Terapija hormonom rasta u osoba s talasemijom
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Trial design: randomised controlled trial Trial grouping: parallel group | |
| Participants | Baseline characteristics GH group Gender: male (n = 6), female (n = 4) Age (mean (SD): 11.66 (0.96) years Height SDS (mean (SD): ‐3.15 (0.29) Height velocity (mean (SD): 2.47 (0.48) cm/year Height velocity SDS (mean (SD): ‐3.42 (0.79) Bone age (mean (SD): 7.86 (0.52) years Hemoglobin (mean (SD): 10.22 (2.3) g/dL Ferritin (mean (SD): 1466.20 (260.82) ng/mL Plasma zinc (mean (SD): 91.82 (11.22) μg/dL FBG (mean (SD): 70.20 (9.18) mg/dL OGTT sum (mean (SD): 347.70 (39.23) mg/dL Thyroxine (mean (SD):8.3 (1.1)μg/dL TSH (mean (SD): 3.33 (3.1) mU/mL IGF‐1 (mean (SD)): 47.44 (9.96) ng/mL Control group (no GH therapy) Gender: male (n = 10), female (n = 0) Age (mean (SD): 11.17 (0.89) years Height SDS (mean (SD): ‐2.79 (0.17) Height velocity (mean (SD): 2.86 (0.39) cm/year Height velocity SDS (mean (SD): ‐3.32 (0.63) Bone age (mean (SD): 7.85 (0.61) years Hemoglobin (mean (SD): 9.50 (1.82) g/dL Ferritin (mean (SD): 1602.20 (234.15) ng/mL Plasma zinc (mean (SD): 104.10 (20.27) μg/dL FBG (mean (SD): 73.71 (11.82) mg/dL OGTT sum (mean (SD): 340.71 (31.76) mg/dL Thyroxine (mean (SD): 8.14 (2.2) μg/dL TSH (mean (SD): 3.27 (1.2) mU/mL IGF‐1 (mean (SD): 47.79 (11.43) ng/mL Inclusion criteria: homozygous β thalassemia, short stature (height below – 2 SD for age, height velocity below 25th percentile and bone age delay of more than 2 years). Exclusion criteria: biochemical decompensated clinical hypothyroidism (low thyroxine, elevated basal TSH) and glucose intolerance (fasting glucose >115 mg/dL, OGTT sum >400 mg/dL, 2 hour OGTT >140 mg/dL). Pre‐treatment: no major differences between groups with the exception of the gender distribution between both groups. Comment: it was unclear whether the participants fulfilled the diagnostic criteria of having GH deficiency, as their GH status at baseline was not available. | |
| Interventions | GH group: recombinant GH (Genotropin, Pharmacia) administered subcutaneously on a daily basis at a dose of 0.7 IU/kg per week for a duration of 12 months in addition to standard treatment Control group: standard treatment | |
| Outcomes | Height velocity (cm/year): continuous data, fully reported, measured in cm/year Height velocity SDS: continuous data, fully reported Change from baseline to final visit in height velocity SDS: continuous data Height SDS: continuous data Change from baseline to final visit in height SDS: continuous data Number of participants with adverse events (esp glucose tolerance and thyroid function): dichotomous data FBG (mg/dL): continuous data OGTT sum (mg/dL): continuous data | |
| Identification | Sponsorship source: supported in part by Pharmacia‐Upjohn and Ankara Thalassemia Society Country: Turkey Setting: paediatric department of a tertiary hospital Authors name: Ayten Arcasoy (first author) Institution: Department of Pediatrics, Divisions of Pediatric Hematology and Pediatric Endocrinology, Faculty of Medicine, Ankara University, Ankara, Turkey Email: [email protected] (Corresponding author Dr Merih Berberolu) Address: 59. Sokak 10/6 Emek, 06510 Ankara, Turkey | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization of the patients was made with closed envelopes for each patient." Judgement comment: methods of random sequence generation was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Comment: sealed envelopes were used for the concealment of allocation as the authors stated that "Randomization of the patients was made with closed envelopes". However, it was not stated if these envelopes were opaque to fully conceal the allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: although not clearly stated, blinding of participants and personnel appeared highly unlikely, as GH was administered subcutaneously. However, we considered the lack of blinding to be unlikely to affect the growth outcomes in this particular group of patients, as the growth of this group of children was not known to be readily influenced by any form of known co‐intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: it was not stated who the assessors of the growth outcomes were, and whether the assessors were blinded to the allocation status of the participants. However, we considered this as unlikely to influence the growth outcomes, which were objectively measured. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: the authors stated that all 20 participants were followed up till trial completion, although it was unclear whether all data were available for analysis for all participants at all periods of measurement. |
