler technique measure | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 72 | 2 | Children aged 3 to 5 years | Denmark | Instructional video + individual training; delivered to participant | Instructional video | PIF | PIF, IVC, PEFR, FEV1, FVC | |
| 26 | 2 | Adults | Australia | Group B: verbal counselling Group C: verbal counselling + physical demonstration; delivered to participant | Group A: printed materials | 9‐Step Turbuhaler checklist | Inhaler technique | |
| 31 pharmacists, 97 participants | 26 | Adults | Australia | Pharmacist‐participant education + inhaler technique labels; delivered to pharmacist | Peak flow measurement training | 9‐Step checklist | Inhaler technique, asthma severity, peak flow variability, AQOL, perceived control | |
| 49 | 4 | Adolescents aged 12 to 19 years | USA | Telepharmacy counselling; delivered to participant | Written instructions only | 8‐Step MDI checklist | Inhaler technique, participant satisfaction | |
| 89 | 12 | Adults | Not reported | Respiratory therapist education; delivered to participant | Rotuine physician education | Not reported | Inhaler technique, asthma control | |
| 201 | 26 | Adults | Belgium | Pharmacist education; delivered to pharmacist | Usual pharmacy care | 10‐Step MDI + spacer checklist/8‐step DPI checklist | Inhaler technique, ACT, exacerbations, ED/hospital visits, adherence, AQLQ, asthma knowledge | |
| 46 | 8.7 | Adults | Iran | Face‐to‐face education; delivered to participant | Usual care (no further details) | Not reported | ACT, FEV1 | |
| 130 | 12 | Children | Turkey | Face‐to‐face nurse training; delivered to participant | Inhaler package insert only | 10‐Step MDI checklist | Inhaler technique, PAQLQ, spirometry | |
| 90 | 4 | Adults | Iran | Face‐to‐face training (no spacer device); delivered to participant | Usual care (no training) | 11‐Step MDI checklist | Inhaler technique, PEFR | |
| 68 | 8 to 20 | Adults | USA | Face‐to‐face training + demonstration; delivered to participant | Inhaler package insert only | Checklist, converted to score out of 1 (0 if any steps missed) | BAI and MDI competency | |
| 29 | 1 to 16 (mean 6) | Adults | USA | Face‐to‐face pharmacist training; delivered to participant | Inhaler package insert only | 10‐Step checklist | Inhaler technique, "effectiveness of instruction" | |
| 96 | 26 | Children and adolescents (max 15 years) | Canada | Interactive nurse training with feedback; delivered to participant | Pictorial nurse training only | Checklist with scores converted to percentages | Inhaler technique, parental perceptions of treatment, asthma morbidity (e.g. no. of exacerbations, ED visits, hospitalisations, days of schools missed) |
ACT: Asthma Control Test; AQLQ: Asthma Quality of Life Questionnaire; AQOL: asthma quality of life; BAI: breath‐activated inhaler; DPI: dry powder inhaler; ED: emergency department; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; IVC: inspiratory vital capacity; MDI: metered dose inhaler; PAQLQ: paediatric AQLQ; PEFR: peak expiratory flow rate; PIF: peak inspiratory flow
| Study ID | Total n | Study duration (weeks) | Age | Country | Intervention | Control | Inhaler technique measure | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 133 | 4 | Adults | USA | Inhaler technique training video; delivered to participant | Asthma education video | Not reported | "Correct usage" of inhaler | |
| 21 | 13 | Children | Ireland | Inhaler technique DVD; delivered to participant | Individual instruction | New inhaler technique measurement tool | Inhaler technique, self‐efficacy, knowledge acquisition | |
| 36 | 4.3 | Children | UK | Inhaler technique educational computer software ("Space Inhalers"); delivered to participant | Placebo software | 15‐Step checklist | Inhaler technique, asthma knowledge | |
| 91 | 4.3 | Children and adolescents | USA | Inhaler technique video; delivered to participant | Attention control video (about nutrition) | 8‐Step checklist | Inhaler technique, self‐efficacy, ACT | |
| 69 | Outcomes assessed immediately | Adults | UK (Turkish‐ speaking population) | Multi‐media touch screen training; delivered to participant | Patient information leaflet plus verbal training | Inhaler checklist | Inhaler technique | |
| 45 | 2.1 | Adults and children (10 to 71 years) | France | Inhaler technique video OR inhaler technique video + spacer; delivered to participant | Patient information sheet | 4‐Step checklist | Inhaler technique, FEV1 | |
| 110 | Outcomes assessed immediately | Children and adults (12 to 87 years) | UK | Multi‐media touch screen training; delivered to participant | Patient information leaflet | Inhaler checklist, global assessment of technique | Inhaler technique, acceptability | |
| 29 | 1 to 16 (mean 6) | Adults | USA | Video training; delivered to participant | Inhaler package insert only | 10‐Step checklist | Inhaler technique, "effectiveness of instruction" | |
| 50 | Not reported | Not reported | USA | Computer training; delivered to participant | Written training OR no training | "Fixed rubric" used to give a score | Inhaler technique |
ACT: Asthma Control Test; DVD: digital versatile disc; FEV1: forced expiratory volume in one second
| Study ID | Total n | Study duration (weeks) | Age | Country | Intervention | Control | Inhaler technique measure | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 108 | 6 | Adults | UK | Verbal training + 2Tone trainer; delivered to participant | Verbal training alone | PIF | PIF, FEV1, AQLQ | |
| 56 | 6 | Adults and children (4 to 55 years) | UK | Verbal training + 2Tone trainer; delivered to participant | Verbal training alone | PIF | PIF, FEV1, AQLQ/PAQLQ | |
| 80 | 12 | Children | UK | AeroChamber Plus with Flo‐Vu; delivered to participant | AeroChamber Plus alone | PIF | PIF, PAQLQ, ACQ, parent spacer preference | |
| 30 | 6 to 8 | Children | Not reported | Verbal training and use of Trainhaler; delivered to participant | Verbal training alone | PIF | PIF, ACQ | |
| 90 | 4 | Adults | Iran | Face‐to‐face training (with spacer device); delivered to participant | Usual care (no training) | 11‐Step MDI checklist | Inhaler technique, PEFR | |
| 132 | 52 | Children | Australia | Funhaler incentive device; delivered to participant | AeroChamber device | Filter used to measure salbutamol deposition | Inhaler technique, asthma control, quality of life | |
| 76 | 4 | Not reported | Libya | Verbal training + 2Tone trainer; delivered to participant | Verbal training alone | PIF | PIF, FEV1, AQLQ, Jones Morbidity Index (JMI) | |
| 19 pharmacists (101 participants) | 4 | Adults | Australia | Verbal training + quantitative inhaler feedback; delivered to pharmacist | Verbal training alone | Inhaler checklist/proportion with correct technique | Inhaler technique, ACQ | |
| 43 | 4 | Not reported | USA | Face‐to‐face demonstration + In‐Check simulator; delivered to participant | Face‐to‐face demonstration | PIF, inhaler checklist | PIF, inhaler technique |
ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; FEV1: forced expiratory volume in one second; MDI: metered dose inhaler; PAQLQ: paediatric AQLQ; PEFR: peak expiratory flow rate; PIF: peak inspiratory flow
We used the following outcomes in subgroup analyses.
-
Inhaler technique.
-
Asthma control.
-
Asthma exacerbations requiring at least OCS.
We used the formal test for subgroup interactions in RevMan (RevMan 2014). When we found insufficient studies to conduct these analyses formally, we displayed in a table summary information regarding each of these variables.
Sensitivity analysis
We planned the following sensitivity analyses.
-
Exclusion of unpublished data.
-
Exclusion of studies considered at high risk of selection bias.
-
Exclusion of studies that did not use a validated tool to assess inhaler technique (from the inhaler technique outcome).
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table and included the following outcomes: inhaler technique; asthma control; asthma exacerbations; quality of life; adverse events; unscheduled visit to healthcare provider; and absenteeism from work or school.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it related to studies that contributed data to the meta‐analyses for prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we used GRADEpro Guideline Development Tool (GDT) software (GRADEpro GDT). We justified all decisions to downgrade or upgrade the quality of the evidence by using footnotes. We made comments to aid the reader's understanding of the review when necessary.
Results
Description of studies
Results of the search
We identified a total of 803 unique potentially relevant records, including 718 records from the main electronic database search and 85 additional records from the clinical trial registries ClinicalTrials.gov (n = 76) and the World Health Organization International Clinical Trial Registry Platrform (WHO ICTRP; n = 9). After removing duplicates, we reviewed 791 records and excluded 694 by looking at titles and abstracts alone. We reviewed full‐text articles for the remaining 97 records. Fifty‐seven records related to 46 studies did not meet the inclusion criteria, and we excluded them (with reasons ‐ see Figure 1). We deemed 40 records related to 29 studies eligible for this review.

Study flow diagram.
Included studies
Twenty‐nine studies, evaluating 2210 participants (children and adults), met the inclusion criteria for this review (Self 1983; Lirsac 1991; Donateo 1996; Turgeon 1996; Agertoft 1998; Rydman 1999; Bynum 2001; Boone 2002; Savage 2003; Basheti 2005; Goodyer 2006; Al‐Showair 2007; Basheti 2008; Mehuys 2008; Tarsin 2008; Acosta 2009; Nahafizadeh 2010; Ozkaya 2010; Fernandes 2011; Schultz 2012; Ammari 2013; Vitari 2013; Arthurs 2014; Rahmati 2014; Shah 2014; Toumas‐Shehata 2014; Ammari 2015; Ammari 2015a; Carpenter 2015). Detailed descriptions of these studies and risk of bias details can be found in the Characteristics of included studies table.
All included studies were parallel RCTs. The study population in each study ranged from 21 to 201 participants, and the median number of participants was 72. Eleven of the included studies took place in Europe (six in the UK and one in each of the following countries: Belgium; Denmark; France; Ireland; and Italy), seven in USA, four in Australia, three in Asia and one in Africa, and three of the included studies did not report the country of origin. Fourteen studies were hospital based: 11 were conducted in outpatient clinics (Self 1983; Turgeon 1996; Agertoft 1998; Rydman 1999; Al‐Showair 2007; Nahafizadeh 2010; Ozkaya 2010; Schultz 2012; Ammari 2013; Rahmati 2014; Ammari 2015), one took place in a hospital ward (Arthurs 2014) and two did not provide details (Fernandes 2011; Shah 2014). Five studies were conducted in primary care facilities (Bynum 2001; Boone 2002; Savage 2003; Goodyer 2006; Carpenter 2015), four took place in community pharmacies (Basheti 2005; Basheti 2008; Mehuys 2008; Toumas‐Shehata 2014), one was carried out in a comprehensive lung centre (Vitari 2013) and five did not reveal the setting in which they were conducted (Lirsac 1991; Donateo 1996; Tarsin 2008; Acosta 2009; Ammari 2015a).
Population characteristics and inclusion criteria
Seven studies included only children (Agertoft 1998; Boone 2002; Ozkaya 2010; Schultz 2012; Arthurs 2014; Ammari 2015; Ammari 2015a) and 12 only adults (Self 1983; Donateo 1996; Rydman 1999; Goodyer 2006; Al‐Showair 2007; Basheti 2008; Mehuys 2008; Acosta 2009; Nahafizadeh 2010; Fernandes 2011; Rahmati 2014; Toumas‐Shehata 2014). Two of the children's studies also included adolescents (Turgeon 1996; Carpenter 2015), and three included adults and adolescents (Lirsac 1991; Savage 2003; Basheti 2005); these studies were classified as children's and adults' studies, respectively. One study included only adolescents aged 12 to 17 years (Bynum 2001), and one included both children and adults (Ammari 2013). Finally, three studies did not report the age range of participants (Tarsin 2008; Vitari 2013; Shah 2014); none of these three studies contributed to a meta‐analysis. The mean age of the overall population was 28.52 years. The mean ages of adult and children populations were 44.42 and 6.97 years, respectively.
All included studies required a diagnosis of asthma. Other frequently used inclusion criteria were age ranges, type of inhaler used, use of a spacer, absence of recent exacerbations, asthma control, non‐acceptable inhaler technique and specific components of the technique. Two studies did not report inclusion criteria (Nahafizadeh 2010; Shah 2014).
Interventions and comparisons
Interventions assessed in the included trials can be categorised into three broad groups:
-
Enhanced inhaler technique education, delivered by a trained individual to a healthcare professional (who in turn educated the patient) or directly to the patient (Table 1). Twelve studies implemented face‐to‐face verbal training with or without demonstration of appropriate inhaler technique (Self 1983; Turgeon 1996; Agertoft 1998; Rydman 1999; Bynum 2001; Basheti 2005; Basheti 2008; Mehuys 2008; Nahafizadeh 2010; Ozkaya 2010; Fernandes 2011; Rahmati 2014).
-
Multi‐media training (Table 2). Four studies used educational computer applications or games (Boone 2002; Savage 2003; Goodyer 2006; Shah 2014). Five studies used instructional videos with or without written information (Self 1983; Lirsac 1991; Acosta 2009; Arthurs 2014; Carpenter 2015).
-
Training devices providing visual or audio feedback regarding the appropriateness of breathing manoeuvres (Table 3). Patients used these devices for different periods to maintain an acceptable inhaler technique. Three of the included studies used a two‐tone trainer ‐ a training device that looks like an MDI and provides different audio feedback for acceptable versus non‐acceptable inspiratory flow rates (Al‐Showair 2007; Tarsin 2008; Ammari 2013). Trainhaler also provided audible feedback for appropriate breathing manoeuvres with an MDI (Ammari 2015a). AeroChamber Plus with Flow‐Vu (Ammari 2015), a portable hand‐held spirometer, was used to assess breathing manoeuvres associated with the use of different inhaler devices (Toumas‐Shehata 2014), and In‐Check (Vitari 2013) provided visual feedback for appropriateness of breathing manoeuvres with MDI, DPI and different types of inhalers, respectively. Finally Funhaler, a spacer device, provides combined visual and audible feedback for correct inhaler technique with an MDI inhaler (Schultz 2012).
One study used spacer devices to simplify inhalation technique by reducing the need for co‐ordination of actuation and inhalation and increasing deposition in lower airways (Donateo 1996). Another study used a spacer device to implement a complex intervention, which included educational sessions and use of a spacer versus an educational session alone or no intervention (Rahmati 2014).
Comparators used varied among included studies; some used standard inhaler technique verbal instruction; others used written instructions, sham video instructions or no instructions.
Excluded studies
Title and abstract screening of articles identified through our systematic searches revealed 97 potentially relevant records. After reviewing the full texts of these articles, we excluded 57 records describing 46 studies, as described in Figure 1. We classified six studies, described in seven records, as ongoing (ACTRN12610000159055; JPRN‐UMIN000006739; NCT02203266; NCT02283008; NCT02611531; NCT01529697) and two studies as awaiting classification because we did not find adequate details to confirm whether they met the review's inclusion criteria (NCT02062463; Westhus 1998). Finally, we excluded 38 studies (48 citations) because they did not meet the inclusion criteria. Of these, seven studies used the wrong study design for the review (WIlliams 1983; Epstein 2001; Andres Jacome 2003; Schacer 2005; Lee 2010; Sandos Dde 2010; Azouz 2015), 10 studies focused on a wrong study population for the review (McElnay 1989; Verver 1996; Compton 2000; Hesselink 2004; Basheti 2005a; Bosnic‐Anticevich 2010; Jolly 2012; NCT01456494; Jolly 2015; NCT01426581) and 21 studies did not assess the intervention of interest for this review (NCT01641211; NCT02046759; NCT02307669; NCT02363192; NCT02715219; Eriksson 1980; Hodges 1981; Pedersen 1983; Rachelefsky 1986; Reiser 1986; Yoon 1993; Wong 1995; Mulloy 1996; Tuazon 2002; Kritikos 2007; Horner 2008; Garcia‐Cardenas 2013; Fornell 2014; Eakin 2015; Grover 2016; Poureslami 2016).
Risk of bias in included studies
We have presented in Figure 2 an overview of risk of bias in the included studies. We have also provided a summary of possible bias related to each domain. We have given details on the rationale for each judgement of each study's risk in the risk of bias table for each study (see the Characteristics of included studies tables).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Most included studies provided very limited information regarding the two selection bias domains. We deemed 12 of the included studies to be at low risk of bias for random sequence generation (Lirsac 1991; Turgeon 1996; Bynum 2001; Savage 2003; Goodyer 2006; Basheti 2008; Mehuys 2008; Schultz 2012; Ammari 2013; Rahmati 2014; Ammari 2015; Carpenter 2015) and only three to be at low risk for allocation concealment (Lirsac 1991; Mehuys 2008; Carpenter 2015). We deemed one study to be at high risk for allocation concealment bias (Shah 2014), and we rated risk of bias of remaining studies in each selection bias domain as unclear.
Blinding
Blinding of participants and personnel to group allocation is challenging because of the nature of the interventions and comparisons; this posed the most significant risk of bias for the evidence in this review. Only one trial reported blinding of participants and personnel (Boone 2002), and we assessed this study as low risk. We judged another trial involving young children, which used a relatively objective measure (lung function), to be at low risk despite lack of blinding (Agertoft 1998). One trial did not report on blinding of participants and personnel; as a result, we rated risk of performance bias for this study as unclear (Fernandes 2011). We deemed all remaining studies as having high risk of performance bias because they were not blinded.
We deemed risk of detection bias to be low in only seven trials (Donateo 1996; Turgeon 1996; Agertoft 1998; Rydman 1999; Savage 2003; Goodyer 2006; Acosta 2009). In the remaining trials, risk of detection bias was high, with the exception of three trials, which we deemed to have unclear risk (Self 1983; Vitari 2013; Shah 2014).
Incomplete outcome data
Sixteen of the included studies had low and balanced attrition across study arms; we deemed these studies to have low risk of bias for incomplete outcome data (Self 1983; Lirsac 1991; Donateo 1996; Agertoft 1998; Rydman 1999; Savage 2003; Basheti 2005; Goodyer 2006; Al‐Showair 2007; Nahafizadeh 2010; Ammari 2013; Vitari 2013; Rahmati 2014; Toumas‐Shehata 2014; Ammari 2015; Carpenter 2015). We rated risk of attrition bias as high in five of the included trials with high or unequal drop‐out (Turgeon 1996; Bynum 2001; Basheti 2008; Mehuys 2008; Schultz 2012) and as unclear in the remaining studies.
Selective reporting
We rated only one study as having low risk of reporting bias (Carpenter 2015) and found 11 of the remaining trials to be at high risk of reporting bias (Lirsac 1991; Turgeon 1996; Basheti 2005; Tarsin 2008; Acosta 2009; Ozkaya 2010; Fernandes 2011; Vitari 2013; Arthurs 2014; Shah 2014; Ammari 2015a). Finally, we were not able to assign a clear risk of bias to the remaining trials, usually because we could not identify a prospective trial registration or a prepublished protocol.
Other potential sources of bias
We did not identify any other potential sources of bias in the included studies.
Effects of interventions
See: Summary of findings 1 Enhanced education compared with control versus usual care for people with asthma; Summary of findings 2 Multimedia training compared with control versus usual care for people with asthma; Summary of findings 3 Feedback device compared with control versus usual care for people with asthma
Structure of the analysis
After examining the data, we found that included studies fell into three main comparisons. As planned, we analysed studies that recruited children (mean age < 12 years) separately from those that recruited adolescents and adults (mean age ≥ 12 years), resulting in six main comparisons.
-
Comparisons 1 (adults) and 2 (children):enhanced inhaler technique education versus control or usual care. Investigators delivered education directly to the participant or to a healthcare professional (e.g. a pharmacist) and the intervention could be a 'one‐off' intervention or could be repeated one or more times. We explored these differences within comparisons in our prespecified subgroup analyses.
-
Comparisons 3 (adults) and 4 (children):multi‐media training versus control or usual care. This included videos and computer programmes, which, in the case of our included studies, investigators always delivered directly to the participant. Some studies used a one‐off delivery of the intervention, and in others, participants had ongoing access to the resource. We planned to explore this in our prespecified subgroup analysis, but for all studies in adults, this was a one‐off intervention, and for all studies in children, participants had ongoing access at home.
-
Comparisons 5 (adults) and 6 (children):feedback device versus control or usual care. Investigators provided devices that gave audio or visual (or both) feedback to the participant on inhaler technique. All but one of the studies that we included in this comparison allowed participants to use the device at home between study visits; thus this prespecified subgroup analysis was not possible.
In the following section, we present results separately for each comparison, with any relevant subgroup or sensitivity analyses described at the end of each comparison section.