| Selective reporting (reporting bias) | Low risk | Comment: the main outcomes defined in the review methodology (growth response and side effects) were reported in sufficient detail. In terms of growth response, the height and height velocity measurements and SDSs were reported with means and standard deviations after the 12‐month period as defined in the methodology. The side effects which were reported such as effects on glucose metabolism and thyroid function are reasonable given the relatively short period of the trial. |
| Other bias | Unclear risk | Comment: there was a gender imbalance in the 2 groups (10 males in the control group and six boys and four girls in the intervention group) which may raise issues in the applicability of the evidence. The trial was sponsored by a pharmaceutical company but there is no clear evidence that this affect the overall risk of bias in the trial. |
FBG: fasting blood glucose
GH: growth hormone
IGF: insulin‐like growth factor
OGTT: oral glucose tolerance test
SD: standard deviation
SDS: standard deviation score
TSH: thyroid stimulating hormone
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This trial evaluated 2 different types of interventions in which the first group received L‐carnitine therapy and the second group received hormonal therapy (females received conjugated equine oestrogen/medroxyprogesterone, whereas males received long‐acting testosterone). Excluded on the basis of intervention. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral glucose tolerance test sum (mg/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1 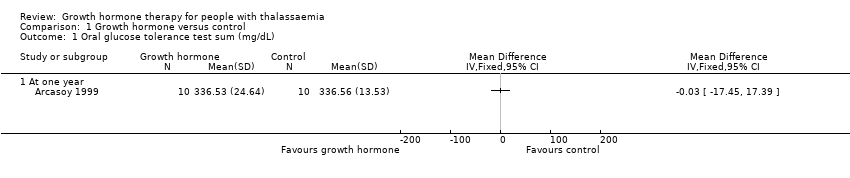 Comparison 1 Growth hormone versus control, Outcome 1 Oral glucose tolerance test sum (mg/dL). | ||||
| 1.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
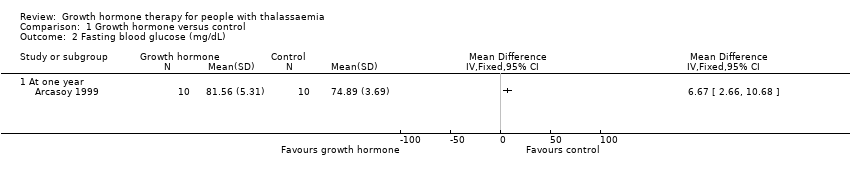
| 2 Fasting blood glucose (mg/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Growth hormone versus control, Outcome 2 Fasting blood glucose (mg/dL). | ||||
| 2.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Height SD score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Growth hormone versus control, Outcome 3 Height SD score. | ||||
| 3.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change from baseline in height SD score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Growth hormone versus control, Outcome 4 Change from baseline in height SD score. | ||||
| 4.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
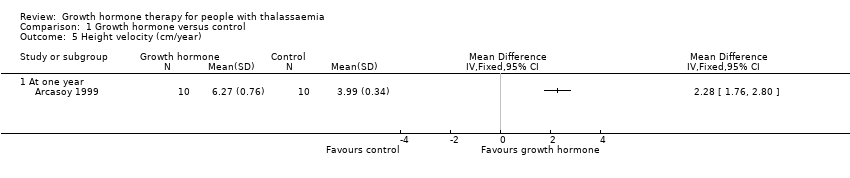
| 5 Height velocity (cm/year) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5 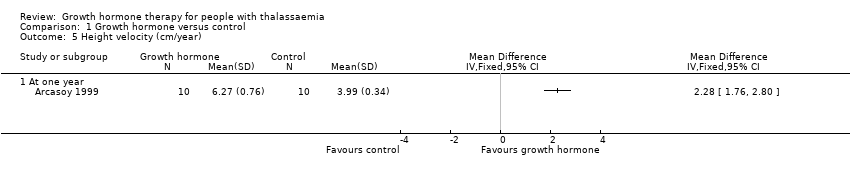 Comparison 1 Growth hormone versus control, Outcome 5 Height velocity (cm/year). | ||||
| 5.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Height velocity SD score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Growth hormone versus control, Outcome 6 Height velocity SD score. | ||||
| 6.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
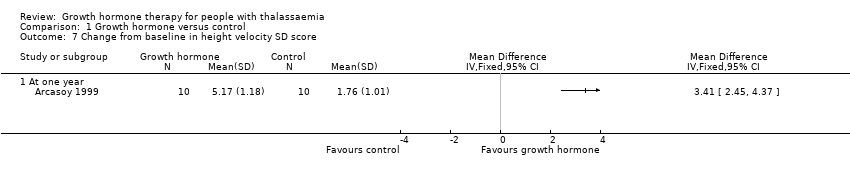
| 7 Change from baseline in height velocity SD score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7 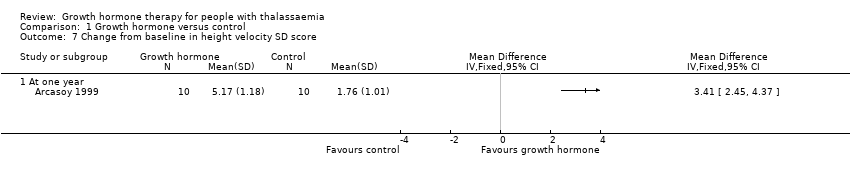 Comparison 1 Growth hormone versus control, Outcome 7 Change from baseline in height velocity SD score. | ||||
| 7.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Serum insulin‐like growth hormone (IGF‐1) (ng/mL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8 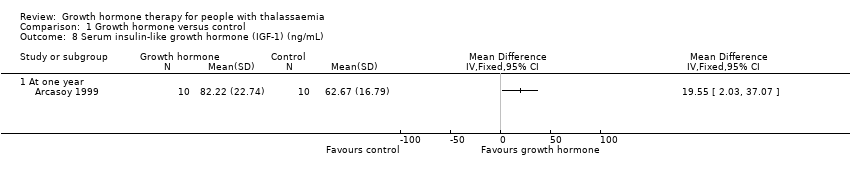 Comparison 1 Growth hormone versus control, Outcome 8 Serum insulin‐like growth hormone (IGF‐1) (ng/mL). | ||||
| 8.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Study flow diagram.