Comparisons 1 and 2: enhanced inhaler technique education versus control or usual care
Nine studies in adults (Self 1983; Rydman 1999; Bynum 2001; Basheti 2005; Basheti 2008; Mehuys 2008; Nahafizadeh 2010; Fernandes 2011; Rahmati 2014) and three studies in children (Turgeon 1996; Agertoft 1998; Ozkaya 2010) contributed to this comparison.
Inhaler technique
Contributing studies measured this in several ways and at different time points. Investigators most commonly used a checklist, which resulted in a score reflecting the number of steps performed correctly, but these results were too varied to pool. Another method was to dichotomise participants into those with and those without 'satisfactory' or 'correct' inhaler technique at follow‐up. Study authors usually defined satisfactory or correct as performing all critical steps correctly.
Among adults, more people in the intervention group than in the control group had correct technique at follow‐up, with moderate to substantial heterogeneity (Analysis 1.1; OR 5.00, 95% CI 1.83 to 13.65; 258 participants; three studies; I² = 57%; moderate‐quality evidence). This equates to 31 out of 100 people having correct technique at 2‐ to 26‐week follow‐up in the control group compared with 69 out of 100 (95% CI 45 to 86) in the active intervention group (Figure 3).
In the control group, 31 out of 100 people had correct inhaler technique after 2 to 26 weeks, compared with 69 (95% CI 45 to 86) out of 100 in the active treatment group.
We considered combining checklist scales using standardised mean difference, but statistical heterogeneity was extremely high and data from individual trials were often heavily skewed. Instead, we have presented effect estimates from the individual studies in data tables. Among adults, when investigators measured performance immediately after delivery of the intervention by using a checklist, they found that inhaler technique education improved inhaler technique over control in most studies (Analysis 1.2). However, these studies have widely different effect estimates and varied precision and provided a similar picture at follow‐up of 2 to 26 weeks (also shown in Analysis 1.2).
Among children, two studies dichotomised participants to those with and without correct technique at follow‐up (between 12 and 26 weeks) and found no significant differences between groups, with confidence intervals including both potential harm and benefit of the intervention (Analysis 2.1; OR 1.29, 95% CI 0.70 to 2.36; 175 participants; two studies; I² = 0%; low‐quality evidence). One small study measured peak inspiratory flow rate immediately after the intervention to assess inhaler technique. It should be noted that participants in this study were young children (three to five years of age) who were being trained in Turbuhaler use, which requires a rapid, forceful inhalation; thus the applicability of this finding to other populations may be limited. Results showed benefit in favour of the educational intervention (Analysis 2.2; MD 10.00, 95% CI 2.16 to 17.84). The same study measured this again at two‐week follow‐up and reported that benefit was maintained (Analysis 2.3; MD 7.60, 95% CI 1.43 to 13.77).
Asthma control
Among adults, researchers measured asthma control using the ACT ‐ "perceived asthma control" ‐ on a 0 to 55 scale and dichotomised results into complete control versus incomplete control on the ACT. None of the included studies of children in this comparison reported asthma control.
We combined ACT and perceived asthma control scores, both measured at 26 weeks, using SMD. Analysis revealed benefit in favour of the educational intervention but with a lower confidence interval, including no difference and high statistical heterogeneity (Analysis 1.3; SMD 0.48, 95% CI ‐0.29 to 1.24; 247 participants; two studies; I² = 88%; very low‐quality evidence). Two small studies reported "complete control" and meta‐analysis favoured the educational intervention (Analysis 1.4; OR 3.18, 95% CI 1.47 to 6.88; 134 participants; two studies; I² = 0%; low‐quality evidence).
Exacerbations
Only one study in adults reported asthma exacerbations at 26 weeks (Mehuys 2008). A similar number of participants in each group experienced an exacerbation requiring at least OCS treatment (10 vs 8), and although more participants in the control group experienced an exacerbation requiring an ED visit or hospital admission (1 vs 5), events were too infrequent to allow investigators to draw a conclusion. We considered the evidence of low quality.
Quality of life
Again, for this comparison, only studies involving adults reported quality of life, and both reported this outcome at 26 weeks. One study used asthma‐related quality of life (AQOL) and the other used the AQLQ. We combined both scales using SMD; although results favoured the educational intervention, the lower confidence interval included no difference and heterogeneity was substantial (Analysis 1.7; SMD 0.52, 95% CI ‐0.04 to 1.09; 247 participants; two studies; I2 = 78%; low‐quality evidence).
Adverse events
One study involving 97 adults (Basheti 2008) reported that no "clinically important adverse events" occurred during the 26‐week follow‐up.
Investigators reported the following outcomes in a way that did not allow meta‐analysis by any of the studies in this comparison: unscheduled visits to healthcare provider; and absenteeism from work or school.
Subgroup analyses
To whom the intervention was delivered: patient versus healthcare professional
We were unable to perform this subgroup analysis for inhaler technique score, as we chose not to perform a meta‐analysis owing to substantial heterogeneity.
We were able to perform a formal test of subgroup differences for dichotomised inhaler technique and asthma control in adults (Analysis 1.8; Analysis 1.9); for both analyses, the subgroup analysis suggests that it may be more effective to deliver the educational intervention to pharmacists rather than directly to patients. However, very few studies contributed to these analyses, and baseline ACT and AQLQ in Mehuys 2008 (a study in which the intervention was delivered to participants) was high, perhaps limiting the scope for improved control among these participants. Therefore, our confidence in this finding is low.
One‐off versus repeated intervention sessions
As above, we could not perform subgroup analysis for inhaler technique score, as we chose not to combine results, and the only studies reporting asthma control delivered repeated sessions. For dichotomised inhaler technique, the formal test for subgroup differences in both adults and children (Analysis 1.10; Analysis 2.4) did not suggest any impact of repeated versus one‐off sessions of education, but as above, very few studies contributed to these analyses.
Comparisons 3 and 4: multi‐media training versus control or usual care
Five studies in adults (Self 1983; Lirsac 1991; Savage 2003; Goodyer 2006; Acosta 2009) and two studies in children (Boone 2002; Carpenter 2015) contributed to this comparison.
Inhaler technique
Again, investigators usually measured inhaler technique by using a checklist, immediately after the intervention or at follow‐up.
Two further linked studies in separate populations reported "global improvement in technique" dichotomously immediately after the intervention and found benefit in favour of the multi‐media intervention when compared with a patient information leaflet and a verbal explanation, but the lower confidence interval of the effect estimate does not rule out benefit for the control group (Analysis 3.1; OR 2.15, 95% CI 0.84 to 5.50; 164 participants; two studies; I² = 49%; moderate‐quality evidence).
One study reporting number with correct use after 15 days found no significant differences between groups, although this study included too small a sample to permit firm conclusions (Analysis 3.2; OR 1.78, 95% CI 0.40 to 7.94; low‐quality evidence).
Two studies in adults reported the mean between‐group difference immediately after the intervention or after follow‐up (or both) as "percent correct use" or as a score out of 20. We decided not to combine these results. Both studies demonstrated benefit of multi‐media training versus usual care (Analysis 3.3).
Two studies in children also reported change from baseline inhaler technique at one‐month follow‐up by using a checklist. We present these effect estimates in a data table and did not include them in a meta‐analysis (Analysis 4.1). Both studies show benefit of multi‐media training.
Asthma control
Only one study in children reported asthma control using the ACT, both as an endpoint score and as change from baseline (Analysis 4.2; Analysis 4.3), and found no significant between‐group differences, with confidence intervals excluding the established minimal clinically important difference (MCID) of 3 (low‐quality evidence).
No studies in this comparison reported the following outcomes in a way that allowed meta‐analysis: exacerbations; quality of life; adverse events; unscheduled visits to a health care provider; and absenteeism from work or school.
Subgroup analysis
We were unable to perform either of our prespecified subgroup analyses for this comparison.
Comparisons 5 and 6: feedback device versus control or usual care
Three studies in adults (Al‐Showair 2007; Ammari 2013; Toumas‐Shehata 2014) and four studies in children (Schultz 2012; Ammari 2013; Ammari 2015; Ammari 2015a) contributed to at least one meta‐analysis (Ammari 2013 recruited both adults and children and presented disaggregated results for quality of life).
Inhaler technique
Study authors assessed inhaler technique in both adults and children using peak inspiratory flow (PIF) rate. DPIs require fast, deep inhalation (high PIF rate), and MDIs require a slower, deep inhalation (low PIF rate).
One small study in adults measured this dichotomously at six weeks with participants classified as achieving or not achieving the optimal rate of < 90 L/min. Results showed benefit in favour of the feedback device, but the effect was very imprecise (Analysis 5.1; OR 18.26, 95% CI 2.22 to 150.13; 71 participants; one study; low‐quality evidence). Another study, in which pharmacists in the intervention group used an inhaler device to give quantitative feedback on inhaler technique to participants, dichotomised participants into those with correct technique and those without incorrect technique at four weeks. Use of an inhaler feedback device in addition to verbal training increased the odds of achieving the correct technique (Analysis 5.2: OR 4.80, 95% CI 1.87 to 12.33; 97 participants; one study; low‐quality evidence). This equates to 51 out of 100 people having correct technique at four‐week follow‐up in the control group compared with 83 out of 100 (95% CI 66 to 93) in the active intervention group.
Two small studies in children assessed PIF rate as a continuous variable (L/min) at follow‐up between 6 and 12 weeks. As these studies were assessing MDI technique, slower inhalation (lower PIF rate) was preferred. Combined results do not show clear benefit of the intervention, although the confidence intervals do not rule out benefit or harm (Analysis 6.1; MD ‐9.22, 95% CI ‐33.71 to 15.27; 98 participants; two studies; I² = 42%; low‐quality evidence).
Asthma control
One study measured asthma control using the ACQ at four weeks and found no differences between groups and confidence intervals, excluding the MCID of 0.5 (Analysis 5.4: MD ‐0.10, 95% CI ‐0.46 to 0.26; 97 participants; one study; low‐quality evidence).
Two studies in children measured this using the ACQ at between 6 and 12 weeks. Investigators noted no between‐group differences, with confidence intervals again excluding the MCID of 0.5 (Analysis 6.2; MD ‐0.02, 95% CI ‐0.35 to 0.32; 98 participants; two studies; I² = 0%; moderate‐quality evidence).
Quality of life
Two studies in adults reported quality of life; both used the mini‐AQLQ and measured this outcome at six weeks. Pooled analysis suggests benefit of device feedback over usual care. However, the mean difference falls below the MCID of 0.5, and the lower confidence interval includes no difference (Analysis 5.5; MD 0.38, 95% CI ‐0.01 to 0.77; 100 participants; two studies; I² = 0%; low‐quality evidence). One of the two studies also reported the mini‐AQLQ as a "responder analysis" (i.e. those experiencing at least a 0.5 point improvement), which shows clear, although imprecise, benefit of the intervention (Analysis 5.6; OR 5.29, 95% CI 1.76 to 15.89; 71 participants; one study; moderate‐quality evidence). This equates to 49 out of 100 people having a greater than 0.5 point improvement at six‐week follow up in the control group compared with 83 out of 100 (95% CI 42 to 94) in the active intervention group (Figure 4).

In the control group, 49 out of 100 people had at least 0.5 unit increase in AQLQ after 6 weeks, compared with 83 (95% CI 42 to 94) out of 100 in the active treatment group.
Among children, three studies reported quality of life ‐ two using the paediatric AQLQ (PAQLQ) and one using the PedsQL Asthma Module. We pooled the two studies reporting PAQLQ as a change from baseline; results show benefit in favour of the feedback device, but the lower confidence interval includes no difference (of note, in one of the studies, reported quality of life deteriorated in both groups, just less so in the feedback device group) (Analysis 6.3; MD 0.25, 95% CI ‐0.07 to 0.58; 91 participants; two studies; I² = 20%; low‐quality evidence). Schultz 2012 reported quality of life on the PedsQL (Asthma Module) at three months and did not detect any significant between‐group differences (Analysis 6.4; MD 41.00, 95% CI ‐76.49 to 158.49; 109 participants; one study), but the result was imprecise.
Researchers did not report the following outcomes in a way that allowed meta‐analysis in this comparison: exacerbations; adverse events; unscheduled visits to a healthcare provider; and absenteeism from work or school.
Subgroup analysis
We were unable to perform either of our prespecified subgroup analyses for this comparison.
Sensitivity analyses
We did not perform prespecified sensitivity analyses for the following reasons.
-
We did not obtain any unpublished data.
-
The only trial deemed at high risk of selection bias (Shah 2014) did not contribute data to any meta‐analysis.
-
Almost all trials that contributed data on inhaler technique used a standard or validated checklist to assess inhaler technique, or they used an objective measure such as PIF rate. For only one contributing study (Acosta 2009), we could not determine how inhaler technique had been assessed. This information was provided as a conference abstract that reported percentage "correct usage" of inhaler; it is not clear whether this refers to the number of individuals who used the device correctly, or to the mean percentage of correct steps followed by each participant. However, as we decided not to pool checklist scores owing to extremely high statistical heterogeneity and heavily skewed data, we determined that the planned sensitivity analysis was not necessary.
Discussion
Summary of main results
This review includes 29 parallel randomised controlled trials (RCTs; n = 2210), although not all trials reported relevant or useable data. All participants had asthma, and some studies specified use of a particular inhaler or spacer, or required a particular level of asthma control. Follow‐up of analysed studies ranged from 2 to 26 weeks. Studies used a variety of scales and did not always use validated scales. Almost all included studies reported some measure of inhaler technique on a range of different checklists. Most studies were at low or unclear risk of selection and attrition biases and at high risk of biases associated with blinding. We considered most of the evidence to be of low quality owing to these biases and to imprecision in the estimates of effect.
Most studies were classified into three comparisons: those assessing an enhanced face‐to‐face training session(s), those using multi‐media to deliver inhaler training (e.g. a video, computer app or game) and those testing devices that give people visual or audio feedback about technique. Despite the large number of included studies, these differences between interventions, as well as differences in age groups and outcome measures, meant that meta‐analyses often could not be performed. This was particularly true for exacerbations, adverse events, unscheduled visits to a healthcare provider and absenteeism from work or school.
Enhanced inhaler technique education and multi‐media training led to improved technique in most studies immediately after the intervention and at follow‐up, although the variety of checklists used meant that this was difficult to assess reliably. For both adults and children, how and when inhaler technique was assessed appeared to affect whether inhaler technique improved and by how much. Analyses of the numbers of people who demonstrated correct or 'good enough' technique were generally more useful; adult studies of enhanced education showed benefit with this metric (odds ratio (OR) 5.00, 95% confidence interval (CI) 1.83 to 13.65; 258 participants; three studies), as did analyses looking at feedback devices (OR 4.80, 95% CI 1.87 to 12.33; 97 participants; one study), but the benefit of multi‐media training for adults was uncertain (OR 2.15, 95% CI 0.84 to 5.50; 164 participants; two studies; I² = 49%). Evidence tended to be less clear for children, usually because results were based on fewer and smaller studies.
Studies found some benefit for quality of life and asthma control, but results generally did not indicate consistent or important benefits of inhaler technique interventions for adults or children. Confidence intervals included no difference or fell under a range that could be considered clinically important. Responder analyses sometimes showed that more people in the intervention groups saw improvement, even though the mean difference between groups was small.
Overall completeness and applicability of evidence
Most of the studies included in this review showed that an intervention to improve inhaler technique improved technique when assessed by a checklist or dichotomously. This was true for both adults and children with asthma and across the three main types of interventions included in the review. However, interventions used in the included studies were variable, even within the three main comparisons that we have identified. Investigators measured outcomes at different time points and in different ways; thus we have limited confidence in how our findings can be applied to the real‐life setting. A checklist score is not necessarily the most useful measure for clinicians; not all items on the checklist are equally important for achieving medication delivery, and this distinction is not clear when mean scores alone are reported. For example, failing to remove an inhaler cap is a critical error, whereas failing to hold the inhaler upright might not be so critical. A more useful measure, reported by a minority of included studies, is a dichotomous outcome that reports the number of people with (or without) a critical inhaler handling error. We know that critical errors are common, ranging in one study from 12% to 44% of users (depending on inhaler type), and are associated with poorer outcomes (Melani 2011).
In addition, many included studies have treated checklist scores as continuous variables and have used parametric statistical tests, but these measures are clearly skewed, and the wisdom of this analysis choice could be questioned. As a result of the skew and the heterogeneity of the measurement tools used, we chose not to perform meta‐analyses of checklist outcomes and instead reported these outcomes in data tables. Our inability to pool inhaler technique measurements for most of the included studies has considerably limited the conclusions that we can reach.
Another limitation to the applicability of our findings is that we were unable to perform most of our planned subgroup and sensitivity analyses, or, when we were able, our subgroups included very few studies, limiting our confidence in the findings. This means that we cannot comment on whether multiple intervention sessions are superior to one‐off sessions, or whether delivering the intervention to a healthcare professional (e.g. a pharmacist) is more or less effective than delivering the intervention directly to the patient. These are important issues, as the feasibility of larger‐scale implementation would be affected by such intervention design details.
Improvements in inhaler technique observed in many of the included studies did not always translate to any meaningful benefit for patient‐important outcomes, such as exacerbations and quality of life. In some cases, these outcomes were simply not measured; in other cases, studies may not have been powered, or were of insufficient duration, to detect a difference. The maximum duration of follow‐up was only 26 weeks (three studies), and many trials had a much shorter duration. Also, as discussed above, although many participants were performing steps more correctly, they still might have been making critical errors that prevented improved drug delivery and therefore limited clinical improvement.