Comparison 1 Growth hormone versus control, Outcome 1 Oral glucose tolerance test sum (mg/dL).
Comparison 1 Growth hormone versus control, Outcome 2 Fasting blood glucose (mg/dL).

Comparison 1 Growth hormone versus control, Outcome 3 Height SD score.

Comparison 1 Growth hormone versus control, Outcome 4 Change from baseline in height SD score.
Comparison 1 Growth hormone versus control, Outcome 5 Height velocity (cm/year).

Comparison 1 Growth hormone versus control, Outcome 6 Height velocity SD score.
Comparison 1 Growth hormone versus control, Outcome 7 Change from baseline in height velocity SD score.

Comparison 1 Growth hormone versus control, Outcome 8 Serum insulin‐like growth hormone (IGF‐1) (ng/mL).
| Growth hormone for people with thalassaemia | ||||||
| Patient or population: people with thalassaemia (any age) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with growth hormone | |||||
| Final height and change in height | The included trial did not assess either of these outcomes. | |||||
| Adverse effects Oral glucose tolerance test (mg/dL) (at one year) | The mean oral glucose tolerance test was 336.56 mg/dL. | MD 0.03 lower (17.45 lower to 17.39 higher). | ‐ | 20 | ⊕⊕⊕⊝ | Fasting blood glucose levels in the growth hormone group were significantly higher than in the control group but both were still within the normal range. |
| Height SDS | The mean height SDS was ‐2.85. | MD 0.09 lower | ‐ | 20 | ⊕⊕⊕⊝ | |
| Change in height SDS (difference between baseline and final visit at one year) | The change in mean height SDS was ‐0.05. | MD 0.26 higher | ‐ | 20 | ⊕⊕⊕⊝ | |
| Height velocity (cm/year) | The mean height velocity was 3.99 cm/year. | MD 2.28 higher | ‐ | 20 | ⊕⊕⊕⊝ | |
| Height velocity SDS | The mean height velocity SDS was ‐1.56. | MD 3.31 higher | ‐ | 20 | ⊕⊕⊕⊝ | |
| Change in height velocity SDS (difference between baseline and final visit at one year) | The change in mean height velocity SDS was 1.76. | MD 3.41 higher | ‐ | 20 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Data contributed by a single trial with small sample and 95% CI is wide. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral glucose tolerance test sum (mg/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Fasting blood glucose (mg/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Height SD score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change from baseline in height SD score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Height velocity (cm/year) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Height velocity SD score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Change from baseline in height velocity SD score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Serum insulin‐like growth hormone (IGF‐1) (ng/mL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