Another important consideration when the applicability of the evidence is assessed is whether such interventions could be delivered realistically in routine clinical practice. Many healthcare systems are under strain, and healthcare professionals may have only a few minutes to spend with each patient. In view of the time‐consuming nature of many of the interventions investigated in this review, it is even more essential for researchers to demonstrate that clear clinical benefit can be expected from this investment of resources. Of note, only 10 of the 29 studies included in this review were published within the past five years, and of these, only six contributed data to at least one meta‐analysis.
An alternative explanation for the lack of clinical improvement would be that very few of the included studies attempted to address adherence; even a participant who can demonstrate correct technique at follow‐up may not have been adherent to the medication regimen during the follow‐up period. Inadequate adherence to prescribed medication is thought to have contributed to approximately one‐third of asthma deaths in the UK over the course of a year (NRAD 2014). An intervention to improve inhaler technique could be considered incomplete unless it also includes an attempt to address adherence. However, our protocol clearly stated that we would include only studies for which improving inhaler technique was the main aim, and adherence is the topic of another Cochrane Review. This resulted in the exclusion of many studies for which inhaler technique training was just one component of a broader self‐management or asthma education intervention. As a result, we may have excluded studies that would have been informative in a 'real‐world' setting, but their inclusion would have further hampered interpretation of findings. It would be very difficult to assess which element of the intervention had led to any observed clinical benefit.
Some included studies specifically recruited people known to have poor inhaler technique or people with poorly controlled asthma. We did not plan to analyse people with poorly controlled asthma separately from those with better controlled asthma. Greater improvements might have been seen in this group than in a more general asthma population, and this might help in terms of targeting a potentially expensive and time‐consuming intervention in clinical practice. Review authors could consider such a subgroup analysis for future updates of this review.
Quality of the evidence
Most of the evidence that could be combined and graded was related to inhaler technique, asthma control and quality of life. For all of these outcomes, risk of performance and detection bias may have led to an overestimation of treatment effects. Although it was not possible to blind the delivery of behavioural interventions within studies, it would have been possible to control for detection bias by blinding those who assessed inhaler technique at the end of the study; in most cases, this was not done. Across comparisons and outcomes, we downgraded evidence quality for this reason unless it was clear that a particular outcome had been assessed independently (e.g. inhaler technique in Goodyer 2006 and Savage 2003). We were more confident in results from studies in which the control group received additional support or an active control intervention, as this would have minimised the effects of performance bias. For outcomes for which risk of bias was our only concern, we had moderate confidence in the results, meaning that the true effect is likely to be close to that estimated. This was true for inhaler technique in adults given enhanced and multi‐media training, and for responder analyses of asthma control and quality of life.
Our confidence in results was also reduced by imprecision of estimates for which confidence intervals did not exclude the possibility of the effect favouring the control group. This took our confidence in the evidence down to low, which was the most common rating across outcomes. This means that our confidence in the effect estimates is limited, and that the true effect may be substantially different from that estimated from the current evidence. When we noted inconsistency between results, we further downgraded the evidence to very low quality; we did this for the effect of enhanced training on asthma control in adults (comparison 1).
We did not apply GRADE criteria to outcomes for which we could not perform a meta‐analysis, including those for which data were available but we considered it unreasonable to pool results. In these cases, particularly for inhaler technique checklist scores, the results are very difficult to interpret owing to inconsistencies in measurement and non‐parametric properties of the data.
Potential biases in the review process
We followed the methods described in the published protocol (Normansell 2016) and recorded any deviations in the section titled Differences between protocol and review. We made some changes to the division of the workload, but the main deviation from the protocol was result of insufficient data, which prevented several planned meta‐analyses. This is discussed in Overall completeness and applicability of evidence.
We did not know in advance how studies would vary, particularly with regard to interventions delivered and the nature of control groups. As a result, the method of grouping studies into comparisons was iterative and was based on the judgement of the review authors. We stated in the protocol that we would conduct meta‐analyses only "if treatments, participants and the underlying clinical question were similar enough for pooling to make sense". We have been transparent about which studies were included in each comparison but we accept that the post hoc nature of this process could have introduced bias.
Usually we would contact study authors to ask for additional outcome data and to clarify uncertainties about risk of bias, so we could be certain that the evidence is reliable and complete. Owing to the large number of studies identified, we did not contact study authors routinely for this information and contacted teams only if we wished to clarify specific issues related to outcome data. We did not request unpublished data, so analyses may be incomplete if studies did not include all of their outcomes or time points in the published reports. We assessed several studies to be at high risk of bias for selective outcome reporting, but we did not strongly suspect that publication bias compromised the meta‐analyses we were able to perform.
Agreements and disagreements with other studies or reviews
Poor inhaler technique is a common and burdensome problem, and several primary studies previously evaluated interventions to improve inhaler technique among both children and adults with asthma. The variety of interventions assessed and outcomes presented in the primary studies posed significant challenges for our review. Previous relevant systematic reviews identified similar issues. Gillette 2016 evaluated educational interventions to improve inhalation technique among children with asthma on the basis of results obtained from 28 studies. Lavorini 2007 assessed the effect of incorrect use of dry powder inhalers on the treatment of patients with asthma and chronic obstructive pulmonary disease (COPD); this review included 47 relevant primary studies. Both of these reviews identified significant variation in the proportion of patients correctly using their inhalers, which was associated with the use of different inhaler devices and different evaluation methods. In our review, we did not assess differences among devices. Both previous reviews also concluded that appropriate inhaler technique instruction leads to significant but short‐lived improvement in inhalation technique. Our review detected a trend towards prolonged benefit compared with control. However, this finding is based on very limited data, and confirmatory trials are required. In the meantime, as suggested by previous systematic reviews, correct inhaler technique should be reassessed and reinforced regularly. Finally, although Lavorini 2007 describes only incorrect inhaler technique and whether or not patients received adequate instruction, Gillette 2016 identified a variety of educational interventions with different efficacy, which is consistent with our findings.
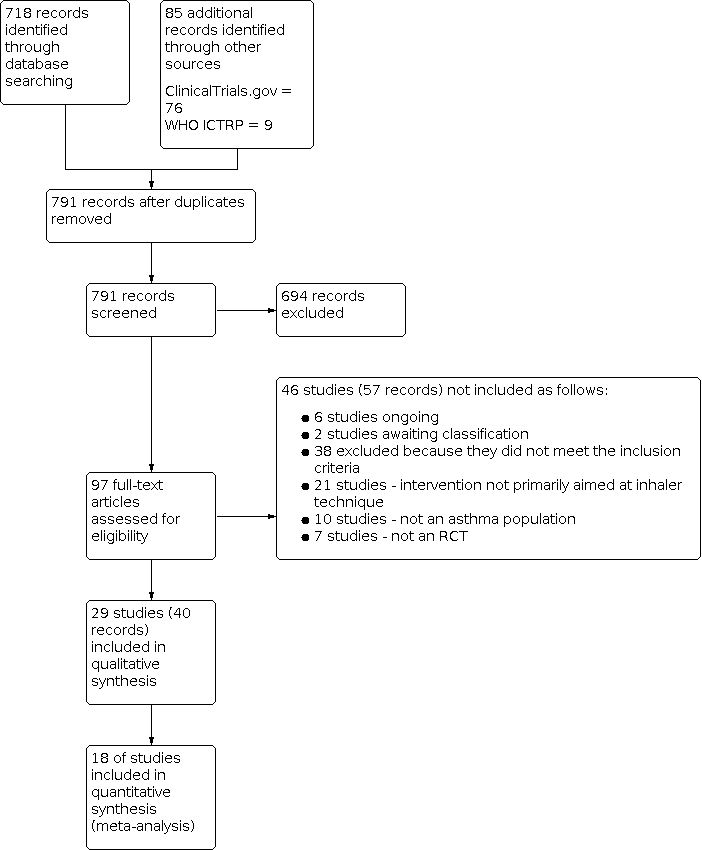
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

In the control group, 31 out of 100 people had correct inhaler technique after 2 to 26 weeks, compared with 69 (95% CI 45 to 86) out of 100 in the active treatment group.

In the control group, 49 out of 100 people had at least 0.5 unit increase in AQLQ after 6 weeks, compared with 83 (95% CI 42 to 94) out of 100 in the active treatment group.

Comparison 1: Adults: enhanced education versus control/usual care, Outcome 1: Correct inhaler technique (at follow‐up)
| Inhaler technique score | |||||
| Study | Time of rating | Intervention mean (SD) | Control mean (SD) | Total N | Scale (higher score= better technique) |
|---|---|---|---|---|---|
| Basheti 2008 | Post‐intervention | ‐ | ‐ | ‐ | ‐ |
| Follow‐up (26 wks) | 2.80 (1.60) | 0.90 (1.40) | 100 | Max 9 (change) | |
| Bynum 2001 | Post‐intervention | 7.33 (0.72) | 5.14 (1.62) | 36 | Max 8 |
| Follow‐up (2 to 4 wks) | 6.73 (1.22) | 4.86 (1.10) | 36 | Max 8 | |
| Mehuys 2008 | Post‐intervention | ‐ | ‐ | ‐ | ‐ |
| Follow‐up (26 wks) | 93.2 (10.7) | 83.7 (22.5) | 150 | Max 100 | |
| Rahmati 2014 | Post‐intervention | 10.8 (0.48) | 5.57 (1.20) | 120 | Max 11 |
| Follow‐up (4 wks) | 10.17 (0.91) | 5.48 (1.27) | 90 | Max 11 | |
| Rydman 1999 | Post‐intervention | 0.68 (0.46) | 0.60 (0.49) | 120 | Max 1 |
| Follow‐up (8 to 20 wks) | 0.72 (0.45) | 0.75 (0.43) | 120 | Max 1 | |
| Self 1983 | Post‐intervention | 16.8 (4.1) | 10.7 (5.4) | 19 | Max 20 |
| Follow‐up | ‐ | ‐ | ‐ | ‐ | |
Comparison 1: Adults: enhanced education versus control/usual care, Outcome 2: Inhaler technique score

Comparison 1: Adults: enhanced education versus control/usual care, Outcome 3: Asthma control

Comparison 1: Adults: enhanced education versus control/usual care, Outcome 4: Asthma control

Comparison 1: Adults: enhanced education versus control/usual care, Outcome 5: Exacerbations requiring at least OCS

Comparison 1: Adults: enhanced education versus control/usual care, Outcome 6: Exacerbations requiring ED/hospitalisation

Comparison 1: Adults: enhanced education versus control/usual care, Outcome 7: Quality of life

Comparison 1: Adults: enhanced education versus control/usual care, Outcome 8: Subgroup analysis: inhaler technique (at follow‐up): to whom intervention is delivered

Comparison 1: Adults: enhanced education versus control/usual care, Outcome 9: Subgroup analysis: asthma control: to whom intervention is delivered

Comparison 1: Adults: enhanced education versus control/usual care, Outcome 10: Subgroup analysis: inhaler technique (at follow‐up): off‐off vs repeated sessions

Comparison 2: Children: enhanced education versus control/usual care, Outcome 1: Correct inhaler technique (at follow‐up)

Comparison 2: Children: enhanced education versus control/usual care, Outcome 2: Inhaler technique (PIF immediately after intervention)

Comparison 2: Children: enhanced education versus control/usual care, Outcome 3: Inhaler technique (PIF at follow‐up)

Comparison 2: Children: enhanced education versus control/usual care, Outcome 4: Subgroup analysis: inhaler technique (at follow‐up): one‐off vs repeated sessions

Comparison 3: Adults: multi‐media training versus control/usual care, Outcome 1: Correct inhaler technique (immediately after intervention)

Comparison 3: Adults: multi‐media training versus control/usual care, Outcome 2: Correct inhaler technique (at follow‐up)
| Inhaler technique score | ||||
| Study | Time of rating | MD (SE) | Total N | Scale (higher score= better technique) |
|---|---|---|---|---|
| Acosta 2009 | Post‐intervention | 14.77 (2.23) | 116 | % correct use |
| Follow‐up (4 wks) | 13.30 (2.33) | 116 | % correct use | |
| Self 1983 | Post‐intervention | 6.2 (2.33) | 20 | Max 20 |
| Follow‐up (4 wks) | Control not followed‐up | ‐ | Max 20 | |
Comparison 3: Adults: multi‐media training versus control/usual care, Outcome 3: Inhaler technique score
| Change in inhaler technique score | |||||
| Study | Time of rating | Intervention mean (SD) | Control mean (SD) | Total N | Scale (higher score= better technique) |
|---|---|---|---|---|---|
| Boone 2002 | Post‐intervention | ‐ | ‐ | ‐ | ‐ |
| Follow‐up (4 wks) | 2.60 (0.60) | 0.50 (0.50) | 36 | Max 15 | |
| Carpenter 2015 | Post‐intervention | 1.12 (1.09) | 0.03 (1.07) | 91 | Max 8 |
| Follow‐up (4 wks) | 0.87 (1.09) | 0.32 (1.14) | 91 | Max 8 | |
Comparison 4: Children: multi‐media training versus control/usual care, Outcome 1: Change in inhaler technique score

Comparison 4: Children: multi‐media training versus control/usual care, Outcome 2: Asthma control (change from baseline)

Comparison 4: Children: multi‐media training versus control/usual care, Outcome 3: Asthma control (endpoint)

Comparison 5: Adults: feedback device versus control/usual care, Outcome 1: Inhaler technique (PIF)

Comparison 5: Adults: feedback device versus control/usual care, Outcome 2: Correct inhaler technique (at follow‐up)
| Inhaler technique score | |||||
| Study | Time of rating | Intervention mean (SD) | Control mean (SD) | Total N | Scale (higher score= better technique) |
|---|---|---|---|---|---|
| Toumas‐Shehata 2014 | Follow‐up (4 wks) | 9.50 (1.00) | 8.90 (1.00) | 97 | Max 10 or 11 |
Comparison 5: Adults: feedback device versus control/usual care, Outcome 3: Inhaler technique score

Comparison 5: Adults: feedback device versus control/usual care, Outcome 4: Asthma control

Comparison 5: Adults: feedback device versus control/usual care, Outcome 5: Quality of life

Comparison 5: Adults: feedback device versus control/usual care, Outcome 6: Quality of life (responders)

Comparison 6: Children: feedback device versus control/usual care, Outcome 1: Inhaler technique (PIF)

Comparison 6: Children: feedback device versus control/usual care, Outcome 2: Asthma control

Comparison 6: Children: feedback device versus control/usual care, Outcome 3: Quality of life (change from baseline)

Comparison 6: Children: feedback device versus control/usual care, Outcome 4: Quality of life (endpoint)
| Enhanced education compared with control/usual care for people with asthma | |||||||
| Patient or population: adults and children with asthma | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | ||
|---|---|---|---|---|---|---|---|
| Risk with control/usual care | Risk with enhanced education | ||||||
| Correct inhaler technique Follow‐up: 2 to 26 weeks (adults) 12 to 26 weeks (children) | Adults | 31 per 100 | 69 per 100 | OR 5.00 (1.83 to 13.65) | 258 | ⊕⊕⊕⊝ MODERATEa | Additional results from technique rating scales could not be combined (Analysis 1.2) |
| Children | 49 per 100 | 55 per 1,00 | OR 1.29 | 175 | ⊕⊕⊝⊝ LOWa,b | One study measured PIF as a marker for inhaler technique and showed benefit (Analysis 2.2; Analysis 2.3) | |
| Asthma control score 4 to 26 weeks | Adults | ‐ | Score in the intervention group was 0.48 SDs higher than in the control group | ‐ | 247 | ⊕⊝⊝⊝ VERY LOWa,b,c | We were not able to calculate a control risk, as the outcome was measured on different scales |
| Asthma control responders 8 to 12 weeks | Adults | 42 per 100 | 70 per 100 | OR 3.18 | 134 | ⊕⊕⊝⊝ LOWd | |
| Exacerbations requiring at least OCS 26 weeks | Adults | 10 per 100 | 13 per 100 (5 to 28) | OR 1.32 (0.49 to 3.55) | 158 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | The same study also reported exacerbations requiring ED/hospitalisation. Events were rare and results imprecise |
| Quality of life 26 weeks | Adults | ‐ | Score in the intervention group was 0.52 SDs higher than in the control group | ‐ | 247 | ⊕⊕⊝⊝ LOWa,c,e | We were not able to calculate a control risk as the outcome was measured on different scales |
| Other outcomes | No results could be analysed for adverse events, unscheduled visits to a healthcare provider or school/work absences | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | |||||||
| GRADE Working Group grades of evidence | |||||||
| aStudies contributing to this outcome were at high risk of bias in several domains (including performance and detection bias). Downgraded once bWide confidence intervals including possible harm and benefit of intervention. Downgraded once cHigh level of statistical inconsistency detected. Downgraded once dThe two small studies contributing to this outcome were identified as abstracts only; it is therefore difficult to assess methodological quality. Studies were considered at high or unclear risk of bias in multiple domains (including selection, performance, detection and reporting biases). Downgraded twice eConfidence interval includes no difference with random‐effects model, driven by statistical heterogeneity. Fixed‐effect sensitivity analysis yields more precise result. Not downgraded | |||||||
| Multimedia training compared with control/usual care for people with asthma | |||||||
| Patient or population: adults and children with asthma | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | ||
|---|---|---|---|---|---|---|---|
| Risk with control/usual care | Risk with multi‐media training | ||||||
| Correct inhaler technique Immediately after intervention | Adults | 30 per 100 | 47 per 100 | OR 2.15 | 164 | ⊕⊕⊕⊝ MODERATEa,b | |
| Correct inhaler technique 2‐Week follow‐up | Adults | 43 per 100 | 57 per 100 | OR 1.78 | 28 | ⊕⊕⊝⊝ LOWa,c | |
| Inhaler technique score | Adults | Not pooled; narrative summary of evidence provided in data table (Analysis 3.3) | ‐ | 136 (2 RCTs) | Not graded | Suggests benefit of multi‐media training over control | |
| Children | Not pooled; narrative summary of evidence provided in data table (Analysis 4.1) | ‐ | 127 (2 RCTs) | Not graded | Suggests benefit of multi‐media training over control | ||
| Asthma control (ACT) 4 weeks | Children | Mean change in control group was 1.2 | Mean change was 0.73 better (‐0.99 worse to 2.45 better) | ‐ | 91 (1 RCT) | ⊕⊕⊝⊝ LOWa,c | |
| Other outcomes | No results could be analysed for quality of life, exacerbations, adverse events, unscheduled visits to a healthcare provider or school/work absences | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | |||||||
| GRADE Working Group grades of evidence | |||||||
| aConfidence intervals include no difference. Downgraded once bAlthough participants in both studies were unblinded, inhaler technique was rated by a blinded assessor, and both groups received an intervention. Not downgraded cHigh risk of bias for performance and detection. Downgraded once | |||||||
| Feedback device compared with control/usual care for people with asthma | |||||||
| Patient or population: adults and children with asthma | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | ||
|---|---|---|---|---|---|---|---|
| Risk with control/usual care | Risk with feedback device | ||||||
| Correct inhaler technique 4‐Week follow‐up | Adults | 51 per 100 | 83 per 100 (66 to 93) | OR 4.80 (1.87 to 12.33) | 97 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | Additional results from technique rating scales could not be combined (Analysis 5.3) |
| Inhaler technique (PIF) Follow‐up: 6 weeks (adults) 6 to 12 weeks (children) | Adults | 66 per 100 | 97 per 100 | OR 18.26 | 71 | ⊕⊕⊝⊝ LOWa,b | |
| Children | Mean PIF was 51.2 litres/min | Mean PIF was 9.22 litres/min better (33.71 better to 15.27 worse) | ‐ | 98 | ⊕⊕⊝⊝ LOWa,c | ||
| Asthma control (ACQ) 6 to 12 weeks | Adults | Mean ACQ score was 1.4 | Mean score in the intervention group was 0.1 better (0.46 better to 0.26 worse) | ‐ | 97 (1 RCT) | ⊕⊕⊝⊝ LOWa,c | |
| Children | Mean ACQ score was 0.7 | Mean score in the intervention group was 0.02 worse | ‐ | 98 | ⊕⊕⊕⊝ MODERATEa | ||
| Quality of life Follow‐up: 6 weeks (adults) 6 to 12 weeks (children) | Adults | Mean score on the mini‐AQLQ was 4.2 | Mean score in the intervention group was 0.38 better | ‐ | 100 | ⊕⊕⊝⊝ LOWa,d | |
| Children | Mean change in quality of life was 0.07 | Mean change was 0.25 better | ‐ | 91 | ⊕⊕⊝⊝ LOWa,d | One study reported endpoint quality of life (Analysis 6.4) | |
| Quality of life (responders) 6 weeks | Adults | 49 per 100 | 83 per 100 | OR 5.29 | 71 | ⊕⊕⊕⊝ MODERATEa | |
| Other outcomes | No results could be analysed for exacerbations, adverse events, unscheduled visits to a healthcare provider or school/work absences | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | |||||||
| GRADE Working Group grades of evidence | |||||||
| aHigh risk of performance and detection bias. Downgraded once bVery wide confidence intervals based on one study. Downgraded once cConfidence intervals include possible harm and benefit of the intervention. Downgraded once dConfidence interval does not exclude no difference, and upper limit exceeds the MCID of 0.5 units. Downgraded once | |||||||
| Study ID | Total n | Study duration (weeks) | Age | Country | Intervention | Control | Inhaler technique measure | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 72 | 2 | Children aged 3 to 5 years | Denmark | Instructional video + individual training; delivered to participant | Instructional video | PIF | PIF, IVC, PEFR, FEV1, FVC | |
| 26 | 2 | Adults | Australia | Group B: verbal counselling Group C: verbal counselling + physical demonstration; delivered to participant | Group A: printed materials | 9‐Step Turbuhaler checklist | Inhaler technique | |
| 31 pharmacists, 97 participants | 26 | Adults | Australia | Pharmacist‐participant education + inhaler technique labels; delivered to pharmacist | Peak flow measurement training | 9‐Step checklist | Inhaler technique, asthma severity, peak flow variability, AQOL, perceived control | |
| 49 | 4 | Adolescents aged 12 to 19 years | USA | Telepharmacy counselling; delivered to participant | Written instructions only | 8‐Step MDI checklist | Inhaler technique, participant satisfaction | |
| 89 | 12 | Adults | Not reported | Respiratory therapist education; delivered to participant | Rotuine physician education | Not reported | Inhaler technique, asthma control | |
| 201 | 26 | Adults | Belgium | Pharmacist education; delivered to pharmacist | Usual pharmacy care | 10‐Step MDI + spacer checklist/8‐step DPI checklist | Inhaler technique, ACT, exacerbations, ED/hospital visits, adherence, AQLQ, asthma knowledge | |
| 46 | 8.7 | Adults | Iran | Face‐to‐face education; delivered to participant | Usual care (no further details) | Not reported | ACT, FEV1 | |
| 130 | 12 | Children | Turkey | Face‐to‐face nurse training; delivered to participant | Inhaler package insert only | 10‐Step MDI checklist | Inhaler technique, PAQLQ, spirometry | |
| 90 | 4 | Adults | Iran | Face‐to‐face training (no spacer device); delivered to participant | Usual care (no training) | 11‐Step MDI checklist | Inhaler technique, PEFR | |
| 68 | 8 to 20 | Adults | USA | Face‐to‐face training + demonstration; delivered to participant | Inhaler package insert only | Checklist, converted to score out of 1 (0 if any steps missed) | BAI and MDI competency | |
| 29 | 1 to 16 (mean 6) | Adults | USA | Face‐to‐face pharmacist training; delivered to participant | Inhaler package insert only | 10‐Step checklist | Inhaler technique, "effectiveness of instruction" | |
| 96 | 26 | Children and adolescents (max 15 years) | Canada | Interactive nurse training with feedback; delivered to participant | Pictorial nurse training only | Checklist with scores converted to percentages | Inhaler technique, parental perceptions of treatment, asthma morbidity (e.g. no. of exacerbations, ED visits, hospitalisations, days of schools missed) | |
| ACT: Asthma Control Test; AQLQ: Asthma Quality of Life Questionnaire; AQOL: asthma quality of life; BAI: breath‐activated inhaler; DPI: dry powder inhaler; ED: emergency department; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; IVC: inspiratory vital capacity; MDI: metered dose inhaler; PAQLQ: paediatric AQLQ; PEFR: peak expiratory flow rate; PIF: peak inspiratory flow | ||||||||
| Study ID | Total n | Study duration (weeks) | Age | Country | Intervention | Control | Inhaler technique measure | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 133 | 4 | Adults | USA | Inhaler technique training video; delivered to participant | Asthma education video | Not reported | "Correct usage" of inhaler | |
| 21 | 13 | Children | Ireland | Inhaler technique DVD; delivered to participant | Individual instruction | New inhaler technique measurement tool | Inhaler technique, self‐efficacy, knowledge acquisition | |
| 36 | 4.3 | Children | UK | Inhaler technique educational computer software ("Space Inhalers"); delivered to participant | Placebo software | 15‐Step checklist | Inhaler technique, asthma knowledge | |
| 91 | 4.3 | Children and adolescents | USA | Inhaler technique video; delivered to participant | Attention control video (about nutrition) | 8‐Step checklist | Inhaler technique, self‐efficacy, ACT | |
| 69 | Outcomes assessed immediately | Adults | UK (Turkish‐ speaking population) | Multi‐media touch screen training; delivered to participant | Patient information leaflet plus verbal training | Inhaler checklist | Inhaler technique | |
| 45 | 2.1 | Adults and children (10 to 71 years) | France | Inhaler technique video OR inhaler technique video + spacer; delivered to participant | Patient information sheet | 4‐Step checklist | Inhaler technique, FEV1 | |
| 110 | Outcomes assessed immediately | Children and adults (12 to 87 years) | UK | Multi‐media touch screen training; delivered to participant | Patient information leaflet | Inhaler checklist, global assessment of technique | Inhaler technique, acceptability | |
| 29 | 1 to 16 (mean 6) | Adults | USA | Video training; delivered to participant | Inhaler package insert only | 10‐Step checklist | Inhaler technique, "effectiveness of instruction" | |
| 50 | Not reported | Not reported | USA | Computer training; delivered to participant | Written training OR no training | "Fixed rubric" used to give a score | Inhaler technique | |
| ACT: Asthma Control Test; DVD: digital versatile disc; FEV1: forced expiratory volume in one second | ||||||||
| Study ID | Total n | Study duration (weeks) | Age | Country | Intervention | Control | Inhaler technique measure | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 108 | 6 | Adults | UK | Verbal training + 2Tone trainer; delivered to participant | Verbal training alone | PIF | PIF, FEV1, AQLQ | |
| 56 | 6 | Adults and children (4 to 55 years) | UK | Verbal training + 2Tone trainer; delivered to participant | Verbal training alone | PIF | PIF, FEV1, AQLQ/PAQLQ | |
| 80 | 12 | Children | UK | AeroChamber Plus with Flo‐Vu; delivered to participant | AeroChamber Plus alone | PIF | PIF, PAQLQ, ACQ, parent spacer preference | |
| 30 | 6 to 8 | Children | Not reported | Verbal training and use of Trainhaler; delivered to participant | Verbal training alone | PIF | PIF, ACQ | |
| 90 | 4 | Adults | Iran | Face‐to‐face training (with spacer device); delivered to participant | Usual care (no training) | 11‐Step MDI checklist | Inhaler technique, PEFR | |
| 132 | 52 | Children | Australia | Funhaler incentive device; delivered to participant | AeroChamber device | Filter used to measure salbutamol deposition | Inhaler technique, asthma control, quality of life | |
| 76 | 4 | Not reported | Libya | Verbal training + 2Tone trainer; delivered to participant | Verbal training alone | PIF | PIF, FEV1, AQLQ, Jones Morbidity Index (JMI) | |
| 19 pharmacists (101 participants) | 4 | Adults | Australia | Verbal training + quantitative inhaler feedback; delivered to pharmacist | Verbal training alone | Inhaler checklist/proportion with correct technique | Inhaler technique, ACQ | |
| 43 | 4 | Not reported | USA | Face‐to‐face demonstration + In‐Check simulator; delivered to participant | Face‐to‐face demonstration | PIF, inhaler checklist | PIF, inhaler technique | |
| ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; FEV1: forced expiratory volume in one second; MDI: metered dose inhaler; PAQLQ: paediatric AQLQ; PEFR: peak expiratory flow rate; PIF: peak inspiratory flow | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Correct inhaler technique (at follow‐up) Show forest plot | 3 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 5.00 [1.83, 13.65] |
| 1.2 Inhaler technique score Show forest plot | 6 | Other data | No numeric data | |
| 1.3 Asthma control Show forest plot | 2 | 247 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.29, 1.24] |
| 1.4 Asthma control Show forest plot | 2 | 134 | Odds Ratio (M‐H, Random, 95% CI) | 3.18 [1.47, 6.88] |
| 1.5 Exacerbations requiring at least OCS Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6 Exacerbations requiring ED/hospitalisation Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.7 Quality of life Show forest plot | 2 | 247 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.04, 1.09] |
| 1.8 Subgroup analysis: inhaler technique (at follow‐up): to whom intervention is delivered Show forest plot | 3 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 5.00 [1.83, 13.65] |
| 1.8.1 Delivered to participants | 2 | 174 | Odds Ratio (M‐H, Random, 95% CI) | 3.03 [1.61, 5.68] |
| 1.8.2 Delivered to pharmacists | 1 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 12.38 [4.04, 37.90] |
| 1.9 Subgroup analysis: asthma control: to whom intervention is delivered Show forest plot | 2 | 247 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.29, 1.24] |
| 1.9.1 Delivered to participants | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.22, 0.42] |
| 1.9.2 Delivered to pharmacists | 1 | 97 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [0.46, 1.30] |
| 1.10 Subgroup analysis: inhaler technique (at follow‐up): off‐off vs repeated sessions Show forest plot | 3 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 5.00 [1.83, 13.65] |
| 1.10.1 One‐off | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 3.50 [0.50, 24.56] |
| 1.10.2 Repeated | 2 | 234 | Odds Ratio (M‐H, Random, 95% CI) | 5.64 [1.40, 22.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Correct inhaler technique (at follow‐up) Show forest plot | 2 | 175 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.70, 2.36] |
| 2.2 Inhaler technique (PIF immediately after intervention) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3 Inhaler technique (PIF at follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4 Subgroup analysis: inhaler technique (at follow‐up): one‐off vs repeated sessions Show forest plot | 2 | 175 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.70, 2.36] |
| 2.4.1 One‐off | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [0.66, 2.83] |
| 2.4.2 Repeated | 1 | 55 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.37, 3.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Correct inhaler technique (immediately after intervention) Show forest plot | 2 | 164 | Odds Ratio (M‐H, Random, 95% CI) | 2.15 [0.84, 5.50] |
| 3.2 Correct inhaler technique (at follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.3 Inhaler technique score Show forest plot | 2 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Change in inhaler technique score Show forest plot | 2 | Other data | No numeric data | |
| 4.2 Asthma control (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.3 Asthma control (endpoint) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Inhaler technique (PIF) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2 Correct inhaler technique (at follow‐up) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.3 Inhaler technique score Show forest plot | 1 | Other data | No numeric data | |
| 5.4 Asthma control Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.5 Quality of life Show forest plot | 2 | 100 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.01, 0.77] |
| 5.6 Quality of life (responders) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Inhaler technique (PIF) Show forest plot | 2 | 98 | Mean Difference (IV, Random, 95% CI) | ‐9.22 [‐33.71, 15.27] |
| 6.2 Asthma control Show forest plot | 2 | 98 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.35, 0.32] |
| 6.3 Quality of life (change from baseline) Show forest plot | 2 | 91 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.07, 0.58] |
| 6.4 Quality of life (endpoint) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |

